- Department of Pathology, National Institute of Infectious Diseases, Tokyo, Japan
The interferon-inducible host restriction factor bone marrow stromal antigen 2 (BST-2/tetherin) blocks the release of HIV-1 and other enveloped viruses. In turn, these viruses have evolved specific antagonists to counteract this host antiviral molecule, such as the HIV-1 protein Vpu. BST-2 is a type II transmembrane protein with an unusual topology consisting of an N-terminal cytoplasmic tail (CT) followed by a single transmembrane (TM) domain, a coiled-coil extracellular (EC) domain, and a glycosylphosphatidylinositol (GPI) anchor at the C terminus. We and others showed that BST-2 restricts enveloped virus release by bridging the host and virion membranes with its two opposing membrane anchors and that deletion of either one completely abrogates antiviral activity. The EC domain also shows conserved structural properties that are required for antiviral function. It contains several destabilizing amino acids that confer the molecule with conformational flexibility to sustain the protein’s function as a virion tether, and three conserved cysteine residues that mediate homodimerization of BST-2, as well as acting as a molecular ruler that separates the membrane anchors. Conversely, the efficient release of virions is promoted by the HIV-1 Vpu protein and other viral antagonists. Our group and others provided evidence from mutational analyses indicating that Vpu antagonism of BST-2-mediated viral restriction requires a highly specific interaction of their mutual TM domains. This interpretation is further supported and expanded by the findings of the latest structural modeling studies showing that critical amino acids in a conserved helical face of these TM domains are required for Vpu–BST-2 interaction and antagonism. In this review, we summarize the current advances in our understanding of the structural basis for BST-2 antiviral function as well as BST-2-specific viral antagonism.
Introduction
As a result of exposure to viral pathogens over millions of years, humans and other mammals evolved intrinsic immunity proteins that provide resistance to infection by directly interfering with different stages of the viral life cycle. These so-called host restriction factors are normally induced by interferon-α (IFN-α) during induction of the innate immune response by viral infection. A case in point is HIV-1, an extensively studied pathogen for which four major restriction factors have been identified: the apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3 (APOBEC3) family of cytidine deaminases (Sheehy et al., 2002); the α-isoform of the tripartite motif-containing protein 5 (TRIM5α; Stremlau et al., 2004); the bone marrow stromal antigen 2 (Neil et al., 2008; Van Damme et al., 2008; BST-2, also known as tetherin or CD317, referred to hereafter as BST-2), which is the subject of this review article; and, more recently, SAMHD1 (Hrecka et al., 2011; Laguette et al., 2011). HIV-1, in turn, evolved countermeasures to overcome the antiviral activity of their host restriction factors, mainly by acquiring a series of trans-acting viral accessory proteins, including Vif and Vpu. Vif blocks the above-described APOBEC3 proteins that mediate extensive deamination of cytosines in single-stranded viral DNA, thus halting HIV replication. Vpu is another viral antagonist of the transmembrane BST-2 protein that blocks the release of enveloped viruses by physically binding the budding viral particles to the membrane of infected cells. Likewise, in HIV-2 and related simian immunodeficiency viruses, Vpx acts as an antagonist of SAMHD1 that blocks HIV-1 replication in dendritic and myeloid cells. It should be noted that HIV-1 is not susceptible to human TRIM5α antiviral action (Stremlau et al., 2004). In this review, we focus on current advances in structure-based analyses of BST-2 and viral antagonists.
BST-2: Molecular Characteristics
BST-2 is an interferon-induced type II membrane glycoprotein of unusual topology (Ishikawa et al., 1995; Kupzig et al., 2003), which efficiently blocks the release of diverse mammalian enveloped viruses by directly tethering viral particles to the membranes of infected cells. Viruses restricted by BST-2 are found among diverse families, including filoviruses, arenaviruses, paramyxoviruses (Jouvenet et al., 2009; Kaletsky et al., 2009; Sakuma et al., 2009a; Radoshitzky et al., 2010), gamma-herpesviruses (Mansouri et al., 2009; Pardieu et al., 2010), rhabdoviruses (Weidner et al., 2010), and a wide array of retroviruses from several mammal host species (Arnaud et al., 2010; Dietrich et al., 2011; Xu et al., 2011). A recent study characterizing a feline BST-2 ortholog reported the protein’s strong activity against FIV particle release in vitro (Dietrich et al., 2011). BST-2 comprises a short, 21-amino-acid cytoplasmic N-terminal tail (CT), followed by an α-helical transmembrane (TM) domain, an extracellular domain (EC) that is predominantly helical and contains an extended parallel coiled-coil, and a C-terminal glycosylphosphatidylinositol (GPI) component that acts as a second anchor linking the protein back to the cell membrane (Kupzig et al., 2003; Figure 1A). This double-anchor topology is extremely unusual and is only shared by an isoform of the prion protein (Moore et al., 1999).
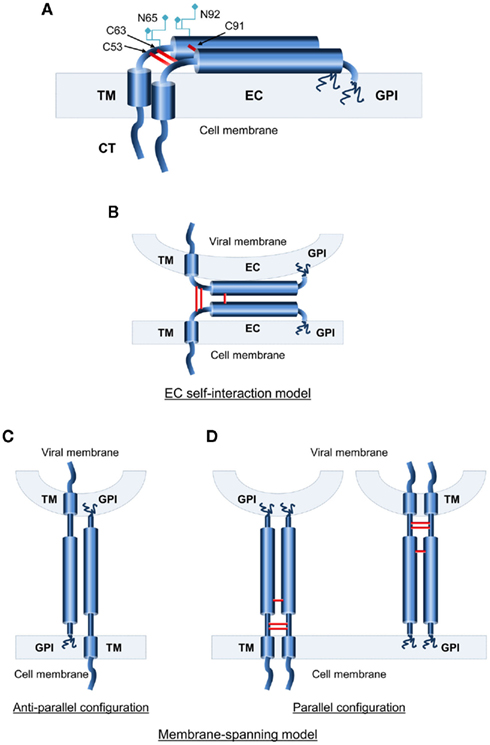
Figure 1. Topological characteristics of human BST-2. (A) Schematic representation of the domain structure of BST-2, a type II transmembrane (TM) protein. BST-2 features a short cytoplasmic N-terminus followed by an α-helical single-pass TM domain and an extended coiled-coil extracellular domain that is linked back to the plasma membrane by a C-terminal GPI anchor. N-glycosylation sites and cysteine residues for disulfide-bond formation in the extracellular domain (EC) are noted. (B–D) Topological models of BST-2’s functional state. (B) The EC self-interaction model, in which individual BST-2 monomers are anchored at both ends to the same membrane, with interaction between the ECs of cell-bound and virion-bound monomers. (C) Membrane-spanning anti-parallel model. Monomers are anchored in both membranes with opposing orientations. (D) Membrane-spanning parallel model. Monomers are anchored in both membranes with the same orientation.
Accumulating evidence supports the view that the structural features of BST-2 are key to its antiviral activity, as discussed in detail in the following sections. In agreement with a direct tethering mechanism, a requirement for both the TM and GPI anchors has been found for BST-2’s antiviral activity (Neil et al., 2008; Iwabu et al., 2009; Perez-Caballero et al., 2009). Additionally, the EC of BST-2 contains a series of important residues that are conserved throughout the protein’s mammalian orthologs, and these residues are essential to the inhibition of viral release (Van Damme et al., 2008; Andrew et al., 2009; Sakuma et al., 2009b). Whereas the stability of BST-2 is maintained by disulfide-links (Hinz et al., 2010; Schubert et al., 2010), the EC forms an extended coiled-coil domain that contains several conserved destabilizing amino acid residues, providing the conformational flexibility necessary for the molecule to sustain its role as a physical tether, as described later. Salient BST-2 structural motifs important for antiviral function are summarized in Table 1.
Based on the identification of these structural features critical for BST-2’s antiviral activity, Perez-Caballero et al. (2009) through domain replacement experiments, were able to show that BST-2’s configuration rather than its primary sequence is critical for antiviral activity. In an elegant demonstration, the authors generated a completely artificial BST-2-like protein made of structurally similar domains from three unrelated heterologous proteins (the TM from the transferrin receptor, the coiled-coil from dystrophia myotonica protein kinase, and the GPI anchor from the urokinase plasminogen activator receptor). Despite its lack of sequence homology with native BST-2, this artificial protein reproduced the latter’s antiviral activity as it was able to inhibit the release of HIV-1 and Ebola virus-like-particles.
Both TM and GPI Anchor are Important for the Restriction of Virus Release
The TM (amino acid positions 22–43) of BST-2 is a short single-pass α-helix that anchors the molecule to the plasma membrane, while the GPI anchor is located at the C-terminal region of the protein (Kupzig et al., 2003). These two membrane anchors in part determine the antiviral function of BST-2. This unusual topology suggests a model that BST-2 directly tethers budding virions to the membrane of infected cells. Indeed, unequivocal support for this model has come from immunoelectron microscopy studies demonstrating that BST-2 is associated with virions and located between the viral and cell membranes as well as between tethered virions (Neil et al., 2008; Fitzpatrick et al., 2010; Hammonds et al., 2010).
As shown in Table 1, two structural elements are absolutely required for BST-2-mediated restriction of viral release; (1) the presence of both the TM and the GPI anchor (Neil et al., 2008; Van Damme et al., 2008; Iwabu et al., 2009; Perez-Caballero et al., 2009); and (2) homodimer formation through EC disulfide-bond interactions (Andrew et al., 2009; Perez-Caballero et al., 2009). The latter is discussed in greater detail in a later section of this review. These two elements form the basis of the two proposed topological models of BST-2. In the “EC self-interaction model (Figure 1B),” individual BST-2 monomers are anchored at both ends to the same membrane (cellular or viral), and interaction between the EC domains of cell-bound and virion-bound monomers is required for the restriction of virus release. The alternative is the “membrane-spanning model (Figures 1C,D),” in which both BST-2 end tails (TM and GPI anchor) are anchored in different membranes (i.e., cellular and viral). Theoretically, the BST-2 monomers in this model can be arranged in either an anti-parallel (Figure 1C) or parallel (Figure 1D) configuration.
The first approach to resolve the topology of BST-2 involves cleavage of the GPI anchor by treatment with the hydrolytic enzyme phosphatidyl inositol-specific phospholipase C (Pi–PLC). However, the enzymatic treatment does not effectively release restricted virions from the cell membrane (Fitzpatrick et al., 2010), supporting either a membrane-spanning anti-parallel configuration (Figure 1C) or the EC self-interaction model (Figure 1B), in which monomers would be able to remain attached to the respective membrane by the TM domain even after cleavage of the GPI anchor.
The second approach is to evaluate the gap between the cellular and viral membranes in electron microscopy studies. If the BST-2 monomers are positioned parallel to the cellular and viral membranes (EC self-interaction model; Figure 1B), virions would be tethered very close to the membrane, less than 3–5 nm, as described in (Hinz et al., 2010). However, imaging studies show larger distances between virions and cells (Neil et al., 2008; Perez-Caballero et al., 2009; Hammonds et al., 2010), thus supporting a membrane-spanning model (Figures 1C,D).
The third approach to this problem has been the systematic determination of BST-2 function in mutational analyses. We have previously shown that the anchoring of BST-2 through both its N-terminal and C-terminal regions is required for antiviral activity (Iwabu et al., 2009). Briefly, mutagenesis studies using GPI-anchor-deleted and CD4 signal peptide chimeric versions of BST-2, in which the protein is linked to the cell membrane only through one of its ends, showed that removal of either end abrogated the antiviral effect of BST-2 on virus production. Therefore, we concluded that membrane binding through both the TM and GPI anchor of BST-2 is critical for its antiviral activity, supporting the model of the membrane-spanning parallel configuration (Figure 1D). Further evidence for this parallel-dimer model comes from the analysis of residual BST-2 found in virions released through proteolytic treatment with subtilisin (Perez-Caballero et al., 2009).
Finally and more importantly, four different groups have combined high-resolution crystallography (1.6–2.8Å), and small-angle X-ray scattering-based modeling to determine the structures of the entire human and murine BST-2 EC, and have shown that BST-2 forms parallel coiled-coil arrangements (Hinz et al., 2010; Schubert et al., 2010; Yang et al., 2010a; Swiecki et al., 2011). Taken together, these observations suggest that the antiviral state of BST-2 present at the cell membrane corresponds to the membrane-spanning parallel configuration model as shown in Figure 1D.
The EC Mediates Homodimerization
The BST-2 EC (amino acid positions 44–160) is predominantly an α-helical coiled-coil structure that contains a series of residues highly conserved among mammalian orthologs: two asparagines that are N-linked glycosylation sites (N65, N92), and three cysteines (C53, C63, C91) responsible for intermolecular disulfide-bonds that result in homodimerization (Figure 1A; Ohtomo et al., 1999; Andrew et al., 2009). Disulfide linkage through these cysteine residues is critical for the restriction of HIV production (Table 1). Mutational analyses demonstrate that partial disulfide-bond formation through at least one such cysteine residue is necessary for the retention of antiviral activity, whereas mutations at all three positions result in the total loss of antiviral function even though expression of the protein at the cell membrane remains unaltered (Andrew et al., 2009; Perez-Caballero et al., 2009; Hinz et al., 2010), although this is not the case for filovirus or arenavirus (Lassa virus) particles (Perez-Caballero et al., 2009; Sakuma et al., 2009a).
Several conserved amino acids within the EC domain, which are also thought to stabilize the dimers through weak coiled-coil domain interactions, include two interhelical salt bridges (E105–K106, and E133–R138) and one interhelical hydrogen bond (N141), and contribute to stabilize the EC domain interface (Hinz et al., 2010). Glycosylation of residues N65 and N92 was shown to contribute to anterograde transport and correct protein folding, but mutations in these positions had no effect on BST-2 antiviral activity (Table 1; Andrew et al., 2009; Sakuma et al., 2009a). In summary, all evidence thus far suggests that BST-2 EC contains a dimeric coiled-coil that is stabilized by C53–C53, C63–C63, and C91–C91 disulfide-bonds, with the conservation of at least one of these, along with weak interactions within the coiled-coil domain, and is required for dimer stability and the antiviral activity of BST-2.
The BST-2 EC Exhibits Conformational Flexibility
The most recent structural studies provide valuable clues to the biological function of the EC while at the same time reconciling the topological models of BST-2 dimer configuration with available electron microscopy data, as outlined above. Resolution of the crystal structure of human BST-2 EC (Hinz et al., 2010; Schubert et al., 2010; Yang et al., 2010a) together with small-angle X-ray scattering data suggest an elongated extracellular domain forming a long rod-like structure and a greatly extended EC separating the two membrane anchors, acting as a molecular ruler with a predicted distance of 170Å (Table 1). This distance would correspond to the predicted separation between membrane-tethered virions and the plasma membrane of the host cells, or between tethered viral particles, and is in agreement with the separation determined in published electron micrographic studies. This finding seems to be consistent with the aforementioned membrane-spanning model (Figure 1D).
The authors of those studies also described the presence of irregularities in the 90-Å coiled-coil motif. The irregularities arise from the introduction of destabilizing residues (see Table 1) that are arranged regularly in core heptad positions, i.e., amino acid residues located at the center of the α–helix. The destabilizing residues loosen regular coiled-coil packing increasing the pitch and radius of the α-helix, accounting for the low stability of BST-2’s coiled-coil under reducing conditions in vitro. These positions are conserved throughout all available BST-2 sequences, and their mutations result in loss of the antiviral function of BST-2 (Hinz et al., 2010). Yet, despite this intrinsic instability, the disulfide-bonds are still able to be formed, restabilizing the EC domains in a dimeric form. These findings suggest that conformational flexibility allows adaptation to the dynamic events of virion budding, while disulfide-bond-mediated dimerization prevents major separation of the coiled-coils. Together, these two properties result in a dynamic structure that permits dimer dissociation and restabilization during the process of virion trapping (Hinz et al., 2010; Swiecki et al., 2011). A high-resolution crystal structure of the full-length mouse BST-2 EC confirmed the presence of an elongated EC characteristically unstable due to the insertion of destabilizing residues (Swiecki et al., 2011). In that study, structural and biophysical analyses of murine and human BST-2 EC domains revealed that an unstable coiled-coil motif is evolutionarily conserved. This evidence provides further support for the aforementioned model of conformational flexibility.
The GPI Anchor Mediates Surface Localization and the CT Is Critical for BST-2 Trafficking
BST-2 localizes both to the plasma membrane and internal compartments, particularly the trans-Golgi network (TGN) and recycling endosomes (Kupzig et al., 2003; Rollason et al., 2007; Dube et al., 2009; Masuyama et al., 2009; Habermann et al., 2010). At the cell surface, BST-2 localizes into cholesterol-enriched lipid rafts, due to its GPI anchor. This localization is implicated in the promotion of clathrin-mediated endocytosis (Rollason et al., 2007; Masuyama et al., 2009) and, importantly, it allows BST-2 to directly interfere with the virion-release process, as lipid rafts are the preferential site of budding of several enveloped viruses (Aloia et al., 1993; Panchal et al., 2003; Waheed and Freed, 2009). This also positions BST-2 at the virological synapse (VS; Casartelli et al., 2010; Jolly et al., 2010; Pais-Correia et al., 2010), but its potential to restrict cell-to-cell viral spread remains controversial. With respect to internalization and cell trafficking, it was previously shown that rodent BST-2 is internalized from the cell surface in a clathrin-dependent manner (Rollason et al., 2007; Masuyama et al., 2009). Internalization requires a non-canonical dual tyrosine motif at amino acid positions 6 and 8 of the protein’s CT (YxY6–8; Table 1). This motif is highly conserved through all mammalian orthologs and sequentially participates in the interaction of BST-2 with the clathrin adaptors AP-2, which mediates internalization by endocytosis, and AP-1, which retrieves BST-2 to the TGN. The CT domain of BST-2 indirectly interacts with the underlying actin cytoskeleton through a series of adaptor proteins (RICH2, EBP50, ezrin), although additional studies are required to understand the implications of these interactions for BST-2 function (Rollason et al., 2009).
Viral Antagonism of BST-2
Since BST-2 targets the lipid bilayer of the host cell, viruses cannot evade it simply by escape mutations. Therefore, enveloped viruses had been obliged to evolve trans-acting countermeasures specifically to overcome BST-2 restriction. Among primate lentiviruses, three different viral gene products are known to antagonize BST-2. In most SIV strains, the viral Nef protein antagonizes primate BST-2, while in HIV-1 and HIV-2, the Vpu protein and the Env glycoprotein, respectively, antagonize human BST-2. Other BST-2 antagonists include the Kaposi’s sarcoma-associated herpesvirus (KSHV) K5 protein and the Ebola virus glycoprotein (GP). With the exception of Ebola GP, all of these viral proteins downregulate BST-2 at the plasma membrane, thus effectively removing it from viral budding sites.
HIV-1 Vpu
Just as the study of HIV-1 Vif led to the discovery of APOBEC3 as a host restriction factor (Sheehy et al., 2002), BST-2 was identified by searching for the host restriction factor antagonized by the accessory viral protein Vpu. This 16-kDa type I transmembrane viral protein is a BST-2 antagonist and as such promotes the release of HIV-1 virions (Cohen et al., 1988; Strebel et al., 1988; Malim and Emerman, 2008). Importantly, Vpu can directly mediate the removal of BST-2 away from its site of action on the cell surface, although the mechanisms remain hotly debated (Van Damme et al., 2008; Iwabu et al., 2009, 2010; Ruiz et al., 2010; Lau et al., 2011). Thus far, it appears that Vpu recruits cellular proteins to remove BST-2 from the surface (Figure 2A). As we and others have shown, BST-2 downregulation by Vpu involves a beta-transducin repeat-containing protein (β-TrCP)-dependent mechanism (Douglas et al., 2009; Iwabu et al., 2009; Mangeat et al., 2009; Mitchell et al., 2009; Dubé et al., 2010; Tokarev et al., 2011); however, this only partially explains the underlying mechanism, since mutations in the β-TrCP-binding motif of Vpu do not entirely abrogate its antagonism of BST-2 (Schubert and Strebel, 1994; Van Damme et al., 2008; Iwabu et al., 2009).
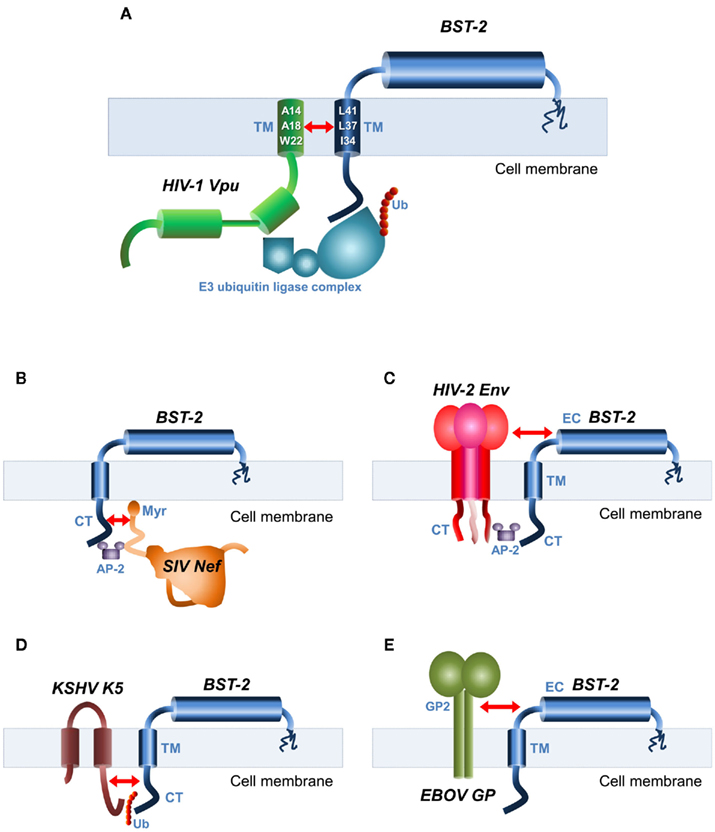
Figure 2. Viral antagonists of BST-2 and their domains of interaction. Schematic representation of BST-2 and its known antagonists. The structural domains of interaction are indicated by red arrows. (A) HIV-1 Vpu and BST-2 interact through their mutual transmembrane (TM) domains. Key amino acid residues involved in the interaction are depicted in the TM helices. Also shown is the E3 ubiquitin (Ub) ligase complex required for BST-2 internalization. (B) SIV Nef recognizes the cytoplasmic (CT) domain of BST-2. The AP-2 clathrin adaptor recruited for BST-2 internalization is also shown. Myr, myristoylation site. (C) The envelope glycoprotein (Env) of HIV-2 and SIVtan binds to BST-2 through their mutual ectodomains (EC), and recruitment of AP-2 by the CT domain of Env required for internalization is also shown. (D) Kaposi’s sarcoma-associated herpesvirus (KSHV) K5 protein that is an ubiquitin ligase ubiquitinates a target lysine motif in the CT domain of BST-2, resulting in its internalization. (E) The antagonistic mechanisms of the Ebola virus (EBOV) glycoprotein (GP) are unclear, but require interaction between GP2 subunit of EBOV–GP and BST-2 EC.
Whereas several reports suggest that BST-2 downregulation in the presence of Vpu is accomplished at least in part through proteasomal degradation (Goffinet et al., 2009; Gupta et al., 2009a; Mangeat et al., 2009), evidence obtained by our group and others supports a model of BST-2 downregulation through lysosomal degradation (Douglas et al., 2009; Iwabu et al., 2009; Mitchell et al., 2009; Janvier et al., 2011). It is proposed that Vpu causes the retention of BST-2 within endosomes by blocking its recycling after endocytosis (Mitchell et al., 2009; Dubé et al., 2010; Lau et al., 2011). Alternatively, it is hypothesized that Vpu inhibits the membrane transport of BST-2 by causing its intracellular sequestration within the TGN (Dubé et al., 2010; Andrew et al., 2011; Lau et al., 2011). We and others suggested that Vpu directly internalizes BST-2 from the cell surface through TM interactions leading to lysosomes (Iwabu et al., 2009, 2010; Janvier et al., 2011; Skasko et al., 2011a). An additional level of complexity in the BST-2 downregulation mechanism stems from a report that in certain cell lines (CEMx174, H9), Vpu overexpression results in the enhancement of virion production, but without effectively reducing the surface levels of BST-2 (Miyagi et al., 2009). Thus, it is not yet clear how Vpu affects the internalization, recycling, or membrane transport, of BST-2.
Regardless of the mechanisms of Vpu-induced BST-2 downregulation, the ability of Vpu to bind to BST-2 is crucial for the antagonism of BST-2-mediated restriction (Figure 2A), as evidenced by data showing that the anti-BST-2 activity of Vpu is abrogated by mutations that disrupt TM-TM interaction. (Gupta et al., 2009a; Iwabu et al., 2009; McNatt et al., 2009; Rong et al., 2009; Skasko et al., 2011a). This interaction is highly specific since single point mutations in either BST-2 (I34, L37, L41; Table 1; Kobayashi et al., 2011) or Vpu (A14, A18, and W22; Vigan and Neil, 2010) render BST-2 resistant to Vpu antagonism. Their structural analyses showed that these residues form both hydrophobic faces of the helices, and therefore presumably contribute to their interacting surfaces. Recently, the aforementioned residues have been shown by NMR spectroscopy to interact directly in a membrane-embedded TM–TM interface (Skasko et al., 2011b).
Importantly, a high degree of species-specificity characterizes this interaction. Even though all primate BST-2 proteins are able to block HIV-1 virion-release, non-human BST-2 proteins are mostly insensitive to Vpu antagonism (Goffinet et al., 2009; Gupta et al., 2009a; Jia et al., 2009; Zhang et al., 2009). Analyses of codon-specific positive selection in the primate lineage showed that a mutation of residue T45 in human BST-2 is sufficient to reduce its sensitivity to Vpu (Gupta et al., 2009a). Likewise, the transfer of amino acid positions 30–45 of the human BST-2 TM domain into rhesus BST-2 was sufficient to render it Vpu-sensitive, while a single I48T mutation in rhesus BST-2 conferred partial Vpu sensitivity (Yoshida et al., 2011). These results suggest that this specificity of HIV-1 Vpu for BST-2 depends on conserved amino acids in the latter’s TM domain (as described above) that are divergent between the human protein and its simian counterparts.
Other BST-2 Antagonists
Most of the primate lentiviruses that do not encode a Vpu protein instead use Nef to counteract BST-2’s antiviral function (Jia et al., 2009; Sauter et al., 2009; Zhang et al., 2009). It should be noted that even though the primate ancestors of HIV-1, SIVcpz, and SIVgor from chimpanzees and gorillas encode Vpu, they also use Nef to antagonize BST-2 (Sauter et al., 2009; Yang et al., 2010b). Analogous to HIV-1 Vpu antagonism of human and chimpanzee, but not other primate BST-2 proteins (Goffinet et al., 2009; McNatt et al., 2009; Hauser et al., 2010), SIV Nef counteracts primate but not human BST-2 orthologs. This selectivity resides in the CT of non-human primate BST-2, which contains a discreet DDIWK14–18 sequence (Table 1) that is required for the response to SIV Nef but is deleted in the protein’s human counterparts (Sauter et al., 2009; Lim et al., 2010; Yang et al., 2010b). Furthermore, antagonism of non-human primate BST-2 is abrogated by mutations in the myristoylation site of SIV Nef (Figure 2B; Jia et al., 2009; Zhang et al., 2009). In addition, SIV Nef mutations that impair CD4 and CD28 downregulation also abrogate BST-2 antagonism, suggesting a similar mechanism of interaction (Zhang et al., 2009). By contrast, BST-2 antagonism by some strains of HIV-2 (as well as SIVtan from Tantalus monkeys) is mediated by the Env glycoprotein (Figure 2C; Bour and Strebel, 1996; Ritter et al., 1996; Abada et al., 2005; Gupta et al., 2009b). Although the exact determinants of interaction are not well understood, an endocytic motif (GYxxΦ) in the cytoplasmic region of gp41 (Boge et al., 1998) is known to be required to bind to AP-2, triggering BST-2 downregulation (Le Tortorec and Neil, 2009), while extracellular domains of HIV-2 Env apparently bind to the EC of BST-2. It was recently reported that an A100D point mutation of BST-2’s EC abrogates the HIV-2 Env-mediated block of BST-2 restriction (Gupta et al., 2009b), supporting a model of interaction between HIV-2 Env and the EC of BST-2.
Other BST-2 antagonists include KSHV K5 protein, which ubiquitinates K18 residue in the CT domain of BST-2 (Table 1), leading to reduced surface and intracellular levels of BST-2, presumably through an endolysosomal process (Figure 2D; Mansouri et al., 2009; Pardieu et al., 2010). The Ebola virus GP2 appears to use a novel non-sequence-specific mechanism, overcoming BST-2’s restriction without significant removal of the protein from the cell surface (Figure 2E; Kaletsky et al., 2009; Lopez et al., 2010; Kühl et al., 2011). Influenza virus is suspected of harboring an unidentified viral antagonist against BST-2, since BST-2 expression was unable to block replication-competent influenza virus production but inhibited the release of influenza virus-like-particles (Watanabe et al., 2011).
Conclusion
Considerable progress was made recently in understanding the structure and function of BST-2, as well as the mechanisms by which viral antagonists counteract its activity. Through a combination of biological studies and structural analyses, the functional state of BST-2 is characterized as that of a parallel dimeric coiled-coil that, via its double-membrane anchors, physically binds budding virions to the infected cell. More importantly, current evidence shows that the unusual structural features of BST-2 determine its antiviral function independently of sequence homology. The EC has a prime role acting as a molecular ruler that separates the membrane anchors, in addition to allowing dimerization of BST-2 and providing conformational flexibility to sustain the protein’s function as a viral particle tether. Likewise, loss of BST-2’s double-membrane anchoring leads to the complete abrogation of the antiviral activity.
Although most of the evidence presented here was obtained from in vitro systems, a recent study using BST-2 knockout mice has shown that BST-2 inhibited the replication and release of a murine retrovirus in vivo, in a manner completely dependent on IFN-α production. Additionally, BST-2 restricted viral pathogenesis and delayed disease progression, suggesting that it has verifiable antiviral activity not only in vitro but also in vivo. (Liberatore and Bieniasz, 2011). Another study using rhesus macaques has confirmed the importance of the antagonism of BST-2 antiviral activity by Vpu in vivo (Shingai et al., 2011). Further investigation of the antiviral mechanisms exerted by host restriction factors, as well as the evolution of viral countermeasures, will not only advance our understanding of AIDS pathogenesis but also lead to the development of therapeutic alternatives.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Ministry of Health, Labor and Welfare of Japan (Research on HIV/AIDS; H21–009), and from the Ministry of Education, Science, Technology, Sports and Culture of Japan.
References
Abada, P., Noble, B., and Cannon, P. M. (2005). Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 79, 3627–3638.
Aloia, R. C., Tian, H., and Jensen, F. C. (1993). Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 90, 5181–5185.
Andrew, A. J., Miyagi, E., Kao, S., and Strebel, K. (2009). The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 6, 80.
Andrew, A. J., Miyagi, E., and Strebel, K. (2011). Differential effects of human immunodeficiency virus type 1 Vpu on the stability of BST-2/tetherin. J. Virol. 85, 2611–2619.
Arnaud, F., Black, S. G., Murphy, L., Griffiths, D. J., Neil, S. J., Spencer, T. E., and Palmarini, M. (2010). Interplay between ovine bone marrow stromal cell antigen 2/tetherin and endogenous retroviruses. J. Virol. 84, 4415–4425.
Boge, M., Wyss, S., Bonifacino, J. S., and Thali, M. (1998). A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273, 15773–15778.
Bour, S., and Strebel, K. (1996). The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J. Virol. 70, 8285–8300.
Casartelli, N., Sourisseau, M., Feldmann, J., Guivel-Benhassine, F., Mallet, A., Marcelin, A.-G., Guatelli, J., and Schwartz, O. (2010). Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 6, e1000955. doi:10.1371/journal.ppat.1000955
Cohen, E. A., Terwilliger, E. F., Sodroski, J. G., and Haseltine, W. A. (1988). Identification of a protein encoded by the vpu gene of HIV-1. Nature 334, 532–534.
Dietrich, I., Hosie, M. J., and Willett, B. J. (2011). The role of BST2/tetherin in feline retrovirus infection. Vet. Immunol. Immunopathol. 143, 255–264.
Douglas, J. L., Viswanathan, K., Mccarroll, M. N., Gustin, J. K., Fruh, K., and Moses, A. V. (2009). Vpu Directs the degradation of the human immunodeficiency virus restriction factor BST-2/tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 83, 7931–7947.
Dubé, M., Bhusan Roy, B., Guiot-Guillain, P., Binette, J., Mercier, J., Chiasson, A., and Cohen, É. A. (2010). Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6, e1000856. doi:10.1371/journal.ppat.1000856
Dube, M., Roy, B. B., Guiot-Guillain, P., Mercier, J., Binette, J., Leung, G., and Cohen, E. A. (2009). Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83, 4574–4590.
Fitzpatrick, K., Skasko, M., Deerinck, T. J., Crum, J., Ellisman, M. H., and Guatelli, J. (2010). Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6, e1000701. doi:10.1371/journal.ppat.1000701
Goffinet, C., Allespach, I., Homann, S., Tervo, H.-M., Habermann, A., Rupp, D., Oberbremer, L., Kern, C., Tibroni, N., Welsch, S., Krijnse-Locker, J., Banting, G., Kräusslich, H.-G., Fackler, O. T., and Keppler, O. T. (2009). HIV-1 Antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5, 285–297.
Gupta, R. K., Hué, S., Schaller, T., Verschoor, E., Pillay, D., and Towers, G. J. (2009a). Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5, e1000443. doi:10.1371/journal.ppat.1000443
Gupta, R. K., Mlcochova, P., Pelchen-Matthews, A., Petit, S. J., Mattiuzzo, G., Pillay, D., Takeuchi, Y., Marsh, M., and Towers, G. J. (2009b). Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U.S.A. 106, 20889–20894.
Habermann, A., Krijnse-Locker, J., Oberwinkler, H., Eckhardt, M., Homann, S., Andrew, A., Strebel, K., and Krausslich, H.-G. (2010). CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84, 4646–4658.
Hammonds, J., Wang, J.-J., Yi, H., and Spearman, P. (2010). Immunoelectron microscopic evidence for tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6, e1000749. doi:10.1371/journal.ppat.1000749
Hauser, H., Lopez, L. A., Yang, S. J., Oldenburg, J. E., Exline, C. M., Guatelli, J. C., and Cannon, P. M. (2010). HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7, 51.
Hinz, A., Miguet, N., Natrajan, G., Usami, Y., Yamanaka, H., Renesto, P., Hartlieb, B., Mccarthy, A. A., Simorre, J.-P., Göttlinger, H., and Weissenhorn, W. (2010). Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 7, 314–323.
Hrecka, K., Hao, C., Gierszewska, M., Swanson, S. K., Kesik-Brodacka, M., Srivastava, S., Florens, L., Washburn, M. P., and Skowronski, J. (2011). Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661.
Ishikawa, J., Kaisho, T., Tomizawa, H., Lee, B. O., Kobune, Y., Inazawa, J., Oritani, K., Itoh, M., Ochi, T., Ishihara, K., and Hirano, T. (1995). Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics 26, 527–534.
Iwabu, Y., Fujita, H., Kinomoto, M., Kaneko, K., Ishizaka, Y., Tanaka, Y., Sata, T., and Tokunaga, K. (2009). HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284, 35060–35072.
Iwabu, Y., Fujita, H., Tanaka, Y., Sata, T., and Tokunaga, K. (2010). Direct internalization of cell-surface BST-2/tetherin by the HIV-1 accessory protein Vpu. Commun. Integr. Biol. 3, 366–369.
Janvier, K., Pelchen-Matthews, A., Renaud, J.-B., Caillet, M., Marsh, M., and Berlioz-Torrent, C. (2011). The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog. 7, e1001265. doi:10.1371/journal.ppat.1001265
Jia, B., Serra-Moreno, R., Neidermyer, W. Jr., Rahmberg, A., Mackey, J., Fofana, I. B., Johnson, W. E., Westmoreland, S., and Evans, D. T. (2009). Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5, e1000429. doi:10.1371/journal.ppat.1000429
Jolly, C., Booth, N. J., and Neil, S. J. D. (2010). Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol. 84, 12185–12199.
Jouvenet, N., Neil, S. J., Zhadina, M., Zang, T., Kratovac, Z., Lee, Y., Mcnatt, M., Hatziioannou, T., and Bieniasz, P. D. (2009). Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83, 1837–1844.
Kaletsky, R. L., Francica, J. R., Agrawal-Gamse, C., and Bates, P. (2009). Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 106, 2886–2891.
Kobayashi, T., Ode, H., Yoshida, T., Sato, K., Gee, P., Yamamoto, S. P., Ebina, H., Strebel, K., Sato, H., and Koyanagi, Y. (2011). Identification of amino acids in the human tetherin transmembrane domain responsible for HIV-1 Vpu interaction and susceptibility. J. Virol. 85, 932–945.
Kühl, A., Banning, C., Marzi, A., Votteler, J., Steffen, I., Bertram, S., Glowacka, I., Konrad, A., Stürzl, M., Guo, J.-T., Schubert, U., Feldmann, H., Behrens, G., Schindler, M., and Pöhlmann, S. (2011). The ebola virus glycoprotein and HIV-1 Vpu employ different strategies to counteract the antiviral factor tetherin. J. Infect. Dis. 204, S850–S860.
Kupzig, S., Korolchuk, V., Rollason, R., Sugden, A., Wilde, A., and Banting, G. (2003). Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4, 694–709.
Laguette, N., Sobhian, B., Casartelli, N., Ringeard, M., Chable-Bessia, C., Segeral, E., Yatim, A., Emiliani, S., Schwartz, O., and Benkirane, M. (2011). SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657.
Lau, D., Kwan, W., and Guatelli, J. (2011). Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J. Virol. 85, 9834–9846.
Le Tortorec, A., and Neil, S. J. D. (2009). Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83, 11966–11978.
Liberatore, R. A., and Bieniasz, P. D. (2011). Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 108, 18097–18101.
Lim, E. S., Malik, H. S., and Emerman, M. (2010). Ancient adaptive evolution of tetherin shaped the functions of vpu and nef in human immunodeficiency virus and primate lentiviruses. J. Virol. 84, 7124–7134.
Lopez, L. A., Yang, S. J., Hauser, H., Exline, C. M., Haworth, K. G., Oldenburg, J., and Cannon, P. M. (2010). Ebola virus glycoprotein counteracts BST-2/tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J. Virol. 84, 7243–7255.
Malim, M. H., and Emerman, M. (2008). HIV-1 accessory proteins ensuring viral survival in a hostile environment. Cell Host Microbe 3, 388–398.
Mangeat, B., Gers-Huber, G., Lehmann, M., Zufferey, M., Luban, J., and Piguet, V. (2009). HIV-1 Vpu neutralizes the antiviral factor tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5, e1000574. doi:10.1371/journal.ppat.1000574
Mansouri, M., Viswanathan, K., Douglas, J. L., Hines, J., Gustin, J., Moses, A. V., and Fruh, K. (2009). Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 83, 9672–9681.
Masuyama, N., Kuronita, T., Tanaka, R., Muto, T., Hirota, Y., Takigawa, A., Fujita, H., Aso, Y., Amano, J., and Tanaka, Y. (2009). HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with α-adaptin. J. Biol. Chem. 284, 15927–15941.
McNatt, M. W., Zang, T., Hatziioannou, T., Bartlett, M., Fofana, I. B., Johnson, W. E., Neil, S. J. D., and Bieniasz, P. D. (2009). Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5, e1000300. doi:10.1371/journal.ppat.1000300
Mitchell, R. S., Katsura, C., Skasko, M. A., Fitzpatrick, K., Lau, D., Ruiz, A., Stephens, E. B., Margottin-Goguet, F., Benarous, R., and Guatelli, J. C. (2009). Vpu antagonizes BST-2–mediated restriction of HIV-1 release via β-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5, e1000450. doi:10.1371/journal.ppat.1000450
Miyagi, E., Andrew, A. J., Kao, S., and Strebel, K. (2009). Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U.S.A. 106, 2868–2873.
Moore, R. C., Lee, I. Y., Silverman, G. L., Harrison, P. M., Strome, R., Heinrich, C., Karunaratne, A., Pasternak, S. H., Chishti, M. A., Liang, Y., Mastrangelo, P., Wang, K., Smit, A. F. A., Katamine, S., Carlson, G. A., Cohen, F. E., Prusiner, S. B., Melton, D. W., Tremblay, P., Hood, L. E., and Westaway, D. (1999). Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292, 797–817.
Neil, S. J., Zang, T., and Bieniasz, P. D. (2008). Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430.
Ohtomo, T., Sugamata, Y., Ozaki, Y., Ono, K., Yoshimura, Y., Kawai, S., Koishihara, Y., Ozaki, S., Kosaka, M., Hirano, T., and Tsuchiya, M. (1999). Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 258, 583–591.
Pais-Correia, A.-M., Sachse, M., Guadagnini, S., Robbiati, V., Lasserre, R., Gessain, A., Gout, O., Alcover, A., and Thoulouze, M.-I. (2010). Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 16, 83–89.
Panchal, R. G., Ruthel, G., Kenny, T. A., Kallstrom, G. H., Lane, D., Badie, S. S., Li, L., Bavari, S., and Aman, M. J. (2003). In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc. Natl. Acad. Sci. U.S.A. 100, 15936–15941.
Pardieu, C., Vigan, R., Wilson, S. J., Calvi, A., Zang, T., Bieniasz, P., Kellam, P., Towers, G. J., and Neil, S. J. D. (2010). The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6, e1000843. doi:10.1371/journal.ppat.1000843
Perez-Caballero, D., Zang, T., Ebrahimi, A., Mcnatt, M. W., Gregory, D. A., Johnson, M. C., and Bieniasz, P. D. (2009). Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139, 499–511.
Radoshitzky, S. R., Dong, L., Chi, X., Clester, J. C., Retterer, C., Spurgers, K., Kuhn, J. H., Sandwick, S., Ruthel, G., Kota, K., Boltz, D., Warren, T., Kranzusch, P. J., Whelan, S. P., and Bavari, S. (2010). Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 84, 10569–10580.
Ritter, G. D. Jr., Yamshchikov, G., Cohen, S. J., and Mulligan, M. J. (1996). Human immunodeficiency virus type 2 glycoprotein enhancement of particle budding: role of the cytoplasmic domain. J. Virol. 70, 2669–2673.
Rollason, R., Korolchuk, V., Hamilton, C., Jepson, M., and Banting, G. (2009). A CD317/tetherin–RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 184, 721–736.
Rollason, R., Korolchuk, V., Hamilton, C., Schu, P., and Banting, G. (2007). Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell. Sci. 120, 3850–3858.
Rong, L., Zhang, J., Lu, J., Pan, Q., Lorgeoux, R.-P., Aloysius, C., Guo, F., Liu, S.-L., Wainberg, M. A., and Liang, C. (2009). The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83, 7536–7546.
Ruiz, A., Hill, M. S., Schmitt, K., and Stephens, E. B. (2010). Membrane raft association of the Vpu protein of human immunodeficiency virus type 1 correlates with enhanced virus release. Virology 408, 89–102.
Sakuma, T., Noda, T., Urata, S., Kawaoka, Y., and Yasuda, J. (2009a). Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83, 2382–2385.
Sakuma, T., Sakurai, A., and Yasuda, J. (2009b). Dimerization of tetherin is not essential for its antiviral activity against Lassa and Marburg viruses. PLoS ONE 4, e6934. doi:10.1371/journal.pone.0006934
Sauter, D., Schindler, M., Specht, A., Landford, W. N., Münch, J., Kim, K.-A., Votteler, J., Schubert, U., Bibollet-Ruche, F., Keele, B. F., Takehisa, J., Ogando, Y., Ochsenbauer, C., Kappes, J. C., Ayouba, A., Peeters, M., Learn, G. H., Shaw, G., Sharp, P. M., Bieniasz, P., Hahn, B. H., Hatziioannou, T., and Kirchhoff, F. (2009). Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6, 409–421.
Schubert, H. L., Zhai, Q., Sandrin, V., Eckert, D. M., Garcia-Maya, M., Saul, L., Sundquist, W. I., Steiner, R. A., and Hill, C. P. (2010). Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations. Proc. Natl. Acad. Sci. U.S.A. 107, 17951–17956.
Schubert, U., and Strebel, K. (1994). Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 68, 2260–2271.
Sheehy, A. M., Gaddis, N. C., Choi, J. D., and Malim, M. H. (2002). Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650.
Shingai, M., Yoshida, T., Martin, M. A., and Strebel, K. (2011). Some human immunodeficiency virus type 1 Vpu proteins are able to antagonize macaque BST-2 in vitro and in vivo: Vpu-negative simian-human immunodeficiency viruses are attenuated in vivo. J. Virol. 85, 9708–9715.
Skasko, M., Tokarev, A., Chen, C.-C., Fischer, W. B., Pillai, S. K., and Guatelli, J. (2011a). BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu: evidence for a post-ER mechanism of Vpu-action. Virology 411, 65–77.
Skasko, M., Wang, Y., Tian, Y., Tokarev, A., Munguia, J., Ruiz, A., Stephens, E. B., Opella, S. J., and Guatelli, J. (2011b). HIV-1 Vpu antagonizes the innate restriction factor BST-2 via lipid-embedded helix-helix interactions. J. Biol. Chem. doi:10.1074/jbc.M111.296772. [Epub ahead of print].
Strebel, K., Klimkait, T., and Martin, M. A. (1988). A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 241, 1221–1223.
Stremlau, M., Owens, C. M., Perron, M. J., Kiessling, M., Autissier, P., and Sodroski, J. (2004). The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in old world monkeys. Nature 427, 848–853.
Swiecki, M., Scheaffer, S. M., Allaire, M., Fremont, D. H., Colonna, M., and Brett, T. J. (2011). Structural and biophysical analysis of BST-2/tetherin ectodomains reveals an evolutionary conserved design to inhibit virus release. J. Biol. Chem. 286, 2987–2997.
Tokarev, A. A., Munguia, J., and Guatelli, J. C. (2011). Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J. Virol. 85, 51–63.
Van Damme, N., Goff, D., Katsura, C., Jorgenson, R. L., Mitchell, R., Johnson, M. C., Stephens, E. B., and Guatelli, J. (2008). The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252.
Vigan, R., and Neil, S. J. D. (2010). Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J. Virol. 84, 12958–12970.
Waheed, A. A., and Freed, E. O. (2009). Lipids and membrane microdomains in HIV-1 replication. Virus Res. 143, 162–176.
Watanabe, R., Leser, G. P., and Lamb, R. A. (2011). Influenza virus is not restricted by tetherin whereas influenza VLP production is restricted by tetherin. Virology 417, 50–56.
Weidner, J. M., Jiang, D., Pan, X. B., Chang, J., Block, T. M., and Guo, J. T. (2010). Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 84, 12646–12657.
Xu, F., Tan, J., Liu, R., Xu, D., Li, Y., Geng, Y., Liang, C., and Qiao, W. (2011). Tetherin inhibits prototypic foamy virus release. Virol. J. 8, 198.
Yang, H., Wang, J., Jia, X., Mcnatt, M. W., Zang, T., Pan, B., Meng, W., Wang, H.-W., Bieniasz, P. D., and Xiong, Y. (2010a). Structural insight into the mechanisms of enveloped virus tethering by tetherin. Proc. Natl. Acad. Sci. U.S.A. 107, 18428–18432.
Yang, S. J., Lopez, L. A., Hauser, H., Exline, C. M., Haworth, K. G., and Cannon, P. M. (2010b). Anti-tetherin activities in Vpu-expressing primate lentiviruses. Retrovirology 7, 13.
Yoshida, T., Kao, S., and Strebel, K. (2011). Identification of residues in the BST-2 TM domain important for antagonism by HIV-1 Vpu using a gain-of-function approach. Front. Microbiol. 2:35. doi:10.3389/fmicb.2011.00035
Keywords: HIV-1, Vpu, BST-2, transmembrane, restriction factor, antagonist, interaction
Citation: Arias JF, Iwabu Y and Tokunaga K (2011) Structural basis for the antiviral activity of BST-2/tetherin and its viral antagonism. Front. Microbio. 2:250. doi: 10.3389/fmicb.2011.00250
Received: 18 November 2011; Accepted: 25 November 2011;
Published online: 12 December 2011.
Edited by:
Akio Adachi, The University of Tokushima Graduate School, JapanReviewed by:
Masako Nomaguchi, The University of Tokushima Graduate School, JapanHirotaka Ode, National Hospital Organization Nagoya Medical Center, Japan
Copyright: © 2011 Arias, Iwabu and Tokunaga. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Kenzo Tokunaga, Department of Pathology, National Institute of Infectious Diseases, Shinjuku-ku, Tokyo 162-8640, Japan. e-mail: tokunaga@nih.go.jp