Corrigendum: Food Allergy and Helicobacter pylori Infection: A Systematic Review
- 1Department of Human Nutrition, University of Otago, Dunedin, New Zealand
- 2School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- 3Department of Medicine, Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX, USA
- 4Department of Environmental and Preventive Medicine, Oita University Faculty of Medicine, Yufu, Japan
Introduction: Based on the hygiene hypothesis, a low prevalence of Helicobacter pylori (H. pylori) infection may explain the recent high prevalence of allergic diseases including food allergy. However, there are very few studies that investigate the relationship between H. pylori and food allergy.
Summary: We searched for PubMed, Ovid Medline and the Cochrane library for relevant articles published in English from inception to November 2015. The inverse relationship between H. pylori and food allergy remains unproven because of contradictory and limited evidence at the moment. Likewise, only limited studies have examined the relationship between CagA; one of H. pylori virulence factor and food allergy. On the other hand, in vitro evidence seems to point out a role of H. pylori in the causation of food allergy. The inconsistent results from epidemiological data may be due to small sample size, heterogeneous populations and unstandardised methods or food allergens.
Conclusion: Available studies do not support the role of H. pylori in food allergy.
Introduction
Helicobacter pylori (H. pylori) is a major cause of various gastroduodenal diseases, including peptic ulcer disease and gastric cancer (Suerbaum and Michetti, 2002). Nearly half of the world population are infected with H. pylori (Peek and Blaser, 2002) with a higher prevalence in developing countries (∼80%; Suerbaum and Michetti, 2002), although it is recognized that some populations have unexpectedly low rates (Lee et al., 2013). Nevertheless, the overall prevalence of H. pylori infection has decreased in recent years because of effective eradication therapy and improvement in hygiene and living environment.
A low prevalence or loss of H. pylori is associated with a reduced risk for gastric cancer (Derakhshan and Lee, 2012; Lee and Derakhshan, 2013), however, the prevalence of allergic diseases may increase if the hygiene hypothesis holds true. The hygiene hypothesis proposed that an improved condition in early life reduces the exposure to various childhood infections, which might promote an increased in the prevalence of atopic disorders (Strachan, 1989). While an improved living condition in early life reduces exposure to various childhood infections, however, atopic disorders may increase and this is the basis of the hygiene hypothesis (Strachan, 1989). Only limited data are available to support the hygiene hypothesis in relation to food allergy.
Most studies focus on the role of H. pylori in allergic diseases such as asthma. However, there are only few studies that investigate the relationship between H. pylori and food allergy. This is despite that food allergy is as common as asthma or other allergic diseases if not more prevalent. The aim of this review was to highlight the increasing prevalence of food allergy in the West and Asia and the role of H. pylori to this increase.
Search Strategy
We searched for PubMed, Ovid Medline and the Cochrane library for relevant articles published in English from inception to November 2015. Various combinations of the following terms were used: Helicobacter pylori, H. pylori, H. pylori infection, food allergy, allergic disease and atopic disease. We also located additional publications from reference lists of the retrieved studies. Relevant articles were selected for full texts after screening through the titles, abstracts and descriptors of the studies which met the eligibility of our review. We selected the studies for review if they investigated the relationship between H. pylori and food allergy in humans. Studies were excluded if they did not use H. pylori and food allergy as an outcome in the analysis.
Epidemiology of Food Allergy
Food allergy is defined as an immunologically mediated process in response to a specific food response (Bruijnzeel-Koomen et al., 1995). Compared to previous epidemiological surveys (Gupta et al., 2007; Mullins, 2007; Branum and Lukacs, 2009), recent figures suggest that food allergy is more prevalent, affecting ∼6% of children and 3–4% of adults (Wang and Sampson, 2011). In the United States, the prevalence of food allergy in children aged 0–17 years had increased from 3.4% in 1997–1999 to 5.1% in 2009–2011 (Jackson et al., 2013).
Relationship Between Food Allergy and H. pylori Infection
Only two studies by the same author reported a positive association between food allergy and H. pylori infection (Table 1). Corrado et al. (2000) reported that children with food allergy had a higher anti-H. pylori IgG concentration than those without food allergy (p < 0.05). An earlier study by, Corrado et al. (1998) reported that 30% of their patients with food allergy had a higher anti-H. pylori IgG concentration.
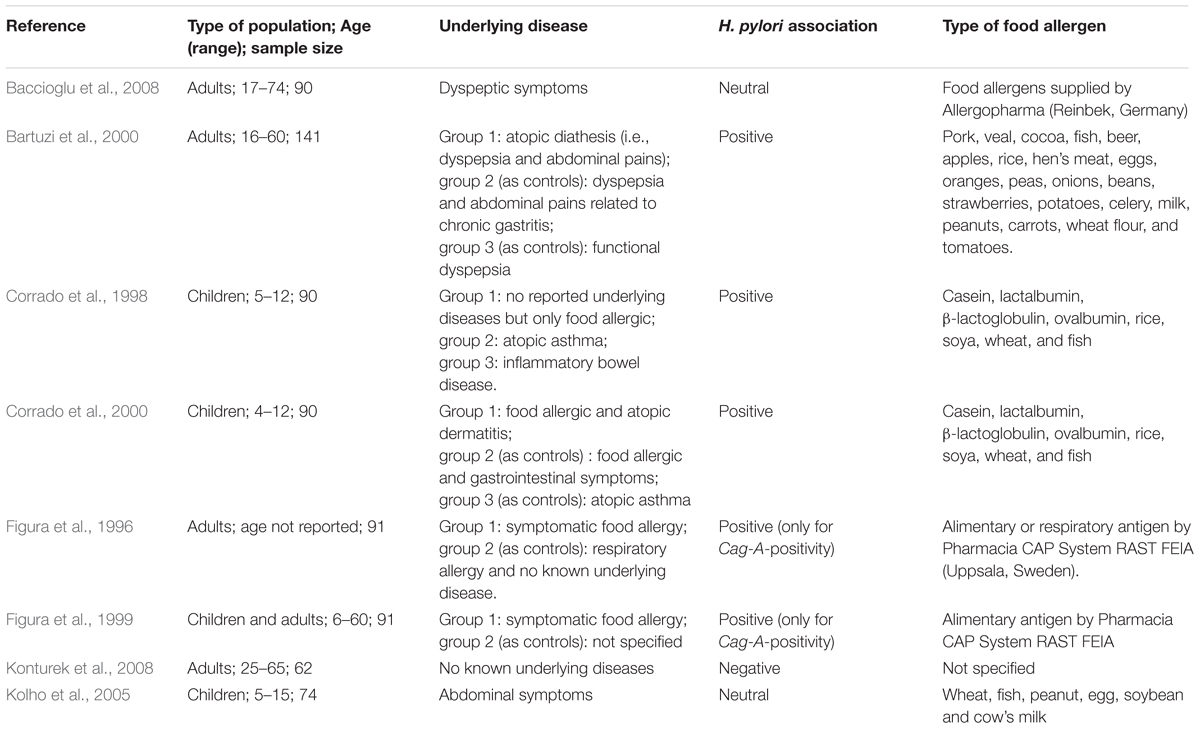
TABLE 1. A summary of studies which investigated the relationship between food allergy and Helicobacter pylori infection.
On the other hand, three studies report no association between food allergy and H. pylori infection (Table 1). Baccioglu et al. (2008) reported no significant difference in the prevalence of food allergy between patients with and without H. pylori infection. In addition, Figura et al. (1996) reported no difference between anti-H. pylori IgG levels between patients with food allergy and controls. Also, a study by Kolho et al. (2005) reported no difference in food specific IgE concentration between H. pylori positive and negative children. Only one study by Konturek et al. (2008) reported that patients with H. pylori infection had a reduced risk of food allergy. However, it is unknown if the reduced risk of food allergy was associated with the presence of other commensal bacteria (Cao et al., 2014).
It is difficult to interpret these contradictory results but many of these studies were limited by small sample sizes (ranged from 62 to 141) and heterogeneous methodologies (Table 1). For example, study by Corrado et al. (1998) enrolled food-allergic patients with unknown underlying disease, and his later study enrolled food-allergic patients with atopic disease and gastrointestinal symptoms (Corrado et al., 2000), raising the possibility for a type 1 error. Where else, Baccioglu et al. (2008) enrolled patients with dyspeptic symptom which might cause a type II error. The age range of patients was broad across studies: children aged 4–17 years and adults aged 18–74 years. One study reported their results of children and adults as a group (Figura et al., 1999). The types of food allergens were also different across studies (Table 1).
How H. pylori Causes Food Allergy?
Food allergen is defined as those specific components of food or ingredients within food that are recognized by allergen-specific immune cells and elicit specific immunologic reaction, resulting in characteristic symptoms (Boyce et al., 2010). A T cell-mediated suppression or ‘oral tolerance’ will usually occur when the GI tract is exposed to food allergens (Faria and Weiner, 2005). However, this mechanism seems to fail in individuals with food allergy. The allergen causes inflammation of the GI mucosa, which subsequently increases the permeability to food antigens (Bock, 1980). Allergic sensitization then occurs and the resultant T-helper-2 (Th2) response leads to increase production of IgE, which then binds to the receptor on the mast cell surfaces located in the skin and the GI tract (Gould and Sutton, 2008).
The process of allergic sensitisation described above seems to be exacerbated by the presence of H. pylori. The passage of intact food proteins is increased across the gastric epithelial barriers if H. pylori is present (Matysiak-Budnik et al., 2004). In addition, gastric mucosa in patients with food allergy may be more hyperaemic and edematous (Ramsay et al., 2010), which further promote H. pylori adhesion. Previous studies have reported a relationship between an increase in food antigens’ absorption by the gut and the development of food allergy in those harboring this bacterium. H. pylori also causes inflammation of the GI mucosa and this results in a greater mucosal permeability which then allows more transition of food allergens (Borch et al., 1998; Fukuda et al., 2001). The food allergens bind to IgE on the mucosal mast cells, which then induce IgE-mediated histamine release from the human basophils in vitro (Aceti et al., 1991). The evidence indicates that infection of H. pylori may exacerbate the severity of IgE-mediated food allergy.
Role of Caga Virulence Factor in Food Allergy
Colonization of H. pylori in the gastric mucosa stimulates release of cytokines such as interleukin (IL)-8 (Takahashi et al., 1998). IL-8 is one of the main mediators of inflammation of gastric mucosa during the course of H. pylori infection. IL-8 synthesis intensifies within the inflamed gastric epithelium (Huang et al., 1995) and cytotoxin-associated gene A (CagA) positivity was found to be an important stimulant for IL-8 (Leonard and Yoshimura, 1990). CagA is a 120–145 kda protein encoded on the Cag pathogenicity island (PAI; Hatakeyama and Higashi, 2005). There is a high prevalence of CagA-positive H. pylori in Asia, particularly in the Southeast Asian countries (93%; Sahara et al., 2012) and China (96%; Zhou et al., 2004).
There are studies which have investigated the relationship between food allergy and virulence factor of H. pylori (Table 1). CagA-positive patients could worsen food allergy as shown by Figura et al. (1996) because CagA stimulates the gastric cells to secrete high levels of inflammatory cytokines (Censini et al., 1996). For example, Figura et al. (1999) reported a higher IgE concentration was found in those with CagA-positive than those with CagA-negative patients. Another study by Figura et al. (1996) reported that CagA seropositivity was significantly higher in patients with food allergy than in controls (P-value = 0.03).
However, some studies (Corrado et al., 2000; Kolho et al., 2005) report no association between CagA positivity and food allergy (Table 1). For example, Kolho et al. (2005) reported no correlation between the food specific IgE and the severity of H. pylori infection as assessed by the presence of CagA antibodies.
There is no clear evidence to support that eradication of H. pylori worsens or improves food allergy reactions. (Figura et al., 1996, 1999; Corrado et al., 2000; Kolho et al., 2005). Very few studies have investigated on this research question. Even though there is a relationship between H. pylori infection and food allergy, the available data do not prove causality.
Food Allergy and H. pylori in Asia
Surveys using self-reported methodology seem to overestimate the prevalence of food allergy in the Asian populations. For example, Leung et al. (2009) reported that the prevalence rate of parent-reported, adverse food reaction in Hong Kong was 6.7%, and this was higher than doctor-diagnosed adverse food reaction (4.6%). However, some Asian countries use oral food challenges to diagnose food allergy in their populations (2013). But the methods used to diagnose food allergy are not standardized across Asian studies. Therefore, it is unclear whether the prevalence of food allergy in Asia reflects the true prevalence of food allergy in their populations.
Nevertheless, food allergy is shown to be on the increase in Asia. For example, Hu et al. (2010) reported that the prevalence of IgE-mediated food allergy has increased from 3.5 to 7.7% in children aged 0–2 years living in China between 1999 and 2009. However, many Asian countries do not have any data or have incomplete data on food allergy (Prescott et al., 2013). A meta-analysis by the National Institute of Allergy and Infectious Disease reported that the estimated prevalence of food allergy is generally between 1 and 10% from available Asian data, with a higher prevalence in children (Boyce et al., 2010). At the moment, there are no reported studies from Asia on the relationship between food allergy and H. pylori.
Recent data indicate a declining prevalence of H. pylori in Asia (Nakajima et al., 2010). This decline may explain the increasing prevalence of food allergy in Asia, if the hygiene hypothesis is true. However, it cannot be ruled out that other factors such as changing of food patterns to a more westernized diet (Prescott et al., 2013) and the interactions between gene and environmental factors (Kauffmann and Demenais, 2012) may also contribute to the increase in prevalence of food allergy in this region.
Treatment Strategies
Currently, there is no cure for food allergy. Allergen avoidance and pharmacological treatments are used to manage food allergy (Sicherer and Sampson, 2014). Antihistamines and mast cell stabilizers are typically used over long term. Patients should be educated about dietary avoidance of causative food allergens in order to minimize the risk of food allergy reactions. At the same time, patients or their parents should be taught on the management of acute food allergic reactions based on the Guidelines for the Diagnosis and Management of Food Allergy in the United States (Boyce et al., 2010; Table 2).

TABLE 2. A summary of the guidelines for the diagnosis and management of food allergy in the United States (Boyce et al., 2010).
Conclusion
Available studies do not fully support an inverse relationship between H. pylori and food allergy. Furthermore, there are no studies that have been published to investigate the relationship since 2008. Although in vitro evidence points to a role of H. pylori in food allergy, the current data on human studies are less convincing. Therefore, further investigations are needed to better understand the role of H. pylori in food allergy which is an increasingly common condition especially among children both in the West and in Asia.
Author Contributions
All authors have contributed substantially to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: ZM wrote the first draft of the manuscript; NM, YY, and YL revised and provided feedbacks for the manuscript. All authors have drafted and revised the work critically for important intellectual contents. All authors approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This paper has been funded by the Universiti Sains Malaysia (USM) Research University Individual (RUI) grant (reference: 1001/PPSP/812151).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aceti, A., Celestino, D., Caferro, M., Casale, V., Citarda, F., Conti, E. M., et al. (1991). Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology 101, 131–137.
Baccioglu, A., Kalpaklioglu, F., Guliter, S., and Yakaryilmaz, F. (2008). Helicobacter pylori in allergic inflammation–fact or fiction? Allergol. Immunopathol. (Madr.) 36, 85–89. doi: 10.1157/13120393
Bartuzi, Z., Zbikowska-Gotz, M., Romanski, B., and Sinkiewicz, W. (2000). Evaluating the profile of selected cytokines in patients with food allergy and chronic gastritis. Med. Sci. Monit. 6, 1128–1135.
Bock, S. A. (1980). Food sensitivity: a critical review and practical approach. Am. J. Dis. Child. 134, 973–982. doi: 10.1001/archpedi.1980.02130220049015
Borch, K., Sjostedt, C., Hannestad, U., Soderholm, J. D., Franzen, L., and Mardh, S. (1998). Asymptomatic Helicobacter pylori gastritis is associated with increased sucrose permeability. Dig. Dis. Sci. 43, 749–753. doi: 10.1023/A:1018809913230
Boyce, J. A., Assa’ad, A., Burks, A. W., Jones, S. M., Sampson, H. A., Wood, R. A., et al. (2010). Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J. Allergy. Clin. Immunol. 126, S1–S58. doi: 10.1016/j.jaci.2010.10.007
Branum, A. M., and Lukacs, S. L. (2009). Food Allergy Among Children in the United States. Pediatrics 124, 1549–1555. doi: 10.1542/peds.2009-1210
Bruijnzeel-Koomen, C., Ortolani, C., Aas, K., Bindslev-Jensen, C., Björkstén, B., Moneret-Vautrin, D., et al. (1995). Adverse reactions to food. Allergy 50, 623–635. doi: 10.1111/j.1398-9995.1995.tb02579.x
Cao, S., Feehley, T. J., and Nagler, C. R. (2014). The role of commensal bacteria in the regulation of sensitization to food allergens. FEBS Lett. 588, 4258–4266. doi: 10.1016/j.febslet.2014.04.026
Censini, S., Lange, C., Xiang, Z., Crabtree, J. E., Ghiara, P., Borodovsky, M., et al. (1996). cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. U.S.A. 93, 14648–14653. doi: 10.1073/pnas.93.25.14648
Corrado, G., Luzzi, I., Lucarelli, S., Frediani, T., Pacchiarotti, C., Cavaliere, M., et al. (1998). Positive association between Helicobacter pylori infection and food allergy in children. Scand. J. Gastroenterol. 33, 1135–1139. doi: 10.1080/00365529850172467
Corrado, G., Luzzi, I., Pacchiarotti, C., Lucarelli, S., Frediani, T., Cavaliere, M., et al. (2000). Helicobacter pylori seropositivity in children with atopic dermatitis as sole manifestation of food allergy. Pediatr. Allergy Immunol. 11, 101–105. doi: 10.1034/j.1399-3038.2000.00028.x
Derakhshan, M. H., and Lee, Y. Y. (2012). Gastric cancer prevention through eradication of helicobacter pylori infection: feasibility and pitfalls. Arch. Iran. Med. 15, 662–663.
Faria, A. M., and Weiner, H. L. (2005). Oral tolerance. Immunol. Rev. 206, 232–259. doi: 10.1111/j.0105-2896.2005.00280.x
Figura, N., Perrone, A., Gennari, C., Orlandini, G., Giannace, R., Lenzi, C., et al. (1999). CagA-positive Helicobacter pylori infection may increase the risk of food allergy development. J. Physiol. Pharmacol. 50, 827–831.
Figura, N., Perrone, A., Gennari, C., Vagliasindi, M., and Rottoli, P. (1996). Food allergy in H. pylori infection. Gut 39, A93.
Fukuda, Y., Bamba, H., Okui, M., Tamura, K., Tanida, N., Satomi, M., et al. (2001). Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion 63(Suppl. 1), 93–96. doi: 10.1159/000051918
Gould, H. J., and Sutton, B. J. (2008). IgE in allergy and asthma today. Nat. Rev. Immunol. 8, 205–217. doi: 10.1038/nri2273
Gupta, R., Sheikh, A., Strachan, D. P., and Anderson, H. R. (2007). Time trends in allergic disorders in the UK. Thorax 62, 91–96. doi: 10.1136/thx.2004.038844
Hatakeyama, M., and Higashi, H. (2005). Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci. 96, 835–843. doi: 10.1111/j.1349-7006.2005.00130.x
Hu, Y., Chen, J., and Li, H. (2010). Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr. Int. 52, 820–824. doi: 10.1111/j.1442-200X.2010.03166.x
Huang, J., O’Toole, P. W., Doig, P., and Trust, T. J. (1995). Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect. Immun. 63, 1732–1738.
Jackson, K. D., Howie, L., and Akinbami, L. J. (2013). Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief 121, 1–8.
Kauffmann, F., and Demenais, F. (2012). Gene-environment interactions in asthma and allergic diseases: challenges and perspectives. J. Allergy Clin. Immunol. 130, 1229–1240. doi: 10.1016/j.jaci.2012.10.038 quiz 1241-1222,
Kolho, K. L., Haapaniemi, A., Haahtela, T., and Rautelin, H. (2005). Helicobacter pylori and specific immunoglobulin E antibodies to food allergens in children. J. Pediatr. Gastroenterol. Nutr. 40, 180–183. doi: 10.1097/00005176-200502000-00018
Konturek, P. C., Rienecker, H., Hahn, E. G., and Raithel, M. (2008). Helicobacter pylori as a protective factor against food allergy. Med. Sci. Monit. 14, Cr452–Cr458.
Lee, Y. Y., and Derakhshan, M. H. (2013). Environmental and lifestyle risk factors of gastric cancer. Arch. Iran. Med. 16, 358–365.
Lee, Y. Y., Raj, S. M., and Graham, D. Y. (2013). Helicobacter pylori Infection – A Boon or a Bane: lessons from studies in a low-prevalence population. Helicobacter 18, 338–346. doi: 10.1111/hel.12058
Leonard, E. J., and Yoshimura, T. (1990). Neutrophil attractant/activation Protein-1 (NAP-1 [Interleukin-8]). Am. J. Respir. Cell. Mol. Biol. 2, 479–486. doi: 10.1165/ajrcmb/2.6.479
Leung, T. F., Yung, E., Wong, Y. S., Lam, C. W., and Wong, G. W. (2009). Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr. Allergy Immunol. 20, 339–346. doi: 10.1111/j.1399-3038.2008.00801.x
Matysiak-Budnik, T., Coffin, B., Lavergne-Slove, A., Sabate, J. M., Megraud, F., and Heyman, M. (2004). Helicobacter pylori increases the epithelial permeability to a food antigen in human gastric biopsies. Am. J. Gastroenterol. 99, 225–232. doi: 10.1111/j.1572-0241.2004.04080.x
Mullins, R. J. (2007). Paediatric food allergy trends in a community-based specialist allergy practice, 1995-2006. Med. J. Aust. 186, 618–621.
Nakajima, S., Nishiyama, Y., Yamaoka, M., Yasuoka, T., and Cho, E. (2010). Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J. Gastroenterol. Hepatol. 25(Suppl. 1), S99–S110. doi: 10.1111/j.1440-1746.2009.06214.x
Peek, R. M. Jr., and Blaser, M. J. (2002). Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37. doi: 10.1038/nrc703
Prescott, S., Pawankar, R., Allen, K., Campbell, D., Sinn, J., Fiocchi, A., et al. (2013). A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 6:21. doi: 10.1186/1939-4551-6-21
Ramsay, D. B., Stephen, S., Borum, M., Voltaggio, L., and Doman, D. B. (2010). mast cells in gastrointestinal disease. Gastroenterol. Hepatol. 6, 772–777.
Sahara, S., Sugimoto, M., Vilaichone, R.-K., Mahachai, V., Miyajima, H., Furuta, T., et al. (2012). Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: a meta-analysis. BMC Infect. Dis. 12:223. doi: 10.1186/1471-2334-12-223
Sicherer, S. H., and Sampson, H. A. (2014). Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 133, 291–307; quiz308. doi: 10.1016/j.jaci.2013.11.020
Strachan, D. P. (1989). Hay fever, hygiene, and household size. BMJ 299, 1259–1260. doi: 10.1136/bmj.299.6710.1259
Suerbaum, S., and Michetti, P. (2002). Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186. doi: 10.1056/NEJMra020542
Takahashi, S., Nakamura, E., and Okabe, S. (1998). Effects of cytokines, without and with Helicobacter pylori components, on mucus secretion by cultured gastric epithelial cells. Dig. Dis. Sci. 43, 2301–2308. doi: 10.1023/A:1026635110099
Wang, J., and Sampson, H. A. (2011). Food allergy. J. Clin. Invest. 121, 827–835. doi: 10.1172/jci45434
Keywords: Helicobacter pylori, atopic disease, food allergy, allergy, food sensitivity
Citation: Ma ZF, Majid NA, Yamaoka Y and Lee YY (2016) Food Allergy and Helicobacter pylori Infection: A Systematic Review. Front. Microbiol. 7:368. doi: 10.3389/fmicb.2016.00368
Received: 21 November 2015; Accepted: 07 March 2016;
Published: 23 March 2016.
Edited by:
Simona Zompi, University of California San Francisco, USAReviewed by:
Paul Fisch, University Medical Center Freiburg, GermanyMaria Cristina Cerquetti, University of Buenos Aires, Argentina
Copyright © 2016 Ma, Majid, Yamaoka and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeong Yeh Lee, justnleeyy@gmail.com
 Zheng Feei Ma
Zheng Feei Ma Noorizan A. Majid2
Noorizan A. Majid2 Yoshio Yamaoka
Yoshio Yamaoka Yeong Yeh Lee
Yeong Yeh Lee