- 1Division of Infectious Diseases, University Medicine Cluster, National University Health System, Singapore, Singapore
- 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
A major global concern is the emergence and spread of systemic life-threatening fungal infections in critically ill patients. The increase in invasive fungal infections, caused most commonly by Candida and Aspergillus species, occurs in patients with impaired defenses due to a number of reasons such as underlying disease, the use of chemotherapeutic and immunosuppressive agents, broad-spectrum antibiotics, prosthetic devices and grafts, burns, neutropenia and HIV infection. The high morbidity and mortality associated with these infections is compounded by the limited therapeutic options and the emergence of drug resistant fungi. Hence, creative approaches to bridge the significant gap in antifungal drug development needs to be explored. Here, we review the potential anti-fungal targets for patient-centered therapies and immune-enhancing strategies for the prevention and treatment of invasive fungal diseases.
Introduction
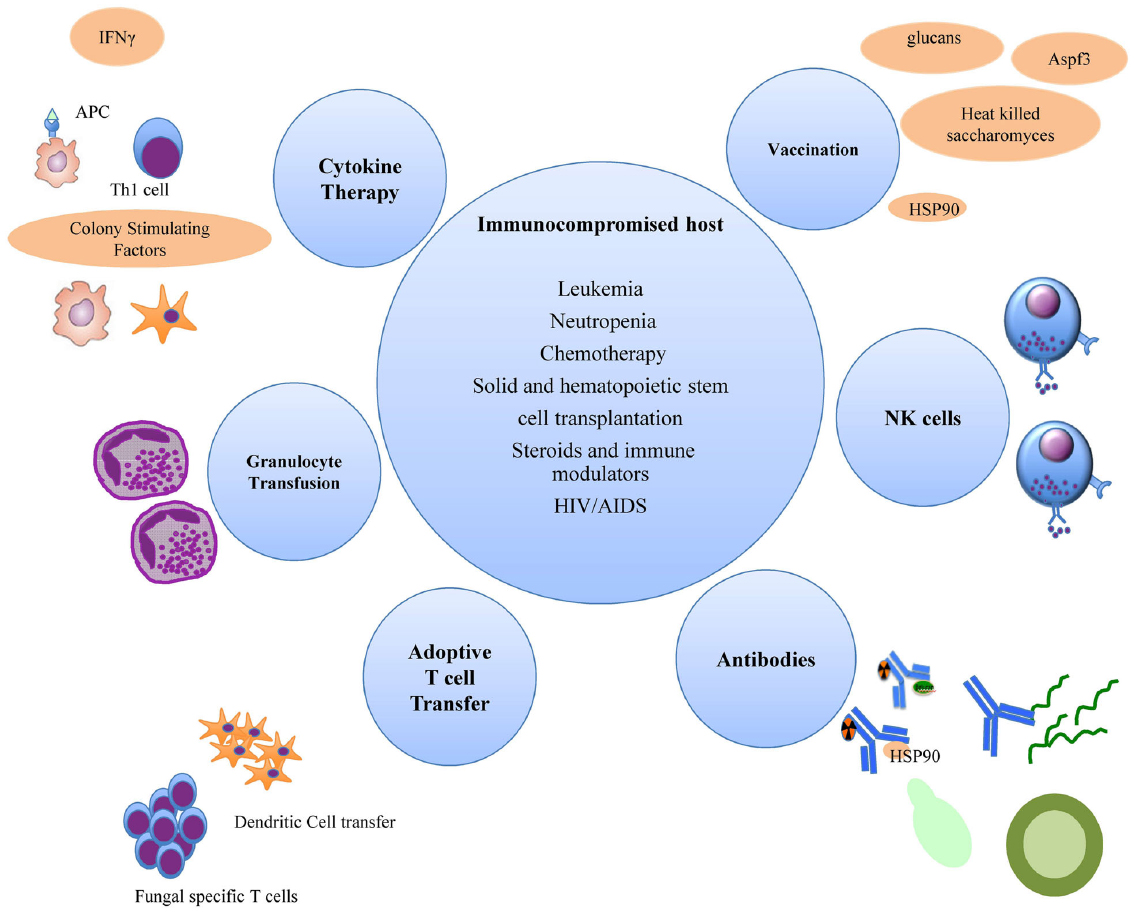
From among more than a million species of fungi present in nature, only a few 100 of them are capable of causing infections in humans (O’Brien et al., 2005). Of these, only a handful can cause diseases in healthy people, which is mostly superficial in nature (Kohler et al., 2015; LIFE at www.life-worldwide.org). Invasive fungal diseases (IFD) usually occur in susceptible individuals who are immunocompromised due to serious illnesses such as leukemia, neutropenia, AIDS, etc. In addition, medical advances have created vulnerable populations such as patients undergoing chemotherapy, solid and hematopoietic stem cell transplantation (HSCT), complex surgeries, immunosuppressive therapies for auto-immune and auto-inflammatory diseases, antibiotic therapies and treatment in intensive care units.
The major fungi responsible for these invasive infections, which kill about one and a half million people every year, are Candida, Aspergillus, and Zygomycetes species. Invasive candidiasis is the fourth and sixth most common nosocomial infection in US and Europe respectively with a high mortality rate ranging from 36 to 63% (Wisplinghoff et al., 2004; Brown et al., 2012; Rodrigues et al., 2014). The risk factors for candidemia include prior antibiotic usage, abdominal surgery, Candida colonization, central lines and parenteral nutrition (Wey et al., 1989; Blumberg et al., 2001) Aspergillus is a ubiquitous filamentous saprophytic mold whose conidia are dispersed in the air. Like the Zygomycetes, these molds cause several invasive diseases in hosts with markedly suppressed immunity and have a mortality rate in excess of 50–60% despite treatment (Herbrecht et al., 2002; Neofytos et al., 2009).
Such is the concern of the impact of IFD in immuno-compromised patients. This is despite the ever wider availability of anti-microbials beyond the conventional amphotericin-B based preparations and in the recent decade especially, the newer generation and classes of anti-fungals like voriconazole, posaconazole, and isavuconazole (of the azole family) and the echinocandins (caspofungin, anidulafungin, and micafungin). Some of the reasons for the high mortality are the difficulties in the early and correct diagnosis of invasive fungal infections (de Pauw and Picazo, 2008; Erjavec et al., 2009) as well as drug resistance profiles among specific fungal pathogens (Perlin, 2007; Verweij et al., 2007; Xie et al., 2014). The main reason for the poor outcomes from invasive disease nonetheless, is the incapacity of the patient’s compromised immune system to respond appropriately to the invading pathogen despite the presence of antimicrobials.
The response to such a challenge faced by the clinician at the bedside has led to exploration of novel therapeutic modalities beyond conventional antimicrobials; specifically, the manipulation and augmentation of the host immune response in the face of IFD. Through understanding how the immune system can detect the fungi, immunotherapeutic strategies may be formulated as adjuncts in the management of IFD.
Immune Recognition and Response by the Host
The susceptibility and outcome of fungal infections depend on two main factors: the pathogen and the host. Pathogen factors may include the dose of the infecting fungi and its virulence. The efficacy of the immune response and the degree of the immune suppression in the patient are the major host determinants. The host defense capacity to fungal infection range from the protective mechanisms provided by skin, mucosa and innate immunity to the humoral response and adaptive immunity (Mueller-Loebnitz et al., 2013). The innate immune system despite its lack of specificity has been considered to bear significant importance in the defense mechanism against fungi. Monocytes, macrophages, neutrophils, and natural killer (NK) cells effect anti-fungal capabilities through phagocytosis, and directed pathogen killing. The fungal cell wall is the first structure encountered by host cells. Fungal cell wall is made up of various polysaccharides that have immune activating and modulatory properties. These pathogen associated molecular patterns (PAMPs); such as alpha and beta glucans, chitins, mannans, β- 1, 2-oligomannosides and galactomannan of varying constitutions in the cell wall of various fungi allow recognition by the innate immune cells; mainly monocytes, macrophages, dendritic cells (DCs) and endothelial cells (Netea et al., 2008). Pathogen recognition receptors (PRRs), a protein family of cellular receptors that mediate recognition of microbial pathogens and subsequent inflammatory response are present on the surface of DCs and macrophages (Hamad, 2012).
Immune Recognition
One of the main PRRs are the Toll-like receptors (TLRs), whose role in the recognition of Aspergillus and Candida has been well documented especially, TLR2, TLR4, and TLR9 (Pasare and Medzhitov, 2005; Takeda and Akira, 2005; Goodridge and Underhill, 2008; Uematsu and Akira, 2008; Loures et al., 2010). The PRRs mounted on the host cells recognize specific fungi cell wall moieties of polysaccharide origin, namely the PAMPs. Fungal PAMPs for cell surface TLRs have been identified mainly through studies involving fungi with cell wall mutations. For instance, fungal phospholipomannans (PLMs), linear beta-1, 2-oligomannosides and glucuronoxylomannan (GXM) are known to bind with TLR2, while, O-linked mannans have been shown to activate TLR4 (Netea et al., 2006). Apart from cell surface PAMPs, nucleic acids released from the fungi in the phagosome also stimulate TLRs and modulate the host responses. TLR 9 activation occurs through interaction of genomic DNA whereas double stranded and single stranded RNA stimulate TLR3 and TLR7 respectively (Bourgeois and Kuchler, 2012).
Recognition of fungal antigen by TLR4 leads to pro-inflammatory cytokine production by NF-κB activation mediated by the adaptor protein Myd88. Bellocchio et al. (2004) supported that TLR4-mediated pro-inflammatory effects are protective against invasive aspergillosis by showing increased susceptibility of TLR4–/– mice to Aspergillus fumigatus infection. Mutation of Asp299Gly in TLR4 is associated with increased incidence of pulmonary aspergillosis (Carvalho et al., 2008). It was subsequently demonstrated that HSCT patients in possession of the D299G/T399I haplotype were at higher risks of invasive aspergillosis (Bochud et al., 2008). TLR2 was shown to influence early recruitment and killing capacity of neutrophils against A. fumigatus (Bellocchio et al., 2004). TLR2–/– mice infected intraperitoneally with Candida albicans were found to have lesser recruitment of neutrophils and monocytes (Tessarolli et al., 2010). However, TLR2–/– mice had decreased fungal burden compared to the control mice accompanied by increased production of interleukin 12 (IL12) and decreased production of IL10. The role of TLR2 is still under debate as studies based on targeted patient genotype of TLR2 did not reveal enhanced susceptibility. TLR9–/– mice are reported to have higher fungal burden than control mice and found to be producing more IL10 and lower IL12 which is in contrast to findings in TLR2–/– mice. Mutations in TLR9 are associated with increased incidence of allergic bronchopulmonary aspergillosis (Carvalho et al., 2008; Mezger et al., 2010). An association of invasive aspergillosis was also seen in patients undergoing HSCT with SNPs in TLR1 and TLR6 (Kesh et al., 2005). It should be noted that TLR response may vary depending on fungal species and morphotype, and route of infection as well as the specific fungal infection (Romani, 2011).
Another family of PRRs that are important in the recognition of fungal PAMPs are C-type lectin receptors, otherwise known as CLRs. β-glucans present on the cell walls of Candida and Aspergillus species activate Dectin-1 receptor, while Dectin-2, and Dectin-3 mainly recognize hyphal α-mannan (Saijo et al., 2010). N-mannan is recognized by mannose receptor while galectin-3 binds to β-mannans. Fungal N- linked mannans also bind to DC-SIGN and mannose binding lectin (MBL) receptors present on phagocytes (Becker et al., 2015).
Dectin-1 is the most widely known CLR associated with fungal recognition. Dectin-1 recognition of β-glucan activates canonical and non-canonical NF-κB activation by two pathways, Syk-CARD9 and RAF pathways, resulting in increase in the pro-inflammatory cytokine production. Stimulation of Dectin-1 also increases IL1β and IL18 production through NLRP3 inflammasome pathway. Dectin-1 also collaborates with TLR2 to trigger pro-inflammatory cytokine production upon recognition of Candida albicans and zymosan. Dectin-1 deficient and CARD 9 deficient mice have predisposition to Candida infections (Ferwerda et al., 2009; Drewniak et al., 2013). Dectin-2 pairs with FcRγ to induce pro-inflammatory cytokine release. Dectin-1 plays an important role in human fungal infections too. It is evident from the polymorphism Y238X noticed in a Dutch family whose members were subject to recurrent vulvovaginal candidiasis and/or onychomycosis, while increased oral and gastrointestinal colonization of Candida was observed in HSCT recipients. In addition, it was noticed that there were defects in the expression of Dectin-1 and β-glucan recognition by phagocytes coupled with decrease in the production of cytokines, especially IL17 (Ferwerda et al., 2009; Plantinga et al., 2009). Similarly CARD9–/– patients show increased susceptibility to chronic mucocutaneous candidiasis and reduced Th17 cells (Glocker et al., 2009). MINCLE, which is mainly expressed by macrophages, also induce NF-κB activation through Syk-CARD9 signaling. Mannose receptors are involved in the phagocytosis of un-opsonized Candida yeasts. Mannose receptor interacts with galectin-3, a PRR which recognizes carbohydrate moieties on fungal cell wall, to induce TNFα production (Esteban et al., 2011; Kawai and Akira, 2011).
Both Candida and Aspergillus also trigger an immune response through activation of the inflammasome—most well described through NLRP3 and caspase-1 activation, with the involvement of the tyrosine kinase Syk and Dectin-1 (Gross et al., 2009; Said-Sadier et al., 2010). The non-canonical caspase-8 pathway is also implicated in the context of Candida (Gringhuis et al., 2012). Both result in the cleaving and production of IL1β, a pivotal mediator of inflammatory response together with interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα). The invocation of a “pro-inflammatory” response necessitates a “counter-regulatory” component which is maintained by IL4 and IL10 and more recently, possibly through the inhibitory group of NLR (nucleotide-binding domain, leucine-rich repeat containing) proteins (Ting et al., 2010). It is believed that it is in the context of such a conventional paradigm of a balance between a “pro- and anti-inflammatory milieu” that host susceptibility and outcome of an IFD episode may be determined (Chai et al., 2011).
Immune Regulation
Role of Neutrophils
The state of neutropenia is a well-established risk factor for invasive aspergillosis (Marr et al., 2002). Neutrophils, being the primary effector cells of innate immune system, efficiently and rapidly kill fungi by various mechanisms. Neutrophils are capable of recognizing fungi by TLR2, TLR4, Dectin-1, and complement receptors such as CR1 and CR3 (Braem et al., 2015). MAP kinase signaling is reported to mediate neutrophil activation, especially ERK signaling pathway, since inhibition of ERK signaling pathway abolishes C. albicans induced neutrophil migration (Wozniok et al., 2008). Once activated, neutrophils are able to release neutrophil extracellular traps as well as an array of cytokines and chemokines. Neutrophils recruitment, activation and survival in inflammatory sites are affected by Th17 controlled pathway in fungal infections. Neutrophils are also the source of pattern recognition molecule, pentraxin 3 (PTX3) which forms complexes on the conidial surface of the fungus and acts as an opsonin, enhancing recognition and phagocytosis of conidia through mechanisms that depend on Fcγ receptor, CD11b and complement (Mantovani et al., 2011; Cunha et al., 2014).
Role of Dendritic Cells/Monocytes/Macrophages
Dendritic cells serve the bridge between innate and adaptive immunity since they can present antigen to T cells, activate both innate and adaptive immune system by release of cytokines and chemokines. DCs can recognize fungal pathogens by the receptors such as Dectin-1, TLR2, and TLR4. Production of CCL20 as well as PTX3 increased with the activation of DCs (Mezger et al., 2008). DCs also mature after phagocytosis of fungal cells and promote the differentiation of naive T cells to CD4+ T cells which are essential for antifungal defense. Aspergillus conidia and hyphae induce NF-κB translocation and release of proinflammatory cytokine TNFα, and MIP2 in TLR2 and TLR4 dependent manner via adaptor protein Myd88.
Monocytes are macrophage and DC precursors; they serve as phagocytes as well as antigen presenting cells. Monocytes produce CCL20 which activates neutrophils, monocytes and naive T cells. Alveolar macrophages destroy Aspergillus conidia via non-oxidative mechanisms. The activity of macrophages can be enhanced by GM-CSF or IFNγ (Mueller-Loebnitz et al., 2013).
Immune Resistance vs Immune Tolerance
T cells act as the immune modulators and master effectors in the immune response against fungal pathogens. Conventionally, Th1 response is associated with TLR4 signaling resulting in secretion of IFNγ and TNFα (for protection against fungal pathogen), while Th2 response is associated with TLR2 signaling resulting in the production of anti-inflammatory cytokines (IL4 and IL10) to regulate the inflammatory response (which unfortunately leads to more susceptibility against fungal infection). Th17 cells have been increasingly recognized to serve one of the central roles in the anti-Candida response especially the mucosal immunity. Th17 has long been attributed to autoimmune diseases while defective Th17 response results in mucocutaneous candidiasis in patients with primary immunodeficiencies (Zelante et al., 2009). In fungal infections, Th17 activation occurs through Syk-CARD9, Myd88 and mannose receptor signaling pathways in DCs and macrophages (Romani, 2011). Activation of IL17 results in the recruitment of neutrophils, defensins and ultimately results in inflammation. However, IL17 activation is also associated with high inflammatory pathology and inhibitory effects on the IFNγ related activation of indolamine 2, 3-dioxygenase (IDO) that is important for immune tolerance function (Romani and Puccetti, 2008). Candida albicans is known to dampen Th17 response resulting in chronic inflammation due to the impairment of IL17 dependent neutrophil recruitment leading to fungal persistence and immune dysregulation (Cheng et al., 2010).
While inflammation and immune response is necessary to eliminate the fungus, it is also important to limit the collateral damage to tissue and restore homeostasis to the environment. IL10, a major suppressive cytokine produced by CD4+ T regulatory cells plays an important role in keeping inflammation under control. However, the delicate balance of IL10/IFNγ needs to be in check since high level of IL10 suppresses the activity of IFNγ which provides the main Th1 defense against fungal infections. IDO which is a product of tryptophan metabolism is also increasingly recognized as the master regulator of immune resistance and tolerance since it can induce T regulatory cells and inhibit Th17. IDO and kynurenines balance immune tolerance and resistance by providing adequate elimination of fungal pathogen while preventing the unacceptable level of inflammation and allergy (Zelante et al., 2009).
Immune Enhancement Strategies
The increased understanding of anti-fungal host responses has facilitated novel approaches into molecular and cell-based immunotherapeutics for invasive fungal infections (Figure 1). Notably, the major protective host response against fungi is the effective induction of Th1 and IFNγ responses, which in turn, activates effector phagocytic cells that kill the fungi. A cautionary note, however, is that this inflammatory response needs to be appropriately regulated or curbed when the pathogen or stimulatory ligand is contained, to minimize progression into a chronic inflammatory state which may induce collateral tissue damage.
Cytokine Therapy
The use of recombinant cytokines such as human granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF) and interferon-gamma (IFNγ) have been explored as immune enhancing agents. GM-CSF, G-CSF, and M-CSF belong to the family of hematopoietic cytokines. They stimulate the proliferation of granulocyte and/or macrophage progenitor cells, induce differentiation and maturation, and stimulate functional activity of mature hematopoietic cells (Pikman and Ben-Ami, 2012). GM-CSF alone or in combination with IFN-γ has been shown to enhance the fungicidal activity of innate phagocytic cells in vitro and in vivo. GM-CSF has been shown to preferentially enhance both the numbers and activity of type 1 DCs and cause upregulation of macrophage dectin-1 expression (Willment et al., 2003; Van de Veerdonk et al., 2010). Human M-CSF enhanced the activity of phagocytic cells and prolonged survival alone or in combination with amphotericin B in immunosuppressed mice with systemic Candida infection (Kuhara et al., 2000). Similarly, M-CSF when added to standard antifungal treatment of 46 stem cell transplantation recipients with progressive fungal infections showed better overall survival rates (Nemunaitis et al., 1993).
G-CSF is widely used in clinical practice during chemotherapy induced neutropenia. While G-CSF clearly reduces neutropenic days and neutropenia-related hospitalization, its efficacy in clinical outcomes including infection and mortality rates remain less clear (Smith et al., 2006). In a review of 925 mucormycosis cases, 15 of 18 patients showed favorable clinical response when given G-CSF adjunctive therapy (Roden et al., 2005). Clinical data on the use of GM-CSF as adjuvant antifungal therapy are scarce. Few case reports or small patient series with drug-refractory invasive aspergillosis infection have been published but provide limited information. Recently, a retrospective assessment of 66 patients was performed in whom GM-CSF was given during antifungal therapy to high-risk cancer patients and stem cell transplant recipients with IFD. A complete or partial response occurred in more than half of the patients treated with GM-CSF despite recent treatment with antineoplastic therapy and presence of other predictors of poor outcomes (Safdar et al., 2013). Further prospective studies to assess CSFs efficacy in the treatment of established fungal disease are needed.
IFNγ, produced by T and NK cells, increases the cytotoxic capacity of antigen presenting cells and intracellular killing. In a recent prospective case series, eight patients with invasive Candida and/or Aspergillus infections were treated with recombinant IFN-γ for 2 weeks in addition to standard antifungal therapy. Recombinant IFN-γ treatment in patients with invasive Candida and/or Aspergillus infections partially restored immune function, as characterized by an increased HLA-DR expression in those patients and an enhanced production of pro-inflammatory cytokines involved in antifungal defense (Delsing et al., 2014). IFNγ is also used in the treatment of recalcitrant aspergillosis (Kelleher et al., 2006; Bandera et al., 2008; Estrada et al., 2012). Further large-scale clinical studies to assess the potential clinical benefit of IFNγ is needed, but the cost of the drug remains a major concern.
Preclinical trials have assessed other pro-inflammatory cytokines that upregulate the antifungal Th1 response such as IL12, IL15, and TNFα as candidate adjuvants. IL12 is required for Candida-induced differentiation of Th1 cells in vivo (Romani et al., 1997) and for the antifungal activity of monocytes against A. fumigatus hyphae in vitro (Roilides et al., 1999). The usefulness of IL12 as immune enhancer is controversial. Invasive mold infections were reported in two autologous stem cell transplantation recipients treated with IL12 (Toren et al., 1997), raising concern that IL12 may paradoxically provoke an immune flare to fungal pathogens.
IL15 is also a potential new drug candidate. This cytokine, shares biological activities with IL2, in enhancing antifungal granulocyte activity in cell cultures (Vazquez et al., 1998; Winn et al., 2003). Neutralization of TNFα, a signature cytokine of Th1 cells, increases the susceptibility of mice to invasive aspergillosis, whereas intratracheal instillation of TNFα agonist peptides confers protection against A. fumigatus conidia (Mehrad et al., 1999). Further preclinical investigation is required not only for these cytokines, but also for IL18 and IL36 belonging to the interleukin 1 family (Gresnigt et al., 2013; Ketelut-Carneiro et al., 2015).
Granulocyte Transfusion
Transfusion of granulocytes from healthy donors has been used anecdotally for immune enhancement in patients with neutropenia who suffer from invasive fungal infections. Earlier attempts were beset by the lower yield and quality of granulocytes recovered from steroid treated donors. However, with advances in apheresis methods, better sedimenting agents and the recent use of recombinant cytokines like G-CSF and IFN-γ1b in addition to steroids, the yield and quality of leukocytes from healthy donors have improved.
The efficacy of granulocyte transfusion has been shown by the increased survival rates following its use in the treatment of cancer patients with candidemia (Price et al., 2000). In an uncontrolled prospective study of 23 patients treated for IFD with granulocyte transfusion, no recurrent infection was observed (Mousset et al., 2005). However, in a Phase III randomized trial of 74 patients with febrile neutropenia, 55 of whom had IFD and 39 had received stem cell transplantation, there was no clear effect of granulocyte transfusion on survival up to day 100 (Seidel et al., 2008). Though major randomized trials are lacking for patients with invasive aspergillosis and mucormycosis, good clinical efficacy and safety using appropriate granulocytes is evident through various small case series and case reports (Dignani et al., 1997; Illerhaus et al., 2002; Slavin et al., 2002; Safdar et al., 2006). Therefore, the use of granulocyte transfusions in patients with severe neutropenia and uncontrolled infection, in spite of appropriate antifungal therapy might be considered as a potential life-saving treatment option.
Antibodies
The era of antibody-based therapy for invasive fungal infections dawned with the discovery of protective monoclonal antibodies (mAbs) against the capsular polysaccharide of Cryptococcus neoformans (Dromer et al., 1987). Subsequently, protective antibodies against Candida albicans (Han and Cutler, 1995; Moragues et al., 2003), Aspergillus fumigatus (Chaturvedi et al., 2005) and other fungi were elucidated.
Two antifungal mAbs have been evaluated in clinical trials. 18B7, a mAb against the capsular polysaccharide of C. neoformans was found to be safe in a Phase I study (Larsen et al., 2005) but there is a lack of efficacy data. Efungumab (Mycograb) is a genetically engineered human recombinant antibody against fungal heat shock protein 90. HSP90 is an immunodominant antigen of the Candida cell wall and is required for its survival. In preclinical studies, Mycograb showed activity against a wide range of Candida species and synergized with antifungal drugs (Matthews et al., 2003; Hodgetts et al., 2008). But the role of Mycograb at the bedside remains still controversial. Results of a double-blind clinical trial in 117 patients with invasive candidiasis receiving liposomal Amphotericin B with or without Mycograb, showed that by day 10, the patient group receiving Mycograb combination (84 vs 48%; p < 0.001) had complete response with more rapid clearance of fungal cultures and reduced Candida-attributable mortality rate (Pachl et al., 2006). However, due to methodological and safety issues (Herbrecht et al., 2006), the drug has not gained licensure yet.
On a similar note, monoclonal antibodies mAb C7 and mAb A9, against Candida cell wall mannoprotein and A. fumigatus cell wall glycoprotein respectively, exhibit direct fungicidal activity (Moragues et al., 2003; Chaturvedi et al., 2005) with reduced fungal burden and increased survival rate in murine models of invasive infection.
Killer anti-idiotypic antibodies, which mimic broad spectrum antimicrobial peptides have been developed. These antibodies, upon intranasal administration to immunosuppressed mice with invasive aspergillosis have resulted in cure and long-term survival (Cenci et al., 2002).
Radioimmunotherapy is another novel antibody-based concept, whereby radiolabeled antibodies that recognize fungal antigens are used to deliver microbicidal radiation with less systemic toxicity (Bryan et al., 2010). It is hoped that radiolabeled mAbs that bind antigens shared by many pathogenic fungi, such as HSP60 and β1, 3 glucan, may act as adjuncts in tandem with conventional antifungals (Bryan et al., 2012).
Vaccination
Antifungal vaccines is an area that has drawn increasing interest and research in recent years. The effective usage of fungal vaccines is limited in the immunocompromised hosts as they not only tend to mount weak protective responses to vaccines but are also at risk from live attenuated formulations. Hence fungal vaccines are often based on standardized cellular subunits which require an adjuvant to induce protective immunity. Heat shock proteins may serve as powerful adjuvants while the immune response may be enhanced by mannosylation of antigens (Spellberg, 2011). Protective immunity arises from both T-cell responses, specifically Th1 and/or Th17 (Wuthrich et al., 2011) and antibody responses.
Preclinical evaluation of vaccines to a number of important fungal pathogens have been performed and at least two have been subject to Phase I clinical trials (Pikman and Ben-Ami, 2012). Universal fungal vaccines may be on the horizon with a conjugate vaccine that evokes antibodies to β-glucans offering cross-protection against three major fungal pathogens: C. albicans, A. fumigatus, and C. neoformans (Torosantucci et al., 2009). Another promising panfungal vaccine preparation originates from heat-killed Saccharomyces and is found to confer protection against Aspergillus, Coccidioides, and Candida infections (Stevens et al., 2011).
Though animal studies with crude A. fumigatus antigens are promising, the ideal dose that can be safely administered to humans is not well understood (Stevens, 2004). Vaccination of mice with a distinct Aspergillus antigen Aspf 3 prior to immunosuppression was shown to confer protection against subsequent inhalational challenge with A. fumigatus (Ito et al., 2006). It was shown that immunization confers cellular rather than humoral immunity since naive mice were protected from invasive aspergillosis by passive transfer of CD4+ cells rather than anti-Aspf 3 antibodies from immunized mice (Diaz-Arevalo et al., 2011). Additional vaccine candidates include secreted protein Pep1p and anchored proteins Gel1p and Crf1p (Bozza et al., 2009) of which, Crf1p proved to be immunogenic with cross-reactivity and protection against C. albicans (Stuehler et al., 2011).
Natural Killer Cell Treatment
Recently the role of NK cells in antifungal immunity is being investigated. It has been found that IL2-primed NK cells are cytotoxic toward A. fumigatus germlings and hyphae, an effect that is not mediated through degranulation of its cytotoxic proteins like perforin, granzymes etc., but mediated by IFNγ and TNFα secretion (Bouzani et al., 2011; Schmidt et al., 2011). NK cells have been shown to be the most important source of IFN-γ in the lungs of neutropenic hosts during the early stages of invasive aspergillosis (Park et al., 2009). It was also shown that the chemokine ligand MCP1/CCL2 mediates recruitment of NK cells resulting in more rapid clearance of Aspergillus from the lungs (Morrison et al., 2003) implicating the potential for NK-based therapeutic applications.
Adoptive T cell Transfer
Defective T-cell immunity is a hurdle in the path to a robust immune response to vaccines and antimicrobial treatment. Conceptually, this problem could be overcome by T-cell-independent vaccination, wherein the CD4+ T-cell-derived factor CD40L, required for DC costimulation of B cells, is replaced (Zheng et al., 2005).
One of the strategies to reduce the risk of invasive aspergillosis is the induction of Th1-type immune response that may be achieved by either transferring Aspergillus-specific Th clones or DCs that have been primed to trigger Aspergillus-specific immunity (Pikman and Ben-Ami, 2012). Adoptive T-cell transfer has been shown to decrease galactomannan levels significantly with higher survival rates as compared with patients who did not receive immunotherapy (Perruccio et al., 2004). Specific Candida cell wall proteins expressed during invasive infection have been synthesized as immunogenic peptide epitope–β-mannan conjugates. DCs pulsed with three of these epitopes conferred protection against disseminated candidiasis in mice. Of note is one epitope, derived from fructose-bisphosphate aldolase, which was shown to induce robust antibody dependent protective responses to C. albicans (Xin et al., 2008).
Various vaccine formulations using DCs to induce adoptive immunity to Aspergillus have been studied. DCs pulsed with live conidia, transfected with conidial RNA or primed with unmethylated CpG oligodeoxynucleotides and pulsed with Aspf16 antigens trigger specific Th1-type responses and protective immunity against invasive aspergillosis in a mouse model. DC infusion was shown to be more effective and superior to that of Aspergillus-specific T cells (Bozza et al., 2002, 2003). Subsequently, it was shown that DCs transfected with IL-12 DNA and pulsed with heat-inactivated A. fumigatus induced protective immunity against invasive pulmonary aspergillosis, as reflected by decreased fungal burden and increased survival (Shao et al., 2005).
Conclusions and Future Perspectives
Despite the advances in our knowledge and understanding in pathogenesis, IFD continues to result in significant morbidity and mortality in immunocompromised patients. The current conventional therapeutic modalities have not been fully effective. In addition, prolonged use of antifungal agents pose the risk of emergence of fungi resistant to conventional drugs.
The urgent need of the hour is to improve treatment options for patients with IFD by the usage of newer and more effective drugs, alone or combined together that can cure the infection. The other promising solution would be the use of immunotherapeutic modalities to improve and enhance the host defense system against fungal pathogens. The increase in knowledge of the pathogenesis of fungal infections has ushered in a new era of immunotherapeutic options. It is of utmost importance that further relevant clinical trials be conducted to explore the various immunotherapeutic strategies that hold promise for the better treatment and control of IFD in the near future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LC has been supported by Clinician Scientist Award (CSA), Individual Research Grant (IRG), Bedside & Bench (B&B) Grant and the Training Fellowship Award from the National Medical Research Council (NMRC), Singapore. He also acknowledges the Aspiration Grant, Bench to Bedside Grant and Seed Funding Grant from the National University Health System, Singapore. LC has received grant support and has been advisor/consultant for Pfizer, Merck Sharp and Dohme (MSD), Gilead and Astellas. WMS has been supported by Clinician Scientist Individual Research Grant - New Investigator Grant (CSIRG-NIG) from NMRC, Singapore.
References
Bandera, A., Trabattoni, D., Ferrario, G., Cesari, M., Franzetti, F., Clerici, M., et al. (2008). Interferon-gamma and granulocyte-macrophage colony stimulating factor therapy in three patients with pulmonary aspergillosis. Infection 36, 368–373. doi: 10.1007/s15010-008-7378-7
Becker, K. L., Ifrim, D. C., Quintin, J., Netea, M. G., and van de Veerdonk, F. L. (2015). Antifungal innate immunity: recognition and inflammatory networks. Semin. Imunopathol. 37, 107–116. doi: 10.1007/s00281-014-0467-z
Bellocchio, S., Montagnoli, C., Bozza, S., Gaziano, R., Rossi, G., Mambula, S. S., et al. (2004). The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172, 3059–3069. doi: 10.4049/jimmunol.172.5.3059
Blumberg, H. M., Jarvis, W. R., Soucie, J. M., Edwards, J. E., Patterson, J. E., Pfaller, M. A., et al. (2001). Risk factors for Candida bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis survey. Clin. Infect. Dis. 33, 177–186. doi: 10.1086/321811
Bochud, P. Y., Chien, J. W., Marr, K. A., Leisenring, W. M., Upton, A., Janer, M., et al. (2008). Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 359, 1766–1777. doi: 10.1056/NEJMoa0802629
Bourgeois, C., and Kuchler, K. (2012). Fungal pathogens—a sweet and sour treat for Toll-like receptors. Front. Cell Infect. Microbiol. 2:142. doi: 10.3389/fcimb.2012.00142
Bouzani, M., Ok, M., McCormick, A., Ebel, F., Kurzai, O., Morton, C. O., et al. (2011). Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-γ release. J. Immunol. 187, 1369–1376. doi: 10.4049/jimmunol.1003593
Bozza, S., Clavaud, C., Giovannini, G., Fontaine, T., Beauvais, A., Sarfati, J., et al. (2009). Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J. Immunol. 183, 2407–2414. doi: 10.4049/jimmunol.0900961
Bozza, S., Gaziano, R., Lipford, G. B., Montagnoli, C., Bacci, A., Di Francesco, P., et al. (2002). Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 4, 1281–1290. doi: 10.1016/S1286-4579(02)00007-2
Bozza, S., Perruccio, K., Montagnoli, C., Gaziano, R., Bellocchio, S., Burchielli, E., et al. (2003). A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102, 3807–3814. doi: 10.1182/blood-2003-03-0748
Braem, S. G., Rooijakkers, S. H., van Kessel, K. P., de Cock, H., Wosten, H. A., van Strijp, J. A., et al. (2015). Effective neutrophil phagocytosis of Aspergillus fumigatus is mediated by classical pathway complement activation. J. Innate Immun. 7, 364–374. doi: 10.1159/000369493
Brown, G. D., Denning, D. W., Gow, N. A., Levitz, S. M., Netea, M. G., and White, T. C. (2012). Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13. doi: 10.1126/scitranslmed.3004404
Bryan, R. A., Guimaraes, A. J., Hopcraft, S., Jiang, Z., Bonilla, K., Morgenstern, A., et al. (2012). Toward developing a universal treatment for fungal disease using radioimmunotherapy targeting common fungal antigens. Mycopathologia 173, 463–471. doi: 10.1007/s11046-011-9476-9
Bryan, R. A., Jiang, Z., Howell, R. C., Morgenstern, A., Brushertseifer, F., Casadevall, A., et al. (2010). Radioimmunotherapy is more effective than antifungal treatment in experimental cryptococcal infection. J. Infect. Dis. 202, 633–637. doi: 10.1086/654813
Carvalho, A., Pasqualotto, A. C., Pitzurra, L., Romani, L., Denning, D. W., and Rodrigues, F. (2008). Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J. Infect. Dis. 197, 618–621. doi: 10.1086/526500
Cenci, E., Mencacci, A., Spreca, A., Montagnoli, C., Bacci, A., Perruccio, K., et al. (2002). Protection of killer antiidiotypic antibodies against early invasive aspergillosis in a murine model of allogeneic T-cell-depleted bone marrow transplantation. Infect. Immun. 70, 2375–2382. doi: 10.1128/IAI.70.5.2375-2382.2002
Chai, L. Y., Vonk, A. G., Kullberg, B. J., and Netea, M. G. (2011). Immune response to Aspergillus fumigatus in compromised hosts: from bedside to bench. Future Microbiol. 6, 73–83. doi: 10.2217/fmb.10.158
Chaturvedi, A. K., Kavishwar, A., Shiva Keshava, G. B., and Shukla, P. K. (2005). Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis. Clin. Diagn. Lab. Immunol. 12, 1063–1068. doi: 10.1128/cdli.12.9.1063-1068.2005
Cheng, S. C., van de Veerdonk, F., Smeekens, S., Joosten, L. A., van der Meer, J. W., Kullberg, B. J., et al. (2010). Candida albicans dampens host defense by downregulating IL-17 production. J. Immunol. 185, 2450–2457. doi: 10.4049/jimmunol.1000756
Cunha, C., Aversa, F., Lacerda, J. F., Busca, A., Kurzai, O., Grube, M., et al. (2014). Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 370, 421–432. doi: 10.1056/NEJMoa1211161
Delsing, C., Gresnigt, M., Leentjens, J., Preijers, F., Allantaz, Frager, F., Kox, M., et al. (2014). Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect. Dis. 14:166. doi: 10.1186/1471-2334-14-166
de Pauw, B. E., and Picazo, J. J. (2008). Present situation in the treatment of invasive fungal infections. Int. J. Antimicrob. Agents 32, 167–171. doi: 10.1016/S0924-8579(08)70020-7
Diaz-Arevalo, D., Bagramyan, K., Hong, T. B., Ito, J. I., and Kalkum, M. (2011). CD4+ T cells mediate the protective effect of the recombinant Aspf3-based anti-aspergillosis vaccine. Infect. Immun. 79, 2257–2266. doi: 10.1128/IAI.01311-10
Dignani, M. C., Anaissie, E. J., Hester, J. P., O’Brien, S., Vartivarian, S. E., Rex, J. H., et al. (1997). Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia 11, 1621–1630. doi: 10.1038/sj.leu.2400811
Drewniak, A., Gazendam, R. P., Tool, A. T., van Houdt, M., Jansen, M. H., van Hamme, J. L., et al. (2013). Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121, 2385–2392. doi: 10.1182/blood-2012-08-450551
Dromer, F., Charreire, J., Contrepois, A., Carbon, C., and Yeni, P. (1987). Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55, 749–752.
Erjavec, Z., Kluin-Nelemans, H., and Verwejj, P. E. (2009). Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 15, 625–633. doi: 10.1111/j.1469-0691.2009.02929.x
Esteban, A., Popp, M. W., Vyas, V. K., Strijbis, K., Ploegh, H. L., and Fink, G. R. (2011). Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. U.S.A. 108, 14270–14275. doi: 10.1073/pnas.1111415108
Estrada, C., Desai, A. G., Chirch, L. M., Suh, H., Seidman, R., Darras, F., et al. (2012). Invasive aspergillosis in a renal transplant recipient successfully treated with interferon-gamma. Case Rep. Transplant. 2012, 5. doi: 10.1155/2012/493758
Ferwerda, B., Ferwerda, G., Plantinga, T. S., Willment, J. A., van Spriel, A. B., Venselaar, H., et al. (2009). Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767. doi: 10.1056/NEJMoa0901053
Goodridge, H., S., and Underhill, D. M. (2008). Fungal recognition by TLR2 and Dectin-1. Handb. Exp. Pharmaco. 183, 87–109. doi: 10.1007/978-3-540-72167-3_5
Glocker, E. O., Hennigs, A., Nabavi, M., Schaffer, A. A., Woellner, C., Salzer, U., et al. (2009). A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735. doi: 10.1056/NEJMoa0810719
Gresnigt, M. S., Rosler, B., Jacobs, C. W., Becker, K. L., Joosten, L. A., van der Meer, J. W., et al. (2013). The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur. J. Immunol. 43, 416–426. doi: 10.1002/eji.201242711
Gringhuis, S. I., Kaptein, T. M., Wevers, B. A., Theelen, B., van der Vlist, M., Boekhout, T., et al. (2012). Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a non-canonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254. doi: 10.1038/ni.2222
Gross, O., Poeck, H., Bscheider, M., Dostert, C., Hannesschlager, N., Endres, S., et al. (2009). Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436. doi: 10.1038/nature07965
Hamad, M. (2012). Innate and adaptive antifungal immune responses: partners on an equal footing. Mycoses 55, 205–217. doi: 10.1111/j.1439-0507.2011.02078.x
Han, Y., and Cutler, J. E. (1995). Antibody response that protects against disseminated candidiasis. Infect. Immun. 63, 2714–2719.
Herbrecht, R., Denning, D. W., Patterson, T. F., Bennett, J. E., Greene, R. E., Oestmann, J. W., et al. (2002). Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347, 408–415. doi: 10.1056/NEJMoa020191
Herbrecht, R., Fohrer, C., and Nivoix, Y. (2006). Mycograb for the treatment of invasive candidiasis. Clin. Infect. Dis. 43, 1083–1084. doi: 10.1086/507547
Hodgetts, S., Nooney, L., Al-Akeel, R., Curry, A., Awad, S., Matthews, R., et al. (2008). Efungumab and caspofungin: pre-clinical data supporting synergy. J. Antimicrob. Chemother. 61, 1132–1139. doi: 10.1093/jac/dkn075
Illerhaus, G., Wirth, K., Dwenger, A., Waller, C. F., Garbe, A., Brass, V., et al. (2002). Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Ann. Hematol. 81, 273–281. doi: 10.1007/s00277-002-0439-6
Ito, J. I., Lyons, J. M., Hong, T. B., Tamae, D., Liu, Y. K., Wilczynski, S. P., et al. (2006). Vaccinations with recombinant variants of Aspergillus fumigatus allergen Aspf3 protect mice against invasive aspergillosis. Infect. Immun. 74, 5075–5084. doi: 10.1128/IAI.00815-06
Kawai, T., and Akira, S. (2011). Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. doi: 10.1016/j.immuni.2011.05.006
Kelleher, P., Goodsall, A., Mulgirigama, A., Kunst, H., Henderson, D. C., Wilson, R., et al. (2006). Interferon-gamma therapy in two patients with progressive chronic pulmonary aspergillosis. Eur. Respir. J. 27, 1307–1310. doi: 10.1183/09031936.06.00021705
Kesh, S., Mensah, N. Y., Peterlongo, P., Jaffe, D., Hsu, K., Van Den Brink, M., et al. (2005). TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive apergillosis after allogeneic stem cell transplantation. Ann. N. Y. Acad. Sci. 1062, 95–103. doi: 10.1196/annals.1358.012
Ketelut-Carneiro, N., Silva, G. K., Rocha, F. A., Milanezi, C. M., Cavalcanti-Neto, F. F., Zamboni, D. S., et al. (2015). IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J. Immunol. 194, 4507–4517. doi: 10.4049/jimmunol.1402321
Kohler, J. R., Casadevall, A., and Perfect, J. (2015). The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 5:a019273. doi: 10.1101/cshperspect.a019273
Kuhara, T., Uchida, K., and Yamaguchi, H. (2000). Therapeutic efficacy of human macrophage colony-stimulating factor, used alone and in combination with antifungal agents, in mice with systemic Candida albicans infection. Antimicrob. Agents Chemother. 44, 19–23. doi: 10.1128/AAC.44.1.19-23.2000
Larsen, R. A., Pappas, P. G., Perfect, J., Aberg, J. A., Casadevall, A., Cloud, G. A., et al. (2005). Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49, 952–958. doi: 10.1128/AAC.49.3.952-958.2005
Loures, F. V., Pina, A., Felonato, M., Araujo, E. F., Leite, K. R., and Calich, V. L. (2010). Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect. Immun. 78, 1078–1088. doi: 10.1128/IAI.01198-09
Mantovani, A., Cassatella, M. A., Costantini, C., and Jaillon, S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531. doi: 10.1038/nri3024
Marr, K. A., Carter, R. A., Boeckh, M., Martin, P., and Corey, L. (2002). Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100, 4358–4366. doi: 10.1182/blood-2002-05-1496
Matthews, R. C., Rigg, G., Hodgetts, S., Carter, T., Chapman, C., Gregory, C., et al. (2003). Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 47, 2208–2216. doi: 10.1128/AAC.47.7.2208-2216.2003
Mehrad, B., Strieter, R. M., and Standiford, T. J. (1999). Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162, 1633–1640.
Mezger, M., Kneitz, S., Wozniok, I., Kurzai, O., Einsele, H., and Loeffler, J. (2008). Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 197, 924–931. doi: 10.1086/528694
Mezger, M., Einsele, H., and Loeffler, J. (2010). Genetic susceptibility to infections with Aspergillus fumigatus. Crit. Rev. Microbiol. 36, 168–177. doi: 10.3109/10408410903530619
Moragues, M. D., Omaetxebarria, M. J., Elguezabal, N., Sevilla, M. J., Conti, S., Polonelli, L., et al. (2003). A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71, 5273–5279. doi: 10.1128/IAI.71.9.5273-5279.2003
Morrison, B. E., Park, S. J., Mooney, J. M., and Mehrad, B. (2003). Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J. Clin. Invest. 112, 1862–1870. doi: 10.1172/JCI18125
Mousset, S., Hermann, S., Klein, S. A., Bialleck, H., Duchscherer, M., Bomke, B., et al. (2005). Prophylactic and interventional granulocyte transfusions in patients with haematological malignancies and life-threatening infections during neutropenia. Ann. Hematol. 84, 734–741. doi: 10.1007/s00277-005-1055-z
Mueller-Loebnitz, C., Ostermann, H., Franzke, A., Loeffler, J., Uharek, L., Topp, M., et al. (2013). Immunological aspects of Candida and Aspergillus systemic fungal infections. Interdiscip. Perspect. Infect. Dis. 2013, 102934. doi: 10.1155/2013/102934
Nemunaitis, J., Shannon-Dorcy, K., Appelbaum, F. R., Meyers, J., Owens, A., Day, R., et al. (1993). Long-term follow-up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood 82, 1422–1427.
Neofytos, D., Horn, D., Anaissie, E., Steinbach, W., Olyaei, A., Fishman, J., et al. (2009). Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter prospective antifungal therapy (PATH) Alliance registry. Clin. Infect. Dis. 48, 265–273. doi: 10.1086/595846
Netea, M. G., Brown, G. D., Kullberg, B. J., and Gow, N. A. (2008). An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78. doi: 10.1038/nrmicro1815
Netea, M. G., Gow, N. A., Munro, C. A., Bates, S., Collins, C., Ferwerda, G., et al. (2006). Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116, 1642–1650. doi: 10.1172/JCI27114
O’Brien, B. L., Parrent, J. L., Jackson, J. A., Moncalvo, J. M., and Vilgalys, R. (2005). Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 71, 5544–5550. doi: 10.1128/AEM.71.9.5544-5550.2005
Pachl, J., Svoboda, P., Jacobs, F., Vandewoude, K., van der Hoven, B., Spronk, P., et al. (2006). A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination withan antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42, 1404–1413. doi: 10.1086/503428
Park, S. J., Hughes, M. A., Burdick, M., Streiter, R. M., and Mehrad, B. (2009). Early NK cell-derived IFN-gamma is essential to host defense in neutropenic invasive aspergillosis. J. Immunol. 182, 4306–4312. doi: 10.4049/jimmunol.0803462
Pasare, C., and Medzhitov, R. (2005). Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18. doi: 10.1007/0-387-24180-9_2
Perlin, D. S. (2007). Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat 10, 121–130. doi: 10.1016/j.drup.2007.04.002
Perruccio, K., Bozza, S., Montagnoli, C., Bellocchio, S., Aversa, F., Martelli, M., et al. (2004). Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 33, 248–255. doi: 10.1016/j.bcmd.2004.08.011
Pikman, R., and Ben-Ami, R. (2012). Immune modulators as adjuncts for the prevention and treatment of invasive fungal infections. Immunotherapy 4, 1869–1882. doi: 10.2217/imt.12.127
Plantinga, T. S., van der Velden, W. J., Ferwerda, B., van Spriel, A. B., Adema, G., Feuth, T., et al. (2009). Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 49, 724–732. doi: 10.1086/604714
Price, T. H., Bowden, R. A., Boeckh, M., Bux, J., Nelson, K., Liles, W. C., et al. (2000). Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood 95, 3302–3309.
Roden, M. M., Zaoutis, T. E., Buchanan, W. L., Knudsen, T. A., Sarkisova, T. A., Schaufele, R. L., et al. (2005). Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41, 634–653. doi: 10.1086/432579
Rodrigues, M. E., Silva, S., Azeredo, J., and Henriques, M. (2014). Novel strategies to fight Candida species infection. Crit. Rev. Microbiol 10, 1–13. doi: 10.3109/1040841X.2014.974500
Roilides, E., Tsaparidou, S., Kadiltsoglou, I., Sein, T., and Walsh, T. J. (1999). Interleukin-12 enhances antifungal activity of human mononuclear phagocytes against Aspergillus fumigatus: implications for a gamma interferon-independent pathway. Infect. Immun. 67, 3047–3050.
Romani, L. (2011). Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288. doi: 10.1038/nri2939
Romani, L., and Puccetti, P. (2008). Immune regulation and tolerance to fungi in the lungs and skin. Chem. Immunol. Allergy 94, 124–137. doi: 10.1159/000154957
Romani, L., Puccetti, P., and Bistoni, F. (1997). Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 10, 611–636.
Safdar, A., Rodriguez, G. H., Lichtiger, B., Dickey, B. F., Kontoyiannis, D. P., Freireich, E. J., et al. (2006). Recombinant interferon gamma-1b immune enhancement in 20 patients with hematologic malignancies and systemic opportunistic infections treated with donor granulocyte transfusions. Cancer 106, 2664–2671. doi: 10.1002/cncr.21929
Safdar, A., Rodriguez, G., Zuniga, J., Akhrass, F., Georgescu, G., and Pande, A. (2013). Granulocyte macrophage colony-stimulating factor in 66 patients with myeloid or lymphoid neoplasms and recipients of hematopoietic stem cell transplantation with invasive fungal disease. Acta Heamatol. 129, 26–34. doi: 10.1159/000342121
Said-Sadier, N., Padilla, E., Langsley, G., and Ojcius, D. M. (2010). Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE 5:e10008. doi: 10.1371/journal.pone.0010008
Saijo, S., Ikeda, S., Yamabe, K., Kakuta, S., Ishigame, H., Akitsu, A., et al. (2010). Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. doi: 10.1016/j.immuni.2010.05.001
Schmidt, S., Tramsen, L., Hanisch, M., Latge, J. P., Huenecke, S., Koehl, U., et al. (2011). Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J. Infect. Dis. 203, 430–435. doi: 10.1093/infdis/jiq062
Seidel, M. G., Peters, C., Wacker, A., Northoff, H., Moog, R., Boehme, A., et al. (2008). Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 42, 679–684. doi: 10.1038/bmt.2008.237
Shao, C., Qu, J., He, L., Zhang, Y., Wang, J., Zhou, H., et al. (2005). Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes Immun. 6, 103–114. doi: 10.1038/sj.gene.6364167
Slavin, M. A., Kannan, K., Buchanan, M. R., Sasadeusz, J., and Roberts, A. W. (2002). Successful allogeneic stem cell transplant after invasive pulmonary zygomycosis. Leuk. Lymphoma 43, 437–439. doi: 10.1080/10428190290006305
Smith, T. J., Khatcheressian, J., Lyman, G. H., Ozer, H., Armitage, J. O., Balducci, L., et al. (2006). 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J. Clin. Oncol. 24, 3187–3205. doi: 10.1200/JCO.2006.06.4451
Spellberg, B. (2011). Vaccines for invasive fungal infections. F1000 Med. Rep. 3, 13. doi: 10.3410/M3-13
Stevens, D. A. (2004). Vaccinate against aspergillosis! A call to arms of the immune system. Clin. Infect. Dis. 38, 1131–1136. doi: 10.1086/382882
Stevens, D. A., Clemons, K. V., and Liu, M. (2011). Developing a vaccine against aspergillosis. Med. Mycol. 49(Suppl. 1), S170–S176. doi: 10.3109/13693786.2010.497775
Stuehler, C., Khanna, N., Bozza, S., Zelante, T., Moretti, S., Kruhm, M., et al. (2011). Cross-protective Th1 immunity against Aspergillus fumigatus and Candida albicans. Blood 117, 5881–5891. doi: 10.1182/blood-2010-12-325084
Takeda, K., and Akira, S. (2005). Toll like receptors in innate immunity. Int. Immunol. 17, 1–14. doi: 10.1093/intimm/dxh186
Tessarolli, V., Gasparoto, T. H., Lima, H. R., Figueira, E. A., Garlet, T. P., Torres, S. A., et al. (2010). Absence of TLR2 influences survival of neutrophils after infection with Candida albicans. Med. Mycol. 48, 129–140. doi: 10.3109/13693780902964339
Ting, J. P., Duncan, J. A., and Lei, Y. (2010). How the inflammasome NLRs function in the innate immune system. Science 327, 286–290. doi: 10.1126/science.1184004
Toren, A., Or, R., Ackerstein, A., and Nagler, A. (1997). Invasive fungal infections in lymphoma patients receiving immunotherapy following autologous bone marrow transplantation (ABMT). Bone Marrow Transplant. 20, 67–69. doi: 10.1038/sj.bmt.1700847
Torosantucci, A., Chiani, P., Bromuro, C., De Bernardis, F., Palma, A. S., Liu, Y., et al. (2009). Protection by anti-beta-glucan antibodies is associated with restricted beta-1, 3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE 4:5392. doi: 10.1371/journal.pone.0005392
Uematsu, S., and Akira, S. (2008). Toll-like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 183, 1–20. doi: 10.1007/978-3-540-72167-3_1
Van de Veerdonk, F. L., Netea, M. G., Joosten, L. A., van der Meer, J. W., and Kullberg, B. J. (2010). Novel strategies for the prevention and treatment of Candida infections: the potential of immunotherapy. FEMS Microbiol. Rev. 34, 1063–1075. doi: 10.1111/j.1574-6976.2010.00232.x
Vazquez, N., Walsh, T. J., Friedman, D., Chanock, S. J., and Lyman, C. A. (1998). Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect. Immun. 66, 145–150.
Verweij, P. E., Mellado, E., and Melchers, W. J. (2007). Multiple triazole resistant aspergillosis. N. Engl. J. Med. 356, 1481–1483. doi: 10.1056/NEJMc061720
Wey, S. B., Mori, M., Pfaller, M. A., Woolson, R. F., and Wenzel, R. P. (1989). Risk factors for hospital acquired candidemia. A matched case-control study. Arch. Intern. Med. 149, 2349–2353. doi: 10.1001/archinte.1989.00390100145030
Willment, J. A., Lin, H. H., Reid, D. M., Taylor, P. R., Williams, D. L., Wong, S. Y., et al. (2003). Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF treated macrophages and are negatively regulated by IL-10, dexamethasone and lipopolysaccharide. J. Immunol. 171, 4569–4573. doi: 10.4049/jimmunol.171.9.4569
Winn, R. M., Gil-Lamaignere, C., Roilides, E., Simitsopoulou, M., Lyman, C. A., Maloukou, A., et al. (2003). Selective effects of interleukin (IL)-15 on antifungal activity and IL-8 release by polymorphonuclear leukocytes in response to hyphae of Aspergillus species. J. Infect. Dis. 188, 585–590. doi: 10.1086/377099
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317. doi: 10.1086/421946
Wozniok, I., Hornbach, A., Schmitt, C., Frosch, M., Einsele, H., Hube, B., et al. (2008). Induction of ERK-kinase signaling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell Microbiol. 10, 807–820. doi: 10.1111/j.1462-5822.2007.01086.x
Wuthrich, M., Gern, B., Hung, C. Y., Ersland, K., Rocco, N., Pick-Jacobs, J., et al. (2011). Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121, 554–568. doi: 10.1172/JCI43984
Xie, J. L., Polvi, E. J., Shekhar-Guturja, T., and Cowen, L. E. (2014). Elucidating drug resistance in human fungal pathogens. Future Microbiol. 9, 523–542. doi: 10.2217/fmb.14.18
Xin, H., Dziadek, S., Bundle, D. R., and Cutler, J. E. (2008). Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. U.S.A. 105, 13526–13531. doi: 10.1073/pnas.0803195105
Zelante, T., De Luca, A., D’Angelo, C., Moretti, S., and Romani, L. (2009). IL-17/Th17 in anti-fungal immunity: what’s new? Eur. J. Immunol. 39, 645–648. doi: 10.1002/eji.200839102
Keywords: invasive fungal infections, immunocompromised, immune regulation, immune enhancement, cytokines
Citation: Ravikumar S, Win MS and Chai LY (2015) Optimizing Outcomes in Immunocompromised Hosts: Understanding the Role of Immunotherapy in Invasive Fungal Diseases. Front. Microbiol. 6:1322. doi: 10.3389/fmicb.2015.01322
Received: 31 August 2015; Accepted: 10 November 2015;
Published: 26 November 2015.
Edited by:
Edvaldo A. R. Rosa, The Pontifical Catholic University of Paraná, BrazilReviewed by:
Dmitri Debabov, NovaBay Pharmaceuticals, USAVishvanath Tiwari, Central University of Rajasthan, India
Copyright © 2015 Ravikumar, Win and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louis Y. A. Chai, chailouis@hotmail.com
 Sharada Ravikumar
Sharada Ravikumar