Long term photoacclimation responses of the coral Stylophora pistillata to reciprocal deep to shallow transplantation: photosynthesis and calcification
- 1Department of Oceanography, The Institute of Earth Sciences, The Hebrew University of Jerusalem, Jerusalem, Israel
- 2H. Steinitz Marine Biology Laboratory, The Interuniversity Institute for Marine Sciences in Eilat, Eilat, Israel
- 3The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel
Reciprocal transplantation of Stylophora pistillata coral fragments between deep (30 m) and shallow sites (3 m) was conducted gradually and resulted with 100% survival. Photoacclimation of transplants at both depths showed two distinct phases: at the first phase, within 2 weeks, zooxanthellae density decreased below (at 3 m) and increased beyond (at 30 m) these of the control values at the new depth, while chlorophyll a per zooxanthellae cell remained as in the original depths, thereby fully adjusting areal chlorophyll concentration. On the second phase, after 6 months, zooxanthellae chlorophyll and their quantum yield (Fv/Fm) were adjusted at both new depths. Such regulated acclimation was also observed in the maximal photosynthesis rate of both transplants, whereas respiration adjustment was rapid. These results differ from previously reported rapid shade and light acclimation strategies hence we suggest that acclimation mechanism changes when certain symbiont type is exposed to depth out of its boundary zone. Despite seemingly having become physiologically acclimated, calcification at both new depths was only half the rate achieved by the controls, suggesting that the coral host requires even longer time than the symbionts to acclimate.
Introduction
The coral and its symbiotic algae, the zooxanthellae, form a unique holobiont that successfully dominates several niches at diverse conditions along the depth gradient along tropical and subtropical shorelines. Corals of the same species living at the extremes of their depth distribution, at the reef flat or in dimly lit depths are physiologically adapted in order to cope with these two stressful environmental conditions, supraoptimal irradiance and too little of it. The intensity of irradiance (PAR and UV-B) appear to be the most significant factors affecting coral physiology (Cohen et al., 2013) and bathymetric distribution (Achituv and Dubinsky, 1990) throughout depth. Corals minimize the potential for photooxidative damage under excess light in shallow reefs or maximize photosynthesis under dim light in deep water by regulating their tri-dimensional morphologies (Mccloskey and Muscatine, 1984), changes in ratios of chlorophyll a and c, carotenoids, peridinin (i.e., Shick et al., 1995; Brown et al., 1999; Stambler and Dubinsky, 2004) and photosynthetic unit size (Dustan, 1982). Furthermore, shallow and deep corals were found to harbor different types (clades) of symbionts (i.e., Iglesias-Prieto et al., 2004).
Experimental transfer of colonies from their original location to the opposite end of their light intensity distribution range, subjects them to stressful perturbations of metabolic patterns, which usually lead to their demise. The photoacclimation strategies of the zooxanthellae exposed to different light regimes and the photo adaptive responses to changes in light quantity and quality, have been documented in many papers (Titlyanov et al., 1980; Chang et al., 1983; Mccloskey and Muscatine, 1984; Parker and Muscatine, 1984; Porter et al., 1984; Kaiser et al., 1993; Masuda et al., 1993; Leletkin et al., 1996). Yet, physiological strategies of acclimation applied by deep corals upon exposure to light at shallow depths in comparison to controls remaining at both extremes are poorly understood. Gattuso (1985) showed in a preliminary study that the saturation intensity of photosynthesis (Ik) of Stylophora pistillata adjusts quicker following shallow rather than deep transfer. Shallow transplantation of low light corals usually results in bleaching (Baker, 2001; Richier et al., 2008) and high mortality rates (Dustan, 1982; Vareschi and Fricke, 1986; Yap et al., 1998; Iglesias-Prieto et al., 2004). Light harvesting properties of the low light acclimated corals can reach 95% of impinging light (Stambler and Dubinsky, 2005). Sudden exposure to high light causes imbalance between the rate of excitation of reaction centers and the relatively slow rate of the dark reactions, limited by low Rubisco to photosynthetic unit content (Fisher et al., 1989). That results in free radical formation and photodynamic damage. That acute stressor is aggravated by UV-B that likewise can impose oxidative stress which can be fatal (Shick et al., 1995). For example, Lang (1973) observed that corals from 14 to 30 m bleach when transferred to 1 m, but not corals from 25 to 50 m when transferred to 15 m. UV protection and damage repair at shallow depths and extensive production of photosynthetic pigments at deeper depths requires energy diverted from essential metabolic functions (Karentz et al., 1994). Hence some corals fail to modify the concentrations of UV absorbing molecules, hindering their acclimation to shallow water (Scelfo, 1986; Vareschi and Fricke, 1986).
This study, beside its importance for understanding photoacclimative mechanisms, is of ecological interest, since coral reefs, as sessile as they are, may be subjected to alteration in their depth. Since 1833, over 35 tsunamis have been recorded around the Indonesian archipelago (Carey et al., 2001). The earthquakes led to these tsunamis sometimes results in extensive reef upheaval (Moore and Moore, 1984, 1988). Movement of the sea bottom may sink reefs to a deeper location (Brown, 2005), but may also uplift corals to a shallower location (Grigg and Jones, 1997; Carey et al., 2001; Yamano et al., 2001; Brown, 2005; Searle, 2006; Liew and He, 2007). In addition to such natural causes, corals are frequently placed in artificial reefs as part of damaged reef remediation efforts, in coral nurseries, research water tables, and underwater observatories, at depths sometimes different from their original ones.
In the present work, we employ gradual, stepwise transfer of S. pistillata from two extreme depths (30 and 3 m) to the reciprocal depth and evaluate the short (14 days) and long (6 months) term acclimative mechanisms of the photosynthetic apparatus and their effect on calcification.
Materials and Methods
Experimental Setup
Five medium sized colonies (15–20 cm diameter) of the hermatypic coral S. pistillata (Esper, 1797) were collected from each, 3 and 30 m depth, along the reef adjacent to the Interuniversity Institute for Marine Science (IUI) at the Gulf of Eilat, the northern Red Sea (29° 30′ N, 34° 56′ E). Each colony was fragmented into 80 branches 3–5 cm long, glued onto a plastic tip and returned to its original depth on underwater rectangular tables (1 × 2 m and 1 m above the ground). Twenty days later, half of the fragments from each colony were gradually transferred to the reciprocal depth. Shallow transfer included 10 intermediate stations with 10 day intervals following the method described in Cohen et al. (2013). The slow ascend of deep fragments was required for avoiding the acute oxidative stress and to increase their survival. Deep transfer requires mainly photo-acclimation and not photo-protection processes to evolve, hence included one intermediate station at 10 m for a period of 14 days before final transplantation to 30 m. The shallow transfer of 30 m corals was initiated on November 6th 2006, and the 3 m corals were transferred to the intermediate station at 10 m depth on the 16th of December 2006. Corals were transferred to their final depth at 30 and 3 m on the 1st of January and 10th of February 2007 respectively. By the time shallow corals were placed at 30 m the deep corals were at 7.5 m depth, hence both treatments were not exposed to their final depth the exact time before the first sampling. The process of shallow transfer started before and ended after the process of deep transfer, this however did not affect the results since the first phase of acclimation occurred within this time frame of a few weeks and the second phase was observed only 6 months later. Fragments from both treatments and controls, which remained at their original depth, were analyzed for photoacclimation of photosynthesis and calcification, 14 days (during February 2007) and 6 months (August 2007) after the final transplantation to 3 m. For convenience of the presentation of the results we used this time although final transplantation of shallow corals in 30 m was a month earlier. For the first sampling, 3–4 random fragments from each colony were tested (n = 16). On April 2007 half of the remaining fragments were lost due to a storm, hence for the second sampling we used only 1–2 fragments from each colony (n = 8). The effect of inner colony variation was tested statistically.
Data Collection
Irradiance and sea temperature were recorded at 3 to 30 meters, at each sampling point (Table 1) by a Pulse Amplitude Modulated instrument (diving- PAM, WALZ instruments, S.N: UWFA0221A, Effeltrich, Germany). The light sensor connected to the PAM was previously calibrated to a Li- Cor-1000 light meter.

Table 1. Physical parameters at 3 and 30 m depth measured 14 days (February) and 6 months (August) after relocation next to the experimented corals.
Quantum Yield of Fluorescence and Non-photochemical Quenching
Maximal quantum yields (Fv/Fm) were measured with the diving PAM at 3 and 30 m depth-in situ, on the same day 20 min after sunset. According to a diel quantum yield cycle made for several days on 3 and 30 m treatments and controls during both sampling points- a few minutes after sunset Fv/Fm reaches a maximal peak which is stable for an hour before a gradual night decline. Since this is the highest Fv/Fm of the day, corals were already dark adapted. In the gulf of Eilat the sun sets behind 200 m high mountains, hence there is still a dim light that enables diving without external light source. In order to accurately measure all samples in one dive only 3 repeats were measured in each treatment. Initial fluorescence (F0) is emitted by the zooxanthellae after weak flashes of wavelength λ > 670 nm, maximal fluorescence (Fm) is achieved after saturating pulse of 5000 μmol photons m2 s−1 generated by the diving PAM for 0.8 s.
Equation 1:
Similar Fv/Fm measurement was performed also at noon in order to calculate NPQ (Equation 2)- the relative decrease in maximal fluorescence during the day (Fm') as a result of shift in dissipation mechanism to heat, compared to night (Fm).
Equation 2:
Photosynthesis
For the first and second sampling points, 4 and 2 respectively, random fragments from each colony were retrieved from their experimental depth covered with an opaque plastic strap to avoid light stress and within 2 min placed in running seawater aquaria, shaded according to the treatment, for a maximum of 4 h before the measurement of photosynthesis. Each specimen was placed in a sealed metabolic chamber containing 150 ml filtered sea water (G/FC Whatman) to prevent non coral related respiration or photosynthesis. The water was thermostatted according to the respective seasonal temperature (20°C on February and 26°C on August) using a circulating water bath (NESLAB, RTE 210). Oxygen electrodes (Oxi 323, WTW), connected to a digital data logger, recorded the changes in dissolved oxygen concentrations. The electrodes were calibrated for their minimal and maximal readings by bubbling N2 or O2 respectively. Since this electrode is temperature compensated we calibrated it in both seasons to air saturation according to 6.6 mg/L at 25°C. The electrodes had minimal oxygen consumption that was corrected by measuring oxygen change of filtered seawater without corals (blank control). The chambers were cleaned each time before placing a new sample in order to prevent biofilm formation. Fragments were dark adapted for 15 min in order to fully oxidize the PQ pool and avoid a time lag in photosynthesis (Moberg et al., 1997). Changes in dissolved oxygen indicated dark respiration during the first 20 min and net photosynthetic rates at a sequence of 9 increasing irradiances at 10-min intervals. Photosynthesis normalized to chlorophyll a was fitted to hyperbolic tangent function (Equation 3) according to Chalker (1981) using nonlinear least squares (nls) of the statistical program Rstudio (version 2.15.0). For this fit we removed all data points that were in photoinhibition since it is not accounted in this model. This formula calculates gross photosynthesis (PG); however for biological interpretations it was easier to compare net photosynthesis between the treatments, so the data in Table 2 are calculated after subtraction of respiration of each specimen.
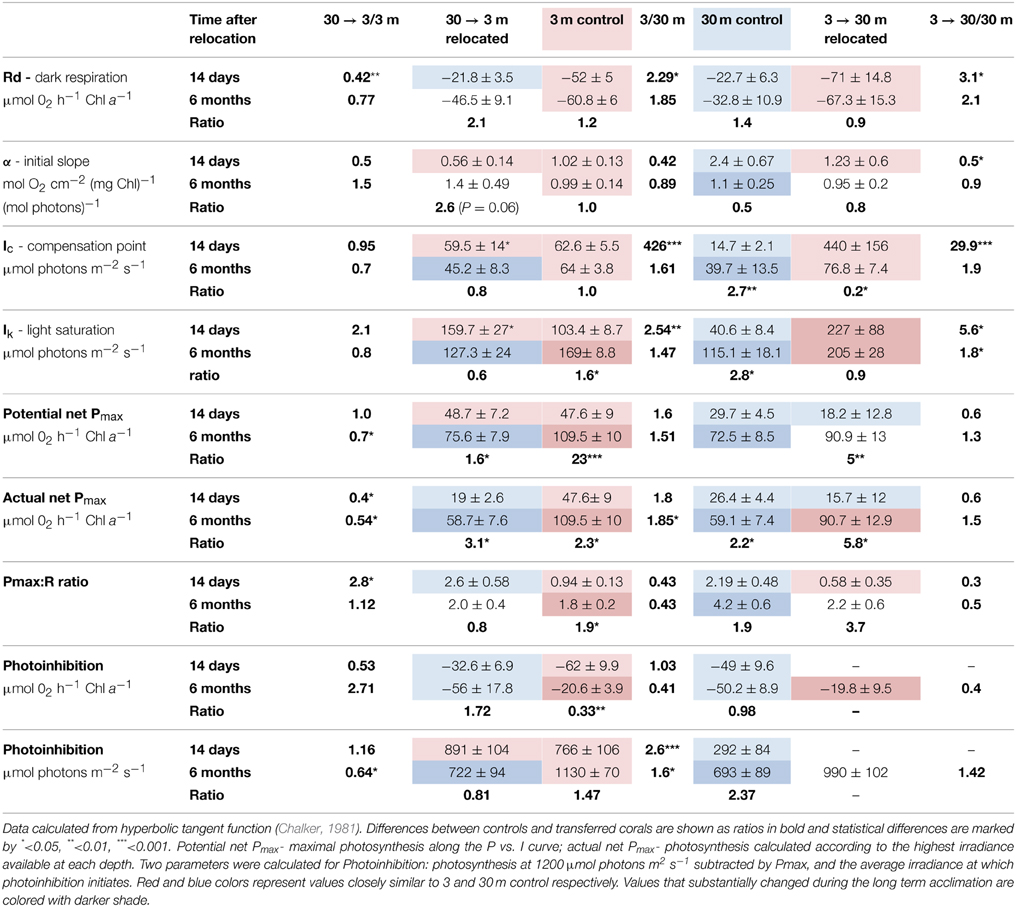
Table 2. Photosynthetic parameters (Mean ± SE) of S. pistillata from 3 and 30 m, and after 14 days (during February) and 6 months (during August) of their relocation to the reciprocal depth (3→30 m and 30→3 m).
Equation 3:
The initial slope- α, compensation irradiance- Ic, Pmax and the saturation irradiance- Ik were calculated using Equation 3.
Zooxanthellae Density and Chlorophyll a Concentration
After the photosynthesis vs. irradiance measurement, tissue of the fragments was removed by a jet of compressed air and filtered (GF/C Whatman) seawater using an artist's air brush. The tissue slurry was homogenized and stirred. Zooxanthellae of each sample were counted 4 times on a hemocytometer grid under light microscope (400×). Number of cells was multiplied by 104 in order to receive the amount in 1 ml, multiplied again by 50 to match the dilution volume and divided by surface area to calculate their areal density as number of zooxanthellae per cm2. The surface area of each fragment was estimated from the weight of aluminum foil covering its surface area according to Marsh (1970).
Chlorophyll a was extracted by filtering 5 ml of the tissue slurry on a GF/C filter. The filter was then homogenized with 5 ml of acetone 90% and kept for 24 h in the dark at 4°C. Chlorophyll a concentration was evaluated with a spectrophotometer (ultrospec 2100 pro, Amersham Pharmacia Biotech) following the equations described by Lorenzen (1967). Concentration of chlorophyll a per zooxanthella cell was calculated by dividing chlorophyll a concentration (μg ml−1) by the number of cells ml−1. Concentration of chlorophyll a cm−2 was calculated by multiplying chlorophyll a (μg ml−1) by the volume of the sample (50 ml) and dividing by surface area of the fragment.
Calcification
Daily percent growth was calculated using the buoyant weight technique (Jokiel et al., 1978). Specimens were weighed with a semi analytic digital scale (SnowRex, LB300 300 × 0.01 g) on December 20th 2006 and at each sampling time (14 days and 6 months after final relocation). During December 2006 the deep corals were still in the process of the gradual shallow transfer and were at 7.5 m, this was necessary however in order to get a good signal when calculating growth rates. The fragments used for each sampling were sacrificed for the rest of the measurements hence the results were independent.
Statistical Analyses
All statistical analysis was done with Rstudio software. Within the repeats of all treatments we measured several fragments from each of the five mother colonies extracted from each 3 and 30 m depth. To assess the significance of the effect of inter-colony variation as well as season and depth on calcification, photosynthesis and symbiosis we used permutated mixed effects model. The permutation test was chosen due to the non- normality of the data according to Shapiro-Wilk normality test. In order to examine the statistical differences between the combine groups of depth and seasons we tested the normality of the mixed effects model residuals using the Shapiro-Wilk normality and performed pairwise Wilcoxon rank sum test. Bonferroni correction was used to account for the multiple comparisons. In each test the fix factors were colony number (1–5), season (winter- 14 days and summer- 6 months) and treatment (3 m, 3→30 m, 30 m, 30→3 m).
Results
The slow and gradual transplantation to both depths resulted in 100% survival of coral fragments, with no partial mortality and no significant sign of stress as excessive mucus release. This allowed us to relate to the results of the treatments as corals in a process of acclimation and not in a process of degradation. All of the parameters used for the statistical tests, except one (Ic) showed that the difference between repeats of the same colony was larger than in between colonies, hence all fragments are accounted as true repeats.
Irradiance levels at noon were 8.8 fold higher at 3 m than at 30 m during February (winter), 14 days after transfer, and only 6.8 fold higher during August (summer) 6 months later (Table 1). Irradiance during August, compared to February, was only 9% higher at 3 m in respect to 41% at 30 m. That difference corresponds to the documented seasonal changes in phytoplankton densities between the stratified, oligotrophic summer and the nutrient enriched winter and results in the vertical light attenuation kd at 30 m increase from its summer minimum of 0.45 m−1 to 0.8 m−1 in winter (Dishon et al., 2012). Temperature mostly changed between seasons: 20°C at both depths during February and between 25 and 27°C during summertime seawater stratification in August.
Quantum Yields
Corals transferred to deep water have increased Fv/Fm values; however values attained 14 days after final transplantation (and 3 months after the gradual transplantation had started) were still below these of the native deep controls. Only 6 months later, Fv/Fm in the deep transplants reached values equal to these of the 30 m controls. Fv/Fm values of the shallow transferred corals decreased already after 14 days (P < 0.05, Figure 1), however, these never reach the low values of the controls. A similar trend emerged in the NPQ values (Figure 1) which changed significantly in both treatments (P < 0.05). It is not surprising that shallow corals decreases NPQ at greater depth, however, these corals attained lower values even than the controls at 30 m after 14 days and 6 months (P < 0.05). During August all corals dissipated a higher percentage of the impinging irradiance (significant only in 30 m and 3→30 m, P < 0.05).
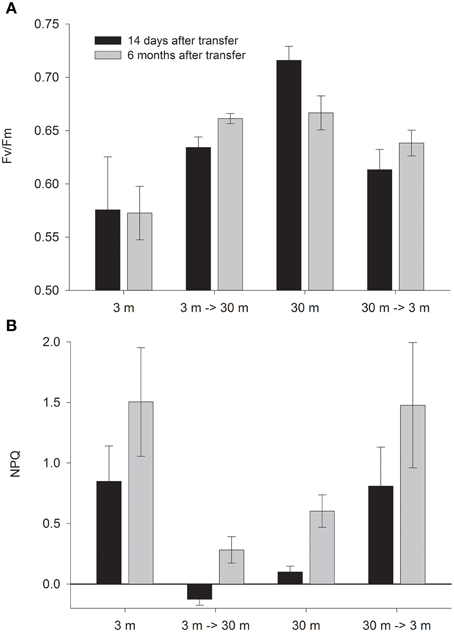
Figure 1. Mean ± SE (n = 3) of (A) Fv/Fm and (B) NPQ measured in situ on control (3 and 30 m) and depth transferred (3→30 m and 30→3 m) S. pistillata, 14 days (February) and 6 months (August) after reciprocal depth transfer.
The formula provided for calculation of NPQ can lead, in some cases, to over or under estimation of values, since it is based on the variable fluorescence proxy values. During an August day, all shallow corals reduced their Fm' to less than one half of those measured at night. As a result NPQ is calculated to be higher than 1- more than 100% of the light appears to dissipate. Conversely at deep water, Fm' values of 3→30 climbed during the day above the values measured in the dark, therefore NPQ seems negative even though it is impossible to release negative amount of heat. We discuss possible reasons for this phenomenon, and explain why although we cannot rely on the PAM NPQ values, the trend of the difference between treatments can be considered genuine.
Photosynthesis and Respiration
Oxygen consumption via respiration of S. pistillata corals was more than twice faster at 3 m than at 30 m (P < 0.05, Table 2), and became greatly modified upon both shallow and deep transfers (P < 0.01 and P < 0.05 respectively). The compensation intensity (Ic) and saturation intensity (Ik) of photosynthesis were 4.3 and 2.5 fold higher respectively at 3 m compared to 30 m corals during February, whereas the irradiance difference during that month at noon was 8.8 fold higher at 3 m. During August, Ic and Ik were only 1.6 and 1.5 fold higher at 3 m compared to 30 m corals whereas irradiance was 6.8 fold higher. Hence photosynthesis, in regard to the available light, is consistently lower in shallow compare to deep corals. Such discrepancy suggests that photoacclimation over a depth range is not linear with light intensity. Compensation irradiance changed in both shallow and deep transfers but was far from attaining the optimal values of controls by the end of the first 2 weeks. Photosynthesis among deep transferred corals compensated their respiration at higher irradiance compared to the 30 m controls, although similar respiration was measured. Shallow transferred corals utilized oxygen at a rate higher than it was produced by the symbionts under most of the light intensities along the P vs. I curves (Figure 2) and reached compensation at irradiance 7 fold higher than was measured for 3 m controls. Only 6 months later, compensation irradiance and the photosynthesis curves under both treatments came to closely resemble the controls at the new depth.
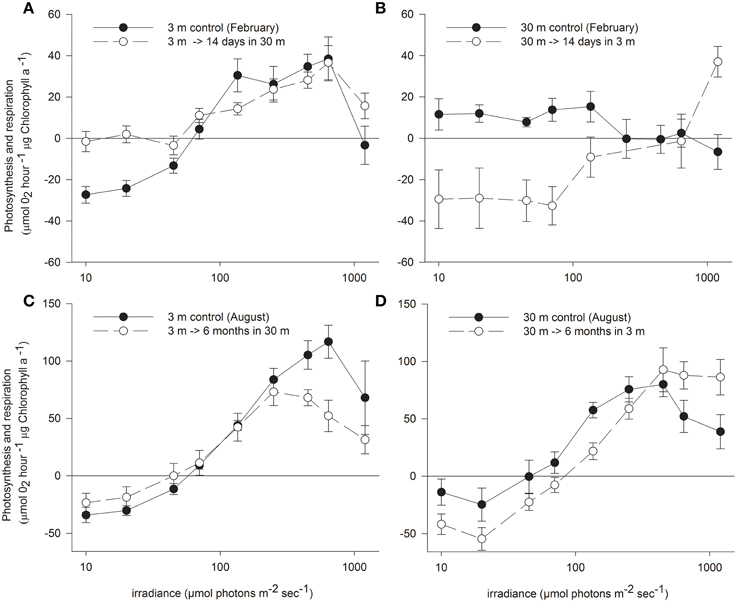
Figure 2. Net photosynthesis vs. irradiance (mean ± SE) of control S. pistillata from (A) 3 m and (B) 30 m depth and their clone fragments 14 days (during February) after transfer to the reciprocal depth (n = 16). Different fragments from the same (C) 3 m and (D) 30 m controls and transferred fragments were measured also 6 months (during August) after the reciprocal transfer (n = 8).
Maximal rates of photosynthesis according to the P vs. I curves were 1.6 fold higher at 3 m than at 30 m regardless of the season, however this represent a potential Pmax at intensities up to 1200 μmol photons m−2 s−1. Irradiance levels at 3 m are higher than the Ik measured for shallow corals, hence the actual photosynthesis of these corals are similar to their potential Pmax. Conversely, S. pistillata at 30 m can increase photosynthesis at irradiances beyond their availability attaining only 0.88 of the actual Pmax in February and 0.8 in August. Hence the actual differences in Pmax are higher in shallow corals than usually calculated. While the potential Pmax is not significantly different between shallow and deep control, the actual Pmax is significantly higher in shallow corals. Two weeks after shallow or deep transfer the potential Pmax was not influenced; significant change within the deep transferred corals was observed only after 6 months.
Photosynthesis was inhibited in shallow fragments at higher irradiance compared to deep fragments at both seasons. However, rate of photosynthesis under the highest experimental illumination decreased similarly in both controls. The resilience to photoinhibition under high irradiance within shallow corals remained high also after 2 weeks of deep transplantation, but resembled the deep control corals after 6 months. In August, both control and transferred corals at 3 m were less photoinhibited by the high light of the P vs. I cure than control and transferred corals at 30 m.
Zooxanthellate Density and Chlorophyll a Concentration
Deep control corals harbor 28% higher zooxanthellae density per cm2 (Figure 3A) and 38% higher chlorophyll a concentrations per zooxanthella cell than shallow corals in February (Figure 3B) This led to twice higher chlorophyll a cm−2 at 30 m than at 3 m controls (P < 0.01). In both treatments, 14 days subsequent to their final depth transfer, a dramatic change was observed in the abundance of zooxanthellae cells whereas chlorophyll a per zooxanthellae cell did not change and resembled the control colonies at the original depth. The 3 → 30 m transfer resulted in 71% increase in zooxanthellae (P < 0.05), 24% higher than in the 30 m control and the 30→3 m samples lost 69% of their zooxanthellae (P < 0.001), remaining with 47% lower than the shallow controls (P = 0.07). Increasing densities of the low chlorophyll zooxanthellae with deep transfer and dilution of the high chlorophyll zooxanthellae upon shallow transfer resulted in significant change in chlorophyll a cm−2 (P < 0.001 with shallow and P < 0.5 with deep transfer), which attained similar values to the controls at both new depths (Figure 3C).
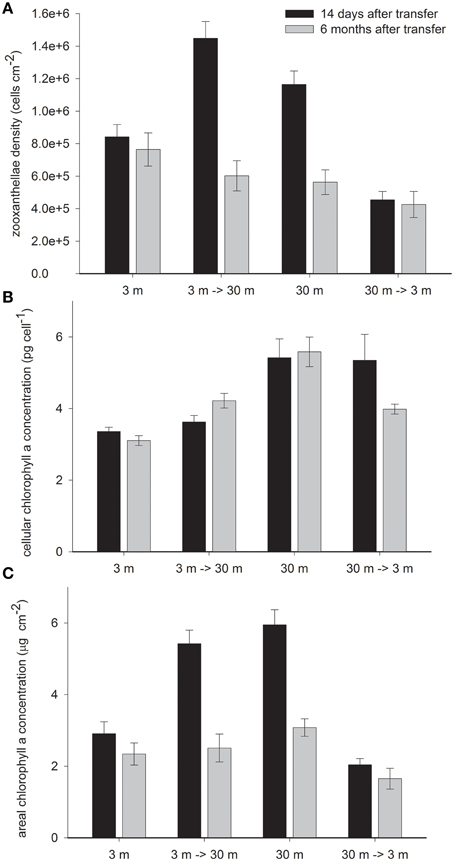
Figure 3. Mean ± SE (n = 16) of (A) density of zooxanthellae cells per 1 cm2 (B) Pico gram chlorophyll a per cell of zooxanthella and (C) μg chlorophyll a per 1 cm2 measured in control S. pistillata fragments from 3 and 30 m depth and in their clones after 14 days (February) and 6 months (August) of reciprocal depth transfer.
Changes in cellular chlorophyll were observed only 6 months after transplantation of both treatments- becoming significantly different from their original depth control (P < 0.05). The 36% increase by deep and 29% decrease by shallow transferred corals closed only some of the gap with the controls in their new habitat. Superimposed on the above described depth related changes, areal chlorophyll concentration decreased in all corals during summer, however significantly only in 30 m (−58%) and 3→30 m (−55%) fragments (both P < 0.001) as a result of substantial decrease in zooxanthellae density (P < 0.05), whereas with no significant concomitant decrease in cellular pigmentation.
Calcification
S. pistillata in this study grew 3.5 times faster at 3 m than at 30 m depth (P < 0.01, Figure 4). The shallow corals reduced growth rate by 86% (P < 0.01) when transferred to 30 m, 48% lower than the deep control after 14 days and remained lower than the deep control even after 6 months. Conversely, deep coral fragments increased their growth rate by 100% (P < 0.05) above their original growth rates when transferred to 3 m. However, they were still 43% lower than the 3 m controls (P < 0.01). Six months later, 30→3 m transplants increased the daily average growth by a further 43%, but were still slightly growing slower (by 14%) than the rates of the 3 m control.
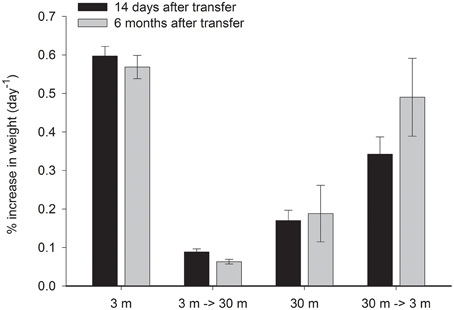
Figure 4. Mean ± SE (n = 14) percent of weight gained per day of control S. pistillata fragments from 3 and 30 m depth and of their clone fragments, 14 days and 6 months after their final transfer to the reciprocal depth.
Discussion
The depths chosen for this study (3 and 30 m) represent two extremes of their bathymetric range- where corals withstand either too much or very low light. Corals grow ideally at 5–10 m depth (Erez, 1978; Gattuso, 1985) which provides 45–60% of subsurface irradiance in the Red Sea (Cohen et al., 2013). Depth acclimation of S. pistillata was evident within all physiological parameters measured in this study however following transfer to a new depth some of these parameters change partially after a few weeks and complete their change only after a few months. Although shallow transfer started earlier than the deep transfer, both treatments were still in the short term acclimation process during our first sampling- zooxanthellae chlorophyll concentrations and the potential Pmax are still similar to their original depth controls.
Quantum Yield and Photosynthesis
Lower respiration with depth and the concomitant dim light, which was shown in many studies (Dustan, 1982; Mccloskey and Muscatine, 1984; Gattuso, 1985; Masuda et al., 1993; Fitt et al., 2000), was one of the initial responses to depth relocation of S. pistillata (Table 2). Respiration can be stimulated by the high photosynthesis and growth rates (Mccloskey and Muscatine, 1984) as seen in shallow corals. However, the high respiration observed in the corals transferred to 3 m could result from chronic photoinhibition (Hoegh-Guldberg and Jones, 1999; Warner et al., 1999) and may assist in avoiding dangerous increase in oxygen concentration in the tissue (Shick et al., 1991).
Unlike respiration, a two step, slow process of acclimation was observed in all parameters of photosynthesis. While differences in α, Ic, Ik, and Pmax in this study were consistent with corals at different depth (Chalker et al., 1983; Gattuso, 1985) or shading (Falkowski and Dubinsky, 1981), some discrepancies were found in the kinetics of depth and shade acclimation. Unlike the rapid photosynthetic adjustment between reciprocal shade and light acclimation reported by Falkowski and Dubinsky (1981) we observed a much longer term process with depth acclimation.
Shallow corals maintain the high reduction potential of the plastoquinone pool by not optimizing the compensation irradiance (Ik) after 14 days at 30 m, resulting in 2.5 fold higher potential Pmax compared to actual Pmax under the intensity of 30 m. This mechanism can prevent further stress under natural cases when corals experience temporarily changes in light conditions. Such mechanism was also responsible for the observed resilience to photoinhibition of the deep transferred corals under the high experimental intensities, therefore indicates that photoacclimation mechanisms, especially to high light, can be highly conservative. After 6 months, deep transferred corals became more susceptible to photoinhibition and their Ik was only slightly higher than the 30 m control (Table 2).
Conversely, Ik and Ic were the first parameters to change upon shallow transfer, probably due to the increased respiration and was even higher than the shallow control. Ik is commonly used as a measure of the adaptation of a plant to its light regime (Steemann Nielsen, 1975). At irradiance levels above Ik, like that which deep corals experience when transferred to shallow water, more energy is trapped by the light reaction centers than can be used by photochemical processers or be disposed harmlessly. This imbalance may lead to photodynamic damage to components of the photosynthetic apparatus (Kyle et al., 1984; Hoegh-Guldberg and Jones, 1999) and to non-photosynthetic components of the electron transport chain (Figueroa et al., 2003). Since photosynthesis among 30 m controls was inhibited at intensity of ~290 μmol photons m−2 s−1, shallow transferred corals would be expected to experience photoinhibition under intensity that can reach ~825 μmol photons m−2 s−1 at 3 m depth. However, the low photosynthesis observed among the shallow transferred corals resulted only from its limited photoacclimation mechanisms to increase net photosynthesis above the vast increase in respiration resulting in photosynthesis below the compensation point throughout most of the P vs. I curve. The dark adapted Fv/Fm of this treatment remained higher than the shallow control, suggesting that the turnover time of PSII repair was higher than the immediate damage. In fact, the Ic and Ik of these corals calculated by the hyperbolic tangent underestimate the real measurements shown in Figure 2 since the increase in oxygen occurred only under high intensities, roughly equivalent to the peak irradiance at 3 m.
Furthermore, the shallow transferred S. pistillata dissipated the excess energy via NPQ at efficiency similar to the shallow controls (Figure 1). High NPQ may indicate on a functional photosynthesis since epoxidation of diadinoxanthin to diatoxanthin during light depends on a pH gradient developed by pumping protons, via non damaged PSII, across the thylakoid membrane into the lumen. However, even damaged reaction centers can continue to trap excitation energy and NPQ may occur simultaneously (Krause, 1988; Falkowski and Kolber, 1995).
Fm' values decreases during the day with activation of NPQ (Gorbunov et al., 2001), however over-reduction of Fm' in corals at 3 m during August resulted in NPQ values higher than 1. This was also shown for plants under high light (Ralph and Gademann, 2005). In contrast, Fm' slightly increased in the deep transferred zooxanthellae hence negative NPQ values were calculated. In such case where the difference in Fm is lower than the error of measurement- the NPQ value is counted as 0 (Schreiber, 2004). Fluorescence may account at most for a few percentage of harvested energy; Therefore, Equation 2 can only approximate thermal dissipation. Although these assessments do not represent the correct absolute values, we presume that the differences between treatments are reflecting their factual behavior.
Potential (and actual) P: R ratios are consistently higher in deeper corals compared with shallow ones. While during winter Pmax is almost equal to respiration of shallow corals, and hence daily photosynthetic energy input is limited. The opposite happens in summer, when the net energy input from photosynthesis is even higher at 3 m than at 30 m, although P: R ratios are lower, since photosynthesis rates are much higher (Table 2, Falkowski et al., 1984).
Zooxanthellate Density and Chlorophyll a Concentration
Chlorophyll per zooxanthellae was higher in deep S. pistillata (Figure 3B) like in other deep corals (Titlyanov et al., 1980; Mccloskey and Muscatine, 1984; Porter et al., 1984; Kaiser et al., 1993; Masuda et al., 1993; Leletkin et al., 1996; Mass et al., 2007; Winters et al., 2009). However, zooxanthellae density with depth increase in some corals (Figure 3B, Drew, 1972; Winters et al., 2009) and decrease in others (Dustan, 1979; Mccloskey and Muscatine, 1984; Masuda et al., 1993; Fitt et al., 2000). Such variation is not specie specific, for example zooxanthellae density in S. pistillata can either increase (Figure 3, Winters et al., 2009) or decrease (Mccloskey and Muscatine, 1984) with depth. However, there might be a correlation between zooxanthellae density and the strategy of acclimation to depth. In studies where zooxanthellae density decreases with depth they were harbored in 4–5 million cells cm−2 in shallow water, and only 2–3 million at greater depth. Mccloskey and Muscatine (1984) suggested that lower zooxanthellae densities in S. pistillata under dim light may minimize self-shading. Conversely, when the density was below 1 million cells cm−2 in shallow thereby higher zooxanthellae densities would assist rather than obstruct light capture.
Optimizing light capture at depth by increasing zooxanthellae densities becomes jeopardize when encountering sudden increase in PAR and UV-B by transfer to shallow depth. ROS could be induced in the host (Nii and Muscatine, 1997) and in the zooxanthellae (Lesser, 1996) and lead to zooxanthellae expulsion (Lesser, 1996; Smith et al., 2005), and trigger cell death (Dunn et al., 2002; Apel and Hirt, 2004; Franklin et al., 2004; Lesser and Farrell, 2004). Such reduction in zooxanthellae densities upon shallow transfer of S. pistillata from 30 m is enhanced by the high UV-B levels (Cohen et al., 2013). Dramatic reductions in algal densities can occur within a very short time scale (24 h) after exposure to high solar radiation (Brown et al., 1994) or within seasons (Figure 3, Mass et al., 2007). In the present study, 14 days after final transplantation of deep S. pistillata to 3 m, zooxanthellae densities were lower than in the control at 3 m, conversely, 3–30 m transfers had higher zooxanthellae densities than the controls at that depth. Overshooting numbers of zooxanthellae in both treatments compensate for the unaffected cellular chlorophyll content, which remained high in the shallow transferred corals and low in the deep transferred corals. As a result, appropriate areal chlorophyll concentrations are maintained in the new light fields (Figure 3C).
The remaining symbionts within shallow transferred fragments were able to efficiently dissipate the excess energy (as explained before) however the inadequate cellular chlorophyll may have impeded optimal photosynthetic capability. Six months after shallow transfer, zooxanthellae densities were still different from control values at the new habitat, however at lower discrepancies. This associates with partial, however significant, change in cellular chlorophyll toward control values at both relocation depths which was probably still an ongoing process. Cellular chlorophyll adjustments in zooxanthellae were complete after 1 month of shade to light acclimation and 3 months of light to shade acclimation (Falkowski and Dubinsky, 1981), and were as fast as 24 h in the free living chlorophyte alga Dunaliella salina (Berner et al., 1989). Hence such progressive response of cellular chlorophyll to depth relocation, observed in this study, probably occurs at a different mechanism. We suggest that the reason for the prolonged two step acclimation observed lays in the difference in zooxanthellae types, which is known to have distribution boundaries set by depth (Iglesias-Prieto and Trench, 1997a; Baker, 2001; Iglesias-Prieto et al., 2004; Winters et al., 2009). Dustan (1979) suggested that corals originating from different depths respond differently to the same conditions in part due to the inherent characteristics of several populations of symbionts. This could explain the differences in zooxanthellae densities and their chlorophyll content between transferred corals and the control at the new depth to adjust areal chlorophyll concentrations. Differences in zooxanthellae densities of control and transferred corals at the same depth resemble the different densities Symbiodinium clades were found under several temperatures (Gillette, 2012). Also, Chang et al. (1983) and (Iglesiasprieto and Trench, 1994, 1997b) isolated several strains of Symbiodinium microadriaticum and showed that their mechanisms of photoadaptation are different although they were kept under similar high or low light conditions. One of these mechanisms is a distinct variation among the chlorophyll-protein complexes (Iglesias-Prieto and Trench, 1997b) as would affect the chlorophyll per cell concentrations suggested here. Hence, while corals transferred to shade or light rapidly adjust densities of zooxanthellae and chlorophyll per cell (Falkowski and Dubinsky, 1981), depth relocation is prolong.
Earlier studies on Montastraea annularis (Rowan et al., 1997) showed that depth relocation caused shift in the clades after 6 months- corresponding to the time response for cellular chlorophyll to adjust (Figure 3). P. verrucosa harboring clade A responded to depth transfer by changing cellular chlorophyll and not symbiont density (Ziegler et al., 2014) as was observed for shade and light acclimation of shallow S. pistillata in the Red Sea (Falkowski and Dubinsky, 1981) which harbors clade A in the shade and also in high light (Winters et al., 2009). In deeper water S. pistillata (20 m), clade A co-exists with the C type (Winters et al., 2009) which responds to higher light by dramatic reduction in its densities (Figure 3). Similar results were observed when P. verrucosa harboring clade C zooxanthellae were transferred to shallow depth (Richier et al., 2008).
Based on these former observations and our results, we suggest in Figure 5 a mechanistic model for short and long term depth acclimation. Days to weeks photoacclimation of the indigenous zooxanthellae clades of the depth of origin is limited by the boundaries of phenotypic flexibility inherent to each clade. The ensuing, months-long complete “acclimation” we documented, is likely to have resulted from the replacement of the zooxanthellae clades by those native to the “new” depths.
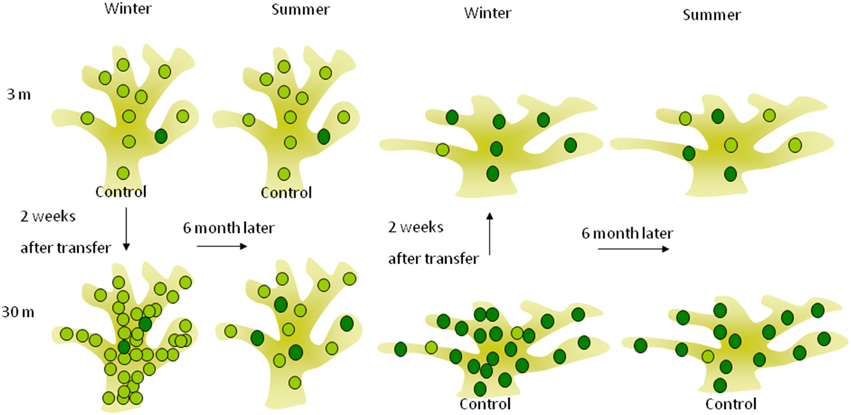
Figure 5. Short and long-term acclimation responses of deep (30 m) and shallow (3 m) S. pistillata corals at the reciprocal depth. The lighter green circles represent symbionts (possibly also represent different clade) containing lower chlorophyll concentration found mostly in shallow corals, while the darker circles are symbionts containing higher chlorophyll concentration found mostly in deep corals. Two weeks subsequent to shallow and deep relocation, symbiont densities were overshot to lower and higher densities (respectively) than the control at the new depth whereas chlorophyll concentration per symbiont did not change, resulting in similar areal chlorophyll concentrations. Six months after depth relocation, both treatments attained closely similar cellular chlorophyll concentrations as the controls at the new depth although seasonal fluctuation in cellular chlorophyll was small.
Natural seasonal bleaching and recovery were repeatedly observed in deep (40–63 m) S. pistillata in the Red Sea from April to September (Nir et al., 2014). This phenomenon was apparent with the 52 and 59% reduction in zooxanthellae cells of control and transplant corals at 30 m, in regard to only 6–10% reduction at shallow. This would suggest that the bleaching phenomenon responds to conditions in the deep environment and is not intrinsic only to corals originated at the mesophotic zone. However, according to the model we suggest, zooxanthellae clade after deep transfer could already be replaced by August, hence the bleaching might still result from an intrinsic response of the deeper clade C to the increased light and temperature of summer. Downs et al. (2002) correlate the drastic decline in cell density at greater depths with increase in levels of oxidative damage products as consequence of raising water temperature.
Calcification
The symbiotic relationship between coral (or Foraminifera) and zooxanthellae is not always reflected by linear rates of their most prominent biological activity- host calcification and symbiont photosynthesis. Understanding the physiological interrelationship between these organisms is a prerequisite for understanding the constraints of this interaction and the conditions where it is most efficient. This notion was already discussed when Goreau and Goreau (1959) and Colombo-Pallotta et al. (2010) showed that calcification of bleached corals can be activated by light, although in reduced rates. In Foraminifera, LEC can proceed in normal rates for 5 h although photosynthesis is inhibited with the PSII blocker DCMU (Erez, 1983). These responses of the host are due to light activation of Ca2+ uptake from the seawater and elevation of pH at the calcifying site (Al-Horani et al., 2003). Photoacclimation of photosynthesis as observed in this study may influence coral calcification by optimizing energy supply (Muscatine et al., 1984) for such active biological processes. Transport of Ca2+ (Chalker, 1976) and carbon (Lucas and Knapp, 1997) are energetically expensive although these ions can also arrive to the site of calcification by a paracellular pathway (Tambutte et al., 2012). Several of the carbohydrates synthesized by the symbionts are transported to growing parts of the polyp to generate ATP (Pearse and Muscatin, 1971; Fang et al., 1989). A good correlation was found between calcification rates in this study: 3 (August) to 3.5 (February) fold higher at 3 m over 30 m fragments (Figure 4), and the translocation of photosynthetically fixed carbon to the S. pistillata host: 38% at 3 m and 11% at 35 m depth (Mccloskey and Muscatine, 1984). Low light corals use most of the limited carbon to sustain essential metabolic functions (Scharf, 1982), whereas at shallow depths, photoinhibition (Muscatine, 1990) in addition to UV light (Jokiel and York, 1982) may also lead to reduced growth rates. S. pistillata grow ideally at 5–10 m depth (Erez, 1978; Gattuso, 1985), however specie specific responses to such perturbations alters the optimum depth for growth (Huston, 1985). Carricart-Ganivet et al. (2007) for example showed that massive Porites corals did not change significantly with increasing depth up to 20 m. Without acclimation, Montastrea annularis collected from 33.5 m decreased calcification when incubated at 9 m for 24 h (Barnes and Taylor, 1973). Physiological acclimation, as observed in chlorophyll concentrations of corals exposed to changes in irradiance can minimize decreases in growth rate. Indeed, calcification was significantly enhanced upon transfer to shallow depth, although the P: R ratio in the transferred corals was much lower (0.58) in respect to the deep control (2.2) during February. This indicates that light can enhance calcification even when photosynthesis is much lower than respiration, a conclusion deviating from the stoichiometric relationship implied by the “light enhanced calcification” model. Mass et al. (2007) also found evidence for such discrepancies in corals at 50–65 m depth. During the first 2 weeks after depth transfer, the corals in both treatments grew only half the rate of the control at their new depth. We suspect that the lower calcification rates of the deep transferred corals (also observed by Yap et al., 1998) stemmed from energy diverted to acclimation process. Interestingly, 6 months after relocation, growth of both transplants was still lower than the control at the new depth although Pmax and P: R ratios were similar, suggesting that acclimation processes of the host are longer than those of the zooxanthellae.
Summary and Conclusions
In this study, reciprocal shallow (3 m) and deep (30 m) transplantation of S. pistillata fragments was performed gradually and resulted with 100% survival rate. Photoacclimation of photosynthesis and calcification to the new depth revealed differences from previously known shade and light acclimation at the same depth and suggest a different mechanism. This acclimation was prolonged and included two distinct phases. The initial response to depth relocation results is a vast change of zooxanthellae densities and not in chlorophyll concentration per cell as would be expected from shade to light acclimation. Such changes were not efficient in modifying photosynthesis at both depths during the short acclimation time but only after 6 months, when cellular chlorophyll concentrations were almost adjusted. Conversely respiration rapidly increased with shallow transfer and decreased with deep transfer. The faster, incomplete adjustment to the “new” depth is assumed to be within the phenotypic plasticity range of the original zooxanthellae population. The subsequent, long-term full adjustment to values in the unmoved controls is likely the result of a slow replacement in symbionts' clade. Such photosynthetic acclimation did not alleviate the drastic reduction in calcification of both transferred treatments. Deep transferred corals grew only half of the unmoved deeper corals, even after 6 months suggesting that the host requires longer than its symbionts for a complete depth acclimation. Interestingly, calcification within shallow transferred corals did increase under the high irradiance although the Pmax: R ratio of these corals was 0.58 during the first 2 weeks after transfer. This suggests that light has a larger role on calcification than photosynthesis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks to Noa Moshkowitz for the assistance with the statistical analyses and to Ofri Mann for creating the hyperbolic tangent model. We are grateful to Dana Nahum Cohen for the assistance in data collection, Ms. A. Goldreich for the meticulous editing and to Oded Ben-Shaprut for planning the dives and to the staff of the Interuniversity institute in Eilat, Israel. This study was performed under permit No. 2004/20265 of the nature reservation authority and was supported by Israel Science Foundation grant number 408/03-17.3, NATO grant number SFP 981883 and by grant EU FP7 European Research Council 309646 and grant Horizon 2020 European Research Council PoC 639304 to Z. D.
References
Achituv, Y., and Dubinsky, Z. (1990). “Evolution and zoogeography of coral reefs,” in Ecosystems of the World 25. Coral Reefs, ed Z. Dubinsky (Amsterdam: Elsevier), 1–19.
Al-Horani, F. A., Al-Moghrabi, S. M., and De Beer, D. (2003). The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426. doi: 10.1007/s00227-002-0981-8.
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Baker, A. C. (2001). Ecosystems - Reef corals bleach to survive change. Nature 411, 765–766. doi: 10.1038/35081151
Barnes, D. J., and Taylor, D. L. (1973). In-situ studies of calcification and photosynthetic carbon fixation in coral Montastrea-annularis. Helgolander Wissenschaftliche Meeresuntersuchungen 24, 284–291. doi: 10.1007/BF01609519
Berner, T., Dubinsky, Z., Wyman, K., and Falkowski, P. G. (1989). Photoadaptation and the package effect in Dunaliella-tertiolecta (chlorophyceae). J. Phycol. 25, 70–78. doi: 10.1111/j.0022-3646.1989.00070.x
Brown, B. E. (2005). The fate of coral reefs in the Andaman Sea, eastern Indian Ocean following the Sumatran earthquake and tsunami, 26 December 2004. Geograp. J. 171, 372–374. doi: 10.1111/j.1475-4959.2005.00175_2.x
Brown, B. E., Dunne, R. P., Ambarsari, I., Le Tissier, M. D. A., and Satapoomin, U. (1999). Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar. Ecol. Prog. Ser. 191, 53–69. doi: 10.3354/meps191053
Brown, B. E., Dunne, R. P., Scoffin, T. P., and Letissier, M. D. A. (1994). Solar damage in intertidal corals. Mar. Ecol. Prog. Ser. 112, 312–312. doi: 10.3354/meps105219
Carey, S., Morelli, D., Sigurdsson, H., and Bronto, S. (2001). Tsunami deposits from major explosive eruptions: an example from the 1883 eruption of Krakatau. Geology 29, 347–350. doi: 10.1130/0091-7613(2001)029<0347:tdfmee>2.0.co;2
Carricart-Ganivet, J. P., Lough, J. M., and Barnes, D. J. (2007). Growth and luminescence characteristics in skeletons of massive Porites from a depth gradient in the central Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 351, 27–36. doi: 10.1016/j.jembe.2007.05.038
Chalker, B. E. (1976). Calcium-transport during skeletogenesis in hermatypic corals. Comp. Biochem. Physiol. A Physiol. 54, 455–459. doi: 10.1016/0300-9629(76)90049-9
Chalker, B. E. (1981). Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar. Biol. 63, 135–141. doi: 10.1007/BF00406821
Chalker, B. E., Dunlap, W. C., and Oliver, J. K. (1983). Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier-Reef, Australia.2. Light saturation curves for photosynthesis and respiration. J. Exp. Mar. Biol. Ecol. 73, 37–56. doi: 10.1016/0022-0981(83)90004-7
Chang, S. S., Prezelin, B. B., and Trench, R. K. (1983). Mechanisms of photoadaptation in 3 strains of the symbiotic dinoflagellate Symbiodinium-microadriaticum. Mar. Biol. 76, 219–229. doi: 10.1007/BF00393021
Cohen, I., Dishon, G., Iluz, D., and Dubinsky, Z. (2013). UV-B as a photoacclimatory enhancer of the hermatypic horal Stylophora pistillata. Open J. Mar. Sci. 3, 15–27. doi: 10.4236/ojms.2013.32A003
Colombo-Pallotta, M. F., Rodríguez-Román, A., and Iglesias-Prieto, R. (2010). Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29, 899–907. doi: 10.1007/s00338-010-0638-x
Dishon, G., Dubinsky, Z., Caras, T., Rahav, E., Bar-Zeev, E., Tzubery, Y., et al. (2012). Optical habitats of ultraphytoplankton groups in the Gulf of Eilat (Aqaba), Northern Red Sea. Int. J. Remote Sens. 33, 2683–2705. doi: 10.1080/01431161.2011.619209
Downs, C. A., Fauth, J. E., Halas, J. C., Dustan, P., Bemiss, J., and Woodley, C. M. (2002). Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 33, 533–543. doi: 10.1016/S0891-5849(02)00907-3
Drew, E. A. (1972). The biology and physiology of alga-invertebrate symbioses. I. The density of symbiotic algal cells in a number of hermatypic hard corals and alcyonarians from various depths. J. Exp. Mar. Biol. Ecol. 9, 71–75. doi: 10.1016/0022-0981(72)90008-1
Dunn, S. R., Bythell, J. C., Le Tissier, M. D. A., Burnett, W. J., and Thomason, J. C. (2002). Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. J. Exp. Mar. Biol. Ecol. 272, 29–53. doi: 10.1016/S0022-0981(02)00036-9
Dustan, P. (1979). Distribution of zooxanthellae and photosynthetic chloroplast pigments of the reef-building coral Montastrea-annularis Ellis and Solander in relation to depth on a West-Indian coral-reef. Bull. Mar. Sci. 29, 79–95.
Dustan, P. (1982). Depth-dependent photoadaptation by zooxanthellae of the reef coral Montastrea-annularis. Mar. Biol. 68, 253–264. doi: 10.1007/BF00409592
Erez, J. (1978). Vital effect on stable-isotope composition seen in foraminifera and coral skeletons. Nature 273, 199–202. doi: 10.1038/273199a0
Erez, J. (1983). “Calcification rates, photosynthesis and light in planktonic foraminifera,” in Biomineralization and Biological Metal Accumulation, eds P. Westbroek and E. W. de Jong (Renesse: Springer), 307–312.
Falkowski, P. G., and Dubinsky, Z. (1981). Light-shade adaptation of Stylophora-pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289, 172–174. doi: 10.1038/289172a0
Falkowski, P. G., Dubinsky, Z., Muscatine, L., and Porter, J. W. (1984). Light and the bioenergetics of a symbiotic coral. Bioscience 34, 705–709. doi: 10.2307/1309663
Falkowski, P. G., and Kolber, Z. (1995). Variations in chlorophyll fluorescence yields in phytoplankton in the world oceans. Aust. J. Plant Physiol. 22, 341–355. doi: 10.1071/PP9950341
Fang, L. S., Chen, Y. W. J., and Chen, C. S. (1989). Why does the white tip of stony coral grow so fast without zooxanthellae. Mar. Biol. 103, 359–363. doi: 10.1007/BF00397270
Figueroa, F. L., Conde-Alvarez, R., and Gomez, I. (2003). Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosyn. Res. 75, 259–275. doi: 10.1023/A:1023936313544
Fisher, T., Shurtzswirski, R., Gepstein, S., and Dubinsky, Z. (1989). Changes in the levels of ribulose-1,5-bisphosphate carboxylase oxygenase (rubisco) in Tetraedron-minimum (chlorophyta) during light and shade adaptation. Plant Cell Physiol. 30, 221–228.
Fitt, W. K., Mcfarland, F. K., Warner, M. E., and Chilcoat, G. C. (2000). Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685. doi: 10.4319/lo.2000.45.3.0677
Franklin, D. J., Hoegh-Guldberg, P., Jones, R. J., and Berges, J. A. (2004). Cell death and degeneration in the symbiotic dinoflagellates of the coral Stylophora pistillata during bleaching. Mar. Ecol. Prog. Ser. 272, 117–130. doi: 10.3354/meps272117
Gattuso, J. P. (1985). “Features of depth effects on Stylophora pistillata, a hermatypic coral in the Gulf of Aqaba (Jordan, Red Sea),” in Procceedings 5th International Coral Reef Congress (Tahiti), 95–100.
Gillette, P. (2012). Intraspecific genetic variability in temperature tolerance in the coral pocillopora damicornis: effects on growth, photosynthesis and survival. Open Access Theses Paper, p. 383.
Gorbunov, M. Y., Kolber, Z. S., Lesser, M. P., and Falkowski, P. G. (2001). Photosynthesis and photoprotection in symbiotic corals. Limnol. Oceanogr. 46, 75–85. doi: 10.4319/lo.2001.46.1.0075
Goreau, T. F., and Goreau, N. (1959). The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. Biol. Bull. 117, 239–250. doi: 10.2307/1538903
Grigg, R. W., and Jones, A. T. (1997). Uplift caused by lithospheric flexure in the Hawaiian Archipelago as revealed by elevated coral deposits. Mar. Geol. 141, 11–25. doi: 10.1016/S0025-3227(97)00069-8
Hoegh-Guldberg, O., and Jones, R. J. (1999). Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Mar. Ecol. Prog. Ser. 183, 73–86. doi: 10.3354/meps183073
Huston, M. (1985). Variation in coral growth-rates with depth at Discovery Bay, Jamaica. Coral Reefs 4, 19–25. doi: 10.1007/BF00302200
Iglesias-Prieto, R., Beltran, V. H., Lajeunesse, T. C., Reyes-Bonilla, H., and Thome, P. E. (2004). Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. B Biol. Sci. 271, 1757–1763. doi: 10.1098/rspb.2004.2757
Iglesiasprieto, R., and Trench, R. K. (1994). Acclimation and adaptation to irradiance in symbiotic dinoflagellates.1. Responses of the photosynthetic unit to changes in photon flux-density. Mar. Ecol. Progr. Ser. 113, 163–175. doi: 10.3354/meps113163
Iglesias-Prieto, R., and Trench, R. K. (1997a). “Photoadaptation, photoacclimation and niche diversification in invertebrate-dinoflagellate symbioses,” Proceedings 8th International Coral Reef Symposium (Panamá), 1319–1324.
Iglesias-Prieto, R., and Trench, R. K. (1997b). Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar. Biol. 130, 23–33. doi: 10.1007/s002270050221
Jokiel, P. L., Maragos, J. E., and Franzisket, L. (1978). Coral growth: buoyant weight technique. Monogr. Oceanogr. Methodol. 5, 529–542.
Jokiel, P. L., and York, R. H. (1982). Solar ultraviolet photobiology of the reef coral Pocillopora-damicornis and symbiotic zooxanthellae. Bull. Mar. Sci. 32, 301–315.
Kaiser, P., Schlichter, D., and Fricke, H. W. (1993). Influence of light on algal symbionts of the deep-water coral Leptoseris-fragilis. Mar. Biol. 117, 45–52. doi: 10.1007/BF00346424
Karentz, D., Bothwell, M. L., Coffin, R. B., Hanson, A., Herndl, G. J., Kilham, S. S., et al. (1994). Impact of UV-B radiation on pelagic freshwater ecosystems: report of working group on bacteria and phytoplankton. Arch. Hydrobiol. Beih. Ergeb. Limnol. 43, 31–69.
Krause, G. H. (1988). Photoinhibition of photosynthesis - an evaluation of damaging and protective mechanisms. Physiol. Plant. 74, 566–574. doi: 10.1111/j.1399-3054.1988.tb02020.x
Kyle, D. J., Ohad, I., and Arntzen, C. J. (1984). Membrane-protein damage and repair - Selective loss of a quinone-protein function in chloroplast membranes. Proc. Natl. Acad. Sci. U.S. A. 81, 4070–4074. doi: 10.1073/pnas.81.13.4070
Lang, J. (1973). Coral reef project - papers in memory of Dr. Thomas F. Goreau. 11. Interspecific aggression by scleractinian corals.2. Why the race is not only to swift. Bull. Mar. Sci. 23, 260–279.
Leletkin, V. A., Titlyanov, E. A., and Dubinsky, Z. (1996). Photosynthesis and respiration of the zooxanthellae in hermatypic corals habitated on different depths of the Gulf of Filat. Photosynthetica 32, 481–490.
Lesser, M. P. (1996). Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 41, 271–283. doi: 10.4319/lo.1996.41.2.0271
Lesser, M. P., and Farrell, J. H. (2004). Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23, 367–377. doi: 10.1007/s00338-004-0392-z
Liew, S. C., and He, J. (2007). Uplift of a coral island in the Andaman Sea due to the 2004 Sumatra earthquake measured using remote sensing reflectance of water. Geosci. Remote Sens. Lett. IEEE 5, 701–704. doi: 10.1109/LGRS.2008.2004208
Lorenzen, C. J. (1967). Determination of chlorophyll and pheo-pigments - spectrophotometric equations. Limnol. Oceanogr. 12, 343–346. doi: 10.4319/lo.1967.12.2.0343
Lucas, J. M., and Knapp, L. W. (1997). A physiological evaluation of carbon sources for calcification in the octocoral Leptogorgia virgulata (Lamarck). J. Exp. Biol. 200, 2653–2662.
Marsh, J. A. (1970). Primary productivity of reef-building calcareous red algae. Ecology 51, 254–263. doi: 10.2307/1933661
Mass, T., Einbinder, S., Brokovich, E., Shashar, N., Vago, R., Erez, J., et al. (2007). Photoacclimation of Stylophora pistillata to light extremes: metabolism and calcification. Mar. Ecol. Prog. Ser. 334, 93–102. doi: 10.3354/meps334093
Masuda, K., Goto, M., Maruyama, T., and Miyachi, S. (1993). Adaptation of solitary corals and their zooxanthellae to low-light and UV-radiation. Mar. Biol. 117, 685–691. doi: 10.1007/BF00349781
Mccloskey, L. R., and Muscatine, L. (1984). Production and respiration in the red-sea coral Stylophora-pistillata as a function of depth. Proc. R. Soc. Series B Biol. Sci. 222, 215–230. doi: 10.1098/rspb.1984.0060
Moberg, F., Nystrom, M., Kautsky, N., Tedengren, M., and Jarayabhand, P. (1997). Effects of reduced salinity on the rates of photosynthesis and respiration in the hermatypic corals Porites lutea and Pocillopora damicornis. Mar. Ecol. Prog. Ser. 157, 53–59. doi: 10.3354/meps157053
Moore, G. W., and Moore, J. G. (1988). Large scale bedforms in boulder gravel produced by giant waves in Hawaii. Am. Spec. Pap. 229, 101–110. doi: 10.1130/spe229-p101
Moore, J. G., and Moore, G. W. (1984). Deposit from a giant wave on the island of Lanai, Hawaii. Science 226, 1312–1315. doi: 10.1126/science.226.4680.1312
Muscatine, L. (1990). The role of symbiotic algae in carbon and energy flux in reef corals. Coral Reefs 25, 75–87.
Muscatine, L., Falkowski, P. G., Porter, J. W., and Dubinsky, Z. (1984). Fate of photosynthetic fixed carbon in light-adapted and shade-adapted colonies of the symbiotic coral Stylophora-pistillata. Proc. R. Soc. Ser. B Biol. Sci. 222, 181–202. doi: 10.1098/rspb.1984.0058
Nii, C. M., and Muscatine, L. (1997). Oxidative stress in the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943): contribution of the animal to superoxide ion production at elevated temperature. Biol. Bull. 192, 444–456. doi: 10.2307/1542753
Nir, O., Gruber, D. F., Shemesh, E., Glasser, E., and Tchernov, D. (2014). Seasonal mesophotic coral bleaching of Stylophora pistillata in the Northern Red Sea. PLoS ONE 9:e84968. doi: 10.1371/journal.pone.0084968
Parker, G. M., and Muscatine, L. (1984). Photosynthesis-irradiance responses and photosynthetic periodicity in the sea anemone Aiptasia pulchella and its zooxanthellae. Mar. Biol. 82, 225–232. doi: 10.1007/BF00392403
Pearse, V. B., and Muscatin, L. (1971). Role of symbiotic algae (zooxanthellae) in coral calcification. Biol. Bull. 141, 350–363. doi: 10.2307/1540123
Porter, J. W., Muscatine, L., Dubinsky, Z., and Falkowski, P. G. (1984). Primary production and photoadaptation in light-adapted and shade-adapted colonies of the symbiotic coral, Stylophora-pistillata. Proc. R. Soc. Ser. B Biol. Sci. 222, 161–180. doi: 10.1098/rspb.1984.0057
Ralph, P. J., and Gademann, R. (2005). Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat. Bot. 82, 222–237. doi: 10.1016/j.aquabot.2005.02.006
Richier, S., Cottalorda, J.-M., Guillaume, M. M. M., Fernandez, C., Allemand, D., and Furla, P. (2008). Depth-dependant response to light of the reef building coral, Pocillopora verrucosa: implication of oxidative stress. J. Exp. Mar. Biol. Ecol. 357, 48–56. doi: 10.1016/j.jembe.2007.12.026
Rowan, R., Knowlton, N., Baker, A., and Jara, J. (1997). Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269. doi: 10.1038/40843
Scelfo, G. M. (1986). “Relationship between solar radiation and pigments of the coral Montipora verrucosa and its zooxanthellae,” in Coral Reef Population Biology. Technical Report 37, eds P. L. Jokiel, R. H. Richmond and R. A. Rogers (Honolulu: Hawaii Institute of Marine Biology).
Scharf, D. (1982). The Adaptation of the Hermatypic S. Pistillata to Various Light Intensity. Ramat-Gan: Bar-Ilan University.
Schreiber, U. (2004). “Pulse-amplitude (PAM) fluorometry and saturation pulse method,” in Chlorophyll Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration Series, eds G. Papageorgiou and Govindjee (Dordrecht: Kluwer Academic Publishers), 279–319.
Searle, M. (2006). Co-seismic uplift of coral reefs along the western Andaman Islands during the December 26th 2004 earthquake. Coral Reefs 25, 2–2. doi: 10.1007/s00338-005-0051-z
Shick, J. M., Lesser, M. P., Dunlap, W. C., Stochaj, W. R., Chalker, B. E., and Won, J. W. (1995). Depth-dependent responses to solar ultraviolet-radiation and oxidative stress in the zooxanthellate coral Acropora-microphthalma. Mar. Biol. 122, 41–51. doi: 10.1007/BF00349276
Shick, J. M., Lesser, M. P., and Stochaj, W. R. (1991). Ultraviolet-radiation and photooxidative stress in zooxanthellate anthozoa: the sea-anemone Phyllodiscus-semoni and the Octocoral clavularia-sp. Symbiosis 10, 145–173.
Smith, D. J., Suggett, D. J., and Baker, N. R. (2005). Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Global Change Biol. 11, 1–11. doi: 10.1111/j.1365-2486.2004.00895.x
Stambler, N., and Dubinsky, Z. (2004). “Stress effects on metabolism of hermatypic coral,” in Coral Health and Disease, eds E. Rosenberg and Y. Loya (Berlin: Springer-Verlag), 195–215. doi: 10.1007/978-3-662-06414-6_9
Stambler, N., and Dubinsky, Z. (2005). Corals as light collectors: an integrating sphere approach. Coral Reefs 24, 1–9. doi: 10.1007/s00338-004-0452-4
Steemann Nielsen, E. (1975). Marine Photosynthesis with Special Emphasis on the Ecological Aspects. Amsterdam: Elsevier Scientific Publishing Co.
Tambutte, E., Tambutte, S., Segonds, N., Zoccola, D., Venn, A., Erez, J., et al. (2012). Calcein labelling and electrophysiology: insights on coral tissue permeability and calcification. Proc. R. Soc. B Biol. Sci. 279, 19–27. doi: 10.1098/rspb.2011.0733
Titlyanov, E. A., Shaposhnikova, M. G., and Zvalinskii, V. I. (1980). Photosynthesis and adaptation of corals to irradiance.1. Contents and native-state of photosynthetic pigments in symbiotic microalga. Photosynthetica 14, 413–421.
Vareschi, E., and Fricke, H. (1986). Light responses of a scleractinian coral (plerogyra-sinuosa). Mar. Biol. 90, 395–402. doi: 10.1007/BF00428563
Warner, M. E., Fitt, W. K., and Schmidt, G. W. (1999). Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. U.S.A. 96, 8007–8012. doi: 10.1073/pnas.96.14.8007
Winters, G., Beer, S., Ben Zvi, B., Brickner, I., and Loya, Y. (2009). Spatial and temporal photoacclimation of Stylophora pistillata: zooxanthella size, pigmentation, location and clade. Mar. Ecol. Prog. Ser. 384, 107–119. doi: 10.3354/meps08036
Yamano, H., Kayanne, H., and Yonekura, N. (2001). Anatomy of a modern coral reef flat: a recorder of storms and uplift in the late Holocene. J. Sediment. Res. 71, 295–304. doi: 10.1306/082900710295
Yap, H. T., Alvarez, R. M., Custodio, H. M., and Dizon, R. M. (1998). Physiological and ecological aspects of coral transplantation. J. Exp. Mar. Biol. Ecol. 229, 69–84. doi: 10.1016/S0022-0981(98)00041-0
Keywords: photosynthesis, calcification, photoacclimation, survival, Symbiosis
Citation: Cohen I and Dubinsky Z (2015) Long term photoacclimation responses of the coral Stylophora pistillata to reciprocal deep to shallow transplantation: photosynthesis and calcification. Front. Mar. Sci. 2:45. doi: 10.3389/fmars.2015.00045
Received: 01 March 2015; Accepted: 03 June 2015;
Published: 23 June 2015.
Edited by:
Hajime Kayanne, The University of Tokyo, JapanReviewed by:
Juan Pablo Carricart-Ganivet, Universidad Nacional Autónoma de México, MexicoSaki Harii, University of the Ryukyus, Japan
Copyright © 2015 Cohen and Dubinsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Itay Cohen, Department of Oceanography, The Institute of Earth Sciences, The Hebrew University of Jerusalem, Campus of Givat Ram, Balfur St., Jerusalem 91904, Israel, 2itaycohen@gmail.com
 Itay Cohen
Itay Cohen Zvy Dubinsky
Zvy Dubinsky