Microplastics in Seawater: Recommendations from the Marine Strategy Framework Directive Implementation Process
- 1Instituto Español de Oceanografía, Vigo, Spain
- 2Institut Français de Recherche pour l'Exploitation de la Mer, Bastia, France
- 3Lowestoft Laboratory, Centre for Environment, Fisheries and Aquaculture Science, Lowestoft Suffolk, UK
- 4School of Marine Science and Engineering, Plymouth University, Plymouth, UK
Microplastic litter is a pervasive pollutant present in marine systems across the globe. The legacy of microplastics pollution in the marine environment today may remain for years to come due to the persistence of these materials. Microplastics are emerging contaminants of potential concern and as yet there are few recognized approaches for monitoring. In 2008, the EU Marine Strategy Framework Directive (MSFD, 2008/56/EC) included microplastics as an aspect to be measured. Here we outline the approach as discussed by the European Union expert group on marine litter, the technical Subgroup on Marine litter (TSG-ML), with a focus on the implementation of monitoring microplastics in seawater in European seas. It is concluded that harmonization and coherence is needed to achieve reliable monitoring.
Introduction
The ubiquity of plastics in the marine environment and in biota from across the globe has highlighted the prevalence of this contaminant within our oceans. The global mass-production of plastics which started mid last century has been followed by the accumulation of plastic litter in the marine environment (Rochman et al., 2013).
The term “microplastics” (referred to as MPs from hereon) first entered the published literature in 2004 (Thompson et al., 2004), but is now used extensively to describe small fragments of plastic. There is no widely accepted “lower boundary” in size as the limit of detection is dependent on the sensitivity of the sampling technique used (e.g., mesh size of the net or size of the filter).
Microplastics are widely dispersed in the marine environment and are present in the water column, on beaches and on the seabed (Barnes et al., 2009; Law et al., 2010; Browne et al., 2011). Microplastics are a newly recognized type of marine pollution and as such there are few regulations in terms of production, use or emissions.
In the EU, the Marine Strategy Framework Directive (hereinafter MSFD) adopted in 2008 (European Commission, 2008), aims to establish a good environmental status (GES) of the European seas by 2020. The MSFD represents the first instance, worldwide, that MPs in the marine environment have been included in a legislative proposal. In this sense is important to mention that MPs were not included in the Water Framework Directive (WFD), the main EU directive dealing with pollution of river basins.
The main findings of the MSFD marine litter expert group in relation to MPs in seawater are described here. This information may help researchers and governments of EU member states and also other countries to establish legislative tools and to implement programs aimed to study abundance and the impacts of microplastics in the marine environment.
Microplastics in the Marine Environment
Microplastics can enter the marine environment directly as primary MPs (e.g., pre-production pellets and/or granules used as abrasives in cleaning products) or indirectly, as secondary MPs, i.e., the result of progressively fragmentation in the environment of larger items. The relative importance of primary and secondary sources of microplastics to the marine environment is not known (Andrady, 2011).
One of the main threats emanating from MPs is their potential to be taken up by marine organisms. Potentially affected species include primary producers at the base of the food chain through zooplankton, and all the way up to macro invertebrates, fish, and mammals (CBD, 2012). There is limited information on the extent to which microplastics might cause harm in the marine environment. Cell damage, infections, tumor formation, death are just some of the reported toxicity effects by MPs (CBD, 2012).
The MSFD: An Integrated Environmental Policy for the Marine Environment
The European Directive 2008/56/EC (MSFD) is a key element in Europe's actions to protect seas and oceans. The Directive calls for all of the EU's marine regions and sub-regions to achieve or maintain “Good Environmental Status” (GES) by 2020. GES is defined by means of 11 qualitative “descriptors.” The relevant criteria and indicators applicable to those descriptors are defined in the Commission Decision 2010/477/EU (European Commission, 2010).
One of the most important strengths of the MSFD is the aim to provide a holistic, functional approach; it separates the ecosystem into a set of process-related (functional) objectives, and then recombines these, to ensure the integrity of the ecosystem.
Descriptor 10 relating to marine litter, and their formulation according to the MSFD is that “Properties and quantities of marine litter do not cause harm to the coastal and marine environment.” It is the first time that marine litter is addressed, in an integrated way for the protection of the marine environment, in a European directive (Galgani et al., 2013a).
A Technical Subgroup on Marine Litter (TSG-ML) was established in 2010 to support Member States in harmonizing monitoring protocols and streamlining monitoring strategies in the framework of the MSFD (Galgani et al., 2013a,b).
Microplastics in the Context of the MSFD
Microplastics are considered specifically in descriptor 10 of the MSFD [10.1.3 “Trends in the amount, distribution, and where possible, composition of micro-particles (in particular micro- plastics)”], and not directly but implicitly in the indicator related with impacts of litter on marine life. The descriptor will establish baseline quantities, properties, and potential impacts of MPs. It must be noted however that the decision was reviewed recently for changes in order to make it simpler and clearer, to introduce minimum standards and to be coherent with other EU legislation.
Within the process, the TSG-ML suggested that micro-litter be considered as a size fraction integrating micro-litter along with other litter fractions in the matrix related indicators. Not all of the experts support this view, arguing that micro litter is different from other litter types (meso/macro) and that micro-litter may have considerably different effects to those caused by larger items of litter. The idea of merging indicators 10.1.2 (litter at sea, floating and on the sea floor) with indicator 10.1.3 (microplastics) aimed to avoid treating microparticles as a separate issue while measures to combat marine litter need to be formulated covering all size classes.
Finally, the revised decision (article 9/3 and 11/4) kept (the review has been done but not published yet) criteria separated for macro litter (10DC1) and microplastics (D10C3), now defined as “The composition, amount, and spatial distribution of micro-litter in the surface layer of the water column, in sea-floor sediment, and possibly on coastlines, is at a level that does not cause harm to the coastal and marine environment.”
MPs should be categorized according to their physical characteristics including size, shape, and color (see Table 1). It is also important to obtain information on polymer type.
The size definition of MPs according to the TSG-ML (Galgani et al., 2013b) is in line with the NOAA definition. We strongly suggest using this size (<5 mm) as an international standard. One aspect that should be refined is the definition of the lower size boundary for MPs in the MSFD. The lower size has not been defined strictly and nanoparticles have not been considered as a category despite their potential relevance (Galgani et al., 2013a).
Sampling of MPs in the different marine compartments (sea water, sediment, and biota) requires different approaches: samples can be selective, bulk, or volume-reduced (see e.g., Hidalgo-Ruz et al., 2012). Selective sampling in the field involves visual identification and manual sorting of fragments from different matrices and is not very effective for MPs due to difficulties in handling small size items. The subsequent identification of plastic particles in the matrix follows similar procedures (section Quantification and nature of MPs).
Bulk samples refer to samples where the entire volume of the sample is taken without reducing it during the sampling process. Bulk samples are most appropriate when MPs cannot be easily identified visually because in the field because (i) they are covered by sediment particles, (ii) their abundance is small requiring sorting/filtering of large volumes of sediment/water, or (iii) they are too small to be identified with the naked eye (Hidalgo-Ruz et al., 2012).
Volume-reduced samples, in seawater, refers to sampling where the bulk volume of the sample is reduced during sampling, preserving only that portion of the sample that is of interest for further processing. While on board a vessel seawater samples can be volume-reduced by filtering water through nets or screens.
A Need for Standardization: The Exemplary Case of Sampling Seawater
In the last years studies determining the global quantity of plastic particles in the ocean have been published (Eriksen et al., 2014; Cózar et al., 2014, 2015). In order to ensure inter-comparability between these studies to evaluate when (seasonality) and where (space) contamination is taking place, harmonization is urgently needed.
Seawater samples for MPs are mostly taken by nets. The main advantage of using a net is that large volumes of water can be sampled quickly, only retaining the volume-reduced sample. Most studies have been from surface water using neuston nets (Hidalgo-Ruz et al., 2012); manta and bongo nets have also been used at the sea surface. Since most plastics are buoyant they are likely to accumulate at the sea surface. Another instrument, that is widely deployed on a global scale and that has also been used for MPs sampling is the Continuous Plankton Recorder (CPR) (Thompson et al., 2004). Some instruments, including bongo and the CPR, are used sub surface making direct comparison rather difficult (Hidalgo-Ruz et al., 2012; Frias et al., 2014).
The most relevant characteristics of the sampling nets used are the mesh size and the net opening. Mesh sizes used for microparticle sampling range from 0.053 to 3 mm, with a majority of the studies (rather than individuals samples collected) ranging from 0.30 to 0.39 mm (Hidalgo-Ruz et al., 2012). The net aperture for rectangular openings of neuston nets (sea surface) ranged from 0.03 to 2.0 m2.
Techniques using apparatus to collect surface seawater and pass it through a filter on-board ship are being developed for example by CEFAS, UK (T. Maes; personal communication). They use the ships water inlet, collecting seawater from the side at specified depths, mostly ranging between 4 and 1 m depth. The seawater is being passed along a set of sieves or nets after which the sieves or nets can be removed and analyzed for MPs in the laboratory (Pitois et al., 2016).
The advantage of such systems is that it can collect marine litter samples from the water column while steaming and thus long transects over several kilometers can be collected autonomous in connection with in-line analytical systems for other parameters like nutrients or oxygen. The development of filtration systems for the quantification of MPs appears promising (Lusher et al., 2014).
The recommendation from the TSG-ML is to obtain samples from sea water wherever possible, and to ensure the following details are recorded to accompany each sample: type of net (preferably Manta net), aperture (usually 60 cm), and mesh size (preferably 333 μm). It is also important to record the following parameters: depth (preferably either at the sea surface or within surface 10 m, for greatest inter-comparability among sampling programmes) distance towed, location of tow (in/out of water) and volume of water filtered (with a current meter).
Also prevailing weather conditions and sea state, together with any relevant information on the volume of plankton or other particulates sampled, for example if there is concern that the net may have become clogged due to high concentration of plankton, must be recorded. Samples should be stored in glass jars. MPs are determined as the total quantity of items per volume of seawater captured by the net during the period it is deployed.
Samples in seawater can be passed through a 500 μm sieve, and liquid passing through the sieve then filtered through a filter paper using a Buckner funnel. Filter papers can then be examined under a dissecting microscope to quantify microplastics below 5 mm. Sample on CPR silk filter screens can be examined directly under the microscope.
At present and from the experience in the implementation of the MSFD discussed in the TSG-ML, it is not appropriate to recommend one approach over all others. As an example, in Table 2 are shown MPs values available for the Mediterranean with sampling details (mesh size, net). Each approach has advantages and disadvantages and may be preferable according to local availability/sampling opportunities, the characteristics of the area to be sampled and other factors. The mesh size and water volume are important if one wants to compare different surveys and thus harmonization between these parameters is recommended.
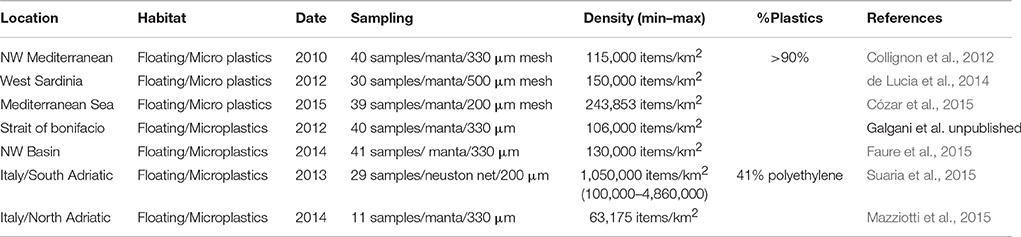
Table 2. Summary of some available data for microplastics in surface waters in the Mediterranean Sea.
Quantification and Nature of MPs
Once MPs have been separated from their environmental matrix (seawater, sediments or biota) they must be quantified and identified.
Identification of MPs
The identification of MPs polymers is achieved by comparing the spectra from the unknown sample against that of a known standard polymer in a database. We encourage consulting Hummel (2002) for more details on this methodology. It should be noted that this method is only definitive where a good match is obtained and this is not always possible. Due to biofouling and degradation processes of microplastics in the environment, their spectra are not totally similar to spectra from the virgin material in the library.
If formal identification of particles using Fourier Transformed- Infra Red (FT-IR) or Raman Spectroscopy is applied then polymer type should also be recorded. Spectroscopy is not critical for routine monitoring of larger fragments > 500 μm. However, it should be considered essential for fragments > 50 μm and a proportion (5–10%) of all samples should be routinely checked to confirm the relative accuracy of any visual examination.
A suitable approach proposed by the TSG-ML would be to automatically accept any match >70% similarity (Frias et al., 2016), to individually examine matches between 60 to 70% similarity rejecting any samples which do not show clear evidence of peaks corresponding to known synthetic materials and to routinely reject (as synthetic) any samples which produce spectra with a match <60%).
It is advocated that when analyzing particles in the range 1–100 μm to subject them to further spectroscopic analysis to confirm polymer identity (e.g., using FT-IR). For particles in the size range 101 μm–4.99 mm we recommend that a proportion (10% of the material in each size class, up to a maximum of 50 items per year or sampling occasion whichever is the least frequent) of the items considered to be MPs is subjected to further spectroscopic analysis to confirm identity (e.g., using FT-IR). This step is important in order to; (1) ensure quality control of visual identification and (2) gain information on the relative abundance of different polymer types which can inform on sources.
One important issue is to mitigate contamination of samples, as plastics are present in our daily lives (in clothes, scrubbers) and in labs (labware). People undertaking the sampling and working in the lab should minimize any synthetic clothing. As procedural controls to check ambient cleanliness, place unused clean filter papers in Petri dishes. Remove the lid and leave the Petri-dish open for a fixed time period relevant to the time period for which samples might be exposed to the air during examination. Procedural contamination should be <10% of the average values determined form the samples themselves.
Required Reporting Units
For MPs in seawater items/m3 seawater, average size of particles, relative abundance of main colors and shape are suggested as units. Relating quantities of MPs to volume is relevant when considering the sampling of water column through filtration. Expressing quantities by volume also allow to link field studies directly with exposure experiments in the laboratory. The estimation of volumes is however impossible when using neuston/manta nets as the trawl frames are permanently moving vertically at the surface of the sea, complicating correct calculation of the sampled water height covered during tows. For this reason, the sampling of the surface density most often rely on items/m2, a more relevant estimation of the sample covered. It should be stressed that when possible, more info should be recorded to facilitate reporting in several units in order to ensure comparison with other studies. If FT-IR or Raman is used then polymer type should also be recorded together with shape and color.
Final Remarks
When comparing reported abundances of MPs in the water column it is important to keep in mind that even though most surveys are conducted using a neuston net, the mesh size of these nets often differ. In addition, despite recommendations for the definition of MPs as particles smaller of 5 mm (Arthur et al., 2009; Galgani et al., 2013a), many authors worldwide are using other size limits e.g., 1 mm (Costa et al., 2010; Van Cauwenberghe et al., 2013). Furthermore, sometimes it is not possible to compare density values due to different methodologies used for sampling (items/km2 vs. items/km3). Hence, comparison between studies is quite complex.
There is a need for research to develop and subsequently validate new methods to rapidly and inexpensively identify and quantify MPs. These methods could include image recognition equipment to facilitate rapid identification as is currently used for plankton and particulate characterisation (Sieracki et al., 2009) and separation. Development of bulk chemical approaches to provide either an absolute value or an index of extent to which a water sample is contaminated with MPs and to indicate the type of particles (as e.g., polymer type) could also prove useful. It is also important to note that methods for detecting nanoparticles in the marine environment should be developed in the coming years.
As this is an emerging field and our understanding of the rates of accumulation and the extent to which MPs might cause harm in the environment is very scarce. Therefore, the experts of TSG-ML advocate a precautionary approach and recommends the development and calibration of methods and initiation of wider scale monitoring should commence straight away.
In our view one of the most important long term needs for the MSFD beyond 2020 are to gain a holistic understanding of marine litter by integrating MPs data collected from waters, sediments, and biota with other litter data and by integrating knowledge of temporal and spatial trends across types and sizes of marine litter (Van Franeker and Law, 2015).
Some of the monitoring approaches for the MSFD are still under development, so the implementation and improvement of monitoring will require continuous collaborative efforts. To achieve the greatest efficiency, MPs in seawater should be sampled alongside other routine sampling programmes. Similarly sampling of sea water column could also be incorporated into other monitoring programmes. A key consideration in collecting seawater samples is the cost of ship time. Hence the potential to sample during existing cruises or programmes is well worth considering.
The comparable quantification of MPs, by the use of common methodologies, is also important for identification of the sources, planning of measures against marine litter and for checking the efficiency of these counter-measures under the umbrella of the MSFD.
Author Contributions
JG write the paper and took part in discussions. FG, TM, and RT contribute to the paper discussions.
Funding
Participation of JG was financed by Spanish minister of Environment under Project 3-ESMARAC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article is based on the activities of the GES-Technical subgroup on Marine litter (2012–2015), specifically on microplastics. We want to express our gratitude to all members of this group. We are very great full to the three reviewers that have made a number of good suggestions to improve this paper.
References
Andrady, A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. doi: 10.1016/j.marpolbul.2011.05.030
Arthur, C., Baker, J., and Bamford, H. (2009). Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris. NOAA Technical Memorandum NOS-OR&R30.
Barnes, D. K. A., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1985–1998. doi: 10.1098/rstb.2008.0205
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 45, 9175–9179. doi: 10.1021/es201811s
CBD (Secretariat of the Convention on Biological Diversity the Scientific the Technical Advisory Panel GEF) (2012). Impacts of Marine Debris on Biodiversity: Current Status and d Potential Solutions. Technical Series, No. 67. Montreal, QC.
Collignon, A., Hecq, J.-H., Galgani, F., Voisin, P., Collard, F., and Goffart, A. (2012). Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 64, 861–864. doi: 10.1016/j.marpolbul.2012.01.011
Costa, M. F., Ivar do Sul, J. A., Silva-Cavalcanti, J. S., Araúja, M. C. B., Spengler, A., and Tourinho, P. S. (2010). On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a Brazilian beach. Environ. Monit. Assess. 168, 299–304. doi: 10.1007/s10661-009-1113-4
Cózar, A., Echevarría, F., González-Gordillo, I., Irigoien, X., Úbeda, B., Hernández-León, S., et al. (2014). Plastic debris in the open ocean. Proc. Natl. Acad. Sci. U.S.A. 111, 10239–10244. doi: 10.1073/pnas.1314705111
Cózar, A., Sanz-Martín, M., Martí, E., González-Gordillo, J., Ubeda, B., Gálvez, J., et al. (2015). Plastic accumulation in the Mediterranean Sea. PLoS ONE 10:e0121762. doi: 10.1371/journal.pone.0121762
de Lucia, G. A., Caliani, I., Marra, S., Camedda, A., Coppa, S., Alcaro, L., et al. (2014). Amount and distribution of neustonic micro-plastic off the Western Sardinian coast (Central-Western Mediterranean Sea). Mar. Environ. Res. 100, 10–16. doi: 10.1016/j.marenvres.2014.03.017
European Commission (2008). Directive 2008/56/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Brussels: Official Journal of the European Union L164, 19–40.
European Commission (2010). Commission Decision of 1 September 2010 on Criteria and Methodological Standards on Good Environmental Status of Marine Waters (Notified under Document C(2010) 5956)(2010/477/EU). Brussels: Official Journal of the European Union L232, 12–24.
Eriksen, M., Lebreton, C. M., Carson, H. S., Thiel, M., Moore, C. J., Borrero, J. C., et al. (2014). Plastic pollution in the World's Oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9:e111913. doi: 10.1371/journal.pone.0111913
Faure, F., Saini, C., Potter, G., Galgani, F., Felippe de Alencastroand, L., and Hagmann, P. (2015). An evaluation of surface micro- and mesoplastic pollution in pelagic ecosystems of the Western Mediterranean Sea. Environ. Sci. Pollut. Res. 22, 12190–12197. doi: 10.1007/s11356-015-4453-3
Frias, J. P., Gago, J., Otero, V., and Sobral, P. (2016). Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 114, 24–30. doi: 10.1016/j.marenvres.2015.12.006
Frias, J. P., Otero, V., and Sobral, P. (2014). Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 95, 89–95. doi: 10.1016/j.marenvres.2014.01.001
Galgani, F., Hanke, G., Werner, S., and De Vrees, L. (2013a). Marine litter within the European marine Strategy Framework Directive. ICES J. Mar. Sci. 70, 1055–1064. doi: 10.1093/icesjms/fst122
Galgani, F., Hanke, G., Werner, S., Oosterbaan, L., Nilsson, P., Fleet, D., et al. (2013b). “Guidance on Monitoring of Marine Litter in European Seas,” in EUR – Scientific and Technical Research Series – ISSN 1831-9424 (Online), eds G. Hanke, S. Werner, F. Galgani, J. M. Veiga, and M. Ferreira (Luxembourg: Publications Office of the European Union).
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., and Thiel, M. (2012). Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 46, 3060–3075. doi: 10.1021/es2031505
Hummel, D. O. (2002). Atlas of Plastics Additives. Analysis by Spectrometric Methods. Berlin: Springer-Verlag.
Law, K. L., Morét-Ferguson, S., Maximenko, N. A., Proskurowski, G., Peacock, E. E., Hafner, J., et al. (2010). Plastic Accumulation in the North Atlantic Subtropical Gyre. Science 329, 1185–1188. doi: 10.1126/science.1192321
Lusher, A. L., Burke, A., O'Connor, I., and Officer, R. (2014). Microplastic pollution in the Northeast Atlantic Ocean: validated and opportunistic sampling. Mar. Pollut. Bull. 88, 325–333. doi: 10.1016/j.marpolbul.2014.08.023
Mazziotti, C., Bertaccini, E., Benzi, M., Martini, S., Lera, S., Silvestri, C., et al. (2015). “Sea surface microplastics distribution on Emilia Romagna coast: defishgear project preliminary results. Micro 2015,” in Seminar of the Defishgear Project, Abstract Book (Piran), 41.
Pitois, S. G., Bouch, P., Creach, V., and Van der Kooij, J. (2016). Comparison of zooplankton data collected by a continuous semi-automatic sampler (CALPS) and a traditional vertical ring net. J. Plankton Res. 38, 931–943. doi: 10.1093/plankt/fbw044
Rochman, C. M., Browne, M. A., Halpern, B. S., Hentschel, B. T., Hoh, E., Karapanagioti, H., et al. (2013). Policy: classify plastic waste as hazardous. Nature 494, 169–171. doi: 10.1038/494169a
Sieracki, M. E., Benfield, M., Hanson, A., Davis, C., Pilskaln, C. H., Checkley, D., et al. (2009). “Optical plankton imaging and analysis systems for ocean observation,” in Procceedings in OceanObs'09: Sustained Ocean Observations and Information for Society (2010), Vol. 2., eds J. Hall, D. E. Harrison, and D. Stammer (Venice: ESA Publication), WPP 306, 21–25.
Suaria, G., Avio, C., Lattin, G., Regoli, F., and Aliani, S. (2015). “Neustonic microplastics in the Southern Adriatic Sea. Preliminary results. Micro 2015,” in Seminar of the Defishgear Project, Abstract Book (Piran), 42.
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at sea: where is all the plastic? Science 304, 838. doi: 10.1126/science.1094559
Van Cauwenberghe, L., Vanreusel, A., Mees, J., and Janssen, C. (2013). Microplastic pollution in deep-sea sediments. Environ. Pollut. 182, 495–499. doi: 10.1016/j.envpol.2013.08.013
Keywords: marine debris, plastics, microplastics, monitoring
Citation: Gago J, Galgani F, Maes T and Thompson RC (2016) Microplastics in Seawater: Recommendations from the Marine Strategy Framework Directive Implementation Process. Front. Mar. Sci. 3:219. doi: 10.3389/fmars.2016.00219
Received: 13 June 2016; Accepted: 24 October 2016;
Published: 08 November 2016.
Edited by:
Maria C. Uyarra, AZTI Tecnalia, SpainReviewed by:
Mario Barletta, Federal University of Pernambuco, BrazilStefano Aliani, National Research Council, Italy
Copyright © 2016 Gago, Galgani, Maes and Thompson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesus Gago, jesus.gago@vi.ieo.es
 Jesus Gago
Jesus Gago Francois Galgani
Francois Galgani Thomas Maes
Thomas Maes Richard C. Thompson4
Richard C. Thompson4