The Effect of Essential Fatty Acids for the Somatic Growth in Nauplii of Calanus finmarchicus
- 1Department of Biology, NTNU Norwegian University of Science and Technology, Trondheim, Norway
- 2Planktonic AS, Trondheim, Norway
- 3Department of Biotechnology, NTNU Norwegian University of Science and Technology, Trondheim, Norway
The growth of Calanus finmarchicus nauplii was studied through three spawning seasons (2007, 2009, and 2011) using flow-through tubes. Natural seston was collected every second day and added as food source using a peristaltic pump. A mixture of cultured microalgae supplied in surplus concentration was used as a control treatment. No significant correlation between growth and food concentration measured as Chlorophyll a (Chla) or particulate organic carbon (POC) was detected, but the growth rate was significantly related to the content of EPA (20:5n-3) and DHA (22:6n-3) in the seston. The growth rate was overall higher for nauplii fed cultured microalgae compared to the nauplii fed natural seston. Although the nauplii fed algae cultures were fed surplus food, the growth did vary between the growth periods. Furthermore, the growth rate for nauplii fed natural seston and for nauplii fed cultured algae were positively related, suggesting that the maternal condition and the food quality experienced by the mothers could explain some of the variation in naupliar growth rate. We present lipid class data on C. finmarchicus eggs from field samples that, contrary to previous studies, showed a high content of wax esters. Fatty acid analyzes of eggs, nauplii stages, and copepodites from field samples showed that eggs and nauplii have a similar fatty acid composition and that the main increase in the content and share of DHA and EPA was from nauplii to copepodite. The secondary production measured as naupliar growth was compared to the secondary production measured as carbon specific female egg production rate. The secondary production measured as egg production was generally higher than the secondary production measured as naupliar growth early in the spring, whereas the opposite situation was observed during post-bloom situations in late spring/early summer.
Introduction
Calanus finmarchicus is the dominating copepod in the North Atlantic and Barents Sea (Conover, 1988). It is a vital link between marine primary production and higher trophic levels as an important food source for planktivorous fishes, whales, gelatinous zooplankton, carnivorous zooplankton and bottom dwellers like sponges, and corals (Payne et al., 1990; Dommasnes et al., 2004; Tönnesson et al., 2006; Blachowiak-Samolyk et al., 2007; Watling, 2007; Dalpadado et al., 2008; Ohman et al., 2008; Dodds et al., 2009). Eggs and nauplii stages of copepods are the most important food source of many larval fishes and therefore of great significance in their recruitment (Kane, 1984; Runge, 1988; Planque and Batten, 2000).
In order to quantify food transfer in trophic levels, estimation of zooplankton secondary production is inevitable. This is done mainly by studying cohort development; either by sorting individual nauplii or by creating an artificial cohort based on size-fractionation (Winberg, 1971). Measurement of egg production can be used to estimate secondary production, under the assumption that female copepods use all their assimilated energy to create offspring. The energy incorporated into produced eggs thus is a direct measure of secondary production (Kiørboe and Johansen, 1986; Poulet et al., 1995).
The stages of egg production, hatching success, and subsequent growth of naupliar stages are critical stages for development for calanoid copepods, and high rates of egg production are therefore not always followed by an increase in copepodite abundance (Jonasdottir et al., 2008). Different mechanisms are proposed to explain this, including cannibalism (Bonnet et al., 2004; Basedow and Tande, 2006), predation (Eiane et al., 2002; Ohman et al., 2004, 2008), food limitation (Koski et al., 2010), and toxic effects from diatoms (Ianora et al., 2003). The nutritional value of the food, and specifically the content of long-chain polyunsaturated n-3 fatty acids (LC-n-3 PUFAs) in the food, particularly eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), have been shown to be beneficial for reproductive rates of copepods (Pond et al., 1996; Jonasdottir et al., 2002, 2005; Evjemo et al., 2008).
The hatching and the growth through the two first nauplii stages is also sensitive for maternal effects. Higher hatching success and protein content are found in offspring of females subjected to high food availability and high contents of essential fatty acids in their food (Koski et al., 2012). The reproduction rate of C. finmarchicus in the Trondheimsfjord was found to be closely linked to food concentration, essential fatty acid content of the seston and also to the content of specific fatty acids (DHA, EPA) in female copepods (Leiknes, 2016).
The oil sac in female copepods is in close proximity to the gonads, and the lipid content of females generally decreases as they produce eggs. The eggs are known to have a high number of yolk granules with lipovitellin (peptides, phospholipids, and cholesterol), and lipid droplets (wax esters or triacylglycerols). The embryo utilizes these yolk granules for energy and biosynthesis of membranes and hormones (Lee and Walker, 1995). However, previous studies on lipid class composition of eggs from Calanus helgolandicus and C. finmarchicus are scarce and have shown substantial variability (Lee et al., 1972b; Gatten et al., 1980; Ohman and Runge, 1994).
The first two naupliar stages of C. finmarchicus do not feed at all and depends solely on egg yolk for its nourishment. Hence, the growth measured as individual dry weight or carbon content is negative (e.g., Harris et al., 2000). They spend most of their egg yolk and some lipid droplets in their first molts, but start to gain weight from nauplii stage III onwards. The main storage lipid increments take place during copepodite stages CI–CV. The growth stages of C. finmarchicus has been mainly studied in laboratory (Corkett et al., 1986; Tande, 1988; Campbell et al., 2001b) and mesocosm experiments (Harris et al., 2000; Hygum et al., 2000a,b). The growth rate was shown to be affected by temperature and food availability (Møller et al., 2012), although it appears that naupliar stages are less sensitive to low food concentrations than copepodites (Hygum et al., 2000a). Although the conversion of storage lipids into eggs by female copepods and subsequent growth and development of nauplii has shown to be sensitive for the availability of specific polyunsaturated fatty acids, relatively little work is done on lipid and fatty acid composition of copepod eggs and early nauplii stages (Kattner et al., 2007).
This study aims at evaluating effect of food quantity and quality on growth of C. finmarchicus nauplii. Since previous studies has shown that development of eggs, nauplii, and copepodites are sensitive to fatty acid composition via maternal effects and food, a further aim for the study was to survey the development of fatty acid content and composition in C. finmarchicus eggs, nauplii, and copepodites, and lipid class composition for C. finmarchicus eggs. A high content of essential fatty acids in eggs, nauplii, and copepodites could further indicate a high affinity for these fatty acids. As previously mentioned, the secondary production can be measured both from somatic growth and from egg production experiments. We wanted to compare the two zooplankton production measurements to discuss whether or not the results are comparable.
Materials and Methods
C. finmarchicus females were collected through three spring seasons; 2007, 2009 and 2011, from two locations in the Trondheimsfjord, sampling stations Munkholmen (N 63°27′, E 10°20′; 2007) and Trollet (N 63°29′, E 10°18′; 2009 and 2011; Table 2). Samples for fatty acid analysis of Calanus eggs, nauplii, and copepodites were collected during the same cruises, but with additional sampling during cruises in 2007 and 2010 (Tables 1, 2). During cruises in 2007, we used two different plankton nets; a plankton net with mesh size 500 μm (diameter 1.5 m, length 10 m) with a non-filtering cod end and a plankton net with 70 μm mesh size (diameter 1 m, length 7 m). The nets were hauled horizontally at 10 m depth at a speed of ~0.5 m s−1 for 20 min. The coarse-meshed net was used to collect female C. finmarchicus and the fine-meshed net was used for collecting eggs and nauplii. In 2009 and 2011, females were sampled with repeated vertical net hauls from 50 m depth to surface using a modified Nansen net with 200 μm mesh size and a large, non-filtering cod end. In 2007 and 2010 smaller stages of copepods were collected with 70 μm net. The copepodites and nauplii were carefully transferred to 25 L tanks containing surface water and brought to the laboratory for further sorting within 1 h after sampling.
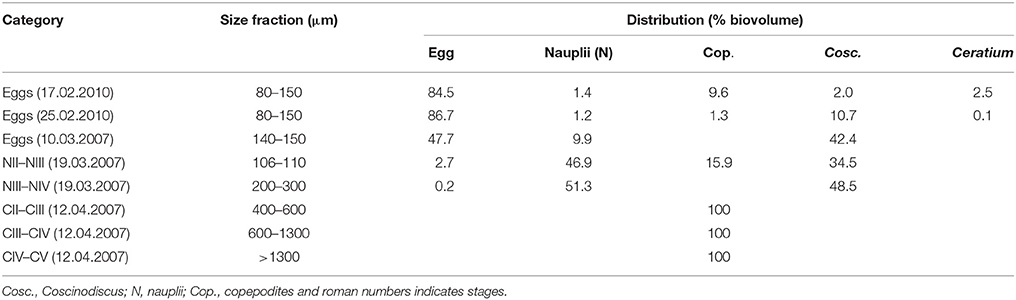
Table 1. Size fractions and the percent distribution by biovolume for the fractions selected for fatty acid analysis.
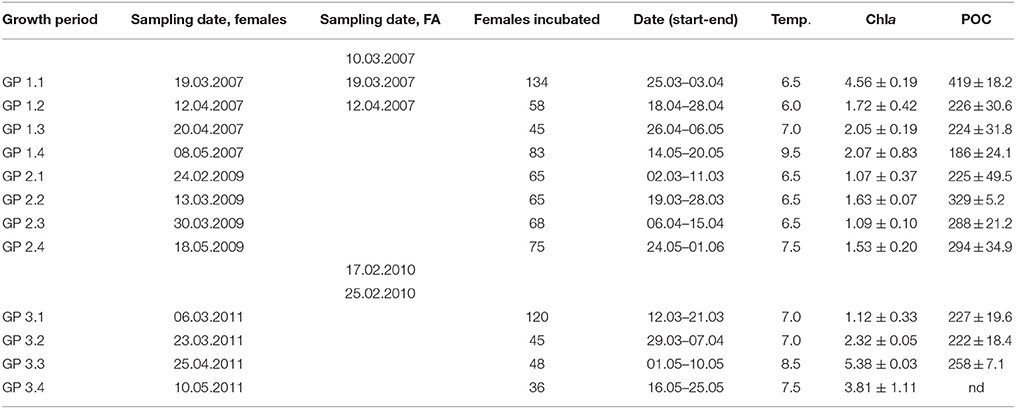
Table 2. Sampling dates for females and fatty acids (FA), number of females incubated, dates for growth experiments, incubation temperatures (°C) and concentration of Chla (μg L−1), and particulate organic carbon (POC, μg C L−1).
The various stages of C. finmarchicus for lipid analysis were separated by successive filtration of the material collected with 70 μm net. A filtration tower with 25 different mesh sizes of polyethylene sieves (diameter 10 cm) were used for this. The sample (>70 μm) was poured into the successive filtration device at the top and thoroughly washed with 10 μm filtered seawater. The major part of the content in each tube was allowed to drip of. The material was transferred into sterile plastic vials (20 mL) and stored at −80°C under N2 atmosphere for further analysis. In order to estimate biovolume of organisms present in the fatty acid samples, a subsample taken from each sieve was fixed with acidic Lugol (1% final concentration). The abundance and biovolume of different organisms present was calculated from microscope counts using either a stereoscopic microscope (Leica MZ6) or an inverted microscope (Leica DM IRB), depending on the size of the dominating organisms. From the sampling in 2007, our experience was that many of the sieves contained similar content. We therefore used a simplified filtration setup with fewer sieves to separate eggs in 2010 (Table 1).
Analysis of total lipids and fatty acid methyl esters (FAME) in the seston and in copepods was done according to Bergvik et al. (2012a) and analysis of lipid classes according to Bergvik et al. (2012b). During the sampling in 2010, we were able to obtain an almost pure sample of copepod eggs (Table 1) in quantities sufficient for analysis of both FAME and lipid classes (minimum 20 mg dry weight).
For egg production rate experiments; active, undamaged female copepods (n = 36–134, Table 2) were selected under stereomicroscope and incubated individually in petri dishes (diameter 55 mm) containing 20 mL GF/F-filtered seawater. The copepods were incubated in darkness in a temperature controlled room at 15°C for 48 h. The petri dishes were inspected for copepod eggs every 24 h. In order to avoid egg cannibalism, females were moved to a new petri dish if eggs were present. The individual Calanus females were fixed in ethanol, and we separated C. finmarchicus from C. helgolandicus based on the curvature of the fifth pair of swimming legs (Fleminger and Hulsemann, 1977). C. helgolandicus never constituted more than 10% of the total number of Calanus females incubated. The C. helgolandicus females were removed from the calculations, but were too few to be included as a separate species in our experiments. The eggs collected from female C. finmarchicus were further incubated for 96 h before enumerating nauplii.
The nauplii were mixed homogenously and transferred in equal numbers to flow-through Plexiglas tubes, for growth experiments. We used plexiglas-tubes (3 × 21 cm, volume 142 mL) submerged in a temperature controlled water bath and a multichannel peristaltic pump (Ismatech IPC) to supply water. The upper part of each plexiglas-tube (1 cm) was kept above water level. While water was added at the top of each tube, the base was fitted with a 20 μm plankton mesh to prevent nauplii from escaping. The overflow from the water bath was drained through a tube placed at the surface of the water bath. The flow rate of food suspension added to each tube was adjusted at 960 mL day−1. The nauplii were supplied with food from natural seawater collected at 3 m depth at Trondhjem Biological Station pier (N 63° 26′, E 10° 20′). Some nauplii cultures were fed cultured microalgae (see below) suspended in filtered sea water. The food was supplied from 10 L Pyrex bottles, and each fresh batch was prepared every second day. The incubation temperature of the experiment was adjusted to mimic in situ temperature at 3 m depth, measured at the start of the incubation.
To remove any stray eggs or nauplii from natural seston suspension offered as food in our experimental set up, water was reverse filtered with 55 μm plankton mesh. Subsamples of the screened natural seawater were filtered onto GF/F-filters for further analyses of Chla, particulate organic carbon (POC) and nitrogen (PON). Chla was extracted in methanol and quantified using a fluorometer (Turner Designs) according to Strickland and Parsons (1972). POC and PON were analyzed with CN-analyzer (Costech ECS model 44010). We also collected seston sample (< 55 μm) for fatty acid analysis by means of a flow-through centrifuge. A complete description of the sampling method of seston and lipid analyses are described elsewhere (Evjemo et al., 2008; Bergvik et al., 2012a).
During the last two sampling seasons (2009 and 2011) we included a separate treatment where the nauplii were fed a mixture of equal carbon amounts of Rhodomonas baltica, Isochrysis galbana, and Dunaliella tertiolecta. The algae were kept in exponential growth phase on F/2-medium by replacing 50% of the medium each day (Guillard, 1975). The total biomass of added algal mixture was 150 μg C L−1. This mixture of microalgae was chosen because it is used to maintain a multi-generation culture of C. finmarchicus at NTNU Sealab (Hansen et al., 2007).
The growth rate of nauplii was calculated by measuring change in biovolume with time. Incubated nauplii were sedated with carbon dioxide, by adding a solution of crystallized Na2CO3 in filtered seawater. The solution was administered drop by drop to petri dishes containing nauplii until they were sedated. Pictures were taken using a digital camera (Sony DFW-700) fitted to an inverted microscope (Leica DRM). The pictures of dorsal side of the nauplii were taken; length (L) and width (W) were measured to calculate the biovolume using the standard formula of a half elliptic sphere:
Instantaneous specific growth rates of nauplii (IGRnau, d−1) were calculated from average biovolume at the beginning () and the end () of the growth periods. Time (t) in Equation (2) refers to the incubation time in days.
To compare secondary production as somatic growth of nauplii with secondary production measured by egg production rate, we used data from same sampling dates. The carbon-specific secondary production was calculated, a carbon-content of 45% of dry matter for female C. finmarchicus (Båmstedt, 1986), and an average egg carbon content of 0.23 μg C egg−1 (Hirche, 1990). Instantaneous adult growth rates (IGRfem, day−1) were calculated using the equation of Hopcroft and Roff (1998):
WFemale and WEggs are the carbon specific masses of females and eggs, respectively and t is the incubation time in days.
The Shapiro-Wilk test was used to test for normality. Since an overall goal of the study was to evaluate effect of food quantity and food quality on the growth of C. finmarchicus nauplii, variables assumed to influence the growth were added to a multiple regression model (Table 3). A generalized linear model (GLM) with forward selection was used to define the variables best explaining growth rate. The model was run separately both with original growth data and with temperature-normalized data using a Q10-value of 2.7 (Hirst and Bunker, 2003). Systat 13 was used for the GLM analyzes, Sigmaplot 13 was used for correlation analysis, regression analysis and graphs.
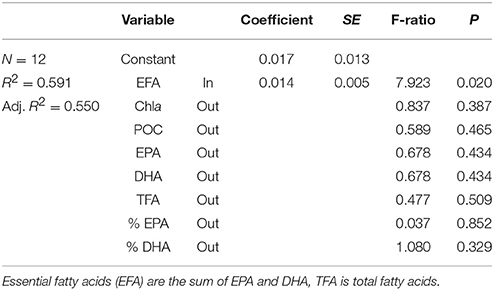
Table 3. Multiple regression coefficients and their significance for the model describing factors affecting the growth rate of nauplii [growth = constant + EFA (mg g−1 DW)].
Results
The higher Chla concentration were observed during growth periods (GP) 1.1, GP 3.3, and GP 3.4, with concentrations of 4.6, 5.4, and 3.9 μg Chla L−1, respectively (Table 2). During the remaining growth periods, the Chla concentration fluctuated between 1.1 and 2.3 μg Chla L−1. The POC- concentration showed no correlation with the Chla-concentration (P > 0.05). The total lipid content (TL) and total fatty acid (TFA) concentrations of seston < 55 μm showed pronounced variations between growth periods (Figure 1A). The TL was highest during GP 1.1 and 3.4, and lowest during GP 3.1 and 3.2. The TFA concentrations also followed a similar pattern, and overall averages for TFA and TL were 16.0 ± 2.9 and 70.5 ± 8.6 (mg g−1 DW), respectively. The dominating fatty acids of the seston were 14:0, 16:0, 18:0, 16:1n-7, 18:3n-3, 18:4n-4, 20:5n-3 (EPA), and 22:6n-3 (DHA). Some taxonomic group specific fatty acids, like the diatom fatty acid 16:1n-7 varied from 0.26 (GP 1.3) to 13.0 mg g−1 DW (GP 3.4), and the flagellate fatty acid 18:4n-3 varied from 0.22 (GP 3.1) to 2.1 mg g−1 DW (GP 3.4). The highly unsaturated fatty acids EPA and DHA constituted on the average 13.6 and 7.5% of TFA, respectively (Figures 1B,C). The non-fatty acid fraction was not further analyzed, but microalgae normally contain variable amounts of fatty alcohols (Antia et al., 1970), sterols (Patterson, 1971), pigments, and glycolipids (Meireles et al., 2003).
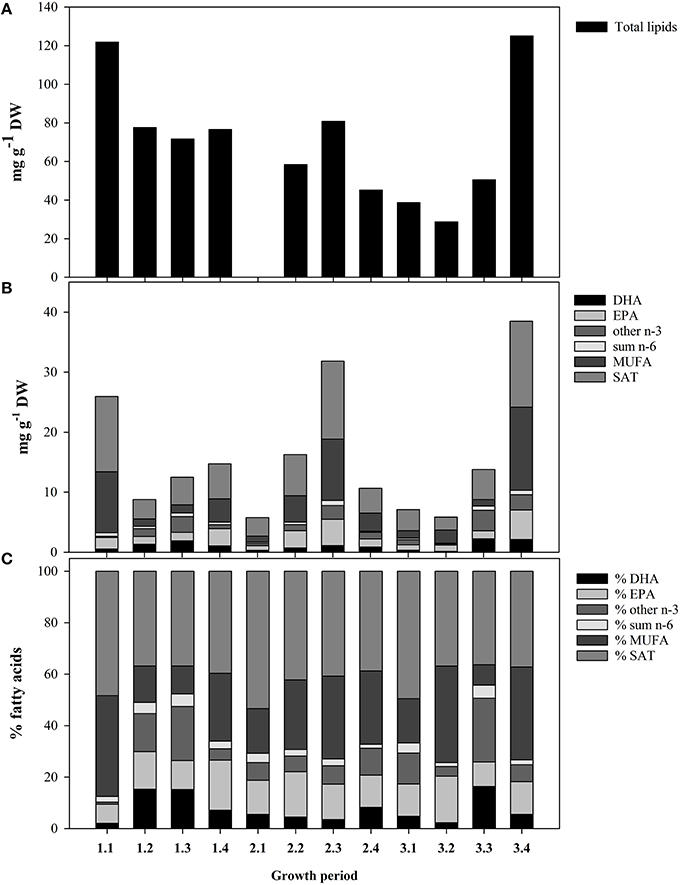
Figure 1. Fatty acid profiles of the seston, < 55 μm for the different growth periods listed in Table 2. (A) Total lipids content. (B) Quantitative content (mg g−1 DW) of different groups of fatty acids. Important essential fatty acids (EFAs; DHA; and EPA) are separated. The total height of the bars represents the total fatty acid content. (C) Relative content of the different groups of fatty acids (% of total fatty acids).
It was difficult to obtain pure samples of eggs and nauplii of C. finmarchicus after the onset of the spring bloom. Therefore, all lipid and fatty acid data for nauplii are from 19.03.2007, whereas egg samples were collected even earlier in the season (Table 1). The data for copepodites are from 12.04.2007. The concentrations of TL and TFA of eggs and nauplii differed between sampling dates. The eggs from 25.02 contained almost twice the amount of TL and TFA as those from 17.02 and 10.03 (Figure 2). The nauplii stages NII–III showed a slightly higher TL- and TFA-content than NIII–IV and copepodite stages CII–III, but the TL- and TFA-content increased in the later stages CIII–IV and CIV–V.
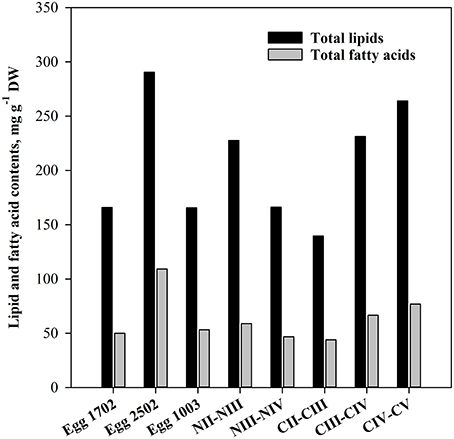
Figure 2. Total lipids and total fatty acids content (mg g−1 DW) in eggs, nauplii, and copepodites of C. finmarchicus.
The nauplii and eggs showed a variable TFA-content and a variable content of fatty acids. The fatty acid composition of different nauplii stages was similar to those of eggs, but TFA- EPA and DHA-contents were much higher in the copepodite stages. The average content of EPA and DHA in nauplii was 10.2 and 5.7 mg g−1 DW in NII–III, and increased to 18.8 and 13.0 mg g−1 DW in CIV–V (Figure 3).
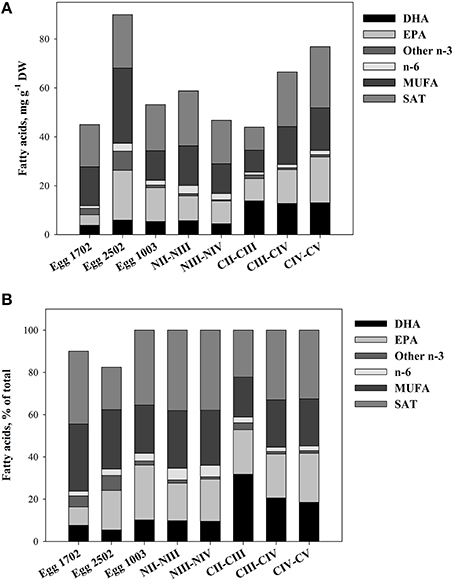
Figure 3. Fatty acid profiles of eggs, nauplii, and copepodites of C. finmarchicus. (A) Quantitative fatty acid content (mg g−1 DW). (B) Relative content (% of total fatty acids). The samples from 17/2 and from 25/2 contained a fraction of unknown fatty acids not included in the figure.
The lipid class analyses of C. finmarchicus eggs showed pronounced differences between sampling days, with highest content of most lipid classes in the eggs sampled at the 25.02 (Figure 4). Both samples contained high amounts (Figure 4A) and percentage fractions of WE (Figure 4B, >80% of TL), variable amounts and fractions of TAG; less and more stable amounts and fractions of phosphatidylethanolamine (PE), phosphatidylcholine (PC) and free fatty acids (FFA). The variability was accordingly most pronounced for neutral storage lipids.
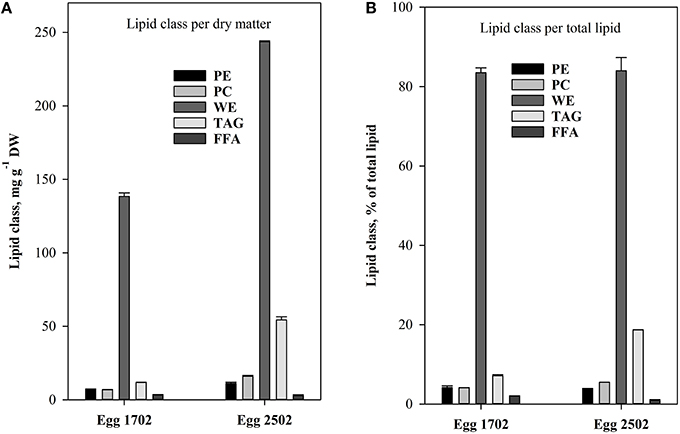
Figure 4. Content of the different lipid classes: phosphatidylethanolamine (PE), phosphatidylcholine (PC), wax ester (WE), triacylglycerol (TAG), and free fatty acids (FFA) in C. finmarchicus eggs. (A) Quantitative content (mg g−1 DW). (B) Relative content (% of total lipids). Error bars equals standard error (n = 2).
The specific growth rate of C. finmarchicus nauplii exhibited some variability, but showed an increase through the growth season, with growth rates close to zero in early March, average values around 0.08 day−1 in late March, and 0.12 ± 0.02 day−1 in May (Figure 5). For all growth periods except GP 2.4 and 3.4, average growth rate was significantly higher for nauplii fed with cultured microalgae than for nauplii fed on natural food source alone (pairwise T-test, p < 0.05, Figure 5).
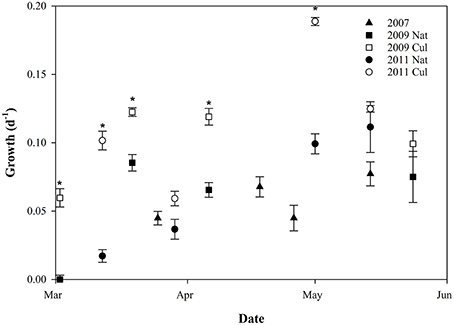
Figure 5. Scatterplot of biovolume-specific growth rates (day−1) through three seasons (mean ± SE). Filled symbols indicate nauplii fed natural seston (Nat), open symbols indicate nauplii fed cultured algae (Cul). Asterisk indicates growth periods where there is a difference between growth of nauplii fed natural seston and cultured microalgae (Student T-test, p < 0.05).
Higher content of green matter was observed in guts of C. finmarchicus nauplii fed with surplus microalgae compared to those fed on natural seston. The growth rates in the nauplii fed cultured microalgae in excess were found to be different between growth periods (p < 0.001, one-way ANOVA). Nauplii from GP 3.3 fed surplus food had the highest growth rate (0.19 ± 0.003 d−1), whereas nauplii from GP 2.1 and 3.2 had the lowest growth rates for the nauplii fed surplus food, both with a growth rate of 0.060 d−1. The growth rate of the nauplii fed cultured microalgae increased with increasing growth in nauplii fed natural seawater (r2 = 0.670, slope 0.924 ± 0.265, p = 0.013, Figure 6), suggesting that 67% of the variability of the growth rate in nauplii fed cultured microalgae was explained by the recent feeding history of the mothers.
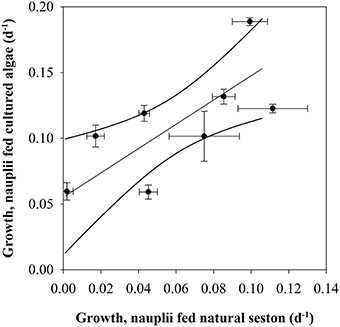
Figure 6. Growth in nauplii fed natural seston (d−1) vs. nauplii fed cultured microalgae (d−1). Dots indicate average, error bars standard error. Lines indicate linear regression line with 95% confidence interval.
The somatic growth in nauplii was best predicted from the content of essential fatty acids (EFA) in the seston (Table 3). Temperature-normalized data gave a slightly better fit for the regression between EFA and growth, but the model could only describe 59% of the variation in growth.
Both the effect on growth of EPA, DHA and EFA in the food could be described by a saturation hyperbola [EPA: r2 = 0.493, half-saturation constant (K) = 2.25 ± 1.75, p = 0.0109, Figure 8A, DHA: r2 = 0.508, half-saturation constant (K) = 0.778 ± 0.643, p = 0.0093, Figure 8B, EFA: r2 = 0.656, half-saturation constant (K) = 6.006 ± 5.033, p = 0.0014, Figure 8C].
There was a tendency for higher naupliar growth with higher Chla-concentration (Figure 7), but there were no significant relationships between growth and the concentration of Chla (Pearson coefficient 0.497, p = 0.103) or POC (Pearson coefficient 0.163, p = 0.632). The growth rates of the nauplii showed pronounced variability both for low and high female growth rates, and the values deviated from the 1:1 line in many growth periods (Figure 9). The growth rates of mothers and offspring were accordingly not significantly correlated (Pearson correlation coefficient 0.129, p = 0.69).
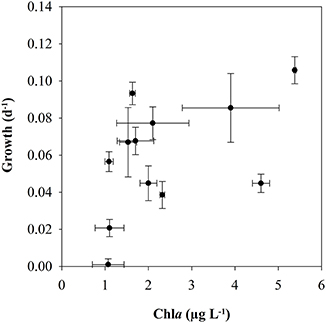
Figure 7. Biovolume-specific growth rate (d−1) of nauplii of C. finmarchicus vs. Chla < 55 μm (Pearson correlation coefficient 0.497, p = 0.103).
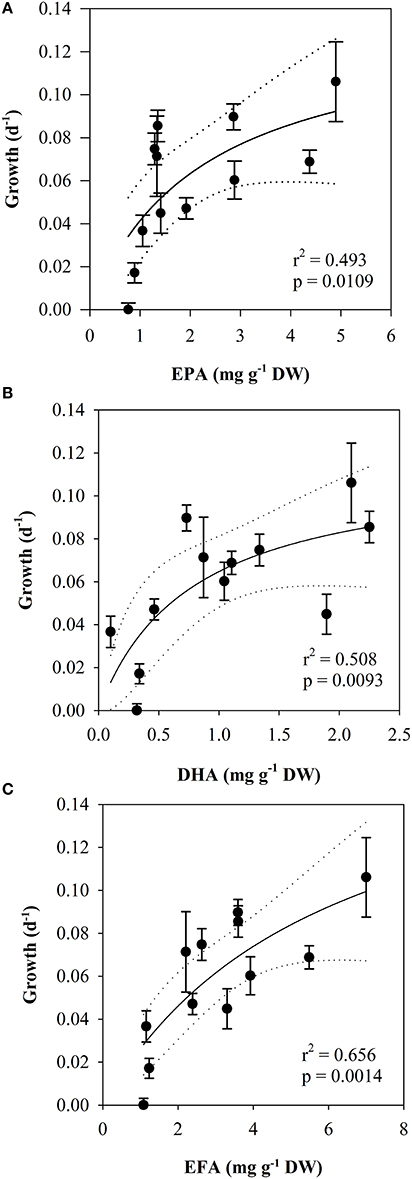
Figure 8. Scatterplot of temperature-normalized growth rates (day−1, mean ± SE) of nauplii vs. (A): the content of EPA, (B): the content of DHA and (C): the content of EFA in the seston (mg g DW−1). Lines indicate regressions (2-parameter hyperbola) with 95% confidence intervals.
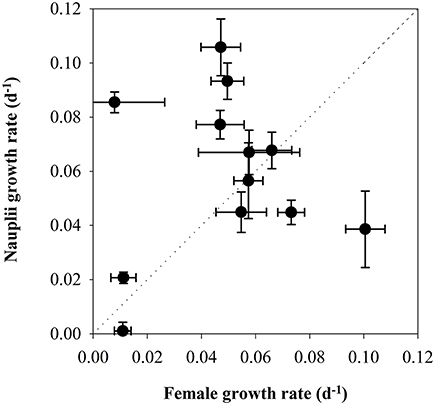
Figure 9. Scatterplot of the secondary production in C. finmarchicus calculated from somatic growth in nauplii (d−1) vs. secondary production calculated from egg production (d−1).
Discussion
One main conclusion from our study was that food quantity and/or food quality limited the instantaneous growth rate of nauplii fed natural seston (IGRnau, d−1). The IGRnau increased significantly (p < 0.05, Figure 8) with increasing content of DHA and EPA in food. To our knowledge, this has not been reported for nauplii of C. finmarchicus in previous investigations. The contents of DHA and EPA in the food have repeatedly been shown to have a positive effect on rate of egg production of later stages of copepods (e.g., Evjemo et al., 2008; Jonasdottir et al., 2009). The variability in DHA- and EPA-contents of suspended particulate matter (< 55 μm) is mainly results from species composition of plankton. Diatoms generally have low DHA and high EPA contents, whereas dinoflagellates and smaller pigmented flagellates have high DHA and variable concentrations of EPA (Ackman et al., 1968; Hallegraeff et al., 1991; Reitan et al., 1994; St. John and Lund, 1996; Mansour et al., 1999). In addition to these, taxon-specific differences, sensitivity of microalgae to inorganic nutrients can also determine the content of DHA and EPA (Reitan et al., 1994).
Another factor, not further evaluated, is varying concentrations and consumption of detritus particles. When comparing measured POC-concentrations with carbon in microalgae calculated from Chla-concentrations (C:Chla conversion factor of 64 μg C:μg Chla, Vadstein et al., 2004); Chla-containing fractions were varying from 24 to 72% of total POC-concentration. There are, to our knowledge, no published papers on C. finmarchicus nauplii feeding on detritus particles and previous reports on Calanus spp. adults feeding on detritus are scarce and the results are contradictory (Paffenhöfer and Strickland, 1970; Carlotti and Radach, 1996; Dilling et al., 1998). We therefore suggest that nauplii grazing selectively on phytoplankton and ciliates (Turner et al., 2001; Irigoien et al., 2003) might have experienced higher DHA- and EPA-concentrations in their actual food than what we measured in the seston samples, because dead matter is likely lower in these fatty acids than live plankton (Suroy et al., 2014).
Contrary to other studies (Campbell et al., 2001b) IGRnau was not significantly correlated with food concentration measured as Chla or POC in the present study, in agreement with the suggestion that the nauplii were mainly DHA- and EPA-limited. Moreover, IGRnau of nauplii fed cultured algae were throughout higher than those of the nauplii fed natural seston. The IGRnau of nauplii fed cultured algae was typically 12–493% higher than the IGRnau for nauplii fed natural seston (Figure 5). The mixture of cultured algae was not analyzed for fatty acids, but contained algae with known and complementary fatty acid composition. Rhodomonas balticum has a high amount of EPA, DHA, 18:3-n3, and 18:4-n3 (Olsen et al., 2014), Isochrysis galbana has a high content of DHA, 18:2n-6, 18:1, and 16:1 (Custódio et al., 2014), and Dunaliella tertiolecta has a high content of 18:3-n3, 16:4-n3, 18:1, and 16:0 (Lee et al., 2014).
We found that the DHA-content was similar in eggs and nauplii NII–III of C. finmarchicus, on average 5.4 ± 0.20 (mean ± SE) mg DHA g−1 DW (9.8% of TFAs, Figure 3). In copepodites CII–III, the average DHA-content had increased to 13.8 ± 0.83 mg DHA g−1 DW (31.8% of TFAs, Figure 3), in agreement with earlier results for this stage of C. finmarchicus (Evjemo et al., 2003). There was no further increase in quantitative DHA content with increasing developmental stage beyond CII–III, but TL and TFA contents were steadily increasing. The fatty acid composition for copepodite stage V throughout the reproductive season is reported elsewhere (Bergvik et al., 2012a). The main pattern of variation in absolute and relative DHA contents showed an increase in DHA through the reproductive season that was related to fatty acid composition of the feed. DHA and EPA are normally not synthesized in significant rates in C. finmarchicus (Sargent and Whittle, 1981; Bell et al., 2007). A low capacity of synthesis combined with a high content of DHA reflects high dietary requirements for DHA, and a variable content of DHA in the feed makes it likely for DHA to become a critically essential component for the animals. We therefore suggest that in the present study, availability of DHA in food of C. finmarchicus nauplii limited the growth rate of nauplii. This has been shown for other copepods (Breteler et al., 2005) and fish larvae, which are classified as carnivore zooplankton (Ruyter et al., 2000; Tocher et al., 2001).
The treatment that involved use of cultured algae as food for nauplii was intended to serve as a positive control. The added food was always kept at concentrations assumed to be above saturation for C. finmarchicus nauplii (150 μg C L−1, Campbell et al., 2001b). As temperature did not vary widely between sampling dates, we expected that IGRnau was similar for experiments with nauplii fed surplus cultured food. However, IGRnau was not equal for different growth periods (Figure 5), and we observed that there was a significant relationship between IGRnau of nauplii fed cultured algae and those fed natural seston (r2 = 0.67, p = 0.013, Figure 6). This suggests that variation in maternal condition and the food quality experienced by the mothers explain some of the variations in naupliar growth rates. Our presented results on lipid class and fatty acid compositions of C. finmarchicus eggs suggested that both the content of TL and TFA can vary quite strongly and that this might reflect variable nutritional states of the females. The lipid classes forming lipid droplets in the eggs are wax esters and/or triacylglycerides (Lee et al., 2006). In our study we found both WE and TAG in eggs of C. finmarchicus, whereas previous investigations have reported phospholipids (Ohman and Runge, 1994) and TAG (Lee et al., 1972b; Gatten et al., 1980) as the main lipid classes. Another study found PL as the main lipid class, and PL is the main lipid class in lipovitellin (Lee and Walker, 1995). There could potentially be some contamination from copepodites and Coscinodiscus present in egg samples used for lipid class analyzes (Table 1). However, the copepodites consisted of early copepodites of small copepod species (Pseudocalanus sp. and Microcalanus sp.) known to have a very low content of wax esters (Lischka and Hagen, 2007). Marine microalgae in general do not contain wax esters (Lee et al., 1972a). Therefore, copepodites and Coscinodiscus in egg lipid class samples would have a diluting effect rather than additions to the total WE content observed.
The high variability in lipid content and storage lipids in eggs could be a result of different nutritional states of females. The egg samples from our study were from February and early March before females started feeding. The storage lipids in these females must therefore have originated from previous season in the form of WE (Sargent and Falk-Petersen, 1988). The egg samples in the studies of Gatten et al. (1980), Lee et al. (1972b), and Ohman and Runge (1994) were sampled during summer when females had been fed satiated food concentrations. It is likely that these females had low WE contents and that they therefore transferred less WE to the eggs. As indicated in Table 1, both egg lipid class samples could be contaminated, mainly by copepods (17.02.2010) and the diatom Coscinodiscus sp. (25.02.2010). The copepods present in the egg sample were mainly smaller copepodites of Pseudocalanus sp and Microcalanus sp. known to contain WE (Norrbin et al., 1990; Schnack-Schiel, 2001; Peters et al., 2006; Parrish et al., 2012), but diatoms in general do not contain WE (Lee et al., 1971). A simple calculation assuming a WE content of 60% of TL for the copepodites (Peters et al., 2006) and 0% for nauplii, Coscinodiscus sp. and Ceratium sp. show that the WE content in the Calanus eggs could be >90% of TL for both samples. This is higher than in previous studies and even high compared to copepodites of various calanoid species (Lee et al., 1972b; Sargent and Henderson, 1986; Schnack-Schiel, 2001).
It has been shown that WEs are converted to TAG for reproductive needs when copepods leave diapause (Jonasdottir, 1999; Richardson et al., 1999). This is likely to happen, but our results suggested that WE can also be transferred directly to eggs, at least before the spring bloom. During this period the females usually have low food availability and high lipid reserves in the form of WE. The WE contents of eggs may influence mortality and growth rates through egg and first nauplii stages. We nevertheless suggest that the high variability of lipid and lipid class composition in eggs could be critical factors for hatching success and mortality of nauplii and needs to be further investigated.
The measured growth rates obtained for C. finmarchicus nauplii were in the lower range of what has been reported in previous studies (Hygum et al., 2000a; Campbell et al., 2001b), but were similar to those found for Centrophages typicus nauplii (Calbet et al., 2000) and for C. finmarchicus copepodites obtained for shipboard incubations (Campbell et al., 2001a). Our relatively low IGRnau values may originate from a varying efficiency in ingestion and assimilation of food particles. In earlier feeding selectivity experiments (Turner et al., 2001; Irigoien et al., 2003; Castellani et al., 2008), nauplii of C. finmarchicus were found to select among food particles smaller than 55 μm. We therefore assume that we did not remove any potential food particles for nauplii by screening water on 55 μm. In a feeding study of nauplii of C. helgolandicus (Rey et al., 2001), the ingestion rate was higher for nauplii offered big algae (Prorocentrum micans, ESD 26–27 μm) than smaller algae (Isochrysis galbana, ESD 4–5 μm), however growth rate was higher in nauplii fed the smaller algae. Others have shown that C. finmarchicus nauplii in stages NIV to NVI can select among diatoms, ciliates and dinoflagellates, depending on the species composition of the microplankton community (Turner et al., 2001; Irigoien et al., 2003; Castellani et al., 2008). Field data from a nearby sampling station show that the microplankton community in general was dominated by diatoms during the spring bloom whereas small flagellates, ciliates, and dinoflagellates were dominant during the post bloom periods (Leiknes, 2016). This suggests that the nauplii offered natural seston could experience low availability of smaller food particles although chlorophyll a and POC-data indicated surplus food.
The observations of reproductive rates of C. finmarchicus females together with somatic growth of nauplii made it possible to compare the two different methods for estimating secondary production. It was then kept in mind that the start of the somatic growth period was 5 days after the sampling date of females. There was considerable scatter around the 1:1 line. The secondary production by female C. finmarchicus was higher than the naupliar growth early in the production season during the spring bloom, whereas naupliar growth was higher during post-bloom situations in May. This might suggest that females utilized different food items than nauplii, as suggested by other authors (Hansen et al., 1994; Gismervik et al., 1996), and/or that the females were fueling the reproduction by internal lipid stores. It is noticeable that the four dates with the highest IGRnau in nauplii fed natural seawater were situations dominated by small flagellates (Leiknes, 2016).
In summary, the specific growth rate of nauplii varied during seasons because of variations in food quality. The contents of EPA and DHA in seston were the variables that had the strongest effect on the naupliar growth rate in our study, but no variable explained more than 66% of variation in naupliar growth rate. The regression analysis indicated that both the EPA-content and DHA-content (and hence, the EFA-content) in the food reached saturation (Figure 8). The pronounced difference in IGR between the nauplii fed natural seston compared to cultured algae in periods of the productive season indicated periods of food limitation, either by food quantity or food quality. Food availability, measured by Chla or POC, could not explain the variability in growth rate.
Our lipid class analyses on eggs from the early spawning of C. finmarchicus showed high WE contents and differed from earlier reports on lipid class compositions of eggs. We therefore propose that C. finmarchicus females can pass on WE to eggs directly without converting WE to TAG, contrary to what is previously reported (Lee et al., 1972b). Since the previously published lipid class analyzes originate from summer samples, it would be very interesting to follow the lipid class composition on C. finmarchicus egg through the spawning season. We also suggest that further studies should try to evaluate to what extent growth and mortality of C. finmarchicus nauplii is affected by such maternal effects, or if food quality or quantity of first feeding nauplii stages is most important.
There were at times big differences between secondary production estimated by egg production measurements and by somatic growth. This suggests that it is not sufficient to use a single approach to assess the state of entire copepod community, but that both approaches should be applied simultaneously. In addition, wide range of growth rates found in a narrow temperature variation (6.5–9.5°C) suggested that other factors should be considered in production models.
Author Contributions
SE and NT provided data from experiments run in 2007, whereas ØL performed experiments in 2009 and 2011. The analytical work was performed by ØL, MB, and SE. ØL wrote the paper with comments from the other authors. YO and OV also contributed to the planning of the experiments.
Funding
We thank the Research Council of Norway for funding through the project Harvest (project number 178447).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the captain and crew of R/V Gunnerus and Calanus for smooth cruise operations and Kjersti Rennan Dahl for technical assistance. We thank M. K. Avarachen and the two anonymous reviewers whose comments helped improve and clarify this manuscript.
References
Ackman, R. G., Tocher, C. S., and McLachlan, J. (1968). Marine phytoplankter fatty acids. J. Fish. Res. Board Can. 25, 1603–1620. doi: 10.1139/f68-145
Antia, N. J., Desai, I. D., and Romilly, M. J. (1970). The tocopherol, vitamin K, and related isoprenoid quinone composition of a unicellular red alga (Porphyridium cruentum). J. Phycol. 6, 305–312.
Båmstedt, U. (1986). “Chemical composition and energy content,” in The Biological Chemistry of Marine Copepods, eds E. D. S. Corner and S. C. M. O'Hara (Oxford: Clarendon Press), 1–58.
Basedow, S. L., and Tande, K. S. (2006). Cannibalism by female Calanus finmarchicus on naupliar stages. Mar. Ecol. Prog. Ser. 327, 247–255. doi: 10.3354/meps327247
Bell, M. V., Dick, J. R., Anderson, T. R., and Pond, D. W. (2007). Application of liposome and stable isotope tracer techniques to study polyunsaturated fatty acid biosynthesis in marine zooplankton. J. Plankton Res. 29, 417–422. doi: 10.1093/plankt/fbm025
Bergvik, M., Leiknes, Ø., Altin, D., Dahl, K. R., and Olsen, Y. (2012a). Dynamics of the lipid content and biomass of Calanus finmarchicus (copepodite V) in a Norwegian Fjord. Lipids 47, 881–895. doi: 10.1007/s11745-012-3700-3
Bergvik, M., Overrein, I., Bantle, M., Evjemo, J. O., and Rustad, T. (2012b). Properties of Calanus finmarchicus biomass during frozen storage after heat inactivation of autolytic enzymes. Food Chem. 132, 209–215. doi: 10.1016/j.foodchem.2011.10.058
Blachowiak-Samolyk, K., Kwasniewski, S., Dmoch, K., Hop, H., and Falk-Petersen, S. (2007). Trophic structure of zooplankton in the Fram Strait in spring and autumn 2003. Deep Sea Res. II Top. Stud. Oceanogr. 54, 2716–2728. doi: 10.1016/j.dsr2.2007.08.004
Bonnet, D., Titelman, J., and Harris, R. (2004). Calanus the cannibal. J. Plankton Res. 26, 937–948. doi: 10.1093/plankt/fbh087
Breteler, W. C. M. K., Schogt, N., and Rampen, S. (2005). Effect of diatom nutrient limitation on copepod development: role of essential lipids. Mar. Ecol. Prog. Ser. 291, 125–133. doi: 10.3354/meps291125
Calbet, A., Trepat, I., and Arin, L. (2000). Naupliar growth versus egg production in the calanoid copepod Centropages typicus. J. Plankton Res. 22, 1393–1402. doi: 10.1093/plankt/22.7.1393
Campbell, R. G., Runge, J. A., and Durbin, E. G. (2001a). Evidence for food limitation of Calanus finmarchicus production rates on the southern flank of Georges Bank during April 1997. Deep Sea Res. II Top. Stud. Oceanogr. 48, 531–549. doi: 10.1016/S0967-0645(00)00089-8
Campbell, R. G., Wagner, M. M., Teegarden, G. J., Boudreau, C. A., and Durbin, E. G. (2001b). Growth and development rates of the copepod Calanus finmarchicus reared in the laboratory. Mar. Ecol. Prog. Ser. 221, 161–183. doi: 10.3354/meps221161
Carlotti, F., and Radach, G. (1996). Seasonal dynamics of phytoplankton and Calanus finmarchicus in the North Sea as revealed by a coupled one-dimensional model. Limnol. Oceanogr. 41, 522–539. doi: 10.4319/lo.1996.41.3.0522
Castellani, C., Irigoien, X., Mayor, D. J., Harris, R. P., and Wilson, D. (2008). Feeding of Calanus finmarchicus and Oithona similis on the microplankton assemblage in the Irminger Sea, North Atlantic. J. Plankton Res. 30, 1095–1116. doi: 10.1093/plankt/fbn074
Conover, R. J. (1988). Comparative life histories in the genera Calanus and Neocalanus in high-latitudes of the northern hemisphere. Hydrobiologia 167, 127–142. doi: 10.1007/BF00026299
Corkett, C. J., McLaren, I. A., and Sevigny, J.-M. (1986). The rearing of the marine calanoid copepods Calanus finmarchicus (Gunnerus), C. glacialis Jaschnov and C. hyperboreus Krøyer, with comment on the equiproportional rule. Sylleogeus 58, 539–546.
Custódio, L., Soares, F., Pereira, H., Barreira, L., Vizetto-Duarte, C., Rodrigues, M., et al. (2014). Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 26, 151–161. doi: 10.1007/s10811-013-0098-0
Dalpadado, P., Yamaguchi, A., Ellertsen, B., and Johannessen, S. (2008). Trophic interactions of macro-zooplankton (krill and amphipods) in the Marginal Ice Zone of the Barents Sea. Deep Sea Res. II Top. Stud. Oceanogr. 55, 2266–2274. doi: 10.1016/j.dsr2.2008.05.016
Dilling, L., Wilson, J., Steinberg, D., and Alldredge, A. (1998). Feeding by the euphausiid Euphausia pacifica and the copepod Calanus pacificus on marine snow. Mar. Ecol. Prog. Ser. 170, 189–201. doi: 10.3354/meps170189
Dodds, L. A., Black, K. D., Orr, H., and Roberts, J. M. (2009). Lipid biomarkers reveal geographical differences in food supply to the cold-water coral Lophelia pertusa (Scleractinia). Mar. Ecol. Prog. Ser. 397, 113–124. doi: 10.3354/meps08143
Dommasnes, A., Melle, W., Dalpadado, P., and Ellertsen, B. (2004). Herring as a major consumer in the Norwegian Sea. Ices J. Mar. Sci. 61, 739–751. doi: 10.1016/j.icesjms.2004.04.001
Eiane, K., Aksnes, D. L., Ohman, M. D., Simon, W., and Martinussen, M. B. (2002). Stage-specific mortality of Calanus spp. under different predation regimes. Limnol. Oceanogr. 47, 636–645. doi: 10.4319/lo.2002.47.3.0636
Evjemo, J. O., Reitan, K. I., and Olsen, Y. (2003). Copepods as live food organisms in the larval rearing of halibut larvae (Hippoglossus hippoglossus L.) with special emphasis on the nutritional value. Aquaculture 227, 191–210. doi: 10.1016/S0044-8486(03)00503-9
Evjemo, J. O., Tokle, N., Vadstein, O., and Olsen, Y. (2008). Effect of essential dietary fatty acids on egg production and hatching success of the marine copepod Temora longicornis. J. Exp. Mar. Biol. Ecol. 365, 31–37. doi: 10.1016/j.jembe.2008.07.032
Fleminger, A., and Hulsemann, K. (1977). Geographical range and taxonomic divergence in North Atlantic Calanus (Calanus helgolandicus, Calanus finmarchicus and Calanus glacialis). Mar. Biol. 40, 233–248. doi: 10.1007/BF00390879
Gatten, R. R., Sargent, J. R., Forsberg, T. E. V., O'Hara, S. C. M., and Corner, E. D. S. (1980). On the nutrition and metabolism of zooplankton. XIV. Utilization of lipid by Calanus helgolandicus during maturation and reproduction. J. Mar. Biol. Assoc. U.K. 60, 391–399. doi: 10.1017/S0025315400028411
Gismervik, I., Andersen, T., and Vadstein, O. (1996). Pelagic food webs and eutrophication of coastal waters: impact of grazers on algal communities. Mar. Pollut. Bull. 33, 22–35. doi: 10.1016/S0025-326X(96)00134-8
Guillard, R. R. L. (ed.). (1975). Culture of Phytoplankton for Feeding Marine Invertebrates. New York, NY: Plenum Press.
Hallegraeff, G. M., Nichols, P. D., Volkman, J. K., Blackburn, S. I., and Everitt, D. A. (1991). Pigments, fatty-acids, and sterols of the toxic dinoflagellate Gymnodinium catenatum. J. Phycol. 27, 591–599. doi: 10.1111/j.0022-3646.1991.00591.x
Hansen, B. H., Altin, D., Nordtug, T., and Olsen, A. J. (2007). Suppression subtractive hybridization library prepared from the copepod Calanus finmarchicus exposed to a sublethal mixture of environmental stressors. Comp. Biochem. Physiol. D Genomics Proteomics 2, 250–256. doi: 10.1016/j.cbd.2007.04.006
Hansen, B. W., Bjørnsen, P. K., and Hansen, P. J. (1994). The size ratio between planktonic predators and their prey. Limnol. Oceanogr. 39, 395–403. doi: 10.4319/lo.1994.39.2.0395
Harris, R. P., Irigoien, X., Head, R. N., Rey, C., Hygum, B. H., Hansen, B. W., et al. (2000). Feeding, growth, and reproduction in the genus Calanus. Ices J. Mar. Sci. 57, 1708–1726. doi: 10.1006/jmsc.2000.0959
Hirche, H. J. (1990). Egg-production of Calanus finmarchicus at low temperature. Mar. Biol. 106, 53–58. doi: 10.1007/BF02114674
Hirst, A. G., and Bunker, A. J. (2003). Growth of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnol. Oceanogr. 48, 1988–2010. doi: 10.4319/lo.2003.48.5.1988
Hopcroft, R. R., and Roff, J. C. (1998). Zooplankton growth rates: the influence of female size and resources on egg production of tropical marine copepods. Mar. Biol. 132, 79–86. doi: 10.1007/s002270050373
Hygum, B. H., Rey, C., and Hansen, B. W. (2000a). Growth and development rates of Calanus finmarchicus nauplii during a diatom spring bloom. Mar. Biol. 136, 1075–1085. doi: 10.1007/s002270000313
Hygum, B. H., Rey, C., Hansen, B. W., and Carlotti, F. (2000b). Rearing cohorts of Calanus finmarchicus (Gunnerus) in mesocosms. Ices J. Mar. Sci. 57, 1740–1751. doi: 10.1006/jmsc.2000.0956
Ianora, A., Poulet, S. A., and Miralto, A. (2003). The effects of diatoms on copepod reproduction: a review. Phycologia 42, 351–363. doi: 10.2216/i0031-8884-42-4-351.1
Irigoien, X., Titelman, J., Harris, R. P., Harbour, D., and Castellani, C. (2003). Feeding of Calanus finmarchicus nauplii in the Irminger Sea. Mar. Ecol. Prog. Ser. 262, 193–200. doi: 10.3354/meps262193
Jonasdottir, S. H. (1999). Lipid content of Calanus finmarchicus during overwintering in the Faroe-Shetland Channel. Fish. Oceanogr. 8, 61–72. doi: 10.1046/j.1365-2419.1999.00003.x
Jonasdottir, S. H., Gudfinnsson, H. G., Gislason, A., and Astthorsson, O. S. (2002). Diet composition and quality for Calanus finmarchicus egg production and hatching success off south-west Iceland. Mar. Biol. 140, 1195–1206. doi: 10.1007/s00227-002-0782-0
Jonasdottir, S. H., Richardson, K., Heath, M. R., Ingvarsdottir, A., and Christoffersen, A. (2008). Spring production of Calanus finmarchicus at the Iceland-Scotland ridge. Deep Sea Res. I Oceanogr. Res. Papers 55, 471–489. doi: 10.1016/j.dsr.2007.12.009
Jonasdottir, S. H., Trung, N. H., Hansen, F., and Gartner, S. (2005). Egg production and hatching success in the calanoid copepods Calanus helgolandicus and Calanus finmarchicus in the North Sea from March to September 2001. J. Plankton Res. 27, 1239–1259. doi: 10.1093/plankt/fbi091
Jonasdottir, S. H., Visser, A. W., and Jespersen, C. (2009). Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Mar. Ecol. Prog. Ser. 382, 139–150. doi: 10.3354/meps07985
Kane, J. (1984). The feeding-habits of co-occurring cod and haddock larvae from Georges Bank. Mar. Ecol. Prog. Ser. 16, 9–20. doi: 10.3354/meps016009
Kattner, G., Hagen, W., Lee, R. F., Campbell, R., Deibel, D., Falk-Petersen, S., et al. (2007). Perspectives on marine zooplankton lipids. Can. J. Fish. Aquat. Sci. 64, 1628–1639. doi: 10.1139/f07-122
Kiørboe, T., and Johansen, K. (1986). Studies of a larval herring (Clupea harengus L.) patch in the Buchan area. IV. Zooplankton distribution and productivity in relation to hydrographic features. Dana 6, 37–51.
Koski, M., Dutz, J., Breteler, W. K., Rampen, S., and Noordeloos, A. (2010). Seasonal changes in food quantity and quality of the common North Sea copepods Temora longicornis and Pseudocalanus elongatus: a bioassay approach. Mar. Ecol. Prog. Ser. 399, 141–155. doi: 10.3354/meps08357
Koski, M., Yebra, L., Dutz, J., Jonasdottir, S. H., Vidoudez, C., Jakobsen, H. H., et al. (2012). The effect of egg versus seston quality on hatching success, naupliar metabolism and survival of Calanus finmarchicus in mesocosms dominated by Phaeocystis and diatoms. Mar. Biol. 159, 643–660. doi: 10.1007/s00227-011-1843-z
Lee, R. F., Hagen, W., and Kattner, G. (2006). Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 307, 273–306. doi: 10.3354/meps307273
Lee, R. F., Hirota, J., Nevenzel, J. C., Sauerheber, R., Benson, A. A., and Lewis, A. (1972a). Lipids in the marine environment. Calif. Coop. Oceanic Fish. Invest. Rep. 16, 95–102.
Lee, R. F., Nevenzel, J. C., and Paffenhöfer, G.-A. (1971). Importance of wax esters and other lipids in marine food chain - phytoplankton and copepods. Mar. Biol. 9, 99–108. doi: 10.1007/BF00348249
Lee, R. F., Nevenzel, J. C., and Paffenhöfer, G.-A. (1972b). The Presence of wax esters in marine planktonic copepods. Naturwissenschaften 59, 406–411. doi: 10.1007/BF00623130
Lee, R. F., and Walker, A. (1995). Lipovitellin and lipid droplet accumulation in oocytes during ovarian maturation in the blue crab, Callinectes sapidus. J. Exp. Zool. 271, 401–412. doi: 10.1002/jez.1402710510
Lee, S.-Y., Kim, S.-H., Hyun, S.-H., Suh, H. W., Hong, S.-J., Cho, B.-K., et al. (2014). Fatty acids and global metabolites profiling of Dunaliella tertiolecta by shifting culture conditions to nitrate deficiency and high light at different growth phases. Process Biochem. 49, 996–1004. doi: 10.1016/j.procbio.2014.02.022
Leiknes, Ø. (2016). The Effect of Nutrition on Important Life-History Traits in the Marine Copepod Calanus finmarchicus. Ph.D. Norwegian University of Science and Technology (NTNU).
Lischka, S., and Hagen, W. (2007). Seasonal lipid dynamics of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Mar. Biol. 150, 443–454. doi: 10.1007/s00227-006-0359-4
Mansour, M. P., Volkman, J. K., Jackson, A. E., and Blackburn, S. I. (1999). The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 35, 710–720. doi: 10.1046/j.1529-8817.1999.3540710.x
Meireles, L. A., Guedes, A. C., and Malcata, F. X. (2003). Lipid class composition of the microalga Pavlova lutheri: eicosapentaenoic and docosahexaenoic acids. J. Agric. Food Chem. 51, 2237–2241. doi: 10.1021/jf025952y
Møller, E. F., Maar, M., Jónasdóttir, S. H., Nielsen, T. G., and Tönnesson, K. (2012). The effect of changes in temperature and food on the development of Calanus finmarchicus and Calanus helgolandicus populations. Limnol. Oceanogr. 57, 211–220. doi: 10.4319/lo.2012.57.1.0211
Norrbin, M. F., Olsen, R. E., and Tande, K. S. (1990). Seasonal variation in lipid class and fatty acid composition of two small copepods in Balsfjorden, northern Norway. Mar. Biol. 105, 205–211. doi: 10.1007/BF01344288
Ohman, M. D., Durbin, E. G., Runge, J. A., Sullivan, B. K., and Field, D. B. (2008). Relationship of predation potential to mortality of Calanus finmarchicus on Georges Bank, Northwest Atlantic. Limnol. Oceanogr. 53, 1643–1655. doi: 10.4319/lo.2008.53.4.1643
Ohman, M. D., Eiane, K., Durbin, E. G., Runge, J. A., and Hirche, H. J. (2004). A comparative study of Calanus finmarchicus mortality patterns at five localities in the North Atlantic. Ices J. Mar. Sci. 61, 687–697. doi: 10.1016/j.icesjms.2004.03.016
Ohman, M. D., and Runge, J. A. (1994). Sustained fecundity when phytoplankton resources are in short supply: omnivory by Calanus finmarchicus in the Gulf of St. Lawrence. Limnol. Oceanogr. 39, 21–36. doi: 10.4319/lo.1994.39.1.0021
Olsen, Y., Evjemo, J. O., Kjorsvik, E., Larssen, H., Li, K. S., Overrein, I., et al. (2014). DHA content in dietary phospholipids affects DHA content in phospholipids of cod larvae and larval performance. Aquaculture 428, 203–214. doi: 10.1016/j.aquaculture.2014.03.002
Paffenhöfer, G.-A., and Strickland, J. H. D. (1970). A note on feeding of Calanus helgolandicus on detritus. Mar. Biol. 5, 97–99. doi: 10.1007/BF00352591
Parrish, C. C., French, V. M., and Whiticar, M. J. (2012). Lipid class and fatty acid composition of copepods (Calanus finmarchicus, C. glacialis, Pseudocalanus sp., Tisbe furcata and Nitokra lacustris) fed various combinations of autotrophic and heterotrophic protists. J. Plankton Res. 34, 356–375. doi: 10.1093/plankt/fbs003
Patterson, G. W. (1971). The distribution of sterols in algae. Lipids 6, 120–127. doi: 10.1007/BF02531327
Payne, P. M., Wiley, D. N., Young, S. B., Pittman, S., Clapham, P. J., and Jossi, J. W. (1990). Recent fluctuations in the abundance of baleen whales in the southern Gulf of Maine in relation to changes in selected prey. Fish. Bull. 88, 687–696.
Peters, J., Renz, J., Justus van, B., Boersma, M., and Hagen, W. (2006). Trophodynamics and seasonal cycle of the copepod Pseudocalanus acuspes in the Central Baltic Sea (Bornholm Basin): evidence from lipid composition. Mar. Biol. 149, 1417–1429. doi: 10.1007/s00227-006-0290-8
Planque, B., and Batten, S. D. (2000). Calanus finmarchicus in the North Atlantic: the year of Calanus in the context of interdecadal change. Ices J. Mar. Sci. 57, 1528–1535. doi: 10.1006/jmsc.2000.0970
Pond, D., Harris, R., Head, R., and Harbour, D. (1996). Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK. Mar. Ecol. Prog. Ser. 143, 45–63. doi: 10.3354/meps143045
Poulet, S. A., Ianora, A., Laabir, M., and Breteler, W. (1995). Towards the measurement of secondary production and recruitment in copepods. Ices J. Mar. Sci. 52, 359–368. doi: 10.1016/1054-3139(95)80051-4
Reitan, K. I., Rainuzzo, J. R., and Olsen, Y. (1994). Effect of nutrient limitation on fatty-acid and lipid-content of marine microalgae. J. Phycol. 30, 972–979. doi: 10.1111/j.0022-3646.1994.00972.x
Rey, C., Harris, R., Irigoien, X., Head, R., and Carlotti, F. (2001). Influence of algal diet on growth and ingestion of Calanus helgolandicus nauplii. Mar. Ecol. Prog. Ser. 216, 151–165. doi: 10.3354/meps216151
Richardson, K., Jónasdóttir, S. H., Hay, S. J., and Christoffersen, A. (1999). Calanus finmarchicus egg production and food availability in the Faroe–Shetland Channel and northern North Sea: October–March. Fish. Oceanogr. 8, 153–162. doi: 10.1046/j.1365-2419.1999.00007.x
Runge, J. A. (1988). Should we expect a relationship between primary production and fisheries? The role of copepod dynamics as a filter of trophic variability. Hydrobiologia 167, 61–71. doi: 10.1007/BF00026294
Ruyter, B., Rosjo, C., Masoval, K., Einen, O., and Thomassen, M. S. (2000). Influence of dietary n-3 fatty acids on the desaturation and elongation of [1-14C] 18:2 n-6 and [1-14C] 18:3 n-3 in Atlantic salmon hepatocytes. Fish Physiol. Biochem. 23, 151–158. doi: 10.1023/A:1007893317923
Sargent, J. R., and Falk-Petersen, S. (1988). The lipid biochemistry of calanoid copepods. Hydrobiologia 167, 101–114. doi: 10.1007/BF00026297
Sargent, J. R., and Henderson, R. J. (1986). The Biological Chemistry of Marine Copepods. Oxford: Clarendon Press.
Sargent, J. R., and Whittle, K. J. (1981). “Lipids and hydrocarbons in the marine food web,” in Analysis of Marine Ecosystems, ed A. R. Longhurst (London: Academic Press), 491–533.
Schnack-Schiel, S. B. (2001). Aspects of the study of the life cycles of Antarctic copepods. Hydrobiologia 156, 9–24. doi: 10.1023/A:1013195329066
St. John, M. A., and Lund, T. (1996). Lipid biomarkers: linking the utilization of frontal plankton biomass to enhanced condition of juvenile North Sea cod. Mar. Ecol. Prog. Ser. 131, 75–85. doi: 10.3354/meps131075
Strickland, J. H. D., and Parsons, T. R. (1972). A Practical Handbook of Seawater Analysis. Ottawa, ON: Fisheries Research Board of Canada.
Suroy, M., Moriceau, B., Boutorh, J., and Goutx, M. (2014). Fatty acids associated with the frustules of diatoms and their fate during degradation—A case study in Thalassiosira weissflogii. Deep Sea Res. I Oceanogr. Res. Papers 86, 21–31. doi: 10.1016/j.dsr.2014.01.001
Tande, K. S. (1988). Aspects of developmental and mortality-rates in Calanus finmarchicus related to equiproportional development. Mar. Ecol. Prog. Ser. 44, 51–58. doi: 10.3354/meps044051
Tocher, D. R., Bell, J. G., MacGlaughlin, P., McGhee, F., and Dick, J. R. (2001). Hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition of liver in salmonids: effects of dietary vegetable oil. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 130, 257–270. doi: 10.1016/S1096-4959(01)00429-8
Tönnesson, K., Nielsen, T. G., and Tiselius, P. (2006). Feeding and production of the carnivorous copepod Pareuchaeta norvegica in the Skagerrak. Mar. Ecol. Prog. Ser. 314, 213–225. doi: 10.3354/meps314213
Turner, J. T., Levinsen, H., Nielsen, T. G., and Hansen, B. W. (2001). Zooplankton feeding ecology: grazing on phytoplankton and predation on protozoans by copepod and barnacle nauplii in Disko Bay, West Greenland. Mar. Ecol. Prog. Ser. 221, 209–219. doi: 10.3354/meps221209
Vadstein, O., Stibor, H., Lippert, B., Loseth, K., Roederer, W., Sundt-Hansen, L., et al. (2004). Moderate increase in the biomass of omnivorous copepods may ease grazing control of planktonic algae. Mar. Ecol. Prog. Series 270, 199–207. doi: 10.3354/meps270199
Watling, L. (2007). Predation on copepods by an Alaskan cladorhizid sponge. J. Mar. Biol. Assoc. U.K. 87, 1721–1726. doi: 10.1017/s0025315407058560
Keywords: secondary production, growth rate, Calanus finmarchicus, DHA, EPA, food concentration, zooplankton
Citation: Leiknes Ø, Etter SA, Tokle NE, Bergvik M, Vadstein O and Olsen Y (2016) The Effect of Essential Fatty Acids for the Somatic Growth in Nauplii of Calanus finmarchicus. Front. Mar. Sci. 3:33. doi: 10.3389/fmars.2016.00033
Received: 17 November 2015; Accepted: 04 March 2016;
Published: 21 March 2016.
Edited by:
Marty Riche, Florida Atlantic University, USAReviewed by:
Sigrun Huld Jonasdottir, Technical University of Denmark, DenmarkValeria Mamouridis, Institut de Ciències del Mar (ICM-CSIC), Spain
Copyright © 2016 Leiknes, Etter, Tokle, Bergvik, Vadstein and Olsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Øystein Leiknes, oystein.leiknes@ntnu.no
 Øystein Leiknes
Øystein Leiknes Siv A. Etter
Siv A. Etter Nils E. Tokle
Nils E. Tokle Maria Bergvik
Maria Bergvik Olav Vadstein
Olav Vadstein Yngvar Olsen
Yngvar Olsen