- 1Institute for Landscape Biogeochemistry, Leibniz Centre for Agricultural Landscape Research, Müncheberg, Germany
- 2Plant Production Department, Faculty of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
- 3Institute of Environmental Biotechnology, Graz University of Technology, Graz, Austria
The application of biochar to soil is considered to have the potential for long-term soil carbon sequestration, as well as for improving plant growth and suppressing soil pathogens. In our study we evaluated the effect of biochar on the plant growth of soybeans, as well as on the community composition of root-associated bacteria with plant growth promoting traits. Two types of biochar, namely, maize biochar (MBC), wood biochar (WBC), and hydrochar (HTC) were used for pot experiments to monitor plant growth. Soybean plants grown in soil amended with HTC char (2%) showed the best performance and were collected for isolation and further characterization of root-associated bacteria for multiple plant growth promoting traits. Only HTC char amendment resulted in a statistically significant increase in the root and shoot dry weight of soybeans. Interestingly, rhizosphere isolates from HTC char amended soil showed higher diversity than the rhizosphere isolates from the control soil. In addition, a higher proportion of isolates from HTC char amended soil compared with control soil was found to express plant growth promoting properties and showed antagonistic activity against one or more phytopathogenic fungi. Our study provided evidence that improved plant growth by biochar incorporation into soil results from the combination of a direct effect that is dependent on the type of char and a microbiome shift in root-associated beneficial bacteria.
Introduction
Biochar is a fine-grained charcoal that is rich in organic carbon, produced by pyrolysis or by heating biomass in a low oxygen environment and has been used worldwide as a soil amendment to increase soil fertility (Lehmann and Joseph, 2009; Schomberg et al., 2012). However, biochar application is a very old method of improving soil quality and plant growth, as reported by the Amazonian Dark Earths (ADE) or Terra Preta de Índio formed in the past by pre-Columbian populations (Barbosa Lima et al., 2015). Presently, there are extensive literature reviews about the use of biochar and hydrochar to mitigate climate change by increasing carbon storage in soils (Lehmann et al., 2011). Other topics are about improving soil nutrient availability and the growth and development of agriculturally important crops, inducing systemic resistance in plants against soil borne fungal pathogens (Elad et al., 2010). Improvements in plant growth and yield following biochar application have been reported under field and greenhouse conditions for a variety of crops, including legumes such as soybean (Glycine max L.; Tagoe et al., 2008) and common bean (Phaseolus vulgaris; Rondon et al., 2007). Suppadit et al. (2012) reported an increased number of nodules, plant height, dry weight, yield and nutrient uptake in soybeans by quail litter biochar. Reibe et al. (2015a) observed that plant growth and development were affected by the type of char and rates of application, e.g., increasing amounts of fermented hydrochar (HTC) increased shoot biomass and the shoot/root ratio in case of spring wheat. Whereas the agricultural benefits of incorporating biochar into soils are frequently reported, there is little and incomplete evidence concerning the mechanisms of plant growth stimulation or the protection of plants from fungal pathogens by biochar. There are several studies explaining an indirect effect of biochar on root growth and development by altering soil properties, such as porosity and pore size distribution, water holding capacity, mechanical stability, sorption properties and the bioavailability of nutrients and trace elements (Laird et al., 2010; Spokas et al., 2010), but the functional response of soil microbial populations after biochar amendments are not well-understood (Lehmann et al., 2011). Anders et al. (2013) stated that the change in the structure of the microbial community by biochar application is an indirect effect and depends on soil nutrient status. Barbosa Lima et al. (2015) revealed that soil type contributes to the composition of bacterial communities in studies of forest sites (Mimosa debilis) and open areas (Senna alata) in the Amazon region. However, despite numerous reports on microbial changes induced by biochar application in various cropping systems, there have been no studies on how biochar affects the diversity and physiological activity of plant growth stimulating rhizobacteria, especially in legumes.
Most members of root-associated microbes are capable to promote plant growth and are commonly studied for their ability to stimulate plant yield, nutrient uptake, stress tolerance, and biological control of soil borne disease (Egamberdieva et al., 2008, 2011; Argaw, 2012; Berg et al., 2013a). The composition of rhizosphere bacteria is influenced not only by the plant species but also by the soil type (Berg and Smalla, 2009). The mechanisms involved in the beneficial effects conferred to plants include the production of phytohormones (Spaepen, 2015), the solubilization of insoluble phosphorus into solution available for plant use (Oteino et al., 2015), ACC deaminase enzymes, which effectively reduce plant ethylene levels in the root system (Glick, 2014), siderophores to competitively acquire ferric iron (Solanki et al., 2014), antifungal activity against a variety of plant-pathogenic fungi (Köberl et al., 2013), cell wall degrading enzymes and competition for nutrients and niches (Egamberdieva et al., 2011). Recently, a microbiome shift induced by rhizobacteria was identified as a novel mode of action for biocontrol agents (Schmidt et al., 2012; Erlacher et al., 2014).
In our study, we focused on soybean (Glycine max L.) as an important grain legume because it is a source of protein, oil, animal feed, and biodiesel in many countries worldwide, with an annual production of 276.4 Mio t1. Improved growth and production of soybeans after biochar application have been reported by Suppadit et al. (2012) and Mete et al. (2015) but mechanisms remain mostly unresolved. We hypothesized that improved growth induced by biochar amendment is strongly linked to interactions with root-associated soil microbes because biochar would promote favorable conditions for microbial proliferation in the rhizosphere. Thus, the main objectives of our study were (i) to evaluate the growth of soybean plants in response to the application of different concentrations of biochar and hydochar, and (ii) to reveal whether char incorporation into soil influences interactions between plants and root-associated microbes that are linked with plant fitness.
Materials and Methods
Plant Growth Under Greenhouse Conditions
The soil used for pot experiments was from an experimental arable field under irrigation (V4) operated by the Experimental Field Station of Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany. The selected chemical and physical properties of soil are as follows: clay and fine silt, 7%; coarse and medium silt, 19%; sand, 74%; Corg – 570 mg 100 g−1; pH, 6.2; organic C content, 0.55%; total N content, 0.07%; P content, 32.0 mg (100 g soil)−1; K content, 1.25 g (100 g soil)−1; and Mg content, 0.18 g (100 g soil−1).
The three types of char were supplied from the Leibniz-Institut for Agrartechnik Potsdam-Bornim e.V. (ATB) and used for pot experiments (Reibe et al., 2015a,b): (i) pyrolysis biochar from maize (MBC, 600°C for 30 min), (ii) pyrolysis biochar from wood (WBC, 850°C for 30 min), and (iii) hydrochar from maize silage (HTC char, processed by batch-wise hydrothermal carbonization at 210°C and 23 bar for 8 h). The chemical composition of the chars is presented in Table 1.
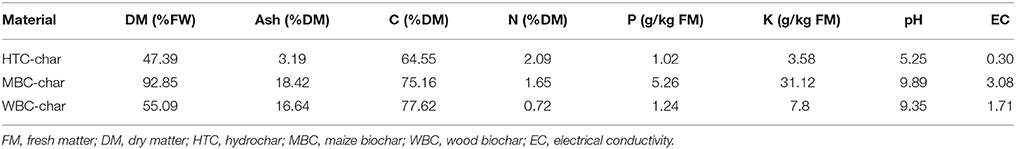
Table 1. Characterization of chars (Reibe et al., 2015b).
The soil was mixed with crushed chars (particle size < 3 mm) at increasing rates of 1, 2, and 3% (w/v) just before planting pre-germinated soybean seeds. All pots were arranged in a randomized block design. The soybean seeds (Glycine max. cv. Sultana, Naturland Markt, Berlin, Germany) were surface-sterilized using 10% v/v NaOCl for 5 min and 70% ethanol for 5 min, and then rinsed five times with sterile distilled water. Surface-sterilized seeds were transferred on paper tissue soaked in 0.5 mM CaSO4 and germinated for 5 days in a dark room at 25°C. The germinated seeds were transferred to pots containing 800 g of soil with four replicates. The treatments were control plants without biochar, soil amended with biochar (MBC, WBC) and HTC char at rates of 1, 2, and 3% (w/v). The plants were grown under greenhouse conditions (day/night temperature 24°C/16°C; humidity 50–60%; day length 12 h) and were watered when necessary. After 6 weeks, the plants were harvested, the roots were separated from shoots and the dry weight was determined.
Isolation of Rhizosphere Bacteria
Among the biochar types under study, HTC char showed stimulatory effects on soybean plants in previous experiments and thus was used for further study. Three plants from each treatment, soil without biochar and soil amended with HTC char (2%) were collected for bacterial isolation. Excess soil was removed from the root by shaking, and only tightly adherent soil remained for study. The root samples (10 g for each treatment) were added to 100 ml of PBS buffer (PBS; 20 mM sodium phosphate, 150 mM NaCl, pH 7.0) supplemented with cycloheximide (Sigma, St. Louis, USA) at a final concentration of 100 μg ml −1 and were shaken for 1 h. Serial dilutions (up to 10−3) were prepared, and 100 μl from appropriate dilutions was dispensed on Tryptic Soy Agar (TSA, Difco Laboratories, Detroit, USA) for bacterial culture and Peptone dextrose agar (PDA, Difco Laboratories, Detroit, USA) for fungal culture. The plates were incubated at 28°C for 2 days, and the total numbers of bacteria and fungi were counted. The colonies of bacteria that displayed differentiable colony morphologies were picked from plates and were re-streaked on fresh agar plates for purification. One hundred bacterial cultures were selected from each treatment and maintained at 4°C for further study.
Plant Growth Stimulation
To test whether bacterial isolates were capable of stimulating plant growth, a pot experiment was conducted in the greenhouse using loamy sand. The seeds were surface-sterilized and inoculated with bacterial strains as described above. The sterility of the seeds was previously tested on TSA agar by incubating the plates for 3 days at 28°C. No contaminants were found, indicating that the surface-sterilization was effective. Two hundred bacterial strains isolated from the rhizosphere of soybeans were grown overnight in Tryptic Soy Broth (TSB), and one milliliter of each culture was pelleted by centrifugation (10,000 × g for 10 min); the supernatant was discarded. Non-inoculated plants were used as negative controls. Cell pellets were washed with 1 ml of PBS and re-suspended in PBS. Cell suspensions corresponded to a cell density of 107 cells/ml. Germinated seeds were placed in the bacterial suspension with sterile forceps and shaken gently. After approximately 10 min, the inoculated seeds were aseptically planted into the potting soil. Three seeds were sown per plastic pot (12 cm diameter; 10 cm deep) to a depth of approximately 1.5 cm. After germination, plants were thinned to one per pot, and pots were set-up in a randomized design with six replications. The plants were grown under greenhouse conditions (day temperature 24°C/night 16°C; humidity 50–60%, day length 12 h) for 1 month. At harvest, the plants were removed from the pots, and the dry weights of roots and shoots were determined. A total 32 strains from control soil and 43 strains from HTC char amended soil were selected based on their plant growth promoting abilities and were further identified and characterized.
Identification of Beneficial Plant Strains
The identification of isolated strains was performed using whole-cell matrix assisted laser desorption/ionization (MALDI)–time of flight (TOF) mass spectrometry. Sample preparation was carried out according to the ethanol/formic acid extraction protocol recommended by Bruker Daltonics (Bremen, Germany). The isolates were cultured on tryptic soy agar (TSA, Difco Laboratories, Detroit, Michigan, USA) for 24 h, and approximately 10 mg of cell mass was suspended in 300 μL of water and vortexed to generate a homogenous suspension. The suspension was mixed with 900 μL of ethanol and centrifuged. The pellet was resuspended in 50 μL of 70% formic acid and subsequently carefully mixed with 50 μL of acetonitrile. After centrifugation, aliquots of 1 μL of supernatant were placed immediately on spots of a MALDI target. Each spot was allowed to dry and subsequently overlaid with 1 μL of matrix (α-ciano-4-hydroxycinnamic acid in 50% aqueous acetonitrile containing 2.5% trifluoroacetic acid). Mass spectra were acquired using a MALDI-TOF MS spectrometer in a linear positive mode (Microflex™LT, Bruker Daltonics, Bermen, Germany) in a mass range of 2–20 kDa. A bacterial test standard (BTS, Bruker Daltonics, Bremen, Germany) was used for instrument calibration. The raw spectra were imported into MALDI Biotyper™ software (Bruker Daltonics, Germany) and then processed and analyzed using standard pattern matching against the reference spectra in the MALDI Biotyper™ reference database (version 3.0, Bruker Daltonics, Germany).
In vitro Screening of Bacterial Isolates for their PGP Activities
Indole 3-acetic Acid Production
Production of IAA (indole 3-acetic acid) was determined as described by Bano and Musarrat (2003). Briefly, bacterial strains were grown in TSB medium. After 3 days, 1 ml of each culture was pelleted by centrifugation, and the supernatant was discarded. Cell pellets were washed with 1 ml of PBS and re-suspended in PBS. One milliliter of cell suspension (corresponding to a cell density of 107cells/ml) was added to 10 ml of TSB amended with tryptophan (100 μg/ml). After 3 days of cultivation, 2 ml aliquots of bacterial cultures were centrifuged at 13.000 × g for 10 min. One milliliter of supernatant was transferred to a fresh tube to which 100 μg/ml of 10 mM orthophosphoric acid and 2 ml of reagent (1 ml of 0.5 M FeCl3 in 50 ml of 35% HClO4) were added. After 25 min, the absorbance of the developed pink color was read at 530 nm. The IAA concentration in culture was calculated using a calibration curve of pure IAA as a standard.
Phosphate solubilization
The phosphate-solubilizing activity of the bacterial strains was determined on Pikovskaya agar (Pikovskaya, 1948) containing precipitated tricalcium phosphate. The bacterial culture grown in TSA medium for 2 days was streaked on the surface of Pikovskaya agar plates and incubated for 3 days. The presence of a clearing zone around bacterial colonies was considered to be an indicator of positive P-solubilization.
Production of Cell Wall Degrading Enzymes
The cellulose-degrading ability of bacterial isolates was analyzed by streaking inocula on cellulose Congo-Red agar media, as described by Gupta et al. (2012). Zones of clearance around and beneath the colony were detected, indicating enzymatic degradation of cellulose. Lipase activity of the bacterial strains was determined by the Tween lipase indicator assay. Bacterial strains were grown in LA (Luria Agar) containing 2% Tween 80 at 28°C (Howe and Ward, 1976). Protease activity was determined using 5% skimmed milk agar (Brown and Foster, 1970), and pectinase activity was determined using 0.5% pectine amended in M9 medium agar (Kumar et al., 2005).
HCN Production
For testing HCN production by bacterial strains, the isolates were grown in Kings' B agar medium (KB). A sterilized filter paper saturated with a 1% solution of picric acid and 2% sodium carbonate was placed in the upper lid of the Petri plate. The Petri plate was sealed with Parafilm® M and incubated at 28°C for 3 days. The change in the paper color from yellow to dark blue was recorded as an index of HCN production (Castric, 1975).
In vitro Antibiosis Assay
The bacterial isolates were tested in vitro for their antagonistic activities against the pathogenic fungi Fusarium solani, F. culmorum, F. graminearum, Alternaria infectoria, and A. teniussima. The bacterial isolates were grown in TSB broth for 3 days and 50 μL of bacterial culture was dropped into the hole of a PDA plate (4 mm in diameter). Fungal strains were grown in peptone dextrose agar (PDA) plates at 28°C for 5 days, and disks of fresh fungal culture (5 mm diameter) were cut out and placed 2 cm from the hole filled with bacterial filtrate. The plates were sealed with Parafilm® M and incubated at 28°C in darkness until the fungi had grown over the control plates without bacteria. Antifungal activity was recorded as the width of the zone of growth inhibition between the fungus and the test bacterium.
Statistical Analyses
Data were tested for statistical significance using the analysis of variance package included in Microsoft Excel 2007. Comparisons were performed using Student's t-test. Mean comparisons were conducted using a least significant difference (LSD) test (P = 0.05).
Results
Response of Soybeans to the Type and Concentration of Biochar
The response of the soybeans to the type of biochar and to different concentrations was investigated under greenhouse conditions. Our study showed that shoot and root biomass of soybeans were not significantly affected by either MBC or WBC amendments in all concentrations (1, 2, and 3%; Figures 1A,B). However, there was a slight but not significant increase in shoot and root growth in the soybeans grown in soil amended with MBC at 1 and 2% concentrations compared with control plants (Figure 1A). In contrast, the root dry weight of soybeans was significantly increased up to 34–41%, and the shoot dry weight was increased up to 24–28% by HTC char amendment at 1 and 2% concentrations, respectively (Figure 1C).
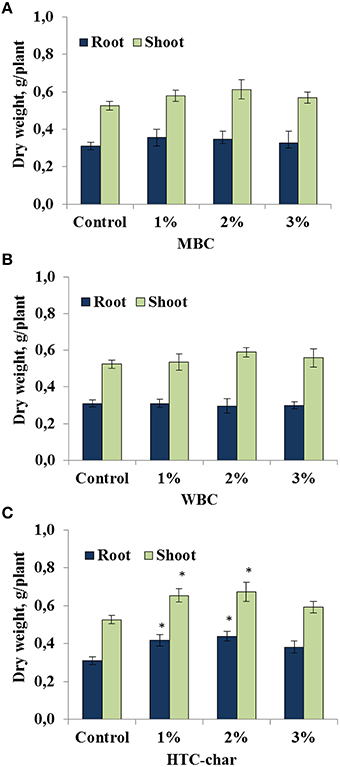
Figure 1. Root and shoot dry weights of soybeans grown in a greenhouse for 30 days under three maize biochar (MBC) (A), wood biochar (WBC) (B), and HTC-char (C) concentrations (1, 2, and 3%). Columns represent means for six plants (N = 6) with error bars showing the standard deviation. Columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05.
Enumeration of Microbes and Isolation of Root-Associated Bacteria
The results of the pot experiments showed that HTC char at a concentration of 2% stimulated the growth of soybeans and thus was used for the characterization of root-associated plant growth promoting bacteria. The bacteria were enumerated after 48 h in the plate count agar and fungi after 5 days in PDA medium. The total numbers of cultivable bacteria isolated from the rhizosphere of plants grown in soil without biochar were 1.5 × 107 CFU (colony-forming units, per gram fresh weight) and 5.3 × 107 CFU (per gram fresh weight) in soil with 2% HTC char. Furthermore, a remarkably greater number of fungi (1.8 × 104 CFU per gram fresh weight) were observed in the rhizosphere of the plants grown in soil without biochar compared with the plants grown in soil amended with 2% HTC char (0.9 × 104 CFU per gram fresh weight).
In total, 200 bacterial strains were isolated from the rhizosphere of soybeans. Among these, 90 isolates were selected from plants grown in control soil, and 110 isolates, from plants grown in HTC char amended soil. All strains were tested for their abilities to stimulate root and shoot growth of soybeans under greenhouse conditions in loamy sand soil. Root and shoot growth stimulating abilities (>20%) were observed in 27–32% of isolates from plants grown in soil without biochar and in 45–57% of isolates from soil amended with 2% HTC char, respectively. A total of 32 isolates from the control plants and 43 isolates from the HTC amended soil induced stimulatory effects on plant growth compared with the non-treated control plants.
Identification of Plant Growth Promoting Bacteria by MALDI-TOF MS
A total of 35 pure isolates from the rhizosphere of control plants and 43 isolates from the rhizosphere of soybeans grown in HTC char amended soil showing plant growth stimulation ability were taxonomically analyzed by MALDI-TOF MS. As shown in Tables 2A,B and Figure 2, there are considerable differences in the diversity of strains isolated from the HTC char amended soil and the control soil. In the rhizosphere of soybeans grown in the control soil, isolates were affiliated with seven genera, whereas 24 isolates were identified at the species level. Bacillus was the predominant genus, which was followed by the genera Arthrobacter and Rhizobium. Furthermore, isolates affiliated with the genera Cellulosimicrobium, Enterobacter and Pseudomonas were also found. The most abundant species were identified as Rhizobium radiobacter (C14, C53, C19, C87), followed by the species Arthrobacter globiformis (C3, C16), Bacillus megaterium (C32, C38), Cellulosimicrobium cellulans (C29, C42), Enterobacter asburiae (C46, C50), and Pseudomonas chlororaphis (C28, C44). Only one isolate was identified as Burkholderia terricola (Figure 2).
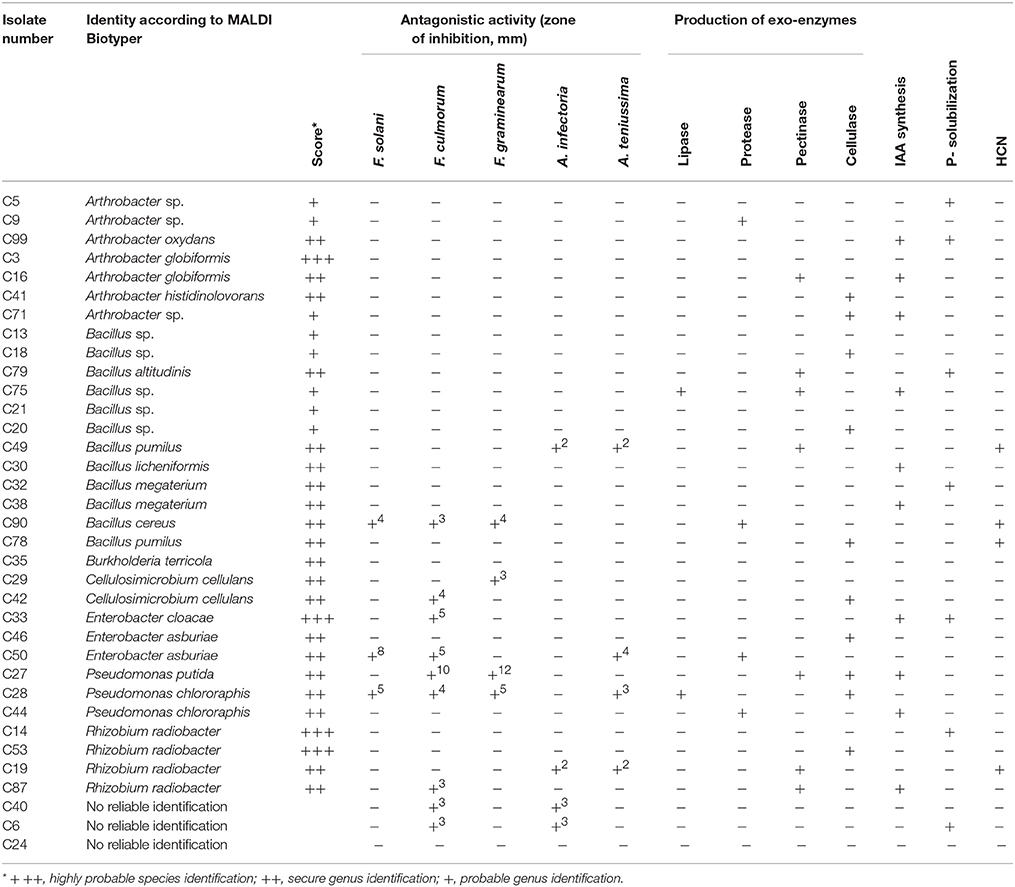
Table 2A. Plant growth promoting traits of strains isolated from the rhizosphere of soybeans grown in soil without hydrochar.
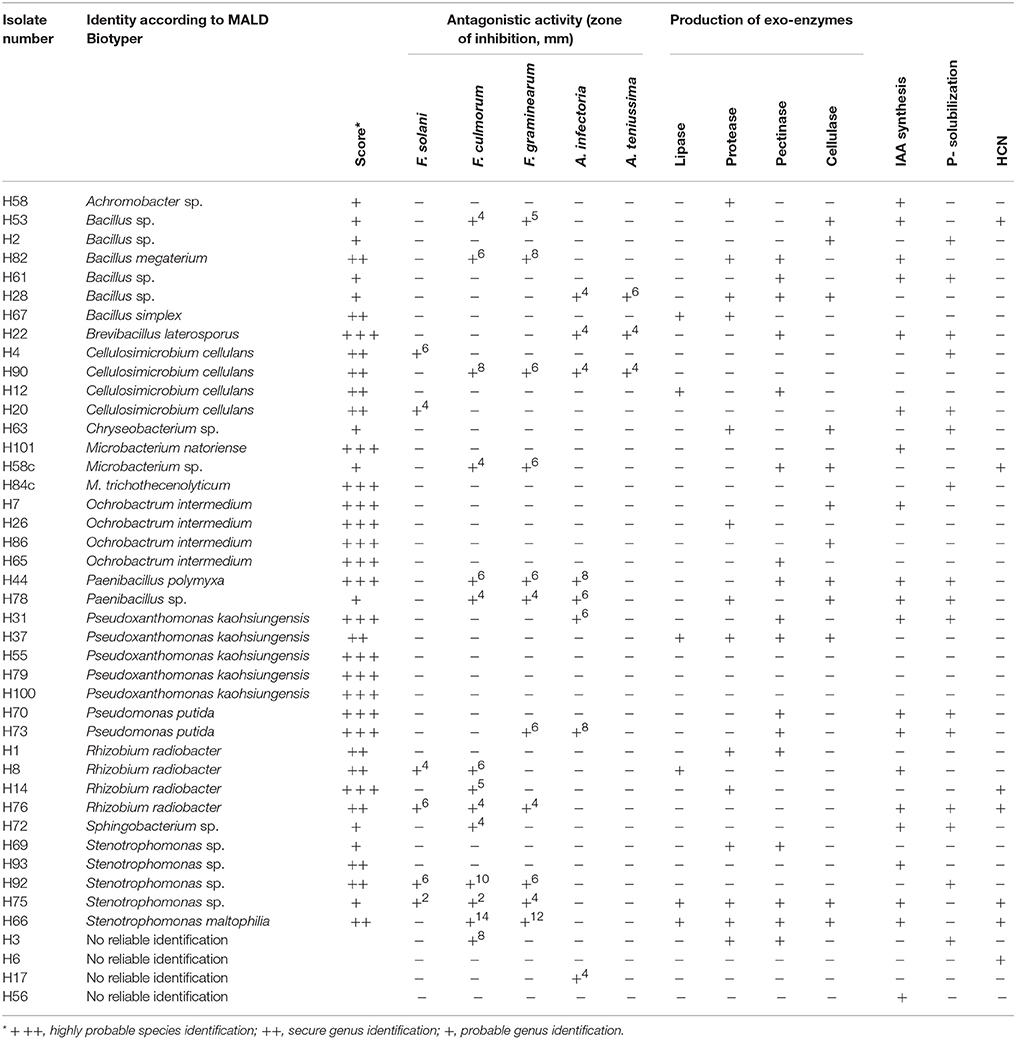
Table 2B. Plant growth promoting traits of strains isolated from the rhizosphere of soybeans grown in soil amended with 2% HTC char.
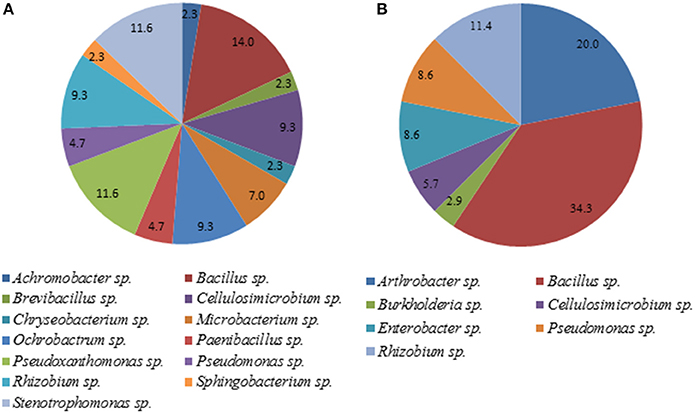
Figure 2. Diversity of cultivable bacteria in the rhizosphere of soybeans grown in control soil (without char) (A) and HTC-char amended soil (B). Numbers indicate the relative abundance, expressed as a percentage of the total number of isolates.
A total of 13 bacterial genera were isolated from the rhizosphere of soybeans grown in HTC char amended soil, whereas 12 isolates were identified at the species level (Table 2B). The isolates from biochar amended soil showed a greater diversity compared with the isolates originating from the plant rhizosphere of the control soil. The most abundant isolates were identified as Cellulosimicrobium cellulans (H4, H90, H12, H20), Ochrobactrum intermedium (H7, H26, H86, H65), Pseudoxanthomonas kaohsiungensis (H31, H37, H55, H79, H100), and Stenotrophomonas sp. (H69, H93, H92, H75). Members of the genera Achromobacter, Brevibacillus, Chryseobacterium, Microbacterium, Ochrobactrum, Paenibacillus, Pseudoxanthomonas, Sphingobacterium and Stenotrophomonas were not found among isolates from the control soil.
In vitro Plant Growth Promoting Traits
All bacterial strains isolated from the rhizosphere of soybeans grown in HTC char amended soil and without biochar were screened for multiple plant growth promoting traits. Most of the bacterial isolates exhibited one or more plant growth-promoting activities (Tables 2A,B).
The production of the phytohormone IAA by bacterial isolates is shown in Table 2A. A large amount of the rhizosphere isolates (48%) from HTC char amended soil produced IAA, whereas only 28% of the isolates from control soil showed IAA production. Most of the IAA producing isolates from control soil belonged to the genera Arthrobacter (C99, C16, C71) and Bacillus (C21, C32, C90). Three isolates belonging to the genus Stenotrophomonas (H93, H75, H66) from HTC char amended soil showed IAA activity, followed by the genera Cellulosimicrobium (H90, H20), Pseudomonas (H70, H73) and Rhizobium (H8, H76).
Positive P-solubilization was observed in 7 strains from 4 genera (20%) originating from plants grown in control soil and 16 strains from 11 genera (37%) originating from HTC char amended soil. All bacterial isolates were screened for their ability to suppress plant pathogenic fungi, such as Fusarium solani, F. culmorum, F. graminearum, Alternaria infectoria, and A. teniussima. The proportions of isolates with antagonistic activity to one or more pathogens was higher for the HTC char amended soil (51%) than for the control soil (28%). As shown in Table 2A, two Pseudomonas chlororaphis strains, C27 and C28, from control soil and six isolates, Stenotrophomonas maltophilia H66, Stenotrophomonas sp. H92, Cellulosimicrobium cellulans H90, Bacillus megaterium H82, Paenibacillus polymyxa C44, and Pseudomonas putida 73, from HTC char amended soil exerted the highest inhibition of mycelial growth of the genus Fusarium.
The ability of isolates to produce cell wall degrading enzymes, as well as proteases and lipases, was also determined. The isolates from the HTC char amended soil exhibited a higher proportion of enzyme producers than the control soil, where lipase, protease, pectinase and cellulase activity were detected in 14, 33, 40, and 26% of the isolates, respectively. The percentage of enzyme producing bacteria isolated from control soil was lower, where only 6% of isolates exhibited lipase, 11% protease, 20% pectinase, and 29% cellulase activity. Out of isolates that exhibited plant growth-promoting activities in vitro, eight isolates (H66, H75, H72, H76, H73, H44, H22, and H90) originating from HTC char amended soil and six isolates (C99, C28, C46, C78, C30, and C87) originating from control soil were selected for plant growth stimulation under greenhouse conditions.
Plant Growth Stimulation
All 14 selected bacterial strains were screened for plant growth stimulating abilities in pots under greenhouse conditions. The results showed that six strains isolated from plants grown in the control soil without biochar significantly increased root or shoot dry weight compared with the untreated controls (Figures 3A,B). The root dry weight increased up to 51% after inoculation with Pseudomonas chlororaphis (C28) and the shoot dry weight increased up to 44% with Enterobacter asburiae (C46; Figure 3A). Significant increases (between 28 and 63%) in plant dry weight relative to non-inoculated controls were observed with isolates from HTC char amended soil. The isolates Cellulosimicrobium cellulans (H90), Pseudomonas putida (H73), Stenotrophomonas maltophilia (H66) and Stenotrophomonas sp. (H75) showed significantly higher plant growth stimulation, from 40 to 63% (Figure 3B).
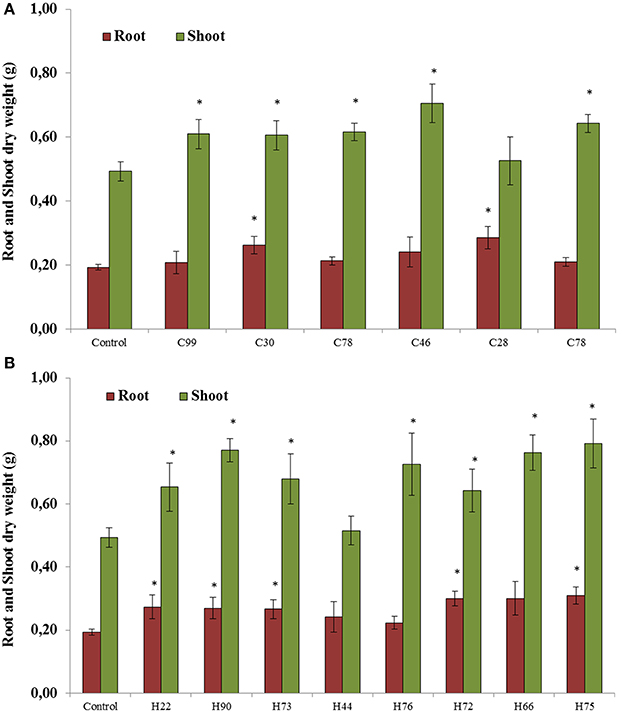
Figure 3. Root and shoot dry weight of soybeans when seedlings were inoculated with bacterial strains isolated from soybeans grown in soil without char (A) and in 2% HTC-char (B) amended soil. Columns represent the means for six plants (N = 6), with error bars showing the standard deviation. Columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05.
Discussion
Biochar incorporation into soil has been shown to enhance plant growth, to sequester carbon and to improve soil fertility, and moreover to protect plants from various soil borne pathogens (Lehmann and Joseph, 2009; Zimmerman, 2010). The increase in plant growth with biochar application has been reported for various species such as pine and alder (Robertson et al., 2012), peanut (Agegnehu et al., 2015), tomato (Vaccari et al., 2015), wheat (Akhtar et al., 2015) and also soybean (Sanvong and Nathewet, 2014)—however, several other studies reported no significant effect on plant growth (Chan et al., 2007; Van Zwieten et al., 2010). In summary, these observations indicate that effects of biochar on plant growth depend on the type of biochar, the application rate, and soil properties (Alburquerque et al., 2014) but mechanisms behind effects mostly remain unresolved. In our study, we confirmed a positive impact of HTC char treatment on the growth of soybean, but not in case of either MBC or WBC amendments. Similar observations were reported by Reibe et al. (2015b), when Pyro-char (MBC) and HTC-char applications resulted in significantly higher dry matter yields of wheat after 6 weeks of growth in rhizoboxes, as compared to Pyreq-char (WBC) or a control. There are several possible reasons why hydrochar might increase plant growth and enhance nutrient acquisition. Hyrdochar contains a higher amount of labile carbon fractions (Cao et al., 2010), which may stimulate microbial activity and thereby improve soil nutrient cycling (Kolb et al., 2009). Furthermore, hydrochars were found to reduce nitrogen losses from soil by immobilization and may provide nitrogen in plant-available form (Libra et al., 2011), whereas Pyro-chars contain less nitrogen with a decreased availability to plants (Gaskin et al., 2010).
Furthermore, HTC biochar amendment showed an impact on root associated microbes and on microbial interactions with plants, which were previously rarely studied in this context. Our findings are confirmed by the results of Kolton et al. (2011), who showed a clear shift in the total root-associated microbial community composition of mature sweet pepper (Capsicum annuum L.) after amendment with biochar from citrus wood. In our study, the analysis of cultivable root associated bacteria demonstrated that HTC char amendments increased bacterial populations in the rhizosphere of soybeans compared with control plants, whereas fungal growth was decreased over the control, in agreement with Chen et al. (2013). An increased microbial activity in the rhizosphere after the addition of hydrochar could be explained as a result of changes in soil chemical and physical properties in the root surface area. Prendergast-Miller et al. (2014) observed that biochar-amended soils had larger rhizosphere zones than the control. Moreover, the rhizosphere contained biochar particles providing additional labile carbon, nitrogen and phosphorus sources and also habitat niches, supporting bacterial proliferation and persistence in the rhizosphere.
In all rhizosphere samples from soybeans, we found a high diversity of potential plant growth promoting rhizobacteria. However, the species composition in the treated and non-treated plants was different. The most abundant species isolated from soybeans grown in control soil were Rhizobium radiobacter, Arthrobacter globiformis, and Bacillus megaterium, whereas in HTC char amended soil, Cellulosimicrobium cellulans, Ochrobactrum intermedium, Pseudoxanthomonas kaohsiungensis, and Stenotrophomonas sp. were dominant. The species identified in our study are already known for their plant growth promoting abilities, e.g., R. radiobacter stimulated growth of barley (Hordeum vulgare; Humphry et al., 2007), and B. megaterium stimulated growth of bean (Phaseolus vulgaris; Ortíz-Castro et al., 2005). Furthermore, a strain of the species C. cellulans (KUGr3) is able to form IAA, solubilize phosphate and stimulated growth of chili plants (Capsicum annuam; Chatterjee et al., 2009). O. intermedium increased seed germination, root and shoot length, and grain yield in lentil (Lens esculenta; Faisal, 2013). Several Stenotrophomonas sp. strains increased root and shoot growth and the nutrient uptake of soybean (Glycine max), cucumber (Cucumis sativus), and tomato (Solanum lycopersicum; Egamberdieva et al., 2011; Berg and Martinez, 2015).
In addition, the beneficial properties of species in treated and non-treated plants were different. Compared with control soil, a higher proportion of isolates from the HTC char amended soil was found to produce IAA, HCN and cell wall degrading enzymes. Furthermore, a higher proportion of bacterial isolates was capable of hydrolyzing organic and inorganic phosphorus from insoluble compounds and showed antagonistic activity to one or more pathogens. In the present study, a decrease in fungal populations (~50% reduction) was observed after HTC char addition. The increased proportion of bacteria capable of inhibiting fungal pathogens following amendment of HTC char suggests that the observed suppression of the fungal population was due to antagonistic interactions of microbes. The phytohormone IAA is a naturally occurring auxin which has a major role in the regulation of plant growth. The stimulation of the growth of various plants by inoculation with PGPR and IAA producing ability is well-documented (Egamberdieva, 2009, 2012; Berg et al., 2010). Phytohormones produced by root-associated bacteria will be taken up by plant cells, stimulate cell proliferation, and enlarge the root system so that nutrients and water can be taken up more efficiently. For example, IAA producing Stenotrophomonas rhizophila significantly affected plant growth, N and P uptake and the number of nodules in soybean (Egamberdieva et al., 2015). Similarly, multiple isolates from the rhizosphere that suppress fungal growth by the production of HCN, cell wall degrading enzymes or antifungal compounds were used to prevent and control fungal diseases (Berg et al., 2013b; Maurer et al., 2013). Seed coating with Pseudomonas strains antagonistic to soilborne pathogens, such as Sclerotium rolfsii, Fusarium oxysporum, and Rhizoctonia solani, produced siderophores, chitinase, and HCN and were therefore able to suppress infections in soybean seedlings by fungal pathogens (Susilowati et al., 2011). In another study, the charcoal root rot of soybean caused by Macrophomina phaseolina was attenuated by the antagonistic bacterial strains P. agglomerans and Bacillus sp. under greenhouse conditions (Vasebi et al., 2013). The mechanisms involved in plant growth stimulation and the biological control of plant pathogens were also observed for bacterial isolates in our study and were thus further evaluated for their impact on plant growth promotion of soybeans under greenhouse conditions. Indeed, inoculation of soybeans with these isolates led to significant increases in plant growth and development. In previous studies, PGPR Stenotrophomonas rhizophila was able to stimulate root and shoot growth, nodulation and nutrient uptake of soybeans under greenhouse conditions (Egamberdieva et al., 2015). Similarly, Aung et al. (2013) found a significant increase in shoot and root biomass, as well as nodulation in soybeans inoculated with Azospirillum sp., compared to non-inoculated controls under pot conditions.
From our study, we conclude that increased plant growth in response to soil amendment with biochar is based on the type of char, i.e., HTC application increased growth of soybean but not in case of either MBC or WBC. Moreover, HTC application was shown to alter the community composition of root associated microbes exhibiting plant growth-promoting activities in vitro such as phytohormone production and suppression of fungal pathogens. Thus, we provided evidence that improved plant growth by hydrochar incorporation into soil is mostly an indirect rather than a direct effect that depends on the type of char and the activity of plant-associated beneficial soil bacteria. The stimulation of certain plant-beneficial bacteria by biochar also suggests the possibility of developing combined approaches of biochar treatment and biological control solutions (Berg et al., 2013b).
Author Contributions
DE, SW, and GB did experimental design work. DE and UB conducted experiments. EA analyzed the data. DE, SW, and GB wrote the manuscript. All authors read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Georg Forster Research Fellowship (HERMES), the Alexander von Humboldt Foundation for DE. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this Research group no. (RG-1435-014).
Footnotes
1. ^FAOstat (2013). Available online at: http://faostat.fao.org/ (2013-02-25).
References
Agegnehu, G., Bass, A. M., Nelson, P. N., Muirhead, B., Wright, G., and Birda, M. I. (2015). Biochar and biochar-compost as soil amendments: effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 213, 72–85. doi: 10.1016/j.agee.2015.07.027
Akhtar, S. S., Andersen, M. N., and Liu, F. (2015). Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 158, 61–68. doi: 10.1016/j.agwat.2015.04.010
Alburquerque, J. A., Calero, J. M., Barrón, V., Torrent, J., del Campillo, M. C., Gallardo, A., et al. (2014). Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 177, 16–25. doi: 10.1002/jpln.201200652
Anders, E., Watzinger, A., Remt, F., Kitzler, B., Wimmer, B., Zehetner, F., et al. (2013). Biochar affects the structure rather than the total biomass of microbial communities in temperate soils. Agric. Food Sci. 22, 404–423.
Argaw, A. (2012). Evaluation of co-inoculation of Bradyrhizobium japonicum and phosphate solubilizing Pseudomonas spp. effect on soybean (Glycine max L. (Merr.) in Assossa Area. J. Agric. Sci. Tech. 14, 213–224.
Aung, T. T., Tittabutr, P., Boonkerd, N., Herridge, D., and Teaumroong, N. (2013). Co-inoculation effects of Bradyrhizobium japonicum and Azospirillum sp. on competitive nodulation and rhizosphere eubacterial community structures of soybean under rhizobia-established soil conditions. Afr. J. Biotech. 12, 2850–2862. doi: 10.5897/AJB12.2557
Bano, N., and Musarrat, J. (2003). Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microb. 46, 324–328. doi: 10.1007/s00284-002-3857-8
Barbosa Lima, A., Cannavan, F. S., Navarrete, A. A., Teixeira, W. G., Kuramae, E. E., and Tsai, S. M. (2015). Amazonian dark Earth and plant species from the Amazon region contribute to shape rhizosphere bacterial communities. Microb. Ecol. 69, 855–866. doi: 10.1007/s00248-014-0472-8
Berg, G., Alavi, M., Schmidt, C. S., Zachow, C., Egamberdieva, D., Kamilova, F., et al. (2013a). “Biocontrol and osmoprotection for plants under saline conditions,” in Molecular Microbial Ecology of the Rhizosphere, ed F. J. de Bruijn (Hoboken, NJ: John Wiley & Sons, Inc.). doi: 10.1002/9781118297674.ch55
Berg, G., Egamberdieva, D., Lugtenberg, B., and Hagemann, M. (2010). “Symbiotic plant-microbe interactions: stress protection, plant growth promotion and biocontrol by Stenotrophomonas,” in Symbioses and Stress, Cellular Origin, Life in Extreme Habitats and Astrobiology, eds J. Seckbach and M. Grube (Dordrecht: Springer Science+Business Media B.V.), 445–460.
Berg, G., and Martinez, J. L. (2015). Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front. Microbiol. 6:241. doi: 10.3389/fmicb.2015.00241
Berg, G., and Smalla, K. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. doi: 10.1111/j.1574-6941.2009.00654.x
Berg, G., Zachow, C., Müller, H., Philipps, J., and Tilcher, R. (2013b). Next-generation bio-products sowing the seeds of success for sustainable agriculture. Agronomy 3, 648–656. doi: 10.3390/agronomy3040648
Brown, M. R. W., and Foster, J. H. S. (1970). A simple diagnostic milk medium for Pseudomonas aeruginosa. J. Clin. Pathol. 23, 172–176. doi: 10.1136/jcp.23.2.172
Cao, X., Ro, K. S., Chappell, M., Li, Y., and Mao, J. (2010). Chemical structure of swine-manure chars with different carbonization conditions using advanced solid-state 13C NMR spectroscopy. Energy Fuels 25, 388–397. doi: 10.1021/ef101342v
Castric, P. A. (1975). Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 21, 613–618. doi: 10.1139/m75-088
Chan, K. Y., Van Zwieten, L., Meszaros, I., Downie, A., and Joseph, S. (2007). Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 45, 629–634. doi: 10.1071/SR07109
Chatterjee, S., Sau, G. B., and Mukherjee, S. K. (2009). Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J. Microb. Biotech. 25, 1829–1836. doi: 10.1007/s11274-009-0084-5
Chen, J., Liu, X., Zheng, J., Zhang, B., Lu, H., Chi, Z., et al. (2013). Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 71, 33–44. doi: 10.1016/j.apsoil.2013.05.003
Egamberdieva, D. (2009). Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 31, 861–864. doi: 10.1007/s11738-009-0297-0
Egamberdieva, D. (2012). Pseudomonas chlororaphis: a salt tolerant bacterial inoculant for plant growth stimulation under saline soil conditions. Acta Physiol. Plant. 34, 751–756. doi: 10.1007/s11738-011-0875-9
Egamberdieva, D., Jabborova, D., and Berg, G. (2015). Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, nodulation and nutrition of soybean under salt stress. Plant Soil 1–11. doi: 10.1007/s11104-015-2661-8
Egamberdieva, D., Kamilova, F., Validov, S., Gafurova, L., Kucharova, Z., and Lugtenberg, B. (2008). High incidence of plant growth stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 10, 1–9. doi: 10.1111/j.1462-2920.2007.01424.x
Egamberdieva, D., Kucharova, Z., Davranov, K., Berg, G., Makarova, N., Azarova, T., et al. (2011). Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fert. Soils 47, 197–205. doi: 10.1007/s00374-010-0523-3
Elad, Y., Rav David, D., Meller Harel, Y., Borenshtein, M., Ben Kalifa, H., Silber, A., et al. (2010). Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 9, 913–921. doi: 10.1094/PHYTO-100-9-0913
Erlacher, A., Cardinale, M., Grosch, R., Grube, M., and Berg, G. (2014). The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 5:175. doi: 10.3389/fmicb.2014.00175
Faisal, M. (2013). Inoculation of plant growth promoting bacteria Ochrobactrum intermedium, Brevibacterium sp. and Bacillus cereus induce plant growth parameters. J. Appl. Biotech. 1, 45–53. doi: 10.5296/jab.v1i1.3698
Gaskin, J. W., Speir, R. A., Harris, K., Das, K. C., Lee, R. D., Morris, L. A., et al. (2010). Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 102, 623–633. doi: 10.2134/agronj2009.0083
Glick, B. R. (2014). Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169, 30–39. doi: 10.1016/j.micres.2013.09.009
Gupta, P., Samant, K., and Sahu, A. (2012). Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012:578925. doi: 10.1155/2012/578925
Howe, T. G., and Ward, J. M. (1976). The utilization of tween 80 as carbon source by Pseudomonas. J. Gen. Microbiol. 92, 234–235. doi: 10.1099/00221287-92-1-234
Humphry, D. R., Andrews, M., Santos, S. T., James, E. K., Vinogradova, L. V. Perin. L., et al. (2007). Phylogenetic assignment and mechanism of action of a crop growth promoting Rhizobium radiobacter strain used as a biofertiliser on graminaceous crops in Russia. Antonie van Leeuwenhoek 9, 105–113. doi: 10.1007/s10482-006-9100-z
Köberl, M., Schmidt, R., Ramadan, E. M., Bauer, R., and Berg, G. (2013). The microbiome of medicinal plants: diversity and importance for plant growth, quality and health. Front. Microbiol. 4:400. doi: 10.3389/fmicb.2013.00400
Kolb, S. E., Fermanich, K. J., and Dornbush, M. E. (2009). Effect of charcoal quantity on microbial biomass and activity in temperate soils. SSSAJ 73, 1173–1181. doi: 10.2136/sssaj2008.0232
Kolton, M., Meller Harel, Y., Pasternak, Z., Graber, E. R., Elad, Y., and Cytryn, E. (2011). Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microb. 77, 4924–4930. doi: 10.1128/AEM.00148-11
Kumar, R. S., Ayyadurai, N., Pandiaraja, P., Reddy, A. V., Venkatesvarlu, Y., Prsakash, O., et al. (2005). Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad spectrum antifungal activity and biofertilizing traits. J. Appl. Microbiol. 98, 145–154. doi: 10.1111/j.1365-2672.2004.02435.x
Laird, D. A., Fleming, P. D., Davis, D. D., Horton, R., Wang, B., and Karlen, D. L. (2010). Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158, 443–449. doi: 10.1016/j.geoderma.2010.05.013
Lehmann, J., and Joseph, S. (2009). “Biochar for environmental management: an introduction,” in Biochar for Environmental Management: Science and Technology, eds J. Lehmann and S. Joseph (London: Earthscan), 1–12.
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., and Crowley, D. (2011). Biochar effects on soil biota – a review. Soil Biol. Biochem. 43, 1812–1836. doi: 10.1016/j.soilbio.2011.04.022
Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D., Neubauer, Y., et al. (2011). Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2, 71–106. doi: 10.4155/bfs.10.81
Maurer, K. A., Zachow, C., Seefelder, S., and Berg, G. (2013). Initial steps towards biocontrol in hops: Successful colonization and plant growth promotion by four bacterial biocontrol agents. Agronomy 3, 583–594. doi: 10.3390/agronomy3040583
Mete, F. Z., Mia, S., Dijkstra, F. A., Abuyusuf, M., and Iqbal Hossain, A. S. M. (2015). Synergistic effects of biochar and NPK Fertilizer on soybean yield in an alkaline soil. Pedosphere 25, 713–719. doi: 10.1016/S1002-0160(15)30052-7
Ortíz-Castro, R., Valencia-Cantero, E., and López-Bucio, J. (2005). Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 3, 263–265. doi: 10.4161/psb.3.4.5204
Oteino, N., Lally, R. D., Kiwanuka, S., Lloyd, A., Ryan, D., Germaine, K. J., et al. (2015). Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 6:745. doi: 10.3389/fmicb.2015.00745
Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologiya 17, 362–370.
Prendergast-Miller, M. T., Duvall, M., and Sohi, S. P. (2014). Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 65, 173–185. doi: 10.1111/ejss.12079
Reibe, K., Götz, K. P., Ross, C. L., Doering, T. F., Ellmer, F., and Ruess, L. (2015a). Impact of quality and quantity of biochar and hydrochar on soil collembola and growth of spring wheat. Soil Biol. Biochem. 8, 84–87. doi: 10.1016/j.soilbio.2015.01.014
Reibe, K., Roß, C. L., and Ellmer, F. (2015b). Hydro-/Biochar application to sandy soils: impact on yield components and nutrients of spring wheat in pots. Arch. Agron. Soil Sci. 61, 1055–1060. doi: 10.1080/03650340.2014.977786
Robertson, S. J., Rutherford, P. M., Lopez-Gutierrez, J. C., and Massicotte, H. B. (2012). Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can. J. Soil Sci. 92, 329–340. doi: 10.4141/cjss2011-066
Rondon, M. A., Lehmann, J., Ramirez, J., and Hurdalo, M. (2007). Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with biochar additions. Biol. Fertil. Soils 43, 699–708. doi: 10.1007/s00374-006-0152-z
Sanvong, C., and Nathewet, P. (2014). A comparative study of pelleted broiler litter biochar derived from lab-scale pyrolysis reactor with that resulted from 200-liter-oil drum kiln to ameliorate the relations between physicochemical properties of soil with lower organic matter soil and soybean yield. Environment Asia 7, 95–100. doi: 10.14456/ea.2014.13
Schmidt, C. S., Alavi, M., Cardinale, M., Müller, H., and Berg, G. (2012). Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol. Fertil. Soils 48, 947–960. doi: 10.1007/s00374-012-0688-z
Schomberg, H. H., Gaskin, J. W., Harris, K., Das, K. C., Novak, J. M., Busscher, W. J., et al. (2012). Influence of biochar on nitrogen fractions in a coastal plain soil. J. Environ. Qual. 41, 1087–1095. doi: 10.2134/jeq2011.0133
Solanki, M. K., Singh, R. K., Srivastava, S., Kumar, S., Kashyap, P. L., Srivastava, A. K., et al. (2014). Isolation and characterization of siderophore producing antagonistic rhizobacteria against Rhizoctonia solani. J. Basic Microb. 54, 585–597. doi: 10.1002/jobm.201200564
Spaepen, S. (2015). “Plant hormones produced by microbes,” in Principles of Plant-Microbe Interactions, ed B. Lugtenberg (Basel: Springer International Publishing AG), 247–256.
Spokas, K. A., Baker, J. M., and Reicosky, D. C. (2010). Ethylene: potential key for biochar amendment impacts. Plant Soil 333, 443–452. doi: 10.1007/s11104-010-0359-5
Suppadit, T., Phumkokrak, N., and Poungsuk, P. (2012). The effect of using quail litter biochar on soybean (Glycine max [L.] Merr.) production. Chil. J. Agric. Res. 72, 244–251. doi: 10.4067/S0718-58392012000200013
Susilowati, A., Wahyudi, A. T., Lestari, Y., Suwanto, A., and Wiyono, S. (2011). Potential of Pseudomonas isolated from soybean rhizosphere as biocontrol against soilborne phytopathogenic fungi. HAYATI. J. Biosci. 18, 51–56. doi: 10.4308/hjb.18.2.51
Tagoe, S., Horiuchi, T., and Matsui, T. (2008). Effects of carbonized and dried chicken manures on the growth, yield, and N content of soybean. Plant Soil 306, 211–220. doi: 10.1007/s11104-008-9573-9
Vaccari, F. P., Maienza, A., Miglietta, F., Baronti, S., Di Lonardo, S., Giagnoni, L., et al. (2015). Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric Ecosyst. Environ. 207, 163–170. doi: 10.1016/j.agee.2015.04.015
Van Zwieten, L., Kimber, S., Morris, S., Chan, K. Y., Downie, A., Rust, J., et al. (2010). Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327(1–2), 235–246. doi: 10.1007/s11104-009-0050-x
Vasebi, Y., Safaie, N., and Alizadeh, A. (2013). Biological control of soybean charcoal root rot disease using bacterial and fungal antagonists in vitro and greenhouse condition. J. Crop Prot. 2, 139–150.
Keywords: soybean, rhizosphere, plant growth promoting rhizobacteria, biochar
Citation: Egamberdieva D, Wirth S, Behrendt U, Abd_Allah EF and Berg G (2016) Biochar Treatment Resulted in a Combined Effect on Soybean Growth Promotion and a Shift in Plant Growth Promoting Rhizobacteria. Front. Microbiol. 7:209. doi: 10.3389/fmicb.2016.00209
Received: 10 December 2015; Accepted: 08 February 2016;
Published: 25 February 2016.
Edited by:
Suhelen Egan, The University of New South Wales, AustraliaReviewed by:
Hovik Panosyan, Yerevan State University, ArmeniaAdriano Reis Lucheta, Netherlands Institute of Ecology, Netherlands
Copyright © 2016 Egamberdieva, Wirth, Behrendt, Abd_Allah and Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilfuza Egamberdieva, egamberdieva@yahoo.com
 Dilfuza Egamberdieva
Dilfuza Egamberdieva Stephan Wirth
Stephan Wirth Undine Behrendt
Undine Behrendt Elsayed F. Abd_Allah
Elsayed F. Abd_Allah Gabriele Berg
Gabriele Berg