Cathodic stripping voltammetric determination of chromium in coastal waters on cubic Nano-titanium carbide loaded gold nanoparticles modified electrode
- 1Key Laboratory of Coastal Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai, China
- 2The Key Lab in Molecular and Nano-materials Probes of the Ministry of Education of China, College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan Shandong, China
The novel cubical nano-titanium carbide loaded gold nanoparticles modified electrode for selective and sensitive detection of trace chromium (Cr) in coastal water was established based on a simple approach. Nano-titanium carbide is used as the typical cubical nanomaterial with wonderful catalytic activity toward the reduction of Cr(VI). Gold nanoparticles with excellent physical and chemical properties can facilitate electron transfer and enhance the catalytic activity of the modified electrode. Taking advantage of the synergistic effects of nano-titanium carbide and gold nanoparticles, the excellent cathodic signal responses for the stripping determination of Cr(VI) can be obtained. The limit of detection of this method is calculated as 2.08 μg L−1 with the linear calibration curve ranged from 5.2 to 1040 μg L−1. This analytical method can be used to detect Cr(VI) effectively without using any complexing agent. The fabricated electrode was successfully applied for the detection of chromium in coastal waters collected from the estuary giving Cr concentrations between 12.48 and 22.88 μg L−1 with the recovery between 96 and 105%.
Introduction
Chromium (Cr) is an ecotoxic trace metallic element that exists in soils, waters, and air. Due to the large discharge of Cr from various industrial processes such as electroplating (Zhang et al., 2012) and pigments (Kendig et al., 2001), the Cr speciation in terrestrial and oceanic systems has attracted considerable attention over the past several years. Cr is present in natural waters in two most stable oxidation states: Cr(VI) and Cr(III), which have different toxicities. Cr(VI) is a suspected carcinogen and soil, surface water, and seawater contaminant, toxic to humans and other mammals, while Cr(III) is an essential nutrient element for living organism (Zhou et al., 2012; Espada-Bellido et al., 2013). The seawater contaminant of Cr(VI) will produce serious impact to marine organism, even the whole marine ecosystem. It has been reported that by exposure of sample waters containing Cr(VI) and Cr(III) to UV light, Cr(III) could be oxidized to Cr(VI), with Cr(VI) concentration equal to the total Cr (Bobrowski et al., 2004; Bas, 2006). Therefore, determination of Cr(VI) in seawater has been an important task and challenge.
So far, many methods have been developed for the detection of Cr, such as inductively coupled plasma mass spectrometry (Zhang et al., 2008), chemiluminescence (Kanwal et al., 2010), colorimetric (Zhao et al., 2012), fluorescence resonance energy transfer assay (Liu et al., 2013), inductively coupled plasma atomic emission (ICP-AES) (Lai and Tseng, 2011), UV-vis spectroscopy (Jin et al., 2014), high performance liquid chromatography (HPLC) (Posta et al., 1996), and atomic absorption spectroscopy (Gardner and Comber, 2002). However, there are some disadvantages of these methods, for example, time-consuming procedures, tedious sample preparation, and special equipment needed (Zhou et al., 2013). Instead, electrochemical method has been recognized as one of the most attractive alternative methods for trace Cr analysis, benefiting from its advantages of low cost, simple, and convenient operation, fast experimental process, high sensitivity and selectivity, and potential application for on-site monitoring (Rodgers et al., 1999). To increase the performance of electrochemical detection of Cr, numerous of nanomaterials were employed to modify the work electrode.
Metal nanoparticles have been widely used in electroanalysis field due to their serious of advantageous properties. They provide important functions for electroanalysis: improved mass transport, high effective surface area, and catalytic properties (Liu et al., 2008). Gold nanoparticles (AuNPs) show excellent catalytic property toward the electrochemical reduction of Cr(VI) and have been used as the electrode modifier for the determination of Cr (Liu et al., 2007, 2008; Tsai and Chen, 2008). It should be noted that aggregation of AuNPs usually happens if the dip-coating of AuNPs aqueous solution is employed. So, the method based on the self-assembled monolayer of AuNPs was reported (Yang and Zhang, 2004; Zhou et al., 2006). However, it is not very convenient to modify the electrode surface with AuNPs by multi-step process. Therefore, electrodeposition was preferred for the preparation of AuNPs modified electrodes in one step process (Tsai and Chen, 2008). Nevertheless, the AuNPs deposited on the electrode surface usually fall off during the electrochemical detection process. To solve this problem, an appropriate supporter should be selected to load the AuNPs.
Recently, Nano-titanium carbide (Nano-TiC) as one of the nanosized transition metal carbides has attracted significant interest for a number of applications in many fields due to its intrinsic material properties such as high electrical conductivity, low density, high surface area, and catalytic activity (Rodríguez et al., 2009; Flaherty et al., 2010). Besides, the cubic phase also provides Nano-TiC the potential application as the supporter of AuNPs. More importantly, Nano-TiC exhibits interesting electrocatalytic properties.
In this paper, a modified electrode was fabricated using cubical Nano-TiC as the supporter to load AuNPs for the determination of chromium in coastal water samples. The fall off of deposited AuNPs was prevented by loaded on the cubical Nano-TiC. Moreover, based on the synergistic effects of Nano-TiC and AuNPs, such designed AuNPs/Nano-TiC modified glass carbon electrode (AuNPs/Nano-TiC/GCE) can offer remarkably improved sensitivity for voltammetric measurement of Cr(VI). Experimental conditions and analytical performances were systematically investigated. The developed electrode was successfully applied for the analysis of Cr in real coastal water samples with satisfactory results.
Material and Methods
Materials
Chloroauric acid (HAuCl4) was supplied by Sinopharm Chemical Reagent Co., Ltd. Nano-TiC was purchased from Nanjing Emperor Nano Material Co., Ltd., China. All other chemicals are analytical reagents used without further purification. Cr(VI) solution was prepared by dissolving potassium dichromate in deionized water. Deionized water (18.2 MΩ cm specific resistance) obtained from Pall Cascada laboratory water system was used throughout. Coastal water samples were collected from local (Yantai) estuary with different position.
Characterization
The properties of the AuNPs/Nano-TiC/GCE were characterized by scanning electron microscopy (SEM, Hitachi S-4800). All the electrochemical experiments were carried out in a conventional three-electrode cell controlled by CHI 660E Electrochemical Work Station. A modified glassy carbon (GC) disk (3 mm in diameter) was used as the work electrode, with an Ag/AgCl electrode and platinum foil serving as the reference and counter electrodes, respectively.
Fabrication of the AuNPs/Nano-TiC/GCE
Before used, the GCE was firstly mechanically polished over a micro-cloth with 0.05 μm alumina slurry and then was rinsed and ultrasonicated by deionized water. The suspension of 0.5 mg mL−1 Nano-TiC was obtained by dispersing the powders with deionized water, and then ultrasonicated for 10 min. The Nano-TiC/GCE was prepared by dropping above Nano-TiC suspension (10 μL) on the surface of GCE and drying with an infrared lamp. Then the Nano-TiC/GCE was immersed in the 0.5 mol L−1 sulfuric acid (H2SO4) solution containing 1 mmol L−1 HAuCl4, and electrodeposited at the potential of −0.2 V for 3 s under stirring. After cleaning carefully, the AuNPs/Nano-TiC/GCE was obtained. Schematic illustration of the fabrication process is existed (Figure 1). For comparison, AuNPs/GCE was fabricated through electrodepositing AuNPs on GCE directly.
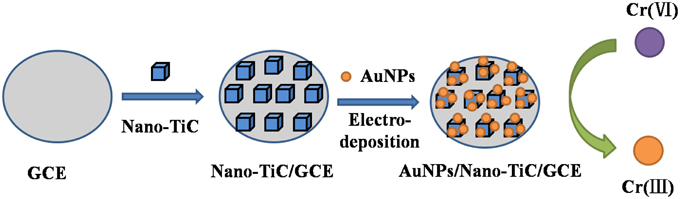
Figure 1. Schematic illustration for the fabrication process of the proposed electrode designed for Cr (VI) detection.
Electrochemical Procedure
The AuNPs/Nano-TiC/GCE was placed in a voltammetric cell with 10 ml electrolyte solution during the electrochemical measurements. Differential pulse voltammetry (DPV) technique was used to investigate the response of AuNPs/Nano-TiC/GCE toward the electroreduction of Cr(VI). DPVs were performed in hydrochloric acid (HCl, pH = 2), if not stated otherwise. The Cr(VI) solutions were spiked into the cell and the accumulation was carried out at 0.5 V with stirring. After a 10 s quilibration period, the voltammogram was recorded by applying a negative going DPV potential scan from 0.65 to 0.1 V (with a step potential of 8 mV and amplitude of 50 mV).
Results
SEM Characterization of the Modified Electrode
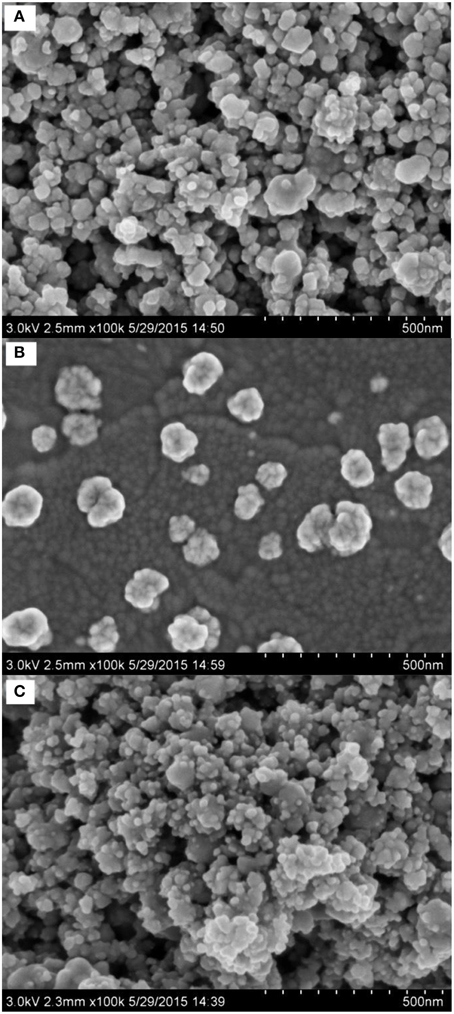
SEM is an efficient tool for the morphology investigation of modified electrodes. The SEM photographs of Nano-TiC/GCE (Figure 2A), AuNPs/GCE (Figure 2B), and AuNPs/Nano-TiC/GCE (Figure 2C) are illustrated here. It can be clearly observed that cubical Nano-TiC on electrode surface aggregates to form the Nano-TiC clusters. AuNPs with about 100 nm in diameter were distributed on the AuNPs/GCE. As to the AuNPs/Nano-TiC/GCE, the morphology of Nano-TiC was similar to that on Nano-TiC/GCE, but the clusters are covered with numerous of AuNPs with a smaller diameter (about 15 nm).
Electrochemical Properties of the Modified Electrode
To investigate the electrochemical properties of the modified electrode, cyclic voltammograms (CVs) of different electrodes in 0.5 mol L−1 H2SO4 from 0.2 to 1.2 V with a scan rate of 100 mV s−1 are shown in Figure 3A. As expected, no redox peak can be observed in the CVs of GCE and Nano-TiC/GCE within the scan range. However, a reduction peak at about 0.6 V can be observed on the AuNPs/GCE. As to the AuNPs/Nano-TiC/GCE, the reduction peak appears larger and shifts positively to about 0.65 V. The DPV responses of different electrodes toward Cr(VI) in HCl (pH = 2) are also explored and shown in Figure 3B. There is no reduction peak at the GCE with 520 μg L−1 Cr(VI). However, the reduction peak of Cr(VI) appears at about 0.22 and 0.42 V for AuNPs/GCE and Nano-TiC/GCE respectively. By contrast, the reduction peak of Cr(VI) at AuNPs/Nano-TiC/GCE is much larger than that of AuNPs/GCE and Nano-TiC/GCE, which is also at about 0.42 V.
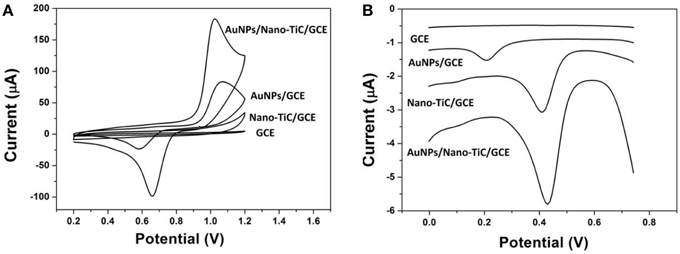
Figure 3. Cyclic voltammograms (A) in 0.5 mol L−1 H2SO4 solution (scan rate: 100 mV s−1) and differential pulse voltammograms (B) in HCl solution (with 520 μg L−1 Cr(VI), pH = 2) of GCE, Nano-TiC/GCE, AuNPs/GCE, and AuNPs/Nano-TiC/GCE.
Optimization for Cr(VI) Detection at AuNPs/Nano-TiC/GCE
The parameters that control the analytical performance of Cr(VI) on the AuNPs/Nano-TiC/GCE have been optimized. Figure 4A shows the effect of Nano-TiC volumes dropped at the GCE surface on the DPV signals for 520 μg L−1 Cr(VI). When 10 μL was adopted, the fabricated electrode has the biggest reduction current. The effect of electrodepositing time on the response of this electrode was shown in Figure 4B. It can be seen that 3 s was the best time for the AuNPs electrodeposition. Figure 4C exhibits the effect of pH (HCl solution) values on the response of the proposed electrode. Clearly, the detection performance of Cr(VI) was best when the pH value of HCl solution was equal to 2.
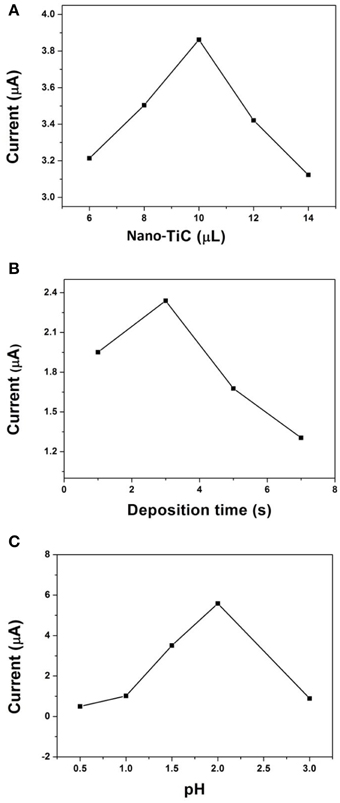
Figure 4. Effect of Nano-TiC volumes (A), electrodepositing time (B), and pH values (C) on the DPV signals for 520 μg L−1 Cr(VI).
Analytical Performance
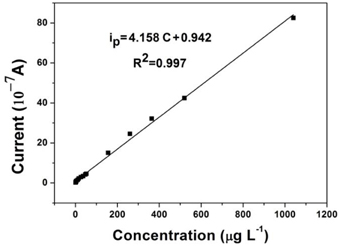
For accurate analysis, a linear calibration curve between reduction current and Cr(VI) concentration is necessary. Figure 5 displays the calibration curve of the AuNPs/Nano-TiC/GCE for increasing Cr(VI) concentrations under the optimum experimental conditions. A linear relationship between the reduction current and Cr(VI) concentration was obtained covering the concentration range from 5.2 to 1040 μg L−1. The linear regression equation was ip = 4.158 C + 0.942 (R2 = 0.997), in which ip as the peak current and C the concentration of Cr(VI). The limit detection was calculated as 2.08 μg L−1 (S/N = 3).
The reproducibility of the AuNPs/Nano-TiC/GCE was evaluated in 520 μg L−1 Cr(VI) by ten independent electrodes prepared in the same way, and the corresponding relative standard deviation (RSD) was 3.79%. The repeatability of the proposed electrode was studied by detecting 520 μg L−1 Cr(VI) using the same electrode for ten measurements, and the RSD was 3.32%. To verify the anti-interference ability of this electrode various foreign species were added into the HCl (pH = 2) solution containing 520 μg L−1 Cr(VI). It was found that additions of 1000 fold excess of Na+, K+, Mg2+, Ca2+, 100 fold excess of Pb2+, Mn2+, Cu2+, Cd2+, Cr3+, and 50 fold excess of Fe3+ did not affect the determination of Cr(VI) (5% current change).
Coastal Water Samples Analysis
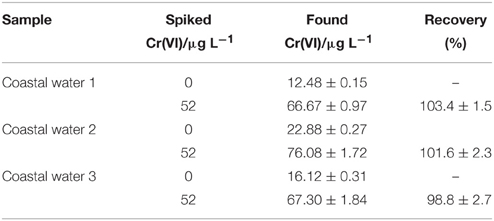
This proposed modified electrode was used for the analysis of coastal water samples. Before detected with the proposed method, all coastal water samples were filtered with 0.45 μm membrane filters and then UV-digested after adjusting the pH to less than 2.0 to make sure that all ligands bound to Cr were released and all Cr species were oxidized to Cr(VI). About 100 μL HCl (1 mol L−1) were added to 9.9 mL above samples solution to adjust the pH value equal to 2. After stirring, the DPV procedure mentioned above were operated. The obtained results are shown in Table 1.
Discussion
From the SEM photographs of different electrodes (Figure 2), it can be concluded that AuNPs are loaded on Nano-TiC uniformly. The reduction of AuNPs diameter was caused by the existence of Nano-TiC, and this can provide more active sites for Cr(VI) electrochemical reduction. The amount of AuNPs was increased long with the reduction of AuNPs diameter.
The CV comparison of different electrodes (Figure 3A) shows that the obtained electrode has the strongest electrochemical activity. Nano-TiC can enhance the background current due to its high specific surface area. For AuNPs/GCE, the reduction peak at about 0.6 V was caused by the AuNPs. As to the AuNPs/Nano-TiC/GCE, the enhancement of the reduction peak of AuNPs may be caused by the reduction of AuNPs diameter and increase of AuNPs amount as well as the electrocatalytic activity of Nano-TiC. Figure 3B illustrates that all Nano-TiC and AuNPs have the electrocatalytic effect on the reduction of Cr(VI). However, due to the synergistic effects of Nano-TiC and AuNPs, AuNPs/Nano-TiC/GCE has the biggest electrocatalytic response.
Figure 4 shows the parameters that control the analytical performance of Cr(VI) on AuNPs/Nano-TiC/GCE. 10 μL Nano-TiC (0.5 mg mL−1), 3 s electrodepositing time, and pH = 2 HCl solution are the optimized parameters. A larger Nano-TiC volume, the response signal started to decrease. A possible explanation to this phenomenon is that the Nano-TiC film is so thick to prevent electrons transfer. As mentioned above, the response of this electrode is due to the synergistic effects of Nano-TiC and AuNPs, and the effect of Nano-TiC is remarkable. So, if it is much more than Nano-TiC, AuNPs will manage the electrocatalytic response of this electrode. However, the effect of AuNPs is less than the synergistic effects of Nano-TiC and AuNPs. This is why 3 s is the best electrodepositing time. According to literature (Liu et al., 2008), Cr(VI) reduction is a proton-dependent process, so the pH is an important parameter. In this proposed method, the electrode has the largest response toward the Cr(VI) reduction when the pH of HCl solution was equal to 2.
The analytical performance of this proposed method is satisfactory. The linear range of this method spans the concentration of Cr(VI) from 5.2 to 1040 μg L−1, which is wider than that at AuNPs/GCE and graphite modified screen-printed electrode (graphite/SPE) (Liu et al., 2008; Hallam et al., 2010). The limit of detection is 2.08 μg L−1, which is also better than that at AuNPs/SPE, Au/SPE, and Au microchip electrode (Liu et al., 2007; Metters et al., 2012; Li et al., 2013). The comparison of performances of different modified electrodes for the determination of Cr(VI) is shown in Table 2. Moreover, the reproducibility, repeatability, and anti-interference ability of this electrode are also satisfactory, and this makes it possible for the analysis of coastal water samples.
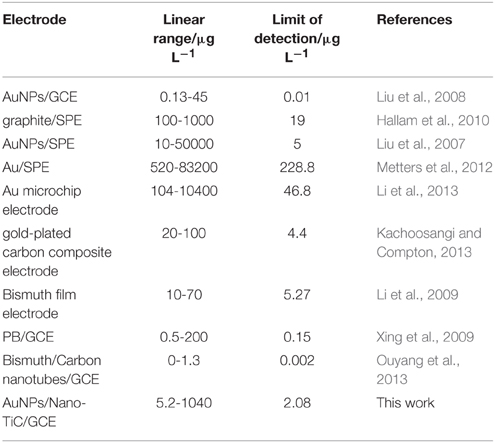
Table 2. Comparison of the performances of different modified electrodes for the determination of Cr(VI).
As to the practical analysis of coastal water, the Cr(VI) concentration from 12.48 to 22.88 μg L−1 can be detected in the estuarine water samples. To verify the feasibility of this method for coastal water detection, a recovery test was performed by adding known concentration (52 μg L−1) of Cr(VI) into the estuarine water samples. The percentages of recovery between 96 and 105% were obtained.
Conclusions
The novel modified electrode based on cubical Nano-TiC and AuNPs was fabricated for the determination of chromium in coastal water samples. AuNPs were loaded on Nano-TiC, and their diameter was decreased due to the existence of Nano-TiC. Meanwhile, the fall off of deposited AuNPs was prevented by loaded on the cubical Nano-TiC. Due to the synergistic effects of Nano-TiC and AuNPs, this fabricated AuNPs/Nano-TiC/GCE shows an excellent response toward the reduction of Cr(VI). The developed electrode was successfully applied for the analysis of Cr in real coastal water samples with satisfactory results. The limit of detection of this method is calculated as 2.08 μg L−1 with the linear calibration curve ranged from 5.2 to 1040 μg L−1. Cr(VI) with the concentration from 12.48 to 22.88 μg L−1 were detected in different estuarine water samples with this method. The percentages of recovery between 96 and 105% were obtained.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (41276093), the Youth Innovation Promotion Association (2011170) and the Outstanding Young Scientists Program of CAS. We would like to thank W. Wang for the SEM characterization of the modified electrode.
References
Bas, B. (2006). Refreshable mercury film silver based electrode for determination of chromium(VI) using catalytic adsorptive stripping voltammetry. Anal. Chim. Acta 570, 195–201. doi: 10.1016/j.aca.2006.04.013
Bobrowski, A., Mocak, J., Dominik, J., Pereira, H., Bas, B., and Knap, W. (2004). Metrological characteristics and comparison of analytical methods for determination of chromium traces in water samples. Acta Chim. Slov. 51, 77-93.
Espada-Bellido, E., Bi, Z., and van den Berg, C. M. G. (2013). Determination of chromium in estuarine waters by catalytic cathodic stripping voltammetry using a vibrating silver amalgam microwire electrode. Talanta 105, 287–291. doi: 10.1016/j.talanta.2012.10.031
Flaherty, D., May, R., Berglund, S., Stevenson, K., and Mullins, C. (2010). Low temperature synthesis and characterization of nanocrystalline titanium carbide with tunable porous architectures. Chem. Mater. 22, 319–329. doi: 10.1021/cm902184m
Gardner, M., and Comber, S. (2002). Determination of trace concentrations of hexavalent chromium. Analyst 127, 153–156. doi: 10.1039/b109374f
Hallam, P. M., Kampouris, D. K., Kadara, R. O., and Banks, C. E. (2010). Graphite screen printed electrodes for the electrochemical sensing of chromium(VI). Analyst 135, 1947–1952. doi: 10.1039/c0an00228c
Jin, W., Wu, G., and Chen, A. (2014). Sensitive and selective electrochemical detection of chromium(VI) based on gold nanoparticle-decorated titania nanotube arrays. Analyst 139, 235–241. doi: 10.1039/C3AN01614E
Kachoosangi, R., and Compton, R. (2013). Voltammetric determination of Chromium(VI) using a gold film modified carbon composite electrode. Sens. Actuators B 178, 555–562. doi: 10.1016/j.snb.2012.12.122
Kanwal, S., Fu, X., and Su, X. (2010). Size dependent active effect of CdTe quantum dots on pyrogallol-H2O2 chemiluminescence system for chromium(III) detection. Microchim. Acta 169, 167–172. doi: 10.1007/s00604-010-0334-0
Kendig, M., Jeanjaquet, S., Addison, R., and Waldrop, J. (2001). Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surf. Coat. Tech. 140, 58–66. doi: 10.1016/S0257-8972(01)01099-4
Lai, Y. J., and Tseng, W. L. (2011). Role of 5-thio-(2-nitrobenzoic acid)-capped gold nanoparticles in the sensing of chromium(VI): remover and sensor. Analyst 136, 2712–2717. doi: 10.1039/c1an15162b
Li, D., Li, J., Jia, X., Xia, Y., Zhang, X., and Wang, E. (2013). A novel Au-Ag-Pt three-electrode microchip sensing platform for chromium(VI) determination. Anal. Chim. Acta 804, 98–103. doi: 10.1016/j.aca.2013.10.014
Li, J., Zhang, J., Wei, H., and Wang, E. (2009). Combining chemical reduction with an electrochemical technique for the simultaneous detection of Cr(VI), Pb(II) and Cd(II). Analyst 134, 273–562. doi: 10.1039/B804670K
Liu, B., Lu, L., Wang, M., and Zi, Y. (2008). A study of nanostructured gold modified glassy carbon electrode for the determination of trace Cr(VI). J. Chem. Sci. 120, 493–498. doi: 10.1007/s12039-008-0077-1
Liu, B., Tan, H., and Chen, Y. (2013). Upconversion nanoparticle-based fluorescence resonance energy transfer assay for Cr(III) ions in urine. Anal. Chim. Acta 761, 178–185. doi: 10.1016/j.aca.2012.11.035
Liu, G., Lin, Y. Y., Wu, H., and Lin, Y. (2007). Voltammetric detection of Cr(VI) with disposable screen-printed electrode modified with gold nanoparticles. Environ. Sci. Technol. 41, 8129–8134. doi: 10.1021/es071726z
Metters, J. P., Kadara, R. O., and Banks, C. E. (2012). Electroanalytical sensing of chromium(III) and (VI) utilising gold screen printed macro electrodes. Analyst 137, 896–902. doi: 10.1039/c2an16054d
Ouyang, R., Zhang, W., Zhou, S., Xue, Z. L., Xu, L., Gu, Y., et al. (2013). Improved Bi film wrapped single walled carbon nanotubes for ultrasensitive electrochemical detection of trace Cr(VI). Electrochim. Acta 113, 686–693. doi: 10.1016/j.electacta.2013.09.110
Posta, J., Alimonti, A., Petrucci, F., and Caroli, S. (1996). On-line separation and preconcentration of chromium species in seawater. Anal. Chim. Acta 325, 185–193. doi: 10.1016/0003-2670(96)00030-X
Rodgers, J., Jedral, W., and Bunce, N. (1999). Electrochemical oxidation of chlorinated phenols. Environ. Sci. Technol. 33, 1453–1457. doi: 10.1021/es9808189
Rodríguez, J. A., Feria, L., Jirsak, T., Takahashi, Y., Nakamura, K., and Illas, F. (2009). Role of Au-C Interactions on the Catalytic Activity of Au Nanoparticles Supported on TiC(001) toward Molecular Oxygen Dissociation. J. Am. Chem. Soc. 132, 3177–3186. doi: 10.1021/ja910146g
Tsai, M. C., and Chen, P. Y. (2008). Voltammetric study and electrochemical detection of hexavalent chromium at gold nanoparticle-electrodeposited indium tinoxide (ITO) electrodes in acidic media. Talanta 76, 533–539. doi: 10.1016/j.talanta.2008.03.043
Xing, S., Xu, H., Shi, G., Chen, J., Zeng, L., and Jin, L. (2009). A simple and sensitive method for the amperometric detection of trace Chromium (VI) based on prussian blue modified glassy carbon electrode. Electroanalysis 21, 1678–1684. doi: 10.1002/elan.200904594
Yang, M., and Zhang, Z. (2004). Impediment to heterogeneous electron transfer reactions of redox-active species by alkanedithiol self-assembled monolayers with and without an adlayer of Au nanoparticles. Electrochim. Acta 49, 5089–5095. doi: 10.1016/j.electacta.2004.06.020
Zhang, N., Suleiman, J. S., He, M., and Hu, B. (2008). Chromium(III)-imprinted silica gel for speciation analysis of chromium in environmental water samples with ICP-MS detection. Talanta 75, 536–543. doi: 10.1016/j.talanta.2007.11.059
Zhang, W., Zhuang, L., Tong, L., Lo, I. M. C., and Qiu, R. (2012). Electro-migration of heavy metals in an aged electroplating contaminated soil affected by the coexisting hexavalent chromium. Chemosphere 86, 809–816. doi: 10.1016/j.chemosphere.2011.11.042
Zhao, L., Jin, Y., Yan, Z., Liu, Y., and Zhu, H. (2012). Novel, highly selective detection of Cr(III) in aqueous solution based on a gold nanoparticles colorimetric assay and its application for determining Cr(VI). Anal. Chim. Acta 731, 75–81. doi: 10.1016/j.aca.2012.04.022
Zhou, N., Wang, J., Chen, T., Yu, Z., and Li, G. (2006). Enlargement of gold nanoparticles on the surface of a self-assembled monolayer modified electrode: a mode in biosensor design. Anal. Chem. 78, 5227–5230. doi: 10.1021/ac0605492
Zhou, Y., Li, Y. S., Meng, X. Y., Zhang, Y. Y., Yang, L., Li, Z. H., et al. (2013). Production of a monoclonal antibody and development of an immunoassay for detection of Cr(III) in water samples. Chemosphere 93, 2467–2472. doi: 10.1016/j.chemosphere.2013.08.088
Zhou, Y., Li, Y., Tian, X., Zhang, Y., Yang, L., Zhang, J., et al. (2012). Enhanced ultrasensitive detection of Cr(III) using 5-thio-2-nitrobenzoic acid (TNBA) and horseradish peroxidase (HRP) dually modified gold nanoparticles (AuNPs). Sens. Actuators B 161, 1108–1113. doi: 10.1016/j.snb.2011.12.035
Keywords: chromium, nano-titanium carbide, gold nanoparticles, cathodic stripping voltammetry, coastal water
Citation: Han H, Pan D, Liu D, Hu X, Lin M and Li F (2015) Cathodic stripping voltammetric determination of chromium in coastal waters on cubic Nano-titanium carbide loaded gold nanoparticles modified electrode. Front. Mar. Sci. 2:75. doi: 10.3389/fmars.2015.00075
Received: 23 July 2015; Accepted: 14 September 2015;
Published: 29 September 2015.
Edited by:
Hans Uwe Dahms, Kaohsiung Medical University, TaiwanReviewed by:
Xiaoshou Liu, Ocean University of China, ChinaChin-Chang Hung, National Sun Yat-sen University, Taiwan
Copyright © 2015 Han, Pan, Liu, Hu, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawei Pan, Key Laboratory of Coastal Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, 17th Chunhui Road, Laishan District, Yantai, Shandong 264003, China, dwpan@yic.ac.cn
 Haitao Han
Haitao Han Dawei Pan
Dawei Pan Dongyan Liu
Dongyan Liu Xueping Hu
Xueping Hu Mingyue Lin
Mingyue Lin Fei Li
Fei Li