Evaluating the Impact of Atmospheric Depositions on Springtime Dinitrogen Fixation in the Cretan Sea (Eastern Mediterranean)—A Mesocosm Approach
- 1Israel Oceanographic and Limnological Research, National Institute of Oceanography, Haifa, Israel
- 2Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel
- 3Division of Life Science, Hong Kong University of Science and Technology, Hong Kong, Hong Kong
- 4Department of Microbiology, University of Bergen, Bergen, Norway
- 5Hellenic Centre for Marine Research, Heraklion, Greece
- 6Department of Ocean, Earth and Atmospheric Sciences, Old Dominion University, Norfolk, VA, USA
- 7Laboratory of Environmental Chemistry, Department of Chemistry, University of Athens, Athens, Greece
Large amounts of dust and atmospheric aerosols, originating from surrounding desert areas (e.g., Sahara and Middle East) are deposited annually on the surface of the Eastern Mediterranean Sea. These depositions can provide high amounts of micro (such as Fe, Zn, Co) and macro nutrients (such as P and N) to supplement nutrient-poor surface waters- that typically limit primary productivity and also dinitrogen (N2) fixation in many marine environments. Here, we studied the impact of the atmospheric deposition of dust and aerosols on N2 fixation in the Cretan Sea (Eastern Mediterranean Sea). Mixed polluted aerosols (hereafter A) and Saharan dust (hereafter SD) were added to nine mesocosms (3-m3 each) containing surface mixed layer seawater (~10 m), and N2 fixation was evaluated for 6 days during May 2012 (springtime). The addition of SD triggered a rapid (30 h) and robust (2–4-fold) increase in N2 fixation rates that remained high for 6 days and contributed 3–8% of the primary productivity. The A addition also resulted in higher N2 fixation rates compared to the unamended control mesocosms, although the responses were less profound (1.5–2-fold) and accounted for only 2–4% of the primary productivity. The microbial community responded differently to the two additions. Heterotrophic bacterial N2 fixers dominated the diazotroph community in A and the control mesocosms, while the non-filamentous cyanobacterial group Trichodesmium prevailed in the SD treatment (68% of all the operational taxonomic units, verified by qPCR analyses). Our results indicate that the aerosol source, its route prior to deposition, and its specific chemical composition, can alter the diazotrophic diversity and activity in the Eastern Mediterranean Sea and may thus impact both the N and C dynamics in this impoverished environment.
Introduction
Dinitrogen (N2) fixation is recognized as an important pathway for bioavailable nitrogen inputs in many of the world's oceans (Gruber and Galloway, 2008; Sohm et al., 2011). This process can alter phytoplankton populations and primary production in nitrogen (N) limited marine environments (e.g., Falkowski, 1997; Carpenter et al., 2004; Sohm et al., 2011) and thus fuels the biological pump. Many physical, chemical, and biological factors can affect the magnitude of N2 fixation in a given ecosystem. Where N2 fixers (diazotrophs) occur, they are most often limited by phosphorus (P) (Wu et al., 2000; Sanudo-Wilhelmy et al., 2001; Dyhrman and Haley, 2006), iron (Fe) (Paerl et al., 1987; Berman-Frank et al., 2001, 2007; Moore et al., 2009), or both (Mills et al., 2004).
Wet and dry deposition of atmospheric dust can increase the availability of Fe and P in the surface ocean (Herut et al., 1999, 2005; Bonnet and Guieu, 2006; Guieu et al., 2014) thereby supplementing the requirements of primary producers (mostly N and P, Zohary et al., 2005; Rahav et al., 2016a; Tsiola et al., 2016) and N2-fixers (Gruber and Sarmiento, 1997; Mills et al., 2004; Marañón et al., 2010). Dust enrichment enhanced N2 fixation rates in microcosm experiments from the tropical Atlantic (Mills et al., 2004; Marañón et al., 2010), the North Atlantic (Gruber and Sarmiento, 1997), the Red Sea (Foster et al., 2009), and the tropical and subtropical western North Pacific (Kitajima et al., 2009). Furthermore, a 100-fold increase in the abundance of Trichodesmium, a non-filamentous cyanobacterial diazotroph, and a ~2-fold increase in dissolved organic nitrogen were reported following dust stimulation in the West Florida Shelf (Lenes et al., 2001). However, N2 fixation rates and high abundances of diazotrophs are not always observed in areas of the ocean where dust deposition is high. For example, the oligotrophic Mediterranean Sea receives high annual inputs of dust (20 × 106 to 50 × 106 tons y−1, Guerzoni et al., 1999) characterized by relatively high dissolved Fe concentrations (~42 μmol m−2 y−1, Bonnet and Guieu, 2006). Yet N2 fixation rates are typically low in surface waters and the upper mixed layer (usually < 0.2 nmol N L−1 d−1, Sandroni et al., 2007; Ibello et al., 2010; Bonnet et al., 2011; Rahav et al., 2013a; Benavides et al., 2016) relative to other marine systems (reviewed in Sohm et al., 2011).
Several studies have examined the possibility of low P availability limiting diazotrophy in the Mediterranean Sea but results have been inconclusive (see literature compilation in Table 1). While N2 fixation was enhanced by ~50% after the addition of P to surface waters in an ultraoligotrophic, P-limited anticyclonic eddy in the Eastern Mediterranean Sea during the summer of 2002 (Rees et al., 2006), no significant increases in N2 fixation rates were observed several years later during an identical experiment at the same location and for the same incubation period (24 h) (Ridame et al., 2011). Similarly, N2 fixation rates were not enhanced by P additions in the Levantine Basin (Eastern Mediterranean) during the summer of 2009, including within the cores of cyclonic and anticyclonic eddies (Rahav et al., 2013b).
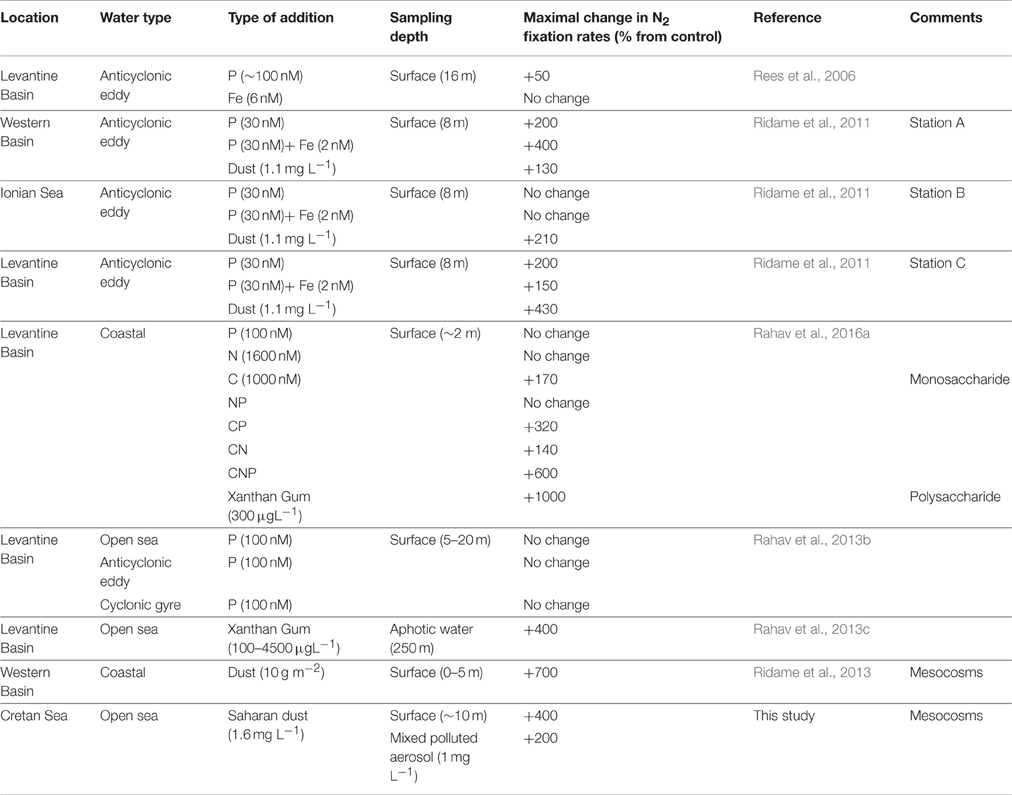
Table 1. Summary of the microcosm/mesocosm experiments performed in the Mediterranean Sea aimed studying the limiting nutrients for diazotrophy.
One possible reason for the variable results from the nutrient amendment experiments above could be the relatively small volumes of the microcosm experiments (each a few liters in total). The ultra-oligotrophic conditions in the study area coupled with the short duration of the experiments (usually less than 48 h) may have been insufficient to detect active diazotrophy. In addition, the low population density may have excluded the representation of the entire microbial food web in the small incubation experiments. In ultraoligotrophic areas such as the Eastern Mediterranean Sea, larger-scale experiments conducted over longer timescales may be required to determine N2 fixation rates and responses to additions of potentially rate-limiting elements. Mesocosms (52 m3 bags) were recently employed to examine diazotrophic responses to dust additions in the western Mediterranean Sea (2008, 2010) (Ridame et al., 2013). During this experiment, addition of a dust analog (manipulated top-soils collected from the Sahara desert) induced a rapid (24–48 h), robust (2–5.3-fold), and lengthy (4–6 days duration) increase in N2 fixation (Table 1), suggesting that dust inputs may stimulate diazotrophic activity over longer timescales (>48 h) than typical microcosm experiments.
In this study, we employed mesocosms to evaluate the impact of atmospheric deposition on diazotrophy and primary productivity during springtime in the Cretan Sea, an oligotrophic region in the Eastern Mediterranean Sea. Treatments included additions of: (1) “pure” Saharan dust (1.6 mg L−1), and (2) mixed polluted and desert origin aerosols (1 mg L−1). Our main objectives were to study how different aerosols affect diazotrophic diversity and N2 fixation in the oligotrophic Cretan Sea during the spring and study whether these atmospheric inputs can relieve the nutrient limitations for diazotrophs. We hypothesized that the aerosol source and the atmospheric route prior to deposition might trigger different diazotrophic responses that would be reflected in both diversity and activity. Specifically, the two deposition types, and their consequent leached nutrients (N, P and the subsequent N:P ratio), might result in different responses of the diazotrophic community that could subsequently impact the microbial food web in the oligotrophic Cretan Sea.
Materials and Methods
Experimental Design
Water was collected using a rotary submersible pump from the surface mixed layer (~10 m) on May 9–10, 2012, from an area located 5 nautical miles north of Heraklion, Crete (35° 24.957 N, 25° 14.441 E). The collected seawater was brought within ~2 h to the Hellenic Centre for Marine Research mesocosm facility (www.cretacosmos.eu), where it was homogenously distributed between pre-cleaned (10% HCl) mesocosms (see Figure 1 in Herut et al., this issue, Pitta et al., 2016). Nine 3-m3 mesocosms were filled and chemical and biological measurements were made to fully characterize the initial properties of the water (Table 2). The mesocosms were prepared from transparent polyethylene bags and were supported by aluminum frames. The mesocosms were deployed within a continuously circulating seawater 350 m3 pool to maintain ambient temperature. Each mesocosm was 3 m deep and the seawater inside it was gently mixed using an air pump. Two experimental amendments were made (triplicate mesocosms per treatment): (1) the addition of “pure” Saharan dust (SD), collected from Heraklion and Tel-Shikmona (Haifa, Israel) during Saharan dust events, and (2) the addition of mixed aerosols (A), collected in Crete and Israel, which contained a natural mixture of desert dust and polluted European particles. The leaching nutrient values of the SD and A particles are described in details in Herut et al., (this issue). In short, the SD leached ~23.5 nmol N per mg of dust and ~2.3 nmol P per mg of dust. The A particles leached ~53.0 nmol N per mg of dust and ~2.8 nmol P per mg of dust. These leached nutrients resulted in significantly different N:P ratio of ~10 (mol:mol) in the SD and ~19 (mol:mol) in the A particles. Three mesocosms were used as controls, with no addition. All mesocosms were sampled daily at 08:30 over a 6 days period. For a detailed description of the mesocosms' setup and analyses, see Tsagaraki et al. (this issue) and Herut et al. (this issue).
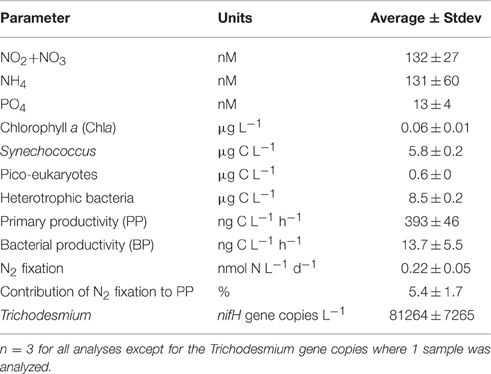
Table 2. The initial chemical and biological properties of the Cretan Sea sub-surface water during May 9th 2012, before amendments were performed.
Nutrient Concentrations
Water samples were collected and analyzed daily to measure phosphate concentrations using the MAGIC method that concentrate the phosphate in the samples, thus allowing a more accurately measures in oligotrophic environments such as the surface waters of the Mediterranean Sea (Rimmelin and Moutin, 2005), nitrite and nitrate concentrations (Strickland and Parsons, 1972), and ammonium concentrations (Ivancic and Deggobis, 1984). The detection limits were 0.8 nM for phosphate, 0.017 μM for nitrate+nitrite, and 0.019 μM for ammonium.
Chlorophyll a
Seawater samples (500 mL) were passed through a Whatman GF/F filter, and extracted overnight in 10 mL of 90% acetone solution in the dark. Chlorophyll a concentrations were determined by the non-acidification method (Welschmeyer, 1994) using a TD700 fluorometer equipped with 436 nm excitation and 680 nm emission filters.
Picophytoplankton and Heterotrophic Bacterial Abundance
Samples for determining picophytoplankton and heterotrophic bacterial abundance were collected every day during the experimental period. The samples were fixed with 0.2 μm filtered glutaraldehyde (0.5% final concentration) and held at 4°C for approximately 45 min, flash-frozen in liquid nitrogen, and then transferred to a −80°C refrigerator until further processing. Frozen samples were thawed at room temperature and sub-samples were stained with SYBR Green I and incubated for 10 min in the dark, according to Marie et al. (1997) and Vaulot and Marie (1999). Samples for picophytoplankton abundance were analyzed based on their auto-fluorescence signals, without pre-staining using a FACSCalibur (Becton Dickinson) flow cytometer equipped with an air-cooled laser at 488 nm and a standard filter set-up (Marie et al., 1997; Vaulot and Marie, 1999). Flow cytometry data were acquired and processed with the Cell Quest Pro software (Becton Dickinson). An average estimated flow rate of 58 μL min−1 was used. The picophytoplankton carbon biomass was calculated from cell counts, assuming 175 fg C cell−1 for Synechococcus cells, 53 fg C cell−1 for Prochlorococcus cells, and 2100 fg C cell−1 for picoeukaryotes (Campbell and Yentsch, 1989).
Primary Productivity (PP)
Photosynthetic carbon fixation rates were estimated using the 14C incorporation method (Nielsen, 1952). Water samples were analyzed in triplicate with dark and zero time controls. Polycarbonate (Nalgene) bottles were filled with 50 mL of sample water, inoculated with 5 μCi of NaH14CO3 tracer (Perkin Elmer, Boston, MA, USA), and incubated for 4 h under natural illumination and temperature. To determine the quantity of the added tracer, 50 μl samples were immediately taken out from each of the polycarbonate bottles and stored with 50 μL of ethanolamine for later analysis. Incubations were terminated by filtering the samples through a GF/F filter using a low vacuum pressure (< 50 mmHg). The filters were then placed in scintillation vials (5 mL) and 50 μl of 32% hydrochloric acid solution were added to each vial in order to remove excess 14C-bicarbonate overnight. After the addition of 3 mL of scintillation cocktail (ULTIMA-GOLD), the samples were counted using a TRI-CARB 2100 TR (PACKARD) scintillation counter. The hourly PP rates were also compared to daily measurements taken using the NaH13CO3 method as described in Mulholland and Bernhardt (2005). The positive (R = 0.62) and significant (P < 0.001) relationship between the two measuring techniques lends credibility to our results and suggest that the use of “potential” PP (4 h incubation and not daily) was accurate enough to characterize the Cretan Sea autotrophic production during the study period.
For the 13C uptake rate measurements, water samples were placed in triplicate clear 4.5 L polycarbonate Nalgene bottles and amended with (99%) NaH13CO3 (Sigma) to obtain 1% of the ambient dissolved inorganic carbon and incubated under the same conditions and bottles as for the N2 fixation measurements (see below more details). Triplicate parallel dark bottles were also incubated and subtracted from the light bottles to correct for dark carbon fixation. Incubations were terminated and analyzed as described for the N2 fixation measurements. The contribution of N2 fixation to the N demand for primary productivity was estimated using the measured particulate C: N ratio obtained for each sample. A comparison between the rates measured using the 13C and 14C methods can be seen in Figure S1.
Bacterial Productivity (BP)
Rates of bacterial productivity (BP) were estimated using the [4,5-3H]-leucine incorporation method (Simon et al., 1990). Triplicate (1.7 mL) samples were incubated with 100 nmol L−1 [4,5-3H]-leucine (Perkin Elmer, Boston, MA, USA) for 4 h at in situ temperatures in the dark, with triplicate trichloroacetic acid (TCA) killed samples serving as controls. Incubations were terminated by adding 100 μL of cold TCA (100%) to the vials and treated according to the micro-centrifugation protocol (Simon et al., 1990). After the addition of 1 mL of scintillation cocktail (ULTIMA-GOLD), the samples were counted using a TRI-CARB 2100 TR (PACKARD) scintillation counter. A conversion factor of 3.1 kg C mol−1 and an isotope dilution factor of 2.0 were used to estimate bacterial productivity (Simon et al., 1989).
Dinitrogen (N2) Fixation Rates
15N2 uptake measurements were performed using the 15N-enriched seawater protocol described by Mohr et al. (2010), with minor modifications for the Eastern Mediterranean Sea (Rahav et al., 2013a,c). Enriched seawater was prepared daily by degassing filtered (0.2-μm) natural seawater using a degassing membrane (Liqui-Cel, MiniModule® G542) for ~1 h. Then 1 ml of 15N2 gas (99%) was added for every 100 ml of the degassed seawater and shaken vigorously until the bubbles disappeared (~30 min). Aliquots of this 15N2- sea enriched water were then added to the incubation bottles, with the enriched water constituting 5% of the total volume of the sample (i.e., 225 mL). Similar enriched seawater additions collected from the oligotrophic North Pacific Subtropical Gyre (NPSG) resulted in a final 15N2 enrichment of 1.5 atom%, following the addition of 50 ml of 15N2-enriched water to a 4.5 L bottle (Wilson et al., 2012). The bottles were then shaken and incubated under ambient surface seawater temperatures. Incubation bottles were either covered with neutral density screening to simulate ambient light or were kept under complete darkness for 24 h (see Rahav et al., 2013b). The incubations under ambient irradiance (representative of a full diel cycle having both light and dark cycles) recorded the activities of both autotrophic and heterotrophic diazotrophs, whereas the dark incubations reflected the activity of mainly heterotrophic diazotrophs who do not require light energy for fixing N2 (Rahav et al., 2013b). We estimated the heterotrophic contribution to N2 fixation by comparing the dark incubations to the bottles incubated under ambient diel irradiance.
The incubations were terminated by filtering water through pre-combusted 25 mm GF/F filters (with a nominal pore size of 0.7 μm). The filters were then dried in an oven at 60°C and stored in a desiccator until analysis. In the laboratory, samples were pelletized in tin disks and analyzed with a Europa 20/20 mass spectrometer equipped with an automated carbon and nitrogen analyzer. For isotope ratio mass spectrometry, standard curves were performed with each sample run to determine N mass. Samples were run only when the standard curves had R2 values >0.99. At masses >4.7 μg N, the precision for the atom % 15N measurement was < 0.0001%, based on daily calibrations made in association with sample runs and with calibrations averaged made throughout several years. For most of the results reported here, the masses were >4.7 μg N. However, samples with < 4.7 μg N were only used if the precision was 0.0001% for that sample run. Standard masses ranged between 1.2 and 100 μg N. In addition to the daily standard curves, reference standards and standards processed as samples were run every 6 to 8 samples.
DNA Sample Collection and Extraction
Seawater (3–8 L) was filtered through GF/D glass microfiber filters (2.7 μm, Whatman International Ltd.) and 0.2 μm pore-sized polyethersulfone membrane filters (Supor-200, Pall Corp.) using a peristaltic pump for a duration of 40 min. Total genomic DNA was recovered from biomass left on the membrane filters using PureLink Genomic DNA Kits (Invitrogen, Carlsbad, CA) (Kong et al. (2013). Extracted DNA was eluted into 50–60 μl of TE buffer (Tris and EDTA) and stored at −20°C until further analysis.
Nested PCR and 454-Pyrosequencing
Samples collected onto 0.2 μm filters were sequenced using a 454-pyrosequencing at −48, 48, and, 144 h after the atmospheric additions were carried out. The Genomic DNA samples collected from each of the different treatments were pooled into one sample prior to pyrosequencing, granting a representative view of the microbial microorganisms of each mesocosm type. nifH gene fragments were amplified from the genomic DNA samples following the nested polymerase chain reaction (PCR) protocol (Zehr and Turner, 2001). The nested PCR reaction was performed in triplicate using a Platinum Taq DNA polymerase PCR system in a volume of 12.5 μL (Invitrogen, Carlsbad, CA), which contained a 1X rxn PCR buffer, 4 mM MgCl2, 400 μM dNTPs, 1 μM of primers (nifH 3 and nifH 4 for the first round and nifH 1 and nifH 2 for the second round of the nest PCR), 1 unit of Platinum Taq polymerase and 1 μL of total genomic DNA. Thirty cycles were performed for each of the nested PCR rounds. After the nested PCR cycles ended, 1 μL of the PCR products were used to run 10 cycles of PCR with sample-specific multiplex identifiers (MIDs) and adaptor-attached primers (Farnelid et al., 2011). The PCR condition was the same as that used for the nested PCR. The PCR products were then gel-purified with a Quick gel purification kit (Invitrogen, Carlsbad, CA). For 454-pyrosequencing, the MID-adaptor-labeled PCR products were mixed in the same concentrations to construct an amplicon library following the Rapid Library construction protocols (Roche, 454 Life Science). The DNA library attached beads were loaded onto a Pico TiterPlate and sequenced with a GS Junior System.
Quantitative PCR (qPCR)
Trichodesmium sp. nifH gene copies were quantified using a TaqMan probe qPCR analysis as described in Moisander et al. (2012). No other cyanobacterial diazotrophs were detected in any of the mesocosm bags. The qPCR reaction was 10 μL in volume, contained 1 X Premix Ex Taq (Takara), 1 μL genomic DNA or RNA, 0.5 μM reverse and forward primers and 0.25 μM probes (labled with 5′-FAM and 3′-TAMRA). The qPCR reactions were run in triplicate using the Applied Biosystems' 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA) (Moisander et al., 2012). The linear regression r2 value of the standard curve was 0.99 for all the reactions and the efficiency of the qPCR reaction was 101%. The detection limit was 10 nifH gene copies L−1.
Sequences Quality Control and Analysis
The sequencing quality control and analysis were conducted with Mothur (Schloss et al., 2009). Low quality sequences were removed, including short sequences (< 300 bases in length), ambiguous base-containing sequences and chimeric sequences. The trimmed sequences were de-noised with 0.01 sigma value in order to reduce the effects of the PCR bias and were then aligned with the nifH DNA database of the Ribosomal Database Project (Wang et al., 2013). The distances between these high quality DNA sequences were then calculated and clustered at 95% similarity. Based on this similarity clustering, operational taxonomic units (OTUs), representative sequences of each OTU, the Shannon diversity index, and the Chao richness estimator were generated using Mothur. The OTUs that contained more than 10 sequences were selected for the above analysis, whereas the rest of the OTUs were grouped as “other” species. Note that the rarefaction curves of our samples did not reach a plateau (not shown), suggesting that the sequencing depth of our study was insufficient to look at rare diazotrophic species. We therefore set a cutoff of 10 sequences to filter the less abundant OTUs (e.g., Farnelid et al., 2013). The OTU representative DNA sequences were translated into amino acid (aa) sequences using the FrameBOT pipeline in which the frameshift errors were corrected (Wang et al., 2013). The OTU representative sequences were used to search the protein sequence database in the National Center of Biotechnology Information (NCBI) via the protein BLAST webpage (McGinnis and Madden, 2004). The OTU representative sequences and the affiliated references were used to construct a Neighbor-joining phylogenetic tree (p-distance) with MEGA 6.0 (Tamura et al., 2013). The OTUs affiliated with the same reference sequences were grouped and these groups were then named with the species name of the reference sequences. To display the diazotrophic community structures, the relative abundances of these groups were calculated. For the relationships between the samples, the samples were clustered with a Thetayc calculator using an Unweighted Pair Group Method with an Arithmetic Mean (UPGMA) algorithm using Mothur. Sequence data was deposited in NCBI under accession number SRP075730.
Statistical Analysis
Data are displayed as averages; error bars signify one standard deviation (n = 3). A repeated measures analysis of variance (ANOVA) was used to compare differences between the control and the SD or A mesocosm treatments. Prior analyses, the ANOVA assumptions, namely the normally and the heterogeneity of variances of the data, were examined. At selected time-points throughout the experiment, a one-way ANOVA followed by Tukey's post-hoc test was applied (P < 0.05). The relationship between the N2 fixation and the autotrophic and heterotrophic variables was determined with a Pearson correlation test (n = 3, P < 0.05). All tests were performed using the XLSTAT software.
Results and Discussion
Characteristics of Cretan Sea Surface Water
The surface Cretan Sea water used for the mesocosm study exhibited typical low-nutrient, low-chlorophyll, oligotrophic Eastern Mediterranean Sea characteristics (Table 2; Pitta et al., 2016). The dissolved inorganic nitrite+nitrate, ammonium, and phosphorus (P) concentrations were close to their detection limits (132 ± 27, 131± 60, and 13 ± 4 nM, respectively), resulting in a high N:P ratio (~20:1). Correspondingly, low surface chlorophyll-a was measured prior to any addition (0.06 ± 0.01 μg L−1). The picophytoplankton biomass was within the range previously reported for the Cretan Sea (Ignatiades et al., 2002; Tsiola et al., 2016), with Synechococcus the predominant autotrophic picoplankton (5.8 ± 0.2 μg C L−1), followed by pico-eukaryotes (0.6 ± 0.2 μg C L−1). The heterotrophic bacterial biomass was higher than that of the autotrophic bacterioplankton (8.5 ± 0.2 μg C L−1), although it was still at the lower end of that reported for oligotrophic oceans (Cho and Azam, 1988 and see Figure 3 in Herut et al., this issue). The surface primary and bacterial productivity rates (PP and BP) were also low (393 ± 46 ng C L−1 h−1 for PP and 13.7 ± 5.5 ng C L−1 h−1 for BP), and were consistent with previous studies performed in the Cretan Sea (Gotsis-Skretas et al., 1999; Psarra et al., 2000; Tsiola et al., 2016).
Dinitrogen (N2) fixation rates, measured using the enriched seawater method (Mohr et al., 2010), were low (0.22 ± 0.05 nmol N L−1 d−1), and similar to the rates measured the previous spring in surface waters in the Levantine Basin using identical methodology (Rahav et al., 2013a). Two days prior to the experiment, N2 fixation contributed 5.4 ± 1.7% to PP (Table 2), a slightly higher contribution than those estimated during the previous spring (~2%, Rahav et al., 2013a) and summer (~0.5–2%, Yogev et al., 2011; Rahav et al., 2013b) in the Eastern Mediterranean Sea. We postulate that this may have been due to the relatively high number of nifH gene copies observed from the non-filamentous photoautotrophic cyanobacteria Trichodesmium (>80,000 gene copies L−1, Table 2) found in the Cretan water prior to the experiment. To date, only one sporadic bloom of Trichodesmium has been recorded in the EMS (Spatharis et al., 2012), and the reason for its rare occurrence in this warm, N-impoverished environment is unknown (Berman-Frank and Rahav, 2012). Our findings here combined with the published literature of N2 in the Eastern Mediterranean Sea (e.g., Ibello et al., 2010; Bonnet et al., 2011; Yogev et al., 2011; Rahav et al., 2013a,b,c; Raveh et al., 2015; Rahav et al., 2016a) suggest patchy spatial and temporal occurrence of cyanobacterial (and other) diazotrophs with variable contribution to PP in the Cretan Sea.
The Response of Diazotrophy to Saharan Dust and Mixed Aerosol Amendments
The addition of Saharan dust (SD) triggered a rapid (30 h after enrichment) response, with a ~2-fold increase in N2 fixation compared to the unamended controls (0.42 ± 0.08 and 0.24 ± 0.04 nmol N L−1 d−1, respectively, one-way ANOVA, P = 0.02) (Figure 1). At T48, N2 fixation rates declined in all mesocosms, yet remained higher in the SD and A mesocosms than in the controls (Figure 1). This decline in all mesocosms may suggest a weak, yet notable, bottle effect imposed by the mesocosm bags (Calvo-Díaz et al., 2011), low % enrichment of the enriched seawater used for the N2 fixation measurements at this specific time point, or competition between the ambient surface microbial populations and diazotrophs that preclude diazotrophs from thriving. The marked and significant differences between the treatments throughout most samplings suggest that even if an experimental artifact occurred at T48, its impact on the measured rates was weak overall. After 78 h, N2 fixation declined in both SD treatments and control incubations. Yet, N2 fixation remained 4 times higher in the SD treatments relative to the controls (~0.25 ± 0.04 and ~0.07 ± 0.01 nmol N L−1 d−1, respectively, one-way ANOVA, P = 0.004). By day 5 (120 h), N2 fixation rates in both SD treatments and control incubations were near the limit of analytical detection (~0.10 nmol N L−1 d−1, one-way ANOVA, P > 0.05, Figure 1). Similarly, the mixed aerosol (A) addition led to an overall increase in N2 fixation rates in the short term (30 h post addition, Figure 1), although the responses were moderate (~1.5–2-fold higher rates in treatment incubations relative to controls) and differences between treatments and controls were significant only at the 2 days time point. Similar to the SD treatments, N2 fixation rates in the A treatments decreased to near the limits of analytical detection and were not significantly different from controls after 144 h (Figure 1). The observed changes in N2 fixation following the SD or A addition can be explained by the enhanced concentrations of essential (and limiting in the unamended seawater) nutrients from the additives such as P (2–3 nmol P per mg dust/aerosol, Herut et al., this issue). It is also possible that leached Fe in the SD mesocosms (net change of ~6 nM in dissolved Fe 3 h after SD addition), and the insignificant change in the A mesocosms (Herut et al., this issue) could explain the observed increase in N2 fixation. Despite the increase in N2 fixation rates in the 30–48 h following the SD or A additions, absolute N2 fixation rates remained low overall throughout the experiment (Figure 1). This could be due to limited abundances of diazotrophs in the initial conditions so that overall rates remained low. Furthermore, after the initial enhanced response of the diazotrophs to the added dust- or aerosol-borne nutrients and/or trace elements, competition by more efficient non-diazotrophic phytoplankton or bacteria could have maintained low overall N2 fixation rates.
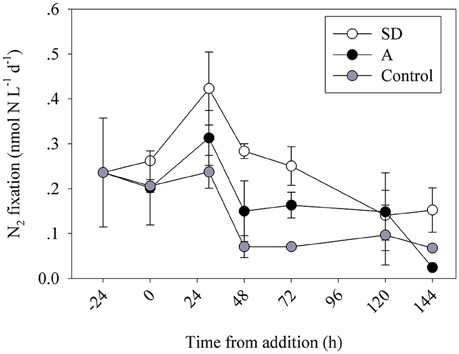
Figure 1. Temporal changes in volumetric N2 fixation rates during the mesocosm enrichment experiments. Data are averages of 3 replicate bottles per treatment of the control (gray), SD (white) and A (black) mesocosms during May 2012.
The short-term enhancement of N2 fixation rates measured after the addition of SD or A were similar to those observed previously in mesocosm experiments amended with a Saharan dust analog performed in the Western Mediterranean Sea (WMS) during the summer of 2008 and 2010 (2–5.3-fold and 4–6 days, Ridame et al., 2013). This may suggest common limiting factors for diazotrophy in these two contrasting systems. Elements leached off dust can introduce other nutrients and trace elements to the surface water in different quantities, chemical forms, concentrations and stoichiometry, depending on the dust/aerosol origin (Léon et al., 2015). In addition, atmospheric deposition can be a source of viable diazotrophic microbes as demonstrated from aerosols that originated in the Sahara desert, collected in Israel, and dissolved in seawater from the east Mediterranean Sea (Rahav et al., 2016b). N2 fixation by these airborne diazotrophs delivered with atmospheric deposition comprised ~10–15% (~0.03 nmol N L−1 d−1) of the “typical-average” rates measured in the EMS (0.10–0.20 nmol N L−1 d−1), suggesting that atmospheric deposition can supply not only nutrients and trace metals to stimulate in situ N2 fixation but may potentially deliver diazotrophs (Rahav et al., 2016b). Yet, the role of airborne diazotrophs should be extensively studied in the Mediterranean Sea as well as in other marine environments exposed to high atmospheric depositions such as the North Atlantic Ocean and the China Sea. In the present study, we did not determine the contribution of diazotrophs or other bacteria originating from our SD and A additions (i.e., measure N2 fixation and BP, PP in SD or A added to sterile seawater). Other factors that regulating diazotrophs and N2 fixation rates include top–down effects by grazing (Stukel et al., 2014), viral cell lysis (Hewson et al., 2009), competition with non-diazotrophic primary producers such as Prochlorococcus (Moisander et al., 2012) or bacterial heterotrophs (Thingstad et al., 2005), and other nutritional constraints such as carbon for heterotrophs (Rahav et al., 2016a) or vitamin B12 (Bonnet et al., 2010) may further limit production. Top–down grazing of diazotrophs probably did not greatly impact the diazotrophic populations in our experiments. Zooplankton abundance did not vary significantly between the mesocosms (M. Lou personal communication). Specifically, no known diazotroph grazers such as Macrosetella gracilis that feed on Trichodesmium cells were found in our mesocosms nor observed in the few surveys performed in the EMS (Kimor and Wood, 1975; Zakaria, 2015). In contrary, we hypothesize that airborne microbes or viruses can be important in regulating surface N2 fixation in the Mediterranean Sea. Such interactions may occur especially in light of the high amount of aerosols deposited to the surface waters of Mediterranean Sea (Guerzoni et al., 1999), which potentially brings ~107 viruses per m3 of air (Prospero et al., 2005; Womack et al., 2010; Polymenakou, 2012). Yet, examination of these factors was beyond the scope of the present study and warrants more research.
Both heterotrophic and cyanobacterial diazotrophs were present in this mesocosm study (Figure 2) with the Shannon diversity index ranging between 0.7 and 2.5 throughout the entire experiment (Figure 2). Based on the relative abundance of the nifH OTUs, most diazotrophs present in the original Cretan Sea water samples grouped into known nifH clusters (Figure 3; Chien and Zinder, 1996; Zehr et al., 2003). After 2 days (48 h), the diazotroph community in the control treatments was dominated by Sideroxydans (30%) and Acrobacter (20%), whereas in the SD and A treatments Trichodesmium (68%) and Pseudomonas (41%) respectively dominated the diazotroph populations. At the conclusion of the experiment, the diazotrophic communities in the control mesocosms had shifted to Azorhizobium and Novosphingobium, Azorhizobium in the SD treatments, and Burkholderia in the A amended mesocosms (Figure 3). By the end of the experiment (144 h), the Shannon diversity dropped to 0.5–1.5. One explanation for this convergence in microbial populations is the potential of those OTUs to successfully utilize heavy metal concentrations as those found in the SD and A additions (see Table 4 in Herut et al., this issue).
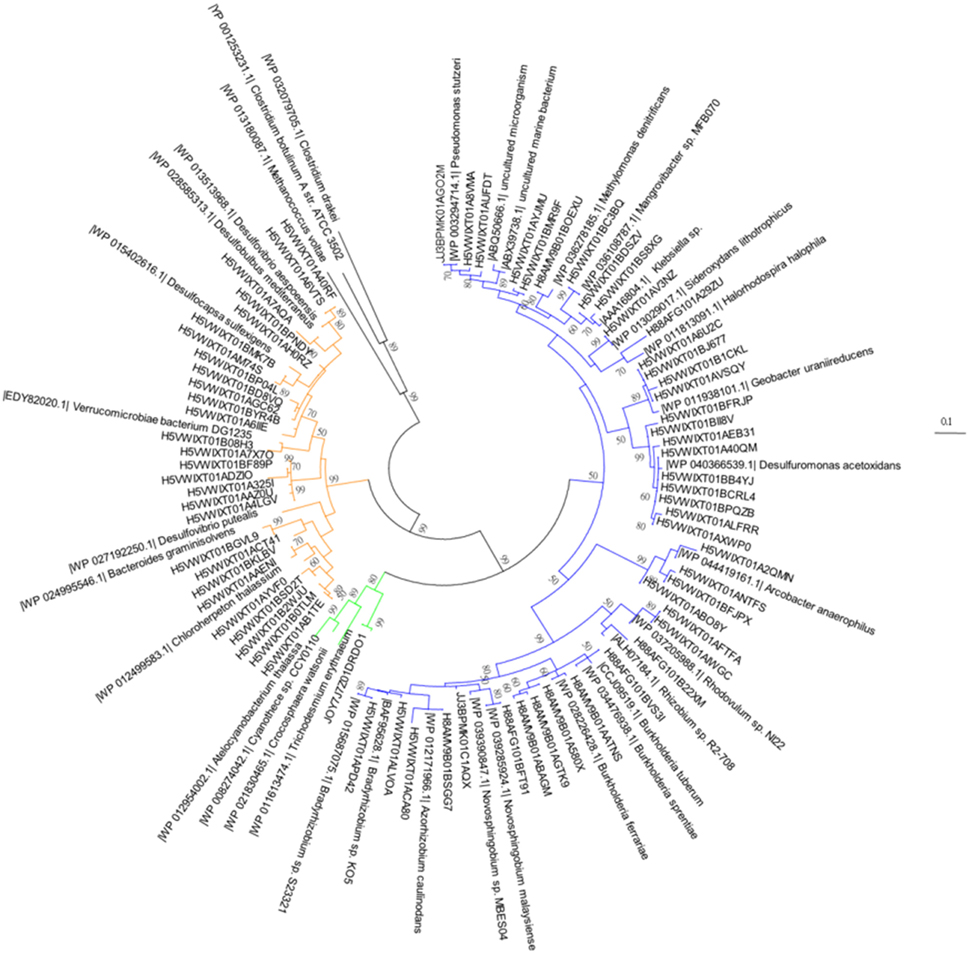
Figure 2. A neighbor-joining phylogentic tree constructed with nifH gene amino acid sequences. Samples were taken 2 days prior to the addition of the SD or A addition. The differently-colored sequences are representative sequences of the OTUs revealed in this study. The sequences fall into Cluster I proteobacteria (blue), Cluster III (yellow), and cyanobacteria (green). The accession numbers of each reference sequence are shown in front of the sequence names.
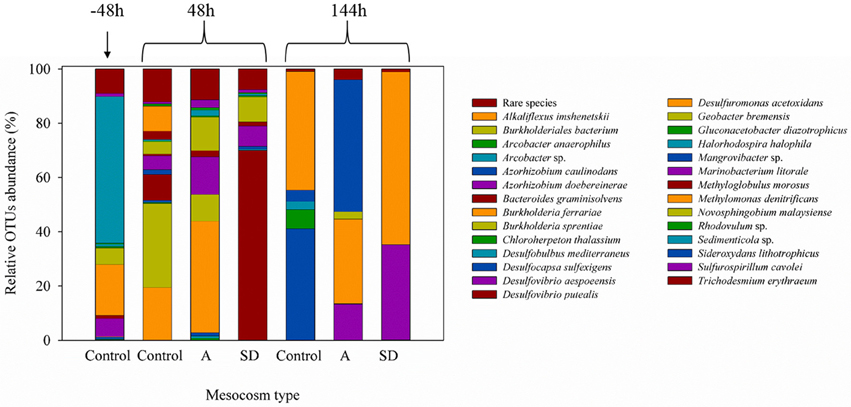
Figure 3. The relative abundance (%) of the nifH DNA phyla during the mesocosm enrichment experiment of May 2012.
While some cyanobacteria, including Trichodesmium, were present, most nifH OTUs retrieved in this experiment belonged to Proteobacteria. The overall predominance of non-cyanobacterial heterotrophic bacteria over autotrophic diazotrophs, highlights the importance of heterotrophic diazotrophy in the EMS waters as recorded in the Levantine Basin (Man-Aharonovich et al., 2007; Yogev et al., 2011; Rahav et al., 2013c) and also across the Mediterranean (Benavides et al., 2016). A greater contribution of heterotrophic diazotrophs is now recognized also from many other oceanic environments (reviewed in Riemann et al., 2010) including the Red Sea (Foster et al., 2009; Rahav et al., 2015), the western North Atlantic Ocean (Mulholland et al., 2012) and the English Channel (Rees et al., 2009, 2016).
To further estimate the role of heterotrophic diazotrophs in our system, we simultaneously incubated light and dark 4.6 L bottles (microcosms) from each treatment (Table 3). We assumed that dark incubations mainly reflect the activity of heterotrophic diazotrophs that do not require light energy for N2 fixation, and estimated the heterotrophic contribution to N2 fixation by comparing the dark incubations to light bottles incubated under the ambient surface irradiance. When we compared our light vs. dark bottle incubations, we found that the light:dark N2 fixation ratio in the control mesocosms was usually ~1 (average 1.7 ± 1.6, Table 3), suggesting N2 was fixed by both autotrophic and heterotrophic diazotrophs. Enrichment by SD or A caused the light:dark ratio to decline (an average of 0.8 ± 0.7 for SD and 0.8 ± 0.3 for A, Table 3), implying that the aerosol additions disproportionately stimulated heterotrophic N2 fixation. Similar comparisons between ambient light and dark bottle N2 fixation rates showed the same trend in the Mediterranean (also in the spring) and the Gulf of Aqaba/Northern Red Sea (in summertime), highlighting the important role of heterotrophs in fixing dinitrogen in ultra-oligotrophic systems (Bonnet et al., 2013; Rahav et al., 2013a, 2015).
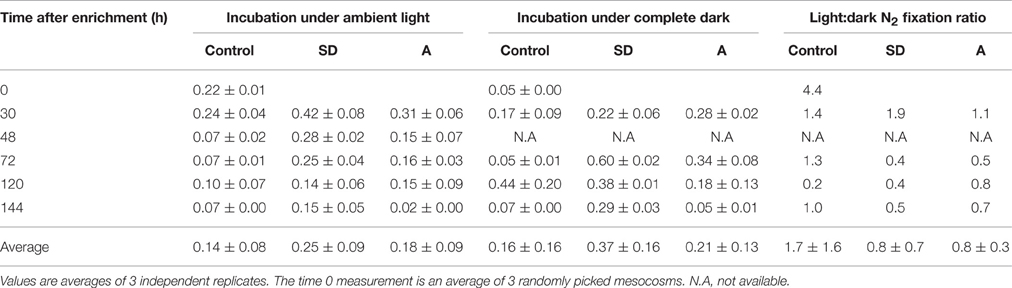
Table 3. The N2 fixation rates of Cretan sub-surface water in experimental mesocosms (controls, +SD, +A), for bottles incubated under ambient lighting (L) and in complete darkness (D) for 24 h.
The Contribution of N2 Fixation to Primary Productivity in the Cretan Sea during Spring
To estimate the contribution of N2 fixation to primary productivity, we calculated the contribution of the fixed N (as particulate organic nitrogen, PON) to the total particulate organic carbon (POC) from the mesocosms. An average POC:PON ratio of ~8 concurs with previous measurements from the open-waters of the Levantine basin (Yogev et al., 2011; Rahav et al., 2013b). We subsequently used this average to calculate the percent contribution of N2 fixation to PP. Our results show that N2 fixation contributed 2–4% (P > 0.05) of PP following the mixed aerosol additions and 3–8% (P = 0.04) after SD additions (Figure 4). These estimations (2–8%) are in line with other estimates from the Mediterranean Sea (Garcia et al., 2006; Bonnet et al., 2011; Yogev et al., 2011; Rahav et al., 2013b). However, this is not surprising as heterotrophic diazotrophs comprised the bulk of the retrieved nifH OTUs and the dark N2 fixation was generally higher than the rates measured under ambient light (Table 3 and discussion above). The positive coupling between N2 fixation and bacterial abundance (BA) or bacterial production (BP), while no distinct pattern was observed with Trichodesmium nifH gene expression (Figure 5), further supports the importance of heterotrophic bacterial diazotrophs in this system (Rahav et al., 2013a, 2015).
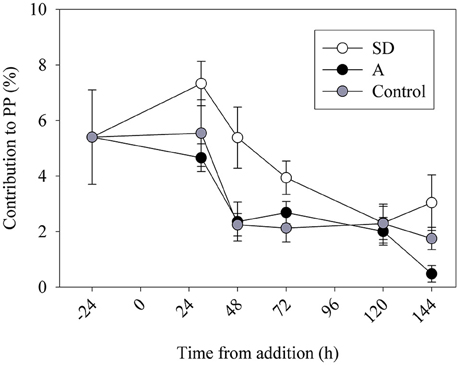
Figure 4. The contribution of N2 fixation to primary productivity (PP) in the control (gray), SD (white), and A (black) mesocosms.
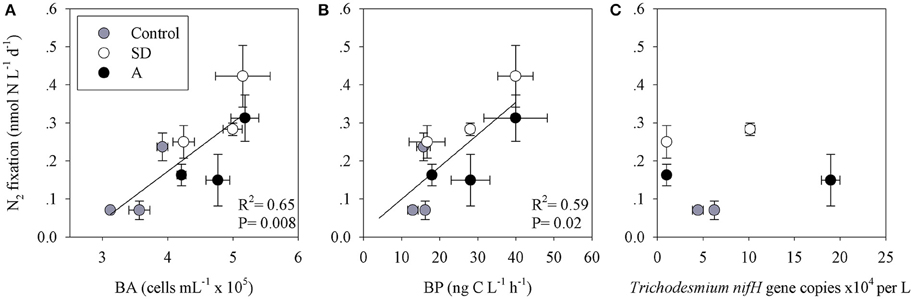
Figure 5. The relationship between N2 fixation and (A) bacterial abundance; BA, (B) bacterial production; BP, and (C) Trichodesmium nifH copies, in the first 144 h following the Saharan dust (SD) and mixed aerosol (A) enrichments.
Higher contributions of diazotrophs to primary productivity are typically found where filamentous cyanobacterial N2-fixers predominate. For example, the contribution of Trichodesmium to PP was 11.6% in the outer Sanya Bay of the China Sea (Dong et al., 2008), 22 and 44% in the equatorial Atlantic and South Atlantic Gyre, respectively (Fernández et al., 2010), and 47% of the total depth-integrated PP in the North Atlantic Ocean (Carpenter et al., 2004).
In cases where EMS surface water receives Saharan dust inputs (as tested here), Trichodesmium-derived PP may be enhanced if there is a seed population that can respond to the addition. Yet, as previously observed, the diazotrophs that characterize the EMS are predominantly small-sized bacterial phylotypes (Figure 3; Man-Aharonovich et al., 2007; Yogev et al., 2011), and Trichodesmium is uncommon (Yogev et al., 2011; Spatharis et al., 2012).
Finally, our results demonstrate that intense dust and aerosol deposition events (as frequently occur in the Mediterranean Sea, Herut et al., 2002; Bonnet and Guieu, 2006) may potentially fuel diazotrophy and alter the composition of diazotrophic communities. Furthermore, these depositions to the Mediterranean Sea can also enrich surface waters with bioavailable N sources (Carbo et al., 2005; Herut et al., 2005; and see Table S1 in Rahav et al., 2016c). The higher N concentrations can stimulate new production and induce changes in community structure such as a shift from populations of picophytoplankton to larger-size class microphytoplankton such as diatoms and dinoflagellates (Guieu et al., 2014), which, in turn, may enhance organic matter export and reduce the high recycling rates typical for the microbial food web of the EMS.
Author Contributions
Conceived and designed the experiments: ER, BH, IB-F. Performed the experiments: ER, BH, IB-F, GC, HL, TT, AG, AT, SP, AL, MM, ES, PP. Analyzed the data: ER, BH, IB-F, CS. Contributed reagents/materials/analysis tools: BH, IB-F, MM, PP Wrote the paper: ER, BH, IB-F.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Dr. P. Divanach for his valuable advice on technical matters, G. Piperakis for his inspired technical assistance, S. Zivanovic and E. Dafnomili for assisting with chemical analyses, A. Konstantinopoulou for assisting with bacterial production analyses, D. Podaras and S. Diliberto for assisting during the experiment, and N. Sekeris for his help with constructions and with ideas regarding technical solutions. The captain and the crew of the R/V Philia are also thanked for their assistance during the transportation of water from the sea to the mesocosms. This study was in partial fulfillment of a Ph.D. thesis for ER from Bar Ilan University. This work was financed by the European Union Seventh Framework Program (FP7/2007–2013) under grant agreement no. 228224, “MESOAQUA: Network of leading MESOcosm facilities to advance the studies of future AQUAtic ecosystems from the Arctic to the Mediterranean” through grants to ER, GC, HL, BH, and IB-F, by the EU and Greek-national-funded Program THALES-ADAMANT and the Israel Science Foundation grants (996/08) to IB-F and BH.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2016.00180
References
Benavides, M., Bonnet, S., Hernández, N., Martínez-Pérez, A. M., Nieto-Cid, M., Álvarez-Salgado, X. A., et al. (2016). Basin-wide N2 fixation in the deep waters of the Mediterranean Sea. Global Biogeochem. Cycles. 30, 952–961. doi: 10.1002/2015GB005326
Berman-Frank, I., Cullen, J. T., Shaked, Y., Sherrell, R. M., and Falkowski, P. G. (2001). Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 46, 1249–1260. doi: 10.4319/lo.2001.46.6.1249
Berman-Frank, I., Quigg, A., Finkel, Z. V., Irwin, A. J., and Haramaty, L. (2007). Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol. Oceanogr. 52, 2260–2269. doi: 10.4319/lo.2007.52.5.2260
Berman-Frank, I., and Rahav, E. (2012). “Nitrogen fixation as a source for new production in the Mediterranean Sea: a review,” in Life in the Mediterranean Sea: A Look at Habitat Changes, ed N. E. Stambler (New York, NY: Nova Science Publishers), 199–226.
Bonnet, S., Dekaezemacker, J., Turk-Kubo, K. A, Moutin, T., Hamersley, R. M., Grosso, O., et al. (2013). Aphotic N2 fixation in the Eastern Tropical South Pacific Ocean. PLoS ONE 8:e81265. doi: 10.1371/journal.pone.0081265
Bonnet, S., Grosso, O., and Moutin, T. (2011). Planktonic dinitrogen fixation along a longitudinal gradient across the Mediterranean Sea during the stratified period (BOUM cruise). Biogeosciences 8, 2257–2267. doi: 10.5194/bg-8-2257-2011
Bonnet, S., and Guieu, C. (2006). Atmospheric forcing on the annual iron cycle in the western Mediterranean Sea: a 1-year survey. J. Geophys. Res. Oceans 111, 1–13. doi: 10.1029/2005jc003213
Bonnet, S., Webb, E. A., Panzeca, C., Karl, D. M., Capone, D. G., and Sañudo-Wilhelmy, S. A. (2010). Vitamin B12 excretion by cultures of the marine cyanobacteria Crocosphaera and Synechococcus. Limnol. Oceanogr. 55, 1959–1964. doi: 10.4319/lo.2010.55.5.1959
Calvo-Díaz, A., Díaz-Pérez, L., Suárez, L. Á., Morán, X. A. G., Teira, E., and Marañón, E. (2011). Decrease in the autotrophic-to-heterotrophic biomass ratio of picoplankton in oligotrophic marine waters due to bottle enclosure. Appl. Environ. Microbiol. 77, 5739–5746. doi: 10.1128/AEM.00066-11
Campbell, J. W., and Yentsch, C. M. (1989). Variance within homogeneous phytoplankton populations, III: analysis of natural populations. Cytometry 10, 605–611. doi: 10.1002/cyto.990100516
Carbo, P., Krom, M. D., Homoky, W. B., Benning, L. G., and Herut, B. (2005). Impact of atmospheric deposition on N and P geochemistry in the southeastern Levantine basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 52, 3041–3053. doi: 10.1016/j.dsr2.2005.08.014
Carpenter, E. J., Subramaniam, A., and Capone, D. G. (2004). Biomass and productivity of the cyanobacterium, Trichodesmium spp. in the tropical North Atlantic Ocean. Deep Sea Res. I 51, 173–203. doi: 10.1016/j.dsr.2003.10.006
Chien, Y. T., and Zinder, S. H. (1996). Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227. J. Bacteriol. 178, 143–148.
Cho, B. C., and Azam, F. (1988). Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 332, 441–443. doi: 10.1038/332441a0
Dong, J., Zhang, Y., Wang, Y., Zhang, S., and Wang, H. (2008). Spatial and seasonal variations of cyanobacteria and their nitrogen fixation rates in Sanya Bay, South China Sea. Sci. Mar. 72, 239–251. doi: 10.3989/scimar.2008.72n2239
Dyhrman, S. T., and Haley, S. T. (2006). Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl. Environ. Microbiol. 72, 1452–1458. doi: 10.1128/AEM.72.2.1452-1458.2006
Falkowski, P. G. (1997). Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387, 272–275. doi: 10.1038/387272a0
Farnelid, H., Andersson, A. F., Bertilsson, S., Al-Soud, W. A., Hansen, L. H., Sørensen, S., et al. (2011). Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PLoS ONE 6: e19223. doi: 10.1371/journal.pone.0019223
Farnelid, H., Bentzon-tilia, M., Andersson, A. F., and Bertilsson, S. (2013). Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J. 7, 1413–1423. doi: 10.1038/ismej.2013.26
Fernández, A., Mouriño-Carballido, B., Bode, A., Varela, M., and Marañón, E. (2010). Latitudinal distribution of Trichodesmium spp. and N2 fixation in the Atlantic Ocean. Biogeosciences 7, 2195–2225. doi: 10.5194/bgd-7-2195-2010
Foster, R. A., Paytan, A., and Zehr, J. P. (2009). Seasonality of N2 fixation and nifH gene diversity in the Gulf of Aqaba (Red Sea). Limnol. Oceanogr. 54, 219–233. doi: 10.4319/lo.2009.54.1.0219
Garcia, N., Raimbault, P., Gouze, E., and Sandroni, V. (2006). Nitrogen fixation and primary production in Western Mediterranean. C. R. Biol. 329, 742–750. doi: 10.1016/j.crvi.2006.06.006
Gotsis-Skretas, O., Pagou, K., and Moraitou-Apostolopoulou, M. (1999). Seasonal horizontal and vertical variability in primary production and standing stocks of phytoplankton and zooplankton in the Cretan Sea and the Straits of the Cretan Arc (March 1994-Jannuary 1995). Prog. Oceanogr. 44, 625–649. doi: 10.1016/S0079-6611(99)00048-8
Gruber, N., and Galloway, J. N. (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296. doi: 10.1038/nature06592
Gruber, N., and Sarmiento, J. L. (1997). Global patterns of marine nitrogen fixation and denitrification. Glob. Biogeochem. Cycles 11, 235–266. doi: 10.1029/97GB00077
Guerzoni, S., Chester, R., Dulac, F., Measures, C., Migon, C., Molinaroli, E., et al. (1999). The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog. Oceanogr. 44, 147–190. doi: 10.1016/S0079-6611(99)00024-5
Guieu, C., Aumont, O., Paytan, A., Bopp, L., Law, C. S., Mahowald, N., et al. (2014). Global biogeochemical cycles deposition to low nutrient Low chlorophyll regions. Global Biogeochem. Cycles 28, 1179–1198. doi: 10.1002/2014GB004852
Herut, B., Collier, R., and Krom, M. D. (2002). The role of dust in supplying nitrogen and phosphorus to the Southeast Mediterranean. Limnol. Oceanogr. 47, 870–878. doi: 10.4319/lo.2002.47.3.0870
Herut, B., Krom, M. D., Pan, G., and Mortimer, R. (1999). Atmospheric input of nitrogen and phosphorus to the Southeast Mediterranean: sources, fluxes, and possible impact. Limnol. Oceanogr. 44, 1683–1692. doi: 10.4319/lo.1999.44.7.1683
Herut, B., Zohary, T., Krom, M. D., Mantoura, R. F. C., Pitta, P., Psarra, S., et al. (2005). Response of East Mediterranean surface water to Saharan dust: On-board microcosm experiment and field observations. Deep Sea Res. Part II Top. Stud. Oceanogr. 52, 3024–3040. doi: 10.1016/j.dsr2.2005.09.003
Hewson, I., Poretsky, R. S., Dyhrman, S. T., Zielinski, B., White, A. E., Tripp, H. J., et al. (2009). Microbial community gene expression within colonies of the diazotroph, Trichodesmium, from the Southwest Pacific Ocean. ISME J. 3, 1286–1300. doi: 10.1038/ismej.2009.75
Ibello, V., Cantoni, C., Cozzi, S., and Civitarese, G. (2010). First basin-wide experimental results on N2 fixation in the open Mediterranean Sea. Geophys. Res. Lett. 37, 1–5. doi: 10.1029/2009GL041635
Ignatiades, L., Psarra, S., and Zervakis, V. (2002). Phytoplankton size-based dynamics in the Aegean Sea (Eastern Mediterranean). J. Mar. Syst. 36, 11–28. doi: 10.1016/S0924-7963(02)00132-X
Ivancic, I., and Deggobis, D. (1984). An optimal manual procedure for ammonia analysis in natural waters by indophenol blue method. Water Res. 18, 1143–1147. doi: 10.1016/0043-1354(84)90230-6
Kimor, B., and Wood, E. J. F. (1975). Plankton study in Eastern Mediterranean Sea. Mar. Biol. 29, 321–333. doi: 10.1007/BF00388852
Kitajima, S., Furuya, K., Hashihama, F., Takeda, S., and Kanda, J. (2009). Latitudinal distribution of diazotrophs and their nitrogen fixation in the tropical and subtropical western North Pacific. Limnol. Oceanogr. 54, 537–547. doi: 10.4319/lo.2009.54.2.0537
Kong, L., Jing, H., Kataoka, T., Buchwald, C., and Liu, H. (2013). Diversity and spatial distribution of hydrazine oxidoreductase (hzo) gene in the oxygen minimum zone off Costa Rica. PLoS ONE 8:e78275. doi: 10.1371/journal.pone.0078275
Lenes, J. M., Darrow, B. P., Cattrall, C., Heil, C. A., Callahan, M., Vargo, G. A., et al. (2001). Iron fertilization and the Trichodesmium response on the West Florida shelf. Limnol. Oceanogr. 46, 1261–1277. doi: 10.4319/lo.2001.46.6.1261
Léon, J.-F., Augustin, P., Mallet, M., Bourrianne, T., Pont, V., Dulac, F., et al. (2015). Aerosol vertical distribution, optical properties and transport over Corsica (western Mediterranean). Atmos. Chem. Phys. Discuss. 15, 9507–9540. doi: 10.5194/acpd-15-9507-2015
Man-Aharonovich, D., Kress, N., Zeev, E. B., Berman-Frank, I., and Béjà, O. (2007). Molecular ecology of nifH genes and transcripts in the eastern Mediterranean Sea. Environ. Microbiol. 9, 2354–2363. doi: 10.1111/j.1462-2920.2007.01353.x
Marañón, E., Fernández, A., Mouriño-Carballido, B., Martínez-García, S., Teira, E., Cermeño, P., et al. (2010). Degree of oligotrophy controls the response of microbial plankton to Saharan dust. Limnol. Oceanogr. 55, 2339–2352. doi: 10.4319/lo.2010.55.6.2339
Marie, D., Partensky, F., Jacquet, S., and Vaulot, D. (1997). Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR I. Appl. Environ. Microbiol. 63, 186–193.
McGinnis, S., and Madden, T. L. (2004). BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 32, 20–25. doi: 10.1093/nar/gkh435
Mills, M. M., Ridame, C., Davey, M., La Roche, J., and Geider, R. J. (2004). Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294. doi: 10.1038/nature02550
Mohr, W., Großkopf, T., Wallace, D. W. R., and Laroche, J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 49:e12583. doi: 10.1371/journal.pone.0012583
Moisander, P. H., Zhang, R., Boyle, E. A., Hewson, I., Montoya, J. P., and Zehr, J. P. (2012). Analogous nutrient limitations in unicellular diazotrophs and Prochlorococcus in the South Pacific Ocean. ISME J. 6, 733–744. doi: 10.1038/ismej.2011.152
Moore, M. C., Mills, M. M., Achterberg, E. P., Geider, R. J., LaRoche, J., Lucas, M. I., et al. (2009). Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat. Geosci. 2, 867–871. doi: 10.1038/ngeo667
Mulholland, M. R., and Bernhardt, P. W. (2005). The effect of growth rate, phosphorus concentration, and temperature on N2 fixation, carbon fixation, and nitrogen release in continuous cultures of Trichodesmium IMS101. Limnol. Oceanog. 50, 839–849. doi: 10.4319/lo.2005.50.3.0839
Mulholland, M. R., Bernhardt, P. W., Blanco-Garcia, J. L., Mannino, A., Hyde, K., Mondragon, E., et al. (2012). Rates of dinitrogen fixation and the abundance of diazotrophs in North American coastal waters between Cape Hatteras and Georges Bank. Limnol. Oceanogr. 57, 1067–1083. doi: 10.4319/lo.2012.57.4.1067
Nielsen, E. (1952). The use of radioactive carbon (14C) for measuring organic production in the sea. Conseil Permanent International pour l'Exploration de la Mer 18, 117–140. doi: 10.1093/icesjms/18.2.117
Paerl, H. W., Crocker, K. M., and Prufert, L. E. (1987). Limitation of N2 fixation in coastal marine waters: relative importance of molybdenum, iron, phosphorus, and organic matter availability. Limnol. Oceanogr. 32, 525–536. doi: 10.4319/lo.1987.32.3.0525
Pitta, P., Nejstgaard, J. C., Tsagaraki, T. M., Zervoudaki, S., Egge, J. K., Frangoulis, C., et al. (2016). Confirming the “Rapid phosphorus transfer from microorganisms to mesozooplankton in the Eastern Mediterranean Sea” scenario through a mesocosm experiment. J. Plankton Res. 0, 1–20. doi: 10.1093/plankt/fbw010
Polymenakou, P. N. (2012). Atmosphere: a source of pathogenic or beneficial microbes? Atmosphere (Basel) 3, 87–102. doi: 10.3390/atmos3010087
Prospero, J. M., Blades, E., Mathison, G., and Naidu, R. (2005). Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia (Bologna) 21, 1–19. doi: 10.1007/s10453-004-5872-7
Psarra, S., Tselepides, A., and Ignatiades, L. (2000). Primary productivity in the oligotrophic Cretan Sea (NE Mediterranean): seasonal and interannual variability. Prog. Oceanogr. 46, 187–204. doi: 10.1016/S0079-6611(00)00018-5
Rahav, E., Bar-Zeev, E., Ohayon, S., Elifantz, H., Belkin, N., Herut, B., et al. (2013c). Dinitrogen fixation in aphotic oxygenated marine environments. Front. Microbiol. 4:227. doi: 10.3389/fmicb.2013.00227
Rahav, E., Giannetto, J. M., and Bar-Zeev, E. (2016a). Contribution of mono and polysaccharides to heterotrophic N2 fixation at the eastern Mediterranean coastline. Sci. Rep. 6:27858. doi: 10.1038/srep27858
Rahav, E., Herut, B., Levi, A., Mulholland, M. R., and Berman-Frank, I. (2013a). Springtime contribution of dinitrogen fixation to primary production across the Mediterranean Sea. Ocean Sci. 9, 489–498. doi: 10.5194/os-9-489-2013
Rahav, E., Herut, B., Mulholland, M., Belkin, N., Elifantz, H., and Berman-Frank, I. (2015). Heterotrophic and autotrophic contribution to dinitrogen fixation in the Gulf of Aqaba. Mar. Ecol. Prog. Ser. 522, 67–77. doi: 10.3354/meps11143
Rahav, E., Herut, B., Stambler, N., Bar-Zeev, E., Mulholland, M. R., and Berman-Frank, I. (2013b). Uncoupling between dinitrogen fixation and primary productivity in the eastern Mediterranean Sea. J. Geophys. Res. Biogeosci. 118, 195–202. doi: 10.1002/jgrg.20023
Rahav, E., Ovadia, G., Paytan, A., and Herut, B. (2016b). Contribution of airborne microbes to bacterial production and N2 fixation in seawater upon aerosol deposition. Geophys. Res. Lett. 43, 1–9. doi: 10.1002/2015GL066898
Rahav, E., Paytan, A., Chien, C.-T., Ovadia, G., Katz, T., and Herut, B. (2016c). The impact of atmospheric dry deposition associated microbes on the southeastern Mediterranean Sea surface water following an intense dust storm. Front. Mar. Sci. 3:127. doi: 10.3389/fmars.2016.00127
Raveh, O., David, N., Rilov, G., and Rahav, E. (2015). The temporal dynamics of coastal phytoplankton and bacterioplankton in the Eastern Mediterranean Sea. PLoS ONE 10:e0140690. doi: 10.1371/journal.pone.0140690
Rees, A. P., Gilbert, J. A., and Kelly-Gerreyn, B. A. (2009). Nitrogen fixation in the western English Channel (NE Atlantic Ocean). Mar. Ecol. Prog. Ser. 374, 7–12. doi: 10.3354/meps07771
Rees, A. P., Law, C. S., and Woodward, E. M. S. (2006). High rates of nitrogen fixation during an in-situ phosphate release experiment in the Eastern Mediterranean Sea. Geophys. Res. Lett. 33:L10607. doi: 10.1029/2006GL025791
Rees, A. P., Tait, K., Widdicombe, C. E., Quartly, G. D., McEvoy, A. J., and Al-Moosawi, L. (2016). Metabolically active, non-nitrogen fixing, Trichodesmium in UK coastal waters during winter. J. Plankton Res. 0:fbv123. doi: 10.1093/plankt/fbv123
Ridame, C., Guieu, C., and L'Helguen, S. (2013). Strong stimulation of N2 fixation in oligotrophic Mediterranean Sea: Results from dust addition in large in situ mesocosms. Biogeosciences 10, 7333–7346. doi: 10.5194/bg-10-7333-2013
Ridame, C., Le Moal, M., Guieu, C., Ternon, E., Biegala, I. C., L'Helguen, S., et al. (2011). Nutrient control of N2 fixation in the oligotrophic Mediterranean Sea and the impact of Saharan dust events. Biogeosciences 8, 2773–2783. doi: 10.5194/bg-8-2773-2011
Riemann, L., Farnelid, H., and Steward, G. (2010). Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat. Microb. Ecol. 61, 235–247. doi: 10.3354/ame01431
Rimmelin, P., and Moutin, T. (2005). Re-examination of the MAGIC method to determine low orthophosphate concentration in seawater. Anal. Chim. Acta 548, 174–182. doi: 10.1016/j.aca.2005.05.071
Sandroni, V., Raimbault, P., Migon, C., Garcia, N., and Gouze, E. (2007). Dry atmospheric deposition and diazotrophy as sources of new nitrogen to northwestern Mediterranean oligotrophic surface waters. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 1859–1870. doi: 10.1016/j.dsr.2007.08.004
Sañudo-Wilhelmy, S., Kustka, A. B., Gobler, C. J., San, S. A., Hutchins, D. A., Yang, M., et al. (2001). Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411, 66–69. doi: 10.1038/35075041
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Simon, M., Alldredge, A., and Azam, F. (1989). Protein-content and protein-synthesis rates of planktonic marine-bacteria. Mar. Ecol. Prog. Ser. 51, 201–213. doi: 10.3354/meps051201
Simon, M., Alldredge, A., and Azam, F. (1990). Bacterial carbon dynamics on marine snow. Mar. Ecol. Prog. Ser. 65, 205–211. doi: 10.3354/meps065205
Sohm, J. A., Webb, E. A., and Capone, D. G. (2011). Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508. doi: 10.1038/nrmicro2594
Spatharis, S., Skliris, N., and Meziti, A. (2012). First record of a Trichodesmium erythraeum bloom in the Mediterranean Sea. Can. J. Fish. Aquat. Sci. 69, 1444–1455. doi: 10.1139/f2012-020
Strickland, J. D. H., and Parsons, T. R. (1972). Bulletin of the Fisheries Research Board of Canada, Vol. 167, 2nd Edn. Ottawa, ON: Fisheries Research Board of Canada.
Stukel, M. R., Coles, V. J., Brooks, M. T., and Hood, R. R. (2014). Top-down, bottom-up and physical controls on diatom-diazotroph assemblage growth in the Amazon River plume. Biogeosciences 11, 3259–3278. doi: 10.5194/bg-11-3259-2014
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Thingstad, T. F., Krom, M. D., Mantoura, R. F. C., Flaten, G. A. F., Groom, S., Herut, B., et al. (2005). Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 309, 1068–1071. doi: 10.1126/science.1112632
Tsiola, A., Pitta, P., Fodelianakis, S., Pete, R., Magiopoulos, I., Mara, P., et al. (2016). Nutrient limitation in surface waters of the oligotrophic Eastern Mediterranean Sea: an Enrichment Microcosm Experiment. Microb. Ecol. 71, 575–588. doi: 10.1007/s00248-015-0713-5
Vaulot, D., and Marie, D. (1999). Diel variability of photosynthetic picoplankton in the equatorial Pacific. Appl. Environ. Microbiol. 104, 3297–3310. doi: 10.1029/98jc01333
Wang, Q., Iii, J. F. Q., and Fish, J.A. (2013). Ecological patterns of nifH genes in four terrestrial climatic zones. MBio 4, 1–9. doi: 10.1128/mBio.00592-13
Welschmeyer, N. A. (1994). Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39, 1985–1992. doi: 10.4319/lo.1994.39.8.1985
Wilson, S. T., Böttjer, D., Church, M. J., and Karl, D. M. (2012). Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic north pacific ocean. Appl. Environ. Microbiol. 78, 6516–6523. doi: 10.1128/AEM.01146-12
Womack, A. M., Bohannan, B. J. M., and Green, J. L. (2010). Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365, 3645–3653. doi: 10.1098/rstb.2010.0283
Wu, J., Sunda, W., Boyle, E. A., and Karl, D. M. (2000). Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762. doi: 10.1126/science.289.5480.759
Yogev, T., Rahav, E., Bar-Zeev, E., Man-Aharonovich, D., Stambler, N., Kress, N., et al. (2011). Is dinitrogen fixation significant in the Levantine Basin, East Mediterranean Sea? Environ. Microbiol. 13, 854–871. doi: 10.1111/j.1462-2920.2010.02402.x
Zakaria, H. Y. (2015). Lessepsian migration of zooplankton through Suez Canal and its impact on ecological system. Egypt. J. Aquat. Res. 41, 129–144. doi: 10.1016/j.ejar.2015.04.001
Zehr, J. P., Jenkins, B. D., Short, S. M., and Steward, G. F. (2003). Minireview Nitrogenase gene diversity and microbial community structure : a cross-system comparison. Environ. Microbiol. 5, 539–554. doi: 10.1046/j.1462-2920.2003.00451.x
Zehr, J. P., and and, Turner, P. J. (2001). “Nitrogen fixation: nitrogenase genes and gene expression,” in Methods in Marine Microbiology, ed J. H. Paul (New York, NY: Academic Press), 271–286.
Keywords: N2 fixation, primary productivity, bacterial productivity, Saharan dust, aerosols
Citation: Rahav E, Shun-Yan C, Cui G, Liu H, Tsagaraki TM, Giannakourou A, Tsiola A, Psarra S, Lagaria A, Mulholland MR, Stathopoulou E, Paraskevi P, Herut B and Berman-Frank I (2016) Evaluating the Impact of Atmospheric Depositions on Springtime Dinitrogen Fixation in the Cretan Sea (Eastern Mediterranean)—A Mesocosm Approach. Front. Mar. Sci. 3:180. doi: 10.3389/fmars.2016.00180
Received: 15 May 2016; Accepted: 06 September 2016;
Published: 23 September 2016.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Robinson W. (Wally) Fulweiler, Boston University, USAPatrick Georges Gillet, UCO Angers, France
Copyright © 2016 Rahav, Shun-Yan, Cui, Liu, Tsagaraki, Giannakourou, Tsiola, Psarra, Lagaria, Mulholland, Stathopoulou, Paraskevi, Herut and Berman-Frank. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyal Rahav, eyal.rahav@ocean.org.il
 Eyal Rahav
Eyal Rahav Cheung Shun-Yan3
Cheung Shun-Yan3  Hongbin Liu
Hongbin Liu Antonia Giannakourou
Antonia Giannakourou Anastasia Tsiola
Anastasia Tsiola Stella Psarra
Stella Psarra Anna Lagaria
Anna Lagaria Margaret R. Mulholland
Margaret R. Mulholland Pitta Paraskevi
Pitta Paraskevi Barak Herut
Barak Herut Ilana Berman-Frank
Ilana Berman-Frank