- 1 Department of Pathology, National Institute of Infectious Diseases, Tokyo, Japan
- 2 Military Medicine Research Unit, Japan Ground Self Defense Force, Tokyo, Japan
Kaposi’s sarcoma-associated herpesvirus (KSHV; human herpesvirus 8) is a human herpesvirus, classified as a gamma-herpesvirus. KSHV is detected in Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and some cases of multicentric Castleman’s disease (MCD). Similar to other herpes viruses, there are two phases of infection, latent and lytic. In KSHV-associated malignancies such as KS and PEL, KSHV latently infects almost all tumor cells. Quantitative PCR analysis revealed that each tumor cell contains one copy of KSHV in KS lesions. The oncogenesis by KSHV has remained unclear. Latency-associated nuclear antigen (LANA)-1 plays an important role in the pathogenesis of KSHV-associated malignancies through inhibition of apoptosis and maintenance of latency. Because all KSHV-infected cells express LANA-1, LANA-1 immunohistochemistry is a useful tool for diagnosis of KSHV infection. KSHV encodes some homologs of cellular proteins including cell-cycle regulators, cytokines, and chemokines, such as cyclin D, G-protein-coupled protein, interleukin-6, and macrophage inflammatory protein-1 and -2. These viral proteins mimic or disrupt host cytokine signals, resulting in microenvironments amenable to tumor growth. Lytic infection is frequently seen in MCD tissues, suggesting a different pathogenesis from KS and lymphoma.
Introduction
The 1994 discovery of Kaposi’s sarcoma-associated herpesvirus (KSHV, human herpesvirus 8, HHV-8) in Kaposi’s sarcoma (KS) tissues had a huge impact, not only in the field of virology, but also on bioscience generally (Chang et al., 1994; Ganem, 2005). Before the discovery of KSHV, almost all viruses had been identified using conventional virus isolation methods with cell cultures. DNA fragments of KSHV were identified in KS tissues by representational difference analysis, which is a subtraction PCR-based method to purify restriction-endonuclease-digested fragments present in one population of DNA fragments but not in others (Chang et al., 1994). Thus, KSHV is the first virus whose fragments were identified directly by the PCR method before any cell culture methods. In 1996, KSHV-infected cell lines were established, based on the fragments’ DNA sequences (Renne et al., 1996b). Herpesvirus-like particles of this virus were found in lymphoma cells by electron microscopic analysis. Finally, KSHV’s full DNA sequence was determined (Russo et al., 1996). Over the 15-years since the discovery of KSHV, it has been established as a tumor virus (Ganem, 2005). Some KSHV-encoded genes are homologous to oncogenes or cell-cycle-associated genes (Russo et al., 1996); some are transformational genes, able to transform human cells (Gao et al., 1997; Bais et al., 1998; Lee et al., 1998; Muralidhar et al., 1998). However, expression of KSHV-encoded genes is severely restricted; only a few viral genes are expressed in KSHV-infected cells. The KSHV-encoded latency-associated nuclear antigen 1 (LANA-1) is the only protein whose expression is stably detected by immunohistochemistry in KSHV-infected cells (Dupin et al., 1999; Katano et al., 2000b). LANA-1 is a multifunctional protein, but has no full transforming activity. In comparison, Epstein–Barr virus (EBV) encodes a full oncogenic protein, latent membrane protein-1 (LMP1), which is expressed in a subset of EBV-latently infected cells (Cohen, 2000). Thus, KSHV oncogenesis is not simple. Many KSHV-encoded non-transforming proteins apparently collaborate to establish and maintain oncogenesis in KSHV-infected cells. In this review, the pathological aspects of KSHV infection and KSHV-associated diseases are summarized.
Virus and Its Gene Expression
Usually, viral particles are not observed in KS samples by electron microscope because of the small number of KSHV copies. However, they can be seen in primary effusion lymphoma (PEL) cell lines stimulated by 12-O-tetradecanoylphorbol-13-acetate (TPA). A complete viral particle of KSHV, consisting of a capsid and an envelope (Renne et al., 1996b; Said et al., 1996, 1997; Orenstein et al., 1997; Ohtsuki et al., 1999), is 150–200 nm in diameter, which is similar to other human herpes viruses and indistinguishable from other herpes viruses. The unenveloped capsid is produced in the host nucleus and is 100 nm in diameter. It contains a central DNA core, which appears to have a high electron density. The envelope is derived from the inner nuclear membrane, as viral particles bud into the cytoplasm from the nucleus. The tegument protein fills the space between the nucleocapsid and envelope. This feature of viral particles is apparently quite similar among herpes viruses, but related structures forming in infected cells seem to depend on the type of virus.
The KSHV genome consists of linear, double-stranded DNA of about 170 kbp (Renne et al., 1996a; Russo et al., 1996). The KSHV genome consists of a long unique region (LUR) and a terminal repeat (TR) at both termini, which resembles the herpes virus saimiri structure (Russo et al., 1996). The TRs consist of 801-bp direct repeat units having 84.5% GC content. The number of repeats in TRs may vary. The LUR is 140.5 kbp and has 53.5% GC content. KSHV encodes more than 80 viral proteins on LUR. KSHV also encodes 17 microRNAs (miRNAs), which are derived by processing from 12 pre-miRNAs (Cai et al., 2005). Kinetics of KSHV-encoded genes were mainly investigated in KSHV-infected PEL cell lines stimulated with phorbol ester such as TPA (Sun et al., 1999). Like other herpesviruses, viral genes were categorized into lytic and latent genes, and also into immediate-early (IE), early (E), and late (L) genes based on their expressions. The function of each KSHV-encoded gene was summarized in the Table 1. Open reading frame 50 (ORF50) is an IE gene that is a homolog of Rta, a transcriptional activator encoded by EBV (Lukac et al., 1999; Seaman et al., 1999; Sun et al., 1999; Zhu et al., 1999). Transcription of ORF50 results in its expression within 4 h after stimulation by TPA. This expression could not be blocked by phosphonoacetic acid (a herpesvirus-DNA polymerase inhibitor) nor cycloheximide (a protein synthesis inhibitor). Transfection of ORF50 to KSHV-infected cells resulted in the activation of lytic gene expression (Lukac et al., 1999). Thus, ORF50 protein is a lytic switch protein. Expression of ORF50 protein is required for expression of many KSHV-encoded lytic genes such as K3, and K5 (homologs of the IE gene of BHV-4), viral interleukin-6 (vIL-6), viral macrophage inflammatory proteins (vMIPs), polyadenylated nuclear RNA (PAN), vBcl-2, K12, viral G-protein-coupled receptor (vGPCR), viral dihydrofolate reductase (vDHFR), DNA replication factors, and thymidylate synthase (Sarid et al., 1998). ORF50 protein also induces expression of K8 (K-bZIP, a positional homolog of EBV BZLF1) protein, an early protein. K8 protein plays a role as transactivation repressor for ORF50 protein, leading to a negative autoregulation system during lytic infection (Liao et al., 2003). Late genes, including tegument proteins, and virion-associated protein are then expressed (Table 1).
Latent infection is predominant in KSHV infection. KSHV codes a latency-associated gene cluster including ORF73 (LANA-1, LNA, or LNA-1), v-cyclin (ORF72), viral FLICE-inhibitory protein (K13, v-FLIP), Kaposin (K12), and viral-encoded miRNAs. LANA-1 is always detected as a dot-like staining pattern in KSHV-infected cells by immunohistochemistry. KSHV-encoded 17 miRNAs, which are derived by processing from 12 pre-miRNAs, are expressed during viral latency (Cai et al., 2005; Samols et al., 2005).
KSHV Oncogenesis
The first evidence of transformation activity by KSHV came from a report describing that human umbilical vein endothelial cells (HUVEC) were transformed and immortalized by KSHV infection in vitro (Flore et al., 1998). However, such KSHV-infected HUVEC did not express any KSHV gene, and the immortalization by KSHV infection was not confirmed by any other groups (Gao et al., 2003; Tang et al., 2003). KSHV efficiently infects primary cultures of human endothelial cells in vitro (Sakurada et al., 2001; Gao et al., 2003). KSHV-infected cells express LANA-1 within several hours after infection. One week after infection, a large portion of culture cells will be infected by KSHV and expressing LANA-1. Interestingly, expression of any lytic proteins encoded by KSHV is not observed at that time. Latent infection is dominant in KSHV-infected cells in vivo and in vitro. Although some KSHV-encoded proteins such as K1 and vGPCR are shown to have a transformation activity on mammalian cells, these transforming proteins are not usually expressed in KSHV-infected cells (Bais et al., 1998; Lee et al., 1998; Montaner et al., 2003). However, LANA-1, a major KSHV-encoded latency protein, is always expressed in KSHV-infected cells both in vivo and in vitro (Dupin et al., 1999; Katano et al., 1999b; Kellam et al., 1999). Moreover, latency is maintained during the presence of KSHV in the cells. Thus, LANA-1 clearly plays an important role in the pathogenesis of KSHV infection, and has been shown to be a multifunctional protein. Probably the most important role of LANA-1 is to establish and maintain the latency in KSHV-infected cells by tethering KSHV DNA to host chromosomes (Ballestas et al., 1999). LANA-1 binds directly to TR sequences of the KSHV genome, and recruits it to the host chromosome (Figure 1E). The DNA of KSHV is replicated during host cell divisions using host DNA replicative machinery (Sakakibara et al., 2004). Thus, daughter cells inherit KSHV genome without any virus particle. LANA-1 is also associated with signal transduction in KSHV-infected cells. LANA-1 binds directly to p53, a major tumor repressor and anti-apoptotic factor (Friborg et al., 1999). Viral infection usually induces p53 expression and p53-dependent apoptosis as self-defense system. Direct interaction with p53 by LANA-1 results in inhibition of p53-dependent apoptosis in KSHV-infected cells. Moreover, LANA-1 stabilizes β-catenin by binding to the negative regulator GSK-3β, promoting cell-cycle induction by nuclear accumulation of GSK-3β (Fujimuro et al., 2003). Thus, LANA-1 plays a central role in the pathogenesis of KSHV infection, but LANA-1 itself does not have any full transformation activity. Many other factors besides LANA-1 are required to establish KSHV oncogenesis.
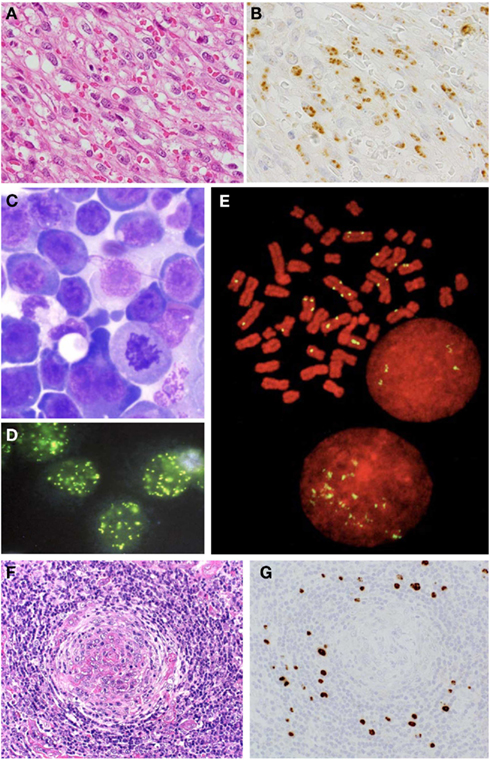
Figure 1. Histological analysis on KSHV-associated diseases. (A) Nodular stage of KS; HE staining. (B) LANA-1 immunohistochemistry of KS. (C) Giemsa staining of PEL. (D) LANA-1 immunofluorescence staining in mitosis of PEL cells. (E) LANA-1 immunostaining of mitosis of PEL cell line, TY-1. Yellow signals indicate LANA-1. Red is counter staining of chromosome. (F) HE staining of MCD. (G) LANA-1 immunohistochemistry of MCD.
Another important factor in KSHV oncogenesis is that KSHV encodes many homologs of human genes. The viral genes of human gene homologs cooperate to establish suitable growth conditions for KSHV-infected cells. Among them, vIL-6 is the most important factor for KSHV pathogenesis. vIL-6 induces angiogenesis by vascular endothelial cell growth factor (VEGF) expression (Aoki et al., 1999), and stimulates the constitutive Jak-Stat pathway through the Stat3 signal, resulting in cell growth (Aoki et al., 2003). In addition, vIL-6 represses the anti-viral function of interferon by binding to a subunit of human IL-6 receptor and suppressing p21 expression (Chatterjee et al., 2002). KSHV-encoded vMIP-1, vMIP-2, vBcl-2, vIRF-1, v-cyclin D, and v-FLIP mimic their human homologs, and work sometimes as inhibitors and sometimes as mimics, resulting in growth of KSHV-infected cells. Because almost all these mimics are lytic proteins, their expression is not usually observed. However, some cytokines may induce their expression independently to lytic and latent infection as necessary. Thus, KSHV oncogenesis is established by cooperation of many viral proteins such as LANA-1 and by the mimic, rather than the primary functions of oncogenetic transformation genes encoded by the virus.
Recently, miRNA has been shown to affect tumor biology. Several KSHV miRNAs were shown to modulate host gene expression, suggesting some roles for miRNA in the pathogenesis of KSHV-induced malignancies. Thrombospondin 1, a potent inhibitor of angiogenesis that is reportedly downregulated in KS lesions, is targeted by multiple miRNAs (Samols et al., 2007). The target of miR-K5 is Bcl2-associated factor BCLAF1, which promote apoptosis (Lei et al., 2010). MiR-K1 targets IκBα, an inhibitor of NF-κB. NF-κB inhibits the activation of lytic viral promoters. By activating NF-κB, miR-K1 suppresses viral lytic replication, maintaining latent infection (Ziegelbauer et al., 2009). So far, miRNAs’ roles in viral infection and replication remain unclear.
Epidemiology
Serological studies have revealed that KSHV-infected individuals are found all over the world. Serum antibody to KSHV is detected with ELISA using lysate of KSHV viral particles or recombinant viral proteins as antigens, or immunofluorescence assay using KSHV-infected cells. The seroprevalence of KSHV infection differs among regions/countries. Among the general population, KSHV seropositivity is less than 10% in northern Europe, America, and Asia, 10–30% in the Mediterranean region, and more than 50% in most of sub-Saharan Africa (Davis et al., 1997; Kedes et al., 1997; Chatlynne et al., 1998; Mayama et al., 1998; Rabkin et al., 1998; Katano et al., 2000a). The homosexual population exhibits higher positivity (8–25%) than the general population (Grulich et al., 2005; Casper et al., 2006; Engels et al., 2007). Although the transmission modes of KSHV have not yet been clarified, transmission though saliva is likely (Pauk et al., 2000), because high KSHV copy numbers are detected in saliva of seropositives. Horizontal transmission through the saliva transmission is suggested among children in endemic countries, while sexual transmission may be predominant among homosexual men in non-endemic countries. Organ transplantation can transmit KSHV (Regamey et al., 1998). Transmission of KSHV through blood transfusion is controversial. While KSHV seroconversion was found in US transfusion recipients (Hladik et al., 2006), later studies found no significant association of KSHV infection between transfusion groups and non-transfusion groups (Cannon et al., 2009).
Genotypes of KSHV are categorized based on sequences of the hypervariable regions in its K1 gene (Meng et al., 1999; Zong et al., 1999; Biggar et al., 2000; Kazanji et al., 2005; Hayward and Zong, 2007; Kanno et al., 2010). The KSHV K1 genes are classified into at five groups: A, B, C, D, and E (Table 2). Geographical differences in KSHV genotypes may reflect the history of migration of human populations (Zong et al., 1999). Subtypes A and C were detected in Japan and subtype A was seen more frequently in AIDS-associated cases than non-AIDS patients (Kanno et al., 2010). There is no correlation between genotype and KSHV-related disease, including KS, PEL, and multicentric Castleman’s disease (MCD).
KSHV-Related Diseases
Fragments of the KSHV genome have been detected in DNA samples extracted from various diseases by PCR. However, the only diseases whose associations with KSHV infection are widely accepted among researchers in this field are KS, PEL, and MCD (Table 3). KSHV is distributed all over the world, and there are many individuals with KSHV infections. Therefore, a low KSHV titer, as detected by PCR, does not mean that a disease is associated with KSHV infection. Because KSHV LANA-1 is always expressed in KSHV-infected cells, LANA-1 immunohistochemistry is a powerful and confirmative tool to detect KSHV-infected cells in pathological samples, and the association with KSHV infection in diseases should be examined by LANA-1 immunohistochemistry on tissue samples.
Primary KSHV Infection
A mass study of immunocompetent children in Egypt, where KSHV infection is common, suggested that a febrile maculopapular skin rash was associated with primary KSHV infection (Andreoni et al., 2002). Seroconversion for KSHV was confirmed in those patients and transmission through saliva was implied by DNA sequences in saliva. A study of homosexual men without HIV infection suggested that diarrhea, fatigue, localized skin rash, and lymphadenopathy were also symptoms of primary KSHV infection (Wang et al., 2001). Moreover, active KSHV infection may be associated with non-malignant illnesses such as fever, cutaneous rash, and hepatitis after peripheral blood stem cell/bone marrow transplantation (Luppi et al., 2000).
Kaposi's Sarcoma
Kaposi’s sarcoma is most important and common of KSHV-associated diseases. Four clinical subtypes have been recognized: classic, AIDS-associated, post-transplantational (iatrogenic or immunodeficient), and African (endemic) subtypes (Antman and Chang, 2000). These four subtypes of KS are histologically indistinguishable. In the AIDS–KS subtype, KS occurs only in homosexual men. KS occurs in the skin, oral cavity, gastrointestinal tract, lung, liver, lymph node, etc. Skin lesions of KS are most common; they are clinically classified as patchy, plaque, and nodular stages. In the patchy stage, small red flat lesions are observed on the skin. Histologically, dilated, abnormally shaped blood vessels with extravasated red blood cells and edema are found in KS lesions. In the plaque stage, patchy lesions fuse together to form plaque lesions. Proliferation of the spindle-shaped cells is seen around vessels in the plaque stage. In the final nodular stage, brown nodular, and elevated lesions are observed. Histologically, proliferation of spindle cells with slit-like vascular spaces is found (Figure 1A). Multiple KS lesions in the extremities or face are often complicated with lymphedema. Pulmonary lesions may lead to fatal respiratory compromise.
Kaposi’s sarcoma should be diagnosed with histology and immunohistochemistry. Immunohistochemical staining with anti-LANA-1 antibody shows that the viral protein is expressed in KS cells, irrespective of clinical type or disease stage (Dupin et al., 1999; Katano et al., 1999b). Expression of LANA-1 can be seen in nuclei of KS spindle cells with a speckled pattern (Figure 1B). The lymphatic marker, podoplanin (D2-40), is also expressed in KS cells (Weninger et al., 1999). In addition to histological investigation, PCR analysis is useful for the KS diagnosis. Because each KS cell contains about one copy of the KSHV genome, KSHV DNA fragments are consistently detected by PCR, even in formalin-fixed paraffin-embedded KS tissues (Asahi-Ozaki et al., 2006). PCR sometimes, but not always, detects KSHV DNA in the sera of KS patients. Serum antibody to KSHV is usually positive in KS patients.
Highly active anti-retroviral therapy (HAART) is effective on KS. Incidence of KS in HIV-infected persons has dramatically decreased in the HAART era. Regression of KS is often observed in patients administrated with HAART. In patients with low CD4 counts, KS progresses earlier than in patients with high CD4 counts. These data suggest that KS progression depends on the host’s immune status (Bower et al., 2009). Recently, patients with KS were administrated with HAART. Patients with aggressive KS received a combination therapy of HAART and chemotherapy of pegylated liposomal doxorubicin (Martin-Carbonero et al., 2008). Irradiation or surgical resection is also performed for the case of small skin lesion in addition to HAART. There is no effective anti-KSHV therapy for KS. Although vaccine is the most effective method to prevent viral diseases, no vaccine against KSHV is commercially available at present.
The pathological roles of KSHV in KS have been intensely investigated for a long time. The origin of KS cells is thought to be endothelial cells. However, cellular protein expression in KS cells is very different from those of endothelial cells. Infection by KSHV induces a dynamic alteration of gene expression in endothelial cells (Hong et al., 2004; Wang et al., 2004). Analysis via DNA array revealed that endothelial cells reduce expression of blood vascular genes and induce markers of lymphatic endothelial cells after KSHV infection in vitro. Thus, KSHV can affect the expression level of cellular proteins in endothelial cells. LANA-1 is expressed in the nucleus by almost all KS spindle shaped cells (Figure 1B), whereas the expression of lytic proteins is limited in KS lesions. Therefore, it is likely that latent infection by KSHV is important for the pathogenesis of KS. As described above, LANA-1 plays a central role in the establishment and maintenance of latency. In addition to LANA-1, cytokines are important for KS pathogenesis. Some cytokines have been detected in the sera of KS patients at high levels. It has been demonstrated that bFGF, IL-6, oncostatin M (OSM), and tumor necrosis factor (TNF)-alpha are required for growth of KS cells in vitro (Liu et al., 1997; Faris et al., 1998; Murakami-Mori et al., 1998). IL-6 is known to be an important growth factor of KS cells especially in vitro. KSHV-encoded vIL-6 interacts with the receptor of human IL-6, mimics its function partially, and contributes to immune escape mechanism by KS cells as described above. KSHV-infected cells have several immune escape mechanisms besides that of vIL-6. K5, a lytic protein of KSHV, down-regulates MHC class I and co-activation molecules, enabling productively infected cells to escape both cytotoxic T cell and NK cell responses (Ishido et al., 2000). In addition, latently infected cells are also resistant to cytotoxic T cell responses owing to reduced levels of MHC class I molecules, impaired antigen processing, and expression of the anti-apoptotic KSHV ORF-K13/viral FLICE-inhibitory protein (v-FLIP; Thome et al., 1997).
Primary Effusion Lymphoma
Primary effusion lymphoma is a rare disease occurring mainly in immunosuppressed patients, in particular HIV-infected homosexual males (Cesarman et al., 1995; Nador et al., 1996). PEL appears as lymphomatous effusions occurring in the pleural, abdominal, or pericardial effusion in the absence of a contiguous tumor mass. Some patients with PEL secondarily develop solid tumors in adjacent structures such as the pleura; these solid tumors have been termed extracavity PEL (Chadburn et al., 2004). About half of PEL patients have KS. These tumors always carry KSHV and are commonly co-infected by EBV. Histologically, the tumor cells exhibit various appearances, from large immunoblastic or plasmablastic cells to cells with more anaplastic morphology (Figure 1C). Nuclei vary from large and round to more irregular in shape, with prominent nucleoli. The cytoplasm can be abundant and is deeply basophilic with vacuoles in occasional cells. Binucleated or multinucleated cells resembling Reed–Sternberg cells can be seen. Mitotic figures are typically numerous. PEL cells are derived from post-germinal center B-cells (Jenner et al., 2003). Their immunophenotypes are undetermined, i.e., CD45 (+), CD138 (+), B-cell markers (−), T cell markers (−); however, their immunoglobulin genes are clonally rearranged and hypermutated. PEL cells contain high copy numbers (about 50 copies/cells) of KSHV DNA (Cesarman et al., 1995; Asahi-Ozaki et al., 2006). PEL cells are sometimes co-infected with EBV, while others are infected only with KSHV. However, expressions of LMPs and EBNAs are suppressed in PEL cells. Several KSHV-infected cell lines have been established from PEL cells (Carbone et al., 2010). A KSHV+/EBV− cell line, TY-1, was even established from EBV+ and KSHV+ PEL cases, suggesting that KSHV plays an essential role in the pathogenesis of PEL (Katano et al., 1999a). Infection by KSHV is predominantly latent in PEL cells, which has made PEL cell lines the most widely studied models for KSHV latency. PEL cells express latent genes coded in the latent cluster in KSHV genome (Figure 1D). However, it is not easy to detect latent viral protein expressions other than LANA-1. The expression pattern of KSHV-encoded proteins is almost the same as KS, except that PEL cell express LANA-2 protein (Rivas et al., 2001). Most PEL lines display a very small subpopulation of cells that stain for markers of lytic reactivation such as ORF50, ORF59, and K8.1 (Katano et al., 2000b). Although KSHV-encoded vIL-6 is thought as a lytic protein, vIL-6 is detected more frequently in PEL cells than other lytic proteins. It has been demonstrated that vIL-6 is a multifunctional protein; vIL-6 can bind to IL-6 receptor gp130 in the absence of another subunit of IL-6 receptor, gp80, suggesting vIL-6 can induce cytokine signals in a broader range of cell types (Chatterjee et al., 2002). The signal from gp130 often secretes human IL-6 itself, raising the possibility of an autocrine loop. vIL-6 also induces VEGF expression, resulting in an indirect proliferation effect on PEL cells (Aoki et al., 1999).
Multicentric Castleman’s Disease
Multicentric Castleman’s disease is characterized by plasmacytic lymphoadenopathy with polyclonal hyperimmunoglobulinemia and high levels of IL-6 in the serum. Histologically, follicular hyperplasia with proliferation of plasma cells and hyaline vascular alterations are observed in the lymph nodes (Figure 1F). Two distinct histopathologic subtypes have been reported; the hyaline vascular type (HV type) and the plasma cell type (PC-type). The HV type is characterized by enlarged lymphoid follicles, hyalinized germinal centers within an expanded mantle zone, and a highly vascularized interfollicular area. In contrast, in the PC-type, remarkable infiltration of plasma cells is observed in the interfollicular area. Among these mantle zone cells, there are variable numbers of the larger cells, which are approximately twice the size of mantle zone lymphocytes. These cells are characterized by a moderate amount of amphophilic cytoplasm and a large vesicular nucleus containing one or sometimes two prominent nucleoli. These cells have been called plasmablasts, although they frequently have immunoblastic features (Dupin et al., 2000). The plasmablasts are also found in the interfollicular area of PC-type MCD frequently. In some, but not all cases of MCD, KSHV is detected (Soulier et al., 1995). Using PCR, KSHV is frequently detected in tissues obtained from patients with MCD associated with HIV infection, but is very rare in MCD cases without HIV infection (Suda et al., 2001). KSHV was also detected with high frequency in MCD complicated with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome (Belec et al., 1999). Immunohistochemistry for LANA-1 revealed that KSHV-infected cells are localized in the mantle zone of lymphoid follicles (Figure 1G). Besides LANA-1, other KSHV-encoded lytic proteins such as vIL-6, K8, and K8.1 are also detected in these cells, suggesting KSHV+ MCD is associated with KSHV-lytic infection (Dupin et al., 1999; Katano et al., 2000b). The KSHV-encoded vIL-6 plays a role in the proliferation of plasma cells, and is also detected in patients’ sera at high levels, suggesting high levels of vIL-6 are associated with MCD pathogenesis (Parravicini et al., 1997). High levels of KSHV DNA are also detected in the serum, which can be a marker of progressive MCD.
Large B-Cell Lymphoma Arising in KSHV-Associated MCD
Large B-cell lymphoma arising in KSHV-associated MCD is characterized by a monoclonal proliferation of KSHV-infected lymphoid cells resembling plasmablasts expressing IgM, arising in the setting of MCD (Dupin et al., 2000; Oksenhendler et al., 2002). The small confluent sheets of LANA-1+ plasmablasts are seen in the interfollicular zone of KSHV-associated MCD. This type of lymphoma occurs in the lymph node or spleen with generalized lymphadenitis and/or massive splenomegaly. Plasmablasts show stippled nuclear staining for LANA-1 and cytoplasmic staining for vIL-6, and strongly express cIgM with λ light-chain restriction.
Conclusion
Since the discovery of KSHV, 16 years have passed. During the period, some useful diagnostic tools have been developed for pathological examination. Anti-LANA-1 antibody is the most powerful tool for diagnosis of pathological samples of KSHV infection. LANA-1 expression is specific to KSHV infection, because all KSHV-infected cells express LANA-1. Real-time PCR is also a powerful tool for diagnosis. Thus, it is not difficult to diagnose KSHV infection in pathological samples. On the other hand, the pathogenesis, and especially the oncogenesis, of KSHV remain unknown. Although many KSHV-encoded proteins have been characterized and their in vitro functions revealed, it is still not clear if KSHV can fully transform or immortalize endothelial cells. It has been shown that LANA-1 plays a central role in KSHV pathogenesis. However, LANA-1 is not enough for KSHV oncogenesis. KSHV-encoded non-transforming proteins may collaborate to establish and maintain appropriate environment for KSHV-infected cells. Further studies should reveal the mechanism of the collaboration by KSHV-encoded proteins.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ms. Yuko Sato for her excellent technical assistance. This study was partly supported by a grant for Research on Publicly Essential Drugs and Medical Devices from the Japan Health Sciences Foundation (to Harutaka Katano and Takayuki Kanno, No. SAA4832); Health and Labor Sciences Research Grants (to Harutaka Katano, No. H21-AIDS-Ippan-006, H22-AIDS-Ippan-002, H23-AIDS-Ippan-002, H21-Shinko-Ippan-009) from the Ministry of Health, Labour and Welfare; and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Harutaka Katano, No. 21590520 and 22390243).
References
Andreoni, M., Sarmati, L., Nicastri, E., El Sawaf, G., El Zalabani, M., Uccella, I., Bugarini, R., Parisi, S. G., and Rezza, G. (2002). Primary human herpesvirus 8 infection in immunocompetent children. JAMA 287, 1295–1300.
Aoki, Y., Feldman, G. M., and Tosato, G. (2003). Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101, 1535–1542.
Aoki, Y., Jaffe, E. S., Chang, Y., Jones, K., Teruya-Feldstein, J., Moore, P. S., and Tosato, G. (1999). Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93, 4034–4043.
Asahi-Ozaki, Y., Sato, Y., Kanno, T., Sata, T., and Katano, H. (2006). Quantitative analysis of Kaposi sarcoma-associated herpesvirus (KSHV) in KSHV-associated diseases. J. Infect. Dis. 193, 773–782.
Bais, C., Santomasso, B., Coso, O., Arvanitakis, L., Raaka, E. G., Gutkind, J. S., Asch, A. S., Cesarman, E., Gershengorn, M. C., and Mesri, E. A. (1998). G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391, 86–89.
Ballestas, M. E., Chatis, P. A., and Kaye, K. M. (1999). Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284, 641–644.
Belec, L., Mohamed, A. S., Authier, F. J., Hallouin, M. C., Soe, A. M., Cotigny, S., Gaulard, P., and Gherardi, R. K. (1999). Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood 93, 3643–3653.
Biggar, R. J., Whitby, D., Marshall, V., Linhares, A. C., and Black, F. (2000). Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype. J. Infect. Dis. 181, 1562–1568.
Bower, M., Weir, J., Francis, N., Newsom-Davis, T., Powles, S., Crook, T., Boffito, M., Gazzard, B., and Nelson, M. (2009). The effect of HAART in 254 consecutive patients with AIDS-related Kaposi’s sarcoma. AIDS 23, 1701–1706.
Cai, X., Lu, S., Zhang, Z., Gonzalez, C. M., Damania, B., and Cullen, B. R. (2005). Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. U.S.A. 102, 5570–5575.
Cannon, M. J., Operskalski, E. A., Mosley, J. W., Radford, K., and Dollard, S. C. (2009). Lack of evidence for human herpesvirus-8 transmission via blood transfusion in a historical US cohort. J. Infect. Dis. 199, 1592–1598.
Carbone, A., Cesarman, E., Gloghini, A., and Drexler, H. G. (2010). Understanding pathogenetic aspects and clinical presentation of primary effusion lymphoma through its derived cell lines. AIDS 24, 479–490.
Casper, C., Carrell, D., Miller, K. G., Judson, F. D., Meier, A. S., Pauk, J. S., Morrow, R. A., Corey, L., Wald, A., and Celum, C. (2006). HIV serodiscordant sex partners and the prevalence of human herpesvirus 8 infection among HIV negative men who have sex with men: baseline data from the EXPLORE study. Sex. Transm. Infect. 82, 229–235.
Cesarman, E., Chang, Y., Moore, P. S., Said, J. W., and Knowles, D. M. (1995). Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191.
Chadburn, A., Hyjek, E., Mathew, S., Cesarman, E., Said, J., and Knowles, D. M. (2004). KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am. J. Surg. Pathol. 28, 1401–1416.
Chang, Y., Cesarman, E., Pessin, M. S., Lee, F., Culpepper, J., Knowles, D. M., and Moore, P. S. (1994). Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266, 1865–1869.
Chatlynne, L. G., Lapps, W., Handy, M., Huang, Y. Q., Masood, R., Hamilton, A. S., Said, J. W., Koeffler, H. P., Kaplan, M. H., Friedman-Kien, A., Gill, P. S., Whitman, J. E., and Ablashi, D. V. (1998). Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi’s sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood 92, 53–58.
Chatterjee, M., Osborne, J., Bestetti, G., Chang, Y., and Moore, P. S. (2002). Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 298, 1432–1435.
Davis, D. A., Humphrey, R. W., Newcomb, F. M., O’Brien, T. R., Goedert, J. J., Straus, S. E., and Yarchoan, R. (1997). Detection of serum antibodies to a Kaposi’s sarcoma-associated herpesvirus-specific peptide. J. Infect. Dis. 175, 1071–1079.
Dupin, N., Diss, T. L., Kellam, P., Tulliez, M., Du, M. Q., Sicard, D., Weiss, R. A., Isaacson, P. G., and Boshoff, C. (2000). HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95, 1406–1412.
Dupin, N., Fisher, C., Kellam, P., Ariad, S., Tulliez, M., Franck, N., Van Marck, E., Salmon, D., Gorin, I., Escande, J. P., Weiss, R. A., Alitalo, K., and Boshoff, C. (1999). Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. U.S.A. 96, 4546–4551.
Engels, E. A., Atkinson, J. O., Graubard, B. I., Mcquillan, G. M., Gamache, C., Mbisa, G., Cohn, S., Whitby, D., and Goedert, J. J. (2007). Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J. Infect. Dis. 196, 199–207.
Faris, M., Ensoli, B., Kokot, N., and Nel, A. E. (1998). Inflammatory cytokines induce the expression of basic fibroblast growth factor (bFGF) isoforms required for the growth of Kaposi’s sarcoma and endothelial cells through the activation of AP-1 response elements in the bFGF promoter. AIDS 12, 19–27.
Flore, O., Rafii, S., Ely, S., O’Leary, J. J., Hyjek, E. M., and Cesarman, E. (1998). Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature 394, 588–592.
Friborg, J. Jr., Kong, W., Hottiger, M. O., and Nabel, G. J. (1999). p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402, 889–894.
Fujimuro, M., Wu, F. Y., Aprhys, C., Kajumbula, H., Young, D. B., Hayward, G. S., and Hayward, S. D. (2003). A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpesvirus latency. Nat. Med. 9, 300–306.
Ganem, D. (2005). Kaposi’s Sarcoma-Associated Herpesvirus. Philadelphia: Lippincott Williams & Wilkins.
Gao, S. J., Boshoff, C., Jayachandra, S., Weiss, R. A., Chang, Y., and Moore, P. S. (1997). KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15, 1979–1985.
Gao, S. J., Deng, J. H., and Zhou, F. C. (2003). Productive lytic replication of a recombinant Kaposi’s sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77, 9738–9749.
Grulich, A. E., Cunningham, P., Munier, M. L., Prestage, G., Amin, J., Ringland, C., Whitby, D., Kippax, S., Kaldor, J. M., and Rawlinson, W. (2005). Sexual behaviour and human herpesvirus 8 infection in homosexual men in Australia. Sex. Health 2, 13–18.
Hayward, G. S., and Zong, J. C. (2007). Modern evolutionary history of the human KSHV genome. Curr. Top. Microbiol. Immunol. 312, 1–42.
Hladik, W., Dollard, S. C., Mermin, J., Fowlkes, A. L., Downing, R., Amin, M. M., Banage, F., Nzaro, E., Kataaha, P., Dondero, T. J., Pellett, P. E., and Lackritz, E. M. (2006). Transmission of human herpesvirus 8 by blood transfusion. N. Engl. J. Med. 355, 1331–1338.
Hong, Y. K., Foreman, K., Shin, J. W., Hirakawa, S., Curry, C. L., Sage, D. R., Libermann, T., Dezube, B. J., Fingeroth, J. D., and Detmar, M. (2004). Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36, 683–685.
Ishido, S., Wang, C., Lee, B. S., Cohen, G. B., and Jung, J. U. (2000). Downregulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74, 5300–5309.
Jenner, R. G., Maillard, K., Cattini, N., Weiss, R. A., Boshoff, C., Wooster, R., and Kellam, P. (2003). Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc. Natl. Acad. Sci. U.S.A. 100, 10399–10404.
Kanno, T., Sato, Y., Nakamura, T., Sakamoto, K., Sata, T., and Katano, H. (2010). Genotypic and clinicopathological characterization of Kaposi’s sarcoma-associated herpesvirus infection in Japan. J. Med. Virol. 82, 400–406.
Katano, H., Hoshino, Y., Morishita, Y., Nakamura, T., Satoh, H., Iwamoto, A., Herndier, B., and Mori, S. (1999a). Establishing and characterizing a CD30-positive cell line harboring HHV-8 from a primary effusion lymphoma. J. Med. Virol. 58, 394–401.
Katano, H., Sato, Y., Kurata, T., Mori, S., and Sata, T. (1999b). High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi’s sarcoma. Am. J. Pathol. 155, 47–52.
Katano, H., Iwasaki, T., Baba, N., Terai, M., Mori, S., Iwamoto, A., Kurata, T., and Sata, T. (2000a). Identification of antigenic proteins encoded by human herpesvirus 8 and seroprevalence in the general population and among patients with and without Kaposi’s sarcoma. J. Virol. 74, 3478–3485.
Katano, H., Sato, Y., Kurata, T., Mori, S., and Sata, T. (2000b). Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology 269, 335–344.
Kazanji, M., Dussart, P., Duprez, R., Tortevoye, P., Pouliquen, J. F., Vandekerkhove, J., Couppie, P., Morvan, J., Talarmin, A., and Gessain, A. (2005). Serological and molecular evidence that human herpesvirus 8 is endemic among Amerindians in French Guiana. J. Infect. Dis. 192, 1525–1529.
Kedes, D. H., Ganem, D., Ameli, N., Bacchetti, P., and Greenblatt, R. (1997). The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA 277, 478–481.
Kellam, P., Bourboulia, D., Dupin, N., Shotton, C., Fisher, C., Talbot, S., Boshoff, C., and Weiss, R. A. (1999). Characterization of monoclonal antibodies raised against the latent nuclear antigen of human herpesvirus 8. J. Virol. 73, 5149–5155.
Lee, H., Veazey, R., Williams, K., Li, M., Guo, J., Neipel, F., Fleckenstein, B., Lackner, A., Desrosiers, R. C., and Jung, J. U. (1998). Deregulation of cell growth by the K1 gene of Kaposi’s sarcoma-associated herpesvirus. Nat. Med. 4, 435–440.
Lei, X., Bai, Z., Ye, F., Xie, J., Kim, C. G., Huang, Y., and Gao, S. J. (2010). Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat. Cell Biol. 12, 193–199.
Liao, W., Tang, Y., Lin, S. F., Kung, H. J., and Giam, C. Z. (2003). K-bZIP of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) binds KSHV/HHV-8 Rta and represses Rta-mediated transactivation. J. Virol. 77, 3809–3815.
Liu, Z. Y., Ganju, R. K., Wang, J. F., Ona, M. A., Hatch, W. C., Zheng, T., Avraham, S., Gill, P., and Groopman, J. E. (1997). Cytokine signaling through the novel tyrosine kinase RAFTK in Kaposi’s sarcoma cells. J. Clin. Invest. 99, 1798–1804.
Lukac, D. M., Kirshner, J. R., and Ganem, D. (1999). Transcriptional activation by the product of open reading frame 50 of Kaposi’s sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73, 9348–9361.
Luppi, M., Barozzi, P., Schulz, T. F., Trovato, R., Donelli, A., Narni, F., Sheldon, J., Marasca, R., and Torelli, G. (2000). Nonmalignant disease associated with human herpesvirus 8 reactivation in patients who have undergone autologous peripheral blood stem cell transplantation. Blood 96, 2355–2357.
Martin-Carbonero, L., Palacios, R., Valencia, E., Saballs, P., Sirera, G., Santos, I., Baldobi, F., Alegre, M., Goyenechea, A., Pedreira, J., Gonzalez Del Castillo, J., Martinez-Lacasa, J., Ocampo, A., Alsina, M., Santos, J., Podzamczer, D., and Gonzalez-Lahoz, J. (2008). Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin. Clin. Infect. Dis. 47, 410–417.
Mayama, S., Cuevas, L. E., Sheldon, J., Omar, O. H., Smith, D. H., Okong, P., Silvel, B., Hart, C. A., and Schulz, T. F. (1998). Prevalence and transmission of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int. J. Cancer 77, 817–820.
Meng, Y. X., Spira, T. J., Bhat, G. J., Birch, C. J., Druce, J. D., Edlin, B. R., Edwards, R., Gunthel, C., Newton, R., Stamey, F. R., Wood, C., and Pellett, P. E. (1999). Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology 261, 106–119.
Montaner, S., Sodhi, A., Molinolo, A., Bugge, T. H., Sawai, E. T., He, Y., Li, Y., Ray, P. E., and Gutkind, J. S. (2003). Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3, 23–36.
Murakami-Mori, K., Mori, S., and Nakamura, S. (1998). Endogenous basic fibroblast growth factor is essential for cyclin E-CDK2 activity in multiple external cytokine-induced proliferation of AIDS-associated Kaposi’s sarcoma cells: dual control of AIDS-associated Kaposi’s sarcoma cell growth and cyclin E-CDK2 activity by endogenous and external signals. J. Immunol. 161, 1694–1704.
Muralidhar, S., Pumfery, A. M., Hassani, M., Sadaie, M. R., Kishishita, M., Brady, J. N., Doniger, J., Medveczky, P., and Rosenthal, L. J. (1998). Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J. Virol. 72, 4980–4988.
Nador, R. G., Cesarman, E., Chadburn, A., Dawson, D. B., Ansari, M. Q., Sald, J., and Knowles, D. M. (1996). Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 88, 645–656.
Ohtsuki, Y., Iwata, J., Furihata, M., Takeuchi, T., Sonobe, H., and Miyoshi, I. (1999). Ultrastructure of Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus-8 (HHV-8) in a primary effusion lymphoma cell line treated with tetradecanoyl phorbol acetate (TPA). Med. Electron Microsc. 32, 94–99.
Oksenhendler, E., Boulanger, E., Galicier, L., Du, M. Q., Dupin, N., Diss, T. C., Hamoudi, R., Daniel, M. T., Agbalika, F., Boshoff, C., Clauvel, J. P., Isaacson, P. G., and Meignin, V. (2002). High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 99, 2331–2336.
Orenstein, J. M., Alkan, S., Blauvelt, A., Jeang, K. T., Weinstein, M. D., Ganem, D., and Herndier, B. (1997). Visualization of human herpesvirus type 8 in Kaposi’s sarcoma by light and transmission electron microscopy. AIDS 11, F35–F45.
Parravicini, C., Corbellino, M., Paulli, M., Magrini, U., Lazzarino, M., Moore, P. S., and Chang, Y. (1997). Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am. J. Pathol. 151, 1517–1522.
Pauk, J., Huang, M. L., Brodie, S. J., Wald, A., Koelle, D. M., Schacker, T., Celum, C., Selke, S., and Corey, L. (2000). Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343, 1369–1377.
Rabkin, C. S., Schulz, T. F., Whitby, D., Lennette, E. T., Magpantay, L. I., Chatlynne, L., and Biggar, R. J. (1998). Interassay correlation of human herpesvirus 8 serologic tests. HHV-8 Interlaboratory Collaborative Group. J. Infect. Dis. 178, 304–309.
Regamey, N., Tamm, M., Wernli, M., Witschi, A., Thiel, G., Cathomas, G., and Erb, P. (1998). Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N. Engl. J. Med. 339, 1358–1363.
Renne, R., Lagunoff, M., Zhong, W., and Ganem, D. (1996a). The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70, 8151–8154.
Renne, R., Zhong, W., Herndier, B., Mcgrath, M., Abbey, N., Kedes, D., and Ganem, D. (1996b). Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2, 342–346.
Rivas, C., Thlick, A. E., Parravicini, C., Moore, P. S., and Chang, Y. (2001). Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75, 429–438.
Russo, J. J., Bohenzky, R. A., Chien, M. C., Chen, J., Yan, M., Maddalena, D., Parry, J. P., Peruzzi, D., Edelman, I. S., Chang, Y., and Moore, P. S. (1996). Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. U.S.A. 93, 14862–14867.
Said, J. W., Chien, K., Tasaka, T., and Koeffler, H. P. (1997). Ultrastructural characterization of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) in Kaposi’s sarcoma lesions: electron microscopy permits distinction from cytomegalovirus (CMV). J. Pathol. 182, 273–281.
Said, W., Chien, K., Takeuchi, S., Tasaka, T., Asou, H., Cho, S. K., De Vos, S., Cesarman, E., Knowles, D. M., and Koeffler, H. P. (1996). Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood 87, 4937–4943.
Sakakibara, S., Ueda, K., Nishimura, K., Do, E., Ohsaki, E., Okuno, T., and Yamanishi, K. (2004). Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78, 7299–7310.
Sakurada, S., Katano, H., Sata, T., Ohkuni, H., Watanabe, T., and Mori, S. (2001). Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J. Virol. 75, 7717–7722.
Samols, M. A., Hu, J., Skalsky, R. L., and Renne, R. (2005). Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 79, 9301–9305.
Samols, M. A., Skalsky, R. L., Maldonado, A. M., Riva, A., Lopez, M. C., Baker, H. V., and Renne, R. (2007). Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 3, e65. doi: 10.1371/journal.ppat.0030065
Sarid, R., Flore, O., Bohenzky, R. A., Chang, Y., and Moore, P. S. (1998). Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72, 1005–1012.
Seaman, W. T., Ye, D., Wang, R. X., Hale, E. E., Weisse, M., and Quinlivan, E. B. (1999). Gene expression from the ORF50/K8 region of Kaposi’s sarcoma-associated herpesvirus. Virology 263, 436–449.
Soulier, J., Grollet, L., Oksenhendler, E., Cacoub, P., Cazals-Hatem, D., Babinet, P., D’Agay, M. F., Clauvel, J. P., Raphael, M., Degos, L., and Sigaux, F. (1995). Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86, 1276–1280.
Suda, T., Katano, H., Delsol, G., Kakiuchi, C., Nakamura, T., Shiota, M., Sata, T., Higashihara, M., and Mori, S. (2001). HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman’s disease. Pathol. Int. 51, 671–679.
Sun, R., Lin, S. F., Staskus, K., Gradoville, L., Grogan, E., Haase, A., and Miller, G. (1999). Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 73, 2232–2242.
Tang, J., Gordon, G. M., Muller, M. G., Dahiya, M., and Foreman, K. E. (2003). Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77, 5975–5984.
Thome, M., Schneider, P., Hofmann, K., Fickenscher, H., Meinl, E., Neipel, F., Mattmann, C., Burns, K., Bodmer, J. L., Schroter, M., Scaffidi, C., Krammer, P. H., Peter, M. E., and Tschopp, J. (1997). Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386, 517–521.
Wang, H. W., Trotter, M. W., Lagos, D., Bourboulia, D., Henderson, S., Makinen, T., Elliman, S., Flanagan, A. M., Alitalo, K., and Boshoff, C. (2004). Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36, 687–693.
Wang, Q. J., Jenkins, F. J., Jacobson, L. P., Kingsley, L. A., Day, R. D., Zhang, Z. W., Meng, Y. X., Pellett, P. E., Kousoulas, K. G., Baghian, A., and Rinaldo, C. R. Jr. (2001). Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins. Blood 97, 2366–2373.
Weninger, W., Partanen, T. A., Breiteneder-Geleff, S., Mayer, C., Kowalski, H., Mildner, M., Pammer, J., Sturzl, M., Kerjaschki, D., Alitalo, K., and Tschachler, E. (1999). Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab. Invest. 79, 243–251.
Zhu, F. X., Cusano, T., and Yuan, Y. (1999). Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 73, 5556–5567.
Ziegelbauer, J. M., Sullivan, C. S., and Ganem, D. (2009). Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat. Genet. 41, 130–134.
Zong, J. C., Ciufo, D. M., Alcendor, D. J., Wan, X., Nicholas, J., Browning, P. J., Rady, P. L., Tyring, S. K., Orenstein, J. M., Rabkin, C. S., Su, I. J., Powell, K. F., Croxson, M., Foreman, K. E., Nickoloff, B. J., Alkan, S., and Hayward, G. S. (1999). High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi’s sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73, 4156–4170.
Keywords: Kaposi’s sarcoma-associated herpesvirus, HHV-8, latency-associated nuclear antigen, LANA-1, primary effusion lymphoma
Citation: Fukumoto H, Kanno T, Hasegawa H and Katano H (2011) Pathology of Kaposi’s sarcoma-associated herpesvirus infection. Front. Microbio. 2:175. doi: 10.3389/fmicb.2011.00175
Received: 30 June 2011; Paper pending published: 21 July 2011;
Accepted: 09 August 2011; Published online: 25 August 2011.
Edited by:
Keiji Ueda, Osaka University Graduate School of Medicine, JapanReviewed by:
Hiroki Isomura, Aichi Cancer Center Research Institute, JapanKeiji Ueda, Osaka University Graduate School of Medicine, Japan
Copyright: © 2011 Fukumoto, Kanno, Hasegawa and Katano. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Harutaka Katano, Department of Pathology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan. e-mail: katano@nih.go.jp
 Hitomi Fukumoto1,2
Hitomi Fukumoto1,2