- 1Department of Soil and Environmental Sciences, Faculty of Agriculture, The University of Poonch, Rawalakot, Azad Jammu and Kashmir, Pakistan
- 2Technical Services Division, Nuclear Institute of Biotechnology and Genetic Engineering, Faisalabad, Pakistan
Introduction and exploitation of plant growth promoting rhizobacteria (PGPR) in agro-ecosystems enhance plant–microbes interactions that may affect ecosystems sustainability, agricultural productivity, and environmental quality. The present study was conducted to isolate and identify PGPRs associated with maize (Zea mays L.) from twenty sites of Himalayan region of Hajira-Rawalakot, Azad Jammu and Kashmir (AJK), Pakistan. A total of 100 isolates were isolated from these sites, out of which eight (HJR1, HJR2, HJR3, HJR4, HJR5, MR6, HJR7, HJR8) were selected in vitro for their plant growth promoting ability (PGPA) including phosphorus solubilization, indole-3-acetic acid (IAA) production and N2 fixation. The 16S rRNA gene sequencing technique was used for molecular identity and authentication. Isolates were then further tested for their effects on growth and nutrient contents of maize (Z. mays L.) under pouch and pot conditions. The 16S rRNA gene sequencing and phylogenetic analysis identified these isolates belong to Pseudomonas and Bacillus genera. The isolates promoted plant growth by solubilizing soil P which ranged between 19.2 and 35.6 μg mL-1. The isolates HJR1, HJR2, HJR3, and HJR5 showed positive activity in acetylene reduction assay showing their N2-fixation potential. All eight isolates showed the potential to produce IAA in the range of 0.9–5.39 μg mL-1 and promote plant growth. Results from a subsequent pot experiment indicated PGPRs distinctly increased maize shoot and root length, shoot and root dry weight, root surface area, leaf surface area, shoot and root N and P contents. Among the eight isolates, HR3 showed a marked P-solubilizing activity, plant growth-promoting attributes, and the potential to be developed as a biofertilizers for integrated nutrient management strategies.
Introduction
Intensive farming practices that achieve high yield require continuous application of chemical fertilizers in our agro-ecosystems. However, the prices and availability of these chemical fertilizers become the limiting factor for crop production especially in developing countries around the world. Continuous application of N fertilizers may result in negative impacts on agro-ecosystem such as leaching, pollution of water resources, gaseous emissions to atmosphere thus causing irreparable damage to the overall ecosystem and environment. Similarly, phosphorus (P) is one of the major essential macronutrients for biological growth and application of P fertilizers is indispensable component for crop production. However, the availability of P to plants is a serious issue because of its fixation and precipitation behavior in soil which lowers the efficiency of added P. It has been reported that more than 80% of applied P as fertilizers precipitates in the presence of metal ion complexes in soil (Ca2+ in calcareous soils and Fe3+ and Al3+ in acidic soils; Qureshi et al., 2012). In addition to these constraints, the prices of P fertilizers jumped up several folds during recent years making P fertilizers not-affordable to a common resource poor farmer.
Introduction of plant growth promoting rhizobacteria (PGPR) including phosphate solubilizing bacteria (PSB) as biofertilizers is suggested as a sustainable option for the improvement of nutrient availability, plant growth, and yields (Vessey, 2003). Use of microbial consortia in the form of biofertilizers for reducing the use of chemical fertilizers without compromising yield is presently an important feature of research in the field of agriculture, microbiology, and biotechnology (Minorsky, 2008). The search for diverse PGPRs is gaining serious attention and efforts are made to exploit them as biofertilizers for various economically important crops.
During the last two decades use of microbial techniques and introduction of rhizobacteria in agriculture has increased tremendously due to their potential for N2-fixation and P solubilization thus increasing N and P uptake by the plants and therefore yields (Vessey, 2003). Successful results of using PGPR species including Azospirillum, Bacillus, Pseudomonas and Enterobacteria on maize, canola, wheat, and other horticultural crops have been achieved both in the laboratory and in the field under variable ecological conditions (Abbasi et al., 2011; Almaghrabi et al., 2013; Hussain et al., 2013; El-Sayed et al., 2014; Lavakush et al., 2014; Mehta et al., 2014). However, soil–plant–microbe interactions are complex phenomena and detailed explanation had been reported in literature that shows how this interaction influences the plant health and productivity (Mehta et al., 2014). Studies have shown that PGPR strains vary widely and their growth promoting ability may be highly specific to certain plant species, cultivar, soil, and genotype (Lucy et al., 2004). Under such conditions, knowledge of native bacterial population and their identification is important for understanding their distribution and diversity.
It is important to explore and identify region specific microbial strains which can be used as potential plant growth promoters to achieve higher yields under specific ecological and environmental conditions (Fischer et al., 2007). Easy and rapid molecular techniques can be developed to perform microbial characterization. DNA sequences in 16S–23S are known to exhibit a great deal of sequences and length variation which are used to differentiate genera, species up to strain level. Sequence analysis of 16S rRNA gene is more exclusively studied and well-established method for phylogenetic and taxonomic studies (Tan et al., 2001).
Knowledge of the native bacterial population, their characterization and identification is required for understanding the distribution and diversity of indigenous bacteria (Chahboune et al., 2011). With the increasing awareness about the economic and environmental consequences of the use of chemical fertilizers, it is important to explore region specific microbial strains which can be used as a potential plant growth promoter to achieve desired results. The use of indigenous PGPR can be an added advantage since it can easily acclimatize to the natural conditions and enhance the plant–microbe interactions (Verma et al., 2013). Presently, there is very little documentation on the occurrence or utilization of PGPRs in the study region. Therefore, the objectives of this study were (i) to isolate native bacterial strains from the maize (Zea mays L.) rhizosphere under in vitro conditions and to characterize these isolates on the basis of morphological and physiological attributes as well as by 16S rRNA sequence analysis (ii) to assess the PGPAs of these isolates in vivo and their effect on the nutrient contents (N and P) of maize plants at early growth stages.
Materials and Methods
Sample Collection, Bacterial Isolation, and Physiological Characterization
The soil used in this study was collected from twenty different maize growing sites of Hajira-Rawalakot, AJK, Pakistan. The soil in the study site was Humic Lithic Eutrudepts (Inceptisols). Soil samples were collected from 0 to 20 cm depth at random from five different locations at each site and mixed well. The field-moist soil was passed through a 4 mm sieve to eliminate coarse rock and plant material, thoroughly mixed to ensure uniformity and stored at 4°C prior to use. A sub-sample of about 0.5 kg was air- dried and passed through 2-mm sieve and used for the determination of physical and chemical characteristics (Table 1). Soil pH was determined in distilled water with a glass electrode (soil:H2O ratio 1:2.5 w/v). Soil total N was determined by the Kjeldahl method (Bremner and Mulvaney, 1982). Soil organic matter was determined using a modified Mebius method (Nelson and Sommers, 1982). Available P from soil samples was determined according to Soil and Plant Analysis Laboratory Manual (Ryan et al., 2001) using AB-DTPA method modified by Soltanpour and Workman (1979). Exchangeable K was determined using a flame photometer following soil extraction with 1 N ammonium acetate (COOCH3NH4; Simard, 1993). The bacterial population was estimated by most probable numbers (PMN) count according to method described by Mirza et al. (2001).
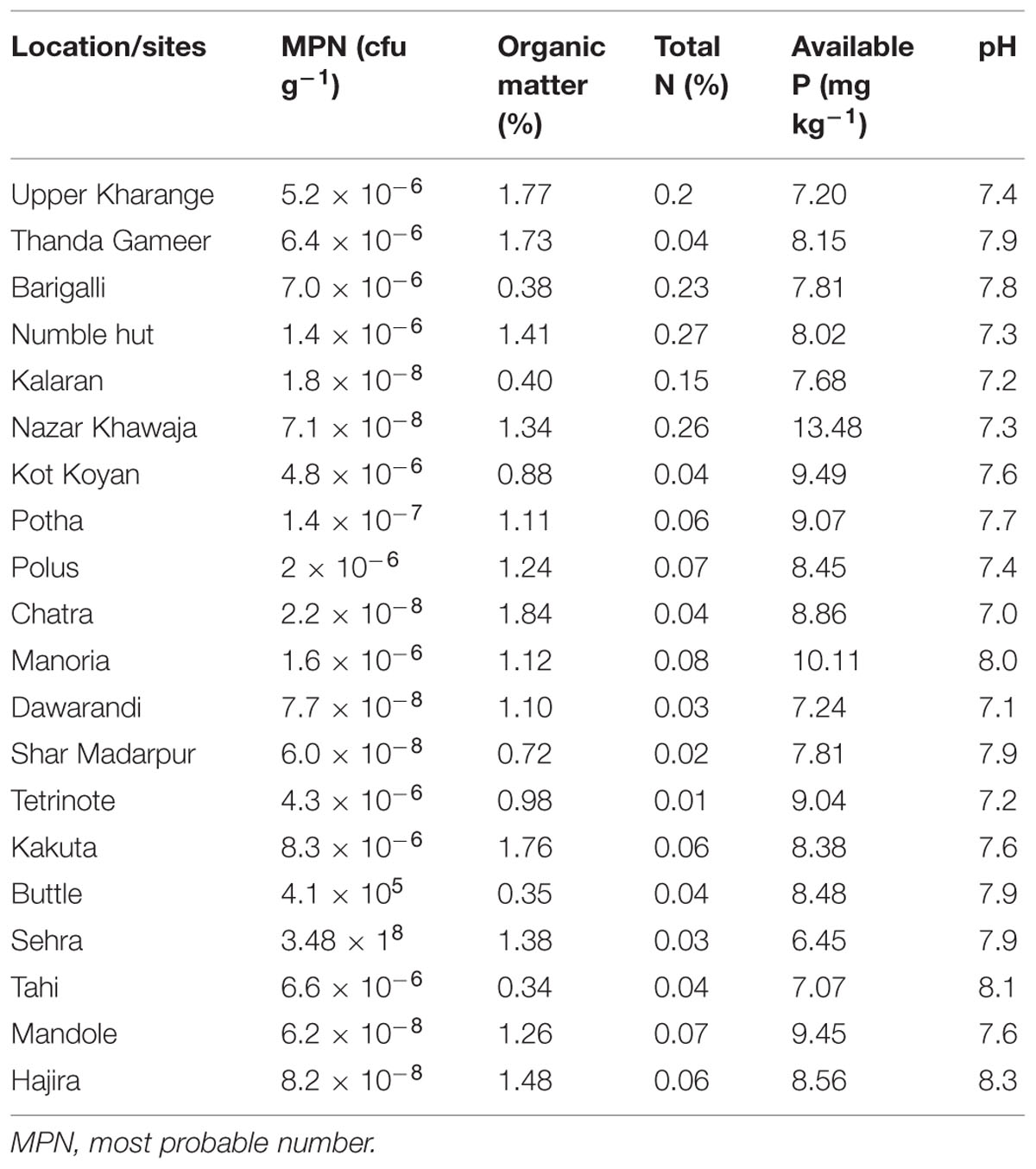
TABLE 1. Physico-chemical properties of soil samples collected for isolation studies from different sites of maize growing areas in Hajira, Rawalakot, Azad Jammu and Kashmir, Pakistan.
The soil samples were processed at National Institute of Biotechnology and Genetic Engineering (NIBGE) Faisalabad, Pakistan for bacterial isolation. For this purpose, plastic pots of about 1 kg capacity were filled with 1 kg soil. Five to six surface sterilized seed of maize (Z. mays L.) were sown in each pot. After 40 days of germination, plants were harvested. Rhizosphere-associated bacteria were isolated by taking 1 g of roots with tightly adhering soil using serial dilution plating technique on LB agar plates (Hameed et al., 2004). The suspension was spread on LB agar plates and incubated at 28 + 2°C till the appearance of bacterial colonies. Individual colonies were picked and streaked on LB plates for further purification. Differences in cell morphology, i.e., cell shape, motility, acid/alkali production, and gram staining were performed by using phase contrast light microscope as described by Vincent (1970). The ability of the isolates to grow in diverse temperature range was carried out by growing isolates in nutrient broth and incubated at different temperatures ranged from 20 to 40°C. Growth was recorded every 24 h up-to 96 h. The ability of the isolates to grow in alkaline or acidic media was tested in nutrient agar plates in which the pH was adjusted from 5.0 to 8.0 and incubated at 30°C for 3 days.
The cell number was calculated from a calibration curve that relates OD values of a series of culture of known cell density. The OD values of cultures containing about ≤0.4 × 109 cell/mL and designated as slow growth (+) ranged from 0.1 to 0.5. The OD values categorized as optimum growth and denoted by (++) ranged from 0.6 to 0.9 while the OD values containing about ≤0.8–2 × cell/mL and designated as maximum growth and is denoted by (+++) ranged ≥1.0 as shown in Table 2.
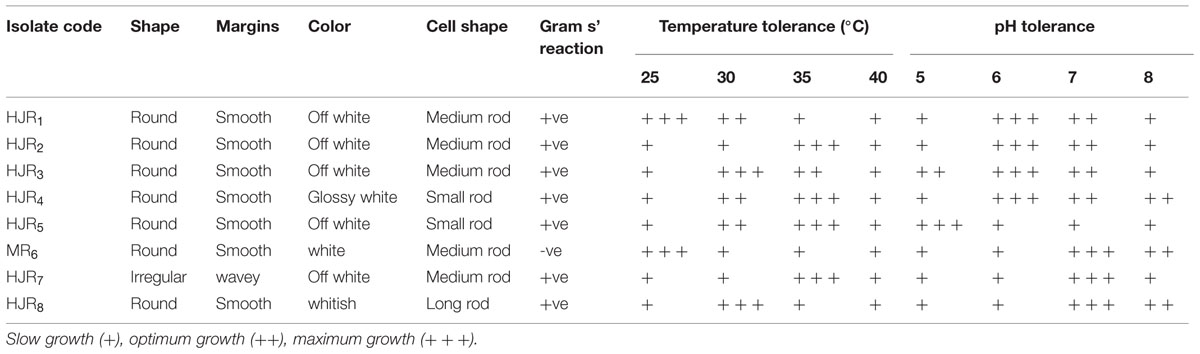
TABLE 2. Colony and cell morphology of selected bacterial isolates and their temperature and pH tolerance ability.
Characterization of Bacteria for Plant Growth Promoting Potential
A total of 100 bacterial isolates were screened for their ability to produce indole-3-acetic acid (IAA), phosphate solubilization, and N2 fixation. For IAA production, individual bacterial cultures were grown in LB broth supplemented with tryptophan (100 mg/L) as a precursor of IAA at 28 ± 2°C with constant shaking (Okon et al., 1977). After 1 week of growth, IAA was extracted from acidified cell-free supernatant using ethyl acetate and analyzed on high-performance liquid chromatograph equipped with Turbochrom software (Perkin Elmer, USA) and C-18 column at a flow rate of 0.5 ml min-1 (Malik et al., 1994). Isolates were categorized into two groups based on their ability to produce IAA in vitro as low IAA producers (1–4 μg mL-1) and medium IAA producers (5–10 μg mL-1) as reported earlier (Khalid et al., 2004). Pure bacterial colonies were inoculated into NFM (Nitrogen Free Malate medium) semisolid medium in vials and incubated at 28 + 2°C for 48 h. Acetylene (10% v/v) was injected to the vials, incubated at room temperature for 16 h and 100 μL of gas sample from each vial was analyzed on a Gas Chromatograph (Thermoquest, Trace G.C, Model K, Rodono, Milan, Italy) equipped with a Porapak Q column and a H2 -flame ionization detector (FID).
To determine phosphate solubilization ability, each bacterial culture was spot inoculated on Pikovskaya (1948) agar plates containing tricalcium phosphate as insoluble phosphate source. The plates were incubated at 28 + 2°C for 7–10 days. Appearance of a clear zone around bacterial colonies indicated the P-solubilization ability of the isolate. Total solubilized phosphate was measured by estimating the available phosphorus in the cell-free supernatant by phospho-molybdate blue color method using spectrophotometer (Halder et al., 1990).
Identification of Potent PGPR based on 16S rRNA Sequencing
Total genomic DNA was extracted by alkaline lysis method (Maniatis et al., 1982). Eubacterial primers fD1 (5′-AGA- GTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGGAGGTGAT- CCAGCC-3′) which correspond to Escherichia coli 16S rRNA gene were used for PCR amplification as described by Weisburg et al. (1991). Amplified PCR products were resolved on 1% agarose gel. Purification of amplified products was done by using PurelinkTM Quick Gel Extraction Kit (Invitrogen) according to manufacturer protocol. The PCR were sequenced commercially by Macrogen (Korea). The gene sequences were compared with others in the GenBank database using the NCBI BLASTn. Multiple sequence alignments were performed by ClustalX and phylogeny was determined by neighbor-joining method (Saitou and Nie, 1987).Tree topology based on re-samplings of 1000 times of the neighbor joining data set was evaluated by boot strap analysis (Felsenstein, 1985).
Plant Inoculation Experiment
Influence of various PGPR isolates on growth and N and P concentration on maize (Z. mays L.) plant was examined in pots under greenhouse conditions. Eight PGPR strains isolated from the 100 screened isolates (HJR1, HJR2, HJR3, HJR4, HJR5, MR6, HJR7, and HJR8) along with un-inoculated control and two levels of N and P fertilizer (½toolNP and full NP fertilizer) were selected. The surface sterilized seeds were inoculated by immersion in the PGPR suspension for 1 h. Cleaned earthen pots of 20 cm height and 15 cm depth were used. A combination of about 4 kg soil and 1 kg sand was used for filling in each pot. Four to five air dried surface sterilized seeds were sown in each pot. The experiment was laid down in a completely randomized design with 11 treatments, three replications and total of 33 pots were used in the experiment. Treatments included: (1) HJR1+½toolNP (2) HJR2+½toolNP (3) HJR3+½toolNP (4) HJR4+½toolNP (5) HJR5+½toolNP (6) MR6+½toolNP (7) HJR7+½toolNP (8) HJR8+½toolNP (9) control (without NP and inoculation; 10) ½toolNP (11) Full NP. Nitrogen and P were applied at the rate of 60 and 45 mg kg-1 (full dose) in the form of urea and single super phosphate, respectively. Pots were kept under greenhouse conditions and equally irrigated when needed. The plants were harvested at 30, and 60 days after germination and following measurements were taken. Morphological characteristics and nutrient contents were determined such as shoot and root length, shoot and root dry weight, root surface area (Scanned image analysis program software), leaf surface area (electronic planimeter), N and P contents in shoot and root. The N and P analysis in shoot and root were carried out using the methods described by Winkleman et al. (1990).
Statistical Analysis
The data were subjected to analysis of variance using statistical program (MSTATC, 1990). The differences among various treatment means were compared using the least significant differences test (LSD) at 5% (P ≤ 0.05) probability level (Steel and Torri, 1980).
Results and Discussion
Isolation and Characterization of PGPR
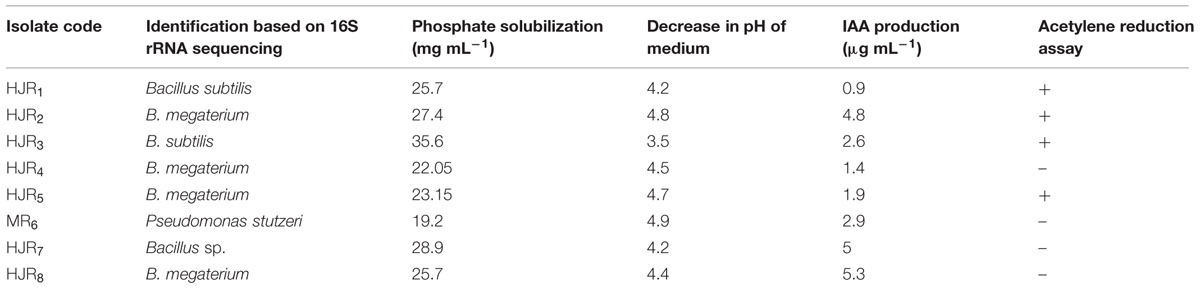
Among the 100 bacterial isolates from the twenty maize growing sites, eight named as HJR1, HJR2, HJR3, HJR4, HJR5, MR6, HJR7, and HJR8 were selected based on their ability to produce phytohormone IAA, solubilize insoluble phosphate, or fix N2. These bacterial isolates were characterized based on their morphological features such as shape, margins, color, and pigmentation. All eight isolates were fast growing, having round to irregular colony shape with raised elevation and smooth surface. No pigmentation was produced by any of the tested isolate on LB medium (Table 2). Isolates HJR4 and HJR5 were small rod; HJR1, HJR2, HJR3, MR6, and HJR7 were medium rods; and HJR8 showed long rods. Except MR6, all isolates were Gram positive. The isolates were grown in ranges of temperature and pH to examine their tolerance to pH and temperature extremes. The results indicated that almost all isolates were able to grow in temperatures ranging between 25 and 40°C, and pH ranging between 5 and 8, with an optimum temperature of 35°C and pH of 7.0 (Table 2). Our results are similar to those reported for PGPR isolated from apple rhizosphere in Himachal Pradesh, India (Mehta et al., 2014). The apple rhizosphere isolates reported by Mehta et al. (2014) had an optimum growth temperature of 30°C and pH of 7.0 with similar phenotypic characteristics as observed in our study.
Characterization of Bacterial Isolates
The sequence analysis of 1.5 kb fragment of 16S rRNA gene of all eight bacterial isolates was analyzed by nucleotide Blast analysis. The sequence of isolates HJR1 and HJR3 showed 99% similarity with Bacillus subtilis and were submitted to GenBank under accession number HQ700330 and HQ700332, respectively. Isolates HJR2, HJR4, and HJR8 had 99% sequence match with that of Bacillus megaterium. These isolates were submitted to GenBank under accession number HQ700331, HQ700333, and HQ700334, respectively. Isolate MR6 showed similarity with genus Pseudomonas and was identified as Pseudomonas stutzeri. These sequences were aligned with sequence of some PGPR of different genera and species within genera from the GenBank database. The phylogenetic tree of these strains constructed by using their 16S rRNA sequences (Figure 1) showed that the selected isolates were members of genus Pseudomonas and Bacillus.

FIGURE 1. Phylogenetic tree of 16S rRNA gene sequences showing the relationships among the isolates isolated from the soils of Himalayan region of Hajira Rawalakot, Azad Jammu and Kashmir (AJK), Pakistan and the related genera. The data of type strains of related species were from GenBank database (the accession numbers are given in parentheses).
Bacillus and Pseudomonas are the most commonly reported genera and represent the dominant isolates in many plant studies (Hallmann and Berg, 2006). The number of isolates belonging to the genus Bacillus was higher in this study similar to those reported earlier explaining that Bacillus spp. are dominant in root adhering soil (Laguerre et al., 1994). Studies on the diversity of root-associated bacteria in maize carried out in different geographical regions, revealed extensive colonization by Bacillus sp. during the active growth stage of the plants (Lambert et al., 1987; Lalande et al., 1989). Both these studies also reported a substantial or rather predominant fraction of Pseudomonas present in the isolation pool (reported in Grönemeyer et al., 2012).
Characterization for Plant Growth Promoting Traits
The isolates associated with the roots of maize crop were tested for features known to contribute to plant growth promotion or biocontrol (Table 3).
Production of Indole-3-Acetic Acid
All Bacillus and Pseudomonas spp. produced IAA in vitro in tryptophan supplemented LB medium. Isolates HJR2, HJR7, and HJR8 were medium producers of IAA (4.8–5.3 μg ml-1) while HJR1, HJR4, HJR5, HJR6 designated as weak producers (0.9–2.9 μg ml-1; Table 3). The amount of IAA produced by these isolates was substantially lower than that reported earlier from other regions (Fischer et al., 2007; Tahir et al., 2013) but similar to that reported under similar environmental conditions from this region (Abbasi et al., 2011; Zhang et al., 2012). However, it has been reported that the amount of indole compounds produced in vitro depends on the particular bacterial species, strain, or the conditions of the culture such as oxygenation and pH (Radwan et al., 2002). The variation among PGPRs to produce IAA found in the present study had also been reported earlier (Zahir et al., 2000; Abbasi et al., 2011). This variation is attributed to the various biosynthetic pathways, location of the genes involved, regulatory sequences, and the presence of enzymes to convert active free IAA into conjugated forms (Patten and Galick, 1996). A high level of IAA production was recorded in different strains of bacteria with the members of the genera Pseudomonas spp., Bacillus spp., Rhizobium, and Mesorhizobium spp. by other workers (Ahmad et al., 2008; Verma et al., 2012, 2013).
Solubilization of Inorganic Phosphates
The results showed that all isolates had P solubilization potential ranging between 19.2 and 35.6 μg mL-1 (Table 3). The highest P solubilization was measured for bacterial isolate HJR3 (35.6 μg mL-1) fallowed by HJR7 (28.9 μg mL-1) and HJR2 (27.4 μg mL-1). The solubilization of TCP by different isolates was accompanied by a significant drop in pH (4.9–3.5) from an initial pH of 7.0. The maximum drop in culture pH of 3.5 was associated with isolate HJR3. P-solubilization activity was associated with the release of organic acids and a drop in pH. Pikovskaya’s medium indicated the efficacy of tested isolates to solubilize the unavailable P (Mehta et al., 2014). It has been reported that P solubilization is mainly due to the production of microbial metabolites including organic acids which decrease the pH of the culture media (Puente et al., 2004; Sahin et al., 2004).
The ability of PGPR strains to solubilize insoluble P and convert it to plant available form is an important characteristic under conditions where P is a limiting factor for crop production. In general, out of the 100 isolates tested, only eight were able to show P solubilization. Such low percentage of isolates that show P solubilization ability is not unique to our study as other studies also show limited numbers. Two separate studies in pearl millet and rice paddy field indicated that only 5% of the 207 total tested strains in of the studies and 23.5% in the other possessed P solubilizing ability (Hameeda et al., 2006; Rashedui et al., 2010). The presence of P-solubilizing microbial population in soils may be considered a positive indicator of utilizing the microbes as biofertilizers for crop production and beneficial for sustainable agriculture.
Based on the acetylene reduction assay, isolates HJR1, HJR2, HJR3, and HJR5 showed the ability to fix N2. The remaining isolates did not show any potential for N2 fixation (Table 3). The presence of N2 fixing bacteria in soil and its isolation and conversion into PGPR biofertilizer is an important strategy reducing the use of expensive chemical fertilizers especially in nutrient poor and degraded soils. Biological N2 fixation provides a major source of N for plants as part of environmental friendly agricultural practices (Cakmakci et al., 2006).
Plant Growth Promotion
Co-inoculation of these isolates along-with 50% reduced fertilizer dose (½toolNP) was tested against un-inoculated/unfertilized control and with two levels of NP fertilizer (½toolNP and full NP fertilizer) to evaluate their potential as PGPR in maize (Table 4). Results indicated that all isolates significantly (P ≤ 0.05) increased the growth of maize compared to the control and in some cases to that recorded under ½toolNP treatment. The shoot length, root length, shoot fresh weight, and root fresh weight were highest in HR3+½toolNP and full NP treatments. Root dry weight and leaf area were significantly (P ≤ 0.05) higher in HR3+½toolNP compared to the remaining treatments including full NP. Root area was maximum in HR8+½toolNP. Most of the isolates when combined with ½toolNP showed significantly higher growth characteristics compared to the treatments supplemented with ½toolNP. The difference in growth between isolates+½toolNP and ½toolNP treatments was due to the addition of PGPR isolates. The results indicated that the growth traits recorded under HJR3+½toolNP treatment were significantly (P ≤ 0.05) higher than those recorded under other isolates, ½toolNP and control treatments but at par with those recoded under full NP fertilizer treatment showing the dominating and promising effect of isolate HJR3 over the remaining isolates (Table 4).
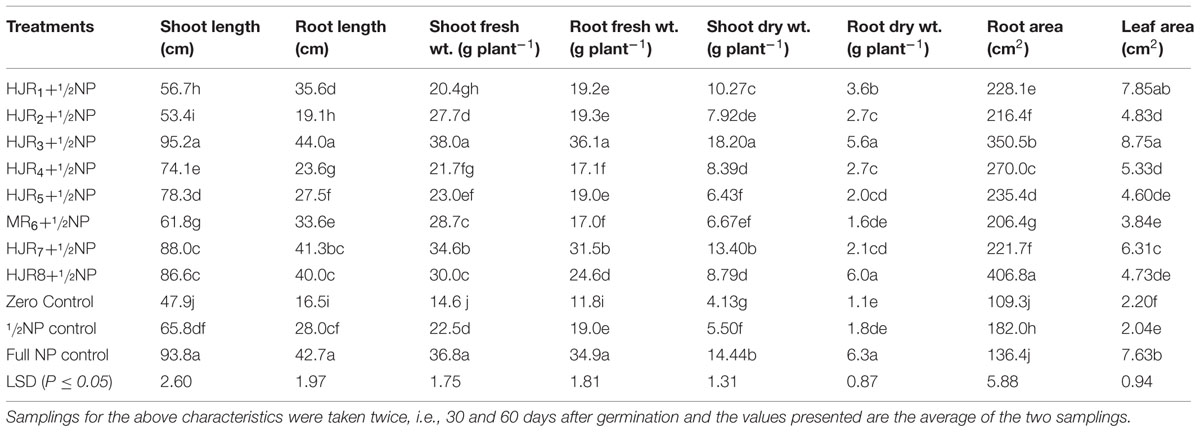
TABLE 4. Effect of inoculation with plant growth promoting rhizobacteria on the growth of maize (Zea mays L.) grown in pots under greenhouse conditions.
Plant growth promotion in response to PGPRs applied alone or with N or P fertilizers has been reported recently for different crops under different ecological and environmental conditions (Lee et al., 2012; Krey et al., 2013; Verma et al., 2013; Lavakush et al., 2014; Mehta et al., 2014). The PGPRs may promote the plant growth either by using their own metabolism (solubilizing phosphates, producing hormones, or fixing nitrogen) or directly affecting the plant metabolism (increasing the uptake of water and minerals), enhancing root development, increasing the enzymatic activity of the plant or “helping” other beneficial microorganisms to enhance their action on the plants, or by suppressing plant pathogens (Pérez-Montaño et al., 2014). The PGPRs tested in this study possessed multiple plant growth promoting traits including P-solubilization, IAA production, and N2 fixation.
N and P Concentration in Maize Plants
The N and P contents of both shoot and root of plants in response to PGPR isolates, NP fertilizer and control treatments is presented in Figure 2. The N and P contents in both shoot and root showed similar trend in response to different treatments; hence, the N and P contents is presented here as a total of the shoot and root. The total N in plants received the control treatment was 7.4 g kg-1 compared with 14.8–47.7 g kg-1 for plants those received the PGPRs and NP fertilizers, showing a 2–6 fold increase. Among the different amendments, co-inoculation with isolate HJR3+½toolNP displayed superiority over the remaining treatments including full NP treatment. The highest total N content (in shoot+root) of 47.7 g kg-1 was recorded in plants grown under HJR3+½toolNP followed by 34.6, 33.8, and 33.1 g kg-1 N under HJR1+½toolNP, full NP and HJR2+½toolNP treatments, respectively. The relative increase in total N contents under HJR3+½toolNP over HJR1+½toolNP, full NP and HJR2+½toolNP was 38, 41, and 44%, respectively. The N contents observed in HJR3+½toolNP was almost double to those recorded under ½toolNP treatment showing that the additional N in plant was eventually because of isolate HJR3.
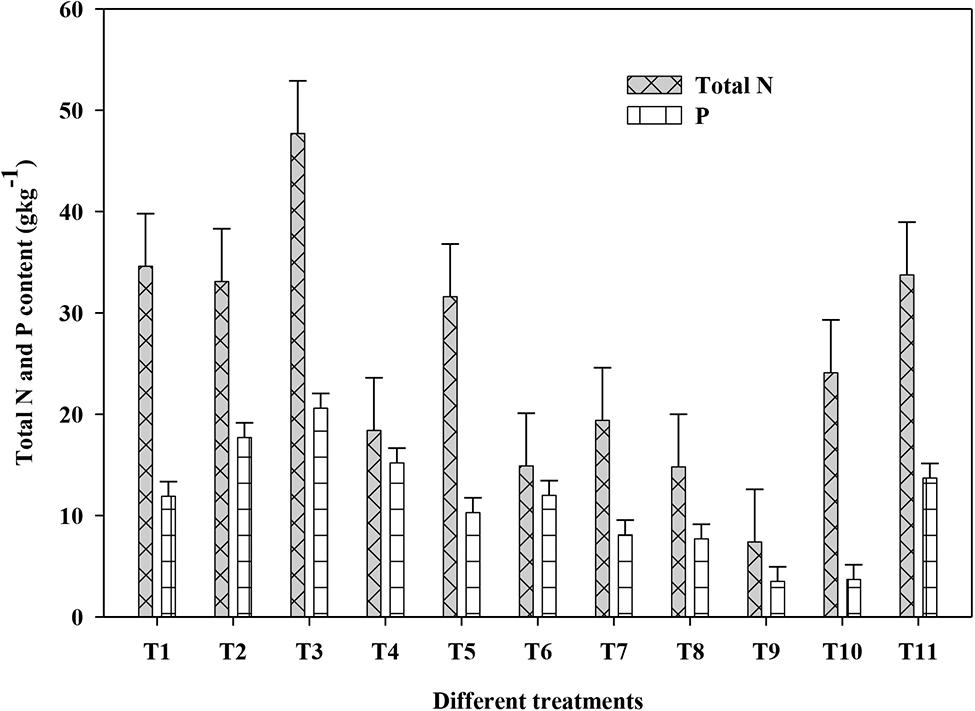
FIGURE 2. Effect of inoculation with Bacillus and Pseudomonas on the shoot and root N and P contents of maize plant grown under greenhouse condition after 60 days. Treatments included T1, HJR1+½toolNP; T2, HJR2+½toolNP; T3, HR3+½toolNP; T4, HJR4+½toolNP, T5, HJR5+½toolNP; T6, MR6+½toolNP; T7, HJR7+½toolNP; T8, HJR8+½toolNP; T9, Control; T10, ½toolNP; T11, Full NP. The vertical lines on each bar represent the standard error of mean (SEM, n = 3).
Total P contents both in shoot and root also showed similar trend as that of total N (Figure 2). The minimum total P content of 3.5 and 3.7 g kg-1 was found under the control and ½toolNP fertilizer treatments. Application of different PGPRs with ½toolNP significantly increased plant P contents to between 7.7 and 20.6 g kg-1 showing a 2–6 fold increase due to inoculation with PGPRs. The highest P content of 20.6 g kg-1 was recorded under HJR3+½toolNP treatment followed by 17.7 g kg-1 P under HJR2+½toolNP. The full NP fertilizer treatment had 13.7 g kg-1 total P content. The relative increase in P contents in plants grown under HJR3+½toolNP and HJR2+½toolNP over full NP was 50 and 29%, respectively.
These results show the efficient transfer of N and P to plants by the PGPR strains as reported earlier rice (Islam et al., 2009), wheat (Abbasi et al., 2011), common bean (Phaseolus vulgaris L.; Tajini et al., 2012), and chickpea (Cicer arietinum L.; Verma et al., 2013). This increase in NP concentration in plant tissues is associated with N2 fixation and P solubilization potential of applied PGPRs. The highest N concentration was recorded in plants supplemented with isolates HJR1, HJR2, HJR3, and HJR5. All of these four isolates showed nitrogenase activity (N2 fixation; Table 3) signifying a close association between plant N concentration and N2 fixation. In our previous study, the N content in wheat shoot under control was 1.2% that significantly increased to 1.7–2.43% by the application of different PGPR strains (Abbasi et al., 2011).
The increase in plant P concentration in response to PGPRs is attributed to the fact that PGPRs have the ability to solubilize insoluble phosphates, making it available for plant uptake through different mechanisms such as acidification, chelation, and ion-exchange reactions (Coutinho et al., 2012). The results presented in Table 3 indicate a substantial potential of applied strains to solubilize P and increase P concentration in plants. The increased concentration of N and P in plants supplemented with PGPRs suggest that a positive interaction exists between root colonization, N and P concentration and growth promotion. Further, this study suggests that plant N derived from N2-fixation and P concentration as a result of P-solubilization by phosphate solubilizing microorganism is substantially enhanced above those of uninoculated control plants.
The significant increase in growth and NP level both in shoot and root upon isolates application is a clear indication that bacterial isolates are able to provide better nutrient flux to the plant and result in increased plant biomass. The increase in root length and mass due to the applied isolates may also be a factor that contributes to the increase in N and P concentration in plant shoot and root.
Conclusion
This study indicates the presence of some efficient and effective species of bacteria in the soils of mountainous region of Rawalakot, AJK, Pakistan. Our results demonstrate that efficient N2 fixing and P solubilizing isolates are present among natural population of rhizobacteria. These characteristics are important growth promoting traits for plants growing in the region under continuous threat of soil erosion and soil degradation. The combination of Bacillus spp. with ½toolNP fertilizer resulted in plant growth equivalent to full NP fertilizer treatment while the N and P concentration in plant biomass in this combined treatment was even higher than that recorded under full NP treatment. The effectiveness of PGPR isolates with NP fertilizers clearly indicates that the chemical fertilizers rate or dose could be reduced through combination of PGPR isolates with fertilizers that may be an eco-friendly and cost effective management strategy. These native isolates may be used as efficient bio-inoculants for integrated nutrient management in the uplands soils facing severe threat of erosion and degradation. Therefore, these isolates might have potential in future field applications as plant growth promoters. The future studies should be focused on the functional characterization of PGPR for practical applications in the field.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research work was kindly supported by the NIBGE, Faisalabad and the University of AJK, Pakistan. The authors are grateful to Dr. Haile Tewolde, Research Agronomist, United States Department of Agriculture, Stoneville, MS, USA for proof reading of this manuscripts. The contribution of Dr. Asma Imran, Senior Scientist, Microbial Ecology Lab, Soil and Environmental Biotechnology Division, NIBGE, Faisalabad for improving the draft of this manuscript is highly acknowledged. The technical staff of the Department of Soil and Environmental Sciences, Faculty of Agriculture, Rawalakot-AJK is appreciated for their technical assistance and help in collecting soil samples.
References
Abbasi, M. K., Sharif, S., Kazmi, M., Sultan, T., and Aslam, M. (2011). Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst. 145, 159–168. doi: 10.1080/11263504.2010.542318
Ahmad, F., Ahmad, I., and Khan, M. S. (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163, 173–181. doi: 10.1016/j.micres.2006.04.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Almaghrabi, O. A., Massoud, S. I., and Abdelmoneim, T. S. (2013). Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J. Biol. Sci. 20, 57–61. doi: 10.1016/j.sjbs.2012.10.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bremner, J. M., and Mulvaney, C. S. (1982). “Nitrogen–Total,” in Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, eds A. L. Page, R. H. Miller, and D. R. Keeney (Madison, WI: SSSA), 595–624.
Cakmakci, R., Donmez, F., Aydın, A., and Sahin, F. (2006). Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 38, 1482–1487. doi: 10.1016/j.soilbio.2005.09.019
Chahboune, R., Barrijal, S., Moreno, S., and Bedmar, E. J. (2011). Characterization of Bradyrhizobium species isolated from root nodules of Cytisus villosus grown in Morocco. Syst. Appl. Microbiol. 34, 440–445. doi: 10.1016/j.syapm.2011.04.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Coutinho, F. P., Felix, W. P., and Yano-Melo, A. M. (2012). Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 42, 85–89. doi: 10.1016/j.ecoleng.2012.02.002
El-Sayed, W. S., Akhkha, A., El-Nagga, M., and Elbadry, M. (2014). In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 5:651. doi: 10.3389/fmicb.2014.00651
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.2307/2408678
Fischer, S. E., Fischer, S. I., Margis, S., and Mori, G. B. (2007). Isolation and characterization of bacteria from rhizosphere of wheat. World J. Microbiol. Biotechnol. 23, 895–903. doi: 10.1007/s11274-006-9312-4
Grönemeyer, J. L., Burbano, C. S., Hurek, T., and Reinhold-Hurek, B. (2012). Isolation and characterization of root-associated bacteria from agricultural crops in the Kavango region of Namibia. Plant Soil 356, 67–82. doi: 10.1007/s11104-011-0798-7
Halder, A. K., Mishra, K. A., Bhattacharyya, P., and Chakrabartty, K. P. (1990). Solubilization of rock phosphate by Rhizobium and Brady-Rhizobium. J. Gen. Appl. Microbiol. 36, 81–92. doi: 10.2323/jgam.36.81
Hallmann, J., and Berg, G. (2006). “Spectrum and population dynamics of bacterial root endophytes,” in Microbial Root Endophytes, eds B. Schulz, C. Boyle, and T. Sieber (Heidelberg: Springer), 15–31.
Hameed, S., Yasmin, S., Malik, K. A., Zafar, Y., and Hafeez, F. Y. (2004). Rhizobium, Bradyrhizobium and Agrobacterium strains isolated from cultivated legumes. Biol. Fertil. Soils 39, 179–185. doi: 10.1007/s00374-003-0697-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hameeda, B., Rupela, O. P., Reddy, G., and Satyavani, K. (2006). Application of plant growth-promoting bacteria associated with composts and macrofauna for growth promotion of pearl millet (Pennisetum glaucum L.). Biol. Fertil. Soils 43, 221–227. doi: 10.1007/s00374-006-0098-1
Hussain, M. I., Asghar, H. N., Akhtar, M. J., and Arshad, M. (2013). Impact of phosphate solubilizing bacteria on growth and yield of maize. Soil Environ. 32, 71–78.
Islam, M. R., Madhaiyan, M., Boruah, H. P. D., Yim. W., Lee, G., Saravanan, V. S.,et al. (2009). Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J. Microbiol. Biotechnol. 19, 1213–1222. doi: 10.4014/jmb.0903.3028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Khalid, A., Arshad, M., and Zahir, Z. A. (2004). Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 96, 473–480. doi: 10.1046/j.1365-2672.2003.02161.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krey, T., Vassilev, N., Baum, C., and Eichler-Löbermann, B. (2013). Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur. J. Soil Biol. 55, 124–130. doi: 10.1016/j.ejsobi.2012.12.007
Laguerre, G., Attard, M. R., Revoy, F., and Amarger, N. (1994). Rapid identification of Rhizobia by restriction fragment length polymorphism analysis of PCR amplified 16S rRNA genes. Appl. Environ. Microbiol. 60, 56–63.
Lalande, R., Bissonnette, N., Coutlée, D., and Antoun, H. (1989). Identification of rhizobacteria from maize and determination of their plant-growth promoting potential. Plant Soil 115, 7–11. doi: 10.1007/BF02220688
Lambert, B., Leyns, F., Van Rooyen, L., Gossele, F., Papon, Y., and Swings, J. (1987). Rhizobacteria of maize and their antifungal activities. Appl. Environ. Microbiol. 53, 1866–1871.
Lavakush, Yadav, J., Verma, J. P., Jaiswal, D. K., and Kumar, A. (2014). Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 62, 123–128. doi: 10.1016/j.ecoleng.2013.10.013
Lee, S., Ka, J., and Song, H. (2012). Growth promotion of Xanthuim italicum by application on rhizobacterial isolates of Bacillus aryabhattai in microcosom soil. J. Microbiol. 50, 45–49. doi: 10.1007/s12275-012-1415-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lucy, M., Reed, E., and Glick, B. R. (2004). Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86, 1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e
Malik, K. A., Rasul, G., Hassan, U., Mehnaz, S., and Ashraf, M. (1994). “Role of N2-fixing and growth hormone producing bacteria in improving growth of wheat and rice,” in Nitrogen Fixation with Non-Legumes, eds N. A. Hegazi, M. Fayez, and M. Monib (Ismaïlia: The American University in Cairo Press), 409–422.
Maniatis, T., Fritsch, E. F., and Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual. New York, NY: Cold Spring Harbor Laboratory, 545.
Mehta, P., Walia, A., Kulshrestha, S., Chauhan, A., and Shirkot, C. K. (2014). Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microbiol. 53, 1–12. doi: 10.1002/jobm.201300562
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mirza, M. S., Waseem, A., Farooq, L., Jacqueline, H. B., Rene, N. P., and Malik, K. A. (2001). Isolation, partial characterization, and the effect of plant growth promoting bacteria (PGPB) on micro propagated sugarcane in vitro. Plant Soil 237, 47–54. doi: 10.1023/A:1013388619231
MSTATC. (1990). A Microcomputer Program for the Design, Management, and Analysis of Research Agronomic Experiments. East Lansing, MI: Michigan State University.
Nelson, D. W., and Sommers, L. E. (1982). “Total carbon, organic carbon and organic matter,” in Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, eds A. L. Page, R. H. Miller, and D. R. Keeney (Madison WI: SSSA), 539–577.
Okon, Y., Albercht, S. L., and Burris, R. H. (1977). Methods for growing Spirillum lipoferum and for counting it in pure culture and in association with plants. Appl. Environ. Microbiol. 22, 85–88.
Patten, C., and Galick, B. R. (1996). Bacterial biosenthesis of indole-3-acetic acid. Can. J. Microbiol. 42, 207–220. doi: 10.1139/m96-032
Pérez-Montaño, F., Alías-Villegas, C., Bellogín, R. A., del Cerro, P., Espuny, M. R., Jiménez-Guerrero, I.,et al. (2014). Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol. Res. 169, 325–336. doi: 10.1016/j.micres.2013.09.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil in connection with thevital activity of some microbial species. Mikrobiologiya 17, 362–370.
Puente, M. E., Li, C. Y., and Bashan, Y. (2004). Microbial populations and activities in rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings. Plant Biol. 6, 643–650. doi: 10.1055/s-2004-821101
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Qureshi, M. A., Ahmad, Z. A., Akhtar, N., and Iqbal, A. (2012). Role of phosphate solubilizing bacteria (PSB) in enhancing P-availability and promoting cotton growth. J. Anim. Plant Sci. 22, 204–210.
Radwan, T. E. E., Mohamed, Z. K., and Reis, V. M. (2002). Production of indole-3-acetic acid by different strains of Azospirillum and Herbaspirillum spp. Symbiosis 32, 39–54.
Rashedui, M., Madhaiyan, M., Boruah, H. P. D., Yim, W., Lee, G., Saravanan, V. S.,et al. (2010). Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J. Microbiol. Biotechnol. 19, 1213–1222.
Ryan, J., Estefan, G., and Rashid, A. (2001). Soil and Plant Analysis Laboratory Manual. (Aleppo: International Center for Agricultural Research in the Dry Areas ICARDA), 172.
Sahin, F., Cakmakci, R., and Kanta, F. (2004). Sugar beet and barley yields in relation to inoculation with N2-fixing and phosphate solubilizing bacteria. Plant Soil 265, 123–129. doi: 10.1007/s11104-005-0334-8
Saitou, N., and Nie, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Bio. Evol. 24, 1596–1599.
Simard, R. R. (1993). “Ammonium acetate-extractable elements,” in Soil Sampling and Methods of Analysis, ed. M. R. Carter (Boca Raton, FL: Lewis Publisher), 39–42.
Soltanpour, P. N., and Workman, S. (1979). Modification of the NaHCO3 DTPA soil test to omit carbon black. Commun. Soil Sci. Plant Anal. 10, 1411–1420. doi: 10.1080/00103627909366996
Steel, R. G. D., and Torri, J. H. (1980). Principles and Procedures of Statistics, 2nd Edn. New York: McGraw Hill Book Co. Inc.
Tahir, M., Sajjad, M. M., Zaheer, A., Dimitrov, M. R., Smidt, H., and Hameed, S. (2013). Isolation and identification of phosphate solubilizer Azospirillum, Bacillus and Enterobacter strains by 16SrRNA sequence analysis and their effect on growth of wheat (Triticum aestivum L.). Aust. J. Crop Sci. 7, 1284–1292.
Tajini, F., Mustapha Trabelsi, M., and Drevon, J. J. (2012). Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 19, 157–163. doi: 10.1016/j.sjbs.2011.11.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tan, Z. Y., Khan, G. S., Pang, E. T., Wang, B., and Chen, W. X. (2001). Rhizobium yangligense spp. nov. isolated from arid and semi arid region of China. Systemic Bacteriol. 51, 909–914.
Verma, J. P., Yadav, J., and Tiwari, K. N. (2012). Enhancement of nodulation and yield of chickpea by co-inoculation of indigenous Mesorhizobium spp. and plant growth-promoting rhizobacteria in eastern Uttar Pradesh. Commun. Soil Sci. Plant Anal. 43, 605–621. doi: 10.1080/00103624.2012.639110
Verma, J. P., Yadav, J., Tiwari, K. N., and Kumar, A. (2013). Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 51, 282–286. doi: 10.1016/j.ecoleng.2012.12.022
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893
Vincent, J. M. (1970). A Manual for the Practical Study of the Root Nodule Bacteria. International Biological Programme. Handbook, Vol. 15. Oxford: Blackwell.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703.
Winkleman, G. E., Amin, R., Rice, W. A., and Tahir, M. B. (1990). Methods Manual Soil Laboratory. BARD Project. Islamabad: PARC.
Zahir, A., Abbas, S. A., Khalid, M., and Arshad, M. (2000). Structure dependent microbially derived plant hormone by improving growth of maize seedlings. Pak. J. Biol. Sci. 3, 289–291. doi: 10.3923/pjbs.2000.289.291
Zhang, J., Liu, J., Meng, L., Ma, Z., Tang, X., Cao, Y.,et al. (2012). Isolation and characterization of plant growth-promoting rhizobacteria from wheat roots by wheat germ agglutinin labeled with fluorescein isothiocyanate. J. Microbiol. 50, 191–198. doi: 10.1007/s12275-012-1472-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: biofertilizer, growth attributes, isolation, PGPR, rhizobacteria, 16S rRNA gene sequencing
Citation: Zahid M, Abbasi MK, Hameed S and Rahim N (2015) Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front. Microbiol. 6:207. doi: 10.3389/fmicb.2015.00207
Received: 16 November 2014; Accepted: 27 February 2015;
Published online: 17 March 2015.
Edited by:
Brigitte Mauch-Mani, Université de Neuchâtel, SwitzerlandReviewed by:
Hossein Borhan, Agriculture and Agri-Food Canada, CanadaNai-Chun Lin, National Taiwan University, Taiwan
Copyright © 2015 Zahid, Abbasi, Hameed and Rahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:M. Kaleem Abbasi, Department of Soil and Environmental Sciences, Faculty of Agriculture, The University of Poonch, Rawalakot, Azad Jammu and Kashmir, Pakistan mkaleemabbasi@gmail.com
 Mahwish Zahid
Mahwish Zahid M. Kaleem Abbasi1*
M. Kaleem Abbasi1*