- 1Department of Biosciences, Molecular Cell Biology of Plants, Goethe University, Frankfurt am Main, Germany
- 2Cluster of Excellence Frankfurt, Goethe University, Frankfurt am Main, Germany
- 3Center of Membrane Proteomics, Goethe University, Frankfurt am Main, Germany
- 4Buchmann Institute of Molecular Life Sciences, Goethe University, Frankfurt am Main, Germany
Cyanobacteria are photosynthetic prokaryotes important for many ecosystems with a high potential for biotechnological usage e.g., in the production of bioactive molecules. Either asks for a deep understanding of the functionality of cyanobacteria and their interaction with the environment. This in part can be inferred from the analysis of their genomes or proteomes. Today, many cyanobacterial genomes have been sequenced and annotated. This information can be used to identify biological pathways present in all cyanobacteria as proteins involved in such processes are encoded by a so called core-genome. However, beside identification of fundamental processes, genes specific for certain cyanobacterial features can be identified by a holistic genome analysis as well. We identified 559 genes that define the core-genome of 58 analyzed cyanobacteria, as well as three genes likely to be signature genes for thermophilic and 57 genes likely to be signature genes for heterocyst-forming cyanobacteria. To get insights into cyanobacterial systems for the interaction with the environment we also inspected the diversity of the outer membrane proteome with focus on β-barrel proteins. We observed that most of the transporting outer membrane β-barrel proteins are not globally conserved in the cyanobacterial phylum. In turn, the occurrence of β-barrel proteins shows high strain specificity. The core set of outer membrane proteins globally conserved in cyanobacteria comprises three proteins only, namely the outer membrane β-barrel assembly protein Omp85, the lipid A transfer protein LptD, and an OprB-type porin. Thus, we conclude that cyanobacteria have developed individual strategies for the interaction with the environment, while other intracellular processes like the regulation of the protein homeostasis are globally conserved.
Introduction
Cyanobacteria are ancient, multifarious, photosynthetic prokaryotes. They are of biotechnological importance and are used for approaches to produce bioactive molecules, biofuels or other energy sources (Jones and Mayfield, 2012; Neilan et al., 2013; Wijffels et al., 2013; Oliver and Atsumi, 2014). In addition, cyanobacteria are considered as model organisms to study general aspects of bacteria and cellular processes. In focus are the analysis of the function and evolution of photosynthetic systems (Shih et al., 2013; Croce and van Amerongen, 2014), nitrogen fixation (Bothe et al., 2010; Zehr, 2011), cell to cell communication (Flores and Herrero, 2010; Hahn and Schleiff, 2014), cell differentiation (Muro-Pastor and Hess, 2012), and cell wall function (Nicolaisen et al., 2009; Singh and Montgomery, 2011) to name just a few examples. However, most of the information was established for selected model cyanobacteria and still need to be generalized.
Aside from being of biotechnological importance, cyanobacteria are part of the phytoplankton (Sommer, 2005), but inhabit a diverse range of environments like rocks, lakes and deserts as well (e.g., Mur et al., 1999). It is estimated that all cyanobacteria on earth reach a total biomass of 1015g (Garcia-Pichel et al., 2003), which marks these bacteria as an important component of ecosystems. Moreover, due to their high acclimation capacity in fluctuating environments, some cyanobacterial species are thought to show a higher adaptability to climate changes compared to other species. It is discussed that this can result in overgrowing other phytoplankton species within the communities (Carey et al., 2012; Elliott, 2012). The latter requires an efficient uptake of nutrients as well as efficient mechanisms to compete for trace elements. The uptake of solutes depends on outer membrane proteins (OMP; Mirus et al., 2010). Most OMPs are β-barrel proteins, which act in the recognition and transport of solutes, metabolites and proteins (e.g., Nicolaisen et al., 2009; Mirus et al., 2010). Such β-barrel proteins are characteristic for the outer membrane of Gram-negative bacteria, mitochondria and chloroplasts (Sommer et al., 2011). While the transporters of the inner membrane were studied in some detail, not much, however, is known about the existence and function of the outer membrane β-barrel proteins of cyanobacteria (Hahn and Schleiff, 2014).
One measure to generalize the findings and to learn more about cyanobacteria is the pan- and core-genome determination. The pan-genome describes the entire gene set composed of all genes of all strains analyzed (Medini et al., 2005; Collingro et al., 2011). Therefore, it can be determined for an entire phylum like the cyanobacterial phylum (spelled in capitals below to emphasize that the entire phylum is analyzed: PAN-GENOME), or for a reduced set of organisms within the cyanobacterial phylum (spelled in small letters below to indicate that only a part of the PAN-GENOME is assigned: pan-genome). A pan-genome includes a core-genome, a dispensable-genome as well as unique genes (Reno et al., 2009). The dispensable-genome is the set of genes, which occurs in an intersection of at least two, but not all analyzed genomes. Unique genes are found in a single genome only. The core-genome includes those sets of genes that exist in each of the strains analyzed (Kettler et al., 2007). Again, we use capital letters (CORE-GENOME) in case the whole phylum is analyzed and small letters (core-genome) for the analysis of selected cyanobacteria only.
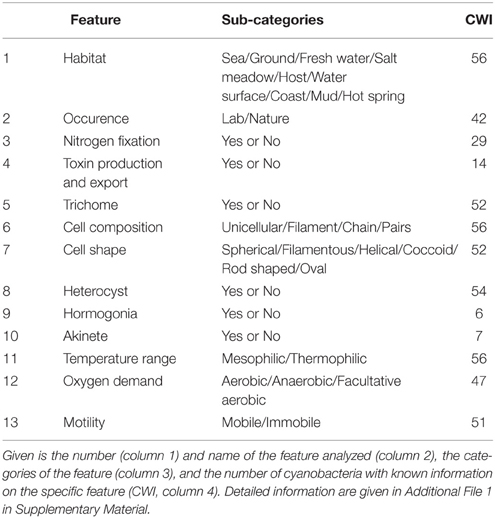
The selection of a subset of strains (clade) for core- and pan-genome analysis can be based on their phylogenetic positioning according to 16S rRNA sequence analysis (e.g., Valério et al., 2009) or traditional morphological features (e.g., Komárek and Anagnostidis, 1986, 1989; Anagnostidis and Komárek, 1987, 1990). In addition, classification of cyanobacteria with respect to their growth habitat offers the opportunity to determine feature-specific sets of genes. The prerequisite for this classification is the definition of morphological, biochemical and physiological features as well as of the typical growth habitat for each strain. Most of this information is deposited in the Integrated Microbial Genomes database (Markowitz et al., 2012). Based on this information, and refined by an exhaustive literature search, we classified the cyanobacterial strains according to 13 distinct features (Table 1, Additional File 1 in Supplementary Material).
Previous studies of gene sets have focused on the identification of intra-species gene sets needed to fully describe a species (Medini et al., 2005). The pan-genome analysis was developed as a consequence of the expanding number of sequenced genomes (Medini et al., 2005; Tettelin et al., 2008). Subsequently, this analysis was applied to study single genera like Prochlorococcus (Kettler et al., 2007), Legionella (D'Auria et al., 2010), or Streptococcus (Donati et al., 2010). Today, pan-genome analysis is used to define core-genomes for model organisms like human (Li et al., 2010) or yeast (Dunn et al., 2012). Similarly, core-genome definition of inter-species comparisons in a single phylum was used to gain information on sequence similarity (Tettelin et al., 2005), phylogenetic relations (Kettler et al., 2007) or evolutionary relations, as for example in Chlamydiae (Collingro et al., 2011) or cyanobacteria (Beck et al., 2012). Based on core-genome determination for a specific clade of species, the term “signature genes” has been introduced to denote genes with a limited phylogenetic distribution (Dutilh et al., 2008). Core-genome and signature gene definition was used to define a set of genes specific for cyanobacteria against eucaryotes containing chloroplasts (Martin et al., 2003) or specific for the various clades of cyanobacteria (Gupta and Mathews, 2010). This approach has contributed to our knowledge on the origin of photosynthesis (Mulkidjanian et al., 2006) and diversity of metabolism (Beck et al., 2012).
Interestingly, pan- and core-genome analysis was not used to identify feature-specific gene sets yet. Therefore, we investigated gene sets for specific features based on 58 cyanobacterial genomes. We confirmed that the selected genomes are sufficient to define the cyanobacterial CORE-GENOME. In addition, for each genome we determined the genes part of the dispensable-genome and unique genes. Subsequently, cyanobacteria were clustered according to their sequence or feature similarities and we defined the pan- and core-genomes of different clades. This analysis yielded the identification of some genes specific for thermophilic cyanobacteria and for heterocyst forming cyanobacteria. To study the conservation and diversity of the outer membrane proteome, we developed a method for identification of genes coding for β-barrel proteins. The majority of OMPs identified in the PAN-GENOME is not present in the CORE-GENOME. The core-set of β-barrel OMPs in all 58 cyanobacteria is composed of only three proteins, while the majority of the β-barrel OMPs is strain-specific or shared by a small fraction of up to 15 cyanobacteria only. We conclude that the outer membrane proteome is largely adapted to the individual live style and environment of each cyanobacterial strain.
Materials and Methods
Ortholog Search and pan-Genome Construction
Literature and databases were searched for completely sequenced cyanobacterial genomes or assembled drafts. The respective literature is cited in the Section Introduction. Cyanobacterial nucleotide and protein sequences and other relevant information was taken from Cyanobase (Nakao et al., 2010) and the Integrated Microbial Genomes database of the Joint Genome Institute (Markowitz et al., 2012). The ORFs for each strain were categorized in known and hypothetical based on the deposited description. For the construction of the PAN- and CORE-GENOME, the dispensable-genome and the unique genes we used the complete proteomes of all 58 cyanobacteria. We used OrthoMCL (Chen et al., 2006) for prediction of CLiques of Orthologous Genes (CLOGs). OrthoMCL excluded poor-quality sequences with a length below 10 amino acids or a stop codon frequency higher than 20%. By this approach, all CLOGs containing at least two sequences were detected. Sequences not assigned to a cluster by OrthoMCL were subsequently determined as single-sequence clusters (CLOGs of unique genes).
CLOGs defined by OrthoMCL were evaluated by the Pan-Genome Analysis Pipeline (PGAP) to construct CLOGs of different orders containing more than one strain in their respective orthologous groups (Zhao et al., 2012). The PGAP implemented algorithm used (–method MP) is based on the combination of InParanoid and MultiParanoid (Ostlund et al., 2010). The input files of PGAP had to fulfill the following criteria: (i) a 3:1 relation between the CoDing Sequence (CDS) and protein sequence length had to exist to avoid wrongly annotated protein sequences; (ii) the same amount of CDS to protein sequences for each annotated gene was expected; (iii) the identifier had to be unique. In the end, pan-genomes for Nostocales, Prochlorales, Chroococcales, and Oscillatoriales were created using the parameters for clustering and pan-genome construction (–cluster; –pan-genome). For the PAN-GENOME assignment we used the results of OrthoMCL.
For confirmation of feature specific cyanobacterial signature genes we used all available genomes for Viridiplantae and bacteria (except cyanobacteria) available at NCBI non-redundant (nr) database. We used the sequences of the proteins found in Thermosynechococcus elongatus BP-1 (thermophile habitat) or Anabaena sp. PCC 7120 (soil living, heterocysts) to blast for similar sequences with at least 80% coverage of the bait sequence and an e-value of 1.0 e−10 or smaller.
To determine the putative function of each CLOG we assigned a functional classification to each sequence of the cyanobacteria (Tatusov et al., 1997) by the Bacterial Annotation System (BASys; van Domselaar et al., 2005) and the information from the WEBserver for Meta-Genome Analysis (WebMGA; Wu et al., 2011).
Construction of the Tanimoto-Like Index and Clustering
The Tanimoto-like index (e.g., Cooper et al., 1993) was used to transform the different features of the cyanobacteria (Additional File 1 in Supplementary Material) in a binary code (bit strings) and calculate the similarity and distance (the latter equals 1-similarity) between two cyanobacteria (Additional File 2 in Supplementary Material). The Tanimoto-like index consists of the sum of bit strings per feature. Each feature may contain more than one subcategory (e.g., habitat: sea, soil, freshwater, host, mud, hot spring, salt marsh) and the amount of subcategories determines the length of each feature bit string. Each subcategory was classified as present (1) or absent (0) based on literature (Additional File 1 in Supplementary Material). Features with no available information were classified as unknown (u). By comparison of two strains we determined whether the feature is (i) unknown in both strains, (ii) known in one strain or (iii) known in both strains. The first case was excluded from further calculations, whereas in the second case the denominator value was increased by 0.5. For the third case we added the sum of ones in the intersection to the numerator and the sum of ones in the union to the denominator (Additional File 2 in Supplementary Material).
Tree Construction
The Tanimoto-like index was used to calculate pair wise distances between strains based on 13 different features (Additional File 3 in Supplementary Material). The distance matrix was used to create the neighbor-joining feature tree (Additional File 4 in Supplementary Material). The CLOG distance neighbor-joining tree (Additional File 4 in Supplementary Material) was based on the CLOG distances (equals 1-similarity) between two strains. The CLOG similarity between two strains was calculated by dividing the number of all shared CLOGs by the number of CLOGs which contained at least one sequence of the two strains. Furthermore, 16S rRNA and average amino acid identity (AAI) neighbor-joining trees were calculated (Additional File 5 in Supplementary Material). The 16S rRNA neighbor-joining tree was based on a multiple alignment via Multiple Alignment using Faster Fourier Transform (MAFFT; Katoh and Standley, 2013). The AAI neighbor-joining tree was built using the 420 CLOGs of the CORE-GENOME that contained one orthologous sequence per strain only. Pairwise global alignments between strains were calculated for each CLOG and the AAI over all CLOGs per pair of strains determined. Neighbor-joining trees were built with the molecular evolutionary genetics analysis package 6 (MEGA6; Tamura et al., 2013). The tree morphology was compared by calculating the patristic distance correlation (between 1 correlation and -1 anti-correlation) using the Mesquite software (Maddison and Maddison, 2011; http://mesquiteproject.org).
β-Barrel Protein Prediction and Clustering
The first step of Trans-Membrane Beta-barrel Prediction (TMBp) was based on the Beta-barrel Outer Membrane protein Predictor (BOMP; Berven et al., 2004), the K-Nearest Neighbor method based predictor (KNN; Hu and Yan, 2008) and the Trans-Membrane Beta-barrel Discriminator (TMBetaDisc; Ou et al., 2008) that are based on physicochemical features and the primary amino acid sequence. The TMBp approach was supported by a program established in our group (Mirus and Schleiff, 2005) in combination with TMHMM (Moller et al., 2001). Sequences detected as β-barrel proteins by more than one predictor were called probable β-barrel proteins.
The second step of β-barrel prediction was based on a Profile Hidden Markov Model (pHMM)-approach using the program HMMer (Eddy, 2011). We used the Protein Family (Pfam) database (Finn et al., 2014), OPM (Lomize et al., 2012), OMPdb (Tsirigos et al., 2011), which provide information on domain architecture and structures of β-barrel OMPs to build HMM profiles for each known β-barrel OMP family. These profiles were used to search for β-barrel OMPs in all cyanobacterial proteomes. Protein sequences with at least one detected β-barrel domain were considered as probable β-barrel OMP.
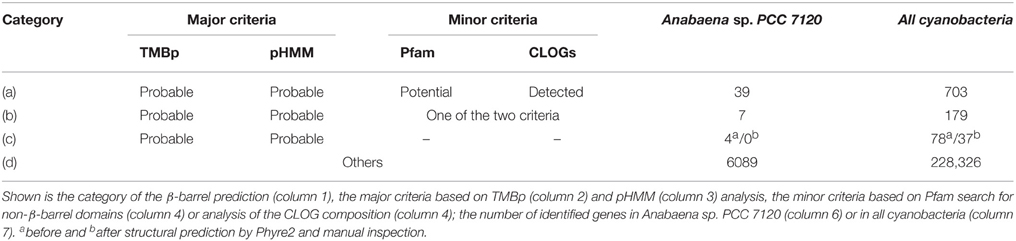
In the third step we defined two minor criteria. First, other domains than β-barrel OMP characterizing domains were identified by searching against the complete Pfam database (Finn et al., 2014). A protein was assigned to have the potential to be β-barrel OMP if an amino acid stretch longer than 79 amino acids was not characterized by such a Pfam domain. Secondly, we analyzed the CLOGs containing sequences representing β-barrel OMPs. If more than 50% of all sequences of a CLOG have been assigned as β-barrel OMP by TMBp and pHMM, the assigned proteins were considered as detected.
All proteins were subsequently classified (Table 2), namely in proteins detected by all four criteria [category (a)], proteins which fulfill the two main criteria and at least one minor criterion [category (b)], proteins which fulfill the two main criteria only [category (c)] and all other proteins [category (d)]. For all sequences of category (c) we performed in silico 3D structure analyzes with Phyre2 (Kelley and Sternberg, 2009). The results were manually inspected resulting in 37 putative β-barrel proteins [category (c); Table 2].
Results and Discussion
The General Composition of Cyanobacterial Genomes
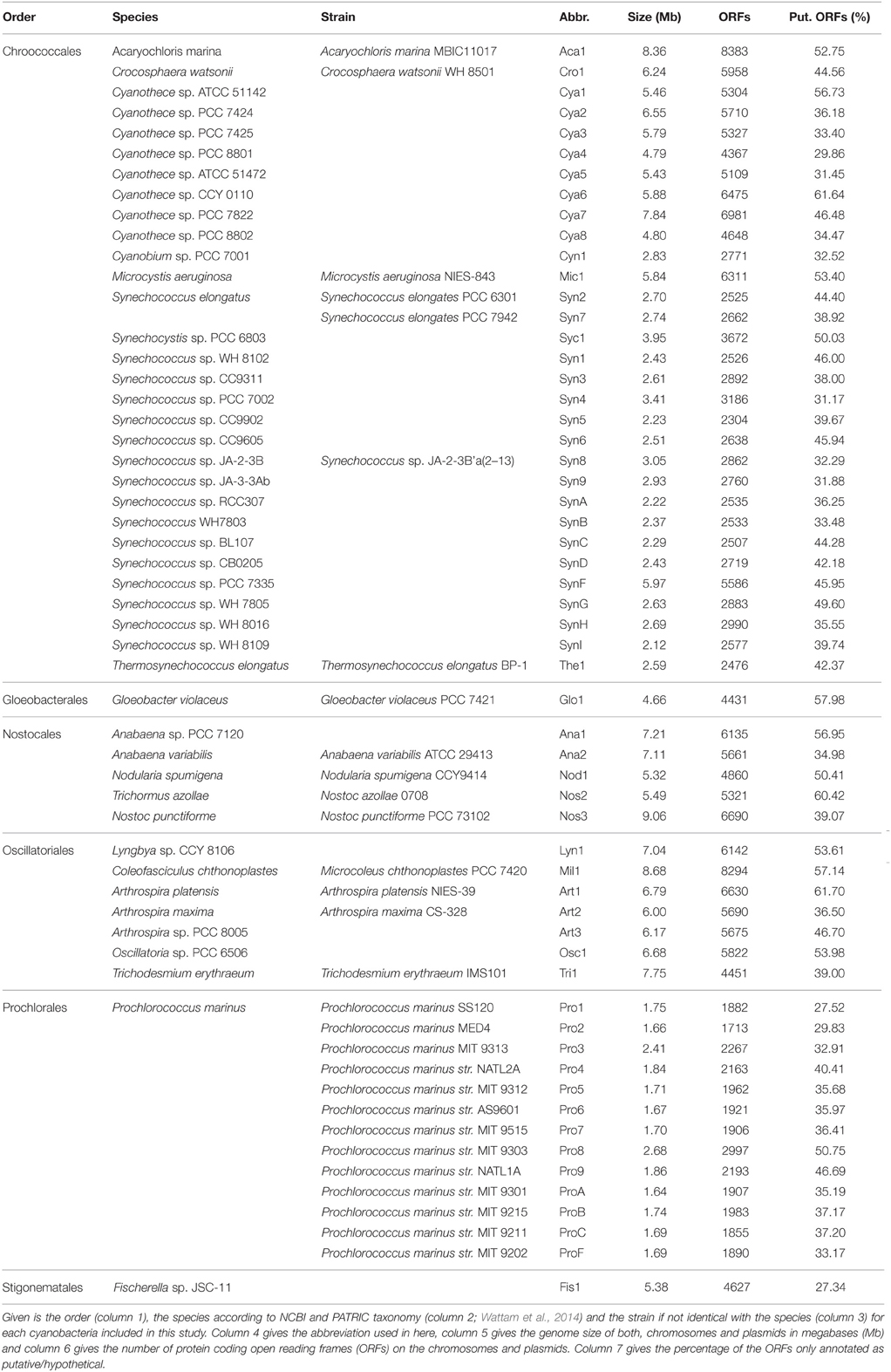
Sequenced and annotated genomes of 58 cyanobacterial strains representing 45 species from six cyanobacterial orders were used to build the PAN-GENOME (Table 3). We used the amino acid sequences of the proteins encoded by all annotated genes present in the according genome and determined the CLiques of Orthologous Genes (CLOGs). CLOGs with sequences of only one cyanobacterial genome and genes not assigned to any CLOG were classified as “CLOGs of unique genes” for unification of the nomenclature. CLOGs with sequences from a certain set of strains (range from two to 57 strains) were annotated as “CLOGs of the dispensable-genome,” and CLOGs with at least one sequence from each of the 58 strains as “CLOGs of the CORE-GENOME.” We identified 44,831 CLOGs in total. 28,520 of all CLOGs are “CLOGs of unique genes” (Figure 1A). However, it needs to be mentioned that uncertain annotations of hypothetical ORFs can cause a high number of unique genes. Indeed, in Cya7, Cya6, Cya5, Cya4, Cya3, Cya2, Cya1, ProC, Tri1, Mic1, Cya8, Nod1, Glo1 genomes more than 50% of all genes are annotated as “hypothetical”. The outcome of this is that 23,781 “CLOGs of unique genes” are “hypothetical” based on the protein sequence description. Moreover, 1725 of the “CLOGs of unique genes” contain two or more sequences from one strain representing putative paralogs. 15,752 are CLOGs of the dispensable-genome, but most of these CLOGs contain only sequences from up to 10 strains (Figure 1A). Finally, 559 CLOGs of the CORE-GENOME (Additional File 6 in Supplementary Material) were identified as they contain sequences of all 58 cyanobacterial strains (Figure 1A). This is consistent with the earlier postulation that the CORE-GENOME of cyanobacteria has a size of 500–600 genes (Beck et al., 2012).
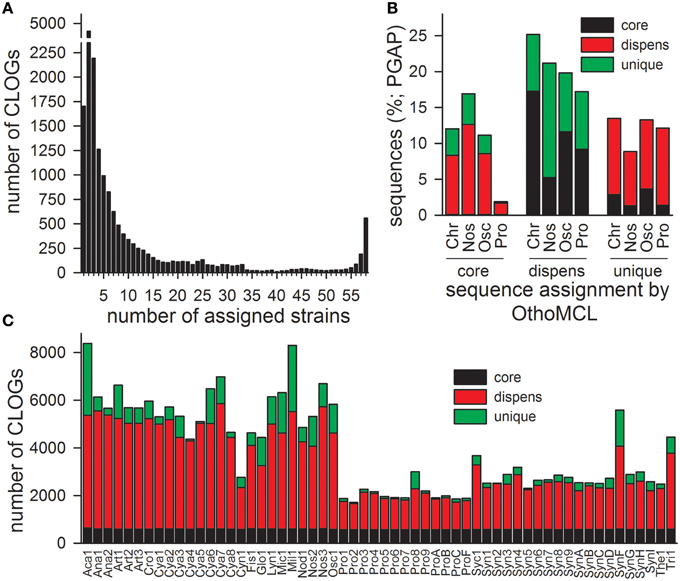
Figure 1. CLOG distribution of the 58 cyanobacteria. (A) The numbers cyanobacterial strains from which sequences are included in an individual CLOG was determined. The number of CLOGs containing genes from a given number of strains is shown. (B) CLOGs representing unique, dispensable (dispens) or CORE-genes (core) were determined by OrthoMCL for all genomes or by PGAP for the genome of the cyanobacterial order Chroococcales, Nostocales, Oscillatoriales, and Prochlorales. Shown is the frequency of assignment of genes of a certain CLOG category detected by OrthoMCL to another CLOG category by PGAP (CORE-gene: black; dispensable gene: red; unique gene: green). (C) Shown is the number of sequences of the individual cyanobacteria represented by a CLOG of the CORE-GENOME (black), by a CLOG of the dispensable-genome (red; dispens), and by a CLOG of unique genes (green).
The distribution of the sequences in the different CLOG categories is by large comparable to the results of the PGAP analysis, which created individual pan-genomes of different cyanobacterial orders (Figure 1B, Zhao et al., 2012). The discrepancy of about 10% observed by the two approaches is expected, because for CLOG definition by OrthoMCL all genomes were analyzed, while due to computational limitations for the PGAP analysis only the genomes of strains of one order could be used.
With respect to the strains we realized that the majority of the genes of each individual strain was assigned to CLOGs of the dispensable-genome (Figure 1C; red). The total number of genes identified in CLOGs of unique genes varies between the different strains (Figure 1C; green) and is primarily related to the genome size (Table 1). This is expected, because smaller genomes generally code for a lower number of proteins (Table 3) and thus, the portion of the genes found in CLOGs of the CORE-GENOME and of the dispensable-genome is larger. However, this rule does not apply to Prochlorococcus marinus strain MIT 9303 (Pro8). Nevertheless, the strain MIT 9303 has the largest genome with most annotated ORFs of all P. marinus strains, which might explain the larger portion of unique genes. The “additional” genes in P. marinus str. MIT 9303 by large encode proteins with putative functions in membrane synthesis and transport (Kettler et al., 2007), which might hint to specific features of this strain when compared to other strains of P. marinus.
Further, exceptions from the rule are Cyanothece sp. PCC 8801 (Cya4), Cyanothece sp. ATCC 51472 (Cya5) and Cyanothece sp. PCC 8802 (Cya8), which have the smallest genome as well as assigned proteome of all Cyanothece species (Table 3). These three species show a large content of genes assigned either to the CORE-GENOME or the dispensable-genome, but a small content of unique genes when compared to other Cyanothece species. Thus, the genome of these three strains might be composed of genes for the basic functions of Cyanothece only.
The Size of the Cyanobacterial Core- and PAN-Genome
Based on the analysis of the 58 cyanobacterial strains a CORE-GENOME size of 559 genes was observed. To judge whether the 45 species represented by the 58 strains are sufficient to define the CORE-GENOME of cyanobacteria, we determined the CORE-GENOME size dependence on the number of genomes analyzed. We determined the size of the core-genome for a given number of randomly selected genomes from the 58 organisms. The random selection was 1000 times repeated and the average calculated (Figure 2A). The number of sequences found in the core-genome changed only little when more than 40 cyanobacterial strains were considered. The result was not dependent on number of repetitions, as for only 100 or even 10,000 random selections the same result was observed (Additional File 7 in Supplementary Material).
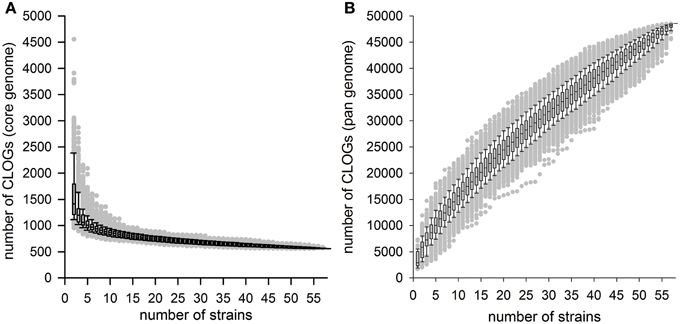
Figure 2. The cyanobacterial core- and pan-genome. (A,B) The number of CLOGs of the cyanobacterial (A) core-genome or (B) pan-genome for a given number of organisms is shown. The box plots were created for the results of 1000 different random selections of different cyanobacterial strains. Further simulations are shown in Additional File 6 in Supplementary Material.
The robustness of our result prompted us to compare the CORE-GENOME determined in here with the CORE-GENOMES defined earlier analyzing eight (Martin et al., 2003; 179 CORE-GENES asssigned), 15 (Mulkidjanian et al., 2006; 1044 CORE-GENES asssigned) or 16 cyanobacterial genomes (Beck et al., 2012; 704 CORE-GENES asssigned). The overlap between previously assigned CORE-GENOMES and the one defined in here consists of 520 and 526 sequences for the two larger studies, respectively. On the one hand, this shows that almost all genes of the CORE-GENOME identified in here are present in the previous CORE-GENOME sets, on the other hand it documents that the low number was not sufficient, which is consistent with our simulation (Figure 2A). Both conclusions support the notion that the CORE-GENOME of cyanobacteria most likely covers about 500 genes.
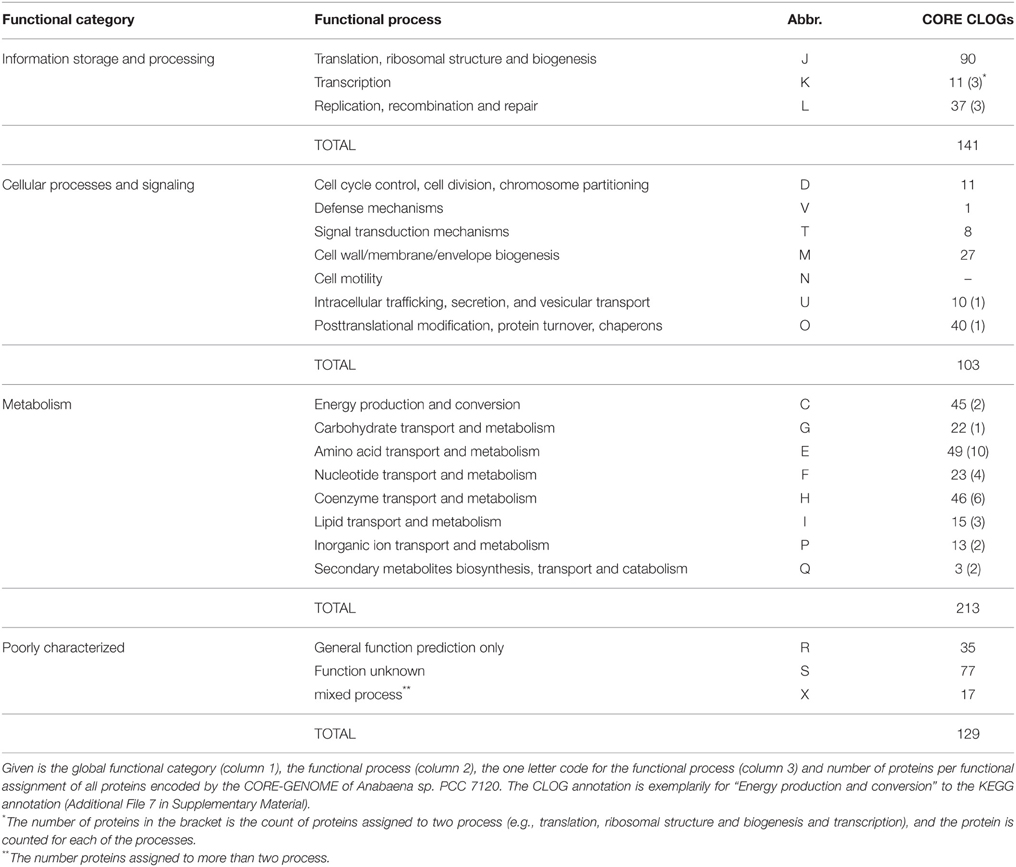
We determined the functional categories based on the sequences of Anabaena sp. PCC 7120 for the CORE-GENOME. Here we used the functional annotation previously established for clusters of orthologous groups (COG) for seven complete genomes from five major phylogenetic lineages (Tatusov et al., 1997). In part, the result was manually compared to the KEGG annotations (Kanehisa and Goto, 2000). We realized that proteins encoded by 231 sequences of the CORE-GENOME (representing ~40%) are involved in metabolic processes in Anabaena sp. PCC 7120 (Table 4). Thereof, 59 proteins are assigned to be involved in amino acid transport and metabolism (category E), 52 as coenzyme transport and metabolism (category H) and 47 in energy production and conversion (category C). The observation that not all components of the photosystems are encoded by the CORE-GENOME was confirmed by the analysis of the distribution of the proteins involved in oxidative phosphorylation, photosynthesis and antenna proteins annotated by KEGG (Additional File 8 in Supplementary Material). In addition, 90 proteins coded by the CORE-GENOME genes in Anabaena sp. PCC 7120 are assigned to be involved in translation, ribosomal structure and biogenesis (category J), while 41 encoded proteins function in posttranslational modification, protein turnover and chaperones and 40 in replication, recombination and repair (Table 4).
Next, we investigated the PAN-GENOME formed by the 44,831 CLOGs observed for the 58 strains defined. Again, we randomly selected the genes of a given number of strains for the determination of the pan-genome and this random selection was repeated 100, 1000, and 10,000 times (Figure 2B; Additional File 7 in Supplementary Material). As for the core-genome analysis, the result was not dependent on the number of random selections used in here. Previously it was postulated that increase of the PAN-GENOME follows the power law with respect to number of genomes included (Tettelin et al., 2008; Figure 2B). For P. marinus it was reported that addition of new strains into the analysis would always yield an increase of the pan-genome size (a so called “open pangenome”), however with a low rate (the according factor is α = 0.80 suggesting a low increase of the PAN-GENOME size by addition of the genomic information of an additional strain; Tettelin et al., 2008). For all cyanobacteria we obtained an α of 0.35 ± 0.07. This suggests that the PAN-GENOME of all cyanobacteria is (i) a so called open PAN-GENOME and increases with addition of new cyanobacterial strains, because only for α > 1 a limit exists, and (ii) the PAN-GENOME of all cyanobacteria increases more rapidly by addition of new genomes as the pan-genome for a single species of cyanobacteria like P. marinus.
Habitat Specific Cyanobacterial Proteins
We gathered information about ecological, morphological and physiological features for all analyzed strains from the Integrated Microbial Genomes database of the Joint Genome Institute (Markowitz et al., 2012) and from selected publications (Additional File 1 in Supplementary Material; Huber, 1985; Stal and Krumbein, 1985; Jones, 1992; Cohen et al., 1994; Rouhiainen et al., 1995; Kaneko and Tabata, 1997; Gruber and Bryant, 1998; Nakamura et al., 2002; Zhou and Wolk, 2002; El-Shehawy et al., 2003; Lesser, 2003; Urmeneta et al., 2003; Tuit et al., 2004; Araoz et al., 2005; Allewalt et al., 2006; Dworkin et al., 2006; Su et al., 2006; Takaichi et al., 2006; Gao et al., 2007; Kaneko et al., 2007; Kettler et al., 2007; Kim et al., 2007; Campbell et al., 2008; Stockel et al., 2008; Swingley et al., 2008; Bolhuis et al., 2010; Fujisawa et al., 2010; Mejean et al., 2010; Ran et al., 2010; Scott et al., 2010; Carrieri et al., 2011; Larsson et al., 2011; Ploug et al., 2011; Markowitz et al., 2012; Nguyen et al., 2012; Stewart et al., 2012) and extracted 13 different features (Table 1, Additional file 1 in Supplementary Material). In some cases information was logically assumed. For example, unicellular organisms should not contain features characterizing multicellular cyanobacteria like heterocysts, akinetes or hormogonia.
Next, we determined genes specific for a subset of cyanobacterial strains with either thermophilic character, with common growth environment or the capability to differentiate heterocysts, because for the remaining features the assignment for the cyanobacteria is laregely incomplete (Additional file 1 in Supplementary Material). For the identification of such genes we searched for CLOGs containing exclusively sequences of cyanobacterial strains with a certain feature. Subsequently, only the CLOGs of the latter pool with sequences of all cyanobacterial strains with this feature were selected. In our set of organisms we had three thermophilic cyanobacteria, namely T. elongatus BP-1 (The1), Synechococcus sp. JA-3-3Ab (Syn9), and Synechococcus sp. JA-2-3B'a(2–13) (Syn8). We obtained four CLOGs with genes of these three strains only. In T. elongatus BP-1 these genes are tlr0324, tlr0548, tlr0547, and tsr0549 (Nakamura et al., 2002, 2003). The protein tlr0324 putatively contains a DNAJ-domain and is predicted to be a Heat shock protein (HSP), while the proteins encoded by the second gene cluster, tlr0548, tlr0547, and tsr0549, are of unknown function. Next we analyzed whether the identified genes are specific to cyanobacteria by searching for similar sequences in plants and bacteria (see Materials and Methods). Sequences with similarity to tlr0548 have been identified in bacterial strains with extreme habitats of the genus Acidithiobacillus (5) and the species Haliangium ochraceum (1), Halothiobacillus neapolitanus (1), Sorangium cellulosum (2), or Thiothrix nivea (1), but not in plants. In turn, we did not identify sequences with similarity to tlr0324, tlr0547, and tsr0549 in the bacterial or plant genomes by the approach applied (see Materials and Methods). Thus, these three genes likely represent “signature genes” for thermophilic cyanobacteria.
With respect to the growth habitat we obtained 34 cyanobacterial strains assigned to live in salt water, 15 in fresh water, three in fresh water as well as on soil, three require a host organism, one is exclusively soil-living and one occurrs in both salt and fresh water (Additional File 1 in Supplementary Material). However, we did not find a CLOG including sequences of all cyanobacteria growing in salt or fresh water. The same holds true for the three host-living cyanobacteria. Thus, either a habitat-specific core-genome does not exist with respect to salt/fresh water and host-living strains, or for some of the strains the assignment found in literature is incomplete.
Five CLOGs for the cyanobacterial strains assigned as capable of soil-living (Anabaena sp. PCC 7120, Anabaena variabilis ATCC 29413, Gloeobacter violaceus PCC 7421, Nostoc punctiforme PCC 73102) were identified. We again aimed for confirmation of the specificity of the identified genes for cyanobacteria. However, similar sequences to the identified oxidoreductase (encoded by all0827 in Anabaena sp. PCC 7120) was found in many other plant and bacterial genomes. Similarily, sequences with similarity to the protein with similarity to acetyltransferases (encoded by alr3061), the membrane-spanning subunit DevC of the heterocyst-specific ABC transporter (encoded by alr4974) and the six-bladed β-propeller TolB-like domain containing protein (encoded by all0351) were identified in many bacterial genomes. Only for the protein of unknown function encoded by alr7204 sequences with similarity could not be identified in the analyzed eucaryotic or prokaryotic genomes. Summing up, we propose the existence of at least three signature genes for thermophilic and one signature gene for soil-living cyanobacteria, while we could not identify signature genes for salt or fresh water living cyanobacteria.
Heterocyst Specific Cyanobacterial Proteins
We aimed for the detection of CLOGs unifying heterocyst-forming cyanobacteria. In our set six cyanobacteria are assigned as heterocyst-forming (Additional File 1 in Supplementary Material; Anabaena sp. PCC 7120, Anabaena variabilis ATCC 29413; Fischerella sp. JSC-11; Nodularia spumigena CCY9414; Nostoc azollae 0708; Nostoc punctiforme PCC 73102), while for four cyanobacteria information was not available (Oscillatoria sp. PCC 6506, Synechococcus sp. WH 8016; Synechococcus elongates PCC 6301; Synechococcus sp. CB0205). To judge whether we have to include the latter four as heterocyst forming, we inspected CLOGs containing genes known to be essential for heterocyst differentiation, but not related to global families like the ABC transporters. We selected 17 of such genes (Figure 3A). Sequences of all confirmed heterocyst-forming cyanobacteria (Additional File 1 in Supplementary Material; Ana1, Ana2, Fis1, Nod1, Nos2, Nos3) are in 14 of the 17 CLOGs formed by the selected heterocyst marker genes (Figure 3B, red bars). Only PatS (asl2301, Anabaena sp. PCC 7120), HetN (alr5358, Anabaena sp. PCC 7120), and PbpB (alr5101, Anabaena sp. PCC 7120) could not be detected in all strains by the method applied.
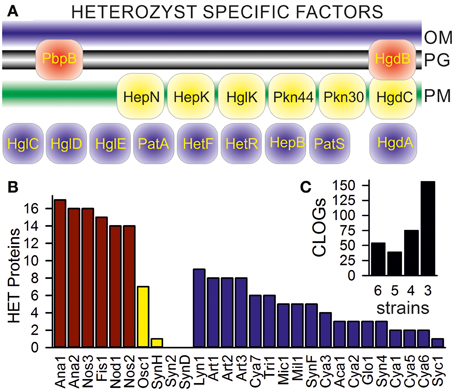
Figure 3. CLOGs of genes involved in heterocyst formation. (A) A scheme of the localization of the 17 selected heterocyst specific proteins is shown: the penicillin-binding protein 2 (alr5101 in Anabaena PCC 7120, PbpB; Lazaro et al., 2001), the pentapeptide-repeat protein HglK (all0813; Black et al., 1995), the oxidoreductase HgdA (all5345; Nicolaisen et al., 2009), the glycolipid deposition proteins HgdB and HgdC (all5347 and all5346; Nicolaisen et al., 2009), the HstK family proteins with two-component sensor domain Pkn44 and Pkn30 (all1625 and all3691; Shi et al., 2007), the sensory protein-histidine kinase of a two-component regulatory system (all4496; HepK; Golden and Yoon, 2003), the ketoreductase HetN (alr5358; Corrales-Guerrero et al., 2014), the heterocyst differentiation control proteins HetR (alr2339; Du et al., 2012) and HetF (alr3546; Ionescu et al., 2010), the poly-peptides controlling heterocyst pattern formation PatA (all0521; Zhang et al., 2007) and PatS (asl2301; Nicolaisen et al., 2009), the heterocyst envelope polysaccharide synthesis factor HepB (alr3698; Wang et al., 2007) and the heterocyst glycolipid synthases HglC, HglD, and HglE (alr5355, alr5354, and alr5351; Fan et al., 2005). (B) CLOGs including Anabaena sp. PCC 7120 sequences mentioned in the text have been analyzed concerning the cyanobacteria the sequences originated from. The number of detected proteins known to be involved in heterocyst formation/function in cyanobacteria known to form heterocysts (red), not to form heterocysts (yellow) or for which information about heterocysts formation is not available (blue) is shown. (C) The inset on the right shows the number of CLOGs with the sequences of the six heterocyst-forming cyanobacteria only.
Nine CLOGs of genes known to be essential to heterocyst differentiation contain sequences of the filamentous Lyngbya sp. CCY 8106; and eight CLOGs contain sequences of each of the Arthrospira strains, though for these cyanobacteria heterocyst formation is not reported (Figure 3B, blue bars, Additional File 1 in Supplementary Material). These eight CLOGs represent PbpB, HglK, HgdA, HetR, HetF, Pkn44, Pkn30, and HepK. The meaning of this observation needs to be explored in future.
Of the four strains with unknown assignment to heterocyst formation, sequences of the filamentous Oscillatoria sp. PCC 6506 are present in seven of the 17 CLOGs of the selected heterocyst specific genes (Figure 3B, yellow bar). As expected sequences of the three most likely unicelluar strains (Syn2, SynD, SynH) are detectable in at most one of the 17 CLOGs (Figure 3B, yellow bar). Consequently, from our inspection of the distribution of genes specific for heterocysts we conclude that only the six confirmed heterocyst forming cyanobacteria should be included in the analysis of the core-genome of genes specific for heterocyst forming cyanobacteria.
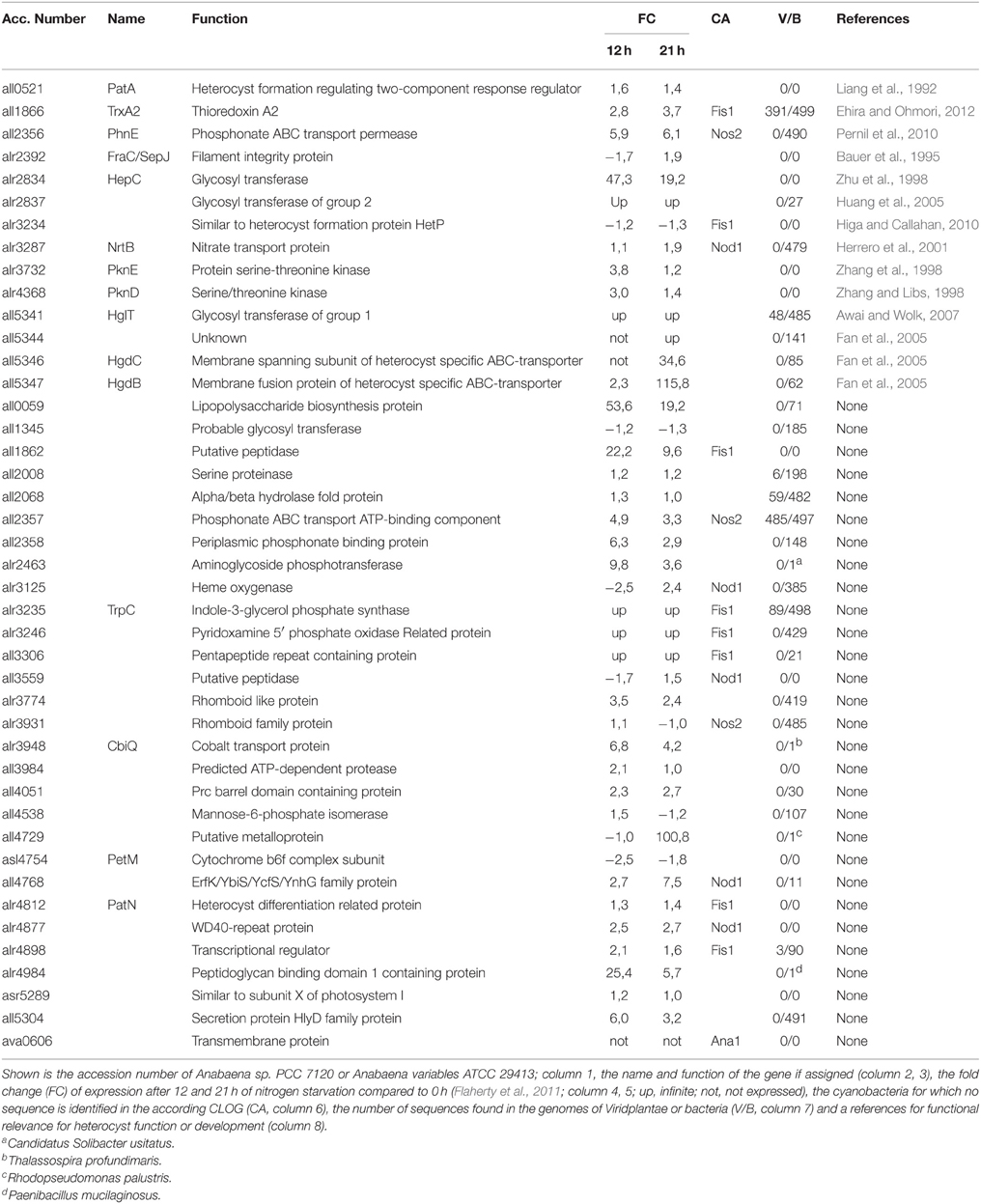
At first we identified all CLOGs with sequences from the six heterocyst-forming strains only. We observed 54 CLOGs with sequences from all six strains, 39 with sequences from five, 75 from four and 156 from three heterocyst-forming cyanobacteria (Figure 3C). The number of CLOGs with sequences of only five strains prompted us to consider the 93 genes of the CLOGS containing sequences of at least five of the six strains as core-genome of heterocyst-forming cyanobacteria (Tables 5, 6). Fourteen of these 93 genes have been experimentally charactarized and for 28 a function can be predicted (Table 5), while for 51 genes a function is not assigned (Table 6). Eight of the 93 genes were shown to be exclusively expressed upon nitrogen starvation in Anabaena PCC 7120, while another 48 genes are at least two-fold higher expressed either 12 or 21 h after nitrogen step-down (Tables 5, 6, Flaherty et al., 2011). In turn, only one gene is not expressed in Anabaena PCC 7120 after nitrogen starvation (asl1933) and one is significantly downregulated (asr1289; Table 5, Flaherty et al., 2011).
We inspected whether the genes identified are heterocyst specific signature genes of cyanobacteria. We realized that six of the experimentally characterized genes and eight genes with putative function are indeed specific for cyanobacteria based on our criteria (see Materials and Methods), because sequences with similarity could not be identified in the analyzed plants and bacteria (Table 5). In addition, for four proteins encoded by the genes identified in the CLOGs formed by heterocyst forming cynobacteria only one other bacterial strain containing a similar sequence was detected (Table 5). In addition, for 44 of the not yet characterized factors similar sequences could not be detected in any of the analyzed genomes, while for additional four only one or two sequences with similarity could be detected (Table 6). We therefore propose that eight of the identified genes are highly specific for heterocyst forming cyanobacteria, while 58 represent heterocyst specific cyanobacterial signature genes. It is worth mentioning, the majority thereof have not yet been characterized.
The Composition of the Core-Genomes of the Different Clades of Cyanobacteria
We calculated a Tanimoto-like index for each pair of cyanobacteria (see Materials and Methods, Additional File 2 in Supplementary Material), which at first transfers the obtained information on cyanobacterial features into a binary code and subsequently determines the similarity of two strains. These indices were used to group the strains (Additional File 3 in Supplementary Material) and to calculate a neighbor-joining tree (Figure 4B, Additional File 4 in Supplementary Material). In parallel, we used the determined CLOGs to calculate the difference between two cyanobacterial strains and used this “pairwise CLOG distance” for calculation of a second neighbor-joining tree (Figure 4A, Additional File 4 in Supplementary Material).
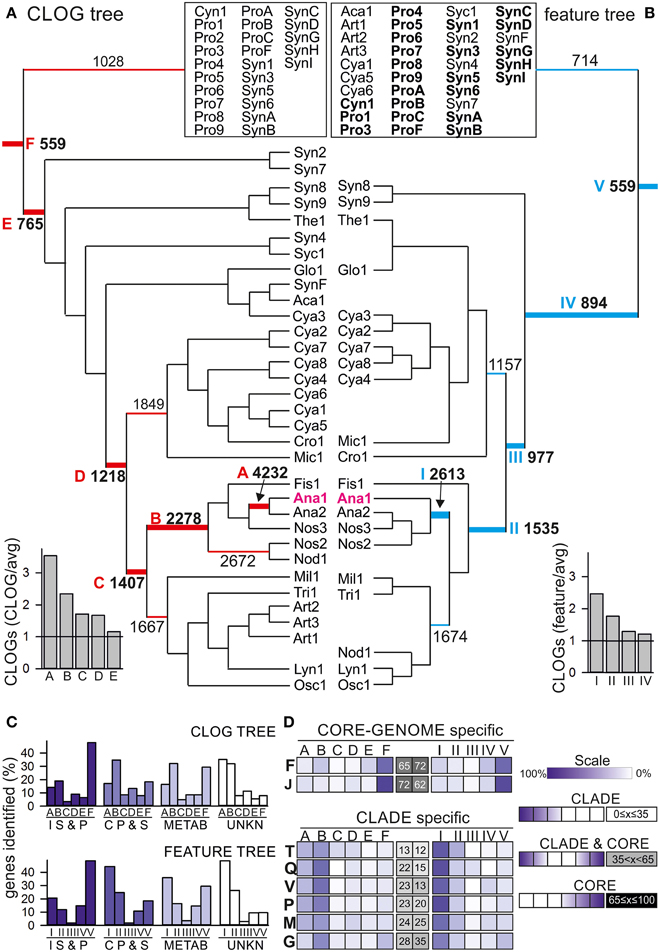
Figure 4. Feature and shared CLOG correlation tree. (A, B) The neighbor-joining tree of the 58 cyanobacteria based (A) on pair-wise shared CLOGs as distances or (B) on the similarities in the 13 selected features as distances was calculated. The root for the different branches from the deepest root (CORE-GENOME) to Anabaena sp. PCC 7120 are marked by letter in A (F–A) or roman numerals in B (I–VI), and the number of CLOGs defining the core-genome for the branch with this root is given. The ratio of the core-genomes of the branches with different roots to the average size of the core-genome expected for this number (Figure 2) is indicated on the bottom left. For simplicity, only branches discussed are shown, while all strains of the remaining part of the tree are clustered in the box on top. The full tree is shown in Additional File 4 in Supplementary Material.(C) Each core-genome with the root indicated in (A,B) was determined and the number of proteins of a specific category/process (Table 4) additionally found to the core-genome of the deeper roots was counted and is deposited in Additional Files 8, 9 in Supplementary Material. Shown is the occurrence of unique proteins (in percent of all identified proteins) assigned to the four categories “Information storage and processing” (I, S, and P), “Cellular processes and signaling” (C, P, and S), “Metabolism“ (METAB) and unknown (UNKN) in the different clade specific core genomes defined for the CLOG tree (top) and feature tree (bottom). (D) Shown is the occurrence of unique proteins assigned to the individual processes (indicated by one letter code shown in Table 4). The distribution for proteins for each process is shown as color code indicated on the right (Scale). For each distribution the profile was analyzed by an inversed gaussian distribution and the position of the minimum was used to assign the process as CLADE specific defined, CLADE and CORE-GENOME defined or CORE genome defined (scale is shown on the right, position of the minimum is given in percent: 0% = exclusive detection in core genome of CLADE A or I, 100% = exclusive detection in CORE-GENOME. The results for equally distributed (CORE and CLADE) genes are shown in Additional File 10 in Supplementary Material.
By large, the two trees show a comparable branching (patristic distance correlation coefficient: 0.51). This suggests a correlation between the proteome setup and the analyzed cyanobacterial features. For further verification we compared the CLOG and feature tree with a tree based on the 16S rRNA and the average amino acid identity (AAI) (Additional File 5 in Supplementary Material). As expected, the correlation between CLOG and IAA tree is the highest with a coefficient of 0.83, while the correlation between the feature tree and the two trees was lower but still detectable (correlation of 0.65 and 0.55, respectively). However, some alterations were observed (Figure 4). The CLOG assignment relates the filamentous Nodularia spumigena CCY9414 (Nod1) to Nostocales, whereas the feature assignment introduces a shift to Oscillatoriales (Osc1 and Lyn1), because they show similarity in growth habitat, trichome formation and toxin production (Figure 4, Additional File 1 in Supplementary Material). As expected the filamentous Arthrospira (Art1–Art3) clustered with Oscillatoriales in the CLOG tree, but not in the feature tree. This shift most likely reflects the assignment of Arthrospira as not nitrogen fixing, facultative aerobic, cells with helical cell shape and fresh water living, which is distinct from other Oscillatoriales (Additional File 1 in Supplementary Material). Finally, two Prochlorales strains (P. marinus MIT 9313, Pro3; P. marinus str. MIT 9303, Pro8) are not assigned to Prochlorales, but to the Chroococcales in the CLOG tree (Additional File 4 in Supplementary Material). For P. marinus MIT 9313 which has the second largest genome of all analyzed P. marinus strains, we speculate that observed clustering in the CLOG tree results from the large number of genes in “CLOGs of dispensable genes” that contain many genes from other species than P. marinus (Figure 1).
We used the two defined trees (Figure 4) to analyze the branch-specific core-genomes with focus on branches including the model system Anabaena sp. PCC 7120 (Ana1). At first we compared the size of the core-genomes of the different branches to the expected random average size of core-genomes with the same number of strains (Figure 2A). We realized that the core genome for the strains in clade I (Figure 4A), A and B (Figure 4B) is two-fold larger than expected from our analysis. This could be due the large cyanobacterial genomes in this clade (>5 Mb) when compared to the small genomes from Chroococcales included in the CORE-GENOME calculation. However, this is in agreement with the close relation of the cyanobacteria in these clades. Next, we determined the functional categories based on the sequences of Anabaena sp. PCC 7120 for the core-genomes of different branches defined by the indicated roots (Figure 4) of the CLOG (Additional Files 4, 8 in Supplementary Material) and feature-based tree (Additional Files 4, 9 in Supplementary Material) by the strategy described for the CORE-GENOME classification.
We inspected the distribution of the genes of the four functional categories (Figure 4C). For proteins involved in the metabolism (METAB) we found a comparable number in the CORE-GENOME (root F, V; entire tree) as in the clade specific core-genome (root A, B, I, II), while most of the proteins assigned as “Information storage and processing (IS and P)” are found already in the CORE-GENOME (root F, V; Figure 4C). Proteins of unknown function (UNKN) and of “Cellular processes and signaling (CP and S)” are largely found in the clade specific core genomes (root A, B, I, II, Figure 4C). On the one hand this suggests that many strain specific processes have not yet been characterized, on the other hand it can be postulated that cyanobacterial signaling strategies are largely strain specific.
To substantiate the latter notion, we analyzed the distribution of the proteins assigned to the various biological processes (Table 1) in the different clade specific core-genomes. We realized that proteins of most categories are found in the CORE-GENOME of all cyanobacteria as well as in clade specific core genomes (Additional Files 9–11 in Supplementary Material). Only proteins of category N (cell motility) are not represented by the CORE-GENOME, but the detected proteins are equally found in all clade specific core-genomes (Additional Files 11 in Supplementary Material). However, we observed two processes for which most of the proteins are encoded by the CORE-GENOME, namely translation, ribosomal structure and biogenesis (category J), as well as in nucleotide metabolism and transport (category F; Figure 4D). This finding is not unexpected as the process of protein synthesis and nucleotide metabolism were previously identified to be very ancient even existing in the last universal common ancestor (e.g., Poole et al., 1999; Armenta-Medina et al., 2014). In contrast, many proteins classified to be involved in signal transduction and defense mechanisms show a clade specific occurrence (categories V and T, Figure 4D). This supports the above formulated notion that cyanobacterial signaling strategies are largely strain specific.
In addition, proteins involved in inorganic ion, secondary metabolite and carbohydrate metabolism and transport (categories G, P, and Q) as well as in cell wall and cell envelope biogenesis (category M; Figure 4D) are largely CLADE specific. This finding suggests that not only signaling strategies, but also the mechanisms to interact with the environment are specific for small clades of cyanobacteria and even for individual strains.
The β-Barrel Proteins in Cyanobacteria
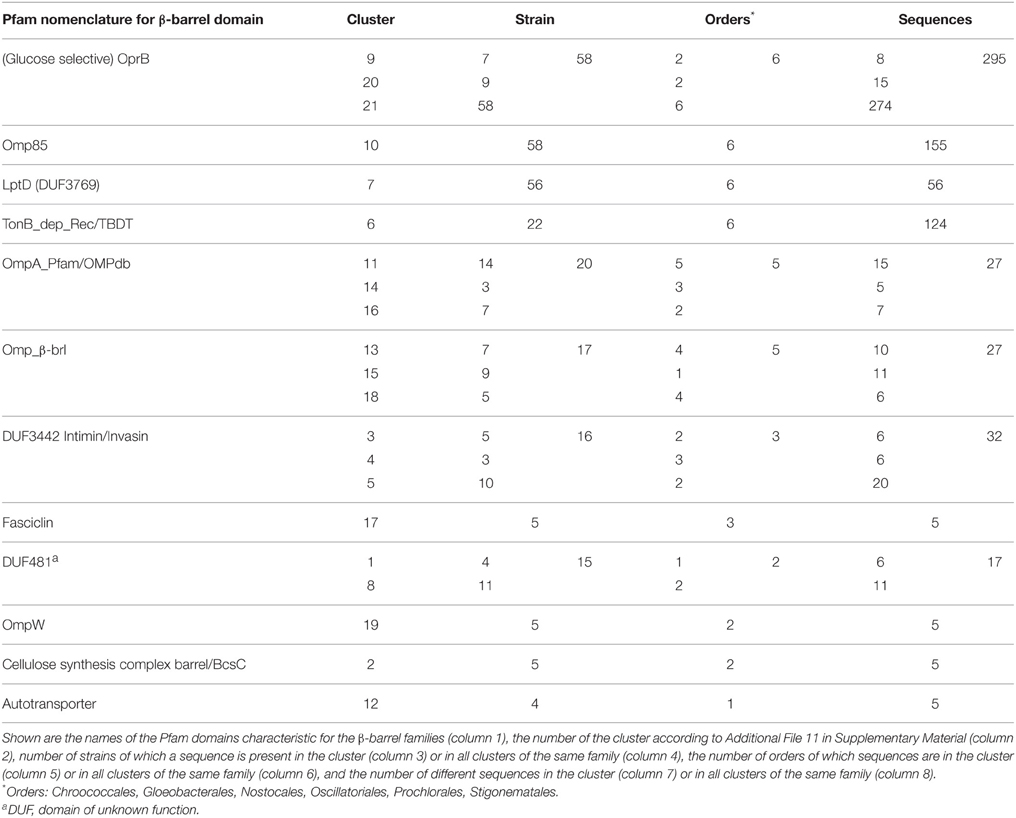
To confirm the notion that the proteome for the interaction with the environment, particularly for the uptake and secretion of molecules is highly clade specific, we aimed for the identification of putative OMPs as they are involved in such processes. We focused on proteins characterized by a membrane-embedded β-barrel domain as representative subset of the outer membrane proteome. We developed a consensus approach for the prediction of β-barrel OMPs in the cyanobacterial proteomes (see Materials and Methods). This approach yielded 703 putative β-barrel proteins detected by all criteria [category (a); Table 2], 179 which fulfill the two main criteria and at least one minor criterion [category (b); Table 2] and 37 which fulfill the two main criteria only, but are confirmed by tertiary structure prediction [category (c); Table 2]. All other proteins were not considered as putative β-barrel proteins [category (d); Table 2]. We clustered the sequence stretches representing the putative β-barrel domains of all selected proteins to assign functional properties as previously established (Mirus et al., 2009). We detected 21 clusters of β-barrel proteins with more than four sequences, which represent 12 functional groups based on domains defined by Pfam (Table 7, Additional File 12 in Supplementary Material).
Sequences of three β-barrel protein families are found in almost all strains analyzed, namely the OMP of 85 kDa (Omp85; Pfam: Bac_surface_Ag; Moslavac et al., 2005), the lipopolysaccharide transport protein D (LptD; Pfam: DUF3769; Haarmann et al., 2010), and the carbohydrate-selective porin (Pfam: OprB–OMP from Pseudomonas aeruginosa; Table 7). Omp85 and LptD are the two central proteins of outer membrane biogenesis of Gram-negative bacteria and belong to the most ancient outer membrane proteins (e.g., Bredemeier et al., 2007; Hahn and Schleiff, 2014), while a porin like OprB is generally required for solute transport. However, only Omp85 is a true component of the CORE-GENOME of cyanobacteria (Figure 5), because orthologs to LptD could not be identified Acaryochloris marina and Synechococcus sp. CB0205, although proteins with low similarity exist. For OprB we realized that the identified sequences cluster in different CLOGs, which is consistent with the detection of the protein family in all strains but the absence in the CORE-GENOME.
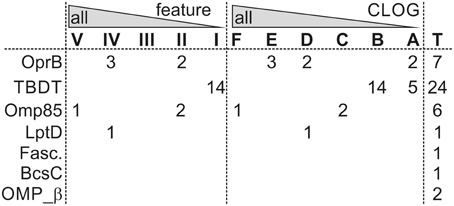
Figure 5. β-barrel proteins in various core-genomes. Given are the numbers of OMPs characterized by the indicated domains (Table 7) found in Anabaena sp. PCC 7120, which are present in the indicated core-genome of the feature or CLOG tree (Figure 3). T indicates the total number of identified sequences.
In addition, sequences with the broad signature for outer membrane localized β-barrel proteins (OmpA_Pfam/OmpA_OMPdb/OMP-β-brl; cluster 11, 13–16, and 18, Table 7, Additional File 11 in Supplementary Material) are found in the genome of 33 strains of all six cyanobacterial orders, which suggests that most of the cyanobacterial strains have additional outer envelope transporters to OrpB. However, they appear to be strain specific as they are not encoded by any clade specific core genome (Figure 5). The same holds true for the TonB dependent transporter involved in metal transport (Mirus et al., 2009), which was identified in all cyanobacterial orders, but only in 22 strains (Table 7).
All other identified β-barrel protein families are restricted to a lower number of strains and cyanobacterial orders. For example, proteins with a domain characteristic of autotransporters are specific for Synechococcus strains (Table 7). Moreover, β-barrel proteins with the INTIMIN/INVASIN domain are only found in five strains of the Prochlorales, in nine Synechococcus strains, in Acaryochloris marina MBIC11017 and in Microcoleus chthonoplastes PCC 7420. Such domains are usually found in virulence factors of enteropathogenic bacteria, mediating invasion into and adherence to host cells (Bodelon et al., 2013). All strains with such proteins are unicellular (except M. chthonoplastes PCC 7420) and live in the sea, which might require proteins with such domain for the association of cells to other organisms of the community.
Furthermore, OMPs with a domain characteristic for the cellulose synthase subunit with β-barrel (BcsC) or a FASCLINE domain are found in only eight strains, namely the heterocyst-forming Anabaena sp. PCC 7120 (both proteins), Anabaena variabilis ATCC 29413 (BcsC), Nostoc punctiforme PCC 73102 (both), Fischerella sp. JSC-11 (FASCLINE), Nodularia spumigena CCY9414 (FASCLINE) as well as in Acaryochloris marina MBIC11017 (BscC), Synechococcus sp. PCC 7002 (BscC) and Oscillatoria sp. PCC 6506 (FASCLINE). BcsC is involved in poly-β-1,6-N-acetyl-D-glucosamine or cellulose export (Keiski et al., 2010). Thus, such a protein might be involved in the formation of the heterocyst specific glycolipid layer and the heterocyst polysaccharide envelope (e.g., Nicolaisen et al., 2009). The FASCICLIN domain is an ancient cell adhesion domain (Borner et al., 2002) that might link the heterocyst specific layer to the outer membrane. In line, the gene of Anabaena sp. PCC 7120 (alr3754) with the BscC domain is highly induced (~10-fold) by nitrogen starvation (Flaherty et al., 2011) and the protein with FASCLINE domain was found in heterocyst membrane proteome (Moslavac et al., 2007). Thus, we propose that the function of two OMP families with BcsC or FASCICLIN domains identified in cyanobacteria is most likely related to heterocyst formation, although the experimental evidence is still missing.
From the inspection of the β-barrel proteome we conclude that the basic set for fundamental processes of outer membrane biogenesis represented by Omp85 and LptD and the basic principle of solute exchange represented by OprB are indeed globally conserved, while the majority of the β-barrel OMPs has evolved clade or strain specific to adapt to environmental situations. The large number of proteins with a membrane anchoring domain with general β-barrel signature in various analyzed strains (Table 7: OmpA, Omp_β, DUF481, and OmpW), but with distinct properties leading to a distinct CLOG assignment (Figure 5) supports the above formulated notion that mechanisms to interact with the environment are specific for small clades of cyanobacteria and even for individual strains.
Conclusion
The analysis of the protein sequences of 58 cyanobacterial strains of six different orders (Table 3) revealed a PAN-GENOME of about 44,831 genes (Figure 2). The cyanobacterial PAN-GENOME is considered to be open, which means that it will increase with each additional genome. In contrast, the CORE-GENOME of the 58 organisms is composed of 559 genes, and it is expected to level off at about 500 sequences (Figure 2). Roughly 20% of the CORE-GENOME is composed of genes involved in protein homeostasis, whereas most of the other genes perform housekeeping functions (Table 4). The individual genomes of cyanobacteria are largely composed of genes of the so-called dispensable-genome genomes, while unique genes are the minority (Figure 1). Based on the comparability of the trees calculated on the base of the genetic information or on features of the cyanobacteria (Figure 3, Table 1) we confirm that features dominate the genomic content. On the one hand, this is supported by the observation that for some features like “heterocyst formation” specific genes can be assigned (Tables 5, 6). On the other hand, analysis of clade specific core-genomes shows the ancient occurrence of processes like translation, ribosomal biogenesis and nucleotide metabolism, while processes involved in reactions to the environment like signal transduction and cell wall biogenesis are highly clade specific (Figure 4). The latter is also supported by the analysis of a specific protein family, namely the β-barrel shaped OMPs. Proteins involved in fundamental processes like outer membrane biogenesis (Omp85, LptD, Figure 5, Table 7) are globally conserved, while the majority of the β-barrel proteins are rather specific for clades of common features or even strain specific (Figure 5). Thus, while the CORE-GENOME describes the housekeeping and protein homeostasis functions, the proteins involved in environment response mechanisms are largely individualized for the various cyanobacteria.
Author Contributions
ES conceptualized, designed and headed the project. SS and MK performed the literature survey, the computational pan-genome and core-genome analysis. SS and MS implemented the β-barrel prediction approach. All authors were involved in analyzing the in silico results. ES, MK, and SS were involved in writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our colleagues for careful reading of the Manuscript, particularly B. Weis. The work was supported by grants from the Deutsche Forschungsgemeinschaft DFG SCHL 585-3 and 585-7 to ES. We thank Nadine Flinner, Oliver Mirus, Sotirios Fragkostefanakis, and Mara Stevanovic for critical discussion of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2015.00219/abstract
Supplementary File 1 (Table)—Features of the 58 Cyanobacterial Strains
For all 58 cyanobacteria information on 13 selected features is presented. For better readability, the information is split in three sub-tables. In Tables S1A, S1B the abbreviation assigned to each strain (Table 1) is given (column 1) and the following columns list the information on growth habitat, growth temperature, cultivation in the Lab or collected from nature, cell shape, cell order, mobility and toxin production (Table S1A), as well as on the ability to form Heterocysts, Akinetes, Hormogonia, or Trichome, on the ability to fix nitrogen as well as on their oxygen demand (Table S1B). The source of the information is represented in brackets and the reference is given in Table S1C.
Supplementary File 2 (Figure)—Calculation of the Tanimoto-Like Index
The feature similarity between two strains was calculated by a Tanimoto-like index (see Materials and Methods). Each feature is divided in categories (rectangles) (like feature habitat is divided in the subcategories: mud; fresh water; sea etc.). For each subcategory in each feature a value for present (1), not present (0), or unknown (u) is added. For each feature three different cases could occur: (I) unknown feature in one of two strains (1. feature) counts 0.5 in the denominator, (II) unknown feature of both strains (2. feature) is excluded from counting, and (III) known feature in both strains (3. feature) counts as the quotient of the intersection in the numerator and union in the denominator.
Supplementary File 3 (Figure)—Heat Map of Feature-Based Distances of Cyanobacteria
Shown is the heat map of the distance of the 58 cyanobacterial strains analyzed in here based on the Tanimoto-like index for the 13 different features. The pair-wise distance is represented in a color code based on percentage calculated by the Tanimoto-like index. Black, 0% distance—related to each other with respect to the features analyzed; white, 100% distance—not related to each other with respect to the features analyzed.
Supplementary File 4 (Figure)—Neighbor-Joining Trees of Figure 3
(A, B)—The neighbor-joining tree of the 58 cyanobacterial organisms is based on their pairwise shared CLOGs (A) or the feature distance (B). In A the number of shared CLOGs including two organisms is used for distance calculation. In B, the feature distance was calculated by a pairwise Tanimoto-like index based on the intersection of 13 features. The patristic distance correlation had a value of 0.51.
Supplementary file 5 (Figure)—Neighbor-Joining Trees of 16s rRNA and AAI
(A, B)—The neighbor-joining tree of the 58 cyanobacterial strains is based on their alignment of 16S rRNA sequences (A) or average amino acid identity (AAI) (B). In A the 16S rRNA sequences were multiple aligned by MAFFT. In B, 420 CLOGs of the CORE-GENOME with a single ortholog per strain were pairwise globally aligned the average over the CLOGs calculated to define a distance for each pair of strains. The patristic distance correlation between both trees is 0.76 meaning a strong correlation.
Supplementary File 6 (Table)—Clogs of the Core-Genome
Shown are the groups of the OrthoMCL ortholog search representing the CLOGs of the CORE-GENOME (column 1) and for each cyanobacterial strain the gene accessions (column 2–59).
Supplementary File 7 (Figure)—Core- and PAN-Genome Size Dependence on the Number of Analyzed Strains
Shown are the numbers of total CLOGs in the core-genome (A) or the pan-genome (B) derived from the analysis of the given number of organisms (x-axis), which have been randomly selected 100 (left) or 10,000 times (right). The results are plotted as box-plots. Values for 1000 iterations are shown in Figure 2.
Supplementary File 8 (Table)—Distribution of Anabaena sp. PCC 7120 Proteins Involved in Oxidative Phosphorylation and Photosynthesis According to KEGG Assignment in the Core-Genomes of Different Clades of the Feature Based Tree
The table gives: the root of the clade of the feature based tree for which the core genome was defined (column 1), the KEGG number of the protein (column 2), the name of the protein (column 3), the accession number of the according gene in Anabaena sp. PCC 7120 (column 4) and the functional category according to KEGG (column 6) and the functional category according to COG (column 7: Energy prod, energy production and conversion; non, no functional assignment in COG, other, a functional assignment distinct from energy production and conversion).
Supplementary File 9 (Table)—Functional Categories of the Core-Genomes Based on the Clog-Based Tree Exemplified for Anabaena sp. PCC 7120
Given is the functional category (column 1), the abbreviation of the COG of the functional process (column 2) and the number of sequences of Anabaena sp. PCC 7120 assigned to the different core-genomes (columns 3–8) based on the CLOG tree (Figure 4A).
Supplementary File 10 (Table)—Functional Categories of the Core-Genomes Based on the Feature Tree Exemplified for Anabaena sp. PCC 7120
Given is the functional category (column 1), the abbreviation of the COG of the functional process (column 2) and the number of sequences of Anabaena sp. PCC 7120 assigned to the different core-genomes (columns 3–8) based on the feature tree (Figure 4B).
Supplementary File 11 (Figure)—Proteins Found in Core and Clade Genes
Shown is the occurrence of unique proteins assigned to the individual processes (indicated by one letter code shown in Table 2). The distribution for proteins for each process is shown as color code indicated in Figure 4D. For each distribution the profile was analyzed by an inversed Gaussian distribution and the position of the minimum was used to assign the process as CLADE and CORE-GENOME defined.
Supplementary File 12 (Figure)—Clustering of Predicted β-Barrel Proteins
Shown are clusters of amino acid sequences sections of putative cyanobacterial β-barrel proteins of category (a), (b), and (c) (Table 2) via CLANS. The clusters were numbered and colored according to their predicted function (Table 7). Distances below 1.0 × e−20 are shown and contain the same functional or domain annotation.
References
Allewalt, J. P., Bateson, M. M., Revsbech, N. P., Slack, K., and Ward, D. M. (2006). Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the octopus spring microbial mat community of Yellowstone National Park. Appl. Environ. Microbiol. 72, 544–550. doi: 10.1128/AEM.72.1.544-550.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anagnostidis, K., and Komárek, J. (1987). Modern approach to the classification system of Cyanophytes. 3. Oscillatoriales. Algol. Stud. 50–53, 327–472.
Anagnostidis, K., and Komárek, J. (1990). Modern approach to the classification system of Cyanophytes. 5. Stigonematales. Algol. Stud. 59, 1–73
Araoz, R., Nghiem, H. O., Rippka, R., Palibroda, N., De Marsac, N. T., and Herdman, M. (2005). Neurotoxins in axenic oscillatorian cyanobacteria: coexistence of anatoxin-a and homoanatoxin-a determined by ligand-binding assay and GC/MS. Microbiology 151, 1263–1273. doi: 10.1099/mic.0.27660-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Armenta-Medina, D., Segovia, L., and Perez-Rueda, E. (2014). Comparative genomics of nucleotide metabolism: a tour to the past of the three cellular domains of life. BMC Genomics 15:800. doi: 10.1186/1471-2164-15-800
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Awai, K., and Wolk, C. P. (2007). Identification of the glycosyl transferase required for synthesis of the principal glycolipid characteristic of heterocysts of Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 266, 98–102. doi: 10.1111/j.1574-6968.2006.00512.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bauer, C. C., Buikema, W. J., Black, K., and Haselkorn, R. (1995). A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J. Bacteriol. 177, 1520–1526.
Beck, C., Knoop, H., Axmann, I. M., and Steuer, R. (2012). The diversity of cyanobacterial metabolism: genome analysis of multiple phototrophic microorganisms. BMC Genomics 13:56. doi: 10.1186/1471-2164-13-56
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Berven, F. S., Flikka, K., Jensen, H. B., and Eidhammer, I. (2004). BOMP: a program to predict integral beta-barrel outer membrane proteins encoded within genomes of Gram-negative bacteria. Nucleic Acids Res. 32, W394–W399. doi: 10.1093/nar/gkh351
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Black, K., Buikema, W. J., and Haselkorn, R. (1995). The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 177, 6440–6448.
Bodelon, G., Palomino, C., and Fernandez, L. A. (2013). Immunoglobulin domains in Escherichia coli and other enterobacteria: from pathogenesis to applications in antibody technologies. FEMS Microbiol. Rev. 37, 204–250. doi: 10.1111/j.1574-6976.2012.00347.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bolhuis, H., Severin, I., Confurius-Guns, V., Wollenzien, U. I., and Stal, L. J. (2010). Horizontal transfer of the nitrogen fixation gene cluster in the cyanobacterium Microcoleus chthonoplastes. ISME J. 4, 121–130. doi: 10.1038/ismej.2009.99
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Borner, G. H., Sherrier, D. J., Stevens, T. J., Arkin, I. T., and Dupree, P. (2002). Prediction of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A genomic analysis. Plant Physiol 129, 486–499. doi: 10.1104/pp.010884
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bothe, H., Schmitz, O., Yates, M. G., and Newton, W. E. (2010). Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 74:529–551. doi: 10.1128/MMBR.00033-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bredemeier, R., Schlegel, T., Ertel, F., Vojta, A., Borissenko, L., Bohnsack, M. T., et al. (2007). Functional and phylogenetic properties of the pore-forming β-barrel transporters of the Omp85 family. J. Biol. Chem. 282, 1882–1890. doi: 10.1074/jbc.M609598200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Campbell, E. L., Christman, H., and Meeks, J. C. (2008). DNA microarray comparisons of plant factor- and nitrogen deprivation-induced Hormogonia reveal decision-making transcriptional regulation patterns in Nostoc punctiforme. J. Bacteriol. 190, 7382–7391. doi: 10.1128/JB.00990-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carey, C. C., Ibelings, B. W., Hoffmann, E. P., Hamilton, D. P., and Brookes, J. D. (2012). Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 46, 1394–1407. doi: 10.1016/j.watres.2011.12.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carrieri, D., Ananyev, G., Lenz, O., Bryant, D. A., and Dismukes, G. C. (2011). Contribution of a sodium ion gradient to energy conservation during fermentation in the cyanobacterium Arthrospira (Spirulina) maxima CS-328. Appl. Environ. Microbiol. 77, 7185–7194. doi: 10.1128/AEM.00612-11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, F., Mackey, A. J., Stoeckert, C. J. Jr., and Roos, D. S. (2006). OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 34, D363–D368. doi: 10.1093/nar/gkj123
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cohen, M. F., Wallis, J. G., Campbell, E. L., and Meeks, J. C. (1994). Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology 140, 3233–3240. doi: 10.1099/13500872-140-12-3233
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Collingro, A., Tischler, P., Weinmaier, T., Penz, T., Heinz, E., Brunham, R. C., et al. (2011). Unity in variety—the pan-genome of the Chlamydiae. Mol. Biol. Evol. 28, 3253–3270. doi: 10.1093/molbev/msr161
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cooper, D. L., Mort, K. A., Allan, N. L., Kinchington, D., and McGuigan, C. (1993). Molecular similarity of anti-HIV phospholipids. J. Am. Chem. Soc. 115, 12615–12616. doi: 10.1021/ja00079a063
Corrales-Guerrero, L., Mariscal, V., Nurnberg, D. J., Elhai, J., Mullineaux, C. W., Flores, E., et al. (2014). Subcellular localization and clues for the function of the HetN factor influencing heterocyst distribution in Anabaena sp. strain PCC 7120. J. Bacteriol. 196, 3452–3460. doi: 10.1128/JB.01922-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Croce, R., and van Amerongen, H. (2014). Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501. doi: 10.1038/nchembio.1555
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
D'Auria, G., Jimenez-Hernandez, N., Peris-Bondia, F., Moya, A., and Latorre, A. (2010). Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 11:181. doi: 10.1186/1471-2164-11-181
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Donati, C., Hiller, N. L., Tettelin, H., Muzzi, A., Croucher, N. J., Angiuoli, S. V., et al. (2010). Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 11:R107. doi: 10.1186/gb-2010-11-10-r107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Du, Y., Cai, Y., Hou, S., and Xu, X. (2012). Identification of the HetR recognition sequence upstream of hetZ in Anabaena sp. strain PCC 7120. J. Bacteriol. 194, 2297–2306. doi: 10.1128/JB.00119-12
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dunn, B., Richter, C., Kvitek, D. J., Pugh, T., and Sherlock, G. (2012). Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 22, 908–924. doi: 10.1101/gr.130310.111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dutilh, B. E., Snel, B., Ettema, T. J., and Huynen, M. A. (2008). Signature genes as a phylogenomic tool. Mol. Biol. Evol. 25, 1659–1667. doi: 10.1093/molbev/msn115
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., and Stackebrandt, E. (eds.). (2006). The Prokaryotes: Vol. 3: Archaea. Bacteria: Firmicutes, Actinomycetes. New York, NY: Springer Science and Business Media.
Eddy, S. R. (2011). Accelerated Profile HMM Searches. PLoS Comput. Biol. 7:e1002195. doi: 10.1371/journal.pcbi.1002195
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ehira, S., and Ohmori, M. (2012). The redox-sensing transcriptional regulator RexT controls expression of thioredoxin A2 in the cyanobacterium Anabaena sp. strain PCC 7120. J. Biol. Chem. 287, 40433–40440. doi: 10.1074/jbc.M112.384206
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Elliott, J. A. (2012). Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 46:1364, 1371. doi: 10.1016/j.watres.2011.12.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
El-Shehawy, R., Lugomela, C., Ernst, A., and Bergman, B. (2003). Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum. Microbiology 149, 1139–1146. doi: 10.1099/mic.0.26170-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fan, Q., Huang, G., Lechno-Yossef, S., Wolk, C. P., Kaneko, T., and Tabata, S. (2005). Clustered genes required for synthesis and deposition of envelope glycolipids in Anabaena sp. strain PCC 7120. Mol. Microbiol. 58, 227–243. doi: 10.1111/j.1365-2958.2005.04818.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Finn, R. D., Bateman, A., Clements, J., Coggill, P., Eberhardt, R. Y., Eddy, S. R., et al. (2014). Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230. doi: 10.1093/nar/gkt1223
Flaherty, B. L., van Nieuwerburgh, F., Head, S. R., and Golden, J. W. (2011). Directional RNA deep sequencing sheds new light on the transcriptional response of Anabaena sp. strain PCC 7120 to combined-nitrogen deprivation. BMC Genomics 12:332. doi: 10.1186/1471-2164-12-332
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Flores, E., and Herrero, A. (2010). Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8, 39–50. doi: 10.1038/nrmicro2242
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fujisawa, T., Narikawa, R., Okamoto, S., Ehira, S., Yoshimura, H., Suzuki, I., et al. (2010). Genomic structure of an economically important cyanobacterium, Arthrospira (Spirulina) platensis NIES-39. DNA Res. 17, 85–103. doi: 10.1093/dnares/dsq004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gao, K., Yu, H., and Brown, M. T. (2007). Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. J. Photochem. Photobiol. B. 89, 117–124. doi: 10.1016/j.jphotobiol.2007.09.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garcia-Pichel, F., Johnson, S. L., Youngkin, D., and Belnap, J. (2003). Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado plateau. Microb. Ecol. 46, 312–321. doi: 10.1007/s00248-003-1004-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Golden, J. W., and Yoon, H. S. (2003). Heterocyst development in Anabaena. Curr. Opin. Microbiol. 6, 557–563. doi: 10.1016/j.mib.2003.10.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gruber, T. M., and Bryant, D. A. (1998). Characterization of the alternative sigma-factors SigD and SigE in Synechococcus sp. strain PCC 7002. SigE is implicated in transcription of post-exponential-phase-specific genes. Arch. Microbiol. 169, 211–219. doi: 10.1007/s002030050563
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, R. S., and Mathews, D. W. (2010). Signature proteins for the major clades of Cyanobacteria. BMC Evol. Biol. 10:24. doi: 10.1186/1471-2148-10-24
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haarmann, R., Ibrahim, M., Stevanovic, M., Bredemeier, R., and Schleiff, E. (2010). The properties of the outer membrane localized Lipid A transporter LptD. J. Phys. Condens. Matter 22, 454124. doi: 10.1088/0953-8984/22/45/454124
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hahn, A., and Schleiff, E. (2014). “The cell envelope,” in The Cell Biology of Cyanobacteria, eds E. Flores and A. Herrero (Norfolk: Caister Academic Press), 29–87.
Herrero, A., Muro-Pastor, A. M., and Flores, E. (2001). Nitrogen control in cyanobacteria. J. Bacteriol. 183, 411–425. doi: 10.1128/JB.183.2.411-425.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Higa, K. C., and Callahan, S. M. (2010). Ectopic expression of hetP can partially bypass the need for hetR in heterocyst differentiation by Anabaena sp. strain PCC 7120. Mol. Microbiol. 77, 562–574. doi: 10.1111/j.1365-2958.2010.07257.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, J., and Yan, C. (2008). A method for discovering transmembrane beta-barrel proteins in Gram-negative bacterial proteomes. Comput. Biol. Chem. 32, 298–301. doi: 10.1016/j.compbiolchem.2008.03.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huang, G., Fan, Q., Lechno-Yossef, S., Wojciuch, E., Wolk, C. P., Kaneko, T., et al. (2005). Clustered genes required for the synthesis of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J Bacteriol 187, 1114–1123. doi: 10.1128/JB.187.3.1114-1123.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huber, A. L. (1985). Factors affecting the germination of akinetes of Nodularia spumigena (Cyanobacteriaceae). Appl. Environ. Microbiol. 49, 73–78.
Ionescu, D., Voss, B., Oren, A., Hess, W. R., and Muro-Pastor, A. M. (2010). Heterocyst-specific transcription of NsiR1, a non-coding RNA encoded in a tandem array of direct repeats in cyanobacteria. J. Mol. Biol. 398, 177–188. doi: 10.1016/j.jmb.2010.03.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, C. S., and Mayfield, S. P. (2012). Algae biofuels: versatility for the future of bioenergy. Curr. Opin. Biotechnol. 23, 346–351. doi: 10.1016/j.copbio.2011.10.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, K. (1992). Diurnal nitrogen fixation in tropical marine cyanobacteria: a comparison between adjacent communities of non-heterocystous Lyngbya sp. and heterocystous Calothrix sp. Br. Phycol. J. 27, 107–118. doi: 10.1080/00071619200650121
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaneko, T., Nakajima, N., Okamoto, S., Suzuki, I., Tanabe, Y., Tamaoki, M., et al. (2007). Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Res. 14, 247–256. doi: 10.1093/dnares/dsm026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaneko, T., and Tabata, S. (1997). Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 38, 1171–1176. doi: 10.1093/oxfordjournals.pcp.a029103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keiski, C. L., Harwich, M., Jain, S., Neculai, A. M., Yip, P., Robinson, H., et al. (2010). AlgK is a TPR-containing protein and the periplasmic component of a novel exopolysaccharide secretin. Structure 18, 265–273. doi: 10.1016/j.str.2009.11.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kelley, L. A., and Sternberg, M. J. (2009). Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371. doi: 10.1038/nprot.2009.2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kettler, G. C., Martiny, A. C., Huang, K., Zucker, J., Coleman, M. L., Rodrigue, S., et al. (2007). Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 3:e231. doi: 10.1371/journal.pgen.0030231
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, C. J., Jung, Y. H., and Oh, H. M. (2007). Factors indicating culture status during cultivation of Spirulina (Arthrospira) platensis. J. Microbiol. 45, 122–127.
Komárek, J., and Anagnostidis, K. (1986). Modern approach to the classification system of Cyanophytes. 2. Chroococcales. Algol. Stud. 43, 157–226.
Komárek, J., and Anagnostidis, K. (1989). Modern approach to the classification system of Cyanophytes. 4. Nostocales. Algol. Stud. 56, 247–345.
Larsson, J., Nylander, J. A., and Bergman, B. (2011). Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 11:187. doi: 10.1186/1471-2148-11-187
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lazaro, S., Fernandez-Pinas, F., Fernandez-Valiente, E., Blanco-Rivero, A., and Leganes, F. (2001). pbpB, a gene coding for a putative penicillin-binding protein, is required for aerobic nitrogen fixation in the cyanobacterium Anabaena sp. strain PCC7120. J Bacteriol 183, 628–636. doi: 10.1128/JB.183.2.628-636.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lesser, M. (2003). Advances in Marine Biology cumulative index volumes 20–44. Adv. Mar. Biol. 45, 9–312. doi: 10.1016/S0065-2881(03)45002-5
Li, R., Li, Y., Zheng, H., Luo, R., Zhu, H., Li, Q., et al. (2010). Building the sequence map of the human pan-genome. Nat. Biotechnol. 28, 57–63. doi: 10.1038/nbt.1596
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liang, J., Scappino, L., and Haselkorn, R. (1992). The patA gene product, which contains a region similar to CheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. U.S.A. 89, 5655–5659. doi: 10.1073/pnas.89.12.5655
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I., and Lomize, A. L. (2012). OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376. doi: 10.1093/nar/gkr703
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maddison, W. P., and Maddison, D. R. (2011). Mesquite: A Modular System for Evolutionary Analysis. Version 2.75. Available online at: http://mesquiteproject.org. (725 modules)
Markowitz, V. M., Chen, I. M., Palaniappan, K., Chu, K., Szeto, E., Grechkin, Y., et al. (2012). IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res. 40, D115–D122. doi: 10.1093/nar/gkr1044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin, K. A., Siefert, J. L., Yerrapragada, S., Lu, Y., McNeill, T. Z., Moreno, P. A., et al. (2003). Cyanobacterial signature genes. Photosyn. Res. 75, 211–221. doi: 10.1023/A:1023990402346
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Medini, D., Donati, C., Tettelin, H., Masignani, V., and Rappuoli, R. (2005). The microbial pan-genome. Curr. Opin. Genet. Dev. 15, 589–594. doi: 10.1016/j.gde.2005.09.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mejean, A., Mazmouz, R., Mann, S., Calteau, A., Medigue, C., and Ploux, O. (2010). The genome sequence of the cyanobacterium Oscillatoria sp. PCC 6506 reveals several gene clusters responsible for the biosynthesis of toxins and secondary metabolites. J Bacteriol 192, 5264-5265. doi: 10.1128/JB.00704-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mirus, O., Hahn, A., and Schleiff, E. (2010). “Outer membrane proteins,” in Prokaryotic Cell Wall Compounds. Structure and Biochemistry, eds H. König, H. Claus, and A. Varma (Berlin; Heidelberg: Springer-Verlag), 175–230. doi: 10.1007/978-3-642-05062-6_6
Mirus, O., and Schleiff, E. (2005). Prediction of beta-barrel membrane proteins by searching for restricted domains. BMC Bioinformatics 6:254. doi: 10.1186/1471-2105-6-254
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mirus, O., Strauss, S., Nicolaisen, K., Von Haeseler, A., and Schleiff, E. (2009). TonB-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 7:68. doi: 10.1186/1741-7007-7-68
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moller, S., Croning, M. D., and Apweiler, R. (2001). Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17, 646–653. doi: 10.1093/bioinformatics/17.7.646
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moslavac, S., Mirus, O., Bredemeier, R., Soll, J., Von Haeseler, A., and Schleiff, E. (2005). Conserved pore-forming regions in polypeptide-transporting proteins. FEBS J. 272, 1367–1378. doi: 10.1111/j.1742-4658.2005.04569.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moslavac, S., Reisinger, V., Berg, M., Mirus, O., Vosyka, O., Ploscher, M., et al. (2007). The proteome of the heterocyst cell wall in Anabaena sp. PCC 7120. Biol. Chem. 388, 823-829. doi: 10.1515/BC.2007.079
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mulkidjanian, A. Y., Koonin, E. V., Makarova, K. S., Mekhedov, S. L., Sorokin, A., Wolf, Y. I., et al. (2006). The cyanobacterial genome core and the origin of photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 13126–13131. doi: 10.1073/pnas.0605709103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mur, L. R., Skulberg, O. M., and Utkilen, H. (1999). “Cyanobacteria in the environment,” in Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management, Chapter 2, eds I. Chorus and J. Bartram (London; New York: E&FN Spon), 15–40.
Muro-Pastor, A. M., and Hess, W. R. (2012). Heterocyst differentiation: from single mutants to global approaches. Trends Microbiol. 20, 548–557. doi: 10.1016/j.tim.2012.07.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakamura, Y., Kaneko, T., Sato, S., Ikeuchi, M., Katoh, H., Sasamoto, S., et al. (2002). Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1 (supplement). DNA Res. 9, 135–148. doi: 10.1093/dnares/9.4.135
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakamura, Y., Kaneko, T., Sato, S., Mimuro, M., Miyashita, H., Tsuchiya, T., et al. (2003). Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids (supplement). DNA Res. 10, 181–201. doi: 10.1093/dnares/10.4.181
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakao, M., Okamoto, S., Kohara, M., Fujishiro, T., Fujisawa, T., Sato, S., et al. (2010). CyanoBase: the cyanobacteria genome database update 2010. Nucleic Acids Res. 38, D379–D381. doi: 10.1093/nar/gkp915
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neilan, B. A., Pearson, L. A., Muenchhoff, J., Moffitt, M. C., and Dittmann, E. (2013). Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 15, 1239–1253. doi: 10.1111/j.1462-2920.2012.02729.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nguyen, T. A., Brescic, J., Vinyard, D. J., Chandrasekar, T., and Dismukes, G. C. (2012). Identification of an oxygenic reaction center psbADC operon in the cyanobacterium Gloeobacter violaceus PCC 7421. Mol. Biol. Evol. 29, 35–38. doi: 10.1093/molbev/msr224
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nicolaisen, K., Hahn, A., and Schleiff, E. (2009). The cell wall in heterocyst formation by Anabaena sp. PCC 7120. J Basic Microbiol 49, 5–24. doi: 10.1002/jobm.200800300
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oliver, J. W., and Atsumi, S. (2014). Metabolic design for cyanobacterial chemical synthesis. Photosyn. Res. 120, 249–261. doi: 10.1007/s11120-014-9997-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ostlund, G., Schmitt, T., Forslund, K., Kostler, T., Messina, D. N., Roopra, S., et al. (2010). InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 38, D196–D203. doi: 10.1093/nar/gkp931
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ou, Y. Y., Gromiha, M. M., Chen, S. A., and Suwa, M. (2008). TMBETADISC-RBF: discrimination of beta-barrel membrane proteins using RBF networks and PSSM profiles. Comput. Biol. Chem. 32, 227–231. doi: 10.1016/j.compbiolchem.2008.03.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pernil, R., Herrero, A., and Flores, E. (2010). Catabolic function of compartmentalized alanine dehydrogenase in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 192, 5165–5172. doi: 10.1128/JB.00603-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ploug, H., Adam, B., Musat, N., Kalvelage, T., Lavik, G., Wolf-Gladrow, D., et al. (2011). Carbon, nitrogen and O(2) fluxes associated with the cyanobacterium Nodularia spumigena in the Baltic Sea. ISME J. 5, 1549–1558. doi: 10.1038/ismej.2011.20
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Poole, A., Jeffares, D., and Penny, D. (1999). Early evolution: prokaryotes, the new kids on the block. Bioessays. 21, 880–889.
Ran, L., Larsson, J., Vigil-Stenman, T., Nylander, J. A., Ininbergs, K., Zheng, W. W., et al. (2010). Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLoS ONE 5:e11486. doi: 10.1371/annotation/835c5766-5128-41c4-b636-adfe0c503103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reno, M. L., Held, N. L., Fields, C. J., Burke, P. V., and Whitaker, R. J. (2009). Biogeography of the Sulfolobus islandicus pan-genome. Proc. Natl. Acad. Sci. U.S.A. 106, 8605–8610. doi: 10.1073/pnas.0808945106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rouhiainen, L., Sivonen, K., Buikema, W. J., and Haselkorn, R. (1995). Characterization of toxin-producing cyanobacteria by using an oligonucleotide probe containing a tandemly repeated heptamer. J. Bacteriol. 177, 6021–6026.
Scott, N. L., Xu, Y., Shen, G., Vuletich, D. A., Falzone, C. J., Li, Z., et al. (2010). Functional and structural characterization of the 2/2 hemoglobin from Synechococcus sp. PCC 7002. Biochemistry 49, 7000–7011. doi: 10.1021/bi100463d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shi, L., Li, J. H., Cheng, Y., Wang, L., Chen, W. L., and Zhang, C. C. (2007). Two genes encoding protein kinases of the HstK family are involved in synthesis of the minor heterocyst-specific glycolipid in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189, 5075–5081. doi: 10.1128/JB.00323-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shih, P. M., Wu, D., Latifi, A., Axen, S. D., Fewer, D. P., Talla, E., et al. (2013). Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA. 110, 1053–1058. doi: 10.1073/pnas.1217107110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Singh, S. P., and Montgomery, B. L. (2011). Determining cell shape: adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends Microbiol. 19, 278–285. doi: 10.1016/j.tim.2011.03.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sommer, M. S., Daum, B., Gross, L. E., Weis, B. L., Mirus, O., Abram, L., et al. (2011). Chloroplast Omp85 proteins change orientation during evolution. Proc. Natl. Acad. Sci. U.S.A. 108, 13841–13846. doi: 10.1073/pnas.1108626108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stal, L. J. K., and Krumbein W. E. (1985). Nitrogenase activity in the non-heterocystous cyanobacterium Oscillatoria sp. grown under alternating light-dark cycles. Arch. Microbiol. 143, 67–71. doi: 10.1007/BF00414770
Stewart, I., Eaglesham, G. K., McGregor, G. B., Chong, R., Seawright, A. A., Wickramasinghe, W. A., et al. (2012). First report of a toxic Nodularia spumigena (Nostocales/ Cyanobacteria) bloom in sub-tropical Australia. II. Bioaccumulation of nodularin in isolated populations of mullet (Mugilidae). Int. J. Environ. Res. Public Health 9, 2412–2443. doi: 10.3390/ijerph9072412
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stockel, J., Welsh, E. A., Liberton, M., Kunnvakkam, R., Aurora, R., and Pakrasi, H. B. (2008). Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl. Acad. Sci. U.S.A. 105, 6156–6161. doi: 10.1073/pnas.0711068105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Su, Z., Mao, F., Dam, P., Wu, H., Olman, V., Paulsen, I. T., et al. (2006). Computational inference and experimental validation of the nitrogen assimilation regulatory network in cyanobacterium Synechococcus sp. WH 8102. Nucleic Acids Res. 34, 1050–1065. doi: 10.1093/nar/gkj496
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Swingley, W. D., Chen, M., Cheung, P. C., Conrad, A. L., Dejesa, L. C., Hao, J., et al. (2008). Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc. Natl. Acad. Sci. U.S.A. 105, 2005–2010. doi: 10.1073/pnas.0709772105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takaichi, S., Mochimaru, M., and Maoka, T. (2006). Presence of free myxol and 4-hydroxymyxol and absence of myxol glycosides in Anabaena variabilis ATCC 29413, and proposal of a biosynthetic pathway of carotenoids. Plant Cell Physiol. 47, 211–216. doi: 10.1093/pcp/pci236
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tatusov, R. L., Koonin, E. V., and Lipman, D. J. (1997). A genomic perspective on protein families. Science 278, 631–637. doi: 10.1126/science.278.5338.631
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tettelin, H., Masignani, V., Cieslewicz, M. J., Donati, C., Medini, D., Ward, N. L., et al. (2005). Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome.” Proc. Natl. Acad. Sci. U.S.A. 102, 13950–13955. doi: 10.1073/pnas.0506758102
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tettelin, H., Riley, D., Cattuto, C., and Medini, D. (2008). Comparative genomics: the bacterial pan-genome. Curr. Opin. Microbiol. 11, 472–477. doi: 10.1016/j.mib.2008.09.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tsirigos, K. D., Bagos, P. G., and Hamodrakas, S. J. (2011). OMPdb: a database of {beta}-barrel outer membrane proteins from Gram-negative bacteria. Nucleic Acids Res. 39, D324–D331. doi: 10.1093/nar/gkq863
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tuit, C., Waterbury, J., and Ravizza, G. (2004). Diel variation of moldybdenum and iron in marine diazotrophic cyanobacteria. Limn. Oceanogr. 49, 978–990. doi: 10.4319/lo.2004.49.4.0978
Urmeneta, J., Navarrete, A., Huete, J., and Guerrero, R. (2003). Isolation and characterization of cyanobacteria from microbial mats of the Ebro Delta, Spain. Curr. Microbiol. 46, 199–204. doi: 10.1007/s00284-002-3856-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Valério, E., Chambel, L., Paulino, S., Faria, N., Pereira, P., and Tenreiro, R. (2009). Molecular identification, typing and traceability of cyanobacteria from freshwater reservoirs. Microbiology 155, 642–656. doi: 10.1099/mic.0.022848-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Domselaar, G. H., Stothard, P., Shrivastava, S., Cruz, J. A., Guo, A., Dong, X., et al. (2005). BASys: a web server for automated bacterial genome annotation. Nucleic Acids Res. 33, W455–W459. doi: 10.1093/nar/gki593
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Y., Lechno-Yossef, S., Gong, Y., Fan, Q., Wolk, C. P., and Xu, X. (2007). Predicted glycosyl transferase genes located outside the HEP island are required for formation of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 189, 5372–5378. doi: 10.1128/JB.00343-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wattam, A. R., Abraham, D., Dalay, O., Disz, T. L., Driscoll, T., Gabbard, J. L., et al. (2014). PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 42, D581–D591. doi: 10.1093/nar/gkt1099
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wijffels, R. H., Kruse, O., and Hellingwerf, K. J. (2013). Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 24, 405–413. doi: 10.1016/j.copbio.2013.04.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wu, S., Zhu, Z., Fu, L., Niu, B., and Li, W. (2011). WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics 12:444. doi: 10.1186/1471-2164-12-444
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zehr, J. P. (2011). Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173. doi: 10.1016/j.tim.2010.12.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, C. C., Friry, A., and Peng, L. (1998). Molecular and genetic analysis of two closely linked genes that encode, respectively, a protein phosphatase 1/2A/2B homolog and a protein kinase homolog in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 180, 2616–2622.
Zhang, C. C., and Libs, L. (1998). Cloning and characterisation of the pknD gene encoding an eukaryotic-type protein kinase in the cyanobacterium Anabaena sp. PCC 7120. Mol. Gen. Genet. 258, 26–33. doi: 10.1007/s004380050703
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, W., Du, Y., Khudyakov, I., Fan, Q., Gao, H., Ning, D., et al. (2007). A gene cluster that regulates both heterocyst differentiation and pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 66, 1429–1443. doi: 10.1111/j.1365-2958.2007.05997.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, Y., Wu, J., Yang, J., Sun, S., Xiao, J., and Yu, J. (2012). PGAP: pan-genomes analysis pipeline. Bioinformatics 28, 416–418. doi: 10.1093/bioinformatics/btr655
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, R., and Wolk, C. P. (2002). Identification of an akinete marker gene in Anabaena variabilis. J. Bacteriol. 184, 2529–2532. doi: 10.1128/JB.184.9.2529-2532.2002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: cyanobacteria, Anabaena sp. PCC 7120, core-genome, genotypic and phenotypic differences, ortholog search, comparative genomics
Citation: Simm S, Keller M, Selymesi M and Schleiff E (2015) The composition of the global and feature specific cyanobacterial core-genomes. Front. Microbiol. 6:219. doi: 10.3389/fmicb.2015.00219
Received: 04 November 2014; Accepted: 04 March 2015;
Published: 19 March 2015.
Edited by:
Eamonn P. Culligan, University College Cork, IrelandReviewed by:
Loren John Hauser, Oak Ridge National Laboratory, USAWolfgang R. Hess, University of Freiburg, Germany
Copyright © 2015 Simm, Keller, Selymesi and Schleiff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enrico Schleiff, Department of Biosciences, Molecular Cell Biology of Plants, Goethe University, Max von Laue Str. 9, Frankfurt am Main, 60438, Germany schleiff@bio.uni-frankfurt.de
 Stefan Simm1
Stefan Simm1 Mario Keller
Mario Keller Enrico Schleiff
Enrico Schleiff