- 1Institute of Environmental Biotechnology, Graz University of Technology, Graz, Austria
- 2Institute of Plant Sciences, University of Graz, Graz, Austria
- 3Leibniz-Institute of Vegetable and Ornamental Crops, Grossbeeren, Germany
Lettuce belongs to the most commonly raw eaten food worldwide and its microbiome plays an important role for both human and plant health. Yet, little is known about the impact of potentially occurring pathogens and beneficial inoculants of the indigenous microorganisms associated with lettuce. To address this question we studied the impact of the phytopathogenic fungus Rhizoctonia solani and the biological control agent Bacillus amyloliquefaciens FZB42 on the indigenous rhizosphere and phyllosphere community of greenhouse-grown lettuce at two plant stages. The rhizosphere and phyllosphere gammaproteobacterial microbiomes of lettuce plants showed clear differences in their overall and core microbiome composition as well as in corresponding diversity indices. The rhizosphere was dominated by Xanthomonadaceae (48%) and Pseudomonadaceae (37%) with Rhodanobacter, Pseudoxanthomonas, Dokdonella, Luteimonas, Steroidobacter, Thermomonas as core inhabitants, while the dominating taxa associated to phyllosphere were Pseudomonadaceae (54%), Moraxellaceae (16%) and Enterobacteriaceae (25%) with Alkanindiges, Pantoea and a group of Enterobacteriaceae unclassified at genus level. The preferential occurrence of enterics in the phyllosphere was the most significant difference between both habitats. Additional enhancement of enterics on the phyllosphere was observed in bottom rot diseased lettuce plants, while Acinetobacter and Alkanindiges were identified as indicators of healthy plants. Interestingly, the microbial diversity was enhanced by treatment with both the pathogen, and the co-inoculated biological control agent. The highest impact and bacterial diversity was found by Rhizoctonia inoculation, but FZB42 lowered the impact of Rhizoctonia on the microbiome. This study shows that the indigenous microbiome shifts as a consequence to pathogen attack but FZB42 can compensate these effects, which supports their role as biocontrol agent and suggests a novel mode of action.
Introduction
Plants host a broad range of ubiquitous but also highly adapted and specific bacterial communities that colonize their epi- and endophytic compartments (Berg and Smalla, 2009; Berendsen et al., 2012; Bulgarelli et al., 2012). Due to their complexity, specific morphology, and production of secondary metabolites, the structure and function of plant-associated microbial communities are specific in these habitats but also at plant species and cultivar levels (Smalla et al., 2001; Berg et al., 2002; Berg and Smalla, 2009; Raaijmakers et al., 2009). The rhizosphere has already been investigated as a microbial habitat for more than one century (Hartmann et al., 2008), while the phyllosphere microbiome is only partly understood. Recent work suggests that the long-term colonization of phyllosphere is preferred by specific bacteria, while short-time colonization comprises many ubiquitous bacteria (Vorholt, 2012). All plant-associated habitats contain a high proportion of plant-beneficial microorganisms such as antagonists, diazotrophs, and plant growth promoting bacteria (PGPB) but also plant pathogens as well as potential human pathogens (Berg et al., 2005; Mendes et al., 2013). While the modes of action are often understood for single beneficial as well as pathogenic strains and species, less is known about the microbial community impact of single strains. Risk assessment and colonization studies for specific biocontrol agents showed minor and only transient effects on the rhizosphere community (Scherwinski et al., 2007; Adesina et al., 2009; Chowdhury et al., 2013; Schmidt et al., 2014), while impacts of pathogens on the indigenous microbiome are severely underexplored.
Soil-borne plant pathogens cause crucial damage to crops. The phytopathogenic fungus Rhizoctonia solani Kühn [teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk; basidiomycetes] is subdivided into anastomosis groups (AGs) according to their hyphal anastomosis reactions (Carling et al., 2002). The fungus causes a wide range of commercially significant plant diseases, such as Brown patch, damping off in seedlings, root rot and belly rot. R. solani strains are characterized by a distinct degree of host specificity as well as by different virulence levels to their plant host. Rhizoctonia strains occur almost ubiquitously in soils but isolates AG1-IB (Germany), AG2-1 (UK, the Netherlands) and AG4 (the Netherlands, UK, USA) have been isolated from diseased lettuce plants (Grosch et al., 2004). Strains belonging to AG1-IB were responsible for up to 70% yield loss of field-grown lettuce (Davis et al., 1997; Wolf and Verreet, 1999). One strain 7/3/14 of the supposed diploid and heterokaryotic R. solani AG1-IB, which was already sequenced, shows a large genome with many unique and unknown features in comparison with other Rhizoctonia strains and phylogenetically related fungi (Wibberg et al., 2013). Due to the low degree of host specificity, Rhizoctonia strains of different AGs can lower the general fitness of the plant during colonization, which results in higher sensitivity for additional pathogens such as spoilage enterobacteria (Berg et al., 2005). Interestingly, Adesina et al. (2009) could demonstrate direct changes caused by R. solani to the fungal and bacterial community patterns using molecular fingerprinting. All Rhizoctonia diseases, and subsequent secondary infections, in plants are difficult to control. In the past, only methyl bromide (MeBr) was effectively used. However, this fumigant has been banned for its ozone-depleting and toxic effects (UNEP, 1999). Alternative and environmentally friendly methods to suppress Rhizoctonia comprise naturally occurring antagonists such as Serratia plymuthica and Pseudomonas jessenii (Faltin et al., 2004; Grosch et al., 2005; Scherwinski et al., 2007; Adesina et al., 2009). In addition, Bacillus amyloliquefaciens FZB42, a long-time established plant strengthener was successfully applied to suppress R. solani on lettuce (Chowdhury et al., 2013). Genome sequencing of FZB42 revealed a high capacity of metabolite production with antimicrobial and antifungal activity, which suggested direct antifungal effects (Chowdhury et al., 2013). However, some reports suggest additional impacts of individual strains on the microbial community (Scherwinski et al., 2007; Schmidt et al., 2012). We therefore hypothesize that both beneficials as well as pathogens can cause significant shifts in the plant-associated microbiome.
The aim of this study was to identify the impact of the phytopathogenic fungus R. solani and the biological control agent B. amyloliquefaciens FZB42 on the indigenous rhizosphere and phyllosphere community of lettuce, cultivated under controlled conditions in a growth chamber. In our study we focus on the human health relevant group of Gammaproteobacteria, which was studied by analyzing specific amplicon libraries together with corresponding bioinformatic and statistical analysis. Gammaproteobacteria belong to the plant microbiome in general (Brandl, 2006), and are especially a substantial fraction of the lettuce-associated microbiome (Rastogi et al., 2012, 2013). However, they also comprise several species which were frequently identified to cause severe foodborne outbreaks (Teplitski et al., 2011).
Material and Methods
Inoculants Used in This Study
The effect of R. solani and B. amyloliquefaciens FZB42 on lettuce growth and health was evaluated in this study. All experiments were performed with the product Rhizovital® 42 liquid (ABiTEP GmbH, Berlin, Germany), which is based on vital spores of FZB42 (Chowdhury et al., 2013). The bottom rot pathogen R. solani AG1-IB (isolate 7/3) was obtained from the strain collection of the Leibniz Institute of Vegetable and Ornamental Crops (Großbeeren, Germany) (Grosch et al., 2004).
Experimental Design of Pot Experiments
The effect of FZB42 and the pathogen R. solani on the microbial community of lettuce was studied by 454-amplicon sequencing analysis. Seeds (cv. Tizian, Syngenta, Bad Salzuflen, Germany) were germinated at 18°C in a seedling tray (92 holes) filled with a non-sterile mixture of quartz sand and substrate [Fruhstorfer Einheitserde Typ P, Vechta, Germany; chemical analysis (mg per l): N = 120, P = 120, K = 170, Mg = 120, S = 100, KCl = 1, organic substance = 167, peat = 309; pH 5.9] at a 1:1 ratio (v/v). The seedlings were further cultivated at 20/15°C until planting in a growth chamber (York, Mannheim, Germany; 16 h/8 h day/night cycle, 500 μmol m−2 s−1, 60/80% relative humidity). Lettuce was planted at two-leaf stage into pots (500 ml) filled with the same substrate sand mixture as mentioned above inoculated and non-inoculated with the pathogen R. solani AG1-IB and grown at 22/15°C for 4 weeks. In the treatments with pathogen inoculation, the substrate mixture was inoculated with 10 R. solani-infested barley kernels and incubated at 25°C for 1 week until planting of lettuce into the pots.
For application of the inoculant FZB42 each lettuce plant was drenched with 20 ml spore solution (107 spores ml−1) 3 days before and at planting time respectively. The pots were watered lightly each day to maintain the substrate moisture, and fertilized weekly (0.2% Wuxal TOP N, Wilhelm Haug GmbH & Co. KG, Düsseldorf, Germany). All pot experiments were done at the Leibniz Institute of Vegetable and Ornamental Crops.
An overview about the sampling design is presented in Table 1. Here, abbreviations for compartments and treatments used throughout the manuscript were explained: P, phyllosphere; R, rhizosphere; Y, young; M, mature; RS, R. solani; C, untreated (control); FZB42RS, FZB42 and R. solani co-inoculation; G, healthy; K, diseased.
Sample Collection and DNA Isolation
Sampling was carried out 2 weeks after planting (young plants) for treatments with and without FZB42 application, followed by a second sampling 4 weeks after planting (mature plants) for control and co-inoculated treatments with FZB42 and R. solani. The total community DNA was extracted per treatment, and habitat from two young plants and three mature plants (two independent DNA extractions were performed for each plant and the DNA was pooled prior to PCR), according to Bragina et al. (2011). Briefly, 5 g of plant material were physically disrupted with sterile pestle and mortar and resuspended in 10 ml of 0.85% NaCl. Two ml of suspension were centrifuged (16,500 × g, 20 min, 4°C) and the obtained pellets were used for isolation of the total-community DNA with the FastDNA® SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA). For mechanical lysis, the cells were homogenized twice in a FastPrep® FP120 Instrument (Qbiogene, BIO101, Carlsbad, CA, USA) for 30 s at a speed of 5.0 m s−1 and treated according to the manufacturer's protocol.
Barcoded Deep 454-Pyrosequening of 16S rRNA Gene Amplicon
The 16S rRNA genes of 24 samples (details are provided in Table 1) were amplified (two technical replicates for each sample) in a nested PCR approach with the Gammaproteobacteria primer set 395f (5′-CMA TGC CGC GTG TGT GAA-3′) and 871r (5′-ACT CCC CAG GCG GTC DAC TTA-3′) (Mühling et al., 2008). The PCR reaction mixture (20 μ l) contained 5 × Taq-&GO Ready-to-use PCR Mix (MP Biomedicals, Germany), 0.25 μM of each primer, 25 mM MgCl2 and 1 μl of template DNA (96°C, 4 min; 32 cycles of 96°C, 1 min; 57°C, 1 min; 74°C, 1 min; and final elongation at 74°C, 10 min). In a second PCR, 1 μl of the amplicon (1:10 diluted phyllosphere and 1:100 diluted rhizosphere derived PCR products) was used. 16S rRNA gene sequences were amplified by using the forward primer Unibac-II-515f (5′-GTG CCA GCA GCC GC-3′) containing the 454-pyrosequencing adaptors and the reverse primer Gamma871r_454 (5′-CTA TGC GCC TTG CCA GCC CGC TCA GAC TCC CCA GGC GGT CDA CTT A-3′). The reaction mixture for the second PCR (30 μ l) contained 5 × Taq-&GO Ready-to-use PCR Mix, 0.25 μ M of each primer (96°C, 4 min; 32 cycles of 96°C, 1 min; 66°C, 1 min; 74°C, 1 min; and final elongation at 74°C, 10 min). PCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, USA). The technical replicates per sample were pooled and the partial 16S rRNA gene fragments were sequenced using 454 Roche GS FLX (MWG Eurofins, Germany) and 454 Roche GS FLX Titanium (Macrogen Korea, South Korea) pyrosequencer. The nucleotide sequences obtained in this work were submitted to the European Nucleotide Archive (www.ebi.ac.uk/ena) and are available under the accession number PRJEB6022.
DNA Sequence Analysis and Taxonomical Identification
Sequences were analyzed with the Qiime software version 6.0 (Caporaso et al., 2010). Replicates from sequencing of each treatment and habitat were bioinformatically pooled during the Qiime analysis for data evaluation. MID-, primer and adapter sequences were removed, length filtered (≥350 nt), quality filtered (score: 50), denoised, chloroplast removed and singletons adjusted. The cut-off level was set to 97% sequence identity. Chimeras were detected with Chimera Slayer and then removed. To compute alpha and beta diversity, the dataset was normalized to 5920 reads per sample. Ring-charts were created using the Krona software package version 2.2 (Ondov et al., 2011) and the profile network was constructed using Cytoscape version 3.0.2 (Shannon et al., 2003). Statistical tests based on the operational taxonomic units (OTUs) table were performed with the nonparametric ANOVA Kruskal Wallis test. This test is functionally an expansion of ANOVA to cases where the sample means are unequal and the distribution is not normal.
Results
The Gammaproteobacterial Microbiome of Lettuce
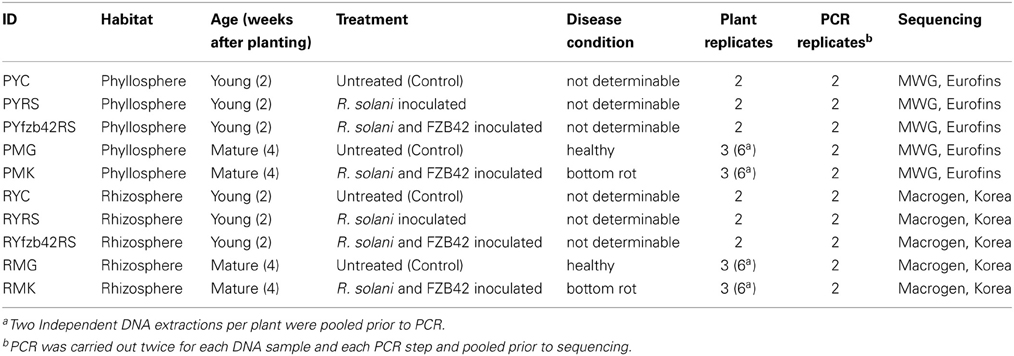
Two sequential batches of 16S rRNA gene amplicon sequencing resulted in a total of 242,022 reads. After removing chimeras, singletons, and chloroplast sequences, 8233 quality mean reads per sample remained with a median absolute deviation of 1842.5 sequence reads. We analyzed the gammaproteobacterial fraction subjected to different treatments, separately for the phyllosphere and rhizosphere (Figure S1). Twenty-four samples (12 from per habitat) yielded in a total of 4.909 distinct OTUs, 1.102 were statistically different (Nonparametric ANOVA Kruskal Wallis Test, p ≤ 0.05) between both habitats. The gammaproteobacterial microbiome from whole lettuce plants contained mainly taxa from Pseudomonadales, followed by Xanthomonadales, Enterobacteriales and Legionellales (Figure 1). The rhizosphere was dominated by Xanthomonadaceae (48%) and Pseudomonadaceae (37%) while the dominating taxa associated to phyllosphere were Pseudomonadaceae (54%), Moraxellaceae (16%) and Enterobacteriaceae (25%). The genus Pseudomonas was almost exclusively assigned to the family Pseudomonadaceae (98%) associated to foliage. The most abundant genus of the root associated microbiome was Rhodanobacter (27%) followed by Pseudomonas (24%), while 8% of the rhizosphere associated reads could not be taxonomically assigned (Figure 1). In addition, differences were found between both plant development stages as well as between the different treatments (Figure S1). Interestingly, comparing only the phyllosphere samples of mature plants with bottom rot disease against the untreated control, we found 371 distinct OTUs with 99 statistically distinct differences.
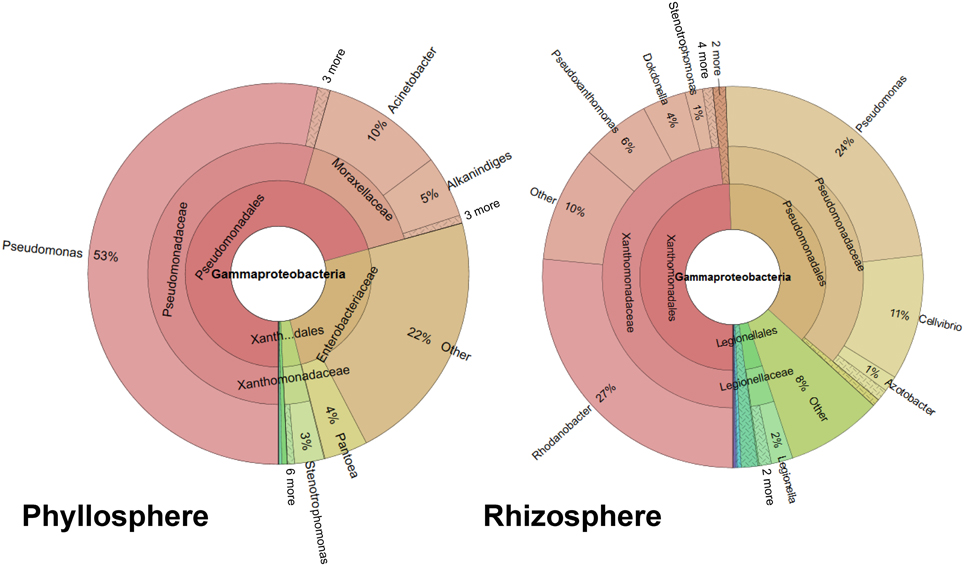
Figure 1. Ring-charts showing the gammaproteobacterial community structure associated with investigated lettuce habitats. The RDP classifier analysis is shown from the mean of 12 samples per habitat. The rings represent different taxonomic ranks (order, family, and genus), and the columns represent distinct taxa. Minor taxa are not specified.
The Core Gammaproteobacterial Community and Its Diversity
A profile clustering network based on all 454-amplicon Gammaproteobacteria libraries was applied to visualize relationships of lettuce-associated bacterial genera and RDP-classified conjoined taxa. The network presented in Figure 2 can be used to identify the core microbiome of lettuce but also to connect specific taxa with different treatments, plant stages or with healthy and diseased plants. Our study revealed that plants belonging to different growth stages harbor several shared genera (at least 0.1% abundant in all samples compared). From 46 taxonomical assignments, 19 taxa could be assigned to a particular core microbiome structure of lettuce. Only Alkanindiges, Pantoea, and a lineage of Enterobacteriaceae (not classified at genus level) were exclusively assigned to the phyllosphere; they are indicated as phyllosphere core microbiome (Figure 2, surrounded by green color). For the rhizosphere a more diverse core microbiome was identified (red color): core taxa were assigned to Rhodanobacter, Pseudoxanthomonas, Dokdonella, Luteimonas, Steroidobacter, Thermomonas, Legionella, and Haliea. Additional OTUs were identified at family level as Xanthomonadaceae, Legionellaceae, Pseudomonadales, and Gammaproteobacteria. The highly abundant Pseudomonas, Acinetobacter, and Stenotrophomonas occurred in the core of both habitats (blue color). There are also other taxa found only associated to a specific habitat but did not account to any core group, due to low abundance or not comparable occurrence in the different treatments. In addition, detailed differences in the composition between the particular treatments are displayed by the network structure. For example, Cellvibrio was found preferentially in higher abundances in the rhizosphere of R. solani treated young plants and decreased in the rhizosphere of mature plants of the same treatment. Comparing all samples which were inoculated with R. solani (RS: PMK, PYRS, PYfzb42RS, n = 7) with the non-inoculated (C: PMG, PYC, n = 5), the highly abundant group of Enterobacteriaceae was dominantly retrieved from phyllosphere samples inoculated with R. solani (RS: 34.5% to C: 12.2% mean abundance), while Moraxellaceae including Acinetobacter and Alkanindiges were more abundant on the foliage of healthy plants (C: 30% to RS: 5.7% mean abundance). Similar gammaproteobacterial patterns could be observed on young plants inoculated only with R. solani compared to plantlets inoculated additionally with FZB42.
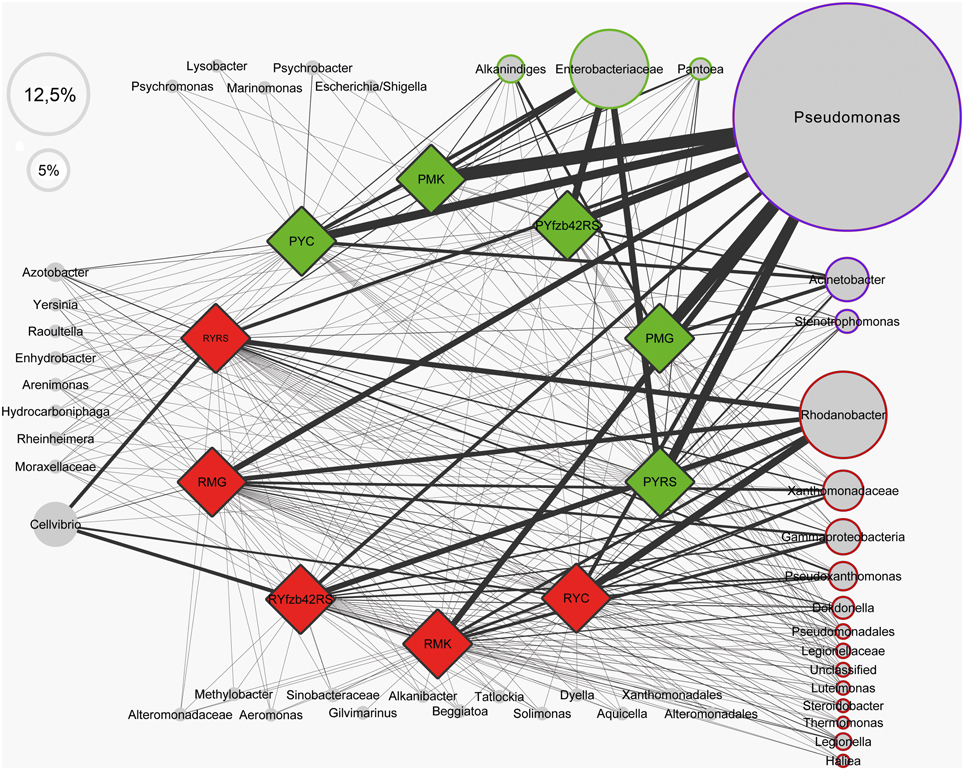
Figure 2. Profile clustering network visualizing the investigated groups of lettuce (var. Tizian) and conjoined taxa. Red diamonds indicate samples derived from the roots and green diamonds from the lettuce foliage, respectively. Nodes represent taxa derived from the RDP classifier and the node sizes correspond to the mean relative abundance of all samples. The abundance of a certain taxa correlated to a particular investigated group is visualized trough the line width of the corresponding connection. The frame color represents the affiliation to the observed core taxa (green—phyllosphere, red—rhizosphere, blue—occurrence in both habitats and all samples, white—not a core taxon). Taxa less abundant than mean ≤0.5% are displayed without size-correlation. Abbreviations: P, phyllosphere; R, rhizosphere; Y, young; M, mature; RS, R. solani; C, untreated (control); FZB42RS, FZB42 and R. solani co-inoculation; G, healthy; K, diseased.
Beta diversity (pairwise sample dissimilarity) indices based on weighted UniFrac distances revealed clear differences between the habitats, but also between young and mature plants infected by R. solani with bottom rot disease compared to non-inoculated healthy young and mature plants (Figure 3, Table S1). A general higher variation was observed among samples derived from the phyllosphere. Alpha diversity indices (Table S1) based on the observed species metric showed a higher diversity of Gammaproteobacteria associated with plants inoculated with R. solani, but the co-inoculation with FZB42 seems to reduce this effect (Figure 4). This was observed across both investigated habitats, and additionally a slight increase of the overall diversity correlated with the maturity state.
Figure 3. Beta diversity metrics of bacterial 16S rRNA genes reveal distinctly clustered Gammaproteobacteria communities structured between healthy plants and plants affect by Rhizoctonia solani. Beta diversity community clustering is observed for phylogenetic beta diversity metrics (weighted UniFrac). In the panel, each point corresponds to a sample from either the lettuce rhizosphere (black) or the phyllosphere (green and red). Red—samples inoculated with R. solani; green—untreated control group. The percentage of variation explained by the plotted principal coordinates is indicated on the axes.
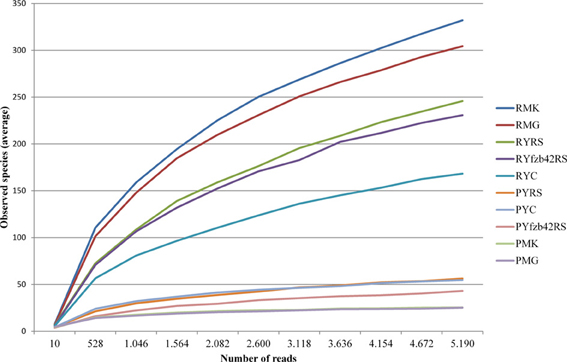
Figure 4. Rarefaction analysis comparing overall diversity of the indigenous microbiota of the investigated lettuce samples (var. Tizian). Prior to rarefaction analysis, rhizosphere, and phyllosphere libraries were pairwise combined corresponding to the particular treatments. Rarefaction curves show saturation of the combined datasets that were clustered at 97% sequence similarity. The curves are supported by 95% confidence intervals. The overall diversity was higher affected in the rhizosphere. In both habitats plants treated with Rhizoctonia solani showed higher diversity than plants treated additionally with FZB42 or untreated plants. Abbreviations: P, phyllosphere; R, rhizosphere; Y, young; M, mature; RS, R. solani; C, untreated (control); FZB42RS, FZB42 and R. solani co-inoculation; G, healthy; K, diseased.
Discussion
Our study gave new insights into the general structure of the lettuce microbiome as well as showed the impact of the plant pathogen R. solani AG1-IB and its antagonistic counterpart B. amyloliquefaciens FZB42. Sequencing of 16S rRNA gene amplicons provided especially a deeper look into the fraction of often health relevant Gammaproteobacteria in the lettuce-associated microbiome, down to the taxonomic rank of genera. While R. solani is a serious lettuce pathogen (Wolf and Verreet, 1999), Chowdhury et al. (2013) demonstrated that FZB42 is an efficient biocontrol agent. FZB42 was able to effectively reduce the disease severity of bottom rot caused by R. solani in pot and field experiments. In our study we showed that both microorganisms have not only a significant impact on plant health, they also significantly influence the structure of the plant-associated microbiome.
The rhizosphere and phyllosphere gammaproteobacterial microbiomes of healthy lettuce plants showed significant differences in their overall composition, their core, and diversity indices. This can be explained by the completely different abiotic conditions in both plant habitats (Raaijmakers et al., 2009; Vorholt, 2012). Here, the most significant and surprising difference we found was the preferential occurrence of enterics in the phyllosphere. Our results are in accordance with the principal findings of Rastogi et al. (2012), who analyzed spatiotemporal variation in bacterial community composition on field-grown lettuce in California. The general composition of phyllosphere bacteria was similar, and Enterobacteriacaea were a substantial fraction also in this study. In general, plant microhabitats are a reservoir for Enterobacteriaceae including potentially human pathogenic bacteria such as human enteric pathogens (Brandl, 2006). Especially after intermediate disturbances such as plant diseases, their abundance was enhanced (Erlacher et al., unpublished data). Due to their impact on human health as either pathogens or immunostimulants, this is an observation that could be of considerable importance for health concerns. Hanski et al. (2012) could show correlation between bacterial diversity and atopy, suggesting significant interactions with Gammaproteobacteria. These authors further showed a positive association between the abundance of Acinetobacter, found abundantly in healthy lettuce in our study and Interleukin-10 expression in peripheral blood mononuclear cells in healthy human individuals. Interleukin-10 is an anti-inflammatory cytokine and plays a central role in maintaining immunologic tolerance to harmless substances (Eskdale et al., 1997; Lloyd and Hawrylowicz, 2009).
In general, members of the genus Pseudomonas play a prominent role in the lettuce gammaproteobacterial microbiome. They were the dominant inhabitants of the phyllosphere; at family level pseudomonads present 54% and at genus level 53% of the microbiome. Also in the rhizosphere they represent 37% of the OTUs at family level. Altogether, Pseudomonas was the most dominant member of the lettuce core microbiome. Pseudomonas is a model organism to study beneficial plant-microbe interactions (Haas and Défago, 2005). Interestingly, in our study, Pseudomonas was not only related to healthy plants, there was also strong connection to diseased plants. The fact is not surprising because pathogenic pseudomonads are well-known but shows the limitation of the applied method. Using amplicon sequencing we can identify the genus or species but not their functional traits. For functional analysis metagenomic/transcriptomic techniques are required.
Plant-microbe interactions are highly complex and changes in the abundance of individual strains, either pathogens or beneficials can result in non-linear alterations of the entire microbiome composition. Such alterations may lead to negative effects to plants and humans as consumers (Berg et al., 2005). However, microbiome shifts can hardly be predicted and must be traced by thorough screening using culture-independent and sequencing-based approaches. Adesina et al. (2009) used DGGE fingerprint to study microbiome shifts and showed that R. solani AG1-IB inoculation severely affected the bacterial and fungal community structure in the rhizosphere of lettuce and that these effects were much less pronounced in the presence of the antagonistic counterpart P. jessenii RU47. In our study we used amplicon sequencing, which allowed a deep insight into the composition of Gammaproteobacteria. The human health relevant group of Enterobacteriaceae was affected by the R. solani; we found a significant increase in gammaproteobacterial diversity due to the pathogen outbreak. The overall enhancement of diversity after biotic stimulation by a pathogen agrees well with the intermediate disturbance hypothesis known to ecology from studies of higher plants or coral reefs (Connell, 1978). However, together with FZB42 this increase was less distinct. Until today, the mechanisms described for biocontrol agents focus on direct antagonistic effects against a pathogen or an interaction via the plant's immune system (Doornbos et al., 2012). In this study we showed a selective compensation of the impact of a pathogen on the indigenous plant-associated microbiome by the biocontrol agent, which is an interesting effect of the beneficial aspect of the inoculant.
Biocontrol of plant pathogens is a promising solution for sustainable agriculture. Molecular techniques, which allowed a deeper insight into the crop-associated microbiome, can also be applied to develop new biocontrol strategies (Berg et al., 2013). Using a profile clustering network in our study, Acinetobacter and Alkanindiges were identified as indicators of healthy lettuce plants. Therefore, they could be promising biocontrol agents. An endophytic Acinetobacter strain isolated from healthy stems of the plant Cinnamomum camphora was already used as biocontrol strain against fungal diseases (Liu et al., 2007) but nothing is known about any biocontrol activity of Alkanindiges. In contrast, in the study of Rastogi et al. (2012), the foliar presence of Xanthomonas campestris pv. vitians, which is the causal agent of bacterial leaf spot of lettuce, correlated positively with the relative representation of bacteria from the genus Alkanindiges. Here, more research is needed for understanding bacterial networking on plants, which is an essential step toward predictable biocontrol. In addition, beneficial Pseudomonas strains could be other interesting candidates for biocontrol because we found a strong connection to healthy lettuce plants. This was shown already successfully for P. jessenii RU47 by Adesina et al. (2009). Altogether, new results favorite diverse bacterial cocktails to control plant diseases (Berg et al., 2013); for lettuce they could contain Acinetobacter, Bacillus, Pseudomonas, and Serratia strains as well.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Tomislav Cernava (Graz) is acknowledged for the kind help in sampling and during the first steps of sample processing. Moreover, we thank Helmut Junge (Berlin) for providing B. amyloliquefaciens FZB42 Rhizovital® 42 liquid (ABiTEP GmbH) for this study. We also would like to thank Irene and Eberhard Gerstädt (Potsdam) for the safe transport of the lettuce samples to Graz. The project was funded by the European Funds for Regional Development (EFRE), co-supported by the regional government of Styria (Austria).
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2014.00175/abstract
References
Adesina, M. F., Grosch, R., Lembke, A., Vatchev, T. D., and Smalla, K. (2009). In vitro antagonists of Rhizoctonia solani tested on lettuce: rhizosphere competence, biocontrol efficiency and rhizosphere microbial community response. FEMS Microbiol. Ecol. 69, 62–74. doi: 10.1111/j.1574-6941.2009.00685.x
Berendsen, R. L., Pieterse, C. M., and Bakker, P. A. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. doi: 10.1016/j.tplants.2012.04.001
Berg, G., Eberl, L., and Hartmann, A. (2005). The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7, 1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x
Berg, G., Roskot, N., Steidle, A., Eberl, L., Zock, A., and Smalla, K. (2002). Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl. Environ. Microbiol. 68, 3328–3338. doi: 10.1128/AEM.68.7.3328-3338.2002
Berg, G., and Smalla, K. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. doi: 10.1111/j.1574-6941.2009.00654.x
Berg, G., Zachow, C., Müller, H., Philipps, J., and Tilcher. R. (2013). Next-Generation bio-products sowing the seeds of success for sustainable agriculture. Agronomy 3, 648–656. doi: 10.3390/agronomy3040648
Bragina, A., Berg, C., Cardinale, M., Shcherbakov, A., Chebotar, V., and Berg, G. (2011). Sphagnum mosses harbour highly specific bacterial diversity during their whole lifecycle. ISME J. 6, 802–813. doi: 10.1038/ismej.2011.151
Brandl, M. T. (2006). Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44, 367–392. doi: 10.1146/annurev.phyto.44.070505.143359
Bulgarelli, D., Rott, M., Schlaeppi, K., van Themaat, E. V. L., Ahmadinejad, N., Assenza, F., et al. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488, 91–95. doi: 10.1038/nature11336
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Carling, D. E., Kuninaga, S., and Brainard, K. A. (2002). Hyphal anastomosis reactions, rDNA-internal transcribed spacer sequences, and virulence levels among subsets of Rhizoctonia solani anastomosis group-2 AG-2 and AG-BI. Phytopathology 92, 43–50. doi: 10.1094/PHYTO.2002.92.1.43
Chowdhury, S. P., Dietel, K., Rändler, M., Schmid, M., Junge, H., Borriss, R., and Grosch, R. (2013). Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its Impact on the rhizosphere bacterial community. PLoS ONE 8:e68818. doi: 10.1371/journal.pone.0068818
Connell, J. H. (1978). Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310. doi: 10.1126/science.199.4335.1302
Davis, R. M., Nunez, J. J., and Subbarao, K. V. (1997). Benefits of cotton seed treatments for the control of seedling diseases in relation to inoculum densities of Pythium species and Rhizoctonia solani. Plant Dis. 81, 766–768. doi: 10.1094/PDIS.1997.81.7.766
Doornbos, R. F., van Loon, L. C., and Bakker, P. A. (2012). Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. Rev. Agron. Sustain. Dev. 32, 227–243. doi: 10.1007/s13593-011-0028-y
Eskdale, J., Kube, D., Tesch, H., and Gallagher, G. (1997). Mapping of the human IL10 gene and further characterization of the 5′flanking sequence. Immunogenetics 46, 120–128. doi: 10.1007/s002510050250
Faltin, F., Lottmann, J., Grosch, R., and Berg, G. (2004). Strategy to select and assess antagonistic bacteria for biological control of Rhizoctonia solani Kühn. Can. J. Microbiol. 50, 811–820. doi: 10.1139/w04-063
Grosch, R., Lottmann, J., Faltin, F., and Berg, G. (2005). Use of bacterial antagonists to control diseases caused by Rhizoctonia solani. Gesunde Pflanzen 57, 199–205. doi: 10.1007/s10343-005-0096-5
Grosch, R., Schneider, J. H. M., and Kofoet, A. (2004). Characterisation of Rhizoctonia solani anastomosis groups causing bottom rot in field-grown lettuce in Germany. Eur. J. Plant. Pathol. 110, 53–62. doi: 10.1023/B:EJPP.0000010137.69498.10
Haas, D., and Défago, G. (2005). Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3, 307–319. doi: 10.1038/nrmicro1129
Hanski, I., von Hertzen, L., Fyhrquist, N., Koskinen, K., Torppa, K., Laatikainen, T., et al. (2012). Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. U.S.A. 109, 8334–8339. doi: 10.1073/pnas.1205624109
Hartmann, A., Rothballer, M., and Schmid, M. (2008). Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil. 312, 7–14. doi: 10.1007/s11104-007-9514-z
Liu, C. H., Chen, X., Liu, T. T., Lian, B., Gu, Y., Caer, V., et al. (2007). Study of the antifungal activity of Acinetobacter baumannii LCH001 in vitro and identification of its antifungal components. Appl. Microbiol. Biotechnol. 76, 459–466. doi: 10.1007/s00253-007-1010-0
Lloyd, C. M., and Hawrylowicz, C. M. (2009). Regulatory T cells in asthma. Immunity 31, 438–449. doi: 10.1016/j.immuni.2009.08.007
Mendes, R., Garbeva, P., and Raaijmakers, J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37, 634–663. doi: 10.1111/1574-6976.12028
Mühling, M., Woolven-Allen, J., Murrell, J. C., and Joint, I. (2008). Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2, 379–392. doi: 10.1038/ismej.2007.97
Ondov, B., Bergman, N., and Phillippy, A. (2011). Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12:385. doi: 10.1186/1471-2105-12-385
Raaijmakers, J. M., Paulitz, T. C., Steinberg, C., Alabouvette, C., and Moënne-Loccoz, Y. (2009). The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 321, 341–361. doi: 10.1007/s11104-008-9568-6
Rastogi, G., Coaker, G. L., and Leveau, J. H. (2013). New insights into the structure and function of phyllosphere microbiota through high−throughput molecular approaches. FEMS Microbiol. Lett. 348, 1–10. doi: 10.1111/1574-6968.12225
Rastogi, G., Sbodio, A., Tech, J. J., Suslow, T. V., Coaker, G. L., and Leveau, J. H. (2012). Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 6, 1812–1822 doi: 10.1038/ismej.2012.32
Scherwinski, K., Wolf, A., and Berg, G. (2007). Assessing the risk of biological control agents on the indigenous microbial communities: Serratia plymuthica HRO-C48 and Streptomyces sp. HRO-71 as model bacteria. BioControl 52, 87–112. doi: 10.1007/s10526-006-9006-8
Schmidt, C. S., Alavi, M., Cardinale, M., Müller, H., and Berg, G. (2012). Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol. Fert. Soils 48, 947–960. doi: 10.1007/s00374-012-0688-z
Schmidt, R., Köberl, M., Mostafa, A., Ramadan, E. M., Monschein, M., Jensen, K. B., et al. (2014). Effects of bacterial inoculants on the indigenous microbiome and secondary metabolites of chamomile plants. Front. Microbiol. 5:64. doi: 10.3389/fmicb.2014.00064
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Smalla, K., Wieland, G., Buchner, A., Zock, A., Parzy, J., Kaiser, S., et al. (2001). Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 67, 4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001
Teplitski, M., Warriner, K., Bartz, J., and Schneider, K. R. (2011). Untangling metabolic and communication networks: interactions of enterics with phytobacteria and their implications in produce safety. Trends Microbiol. 19, 121–127. doi: 10.1016/j.tim.2010.11.007
United Nations Environment Programme (UNEP) Division of Technology, Industry & Economics (DTIE) Ozon Action Programme (1999). Towards Methyl Bromide Phase Out: A Handbook for National Ozone Units - New Publication - Information Release. Available online at: http://www.uneptie.org/ozonaction/information/mmcfiles/4329-e-pr0899mbrhbk.pdf
Vorholt, J. A. (2012). Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840. doi: 10.1038/nrmicro2910
Keywords: lettuce microbiome, Gammaproteobacteria, soil-borne pathogens, 16S rRNA gene pyrosequencing, phyllosphere, rhizosphere, Lactuca sativa
Citation: Erlacher A, Cardinale M, Grosch R, Grube M and Berg G (2014) The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 5:175. doi: 10.3389/fmicb.2014.00175
Received: 31 October 2013; Accepted: 31 March 2014;
Published online: 21 April 2014.
Edited by:
Kornelia Smalla, Julius Kühn-Institut, GermanyReviewed by:
Zuhua He, Chinese Academy of Sciences, ChinaFranz Narberhaus, Ruhr University Bochum, Germany
Kornelia Smalla, Julius Kühn-Institut, Germany
Monica Höfte, Ghent University, Belgium
Copyright © 2014 Erlacher, Cardinale, Grosch, Grube and Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Berg, Institute of Environmental Biotechnology, Graz University of Technology, Petersgasse 12, Graz 8010, Austria e-mail: gabriele.berg@tugraz.at
 Armin Erlacher
Armin Erlacher Massimiliano Cardinale
Massimiliano Cardinale Rita Grosch
Rita Grosch Martin Grube
Martin Grube Gabriele Berg
Gabriele Berg