Ascidians from Rocas Atoll, northeast Brazil
- 1Graduate Program in Tropical Marine Sciences, Universidade Federal do Ceará, Fortaleza, Brazil
- 2Departament of Biological Oceanography, Instituto Oceanográfico, Universidade de São Paulo, São Paulo, Brazil
Rocas Atoll is the only one of its kind in the South Atlantic—and the first Brazilian marine biological reserve. This is the first report about the ascidians from Rocas. A total of 12 species were found, 5 of them not hitherto described: Ascidia viridina sp. nov., Didemnum rochai sp. nov., Leptoclinides crocotulus sp. nov., Polysyncraton maurizeliae sp. nov., and Trididemnum rocasensis sp. nov. One Caribbean species, Didemnum halimedae, was also discovered in the region for the first time. Further, this is the first record of Didemnum digestum in the Atlantic. The results indicate a high degree of endemism in the ascidian fauna from Rocas Atoll, where didemnids are presently the most important members.
Introduction
Ascidians are benthic marine invertebrates that are present throughout the marine environments from the intertidal to the deep sea. Currently, over 3000 species have been formally described (Lambert, 2005), but only 461 have been found in the Atlantic Ocean (Rocha et al., 2012).
Despite their importance as one of the major components of benthic communities, their presence is still ignored in many regions due to the lack of collections or specialists. In Brazil, the knowledge of ascidians has improved considerably in the past two decades, but there are still locations with barely any information about the group. The Brazilian oceanic islands are in such condition, with only two studies available for the Fernando de Noronha Archipelago, by Millar (1977) and Eston et al. (1986), which recorded only 7 species in total.
The Rocas Atoll is the only atoll in the South Atlantic Ocean, and though it is Brazil's first marine biological reserve, an inventory of ascidians from this small oceanic formation is still not available. Rocas is located in the equatorial Atlantic, 266 km off the northeastern Brazilian coast, and its relative isolation has resulted in a moderate degree of endemism, shared with the Fernando de Noronha archipelago (Floeter et al., 2001).
This is the first account of ascidians from the Rocas Atoll, resulting from expeditions aiming to provide information about this fauna.
Materials and Methods
Study Area
The Rocas Atoll is located at 03°51′00″S and 033°49′00″W (Figure 1), atop a submarine mountain chain known as the Fernando de Noronha Fracture Zone (Kikuchi and Leão, 1997). The atoll, situated 150 km west of the Fernando de Noronha Archipelago and 266 km from Natal (RN), is directly under the influence of the South Equatorial Current. It is relevant for the conservation of many marine species, including sea turtles, and it hosts the largest density of tropical seabirds in the Western Atlantic (Fischer et al., 2007). As part of the conservation unit system in Brazil, UNESCO also considers Rocas a World Heritage Site.
Field and Laboratory Work
The specimens were collected during three 30-day expeditions to the atoll between January and December 2011. Ascidians were collected manually while snorkeling during the low tide in eight tidepools (Abrolhos, Âncoras, Barretinha, Barreta falsa, Cemitério, Donzelinha, Farol II, Salãozinho) and on the reef near Cemitério Island. The collection was authorized by license No. 24071-1, which was granted by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio).
The animals were removed from the substrate with the aid of a scraper and kept in plastic bags with seawater and tricaine until properly relaxed. Tissues or parts of the colonies were preserved in 95% ethanol for DNA extraction while the specimens were fixed in 4% saline formalin. In the laboratory all specimens were transferred to 70% ethanol and analyzed following standard dissection and preparation methods (Monniot and Monniot, 1972). Spicules, when present, were analyzed through scanning electron microscopy at the Instituto de Biociências, Universidade de São Paulo and Universidade Federal do Paraná. The holotypes are deposited at the Museu de Zoologia of the Universidade de São Paulo (MZUSP), paratypes and additional material are held at the biological collection of the Instituto Oceanográfico, Universidade de São Paulo (ColBIO).
DNA Extraction and Amplification
The tissues (piece of the atrial siphon for solitary species, dissected zooids for colonials) were processed for DNA extraction using Qiagen QIAamp Micro Kit, according to the manufacturer instructions, with a 3–4 h digestion with proteinase K. Amplifications of cytochrome c oxidase subunit I gene fragments (COI) were performed with Life Technologies Amplitaq Gold 360 mastermix in a Biorad C1000 thermal cycler. Different sets of primers were used in attempts to attain good COI sequences, including LCO1490 and HCO2198 (Folmer et al., 1994), as well as Tun_forward and Tun_reverse2 (Stefaniak et al., 2009). PCR cycle conditions were: initial denaturation of 4 min at 95°C, 1 min at 94°C, 1 min at 39°C, 1.5 min at 72°C for 40 cycles, and a final extension for 10 min at 72°C.
PCR products were analyzed through agarose gel electrophoresis, purified with exonuclease (ExoSAP-IT), and sent for sequencing at Macrogen Inc. facilities in Korea. Obtained electropherograms and sequences were analyzed, paired and edited with Geneious Pro R7 software. Effective COI sequencing was obtained for three species only, as most species were rare at the reefs, and only small amounts of tissue were available. Even for species with good DNA extractions, amplification was not straightforward and most attempts were unsuccessful. All successful amplifications were attained with the use of Tun_forward/Tun_reverse2 primers.
Results
Descriptions
Order Aplousobranchia Lahille, 1887
Family Didemnidae Verrill, 1871
Genus Didemnum Savigny, 1816
Didemnum digestum Sluiter, 1909 (Figure 2)
Material examined
ColBIO-TL-53, ColBIO-TL-435, ColBIO-TL-442, ColBIO-TL-450, ColBIO-TL-451, all collected at Abrolhos tidepool, 1.5 m deep, on 20/i/2011, 8/xii/2011, and 9/xii/2011.
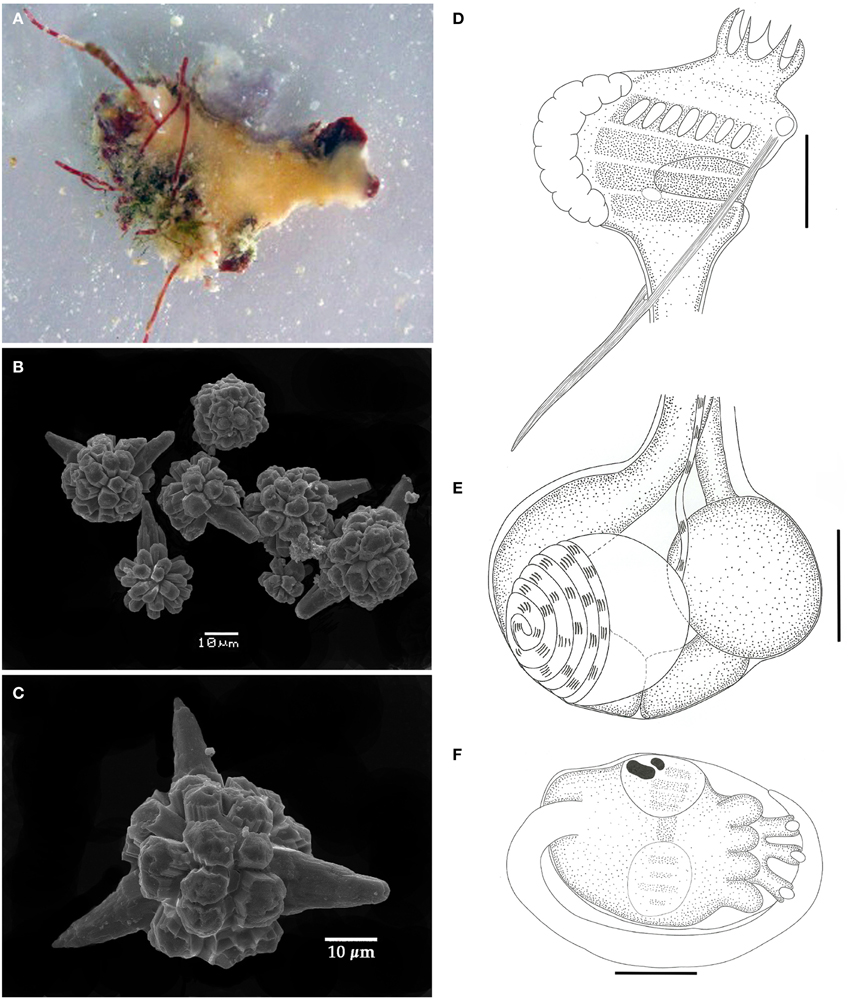
Figure 2. Didemnum digestum. (A) Photograph of the whole colony when alive. (B) Scanning electron micrograph of spicules. (C) Scanning electron micrograph of typical spicule. (D) Illustration of the thorax. (E) Illustration of the abdomen. (F) Illustration of the larva. Illustration scale bars = 125 μm.
Description
Colonies incrusting, thin and brittle, up to 2 cm in length and 0.6 mm thick, with pale orange color. Tunic surface smooth. Spicules abundant throughout the tunic, except for the thin bladder cell layer on the surface. Spicules spherical, with short and blunt rays emerging from polygonal bases. Some spicules have one to three conical rays notably longer than the others. Central portion of the spicules without the longer rays measuring around 25 μm, and on those with long rays it ranges from 30 to 60 μm.
Zooids yellowish colored, around 1 mm in length. Thorax with almost the same size of abdomen, slightly triangular, with the branchial portion narrowing down toward the esophageal neck. Branchial siphon with a short base and 6 long, pointy lobes, measuring 3–4 times the size of the rim in some zooids. Branchial sac with 4 stigmatal rows and 7 stigmata on the first half row. Atrial opening located at the third row position and exposing just a small portion of the branchial sac. Lateral organ round and small, close to de edge of the atrial opening at the level of the third row. Thorax with strong longitudinal muscles on both sides, originating at the dorsal region and passing through the atrial opening to form the muscular process just after the thorax. Esophageal neck short and straight. Stomach globular, smooth, and yellowish colored. Midgut with 2 posterior constrictions. Posterior stomach present. Gonads within the gut loop, with a single testis and spiraled vas deferens with 5–6 counterclockwise coils. Ovary with a single developed oocyte.
Larvae gemmiparous, oval-shaped, whitish, measuring over 330 μm, with 3 papillae and 4 ectodermic ampullae. Ocelus and statocyst present. Tail coiled around 3/4 of the trunk.
Remarks
Didemnum digestum has its type locality in Indonesia (Sluiter, 1909), with further records in Fiji (Kott, 1981), Moorea and Tahiti (Monniot and Monniot, 1987), and Guam (Lambert, 2003). All records are restricted to the Indo-Pacific.
The main diagnostic character for the species is spicules with one to three notably longer rays. The same type of spicule is found in Didemnum uturoa Monniot and Monniot, 1987, which in turn has two testicular follicles. Didemnum sphaericum Tokioka, 1967 also has spicules resembling those of D. digestum, especially the polygonal hollow bases of the spicule rays, but it does not have the typical longer rays (Kott, 1981). Kott (1981) described the specimens from Fiji with only 4 stigmata on the first half row, but Monniot and Monniot (1987) mentioned 7 stigmata, as observed in the specimens here described. The difference is not usual for didemnids, and the two forms might in fact be different species. The type material was not examined, but according to Kott (1981) the holotype is immature, and one of the designated types was included in the description of Didemnum multispirale Kott, 2001.
The specimens from Rocas Atoll fit into the description of D. digestum in every aspect despite the great distance from its previously known area of occurrence. In this regard, this species may well have a more widespread distribution, and be present in many tropical areas that have not been properly investigated.
Didemnum granulatum Tokioka, 1954 (Figure 3)
Material examined
ColBIO TL-49, TL-439, Abrolhos tidepool, 1.5 m, 20/i/2011; and 8/xii/2012; ColBIO TL 468, Barreta Falsa tidepool, 1.5 m, 11/xii/2011; ColBIO TL 456-461, Farol II tidepool, 1.6 m, 10/xii/2011; ColBIO TL 477, Salãozinho tidepool, 2 m, 12/xii/2011.
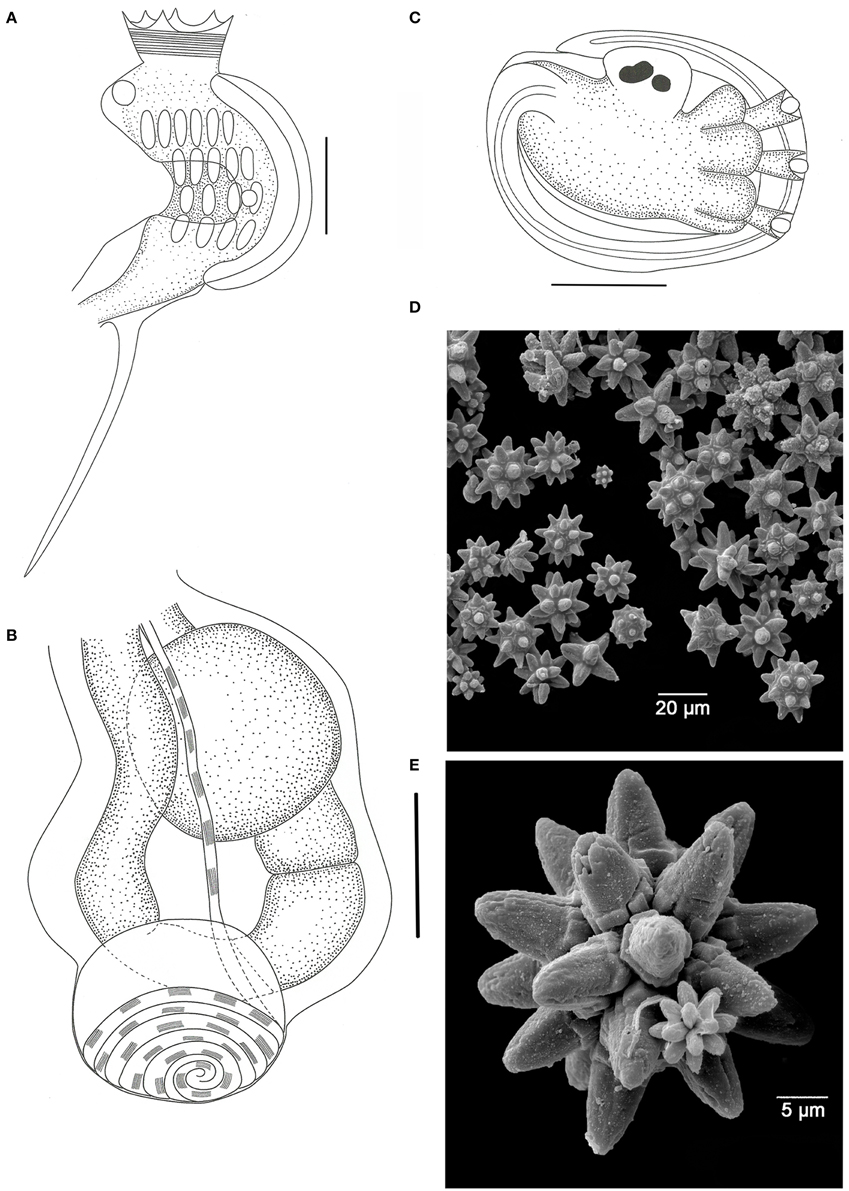
Figure 3. Didemnum granulatum. (A) Illustration of the thorax. (B) Illustration of the abdomen. (C) Illustration of the larva. (D) Scanning electron micrograph of spicules. (E) Scanning electron micrograph of a typical spicule. Illustration scale bars = 125 μm.
Description
Colonies incrusting, thin and brittle, up to 2.5 cm in length and 0.8 mm thick. Bright orange color when alive, fading to pale orange in formalin. Colony surface rough, granulated, marked by microscopic papillae uniformly distributed. Spicules densely packed throughout the tunic, of three types: the most abundant is star-shaped, 30–50 μm in diameter, 5–8 long conic rays in the same optical plane; second type also star shaped, 25–40 μm in diameter, but frequently 8 short conical rays in the same plane; third type tetrahedral, about 50 μm and rays measuring 25 μm.
Zooids pale orange, 0.6 mm in length. Thorax triangular and slightly larger than the abdomen. Branchial sac with 4 stigmatal rows and 6-5-4-4 stigmata per half row. Branchial siphon short, wide, with 6 small pointy lobes and strong circular muscles. Atrial opening wide, exposing most of the branchial sac. Lateral organ small and round, at the fourth row position. Muscular process long, larger than the abdomen, attached to the base of the thorax by two muscle bands. Esophageal neck equivalent to 1/3–1/4 of the zooid length. Stomach globular, smooth, and yellowish. Gut with 2 midgut constrictions. Gonads included in the gut loop. Single testis, with 6 counterclockwise coils in the vas deferens. Ovary with a single developed oocyte.
Remarks
Didemnum granulatum has the Tokara Islands (Japan) as type locality and was originally described as a subspecies of Didemnum moseleyi Herdman, 1886 (Tokioka, 1954). Monniot and Monniot (1994) presented the first record in the Atlantic, in the Madeleine Islands, in Senegal. One year later it was also reported in southeastern Brazil (Rocha and Monniot, 1995), and it was then detected along the country's tropical coast (Lotufo, 2002). Rocha and Kremer (2005) recorded D. granulatum on the southern coast of Brazil and considered the species as non-native in the Atlantic.
The specimens were usually found on shadowed crevices in vertical walls inside the tidepools. Indeed, the colonies are not large compared to those observed in coastal areas, which is probably due to ecological constraints on their growing pattern.
The analyzed specimens match the available descriptions (Tokioka, 1954; Monniot, 1995; Monniot and Monniot, 2001; Lotufo, 2002; Rocha et al., 2005; Lotufo and Silva, 2006), but none of the descriptions mention the presence of tetrahedral spicules, which despite being rare, were seen in all analyzed colonies.
Didemnum halimedae Monniot, 1983 (Figure 4)
Material examined
ColBIO TL 54-56, 436, 440, and 452-454, Abrolhos tidepool, 1.5 m, 20/i/2011, 8/xii/2011 and 9/xii/2011. ColBIO TL-484, Âncoras tidepool, 1.5 m, 13/xii/2011.
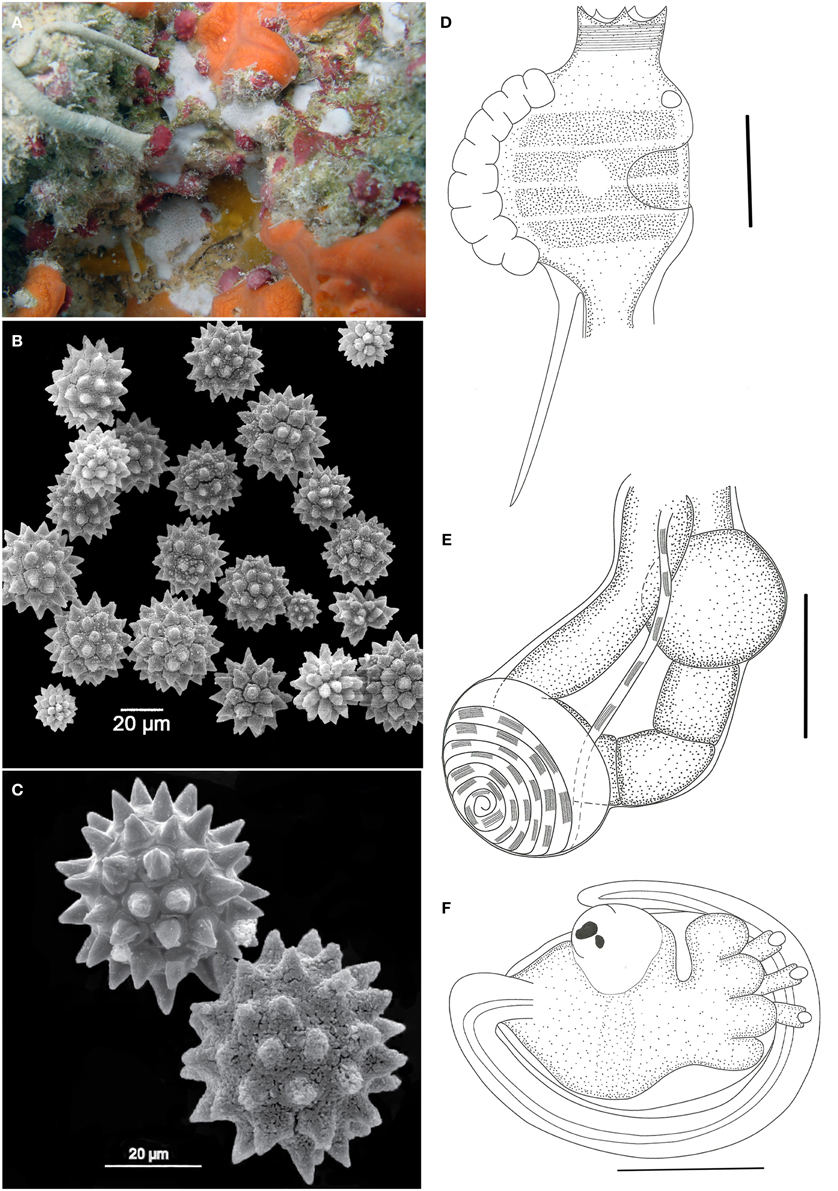
Figure 4. Didemnum halimedae. (A) Underwater photograph of the living colony. (B) Scanning electron micrograph of spicules. (C) Scanning electron micrograph of spicules in detail. (D) Illustration of a thorax. (E) Illustration of an abdomen. (F) Illustration of a larva. Illustration scale bars = 125 μm.
Description
Colonies incrusting, very thin (<1 mm) and brittle, measuring up to 1 cm. Tunic surface smooth, without visible cloacal openings. Color white, with spicules packed throughout the tunic, of 3 different types: the more common is spherical, 25–60 μm in diameter, with about 14 short conical rays in the same optical plane; star-shaped, 25–60 μm, with 10 long conical rays in the same plane; spherical, 12–35 μm in size and many cylindrical rays with rounded tips.
Zooids are whitish, measuring around 0.6 mm in length. Thorax smaller than the abdomen. Branchial siphon short and narrow, with 6 pointy lobes and strong circular muscles. Atrial opening generally small, depending on the contraction state of the zooid, and exposing only a small part of the second and third stigmata rows. Lateral organ as a round depression, located in a central position between the second and third stigmata row. Muscular process about the same size as the thorax, attached to the base of the thorax or sometimes farther on the esophageal neck.
Esophageal neck corresponding to 1/4 of the total zooid length. Stomach globular, smooth, and yellow colored. Abdomen flexed under the thorax, assuming a near horizontal position. Gut marked by two constrictions after the stomach, on the first half of the intestine. Posterior stomach present. Gonads included in the gut loop. Single testis, with a vas deferens with 6–7 counterclockwise coils. Ovary with a single developed oocyte, slightly larger than the testis.
Larvae unremarkable, oval and whitish, measuring around 320 μm, with 3 papillae and 4 large and round ectodermic ampullae. Oozooid in a more posterior position, Ocellus and statocyst present. Tail coiled around 3/4 of the body.
Remarks
Didemnum halimedae was known solely from Caribbean waters. Described by Françoise Monniot (1983a) based on specimens from Guadeloupe, the species got its name because it was frequently found growing over green calcareous algae (Halimeda) or dead coral. Goodbody (2003) recorded the species in Jamaica, attached to old shipwrecks and not associated with Halimeda. At Rocas, it was found over the same substrates used by other species, mainly crustose coralline algae. Didemnum halimedae resembles Didemnum algadescens Monniot and Monniot, 2001 from the Pacific, which is also found over Halimeda, but differs in spicule shape.
The Rocas specimens were more variable in terms of the spicule's shapes, but the most abundant type match the holotype description. The zooids of the Brazilian specimens were slightly smaller, and the muscular process was mostly attached to the base of the thorax, but this is related to the contraction state. The lateral organ of Rocas's specimens is also larger than described by Monniot (1983a). However, there were no other distinctive characters to separate it from D. halimedae. This is the first record of the species outside of Caribbean waters.
Didemnum rochai sp. nov. (Figure 5)
Nomenclatural act recorded at Zoobank: AD084BB8-1A52-457D-B6FB-F82CD6957A55
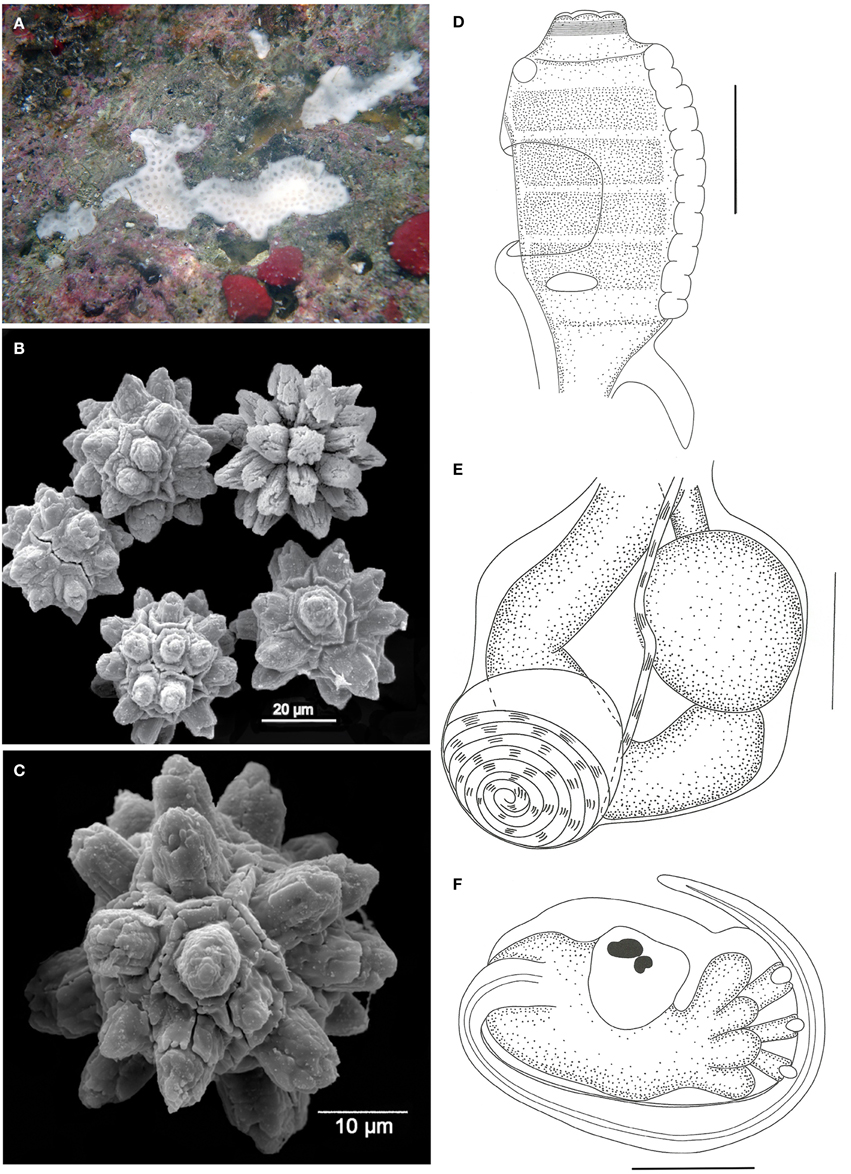
Figure 5. Didemnum rochai sp. nov. (A) Underwater photograph of a colony (white). (B) Scanning electron micrograph of spicules. (C) Scanning electron micrograph of typical spicule. (D) Illustration of a thorax. (E) Illustration of an abdomen. (F) Illustration of a larva. Illustration scale bars = 125 μm.
Material examined
Holotype: MZUSP 00089, 1 colony, Âncoras tidepool, 1.6 m, 13/xii/2011. Paratypes: MZUSP 00090-00094, Cemitério Island sandstone reefs, 1 m, 30/i/2011 and 15/xii/2011.
Etymology
The species was named after Dr. Rosana Moreira da Rocha, in recognition of her contributions to the knowledge of the group.
Description
Colonies white when alive, incrusting, thin (1.2 mm thick) and brittle, measuring up to 1 cm in length, generally growing on small crevices. Tunic surface smooth, devoid of bladder cells, branchial siphon positions marked by slight elevations. One single inconspicuous cloacal opening on each colony. Cloacal channels wide, at the thoracic level, right under a thin superficial tunic layer. Zooids positioned vertically. Spicules abundant in the whole tunic, of two different types: the most common measures 35–45 μm in diameter, star-shaped, with 10–13 thick rays with rounded tips per optical section; second type spherical, 25–50 μm in diameter, with 10 short conical rays on the same plane.
Zooids whitish, about 0.8 mm long, embedded vertically in the colony. Thorax long and barrel shaped, measuring about 300 μm in length. Branchial sac with 4 stigmatal rows, with 9–10 stigmata per half row. Branchial siphon short, with conspicuous circular muscles and 6 small rounded lobes. Atrial opening at the 2nd and 3rd row position, exposing part of the branchial sac. Lateral organ small and flat, at the 4th row position, close to the atrial opening. Muscular process very short and strong, attached to the base of the thorax or first half of the esophageal neck.
Esophageal neck about 1/4 of the total zooid length. Stomach rounded, smooth, and yellow. Midgut with one constriction right after the stomach, and a second one at the distal portion of the abdomen. Gonads included in the gut loop, with a single large and spherical testis and a slightly smaller oocyte. A small mass of cells is usually observed over the testis, close to the ovary. Vas deferens spiraled with 6–7 counterclockwise coils.
Larvae oval and whitish, with 360–375 μm in length, with 3 papillae and 4 pairs of large and rounded ectodermal ampullae, incubated in the basal portion of the colony. Ocellus and statocyst present in the mid portion of the trunk. Tail coiled around 3/4 of the larvae.
Remarks
Overall, the analyzed specimens resemble the description of Didemnum speciosum (Herdman, 1886) in terms of general colony aspect and structure (Rocha and Monniot, 1995; Lotufo, 2002) but differ in the spicules' size and shape. (Millar's, (1977) description of D. speciosum may well match the present species, but the author was certainly mixing different species into one description, as later corrected by Rocha and Monniot (1995). Didemnum ahu Monniot and Monniot, 1987 is also similar to the present species in many aspects, especially zooid morphology. However, specimens from New Caledonia and French Polynesia have spicules of similar size but with needle-stack rays (Monniot and Monniot, 1987; Monniot, 1995). Specimens from Palau, in particular, have spicules measuring up to 500 μm, with short and thick rays (Monniot and Monniot, 2008). Also, in descriptions from both Palau and French Polynesia, the cloacal channels are seen on the colony surface, a character not present in D. rochai. Further, Brazilian D. ahu specimens are yellowish or cream colored, with spicules restricted to the superficial tunic layer (Rocha and Monniot, 1995; Lotufo, 2002).
The species Didemnum algasedens Monniot and Monniot, 2001 from the West Pacific is also similar to D. rochai in terms of the colony structure, spicules, and a few aspects of the zooid morphology, such as gonads, but it has long pointy lobes around the branchial siphon, a long muscular process, a long esophageal neck, and pigmented larvae.
Although most features are commonly found in other white Didemnum species, the combination of characters does not allow the identification as any known species.
Genus Leptoclinides Bjerkan, 1905
Leptoclinides crocotulus sp. nov. (Figure 6)
Nomenclatural act recorded at Zoobank: 3A06FEB4-E0AC-4294-B0BB-E68CB4E9E4F1
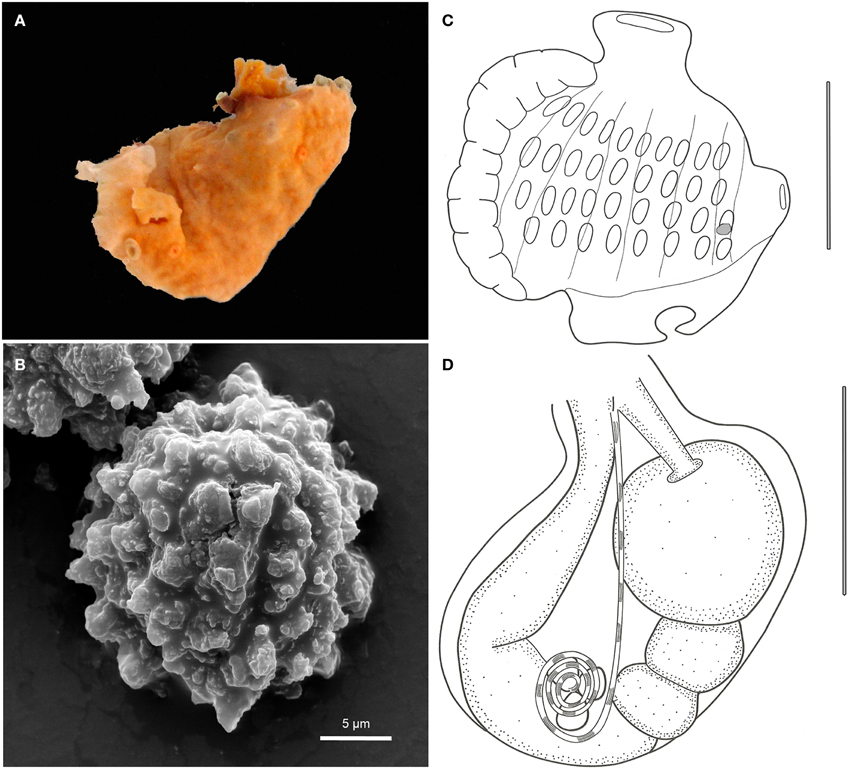
Figure 6. Leptoclinides crocotulus sp. nov. (A) Photograph of the colony right after collection. (B) Scanning electron micrograph of a typical spicule. (C) Illustration of a thorax. (D) Illustration of an abdomen. Illustration scale bars = 250 μm.
Material examined
Holotype: MZUSP 00104, 1 colony, Salãozinho tidepool, 21/i/2011, 3 m.
Etymology
The name refers to the bright orange color of the colony.
Description
Colonies bright orange colored, incrusting, moderatelly thick (1.6 mm), up to 1.2 cm in length. Tunic surface smooth, with a bladder cell layer. Colony consistency soft, with zooids not arranged in any particular pattern. Cloacal openings small, three on the type specimen. Cloacal channels narrow, in thoracic position, sometimes reaching spaces under the zooids. Spicules small and spherical, concentrated on the superficial layer of the tunic, sparsely distributed on the interior of the colony, measuring around 12 μm, with numerous irregular, thin and short conic rays. Spicules form also a thin layer on the underside of the colony.
Zooids yellowish, up to 1.0 mm in length. Branchial sac with 10-9-9-8 stigmata per half row. Branchial siphon small, narrow, and devoid of conspicuous lobes. Atrial opening small and tubular, with smooth rim, located at the 2nd and 3rd stigmata row level and directed dorsally. Lateral organ very small, located at the base of the atrial siphon. Thorax with strong longitudinal muscle bands. Muscular process absent.
Esophageal neck short. Stomach globular, smooth, and yellow. Midgut with two constrictions on the proximal third. Posterior stomach present. Gonads included in the gut loop, with 3 testicular follicles and a spiraled vas deferens with 2–3 counterclockwise coils. Oocytes or larvae were not present.
Remarks
The genus has few species in the Atlantic: Leptoclinides brasiliensis Michaelsen, 1923, Leptoclinides faeroensis Bjerkan, 1905, Leptoclinides latus Monniot, 1983, and Leptoclinides torosus Monniot, 1983 (Rocha et al., 2012). Among them, L. latus is the most similar to the present species, originally described from specimens from Guadeloupe, with a single record for southern Brazil (Rocha et al., 2005). However, L. latus zooids usually have a bifid atrial languet as well as a pyloric gland around the gut on the posterior side of the stomach (Monniot, 1983a), characters not seen in Leptoclinides crocotulus.
Leptoclinides robiginis Monniot, 1989, with the type locality of New Caledonia, also presents spicules concentrated on the superficial layer of the colony and a vas deferens with 2–3 coils, but differs in spicule size, color pattern, branchial siphon lobes, and a bifid atrial languet, as in L. latus (Monniot, 1983a, 1989).
Therefore, Leptoclinides crocotulus does not fit into any available description.
Genus Polysyncraton Nott, 1892
Polysyncraton maurizeliae sp. nov. (Figure 7)
Nomenclatural act recorded at Zoobank: 5B313C77-E2CB-4B57-86F2-F59ED2900A9
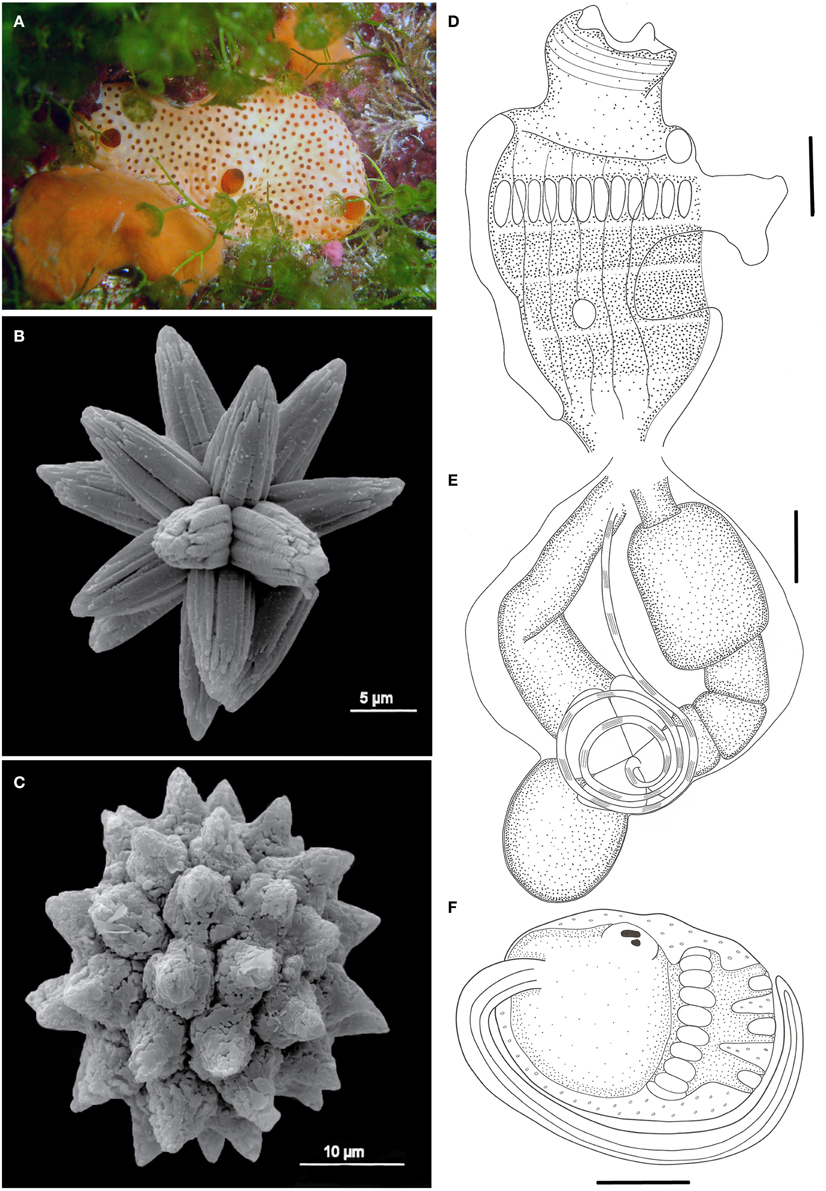
Figure 7. Polysyncraton maurizeliae sp. nov. (A) Underwater photograph of a colony. (B) Scanning electron micrograph of the second spicule type. (C) Scanning electron micrograph of the most common spicule type. (D) Illustration of a thorax. (E) Illustration of an abdomen. (F) Illustration of a larva. Illustration scale bars = 125 μm.
GenBank COI accession number: KR604727
Material examined
Holotype: MZUSP 00095, Âncoras tidepool, 1.6 m, 13/xii/2011; Paratypes: MZUSP 00099-00101, Barreta Falsa tidepool, 1.6 m, 11/xii/2011; MZUSP 00103, Salãozinho tidepool, 3 m, 21/i/2011.
Etymology
The species is named after Maurizélia de Brito Silva due to her tireless work managing and protecting the Rocas Biological Reserve.
Description
Colonies thin (~1.4 mm thick), small, and incrusting, up to 1.0 cm in length. When alive, tunic orange colored on the surface and bright red inside. Tunic surface smooth when the colony is turgid, becoming wrinkled when removed from substrate. Two or three cloacal openings in each colony. Common cloacal cavity short and restricted to thoracic level, but occasionally also at abdominal level. Spicules densely packed on the superficial layer, becoming rare inside the colony. Spicules small, 10–20 μm, of two types: most common type spherical, with over 10 small conic rays on the same plane (Figure 7C); second type star-shaped, with 7 long conic rays per optical section (Figure 7B).
Zooids yellowish, measuring around 1.5 mm. Thorax and abdomen of equal sizes. Branchial sac with 4 stigmatal rows and 12 stigmata on the first half row. Branchial siphon wide, about 1/3 the size of the branchial sac, with 6 small sharp lobes and conspicuous circular musculature. Atrial opening wide, at the middle of the thorax. Atrial languet single, flap-like, absent in most zooids. Lateral organ very small and circular, positioned at the third row level. Thorax with about 5 longitudinal muscle bands, equally spaced. Muscular process absent.
Esophageal neck short, less than 1/3 the total zooid length. Stomach globular, smooth-walled, yellow. Midgut with constrictions defining a posterior stomach. Gonads inside the gut loop, with 3–4 testicular follicles and a vas deferens with 3 counterclockwise coils. Ovary with a single developed oocyte.
Larvae oval-shaped, with few granular cells present on the external epithelium, yellowish, 860 μm in length, with 3 papillae and 27 rounded ectodermal ampullae disposed as a crown. Statocyst and ocellus present at the half of the trunk. Tail coiled around 1/2 the trunk.
Remarks
The present species bears no resemblance with any other Polysyncraton recorded from Brazilian waters. The most common species of this genus in Brazil is Polysyncraton aff. amethysteum (Lotufo, 2002; Dias et al., 2012), which has a different color pattern, spicules, and larvae. However, other Atlantic species seem more related to the one described herein, such as Polysynrcaton bilobatum Lafargue, 1968 regarding the color and spicule shape, and Polysyncraton reedi Monniot and Monniot, 1994, which is similar in terms of the testis structure, but has a wider variation interval in the number of follicles (3–6) and coils in the vas deferens (2–3).
The most noticeable character of the colony is its wrinkled aspect after removal from the substrate, a feature also present in Polysyncraton rugosum Monniot, 1993, which also has a similar color and does not have a muscular process. The differences are in the distribution of spicules in the tunic and the structure of the cloacal openings, with dark edges devoid of spicules. In addition, the size of the larvae (1.1 mm) is also a character that clearly allows the distinction (Monniot, 1993).
Although the wrinkled aspect of the colony is also present in other species, such as Polysyncraton asterix Monniot, 1974 and Polysyncraton pseudorugosum Monniot, 1993, there are important differences related to the size and shape of the spicules and the presence of a muscular process (Monniot, 1974a, 1993).
Genus Trididemnum Della Valle, 1881
Trididemnum maragogi Rocha, 2002 (Figure 8)
GenBank COI accession number: KR604728
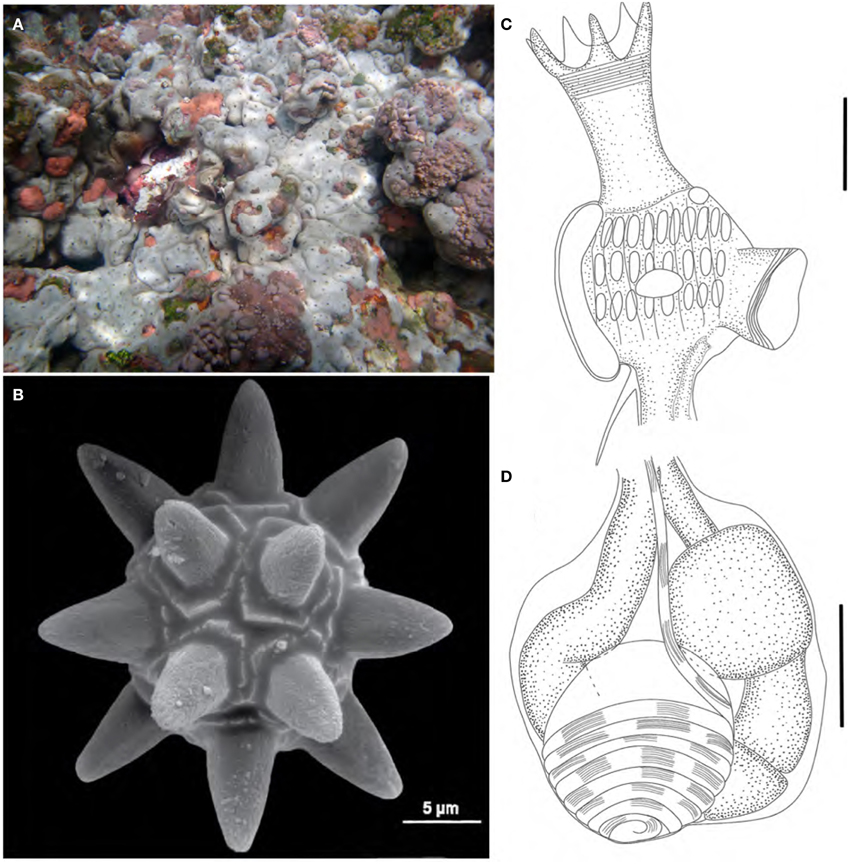
Figure 8. Trididemnum maragogi. (A) Underwater photograph of colonies clustered together. (B) Scanning electron micrograph of a typical spicule. (C) Illustration of a thorax. (D) Illustration of an abdomen. Illustration scale bars = 125 μm.
Material examined
ColBIO TL 52, 437, 438, 447-449, Abrolhos tidepool, 1.6 m, 20/i/2011, 8/xii/2011, and 9/xii/2011; ColBIo TL 514, Cemitério tidepool, 1 m, 11/xi/2011; ColBIO TL 47-48, Donzelinha tidepool, 1 m, 18/i/2011; ColBIO TL 58-60, 473-476, Salãozinho tidepool, 4 m, 21/i/2011 and 12/xii/2011.
Description
Colonies easily removed from substrate, incrusting, up to 2.5 cm in length and 5 mm thick. Color varying from green-brown and reddish-brown on shaded areas to gray-white on light exposed spots. Colonies have rounded edges and grow clustered together, giving them a jigsaw aspect. Five to seven cloacal openings on each colony. Tunic firm and rigid, somewhat brittle, with spicules packed in the whole extension, except for a thin superficial layer. Spicules large, sizes varying from 30 to 75 μm, but most are 50 μm, with around 9 short conical rays on the same optical plane.
Zooids white, 1.0 mm long. Thorax larger than abdomen. Branchial sac with 9–11 stigmata on the first two rows and 6–8 in the third and last row. Branchial siphon long, about the size of the branchial sac, with 6 long and sharp lobes, frequently pigmented with a dark squamous epithelium. Atrial opening small and tubular, about 1/2–1/3 the size of the branchial siphon, inserted at the second stigmata row level. Few zooids may present a wider atrial opening. Thorax with 7 longitudinal muscle bands, and siphons with marked circular muscles and thin longitudinal muscles. Lateral organ large and circular, positioned at the second or third row level. Muscular process short, inserted at the base of the thorax.
Esophageal neck about 1/3 the total zooid length. Stomach small, globular, smooth and yellowish. Midgut with 2 constrictions. Gonads inside the gut loop. Single testis large, with 7–8 counterclockwise coils in the vas deferens. A single oocyte developed on each mature zooid.
Larvae large, measuring 1.0 mm, rounded, with 3 papillae and 11 pairs of ampullae.
Remarks
There are few Trididemnum species recorded from the Brazilian shores: Trididemnum maragogi, Trididemnum orbiculatum (Van Name, 1902), and Trididemnum solidum Van Name, 1902; (Van Name, 1945; Rocha et al., 2012).
Trididemnum maragogi was described based on specimens from Alagoas (Rocha, 2002), and this is the only record for Brazil. The species was also registered in Panama (Rocha et al., 2005). It is strikingly similar to T. solidum, but the distinctive character pointed out by Rocha (2002) regarding the size of the branchial siphon, number of stigmata per row, and vas deferens coils were also noticed in the animals from Rocas. However, it seems reasonable to investigate the validity of this species with an analysis of a greater number of colonies from different locations, along with a genetic study to determine whether the differences between these two species simply reflect intraspecific variation.
Trididemnum rocasensis sp. nov. (Figure 9)
Nomenclatural act recorded at Zoobank: 35A2A8F2-E9D2-4E7B-A78F-287B5F8719B5
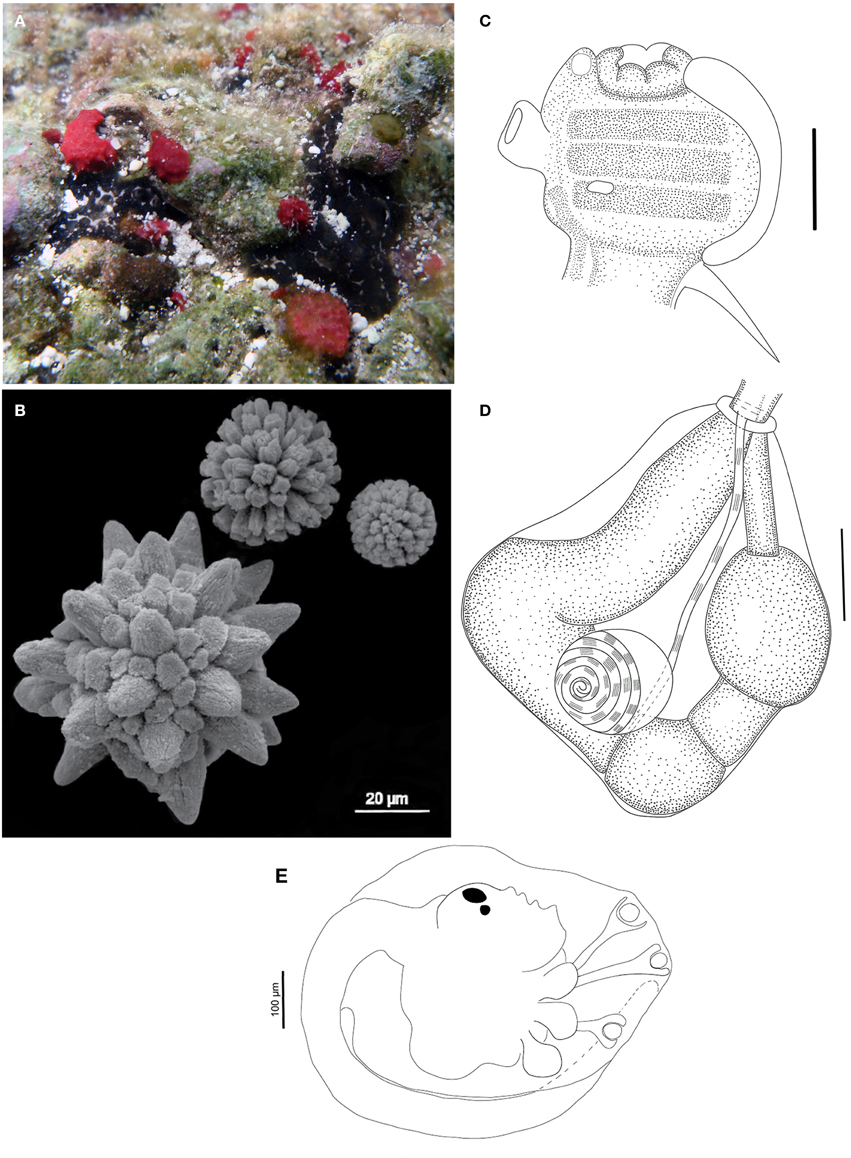
Figure 9. Trididemnum rocasensis sp. nov. (A) Underwater photograph of a colony (black). (B) Scanning electron micrograph of spicules. (C) Illustration of a thorax. (D) Illustration of an abdomen. (E) Illustration of a larva. Illustration scale bars (C,D) = 125 μm; (E) = 100 μm.
Material examined
Holotype: MZUSP 00105, Cemitério Island sandstone reefs, 1 m, 10/xii/2011. Paratypes: MZUSP 00106, Abrolhos tidepool, 1.6 m, 9/xii/2011; MZUSP 00115, Barretinha tidepool, 1.5 m, 23/i/2011; MZUSP 00107-00112, Farol II tidepool, 1 m, 10/xii/2011; MZUSP 00113-00114, Cemitério Island sandstone reefs, 1 m, 15/xii/2011.
Etymology
The species received the epithet from its type locality.
Description
Colonies small and incrusting, up to 2.7 cm in length and 1.5 mm thick. Color black or dark gray, with white spots marking the spicules around the cloacal openings. Tunic fragile and soft, with spherical or elongated brown pigmented cells, mainly on its surface and lining the common cloacal cavity. Cloacal openings large, 1–3 per colony. Common cloacal cavity at the thoracic level, sometimes extending into a posterior abdominal cavity. Spicules absent on the tunic superficial layer, except around the cloacal openings, and abundant on the middle layers, mainly lining the common cloacal cavity, becoming scarce at the basal portion. Spicules of three different types: the most common larger and spherical, 75 μm and short conical rays; another 50 μm and cylindrical rays; last type smaller, with 25 μm and long, thin rays.
Zooids measuring around 1.0 mm, covered with squamous epithelial cells giving it a green-brown color. Thorax slightly smaller than the abdomen. Branchial sac with three stigmata rows. Branchial siphon short, large, and pigmented, generally with 6 lobes, usually rounded, but a few zooids have more pointed ones. Atrial opening tubular, small, devoid of squamous cells, and slightly longer than the branchial siphon, with a few zooids presenting a wider opening. Muscular process strong, about the size of the thorax, formed by 7 converging muscle bands.
Esophageal neck short and wide, typically with a muscular ring in the transition to the abdomen. Stomach rounded, smooth and yellowish. Midgut with two constrictions and posterior stomach present. Gonads with a single testis and 6–7 counterclockwise coils in the vas deferens; a single well-developed oocyte, sometimes with a second smaller oocyte in development.
Larvae oval-shaped, white, 700 μm long, with 3 long stalked papillae and 3 pairs of drop shaped ectodermal ampullae. Statocyst and ocellus present at the limit of the posterior half of the trunk. Tail coiled around 3/4 of the larval trunk.
Remarks
Trididemnum rocasensis sp. nov. is the most widespread and abundant species in Rocas' shallow waters, where it is usually found in exposed spots on the reef as well as in small crevices inside tidepools. The species resembles Trididemnum banneri Eldredge, 1966 in colony color, spicule size, and overall larvae morphology (Eldredge, 1966). However, T. banneri larvae may present 3 or 4 pairs of ampullae, and the lobed atrial opening and larger number of coils in the vas deferens also distinguish this species from T. rocasensis (Monniot and Monniot, 1987; Monniot, 1990/1991).
Trididemnum areolatum Herdman, 1906, Trididemnum cerebriforme Hartmeyer, 1913, and Trididemnum hians Monniot, 1983, among many others, also have dark pigmented cells in the tunic, but differ in the spicule size or shape, endostyle pigmentation, number of coils in the vas deferens, and larvae morphology (Kott, 1981, 2001; Monniot, 1983a).
Trididemnum hians is also similar to T. rocasensis sp. nov. in colony pigmentation and zooid structure, but it has a larger number of ampullae in the larvae (5 pairs) and different spicules.
Family Euherdmaniidae Ritter, 1904 (sensu Kott, 1992)
Genus Euherdmania Ritter, 1904
Euherdmania sp. (Figure 10)
Material examined
ColBIO TL 487, Barreta Falsa tidepool, 1.5 m, 14/ xii/2011.
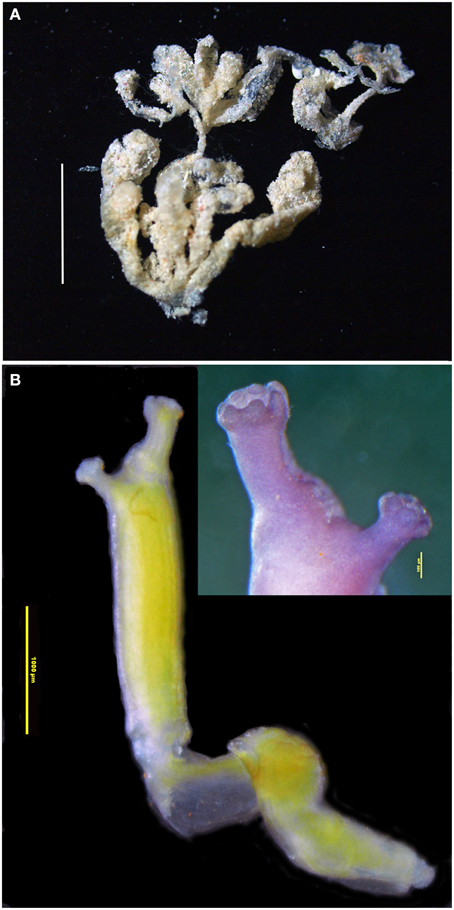
Figure 10. Euherdmania sp. (A) Photograph of the colony, scale bar = 1 cm. (B) Microphotograph of a zooid (scale bar = 1 mm), in the detail: branquial and atrial siphons (scale bar = 100 μm).
Description
Colony small, about 2 cm, formed by elongated finger-like projections, ramified and united at the base. Each projection measuring 0.5–1.5 cm long and about 1.5 mm in diameter, containing a single zooid. Tunic transparent, thin, and delicate, with a small amount of sand incrusted.
Zooids opaque, contracted, yellowish, size varying between 5 and 10 mm long. Branchial siphon slightly larger than the atrial, with 6 rounded lobes, one larger than the other 5, at the more dorsal position. Sometimes the larger dorsal lobe is divided in two, making 7 branchial lobes in total. Atrial siphon in lateral position, with 6 rounded lobes. Thorax long, branchial sac with 16–20 stigmata rows. Longitudinal muscles strong and evident, forming bands along the zooid. Dorsal tubercle protruding, with a vertical slit opening. Oral tentacles numerous, of variable sizes, generally long and coiled. Abdomen and post-abdomen together of the same size of the thorax or smaller. Esophagus long, connected to a cylindrical stomach with 12 internal folds. Post-abdomen in continuation of the abdomen, carrying about 8 male follicles. Ovaries not seen in the zooids. Four to five larvae arranged in a line parallel to the gut.
Remarks
This species is very similar to Euherdmania fasciculata Monniot, 1983, originally described from Guadeloupe (Monniot, 1983b). There are differences in the zooid size, especially in the post-abdomen, which is smaller in the Rocas specimen, and also in the number of folds in the stomach, which is 10 in the original description. The post-abdomen size usually varies considerably depending on the zooid development and contraction state. As only a single small colony was retrieved from Rocas, we were unable to ascertain the species.
This specimen is also very similar to a few other species from this genus recorded in the tropical Atlantic. Euherdmania morgani Millar and Goodbody, 1974, found in Jamaica, has a different colony structure and a larger number of stigmata rows. Euherdmania areolata Millar, 1978, reported on the Guyana shelf, is very similar to E. fasciculata, differing mainly by the areolations in the stomach wall.
Order Phlebobranchia Lahille, 1887
Family Ascidiidae Adams, 1858
Genus Ascidia Linnaeus, 1767
Ascidia viridina sp. nov. (Figure 11)
Nomenclatural act recorded at Zoobank: CE62C50C-CC98-4C5D-AB94-80C18CD9FD32
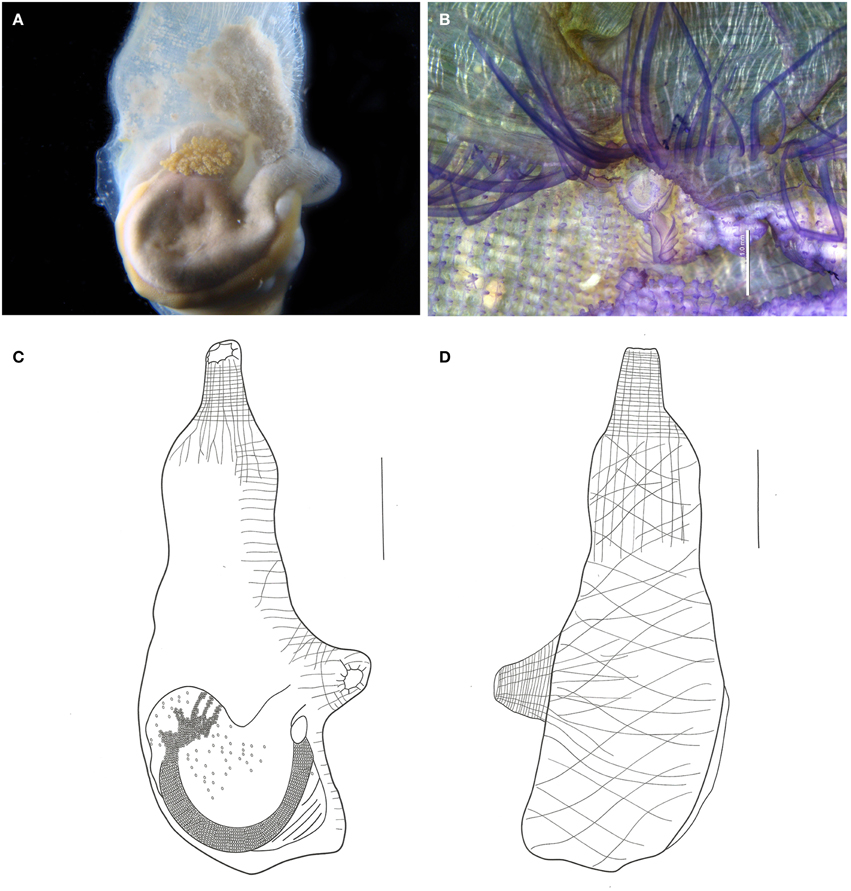
Figure 11. Ascidia viridina sp. nov. (A) Photograph of the left side of the animal (tunic removed), showing the gut and gonads. (B) Micrograph of the anterior portion of the pharynx, showing tentacles and dorsal tubercle, scale bar = 1 mm. (C) Illustration of the whole individual, left side (tunic removed). (D) Illustration of the whole individual, right side (tunic removed). Illustration scale bars = 1 cm.
GenBank COI accession number: KR604726
Material examined
Holotype: MZUSP 00116, Âncoras tidepool, 1.6 m, 13/xii/2011. Paratypes: MZUSP 00119, Abrolhos tidepool, 1.5 m, 20/i/2011; MZUSP 00117-00118, 00120-00121, Barreta Falsa tidepool, 1.5 m, 14/xii/2011 and 11/xii/2011.
Etymology
The name comes from the greenish color of the living animal.
Description
When alive, the animals are gray, and have a greenish tinct. The tunic is translucid, yellowish, wrinkled, and of firm consistency, with many dark vessels visible, especially around the siphons.
Body elongated, 5 cm long. Body wall opaque with brown pigment, especially at the siphons. Many strong circular and longitudinal muscle fibers along the siphons. Right side muscles form a loose network of oblique fibers. Left side muscles have longitudinal fibers departing from the branchial siphon but not reaching the primary gut loop. Dorsal fibers short, regularly spaced, and parallel to each other.
Both siphons with 10 indentated lobes, with dark ocelli between them. Small rounded papillae lining the prepharyngeal area. Prepharyngeal groove double, with many regularly spaced minute filiform projections, including around the dorsal tubercle. Many papillae present in the 2.0 mm band between the prepharyngeal groove and the oral tentacles. Oral tentacles simple, from 60 to 76 in number, of three different orders. Longer tentacles 3.0–3.5 mm long, Intermediate 2.0–2.5 mm long and shorter 1.2–1.5 mm. Dorsal tubercle U-shaped. Dorsal lamina double in the first 1.5–2.0 mm, then becoming a single blade, folded to the left, edge almost smooth with minute indentations following the connective tissue at the transverse vessels level. Branchial sac with 46–60 longitudinal vessels on the right side and 46–53 on the left. Approximately 100 transversal vessels and 4–6 stigmata per mesh. Papillae simple at the vessel crossings, intermediary papillae absent.
Digestive tract occupying the posterior half of the left side of the body. Stomach elongated, with about 10 internal longitudinal pleats. Rectum with a sac-like dilatation. Anus bilobed, with smooth rim. Ovary lobed and restricted to the primary gut loop. Testis with numerous whitish follicles ramified over the gut, but more contained in the secondary gut loop.
Remarks
According to Bonnet and Rocha (2011) Ascidia interrupta Heller, 1878 and Ascidia panamensis Bonnet and Rocha, 2011 are the only Ascidia species in the Atlantic with dark vessels around the siphons. In addition to the dark vessels, A. interrupta is also similar to A. viridina in terms of the muscles, papillae between the tentacles and the ciliated ring, number of tentacles, dilatation of the terminal portion of the intestine, and branchial structure. Despite the similarities, A. interrupta typically has rounded projections on the tunic, the main papillae are trilobed, and the dorsal lamina has small papillae on the right side, near the esophagus (Bonnet and Rocha, 2011).
The type material of A. panamensis was examined. The holotype is a single specimen without pigments in the tunic, but with a dark pigmentation in the whole oral siphon, stopping at the tentacles, which are also dark brown. It may be distinguished from the present species also by the smooth edge of the siphons' lobes, the area between rings without papillae, and because the main papillae from the brachial sac are bilobed. The paratypes designated along with the holoype were not dissected, and certainly were not used in the original description. Although similar in many aspects to the two mentioned species, the differences are based on important taxonomic characters.
Ascidia sp. (Figure 12)
Material examined
ColBIO TL 1076, Cemitério Island sandstone reef, 1 m, 16/i/2011.
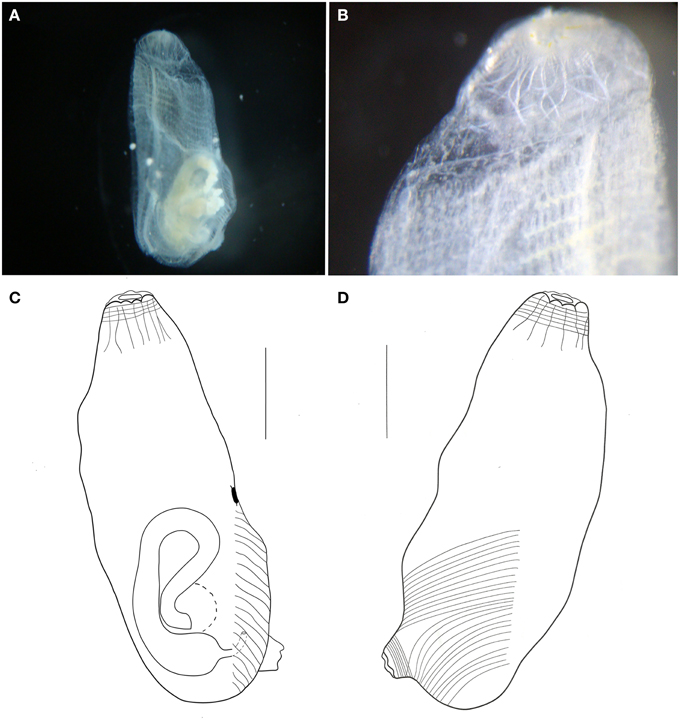
Figure 12. Ascidia sp. (A) Photograph of the left side of the animal (tunic removed). (B) Photograph of the branchial siphon and anterior portion of the branchial sac. (C) Illustration of the whole individual, left side (tunic removed). (D) Illustration of the whole individual, right side (tunic removed). Illustration scale bars = 1 mm.
Description
Individual small, about 3.8 mm long, with a thin transparent tunic. Body wall also transparent, with few longitudinal muscle fibers on the right side, running from the branchial siphon. Transversal muscle fibers starting at the atrial siphon and reaching half of the body. Muscles on the left side almost absent, with few short fibers disposed perpendicularly to the dorsal margin, but present only by the gut. Circular muscle fibers visible in both siphons.
Branchial siphon short, with 8 lobes. Atrial siphon also short, with 6 lobes, positioned at the posterior region. Prepharyngeal groove simple, without papillae in the whole prepharyngeal area. Oral tentacles simple, long, and thin, 21 in total, of 3 orders. Dorsal tubercle small and rounded, 0.3 mm from the tentacles ring, and 1.2 mm from the neural gland. Dorsal lamina smooth-edged. Branchial sac with 18 longitudinal vessels on the right side, 15 on the left, and 27 transverse vessels and 2 stigmata per mesh. Primary papillae simple, but intermediary papillae and parastigmatic vessels absent. Numerous renal vesicles over the stomach and ascending portion of the gut. Hindgut dilated. Immature specimen.
Remarks
The present species resembles Ascidia molguloides Monniot, 1974 in regard to the minute size and body muscles as well as the number of oral tentacles (25). The number of longitudinal vessels on the branchial sac, on the other hand, if far greater in A. molguloides (27–30 on the left side, 29–33 on the right side). There are also differences in the gut loop, which is wider in the North Atlantic species, as well as the tunic completely covered by sand (Monniot, 1974b). All these differences point to a new species, but as the single specimen was immature, we opted not to name it.
Order Stolidobranchia Lahille, 1887
Family Styelidae Sluiter, 1895
Genus Eusynstyela Michaelsen, 1904
Eusynstyela tincta (Van Name, 1902) (Figure 13)
Material examined
ColBIO TL 434, Abrolhos tidepool, 1.6 m, 8/xii/2011; ColBIO TL 43, 68, Cemitério Island sandstone reefs, 1 m, 16/i/2011 and 30/i/2011.
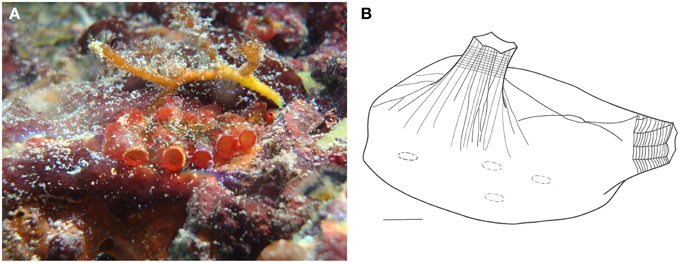
Figure 13. Eusysntyela tincta. (A) Underwater photograph of a colony. (B) Illustration of a zooid, scale bar = 1 mm.
Description
Colonies small, up to 3 cm long, with few zooids, bright red color when alive. Zooids clustered irregularly, more or less individualized, but covered by a common tunic. Tunic firm, rough, and resistant.
Zooids 1 cm long when removed from tunic, dorsoventrally flattened. Body wall yellowish, translucid, with endocarps present. Both siphons smooth-edged and of equal sizes. Atrial siphon with pronounced longitudinal muscles extending to half the body length. Branchial siphon vellum with grooves, giving it a wrinkled aspect. Branchial sac with 4-folds on each side. Oral tentacles simple, filiform, around 30 in total, of 3 different size orders. Dorsal tubercle oval-shaped. Dorsal lamina smooth-edged. Branchial sac with over 62 longitudinal vessels, about 30 on the left side and 32 on the right side. Approximately 20 transverse vessels, and 10–12 stigmata per mesh. Stomach large, orange, barrel-shaped, with about 13-folds and a thumb-shaped cecum curved toward the intestine. Gonads at the ventral region, but strongly attached to the tunic. Gonads composed of 2 oval testicular lobes, surrounded by numerous round oocytes.
Remarks
This is an abundant species in Rocas and is found as individual zooids or small clusters on the underside of loose stones around the reef at Cemitério Island.
The species is widely distributed along the Atlantic, occurring from Florida to southern Brazil (Rocha et al., 2012). The records are concentrated in the Caribbean region, where it seems to be very common. It was found in Belize (Goodbody, 2000), Bermuda (Berril, 1932; Monniot, 1972), Cuba (Hernández-Zanuy and Carballo, 2001), Curaçao (Goodbody, 1984), Guadeloupe (Monniot, 1983c; Monniot and Monniot, 1985), Jamaica (Goodbody, 2003), and Panama (Rocha et al., 2012). The species was also recorded in Senegal, West Africa (Pérès, 1951) and Inhaca Island, Mozambique, in the Indian Ocean (Millar, 1961).
Discussion
The diversity of ascidians in Rocas Atoll may be considered low, when compared with other tropical islands throughout the Atlantic. For instance, Guadeloupe and Pelican Cays have substantially larger species richness, with 93 and 70 species, respectively (Monniot and Monniot, 1985; Goodbody, 2000). A more recent inventory of La Restinga National Park, Isla Margarita (Venezuela) revealed a total of 29 species (Rocha et al., 2010), still more than twice the number of species from Rocas. It is worth mentioning that the atoll is very small at only 7.1 km2, and it does not provide a diversity of habitats, only a typical reef environment. Also, the outer portion of the atoll was not surveyed due to legal and logistical restrictions, but it comprises typical underwater reef areas. Other insular areas may host more species not only for historical reasons but also because they are larger and provide different kinds of habitats that may suit a larger set of species.
For Rocas Atoll, the nearest insular area in the equatorial Atlantic is Fernando de Noronha Archipelago, which remains poorly known in terms of ascidian fauna. Previous accounts of Fernando de Noronha have totaled 7 species (Millar, 1977; Eston et al., 1986), but considering our recent surveys in the area (data not yet published), this number is a gross underestimation.
In terms of the assemblage composition, the number of putative endemic species is striking, comprising half of the recorded species. Of the six known species, three are new records for the south Atlantic, while the other three are commonly found along the tropical Brazilian coast. These results highlight the relevance of the Rocas Atoll to the conservation of marine biodiversity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Maurizélia Brito and Amarílis de Paiva, for their help and support during the expeditions. We also thank Nadia Bonnet for her help with the Ascidiidae, and Drs. Rosana Rocha and Antonio Carlos Marques for allowing the use of the SEMs at their institutions. This research was financially supported by CNPq grant 572341/2008-3. R.R.O.F. was sponsored by a CAPES scholarship.
The LSID for this publication is: urn:lsid:zoobank.org:pub:037 26525-0590-4B1D-A802-E303854D0EE4.
References
Bjerkan, P. (1905). Ascidian von dem norwegischen Fishereidampfer “Michael Sars” in den Jahren 1900-1904 gesammelt. Bergens Mus. Aarbog. 5, 1–29.
Bonnet, N. Y. K., and Rocha, R. M. (2011). The family Ascidiidae Herdman (Tunicata: Ascidiacea) in Bocas del Toro, Panama. Description of six new species. Zootaxa 33, 1–33. doi: 10.11646/zootaxa.3691.3.4
Dias, G. M., Rocha, R. M., Lotufo, T. M. C., and Kremer, L. P. (2012). Fifty years of ascidian biodiversity research in São Sebastião, Brazil. J. Mar. Biol. Assoc. U.K. 93, 273–282. doi: 10.1017/S002531541200063X
Eldredge, L. (1966). A taxonomic review of indo-pacific didemnid ascidians and descriptions of twenty-three central pacific species. Micronesica 2, 161–261.
Eston, V. R., Migotto, A. E., Oliveira Filho, E. C., Rodrigues, S. A., and Freitas, J. C. (1986). Vertical distribution of benthic marine organisms on rocky coasts of the Fernando de Noronha Archipelago (Brazil). Bol. Inst. Oceanogr. 34, 37–53.
Fischer, C. F., Avelar, J. C. L., Silva, M. B., Grosman, A., Carvalho, D. A., Carneiro, C. L., et al. (2007). Plano de Manejo Para a Reserva Biológica do Atol das Rocas. Brasília: Ministério do Meio Ambiente/Instituto Chico Mendes de Conservação da Biodiversidade.
Floeter, S. R., Guimarães, R. Z. P., Rocha, L. A., Ferreira, C. E. L., Rangel, C. A., and Gasparini, J. L. (2001). Geographic variation in reef-fish assemblages along the Brazilian coast. Glob. Ecol. Biogeogr. 10, 423–431. doi: 10.1046/j.1466-822X.2001.00245.x
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299. doi: 10.1371/journal.pone.0013102
Goodbody, I. (1984). The ascidian fauna of two contrasting lagoons in the Netherlands Antilles: Piscadera Baai, Curaçao, and the Lac of Bonaire. Stud. Fauna Curaçao Other Caribb. Isl. 67, 21–61.
Goodbody, I. (2000). Diversity and distribution of ascidians (Tunicata) in the Pelican Cays, Belize. Atoll Res. Bull. 480, 301–333. doi: 10.5479/si.00775630.480
Goodbody, I. (2003). The ascidian fauna of Port Royal, Jamaica I. Harbor and magrove dwelling species. Bull. Mar. Sci. 73, 457–476.
Hartmeyer, R. (1913). “Tunicata,” in Zoologische und Anthropologische Ergebnisse einer Forschungsreise im Westlichen und Zentralen Sudafrika, Vol. 5, eds L. Schultze and Jena (Jena: Gustav Fischer), 125–144.
Herdman, W. A. (1886). “Report on the Tunicata collected during the voyage of H.M.S. Challenger during the years 1873-1876. Part 2, Ascidiae compositae,” in Report on the Scientific Results of the Voyage of H.M.S. Challenger during the Years 1873-1876, Vol. 14, eds C. W. Thompson and J. Murray (London: Her Majesty's Stationery Office), 1–429.
Hernández-Zanuy, A. C., and Carballo, J. L. (2001). Distribution and abundance of ascidian assemblages in Caribbean reef zones of the Golfo de Baranó (Cuba). Coral Reefs 20, 159–162. doi: 10.1007/s003380100154
Kikuchi, R. K. P., and Leão, Z. M. A. N. (1997). Rocas (Southwestern Equatorial Atlantic, Brazil): an atoll built primarily by coralline algae. Proc. 8th Int. Coral Reef Symp. 1, 731–736.
Kott, P. (2001). The Australian ascidiacea. Part 4. Aplousobranchia (3), Didemnidae. Mem. Queensl. Mus. 47, 1–410.
Lafargue, F. (1968). Les Peuplements Sessiles De L'archipel De Glénan II. Les Didemnidae- Systématique—Écologie. Vie et Milieux. 19, 353–446.
Lambert, G. (2005). Ecology and natural history of the protochordates. Can. J. Zool. Can. Zool. 83, 34–50. doi: 10.1139/Z04-156
Lotufo, T. M. C. (2002). Ascidiacea (Chordata: Tunicata) do Litoral Tropical Brasileiro. Doctoral thesis, Universidade de São Paulo, São Paulo.
Lotufo, T. M. C., and Silva, A. M. (2006). “Ascidiacea do litoral cearense,” in Biota Marinha da Costa Oeste do Ceará, eds T. M. C. Lotufo and H. Matthews-Cascon (BrasÃlia: Ministério do Meio Ambiente), 1–248.
Michaelsen, W. (1923). Neue und altbekannte Ascidien aus dem Reichsmuseum zu Stockholm. Mitt. Zool. Mus. Hamburg 40, 1–60.
Millar, R. H. (1961). Ascidians from Mozambique. Ann. Mag. Nat. Hist. Sér. 13, 11–16. doi: 10.1080/00222936108651087
Millar, R. H. (1977). Ascidians (Tunicata: Ascidiacea) from the Northern and Noth-Eastern Brazilian shelf. J. Nat. Hist. 11, 169–223. doi: 10.1080/00222937700770131
Millar, R. H. (1978). Ascidians from the Guyana Shelf. Neth. J. Sea Res. 12, 99–106. doi: 10.1016/0077-7579(78)90027-3
Millar, R. H., and Goodbody, I. (1974). New Species of Ascidian from the West Indies. Stud. Fauna Curaçao Carib. Isl. 45, 142–161.
Monniot, C. (1972). Ascidies Phlébobranches des Bermudes. Bull. Mus. Natl. Hist. Nat. Sér. 3, 939–948.
Monniot, C. (1974b). Ascidies littorales et bathyales récoltées au cours de la campagne Biaçores: Phlébobranches et Stolidobranches. Bull. Mus. Natl. Hist. Nat. Sér. 173, 1327–1352.
Monniot, C. (1983c). Ascidies littorales de Guadaloupe. IV Styelidae. Bull. Mus. Natl. Hist. Nat. Sér. 5, 423–456.
Monniot, C., and Monniot, F. (1972). Clé mondiale des genres d'Ascidies. Arch. Zool. Exp. Gén. 113, 311–367.
Monniot, C., and Monniot, F. (1985). Ascidies Littorales de Guadeloupe. IX. Caracteristiques des populations, écologie, rapports avec la faune mondiale. Téthys 11, 203–213.
Monniot, C., and Monniot, F. (1987). Les Ascidies de Polynésie Française. Bull. Mus. Natl. Hist. Nat. Sér. A, 136, 1–155.
Monniot, C., and Monniot, F. (1994). Additions to the inventory of Eastern tropical Atlantic ascidians: arrival of cosmopolitan species. Bull. Mar. Sci. 54, 71–93.
Monniot, F. (1974a). Ascidies littorales et bathyales récoltées au cours de la campagne Biaçores: Aplousobranches. Bull. Mus. Natl. Hist. Nat. Sér. 173, 1287–1325.
Monniot, F. (1983a). Ascidies littorales de Guadeloupe I. Didemnidae. Bull. Mus. Natl. Hist. Nat. Sér. 5, 5–49.
Monniot, F. (1983b). Ascidies littorales de Guadeloupe III. Polyclinidae. Bull. Mus. Natl. Hist. Nat. Sér. 5, 413–422.
Monniot, F. (1989). Ascidies de Nouvelle-Calédonie. VII. Les genres Atriolum et Leptoclinides dans le lagon sud. Bull. Mus. Natl. Hist. Nat. Sér. 11, 673–691.
Monniot, F. (1990/1991). Ascidies de Nouvelle-Calédonie IX. Le genre Trididemnum. Bull. Mus. Natl. Hist. Nat. Sér. 12, 517–529.
Monniot, F. (1993). Ascidies de Nouvelle-Calédonie XIII. Le genre Polysyncraton (Didemnidae). Bull. Mus. Natl. Hist. Nat. Sér. 15, 3–17.
Monniot, F. (1995). Ascidies de Nouvelle-Calédonie XV. Le genre Didemnum. Bull. Mus. Natl. Hist. Nat. Sér. 16, 299–344.
Monniot, F., and Monniot, C. (2001). Ascidians from the tropical western Pacific. Zoosystema 23, 201–383.
Monniot, F., and Monniot, C. (2008). Compléments sur la diversité des ascidies (Ascidiacea, Tunicata) de l' ouest Pacifique tropical. Zoosystema 30, 799–872.
Pérès, J. M. (1951). Nouvelle contribution à I'étude des Ascidies de la Côte occidentale d' Áfrique. Bull. Inst. Fr. Afr. Noire 4, 1051–1071.
Rocha, R. M. (2002). Trididemnum maragogi sp. nov. (Ascidiacea, Didemnidae) from Alagoas, Northeastern Brazil. Rev. Bras. Zool. 19, 1105–1110. doi: 10.1590/S0101-81752002000400015
Rocha, R. M., Guerra-Castro, E., Lira, C., Pauls, S. M., Hernández, I., Pérez, A., et al. (2010). Inventory of Ascidians (Tunicata, Ascidiacea) from the National Park La Restinga, Isla Margarita, Venezuela. Biota Neotrop. 10, 209–218. doi: 10.1590/S1676-06032010000100021
Rocha, R. M., and Kremer, L. P. (2005). Introduced Ascidians in Paranaguá Bay, Paraná, southern Brazil. Rev. Bras. Zool. 22, 1170–1184. doi: 10.1590/S0101-81752005000400052
Rocha, R. M., and Monniot, F. (1995). Taxonomic and ecological notes on some Didemnum species (Ascidiacea, Didemnidae) from São Sebastião Channel, South-Eastern Brazil. Rev. Bras. Biol. 55, 639–649.
Rocha, R. M., Moreno, T. R., and Metri, R. (2005). Ascídias (Tunicata, Ascidiacea) da Reserva Marinha Arvoredo, Santa Catarina, Brasil. Rev. Bras. Zool. 22, 461–476. doi: 10.1590/S0101-81752005000200024
Rocha, R. M., Zanata, T. B., and Moreno, T. R. (2012). Keys for the identification of families and genera of Atlantic shallow water ascidians. Biota Neotrop. 12, 1–35. doi: 10.1590/s1676-06032012000100022
Sluiter, C. P. (1909). Die Tunicaten der Siboga- Expedition. II: Die merosomen Ascidien. Siboga Expedition 56B, 1–112.
Stefaniak, L., Lambert, G., Gittenberger, A., Zhang, H., Lin, S., and Whitlatch, R. B. (2009). Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat. Invasions 4, 29–44. doi: 10.3391/ai.2009.4.1.3
Tokioka, T. (1954). Contributions to Japanese ascidian fauna. VII. Invertebrate fauna of the intertidal zone of the Tokara Island. VII. Ascidians. Publ. Seto Mar. Biol. Lab. 3, 239–264.
Tokioka, T. (1967). Pacific Tunicata of The United States National Museum. U.S. Nat. Mus. Bull. 261, 1–247. doi: 10.5479/si.03629236.251.1
Keywords: Ascidiacea, Tunicata, biodiversity, coral reef, taxonomy
Citation: Paiva SV, Oliveira Filho RR and Lotufo TMC (2015) Ascidians from Rocas Atoll, northeast Brazil. Front. Mar. Sci. 2:39. doi: 10.3389/fmars.2015.00039
Received: 05 September 2014; Accepted: 24 May 2015;
Published: 16 June 2015.
Edited by:
Wei-Jen Chen, National Taiwan University, TaiwanReviewed by:
Greg W. Rouse, University of California San Diego, USAFrancoise Monniot, Muséum National d'Histoire Naturelle, France
Copyright © 2015 Paiva, Oliveira Filho and Lotufo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tito M. da Cruz Lotufo, Instituto Oceanográfico, Universidade de São Paulo, Pça do Oceanográfico, 191, 05508-120 São Paulo, Brazil, tmlotufo@usp.br
 Sandra V. Paiva
Sandra V. Paiva Ronaldo R. de Oliveira Filho
Ronaldo R. de Oliveira Filho Tito M. da Cruz Lotufo
Tito M. da Cruz Lotufo