Dr. S. Donald (Don) Stookey (1915–2014): Pioneering Researcher and Adventurer
- Corning Incorporated, Corning, NY, USA
Don Stookey, the father of glass–ceramics, was a pioneer in inducing and understanding internal nucleation phenomena in glass. His early work on dense opal glasses and photosensitive precipitation of gold and silver in glass led to an amazing series of inventions: Fotalite®, a photosensitive opal, chemically machined Fotoform® and Fotoceram®, and TiO2-nucleated Pyroceram™ products including missile nosecones and oven-proof cookware. He received a basic patent on glass–ceramics, which was contested and affirmed in court. Don was able to demonstrate a clear photochromic glass that showed reversible darkening for thousands of cycles. This material became a fixture in the ophthalmic industry. He went on to invent a full-color polychromatic glass, capable of yielding a permanent patterned and monolithic stained glass. In his life outside science, Don chaired an interfaith group that founded a home for the elderly in Corning. He was also a wilderness enthusiast, surviving a plane crash in the Arctic and two boat capsizings. Even in his later years, he continued fishing off the coast of Florida and on Lake Ontario and went solo on a trip to the Patagonian Andes. Don Stookey was a special person by any measure: an unassuming optimist, eminent scientist and inventor, adventurer, and a beloved family man.
Introduction
Don Stookey (Figure 1) was a well-known and highly respected scientist at Corning Incorporated, formerly Corning Glass Works, for 50 years (1940–1990). He was responsible for developing an understanding of internal nucleation of crystallites in glass resulting in many products: photosensitive ruby glass, photosensitive opal glass, photochromic glass, polychromatic glass, and glass–ceramics, including Corningware® cookware and Pyroceram™ missile nosecones.
Don was born at Hay Springs, Nebraska on May 23, 1915. His family moved to Cedar Rapids Iowa in 1921. He graduated with a B.A. in Mathematics and Chemistry from Coe College in 1936. He then received a master’s degree in Chemistry from Lafayette College in Easton, PA, USA, in 1938 and went on to earn a Ph.D. in Physical Chemistry from Massachusetts Institute of Technology in 1940. He married his Coe College sweetheart, Ruth Watterson, in the same year. He joined Corning Glass Works in 1940 and began working on dense and opal glasses of interest as the white background in glass thermometers as well as for decorative tableware and translucent lighting globes.
Don was fascinated by the effects of heat treating uncolored glass to yield a ruby glass in some cases and a white material in others. He understood that particles must be forming in the interior of such glasses and from the chemistry of the glass and previous research dating back in some cases to the middle ages, he realized that these particles were gold, silver, or copper in ruby glasses and crystals of titania or fluorides of sodium or calcium in opal glasses.
Photosensitive Glasses
Don’s first invention involved copper ruby glasses that required a heat treatment to bring out the copper particles. Don realized that the traditional added ingredients, such as the oxides of tin and antimony, must play a key role. He reasoned that reheating the glass must initiate a redox reaction where copper is reduced to the metallic state and tin and antimony are oxidized. He then systematically decreased the amount of these additives to the point where the glass remained colorless even after the heat treatment. He then exposed these glasses through a negative to ultraviolet light, and upon subsequent heating, only the exposed areas turned a ruby color. After producing a photosensitive copper ruby glass, Don felt he could do the same with the more beautiful gold ruby glass (Figure 2). The color mechanism here was already understood to be an effect of SnO2 additions to the glass batch, yielding an oxidizing melt and allowing gold to be dissolved as ions. Subsequent reheating of the cold glass caused SnO to reoxidize to SnO2, thus reducing the gold ions allowing the precipitation of tiny gold particles. But, as Don decreased the tin level to the point of zero coloration on reheating, ultraviolet radiation had no effect in retrieving the ruby color through heat treatment. It was then that he tried additions of other multivalent oxides and found that only cerium oxide (CeO2) additions allowed the ultraviolet exposed areas to create ruby patterns. Thereafter, the combination of ceria additions to both gold- and silver-containing glasses became the most important feature in many new photosensitive materials, including photosensitive opal glasses. In this case, Don added silver and ceria and decreased the fluoride level to just below what was required to produce thermal opalization. The silver particles could be formed only in the exposed areas via the photochemical reaction: Ag1+ + Ce4+ → Ag0 + Ce3+, and these nanocrystals were able to act as nuclei for sodium fluoride (NaF) crystals on reheating. Moreover, these NaF crystals grew large enough to scatter light. As a result, his first commercial success, Fotalite®, was produced (Figure 3). This material was used as sheets of white marbleized glass that to this day cover the north face of the United Nations headquarters building in New York City.
Fotoform® and Fotoceram®, the First Glass–Ceramic
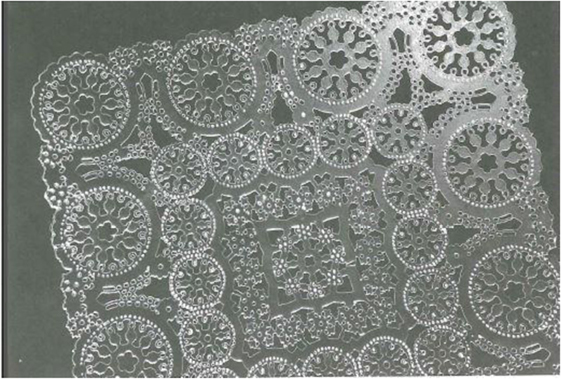
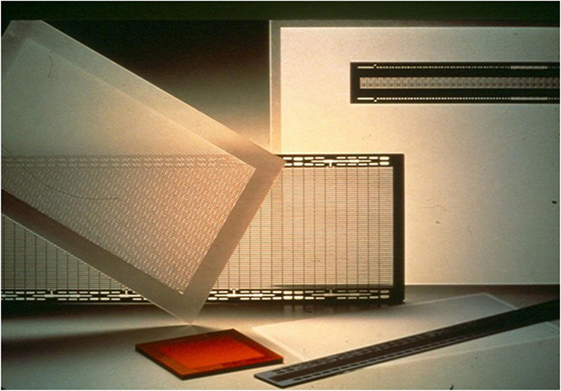
After his discovery of controlled photosensitive precipitation of silver and its role in the nucleation of NaF, Don began to consider whether other crystalline phases could also be internally nucleated in glass. He discovered that actual glass-forming silicate compounds such as barium disilicate and lithium metasilicate could be nucleated, albeit in small amounts. Then, at a meeting in 1947, Dr. J. T. Littleton, Corning’s research director at that time, wondered if glass could somehow be used to make vital aperture masks guiding the electron beams in colored television (Corning already made the bulbs). Don left the meeting without any comment and went ahead testing the photo-precipitated compounds that he had made. To his delight, in one lithium-containing glass, the areas where lithium metasilicate was photo-precipitated could be easily etched with hydrofluoric acid, whereas the untreated glassy areas were resistant. This new photo-etchable material was called Fotoform®, and although it was never used for television apertures, 30 years later it developed into a significant business. It was used in a variety of complexly shaped structures for the electronic and communication industries as well as for decorative items (Figure 4).
The discovery of Fotoform® also led to an even more important albeit accidental discovery. One day, as Don was preparing to heat-treat a plate of pre-exposed Fotoform® glass, the temperature controller became stuck in the “on” position, and instead of remaining at 600°C, the furnace temperature increased to 900°C. Expecting to find a molten pool of glass, Don was amazed to see instead an undeformed opaque and solid plate. Snatching a pair of tongs, he pulled the plate out of the hot furnace, but it slipped from the tongs and fell onto the concrete floor, clanging like steel and remaining unbroken. He had, in fact, converted a glass article to a ceramic article. This first glass–ceramic, called Fotoceram®, was predominantly crystalline (lithium disilicate) and stronger than Fotoform® and could inherit a complex etched shape from Fotoform® through simple flood exposure and reheating (Figure 5).
Explosion of Glass–Ceramic Compositions and Properties
Don Stookey was not satisfied by producing Fotoceram® alone. He was convinced that this was just one example of a conversion of glass to a ceramic and that other glasses of a wide variety of chemical composition would yield crystals of diverse and useful properties. Unfortunately, he found that silver and other metallic nucleating agents were ineffective in most glasses, especially in aluminosilicate formulations. Don knew of crystals of low coefficient of thermal expansion (CTE), such as cordierite (Mg2Al4Si5O18), used in thermal-shock-resistant ceramic kiln ware and lithium aluminosilicates that had been revealed in research at Pennsylvania State University (Penn State) to have low and even negative CTE values. He desperately needed to find an effective nucleating agent for aluminosilicate glasses, and from his knowledge of dense white opal glasses, particularly those based on rutile (TiO2), he decided to add titania to these glass batches. Titania was amazingly effective, easily dissolving into the glasses and producing highly crystalline lithium and magnesium aluminosilicate glass–ceramics upon heat treatment. Surprisingly, little or no deformation was seen during the heating and crystallization process, and even delicate shapes of pressed and blown ware were faithfully retained. Two successful products resulted from this research in the late 1950s: radomes (nose cones for missiles) based on cordierite (Figure 6) and Corningware® (Figures 7 and 8) heat-resistant cookware based on the crystal β-spodumene (LiAlSi2O6–LiAlSi5O12 solid solution). The first glass–ceramic electric cooktop was based on a similar material (Figure 9).

Figure 6. Don Stookey with Ben Allen (engineering manager), Jim Giffen (glass-forming expert), and Bill Armistead (R&D director) with first glass–ceramic radomes (c. 1957).
Don Stookey was also the first to produce transparent glass–ceramics. While experimenting in the late 1950s with various heat treatments of basic Li-aluminosilicate glasses doped with titania, he found that metastable silicate crystals preceded the development of β-spodumene. The X-ray diffraction pattern looked like that of β-eucryptite (LiAlSiO4), a structural derivative of β-quartz, known for its negative CTE. This glass–ceramic was composed of nanocrystals about 50–100 nm in diameter, and because they were smaller than the wavelength of visible light, the glass–ceramic did not scatter light and was transparent. Later, it was found that these crystals were solid solutions of β-eucryptite and β-quartz, similar to the natural mineral: virgilite. Glass–ceramics based on this crystal phase were to become the basis of applications where dimensional stability over a wide spectrum of temperatures (near-zero CTE) is required. These include telescope mirrors (Schott Zerodur®), Visions® transparent cookware, and the largest current glass–ceramic business: dark electric range cooktops.
Photochromic Glass
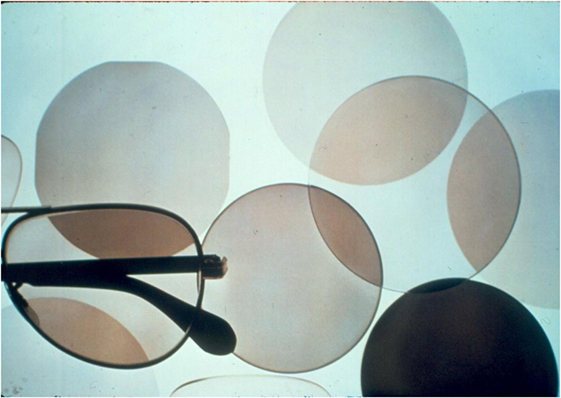
The development of a glass that darkens under sunlight and fades indoors was one of Don Stookey’s finest inventions. It began when Dr. William Armistead (Bill), Corning’s Director of Research and Development at this time, visited a customer who suggested the potential value of reversible sunglasses. Bill then asked Don if he could make such a glass. An accomplished researcher himself, Bill remembered a yellow opal glass containing silver chloride (AgCl) that he had made. This glass appeared to darken under sunlight, so Bill suggested this as a starting point. Don jumped at the idea, and following the procedure that had been successful with photosensitive glasses, he decreased the level of the active agent, in this case AgCl, in the glass until it remained clear after cooling and annealing. Upon further heat treatment below the softening temperature, he was able to produce some opaque and translucent materials that showed some darkening under ultraviolet light, but very little fading. All of the samples that remained transparent were inactive. Remembering his success with ceria as a sensitizer in photosensitive glass, he tried this oxide as well as other multivalent oxide additions to the glass batch. Only copper oxide was effective, not only in producing darkening in transparent samples but also fading as the light source was removed. Surprisingly, this action was reversible and unchanged after thousands of cycles. Transmission electron microscopy later revealed that spherical nanocrystals of Cu-doped AgCl were decorated with metallic silver patches (like continents on the earth) after darkening according to the reversible reaction: Ag1+ + Cu1+ ↔ Ag0 + Cu2+. The patches then disappeared upon removal of the light source, causing fading.
As a result of this work, Don had created the first truly reversible chemical reaction in a solid material. It was not long after this discovery that a multimillion dollar business in photochromic glass for both prescription lenses and sunglasses (Figure 10) became a reality.
Polychromatic Glass
The last of Don Stookey’s major inventions was polychromatic glass, a photosensitive glass that could produce all colors in any patterned form (Figure 11). Don discovered that by including two halides, namely, sodium fluoride (NaF) and either sodium bromide (NaBr) or sodium chloride (NaCl), in the batch of certain compositions related to Fotalite®, a full-color spectrum could be developed in a single glass. To develop the colors, two exposures to ultraviolet light (~300 nm) followed in each case by special heat treatments were required. He found that the first exposure dose (time and/or ultraviolet intensity) controlled the final color, while the second exposure dose controlled the color intensity. The first heat treatment developed the silver nuclei near 475°C and produced both cubic crystals, presumed to be NaF, and crystallites of a peculiar pyramidal or comet-like shape around 525°C. The pyramidal crystals were thought to be mixed silver-doped complex sodium halide- Ag: Na(F,Cl,Br), because without a secondary halide, they could not be formed. The number and density of both silver nuclei and pyramidal halide crystallites increased as a function of the original radiation dose. The second exposure and heat treatment, which could be separated or done simultaneously on a hot plate at about 350°C, caused more silver to precipitate, this time at the tip of the pyramidal crystals. This silver was elongated to a degree dependent upon the density of crystals; the scattered crystals had more and longer silver at the tail, whereas crowded crystals had less and shorter silver tails. The color depended on the aspect ratio of these secondary silver particles at the crystallite tails, green with the longest going through blue, purple, red, orange, and yellow with the shortest.

Figure 11. Evolution of polychromatic glass as formed, after ultraviolet exposure and first heating, and after second ultraviolet exposure and heating.
Polychromatic glass was produced as both clear and opal glasses, depending upon the size of the halide crystals. To the disappointment of Don Stookey, no practical applications for this unique material have yet been found.
Don Stookey’s Research Philosophy
Don has described his research philosophy in a number of quotations which are listed below:
Be observant, diligent, and optimistic. Don was always an optimist, especially in research, but also in his outlook on the world in general.
Science can be fun, and the scientist-inventor need neither be mad, nor a genius, nor confined to an ivory tower. Don was a practical man and believed strongly in the value of useful research. He took a dim view of the idea that pure research was somehow superior to exploratory research and academic research is somehow contaminated by cooperation with industry.
The unique factor in promoting my career has been the proper mix of motivation and imagination, and the opportunity to exercise them. Don was especially grateful for the freedom he was given to do independent research at the Corning Glass Works and had great respect for the Houghton family and R&D director, Dr. William Armistead. He became a lifetime friend of former Corning Chief Executive Officer, Amory Houghton, Jr.
The increasingly popular use of research teams has its place, but original ideas come one at a time. Teamwork applies to later stages of innovation and should not displace the original inventor. Don was unhappy to see an apparent modern trend away from individual thought and accomplishment.
An embryo invention is a fragile flower, easily killed by the pessimism that seems to be a predominant characteristic of most adults. Don did not easily tolerate naysayers.
Meetings, fire calls to solve today’s production problems, and assignments to improve on known inventions are general enemies to the birth of new inventions. Don understood the necessity of continual product development and crises problem-solving; indeed, he admired and gave full credit to those who made contributions in harnessing his inventions. He did, however, believe that those few who were enthusiastic and skilled in fundamental and exploratory research should be allowed to follow their instincts.
Don Stookey’s Fishing Adventures
Don Stookey was an avid fisherman, and it was on fishing vacations that he had many unexpected adventures. One in the 1950s that nearly took his life took place above the Arctic Circle on Great Bear Lake in the Northwest Territories of Canada.
Don and his brother, Dave, were about to land in a float plane near their campsite when the pilot was evidently distracted by the wake of another float plane taking off. The lake was a glassy calm except for these developing waves, and the pilot had difficulty in gaging the distance between his plane and the lake. As a result, he failed to raise the nose of the plane as he hit a wave surface, and the plane flipped over leaving the pilot and passengers upside down and quickly under ice-cold water. Don and the pilot were able to free themselves from their seat belts, and Don was able to open the passenger door against the pressure of the water and hold it open until his brother emerged from the back of the plane. They then hit the water surface and were able to crawl out and stand on the upside-down wing below the water surface, hanging on the floating pontoon on the surface of the lake. They had less than 10 min to survive in the freezing water, but fortunately they were within sight of the Innuit (Eskimos) at the camp, and they were able to reach them by boat in time to save them.
Then there was the time in 1995, when a large group of fishermen including Don, his brother Dave, his two grandsons Dave and Steve, my two sons Doug and Tom, and I were on a fishing trip to Camp Minewan, a native Cree fishing camp about 100 miles east of James Bay in Northern Quebec. One day, we took a portage trail leading to the outlet of a lake where the fishing was so good that large northern pike about 2 ft in length attacked any lure thrown at them in almost every cast. Upon returning along the portage, Don was walking behind me at the end of the group, and I suddenly realized that I could no longer hear his footsteps. The terrain near this trail consisted of typical black spruce taiga with trees separated by a few yards, and it was the same for miles in any direction with no signs of anything beyond the occasional moose trail, one of which Don had evidently mistaken for the portage. Fortunately, my sons had good ears and heard a stick or branch breaking in the distance and off the trail. We were eventually able to find Don, who said he had just realized he was lost and alone when we found him.
Don was a lover of boats and bought a 36-ft Jinx cruiser on his way to Florida when he ran into Hurricane Agnes on Chesapeake Bay and became mired on a reef whose warning buoy had shifted in the storm. He did not know it at the time, but this same storm was to cause flooding in the Corning, NY, USA, area where Don’s house was inundated nearly to the ceiling of the first floor.
Although by now, one might get the impression from these stories that Don was jinxed or prone to accidents, but in fact, he took many other fishing trips in exotic places without incident or apparent danger. He and his family and friends made many fishing trips to Lake Makokibatan on the Albany River in Northern Ontario. Famous for walleye, this lake was the home of an Ojibway Indian family, who supplied Don with an excellent guide who became a friend and companion. The guide returned his friendship by shooting a moose and presenting him with the meat and moccasins made from its hide.
Don also made many expeditions in Florida, one involving catching a 75-pound tarpon off Key West. He also fished in the Nile, catching a giant perch (Figure 12), and more recently took a solo trout-fishing trip in Patagonia when he was in his late 80s.
Don Stookey and the Community
Don was active in community affairs beyond Corning Incorporated. He was a member of the First United Methodist Church, and in this capacity, he chaired an interfaith committee that was responsible for the development of Dayspring, a home for the elderly in the city of Corning.
When asked to spend a year as guest professor at the Ceramic Centre of Alfred University, Don gave classes that were widely attended and that gave students a vivid perspective of the career of a respected scientist and inventor in industry.
Don treated everyone with respect, regardless of social status. He was always optimistic at work and elsewhere. The only thing that seemed to bother him was a negative or pessimistic attitude. He had a great professional relationship with Amory Houghton, Jr. former Chief Executive Officer of Corning Incorporated and multi-term Congressman, and they became lifelong friends. He also met regularly with his long-time technician and friend, Joe Pierson. Both men were present at Don’s receiving of the Phoenix Award (Figure 13) and were regular visitors of Don during his retirement in Florida and in Rochester, NY, USA.

Figure 13. Don Stookey with Phoenix Award (1975) and lifetime friends: technician Joe Pierson (left) and Corning Glass Works CEO and future U.S. Congressman Amory Houghton, Jr.
Don Stookey’s Legacy
Don Stookey was a major positive force on the success of Corning Glass Works, now Corning Incorporated. The current Sullivan Park campus for research, product development, and engineering was built in the 1960s when Don’s inventions, especially Corningware® and photochromic ophthalmic glass were impacting Corning’s earnings. Management well appreciated the impact of Don’s contributions as shown by a cartoon that they presented to him (Figure 14).
Don mentored many younger scientists, including me, and his style was to lead and teach by example. He was certainly both a special friend to me and a masterly guide on how to conduct exploratory research.
Don earned many honors and awards during his distinguished career, including
1. John Price Wetherill Award to the Franklin Institute (1953 and 1962).
2. Coe College Alumni Award of Merit (1955).
3. Ross Coffin Purdy Award of the American Ceramic Society (1960).
4. Coe College Honorary D.Sc. (1963).
5. Toledo Glass and Science Award (1964).
6. Inventor of the Year Award, George Washington University (1970).
7. Award for Creative Invention of the American Chemical Society (1971).
8. Eugene Sullivan Award, Corning Section, American Chemical Society (1971).
9. Myers Achievement Award of Education Foundation in Ophthalmic Optics (1973).
10. Phoenix Award of the Glass Industry (1975).
11. Achievement Award of the Industrial Research Institute (1979).
12. Samuel Giejbeek Award of the American Ceramic Society.
13. Distinguished Inventor Award, Central New York Patent Law Association (1984).
14. Alfred University Honorary D.Sc. (1984).
15. National Medal of Technology presented by President Ronald Reagan (1986).
16. Distinguished Life Member, American Ceramic Society (1989).
17. Wilhelm Eitel Medallion for Excellence in Silicate Science (1993).
18. National Medal of Technology, White House Council (1994).
19. Inductee, National Inventors Hall of Fame (2010).
I believe that Don was most proud of the National Medal of Technology presented to him by President Reagan in 1986 (Figure 15).

Figure 15. Dr. S. Donald Stookey receiving National Medal of Technology from President Ronald Reagan (1986).
Don maintained his interest in Corning Incorporated’s research efforts during his retirement through frequent visits to Sullivan Park set up by Dr. David Morse, Corning’s current executive vice president and chief technical officer. For these occasions, Don always brought a list of ideas for glass-related research. He was the ultimate example for younger scientists to follow, and he always reacted favorably to their ideas. He continued his optimism and good spirit even until his final days.
As David Morse described him: “Don Stookey was fearless; the unknown never daunted him. He was an unassuming and quiet but tough person with an extensive depth of knowledge.”
I believe that these lines by Tennyson reflect Don’s faith and optimism:
Sunset and evening star,
And one clear call for me!
And may there be no moaning of the bar,
When I put out to sea,
For tho’ from out our bourne of Time and Place
The flood may bear me far,
I hope to see my Pilot face to face
When I have crost the bar.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication. In composing this work, the author has relied upon his knowledge of the patents and publications of Dr. S. Donald Stookey, as well as 50 years experience as his colleague and friend.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional Reading
Stookey, S. D. (1985). Journey to the Center of the Crystal Ball. Westerville, OH: The American Ceramic Society, 64.
Stookey, S. D., Beall, G. H., and Pierson, J. E. (1978). Full-color photosensitive glass. J. Appl. Phys. 10, 1978.
Keywords: Stookey, career, invention, research, glass–ceramics, nucleation, photosensitive, photochromic
Citation: Beall GH (2016) Dr. S. Donald (Don) Stookey (1915–2014): Pioneering Researcher and Adventurer. Front. Mater. 3:37. doi: 10.3389/fmats.2016.00037
Received: 26 May 2016; Accepted: 20 July 2016;
Published: 24 August 2016
Edited by:
Wolfram Höland, Ivoclar Vivadent (Liechtenstein), LiechtensteinReviewed by:
Mathieu Hubert, CelSian Glass & Solar, NetherlandsBruno Poletto Rodrigues, Otto-Schott-Institute of Materials Research, Germany
Ralf Mueller, German Institute for Materials Research and Testing, Germany
Copyright: © 2016 Beall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George H. Beall, beallgh@corning.com
 George H. Beall
George H. Beall