- Blackburn Lab, Applied Proteomics and Chemical Biology Group, Division of Medical Biochemistry, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
Ser/Thr/Tyr protein phosphorylation plays a critical role in regulating mycobacterial growth and development. Understanding the mechanistic link between protein phosphorylation signaling network and mycobacterial growth rate requires a global view of the phosphorylation events taking place at a given time under defined conditions. In the present study we employed a phosphopeptide enrichment and high throughput mass spectrometry-based strategy to investigate and qualitatively compare the phosphoproteome of two mycobacterial model organisms: the fast growing Mycobacterium smegmatis and the slow growing Mycobacterium bovis BCG. Cells were harvested during exponential phase and our analysis detected a total of 185 phospho-sites in M. smegmatis, of which 106 were confidently localized [localization probability (LP) = 0.75; PEP = 0.01]. By contrast, in M. bovis BCG the phosphoproteome comprised 442 phospho-sites, of which 289 were confidently localized. The percentage distribution of Ser/Thr/Tyr phosphorylation was 39.47, 57.02, and 3.51% for M. smegmatis and 35, 61.6, and 3.1% for M. bovis BCG. Moreover, our study identified a number of conserved Ser/Thr phosphorylated sites and conserved Tyr phosphorylated sites across different mycobacterial species. Overall a qualitative comparison of the fast and slow growing mycobacteria suggests that the phosphoproteome of M. smegmatis is a simpler version of that of M. bovis BCG. In particular, M. bovis BCG exponential cells exhibited a much more complex and sophisticated protein phosphorylation network regulating important cellular cycle events such as cell wall biosynthesis, elongation, cell division including immediately response to stress. The differences in the two phosphoproteomes are discussed in light of different mycobacterial growth rates.
Introduction
Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), a major health concern worldwide. The current incidence of tuberculosis disease in South Africa is more than 900 cases per 100,000 people per year. Moreover, the World Health Organisation have estimated that roughly one third of the world's population is latently infected with M. tuberculosis. The majority of latently infected individuals will remain asymptomatic throughout their lives, with the risk of developing active TB disease from a latent infection being ~10% per lifetime; in HIV positive individuals though, this risk increases to 10% per year (WHO, 2014). This latent infected population is thus a substantial reservoir of potential new TB cases and is therefore a significant public health concern. The ability of mycobacteria to adapt to, and overcome, hostile environmental conditions in order to survive within host cells depends on a fine-tuned and orchestrated series of events (e.g., response to stress; metabolic remodeling; changes in growth rate) that will ultimately define the success of establishing and maintaining long term infection (McKinney et al., 2000; Marrero et al., 2010). M. tuberculosis infection is often described by two distinct phases: an active phase, in which the microorganism is thought to be growing at or close to its maximum rate; and latent infection, in which the bacilli are thought to persist in a viable but perhaps more dormant-like state with lower or non-existent growth rate. Current thinking suggests that there is most likely a continuum of states between latent TB infection (LTBI), sub-clinical TB and active TB disease, but to date no M. tuberculosis bacilli have been observed in LTBI individuals, so the exact physiological state of M. tuberculosis during a latent infection remains unknown.
Alongside the increasing number of new TB infections there is another matter of great concern, which is the emergence and spread of multi- and extensively drug resistant M. tuberculosis strains. Here unique growth related mechanisms of mycobacteria which facilitate adaptation to different adverse micro-environments are thought to play an important role in the mechanisms of drug tolerance and acquired resistance that are observed during infection (Corper and Cohn, 1933; Wayne and Hayes, 1996). For instance, a recent study showed that diverse growth-limiting stresses trigger a common signal transduction pathway in M. tuberculosis that induces triglyceride synthesis, which is associated with slowing down of growth and reduced antibiotic efficacy (Baek et al., 2011). Therefore, further investigation of the signaling pathways which regulate mycobacterial growth rate might reveal important information regarding the capacity of M. tuberculosis to adapt to its environment and in particular how this relates to drug tolerance and to the ability to establish infection.
In M. tuberculosis, Ser/Thr phosphorylation is essential for growth and environmental adaptation (as reviewed by Kieser and Rubin, 2014). For instance, the Ser/Thr protein kinases (STPKs) PknA and PknB are essential for growth of M. tuberculosis in culture, indicating that phosphorylation plays a pivotal role in the survival of this bacterium (Sassetti et al., 2003; Kang et al., 2005; Fernandez et al., 2006; Molle and Kremer, 2010; Kusebauch et al., 2014). These STPKs are encoded by an operon which regulates genes involved in cell shape determination, cell wall synthesis, and cell division (Deol et al., 2005; Kang et al., 2005; Kusebauch et al., 2014). In addition to PknA and PknB, another group of STPKs comprised of PknG, PknL, and PknF appear to be involved in different aspects of M. tuberculosis growth regulation (Cowley et al., 2004; Deol et al., 2005; Canova et al., 2008). In support of the likely important role played by STPKs in M. tuberculosis, a mass spectrometry-based phosphoproteomic study identified more than 500 Ser/Thr phosphorylation sites on 300 proteins in M. tuberculosis (Prisic et al., 2010) and, more recently, a complementary study detected a number of Tyr phosphorylated proteins in M. tuberculosis (Kusebauch et al., 2014). Notably though, the physiological significance of these findings remains largely unexplored.
In the past years, the use of mycobacterial models such as M. smegmatis and M. bovis BCG have significantly contributed to our current understanding of M. tuberculosis biology and environmental adaptation (as reviewed by Shiloh and Champion, 2010). M. bovis BCG is an attenuated bovine tuberculosis bacillus by a serial passage in the laboratory (Calmette et al., 1921). This mycobacterium is a particular convenient model in part due to it is slow growth rate similar to that observed in M. tuberculosis. On other hand M smegmatis is a fast growing mycobacterial species (with a doubling time of approximately 4 h) that has been widely used to investigate different aspects of mycobacterial physiology (Barry, 2001; Reyrat and Kahn, 2001; Danilchanka et al., 2008). As part of a concerted program to associate protein phosphorylation in mycobacteria with subsequent macromolecular events which determine growth rate and eventual environmental adaption, we have carried out a phosphopeptide enrichment and high throughput mass spectrometry-based study to investigate and compare the phosphoproteome of two model mycobacterial organisms—the fast growing M. smegmatis and the slow growing M. bovis BCG—our goal being to begin to elucidate the phosphorylation events and subsequent signal transduction pathways coordinating differential mycobacterial growth rates, which may in due course lead to important insights into M. tuberculosis physiology during active and latent infection—including aspects of slow growth associated drug resistance.
Materials and Methods
Bacterial Strains
Wild type strains of M. smegmatis mc2155 and M. bovis BCG Pasteur strain 1173P2 were grown in 7H9 Middlebrook (BD, Maryland, USA) broth supplemented with 0.05% Tween 80, OADC (Becton Dickinson) and 0.2% glycerol (v/v). Cells were grown at 37°C with continuous agitation (120 rpm).
Protein Extraction
Cells were harvested during the exponential phase (OD600 ~1.2 and 0.6 for M. smegmatis and M. bovis BCG, respectively) by centrifugation at 4000 g for 15 min at 4°C, washed twice with Phosphate Buffered Saline pH (7.5) (PBS). Cells were snap frozen in liquid nitrogen and stored at -80°C until needed. Frozen pellets were suspended in 800 μl lysis buffer [500 mM Tris-HCl, 0.1% (w/v) SDS, 0.15% sodium deoxycolate, 1× protease inhibitor cocktail, 1× phosphatase inhibitor cocktail (Roche, Mannheim Germany) and 50 μg/ml lysozyme (Repaske, 1956)]. Cells were disrupted by sonication at maximum power for six cycles of 30 s, with 1 min cooling on ice between cycles. Cellular debris was removed by centrifugation at 4000 g for 5 min and the lysate filtered through 20 μm pore size low-protein binding filters (Merck, NJ, USA). Proteins were precipitated using the chloroform-methanol precipitation method as previously described (Wessel and Flügge, 1984). The pellet was re-suspended in urea buffer (6 M urea, 2 M thiourea and 10 mM Tris-HCl, pH 8). Protein concentration was determined using a modified Bradford assay as described by Ramagli (1999).
In-Solution Trypsin Digestion
Five milligram of total protein was reduced with 1 mM DTT for 1 h with agitation and then alkylated with 5.5 mM IAA for 1 h in the dark. Proteins were pre-digested with Lysyl Endopeptidase LysC (Waco, Neuss, Germany) at room temperature for 3 h and diluted 4× with HPLC grade water before adding sequencing grade modified trypsin (Promega, Madison, USA) (1:100 w/w). Proteolysis was carried out at room temperature for 14 h with agitation at 30 rpm. Proteolysis was quenched by addition of trifluoroacetic acid (TFA) (Sigma Aldrich, St Louis, USA).
Phosphopeptide Enrichment with TiO2 Chromatography
Acetonitrile (ACN) (Sigma Aldrich, St Louis, USA) at a final concentration of 30% was added to the tryptic peptides and the pH was adjusted to 2. Ten milligram of Titasphere TiO2 beads (MZ Analysentechnik, Mainz, Germany) in loading buffer [30 mg/ml 2,5 dihydrobenzoic acid (Sigma Aldrich, St Louis, USA), 80% ACN] were added to the sample and incubated at room temperature with rotation for 1 h. The beads were pelleted and the decanted supernatant was further incubated with a fresh batch of 5 mg of beads for 30 min. This step was repeated one further time, giving three fractions of enriched phosphoproteins bound to beads in total. Phosphopeptides bound to the beads were washed vigorously with shaking for 10 min in 1 ml of wash buffer 1 (30% acetonitrile, 3% trifluoroacetic acid) followed by an additional 10 min of vigorous wash with wash buffer 2 (80% acetonitrile, 0.1% trifluoroacetic acid). Phosphopeptides were loaded onto C8 stage tip, washed with wash buffer 2 and then eluted with 3 × 50 μl Elution buffer [40% Mass-spec grade NH4OH (aq, 25% NH3; Sigma-Aldrich, St Louis, USA), 60% acetonitrile (pH 10.5 or higher)]. Solvent was removed in a speed drying vacuum at room temperature and peptides were resuspended in 20 μl Solvent A (2% ACN, 0.1% formic acid).
LC/MS/MS Analysis
Liquid chromatography separation was carried out using a precolumn (100 μm ID × 20 mm) connected to an analytical column (75 μm × 500 mm) packed with C18 Luna 5 μ 100 Å beads (phenomenex 04A-5452). The columns were connected to an Easy n-LC II system (Proxeon). 5 μl of resuspended phosphopeptides were loaded onto the column with starting mobile phase of 2% ACN, 0.1% formic acid. Peptides were eluted with the following gradient 5% ACN to 35% ACN for 120 min the up to 80% ACN over 5 min staying at 80% ACN for 10 min, this was followed by a column wash of 7 to 80% gradient for 30 min, and a column equilibration at 2% ACN for 2 min. The flow rate was held at 300 nL/min.
Mass spectra were acquired on an Orbitrap Q Exactive mass spectrometer in a data-dependent manner, with automatic switching between MS and MS/MS scans using a “Top-10” method. MS spectra were acquired at a resolution of 70,000 with a target value of 3 × 106 ions or a maximum injection time of 250 ms. Peptide fragmentation was performed via higher-energy collision dissociation (HCD) with the energy set at 25 NCE. Intensity threshold for ions selection was set at 1.7e4 with charge exclusion of z = 1 and z > 5. The MS/MS spectra were acquired at a resolution of 17,500, with a target value of 2 × 105 ions or a maximum injection time of 120 ms. The scan range was limited to 300 to 1750 m/z and the isolation window at 4.0 m/z.
Data Processing
Maxquant software package version 1.5.0.3 with integrated Andromeda search engine was used to analyse the raw MS spectra acquired. M. smegmatis (taxonomy 246196) and M. bovis BCG Pasteur (taxonomy 410289) databases obtained from Uniprot were used as the target-decoy databases for the Andromeda search. LysC and Trypsin were defined as proteases and two missed cleavages were allowed. Phosphorylation on serine, threonine, and tyrosine residues, oxidation of methionines and N-terminal acetylation were specified as variable modifications. Carbamidomethylation on cysteines was defined as a fixed modification. We allowed a FTMS MS/MS match tolerance of 20 ppm. False discovery rate was set at 1% for identification of peptides and proteins without re-quantifying. Stringent filtering criteria were employed to validate phospho sites. Phosphopeptides were considered as high confidence phosphorylation events and taken for further analysis only if they had a localization probability of >0.75, had a posterior error probability (PEP) score of <0.001. We further manually validated the phospho sites on the Maxquant viewer for good b- and y- ion series coverage.
Bioinformatics
Predicted Gene Ontology (GO) cellular functions of identified phosphoproteins with high confidence from two mycobacterium strains were obtained from The Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.7) and were grouped into functional categories. To compare the identified phosphoproteomes of these two mycobacterial species, we identified homologs of the M. smegmatis phosphorylated proteins in M. bovis BCG using the University of Cape Town's Computational Biology online bioinformatics tool found at http://galaxy-fen.uct.ac.za/root. For identification of tyrosine-phosphorylation site motifs, we used the Motif-X algorithm (Schwartz, 2005). We defined a sequence window of +/- 10 amino acids on each side of the tyrosine site and generated the sequence logo by Phosphosite logo generator using the algorithm PSP production (Cell signaling Technology). We aligned genomic sequences of both strains using the online tool, obtained from http://www.ebi.ac.uk/Tools/msa/clustalw2/ and respective fasta files were obtained from Uniprot.
Results and Discussion
There is increasing evidence indicating that protein phosphorylation plays an essential role during mycobacterial cell division and environmental adaptation. Understanding the mechanistic connection between such regulatory signaling networks and mycobacterial growth rate requires a global view of the phosphorylation events taking place at a given time under defined conditions. Here, we have carried out a comparative phosphoproteomic analysis of two mycobacterial strains—one fast growing (M. smegmatis), the other slow growing (M. bovis BCG). Our analysis included three rounds of TiO2 chromatography to enrich phosphorylated peptides (see Supplementary Figure S1), followed by subsequent analysis of phosphorylation-events using liquid chromatography coupled with state-of-the-art high resolution tandem mass spectrometry (LC/MS/MS) (Supplementary Figure S2a and S2b), in order to compare the phosphoproteomes of these two model mycobacterium species during exponential growth.
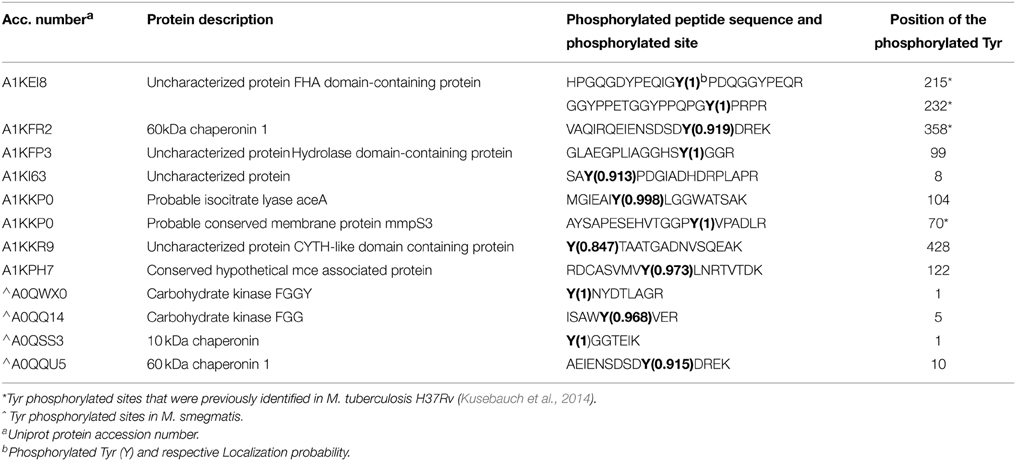
In this study we considered all phosphorylation-events that were detected in any of the biological replicates, and only confidently localized phospho-sites (p-sites) (Figure 1), were considered for qualitative comparative analysis and further discussion. In the two M. bovis BCG replicates, we detected a total of 442 p-sites of which 289 were confidently localized (Localization probability (LP) = 0.75; PEP = 0.01) and 169/289 had a LP = 0.99 (Supplementary Table S1A and Figure S2c). We identified 88,822 MS/MS spectra corresponding to 7784 non-redundant peptide sequences (Supplementary Figure S2d) and 1765 protein groups (402 were identified by a single peptide) (Supplementary Table S1A). The estimated false discovery rate (FDR) was 0.32 at the peptide level, 0.30 at the modification level, and 1.03 at the protein level. Our initial analysis of two biological replicates of M. smegmatis revealed considerable differences in the number of identified p-sites between the two Mycobacterial species, 77 p-sites for M. smegmatis (Supplementary Figure S2e) compared to 289 for M. bovis BCG. To verify that these differences observed were biological, we further analyzed three additional biological replicates for M. smegmatis (Supplementary Table S1B). Phosphoproteomic analysis of five biological replicates of M. smegmatis protein extracts resulted in identification of 180, 396 MS/MS spectra, corresponding to 16, 185 non-redundant peptide sequences (Supplementary Figure S2f) and 2, 462 protein groups (464 were identified by a single peptide) (Supplementary Table S1B). The estimated false discovery rate (FDR) was 0.22 at the peptide level, 0.21 at the modification level, and 0.98 at the protein group level. We detected a total of 185 phospho-sites in M. smegmatis, of which 106 were confidently localized (LP = 0.75; PEP = 0.01) and 64/106 had a LP = 0.99 (Supplementary Table S1B). In detail, for M. bovis BCG, we detected 289 p-sites on 203 phoshoproteins: 35.3% on serine (pSer), 61.6% on threonine (pThr) and 3.1% tyrosine (pTyr). For M. smegmatis we detected 106 p-sites on 76 phosphoproteins: 39.47% on serine (pSer), 57.02% on threonine (pThr) and 3.51% on tyrosine (pTyr). Both phosphoproteomes were biased toward Thr compared with Ser (57–61%; 41–35%), which agrees with previous reports on M. tuberculosis H37Rv (Prisic et al., 2010). Importantly, the percentage of Tyr phosphorylation in M. bovis BCG was closer to that reported in M. tuberculosis (Kusebauch et al., 2014). Although it had previously been suggested that Tyr phosphorylation was non-existent within mycobacterial species, it was recently confirmed that Tyr phosphorylation does in fact occur on a number of diverse M. tuberculosis proteins (Kusebauch et al., 2014). Here, we have confidently identified nine Tyr p-sites in eight proteins in M. bovis BCG (Table 1, Figure 1B and Supplementary Table S1A) and four in M. smegmatis, supporting earlier suggestions that phosphorylation on Tyr residues occur in different mycobacterial species (Kusebauch et al., 2014).
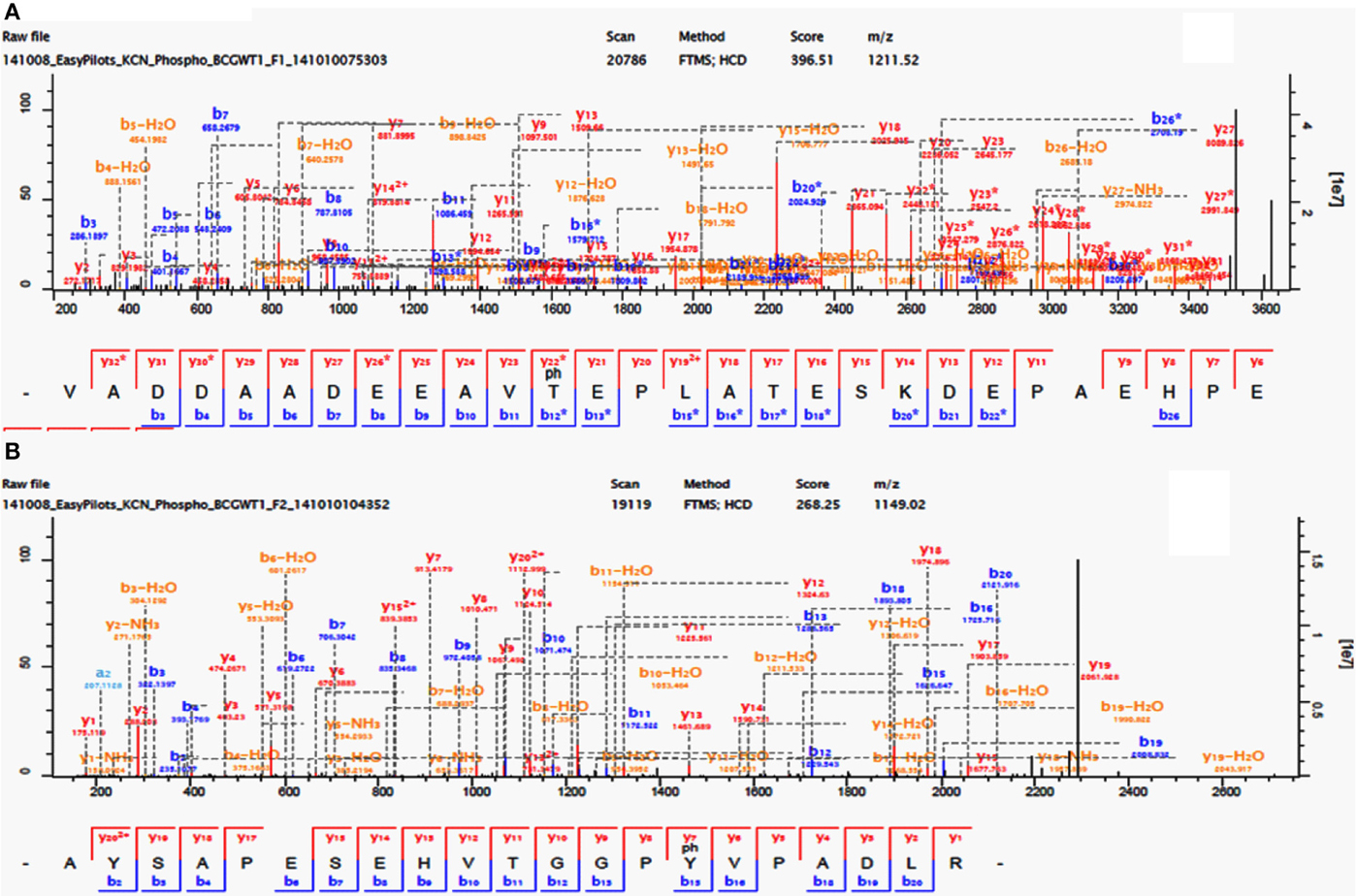
Figure 1. Phosphorylation of M. bovis BCG Cell division FtsQ (Thr24) and probable conserved protein membrane mmpS3 (Tyr70). (A) Identification of phosphorylated residue by mass spectrometry. Fragmentation spectra for modified peptide bearing the phosphorylated Thr24. (B) Fragmentation spectra for modified peptide bearing the phosphorylated Tyr70.
The differences between the compared phosphoproteomes compelled us to investigate whether some of these dissimilarities could be explained by genomic events rather than post-translational control. The multiple sequence alignment of 130 selected M. bovis BCG phosphoproteins with their respective M. smegmatis orthologs (Supplementary S3A) revealed that from 197 M. bovis BCG Ser/Thr/Tyr phosphorylated sites: 12 are conserved across the two mycobacterial species and were found to be phosphorylated in both species, while 94 conserved Ser/Thr/Tyr residues were found to be phosphorylated in M. bovis BCG only. Furthermore, whereas 91 M. bovis BCG phosphorylated residues were aligned with a different non-phosphorylated M. smegmatis residue, 72 of these were aligned with a non-phosphorylated amino acid (Supplementary S3A) and 19 were aligned with different non-phosphorylated Ser/Thr/Tyr residue. Conversely, the multiple sequence alignment of 64 M. smegmatis phosphoproteins with their respective M. bovis BCG orthologs showed that besides the 12 conserved Ser/Thr/Tyr residues phosphorylated in both species, 31 conserved residues were found to be phosphorylated in M. smegmatis only. In this case, while 43 M. smegmatis phosphorylated residues were aligned with a different non-phosphorylated M. bovis BCG amino-acid, 36 of these were aligned with a non-phosphorylated amino acid residue (Supplementary S3B) and seven were aligned with different non-phosphorylated Ser/Thr/Tyr residue (Supplementary S3B). These results point out that some of differences observed between the two phosphoproteomes can be explained by the absence of the corresponding amino acid residue, indicating that during exponential growth phase these two mycobacterial species present an inherently different sub-set of Ser/Thr/Tyr kinase substrates. Additionally, there are some interesting examples in which orthologous proteins were phosphorylated at different p-sites. This suggests that kinase specificities for a substrate could be intimately related with the actual site of phosphorylation. Finally, it is notable that occasions the Ser/Thr/Tyr residue was aligned with different Ser/Thr/Tyr residue (in most cases S for T and vice versa) in some punctual situation the respective residue was phosphorylated (e.g., PknB) but for the majority of the cases these were aligned with non-phosphorylated Ser/Thr/Tyr residue. This intriguing observation leaves open the possibility that Ser/Thr exchange could be a result of an evolutionary processes/environmental adaptation, in which the replacement for the respective residue would probably favor site phosphorylation and therefore the gain of an additional mechanism of protein functional regulation. Although speculative, it would be interesting to explore further in which conditions these sub set of Ser/Thr/Tyr sites are phosphoryalated.
Finally, the number of phosphoproteins/sites identified in M. smegmatis is comparable to those reported in other soil bacteria, e.g., E. coli (Macek et al., 2008; Soares et al., 2013), Bacillus subtilis (Shi et al., 2014) and Pseudomonas putida (Ravichandran et al., 2009), as well as in some pathogenic bacteria such as Pseudomonas aeruginosa (Ravichandran et al., 2009), Streptococcus pneumonia (Sun et al., 2009), Helicobacter pylori (Ge and Shan, 2011), and Klebsiella pneumonia (Lin et al., 2009). Whereas, the number of phosphorylated Ser/Thr/Tyr detected in M. bovis BCG phosphoproteome is comparable to that described in M. tuberculosis H37Rv (Prisic et al., 2010). It is of particular interest that the M. bovis BCG phosphoproteome shows a number of phosphoproteins/sites that are orthologous to those reported in M. tuberculosis H37Rv (Prisic et al., 2010) but also a number that are not conserved. Recently a comparison between the Ser/Thr/Tyr phosphoproteomes of Acinetobacter baumannii reference strain (ATCC17978) and a highly invasive, multidrug resistant clone (AbH12O-A2) demonstrated that, during stationary phase, the multidrug isolate showed twice as many phosphorylation-events as the reference strain (Soares et al., 2014). In contrast to reports on Pseudomonas species (Ravichandran et al., 2009), our current study supports the notion that bacteria within the same genus/species may utilize differing numbers of phosphoproteins.
Phosphoproteomic Analysis Reveals Conserved Ser/Thr/Tyr Phosphorylated Sites Across Mycobacterial Species
Conserved Ser/Thr Phosphorylated Sites
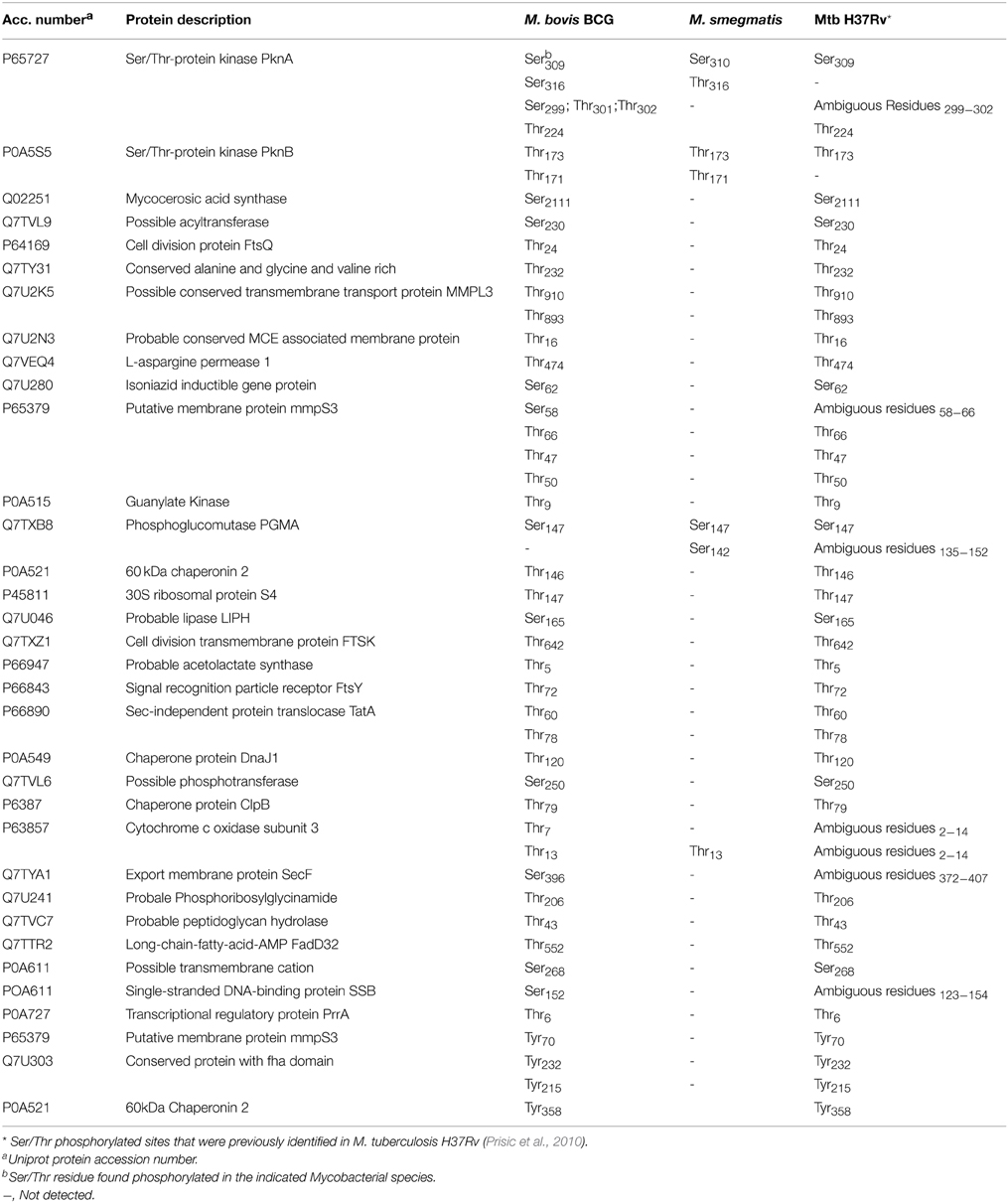
Macek et al. (2008) reported evidence of a possible high degree of conservation within potential bacterial phospho-sites although, as noted by those authors, the conservation of residues does not mean that they are phosphorylated in all species. In fact, as the number of bacterial phosphoproteomic studies increases, it is becoming clearer that the degree of conserved phospho-sites among bacterial species is rather limited and certainly lower than reported within eukaryotic phosphoproteomes (e.g., Freschi et al., 2014). Here, a comparison between the M. smegmatis, M. bovis BCG, and M. tuberculosis H37Rv (Prisic et al., 2010; Kusebauch et al., 2014) phosphoproteomes revealed that these three mycobacterial species share a number of conserved phosphorylated sites (Table 2). Interestingly, we found that M. bovis BCG and M. tuberculosis H37Rv phosphoproteomes share at least 32 Ser/Thr conserved phospho-sites on 27 proteins, of which three were conserved in all three species (Table 2). As pointed out by Freschi et al. (2014), phosphorylation sites that are phosphorylated in different species are more likely to be functional and this conservation criterion could be used to prioritize phosphorylation events for additional characterization.
In the present study we have focused in particular on the STPKs PknB and PknA that have known or predicted functions in cell wall generation and growth in M. smegmatis, M. bovis BCG, and M. tuberculosis (Gee et al., 2012; Kusebauch et al., 2014). We found PknB to be phosphorylated in Thr173 in all three species and in Thr171 in M. smegmatis and M. bovis BCG (Table 2). Previous in vitro assays demonstrated that both Thr173 and Thr171 are conserved auto-phosphorylated residues that lie in the activation loop of PknB (Boitel et al., 2003). Additionally, a M. tuberculosis double mutant Thr171/Thr173 was 300-fold less active than respective wild-type PnkB, suggesting a combined effect of both Thr171 and Thr173 residues on kinase activity. Subsequent studies confirmed that the mutation of these residues had a strong effect not only on PknB kinase activity but also in the process of activation loop-mediated recruitment of its substrates (Villarino et al., 2005). Here we have provided evidence that Thr173 and Thr171 phosphorylation both occur in vivo during the exponential phase, at which PknB is most abundant and is thought be at its maximum activity. Thus, our data reinforces a previous hypothesis suggesting that in vivo this enzyme is regulated through an auto-phosphorylation mechanism involving the phosphorylation state of both Thr173 and Thr171.
Another proposed mechanism of PknB regulation relates to the maintenance of an inactive state via the interaction of the juxtamembrane region with the kinase domain. In this model, the auto-phosphorylation of specific residues in the juxtamembrane sequences releases the inhibition by making the sequence available for further interactions with domains of target proteins (Wybenga-Groot et al., 2001). However, previously it was not clear whether Thr294 and/or Thr309 were the target residues involved so it is notable that our data clearly demonstrate that PknB of M. bovis BCG is in fact phosphorylated on Thr309.
Our analyses indicate that PknA is phosphorylated in at least one conserved residue, Ser309/Ser310 (see Table 2). Intriguingly, in this study M. bovis BCG PknA was found to be phosphorylated on seven different residues (three Ser and four Thr, respectively), all located in the juxtamembrane region. Unlike PknB, in PknA the juxtamembrane region, encompassing residue 269–338 is indispensable not only for auto-phosphorylation of PknA but also for its substrate phosphorylation ability (Thakur et al., 2008). STPks exhibit a wide variety of mechanisms for their regulation. Taking into account the degree of phosphorylation verified here in the juxtamembrane region of M. bovis BCG PknA compared to that observed in M. smegmatis PknA, it is tempting to speculate that this level of phosphorylation of the juxtamembrane region could be in fact limiting the access of PknA to its substrates and this way controlling the action of the enzyme. Importantly, as noted by Chawla et al. (2014), whilst the structure and mode of activation of PknB and PknA have been well established in vitro, the structure-function relationships of the various domains have yet to be investigated in the context of mycobacterial growth (Chawla et al., 2014). Here through a MS based phosphoproteomic approach we have established (at the phospho-site level) the phosphorylation state of different domains for both PknA and PknB in vivo during growth at exponential phase.
Conserved Tyr Phosphorylated Sites
In our study we have identified nine Tyr p-sites (see Table 1), of which four were also found to be phosphorylated in M. tuberculosis (Kusebauch et al., 2014): FHA-domain-containing protein (Tyr215 and Tyr232), 60 kDa chaperonin 1 (Tyr358) and conserved membrane protein mmpS3 (Tyr70) (Figure 1B). FHA-domain-containing protein is a substrate of numerous STPKs, including PknB. Phosphorylation of FHA by PnkB has implication in cell wall synthesis with a possible involvement in mycobacterial virulence (Gupta et al., 2009). Likewise both 60 kDa Chaperonin and conserved membrane protein mmpS3 have been implicated in mycobacterial virulence (Wells et al., 2013). Based on our data, we searched for possible conservation of these peptides across other bacterial species. A sequence motif derived from 60 kDa Chaperonin Tyr358 (RQEIENSDSDYDREKLQERLA) using Seq2Logo revealed an overrepresentation of Tyr358 (Figure 2). A conserved Tyr360 residue on apparently conserved peptide (SDSDYDREKL) was found in three Gram negative pathogenic species, specifically Shigella spp., Klebsiella spp., and Salmonella spp., suggesting that this conserved Tyr phosphorylation site warrants further investigation for possible roles in bacterial pathogenesis.
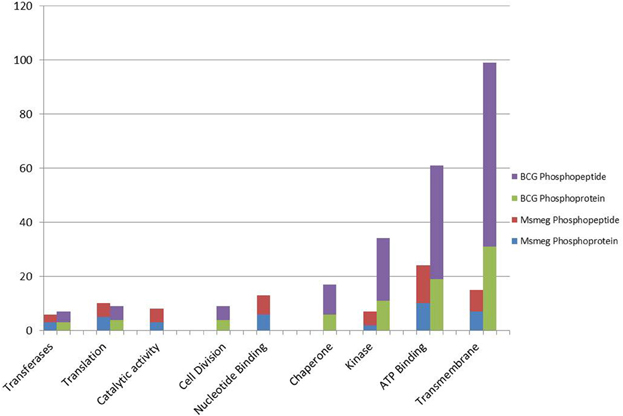
Figure 2. A histogram showing the GO molecular functions of identified phosphoproteins and respective number of phosphopeptides as predicted from their genome annotations.
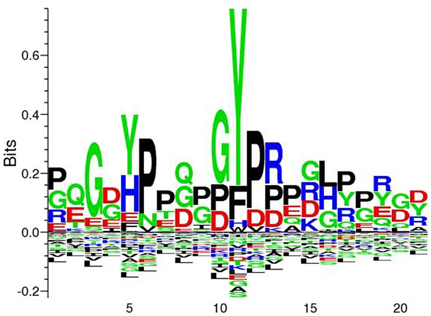
Figure 3. Seq2Logo alignment analysis derived from 60 kDa chaperonin revelead an overrepresentation of Tyr358. Seq2Logo analysis indicate that a conserved Tyr358-360 is found in additional three pathogenic species, specifically Shigella spp., Klebsiella spp., and Salmonella ssp.
Phosphoprotein Functional Enrichment
Gene ontology (GO) terms revealed that in both M. bovis BCG and M. smegmatis, the phosphorproteins/phosphosites were functionally enriched in nine distinct groups, (e.g., ATP binding, translation, kinase activity, cell division, see Figure 2). Of interest, a great deal of phosphorylated proteins in M. bovis BCG was clustered into the Transmembrane group (Figure 2). This included a considerable number of multiple phosphorylated proteins and some phosphorylated in internal as well as external regions, like BCG_3967, it is a probable trans-membrane protein and we found it to be phosphorylated four times, at position 10, which like on the flagellin domain and position 801 and 801, the kinase domain. This suggests that there are transmembrane proteins with a potential role in signal transduction. Additionally, it was visible that M bovis BCG phosphoproteome comprised a notable group of phosphoproteins involved in cell division, possible implications of this is discussed below.
Phosphorylation Events Observed in Proteins that Regulate Mechanisms of Growth and Cell Division
Both PknA and PknB are encoded by genes (pknA and pknB, respectively) located on the same operon as protein phosphatase PstP, RodA (implicated in cell shape control) and PbpA (implicated in peptidoglycan synthesis) (Cole et al., 1998). This locus includes also two FHA (forkhead-associated) domain-containing proteins and in mycobacteria is found near the origin of replication. In M. bovis BCG, all proteins referred to above (except PbpA) were found phosphorylated at a total of 15 p-sites: PknA (7); PknB (3); RodA (1); PstP (1) and FHA domain containing protein (3) (see Supplementary Table S1A). In M. smegmatis, however, only a few proteins were found phosphorylated at a total of 5 p-sites: PknA (2); PknB (2) FHA domain containing protein (1) (see Supplementary Table S1B). These observations suggest that the slow growth of M. bovis BCG preserves this central set of cell division proteins under a tight regulatory network in which key elements are intimately inter-related by an important series of functional (de)phosphorylation events. For example, PknA and PknB are regulated by PstP-mediated phosphorylation (Boitel et al., 2003; Chopra et al., 2003); additionally, recently it has been shown that both PknA and PknB phosphorylate PstP (Sajid et al., 2011). As discussed above, our results showed that both M. smegmatis and M. bovis BCG PknB has conserved phosphorylation on Thr171 and Thr173—both of which sites are known to be substrates for PstP—thus suggesting that in both cases PstP is at least partially inactive. This would make sense considering that during exponential phase PknA and PknB are likely to be at their peak of activity. PstP has been reported to be phosphorylated by PknB on Thr173, Thr141, Thr290, and Thr137 in its cytosolic domain and on Thr174 by PknA (Sajid et al., 2011). Curiously phosphorylated PstP has been reported to be more active than its unphosphorylated form (Sajid et al., 2011). Here, our results indicate that PstP of M. bovis BCG is phosphorylated in vivo on the high confidence p-site, Ser155 (see Supplementary Table S1A). Interestingly, PstP contains three metal-binding centers in its structure (Pullen et al., 2004), sharing the fold and two-metal center of human PP2Cα whilst having a third Mn2+ in a site created by a large shift in a flap domain next to the active site; this Mn2+ occurs at the position of Ser160 so it is plausible that phosphorylation of Ser155 may directly interfere with PstP activity, thus accounting for our deduction here of reduced PstP activity during exponential phase growth.
Overall, M. bovis BCG has 3 times more STPks and nearly 4 times respective p-sites compared to M. smegmatis. Apart from PknA and PknB, the M. bovis BCG phosphoproteome is comprised of PknG (Thr95), PknH (Thr174), PknE (Ser304 and Ser326) PknF (Thr287). Some of these enzymes have previously been directly implicated in mycobacterial growth [e.g., PknG (Fiuza et al., 2008), PknH (Zheng et al., 2007), PknE and PknF (Gupta et al., 2014)] and it is therefore conceivable that some of the proteins comprising the M. bovis BCG phosphoproteome are in fact substrate of some of these phosphorylated protein kinases (vide infra). It is worth noting that our study has also identified several Two Component sensory signal transduction proteins as phosphoproteins (e.g., Two component sensory transduction protein regX3 (Thr151 and Thr153), Two component sensor histidine kinase ppr (Ser446), Two component transcriptional regulatory pprA (Ser20). These results are reminiscient of those previously described in B. subtilis (Jers et al., 2011) and suggest that in M bovis BCG there may be cross talk between Ser/Thr/Tyr phosphorylation and Two component systems, which would add extra complexity to the overall protein phosphorylation signal transduction pathways regulating exponential growth of M. bovis BCG cells.
Phosphorylation Events Observed in Proteins that Regulates Mechanisms of Cell Elongation and Division
In mycobacteria, cell elongation is regulated by a macrocomplex that regulates peptidoglycan remodeling during growth by means of hydrolytic and synthetic roles (as reviewed by (Kieser and Rubin, 2014)). Our data indicate that in M. bovis BCG, three important proteins of the macromolecular elongation complex are phosphorylated during exponential growth, namely Wag31 (Ser245), CwsA (Thr77) and a putative hydrolase (BCG_0021 involved in peptidoglycan catabolic process) (Thr43). In mycobacteria, Wag31 is phosphorylated by PknA and is essential for correct polar localization and biosynthesis (Jani et al., 2010; Lee et al., 2014); in addition, Wag31 is stabilized by the cell wall protein CwsA. Wag31 is thought to be phosphorylated during exponential phase and remains non- or lowly-phosphorylated during stationary phase (Kang et al., 2005; Park et al., 2008). Interestingly, Wag31, CwsA and the putative peptidoglycan hydrolase were not found amongst the M. smegmatis phosphorylated proteins in the present study. Whilst we cannot rule out that our assay did not isolate phosphorylated M. smegmatis Wag31, it is perhaps more likely that in the fast growing M. smegmatis the elongation complex is regulated by alternative non-phosphorylated mechanism.
Another macromolecular complex, named divisome, is responsible for mycobacterial cell division. Assembly and disassembly of this complex is regulated by protein phosphorylation (Kieser and Rubin, 2014). According to the our data, in M. bovis BCG there are five divisome proteins which are phosphorylated, including cell division FtsQ (Thr24), FtsW-like protein (Thr29), CwsA (Thr77) as well as other additional phosphorylated cell division proteins such as RodA (Thr463), cell division transmembrane protein FtsK (Thr325; Thr642) and FtsY (Thr72), strongly suggesting that divisome assembly and indeed cell division in M. bovis BCG is subject to a high level of phosphorylation.
Of interest, in our analysis we have detected Hup, a conserved histidine-like protein, phosphorylated on three different p-sites (Thr45, Thr65, and Ser90). In Mycobacterium sp., the homolog of HU (Mhpl) is implicated in bacterial adaptation to stress response conditions, possible inhibition of cellular metabolism and reduction of bacterial growth rate through nucleoid reorganization (Lee et al., 1998; Matsumoto et al., 1999; Katsube et al., 2007). Apparently, is expressed in exponentially growing cells of M. tuberculosis H37Ra and it is shown to be maximally expressed during stationary phase, while Hup kinases (PknE, PknF, and PknB) were found to be constitutively expressed during exponential phase (Gupta et al., 2014). It has been suggested that the phosphorylation of HupB during the exponential phase by the referred kinases would limit the interaction with DNA (Gupta et al., 2014). In our M. bovis BCG data we have identified all the intervenient proteins involved in the described posttranslational regulation mechanism, including the phosphorylation of phosphosite Hup Thr65. It is therefore appropriate to assume that the same mechanism takes place in M. bovis BCG cells during exponential growth, and although under limited action it remains possible that the rate of unphosphorylated HupB would have an impact on the overall growth rate. In contrast, we did not find any evidence to indicate that similar mechanisms operate in M. smegmatis cells during exponential growth.
Stress Related Proteins
In rich broth during exponential phase, bacteria experience nearly optimal conditions of growth where there is excess nutrients and little accumulation of by products, in addition to scarce competition between bacterial cells. Surprisingly, under these conditions we observed an unexpected number of chaperones and stress related proteins in the M. bovis BCG phosphoproteome: For instance hyperosmotic and heat shock related proteins such as the chaperon protein DnaJ (Thr120) chaperon protein DnaK (Ser558) and GrpE (Ser12 and Thr2), multiply phosphorylated 60 kDa chaperonin, 10 kDa chaperonin, and Copper-sensing transcriptional repressor CsoR (Thr93). On the other hand none of these stress related proteins were found in the M. smegmatis phosphoproteome, which suggests that even under optimal environmental conditions, slow growing mycobacteria such as M. bovis BCG maintain a preventive basal level of stress-related proteins that may act as frontline defense barrier to ensure adequate and prompt response to any sudden change in local environmental conditions. In this scenario protein phosphorylation would keep most of these proteins in an inactive state, whereby dephosphorylation could then immediately recruit these proteins when environmental conditions become unfavorable. A convenient and versatile regulatory mechanism such as this could in fact be a determinant for the survival and persistence of some bacteria.
Conclusion and Prespectives
This study clearly demonstrated that there are major differences between a fast growing and a slow growing mycobacterial phosphoproteome. The M. smegmatis phosphoproteome observed here is in many aspects similar to those reported in other soil-dwelling bacterial models and can be viewed as a minimalist phosphoproteome compared to that of M. bovis BCG. This latter organism presents a much more complex and sophisticated protein phosphorylation network, regulating important cellular cycle events such as cell wall biosynthesis, elongation, and cell division, as well as apparently being involved in regulating response to stress, which over all would allow a quick cellular response to abrupt environmental changes. However, this regulatory advantage might be associated with a cost, reflected by reduced metabolic fitness and slower growth rate.
This study demonstrates M. bovis BCG is a good model to study aspects of mycobacterial phospho-dependent signal transduction pathways, including those involved in persistence and slow growth, including that associated with drug resistance. By contrast, the substantial differences reported here in the phosphoproteomes of M. smegmatis and M. bovis BCG suggest that exponentially growing M. smegmatis cells in vitro are of limited relevance when modeling phosphorylation networks and phospho-regulation events likely to occur in M. tuberculosis at the site of disease during an infection.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the National Research Foundation (NRF) for their financial support of this research and for a PhD bursary to KCN. NCS and JMB thank the NRF for the South African Research Incentive Funding for Rated Researchers and the Research Chair grants respectively. SG thanks the CSIR for a PhD bursary.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2015.00237/abstract
Supplementary Figure S1. Phosphoproteomic workflow diagram. Mycobacterial strains were harvested at exponential phase, lysed. In-solution digestion was then carried out TiO2 phosphor enrichment and data acquisition with LC/MS/MS.
Supplementary Figure S2. (a–f). Total Ion Chromatograms (TIC) and MS summary peptide/phosphopeptides identified in each experiment.
Supplementary S3A. Multiple sequence alignment of M.bovis BCG phosphoproteins with their respective M. smegmatis.
Supplementary S3B. Multiple sequence alignment of M. smegmatis phosphoproteins with their respective M.bovis BCG.
Supplementary Table S1A. Spreadsheet of all the identified protein groups, phosphopeptides and phosphoproteins from M. bovis BCG.
Supplementary Table S1B. Spreadsheet of all the identified protein groups, phosphopeptides and phosphoproteins from M. smegmatis.
References
Baek, S.-H., Li, A. H., and Sassetti, C. M. (2011). Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 9:e1001065. doi: 10.1371/journal.pbio.1001065
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barry, C. E. I. (2001). Mycobacterium smegmatis: an absurd model for tuberculosis? Response from Barry III. Trends Microbiol. 9, 473–474. doi: 10.1016/S0966-842X(01)02169-2
Boitel, B., Ortiz-Lombardía, M., Durán, R., Pompeo, F., Cole, S. T., Cerveñansky, C., et al. (2003). PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis. Mol. Microbiol. 49, 1493–1508. doi: 10.1046/j.1365-2958.2003.03657.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Calmette, A., Guérin, G., Nègre, L., and Boquet, A. (1921). Prémunition des nouveaux-nés contre la tuberculose par le vaccin BCG. Ann. Inst. Pasteur. 40, 89–133.
Canova, M. J., Veyron-Churlet, R., Zanella-Cleon, I., Cohen-Gonsaud, M., Cozzone, A. J., Becchi, M., et al. (2008). The Mycobacterium tuberculosis serine/threonine kinase PknL phosphorylates Rv2175c: mass spectrometric profiling of the activation loop phosphorylation sites and their role in the recruitment of Rv2175c. Proteomics 8, 521–533. doi: 10.1002/pmic.200700442
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chawla, Y., Upadhyay, S. K., Khan, S., Nagarajan, S. N., Forti, F., and Nandicoori, V. K. (2014). Protein Kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J. Biol. Chem. 289, 13858–13875. doi: 10.1074/jbc.M114.563536
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chopra, P., Singh, B., Singh, R., Vohra, R., Koul, A., Meena, L. S., et al. (2003). Phosphoprotein phosphatase of Mycobacterium tuberculosis dephosphorylates serine–threonine kinases PknA and PknB. Biochem. Biophys. Res. Commun. 311, 112–120. doi: 10.1016/j.bbrc.2003.09.173
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544. doi: 10.1038/31159.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corper, H. J., and Cohn, M. L. (1933). The viability and virulence of old cultures of tubercle bacilli: studies on twelve year broth cultures maintained at incubator temperatures. Am. Rev. Tuberc. 28, 856–874.
Cowley, S., Ko, M., Pick, N., Chow, R., Downing, K. J., Gordhan, B. G., et al. (2004). The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 52, 1691–1702. doi: 10.1111/j.1365-2958.2004.04085.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Danilchanka, O., Pavlenok, M., and Niederweis, M. (2008). Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob. Agents Chemother. 52, 3127–3134. doi: 10.1128/AAC.00239-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deol, P., Vohra, R., Saini, A. K., Singh, A., Chandra, H., Chopra, P., et al. (2005). Role of Mycobacterium tuberculosis Ser/Thr kinase PknF: implications in glucose transport and cell division. J. Bacteriol. 187, 3415–3420. doi: 10.1128/JB.187.10.3415-3420.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fernandez, P., Saint-Joanis, B., Barilone, N., Jackson, M., Gicquel, B., Cole, S. T., et al. (2006). The Ser/Thr protein kinase PknB is essential for sustaining mycobacterial growth. J. Bacteriol. 188, 7778–7784. doi: 10.1128/JB.00963-06
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fiuza, M., Canova, M. J., Zanella-Cléon, I., Becchi, M., Cozzone, A. J., Mateos, L. M., et al. (2008). From the characterization of the four serine/threonine protein kinases (PknA/B/G/L) of Corynebacterium glutamicum toward the role of PknA and PknB in cell division. J. Biol. Chem. 283, 18099–18112. doi: 10.1074/jbc.M802615200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Freschi, L., Osseni, M., and Landry, C. R. (2014). Functional divergence and evolutionary turnover in mammalian phosphoproteomes. PLoS Genet. 10:e1004062. doi: 10.1371/journal.pgen.1004062
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ge, R., and Shan, W. (2011). Bacterial phosphoproteomic analysis reveals the correlation between protein phosphorylation and bacterial pathogenicity. Genomics Proteomics Bioinformatics 9, 119–127. doi: 10.1016/S1672-0229(11)60015-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gee, C. L., Papavinasasundaram, K. G., Blair, S. R., Baer, C. E., Falick, A. M., King, D. S., et al. (2012). A Phosphorylated Pseudokinase Complex Controls Cell Wall Synthesis in Mycobacteria. Sci. Signal. 5, ra7. doi: 10.1126/scisignal.2002525
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, M., Sajid, A., Arora, G., Tandon, V., and Singh, Y. (2009). Forkhead-associated domain-containing protein Rv0019c and polyketide-associated protein PapA5, from substrates of serine/threonine protein kinase PknB to interacting proteins of Mycobacterium tuberculosis. J. Biol. Chem. 284, 34723–34734. doi: 10.1074/jbc.M109.058834
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, M., Sajid, A., Sharma, K., Ghosh, S., Arora, G., Singh, R., et al. (2014). HupB, a nucleoid-associated protein of Mycobacterium tuberculosis, is modified by serine/threonine protein kinases in vivo. J. Bacteriol. 196, 2646–2657. doi: 10.1128/JB.01625-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jani, C., Eoh, H., Lee, J. J., Hamasha, K., Sahana, M. B., Han, J.-S., et al. (2010). Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol. 10:327. doi: 10.1186/1471-2180-10-327
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jers, C., Kobir, A., Søndergaard, E. O., Jensen, P. R., and Mijakovic, I. (2011). Bacillus subtilis two-component system sensory kinase DegS is regulated by serine phosphorylation in its input domain. PLoS ONE 6:e14653. doi: 10.1371/journal.pone.0014653
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kang, C. M., Abbott, D. W., Park, S. T., Dascher, C. C., Cantley, L. C., and Husson, R. N. (2005). The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–704. doi: 10.1101/gad.1311105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Katsube, T., Matsumoto, S., Takatsuka, M., Okuyama, M., Ozeki, Y., Naito, M., et al. (2007). Control of Cell Wall Assembly by a Histone-Like Protein in Mycobacteria. J. Bacteriol. 189, 8241–8249. doi: 10.1128/JB.00550-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kieser, K. J., and Rubin, E. J. (2014). How sisters grow apart: mycobacterial growth and division. Nat. Rev. Micro. 12, 550–562. doi: 10.1038/nrmicro3299
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kusebauch, U., Ortega, C., Ollodart, A., Rogers, R. S., Sherman, D. R., Moritz, R. L., et al. (2014). Mycobacterium tuberculosis supports protein tyrosine phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 111, 9265–9270. doi: 10.1073/pnas.1323894111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, B. H., Murugasu-Oei, B., and Dick, T. (1998). Upregulation of a histone-like protein in dormant Mycobacterium smegmatis. Mol. Gen. Genet. MGG 260, 475–479. doi: 10.1007/s004380050919
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, J. J., Kan, C. M., Lee, J. H., Park, K. S., Jeon, J. H., and Lee, S. H. (2014). Phosphorylation-dependent interaction between a serine/threonine kinase PknA and a putative cell division protein Wag31 in Mycobacterium tuberculosis. New Microbiol. 37, 525–533.
Lin, M.-H., Hsu, T.-L., Lin, S.-Y., Pan, Y.-J., Jan, J.-T., Wang, J.-T., et al. (2009). Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol. Cell. Proteomics 8, 2613–2623. doi: 10.1074/mcp.M900276-MCP200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Macek, B., Gnad, F., Soufi, B., Kumar, C., Olsen, J. V., Mijakovic, I., et al. (2008). Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol. Cell. Proteomics 7, 299–307. doi: 10.1074/mcp.M700311-MCP200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marrero, J., Rhee, K. Y., Schnappinger, D., Pethe, K., and Ehrt, S. (2010). Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. U.S.A. 107, 9819–9824. doi: 10.1073/pnas.1000715107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Matsumoto, S., Yukitake, H., Furugen, M., Matsuo, T., Mineta, T., and Yamada, T. (1999). Identification of a Novel DNA-Binding Protein from Mycobacterium bovis Bacillus Calmette-Guérin. Microbiol. Immunol. 43, 1027–1036. doi: 10.1111/j.1348-0421.1999.tb01232.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McKinney, J. D., zu Bentrup, K. H., Munoz-Elias, E. J., Miczak, A., Chen, B., Chan, W.-T., et al. (2000). Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738. doi: 10.1038/35021074
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Molle, V., and Kremer, L. (2010). Division and cell envelope regulation by Ser/Thr phosphorylation: mycobacterium shows the way. Mol. Microbiol. 75, 1064–1077. doi: 10.1111/j.1365-2958.2009.07041.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Park, S. T., Kang, C.-M., and Husson, R. N. (2008). Regulation of the SigH stress response regulon by an essential protein kinase in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 105, 13105–13110. doi: 10.1073/pnas.0801143105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prisic, S., Dankwa, S., Schwartz, D., Chou, M. F., Locasale, J. W., Kang, C.-M., et al. (2010). Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc. Natl. Acad. Sci. U.S.A. 107, 7521–7526. doi: 10.1073/pnas.0913482107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pullen, K. E., Ng, H.-L., Sung, P.-Y., Good, M. C., Smith, S. M., and Alber, T. (2004). An alternate conformation and a third metal in PstP/Ppp, the M. tuberculosis PP2C-Family Ser/Thr protein phosphatase. Structure 12, 1947–54. doi: 10.1016/j.str.2004.09.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ramagli, L. S. (1999). Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol. Biol. 112, 99–103. doi: 10.1385/1-59259-584-7:99
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ravichandran, A., Sugiyama, N., Tomita, M., Swarup, S., and Ishihama, Y. (2009). Ser/Thr/Tyr phosphoproteome analysis of pathogenic and non-pathogenic Pseudomonas species. Proteomics 9, 2764–2775. doi: 10.1002/pmic.200800655
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Repaske, R. (1956). Lysis of gram-negative bacteria by lysozyme. Biochim. Biophys. Acta 22, 189–191. doi: 10.1016/0006-3002(56)90240-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reyrat, J. M., and Kahn, D. (2001). Mycobacterium smegmatis: an absurd model for tuberculosis? Trends Microbiol. 9, 472–473. doi: 10.1016/S0966-842X(01)02168-0
Sajid, A., Arora, G., Gupta, M., Upadhyay, S., Nandicoori, V. K., and Singh, Y. (2011). Phosphorylation of Mycobacterium tuberculosis Ser/Thr phosphatase by PknA and PknB. PLoS ONE 6:e17871. doi: 10.1371/journal.pone.0017871
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sassetti, C. M., Boyd, D. H., and Rubin, E. J. (2003). Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84. doi: 10.1046/j.1365-2958.2003.03425.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwartz, D. G. S. (2005). An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat. Biotechnol. 23, 1391–1398. doi: 10.1038/nbt1146
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shi, L., Pigeonneau, N., Ravikumar, V., Dobrinic, P., Macek, B., Franjevic, D., et al. (2014). Cross-phosphorylation of bacterial serine/threonine and tyrosine protein kinases on key regulatory residues. Front. Microbiol. 5:495. doi: 10.3389/fmicb.2014.00495
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shiloh, M. U., and Champion, P. A. (2010). To catch a killer. what can mycobacterial model teach us about Mycobacterium tuberculosis pathogenesis? Curr. Opin. Microbiol. 13, 86–92. doi: 10.1016/j.mib.2009.11.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Soares, N. C., Spät, P., Krug, K., and Macek, B. (2013). Global dynamics of the Escherichia coli proteome and phosphoproteome during growth in minimal medium. J. Proteome Res. 12, 2611–2621. doi: 10.1021/pr3011843
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Soares, N. C., Spät, P., Méndez, J. A., Nakedi, K., Aranda, J., and Bou, G. (2014). Ser/Thr/Tyr phosphoproteome characterization of Acinetobacter baumannii: comparison between a reference strain and a highly invasive multidrug-resistant clinical isolate. J. Proteomics 102, 113–124. doi: 10.1016/j.jprot.2014.03.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, X., Ge, F., Xiao, C.-L., Yin, X.-F., Ge, R., Zhang, L.-H., et al. (2009). Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. J. Proteome Res. 9, 275–282. doi: 10.1021/pr900612v
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thakur, M., Chaba, R., Mondal, A. K., and Chakraborti, P. K. (2008). Interdomain interaction reconstitutes the functionality of PknA, a eukaryotic type Ser/Thr kinase from Mycobacterium tuberculosis. J. Biol. Chem. 283, 8023–8033. doi: 10.1074/jbc.M707535200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Villarino, A., Duran, R., Wehenkel, A., Fernandez, P., England, P., Brodin, P., et al. (2005). Proteomic identification of M. tuberculosis protein kinase substrates: PknB recruits GarA, a FHA domain-containing protein, through activation loop-mediated interactions. J. Mol. Biol. 350, 953–963. doi: 10.1016/j.jmb.2005.05.049
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wayne, L. G., and Hayes, L. G. (1996). An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64, 2062–2069.
Wells, R. M., Jones, C. M., Xi, Z., Speer, A., Danilchanka, O., Doornbos, K. S., et al. (2013). Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog. 9:e1003120. doi: 10.1371/journal.ppat.1003120
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wessel, D., and Flügge, U. I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143. doi: 10.1016/0003-2697(84)90782-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wybenga-Groot, L. E., Baskin, B., Ong, S. H., Tong, J., Pawson, T., and Sicheri, F. (2001). Structural basis for autoinhibition of the EphB2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745–757. doi: 10.1016/S0092-8674(01)00496-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zheng, X., Papavinasasundaram, K. G., and Av-Gay, Y. (2007). Novel substrates of Mycobacterium tuberculosis PknH Ser/Thr kinase. Biochem. Biophys. Res. Commun. 355, 162–168. doi: 10.1016/j.bbrc.2007.01.122
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: mycobacteria, phosphoproteome, protein phosphorylation, cell division, growth rate
Citation: Nakedi KC, Nel AJM, Garnett S, Blackburn JM and Soares NC (2015) Comparative Ser/Thr/Tyr phosphoproteomics between two mycobacterial species: the fast growing Mycobacterium smegmatis and the slow growing Mycobacterium bovis BCG. Front. Microbiol. 6:237. doi: 10.3389/fmicb.2015.00237
Received: 30 November 2014; Accepted: 10 March 2015;
Published: 08 April 2015.
Edited by:
Ivan Mijakovic, Chalmers University of Technology, SwedenReviewed by:
Chung-Dar Lu, Georgia State University, USAGustavo Antonio De Souza, University of Oslo, Norway
Copyright © 2015 Nakedi, Nel, Garnett, Blackburn and Soares. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan M. Blackburn and Nelson C. Soares, Applied and Chemical Proteomics Group, Division of Medical Biochemistry and Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Anzio, Observatory, Cape Town 7925, South Africa jonathan.blackburn@uct.ac.za; nelson.dacruzsoares@uct.ac.za
 Kehilwe C. Nakedi
Kehilwe C. Nakedi Andrew J. M. Nel
Andrew J. M. Nel Shaun Garnett
Shaun Garnett Jonathan M. Blackburn
Jonathan M. Blackburn Nelson C. Soares
Nelson C. Soares