- 1 Interuniversity Institute for Marine Sciences in Eilat, Eilat, Israel
- 2 Institute of Earth Sciences, Hebrew University of Jerusalem, Jerusalem, Israel
The bioavailability of iron to microorganisms and its underlying mechanisms have far reaching repercussions to many natural systems and diverse fields of research, including ocean biogeochemistry, carbon cycling and climate, harmful algal blooms, soil and plant research, bioremediation, pathogenesis, and medicine. Within the framework of ocean sciences, short supply and restricted bioavailability of Fe to phytoplankton is thought to limit primary production and curtail atmospheric CO2 drawdown in vast ocean regions. Yet a clear-cut definition of bioavailability remains elusive, with elements of iron speciation and kinetics, phytoplankton physiology, light, temperature, and microbial interactions, to name a few, all intricately intertwined into this concept. Here, in a synthesis of published and new data, we attempt to disassemble the complex concept of iron bioavailability to phytoplankton by individually exploring some of its facets. We distinguish between the fundamentals of bioavailability – the acquisition of Fe-substrate by phytoplankton – and added levels of complexity involving interactions among organisms, iron, and ecosystem processes. We first examine how phytoplankton acquire free and organically bound iron, drawing attention to the pervasiveness of the reductive uptake pathway in both prokaryotic and eukaryotic autotrophs. Turning to acquisition rates, we propose to view the availability of various Fe-substrates to phytoplankton as a spectrum rather than an absolute “all or nothing.” We then demonstrate the use of uptake rate constants to make comparisons across different studies, organisms, Fe-compounds, and environments, and for gaging the contribution of various Fe-substrates to phytoplankton growth in situ. Last, we describe the influence of aquatic microorganisms on iron chemistry and fate by way of organic complexation and bio-mediated redox transformations and examine the bioavailability of these bio-modified Fe species.
Introduction
By virtue of its flexible redox chemistry, iron (Fe) plays an integral role in many biological processes such as photosynthesis, respiration, processing of reactive oxygen species, and nutrient acquisition. In view of these functions, it is not surprising that iron inputs and bioavailability in aquatic environments have far reaching repercussions for many natural systems and diverse areas of study as briefly outlined in Box 1. At the basis of these lies the role of iron in controlling phytoplankton growth. Photosynthetic life on Earth originated in reduced, low oxygen aquatic environments where the soluble ferrous iron – Fe(II) – was abundant and freely available. The rise of oxygenic photosynthesis favored the oxidized ferric form – Fe(III) – which rapidly precipitates out of oxic solutions as iron oxides or hydroxides. Modern day oceans and lakes thus cater poorly to the Fe requirements of phytoplankton with surface waters bearing picomolar to nanomolar concentrations of dissolved unchelated inorganic iron, Fe′ (Johnson et al., 1997), the most readily available form of Fe to phytoplankton, be it in ferrous Fe(II)′ or ferric form Fe(III)′ (Morel et al., 2008).
Extensive research on iron bioavailability to phytoplankton has been conducted over recent decades, yet a clear-cut definition of this term remains elusive. Bioavailability may be defined as the degree to which a certain compound can be accessed and utilized by an organism. However this definition may be oversimplistic as elements of iron speciation and kinetics, phytoplankton physiology, light, temperature, and microbial interactions, to name a few, are all intricately intertwined into what we term “bioavailability” (Wells et al., 1995; Worms et al., 2006). Given the complex and interdisciplinary nature of Fe bioavailability, progress in understanding this concept depends on addressing its sub-aspects by means of well-defined questions and multiple analytical techniques. In this contribution, rather than seeking a definition capable of encompassing the multiple aspects and scales of Fe bioavailability to phytoplankton, we attempt to disassemble this concept into its composing facets and explore them further.
At a fundamental level, cellular Fe acquisition or uptake rates are indicative of the availability of any single Fe-substrate to a specific phytoplankton species (Figure 1). Fe uptake rate, in turn, is a function of the uptake pathways expressed by an organism and the chemical compatibility or exchange kinetics of the Fe-substrate with the transport systems (Figure 1). Rates of Fe acquisition can be determined experimentally using model or naturally occurring phytoplankton and Fe-substrates. In the next two sections we discuss the experimental evaluation of Fe uptake pathways and rates and suggest the use of uptake rate constants as a means of comparing between organisms, Fe species, and environments, and gaging the relative contribution of specific Fe-compounds to phytoplankton in a natural setting. Needless to say, the availability of Fe to natural phytoplankton assemblages in oceans and lakes is influenced by many chemical, biological, and physical factors outside the experimental beaker (see Figure 1 for an outline of some of these factors). For example, both Fe speciation and phytoplankton physiology are dynamic in time and space and to complicate matters even further, these two factors are interconnected. Moreover, interactions among the various organisms in the ecosystem, in addition to a host of environmental variables, can strongly impact the ability of phytoplankton to meet their Fe requirements. While a complete description of this added complexity to bioavailability is beyond the scope of this contribution, in the last section we describe how aquatic microorganisms influence iron chemistry and fate by way of organic complexation and bio-mediated redox transformations, emphasizing the resulting effects on Fe bioavailability to phytoplankton.
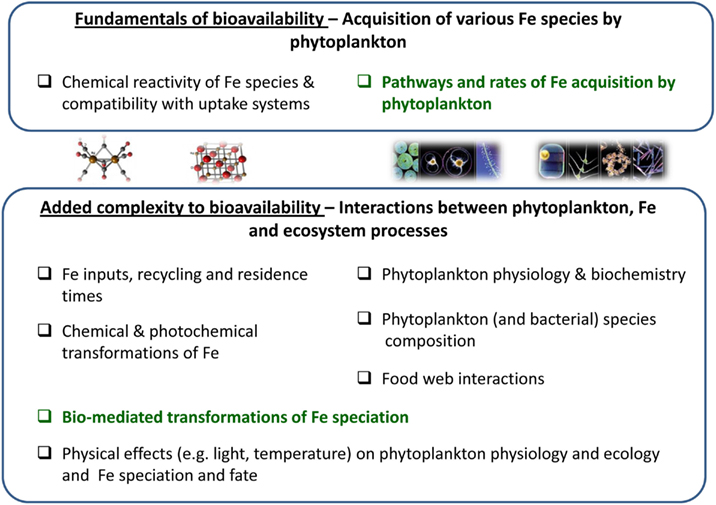
Figure 1. A conceptual diagram disassembling the multivariable concept of iron bioavailability to phytoplankton. The figure outlines the composing facets of Fe bioavailability where green text highlights topics elaborated in the paper. At the most basic level, the availability of an iron species to a phytoplankton species is determined by the rate at which it is acquired by the organism. Fe uptake rate, in turn, is a function of the uptake pathways expressed by an organism and the chemical compatibility or exchange kinetics of the Fe-substrate with the transport systems (upper box). In Sections “Fundamentals of Fe Bioavailability: Phytoplankton Fe Acquisition Systems” and “Fundamentals of Fe Bioavailability: Phytoplankton Fe Acquisition Rates” we discuss the experimental evaluation of Fe uptake rates by laboratory cultures and natural populations. In the environment, many other chemical, biological, and physical factors are important for determining Fe availability to phytoplankton, some of which are detailed in the lower box. In Section “Added Complexity to Bioavailability: Bio-Mediated Transformations of Fe Speciation” we turn to organism–Fe interactions and discuss how secretion of organic compounds and bio-mediated redox processes alter Fe speciation and influence Fe availability.
Box 1. Scope of iron influence on natural systems and research fields.
Aquatic iron biogeochemistry has been in the limelight over the past three decades with numerous studies linking Fe to carbon cycling and global climate (Martin et al., 1990; Watson et al., 2000; Blain et al., 2007; Martinez-Garcia et al., 2011). A particular emphasis has been placed on Fe availability to phytoplankton since over 45% of global photosynthesis occurs in aquatic environments (Falkowski et al., 1998) and photosynthetic systems are heavily dependent on iron (e.g., Raven, 1990; Greene et al., 1991). It is now well established that limited iron availability lowers phytoplankton pigment content and light harvesting capabilities, hinders photosynthesis and growth rates, and subsequently diminishes the production of organic matter and biogenic minerals (CaCO3 and opal) and curtails CO2 drawdown in vast ocean regions (Figure 2; Boyd et al., 2007). Many biogenic gases other than CO2 are important determinants of atmospheric chemistry and climate, but far less is known about the controls iron exerts on the sea-atmosphere fluxes of such gases (Figure 2; Liss, 2007; Buesseler et al., 2008). What is known however, is that phytoplankton growth, death, and decomposition, all of which may be controlled by Fe availability, result in emissions of dimethylsulfide (DMS) and isoprene (cloud formation promoters), N2O and CH4 (potent greenhouse gases), and CO and OH (reactive species influencing the atmosphere oxidation potential; Law and Ling, 2001; Meskhidze and Nenes, 2006). The combined effects of these emissions on atmospheric radiative forcing remain largely unknown (Lampitt et al., 2008).
By controlling phytoplankton standing stocks, Fe availability may also influence the surface ocean light field, and subsequently play a role in the surface ocean heat budget (Figure 2; Manizza et al., 2005). In addition to constraining primary productivity, iron deficiency impedes biogenic element cycling since phytoplankton cannot synthesize the enzymes required for utilizing major nutrients such as nitrate and N2 (Figure 2; Milligan and Harrison, 2000; Kustka et al., 2002; Sohm et al., 2011). Low iron availability may also alter ecosystem structure and function: under Fe limitation smaller phytoplankton are favored, resulting in rapid carbon regeneration and lowered carbon export flux to the deep ocean (Figure 2; Price et al., 1994; Finkel et al., 2010). As limited Fe availability alters nutrient assimilation ratios and phytoplankton species composition, it bares implications for the reconstruction of ocean paleo-productivity and paleo-nutrient distributions. Examples include the intensively studied sedimentary records of diatoms, whose abundance, morphology, and composition is strongly regulated by Fe (Figure 2; Strzepek and Harrison, 2004; Marchetti et al., 2006; Marchetti and Cassar, 2009). An additional, less explored example, is the recently reported effect of Fe limitation on cadmium (Cd) drawdown from seawater by phytoplankton (Lane et al., 2009), Subsequently, Fe limitation may alter seawater Cd:P ratios (Cullen et al., 1999) and thus bias past reconstruction of distributions which is based on seawater Cd:P ratios (Figure 2; Boyle, 1988; Elderfield and Rickaby, 2000). Recent attention has also been drawn to the effect of iron inputs and availability on toxic algal species occurrence and toxin production in oceans and lakes (Figure 2; e.g., Trick et al., 2010; Alexova et al., 2011).
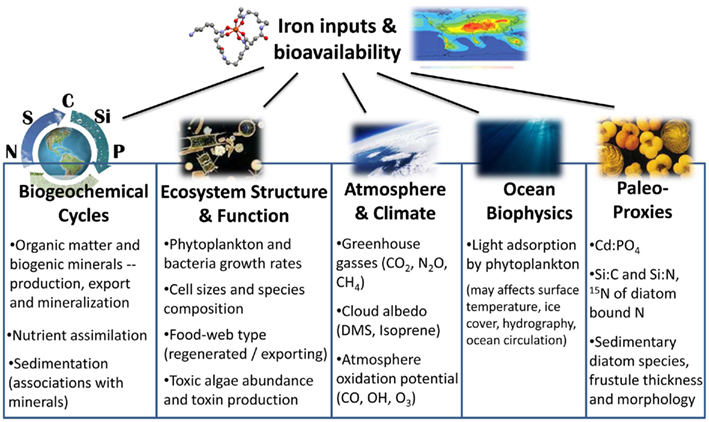
Figure 2. Summary of processes, systems, and research fields which are influenced by iron inputs and bioavailability (see text for details).
Fundamentals of Fe Bioavailability: Phytoplankton Fe Acquisition Systems
In order to determine the effects of Fe inputs on phytoplankton productivity it is essential to identify the relationship between the concentration of various Fe species and their uptake by phytoplankton. As such, phytoplankton iron transport systems, uptake strategies, and rates are important for our understanding of Fe availability. In the next two sections we tackle the question of bioavailability at the organism level and look at mechanistic studies of iron acquisition pathways and rates under controlled conditions – an approach which has provided much insight into Fe bioavailability in natural systems (Hudson and Morel, 1993; Sunda and Huntsman, 1995; Hutchins et al., 1999; Maldonado and Price, 1999; Maldonado et al., 1999; Shaked et al., 2005; Morel et al., 2008). In Section “Uptake Pathways of Aquatic Phytoplankton” we explore two well-studied iron uptake pathways in aquatic phytoplankton – siderophore mediated and reductive Fe uptake – looking at the environmental relevance of each strategy. Since our focus is phytoplankton, we will not cover Fe uptake pathways of heterotrophic bacteria. More on this subject can be found in a recent report by Hopkinson and Barbeau (2012). In Section “Behavioral Patterns of Iron Mining” we briefly mention some behavioral patterns amongst phytoplankton which may be relevant to Fe acquisition from seawater.
Uptake Pathways of Aquatic Phytoplankton
Several uptake pathways for free inorganic iron (Fe′) and organically bound iron (FeL) have been described amongst aquatic phytoplankton. While Fe′ is clearly an important iron source, we focus on FeL, where the transport machinery an organism employs will dictate the accessibility of a specific compound (Morel et al., 2008). To date, two major FeL uptake pathways have been described for phytoplankton: siderophore mediated Fe acquisition (e.g., Goldman et al., 1983; Soria-Dengg et al., 2001) and the reductive iron uptake pathway (Allnutt and Bonner, 1987; Eckhardt and Buckhout, 1998; Maldonado and Price, 2001; Shaked et al., 2005). According to a prevalent paradigm shared by many oceanographers, prokaryotic phytoplankton adopt siderophore-based Fe uptake systems while eukaryotes utilize a reductive strategy (e.g., Hutchins et al., 1999). However, genetic evidence (Webb et al., 2001; Hopkinson and Morel, 2009) as well as results of short term iron uptake experiments (Rose et al., 2005; Lis and Shaked, 2009; Fujii et al., 2010; Kranzler et al., 2011), contradict this paradigm. We propose that the occurrence of siderophore vs. reductive iron uptake can be put down to environmental rather than taxonomic considerations and that reductive iron uptake is a prevalent Fe acquisition strategy amongst phytoplankton (Figure 3; Table A1 in Appendix).
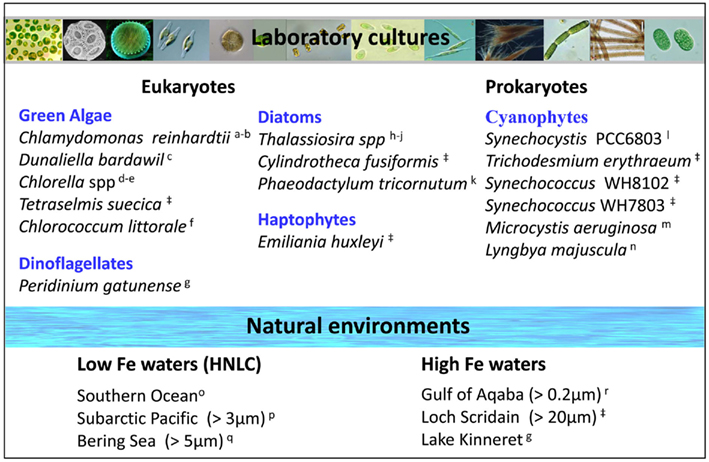
Figure 3. Prevalence of the reductive iron uptake pathway amongst phytoplankton. Listed are laboratory cultures and natural environments for which inhibition of uptake by Fe(II) binding ligands (Ferrozine/BPDS) was observed experimentally and taken to indicate the formation of an Fe(II) intermediate during iron transport. For some species, genomic and proteomic research identified various components of the reductive iron uptake pathway including ferrireductases and multicopper oxidases. See Appendix for supporting data and methodology (Tables A1 and A2 and Figures A1 and A2 in Appendix). Note on locations: The Gulf of Aqaba is located at the northern tip of the Red Sea, Loch Scridain is a sea loch located on the Atlantic coastline of the island of Mull, Scotland, and Lake Kinneret (Sea of Galilee) is a fresh water lake in the north of Israel. References: aEckhardt and Buckhout (1998), bWeger (1999), cKeshtacher et al. (1999), dAllnutt and Bonner (1987), eMiddlemiss et al. (2001), fSasaki et al. (1998), gShaked et al. (2002), hShaked et al. (2005), iJones and Morel (1988), jMaldonado and Price (2001), kSoria-Dengg and Horstmann (1995), lKranzler et al. (2011), mFujii et al. (2010), nRose et al. (2005), oMaldonado et al. (2005), pMaldonado and Price (1999), qShaked et al. (2004), rLis and Shaked (2009), ‡Lis and Shaked, in preparation.
Siderophore mediated Fe uptake involves the synthesis and secretion of ferric iron chelators which are capable of solubilizing, capturing, and delivering Fe(III) to the cell (Kraemer, 2004). The efficiency of this uptake pathway depends on: (a) the probability of the siderophore finding an Fe-substrate and (b) the probability of the ferrisiderophore complex finding its way back to the secreting cell. The build-up and maintenance of an Fe-siderophore diffusion gradient bringing iron back to the host cell is an essential feature of this strategy (Hutchins et al., 1991; Völker and Wolf-Gladrow, 1999). Therefore, siderophore production works best at high cell densities and in quiet waters or contained environments where turbulent disruption is unlikely (e.g., within biogenic aggregates or dense algal colonies). Consequently, siderophore production would be impractical in open waters, with high turbulence, and low cell densities (Hutchins et al., 1991). Indeed, a conspicuous lack of siderophore synthesis or uptake genes amongst open ocean cyanobacteria and eukaryotic phytoplankton (Hopkinson and Morel, 2009) suggests that this strategy may be ill suited to free-living aquatic phototrophs. Given the limitations of siderophore mediated iron uptake in dilute media, particularly open ocean waters, we turn our attention to an alternative strategy better suited to these conditions – Fe acquisition by means of reduction.
Reductive iron uptake offers a practical alternative to siderophore production as demonstrated in the numerical model constructed by Völker and Wolf-Gladrow (1999) comparing the efficiency of these two strategies in the marine environment. Several studies, as well as our own data, support the prevalence of reductive iron uptake amongst a variety of representative phytoplankton from both eukaryotic and prokaryotic taxa as well as in natural phytoplankton communities in high Fe and low Fe environments (Figure 3; Table A1 in Appendix). Reduction operates on both Fe′ and FeL where it involves the dissociation of Fe from its chelating ligand followed by transport of free iron into the cell (Atkinson and Guerinot, 2011). A key feature of this uptake pathway is the formation of an Fe(II) intermediate and thus experimental assays for reductive Fe uptake employ a ferrous iron binding ligand such as ferrozine or bathophenanthrolinedisulfonic acid (BPDS) which competes with the cell for Fe(II) and inhibits Fe uptake (see Figures A1 and A2 in Appendix; Shaked et al., 2004, 2005). On a genetic level, cell surface ferric reductases, similar to those in the yeast Saccharomyces cerevisiae, have been found in green algae (Allen et al., 2007) and ocean dwelling diatoms (Kustka et al., 2007; Bowler et al., 2010). These reductases may operate in conjunction with permease–oxidase complexes which reoxidize the Fe(II) as it is transported into the cell (Maldonado et al., 2006; Chen et al., 2008; Terzulli and Kosman, 2010). Not much is known about the reductive processes only recently shown to exist in aquatic cyanobacteria (Rose et al., 2005; Lis and Shaked, 2009; Kranzler et al., 2011).
The greatest advantage of the reductive strategy is its potential to operate not only on Fe′ but also across a range of organically bound iron complexes, even Fe bound to strong siderophores such as ferrioxamine B (FeDFB; Maldonado et al., 2005; Shaked et al., 2005; Lis and Shaked, 2009; Shi et al., 2010; Kranzler et al., 2011). Phytoplankton equipped with this strategy would be able to integrate iron from a variety of sources, giving them an obvious competitive advantage in Fe acquisition. While we advance the idea of reduction as a prevalent Fe uptake strategy in aquatic systems, we by no means claim it to be exclusive. A single organism may possess both direct FeL uptake pathways (e.g., ferrisiderophore transporters) as well as iron reductases, a classic example being baker’s yeast (Kosman, 2003). Moreover, siderophore mediated and reductive iron uptake do not discount the existence of other Fe uptake pathways, be they under investigation (e.g., Stintzi et al., 2000; Pick et al., 2008; Sutak et al., 2010; Wirtz et al., 2010) or undiscovered.
Behavioral Patterns of Iron Mining
The data covered thus far clearly demonstrates that phytoplankton are not passive in their quest for iron. A major energetic investment is clearly required in order to accumulate intracellular Fe concentrations that are four to six orders of magnitude greater than those in their surrounding environment (Morel and Price, 2003). Some phytoplankton take this a step further and exhibit behavioral patterns which aid in the active mining of iron from the environment. The most striking example is the collection and processing of iron-rich dust particles by colonies of the globally important N2 fixing cyanobacterium Trichodesmium spp. (Rueter et al., 1992; Rubin et al., 2011). The positively buoyant Trichodesmium forms massive blooms at the sea surface where it is likely to encounter dust deposits. Recently, we reaffirmed previous observations on efficient capturing and retention of dust by natural Trichodesmium colonies and documented a specialized ability to actively shuffle dust and iron oxides from the colony periphery to its core (Rubin et al., 2011). Packaging of dust in the colony interior can minimize its detachment and loss and also facilitate its chemical processing within a semi-enclosed microenvironment (Rubin et al., 2011). We found that Trichodesmium colonies were able to mediate dust dissolution, most likely via reduction (Rubin et al., 2011; Shaked, unpublished). Our mechanistic study complements several field observations documenting the ability of Trichodesmium to utilize iron from dust (Moore et al., 2009; Chen et al., 2011). Additionally, large diatoms (such as Rhizosolenia spp. and Ethmodiscus spp.) and dinoflagellates (such as Alexandrium spp. and Gymnodinium spp.) are known to migrate vertically to the nutricline to stock up on nutrients (Villareal et al., 1999; Ralston et al., 2007). While vertical migration was repeatedly proven efficient in nitrogen accumulation (Singler and Villareal, 2005), no clear evidence for iron mining at depth is present (McKay et al., 2000).
Fundamentals of Fe Bioavailability: Phytoplankton Fe Acquisition Rates
The discussion so far has centered on the mechanisms phytoplankton employ for acquiring Fe, knowledge which is crucial for analyzing and predicting their ability to access iron from various Fe pools in the environment (Shaked et al., 2005; Morel et al., 2008). We now focus on a more common approach to determining Fe availability: rate of transport. In this line of research the bioavailability of an Fe-substrate is ascertained by means of growth or short term iron uptake experiments. Although this approach is very promising, it faces significant challenges in terms of quantitative extrapolation to systems outside the experimental framework (be it a beaker or a grow-out incubation). In Section “Experimental Probing of Availability” we discuss the importance of experimental design, stressing the use of well-defined experimental media and organisms in the pursuit of unambiguous data regarding the accessibility of different Fe species to various phytoplankton. However, even when high quality data are obtained, it is very difficult to reach a consensus regarding the availability of any one Fe-compound. The same Fe-substrate, for example, may be available to one organism but not to another, making bioavailability not only a question of “what?” but also of “to whom?”. Moreover, the same organism may employ additional transport pathways upon Fe limitation, further extending the question to “when?” and “where?” in natural environments. In Section “Computing Availability Using Uptake Constants,” we describe the use of uptake rate constants for comparing between studies, organisms, compounds, and environments as well as for gauging the contribution of specific Fe species to phytoplankton growth in situ. We propose that the availability of Fe species to phytoplankton can be viewed as a spectrum rather than an absolute “all or nothing” and establish a relative scale of bioavailability for a range of Fe species and various phytoplankton cultures and natural populations.
Experimental Probing of Availability
Two common experimental approaches for probing the bioavailability of a given Fe-compound are: (1) long term or steady state iron uptake experiments which follow growth rates and/or intracellular Fe quotas of cells grown on a certain Fe-substrate, and (2) short term uptake experiments where the change in intracellular iron is followed over several hours. In order to enable inter- and intra-study comparisons, common grounds in methodology must be established by means of a robust experimental design in which medium and/or organism are well-defined.
Due to the fast hydrolysis and precipitation of Fe(III), an artificial or natural Fe complexing agent is required to keep dissolved iron in solution during experiments. For the study of free inorganic iron (Fe′), EDTA (ethylenediaminetetraacetic acid), and other carboxylic acid compounds are typically used (e.g., Anderson and Morel, 1982). The FeEDTA complex itself is membrane impermeable and not bioavailable (Shaked et al., 2005) and EDTA buffers an easily calculated pool of unchelated iron or Fe′ in the medium (Sunda et al., 2005). There has been some criticism of the applicability of EDTA based studies to natural systems (Gerringa et al., 2000) but the alternative of using uncomplexed FeCl3 when trying to measure free inorganic iron uptake rates has serious pitfalls. When spiking experimental media with FeCl3, the iron is found in two pools – dissolved Fe and freshly precipitated colloids – whose relative proportions and bioavailability fluctuate over time (Wells et al., 1983; Kuma et al., 1996). When examining the bioavailability of organically bound iron (FeL, where L is an organic ligand) many chemical factors such as ligand strength, metal to ligand ratios, FeL equilibration time, and photolability should be taken into consideration. Sufficiently high FeL complex stability and/or a sufficient excess of the ligand compared to Fe are important in order to prevent iron precipitation. On the other hand, high concentrations of free ligand (L) were shown to slow Fe uptake rates down due to competition with the cells for unchelated Fe which is formed during reductive uptake (Maldonado and Price, 2001; Shaked et al., 2005; Lis and Shaked, 2009). Experiments probing the bioavailability of partially complexed, particulate, or colloidal iron require careful support measurements and/or use of chemical speciation modeling software which establish Fe speciation in the experimental medium (e.g., Nodwell and Price, 2001; Rijkenberg et al., 2006, 2008; Hassler et al., 2011a). The concentration of Fe used in short term uptake experiments is often a compromise between environmentally relevant low concentrations and those required for adequate signal. However, it must be noted that in order to compare between experiments Fe concentrations must be sub-saturating (see Computing Availability Using Uptake Constants).
Characterization of the experimental organism is no less important than defining Fe speciation, as physiological status and growth phase can greatly affect experimental outcomes. Iron limitation, for example, is known to cause the upregulation of high affinity Fe acquisition systems (Maldonado and Price, 1999, 2001; Maldonado et al., 2006; Kustka et al., 2007; Allen et al., 2008), allowing access to previously unavailable Fe pools. This high affinity acquisition system allows iron limited diatoms to acquire iron bound to the xenosiderophore ferrioxamine B (FeDFB), whereas this complex is non-accessible to iron replete diatoms (see Figure 5A; Maldonado and Price, 2001). Growth phase may also affect Fe uptake since various transport pathways may be inactivated during different growth phases (Lis and Shaked, unpublished). In addition, the presence of bacteria in uptake assays using non-axenic cultures or natural assemblages may influence the availability of specific compounds to the studied algae or bias the uptake signal through bacterial acquisition of compounds unavailable to the studied organism (Soria-Dengg et al., 2001; Roe et al., 2011). Experimental choices regarding cell density, illumination, temperature, use of pH buffers, and experiment duration, to name a few, may alter uptake rates by influencing both algal physiology and Fe chemistry, and should be carefully evaluated in preliminary experiments. As a rule of thumb, cell density should be kept as low as possible to minimize bio-mediated changes in Fe chemistry (e.g., through the secretion of Fe-binding compounds into the medium), and experimental length should be kept short to avoid changes in the transport systems (Sunda et al., 2005). Short experiments (4–12 h) are preferably conducted in the dark or under red light (which prevents Fe photochemistry while supplying photo-synthetically active irradiation (Kranzler et al., 2011). Many organic pH buffers bind Fe and their effect on uptake rates should be carefully examined (Shi et al., 2009). Whenever possible, data points should be collected several times throughout the experiment rather than at its beginning and end. This ensures the measurement of meaningful rates that can be extrapolated further.
Once answers to fundamental questions of bioavailability have been established using model phytoplankton species and Fe-substrates, the next level of complexity may be added by conducting experiments with either natural aquatic communities or natural ligands (e.g., Maldonado et al., 2005; Shi et al., 2010). This combination of basic laboratory based research and field work has proven to be a powerful tool in unraveling the complexities of natural systems. Experiments of this kind are analytically challenging and involve considerable planning and careful determination of the desired goals. Examples include studying the effect of photochemistry on the availability of model and natural Fe complexes to size fractionated natural phytoplankton communities (Maldonado et al., 2005) and the use of well-studied model organisms to report on changes in the availability of natural Fe-compounds due to variations in pH (Shi et al., 2010).
Computing Availability Using Uptake Constants
As stated above, uptake data are experiment-specific and thus often hard to extrapolate to other organisms or environments. Here we propose a relatively straight forward approach using the uptake rate constant – kup – for comparing between experiments conducted with strongly bound organically complexed Fe (FeL) or unchelated Fe (Fe′). When an Fe-substrate is applied at sub-saturating concentrations, its rate of cellular uptake (ρ) is proportional to its concentration (Eq. 1, Figure 4):
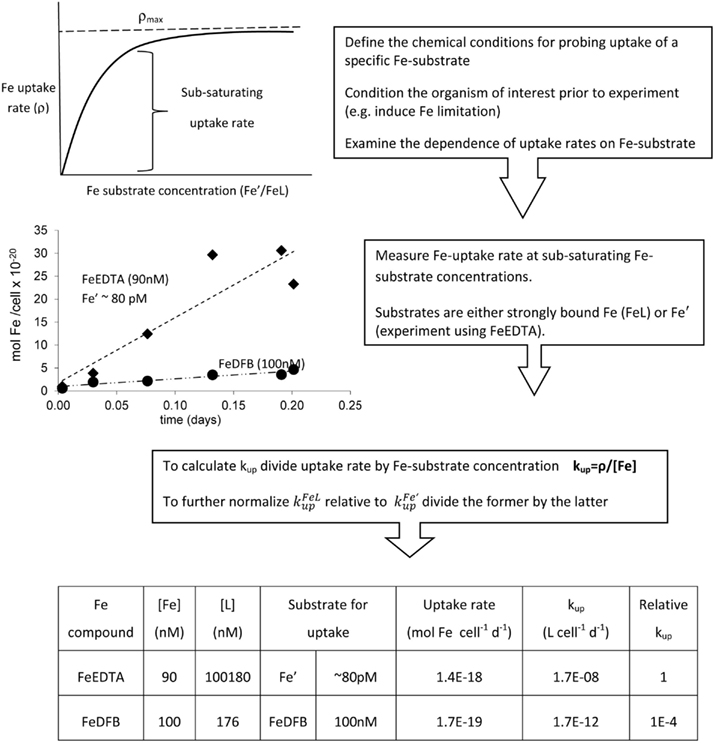
Figure 4. Schematic representation of experimental probing and calculation of the uptake rate constant – kup – exemplified with Fe-limited Emiliania huxleyi uptake data. Note that the complexing ligand L for strong ligands such as DFB should be in sufficient excess of Fe to rule out the presence of Fe′ (which would bias uptake rate since Fe′ is taken up more readily than FeL). But L:Fe should be minimal to prevent ligand from competing with cells for Fe (and thus decreasing uptake rates).
where ρ is cellular Fe uptake rate (mol Fe cell−1 day−1) and [Fe] is Fe-substrate concentration (mol L−1). Under these conditions (linear range in Figure 4), the dependency of cellar uptake rate on Fe concentration is described by the uptake constant – kup with units of L cell−1 day−1 (different units may be used in accordance with the units of uptake rate). Unlike cellular uptake rate (ρ), which varies with Fe concentration, kup is a more faithful representation of the ability of an organism to internalize the iron. In order to convert uptake rates to uptake constants, an action which can also be regarded as normalization, we need to define the concentration of Fe that serves as a substrate for uptake. In experiments probing the uptake of strongly complexed FeL, where Fe′ concentrations are negligible, the substrate for uptake equals the total Fe added to the experiment as precomplexed FeL (Figure 4). In contrast, when probing Fe′ uptake in the presence of the metal buffer EDTA, the total Fe is present predominantly as FeEDTA which is biologically unavailable, while Fe′ – the substrate for uptake is found at minute concentrations (Shaked et al., 2005). The kup calculation cannot be performed for weak FeL complexes as the experimental media contains both Fe′ and FeL. Experiments using FeCl3 are also hard to analyze due to Fe precipitation, unless Fe is added at concentrations lower than its solubility limits in seawater (0.2–0.5 nM; Liu and Millero, 2002). Despite its limitations, the uptake rate constant, kup, is highly useful in comparing the “relative bioavailability” of different Fe-substrates to various phytoplankton, both in the laboratory or in natural environments. Moreover, we can use kup to predict if a specific Fe-compound can support phytoplankton Fe demands in situ. These two applications are described below and in Figures 4 and 5 and Table 1.
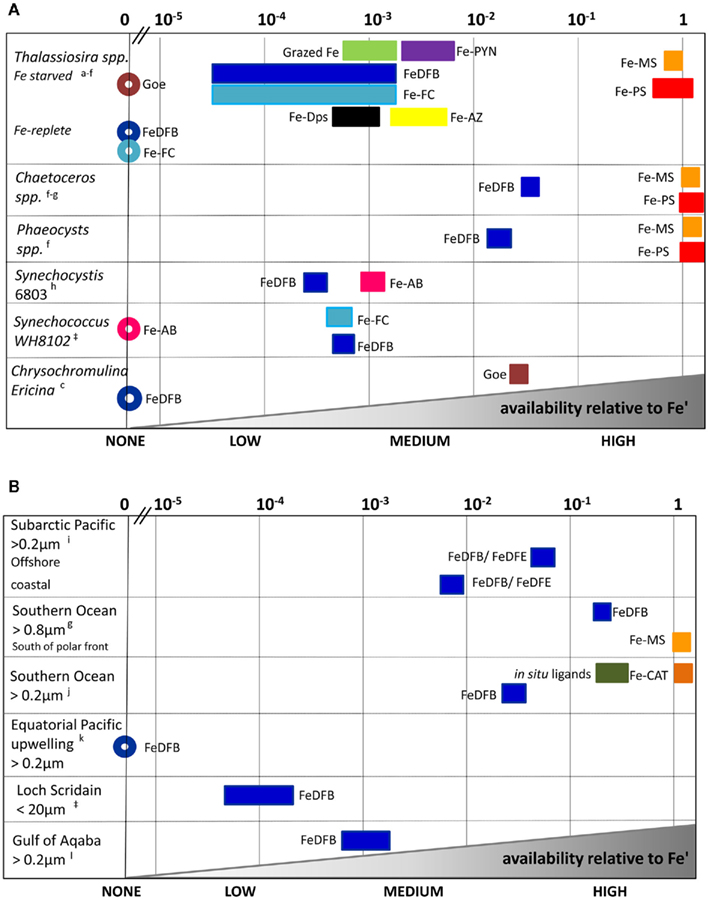
Figure 5. Relative scale of Fe availability established from phytoplankton uptake rates obtained for cultures (A) and natural assemblages (B). By converting experimental data to uptake-rate constants, and normalizing it further relative to Fe’, comparisons across organisms, Fe-substrates and environments are made possible (see text and Figure 4 for details). Different Fe-complexes are presented as different colors, while bar length represents variations among experiments, and circles denote no uptake. Abbreviations: Goe, goethite; DFB, desferrioxamine B; AB, Aerobactin; FC, ferrichrome; PYN, Porphyrin; MS, Monosaccharides; PS, Polysaccharides; AZ, Azotochelin; DPS, DNA binding protein from starved cells (iron storage proteins); CAT, Gallocatechin; DFE, desferrioxamine E. See Appendix for supporting data and Figure 3 for location descriptions. References: aShaked et al. (2005), bChen et al. (2008:1], cNodwell and Price (2001), dKustka et al. (2005), eMaldonado and Price (2001), fHassler and Schoemann (2009), gHassler et al. (2011b), hKranzler et al. (2011), iMaldonado and Price (1999), jMaldonado et al. (2005), kWells et al. (1994); lLis and Shaked (2009), ‡Lis and Shaked, unpublished.

Table 1. A comparison between the minimal daily Fe requirements and the daily FeDFB uptake capacity of Synechococcu s spp.
Relative bioavailability scale as a means of comparing organisms, Fe species, and environments
We first demonstrate the use of kup in comparing the bioavailability of Fe′ and FeDFB (where DFB is the strong siderophore ferrioxamine B) to Fe-limited cultures of the open ocean coccolithophore Emiliania huxleyi (Figure 4). In these experiments we determined Fe uptake rates of E. huxleyi from 100 nM radiolabeled FeDFB (Fe:DFB ratio 1:1.1) to 90 nM radiolabeled FeEDTA (Fe:EDTA ratio 1:1111; Fe′ ∼80 pM). While uptake rate of Fe′ (in the presence of EDTA) is only about twice as fast as for FeDFB, the uptake constants span four orders of magnitude with mol Fe cell−1 day−1 and = 1.7 × 10−12 mol Fe cell−1 day−1 (Figure 4). This marked difference in kup stems from the much lower substrate concentration of Fe′ as compared to FeDFB. While browsing through published uptake data it becomes apparent that kup values for any specific Fe-compound varies among organisms in accordance with their sizes and degree of Fe limitation (Sunda and Huntsman, 1995; Shaked et al., 2005). Seeking a way to present data on many Fe-compounds and many phytoplankton species simultaneously, we chose to establish a relative bioavailability scale which is illustrated in Figure 5. Here, we divide the uptake constants of various Fe complexes by the uptake constant of Fe′ where both constants are obtained from a single study. The resulting ratio represents the availability of model Fe-compounds relative to Fe′, shown on a logarithmic scale for a variety of cultured phytoplankton in Figure 5A. A similar calculation was conducted for natural phytoplankton populations as shown in Figure 5B.
The data presented in Figure 5 clearly show that both in the laboratory and natural environment Fe′ is highly bioavailable as compared to most organically bound iron forms and colloids, as previously suggested by Morel et al. (2008). A notable exception to this is the recently reported high availability of Fe bound to saccharides, tested using model mono-and polysaccharides with several cultured species, and natural phytoplankton (Figures 5A,B and references therein). Iron bound to siderophores such as ferrichrome (FC), ferrioxamine B (DFB), aerobactin (AB), and azotochelin (AZ) is accessible to several cultured phytoplankton at a low to intermediate degree as compared to Fe′. However, when grown under Fe-replete conditions, diatoms from the genus Thalassiosira (and we estimate that this is probably true for other diatoms) are unable to access siderophore bound Fe (Figure 5A). Similarly, ferrioxamine complexes are more accessible to natural phytoplankton assemblages in low Fe than high Fe waters (e.g., Southern Ocean vs. Loch Scridain or offshore vs. coastal stations in the subarctic Pacific, Figure 5B). While centric Thalassiosira spp. diatoms acquire Fe from a wide range of organic substrates, they are unable to internalize stable Fe oxides (Rich and Morel, 1990). On the other hand, mixotrophic dinoflagellates capable of consuming particles (e.g., Chrysochromulina ericina) are able to utilize goethite but not FeDFB (Maranger et al., 1998; Nodwell and Price, 2001). It is important to keep in mind that the source of variation between phytoplankton species and Fe-compounds in Figure 5 may also be attributed to experimental conditions. For example, the relative bioavailability FeDFB may change depending on the amount of excess free DFB present in the experimental medium, where a higher excess makes FeDFB seem less bioavailable. Nonetheless, we find this method of comparison illuminating when considering the bioavailability of different Fe-substrates to aquatic phytoplankton. In this respect, the use of EDTA to buffer known Fe′ concentrations is indispensable since Fe′ is easily calculated despite differences in experimental set-ups.
Gauging the contribution of Fe-substrates to meeting phytoplankton Fe demand in situ
Experimentally obtained uptake rate constants can help examine the availability of various Fe species in natural settings. This is done by multiplying kup by measured or predicted concentrations of a specific Fe-compound. For example, multiplying typical HNLC Fe concentrations of 70 pM chelated Fe (FeL) and 0.07 pM unchelated Fe (Fe′, Rue and Bruland, 1995) by representative uptake constants- ∼ 2 × 10−9 L cell−1 day−1 and ∼ 1 × 10−6 L cell−1 day−1 (Shaked et al., 2005), results in FeL uptake rate of ∼1.4 × 10−19 mol Fe cell−1 day−1 and Fe′ uptake rate of ∼7 × 10−20 mol Fe cell−1 day−1. The overall uptake rate of 2 × 10−19 mol Fe cell−1 day−1, contributed 2/3 by FeL and 1/3 by Fe′, is well within the estimated steady state uptake of natural phytoplankton of 2 × 10−20–4 × 10−18 mol Fe cell−1 day−1 (e.g., Strzepek et al., 2005). Since the kup of most FeL complexes is much lower than that of Fe′ (Figure 5A), 1 pM Fe′ in the ocean can be equated to 100–10000 pM of organically bound Fe, making Fe′ a potentially important Fe source, despite its low concentrations. Therefore, Fe′ formed by processes such as photoreductive dissolution of Fe oxides (Waite and Morel, 1984; Wells et al., 1991; Barbeau and Moffett, 2000) or the degradation of photolabile Fe complexes (Barbeau et al., 2001; Rijkenberg et al., 2006; Amin et al., 2009; Steigenberger et al., 2010) may contribute to a transient yet significant Fe pool which caters, at least partially, to phytoplankton iron requirements in surface waters (Morel et al., 2008).
Such calculations can also be used to examine if a specific compound is likely to support in situ growth of certain phytoplankton species by comparison to the minimal daily Fe requirements. As an example we examine the ability of FeDFB to support Synechococcus spp. growth. Table 1 details the calculation and shows that the minimal theoretical daily Fe requirement of Synechococcus is three orders of magnitude greater than its FeDFB uptake capacity, even given an overshoot in the estimated concentration of FeDFB-like Fe complexes in natural waters. Therefore while Synechococcus is capable of transporting DFB bound iron (i.e., FeDFB can be termed bioavailable should uptake be the single criterion for bioavailability), FeDFB or similar compounds alone are insufficient in meeting the Fe demands of this organism.
Needless to say, care should be taken in the use of kup as a tool of comparison: factors such as experimental conditions and environmental constraints should be taken into account. When synthesizing laboratory and field data it is important to bear in mind that the degree to which a specific organic Fe complex (FeL) supports phytoplankton growth in situ is influenced by factors other than its direct uptake rate. The residence time of FeL in surface water and its tendency to undergo chemical and physical transformations all influence its final availability, an issue we further explore in the next section.
Added Complexity to Bioavailability: Bio-Mediated Transformations of Fe Speciation
Having discussed the fundamentals of bioavailability in the form of uptake pathways and rates – we now turn our attention to the added complexity of Fe–organism interactions (Figure 1). While iron inputs and availability are widely recognized as factors shaping the biology of marine and fresh water environments, biological activities, in turn, exert strong control on iron speciation and cycling in aquatic ecosystems, and may even influence its inputs (Figure 6). Here we explore how Fe–organism interactions can affect iron bioavailability in complex and sometimes unexpected ways due to the many players and intricacies involved in natural systems. We endeavor to address some of these complexities in the present section.
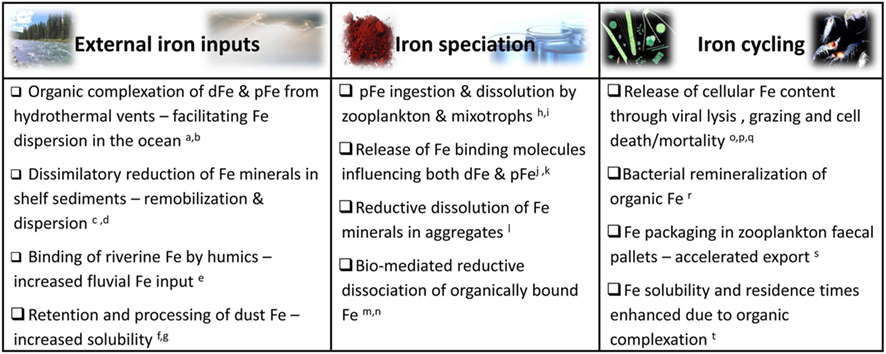
Figure 6. Selected examples of biological interactions with external iron inputs (e.g., aeolian dust deposition, sediment resuspension, fluvial, and hydrothermal Fe) and organism mediated influences on iron speciation and recycling in aquatic environments. While the microbial web in its entirety (from grazers to primary producers, viruses and heterotrophic bacteria) influences iron dynamics and availability to all community members, we focus on resultant Fe bioavailability to photosynthetic microorganisms. Abbreviations: dFe, dissolved iron; cFe, colloidal iron; pFe, particulate Fe. References: aSander and Koschinsky (2011), bWu et al. (2011), cLohan and Bruland (2008), dSevermann et al. (2010), eBatchelli et al. (2010), fBoyd et al. (2010b), gRubin et al. (2011), hBarbeau et al. (1996), iTang et al. (2011), jSato et al. (2007), kBuck et al. (2010), lBalzano et al. (2009), mMaldonado and Price (1999), nShaked et al. (2002), oHiggins et al. (2009), pStrzepek et al. (2005), qTsuda et al. (2007), rBoyd et al. (2010a), sBoyd and Ellwood (2010), tKuma et al. (1996).
Basic life processes significantly impact the inputs, speciation and fate of iron in aquatic ecosystems (Figure 6). Secretion of exopolymers and siderophores, bacterial Fe-oxide respiration, and food web interactions involving bacteria, phytoplankton, grazers, and viruses may all alter iron chemistry and resulting bioavailability (Figure 6; e.g., Poorvin et al., 2004; Boye et al., 2005; Sarthou et al., 2008; Schlosser and Croot, 2009). Thus, far from being slaves to Fe, microorganisms are able to manipulate iron speciation in their surroundings to some extent. Having said this, it should be noted that very few microbial processes influencing iron chemistry are clearly intended to serve iron acquisition purposes and more importantly, not all biological modifications of iron speciation influence its bioavailability favorably. In this section we classify a number of major pathways by which microbial communities alter iron speciation in aquatic environments. Microorganisms are able to mediate Fe redox transformations in their immediate surroundings while biological production and release of Fe-binding ligands are imperative to keeping iron in solution. No less important is the role microbial food web interactions play in the rapid recycling of iron in surface waters. Iron regeneration has received some recent attention (Strzepek et al., 2005; Boyd et al., 2010a) and will not be discussed in detail here. Rather, we will focus on the two former processes and their impact on the Fe pool available to phytoplankton.
Biological Control on Fe Redox Transformations
Iron has two oxidation states of importance to its aquatic chemistry – Fe(II) and Fe(III). Processes of Fe oxidation and reduction, known as redox reactions, take place throughout the water column, across chemical gradients, in sediments, and microenvironments. Redox reactions are central in determining the physical and chemical form of iron and its subsequent chemical and biological reactivity (Figure 7; Table 2). While both abiotic and biotic processes regulate iron redox transformations, we focus on the role biology plays in mediating iron redox cycling and the resulting effects on Fe availability to phytoplankton. We also attempt to distinguish between redox reactions of dissolved inorganic, dissolved organic and colloidal/particulate iron, as the governing factors and the ensuing changes in Fe bioavailability are likely to vary between the different iron species (Figure 7; Table 2).
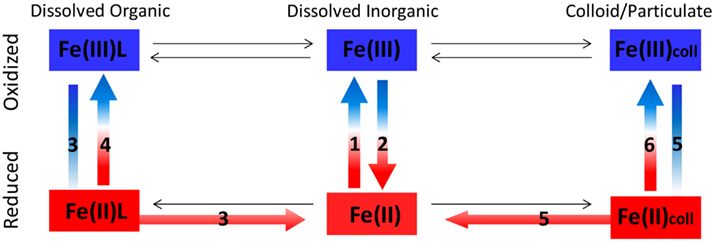
Figure 7. Redox reactions of different Fe species in aquatic environments. See Table 2 below for details on processes 1–6 in the figure.
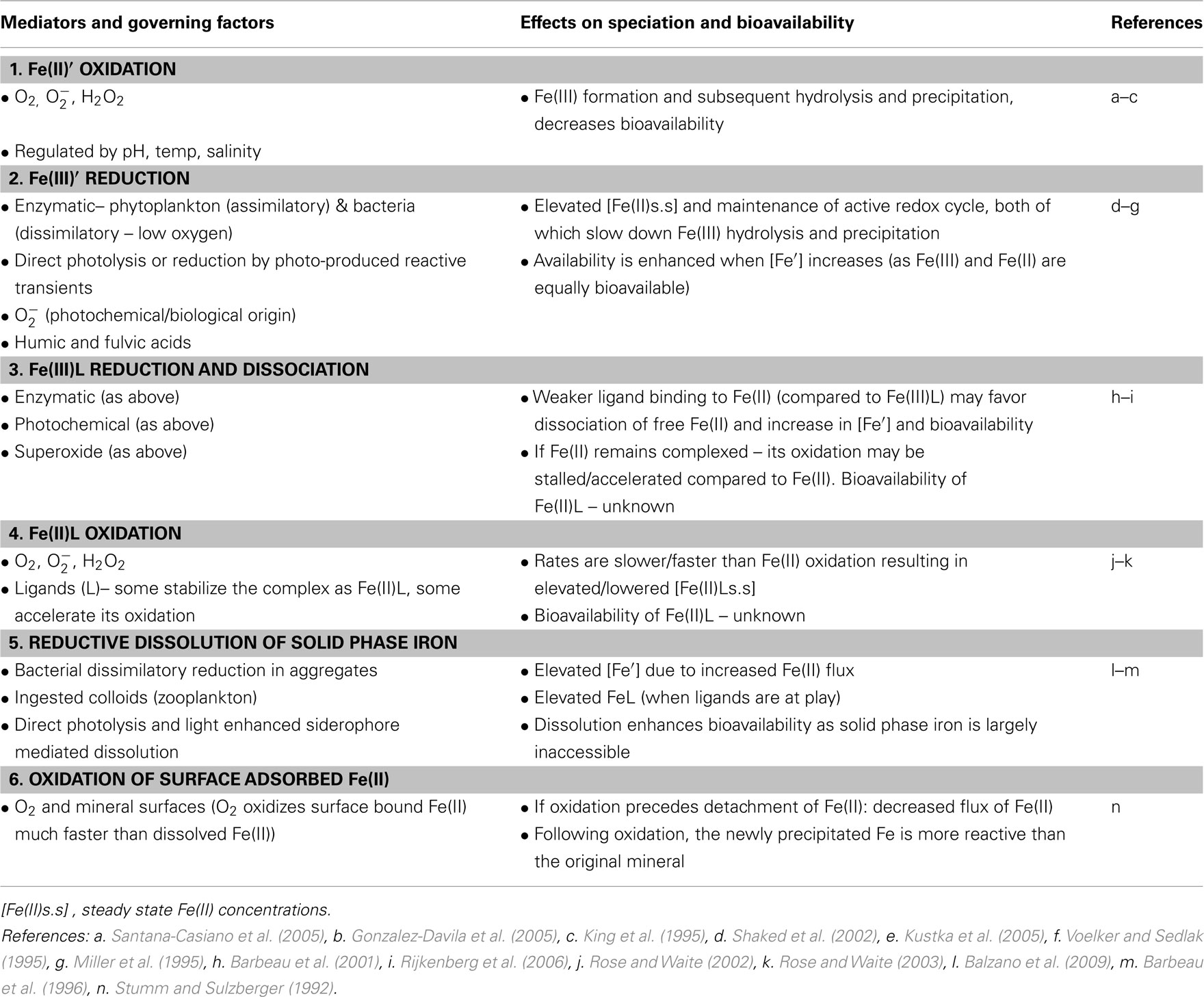
Table 2. Redox reactions of different Fe species in aquatic environments, their governing factors, and potential influence on Fe speciation and bioavailability (this table accompanies Figure 7).
Speciation and reactions
In oxygenated, neutral pH surface waters iron persists predominantly in its oxidized form – Fe(III), as fluxes of the thermodynamically unstable Fe(II) will undergo prompt oxidation by oxygen and hydrogen peroxide (Figure 7; Table 2; Gonzalez-Davila et al., 2005; Santana-Casiano et al., 2005). Fe(II) oxidation rates are not only regulated by the abiotic factors of temperature, pH, and salinity (Santana-Casiano et al., 2006), but also by biology. Bio-generated redox reactive species, oxygen consumption and release via respiration, as well as photosynthesis are able to influence oxidizing agent type and concentration as well as local pH conditions. In environments characterized by high biomass, low turbulence, poor ventilation, or low alkalinity (e.g., marine aggregates, coastal waters, oxygen minimum zones, and lakes) significant biological modifications of the reactants and/or conditions involved in Fe(II) oxidation have been reported (Emmenegger et al., 2001; Shaked et al., 2002; Moffett et al., 2007; Lohan and Bruland, 2008). In pelagic, low biomass surface ocean waters, biology is thought to exert minor controls on Fe(II) oxidation rates (e.g., Miller et al., 1995; Shaked, 2008). However, this view may require reassessment given the biological production of superoxide and hydrogen peroxide recently observed in several new open ocean studies (Rose et al., 2008; Hansard et al., 2010; Vermilyea et al., 2010).
In order for Fe(II) to persist at measurable concentrations in oxygen rich water, continuous Fe(II) production and/or Fe(II) supply must take place (Figure 7; Table 2). Multiple Fe reduction pathways were studied and suggested to operate at varying degrees in surface waters, including direct photochemical reduction, reduction by superoxide of photochemical or biological origin, thermal reduction, reduction by phytoplankton cell surface enzymes, and microbial reduction in isolated suboxic and anoxic microenvironments such as settling fecal pellets and aggregates (Table 2; Alldredge and Cohen, 1987; Kuma et al., 1992; Johnson et al., 1994; Voelker and Sedlak, 1995; Maldonado and Price, 2001; Shaked et al., 2002; Kustka et al., 2005; Rose et al., 2005; Barbeau, 2006; Rijkenberg et al., 2006; Balzano et al., 2009; Roy and Wells, 2011). Fe(II) may be supplied from external sources such as sediments, anoxic or suboxic water, hydrothermal vents, rain and aerosols, or originate from the in situ recycling of cellular iron through grazing and viral lysis (Kieber et al., 2001; Croot et al., 2005; Statham et al., 2005; Buck et al., 2006; Breitbarth et al., 2009). In addition, retardation of Fe(II) oxidation by low temperature, low pH, or complexation by Fe(II) stabilizing organic ligands are thought to contribute to the maintenance of measurable Fe(II) concentrations (Croot et al., 2001, 2008; Roy et al., 2008).
Thanks to the development of rapid and sensitive flow injection based chemiluminescence techniques (FI-CL), Fe(II) datasets in the oxygenated upper waters of oceans and lakes have recently begun to accumulate (Emmenegger et al., 2001; Shaked et al., 2002; Ussher et al., 2007; Shaked, 2008; Hansard et al., 2009; Sarthou et al., 2011). Some of these measurements point to a strong biological control on Fe(II) formation as attested to by the co-variance of chlorophyll and Fe(II) concentrations as well as measurable night-time Fe(II) levels. Commonly, the presence of non-photochemical Fe(II) in the photic zone is suggested to reflect Fe reduction by assimilatory cell surface enzymes of phytoplankton or by biologically produced superoxide (e.g., Sarthou et al., 2011). Assimilatory Fe reduction by cell surface enzymes is not likely to generate high fluxes of Fe(II), as the Fe(II) is probably generated within an enclosed protein complex and subsequently internalized by an adjacent transport protein (Kustka et al., 2005; Shaked et al., 2005). Rather, superoxide, a diffusible reducing agent which has recently been shown to be generated non-photochemically throughout the photic zone (Rose et al., 2008, 2010; Hansard et al., 2010), may be a central player in Fe(II) formation. Similarly, experimental observations of phytoplankton mediated Fe reduction in flow through systems [where Fe(II) is detected downstream of membrane-mounted cells], may be caused by superoxide released from the cells (Milne et al., 2009; Saragosti et al., 2010) in addition to or rather than cell surface enzymes. Despite emerging evidence for non-photochemical Fe(II) formation, photochemistry should not be underestimated as a strong mediator of Fe redox reactions. Currently, it is analytically challenging to detect photochemically produced Fe(II) as it oxidizes completely by the time the water is retrieved and analyzed. Minimizing collection time with high throughput pumps, Shaked (2008) observed a highly active photo-induced redox cycle in the surface waters of the Gulf of Aqaba.
Bioavailability
As Fe(II) is far more soluble than Fe(III), reductive processes generating Fe(II) and/or the occurrence of the thermodynamically unstable Fe(II) in surface waters are commonly linked to enhanced iron bioavailability (e.g., Sunda, 2001). This often justified notion merits careful consideration as some Fe(II) species may not be available and since not all reductive processes increase Fe availability. Aided with Table 2 and Figure 7, we outline the effects of several reductive processes on Fe bioavailability below.
While Fe(II) is potentially the more bioavailable redox state of Fe, several factors will determine whether iron availability is indeed increased by reductive processes. These include the characteristics of the Fe species undergoing reduction, the reductive pathway, the presence of other ligands and oxidants in the immediate surroundings, and ultimately the uptake machinery of the phytoplankton utilizing this iron. As a rule of thumb, redox processes that increase the concentrations of dissolved inorganic Fe (be it Fe(II)’ or Fe(III)’), will generally tend to boost the bioavailability of iron. With this in mind, we discuss the potential for Fe′ release and/or changes to Fe lability occurring in different reductive processes.
Reductive dissolution of solid phase Fe. As solid phase Fe is considered unavailable to phytoplankton (Rich and Morel, 1990; Wells et al., 1991), reductive dissolution of mineral Fe or surface adsorbed Fe generate Fe′, and thus enhance Fe availability (Figure 7; Table 2). Even when the Fe′ formed through reductive dissolution exceeds its solubility limit, it will hydrolyze and form fresh hydroxides which serve as a better iron source than their parent minerals (Stumm and Sulzberger, 1992; Yoshida et al., 2006). When reductive dissolution of mineral Fe is mediated, assisted, or occurs in the presence of organic ligands, it results in FeL (organically bound Fe) rather than Fe′ (Kraemer, 2004; Borer et al., 2005). As stated previously, Fe′ is acquired at faster rates than most studied dissolved organic Fe-complexes (see section “Phytoplankton Fe Acquisition Rates”, Figure 5), and hence the presence of organics may slow uptake down. On the other hand, organic complexation may sustain dissolved Fe in surface waters for longer and at concentrations exceeding its solubility limit (see next section “Biological Production and Release of Iron Binding Ligands”), subsequently providing phytoplankton with Fe over a longer duration.
Reduction of organically bound Fe(III). As reasoned above, reductive release of Fe′ from organic complexes is likely to enhance uptake, at least over short time scales (Figure 7; Table 2). When the ligand (L) undergoes photo-destruction, free, unchelated Fe(II) is liberated and Fe′ increases, as has been extensively demonstrated for Fe(III)EDTA (Hudson and Morel, 1993; Sunda and Huntsman, 1997). In other cases, Fe(II) may remain complexed as Fe(II)L, undergo reoxidation to Fe(III)L or dissociate as unchelated Fe(II) due to reduced ligand affinity for Fe(II) (Harrington and Crumbliss, 2009). Hence, some of these redox transformations will ultimately not alter Fe speciation, but others may temporarily shift Fe(III) from strong complexes into weak complexes, possibly enhancing Fe bioavailability.
Reduction of Fe(III)’. Unlike the former reductive processes which enhance Fe′ concentrations, the total unchelated iron pool remains unchanged when ferric Fe′ transforms into ferrous Fe(II)’ (Figure 7; Table 2). Nonetheless, this reaction mediated by enzymes on the cell surface, is central to the acquisition of Fe′ through the reductive uptake pathway described in Section “Uptake Pathways of Aquatic Phytoplankton,” at least for eukaryotes (Shaked et al., 2005). Fe(III)’ may alternatively undergo reduction by superoxide in the bulk solution (Voelker and Sedlak, 1995), but the effect of this process on Fe availability is disputable. Kustka et al. (2005) found that Fe′ uptake by diatoms remained unaffected by superoxide mediated reduction of Fe(III)’ in the experimental medium, while Rose et al. (2005) reported that superoxide enhanced Fe′ uptake by cyanobacteria. More work is required to evaluate the importance of bulk solution Fe′ reduction in increasing Fe availability.
Thus far we examined the effect of reductive processes on Fe availability and now we turn to the chemical nature and bioavailability of Fe(II), where increasing reports suggest that some of the measured Fe(II) in the ocean is organically bound (Croot et al., 2008; Roy et al., 2008). The existence of Fe(II) binding ligands is deduced from deviations in Fe(II) oxidation kinetics in some natural samples as compared to seawater free of organic matter (Roy et al., 2008). Currently, neither the identity nor the bioavailability of these complexes is known and hence elevated Fe(II) levels are not necessarily indicative of enhanced bioavailability. Indeed, phytoplankton iron stress was not alleviated in Fe fertilized areas where Fe(II) accounted for a significant fraction of the dissolved iron pool (Croot et al., 2008; Roy et al., 2008). Moreover, as the iron in Fe(II)L complexes is already reduced, phytoplankton cannot employ reductive pathways to liberate it from the complex prior to transport as they do for Fe(III)L (see Uptake Pathways of Aquatic Phytoplankton). Thus, if these Fe(II)L are slow to oxidize, we are faced with yet another challenge in determining the transport pathways of Fe(II)L.
Biological Production and Release of Iron Binding Ligands
Over the course of their short life span, aquatic microbes release copious amounts of biomolecules into their immediate environments. These diverse secretions and exudates include waste products, secondary metabolites, sugars, and proteins as well as cellular contents released via grazing, viral attack or programmed cell death (Bidle and Falkowski, 2004; Poorvin et al., 2004; Strzepek et al., 2005; Dalbec and Twining, 2009; Boyd et al., 2010a). While the minority of microbial secretions are synthesized and released with the explicit purpose of binding iron, many do possess an Fe-binding capacity of some kind (Rijkenberg et al., 2008; Hassler et al., 2011a; Levy et al., 2011). It is now well established that the overwhelming majority of dissolved (and in some cases colloidal) iron in most aquatic environments is found as organic complexes (Hunter and Boyd, 2007; Vraspir and Butler, 2009). Research efforts to elucidate the nature and origin of these Fe-binding organic ligands have yielded ambiguous results, reflecting the fact that (a) organic ligands may be introduced into aquatic systems via multiple pathways within and outside the water column and (b) organically bound iron (FeL) may be subjected to further biological and chemical transformations (Figure 7; Table 3). Not all organic Fe complexes are available to all phytoplankton, and even those FeL which are utilized are most likely less available than dissolved inorganic iron (Fe′; Figure 5; Morel et al., 2008). Nonetheless, iron binding ligands are vital for the maintenance of dissolved iron concentrations well above the Fe solubility limit and for retarding Fe aggregation and loss from surface waters (Kuma et al., 1996). Here we provide an abridged overview of some of the organic Fe complexes thought to exist in seawater, highlighting the ways in which organic complexation affects Fe speciation and bioavailability.
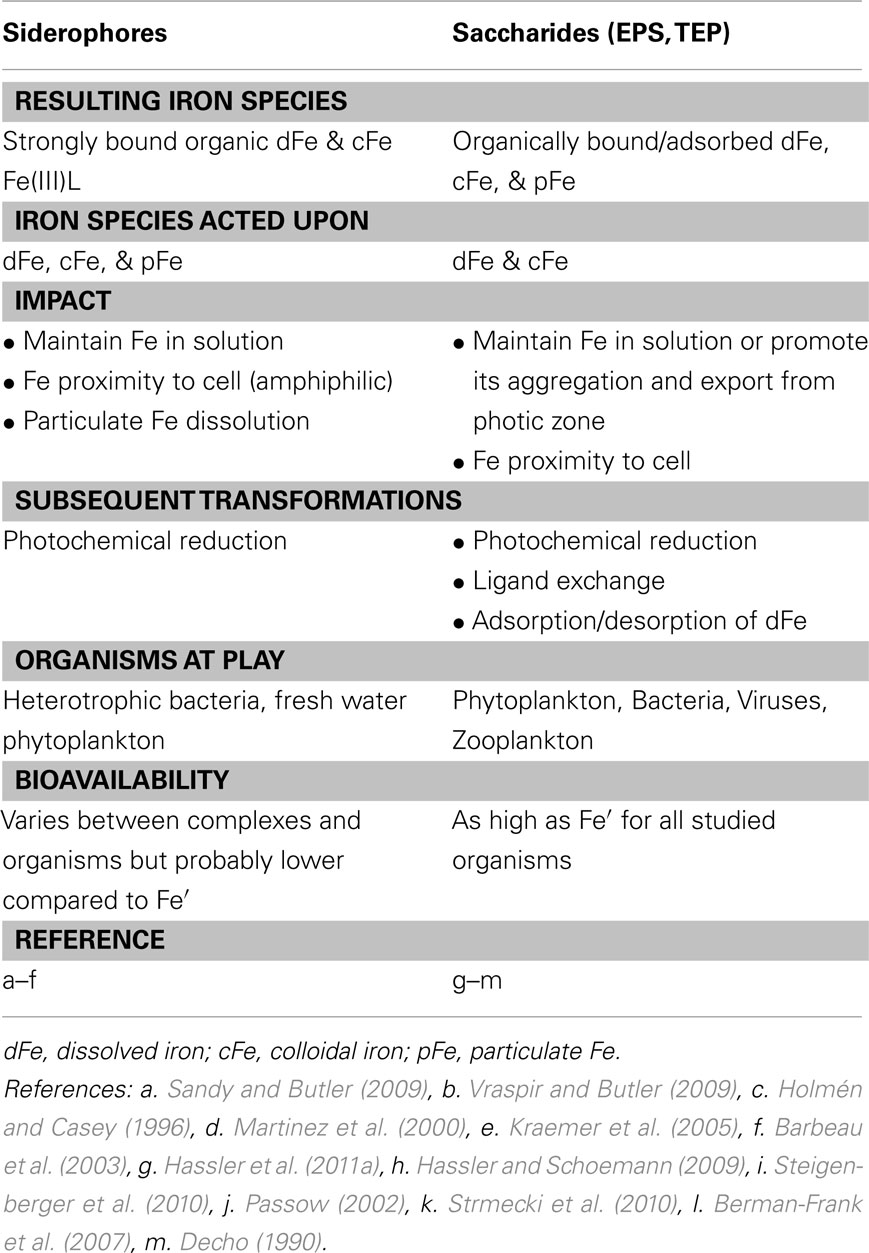
Table 3. The influence of siderophore and saccharide release on iron chemistry and availability in aquatic environments.
Speciation
Organic ligands capable of binding iron (and other metals), were detected in pelagic and coastal ocean waters, estuaries, lakes, and rivers, often in excess of dissolved Fe concentrations (Boye et al., 2001; Nagai et al., 2007; Duckworth et al., 2009; Hassler et al., 2009; Laglera and van den Berg, 2009; Buck et al., 2010). Using competitive ligand exchange (CLE) techniques, researchers have classified two major organic Fe ligand groups based on their stability constants with regards to Fe3+ – the strong L1 and weaker L2 class (e.g., Gledhill and van den Berg, 1994; Ibisanmi et al., 2011). A common view is that the strong L1 ligand class consists of siderophore-like molecules, while the less strongly complexing L2 ligands consist of cellular degradation products. However, the picture is probably more complex and there are additional weaker natural Fe ligands, such as saccharides, overlooked by CLE methods (Town and Filella, 2000; Hunter and Boyd, 2007; Boyd and Ellwood, 2010).
The most studied microbial Fe-binding exudates are siderophores, compounds which have been isolated from both freshwater and seawater (Table 3; Macrellis et al., 2001; Mawji et al., 2008; Velasquez et al., 2011). Typified by an exceptionally high Fe-binding capacity and low molecular weight, siderophores are produced under iron limitation by marine heterotrophic bacteria and some fresh water cyanobacteria (e.g., Haygood et al., 1993; Vraspir and Butler, 2009), while production by marine cyanobacteria remains controversial (Hopkinson and Morel, 2009). Less studied, but widely spread microbial exudates capable of binding iron are exopolymer substances (EPS) and their transparent exopolymer particles (TEP) derivatives (Table 3). These high molecular weight saccharide-rich exopolymers are secreted by most microorganisms, including phytoplankton, bacteria, and zooplankton (Decho, 1990; Passow, 2002; Wotton, 2004; Croot et al., 2007). EPS are thought to weakly bind iron compared to siderophores based on recent data from several model saccharides (Hassler et al., 2011a). TEP, on the other hand, have been shown to have a high affinity for Fe (Quigley et al., 2002). Many other compounds secreted or released due to grazing and lysis contribute to the “Fe ligand soup” in aquatic environments (Boye et al., 2005; Tsuda et al., 2007; Strmecki et al., 2010; Poorvin et al., 2011). The release of iron binding ligands in cultures supplemented with Fe was reported for the marine haptophyte E. huxleyi and two diatom species, qualifying these as non-siderophore Fe-binding ligands (Boye and van den Berg, 2000; Rijkenberg et al., 2008). Some toxins, capable of binding Fe (but not as strongly as siderophores) are known to be synthesized by harmful bloom forming phytoplankton upon Fe limitation (Rue and Bruland, 2001). Secretion of both the neurotoxin domoic acid (a water soluble amino acid produced by Pseudo-nitzschia spp.) and the hepatoxin microcystin (a peptide produced by Microcystis aeruginosa) were shown to improve growth under low Fe conditions (Maldonado et al., 2002; Wells et al., 2005; Alexova et al., 2011). Interestingly, these toxins do not directly provide the cell with Fe, but rather increase copper supply for Pseudo-nitzschia spp. enabling the synthesis of copper containing high affinity iron uptake proteins (Maldonado et al., 2002; Wells et al., 2005), and probably aid M. aeruginosa in oxidative damage protection (Alexova et al., 2011). In addition, terrestrial humics have recently been suggested to be important iron binding ligands in open ocean waters (van den Berg, 1995; Laglera and van den Berg, 2009; Laglera et al., 2011).
Bioavailability
At the cellular level, acquisition of a specific compound depends to a large degree on the uptake machinery possessed by an organism (see Phytoplankton Fe Acquisition Systems). In the natural environment, additional parameters such as the compound residence time in surface waters and its tendency to undergo biological and chemical transformation influence its ability to support growth (Figure 1). In this section we briefly examine the effect of environmental and chemical factors on phytoplankton Fe acquisition from EPS and siderophore bound iron.
At the organism level, recent studies have found that iron bound to model saccharides is highly available to diatoms and natural phytoplankton assemblages (Figure 5; Table 3; Hassler and Schoemann, 2009; Hassler et al., 2011a,b). This was accounted for by the ability of saccharides to stabilize iron in the dissolved and colloidal form and increase the labile Fe pool (Hassler et al., 2011b). In the natural environment, the effect of saccharides on Fe solubility and residence time in the surface water is less clear. EPS and TEP were shown to promote aggregate formation and particle export (Decho, 1990; Passow, 2002), thus possibly shortening the residence time of saccharide bound Fe in surface waters (Berman-Frank et al., 2007). Additionally, iron bound to saccharides may be subjected to chemical and photochemical transformations, further altering its fate and accessibility to phytoplankton. Iron is weakly bound to saccharides and may be exchanged with stronger ligands such as siderophores (Hunter and Boyd, 2007). Photochemical Fe(II) production from saccharide bound iron was recently reported (Steigenberger et al., 2010), but its environmental repercussions are unexplored as yet. Lastly, EPS often create protective microenvironments around microbial consortia, single cells, and cell aggregates (Decho, 1990; Passow, 2002). The formation of such microhabitats impacts all concentration dependent processes and, as such, EPS surrounded cells may be diffusion limited when it comes to acquiring dissolved Fe from the surrounding waters. However, EPS confers two significant advantages on cells: firstly, EPS are highly adsorbent allowing for the storage and easier processing of iron and secondly the confined microenvironment provides protection from diffusive losses of Fe associated with the EPS (Sunda, 2001; Hassler et al., 2011a).
The role of siderophores in supporting phytoplankton growth in situ has received more attention than any other Fe chelator, but in turn raised many new questions (Table 3; Maldonado et al., 2005; Pickell et al., 2009). As the acquisition of siderophores was detailed in Section “Phytoplankton Fe Acquisition Systems,” we briefly discuss two chemical features of siderophores with potential repercussions for iron fate and bioavailability. Firstly, many of the Fe-siderophore complexes isolated from marine bacteria are photolabile (Barbeau et al., 2001, 2003; Vraspir and Butler, 2009). However, due to the fast oxidation of Fe(II) within the complex and since the photoproduct still binds Fe with high affinity, this photo-reactivity does not seem to confer any clear biological advantage (Vraspir and Butler, 2009). This is not always the case however: recently, photo-reduction of iron bound to a newly identified siderophore, vibrioferrin, and the subsequent release of Fe′ was reported to enhance iron uptake by dinoflagellates (Amin et al., 2009). Vibrioferrin undergoes photo-degradation and does not retain significant Fe-binding capacity, thus releasing Fe′. Secondly, many marine siderophore isolates also tend to be amphiphilic and closely associate with bacterial membranes, possibly preventing their loss by diffusion in the marine environment (Vraspir and Butler, 2009). Although the study of amphiphilic siderophore partitioning to membranes is at a very early stage, it may bear interesting implications for the function of siderophores in iron acquisition in the ocean.
Summation
The topic of iron bioavailability has garnered wide spread interest from the scientific community, yet due to its intrinsic complexity a well-rounded understanding of this concept is lacking. In this contribution we have attempted to disassemble bioavailability and address some of its more biologically orientated facets. Of the many topics covered in this synthesis, we conclude with a summary of the principal arguments and perhaps unorthodox perspectives which we hope lend some insights into Fe bioavailability in the aquatic environment.
• Iron acquisition by means of reduction is a widespread Fe uptake strategy in the ocean, common to both eukaryotic and prokaryotic phytoplankton. Both experimental and genomic data challenge the prevalent paradigm of siderophore-based Fe uptake as an exclusive iron acquisition pathway amongst marine cyanobacteria. We propose that the occurrence of siderophore vs. reductive iron uptake can be put down to environmental rather than taxonomic considerations. Organisms residing in densely populated, low turbulence environments (e.g., fecal pellets, marine snow, or colony-consortiums) will favor siderophore-based Fe acquisition, while pelagic phytoplankton will favor the non-specific reductive strategy enabling them to access the dilute, heterogeneous Fe pool.
• The availability of various Fe-substrates to phytoplankton can be viewed as a spectrum rather than an absolute “all or nothing.” A bioavailability scale can be established by comparing substrate normalized uptake rates (by means of the uptake rate constant – kup). We suggest that a further normalization of FeL uptake constants relative to the Fe′ uptake constant is highly useful in the comparison of different studies, organisms and environments. Hence, in order to establish a baseline for the determination of relative bioavailability, we urge for the use of EDTA in order to better regulate Fe′ instead of or in addition to ligand-free FeCl3. Looking beyond short term uptake, kup can be used to gage if a specific compound is likely to be a significant contributor to the Fe requirements of phytoplankton in the environment. This same approach can be extended to natural systems where kup values of natural ligands by known organisms may be compared to model ligands while the kup values of known Fe-substrates by natural communities may be contrasted with those of model organisms.
• For a fuller understanding of iron bioavailability we look beyond phytoplankton iron uptake pathways and rates. Microorganisms are not only influenced by their environment but are themselves agents of change when it comes to Fe bioavailability. Bio-mediated redox transformations, microbial exudates and secretions as well as food web interactions impact iron speciation, residence time in surface waters and ultimate accessibility to phytoplankton be it in a positive or negative manner.
• Due to the greater solubility of Fe(II) compared to Fe(III), the occurrence of reductive processes and/or measurable Fe(II) concentrations is often equated with increased bioavailability. Accumulating evidence suggests that Fe(II) in seawater is organically bound may alter this view, as it is unclear to what degree Fe(II)L is accessible to phytoplankton. Moreover, iron availability does not necessarily increase following its reduction. Factors such as the characteristics of the Fe species undergoing reduction, the reductive pathway, the presence of other ligands and oxidants in the immediate surroundings, and ultimately the uptake machinery of the phytoplankton utilizing this iron, should be considered when evaluating the effect of reductive processes on iron availability.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank M. Maldonado, B. Sunda, B. Hopkinson, F. Morel, P. Croot, and C. Kranzler for their insights prior and during the preparation of this manuscript as well as the reviewers and editor for their valuable comments. This work was supported in part by the Israel Science Foundation grant 933/07, the Israel USA Binational Science Foundation grant 2008097 and the Assemble FP7 research grant awarded to H. Lis. This work is in partial fulfillment of the requirements for a Ph. D thesis to H. Lis from the Hebrew University.
References
Alexova, R., Fujii, M., Birch, D., Cheng, J., Waite, T. D., Ferrari, B. C., and Neilan, B. A. (2011). Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 13, 1064–1077.
Alldredge, A. L., and Cohen, Y. (1987). Can microscale chemical patches persist in the sea – microelectrode study of marine snow, fecal pellets. Science 235, 689–691.
Allen, A. E., Laroche, J., Maheswari, U., Lommer, M., Schauer, N., Lopez, P. J., Finazzi, G., Fernie, A. R., and Bowler, C. (2008). Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc. Natl. Acad. Sci. U.S.A. 105, 10438–10443.
Allen, M. D., Del Campo, J. A., Kropat, J., and Merchant, S. S. (2007). FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryotic Cell 6, 1841–1852.
Allnutt, F. C. T., and Bonner, W. D. J. (1987). Evaluation of reductive release as a mechanism for iron uptake from ferrioxamine B by Chlorella vulgaris. Plant Physiol. 85, 751–756.
Amin, S. A., Green, D. H., Kuepper, F. C., and Carrano, C. J. (2009). Vibrioferrin, an unusual marine siderophore: iron binding, photochemistry, and biological implications. Inorg. Chem. 48, 11451–11458.
Anderson, M. A., and Morel, F. M. M. (1982). The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol. Oceanogr. 27, 789–813.
Atkinson, A., and Guerinot, M. (2011). “Metal transport,” in The Plant Plasma Membrane, eds A. S. Murphy, B. Schulz, and W. Peer (Berlin: Springer), 303–330.
Balzano, S., Statham, P. J., Pancost, R. D., and Lloyd, J. R. (2009). Role of microbial populations in the release of reduced iron to the water column from marine aggregates. Aquat. Microb. Ecol. 54, 291–303.
Barbeau, K. (2006). Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem. Photobiol. 82, 1505–1516.
Barbeau, K., and Moffett, J. W. (2000). Laboratory and field studies of colloidal iron oxide dissolution as mediated by phagotrophy and photolysis. Limnol. Oceanogr. 45, 827–835.
Barbeau, K., Moffett, J. W., Caron, D. A., Croot, P. L., and Erdner, D. L. (1996). Role of protozoan grazing in relieving iron limitation of phytoplankton. Nature 380, 61–64.
Barbeau, K., Rue, E. L., Bruland, K. W., and Butler, A. (2001). Photochemical cycling of iron in the surface ocean mediated by microbial iron (III) -binding ligands. Nature 413, 409–413.
Barbeau, K., Rue, E. L., Trick, C. G., Bruland, K. T., and Butler, A. (2003). Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnol. Oceanogr. 48, 1069–1078.
Batchelli, S., Muller, F. L. L., Chang, K.-C., and Lee, C.-L. (2010). Evidence for strong but dynamic iron-humic colloidal associations in humic-rich coastal waters. Environ. Sci. Technol. 44, 8485–8490.
Berman-Frank, I., Rosenberg, G., Levitan, O., Haramaty, L., and Mari, X. (2007). Coupling between autocatalytic cell death and transparent exopolymeric particle production in the marine cyanobacterium Trichodesmium. Environ. Microbiol. 9, 1415–1422.
Bidle, K. D., and Falkowski, P. G. (2004). Cell death in planktonic, photosynthetic microorganisms. Nat. Rev. Microbiol. 2, 643–655.
Blain, S., Queguiner, B., Armand, L., Belviso, S., Bombled, B., Bopp, L., Bowie, A., Brunet, C., Brussaard, C., Carlotti, F., Christaki, U., Corbiere, A., Durand, I., Ebersbach, F., Fuda, J.-L., Garcia, N., Gerringa, L., Griffiths, B., Guigue, C., Guillerm, C., Jacquet, S., Jeandel, C., Laan, P., Lefevre, D., Lo Monaco, C., Malits, A., Mosseri, J., Obernosterer, I., Park, Y.-H., Picheral, M., Pondaven, P., Remenyi, T., Sandroni, V., Sarthou, G., Savoye, N., Scouarnec, L., Souhaut, M., Thuiller, D., Timmermans, K., Trull, T., Uitz, J., Van Beek, P., Veldhuis, M., Vincent, D., Viollier, E., Vong, L., and Wagener, T. (2007). Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature 446, U1070–U1071.
Borer, P. M., Sulzberger, B., Reichard, P., and Kraemer, S. M. (2005). Effect of siderophores on the light-induced dissolution of colloidal iron(III) (hydr)oxides. Mar. Chem. 93, 179–193.
Bowler, C., Vardi, A., and Allen, A. E. (2010). Oceanographic and biogeochemical insights from diatom genomes. Ann. Rev. Mar. Sci. 2, 333–365.
Boyd, P. W., and Ellwood, M. J. (2010). The biogeochemical cycle of iron in the ocean. Nat. Geosci. 3, 675–682.
Boyd, P. W., Ibisanmi, E., Sander, S. G., Hunter, K. A., and Jackson, G. A. (2010a). Remineralization of upper ocean particles: implications for iron biogeochemistry. Limnol. Oceanogr. 55, 1271–1288.
Boyd, P. W., Mackie, D. S., and Hunter, K. A. (2010b). Aerosol iron deposition to the surface ocean – modes of iron supply and biological responses. Mar. Chem. 120, 128–143.
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., Coale, K. H., Cullen, J. J., De Baar, H. J. W., Follows, M., Harvey, M., Lancelot, C., Levasseur, M., Owens, N. P. J., Pollard, R., Rivkin, R. B., Sarmiento, J., Schoemann, V., Smetacek, V., Takeda, S., Tsuda, A., Turner, S., and Watson, A. J. (2007). Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617.
Boye, M., Nishioka, J., Croot, P. L., Laan, P., Timmermans, K. R., and De Baar, H. J. W. (2005). Major deviations of iron complexation during 22 days of a mesoscale iron enrichment in the open Southern Ocean. Mar. Chem. 96, 257–271.
Boye, M., and van den Berg, C. M. G. (2000). Iron availability and the release of iron-complexing ligands by Emiliania huxleyi. Mar. Chem. 70, 277–287.
Boye, M., Van Den Berg, C. M. G., De Jong, J. T. M., Leach, H., Croot, P., and De Baar, H. J. W. (2001). Organic complexation of iron in the Southern Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 48, 1477–1497.
Boyle, E. A. (1988). Cadmium: chemical tracer of deepwater paleoceanography. Paleoceanography 3, 471–489.
Brand, L. E. (1991). Minimum iron requirements of marine phytoplankton and the implications for biogeochemical control of new production. Limnol. Oceanogr. 36, 1756–1772.
Breitbarth, E., Gelting, J., Walve, J., Hoffmann, L. J., Turner, D. R., Hassellov, M., and Ingri, J. (2009). Dissolved iron (II) in the Baltic Sea surface water and implications for cyanobacterial bloom development. Biogeosciences 6, 2397–2420.
Buck, C. S., Landing, W. M., Resing, J. A., and Lebon, G. T. (2006). Aerosol iron and aluminum solubility in the northwest Pacific Ocean: results from the 2002 IOC cruise. Geochem. Geophys. Geosyst. 7, Q04M07.
Buck, K. N., Selph, K. E., and Barbeau, K. A. (2010). Iron-binding ligand production and copper speciation in an incubation experiment of Antarctic Peninsula shelf waters from the Bransfield Strait, Southern Ocean. Mar. Chem. 122, 148–159.
Buesseler, K. O., Doney, S. C., Karl, D. M., Boyd, P. W., Caldeira, K., Chai, F., Coale, K. H., De Baar, H. J. W., Falkowski, P. G., Johnson, K. S., Lampitt, R. S., Michaels, A. F., Naqvi, S. W. A., Smetacek, V., Takeda, S., and Watson, A. J. (2008). Environment – ocean iron fertilization – moving forward in a sea of uncertainty. Science 319, 162.
Chen, J.-C., Hsieh, S. I., Kropat, J., and Merchant, S. S. (2008). A ferroxidase encoded by FOX1 contributes to iron assimilation under conditions of poor iron nutrition in Chlamydomonas. Eukaryotic Cell 7, 541–545.
Chen, M., and Wang, W. (2008). Accelerated uptake by phytoplankton of iron bound to humic acids. Aquat. Biol. 3, 155–166.
Chen, Y., Tovar-Sanchez, A., Siefert, R. L., Sanudo-Wilhelmy, S. A., and Zhuang, G. (2011). Luxury uptake of aerosol iron by Trichodesmium in the western tropical North Atlantic. Geophys. Res. Lett. 38, L18602.
Croot, P. L., Bluhm, K., Schlosser, C., Streu, P., Breitbarth, E., Frew, R., and Van Ardelan, M. (2008). Regeneration of Fe(II) during EIFeX and SOFeX. Geophys. Res. Lett. 35, L19606.
Croot, P. L., Bowie, A. R., Frew, R. D., Maldonado, M. T., Hall, J. A., Safi, K. A., La Roche, J., Boyd, P. W., and Law, C. S. (2001). Retention of dissolved iron and Fe-II in an iron induced Southern Ocean phytoplankton bloom. Geophys. Res. Lett. 28, 3425–3428.
Croot, P. L., Laan, P., Nishioka, J., Strass, V., Cisewski, B., Boye, M., Timmermans, K. R., Bellerby, R. G., Goldson, L., Nightingale, P., and De Baar, H. J. W. (2005). Spatial and temporal distribution of Fe(II) and H2O2 during EisenEx, an open ocean mescoscale iron enrichment. Mar. Chem. 95, 65–88.
Croot, P. L., Passow, U., Assmy, P., Jansen, S., and Strass, V. H. (2007). Surface active substances in the upper water column during a Southern Ocean Iron Fertilization Experiment (EIFEX). Geophys. Res. Lett. 34, C06015.
Cullen, J. T., Lane, T. W., Morel, F. M. M., and Sherrell, R. M. (1999). Modulation of cadmium uptake in phytoplankton by seawater CO2 concentration. Nature 402, 165–167.
Dalbec, A., and Twining, B. (2009). Remineralization of bioavailable iron by a heterotrophic dinoflagellate. Aquat. Microb. Ecol. 54, 279–290.
Decho, A. (1990). Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Ann. Rev. 28, 73–153.
Duckworth, O. W., Holmstroem, S. J. M., Pena, J., and Sposito, G. (2009). Biogeochemistry of iron oxidation in a circumneutral freshwater habitat. Chem. Geol. 260, 149–158.
Eckhardt, U., and Buckhout, T. J. (1998). Iron assimilation in Chlamydomonas reinhardtii involves ferric reduction and is similar to strategy I higher plants. J. Exp. Bot. 49, 1219–1226.
Elderfield, H., and Rickaby, R. E. M. (2000). Oceanic Cd/P ratio and nutrient utilization in the glacial Southern Ocean. Nature 405, 305–310.
Emmenegger, L., Schonenberger, R. R., Sigg, L., and Sulzberger, B. (2001). Light-induced redox cycling of iron in circumneutral lakes. Limnol. Oceanogr. 46, 49–61.
Falkowski, P. G., Barber, R. T., and Smetacek, V. (1998). Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206.
Finkel, Z. V., Beardall, J., Flynn, K. J., Quigg, A., Rees, T. A. V., and Raven, J. A. (2010). Phytoplankton in a changing world: cell size and elemental stoichiometry. J. Plankton Res. 32, 119–137.
Fujii, M., Rose, A. L., Omura, T., and Waite, T. D. (2010). Effect of Fe(II) and Fe(III) transformation kinetics on iron acquisition by a toxic strain of Microcystis aeruginosa. Environ. Sci. Technol. 44, 1980–1986.
Gerringa, L. J. A., De Baar, H. J. W., and Timmermans, K. R. (2000). A comparison of iron limitation of phytoplankton in natural oceanic waters and laboratory media conditioned with EDTA. Mar. Chem. 68, 335–346.
Gledhill, M., and van den Berg, C. M. G. (1994). Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar. Chem. 47, 41–54.
Goldman, S. J., Lammers, P. J., Berman, M. S., and Sanders-Loehr, J. (1983). Siderophore-mediated iron uptake in different strains of Anabaena sp. J. Bacteriol. 156, 1144–1150.
Gonzalez-Davila, M., Santana-Casiano, J. M., and Millero, F. J. (2005). Oxidation of Iron(II) nanomolar with H2O2 in seawater. Geochim. Cosmochim. Acta 69, 83–93.
Greene, R. M., Geider, R. J., and Falkowski, P. G. (1991). Effect of iron limitation on photosynthesis in a marine diatom. Limnol. Oceanogr. 36, 1772–1782.
Hansard, S. P., Landing, W. M., Measures, C. I., and Voelker, B. M. (2009). Dissolved iron(II) in the Pacific Ocean: measurements from the PO2 and P16N CLIVAR/CO2 repeat hydrography expeditions. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 1117–1129.
Hansard, S. P., Vermilyea, A. W., and Voelker, B. M. (2010). Measurements of superoxide radical concentration and decay kinetics in the Gulf of Alaska. Deep Sea Res. Part I Oceanogr. Res. Pap. 57, 1111–1119.
Harrington, J., and Crumbliss, A. (2009). The redox hypothesis in siderophore-mediated iron uptake. Biometals 22, 679–689.
Hassler, C. S., Alasonati, E., Nichols, C. A. M., and Slaveykova, V. I. (2011a). Exopolysaccharides produced by bacteria isolated from the pelagic Southern Ocean – role in Fe binding, chemical reactivity, and bioavailability. Mar. Chem. 123, 88–98.
Hassler, C. S., Schoemann, V., Nichols, C. M., Butler, E. C. V., and Boyd, P. W. (2011b). Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proc. Natl. Acad. Sci. U.S.A. 108, 1076–1081.
Hassler, C. S., Havens, S. M., Bullerjahn, G. S., Mckay, R. M. L., and Twiss, M. R. (2009). An evaluation of iron bioavailability and speciation in western Lake Superior with the use of combined physical, chemical, and biological assessment. Limnol. Oceanogr. 54, 987–1001.
Hassler, C. S., and Schoemann, V. (2009). Bioavailability of organically bound Fe to model phytoplankton of the Southern Ocean. Biogeosciences 6, 2281–2296.
Haygood, M. G., Holt, P. D., and Butler, A. (1993). Aerobactin production by a planktonic marine Vibrio Sp. Limnol. Oceanogr. 38, 1091–1097.
Henley, W. J., and Yin, Y. (1998). Growth and photosynthesis of marine Synechococcus (Cyanophyceae) under iron stress. J. Phycol. 34, 94–103.
Higgins, J. L., Kudo, I., Nishioka, J., Tsuda, A., and Wilhelm, S. W. (2009). The response of the virus community to the SEEDS II mesoscale iron fertilization. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 2788–2795.
Holmén, B. A., and Casey, W. H. (1996). Hydroxamate ligands, surface chemistry, and the mechanism of ligand-promoted dissolution of goethite [α-FeOOH(s)]. Geochim. Cosmochim. Acta 60, 4403–4416.
Hopkinson, B. M., and Barbeau, K. A. (2012). Iron transporters in marine prokaryotic genomes and metagenomes. Environ. Microbiol. 14, 114–128.
Hopkinson, B. M., and Morel, F. M. M. (2009). The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 22, 659–669.
Hudson, R. J. M., and Morel, F. M. M. (1993). Trace metal transport by marine microorganisms: implications of metal coordination kinetics. Deep Sea Res. A 40, 129–150.
Hunter, K. A., and Boyd, P. W. (2007). Iron-binding ligands and their role in the ocean biogeochemistry of iron. Environ. Chem. 4, 221–232.
Hutchins, D. A., Rueter, J. G., and Fish, W. (1991). Siderophore production and nitrogen fixation are mutually exclusive strategies in Anabaena 7120. Limnol. Oceanogr. 36, 1–12.
Hutchins, D. A., Witter, A. E., Butler, A., and Luther, G. W. (1999). Competition among marine phytoplankton for different chelated iron speciation. Nature 400, 858–861.
Ibisanmi, E., Sander, S. G., Boyd, P. W., Bowie, A. R., and Hunter, K. A. (2011). Vertical distributions of iron-(III) complexing ligands in the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 58, 2113–2125.
Johnson, K. S., Coale, K. H., Elrod, V. A., and Tindale, N. W. (1994). Iron photochemistry in seawater from the equatorial Pacific. Mar. Chem. 46, 319–334.
Johnson, K. S., Gordon, R. M., and Coale, K. H. (1997). What controls dissolved iron concentrations in the world ocean? Mar. Chem. 57, 137–161.
Jones, G. J., and Morel, F. M. M. (1988). Plasmalemma redox activity in the diatom Thalassiosira. Plant Physiol. 87, 143–147.
Keshtacher, L. E., Hadar, Y., and Chen, Y. (1999). Fe nutrition demand and utilization by the green alga Dunaliella bardawil. Plant Soil 215, 175–182.
Kieber, R. J., Williams, K., Willey, J. D., Skrabal, S., and Avery, G. B. Jr. (2001). Iron speciation in coastal rainwater: concentration and deposition to seawater. Mar. Chem. 73, 83–95.
King, D. W., Lounsbury, H. A., and Millero, F. J. (1995). Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ. Sci. Technol. 29, 818–824.
Kraemer, S. M. (2004). Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 66, 3–18.
Kraemer, S. M., Butler, A., Borer, P., and Cervini-Silva, J. (2005). “Siderophores and the dissolution of iron-bearing minerals in marine systems,” in Molecular Geomicrobiology, eds J. E. Banfield, J. Cervini-Silva, and K. H. Nealson (Mineralogical Society of America, Geochemical Society), 53–84.
Kranzler, C., Lis, H., Shaked, Y., and Keren, N. (2011). The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 13, 2990–2999.
Kudo, I., and Harrison, P. J. (1997). Effect of iron nutrition on the marine cyanobacterium Synechococcus grown on different N sources and irradiances. J. Phycol. 33, 232–240.
Kuma, K., Nakabayashi, S., Suzuki, Y., Kudo, I., and Matsunaga, K. (1992). Photo-reduction of iron(III) by dissolved organic substances and existence of iron(II) in seawater during spring blooms. Mar. Chem. 37, 15–27.
Kuma, K., Nishioka, J., and Matsunaga, K. (1996). Controls on iron(III) hydroxide solubility in seawater: the influence of pH and natural organic chelators. Limnol. Oceanogr. 41, 396–407.
Kustka, A., Carpenter, E. J., and Sanudo-Wilhelmy, S. A. (2002). Iron and marine nitrogen fixation: progress and future directions. Res. Microbiol. 153, 255–262.
Kustka, A. B., Allen, A. E., and Morel, F. M. M. (2007). Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatoms. J. Phycol. 43, 715–729.
Kustka, A. B., Shaked, Y., Milligan, A. J., King, D. W., and Morel, F. M. M. (2005). Extracellular production of superoxide by marine diatoms: contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr. 50, 1172–1180.
Laglera, L. M., Battaglia, G., and Van Den Berg, C. M. G. (2011). Effect of humic substances on the iron speciation in natural waters by CLE/CSV. Mar. Chem. 127, 134–143.
Laglera, L. M., and van den Berg, C. M. G. (2009). Evidence for geochemical control of iron by humic substances in seawater. Limnol. Oceanogr. 54, 610–619.
Lampitt, R. S., Achterberg, E. P., Anderson, T. R., Hughes, J. A., Iglesias-Rodriguez, M. D., Kelly-Gerreyn, B. A., Lucas, M., Popova, E. E., Sanders, R., Shepherd, J. G., Smythe-Wright, D., and Yool, A. (2008). Ocean fertilization: a potential means of geoengineering? Philos. Transact. R. Soc. A Math. Phys. Eng. Sci. 366, 3919–3945.
Lane, E. S., Semeniuk, D. M., Strzepek, R. F., Cullen, J. T., and Maldonado, M. T. (2009). Effects of iron limitation on intracellular cadmium of cultured phytoplankton: implications for surface dissolved cadmium to phosphate ratios. Mar. Chem. 115, 155–162.
Law, C. S., and Ling, R. D. (2001). Nitrous oxide flux and response to increased iron availability in the Antarctic Circumpolar Current. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 2509–2527.
Levy, J., Zhang, H., Davison, W., and Groben, R. (2011). Using diffusive gradients in thin films to probe the kinetics of metal interaction with algal exudates. Environ. Chem. 8, 517–524.
Lis, H., and Shaked, Y. (2009). Probing the bioavailability of organically bound iron: a case study in the Synechococcus-rich waters of the Gulf of Aqaba. Aquat. Microb. Ecol. 56, 241–253.
Liss, P. S. (2007). Trace gas emissions from the marine biosphere. Philos. Transact. R. Soc. A Math. Phys. Eng. Sci. 365, 1697–1704.
Lohan, M. C., and Bruland, K. W. (2008). Elevated Fe(II) and dissolved Fe in hypoxic shelf waters off Oregon and Washington: an enhanced source of iron to coastal upwelling regimes. Environ. Sci. Technol. 42, 6462–6468.
Macrellis, H. M., Trick, C. G., Rue, E. L., Smith, G., and Bruland, K. W. (2001). Collection and detection of natural iron-binding ligands from seawater. Mar. Chem. 76, 175–187.
Maldonado, M. T., Allen, A. E., Chong, J. S., Dan Leus, K. L., Karpenko, N., and Harris, S. L. (2006). Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 51, 1729–1743.
Maldonado, M. T., Boyd, P. W., Harrison, P. J., and Price, N. M. (1999). Co-limitation of phytoplankton growth by light and Fe during winter in the NE subarctic Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 46, 2475–2485.
Maldonado, M. T., Hughes, M. P., Rue, E. L., and Wells, M. L. (2002). The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 47, 515–526.
Maldonado, M. T., and Price, N. M. (1999). Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 46, 2447–2473.
Maldonado, M. T., and Price, N. M. (2001). Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J. Phycol. 37, 298–309.
Maldonado, M. T., Strzepek, R. F., Sander, S., and Boyd, P. W. (2005). Acquisition of iron bound to strong organic complexes, with different Fe binding groups and photochemical reactivities, by plankton communities in Fe-limited subantarctic waters. Global Biogeochem. Cycles 19, GB4S23.
Manizza, M., Le Quere, C., Watson, A. J., and Buitenhuis, E. T. (2005). Bio-optical feedbacks among phytoplankton, upper ocean physics and sea-ice in a global model. Geophys. Res. Lett. 32, L05603.
Maranger, R., Bird, D. F., and Price, N. M. (1998). Iron acquisition by photosynthetic marine phytoplankton from ingested bacteria. Nature 396, 248–251.
Marchetti, A., and Cassar, N. (2009). Diatom elemental and morphological changes in response to iron limitation: a brief review with potential paleoceanographic applications. Geobiology 7, 419–431.
Marchetti, A., Maldonado, M. T., Lane, E. S., and Harrison, P. J. (2006). Iron requirements of the pennate diatom Pseudo-nitzschia: comparison of oceanic (high-nitrate, low-chlorophyll waters) and coastal species. Limnol. Oceanogr. 51, 2092–2101.
Martin, J. H., Fitzwater, S. E., and Gordon, R. M. (1990). Iron deficiency limits phytoplankton growth in Antarctic waters. Global Biogeochem. Cycles 4, 5–12.
Martinez, J. S., Zhang, G. P., Holt, P. D., Jung, H. T., Carrano, C. J., Haygood, M. G., and Butler, A. (2000). Self-assembling amphiphilic siderophores from marine bacteria. Science 287, 1245–1247.
Martinez-Garcia, A., Rosell-Mele, A., Jaccard, S. L., Geibert, W., Sigman, D. M., and Haug, G. H. (2011). Southern Ocean dust-climate coupling over the past four million years. Nature 476, 312–315.
Mawji, E., Gledhill, M., Milton, J. A., Tarran, G. A., Ussher, S., Thompson, A., Wolff, G. A., Worsfold, P. J., and Achterberg, E. P. (2008). Hydroxamate Siderophores: occurrence and Importance in the Atlantic Ocean. Environ. Sci. Technol. 42, 8675–8680.
McKay, R. M. L., Villareal, T. A., and La Roche, J. (2000). Vertical migration by Rhizosolenia spp. (Bacillariophyceae): implications for Fe acquisition. J. Phycol. 36, 669–674.
Meskhidze, N., and Nenes, A. (2006). Phytoplankton and cloudiness in the Southern Ocean. Science 314, 1419–1423.
Middlemiss, J. K., Anderson, A. M., Stratilo, C. W., and Weger, H. G. (2001). Oxygen consumption associated with ferric reductase activity and iron uptake by iron-limited cells of Chlorella kessleri (Chlorophyceae). J. Phycol. 37, 393–399.
Miller, W. L., King, D. W., Lin, J., and Kester, D. R. (1995). Photochemical redox cycling of iron in coastal seawater. Mar. Chem. 50, 63–77.
Milligan, A. J., and Harrison, P. J. (2000). Effects of non-steady-state iron limitation on nitrogen assimilatory enzymes in the marine diatom Thalassiosira weissflogii (Bacillariophyceae). J. Phycol. 36, 78–86.
Milne, A., Davey, M. S., Worsfold, P. J., Achterberg, E. P., and Taylor, A. R. (2009). Real-time detection of reactive oxygen species generation by marine phytoplankton using flow-injection chemiluminescence. Limnol. Oceanogr. Methods 7, 705–716.
Moffett, J. W., Goeffert, T. J., and Naqvi, S. W. A. (2007). Reduced iron associated with secondary nitrite maxima in the Arabian Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 1341–1349.
Moore, C. M., Mills, M. M., Achterberg, E. P., Geider, R. J., Laroche, J., Lucas, M. I., Mcdonagh, E. L., Pan, X., Poulton, A. J., Rijkenberg, M. J. A., Suggett, D. J., Ussher, S. J., and Woodward, E. M. S. (2009). Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat. Geosci. 2, 867–871.
Morel, F. M. M., Kustka, A. B., and Shaked, Y. (2008). The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol. Oceanogr. 53, 400–404.
Morel, F. M. M., and Price, N. M. (2003). The biogeochemical cycles of trace metals in the oceans. Science 300, 944–947.
Nagai, T., Imai, A., Matsushige, K., Yokoi, K., and Fukushima, T. (2007). Dissolved iron and its speciation in a shallow eutrophic lake and its inflowing rivers. Water Res. 41, 775–784.
Nodwell, L., and Price, N. (2001). Direct use of inorganic colloidal iron by marine mixotrophic phytoplankton. Limnol. Oceanogr. 46, 755–777.
Passow, U. (2002). Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 55, 287–333.
Pick, U., Paz, Y., Weiss, M., and Katz, A. (2008). A unique mechanism for Fe3+ and Fe2+ acquisition in a halotolerant alga. Plant Biol. (Rockville) 2008, 142.
Pickell, L. D., Wells, M. L., Trick, C. G., and Cochlan, W. P. (2009). A sea-going continuous culture system for investigating phytoplankton community response to macro- and micro-nutrient manipulations. Limnol. Oceanogr. Methods 7, 21–32.
Poorvin, L., Rinta-Kanto, J. M., Hutchins, D. A., and Wilhelm, S. W. (2004). Viral release of iron and its bioavailability to marine plankton. Limnol. Oceanogr. 49, 1734–1741.
Poorvin, L., Sander, S. G., Velasquez, I., Ibisanmi, E., Lecleir, G. R., and Wilhelm, S. W. (2011). A comparison of Fe bioavailability and binding of a catecholate siderophore with virus-mediated lysates from the marine bacterium Vibrio alginolyticus PWH3a. J. Exp. Mar. Biol. Ecol. 399, 43–47.
Price, N. M., Ahner, B. A., and Morel, F. M. M. (1994). The equatorial Pacific Ocean: grazer-controlled phytoplankton populations in an iron-limited system. Limnol. Oceanogr. 39, 520–534.
Quigley, M. S., Santschi, P. H., and Hung, C. C. (2002). Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol. Oceanogr. 47, 367–377.
Ralston, D. K., McGillicuddy, D. J. Jr., and Townsend, D. W. (2007). Asynchronous vertical migration and bimodal distribution of motile phytoplankton. J. Plankton Res. 29, 803–821.
Raven, J. A. (1990). Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol. 116, 1–18.
Rich, H. W., and Morel, F. M. M. (1990). Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 35, 652–662.
Rijkenberg, M. J. A., Gerringa, L. J. A., Carolus, V. E., Velzeboer, I., and De Baar, H. J. W. (2006). Enhancement and inhibition of iron photoreduction by individual ligands in open ocean seawater. Geochim. Cosmochim. Acta 70, 2790–2805.
Rijkenberg, M. J. A., Gerringa, L. J. A., Timmermans, K. R., Fischer, A. C., Kroon, K. J., Buma, A. G. J., Wolterbeek, B. T., and De Baar, H. J. W. (2008). Enhancement of the reactive iron pool by marine diatoms. Mar. Chem. 109, 29–44.
Roe, K. L., Barbeau, K., Mann, E. L., and Haygood, M. G. (2011). Acquisition of iron by Trichodesmium and associated bacteria in culture. Environ. Microbiol.
Rose, A. L., Godrant, A., Furnas, M., and Waite, T. D. (2010). Dynamics of nonphotochemical superoxide production and decay in the Great Barrier Reef lagoon. Limnol. Oceanogr. 55, 1521–1536.
Rose, A. L., Salmon, T. P., Lukondeh, T., Neilan, B. A., and Waite, T. D. (2005). Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ. Sci. Technol. 39, 3708–3715.
Rose, A. L., and Waite, T. D. (2002). Kinetic model for Fe(II) oxidation in seawater in the absence and presence of natural organic matter. Environ. Sci. Technol. 36, 433–444.
Rose, A. L., and Waite, T. D. (2003). Effect of dissolved natural organic matter on the kinetics of ferrous iron oxygenation in seawater. Environ. Sci. Technol. 37, 4877–4886.
Rose, A. L., Webb, E. A., Waite, T. D., and Moffett, J. W. (2008). Measurement and implications of nonphotochemically generated superoxide in the equatorial Pacific Ocean. Environ. Sci. Technol. 42, 2387–2393.
Roy, E. G., and Wells, M. L. (2011). Evidence for regulation of Fe(II) oxidation by organic complexing ligands in the Eastern Subarctic Pacific. Mar. Chem. 127, 115–122.
Roy, E. G., Wells, M. L., and King, D. W. (2008). Persistence of iron(II) in surface waters of the western subarctic Pacific. Limnol. Oceanogr. 53, 89–98.
Rubin, M., Berman-Frank, I., and Shaked, Y. (2011). Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat. Geosci. 4, 529–534.
Rue, E., and Bruland, K. (2001). Domoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar. Chem. 76, 127–134.
Rue, E. L., and Bruland, K. W. (1995). Complexation of iron(III) by natural organic ligands in the central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 50, 117–138.
Rueter, J. G., Hutchins, D. A., Smith, R. W., and Unsworth, N. L. (1992). “Iron nutrition in Trichodesmium,” in Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs, eds E. J. Carpenter, D. G. Capone, and J. G. Rueter (Dordrecht: Kluwer Academic), 289–306.
Sander, S. G., and Koschinsky, A. (2011). Metal flux from hydrothermal vents increased by organic complexation. Nat. Geosci. 4, 145–150.
Sandy, M., and Butler, A. (2009). Microbial iron acquisition: marine and terrestrial siderophores. Chem. Rev. 109, 4580–4595.
Santana-Casiano, J. M., Gonzaalez-Davila, M., and Millero, F. J. (2005). Oxidation of nanomolar levels of Fe(II) with oxygen in natural waters. Environ. Sci. Technol. 39, 2073–2079.
Santana-Casiano, J. M., Gonzalez-Davila, A., and Millero, F. J. (2006). The role of Fe(II) species on the oxidation of Fe(II) in natural waters in the presence of O-2 and H2O2. Mar. Chem. 99, 70–82.
Saragosti, E., Tchernov, D., Katsir, A., and Shaked, Y. (2010). Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS ONE 5, e12508.
Sarthou, G., Bucciarelli, E., Chever, F., Hansard, S. P., Gonzalez-Davila, M., Santana-Casiano, J. M., Planchon, F., and Speich, S. (2011). Labile Fe(II) concentrations in the Atlantic sector of the Southern Ocean along a transect from the subtropical domain to the Weddell Sea Gyre. Biogeosciences 8, 2461–2479.
Sarthou, G., Vincent, D., Christaki, U., Obernosterer, I., Timmermans, K. R., and Brussaard, C. P. D. (2008). The fate of biogenic iron during a phytoplankton bloom induced by natural fertilisation: impact of copepod grazing. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 734–751.
Sasaki, T., Kurano, N., and Miyachi, S. (1998). Induction of ferric reductase activity and of iron uptake activity in Chlococcum littorale cells underextremely high-CO2 and iron-deficient conditions. Plant Cell Physiol. 39, 405–410.
Sato, M., Takeda, S., and Furuya, K. (2007). Iron regeneration and organic iron(III)-binding ligand production during in situ zooplankton grazing experiment. Mar. Chem. 106, 471–488.
Schlosser, C., and Croot, P. L. (2009). Controls on seawater Fe(III) solubility in the Mauritanian upwelling zone. Geophys. Res. Lett. 36, L18606.
Severmann, S., Mcmanus, J., Berelson, W. M., and Hammond, D. E. (2010). The continental shelf benthic iron flux and its isotope composition. Geochim. Cosmochim. Acta 74, 3984–4004.
Shaked, Y. (2008). Iron redox dynamics in the surface waters of the Gulf of Aqaba, Red Sea. Geochim. Cosmochim. Acta 72, 1540–1554.
Shaked, Y., Erel, Y., and Sukenik, A. (2002). Phytoplankton-mediated redox cycle of iron in the epilimnion of Lake Kinneret. Environ. Sci. Technol. 36, 460–467.
Shaked, Y., Kustka, A. B., and Morel, F. M. M. (2005). A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol. Oceanogr. 50, 872–882.
Shaked, Y., Kustka, A. B., Morel, F. M. M., and Erel, Y. (2004). Simultaneous determination of iron reduction and uptake by phytoplankton. Limnol. Oceanogr. Methods 2, 137–145.
Shi, D., Xu, Y., Hopkinson, B. M., and Morel, F. M. M. (2010). Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679.
Shi, D., Xu, Y., and Morel, F. M. M. (2009). Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences 6, 1199–1207.
Singler, H. R., and Villareal, T. A. (2005). Nitrogen inputs into the euphotic zone by vertically migrating Rhizosolenia mats. J. Plankton Res. 27, 545–556.
Sohm, J. A., Webb, E. A., and Capone, D. G. (2011). Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508.
Soria-Dengg, S., and Horstmann, U. (1995). Ferrioxamines B and E as iron sources for the marine diatom Phaeodactylum tricornutum. Mar. Ecol. Prog. Ser. 127, 269–277.
Soria-Dengg, S., Reissbrodt, R., and Horstmann, U. (2001). Siderophores in marine, coastal waters and their relevance for iron uptake by phytoplankton: experiments with the diatom Phaeodactylum tricornutum. Mar. Ecol. Prog. Ser. 220, 73–82.
Statham, P. J., German, C. R., and Connelly, D. P. (2005). Iron (II) distribution and oxidation kinetics in hydrothermal plumes at the Kairei and Edmond vent sites, Indian Ocean. Earth Planet. Sci. Lett. 236, 588–596.
Steigenberger, S., Statham, P. J., Voelker, C., and Passow, U. (2010). The role of polysaccharides and diatom exudates in the redox cycling of Fe and the photoproduction of hydrogen peroxide in coastal seawaters. Biogeosciences 7, 109–119.
Stintzi, A., Barnes, C., Xu, L., and Raymond, K. N. (2000). Microbial iron transport via a siderophore shuttle: a membrane ion transport paradigm. Proc. Natl. Acad. Sci. U.S.A. 97, 10691–10696.
Strmecki, S., Plavsic, M., Steigenberger, S., and Passow, U. (2010). Characterization of phytoplankton exudates and carbohydrates in relation to their complexation of copper, cadmium and iron. Mar. Ecol. Prog. Ser. 408, 33–46.
Strzepek, R. F., and Harrison, P. J. (2004). Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431, 689–692.
Strzepek, R. F., Maldonado, M. T., Higgins, J. L., Hall, J., Safi, K., Wilhelm, S. W., and Boyd, P. W. (2005). Spinning the “Ferrous Wheel”: the importance of the microbial community in an iron budget during the FeCycle experiment. Global Biogeochem. Cycles 19, GB4S26.1–GB4S26.14.
Stumm, W., and Sulzberger, B. (1992). The cycling of iron in natural environments: considerations based on laboratory studies of heterogeneous redox processes. Geochim. Cosmochim. Acta 56, 3233–3257.
Sunda, W., Price, N. M., and Morel, F. M. M. (2005). “Trace metal ion buffers and their use in culture studies,” in Algal Culturing Techniques, ed. R. Anderson (Burlington: Academic Press), 35–63.
Sunda, W. G. (2001). “Bioavailability and bioaccumulation of iron in the sea,” in Biogeochemistry of Fe in Seawater, eds. K. Hunter, and D. Turner (West Sussex: John Wiley & Sons), 41–84.
Sunda, W. G., and Huntsman, S. A. (1995). Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 50, 189–206.
Sunda, W. G., and Huntsman, S. A. (1997). Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390, 389–392.
Sutak, R., Slapeta, J., San Roman, M., Camadro, J.-M., and Lesuisse, E. (2010). Nonreductive iron uptake mechanism in the marine alveolate Chromera velia. Plant Physiol. 154, 991–1000.
Tang, K. W., Glud, R. N., Glud, A., Rysgaard, S., and Nielsen, T. G. (2011). Copepod guts as biogeochemical hotspots in the sea: evidence from microelectrode profiling of Calanus spp. Limnol. Oceanogr. 56, 666–672.
Terzulli, A., and Kosman, D. J. (2010). Analysis of the high-affinity iron uptake system at the Chlamydomonas reinhardtii plasma membrane. Eukaryotic Cell 9, 815–826.
Town, R. M., and Filella, M. (2000). Dispelling the myths: is the existence of L1 and L2 ligands necessary to explain metal ion speciation in natural waters? Limnol. Oceanogr. 45 1341–1357.
Trick, C. G., Bill, B. D., Cochlan, W. P., Wells, M. L., Trainer, V. L., and Pickell, L. D. (2010). Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc. Natl. Acad. Sci. U.S.A. 107, 5887–5892.
Tsuda, A., Takeda, S., Saito, H., Nishioka, J., Kudo, I., Nojiri, Y., Suzuki, K., Uematsu, M., Wells, M. L., Tsumune, D., Yoshimura, T., Aono, T., Aramaki, T., Cochlan, W. P., Hayakawa, M., Imai, K., Isada, T., Iwamoto, Y., Johnson, W. K., Kameyama, S., Kato, S., Kiyosawa, H., Kondo, Y., Levasseur, M., Machida, R. J., Nagao, I., Nakagawa, F., Nakanish, T., Nakatsuka, S., Narita, A., Noiri, Y., Obata, H., Ogawa, H., Oguma, K., Ono, T., Sakuragi, T., Sasakawa, M., Sato, M., Shimamoto, A., Takata, H., Trick, C. G., Watanabe, Y. W., Wong, C. S., and Yoshie, N. (2007). Evidence for the grazing hypothesis: grazing reduces phytoplankton responses of the HNLC ecosystem to iron enrichment in the western subarctic pacific (SEEDS II). J. Oceanogr. 63, 983–994.
Ussher, S. J., Worsfold, P. J., Achterberg, E. P., Laes, A., Blain, S., Laan, P., and De Baar, H. J. W. (2007). Distribution and redox speciation of dissolved iron on the European continental margin. Limnol. Oceanogr. 52, 2530–2539.
van den Berg, C. M. G. (1995). Evidence for organic complexation of iron in seawater. Mar. Chem. 50, 139–157.
Velasquez, I., Nunn, B. L., Ibisanmi, E., Goodlett, D. R., Hunter, K. A., and Sander, S. G. (2011). Detection of hydroxamate siderophores in coastal and Sub-Antarctic waters off the South Eastern Coast of New Zealand. Mar. Chem. 126, 97–107.
Vermilyea, A. W., Hansard, S. P., and Voelker, B. M. (2010). Dark production of hydrogen peroxide in the Gulf of Alaska. Limnol. Oceanogr. 55, 580–588.
Villareal, T. A., Joseph, L., Brzezinski, M. A., Shipe, R. F., Lipschultz, F., and Altabet, M. A. (1999). Biological and chemical characteristics of the giant diatom Ethmodiscus (Bacillariophyceae) in the central North Pacific gyre. J. Phycol. 35, 896–902.
Voelker, B. M., and Sedlak, D. L. (1995). Iron reduction by photoproduced superoxide in seawater. Mar. Chem. 50, 93–102.
Völker, C., and Wolf-Gladrow, D. A. (1999). Physical limits on iron uptake mediated by siderophores or surface reductases. Mar. Chem. 65, 227–244.
Vraspir, J. M., and Butler, A. (2009). Chemistry of marine ligands and siderophores. Ann. Rev. Mar. Sci. 1, 43–63.
Waite, T. D., and Morel, F. M. M. (1984). Photoreductive dissolution of colloidal iron oxides in natural waters. Environ. Sci. Technol. 18, 860–868.
Watson, A. J., Bakker, D. C. E., Ridgwell, A. J., Boyd, P. W., and Law, C. G. (2000). Effect of iron supply on southern ocean CO2 uptake and implications for glacial atmospheric CO2. Nature 407, 730–733.
Webb, E. A., Moffett, J. W., and Waterbury, J. B. (2001). Iron stress in open-ocean cyanobacteria (Synechococcus, Trichodesmium, and Crocosphaera spp.): identification of the IdiA protein. Appl. Environ. Microbiol. 67, 5444–5452.
Weger, H. G. (1999). Ferric and cupric reductase activities in the green alga Chlamydomonas reinhardtii: experiments using iron-limited chemostats. Planta 207, 377–384.
Wells, M. L., Mayer, L. M., Donard, O. F. X., De Souza Sierra, M. M., and Ackelson, S. G. (1991). The photolysis of colloidal iron in the oceans. Nature 353, 248–250.
Wells, M. L., Price, N. M., and Bruland, K. W. (1994). Iron limitation and the cyanobacterium Synechococcus in equatorial Pacific waters. Limnol. Oceanogr. 39, 1481–1486.
Wells, M. L., Price, N. M., and Bruland, K. W. (1995). Iron chemistry in seawater and its relationship to phytoplankton: a workshop report. Mar. Chem. 48, 157–182.
Wells, M. L., Trick, C. G., Cochlan, W. P., Hughes, M. P., and Trainer, V. L. (2005). Domoic acid: the synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 50, 1908–1917.
Wells, M. L., Zorkin, N. G., and Lewis, A. G. (1983). The role of colloid chemistry in providing a source of iron to phytoplankton. J. Mar. Res. 41, 731–746.
Wirtz, N. L., Treble, R. G., and Weger, H. G. (2010). Siderophore-independent iron uptake by iron-limited cells of the cynaobacterium anabaena flos-aquae. J. Phycol. 46, 947–957.
Worms, I., Simon, D. F., Hassler, C. S., and Wilkinson, K. J. (2006). Bioavailability of trace metals to aquatic microorganisms: importance of chemical, biological and physical processes on biouptake. Biochimie 88, 1721–1731.
Wotton, R. S. (2004). The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Sci. Mar. 68, 13–21.
Wu, J., Wells, M. L., and Rember, R. (2011). Dissolved iron anomaly in the deep tropical-subtropical Pacific: evidence for long-range transport of hydrothermal iron. Geochim. Cosmochim. Acta 75, 460–468.
Yoshida, M., Kuma, K., Iwade, S., Isoda, Y., Takata, H., and Yamada, M. (2006). Effect of aging time on the availability of freshly precipitated ferric hydroxide to coastal marine diatoms. Mar. Biol. 149, 379–392.
Appendix
Data in Support of the Prevalence of Reductive Iron Uptake Amongst Phytoplankton (Figure 3)
The laboratory cultures (Table A1) and field populations (Table A2) included in Figure 3 were taken from various studies which demonstrate the presence of a reductive mechanism by one or more of the following techniques:
(a) Colorimetric detection of cell-mediated Fe reduction –measured as accumulation of Fe(II) over time in the presence of cells and a ferrous trapping ligand that binds Fe(II) prior to its oxidation by oxygen or transport. Commonly, cell-mediated Fe reduction is measured via colorimetric methods in the presence of Ferrozine (Fz) or Bathophenanthrolinedisulfonic acid (BPDS). This method requires relatively high micromolar iron concentrations due to its low sensitivity. Shaked et al. (2004) developed a sensitive assay for cell-mediated Fe′ reduction using radiolabeled iron. This reduction assay is conducted simultaneously with Fe′ uptake at physiologically relevant nanomolar iron concentrations, and it was applied for many of the data presented here.
(b) Genetic studies which find components of the reductive uptake system.
(c) EPR – Electron paramagnetic resonance showing Fe reduction.
(d) Inhibition of uptake by Fz/BPDS – Comparing uptake rates in the presence and absence of the membrane impermeable Fe(II) ligands, Ferrozine or BPDS. Here, Fe(II) formed by cell-mediated reduction is trapped and made unavailable for uptake by the cell, hence inhibiting iron uptake by cells employing this strategy. An explanatory illustration of this procedure is found in Figure A1 while Figure A2 provides an example of experimental data using this method.
Data Supporting the Relative Bioavailability Scale (Figure 5)
Tables A3 and A4, outline the data used for constructing Figure 5. Uptake rates obtained from different sources were first normalized to Fe-substrate concentration (either Fe′ for FeEDTA or to FeL for iron bound to ligand L, providing ligand L complexed iron strongly and/or was present in sufficient excess to rule out Fe precipitation) in order to calculate kup according to Eq. 1:
where ρ is Fe uptake rate [mol Fe cell−1 h−1], [Fe] is Fe-substrate concentration [mol L−1]. The calculation applies only to sub-saturating uptake rates. The kup obtained for the different Fe complexes – were further normalized by dividing it by
Note on Fe-substrates
Goe – goethite is an Fe-oxide mineral (FeOOH); DFB – desferrioxamine B is a trihydroxamate siderophore; AB – Aerobactin is hydroxamate photolabile siderophore; FC – ferrichrome is a trihydroxamate siderophore; PYN – Porphyrin; MS – Monosaccharides; PS – Polysaccharides; AZ – Azotochelin is a bis-catecholate siderophore produced by nitrogen-fixing soil bacterium Azotobacter vinelandii; DPS – DNA binding protein from starved cells (iron storage proteins); CAT – Gallocatechin is a polyhydroxybenzene catechol derivative found in green tea; DFE-desferrioxamine E is a trihydroxamate siderophore.
Note on the saccharides uptake data
In the saccharides uptake experiments no Fe′ uptake data was reported. Since we view these as potentially important experiments we chose to normalize the saccharide uptake constant to that of FeCl3 added at concentration of 1 nM. Given a solubility limit of 0.2–0.5 nM (Liu and Millero, 2002), it is possible that some of the Fe precipitated in the experiments. This was demonstrated in control measurements – roughly 30% of the FeCl3 remained in the dissolved fraction, about 12% was in colloidal form and the remainder in the particulate fraction. To calculate we related to the dissolved fraction as the Fe-substrate concentration.
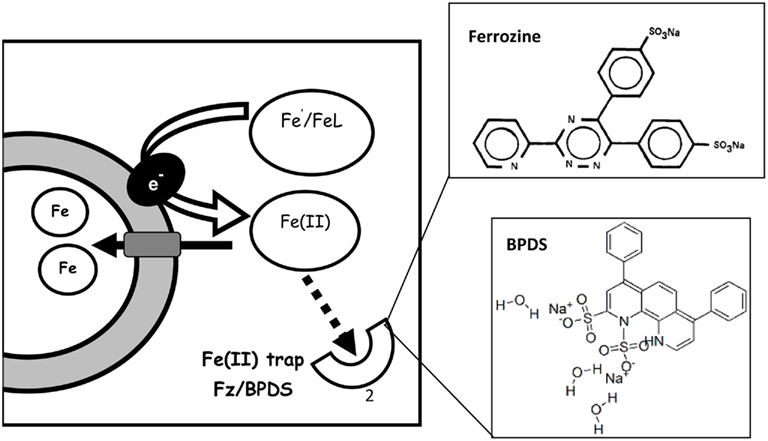
Figure A1. Trapping of ferrous iron formed during Fe′ reduction with ferrozine (Fz) or Bathophenanthrolinedisulfonic acid (BPDS). The Fe(II) trap competes with cells over ferrous iron formed during cell-mediated Fe reduction. The structures of two such traps – ferrozine and BPDS – are shown on the right top and bottom respectively. Fe(II)Fz3 and Fe(II)BPDS3 are detected spectrophotometrically at 562 and 533 nm, respectively. Additionally Fe(II)Fz3 and inhibition of Fe uptake by Fz/BPDS can be detected in radioactive experiments. Figure based on Lis and Shaked (2009).
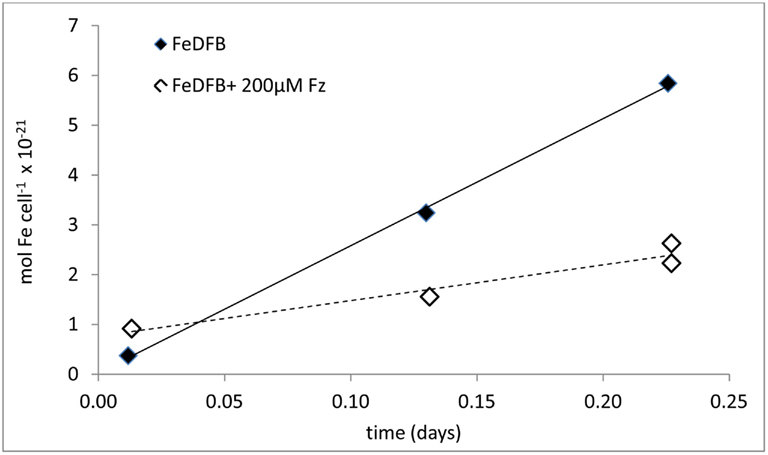
Figure A2. Experimental data of iron uptake by Synechococcus WH8102 in the presence and absence of Ferrozine (Fz), on the basis of which the occurrence of the Fe reductive pathway is deduced. The uptake of iron from 90 nM 55FeDFB by iron limited Synechococcus WH8102 was inhibited in the presence of 200 μM Fz. The inhibitory effect stems from the trapping of Fe(II) formed through cell surface enzymatic reduction during Fe uptake.
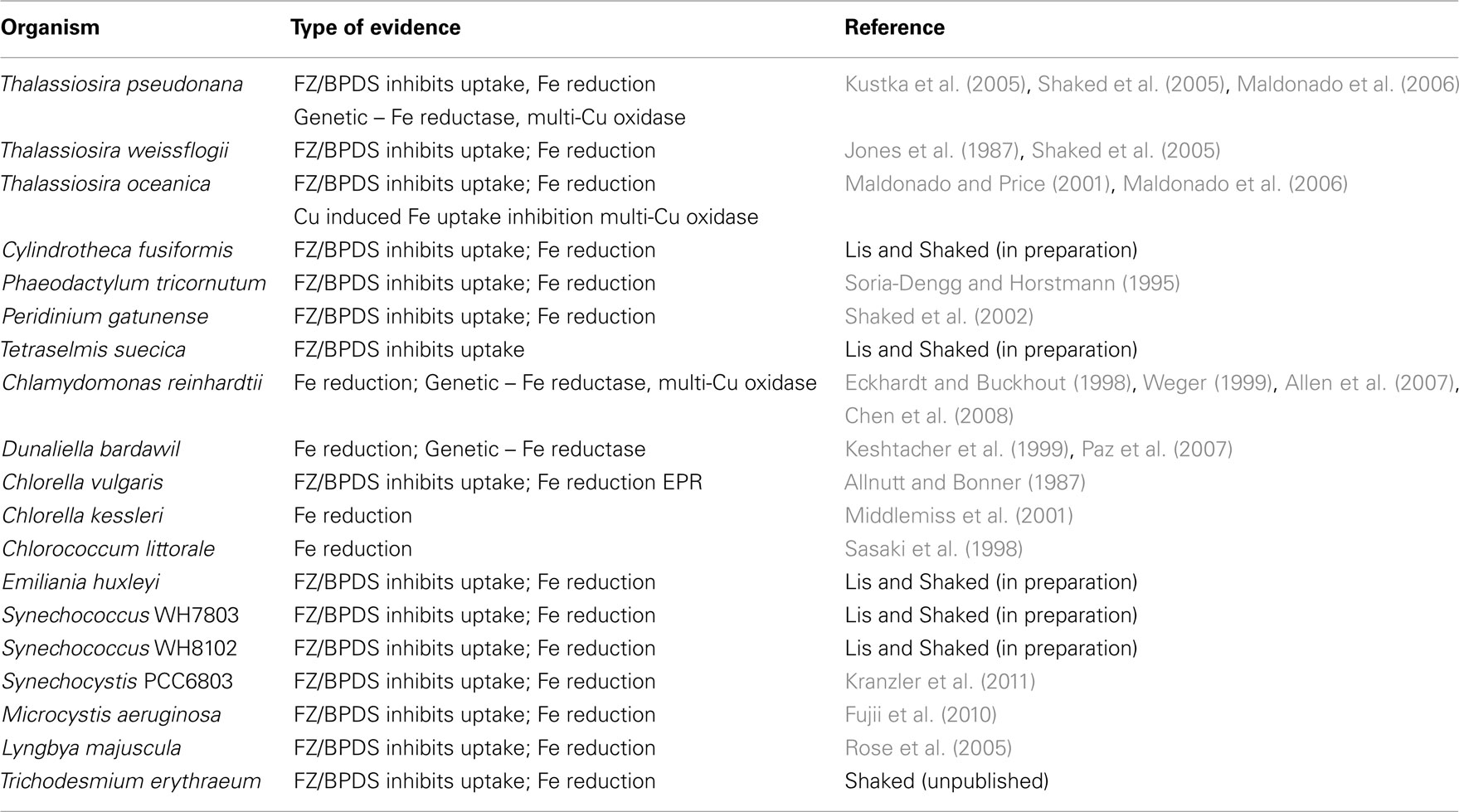
Table A1. Experimental evidence showing the existence of an iron reductive uptake pathway in laboratory cultures.
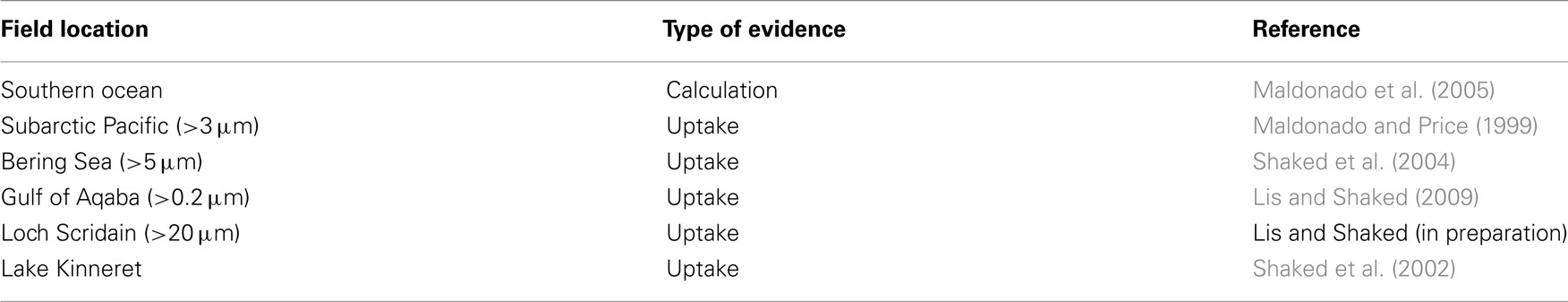
Table A2. Experimental evidence showing the existence of the reductive iron uptake pathway in field populations.
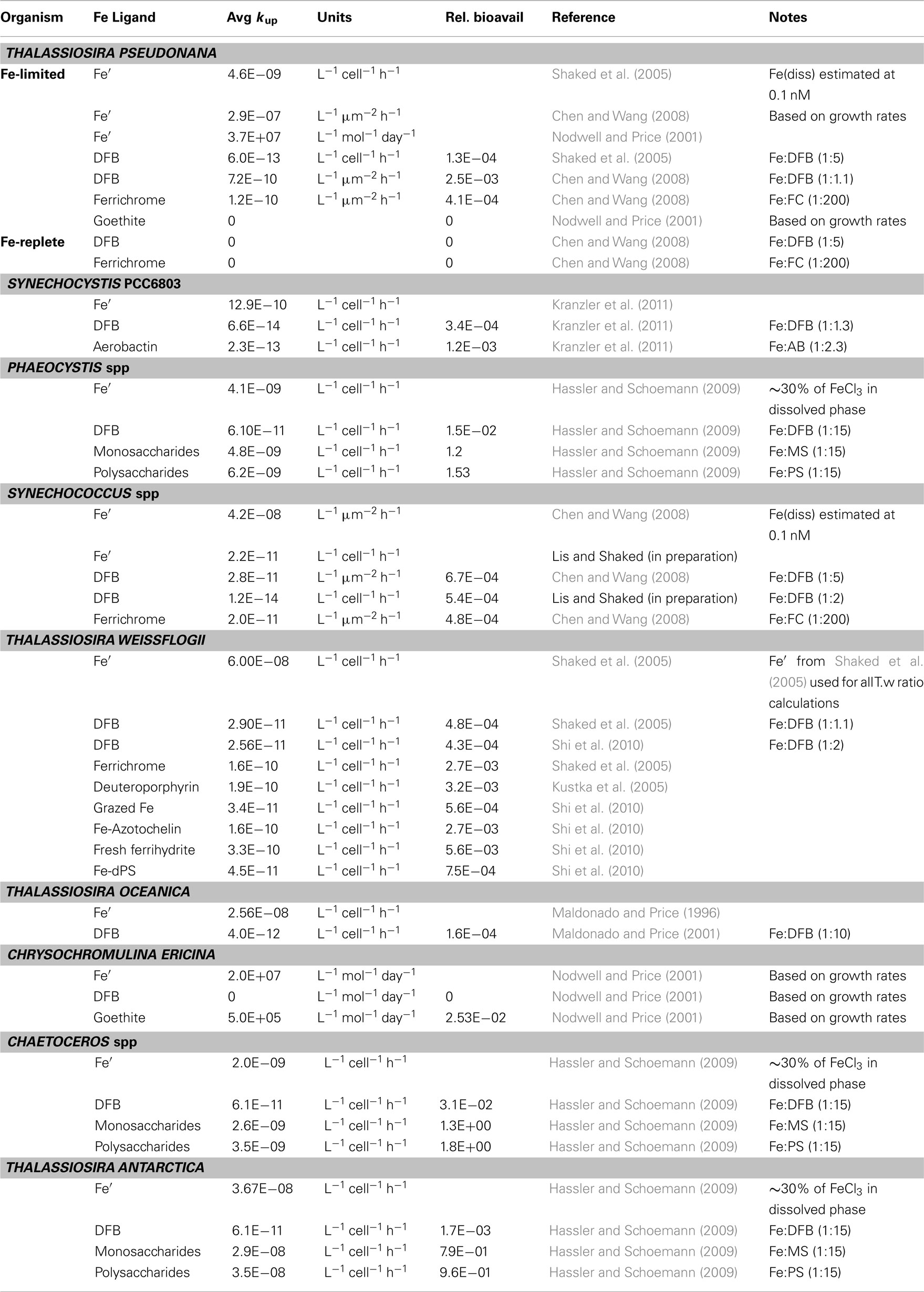
Table A3. Data used in the calculation of relative bioavailability of laboratory cultures (Figure 5A).
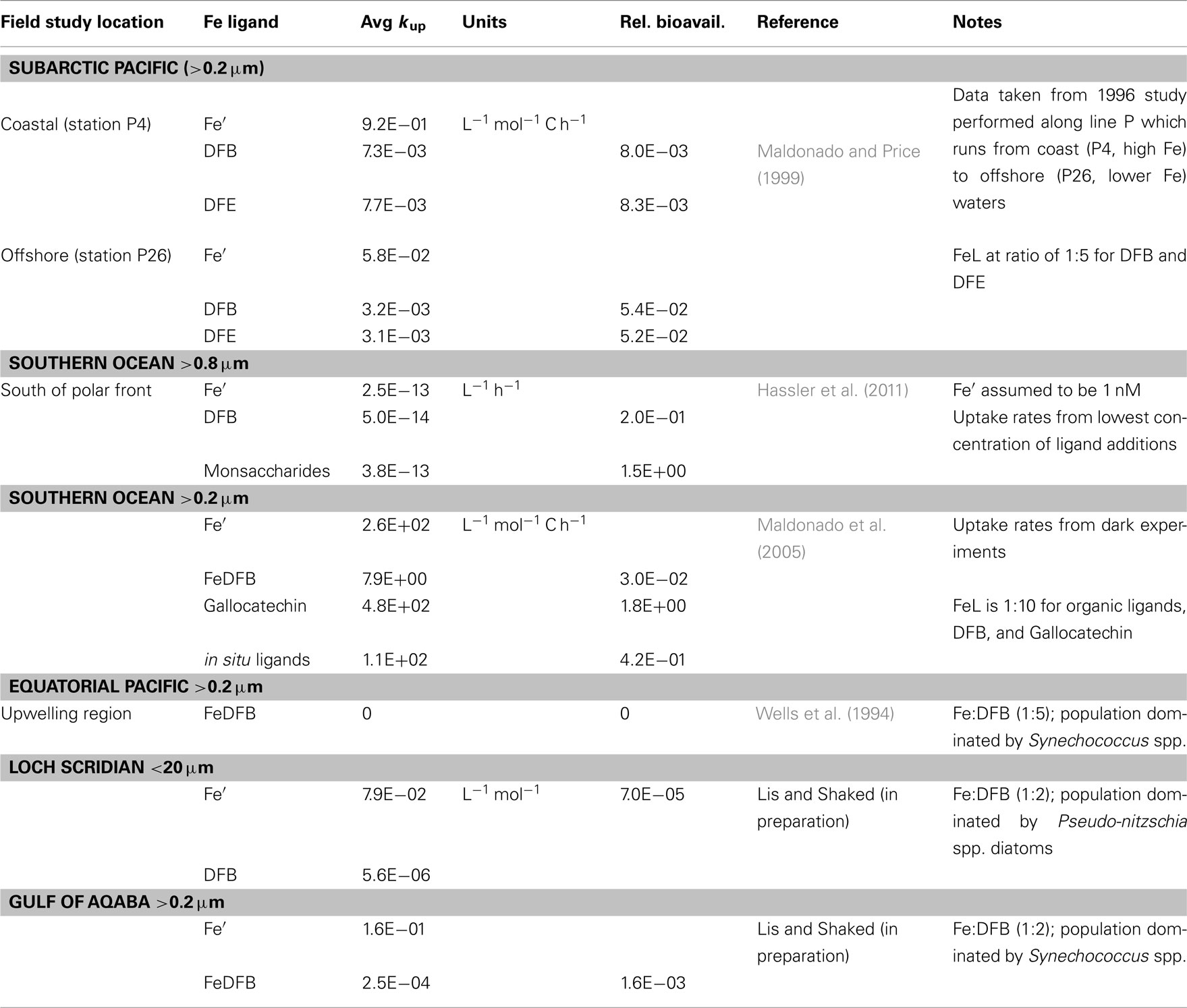
Table A4. Data used in the calculation of relative bioavailability of natural assemblages (Figure 5B).
References
Allen, M. D., Del Campo, J. A., Kropat, J., and Merchant, S. S. (2007). FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryotic Cell 6, 1841–1852.
Allnutt, F. C. T., and Bonner, W. D. J. (1987). Evaluation of reductive release as a mechanism for iron uptake from ferrioxamine B by Chlorella vulgaris. Plant Physiol. 85, 751–756.
Chen, J.-C., Hsieh, S. I., Kropat, J., and Merchant, S. S. (2008). A ferroxidase encoded by FOX1 contributes to iron assimilation under conditions of poor iron nutrition in Chlamydomonas. Eukaryotic Cell 7, 541–545.
Chen, M., and Wang, W. (2008). Accelerated uptake by phytoplankton of iron bound to humic acids. Aquat. Biol. 3, 155–166.
Eckhardt, U., and Buckhout, T. J. (1998). Iron assimilation in Chlamydomonas reinhardtii involves ferric reduction and is similar to strategy I higher plants. J. Exp. Bot. 49, 1219–1226.
Fujii, M., Rose, A. L., Omura, T., and Waite, T. D. (2010). Effect of Fe(II) and Fe(III) transformation kinetics on iron acquisition by a toxic strain of Microcystis aeruginosa. Environ. Sci. Technol. 44, 1980–1986.
Hassler, C. S., and Schoemann, V. (2009). Bioavailability of organically bound Fe to model phytoplankton of the Southern Ocean. Biogeosciences 6, 2281–2296.
Hassler, C. S., Schoemann, V., Nichols, C. M., Butler, E. C. V., and Boyd, P. W. (2011). Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proc. Natl. Acad. Sci. U.S.A. 108, 1076–1081.
Jones, G. J., Palenik, B. P., and Morel, F. F. M. (1987). Trace metal reduction by phytoplankton: the role of plasmalemma redox enzymes. J. Phycol. 23, 237–244.
Keshtacher, L. E., Hadar, Y., and Chen, Y. (1999). Fe nutrition demand and utilization by the green alga Dunaliella bardawil. Plant Soil 215, 175–182.
Kranzler, C., Lis, H., Shaked, Y., and Keren, N. (2011). The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 13, 2990–2999.
Kustka, A. B., Shaked, Y., Milligan, A. J., King, D. W., and Morel, F. M. M. (2005). Extracellular production of superoxide by marine diatoms: contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr. 50, 1172–1180.
Lis, H., and Shaked, Y. (2009). Probing the bioavailability of organically bound iron: a case study in the Synechococcus-rich waters of the Gulf of Aqaba. Aquat. Microb. Ecol. 56, 241–253.
Maldonado, M. T., Allen, A. E., Chong, J. S., Dan Leus, K. L., Karpenko, N., and Harris, S. L. (2006). Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 51, 1729–1743.
Maldonado, M. T., and Price, N. M. (1996). Influence of N substrate on Fe requirements of marine centric diatoms. Mar. Ecol. Prog. Ser. 141, 161–172.
Maldonado, M. T., and Price, N. M. (1999). Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 46, 2447–2473.
Maldonado, M. T., and Price, N. M. (2001). Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J. Phycol. 37, 298–309.
Maldonado, M. T., Strzepek, R. F., Sander, S., and Boyd, P. W. (2005). Acquisition of iron bound to strong organic complexes, with different Fe binding groups and photochemical reactivities, by plankton communities in Fe-limited subantarctic waters. Global Biogeochem. Cycles 19, GB4S23.
Middlemiss, J. K., Anderson, A. M., Stratilo, C. W., and Weger, H. G. (2001). Oxygen consumption associated with ferric reductase activity and iron uptake by iron-limited cells of Chlorella kessleri (Chlorophyceae). J. Phycol. 37, 393–399.
Nodwell, L., and Price, N. (2001). Direct use of inorganic colloidal iron by marine mixotrophic phytoplankton. Limnol. Oceanogr. 46, 755–777.
Paz, Y., Katz, A., and Pick, U. (2007). Multicopper ferroxidase involved in iron binding to transferrins in Dunaliella salina plasma membranes. J. Biol. Chem. 282, 8658–8666.
Rose, A. L., Salmon, T. P., Lukondeh, T., Neilan, B. A., and Waite, T. D. (2005). Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ. Sci. Technol. 39, 3708–3715.
Sasaki, T., Kurano, N., and Miyachi, S. (1998). Induction of ferric reductase activity and of iron uptake activity in Chlococcum littorale cells underextremely high-CO2 and iron-deficient conditions. Plant Cell Physiol. 39, 405–410.
Shaked, Y., Erel, Y., and Sukenik, A. (2002). Phytoplankton-mediated redox cycle of iron in the epilimnion of Lake Kinneret. Environ. Sci. Technol. 36, 460–467.
Shaked, Y., Kustka, A. B., and Morel, F. M. M. (2005). A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol. Oceanogr. 50, 872–882.
Shaked, Y., Kustka, A. B., Morel, F. M. M., and Erel, Y. (2004). Simultaneous determination of iron reduction and uptake by phytoplankton. Limnol. Oceanogr. Methods 2, 137–145.
Shi, D., Xu, Y., Hopkinson, B. M., and Morel, F. M. M. (2010). Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679.
Soria-Dengg, S., and Horstmann, U. (1995). Ferrioxamines B and E as iron sources for the marine diatom Phaeodactylum tricornutum. Mar. Ecol. Prog. Ser. 127, 269–277.
Weger, H. G. (1999). Ferric and cupric reductase activities in the green alga Chlamydomonas reinhardtii: experiments using iron-limited chemostats. Planta 207, 377–384.
Keywords: iron, bioavailability, uptake, phytoplankton, speciation, redox reactions, biogeochemistry, organic complexation
Citation: Shaked Y and Lis H (2012) Disassembling iron availability to phytoplankton. Front. Microbio. 3:123. doi: 10.3389/fmicb.2012.00123
Received: 30 November 2011; Accepted: 14 March 2012;
Published online: 17 April 2012.
Edited by:
Martha Gledhill, University of Southampton, UKReviewed by:
Andrew Rose, Southern Cross University, AustraliaMark Wells, University of Maine, USA
Micha Rijkenberg, Royal Netherlands Institute for Sea Research, Netherlands
Copyright: © 2012 Shaked and Lis. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Yeala Shaked, Interuniversity Institute for Marine Sciences in Eilat, P. O. Box 469, Eilat 88103, Israel. e-mail: yshaked@vms.huji.ac.il
