- 1Department of Medical Microbiology, Tropical Infectious Disease Research and Education Center, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 2Department of Pharmacology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Objectives: Cryptococcus neoformans is an opportunistic fungus that causes fatal meningoencephalitis especially in AIDS patients. There is an increasing need for discovery of new anti-cryptococcal drugs due to emergence of resistance cases in recent years. In this study, we aim to elucidate the antifungal effect of triclosan against C. neoformans.
Methods: Minimal inhibitory concentration (MIC) of triclosan in different C. neoformans strains was first examined. The in vitro interactions between triclosan and two standard anti-fungal drugs (amphotericin B and fluconazole) were further evaluated by microdilution checkerboard assay. Mechanism of triclosan fungicidal activity was then investigated by viewing the cell morphology under transmission electron microscope.
Results: We reported that triclosan potently inhibited the growth of C. neoformans. A combination of triclosan with amphotericin B or with fluconazole enhanced their fungicidal effects. Triclosan-treated C. neoformans displayed characteristics such as nuclear chromatin condensation, extensive intracellular vacuolation and mitochondrial swelling, indicating that triclosan triggered apoptosis-like cell death.
Conclusion: In summary, our report suggests triclosan as an independent drug or synergent for C. neoformans treatment.
Introduction
Infection by Cryptococcus neoformans has become a major cause of mortality following the increased numbers of AIDS patients (Mitchell and Perfect, 1995; Pancharoen et al., 2001) and is estimated to cause approximately 600,000 deaths worldwide annually (Park et al., 2009). C. neoformans exists ubiquitously in the environment, commonly found in decaying wood and bird excreta (Lazera et al., 1996). Most individuals are exposed to C. neoformans through inhalation from the environment during their childhood but stay asymptomatic (Goldman et al., 2001) because C. neoformans is an opportunistic pathogen which rarely progresses to disease in immune competent individuals. However, it can result in life-threatening infection in immunosuppressed or immunocompromised patients (Coelho et al., 2014). Infection of C. neoformans in lung leads to cryptococcal pneumonia whereas systemic dissemination often causes fatal meningoencephalitis (Lin and Heitman, 2006).
In recent years, the widespread use of anti-microbial drugs in the treatment of fungal infection has led to the global emergence of resistant fungal strains. Numerous fungal pathogens including C. neoformans have demonstrated increasing resistance to common antifungal drugs such as amphotericin B and fluconazole (Perfect and Cox, 1999). A recent global study of nearly three thousand C. neoformans isolates shows that >11% of the isolates are resistant to fluconazole (Pfaller et al., 2009). Fluconazole-heteroresistant phenotype of C. neoformans has also been detected in a significant proportion of clinical isolates (Yamazumi et al., 2003). Increased drug resistance in fungal species is mainly attributed to overexpression and hotspot mutations of genes that encode for efflux ATP-binding cassette (ABC) transporters (Sanglard et al., 1995; Looi et al., 2005) such as CDR1, CDR2, and MDR1 (Fling et al., 1991; Prasad et al., 1995; Sanglard et al., 1997). Changes in fungal cell phospholipids and membrane sterol composition can also reduce drug permeation ability (Hitchcock et al., 1986). Alternate usage of other enzymes in the same biosynthetic pathway as a substitute for the drug-targeted enzyme also confers resilience and endurance to the yeasts (Howell et al., 1990). Therefore, studies to discover new drugs or combination of multiple anti-microbial drugs are essential. Some drug combinations demonstrate synergistic effect and superiority to the currently available therapies (Mukherjee et al., 2005; Munoz et al., 2006). For instance, combinational usage of fluconazole plus amphotericin B adds benefit in candidemia treatment (Odds, 2003a).
Triclosan (2,4,4′-trichloro-2′-hydroxydiphenylether, C12H17 Cl3O2) is a chlorinated compound which is widely used for personal care products such as soap, toothpaste and plastics in domestic as well as healthcare settings due to its safety, efficiency and long-lasting effects (Jones et al., 2000). Importantly, various studies using different test systems indicate that triclosan is a non-mutagenic and non-genotoxic agent (Fang et al., 2010). The drug is able to persist for at least 0.7 h (Loftsson et al., 1999) in human fluids, including nasal secretion, serum, urine, and milk after exposure (Syed et al., 2014). Triclosan demonstrates broad-spectrum anti-microbial properties against various species of microorganisms with MICs ranging from 0.1 to 30 mg/l (Schweizer, 2001) including Candida species (Vischer and Regos, 1974; Regos et al., 1979). Previous studies suggest that triclosan likely perturbs cell structure and results in loss of permeability-barrier function (Villalain et al., 2001). It inhibits bacterial and fungal fatty acid synthetic enzyme, by targeting Fab1–encoding NADH-dependent enoyl-acyl-carrier protein reductase (McMurry et al., 1998; Heath et al., 1999; Stewart et al., 1999).
Although the antifungal effect of triclosan has been reported in Candida albicans and several fungal species (Vischer and Regos, 1974; Regos et al., 1979; Yu et al., 2011), at present, the effect of triclosan in C. neoformans has not been investigated. In this study, the inhibitory effect of triclosan as well as the fungicidal synergism between triclosan and standard antifungal drugs against C. neoformans were evaluated. In addition, we examined the mechanism of antifungal action by viewing triclosan-treated cells under electron microscopy.
Materials and Methods
Fungal Isolates and Chemicals
Cryptococcus neoformans H99, C. albicans 90028 and C. albicans SC5314 (MYA2876) were obtained from American Type Culture Collection (ATCC). C. neoformans C14 and C17 strains were isolated from inpatients at the University of Malaya Medical Center. C. neoformans H4, S48B, and S68B environmental strains were isolated from bird droppings at different locations in Klang valley, Malaysia (Tay et al., 2005). All C. neoformans strains used were C. neoformans var. grubii, the predominant serotype A, genotype VNI with an α-mating type (Tay et al., 2006).
Amphotericin B, fluconazole, and triclosan were purchased from Sigma–Aldrich (St. Louis, MO, USA). Stock solutions were prepared at 10 mg/ml by dissolving the amphotericin B and triclosan powder in Dimethyl sulfoxide (DMSO), and fluconazole in water. Drugs were stored at -20°C until use.
Disk Diffusion Assay
Five single colonies were picked and inoculated into Sabouraud dextrose broth (SDB). Cells were grown overnight in a rotary shaker at 200 rpm at 35°C. An aliquot of 100 μl of the yeast suspension at 106 CFU/ml was prepared, applied onto the Sabouraud dextrose agar (SDA) plate and spread uniformly using a cotton swab. Then, 6-mm paper disks impregnated with different concentrations of triclosan (3.125, 6.25, 12.5, 25, 50, and 100 μg) were positioned on a C. neoformans H99 agar plate to evaluate the antifungal effect of triclosan. In the synergy test, 25 μg of a drug alone or in combination were placed onto the agar surface. The synergy test was repeated using sub-inhibitory concentrations of 6.25 μg/ml from AMB and FLU alone and in combination with 6.25, 3.125, and 1.56 μg/ml of triclosan. Dimethyl sulfoxide (DMSO) was used as negative control. After incubation at 37°C for 48 h, the diameters of the growth inhibition zone were measured.
Broth Microdilution Assay
The drug minimum inhibition concentration (MIC) was determined by broth microdilution assay according to Clinical and Laboratory Standards Institute (CLSI) standard (Clinical and Laboratory Standards Institute [CLSI], 2008). The inoculum was prepared by picking five colonies (∼1 mm diameter) from a fresh culture plate. Colonies were resuspended in 2 ml distilled water and vortexed for 15 s. The cell density was adjusted to 75% transmittance at 530 nm wavelength using a spectrophotometer. A working suspension was made by a 1:50 dilution followed by a 1:10 dilution of the stock suspension with RPMI 1640 medium supplemented with 34.53 mg/ml morpholinepropanesulfonic acid (MOPS) at pH 7.0 to yield yeast stock suspension of 1.0 to 5.0 × 103 cells/ml. Antifungal drugs, amphotericin B (32 to 0.017 μg/ml), fluconazole (128 to 0.068 μg/ml), and triclosan (128 to 0.068 μg/ml) were serially diluted in 96-well flat-bottomed microtiter plates. The cells suspension (100 μl) were then seeded into the plate and incubated for 48 h. The microtiter plates for C. albicans and C. neoformans were visually scored after incubation at 37°C for 24 and 48 h, respectively (Tay et al., 2011). Each well was resuspended and agitated for 5 min, and the optical density (OD) at 570 nm wavelength was determined with a spectrophotometer. All samples were run in duplicate. The MIC-1 was defined as the minimal concentration that resulted in 80% growth inhibition while MIC-2 was defined as the minimal concentration that resulted in 50% of growth inhibition (Yu et al., 2011).
Fungicidal Assay
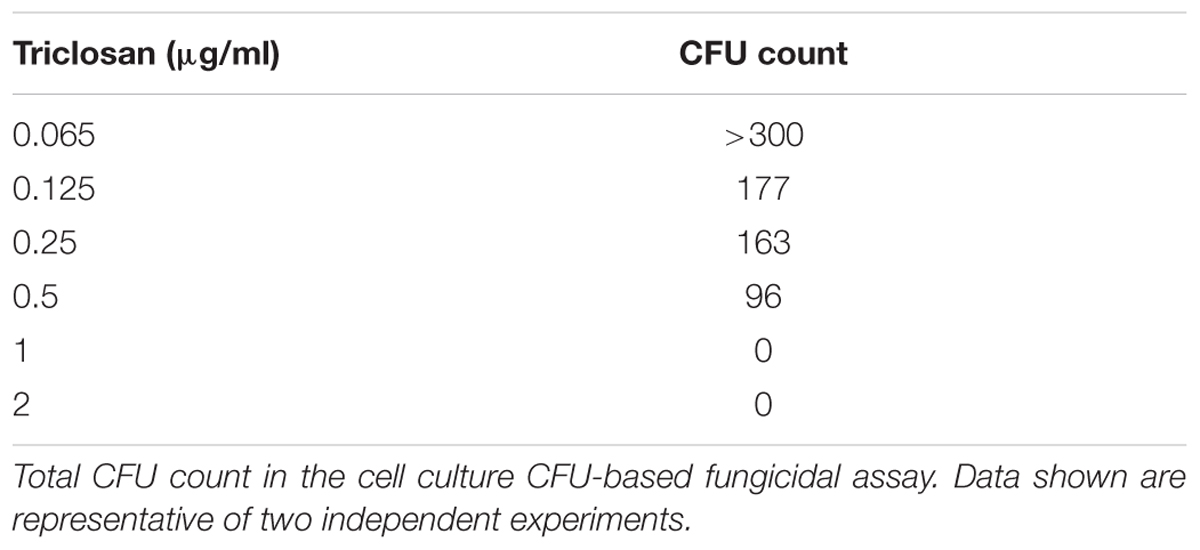
The MFCs of triclosan was determined by conventional culture-based CFU method from microtitration plate in duplicates, as previously described (Meletiadis et al., 2007). Total amount of 20 and 100 μl from all visually clear wells and the first well with the highest drug concentration showing growth (0.5x MIC) were mixed by pipetting up and down several times, washed and subcultured onto SDA plates in duplicates. The SDA plates were incubated at 37°C for 48 h, and the CFU were observed for each drug concentration. The MFCs were defined as the lowest drug concentration yielding no growth using 20 μl (CFU20 MFC) and 100 μl (CFU100 MFC). Triclosan was considered fungicidal when the MFC/MIC ratio is ≤4 and fungistatic when the MFC/MIC ratio is ≥4 (Pfaller et al., 2004; Meletiadis et al., 2007).
Synergy Checkerboard Assay
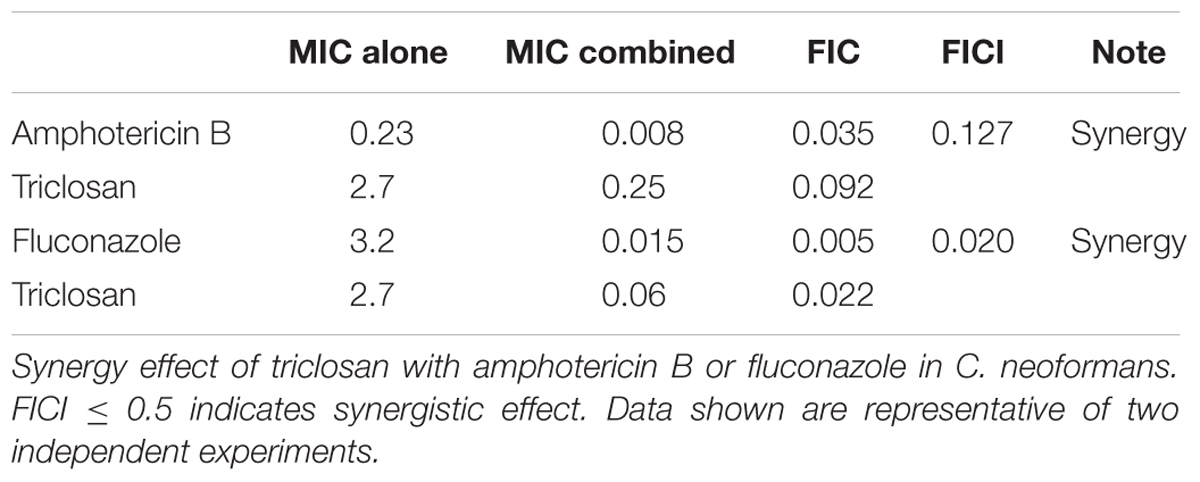
Antibiotic interactions were evaluated using the checkerboard assay as previously described (Rand et al., 1993). Checkerboards were prepared by using serial dilutions of amphotericin B (0.004 to 4.0 μg/ml) and fluconazole (0.0156 to 16.0 μg/ml) in the horizontal wells and triclosan (0.25 to 16.0 μg/ml) in the vertical wells. A fungal suspension was prepared as described in broth microdilution assay (approximately 5 × 103CFU/ml), and 100 μl was inoculated into each well of a 96-well microtiter plate. Plates were read after 48 h of incubation at 35°C, and the wells without visible signs of growth were identified by placing the plate on a mirrored surface. Each well was resuspended and agitated for 5 min, and the OD at 570 nm wavelength was determined with a spectrophotometer. The fractional inhibitory concentration index (FICI) was calculated for each drug using following formula: FICI = FIC (triclosan) + FIC (drug), where FIC equals to MIC-2 of the drug in combination divided by the MIC-2 of the drug alone. FICI ≤ 0.5 indicates synergy, FICI > 4 indicates antagonism whereas 0.5 > FICI > 4 suggests no interaction (additivity/indifference; Odds, 2003b).
Electron Microscopy
Yeast cells were pelleted by centrifugation and resuspended in 5 ml of distilled water. The turbidity of the suspension was adjusted with a spectrophotometer to 75% transmittance at 530 nm. The distilled suspension was diluted 1:50 (0.1 ml plus 4.9 ml) with RPMI 1640 medium. The cell suspension was treated with 0.5 μg/ml of triclosan at 37°C, for 2 h. Cells were then collected and fixed with 4% glutaraldehyde fixative for 120 min. Cells were washed few times with cacodylate buffer and post fixed with OsO4:cacodylate buffer (1:1) for 2 h and kept in cacodylate buffer at 4°C overnight. The samples were washed with distilled water, incubated with uranyl acetate for 10 min before another washing step. Dehydration was performed using 35, 50, 75, and 95% (v/v) ethanol for 10 min each, and followed by 100% ethanol, 15 min for three times. Samples were then incubated with propylene oxide:epon (1:1) for 1 h, propylene oxide:epon (1:3) for 2 h and epon for an overnight incubation. This was followed by embedding the yeast pellet in resin for 5 h at 37°C followed by 60°C overnight. Images were obtained using EFTEM LIBRA 120 transmission electron microscope (Carl Zeiss, Oberkochen, Germany).
Apoptosis Assay
Cell apoptosis was examined by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay using FLOWTAC kit (Trevigen, Gaithersburg, MD, USA). Log-phase cultured cells (107 cells) were treated with 2 mM hydrogen peroxide (H2O2) or 0.5 μg/ml triclosan for 4 h at 37°C. Cells were fixed in 1 ml 3.7% formaldehyde for 10 min followed by CytoninTM permeabilization for 30 min. Cells were then washed and resuspended in labeling reaction mix (TdT dNTP mix, TdT enzyme, 1x Mn2+, 1x TdT labeling buffer) at 37°C for 1 h. Reaction was stopped by adding 1x Stop Buffer followed by staining with 25 μl Strep-Fluorescein for 10 min in dark. Propidium iodide and RNase were added to the cells before analyzed by a FACS Canto cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical Analysis
Data were analyzed with unpaired two-tailed Student’s t-test. Samples were considered significant if P < 0.05.
Results
Triclosan Inhibits the Growth of C. neoformans
To evaluate the antifungal effect of triclosan, 6-mm paper disks impregnated with different concentrations of triclosan (3.125, 6.25, 12.5, 25, 50, and 100 μg) were positioned on a C. neoformans H99 agar plate for an incubation period of 48 h. Control disk (with DMSO) displayed no inhibitory zone. In contrast, we noted that the diameters of the inhibition zones surrounding the triclosan-impregnated disks increased steadily from 7 to 17.6 mm in a dose-dependent manner (Figure 1A). A linear relationship was observed between the inhibition zone size and Log concentration of triclosan (Figure 1B).
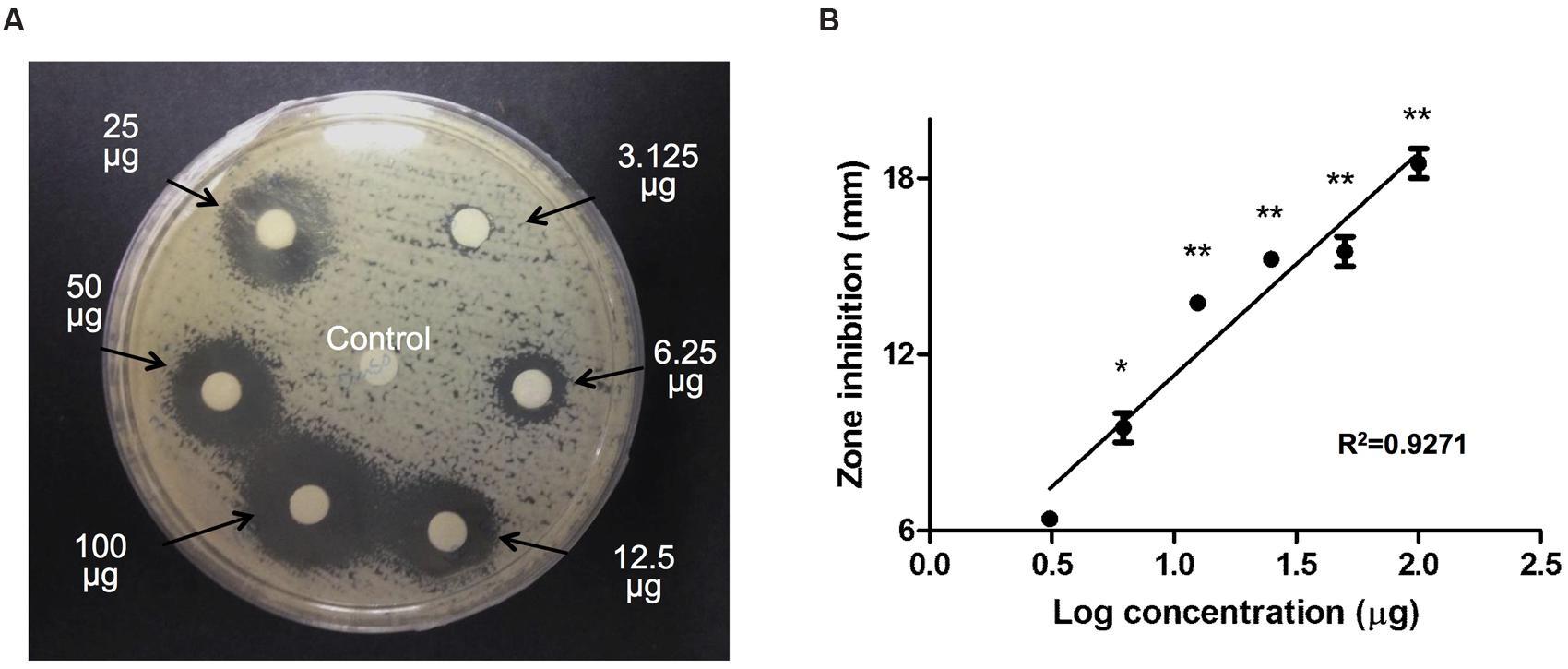
FIGURE 1. Agar disk diffusion assay. (A) Paper disks impregnated with control DMSO or 3.125, 6.25, 12.5, 25, 50, and 100 μg of triclosan were placed on the agar plate containing Cryptococcus neoformans. Diameters of the inhibition zone were measured after 48 h of incubation. Data shown is a representative picture of three independent experiments. (B) Graph shows the inhibition diameter (mm) of C. neoformans plate after triclosan treatment. Data shown are mean ± SD. ∗P < 0.05, ∗∗P < 0.01.
Triclosan MIC-1 and MIC-2 endpoint values, which represent reduction of cell turbidity at 80 and 50%, respectively, were measured by broth microdilution assay (Table 1). Triclosan was fungicidal against C. neoformans H99, with MIC-1 = 3.80 and MIC-2 = 2.70 μg/ml. Local clinical isolates of C. neoformans tested (C14 and C17) showed MIC-1 at 5.197 and 4.875 μg/ml, and MIC-2 at 2.130 and 2.280 μg/ml. Environmental strains C. neoformans H4, S48B and S68B strains showed comparable MIC-1 and MIC-2 values ranging from 0.25 to 1.14 μg/ml and 0.08 to 0.54 μg/ml, respectively (Table 1). Further, we showed that the MIC-1 and MIC-2 of triclosan in two C. albicans (90028 and SC5314) strains were between 16 and 64 μg/ml, consistent with a previous report (Yu et al., 2011). The relatively lower MIC values in C. neoformans suggest that the triclosan is more potent against C. neoformans compared to C. albicans.
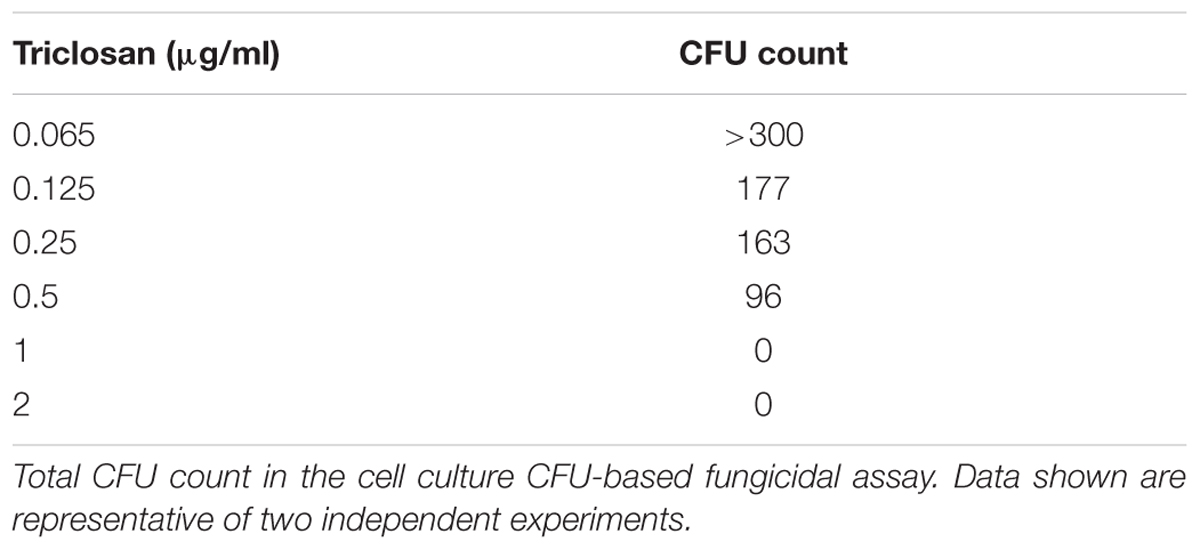
TABLE 1. Minimal inhibitory concentration-1 (80% inhibition) and MIC-2 (50% inhibition) readings of triclosan in different strains of Cryptococcus neoformans and Candida albicans.
A cell culture CFU-based fungicidal assay was then performed to determine if the triclosan was fungicidal or fungistatic (Table 2). CFU formation can be visualized in the wells with low concentrations (≤0.5 μg/ml) but no CFU was detected in the media recovered from the wells treated with ≥1 μg/ml, indicating that MFC for C. neoformans H99 was 1 μg/ml. Thus, our data suggest that triclosan wasfungicidal (MFC/MIC-2 ratio = 0.37, ≤4).
Triclosan Demonstrates a Synergistic Effect with Amphotericin B and Fluconazole
In vitro interactions between triclosan and two standard antifungal agents, amphotericin B and fluconazole were examined using a disk diffusion assay (Figure 2). Inhibition zone for amphotericin B treatment alone was 10 mm. Noticeably, combinational usage of triclosan plus amphotericin B caused enlargement of the inhibition zone to 17 mm (Figure 2A). On the other hand, fluconazole treatment alone showed incomplete inhibition zone of approximately 11 mm, while the combinational usage of triclosan plus fluconazole remarkably augmented the size of inhibition zone to 16 mm (Figure 2B).
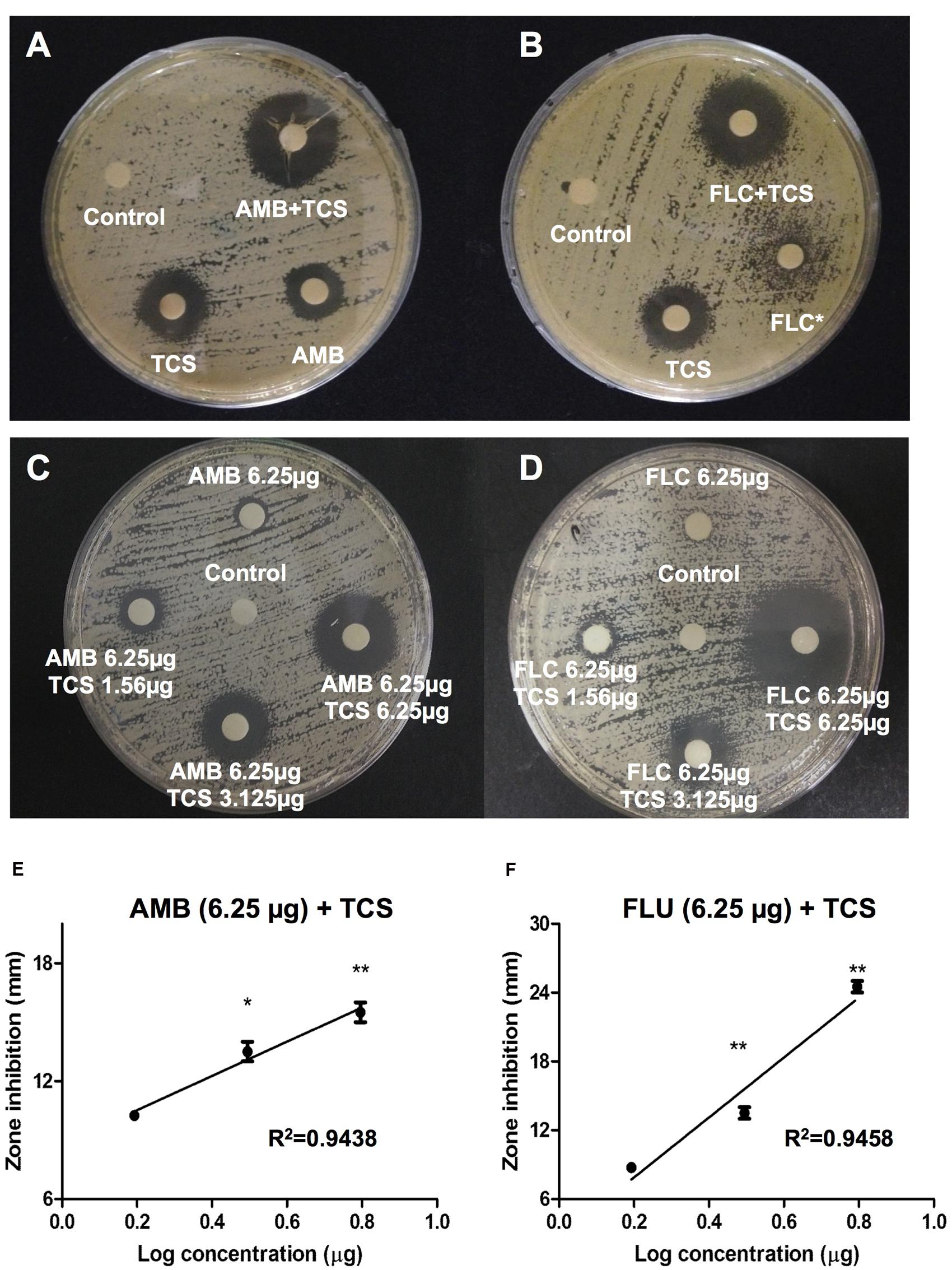
FIGURE 2. The effect of triclosan with amphotericin B and fluconazole. (A,B) Agar disk diffusion assay for triclosan (TCS) in combination with (A) amphotericin B (AMB), or (B) fluconazole (FLC) in C. neoformans H99-containing agar plates. 25 μg of each drug was applied on a disk. (C,D) Agar disk diffusion assay for TCS (1.56, 3.125, and 6.25 μg/ml) in combination with at subinhibitory concentrations (6.25 μg/ml) of (C) AMB and (D) FLU. Diameters of the inhibition zone were measured after 48 h of incubation. Asterisk ∗ indicates partial inhibition. Data shown are representative pictures of three independent experiments. (E,F) Graphs show the inhibition diameter (mm) of C. neoformans plate after triclosan treatment in combination with AMB or FLU. X-axis shows Log concentration of triclosan. Data shown are mean ± SD. ∗P < 0.05, ∗∗P < 0.01.
To further confirm the result, we repeated the experiment using subinhibitory concentrations of drugs. Increasing concentrations of triclosan at 1.56, 3.125, and 6.25 μg/ml were applied on the paper disks containing 6.25 μg amphotericin B (Figure 2C) or fluconazole (Figure 2D) and used for disk diffusion assay. No or minimal inhibition zones were observed in the absence of triclosan while addition of triclosan caused enlargement of inhibition zone in a dose-dependent manner. Both combinations of triclosan with amphotericin B or fluconazole showed linear inhibition patterns (Figures 2E,F). These results suggest that the effect of triclosan with the standard drugs is either synergistic or additive.
The combinational effect of standard drugs and triclosan was further assessed using checkerboard assay to define the median FICI values (Table 3). Both combination of drugs, i.e., triclosan plus amphotericin B (FICI = 0.127), or triclosan plus fluconazole (FICI = 0.020) showed a FICI value <0.5, indicating the synergistic (but not additive) effect of triclosan with both standard drugs.
Triclosan Triggers Apoptotic-Like Cell Death in C. neoformans
To examine the inhibitory mechanism of triclosan on C. neoformans, we processed the control and triclosan-treated (2 h) cells for visualization under electron microscope. Untreated C. neoformans showed an intact cell structure with normal morphologies of nucleus and cytoplasm (Figures 3A,B). In contrast, triclosan-treated C. neoformans demonstrated disrupted cell morphologies including apparent mitochondrial swelling and extensively enlarged cytoplasmic vacuolations (Figures 3C–F). Nuclear chromatin condensation can be also observed. The cell surface fibrilar structure which constitutes polysaccharide component of the cell wall capsule was also disrupted (Pavel and Vasile, 2012). These features collectively suggest that triclosan-treated C. neoformans demonstrated apoptotic-like cell death (ALCD) mechanism.
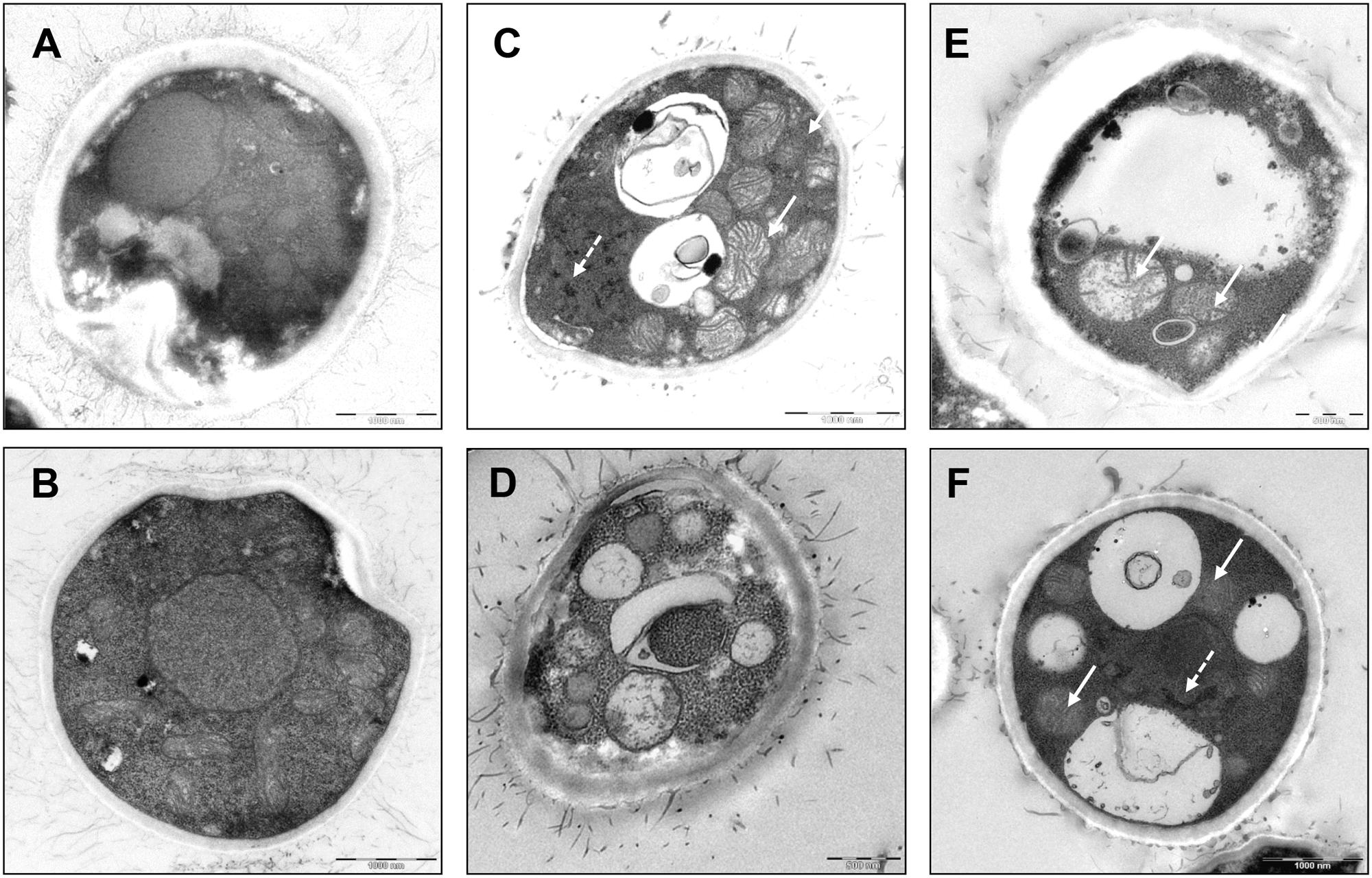
FIGURE 3. Cell morphology of triclosan-treated C. neoformans. Electron microscopic pictures of C. neoformans with or without exposure to triclosan (0.5 μg/ml) for 2 h. (A,B) Untreated control cells showed intact nucleus and cytoplasm. (C–F) Triclosan-treated C. neoformans showed apoptotic morphologies. Note the appearance of apoptotic features such as mitochondrial swelling (arrow) and nuclear chromatin condensation (broken arrow). Besides, intense cytoplasmic vacuolations were formed in the cells. Cell surface fibrilar structures which form capsule polysaccharide component were also disrupted (Pavel and Vasile, 2012).
To further confirm if triclosan triggers apoptosis in C. neoformans cells, we analyzed the cells using TUNEL apoptosis assay followed by flow cytometrical analysis (Figure 4). DNA fragmentation (one of the hallmark of apoptosis) generates free 3′-hydroxyl residues that can be utilized by terminal deoxynucleotidyl transferase to incorporate FITC-tagged dUTP into the blunt ends of double-stranded DNA break. Our data showed that 90.7% triclosan-treated C. neoformans H99 cells were apoptotic (FITC-positive) compared to only 2.1% in the untreated control. Around 71.4% of apoptotic cells were detected in the positive control (H2O2-treated cells). Therefore, triclosan can inhibit C. neoformans by inducing ALCD.
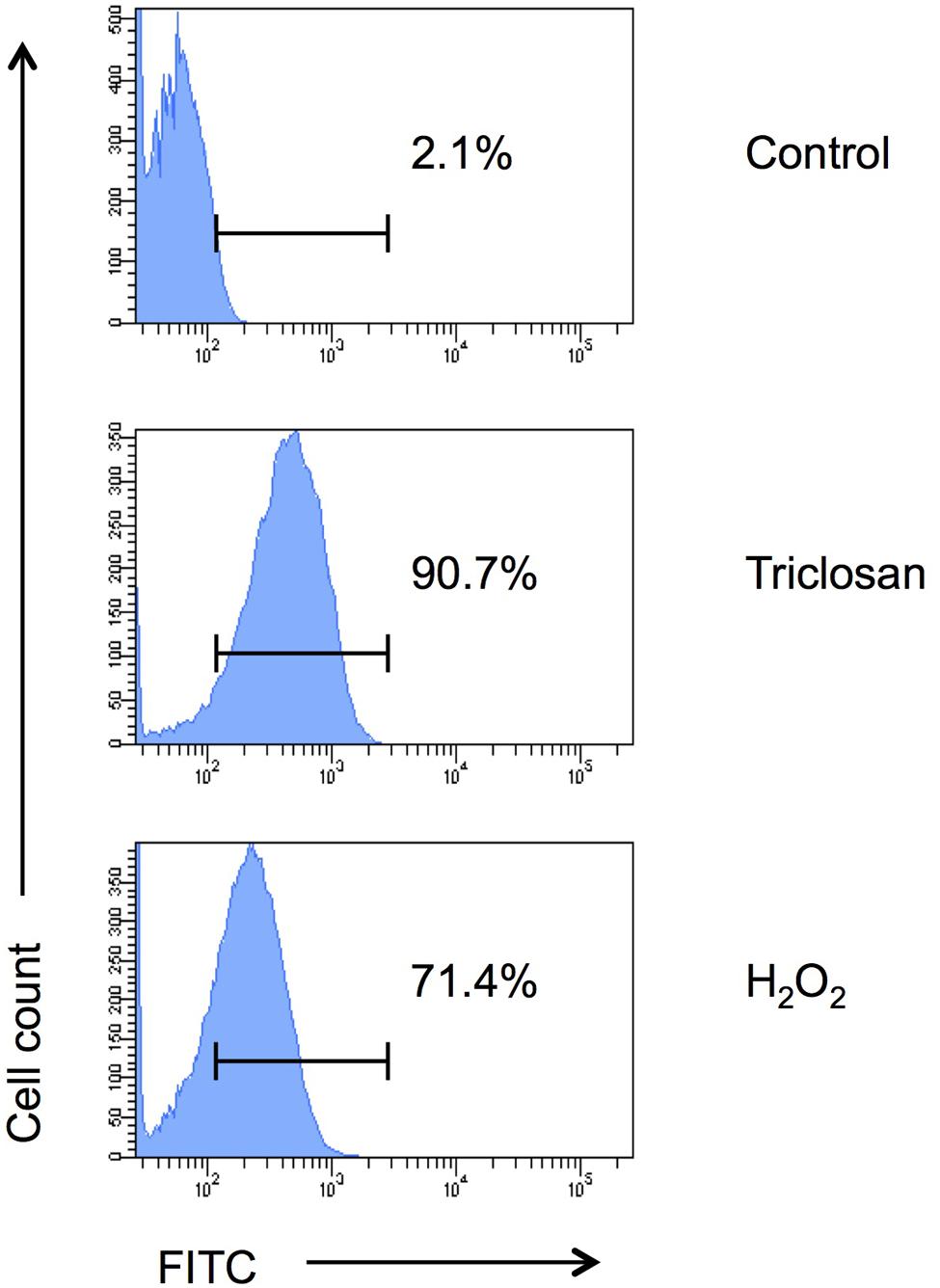
FIGURE 4. TUNEL assay. Apoptosis of triclosan-treated C. neoformans H99 cells was determined by TUNEL assay followed by flow cytometry analysis. C. neoformans H99 cells were non-treated (control), treated with triclosan (0.5 μg/ml) or H2O2 (2 mM) for 4 h. Percentages of the apoptotic cells were indicated. Data shown are representative of two independent experiments.
Discussion
Our study demonstrated the potent antifungal effect of triclosan against C. neoformans. In fact, triclosan treatment exerted a stronger growth inhibition in C. neoformans compared to C. albicans, as evidenced by comparatively lower MIC-1 values (ranged from 0.25 to 3.80 μg/ml) in C. neoformans strains tested, compared to MIC-1 values in C. albicans (ranged from 59.6 to >64 μg/ml).
Combined antibiotic therapy can delay the emergence of microbial resistance by producing desirable synergistic effects in the infection treatment (Adwan and Mhanna, 2008). In our study, synergism between triclosan and two standard drugs (amphotericin B and fluconazole) was established. Fluconazole and amphotericin B are commonly used drugs for candidiasis and cryptococcal diseases. Fluconazole is a member of the azole family that targets the Erg11 enzyme (an essential fungal cytochrome P450 lanosterol 14α-demethylase), thus inhibiting ergosterol biosynthesis (Rodero et al., 2003; Heilmann et al., 2010). Amphotericin B, on the other hand, binds directly to ergosterol and kills yeast cells via ion channel-mediated plasma membrane permeabilization (Gruda and Dussault, 1988). Combinational treatment of triclosan and other drugs has been shown to significantly enhance the drug efficacy against bacteria (Tambe et al., 2001; Sharma et al., 2003). However, two previous reports using C. albicans demonstrated opposing results for combinational usage of triclosan plus fluconazole, whereby one showed synergistic effect (Yu et al., 2011) and another showed antagonistic effect (Higgins et al., 2012). In C. neoformans, we reported that triclosan acts in synergy with fluconazole and amphotericin B.
Administration of standard fungal drugs in AIDS-associated cryptococcal meningoencephalitis shows a successful rate of 34% (fluconazole) in 40% in fluconazole and amphotericin B recipients (Saag et al., 1992). Furthermore, amphotericin B treatment frequently results in adverse effect (nephrotoxicity) in cryptococcal meningitis patients (Perfect et al., 2010), which suggests the need for a more effective treatment strategy. Perhaps a combinational therapy with triclosan could be a good solution. As far as safety is concerned, triclosan is considered non-carcinogenic with low indication of toxicity in both animal and human studies (Bhargava and Leonard, 1996). Triclosan has been impregnated to many of the daily contact products including soaps, toothpastes, disinfection solutions, and medical devices. Upon usage, triclosan can penetrate through cutaneous layer into the blood stream and excreted in the urine or feces (Moss et al., 2000; Sandborgh-Englund et al., 2006). Wound closure with triclosan-coated sutures has been shown to reduce the risk of infection after cerebrospinal fluid surgery (Rozzelle et al., 2008). Triclosan administration also significantly reduced bacterial level in experimental gingivitis (Pancer et al., 2016) and nosocomial infection (Webster et al., 1994).
The mechanism of triclosan-mediated cell disruption has been described in different pathogens. Triclosan triggers changes in bacteria membrane fluidity and function at lower concentration, and leads to cell lysis at a higher concentration (Gomez Escalada et al., 2005). It is also known to block lipid synthesis primarily by targeting the carrier protein of the bacterial type II fatty acid synthesis (FASII) pathway (McMurry et al., 1998). Contradictory, a recent study shows that Fas1 and Fas2 gene overexpression did not alter fungal susceptibility to triclosan and suggests that there may be an alternative target of triclosan in addition to the lipid synthesis pathway (Higgins et al., 2012). Therefore, further investigations are required to elucidate the actual mechanism of action. In mammals, triclosan antagonizes estrogen or androgen receptors, elevates resting cytosolic Ca2+ in primary skeletal myotubules (Ahn et al., 2008) and impairs the excitation contraction coupling of cardiac and skeletal function (Cherednichenko et al., 2012).
From electron microscope pictures, we hypothesize that triclosan induces ALCD, a program cell death mechanism in C. neoformans because various apoptosic subcellular changes can be visualized as early as 2 h post treatment. At an early stage of an apoptotic cell, cell shrinkage occurs as a result of organelle condensation and decreased cytoplasm density (Häcker, 2000). During the apoptosis process, free radicals can modify mitochondrial membrane potential thus inducing mitochondrial swelling and fusion of adjacent mitochondria into megamitochondria (Wakabayashi, 1999). Mitochondria release cytochrome c from storage which subsequently activates the caspase cascade leading to nuclear cleavage (Zhang and Xu, 2000) and extensive plasma membrane blebbing (Coleman et al., 2001). We reported some apoptotic features in triclosan-treated C. neoformans cells including condensed nuclear chromatin, DNA fragmentation and mitochondrial swelling. Whether the H2O2 treatment induces similar pattern of apoptotic features in C. neoformans remains to be investigated. We suspected that the intensive cytoplasmic vacuolation was due to the decreased cytoplasm density and organelles condensation although the cell structure remained intact as a resultant of rigid fungal cell wall. Several pathogenic fungi have been reported to undergo ALCD (Buttner et al., 2006; Ramsdale, 2008; Sharon et al., 2009). For instance, mutation of CDC48 in Saccharomyces cerevisiae shows apoptosis characteristic including chromatin condensation and fragmentation (Madeo et al., 1997). Interestingly, overexpression of mammalian anti-apoptotic Bcl2 rescues fungal cell apoptosis (Longo et al., 1997) while overexpression of pro-apoptotic Bax enhances the cell death effect (Manon et al., 1997; Ligr et al., 1998). ALCD has also been reported in C. neoformans when the cells were stimulated with hydrogen peroxide or cultured in the presence of Staphylococcus aureus (Ikeda and Sawamura, 2008). The apoptosis pathway in C. neoformans is controlled through regulation of apoptosis-inducing factor (Aif1) and metacaspases (Mca1 and Mca2) (Semighini et al., 2011).
TUNEL assay takes advantage of in situ labeling technology, which uses TdT to incorporate FITC-tagged dUTP into blunt ends of double stranded DNA breaks (Denton and Kumar, 2015). TUNEL-labeled cells indicate nuclear DNA fragmentation, an irreversible step in apoptosis (Lawry, 2004). During infection, C. neoformans is able to cause apoptotic cell death of mammalian cells. In the experimental animals with disseminated cryptococcosis, a major constituent of C. neoformans capsular polysaccharide, glucuronoxylomannan (GXM) results in increased TUNEL-labeled cells in both lung and spleen (Chiapello et al., 2003). Studies also show that C. neoformans induces cell death in rat macrophages by promoting inducible nitric oxide synthase expression with nitric oxide production in rat macrophages (Chiapello et al., 2008; Villena et al., 2008) besides interrupting the lysosome and phagosome maturation (Davis et al., 2015; Smith et al., 2015). In addition, C. neoformans induces apoptosis of T lymphocytes through activation of caspase-8 that initiates DNA cleavage activity (Pericolini et al., 2006).
In summary, our study reveals a strong inhibitory effect of triclosan as well as its synergy with amphotericin B and fluconazole in blocking cell growth of the pathogenic fungus C. neoformans. We also report that triclosan maybe fungicidal by inducing the ALCD mechanism in C. neoformans. Our study reveals a potential therapeutic value of triclosan as a novel drug or a synergent in the treatment of cryptococcal infection.
Author Contributions
CYL, STT, and WFW designed the research and analyzed the data. EM, GMYT, KM, and TCY carried out the experiments. EM and WFW wrote the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Malaysian Ministry of Higher Education (UM.C/625/1/HIR/MOE/CHAN/13/4; H-50001-00-A000029) and University of Malaya (RG525-13HTM). EM was supported by a postgraduate research fund (PPP) from University of Malaya.
References
Adwan, G., and Mhanna, M. (2008). Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. Middle-East J. Sci. Res. 3, 134–139.
Ahn, K. C., Zhao, B., Chen, J., Cherednichenko, G., Sanmarti, E., Denison, M. S., et al. (2008). In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosanin bioassay screens: receptor-based bioassay screens. Environ. Health Perspect. 116, 1203–1210. doi: 10.1289/ehp.11200
Bhargava, H. N., and Leonard, P. A. (1996). Triclosan: applications and safety. Am. J. Infect. Control 24, 209–218. doi: 10.1016/S0196-6553(96)90017-6
Buttner, S., Eisenberg, T., Herker, E., Carmona-Gutierrez, D., Kroemer, G., and Madeo, F. (2006). Why yeast cells can undergo apoptosis: death in times of peace, love, and war. J. Cell Biol. 175, 521–525. doi: 10.1083/jcb.200608098
Cherednichenko, G., Zhang, R., Bannister, R. A., Timofeyev, V., Li, N., Fritsch, E. B., et al. (2012). Triclosan impairs excitation–contraction coupling and Ca2+ dynamics in striated muscle. Proc. Natl. Acad. Sci. U.S.A. 109, 14158–14163. doi: 10.1073/pnas.1211314109
Chiapello, L. S., Aoki, M. P., Rubinstein, H. R., and Masih, D. T. (2003). Apoptosis induction by glucuronoxylomannan of Cryptococcus neoformans. Med. Mycol. 41, 347–353. doi: 10.1080/1369378031000137260
Chiapello, L. S., Baronetti, J. L., Garro, A. P., Spesso, M. F., and Masih, D. T. (2008). Cryptococcus neoformans glucuronoxylomannan induces macrophage apoptosis mediated by nitric oxide in a caspase-independent pathway. Int. Immunol. 20, 1527–1541. doi: 10.1093/intimm/dxn112
Clinical and Laboratory Standards Institute [CLSI] (2008). M27-A3 Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard- third edition. Clin. Lab. Stand. Instit. 28, 6–12.
Coelho, C., Bocca, A. L., and Casadevall, A. (2014). The intracellular life of Cryptococcus neoformans. Annu. Rev. Pathol. 9, 219–238. doi: 10.1146/annurev-pathol-012513-104653
Coleman, M. L., Sahai, E. A., Yeo, M., Bosch, M., Dewar, A., and Olson, M. F. (2001). Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3, 339–345. doi: 10.1038/35070009
Davis, M. J., Eastman, A. J., Qiu, Y., Gregorka, B., Kozel, T. R., Osterholzer, J. J., et al. (2015). Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J. Immunol. 194, 2219–2231. doi: 10.4049/jimmunol.1402376
Denton, D., and Kumar, S. (2015). Terminal deoxynucleotidyl transferase (TdT)-mediated dutp nick-end labeling (TUNEL) for detection of apoptotic cells in drosophila. Cold Spring Harb. Protoc. 2015, 568–571. doi: 10.1101/pdb.prot086199
Fang, J. L., Stingley, R. L., Beland, F. A., Harrouk, W., Lumpkins, D. L., and Howard, P. (2010). Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C Environ. Carcinog Ecotoxicol. Rev. 28, 147–171. doi: 10.1080/10590501.2010.504978
Fling, M. E., Kopf, J., Tamarkin, A., Gorman, J. A., Smith, H. A., and Koltin, Y. (1991). Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol. Gen. Genet. 227, 318–329. doi: 10.1007/BF00259685
Goldman, D. L., Khine, H., Abadi, J., Lindenberg, D. J., Pirofski, L., Niang, R., et al. (2001). Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66. doi: 10.1542/peds.107.5.e66
Gomez Escalada, M., Russell, A., Maillard, J. Y., and Ochs, D. (2005). Triclosan–bacteria interactions: single or multiple target sites? Lett. Appl. Microbiol. 41, 476–481. doi: 10.1111/j.1472-765X.2005.01790.x
Gruda, I., and Dussault, N. (1988). Effect of the aggregation state of amphotericin B on its interaction with ergosterol. Biochem. Cell Biol. 66, 177–183. doi: 10.1139/o88-024
Häcker, G. (2000). The morphology of apoptosis. Cell Tissue Res. 301, 5–17. doi: 10.1007/s004410000193
Heath, R. J., Rubin, J. R., Holland, D. R., Zhang, E., Snow, M. E., and Rock, C. O. (1999). Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274, 11110–11114. doi: 10.1074/jbc.274.16.11110
Heilmann, C. J., Schneider, S., Barker, K. S., Rogers, P. D., and Morschhauser, J. (2010). An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 54, 353–359. doi: 10.1128/AAC.01102-09
Higgins, J., Pinjon, E., Oltean, H. N., White, T. C., Kelly, S. L., Martel, C. M., et al. (2012). Triclosan antagonizes fluconazole activity against Candida albicans. J. Dent. Res. 91, 65–70. doi: 10.1177/0022034511425046
Hitchcock, C. A., Barrett-Bee, K. J., and Russell, N. J. (1986). The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J. Gen. Microbiol. 132, 2421–2431.
Howell, S. A., Mallet, A. I., and Noble, W. C. (1990). A comparison of the sterol content of multiple isolates of the Candida albicans darlington strain with other clinically azole-sensitive and -resistant strains. J. Appl. Bacteriol. 69, 692–696. doi: 10.1111/j.1365-2672.1990.tb01564.x
Ikeda, R., and Sawamura, K. (2008). Bacterial and H2O2 stress-induced apoptosis-like events in Cryptococcus neoformans. Res. Microbiol. 159, 628–634. doi: 10.1016/j.resmic.2008.07.006
Jones, R. D., Jampani, H. B., Newman, J. L., and Lee, A. S. (2000). Triclosan: a review of effectiveness and safety in health care settings. Am. J. Infect. Control 28, 184–196. doi: 10.1067/mic.2000.102378
Lazera, M. S., Pires, F. D., Camillo-Coura, L., Nishikawa, M. M., Bezerra, C. C., Trilles, L., et al. (1996). Natural habitat of Cryptococcus neoformans var. neoformans in decaying wood forming hollows in living trees. J. Med. Vet. Mycol. 34, 127–131. doi: 10.1080/02681219680000191
Ligr, M., Madeo, F., Frohlich, E., Hilt, W., Frohlich, K. U., and Wolf, D. H. (1998). Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 438, 61–65. doi: 10.1016/S0014-5793(98)01227-7
Lin, X., and Heitman, J. (2006). The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60, 69–105. doi: 10.1146/annurev.micro.60.080805.142102
Loftsson, T., Leeves, N., Bjornsdottir, B., Duffy, L., and Masson, M. (1999). Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo. J. Pharm. Sci. 88, 1254–1258. doi: 10.1021/js9902466
Longo, V. D., Ellerby, L. M., Bredesen, D. E., Valentine, J. S., and Gralla, E. B. (1997). Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 137, 1581–1588. doi: 10.1083/jcb.137.7.1581
Looi, C. Y., Ec, D. S., Seow, H. F., Rosli, R., Ng, K. P., and Chong, P. P. (2005). Increased expression and hotspot mutations of the multidrug efflux transporter, CDR1 in azole-resistant Candida albicans isolates from vaginitis patients. FEMS Microbiol. Lett. 249, 283–289. doi: 10.1016/j.femsle.2005.06.036
Madeo, F., Frohlich, E., and Frohlich, K. U. (1997). A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139, 729–734. doi: 10.1083/jcb.139.3.729
Manon, S., Chaudhuri, B., and Guerin, M. (1997). Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 415, 29–32. doi: 10.1016/S0014-5793(97)01087-9
McMurry, L. M., Oethinger, M., and Levy, S. B. (1998). Triclosan targets lipid synthesis. Nature 394, 531–532. doi: 10.1038/28970
Meletiadis, J., Antachopoulos, C., Stergiopoulou, T., Pournaras, S., Roilides, E., and Walsh, T. J. (2007). Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob. Agents Chemother. 51, 3329–3337. doi: 10.1128/AAC.00345-07
Mitchell, T. G., and Perfect, J. R. (1995). Cryptococcosis in the era of AIDS–100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8, 515–548.
Moss, T., Howes, D., and Williams, F. M. (2000). Percutaneous penetration and dermal metabolism of triclosan (2,4, 4’-trichloro-2’-hydroxydiphenyl ether). Food Chem. Toxicol. 38, 361–370. doi: 10.1016/S0278-6915(99)00164-7
Mukherjee, P. K., Sheehan, D. J., Hitchcock, C. A., and Ghannoum, M. A. (2005). Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18, 163–194. doi: 10.1128/CMR.18.1.163-194.2005
Munoz, P., Singh, N., and Bouza, E. (2006). Treatment of solid organ transplant patients with invasive fungal infections: should a combination of antifungal drugs be used? Curr. Opin. Infect. Dis. 19, 365–370. doi: 10.1097/01.qco.0000235164.70678.97
Odds, F. C. (2003a). Fluconazole plus amphotericin B combinations are not contraindicated and may add benefit for the treatment of candidemia. Clin. Infect. Dis. 36, 1229–1231. doi: 10.1086/374856
Odds, F. C. (2003b). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob Chemother. 52:1. doi: 10.1093/jac/dkg301
Pancer, B. A., Kott, D., Sugai, J. V., Panagakos, F. S., Braun, T. M., Teles, R. P., et al. (2016). Effects of triclosan on host response and microbial biomarkers during experimental gingivitis. J Clin. Periodontol. doi: 10.1111/jcpe.12519 [Epub ahead of print].
Pancharoen, C., Chindamporn, A., and Thisyakorn, U. (2001). Childhood cryptococcosis: an increasing problem in the era of AIDS. J. Med. Assoc. Thai. 84(Suppl. 1), S86–S90.
Park, B. J., Wannemuehler, K. A., Marston, B. J., Govender, N., Pappas, P. G., and Chiller, T. M. (2009). Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530. doi: 10.1097/QAD.0b013e328322ffac
Pavel, A. B., and Vasile, C. I. (2012). PyElph - a software tool for gel images analysis and phylogenetics. BMC Bioinform. 13:9. doi: 10.1186/1471-2105-13-9
Perfect, J. R., and Cox, G. M. (1999). Drug resistance in Cryptococcus neoformans. Drug Resist. Updat. 2, 259–269. doi: 10.1054/drup.1999.0090
Perfect, J. R., Dismukes, W. E., Dromer, F., Goldman, D. L., Graybill, J. R., Hamill, R. J., et al. (2010). Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 50, 291–322. doi: 10.1086/649858
Pericolini, E., Cenci, E., Monari, C., De Jesus, M., Bistoni, F., Casadevall, A., et al. (2006). Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol. 8, 267–275. doi: 10.1111/j.1462-5822.2005.00619.x
Pfaller, M. A., Diekema, D. J., Gibbs, D. L., Newell, V. A., Bijie, H., Dzierzanowska, D., et al. (2009). Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of non-candidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 47, 117–123. doi: 10.1128/JCM.01747-08
Pfaller, M. A., Sheehan, D. J., and Rex, J. H. (2004). Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17, 268–280. doi: 10.1128/CMR.17.2.268-280.2004
Prasad, R., De Wergifosse, P., Goffeau, A., and Balzi, E. (1995). Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27, 320–329. doi: 10.1007/BF00352101
Ramsdale, M. (2008). Programmed cell death in pathogenic fungi. Biochim. Biophys. Acta 1783, 1369–1380. doi: 10.1016/j.bbamcr.2008.01.021
Rand, K. H., Houck, H. J., Brown, P., and Bennett, D. (1993). Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 37, 613–615. doi: 10.1128/AAC.37.3.613
Regos, J., Zak, O., Solf, R., Vischer, W. A., and Weirich, E. G. (1979). Antimicrobial spectrum of triclosan, a broad-spectrum antimicrobial agent for topical application. II. Comparison with some other antimicrobial agents. Dermatologica 158, 72–79. doi: 10.1159/000250746
Rodero, L., Mellado, E., Rodriguez, A. C., Salve, A., Guelfand, L., Cahn, P., et al. (2003). G484S amino acid substitution in lanosterol 14-alpha demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob. Agents Chemother. 47, 3653–3656. doi: 10.1128/AAC.47.11.3653-3656.2003
Rozzelle, C. J., Leonardo, J., and Li, V. (2008). Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J. Neurosurg. Pediatr. 2, 111–117. doi: 10.3171/PED/2008/2/8/111
Saag, M. S., Powderly, W. G., Cloud, G. A., Robinson, P., Grieco, M. H., Sharkey, P. K., et al. (1992). Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N. Engl. J. Med. 326, 83–89. doi: 10.1056/NEJM199201093260202
Sandborgh-Englund, G., Adolfsson-Erici, M., Odham, G., and Ekstrand, J. (2006). Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health A 69, 1861–1873. doi: 10.1080/15287390600631706
Sanglard, D., Ischer, F., Monod, M., and Bille, J. (1997). Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143(Pt 2), 405–416. doi: 10.1099/00221287-143-2-405
Sanglard, D., Kuchler, K., Ischer, F., Pagani, J. L., Monod, M., and Bille, J. (1995). Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39, 2378–2386. doi: 10.1128/AAC.39.11.2378
Schweizer, H. P. (2001). Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 202, 1–7. doi: 10.1111/j.1574-6968.2001.tb10772.x
Semighini, C. P., Averette, A. F., Perfect, J. R., and Heitman, J. (2011). Deletion of Cryptococcus neoformans AIF ortholog promotes chromosome aneuploidy and fluconazole-resistance in a metacaspase-independent manner. PLoS Pathog. 7:e1002364. doi: 10.1371/journal.ppat.1002364
Sharma, S., Ramya, T. N., Surolia, A., and Surolia, N. (2003). Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge. Antimicrob. Agents Chemother. 47, 3859–3866. doi: 10.1128/AAC.47.12.3859-3866.2003
Sharon, A., Finkelstein, A., Shlezinger, N., and Hatam, I. (2009). Fungal apoptosis: function, genes and gene function. FEMS Microbiol. Rev. 33, 833–854. doi: 10.1111/j.1574-6976.2009.00180.x
Smith, L. M., Dixon, E. F., and May, R. C. (2015). The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell Microbiol. 17, 702–713. doi: 10.1111/cmi.12394
Stewart, M. J., Parikh, S., Xiao, G., Tonge, P. J., and Kisker, C. (1999). Structural basis and mechanism of enoyl reductase inhibition by triclosan. J. Mol. Biol. 290, 859–865. doi: 10.1006/jmbi.1999.2907
Syed, A. K., Ghosh, S., Love, N. G., and Boles, B. R. (2014). Triclosan promotes Staphylococcus aureus nasal colonization. MBio 5:e1015. doi: 10.1128/mBio.01015-13
Tambe, S. M., Sampath, L., and Modak, S. M. (2001). In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J. Antimicrob. Chemother. 47, 589–598. doi: 10.1093/jac/47.5.589
Tay, S., Lim, H., Tajuddin, T., Rohani, M., Hamimah, H., and Thong, K. (2006). Determination of molecular types and genetic heterogeneity of Cryptococcus neoformans and C. gattii in Malaysia. Med. Mycol. 44, 617–622. doi: 10.1080/13693780600857330
Tay, S. T., Abidin, I. A., Hassan, H., and Ng, K. P. (2011). Proteinase, phospholipase, biofilm forming abilities and antifungal susceptibilities of Malaysian Candida isolates from blood cultures. Med. Mycol. 49, 556–560. doi: 10.3109/13693786.2010.551424
Tay, S. T., Chai, H. C., Na, S. L., Hamimah, H., Rohani, M. Y., and Soo-Hoo, T. S. (2005). The isolation, characterization and antifungal susceptibilities of Cryptococcus neoformans from bird excreta in Klang Valley, Malaysia. Mycopathologia 159, 509–513. doi: 10.1007/s11046-005-3091-6
Villalain, J., Mateo, C. R., Aranda, F. J., Shapiro, S., and Micol, V. (2001). Membranotropic effects of the antibacterial agent Triclosan. Arch. Biochem. Biophys. 390, 128–136. doi: 10.1006/abbi.2001.2356
Villena, S. N., Pinheiro, R. O., Pinheiro, C. S., Nunes, M. P., Takiya, C. M., Dosreis, G. A., et al. (2008). Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 10, 1274–1285. doi: 10.1111/j.1462-5822.2008.01125.x
Vischer, W. A., and Regos, J. (1974). Antimicrobial spectrum of Triclosan, a broad-spectrum antimicrobial agent for topical application. Zentralbl Bakteriol. Orig. A 226, 376–389.
Wakabayashi, T. (1999). Structural changes of mitochondria related to apoptosis: swelling and megamitochondria formation. Acta Biochim. Pol. 46, 223–237.
Webster, J., Faoagali, J. L., and Cartwright, D. (1994). Elimination of methicillin-resistant Staphylococcus aureus from a neonatal intensive care unit after hand washing with triclosan. J. Paediatr. Child Health 30, 59–64. doi: 10.1111/j.1440-1754.1994.tb00568.x
Yamazumi, T., Pfaller, M. A., Messer, S. A., Houston, A. K., Boyken, L., Hollis, R. J., et al. (2003). Characterization of heteroresistance to fluconazole among clinical isolates of Cryptococcus neoformans. J. Clin. Microbiol. 41, 267–272. doi: 10.1128/JCM.41.1.267-272.2003
Yu, L., Ling, G., Deng, X., Jin, J., Jin, Q., and Guo, N. (2011). In vitro interaction between fluconazole and triclosan against clinical isolates of fluconazole-resistant Candida albicans determined by different methods. Antimicrob. Agents Chemother. 55, 3609–3612. doi: 10.1128/AAC.01313-10
Keywords: antifungal effect, amphotericin B, apoptosis, Cryptococcus neoformans, fluconazole, synergy, triclosan
Citation: Movahed E, Tan GMY, Munusamy K, Yeow TC, Tay ST, Wong WF and Looi CY (2016) Triclosan Demonstrates Synergic Effect with Amphotericin B and Fluconazole and Induces Apoptosis-Like Cell Death in Cryptococcus neoformans. Front. Microbiol. 7:360. doi: 10.3389/fmicb.2016.00360
Received: 09 July 2015; Accepted: 07 March 2016;
Published: 21 March 2016.
Edited by:
Tzi Bun Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Erin E. McClelland, Middle Tennessee State University, USADennis Michael Lindell, University of Washington, USA
Siddharth Kaushal Tripathi, University of Mississippi, USA
Copyright © 2016 Movahed, Tan, Munusamy, Yeow, Tay, Wong and Looi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Won F. Wong, wonfen@um.edu.my
 Elaheh Movahed
Elaheh Movahed Grace Min Yi Tan
Grace Min Yi Tan Komathy Munusamy1
Komathy Munusamy1 Sun Tee Tay
Sun Tee Tay Won Fen Wong
Won Fen Wong Chung Yeng Looi
Chung Yeng Looi