- Key Laboratory of Advanced Civil Engineering Materials, Tongji University, Ministry of Education, Shanghai, China
The biochemical properties of CaCO3 precipitation induced by Sporosarcina pasteurii, an ureolytic type microorganism, were investigated. Effects of calcium source on the precipitation process were examined, since calcium source plays a key role in microbiologically induced mineralization. Regardless of the calcium source type, three distinct stages in the precipitation process were identified by Ca2+, NH4+, pH and cell density monitoring. Compared with stage 1 and 3, stage 2 was considered as the most critical part since biotic CaCO3 precipitation occurs during this stage. Kinetics studies showed that the microbial CaCO3 precipitation rate for calcium lactate was over twice of that for calcium nitrate, indicating that calcium lactate is more beneficial for the cell activity, which in turn determines urease production and CaCO3 precipitation. X-ray diffraction analysis confirmed the CaCO3 crystal as calcite, although scanning electron microscopy revealed a difference in crystal size and morphology if calcium source was different. The findings of this paper further suggest a promising application of microbiologically induced CaCO3 precipitation in remediation of surface and cracks of porous media, e.g., cement-based composites, particularly by using organic source of calcium lactate.
Introduction
Since the phenomenon that CaCO3 precipitation could be induced by many soil bacteria was revealed (Boquet et al., 1973), research on microbial mineral plugging of porous media has been extensively carried out, especially on the application of bioremediation materials in civil engineering. Compared with traditional repair materials, which include cement grout, mortar, water glass, and epoxy resin, the bio-deposited materials are environmentally friendly and have a better compatibility with civil engineering materials, such as concrete and masonry (De Muynck et al., 2010; Wu et al., 2012). What is more to the point, self-repair can be achieved by using microbial induced deposition (Jonkers et al., 2010).
As a common process in nature, pores or fissures can be filled selectively by microorganisms which generate insoluble compounds inside or outside the cell wall (Ruiz et al., 1988). The mechanism of microbial deposition has been revealed and the feasibility of crack repairing in concrete by Bacillus pasteurii immobilized in polyurethane foam was verified (Stocks-Fischer et al., 1999; Bang et al., 2001; Bachmeier et al., 2002). It has been shown that the capillary water adsorption and gas permeability of concrete can be reduced effectively by surface treatment from microbiological mediated deposition, and the composition of bacterial culture medium has a significant impact on the morphology of CaCO3 (De Muynck et al., 2008a,b; Van Tittelboom et al., 2010; Achal et al., 2011; Xu et al., 2014). In recent years, bacterial induced deposition in using a non-ureolytic pathway has also been found (Jonkers et al., 2010). By mixing spores of these types of bacteria with fresh state concrete, self-healing of concrete cracks is expected since spores will germinate once cracking occurs and CaCO3 precipitation in the open cracks will be triggered by bacterial respiration (Wiktor and Jonkers, 2011; Wang et al., 2014a,b; Xu and Yao, 2014).
For the application of microbiologically induced precipitation in repairing, the primary issue would be the exploration of the biochemical process of the deposition. Since this process involves converting a soluble calcium source into insoluble CaCO3, the effect of the type of calcium source is critical. However, prior works concerning the influence of the type of calcium source on bacterial mineralization process are rare (De Muynck et al., 2008a). In this paper, the effect of two different types of calcium sources on the ureolytic microbiologically induced mineralization precipitation was investigated, which aims at providing guidance for further application.
Experimental Procedures
Bacterial Strains and Growth Conditions
Sporosarcina pasteurii ATCC 11859 was used throughout. Bacteria were cultured in liquid media consisted of 5 g peptone, 3 g meat extract, and 20 g urea per liter of distilled water. Liquid media were sterilized by autoclaving for 20 min at 121°C, then final pH was adjusted to be 9. Cultures were aerobically incubated at 30°C on a water-bath shaker operated at 100 rpm for 24 h. Growth was regularly checked quantitatively under optical microscopy by using a hemocytometer. At the end of the incubation, cultures were washed by repeated centrifugation and resuspension in fresh medium to harvest vegetative cells but to remove dissolved culture constituents and residues. Obtained suspensions were microscopically analyzed to quantify the number of cells present, and the suspension was subsequently kept at 4°C until further use.
Microbiologically Induced CaCO3 Precipitation
Calcium nitrate, instead of calcium chloride, was selected as a representative of inorganic calcium source since chloride ions may be detrimental for the concrete reinforcement. Calcium lactate, which is the most studied organic calcium-containing compound, was selected as a representative of organic calcium source. Groups without bacteria addition were set as control. The composition of liquid medium for each group is shown in Table 1, where letters of N, L, B, and C in group labels represent calcium nitrate, calcium lactate, bacteria, and control, respectively. Immediate abiotic CaCO3 precipitation usually occurs if the initial concentration of calcium ion is high in an alkaline environment, thus the concentration of calcium source was set to 0.025 mol/L.
For each group, triplicate sets of 250 mL Erlenmeyer flasks containing 80 mL of liquid medium were prepared. Each one was inoculated with live bacteria (105 cells/mL). Bacteria were grown at 30°C on the water-bath shaker operated at 100 rpm for 60 h. At regular intervals, three aliquots from each flask were taken to determine the pH, the amount of soluble Ca2+, and the NH4+ concentration, respectively. For pH measurement, a Mettler Toledo pH probe was used. For soluble Ca2+ quantification, the liquid medium was centrifuged and the Ca2+ concentration in the supernatant was measured by the EDTA titration method (APHA, 1989). For NH4+ concentration, an ammonia sensing probe was used. The cell density was measured simultaneously by plating colony-counting method, and the composition of the medium used was the same as the medium for culture growth.
Characterization of Precipitates
The mineralogy of precipitates was determined by powder X-ray diffraction (XRD) using a Rigaku D/max2550VB3+/PC diffractometer. The morphology of the precipitates was studied by scanning electron microscopy (SEM) using a Hitachi S-2360N. The composition of medium before and after precipitation was dried at 80°C and then analyzed by Fourier transform infrared spectroscopy (FT-IR) using a Bruker Hyperion 2000.
Results And Discussion
Biochemical Process of Microbiologically Induced CaCO3 Precipitation
The growth of bacteria in the mineralized medium is shown in Figure 1A. The initial concentration of bacteria in medium was about 105 cells/mL. In the first 22 h of induction period, the cell density showed a little increment of less than 2 × 105 cells/mL. The period of logarithmic growth appeared in 22–26 h and the cell density increased by nearly 100 times. It is observed that bacteria in medium with calcium lactate was more active than in medium with calcium nitrate, for the cell density of group L-B was always two times of that of group N-B. The control groups were not included since no strains were inoculated.
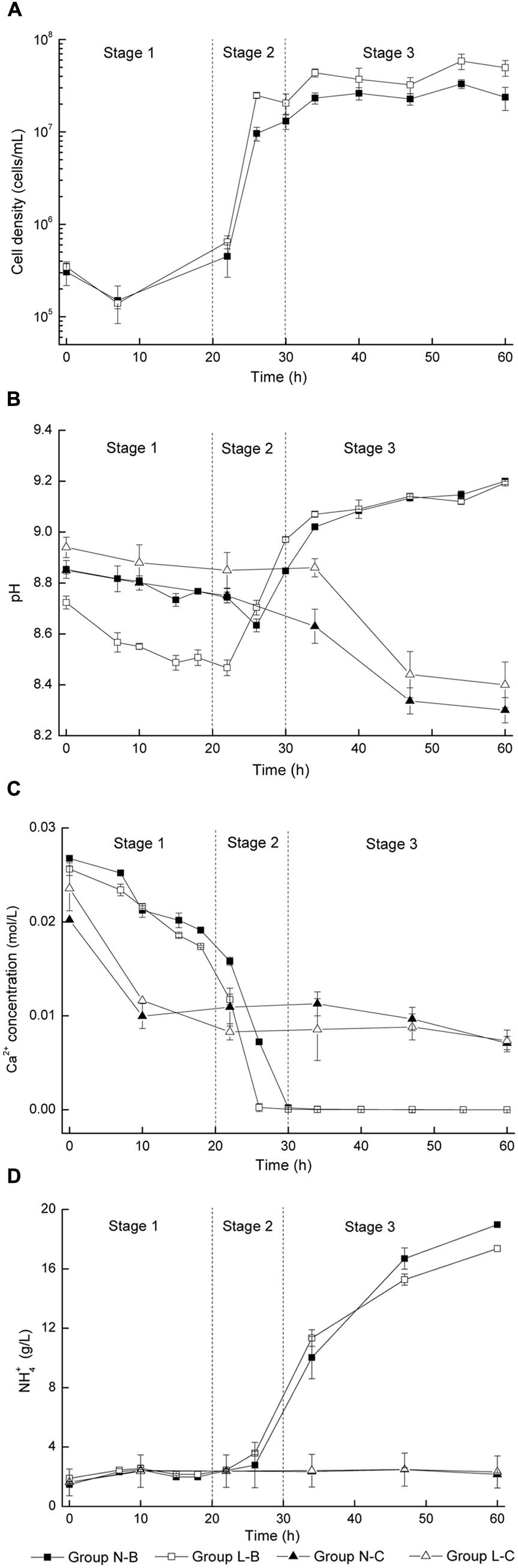
FIGURE 1. Microbiologically induced CaCO3 precipitation: (A) Cell density; (B) pH values; (C) Soluble Ca2+ concentration; (D) NH4+ concentration.
In Figure 1B, pH values of viable bacteria groups decreased for about 0.2 in the first 20–25 h, then rose rapidly for about 0.5 in the next 5–10 h, and finally kept steady. For the control groups, pH value variation less than 0.1 was observed in the first 30 h, followed by a remarkable decline around 0.5 in the later period.
Figure 1C shows the evolution of soluble Ca2+ concentration. The Ca2+ concentration of viable bacteria groups decreased rapidly during 20–30 h for about 0.02 mol/L until the free Ca2+ was completely converted into precipitates. For the control groups, Ca2+ concentration decreased for about 0.01 mol/L in the first 10 h and then kept steady in the following period, indicating that free Ca2+ were not combined in the later stage.
In Figure 1D, the NH4+ concentration of viable bacteria groups was nearly 3 g/L in the first 22 h, and then increased significantly to more than 10 g/L. In contrast, the NH4+ concentration for the control groups always kept constantly at 3 g/L.
The main objective of this paper is to explore the biochemical properties of bacterial induced mineralization, which would be very helpful for its application in structural remediation, particularly in concrete engineering. It was considered that the biochemical process of microbiologically induced CaCO3 precipitation consists of three distinct stages with similar pattern, regardless of the type of calcium source (Figure 1). Details for each stage is discussed as follows:
In the first stage, pH value and Ca2+ concentration dropped gradually while NH4+ concentration and cell density were almost unchanged. The initial alkalinity of the medium was mainly caused by the decomposition of urea during sterilization. In general, Ca2+ ions are apt to precipitate in an alkaline environment, which in turn results in a decrease of pH value. It is interesting to note that a reduction of 0.012 mol/L for Ca2+ occurred during this stage for both the groups with viable cells and control groups (Figure 1B), which indicates that abiotic precipitation mainly contributes to the deposition of CaCO3 in this stage.
At the end of the second stage, the calcium sources were completely converted into CaCO3. The biological-chemical mechanism of this process can be expressed as follows
At this stage, the density of bacteria increased by two orders of magnitude, which corresponds to the logarithmic phase of bacterial growth. Urea was decomposed into NH3 and CO2 by urease, which was produced by bacteria. pH value increased rapidly due to the fact that the large amount of NH3 generated by decomposition of urea. An increase of pH value promoted the dissolution of CO2 and the hydrolysis reaction of HCO-, resulting in an increase of CO32- concentration. In the meantime, positively charged metal ions were bound on bacterial surfaces due to the presence of several negatively charged groups on the cell wall. Such bound metal ions (e.g., Ca2+) may subsequently react with anions (e.g., CO32-) to form an insoluble salt (e.g., CaCO3) (Stocks-Fischer et al., 1999; Bang et al., 2010; De Muynck et al., 2010). Although the duration of stage 2 is short, it should be considered as a critical part in the whole process, and enzymatic hydrolysis by microorganisms is essential in this stage.
For the last stage, depletion of free Ca2+ was observed, while the bacteria density almost remained constant around 107 cells/mL, because of the depletion of nutrients and an increased amount of harmful metabolite. NH3 and CO2 were continuously released due to sustained decomposition of urea. The increase of pH value slowed down because the neutralization effect of CO2 with NH3.
Kinetics of Biotic CaCO3 Precipitation
As presented above, CaCO3 precipitation and ammonia production is correlated with cell growth. It could be noted that the initiation of biotic CaCO3 precipitation and ammonia production for the group L-B was earlier than that of group N-B (Figures 1B,D). In order to further study the effect of calcium source on the biochemical process, kinetics of biotic CaCO3 precipitation as well as ammonia production was examined. In Figure 2, curves corresponding to biotic CaCO3 precipitation (data in stage 1were excluded) were fitted by an exponential logistic equation (Marquardt, 1963)
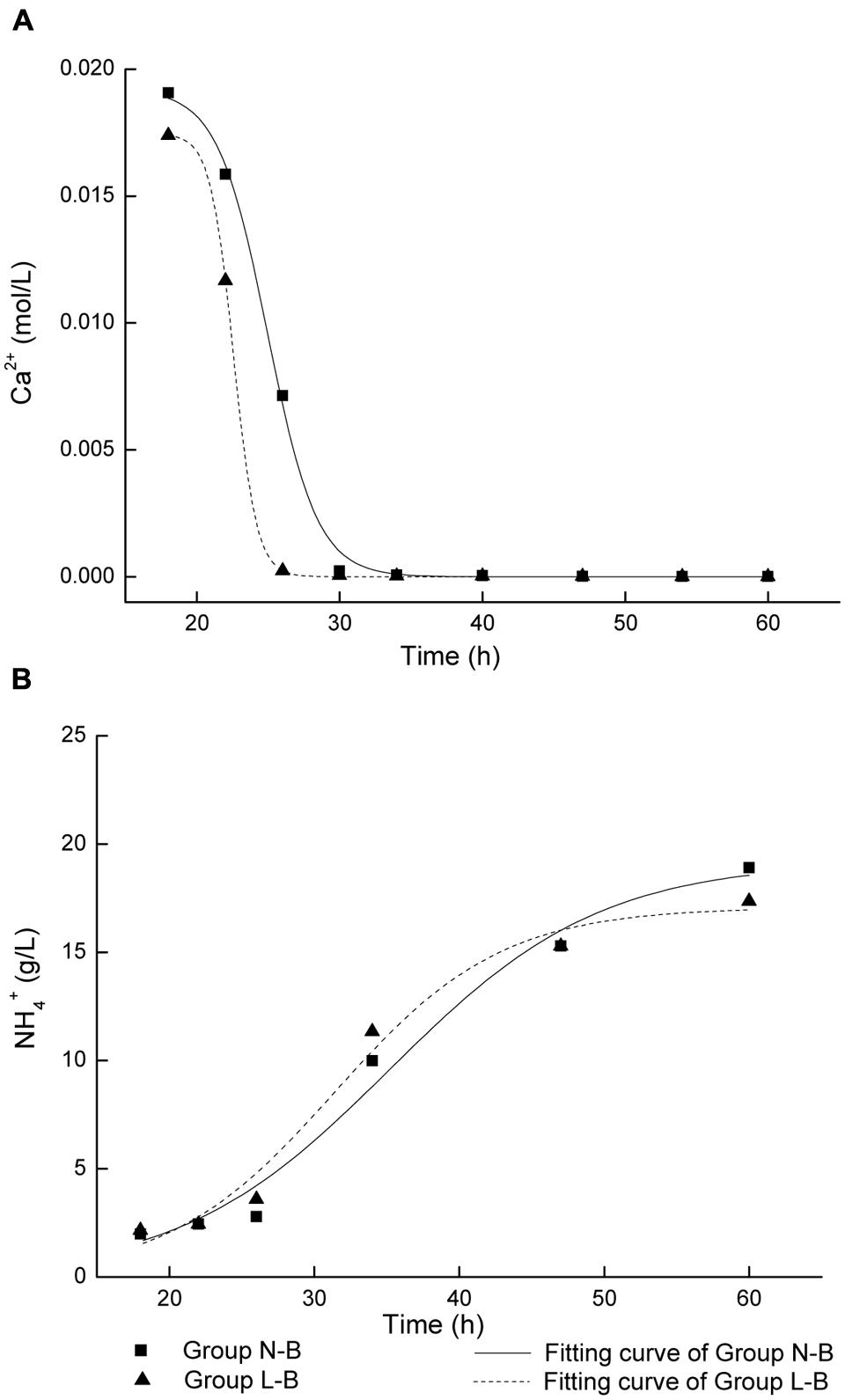
FIGURE 2. Fitting of curves for microbiologically induced precipitation with live cells: (A) CaCO3 precipitation; (B) NH4+ production.
where x is time, y is ion concentration, a is the variation range of y, xc is the time when dy/dx reaches the maximum value, k is the rate constant. The k values of CaCO3 precipitation and ammonia production were calculated from regression analysis, as shown in Table 2.
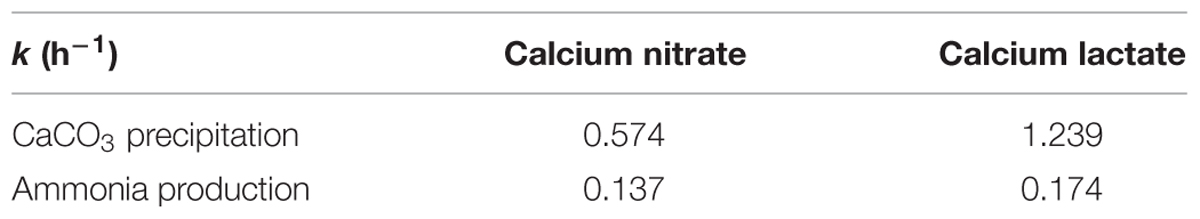
TABLE 2. Rate constants of CaCO3 precipitation and ammonium ions production in different calcium source media.
The rate constants in medium with calcium lactate are larger than that in medium with calcium nitrate. In particular, the k value of CaCO3 precipitation in calcium lactate is over twice of that in calcium nitrate. It suggests that higher cell activity and faster biotic CaCO3 precipitation and ammonia production rate if organic source of calcium lactate was used, which could be due to the bacterial metabolic conversion of calcium lactate according to the following reaction (De Muynck et al., 2010):
Calcium lactate is not only the calcium source, but a kind of carbon source that provides additional nutrition for bacteria. Moreover, extra amounts of CO2 were released if calcium lactate was decomposed by bacterial metabolism. It was reported that other organic calcium compounds have similar bio-reaction (Jonkers, 2011). In this respect, organic calcium source seems more beneficial for microbial mediated CaCO3 precipitation. It is worth to mention that substantial calcium lactate can be obtained from dairy by-products, which can meet the requirements of a potential use of calcium lactate in the field.
Analysis of Precipitates
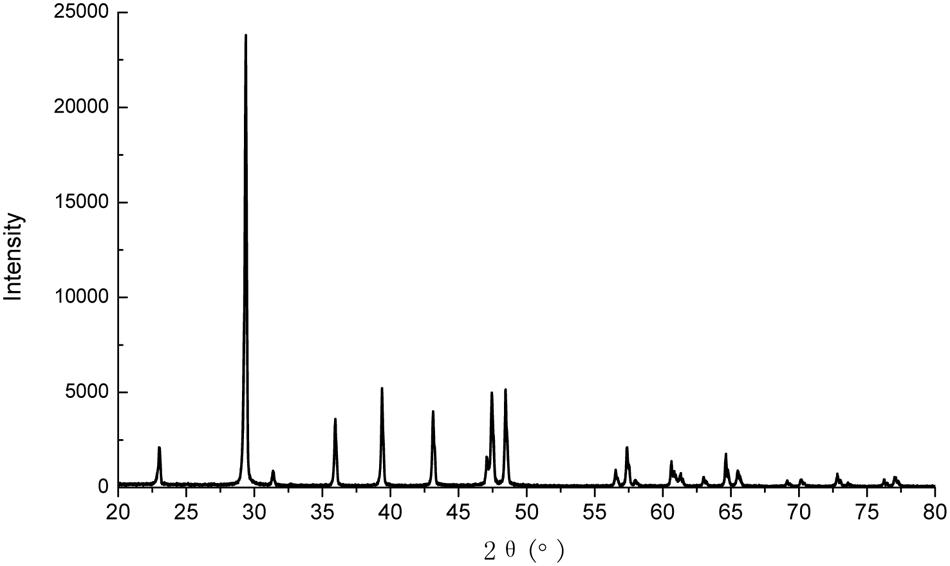
Calcite was precipitated in the liquid medium irrespective of the type of calcium source, as confirmed by XRD analysis (Figure 3). This is consistent with results of other researchers (Bang et al., 2001; De Muynck et al., 2008a).
Figure 4 shows SEM images of calcite precipitates from two kinds of calcium sources. Difference in morphology was observed from calcium nitrate to calcium lactate. The sediments from calcium nitrate are spherical and lamellar particles, with particle size less than 50 μm. In contrast, sediments from calcium lactate are mostly irregular compact lumps or rhombohedral crystals with relatively larger particle size. Imprints of 2–4 μm long and 0.7 μm wide were observed on the surface of crystals. These imprints are likely left by bacteria. Prior findings have already verified that bacterial cells can provide favorable conditions by acting as a nucleus for the formation of crystals (Stocks-Fischer et al., 1999; Bang et al., 2010).
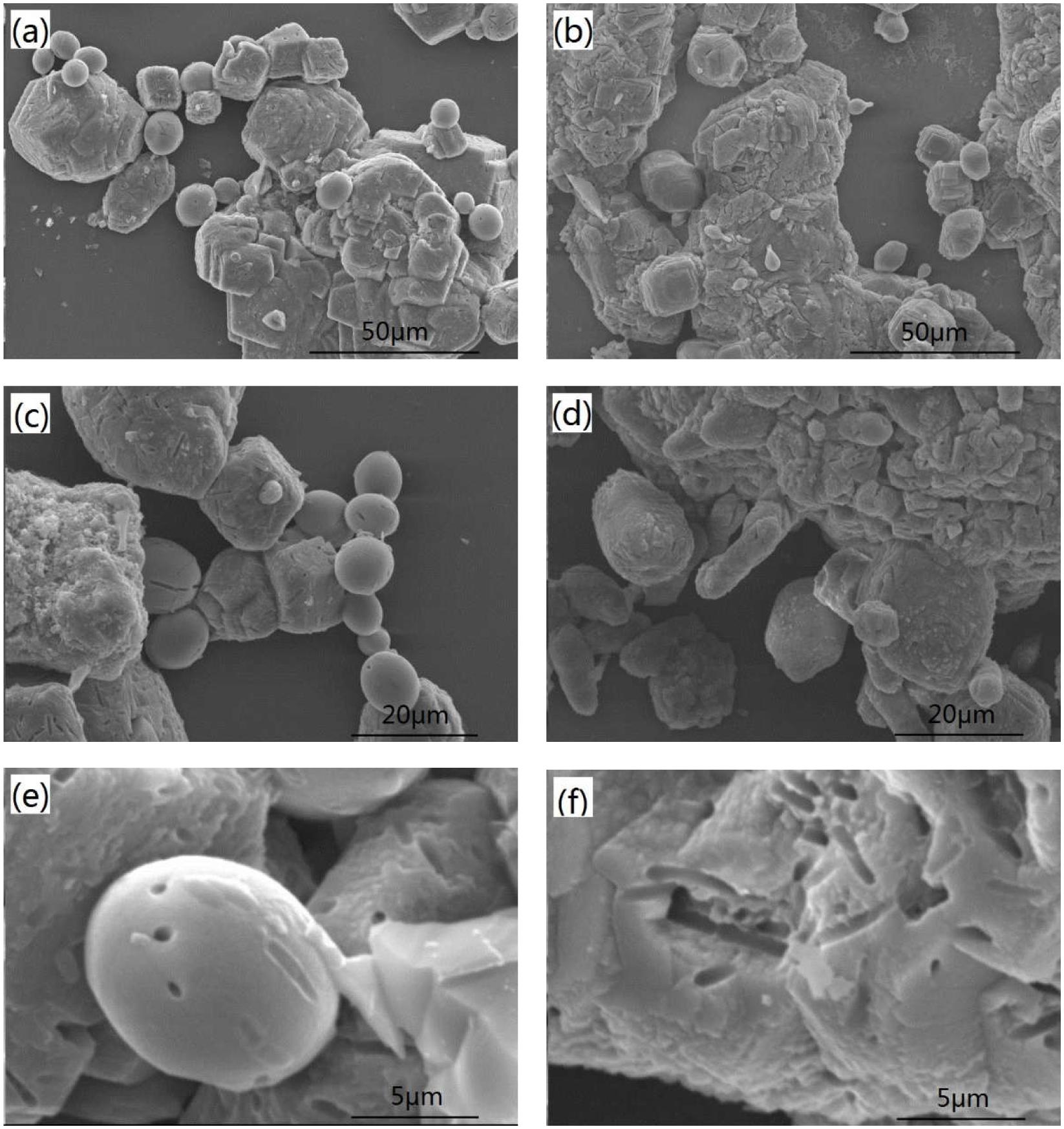
FIGURE 4. Scanning electron microscopy (SEM) images of the precipitates from different calcium source: (a,c,e) calcium nitrate; (b,d) and (f) calcium lactate.
Figure 5 shows the FT-IR spectra of medium with different calcium source before and after precipitation. For the medium before precipitation, the presence of urea was indicated by amino signature at 3345 and 1070 cm-1 and carboxide signature at 1600 cm-1. Calcium nitrate was identified by absorption peak at 1329 cm-1. Calcium lactate was identified by absorption bands at 1451, 1318, and 774 cm-1. For the medium with calcium nitrate after precipitation, a small broad hump at 3263 cm-1 and the absorption band 1315 cm-1 correspond to NH4+, while bands near 1716 cm-1, 1122 cm-1, and 846 cm-1 indicate the presence of CO32-. Bands corresponding to NH4+ and CO32- were also detected for the medium with calcium lactate after precipitation. FT-IR spectra confirms that NH4+ and CO32- were produced by bacterial metabolism.
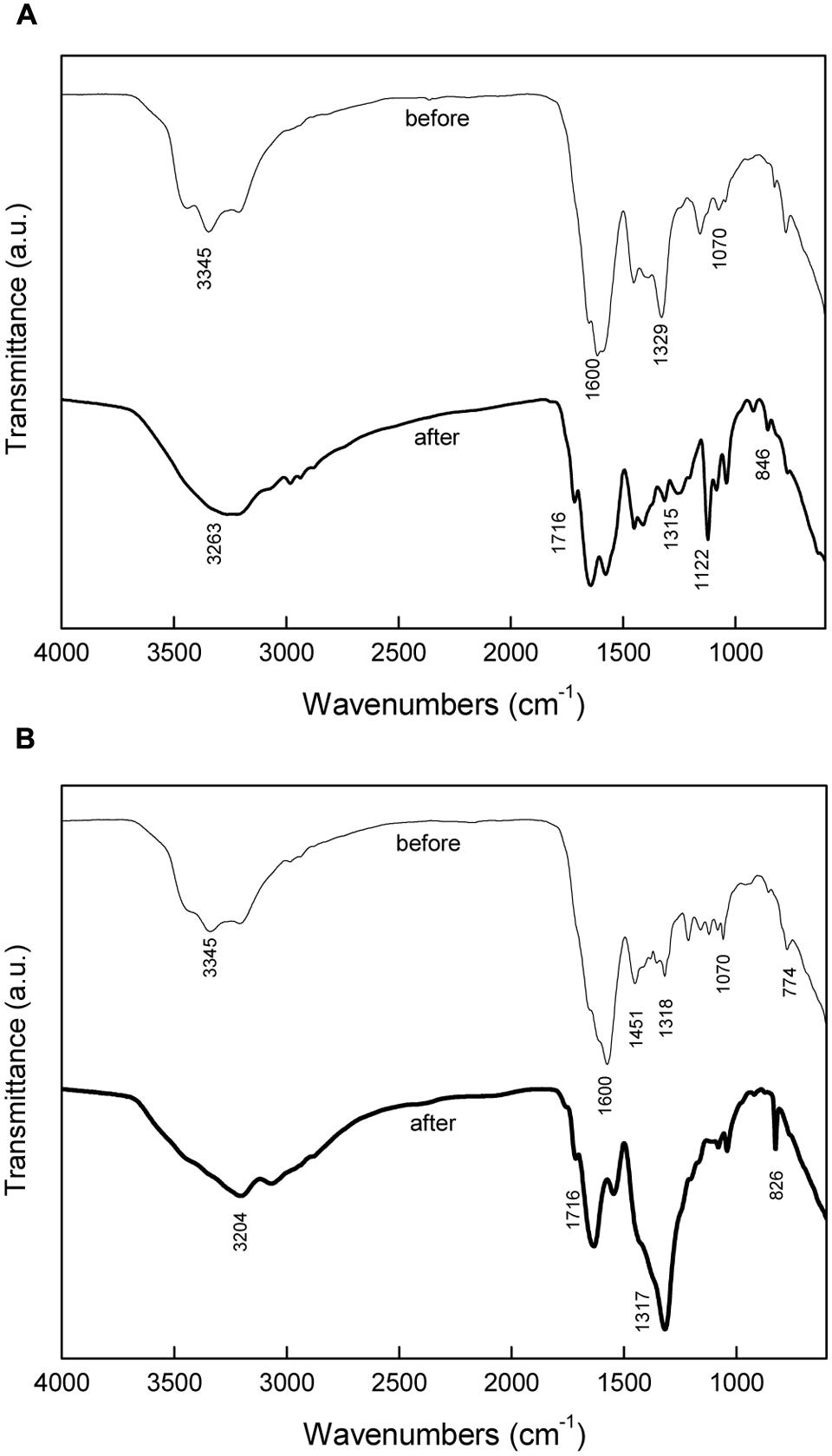
FIGURE 5. Fourier transform infrared spectroscopy (FT-IR) analysis of media before and after precipitation: (A) inorganic calcium source; (B) organic calcium source.
Although calcite was obtained irrelevant to the type of calcium source, different crystal size and morphology were observed for calcium nitrate and calcium lactate. This is consistent with other findings showing that the type of calcium source has a significant impact on the crystallization process (De Muynck et al., 2008a). The crystal growth can be inhibited or altered by the adsorption of organic or inorganic matters to specify crystallographic planes of the growing crystal (Rodriguez-Navarro et al., 2007). On the other hand, differences in crystal morphology could be due to the level of the actual urease activity, which correlates with the cell activity. It further confirms the kinetics results demonstrating that cell activity differs from inorganic calcium nitrate to organic calcium lactate. The influence of conditions other than calcium source type, such as pH, temperature, composition and concentration of nutrients on the morphology of precipitates, are worthy of further research. The influence of morphology and crystal size of on the properties of CaCO3 precipitates also needs assessment in civil engineering remediation application.
Conclusion
The biochemical investigations revealed that the microbiologically induced CaCO3 precipitation consists of three distinct stages and is independent of calcium source. The rate of precipitation for calcium lactate was over twice of that for calcium nitrate, indicating that organic source of calcium lactate may be more beneficial for the cell activity, which directly related to urease production and CaCO3 deposition. The CaCO3 crystal were identified as calcite, although the morphology varied if the type of calcium source was different. It also confirmed that bacterial cells acted as nucleation sites for crystal formation and growth.
Author Contributions
JX, corresponding author and the main contributor of the paper; YD, contribute to the main part of experimental work of the paper; ZJ, provide some ideas and part of funding to the work; AS, provide some ideas and part of testing of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the financial support for this study from the National Natural Science Foundation of China (51378011), the National Basic Research Program of China (2011CB013800), and Tongji-Bayer Academy.
References
Achal, V., Mukherjee, A., and Reddy, M. S. (2011). Microbial concrete: way to enhance the durability of building structures. J. Mater. Civ. Eng. 23, 730–734. doi: 10.1061/(ASCE)MT.1943-5533.0000159
APHA (1989). Standard Methods for the Examination of Water and Wastewater, 17th Edn. Washington, DC: American Public Health Association.
Bachmeier, K. L., Williams, A. E., Warmington, J. R., and Bang, S. S. (2002). Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 93, 171–181. doi: 10.1016/S0168-1656(01)00393-5
Bang, S. S., Galinat, J. K., and Ramakrishnan, V. (2001). Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 28, 404–409. doi: 10.1016/S0141-0229(00)00348-3
Bang, S. S., Lippert, J. J., Yerra, U., Mulukutla, S., and Ramakrishnan, V. (2010). Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int. J. Smart Nano Mater. 1, 28–39. doi: 10.1080/19475411003593451
Boquet, E., Boronat, A., and Ramos-Cormenzana, A. (1973). Production of calcite (calcium carbonate) crystals by soil bacteria is a common phenomenon. Nature 246, 527–529. doi: 10.1038/246527a0
De Muynck, W., Cox, K., De Belie, N., and Verstraete, W. (2008a). Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr. Build. Mater. 22, 875–885. doi: 10.1016/j.conbuildmat.2006.12.011
De Muynck, W., Debrouwer, D., De Belie, N., and Verstraete, W. (2008b). Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 38, 1005–1014. doi: 10.1016/j.cemconres.2008.03.005
De Muynck, W., De Belie, N., and Verstraete, W. (2010). Microbial carbonate precipitation in construction materials: a review. Ecol. Eng. 36, 118–136. doi: 10.1016/j.ecoleng.2009.02.006
Jonkers, H. M. (2011). Healing Agent for Self-Healing Cementious Materials. Patent No: WO 2011/126361 A1.
Jonkers, H. M., Thijssen, A., Muyzer, G., Copuroglu, O., and Schlangen, E. (2010). Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 36, 230–235. doi: 10.1016/j.ecoleng.2008.12.036
Marquardt, D. W. (1963). An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 11, 431–441. doi: 10.1137/0111030
Rodriguez-Navarro, C., Jimenez-Lopez, C., Rodriguez-Navarro, A., Gonzalez-Munoz, M. T., and Rodriguez-Gallego, M. (2007). Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 71, 1197–1213. doi: 10.1016/j.gca.2006.11.031
Ruiz, C., Monteoliva-Sanchez, M., Huertas, F., and Ramos-Cormenzana, A. (1988). Calcium carbonate precipitation by several species of Myxococcus. Chemosphere 17, 835–838. doi: 10.1016/0045-6535(88)90263-9
Stocks-Fischer, S., Galinat, J. K., and Bang, S. S. (1999). Microbiological precipitation of CaCO3. Soil Biol. Biochem. 31, 1563–1571. doi: 10.1016/S0038-0717(99)00082-6
Van Tittelboom, K., De Belie, N., De Muynck, W., and Verstraete, W. (2010). Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 40, 157–166. doi: 10.1016/j.cemconres.2009.08.025
Wang, J., Dewanckele, J., Cnudde, V., Van Vlierberghe, S., Verstraete, W., and De Belie, N. (2014a). X-ray computed tomography proof of bacterial-based self-healing in concrete. Cem. Concr. Compos. 53, 289–304. doi: 10.1016/j.cemconcomp.2014.07.014
Wang, J. Y., Soens, H., Verstraete, W., and De Belie, N. (2014b). Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 56, 139–152. doi: 10.1016/j.cemconres.2013.11.009
Wiktor, V., and Jonkers, H. M. (2011). Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 33, 763–770. doi: 10.1016/j.cemconcomp.2011.03.012
Wu, M., Johannesson, B., and Geiker, M. (2012). A review: self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 28, 571–583. doi: 10.1016/j.conbuildmat.2011.08.086
Xu, J., and Yao, W. (2014). Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem. Concr. Res. 64, 1–10.
Keywords: S. pasteurii, ureolytic, bacterial CaCO3 precipitation, biochemistry, kinetics
Citation: Xu J, Du Y, Jiang Z and She A (2015) Effects of Calcium Source on Biochemical Properties of Microbial CaCO3 Precipitation. Front. Microbiol. 6:1366. doi: 10.3389/fmicb.2015.01366
Received: 30 September 2015; Accepted: 17 November 2015;
Published: 02 December 2015.
Edited by:
Varenyam Achal, East China Normal University, ChinaReviewed by:
Jose M. Bruno-Barcena, North Carolina State University, USAChristopher L. Hemme, University of Rhode Island, USA
Copyright © 2015 Xu, Du, Jiang and She. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Xu, 0610060014@tongji.edu.cn; Zhengwu Jiang, jzhw@tongji.edu.cn
 Jing Xu
Jing Xu Yali Du
Yali Du