- 1 Department of Food Science, Faculty of Agricultural Science, University of Foggia, Foggia, Italy
- 2 Food Quality and Health Research Center, University of Foggia, Foggia, Italy
Essential oils (EOs) are promising and friendly antimicrobials for the prolongation of the shelf life of many foods. They have been extensively used to inhibit spoiling and pathogenic microorganisms of many kinds of products like fruit juices and acidic drinks. Therefore, they could be used successfully to control the germination of spores of Alicyclobacillus acidoterrestris, that finds in these products an optimal environment for growth. This paper reports a brief overview of the literature available, focusing on the effects of EOs toward alicyclobacilli.
Essential Oils: Definition and Perspectives
Definition and Composition
Essential oils (EOs), also called ethereal or volatile oils, are complex mixture extracted from various part of plants (Burt, 2004; Speranza and Corbo, 2010). Apart from a great variability amongst the different oils, EOs are soluble in lipids (i.e., are oils) and possess some compounds, acting as antimicrobials, perfumes, or flavoring (i.e., they contain an essentia, as suggested by Paracelsus von Hohenheim in sixteenth century; Speranza and Corbo, 2010). Actually, ca. 3.000 different EOs are known, but only few hundreds are commonly used as food-grade additives and for the production of soap, perfumes, and cosmetics (Burt, 2004; Speranza and Corbo, 2010).
Essential oils can be produced by distillation, expression, enfleurage, and fermentation, being the hydro-distillation the most used system, and virtually they can be extracted form every part of a plant, i.e., from leaves (e.g., basil, oregano, eucalyptus, lemon grass, rosemary, pepper mint), from flowers (orange, rose, marjoram, clove), peel (lemon, bergamot, orange, tangerine), seeds (anise, cucumin).
Most of the commercially distributed EOs have been studied and characterized and the results published by some International Organizations (European Pharmacopoeia, International Organization for Standardization, World Health Organization, Council of Europe). Chemically, EOs are very complex mixture of 60 or more molecules; however, they contain one or more compounds at high levels and the others in trace. The major component of an EOs is usually labeled “active compound” (Speranza and Corbo, 2010).
Generally active compounds possess a phenolic structure, responsible for the bioactivity of EOs; however, there are some examples of not phenolic compounds, like hexanal.
Bioactivity
There many literature reports on the use of EOs as antimicrobials, both in laboratory media and in foods (Burt, 2004; Bevilacqua et al., 2008c; Speranza and Corbo, 2010); due to the extreme variability in chemical structure, composition, and source, different modes of action have been postulated (Burt, 2004; Speranza and Corbo, 2010):
(1) Due to their solubility in lipids, EOs pass through the cell wall and disrupt membrane, causing its permeabilization, the loss of ions and the reduction of membrane potential, the collapse of the proton pump, and the depletion of the ATP pool (Dorman and Deans, 2000; Walsh et al., 2003).
(2) EOs can coagulate the cytoplasm and cause damages to lipids and proteins (Jerkovic et al., 2001).
(3) In eukaryotic cells, EOs provoke depolarization of the mitochondrial membranes by decreasing the membrane potential, influence ionic Ca++ cycling and other ionic channels, and reduce the pH gradient, affecting (as in bacteria) the proton pump and the ATP pool. (Armstrong, 2006).
What are the most susceptible microorganisms to EOs? Due to the extreme variability of compounds and the various methods proposed in the literature for the evaluation of their antimicrobial potential toward a wide range of microorganisms, it is not possible to build a hierarchy/susceptibility scale; however, following the approach by Speranza and Corbo (2010), it is possible to find a general trend, i.e.:
(1) Gram negative bacteria appear more resistant to EOs than Gram positive ones. This greater resistance could be attributed to the outer membrane surrounding the cell wall, which restricts diffusion of hydrophobic compounds (Speranza and Corbo, 2010).
(2) Among Gram positive bacteria, lactic acid bacteria (LAB) are the most resistant. This resistance was attributed to ATP generation by substrate level phosphorylation (Speranza and Corbo, 2010).
(3) Among the Gram negative bacteria, pseudomonads show high resistance to these antimicrobials (Careaga et al., 2003; Galindo-Cuspinera et al., 2003).
(4) EOs are generally more active against yeasts than they are against Gram positive bacteria (Pauli, 2006; Bevilacqua et al., 2010d).
Essential Oils as Food-Grade Additives
Many EOs and their active compounds are in the list EAFUS (everything added to food in the United States) of the Food and Drug Administration [FDA; Center for Food Safety and Applied Nutrition (CFSAN), 2011; www.cfsan.fda.gov]. As an example, the status of thymol can be reported: thyme EO and thyme are considered generally recognized as safe (21 CFR 180.10 and 180.20), while thymol is labeled as a synthetic food additive (21 CFR 172.515). According to Annex II of Council Regulation 2377/90, the Committee of Experts on Flavoring Substances of the European Commission (EC) has registered thymol in the flavorings in foodstuff list. The same status has been recognized for carvacrol, carvone, cinnamaldehyde, citral, p-cymene, eugenol, limonene, and menthol (Speranza and Corbo, 2010).
Why Use Essential Oils to Inhibit Alicyclobacillus spp.
The use of EOs for the inhibition and/or the control of spore germination was postulated firstly by Chaibi et al. (1997), in the light of a renewed interest in 1990s toward natural antimicrobials and low-impact preservation technologies. Although it is not clear how EOs damage spores, the use of spices, and plant extract was proposed for the inhibition of different spore-former microorganisms (Burt, 2004; Tajkarami et al., 2010). For example, it is well known that many oils are effective against Bacillus spp. and Sofia et al. (2007), based on the results of a disk diffusion assay, proposed the following bioactivity scale (from the strongest oil to the less effective one): clove > mustard > cinnamon > garlic > ginger > mint. Other authors used EOs against Clostridium spp.: Davidson and Naidu (2000) reported the bioactivity of clove oil toward C. perfringens, while Friedman (2007) proposed the extract of black tea for the inactivation of the neurotoxin of C. botulinum. Both Davidson and Naidu (2000) and Friedman (2007) referred to antimicrobial assays performed through different protocols (disk diffusion assay, micro-dilution method).
From these studies to the application of EOs against an emerging spoiling microorganism (Alicyclobacillus acidoterrestris) there is a short distance. Alicyclobacillus spp. have been recognized as an increasing threat in food industry, due to its thermo-resistance and thermo-acidophilic behavior (Bevilacqua et al., 2008c). Silva and Gibbs (2004) proposed A. acidoterrestris as a target to design thermal treatments for fruit juices, since it appeared more heat resistant than other spoiling microorganisms. Heat resistance of spores is greatly variable and relies upon various elements (the strain, the pH and kind of medium, the conditions attained throughout sporulation); Silva and Gibbs (2004) reported that D-value of spores at 95°C in juices could range from 0.06 to 5.3 min and z-value varied from 7.2 to 12.9°C.
Apart from strain variability, it has been reported that alicyclobacilli can survive thermal treatments used by juice producers (90–95°C from second to minutes); a possibility could be the use of a more severe processing, thus affecting the sensorial quality of juices, in terms of color, odor, and nutrient content (Bevilacqua and Corbo, 2010a).
A second possibility could be the use of a friendly approach, i.e., a chemical or a physical treatment able to inactivate spore without or with low effects on the sensorial quality of juices. This approach, based on the green consumerism philosophy (Bevilacqua et al., 2010b), has been proposed successfully for alicyclobacilli, through the use of high pressure processing and high pressure homogenization, ultrasound, microwave an natural antimicrobials (Bevilacqua et al., 2008c).
The use of natural antimicrobials (e.g., lysozyme, nisin, other bacteriocins, and essential oils) has gained an increased interest for the low-impact of these compounds on human health and environment. Essential oils appear as a promising way, as many of them are extracted from fruits and their use in fruit-based products can be friendly for the characteristics of raw material.
Use of Essential Oils Against Alicyclobacillus acidoterrestris: Elements Acting on Their Bioactivity
Despite the increased and renewed interest toward the antimicrobial activity of EOs, only few papers focused on their effectiveness against alicyclobacilli, namely against A. acidoterrestris. The first report was by Takahashi et al. (2004), who proposed the use of leaf extracts and flavonoids from Eucalyptus spp. and found a minimum inhibitory concentration of extracts ranging from 7.8 g/l (E. globosa) to 31 g/l (E. saligna). Then, this topic was addressed exclusively by the research group of Applied Microbiology of University of Foggia; therefore, in the following sheets there is a brief overview of their results, with the aim to give an insight into EOs effect along with the factors that can enhance or limit their bioactivity.
Chemical Structure and Secondary Groups
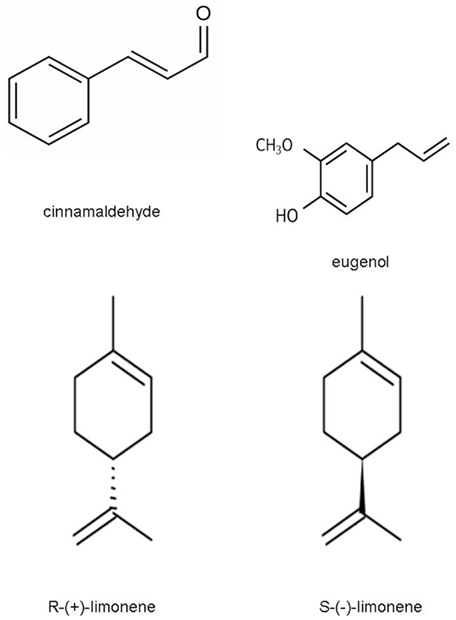
Bevilacqua et al. (2008a) used cinnamaldehyde, eugenol, and limonene (0.05–0.5 g/l; Figure 1 for the chemical structure) against the spores of two different strains of A. acidoterrestris (c8 and Γ4, isolated respectively form soil and spoiled pear juice; Sinigaglia et al., 2003; Bevilacqua et al., 2006) and modeled the data as inhibition index, i.e., as percentage of absorbance at 420 nm referred to the control. Cinnamaldehyde was the most effective compound and a concentration of 500 ppm inhibited completely spore germination for 13 days; otherwise a lower amount of this compound (100 ppm), inhibited the microbial target by 96–97 and 58–70% after 8 and 13 days, respectively.
Eugenol appeared as less effective than cinnamaldehyde, as it inhibited significantly spore germination only at the highest amounts (500 ppm). The stronger effect of cinnamaldehyde was later confirmed by Bevilacqua et al. (2010c), who combined cinnamaldehyde and eugenol to inhibit and/or control the germination of a cocktail of A. acidoterrestris strains and found that 160 ppm of eugenol prolonged the lag phase of the microbial target by 1.5 days, whereas this parameter increased by 4–4.5 days with 80 ppm of cinnamaldehyde added. Bevilacqua et al. (2008a) studied also the bioactivity of limonene, but this oil was not effective in inhibiting spore germination.
The antimicrobial activity of the natural compounds seems to be related to the phenolic rings, but the type of alkyl group has been found to influence the antibacterial effectiveness (Burt, 2004). The results of Bevilacqua et al. (2008a) confirmed this idea: in fact, the phenolic ring is the major part of all the compounds (limonene, eugenol, and cinnamaldehyde). The difference amongst the various antimicrobials is the secondary group, linked to phenol.
Source of the Oil
The composition of EOs is quite variable and depends upon the climate, the location of plants, as well as the method of oil extraction; moreover, the extraction of an oil from different parts of the same plant could result in a strong variability and in a different bioactivity (Burt, 2004).
Bevilacqua et al. (data not published) studied the antimicrobial activity of three essential oils, extracted from various part of citrus and/or lemon, i.e., neroli, lemon extract, and biocitro (a complex citrus extract); the bioactivity of this compound was tested against two different strains of A. acidoterrestris: the isolate DSMZ 2498, purchased from a Public Collection, and the wild strain c8, isolated from soil (Bevilacqua et al., 2006).
Neroli (extracted from Citrus aurantium var. amara or Bigaradia) is a plant oil similar in scent to bergamot; it is produced from the blossom of the bitter orange tree and contains α-pinene, camphene, β-pinene, α-terpinene, neryl acetate, farnesol, geraniol, linalool, nerolidol, linalyl acetate, methyl anthranilate, and indole.
BiocitroLIQUID® is a complex oil purchased from Quinabra (Probena, Spain) and extracted from citrus; the producer reports the following composition for the oil: ascorbic acid and ascorbates (vitamin C), linked with citrus bioflavonoids, 4.0–7.20%; hydrated glycerin linked with other traces of citrus polyphenols, carbohydrates, bio-flavoproteins, pectin, citrus sugars, citric acid, 30.80–36.60%; water, 6.00–11.00%; stabilizer and inert carrier, 50.00%. Chemical analyses revealed that the concentration of ascorbic acid and citrus bioflavonoid is ca. 56,000 ppm; amongst the bioflavonoids, naringin is 6,500 ppm minimum. Finally, the content of limonene is 30,000–50,000 ppm (Bevilacqua et al., 2010d).
Lemon extract (or oil) shows a quite variable composition. Generally it is extracted from the fresh fruit peel by cold expression. The main chemical components of lemon oil are α-pinene, camphene, β-pinene, sabinene, myrcene, α-terpinene, linalool, β-bisabolene, limonene, trans-β-bergamotene, nerol, and neral. This extract was used by several authors (Conte et al., 2007, 2009) as a natural antimicrobials toward the spoiling microflora of fruit and vegetables, showing some promising properties.
Neroli, lemon extract, and citrus extract were used to inhibit the spores of different strains of A. acidoterrestris and a micro-dilution method in a laboratory medium (Malt Extract broth, acidified to pH 4.5), combined with the traditional plate count, was used to point out oil effectiveness (Bevilacqua et al., data not published). Neroli was able to inhibit spore germination only at high concentrations (>500 ppm), not compatible with foods, as the odor of this extract was too strong; on the other hand both biocitro (citrus extract) and lemon extract showed some interesting results, as they were able to reduce spore concentration by 1–2 log cfu/ml after 1 day at 20–80 ppm; this variability in MIC value relied upon the strains, as some isolate appeared more resistant than others.
Besides being effective against A. acidoterrestris, Biocitro was also active against spoiling microflora of juices (Bacillus coagulans, Lactobacillus brevis and L. plantarum, Saccharomyces bayanus, Pichia membranifaciens, and Rhodotorula bacarum) as reported by Bevilacqua et al. (2010d).
Why the oils showed a different bioactivity? Many and different ideas could be suggested; however, the main difference between neroli and biocitro/lemon extract is the source: neroli is generally extracted from flowers of orange tree, otherwise the source of citrus and lemon extract is citrus peel. Thus it could suggested that both the kind of plant and oil source (i.e., part of the plan from which the oil is extracted) play a fundamental role for the antimicrobial activity, as reported also by Speranza and Corbo (2010).
PH of the Medium
A factor playing a fundamental role in the bioactivity of EOs is the pH of the medium; this idea was supported by some papers (Bevilacqua et al., 2008b, 2009b, 2010c,e), addressing this topic and reporting the combinations of cinnamaldehyde (the active compound of cinnamon oil) and eugenol (the main component of clove oil), both in laboratory media and in juices. The use of acidic pH enhanced the effect of active compounds, due to its effect on the partition equilibrium and hydrophobicity; it is generally accepted that EOs constituents are most effective at acidic pHs (Burt, 2004; Ait-Ouazzou et al., 2011); however, that conclusion is mainly based on studies carried out to evaluate their capacity to prevent microbial growth. Regarding bactericidal activity, results varied among studies: Friedman et al. (2004) did not find any influence of acidity when evaluating carvacrol or linalool in apple juice, while Rivas et al. (2010) recovered a higher susceptibility of E. coli to carvacrol or thymol when decreasing the pH to 4. The trend experienced by A. acidoterrestris seemed to confirm this effect, although more data are required to postulate a general hypothesis.
Combination of EOs with Physical Hurdles
A promising way for the inhibition of alicyclobacilli is the use of the combined approach, i.e., a multi-target preservation based on the theory of multiple hurdles (Bevilacqua et al., 2008c). For example, it is possible to combine cinnamaldehyde with a heat treatment: the combination of the antimicrobial at 97 ppm with a thermal treatment at 90°C for 8 min, conducted at pH 4.3, assured the maximal inactivation of A. acidoterrestris in Malt Extract broth (Bevilacqua et al., 2010e). This effect was validated in tomato juice, thus finding that it is possible to control spore germination in juice by mean of a treatment at 90°C for 4 min with 120 ppm of cinnamaldehyde added or through a heat shock at the same temperature for 12 min by reducing the active compound at 40 ppm.
An interesting effect clearly revealed by the cited authors is that it is not convenient to increase processing temperature indefinitely, because a thermal degradation of cinnamaldehyde could occur at a thermal treatment of 12 min/90°C.
Strain Variability
Finally, a major role in the bioactivity of EOs is played by strain variability; this effect was postulated by Bevilacqua et al. (2008a) and Bevilacqua et al. (2009a), with reference to the bioactivity of cinnamaldehyde and eugenol alone or combined with a thermal treatment or homogenization, respectively. This effect was related to a phenomenon due to the isolation source (Bevilacqua et al., 2008a) and to a probable difference in the hydrophobic behavior of the strains (i.e., the different distribution of the fatty acids in the membrane). The more hydrophobic an organism behaves, the more sensitive it will be to a hydrophobic antimicrobial agents, as the active compound of EOs. This idea, however, is only a speculation and needs to be confirmed by molecular and chemical analyses on the genes involved in the susceptibility to EOs constituents and fatty acids distribution in the membrane.
Conclusion and Future Perspectives
The use of EOs against A. acidoterrestris appears as a promising way; however, the research is at the beginning. The reports available in the literature show some interesting directions, but further investigations are required, in order to understand some critical issues:
(1) the real effect of EOs against spore;
(2) the influence of the source of oil and strain variability on the bioactivity of EOs and their active compounds;
(3) the impact of EOs on food shelf life for a prolonged contact time (i.e., months or years);
(4) the ability of EOs to control post-contamination and spore recovery;
(5) the existence of additive effects with some physical hurdles;
(6) the correlation of the sensitivity toward EOs with the membrane.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ait-Ouazzou, A., Cherrat, L., Espina, L., Loràan, S., Rota, C., and Pagàn, R. (2011). The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Trends Food Sci. Technol. 12, 320–329.
Armstrong, J. S. (2006). Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays 28, 253–260.
Bevilacqua, A., and Corbo, M. R. (2010a). Characterization of a wild strain of Alicyclobacillus acidoterrestris: heat resistance and implications for tomato juice. J. Food Sci. 76, M130–M136.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2010b). “Green consumerism and alternative approaches for food preservation: an introduction,” in Application of Alternative Food-Preservation Technologies to Enhance Food Safety & Stability, eds A. Bevilacqua, M. R. Corbo, and M. Sinigaglia (Sharjah: Bentham Publisher), 1–3.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2010c). Combining eugenol and cinnamaldehyde to control the growth of Alicyclobacillus acidoterrestris. Food Control 21, 172–177.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2010d). In vitro evaluation of the antimicrobial activity of eugenol, limonene and citrus extract against bacteria and yeasts, representative of the spoiling microflora of fruit juices. J. Food Prot. 73, 888–894.
Bevilacqua, A., Sinigaglia, M., and Corbo, M. R. (2010e). Use of the surface response methodology and desirability approach to model Alicyclobacillus acidoterrestris spore inactivation. Int. J. Food Sci. Technol. 45, 1219–1227.
Bevilacqua, A., Corbo, M. R., D’Amato, D., Campaniello, D., and Sinigaglia, M. (2006). “Caratterizzazione fenotipica di ceppi di Alicyclobacillus spp. isolati da suolo,” in Ricerche e innovazioni nell’industria alimentare, Vol. VII Atti del VI Congresso Italiano di Scienza e Tecnologia degli Alimenti (Cernobbio-CO-19-20 settembre 2005), ed. S. Porretta (Pinerolo, TO: Chiriotti Editori), 1201–1205.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2008a). Inhibition of Alicyclobacillus acidoterrestris spores by natural compounds. Int. J. Food Sci. Technol. 43, 1271–1275.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2008b). Combined effects of low pH and cinnamaldehyde on the inhibition of Alicyclobacillus acidoterrestris spores in a laboratory medium. J. Food Process. Preserv. 32, 839–852.
Bevilacqua, A., Sinigaglia, M., and Corbo, M. R. (2008c). Alicyclobacillus acidoterrestris: new methods for inhibiting spore germination. Int. J. Food Microbiol. 125, 103–110.
Bevilacqua, A., Corbo, M. R., and Sinigaglia, M. (2009a). “Use of high-pressure homogenization, sodium benzoate and eugenol for the inhibition of Alicyclobacillus acidoterrestris spores,” in Spore-Forming Bacteria in Food, eds D. Sohier, and I. Leguerinel (Quimper, FR: ADRIA Development), 144–146.
Bevilacqua, A., Sinigaglia, M., and Corbo, M. R. (2009b). Effects of pH, cinnamaldehyde and heat-treatment time on spore viability of Alicyclobacillus acidoterrestris. Int. J. Food Sci. Technol. 44, 380–385.
Burt, S. (2004). Essential oils and their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94, 223–253.
Careaga, M., Fernandez, E., Dorantes, L., Mota, L., Jaramillo, M. E., and Hernandez-Sanchez, Z. H. (2003). Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol. 83, 331–335.
Center for Food Safety and Applied Nutrition (CFSAN). (2011). Everything Added to Food in the United States (EAFUS) database that the Food and Drug Administration (FDA) Approved as Food Additives or Affirmed as Generally Recognized As Safe (GRAS). Available at: http://www.accessdata. fda.gov/scripts/fcn/fcnNavigation. cfm?rpt=eafusListing [accessed August 3, 2011].
Chaibi, A., Ababouch, L. H., Belasri, K., Boucetta, S., and Busta, F. F. (1997). Inhibition of germination and vegetative growth of Bacillus cereus T and Clostridium botulinum 62A spores by essential oils. Food Microbiol. 14, 161–174.
Conte, A., Scrocco, C., Sinigaglia, M., and Del Nobile, M. A. (2007). Innovative active packaging systems to prolong the shelf life of mozzarella cheese. J. Dairy Sci. 90, 2126–2131.
Conte, A., Scrocco, C., Sinigaglia, M., and Del Nobile, M. A. (2009). Lemon extract as natural preservative in fruit salad. J. Food Saf. 29, 601–616.
Davidson, H. J. D., and Naidu, A. S. (2000). “Phyto-phenols,” in Natural Food Antimicrobial Systems, ed. A. S. Naidu (Boca Raton, FL: CRC Press), 265–294.
Dorman, H. J. D., and Deans, S. G. (2000). Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88, 308–316.
Friedman, M. (2007). Overview of anti bacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 51, 116–134.
Friedman, M., Henika, P. R., Levin, C. E., and Mandrell, R. E. (2004). Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 52, 6042–6048.
Galindo-Cuspinera, V., Westhoff, D. C., and Rankin, S. A. (2003). Antimicrobial properties of commercial annatto extracts against selected pathogenic, lactic acid, and spoilage organisms. J. Food Prot. 66, 1074–1078.
Jerkovic, I., Mastelic, J., and Milos, M. (2001). The impact of both the season of collection and drying on the volatile constituents of Origanum vulgare L. ssp. hirtum grown wild in Croatia. Int. J. Food Sci. Technol. 36, 649–654.
Pauli, A. (2006). Anticandidal low molecular compounds from higher plants with special reference to compounds from essential oils. Med. Res. Rev. 26, 223–268.
Rivas, L., McDonnell, M. J., Burgess, C. M., O’Brien, M., Navarro-Villa, A., Fanning, S., and Duffy, G. (2010). Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol. 139, 70–78.
Silva, F. V. M., and Gibbs, P. (2004). Target selection in designing pasteurization processes for shelf-stable high-acid fruit products. Crit. Rev. Food Sci. Nutr. 44, 353–360.
Sinigaglia, M., Corbo, M. R., Altieri, C., Campaniello, D., D’Amato, D., and Bevilacqua, A. (2003). Combined effects of temperature, water activity and pH on Alicyclobacillus acidoterrestris spores. J. Food Prot. 66, 2216–2221.
Sofia, P. K., Prasad, R., Vijay, V. K., and Srivastava, A. K. (2007). Evaluation of antibacterial activity of Indian spices against common food borne pathogens. Int. J. Food Sci. Technol. 42, 910–915.
Speranza, B., and Corbo, M. R. (2010). “Essential oils for preserving perishable foods: possibilities and limitations,” in Application of Alternative Food-Preservation Technologies to Enhance Food Safety & Stability, eds A. Bevilacqua, M. R. Corbo, and M. Sinigaglia (Sharjah: Bentham Publisher), 35–57.
Tajkarami, M. M., Ibrahim, S. A., and Cliver, D. O. (2010). Antimicrobial herb and spice compounds in food. Food Control 21, 1199–1218.
Takahashi, T., Kokubo, R., and Sakaino, M. (2004). Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 39, 60–64.
Keywords: Alicyclobacillus acidoterrestris, essential oils, spore inhibition, active compounds, friendly compounds
Citation: Bevilacqua A, Corbo MR and Sinigaglia M (2011) Use of essential oils to inhibit Alicyclobacillus acidoterrestris: a short overview of the literature. Front. Microbio. 2:195. doi: 10.3389/fmicb.2011.00195
Received: 01 July 2011;
Accepted: 02 September 2011;
Published online: 27 September 2011.
Edited by:
Anderson de Souza Sant’Ana, University of São Paulo, BrazilReviewed by:
Juan Martín Oteiza, Centro de Investigaciòn y Asistencia Tècnica a la Industria Agroalimentaria, ArgentinaMaria Crystina Igarashi, University of São Paulo, Brazil
Sun Chul Kang, Daegu University, South Korea
Copyright: © 2011 Bevilacqua, Corbo and Sinigaglia. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Antonio Bevilacqua, Department of Food Science, Faculty of Agricultural Science, University of Foggia, Via Napoli 25, 71122 Foggia, Italy. e-mail: a.bevilacqua@unifg.it; abevi@libero.it