Prognostic value and clinical impact of 18FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy
- 1Department of Nuclear Medicine, University Hospital, Nantes, France
- 2Department of Pediatric Oncology, University Hospital, Nantes, France
- 3Department of Radiology, University Hospital, Nantes, France
- 4U892, CNRS UMR 6299, CRCNA, INSERM, Nantes, France
- 5Department of Biometrics, Cancer Center ICO René Gauducheau, Nantes, France
Objective: Burkitt lymphoma (BL) is a rare and aggressive form of B-cell lymphoma that is curable using intensive chemotherapy. Obtaining a complete response (CR) at the end of induction chemotherapy is a major prognostic factor. This study retrospectively evaluates the potential impact of 18FDG-PET in the management of children with BL after induction chemotherapy, and the prognostic performance of the Deauville criteria.
Methods: Nineteen children with BL treated according to the French LMB2001 protocol between 2005 and 2012 were included. 18FDG-PET and conventional imaging (CI) were performed after induction chemotherapy to confirm CR. 18FDG-PET was interpreted according to Deauville criteria with follow-up and/or histology as the gold standard.
Results: 18FDG-PET was negative in 15 cases, in agreement with CI in 9/15 cases. The six discordant cases confirmed to be negative by histology, were considered as true negative for 18FDG-PET. Negative predictive value (NPV) of CI and 18FDG-PET were 73 and 93%, respectively. The 5-year progression-free survival (PFS) was significantly higher in patients with negative 18FDG-PET than those with positive 18FDG-PET (p = 0.011).
Conclusion: 18FDG-PET interpreted using Deauville criteria can help confirm CR at the end of induction chemotherapy, with a prognostic impact on 5-year PFS. Its high NPV could limit the use of residual mass biopsy. Given the small size of our population, these results need to be confirmed by future prospective studies on a larger population.
Introduction
Burkitt lymphoma (BL) is a highly aggressive B-cell non-Hodgkin lymphoma (NHL). It presents in three distinct clinical forms: endemic (areas of endemic malaria and early acquisition of Epstein–Barr virus infection), sporadic, and immunodeficiency-associated (1). In Europe and North America, BL is mostly sporadic and remains rare with an annual incidence of two per million children under 18 years. Nevertheless, it is the most frequent childhood NHL (30–40%) (2). The most common site of presentation is the abdomen (60–80%), with rapidly growing tumor masses, typically in the ileocecal region (3). Therefore, presenting symptoms include acute abdominal pain, distension, nausea and vomiting, and gastrointestinal bleeding. Authors consider bone marrow involvement occurs in roughly 20% of patients (4).
With the use of intensive multiagent chemotherapy, prognosis rates of children with BL have significantly improved over the past 25 years. Recent studies have shown that chemotherapy alone is effective in low, intermediate, and advanced stage disease (5–8) with 5-year event free survival ranging from 84% in advanced stage, to 92.5% in low-stage disease. Early response to chemotherapy was identified as an important prognostic factor by major United States and European childhood cancer groups, leading to adapted treatment protocols (7–9).
Positron emission tomography using 18F-fluoro-deoxy-glucose (18FDG-PET) is a functional imaging modality widely recommended for staging of FDG-avid lymphomas (10, 11). According to Lugano recommendations (10, 11), 18FDG-PET should also be used for response assessment in all FDG-avid histologies using the five-point scale (Deauville criteria). Nevertheless, while the prognostic value of the 18FDG-PET during treatment course has been strongly demonstrated in adult Hodgkin and aggressive lymphomas (12–15), only a few studies were conducted on 18FDG-PET in children lymphomas especially in BL (16–26). These studies showed that both nodal and extranodal manifestations of BL were detectable with this molecular imaging modality and suggested that 18FDG uptake was reversible after successful treatment (24–26).
This latter issue might be of great interest considering the importance of early response to chemotherapy in the prognosis and management of children BL (7–9). The need to achieve complete response (CR) after induction chemotherapy prior to deciding on further therapies raises the problem of residual mass depicted by conventional imaging (CI) after induction chemotherapy (27). A biopsy of these residual masses is recommended by most of treatment protocols, but it is invasive and only has value if positive. Given its potential to differentiate between necrotic or fibrotic tissue and viable tumor, 18FDG-PET could be an interesting method to characterize residual masses in BL and to avoid biopsy if negative.
The aim of this retrospective study was to demonstrate the potential impact of 18FDG-PET in the management of children with BL after induction chemotherapy. We also evaluated the prognostic performance of the18FDG-PET using the Deauville criteria in this pediatric type of lymphoma.
Materials and Methods
Patients
Nineteen children, diagnosed and treated for histologically proven sporadic BL, at University Hospital of Nantes between 2005 and 2012 were included. All of them were treated according to the French LMB 2001 protocol. Eighteen children were evaluated in first-line treatment. One patient was evaluated in first-line and during two relapses.
Patients with resected stage I and abdominal stage II disease received two courses of cyclophosphamide, vincristine, prednisone, and doxorubicin (COPAD) chemotherapy. Patients with central nervous system and/or bone marrow involvement received 7-day, low-dose, prophase cyclophosphamide, vincristine, and prednisone (COP) therapy. Induction therapy consisted of two cycles of fractionated COPAD and high-dose methotrexate (HD-MTX; COPADM). Consolidation included high-dose and continuous cytarabine with etoposide (CYVE). The other children received 7-day low-dose prophase COP. Their treatment then included two cycles of COPADM, two consolidation cycles of cytarabine and HD-MTX (CYM), and concluded with one maintenance phase of COPADM.
Written and informed consent was obtained from each patient and parents. The local ethics committee approved this study.
Population characteristics are summarized in Table 1.
Conventional Imaging
Response assessment to induction chemotherapy was performed using CI as recommended in the LMB 2001 protocol. CI consisted, in addition to clinical examination, of chest X-ray, of contrast enhanced computed tomography (CT) (Sensation 16, Siemens; Light Speed VCT, GE Medical systems) and of ultrasound (US). MRI was performed for head and neck localizations or when meningeal involvement was suspected. All CI images were evaluated based on 1999 international workshop criteria (IWC) (28). Reviewing was performed in consensus by two experienced pediatric radiologists blinded to the results of other imaging studies but with knowledge of available clinical data. According to LMB 2001 protocol recommendations, only patients with CR according to 1999 IWC criteria were judged to be CI-negative after induction chemotherapy.
18FDG-PET Procedure and Analysis
In addition to the standard procedures, all children were examined with whole-body 18FDG-PET to evaluate response to induction chemotherapy. 18FDG-PET was performed after two courses of chemotherapy for stage II BL patients and after four or five courses for stage III–IV BL patients. In one patient evaluated at initial staging and during two relapses, 18FDG-PET was performed after two courses of chemotherapy in first-line and after four courses at relapses. 18FDG-PET results were not decisional in the patient management. 18FDG-PET could not be systematically performed at diagnosis because of the aggressiveness of the disease. Treatment had to be initiated rapidly and could not be delayed for imaging purposes.
Whole-body 18FDG-PET was acquired on a Discovery LS PET/CT imaging system (GE Medical Systems) 60–80 min after intravenous injection of 5–7 MBq/kg of 18FDG or on a mCT Biograph imaging system (Siemens) after intravenous injection of 3 MBq/kg of 18FDG. Children fasted at least 4 h before 18FDG injection and blood glucose was controlled prior to the injection. Images were reconstructed by OSEM iterative reconstruction algorithm (ordered-subset expectation maximization) with and without attenuation correction. All 18FDG-PET images were retrospectively reviewed on a dedicated workstation (Positoscope; Keosys, France).
18FDG-PET was interpreted visually by at least two nuclear medicine physicians with expertise in lymphoma imaging using the five-point scale (Deauville criteria), as recently recommended by Lugano’s Recommendations in Lymphoma (11). 18FDG-PET was interpreted as follows: 1 = no uptake above background, 2 = uptake equal to or lower than mediastinum, 3 = uptake between mediastinum and liver uptake, 4 = uptake moderately increased compared to the liver, and 5 = uptake markedly increased compared to the liver. Scales 1–3 were considered as 18FDG-PET negative and 4–5 as positive.
Verification of Findings
Surgical biopsy or resection was systematically performed in cases of residual mass on CI. To create a local standard of reference (SOR), all staging examinations, histopathology of biopsies and surgical specimens, and clinical data including the serial follow-up examinations were used for verification of the lesion status. Finally, the results of CI and 18FDG-PET were verified by an interdisciplinary tumor board.
Statistics
Conventional imaging and 18FDG-PET results were compared to the status of the disease determined by SOR and classified as true positive or negative, and false positive or negative allowing determination of sensitivity (Se), specificity (Sp), positive predictive value (PPV), or negative predictive value (NPV).
The end point used to evaluate prognosis impact of 18FDG-PET was progression-free survival (PFS), defined as the time of diagnosis to disease progression, relapse, or death whatever the cause. Survival curves were calculated using Kaplan and Meier analysis. Differences between groups were analyzed using the Breslow test.
Results
Nineteen children (5 female, 14 male) with histologically proven BL were included in this study. The median age was 9 years, and ranged from 2 to 17 years. All children were HIV-negative. A total of 21 18FDG-PET were performed in addition to CI to confirm remission after the induction of chemotherapy. Population follow-up and imaging results are summarized in Table 2. Ten children (52%) did not reach CR on CI after induction chemotherapy. All residual masses were either resected or biopsied before therapeutic modification.
CI and 18FDG-PET Interpretation
Conventional imaging was negative (CR without residual mass on CT) in 11 cases. In the 10 other cases, CT was positive and interpreted as partial response.
According to the gold standard, CI was considered as true negative in eight cases, false negative in three cases, true positive in one case and false positive in nine cases. The Se, Sp, PPV, and NPV of CI were, respectively, of 25, 47, 10, and 73%.
According to the Deauville criteria, 18FDG-PET was negative in 15 cases and positive in 6 cases. 18FDG-PET was in agreement with CI in 9 of the 15 negative cases. The 6 discordant cases (patients no 2, 4, 11, 13, 16, and 19) presented residual masses without significant 18FDG uptake. Resection of these lesions revealed no viable tumor, with necrosis identified on histopathological examination (Figure 1).
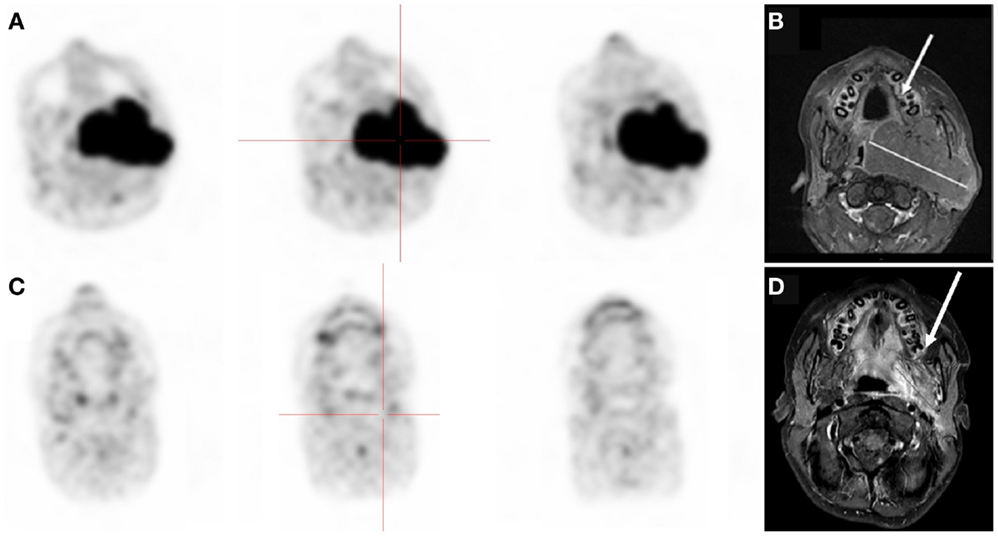
Figure 1. Thirteen-year-old boy with stage III Burkitt’s lymphoma. (A,B)18FDG-PET and MRI at diagnosis showing oropharynx tumor. (C,D) Negative 18FDG-PET but residual disease on MRI after chemotherapy. Biopsy of the residual mass was negative.
One patient (patient no 18) was considered as a complete responder after induction treatment on both 18FDG-PET and CI. This patient experienced an early relapse three months after the end of treatment. For this analysis, we decided to consider 18FDG-PET and CI results as falsely negative.
18FDG-PET was positive in six cases, in agreement with CI in four cases. In the four concordant cases, only one (patient no 17) proved to be a true positive on histopathological analysis, showing residual tumoral cells. In the two discordant cases (patients no 5 and 18 at first relapse), an early progression was confirmed by follow-up and 18FDG-PET was considered as true positive (Figure 2).
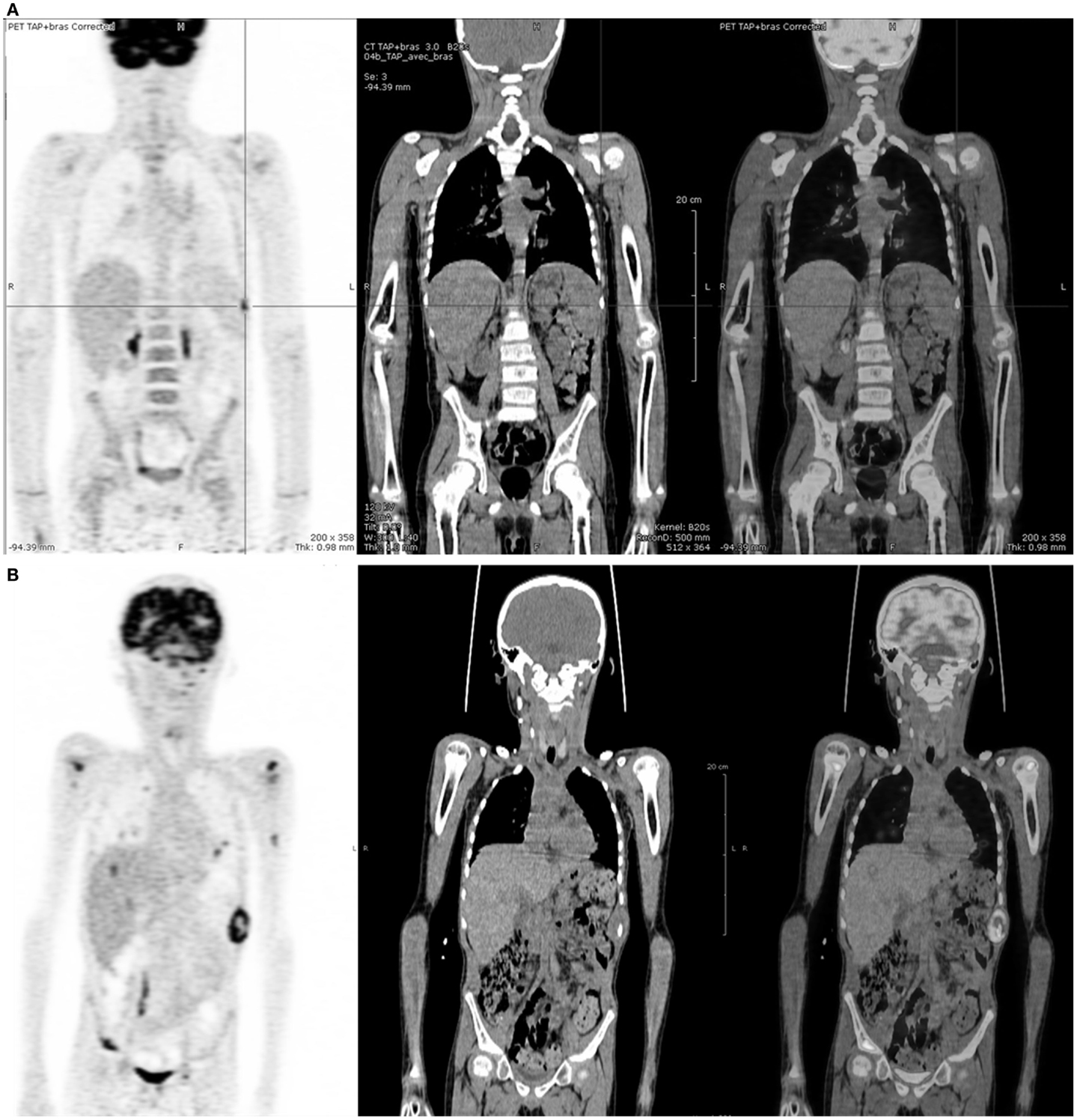
Figure 2. Five-year-old boy with stage IV Burkitt’s lymphoma at relapse. (A)18FDG-PET after induction chemotherapy showing a nodular uptake on spleen whereas CI was negative. (B)18FDG-PET 12 weeks later, showing disease progression.
According to the gold standard, 18FDG-PET was considered as true negative in 14 cases, false negative in 1 case, true positive in 3 cases, and false positive in 3 cases.
The Se, Sp, PPV, and NPV values of 18FDG-PET and CI were, respectively, 75, 82, 50, and 93 versus 25, 47, 10, and 73%. No significant difference was observed.
An overview of the diagnostic values of CI and 18FDG-PET is shown in Table 3.
Prediction of Progression-Free Survival
Median follow-up of patients was 45 months (3–100 months). Of the 18 patients, one relapsed within 3 months and two died after a median delay of 4 months due to lymphoma progression. Except for the false-negative exam outlined above (patient no 18), neither progression nor relapse was observed in patients in the 18FDG-PET negative group.
The Kaplan–Meier survival curves for 5-year PFS according to 18FDG-PET are shown in Figure 3.
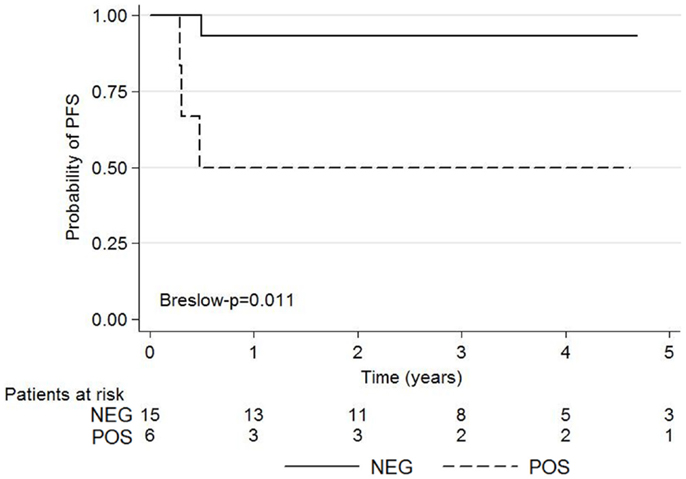
Figure 3. Kaplan–Meier survival graphs show 5-year PFS according to negative and positive post-induction chemotherapy 18FDG-PET using the Deauville criteria.
The 5-year-PFS was significantly higher among patients with negative 18FDG-PET than those with positive 18FDG-PET (p = 0.011). Ninety-three percent (14/15) of patients with score 1, 2, or 3 on Deauville criteria did not experience relapse whereas 50% (3/6) of patients with score 4–5 relapsed or died.
The Kaplan–Meyer survival curves showed no significant difference in PFS among patients with a positive or negative CI (p = 0.356) (Figure 4).
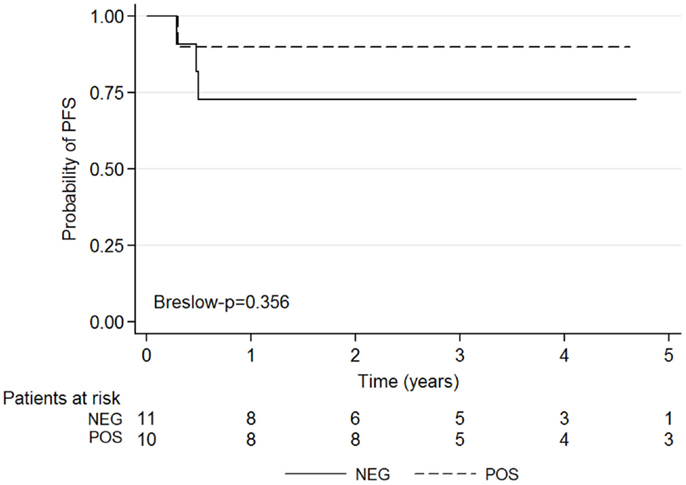
Figure 4. Kaplan–Meier survival graphs show 5-year PFS according to negative and positive post-induction chemotherapy CI results.
Discussion
The few studies previously performed on pediatric lymphomas patients (16–22) demonstrated a good NPV of 18FDG-PET performed during treatment, with values ranging from 85 to 100% suggesting biopsy can be avoided if 18FDG-PET was negative. The recent pediatric study by Furth et al. (17) was conducted on 16 pediatric NHL patients and showed an overall NPV of 85.7%, rising to 100% when considering only BL (n = 7). Similarly, studies dedicated to pediatric BL by Karantanis et al. (23) and Riad et al. (25) reported a 100% NPV of 18FDG-PET after chemotherapy despite residual masses detected on CI. More recently, Carrillo-Cruz et al. analyzed the role of 18FDG-PET at the end of treatment using Deauville criteria. In this heterogeneous study including 13 children and 19 adults and different treatment schemes, the NPV of 18FDG-PET reached 100% (27). Our results are consistent with these data and strengthen the literature on a homogeneous population of pediatric BL, in which all residual lesions were biopsied or resected. Indeed, the NPV of 18FDG-PET was higher than 90 versus 75% for CI. NPV would have reached 100% if we did not decide to classify patient no 18 as FN (relapse 3 month after chemotherapy) despite the fact that he reached CR on both 18FDG-PET and CI, meaning no residual mass was detected. Considering our patients with residual masses detected by CI at the end of induction therapy without significant 18FDG uptake, none have experienced relapse during follow-up. These results suggest that in children BL, biopsy, or surgical resection of residual lesions depicted by CI after induction chemotherapy can be avoided when 18FDG-PET is negative.
As expected, considering the nature of 18FDG, most of the available studies have shown a high false positive rate, leading to a weak PPV. In the Bakhshi et al. study (22), PPV was 41.2% with only 7 patients considered as true positive on 17 positive 18FDG-PET scans. Riad et al. (25) described false-positive 18FDG uptake in four of 28 pediatric patients with abdominal BL. In these studies, abnormal 18FDG uptake was variously defined: uptake greater than background activity in surrounding tissue (19, 23, 24), or the mediastinal blood pool activity (17) or IHP criteria (18) for reference. In our study, 18FDG-PET images were interpreted with the current consensual set of criteria recently recommended by the “Lugano recommendations” (10, 11). We only reported three false positive results related to benign inflammatory processes detected by histopathological examination. If the use of Deauville criteria slightly improves PPV compared to previously described criteria, the PPV remained poor. The PPV of CI is lower in our study than in previously published reports (18, 19, 22). This lower CI PPV can be easily explained: because surgical biopsy or resection was systematically performed in cases of residual masses on CI, only patients with CR according to 1999 IWC criteria (28) were judged to be CI-negative. In other studies, CR and/or unconfirmed complete responders (i.e., with residual masses on CI) were considered to be CI-negative. However, PPV remained poor according to each modality, and biopsy remained essential in 18FDG-PET and CI positive cases.
Recent studies in adult NHL (29) and HL (30) reported high-prognostic value of interim 18FDG-PET (after two or four courses of chemotherapy). These kinds of results were not described by any previous studies of pediatric NHL. Furth et al. (17) revealed no significant difference in PFS neither for interim CI, nor for 18FDG-PET, nor for semi-quantitative analysis using delta SUVmax in 18 children with lymphomas including 7 BL. In this study, 18FDG-PET was performed after two cycles of chemotherapy regardless of the therapeutic scheme or stage of disease, and 18FDG-PET were interpreted visually using IHP criteria. In the Bakhshi et al. study (22), response at interim 18FDG-PET or CI did not predict PFS or overall survival in 34 patients with non-lymphoblastic lymphomas including 28 B-cell lymphomas. In Carrillo-Cruz’s study (27), the Deauville criteria (score ≥4 as positive) did not allow to predict outcome accurately in a heterogeneous population of BL when quantitated at the end of treatment. On the contrary, in our study, the use of Deauville criteria (score ≥4 as positive) improved specificity and PPV and 18FDG-PET was predictive of outcome (p = 0.011) when performed in an earlier setting (after induction chemotherapy).
A very recent study on adult diffuse large B-cell lymphoma, showed that if visual analysis can be employed reliably, computation of semi-quantitative analysis (ΔSUVmax) leads to better outcome prediction and better reproducibility among observers (29) in interim 18FDG-PET (after two and/or four courses of chemotherapy). In the Carrillo-Cruz study (27), NPV reached 100% when ΔSUVmax was <66% of the initial value at the end of treatment but the prognosis value of the semi-quantitative analysis was not studied. Unfortunately, as our study was retrospective, we were unable to complete our data by semi-quantitative analysis for two reasons: unavailable baseline 18FDG-PET for some of our patients with very aggressive disease and media-storage degradation for patients included from 2005 to 2008.
Conclusion
Our study confirms that 18FDG-PET’s very high NPV could limit the use of biopsy of residual masses in sporadic pediatric BL. Our results also suggest that 18FDG-PET interpreted using Deauville criteria can help confirm early CR at the end of induction chemotherapy with prognostic impact on 5-year PFS. However, considering the poor PPV, biopsy remains essential to characterize 18FDG-PET positive residual masses. Nevertheless, given the small size of our study population and the rarity of this lymphoma, future prospective studies on a larger population of children, probably as part of a multicenter study, are highly warranted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program (2009):523–31. doi:10.1182/asheducation-2009.1.523
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt’s lymphoma. Lancet (2012) 379:1234–44. doi:10.1016/S0140-6736(11)61177-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992-2005. Pediatr Blood Cancer (2009) 53:366–70. doi:10.1002/pbc.22047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol (2012) 30:387–93. doi:10.1200/JCO.2010.33.3369
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Atra A, Imeson JD, Hobson R, Gerrard M, Hann IM, Eden OB, et al. Improved outcome in children with advanced stage B-cell non-Hodgkin’s lymphoma (B-NHL): results of the United Kingdom Children Cancer Study Group (UKCCSG) 9002 protocol. Br J Cancer (2000) 82:1396–402. doi:10.1054/bjoc.1999.1083
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Márky I, Björk O, Forestier E, Jónsson OG, Perkkiö M, Schmiegelow K, et al. Intensive chemotherapy without radiotherapy gives more than 85% event-free survival for non-Hodgkin lymphoma without central nervous involvement: a 6-year population-based study from the Nordic society of pediatric hematology and oncology. J Pediatr Hematol Oncol (2004) 26:555–60. doi:10.1097/01.mph.0000139772.98685.d2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood (2007) 109:2773–80. doi:10.1182/blood-2006-07-036673
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Pillon M, Di Tullio MT, Garaventa A, Cesaro S, Putti MC, Favre C, et al. Long-term results of the first Italian association of pediatric hematology and oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92). Cancer (2004) 101:385–94. doi:10.1002/cncr.20382
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, et al. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood (2001) 97:3370–9. doi:10.1182/blood.V97.11.3370
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol (2014) 32:3059–67. doi:10.1200/JCO.2013.54.8800
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol (2014) 32:3048–58. doi:10.1200/JCO.2013.53.5229
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med (2009) 50:527–33. doi:10.2967/jnumed.108.057703
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood (2007) 110:3507–16. doi:10.1182/blood-2007-06-097238
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood (2006) 107:52–9. doi:10.1182/blood-2005-06-2252
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Gallamini A, Fiore F, Sorasio R, Meignan M. Interim positron emission tomography scan in Hodgkin lymphoma: definitions, interpretation rules, and clinical validation. Leuk Lymphoma (2009) 50(11):1761–4. doi:10.3109/10428190903308072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Sioka C. The utility of FDG PET in diagnosis and follow-up of lymphoma in childhood. Eur J Pediatr (2013) 172:733–8. doi:10.1007/s00431-013-1993-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Furth C, Steffen IG, Erdrich AS, Hundsdoerfer P, Ruf J, Henze G, et al. Explorative analyses on the value of interim PET for prediction of response in pediatric and adolescent non-Hodgkin lymphoma patients. EJNMMI Res (2013) 3:71. doi:10.1186/2191-219X-3-71
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. London K, Cross S, Onikul E, Dalla-Pozza L, Howman-Giles R. 18F-FDG PET/CT in paediatric lymphoma: comparison with conventional imaging. Eur J Nucl Med Mol Imaging (2011) 38:274–84. doi:10.1007/s00259-010-1619-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Lopci E, Burnelli R, Ambrosini V, Nanni C, Castellucci P, Biassoni L, et al. (18)F-FDG PET in pediatric lymphomas: a comparison with conventional imaging. Cancer Biother Radiopharm (2008) 23:681–90. doi:10.1089/cbr.2007.0519
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Amthauer H, Furth C, Denecke T, Hundsdoerfer P, Voelker T, Seeger K, et al. FDG-PET in 10 children with non-Hodgkin’s lymphoma: initial experience in staging and follow-up. Klin Padiatr (2005) 217:327–33. doi:10.1055/s-2005-872517
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, et al. Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging (2010) 37:319–29. doi:10.1007/s00259-009-1276-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Bakhshi S, Radhakrishnan V, Sharma P, Kumar R, Thulkar S, Vishnubhatla S, et al. Pediatric non lymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast- enhanced CT for evaluation–a prospective study. Radiology (2012) 262:956–68. doi:10.1148/radiol.11110936
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Karantanis D, Durski JM, Lowe VJ, Nathan MA, Mullan BP, Georgiou E, et al. 18F-FDG PET and PET/CT in Burkitt’s lymphoma. Eur J Radiol (2010) 75:e68–73. doi:10.1016/j.ejrad.2009.07.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Zeng W, Lechowicz MJ, Winton E, Cho S-M, Galt JR, Halkar R. Spectrum of FDG PET/CT findings in Burkitt lymphoma. Clin Nucl Med (2009) 34:355–8. doi:10.1097/RLU.0b013e3181a34552
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Riad R, Omar W, Sidhom I, Zamzam M, Zaky I, Hafez M, et al. False-positive F-18 FDG uptake in PET/CT studies in pediatric patients with abdominal Burkitt’s lymphoma. Nucl Med Commun (2010) 31:232–8. doi:10.1097/MNM.0b013e328334fc14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Sandlund JT. Burkitt lymphoma: staging and response evaluation. Br J Haematol (2012) 156:761–5. doi:10.1111/j.1365-2141.2012.09026.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Carrillo-Cruz E, Marín-Oyaga VA, Rodríguez MS, Borrego-Dorado I, de la Cruz Vicente F, Cantero EQ, et al. Role of 18f-Fdg-Pet/Ct in the management of Burkitt lymphoma. Eur J Haematol (2014). doi:10.1111/ejh.12284
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin lymphomas. J Clin Oncol (1999) 17:1244–53.
29. Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging (2013) 40:1312–20. doi:10.1007/s00259-013-2435-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, et al. Prognostic value of interim FDG PET/CT in Hodgkin’s lymphoma patients treated with interim response-adapted strategy: comparison of international harmonization project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging (2011) 38:1064–71. doi:10.1007/s00259-011-1741-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: Burkitt lymphoma, FDG-PET, pediatric lymphoma, induction chemotherapy, Deauville criteria
Citation: Bailly C, Eugène T, Couec M-L, Strullu M, Frampas E, Campion L, Kraeber-Bodéré F and Bodet-Milin C (2014) Prognostic value and clinical impact of 18FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy. Front. Med. 1:54. doi: 10.3389/fmed.2014.00054
Received: 14 October 2014; Accepted: 02 December 2014;
Published online: 16 December 2014.
Edited by:
Otto C. Boerman, Radboud University Medical Center, NetherlandsReviewed by:
Mark Rijpkema, Radboud University Medical Center, NetherlandsShuang Liu, Purdue University, USA
Copyright: © 2014 Bailly, Eugène, Couec, Strullu, Frampas, Campion, Kraeber-Bodéré and Bodet-Milin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline Bodet-Milin, Service de Médecine Nucléaire, Hôtel Dieu, CHU de Nantes, 1 Place Alexis Ricordeau, Nantes Cedex 01 44093, France e-mail: caroline.milin@chu-nantes.fr
 Clément Bailly
Clément Bailly Thomas Eugène
Thomas Eugène Marie-Laure Couec
Marie-Laure Couec Marion Strullu
Marion Strullu Eric Frampas
Eric Frampas Loïc Campion
Loïc Campion Françoise Kraeber-Bodéré
Françoise Kraeber-Bodéré Caroline Bodet-Milin
Caroline Bodet-Milin