Cyanobacterial Pigments as Natural Anti-Hyperglycemic Agents: An In vitro Study
- 1Division of Salt and Marine Chemicals, Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, Bhavnagar, India
- 2Academy of Scientific and Innovative Research, Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, Bhavnagar, India
Traditional medicines for controlling postprandial hyperglycemia includes herbs and plant extracts as well as synthetic drugs like acarbose. Synthetic drug molecules frequently have side effects such as flatulence and diarrhea. Cyanobacterial pigments have excellent anti-oxidant and free radical scavenging properties. Thus, α-amylase and α-glucosidase inhibiting activities of purified pigments and crude extracts from three cyanobacterial species, Lyngbya, Microcoleus, and Synechocystis sp., were investigated. Lyngbya extract had the highest total anti-oxidant activity (TAC) before digestion (48.26 ± 0.04 μg AAE ml−1) while purified lycopene had the highest TAC after digestion (154.16 ± 0.96 μg AAE ml−1). The Microcoleus extract had the highest ABTS scavenging activity before digestion (98.23 ± 0.25%) while purified C-phycocyanin (C-PC) had the highest ABTS scavenging after digestion (99.69 ± 0.04%). None of the digested or undigested extracts performed better than acarbose in inhibiting α-amylase but the digested Microcoleus extract was able to inhibit its activity by ~35%. The purified pigments gave inhibitory activities ranging from ~8 to 16%. The Lyngbya extract had the highest inhibitory activity against α-glucosidase both before and after digestion (62.22 ± 0.02 and 97.82 ± 0.03%, respectively). Purified C-phycoerythrin (C-PE), C-PC, lycopene and myxoxanthophyll could inhibit α-glucosidase in a range of ~83–96%. Considering the potent inhibitory activities of purified pigments against both α-amylase and α-glucosidase, cyanobacterial pigments could be used as food additives for their dual advantage of anti-oxidant and anti-hyperglycemic activities.
Introduction
Diabetes is a major lifestyle disease which affects several million people worldwide every year. According to reports, 415 million people worldwide were diabetic in 2015, most of them suffering from Type II diabetes (IDF Diabetes Atlas, 2015). Type I diabetes is a result of lack of enough insulin production by the pancreas whereas Type II diabetes results from our body developing immunity against insulin. India stands at second position with respect to the number of type I and II diabetic patients whereas it does not even figure in the top 10 countries for diabetes related expenditure. A major worry is the lack of awareness of the symptoms, which leads to ignorance about the disease until very late.
The management of the disease is an important step for its control which often includes reducing the postprandial increase in blood glucose levels by inhibiting the enzymes, α-amylase, and α-glucosidase, responsible for hydrolysis of carbohydrates to simple sugars such as glucose (Zia-Ul-Haq et al., 2011). α-Amylase, the major form of amylase in mammals including humans, is responsible for the breakdown of complex polysaccharides such as starch into glucose by acting on the α-(1,4) glycosidic linkage (Ramasubbu et al., 2005). α-glucosidase is a type of glucosidase enzyme present in the brush border of the small intestine, which breaks down starch and other complex polysaccharides to glucose and other monosaccharides. It also acts on the α-(1,4) glycosidic linkage to release α-glucose (Flanagan and Forstner, 1978). The released glucose moieties are absorbed by the blood and transported wherever required. Typically, glucose levels in our blood peak after having a heavy meal. Many synthetic drugs are available in the market that aim to manage this hyperglycemic condition by reducing the activities of α-amylase and α-glucosidase, thereby reducing the rate of glucose entering the bloodstream. Examples of such “diabetes pills” include acarbose, voglibose, and miglitol. However, they are not without their side effects. Acarbose, for instance, has been found to cause flatulence and diarrhea in patients due to carbohydrate malabsorption. Acute liver toxicity leading to hepatitis has also been widely reported in various studies (Acarbose, US National Library of Medicine, 2016). Alternate remedies in such cases can include fruits such as berries (Jo et al., 2011), tea and tea pomace extracts (Oh et al., 2015), plant extracts (Zia-Ul-Haq et al., 2011), pine bark (Kim et al., 2004), green tea extracts (Gao et al., 2013), herbal medicines such as Tinospora cordifolia (Sengupta et al., 2009), and vegetables such as bitter gourd (Ahmad et al., 2012).
Cyanobacteria are primitive organisms responsible for photosynthesis and a storehouse of various beneficial biomolecules like polyunsaturated fatty acids (PUFAs), phycobiliproteins (PBPs), carotenoids, sterols, enzymes, and vitamins (Ghosh et al., 2015). Among these compounds, natural pigments like PBPs, and carotenoids have attracted attention due to their fluorescent properties and various uses (Stryer and Glazer, 1985; Kronick, 1986; Sekar and Chandramohan, 2007; Jaswir et al., 2011; Ghosh et al., 2015; Paliwal et al., 2015a; Sudhakar et al., 2015). Moreover, cyanobacteria such as Spirulina sp. has been traditionally consumed by some tribal people living near Lake Chad as far back as the 1940s (Ciferri, 1983). Reports state that Spirulina has been consumed by the Aztec civilization. Due to its nutritional value, it has been recommended by the National Aeronautics and Space Administration (NASA) and the European Space Agency (ESA) as a primary food for long term missions (Deng and Chow, 2010). Considering their nutritional properties, they can be potential candidates for natural alternatives for hyperglycemia. Though not all cyanobacteria are benign; some of them contain potent toxins (Ghosh et al., 2015). To the best of our knowledge, there are almost no reports that utilize cyanobacterial pigments as natural remedies for hyperglycemic conditions. These pigments could be classified into water soluble PBPs or lipid soluble carotenoids. PBPs are water soluble proteins responsible for light harvesting in cyanobacteria, cryptophytes, and some red seaweeds while carotenoids are long chain derivatives of tetra-terpenoids, responsible for photosynthesis as well as protection of photosystems from photo-damage. Apart from light harvesting, PBPs, and carotenoids have also been the focus for their utility as natural food colorants (Jaswir et al., 2011; Manirafasha et al., 2016). A lack of studies and the role of PBPs and carotenoids as food supplements prompted us to investigate their inhibitory effects on α-amylase and α-glucosidase. Further, we also aimed to investigate whether consuming such pigments as part of a regular diet would lead to the same extent of inhibition. Thus, we also digested the cyanobacterial extracts and pigments purified from them in vitro to observe their effects on the activity of said enzymes. This study aims to evaluate potential compounds from cyanobacteria as natural alternatives for managing hyperglycemic conditions, which could be of help to diabetic patients.
Materials and Methods
Extract Preparation
Phycobiliprotein Extract
Lyngbya sp. CCNM 2053 and Microcoleus sp. CCNM 2005 were grown respectively in modified ASN III and Zarrouk's media in a light intensity of 30 μE m−2 s−1 and 12:12 light:dark photoperiod at 25 ± 2°C. The biomass was centrifuged after 18 days and washed to remove salts. The biomass pellets were crushed in 0.1 M phosphate buffer (pH 7.2, 75 mg ml−1 on a wet basis) followed by repeated freezing and thawing at −70 and 25°C to extract the water soluble PBPs. The crude extract was centrifuged at 10,000 g (4°C, 10 min) to remove cell debris and the supernatant was collected. The extract was divided in two fractions; one was used directly and other half was purified using previous methods to obtain purified C-PE and C-PC for experiments (Patel et al., 2005; Mishra et al., 2011). The PBP content was measured according to Bennett and Bogorad (1973):
The UV—Visible spectra of the extracts and the purified PBPs were recorded on a Cary 50 Bio (Agilent, USA) spectrophotometer in a range of 280–800 nm. The fluorescence emission spectra of the crude extracts and the purified PBPs were recorded on an FLS900 fluorescence spectrophotometer (Edinburgh Instruments, UK). The Lyngbya extract and the purified C-PE were excited at 470 nm and the emission spectra were recorded in a range of 480–800 nm while the Microcoleus extract and purified C-PC were excited at 570 nm and their emission spectra were recorded in a range of 575–800 nm. The slit width was kept constant at 2 nm for all measurements. The curves were smoothed using the software provided with the instrument (F900 v7.0.2, Edinburgh Instruments, UK).
Carotenoid Extract
For carotenoid extraction, Synechocystis sp. CCNM 2501 was grown in BG11 medium and harvested by centrifugation (10,000 g, 4°C, 10 min) after 30 days. The supernatant was discarded and the wet biomass was suspended in 1 ml methanol (100 mg ml−1 on a wet basis) and vortexed thoroughly to dissolve the aggregates. The mixture was incubated at 45°C for 24 h followed by centrifugation (10,000 g, 4°C, 10 min) to remove the biomass. The extract was dried under nitrogen stream and subsequently dissolved in 1 mL dimethyl sulfoxide (DMSO). The concentrations were determined using HPLC (Paliwal et al., 2015b). Briefly, lycopene and myxoxanthophyll standards (DHI, Denmark) were run under identical conditions and the retention times and the peak area were recorded. The Synechocystis extract was then run under identical conditions and the fractions corresponding to lycopene and myxoxanthophyll were collected according to their retention times. The purity of both were calculated by comparing their peak areas with the peak areas of their respective standards.
Total Antioxidant Capacity
The total anti-oxidant capacity was measured according to a modified method given in Paliwal et al. (2015a). 200 μl of the undigested or digested extract or purified pigment was mixed with 600 μl of phospho-molybdate reagent (1.1 M H2SO4, 30 mM NaH2PO4, and 4 mM ammonium heptamolybdate). The tubes were incubated at 95°C for 90 min and were cooled to room temperature afterwards. Blank tubes with either 0.1 M phosphate buffer (pH 7.2) or DMSO were prepared in the same way. Absorbances of the samples were read against their respective blanks at 695 nm. Ascorbic acid was used as a standard.
ABTS Scavenging Activity
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), (ABTS) radical scavenging activity was evaluated using the method given by Re et al. (1999). The ABTS radical solution was prepared by adding ABTS and K2S2O8 in MilliQ water to a final concentration of 7 and 2.45 mM, respectively. The solution was kept for 12–16 h at 25 ± 2°C in dark for free radical activation. The resulting dark green solution was diluted using MilliQ water to an absorbance of 0.7 ± 0.05 at 734 nm. 100 μl of cyanobacterial extracts or purified pigments were added to 1 ml of ABTS working solution and incubated for 30 min at room temperature (25 ± 2°C) in the dark. The absorbance was recorded at 734 nm (Cary Varian 50 Bio, Cary, US). The control reaction had 0.1 M phosphate buffer (pH 7.2) or DMSO. The scavenging percentages were calculated according to the Equation:
α-Amylase Inhibitory Activity
Porcine pancreatic α-amylase was purchased from Sigma Aldrich (Sigma, Aldrich, St. Louis, Missouri, USA) while soluble starch was purchased from HiMedia (Himedia Laboratories, Mumbai, India). The enzyme was diluted using 0.1 M phosphate buffer (pH 6.9). The α-amylase inhibitory activity was determined according to a modified procedure described by Zia-Ul-Haq et al. (2011). Briefly, 20 μl of the enzyme solution (1 U ml-1) was pre-incubated with 20 μl of undigested or digested extract or purified pigment, at 37°C for 10 min. After the incubation, 250 μl of 1% starch in 0.1 M phosphate buffer (pH 6.9) was added and the mixture was further incubated at 37°C for 20 min. The reaction was terminated by adding 200 μl of 3,5-dinitrosalicylic acid (DNS) reagent (1% DNS and 12% sodium potassium tartrate in 0.4 M NaOH). The tubes were incubated in a boiling water bath for a few minutes. 5 mL distilled water was added to all the tubes and their absorbance was recorded at 540 nm against a blank devoid of starch and sample. The control reaction, signifying 100% enzyme activity, contained buffer or DMSO instead of the samples while acarbose was used as a positive control. The inhibitory activity was calculated by using the following Equation:
α-Glucosidase Inhibitory Activity
α-Glucosidase from Saccharomyces cerevisiae was purchased from Sigma Aldrich (Sigma, Aldrich, St. Louis, Missouri, USA) while reduced glutathione and p-nitrophenyl α D-glucopyranoside (pNPG) were purchased from HiMedia (Himedia Laboratories, Mumbai, India). The α-glucosidase inhibitory activity was determined according to a modified procedure described by Kim et al. (2004). Briefly, 50 μl of 0.1 M potassium phosphate buffer (pH 6.9) was pre-incubated with 50 μl of reduced glutathione (1 mg ml−1), 20 μl α-glucosidase (1 U ml−1 in 0.1 M phosphate buffer, pH 6.9), and 20 μl of undigested or digested extract or purified pigment at 37°C for 10 min. After the incubation, 20 μl pNPG was added and the mixture was further incubated at 37°C for 30 min. The reaction was terminated by adding 1 ml of 0.1 M sodium carbonate. The absorbance of the samples and control were taken at 405 nm against a blank devoid of pNPG and sample. The control reaction (with 100% enzyme activity) contained buffer or DMSO instead of their respective samples while acarbose was used as a positive control. The inhibitory activity was calculated by using the following Equation:
In vitro Digestion of Cyanobacterial Extracts and Purified Pigments
In vitro digestion of the crude extracts, C-PE, C-PC, myxoxanthophyll, and lycopene were carried out according to Ferranti et al. (2014). 1 ml crude extracts in either 0.1 M phosphate buffer (pH 7.2) or DMSO and the purified pigments in their appropriate solvents (0.1 M phosphate buffer, pH 7.2 for C-PC and C-PE and DMSO for carotenoids) were digested with 90 U ml−1 α-amylase in 4 ml of digestion buffer (120 mM NaCl, 5 mM KCl, 6 mM CaCl2) for 5 min at 37°C. After amylase digestion, 8 ml of the digestion buffer was added and the pH was adjusted to 2.0 using 37% (w/w) HCl. Pepsin (Sigma Aldrich, USA, > 400 U mg−1, final concentration 3 mg ml−1) was added to the solution and the mixture was incubated for 60 min at 37°C. After the incubation, 8 ml of digestion buffer was added to the mixture and the pH was adjusted to 5 using 1.5 M NaHCO3 solution to stop peptic digestion. Duodenal digestion was started by addition of pancreatin (Sigma Aldrich, USA, final concentration 0.4 mg ml−1) and porcine bile extract (SantaCruz Biotechnology, USA, final concentration 2.4 mg ml−1) and the pH was adjusted to 6 using 1.5 M NaHCO3. The mixture was further incubated at 37°C for 300 min. After completion of in vitro digestion, the pH was adjusted to 2 using 37% (w/w) HCl to prevent further digestion of samples during storage. The solutions were centrifuged to remove any particulate matter and stored at −70°C.
Statistical Analysis
All experiments were performed in triplicate and the results are shown in mean ± SD. Analysis of variance was conducted using Fischer LSD test (Info Stat 3.0, Di Rienzo et al., 2011). The differences were calculated to be significant at p < 0.05.
Results
Characterization of Cyanobacterial Extracts and Purified Pigments
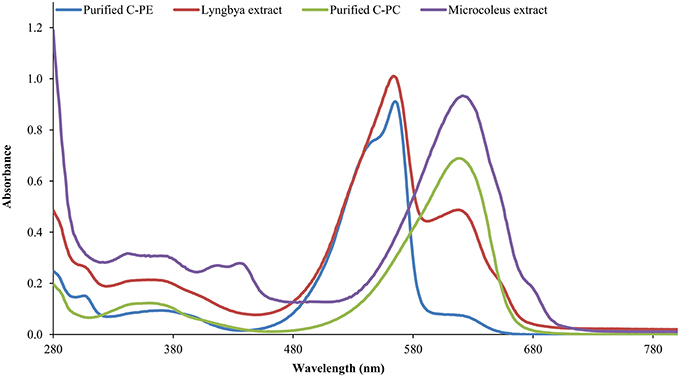
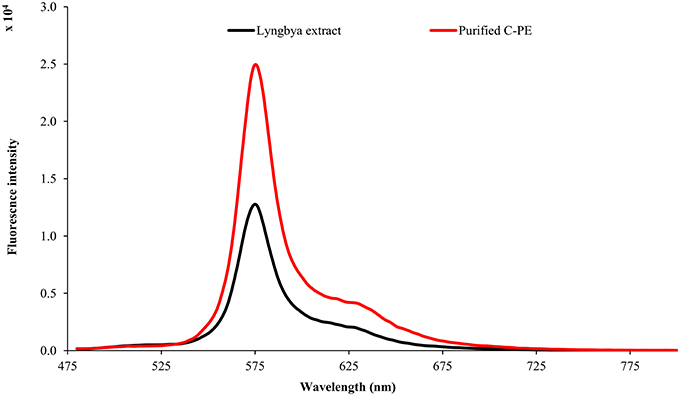
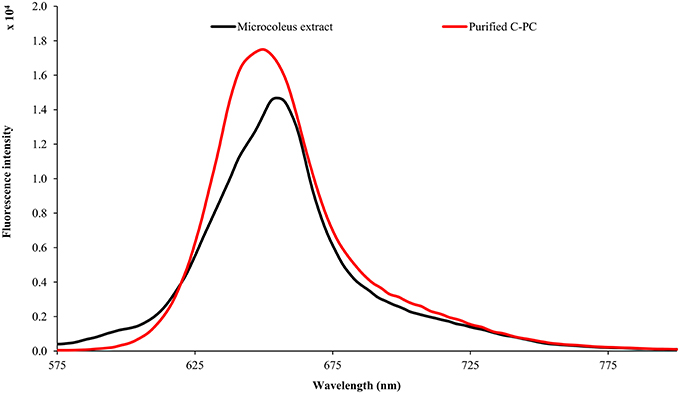
The Lyngbya extract had C-PE as the major pigment (102.48 ± 1.57 mg g−1) while the Microcoleus extract had C-PC as the major pigment (46.58 ± 0.06 mg g−1). Similarly, the Synechocystis extract had myxoxanthophyll as the major carotenoid (2.21 ± 0.43 mg g−1) with a lycopene content of 0.59 ± 0.13 mg g−1. All the values have been reported in triplicate on a dry mass basis.The UV-visible absorbance of the Lyngbya extract and the purified C-PE had characteristic absorption maxima at 565 nm while the Microcoleus extract and purified C-PC had their absorption maxima at 618 nm (Figure 1). Fluorescence emission analysis of the Lyngbya extract and purified C-PE showed an emission maxima at 575 nm (Figure 2). In comparison, the Microcoleus extract and purified C-PC had their emission maxima at 653 nm (Figure 3). The purity ratios for purified C-PE and C-PC were 3.67 and 3.49, respectively.
Total Anti-Oxidant Capacity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
The total antioxidant capacity (TAC) for the undigested and digested extracts as well as purified C-PE, C-PC, myxoxanthophyll, and lycopene are presented in Table 1. TAC values are expressed in terms of ascorbic acid equivalents (AAE). Among the undigested samples, the Lyngbya extract had the highest TAC value (48.26 ± 0.04 μg AAE ml−1) while the extracts from Microcoleus and Synechocystis had similar activities (38.44 ± 0.48 and 34.91 ± 0.48 μg AAE ml−1, respectively), although they were significantly different statistically. Among the purified pigments, C-PE had the highest TAC (15.24 ± 1.47 μg AAE ml−1). If we compare the digested samples, the purified pigments had better TAC values compared to the extracts. The extracts of Lyngbya and Microcoleus had activities of 62.98 ± 0.6 and 110.68 ± 0.2 μg AAE ml−1, respectively while purified C-PE and C-PC had TAC values of 118.97 ± 0.34 and 131.67 ± 0.96 μg AAE ml−1, respectively. Purified lycopene and myxoxanthophyll, after digestion, showed maximum TAC values of 154.16 ± 0.96 and 134.95 ± 0.40 μg AAE ml−1, respectively.
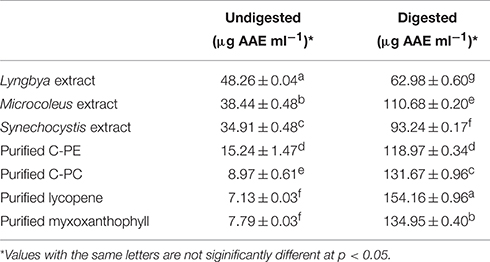
Table 1. Total Anti-oxidant activity of the undigested and digested cyanobacterial extracts and purified pigments.
ABTS Scavenging Activity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
The undigested and digested cyanobacterial extracts and purified pigments were evaluated for ABTS scavenging activity (Table 2). The undigested Lyngbya and Microcoleus extracts had scavenging activities of 91.40 ± 0.75 and 98.23 ± 0.25%, respectively and they were the only samples where the undigested samples had more activity than the digested ones. The undigested Synechocystis extract had an ABTS scavenging activity of 36.26 ± 2.66%. The undigested purified C-PE and C-PC were able to scavenge 94.03 ± 0.91 and 83.57 ± 1.67% of the ABTS free radicals respectively, while purified lycopene and myxoxanthophyll did not show any scavenging activity before digestion. Comparing the digested samples, the Lyngbya and Microcoleus extracts had scavenging activities of 81.08 ± 0.9 and 48.07 ± 0.77%, respectively while the Synechocystis extract had an activity of 77.92 ± 0.74%. In comparison, the scavenging activities of the digested purified pigments was significantly better with all the purified pigments able to scavenge ~99% of the ABTS free radicals.
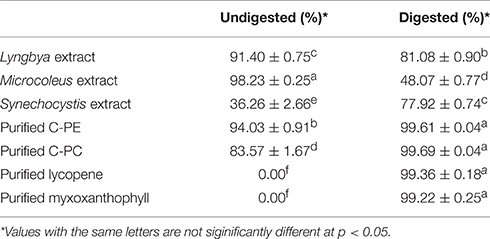
Table 2. ABTS scavenging activity of the undigested and digested cyanobacterial extracts and purified pigments.
α-Amylase Inhibitory Activity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
We investigated the undigested and digested cyanobacterial extracts sourced from three different cyanobacteria as well as purified C-PC, C-PE, myxoxanthophyll, and lycopene for their α-amylase inhibitory activities (Table 3). When we compared the undigested extracts, we observed that acarbose, as the positive control, had the maximum activity (54.23 ± 0.01%) while the Lyngbya and Microcoleus extracts had significantly lesser activities (10.68 ± 0.02 and 8.53 ± 1.65%, respectively). The undigested Synechocystis extract was unable to inhibit the activity of α-amylase in vitro. Purified C-PE and C-PC had almost the same inhibitory activities (14.46 ± 0.26 and 14.06 ± 0.07%, respectively) while purified lycopene and myxoxanthophyll again showed almost no inhibitory effect on α-amylase. In comparison, in vitro digestion had a very minor increase in the inhibitory activities of the extracts and the purified pigments, with the exception of purified C-PC. The greatest enhancement was observed in the activity of the Microcoleus extract (34.89 ± 0.03%) while the Lyngbya and Synechocystis extracts had inhibitory activities of 12.36 ± 0.01 and 14.72 ± 0.05%, respectively. The purified pigments had inhibitory activities ranging from ~8 to 16%. It was also observed that purification had an antagonistc effect on the inhibitory activity of C-PC after in vitro digestion; the inhibitory activity decreased by ~26%.
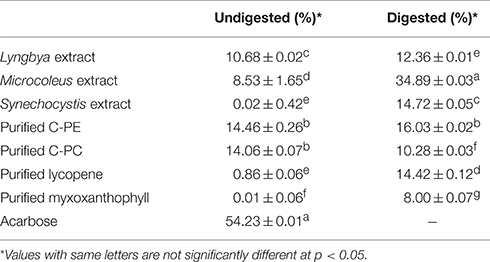
Table 3. α-amylase inhibitory activity of the undigested and digested cyanobacterial extracts and purified pigments.
α-Glucosidase Inhibitory Activity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
The cyanobacterial extracts and purified C-PE, C-PC, lycopene, and myxoxanthophyll were studied for their inhibitory effects on the activity of α-glucosidase (Table 4). On comparing the undigested extracts, we observed that the Lyngbya extract had the highest inhibitory activity against α-glucosidase (62.22 ± 0.02%) whereas the Microcoleus extract had a significantly lower activity (8.53 ± 1.65%). The Synechocystis extract again had no inhibitory activity on α-glucosidase before digestion. The purified pigments also varied widely in their inhibitory activities. Purified C-PE had an activity of 14.46 ± 0.26% while purified C-PC failed to inhibit the enzyme. Purified lycopene and myxoxanthophyll had inhibitory activities in the range of 43.77–46.82%. Acarbose, as the positive control, had an inhibitory effect of 18.62 ± 0.03%. However, the digested samples had a significantly higher inhibitory effect on the enzyme compared to the undigested ones. The Lyngbya and Microcoleus extracts had activities of 97.82 ± 0.03 and 73.95 ± 0.01%, respectively while the Synechocystis extract was able to inhibit the enzyme by 92.46 ± 0.03%. The purified pigments showed inhibitory activities ranging from ~83 to 96%. The activity of purified C-PC and lycopene had higher inhibitory activities compared to purified C-PE and myxoxanthophyll respectively.
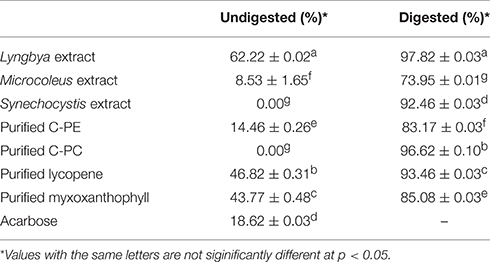
Table 4. α-glucosidase inhibitory activity of the undigested and digested cyanobacterial extracts and purified pigments.
Discussion
Characterization of Cyanobacterial Extracts and Purified Pigments
PBPs are accessory light harvesting pigments in cyanobacteria. They are intensely fluorescent molecules which can capture wavelengths of light not harvested by chlorophyll. A sequential energy transfer mechanism transfers the light energy to the photosystems where the process of photosynthesis is carried out. Due to their fluorescent properties, they have found uses as food colorant, fluorescent probes and markers, neuro and hepato-protective agents and anti-oxidant molecules (Vadiraja et al., 1998; Romay et al., 2003; Sekar and Chandramohan, 2007). The UV-visible spectra of the Lyngbya extract had a characteristic peak at 565 nm which is identical to the absorption maximum of purified C-PE. Additionally, the Lyngbya extract also contains a shoulder peak at 621 nm which signifies the presence of C-PC. The purified C-PE sample does not contain any secondary peaks and its purity ratio was found to be 3.67. Similarly, the absorption maximum of the Microcoleus extract had an absorption maximum at 618 nm which is similar to that of purified C-PC (Figure 1). The purity ratio of the purified C-PC sample was 3.49. The fluorescent property of phycobiliproteins is due to their unique chromophores bound to specific amino acid residues. The chromophores are tetrapyrrole moieties with a high degree of conjugation that gives them their characteristic hues (Ghosh et al., 2015). The fluorescence emission spectrum of the Lyngbya extract and the purified C-PE had an emission maximum at 575 nm due to the presence of the chromophore phycoerythrobilin (Mishra et al., 2011). Moreover, the fluorescence intensity showed a marked increase when the extract was purified to yield C-PE (Figure 2). Similarly, the Microcoleus extract and purified C-PC had their fluorescence emission maxima at 653 nm due to the presence of the chromophore phycoyanobilin (Figure 3; Downes and Hall, 1998). Moreover, we also observed a reduction in the absorbance at 280 nm for both C-PC and C-PE which signified the reduction in contaminating proteins after chromatographic purification.
Carotenoids are accessory pigment molecules in cyanobacteria that are responsible for light harvesting as well as photo-protection of the photosystem and regulation of membrane fluidity (Jaswir et al., 2011). They have been the subject of focused studies on taxonomic classification and identifying the presence of certain microalgae and cyanobacteria in aquatic ecosystems (Mackey et al., 1996; Paliwal et al., 2016). Carotenoids like myxoxanthophyll and lycopene are mainly purified using HPLC techniques. We were able to identify and purify individual carotenoids using TSKGel ODS120T column which partitions carotenoids on the basis of their polarity. The identification of individual carotenoids was achieved by running standard carotenoids from DHI, Denmark. The retention times of the standards of myxoxanthophyll and lycopene were calculated and the respective fractions were collected (Paliwal et al., 2016). The lycopene and myxoxanthophyll fractions were 88 and 86.6% pure, respectively. The purity was calculated based on peak areas of the respective standards and samples. Due to their high degree of stability under adverse conditions of temperature and light and conjugated chemical structures, they are also looked upon as promising candidates for natural food colorants, anti-oxidants, nutrient supplements, feed additives, anti-cancer agents, and nutraceuticals (Jaswir et al., 2011).
Total Anti-Oxidant Capacity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
The total anti-oxidant capacity measures the ability of a compound to reduce Mo6+ ions present in the reagent. The absorbance of the colored compound thus produced is measured at 695 nm wherein the intensity of the color is directly proportional to the anti-oxidant activity of the sample under investigation. PBPs have been reported as excellent anti-oxidant in many studies (Paliwal et al., 2015a; Wu et al., 2015). However, the digested products of the extracts and the purified pigments were found to be better anti-oxidants compared to the undigested samples. The highest TAC value in undigested and digested samples were 48.26 ± 0.04 μg AAE ml−1 (Lyngbya extract) and 154.16 ± 0.96 μg AAE ml−1 (purified lycopene), respectively. In particular, the TAC values of purified C-PC and C-PE were among the highest gainers. The probable reason for this is the generation of peptides with stronger anti-oxidant activity after the digestion process, since there are more accessible amino acid residues after the digestion process (Wu et al., 2015). Similarly, digested purified lycopene and myxoxanthophyll had significantly higher activities (154.16 ± 0.96 and 134.95 ± 0.4 μg AAE ml−1, respectively) as compared to the undigested ones; digested lycopene had the highest activity among all the samples considered. Carotenoids, being hydrophobic in character, are emulsified during digestion to be more accessible for assimilation in a largely aqueous environment of the human digestive system. Thus, the emulsification process can lead to the generation of compounds with higher anti-oxidant activities (Garrett et al., 1999).
ABTS Scavenging Activity of Undigested and Digested Cyanobacterial Extracts and Purified Pigments
ABTS is a common free radical used to assess the scavenging activity of a wide range of compounds. Before use, it is generally reacted with a free radical initiator like potassium persulphate to generate the free radicals. The chief difference between the total anti-oxidant activity and free radical scavenging is that the former tests the power of reduction of substrates while the latter tests for the direct scavenging of free radicals generated through various chemical routes. The scavenging effect of all the cyanobacterial extracts show that their antioxidant activities are directly linked to their pigment content. However, the scavenging activities of both Lyngbya and Microcoleus extracts decrease after in vitro digestion, which suggests that the scavenging agents do not consist of only peptides. To investigate this fact further, we digested the purified C-PE and C-PC proteins wherein we confirmed that the generation of short peptides probably plays a role in free radical scavenging; the activities of the digested samples of purified C-PE and C-PC are greater than their undigested counterparts (~6% for purified C-PE and ~19% for purified C-PC). The exposure of more number of amino acids to the free radical may be responsible for the increased scavenging activity (Wu et al., 2015). Similarly, in vitro digestion of carotenoids is a scarcely researched topic due to the time consuming procedures such as thin layer chromatography; however, rapid techniques like HPLC analysis have since been adapted for the identification and characterization of carotenoids (Nozière et al., 2006). In our observations, we could see that the Synechocystis extract had an increased radical scavenging activity after digestion (~115% increase), while the scavenging activities of purified lycopene and myxoxanthophyll were evident only after the digestion process, possibly due to their micellerization (Garrett et al., 1999). The micellerization process essentially emulsifies the pigments, making them dispersible in an aqueous phase. This process is common to both proteins and carotenoids.
Cyanobacterial Extracts and Purified Pigments As Natural Alternatives for Diabetes Management
Diabetes mellitus is a widespread problem today which necessitates the development of newer molecules for its mitigation or management. Type II diabetes is more common, since it depends on the lifestyle a person leads. Diabetes management is often a combination of different therapies to achieve the best combination. There are three possible ways of combating type II diabetes: increasing the production of insulin in the body, sensitizing the target cells to insulin, or to delay the rate of glucose absorption in blood so that blood glucose levels do not peak abnormally. The last possibility calls for inhibiting various enzymes that are responsible for breakdown of carbohydrates in meals in to simple sugars which are then assimilated by our body. There are a number of published reports that have targeted two different enzymes: pancreatic α-amylase and α-glucosidase, both of which are responsible for digestion of complex carbohydrates through hydrolyzing α-(1,4) glycosidic linkages. Although there are various synthetic drugs available in the market for inhibition of these enzymes, and we have used one of them as our positive control, they are not always free of side effects that mainly result from glucose malabsorption. Alternatives to such molecules usually include herbal supplements and natural plant extracts that have been passed down through generations of experience. Recent reports by various groups indicate that many plants and their fruits have the potential to inhibit both these enzymes in vitro and are potential candidates for in vivo studies. For example, Schizandra chinensis is a type of berry consumed widely in Korea and its fruits and seeds have been used as traditional medicines in different parts of Asia. However, the pulp extract of this berry was found to inhibit α-amylase effectively whereas the seed extract did not show any significant activity (Jo et al., 2011). However, they were able to effectively inhibit the action of α-glucosidase in vitro even at lower concentrations. Low anti α-amylase and higher anti α-glucosidase activities has also been reported in tea fruit peel extracts (Wang et al., 2012) and pulp and skin extracts from eggplants (Kwon et al., 2008). In another study, pomegranate peel extracts were found to be ineffective in inhibiting the activity of α-amylase, although they inhibited α-glucosidase to a great extent (Çam and İçyer, 2013). However, to the best of our knowledge, there are no reports on the effects of cyanobacterial pigments, whether native or digested, on the activity of these enzymes in vitro. Moreover, a balance on the extent of inhibition of α-amylase and α-glucosidase has to be achieved to reduce diarrhea and flatulence linked to undigested starch in the intestines. It also serves as an effective medium for bacterial fermentation processes leading to an increase in production of gases and butyrate (Kwon et al., 2007). Based on these reports and our previous experience of anti-oxidant scavenging studies on digested microalgal pigments, we investigated their ability to inhibit these two enzymes in vitro. Digested samples were able to block enzyme activities much more effectively than native pigments; in some cases, the increase in the inhibitory activity after digestion was as much as 96–97%. Our results also indicated that the tested pigments and digested products had a lower anti α-amylase activity as compared to their corresponding anti α-glucosidase activity. After digestion, purified C-PE was a better inhibitor of α-amylase while purified C-PC was better at inhibiting the activity of α-glucosidase. The carotenoids lycopene and myxoxanthophyll also had significantly higher inhibitory activities against both enzymes after digestion. The probable reason for this increased inhibitory activities may lie in the formation of micelles after digestion of purified pigments (Garrett et al., 1999), which could make them potent active site blockers for the enzymes. Since, the purified products seem to be able to manage hyperglycemia, it might be prudent to consider them as food additives rather than the extracts from Lyngbya, Microcoleus, or Synechocystis sp. The chief reason is the presence of uncharacterized compounds, namely toxins, in these extracts which might have undesired side effects (Ghosh et al., 2015). However, another key feature of such pigments lie not in the extent of inhibition of the enzymes but on their natural origins, which are not expected to have any severe or long term side effects. Promising results and a lack of studies in this area prompts an investigation which could be potentially feasible, both medically and economically. This study could be useful as a primary screen for potentially beneficial metabolites from cyanobacteria for hyperglycemic conditions. Future studies could target the nature and mechanisms of inhibition of the enzymes by the digested products.
Conclusion
The undigested and digested cyanobacterial extracts from Lyngbya, Microcoleus, and Synechocystis sp. and purified C-PE, C-PC, lycopene, and myxoxanthophyll were investigated for their ability to inhibit carbohydrate hydrolysis through enzymatic activity thus helping in the successful management of post-prandial hyperglycemia. It was observed that the digested products were able to successfully inhibit the activities of pancreatic α-amylase and α-glucosidase; the extent of inhibition was better than the undigested samples. The purified pigments were effective blockers of enzymatic activity. In particular, purified C-PE was better able to inhibit α-amylase after digestion while digested purified C-PC was an effective inhibitor of α-glucosidase. Moreover, the digested products also had enhanced free radical scavenging activities compared to the undigested samples, except the Lyngbya and Microcoleus extracts. However, the total anti-oxidant activities increased for all samples after digestion. The results obtained in this study indicate that cyanobacterial pigments and extracts could be considered as potential anti-hyperglycemic agents at par with those obtained from traditional plants and herbs as well as synthetic drugs.
Author Contributions
CP, TG, KB, and RM have performed all the experiments and made substantial contributions to the acquisition and analysis of results. CP, TG, KC, IP, and SM have been involved in drafting and critical check of the entire manuscript. IP, KB, and RM have been involved in the calculation and interpretation of the results obtained. All authors have read and approved the final manuscript.
Funding
TG, CP, and RM gratefully acknowledge CSIR, New Delhi for awarding Senior Research Fellowship. All the authors also acknowledge CSIR and DST for providing the financial support through projects CSC 0203, OLP 0040, OLP 0071, and GAP 2006.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been assigned PRIS number CSIR-CSMCRI—057/2016. The authors would also like to thank Dr. Arvind Kumar (CSIR-CSMCRI, Bhavnagar) for his constant encouragement. The authors would like to thank Dr. Arun Kumar Das, Dr. Parimal Paul, Dr. Arup Ghosh, and Dr. Harshad Brahmabhatt (CSIR-CSMCRI, Bhavnagar) for their timely help during the analysis. TG, CP, and RM wish to acknowledge AcSIR-CSMCRI for Ph.D. enrollment.
References
Ahmad, Z., Zamhuri, K. F., Yaacob, A., Siong, C. H., Selvarajah, M., Ismail, A., et al. (2012). In vitro anti-diabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (Bitter Gourd). Molecules 17, 9631–9640. doi: 10.3390/molecules17089631
Bennett, A., and Bogorad, L. (1973). Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 58, 419–435. doi: 10.1083/jcb.58.2.419
Çam, M., and İçyer, N. C. (2013). Phenolics of pomegranate peels: extraction optimization by central composite design and alpha glucosidase inhibition potentials. J. Food Sci. Technol. 52, 1489–1497. doi: 10.1007/s13197-013-1148-y
Deng, R., and Chow, T.-J. (2010). Hypolipidemic, antioxidant and anti-inflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 28, e33–e45. doi: 10.1111/j.1755-5922.2010.00200.x
Di Rienzo, J., Casanoves, F., Balzarini, M., Gonzalez, L., Tablada, M., and Robledo, C. (2011). InfoStat versión 2011. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online at: http://www.infostat.com.ar
Downes, M. T., and Hall, J. A. (1998). A sensitive fluorometric technique for the measurement of phycobilin pigments and its application to the study of marine and freshwater picophytoplankton in oligotrophic environments. J. Appl. Phycol. 10, 357–363. doi: 10.1023/A:1008085719486
Ferranti, P., Nitride, C., Nicolai, M. A., Mamone, G., Picariello, G., Bordoni, A., et al. (2014). In vitro digestion of Bresaola proteins and release of potential bioactive peptides. Food Res. Int. 63B, 157–169. doi: 10.1016/j.foodres.2014.02.008
Flanagan, P. R., and Forstner, G. G. (1978). Purification of rat intestinal maltase/glucoamylase and its anomalous dissociation either by heat or by low pH. Biochem. J. 173, 553–563. doi: 10.1042/bj1730553
Gao, J., Xu, P., Wang, Y., Wang, Y., and Hochstetter, D. (2013). Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against α-amylase and α-glucosidase in vitro. Molecules 18, 11614–11623. doi: 10.3390/molecules180911614
Garrett, D. A., Failla, M. L., and Sarama, R. J. (1999). Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 47, 4301–4309. doi: 10.1021/jf9903298
Ghosh, T., Paliwal, C., Maurya, R., and Mishra, S. (2015). “Microalgal rainbow colours for nutraceutical and pharmaceutical applications,” in Plant Biology and Biotechnology: Vol. I: Plant Diversity, Organization, Function and Improvement, eds B. Bahadur, M. Venkat Rajam, L. Sahijram, and K. V. Krishnamurthy (New Delhi: Springer India), 777–791.
IDF Diabetes Atlas (2015). International Diabetes Federation. Available online at: http://www.idf.org/idf-diabetes-atlas-seventh-edition (Accessed April 14, 2016).
Jaswir, I., Dedi, N., Reno, F. H., and Fitri, O. (2011). Carotenoids: sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 5, 7119–7131. doi: 10.5897/JMPRX11.011
Jo, S.-H., Ha, K.-S., Moon, K.-S., Lee, O.-H., Jang, H.-D., and Kwon, Y.-I. (2011). In vitro and in vivo anti-hyperglycemic effects of Omija (Schizandra chinensis) fruit. Int. J. Mol. Sci. 12, 1359–1370. doi: 10.3390/ijms12021359
Kim, Y.-M., Wang, M.-H., and Rhee, H.-I. (2004). A novel alpha-glucosidase inhibitor from pine bark. Carbohydr. Res. 339, 715–717. doi: 10.1016/j.carres.2003.11.005
Kronick, M. N. (1986). The use of phycobiliproteins as fluorescent labels in immunoassay. J. Immunol. Methods 92, 1–13. doi: 10.1016/0022-1759(86)90496-5
Kwon, Y. I., Apostolidis, E., Kim, Y. C., and Shetty, K. (2007). Health benefits of traditional corn, beans, and pumpkin: in vitro studies for hyperglycemia and hypertension management. J. Med. Food 10, 266–275. doi: 10.1089/jmf.2006.234
Kwon, Y.-I., Apostolidis, E., and Shetty, K. (2008). In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 99, 2981–2988. doi: 10.1016/j.biortech.2007.06.035
Mackey, M. D., Mackey, D. J., Higgins, H. W., and Wright, S. W. (1996). CHEMTAX - a program for estimating class abundances from chemical markers: application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 144, 265–283. doi: 10.3354/meps144265
Manirafasha, E., Ndikubwimana, T., Zeng, X., Lu, Y., and Jing, K. (2016). Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 109, 282–296. doi: 10.1016/j.bej.2016.01.025
Mishra, S. K., Shrivastav, A., and Mishra, S. (2011). Preparation of highly purified C-phycoerythrin from marine cyanobacterium Pseudanabaena sp. Protein Expr. Purif. 80, 234–238. doi: 10.1016/j.pep.2011.06.016
Nozière, P., Graulet, B., Lucas, A., Martin, B., Grolier, P., and Doreau, M. (2006). Carotenoids for ruminants: from forages to dairy products. Anim. Feed Sci. Technol. 131, 418–450. doi: 10.1016/j.anifeedsci.2006.06.018
Oh, J., Jo, S.-H., Kim, S. J., Ha, K.-S., Lee, J.-Y., Choi, H.-Y., et al. (2015). Selected tea and tea pomace extracts inhibit intestinal α-glucosidase activity in vitro and postprandial hyperglycemia in vivo. Int. J. Mol. Sci. 16, 8811–8825. doi: 10.3390/ijms16048811
Paliwal, C., Ghosh, T., Bhayani, K., Maurya, R., and Mishra, S. (2015a). Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar. Drugs 13, 5384–5401. doi: 10.3390/md13085384
Paliwal, C., Ghosh, T., George, B., Pancha, I., Maurya, R., Chokshi, K., et al. (2016). Microalgal carotenoids: potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 15, 24–31. doi: 10.1016/j.algal.2016.01.017
Paliwal, C., Pancha, I., Ghosh, T., Maurya, R., Chokshi, K., Vamsi Bharadwaj, S. V., et al. (2015b). Selective carotenoid accumulation by varying nutrient media and salinity in Synechocystis sp. CCNM 2501. Bioresour. Technol. 197, 363–368. doi: 10.1016/j.biortech.2015.08.122
Patel, A., Mishra, S., Pawar, R., and Ghosh, P. K. (2005). Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 40, 248–255. doi: 10.1016/j.pep.2004.10.028
Ramasubbu, N., Ragunath, C., Sundar, K., Mishra, P. J., Gyemant, G., and Kandra, L. (2005). Structure-function relationships in human salivary α-amylase: role of aromatic residues. Biology 60, 47–56.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Romay, C., González, R., Ledon, N., Remirez, D., and Rimbau, V. (2003). C-Phy cocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective eff ects. Curr. Prot. Pept. Sci. 4, 207–216. doi: 10.2174/1389203033487216
Sekar, S., and Chandramohan, M. (2007). Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J. Appl. Phycol. 20, 113–136. doi: 10.1007/s10811-007-9188-1
Sengupta, S., Mukherjee, A., Goswami, R., and Basu, S. (2009). Hypoglycemic activity of the antioxidant saponarin, characterized as α-glucosidase inhibitor present in Tinospora cordifolia. J. Enzyme Inhib. Med. Chem. 24, 684–690. doi: 10.1080/14756360802333075
Stryer, L., and Glazer, A. N. (1985). Phycobiliprotein Fluorescent Conjugates. Available online at: http://www.google.co.in/patents/US4542104
Sudhakar, M. P., Jagatheesan, A., Perumal, K., and Arunkumar, K. (2015). Methods of phycobiliprotein extraction from Gracilaria crassa and its applications in food colourants. Algal Res. 8, 115–120. doi: 10.1016/j.algal.2015.01.011
US National Library of Medicine (2016). Acarbose. LiverTox. Available online at: http://livertox.nih.gov/Acarbose.htm (Accessed April 14, 2016).
Vadiraja, B. B., Gaikwad, N. W., and Madyastha, K. M. (1998). Hepatoprotective effect of C-phycocyanin: protection for carbon tetrachloride and R-(+)-Pulegone-mediated hepatotoxicty in rats. Biochem. Biophys. Res. Commun. 249, 428–431. doi: 10.1006/bbrc.1998.9149
Wang, Y., Huang, S., Shao, S., Qian, L., and Xu, P. (2012). Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind. Crop Prod. 37, 520–526. doi: 10.1016/j.indcrop.2011.07.031
Wu, Q., Fu, X.-P., Sun, L.-C., Zhang, Q., Liu, G.-M., Cao, M.-J., et al. (2015). Effects of physicochemical factors and in vitro gastrointestinal digestion on antioxidant activity of R-phycoerythrin from red algae Bangia fusco-purpurea. Int. J. Food Sci. Technol. 50, 1445–1451. doi: 10.1111/ijfs.12775
Keywords: anti-hyperglycemic activity, C-phycocyanin, C-phycoerythrin, myxoxanthophyll, lycopene, in vitro digestion, cyanobacteria
Citation: Ghosh T, Bhayani K, Paliwal C, Maurya R, Chokshi K, Pancha I and Mishra S (2016) Cyanobacterial Pigments as Natural Anti-Hyperglycemic Agents: An In vitro Study. Front. Mar. Sci. 3:146. doi: 10.3389/fmars.2016.00146
Received: 15 April 2016; Accepted: 29 July 2016;
Published: 10 August 2016.
Edited by:
Antonio Trincone, Consiglio Nazionale delle Ricerche, ItalyReviewed by:
Anbarasu Kumarasamy, Bharathidasan University, IndiaJannette Gavillan-Suarez, University of Puerto Rico, USA
Copyright © 2016 Ghosh, Bhayani, Paliwal, Maurya, Chokshi, Pancha and Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandhya Mishra, smishra@csmcri.org
 Tonmoy Ghosh
Tonmoy Ghosh Khushbu Bhayani
Khushbu Bhayani Chetan Paliwal
Chetan Paliwal Rahulkumar Maurya
Rahulkumar Maurya Kaumeel Chokshi
Kaumeel Chokshi Imran Pancha
Imran Pancha Sandhya Mishra
Sandhya Mishra