- 1Inflammation Biology and Cell Signaling Laboratory, National Institute of Pathology, New Delhi, India
- 2Dr. Reddy’s Institute of Life Sciences, University of Hyderabad Campus, Hyderabad, India
- 3Department of Biotechnology, School of Life Sciences, University of Hyderabad, Hyderabad, India
- 4Molecular Infection and Functional Biology Laboratory, Kusuma School of Biological Sciences, Indian Institute of Technology, New Delhi, India
PE/PPE genes, present in cluster with ESAT-6 like genes, are suspected to have a role in antigenic variation and virulence of Mycobacterium tuberculosis. Their roles in immune evasion and immune modulation of host are also well documented. We present evidence that PE32/PPE65 present within the RD8 region are co-operonic, co-transcribed, and co-translated, and play role in modulating host immune responses. Experiments with macrophage cell lines revealed that this protein complex suppresses pro-inflammatory cytokines such as TNF-α and IL-6 whereas also inducing high expression of anti-inflammatory IL-10. Immunization of mice with these recombinant proteins dampens an effective Th1 response as evident from reduced frequency of IFN-γ and IL-2 producing CD4+ and CD8+ T cells. IgG sub-typing from serum of immunized mice revealed high levels of IgG1 when compared with IgG2a and IgG2b. Further IgG1/IgG2a ratio clearly demonstrated that the protein complex manipulates the host immune response favorable to the pathogen. Our results demonstrate that the co-transcribed and co-translated PE32 and PPE65 antigens are involved specifically in modulating anti-mycobacterial host immune response by hampering Th1 response.
Introduction
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis (M. tuberculosis), despite being completely curable, claimed more than 1.5 million human lives in 2013 alone (Zumla et al., 2015) and continue to do so. With over a third of the world population infected with M. tuberculosis, and 9 million new cases each year, the major problems with regard to TB are: (i) lack of proper diagnosis; (ii) unavailability of efficacious vaccine; (iii) nexus with HIV, and (iv) emergence of MDR and XDR strains which is further aggravating the problem. The current protective TB vaccine- a live-attenuated M. bovis Bacillus-Calmette-Guerin (BCG) vaccine is protective against TB and leprosy in young people, but it is not efficient in protecting adults against pulmonary TB or reactivation of the latent TB infection (Mangtani et al., 2014). Proteins unique to these bacteria will be of interest in understanding the pathobiology of this bacterium. Identification of virulence determinants and components that are predicted to interact with host immune system are important to understand the pathogenicity in order to develop new therapeutic interventions.
While mycobacteria underwent reductive evolution as it attained pathogenicity (Ahmed et al., 2008), the only protein family whose presence increased as the genome size decreased was the PE/PPE/PGRS family (Gey van Pittius et al., 2006), present exclusively in this genus (Cole, 1998). Apart from interspecies difference, clinical isolates of even single species depicted genome fluidity as a function of geographic partitioning (Rao et al., 2005). Comparative genomic and proteomic analyses of pathogenic, opportunistic and non-pathogenic mycobacteria revealed an inverse relationship between the number of genes and the pathogenicity/virulence status of the Mycobacterium (Rahman et al., 2014). While M. tuberculosis genome encodes about 175 PE/PPE proteins (Cole, 1998), the recently sequenced ancestral, non-pathogenic and medically relevant M. indicus pranii carries only 66 PE/PPE genes (Saini et al., 2009, 2012). These genes were speculated to be introduced into mycobacteria by mobile elements via lateral gene transfer and later evolved from esx-encoded ancestral PE/PPE copies by gene duplication of this cluster (Gey van Pittius et al., 2006).
Despite representing about 10% of the total genome of M. tuberculosis, the role of these highly expanded and enigmatic protein families is not fully understood (Akhter et al., 2012). Many members of these gene families have been described to have multiple functions (moonlighting) by gene cooption (Singh et al., 2014) as a consequence of genomic reduction. Several PE/PPE genes are co-operonic and are present in clusters along with ESX and/or other proteins (Tundup et al., 2006). ESX, also known as Type VII secretion system, is present in five copies and one of the ESX coding gene cluster has been shown to be essential for virulence (Houben et al., 2014). ESAT-6 and CFP-10, secreted out through the ESX secretory complex interacts with host factors and modulates the immune response (Delogu et al., 2006). This close evolutionary link between ESX clusters and PE/PPE genes along with key difference within this family of proteins, between the virulent (H37Rv) and avirulent (H37Ra) strain, suggests that PE/PPE proteins contribute to infection and pathogenesis (Kohli et al., 2012). These proteins have other myriad functions like, signal transduction (Strong et al., 2006; Deng et al., 2014) role in virulence (Choudhary et al., 2003), immune modulation (Parra et al., 2006), inhibition of phagolysosomal fusion (Jha et al., 2010) immune quorum sensing with subsequent cell death (Tundup et al., 2014), and antigenic variation (Banu et al., 2002; Mohareer et al., 2011). However, antigenic variations found in PE-PGRS family is now attributed to the selection pressure acting on individual PE-PGRS gene and independent of human T-cell recognition (Copin et al., 2014).
Comparative genomics of different species of M. tuberculosis complex revealed differences in few regions of genome, known as Region of Difference (RD). Role of most of the 16 RDs is not clearly understood except for RD1, which has been shown to be the most important factor for virulence (Lewis et al., 2003). RD1 has PE35/PPE68 genes clustered along with ESAT-6 and CFP-10 and ESX secretory system which are known virulent factors of M. tuberculosis (Jackson-Sillah et al., 2013). Another RD harboring PE/PPE genes clustered with ESAT-6 system is RD8, which codes PE32/PPE65, tempting us to speculate that the PE32/PPE65 antigens might be potential virulence determinants. Our results show that PE32/PPE65 are co-operonic and are expressed together at mRNA as well as at protein levels. We used in silico approach to predict the antigenicity of PE32/PPE65 using hydropathy profiling which revealed strong antigenic index for PPE65 as compared to PE32. These in silico findings were experimentally validated in vitro and in vivo in a mouse model. Cytokine and IgG profiling data categorically reveal that these proteins have a role in immune modulation and aid in pathogenicity.
Materials and Methods
Cloning, Expression, and Purification of Recombinant Proteins
PE32 and PPE65 genes were amplified from H37Rv genomic DNA using specific primers (Table 1). The complete ORF encoding PE32 and PPE65 was cloned into BamHI and XhoI site or BamHI and HindIII site of pETDuet-1 vector (Novagen) to construct recombinant expression plasmids pET-PE32 and pET-PPE65, respectively. DNA fragment containing both the genes was PCR amplified from H37Rv genomic DNA using forward primer specific for PE32 and reverse primer specific for PPE65 and the resultant amplicon was cloned into the BamHI and HindIII site of pETDuet vector to generate pET-PE32:PPE65. Recombinant constructs were used to transform Escherichia coli BL21-DE3 strain. Culture was induced with 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) for 3 h at 37°C for the expression of recombinant proteins. Purification of recombinant proteins was carried out by sonicating bacterial cells and solubilizing recombinant protein using 0.3% Sarkosyl in PBS. Recombinant proteins were purified using Ni-NTA affinity column and eluted with 200 mM Imidazole, dialyzed against PBS and finally treated with Polymyxin B beads to remove bacterial endotoxins.
Operonic Organization
DNaseI treated total RNA of H37Rv was used to synthesize first strand cDNA, using specific reverse primer for PPE65. This single strand cDNA was used as template to amplify PE/PPE genes using gene specific primer pairs: (a) PE32 forward and reverse; (b) PPE65 forward and reverse; (c) PE32 forward and PPE65 reverse primer. Table 1 lists the sequence of all the primers used in this study. Genomic DNA was placed as positive control and reaction without reverse transcriptase served as negative control.
To check for co-operonic expression of protein, DNA fragment containing both genes was amplified from H37Rv genomic DNA using forward primer specific for PE32 and reverse primer specific for PPE65. Amplified DNA fragment was cloned in MCS-I of pETDuet-1 vector between BamHI and HindIII sites. BL21-DE3 expression strain of E. coli was transformed with the clone and proteins expressed as mentioned earlier.
Immunization of Mice
Eight to twelve week old C57BL/6j mice were procured from National Institute of Nutrition, Hyderabad, India. All procedures were performed in accordance with Institutional Animal Ethics guidelines. Mice were housed in cages and allowed free access to pellet diet and water with 12 h photoperiod. Period of 7 days was given for acclimatization to the environment and observation for signs of disease. Primary immunization was carried out with 20 μg of recombinant PE/PPE or PE+PPE proteins in PBS, administered subcutaneously at the base of tail. Mice were checked daily for signs of discomfort. Booster was given subcutaneously with 20 μg of recombinant PE/PPE protein at 15th day of immunization. Mice were sacrificed after 4 weeks of primary immunization.
Cell Culture and Estimation of Cytokines by ELISA
RAW264.7 cells were seeded (0.1 million/well) in 96-well plate. Cells were stimulated with different concentration of native or heat inactivated recombinant protein. After 24 h of stimulation, culture supernatant was harvested, and ELISA was performed as described earlier (Ravindran et al., 2014). Splenocytes from immunized and placebo treated mice were also seeded in 96-well plates (0.1 million/well) in DMEM. Protein treatments with different concentration were given for 72 h and supernatants were collected and stored at -20°C.
Secreted cytokine levels in culture supernatant were measured using OptEIA kits (BD Biosciences, San Diego, CA, USA) to determine the levels of mouse TNF-α, IFN-γ, IL-10, and IL-6. ELISA was carried out using manufacturer protocol or as described earlier (Ravindran et al., 2014). Briefly, 96 well ELISA plates were coated with capture antibody in coating buffer (bicarbonate/phosphate buffer) kept at 4°C overnight. Plates were washed with PBS-T (0.05% tween20) thrice. 10% FBS was used as blocking as well as assay diluent. After an hour of blocking, supernatant along with standards were added for 2 h. After 5 washes, detection antibody and enzyme conjugates were added for 1hr. After 7 washes, TMB substrate was added and 2N H2SO4 was added to stop the reaction. Absorbance was taken at 450 nm and curve was plotted along with standards to determine the cytokine levels in test samples.
B-cell response against individual proteins was carried out using specific secondary antibody against IgG1, IgG2a, and IgG2b. Blood was collected by bleeding mice via the lateral tail vein. Sera were collected and stored at -20°C to be used later for ELISA as described earlier (Banerjee et al., 2004). Briefly, 96 well plates were coated by specific proteins in PBS (10 μg/ml) and kept at 4°C overnight. Plate was washed three times with wash buffer and blocked for an hour at room temperature. After 3 washes serum samples in 1:100 dilution was added and kept for 2 h. Secondary conjugate antibody was added in 1:5000, 1:3000, and 1:3000, respectively, for IgG1, 2a and 2b for an hour (Kasturi et al., 2011). Plate was washed at least five times, TMB substrate was added, and reaction was stopped with 2N H2SO4.
MTT Assay
MTT assay was carried out using RAW264.7 cells (1 × 104/well) seeded in 96 well plates in complete DMEM media and treated with proteins in different concentrations for 24 h. Supernatant was harvested and fresh 200 μl media was added. 20 μl of MTT at a concentration of 5 mg/ml was added and incubated at 37°C for 4 h. 100 μl of DMSO was added to each well and mixed well after removing the media completely (Tundup et al., 2014). Absorbance was measured at 595 nm.
Splenocyte Isolation and Single Cell Preparation
Mice were euthanized and spleens were isolated via incision at the left side of the abdomen. Spleens were crushed/perfused using syringe and the suspension was transferred to another tube after passing through cell strainer. Cells were centrifuged, washed and re-suspended in RBC lysis buffer. The cells were centrifuged and finally re-suspended in DMEM containing 10% FBS.
Intracellular Cytokine (ICC) Staining and Extracellular Staining of Surface Markers
For CD4+/CD8+ T cell responses, 1 × 106 Splenocytes/well were seeded in 96-well plate (Ravindran et al., 2014), and re-stimulated with 10 μg/ml concentration of PE32, PPE65 and PE32+PPE65 for 8 h in presence of Golgi plugTM and Golgi stopTM (BD Biosciences, San Diego, CA, USA). Cells were collected, washed with PBS and stained with anti-CD4 and anti-CD8 (FITC for CD4 and CD8). Cells were fixed with 4% paraformaldehyde, permeablized in 0.02% triton X-100, followed by washing and were stained with anti-IFN-γ and anti-IL-2 (APC for IFN-γ, PE for IL-2) for 1 h.
Statistical Analysis
Statistical analysis was performed using Prism 5 software. Results were expressed as mean ± SD and analyzed by one-way ANOVA for calculating p value. Value of p < 0.05 was considered statistically significant.
Results
PE32 and PPE65 Are Organized in an Operon and Transcribed as a Single mRNA
In silico analysis predicted several PE/PPE genes, including the PE32/PPE65 pair to be organized as operons in the genome (Tundup et al., 2006). RT-PCR was carried out using specific reverse and forward primers of PPE65 and PE32 for amplification of PE32 (P1, P2), PPE65 (P3, P4), and PE32+PPE65 (P1, P4) were used (Table 1; Figure 1A). Agarose gel electrophoresis of the amplified product clearly shows amplification fragment of 300 bp corresponding to PE32, 1.2 kb of PPE65, and 1.5 kb of PE32+PPE65 (Supplementary Figure S1). PCR reaction without reverse transcriptase with total RNA did not generate any amplification from genomic DNA contamination (Supplementary Figure S1). These results validated the in silico observation on the co-operonic expression of PE32/PPE65 gene pair.
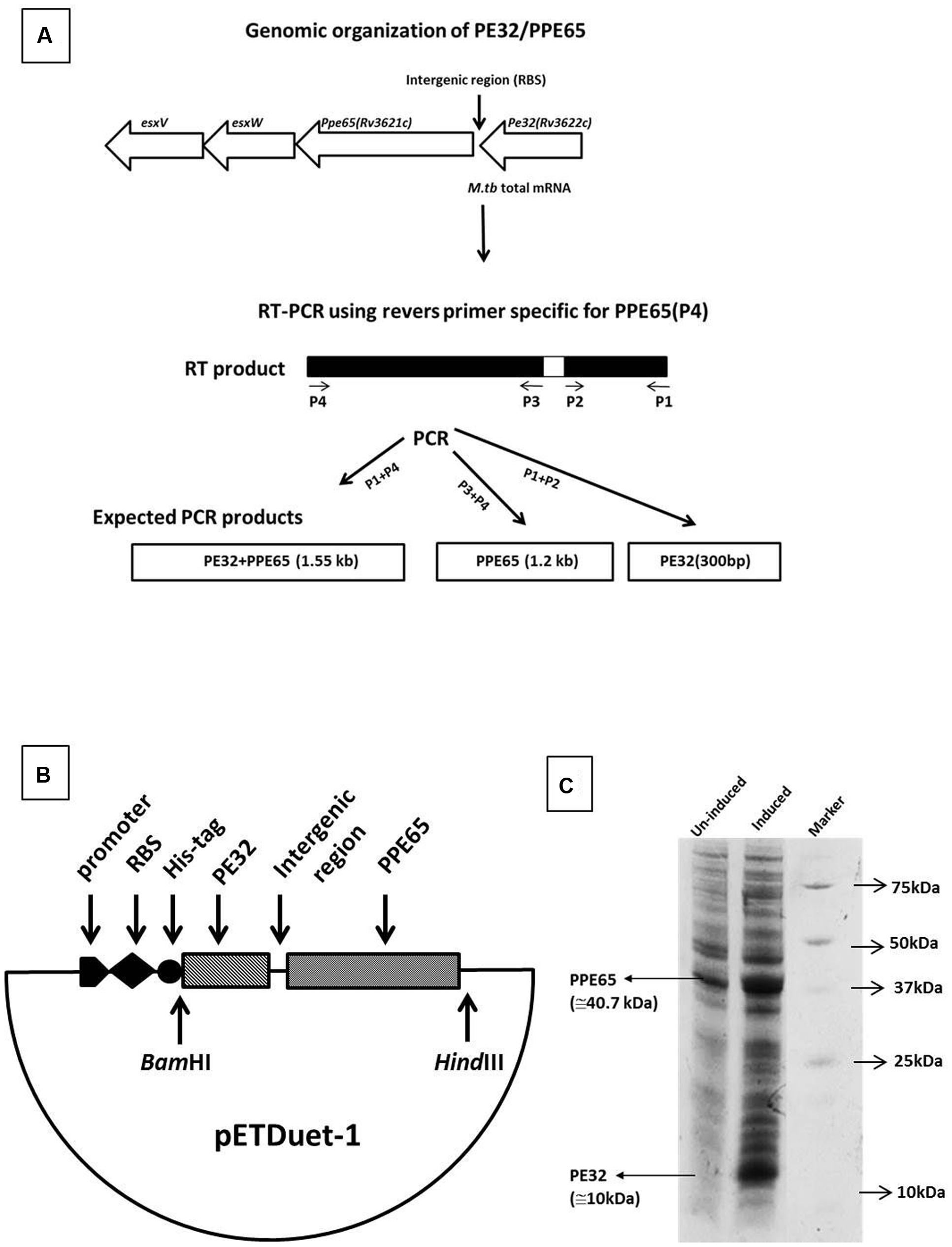
FIGURE 1. Genomic organization and expression analysis reveals PE32 and PPE65 as functionally linked co-operonic gene pair. PE32/PPE65 (Rv3622c/Rv3621c) gene pair is organized in an operon. (A) Schematic representation of co-operonic genes. Primer pair specific to PE32 produced amplicon of the size 300 bp, specific primer pair for PPE65 yielded amplification of 1.2 kb, and forward primer for PE32 and reverse primer for PPE65 revealed an amplification product of 1.5 kb. (B) Schematic representation of clone of PE32 + PPE65 gene pair as single operon. (C) PE32/PPE65 genes are expressed together at protein level. Expression of PE32/PPE65 gene was checked on 10% tricine SDS gel. Lane 1 is un-induced culture, Lane 2 is induced culture showing the expression of PE32 (10 kDa) and PPE65 (40.7 kDa), Lane 3 is protein ladder.
Co-translation of PE32 and PPE65
Co-operonic PE/PPE genes have been earlier shown to be translated together when cloned in a vector having RBS (ribosome binding site) introduced in between (Strong et al., 2006; Tundup et al., 2006). Strong et al employed a co-expression vector that has been modified to include an additional RBS for expression of the second gene. However, we have not disturbed the intergenic region between the two genes, thus utilizing the RBS from vector for the expression of first gene (PE32). The native M. tuberculosis RBS present within the intergenic region was utilized for the second gene (PPE65). A cassette of PE32+intergenic region+PPE65 was cloned under one T7 promoter with RBS and His tag fused to the PE32 gene (Figure 1B). Assuming that a single mRNA will be transcribed for both the genes, RBS present in vector will carry out the translation of PE32 while native RBS present before the PPE65 gene will translate PPE65. This indeed could be seen when the protein expression product present in the supernatant of culture lysate was loaded on 10% tricine SDS gel. Over expression of PE32 (10 kDa) and PPE65 (40.7 kDa) can be observed (Figure 1C) as compared to un-induced culture. These data demonstrate that mRNA transcribed from the co-operonic PE32/PPE65 gene pair was translated using external RBS and the intergenic RBS to give rise to corresponding proteins PE32 and PPE65.
PE32/PPE65 Proteins Reduce Pro-Inflammatory Response and Concomitantly Enhance Anti-Inflammatory Response
Recombinant PE32 and PPE65 were purified with N-terminal Histidine tag, with high purity (Supplementary Figure S2) and treated with Polymyxin B beads to remove endotoxins. Varying concentration of recombinant PE32, PPE65, and PE32+PPE65 proteins were used to stimulate RAW264.7 cells in culture for 24 h. PPE65 was shown to increase the level of anti-inflammatory cytokine IL-10 in a dose dependent manner in RAW264.7 cells (Figure 2A). PE32 alone also increased IL-10 production, though not significantly. Combinations of the two proteins also lead to significant elevation in IL-10 secretion. After observing increase in anti-inflammatory levels we evaluated pro-inflammatory response. IL-6 level was found to decrease (Figure 2B) as a direct function of increasing concentration of recombinant proteins either used singly or together in RAW264.7 cells. TNF-α secretion by RAW264.7 cells was found to decrease with increase in concentration of recombinant proteins (Figure 2C) To rule out cytotoxicity to be the reason for decrease in cytokine level with protein dose, we performed MTT assay which showed no significant death with higher protein dose (Supplementary Figure S3). These results demonstrate that PE32 and PPE65 proteins are immunomodulatory in nature and play role in either establishment or maintenance of infection to favor survival of pathogen by altering the cytokine milieu.
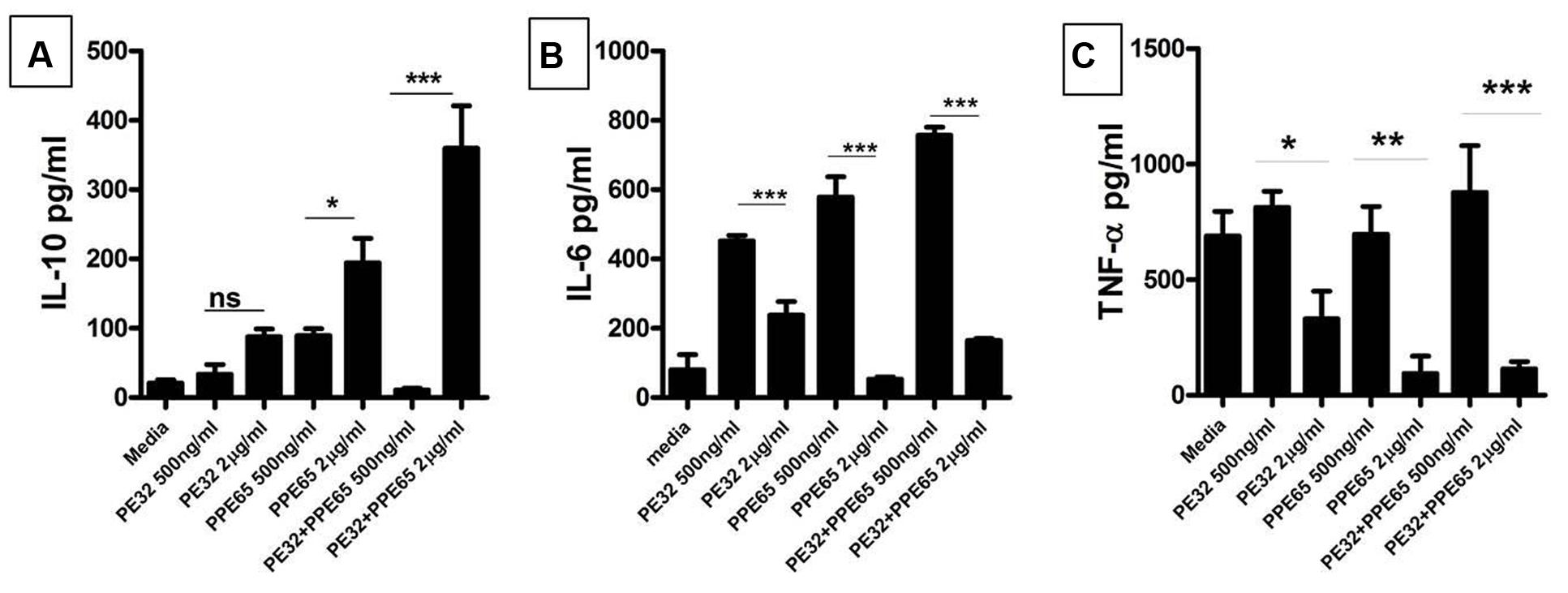
FIGURE 2. PE32 and PPE65 modulate the host immune pathway by suppressing pro inflammatory cytokine production. RAW 264.7 cells were treated with purified recombinant PE/PPE proteins in different concentration for 24 h, culture supernatants were collected for measuring various cytokine levels through sandwich ELISA. (A) PE32, PPE65 induce IL-10 level in RAW 264.7 cells as a direct function of protein concentration whether used singly or together. (B,C) Note the decrease in the level of Th1 cytokine (IL-6 and TNF-α) in RAW 264.7 cells with increase in protein concentration Experiments were performed at least twice; SEM is represented by error bar for biological triplicates. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
B-Cell Response against PPE32/PPE65 in Immunized Mice
Among several operonic PE/PPE proteins, PE had been seen to be less or non-immunogenic (Tundup et al., 2008) in mice as well as in humans. We used DNASTAR prediction tool (Choudhary et al., 2003) and expectedly found that PPE65 has numerous antigenic patches as compared to PE32 which had a few antigenic stretches indicating that PE32 could be less immunogenic (Supplementary Figure S4). In vivo antigenicity assay was carried out by immunizing mice with PE32, PPE65, and PE32+PPE65 followed by booster at 15th day of primary immunization. Serum was collected after 4 weeks of primary immunization. ELISA was performed for IgG1, IgG2a, and IgG2b response against PE32, PPE65, and PE32+PPE65. IgG1 was the predominant subtype in immunized animal (3A) as compared to other subtypes (Figures 3A–C). Higher IgG2a and IgG2b response could also be observed in PPE65 and PE32+PPE65 immunized mice as compared to that of PE32 immunized mice which displayed low levels of all the isotypes (Figures 3B,C). Further, IgG1 and IgG2a ratio revealed that PPE65 and PE32+PPE65 induce Th2 response with no significant result seen for PE32 (Figure 3D). IgG isotype ratio clearly suggested predominance of Th2 response over Th1 that impairs anti-mycobacterial immunity.
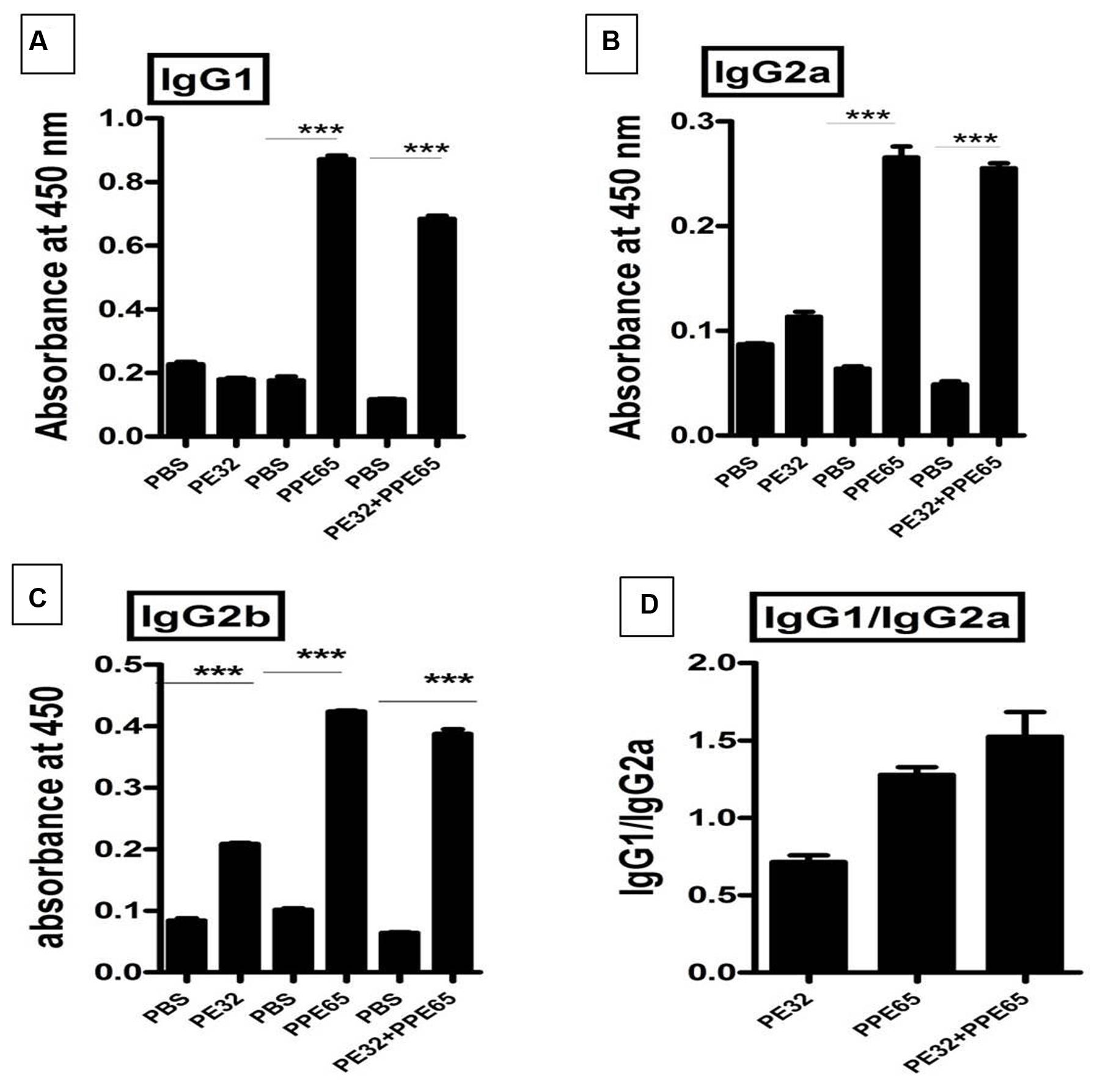
FIGURE 3. Antibody isotype analysis reveals the skewing of immune phenotype toward Th2 type of response. Sera from immunized mice were used to perform ELISA to measure IgG1, IgG2a, and IgG2b. (A) Mice immunized with PE32 show low IgG1 while those immunized with PPE65 and PE32 + PPE65 proteins showed higher levels of IgG1 against respective proteins. (B,C) Similar observation was made for IgG2a and IgG2b. IgG1 vs. IgG2a ratio is important to determine whether the response is Th1 or Th2. (D) The IgG1/IgG2a >1 shows Th2 response for PPE65 and PE32 + PPE65. Error bar represents SEM of average of three animals in biological triplicates. ∗∗∗p < 0.001.
PE32/PPE65 Proteins Suppress Th1 Cytokines Production in Splenocytes
Treatment of macrophage cell lines with PE32/PPE65 proteins demonstrated decrease in pro-inflammatory cytokines along with increase in anti-inflammatory cytokine. Result of IgG subtype responses also corroborated a shift toward Th2 type of immune response. Interestingly, animals immunized with these proteins either singly or in combination also depicted a decrease in IFN-γ level in splenocytes culture supernatant with increasing concentration of PE32, PPE65, and PE32+PPE65 (Figure 4). These finding directly point to the role of these proteins in dampening the anti-mycobacterial Th1 response. Having observed decrease in IFN-γ levels in culture supernatant of splenocytes treated with recombinant proteins, we assayed for T-cell activity by measuring CD4+/CD8+ cells positive for IFN-γ and IL-2 by flow cytometry. A decrease in CD4+ and CD8+ cells positive for IFN-γ and IL-2 could be seen after treatment with these PE/PPE proteins, with maximum decrease observed in splenocytes primed and treated with PE32+PPE65 (Figures 5A,B).
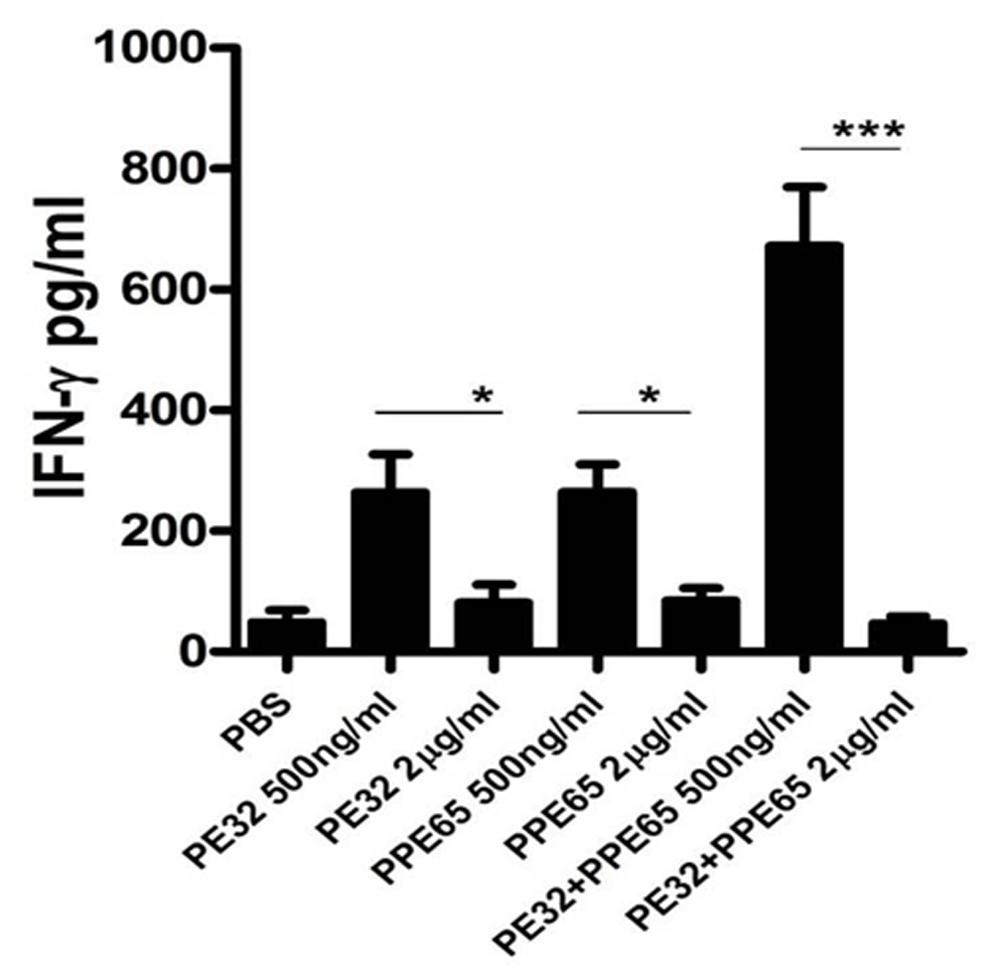
FIGURE 4. PE32 and PPE65 proteins reduce the secretion of IFN-γ in splenocytes of mice immunized with respective proteins. Splenocytes isolated from mice which were immunized with PE32, PPE65, and PE32 + PPE65 proteins were again challenged with corresponding proteins in culture for 72 h, supernatants were harvested and sandwich ELISA for IFNγ was performed. Note the decrease in IFNγ level with increase in protein concentration. Experiments were performed at least twice; SEM is represented by error bar for biological triplicates. ∗p < 0.05, ∗∗∗p < 0.001.
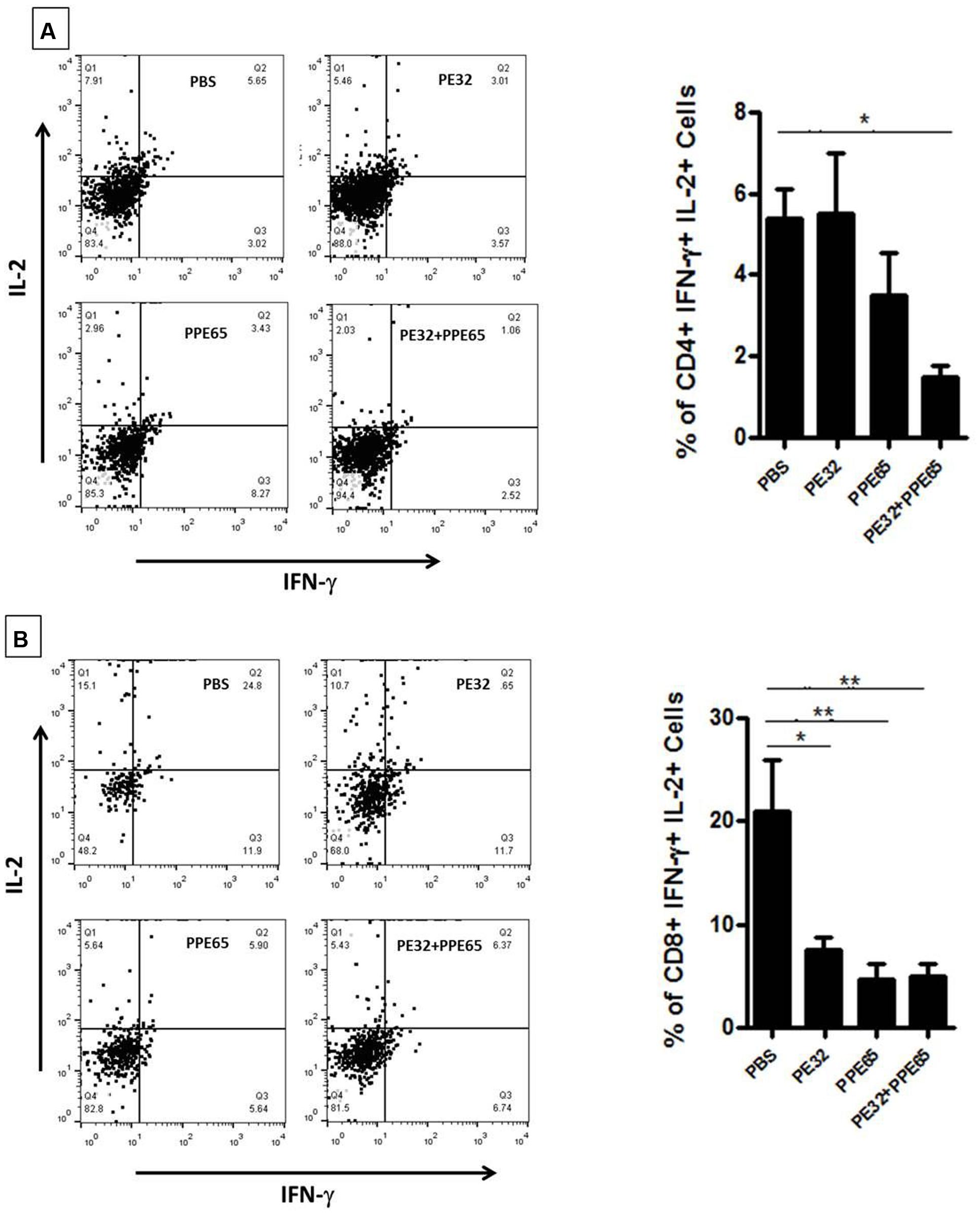
FIGURE 5. PE32 and PPE65 proteins reduce polyfunctional CD4+ and CD8+ cells in immunized mice. Splenocytes isolated from mice which were immunized with PE32, PPE65, and PE32 + PPE65 proteins were again challenged with same proteins in culture for 8 h and stained for intracellular IFN-γ and IL-2. (A) PE32 and PPE65 proteins decrease intracellular IFN-γ and IL-2 level as depicted by graphical representation of change in number of CD4+ cells positive for IFNγ and IL-2. (B) These proteins also decreased frequency of CD8+ cells expressing IFNγ and IL-2 as evident from graphical representation of change in frequency of CD8+ cells positive for IFNγ and IL-2. Dot plots are representative plots from at least four animals for each group. ∗p < 0.05, ∗∗p < 0.01.
The immunological observations described above clearly indicate that this PE/PPE gene pair interacts with host components and thereby modulates critical immune pathways to subvert the immune response for the benefit of pathogen. This alteration of immune phenotype could profoundly impact the course of disease thus implicating this protein family as critical mediators of the host response that finally drives the outcome of infection.
Discussion
The evolution of M. tuberculosis involves genomic reduction (Ahmed et al., 2008) with the exception of PE/PPE/PGRS family of genes which in fact have expanded as a function of evolution toward pathogenicity (Gey van Pittius et al., 2006). The organization of PE/PPE along with ESX family corroborates their role in evolution of pathogenicity and host specificity (Delogu et al., 2006; Tundup et al., 2006). Their unique organization with type VII secretion system makes them important targets of host immune response (Sampson, 2011). Although these proteins are reported to have direct role in pathogenesis and virulence (Ahmed et al., 2015: Fishbein et al., 2015) the actual mechanism is still obscure. We investigated the immunomodulatory behavior of PE32/PPE65 gene complex organized within RD8 segment of M. tuberculosis genome. The operonic organization of these proteins suggests they are functionally linked and thus might have a common function with each protein enhancing the functionality of other one (Tundup et al., 2014). This observation supplements the information available regarding operonic organization of this important pathogen. The operonic organization suggests a functional linkage and thus probably a common biochemical pathways components that could be delineated and targeted to tame this pathogen. It can be speculated that these protein can function independently of each other as well as form a complex to have a gain of function. Our observation of increased effect by using the combination of both suggests that these protein possibly interact in vivo and adjunct the immunomodulatory function of each other.
Macrophages are the first line of defense against M. tuberculosis infection (Clay et al., 2007) but M. tuberculosis employs a plethora of strategies to counteract the host immunity, thereby successfully establishing a niche within macrophages. When RAW264.7 macrophage cell lines were treated with recombinant PE32/PPE65, either singly or in combination, they modulated immune response by decreasing production of pro-inflammatory cytokines and increasing anti-inflammatory cytokine production. Another protein of this family, PPE18 has earlier been reported to increase IL-10 production in THP-1 cells (Nair et al., 2009). Available data also indicate that PE/PPE proteins play important role in subverting innate immune responses and thus help the pathogen in establishment of infection. Apart from immune modulation many PE/PPE proteins have also been implicated in interference of vacuole acidification (Tiwari et al., 2012) which is a vital step for killing of these bacteria. Once bacteria establish itself in host macrophages by subverting innate immune defense it can then modulate the adaptive host immune mechanism for its survival (Goldstone et al., 2009). Apart from modulating the cytokine milieu PE/PPE proteins (PE_PGRS33 and PE_PGRS17) are known to assist in immune evasion by limiting antigen presentation (Delogu et al., 2004), thereby preventing recognition and killing of mycobacteria-infected host cells.
It is now established that B cells can exert influence on T cells and are thus likely to be important in determining the outcome of infection with M. tuberculosis. The IgG2a and the IgG1 isotypes level usually reflect the T cell phenotype. A Th2 biased response favors the production of IgG1 isotype and a Th1 response drives IgG2a production. As PE/PPE family members are a potentially rich source of B cell epitopes (Chakhaiyar et al., 2004). as also predicted from hydropathy profile of PPE65, we determined the levels of antibody isotypes in sera of immunized animals. It has been earlier observed that PE protein is less immunogenic when compared to PPE protein, (Tundup et al., 2008). Our findings also suggest the same for PE32, as PPE65 proved to be a good B-cell antigen with a higher antibody response against PPE65 as compared to PE32 in sera of mice immunized with the respective proteins. Analysis of IgG subtypes revealed that PPE65 induces significant increase in IgG1 as compared to IgG2a (IgG1/IgG2a > 1 = Th2 response) levels which predicts a predominance of Th2 type of immune response (Finkelman et al., 1991). This observation was interesting as a number of PE/PPE proteins may be surface exposed and are likely to be targeted by the humoral response and thus dictate the outcome. This was consistent with an earlier observation of another PPE protein Rv2608 which elicited a high humoral and a low T cell response in TB patients (Chakhaiyar et al., 2004). These observations signify the importance of these proteins and the need to explore further to understand the virulence mechanism.
PE/PPE proteins are also known to encompass a number of T cell epitopes and several of them are being assessed as future vaccines. Contrary to this, the dampening effect of PE32/PP65 on Th1 response highlights a different role for this family and also points to its likely involvement in the immune evasion process. Mice immunized with these proteins were found to have reduced production of IFN-γ from splenocytes as a function of protein concentration, though there was increase in the level of IFN-γ with lower concentrations of proteins which could be possibly the result of basal level of interaction of T cells with the antigens. On increasing antigen concentration, the immunomodulatory function comes into play and determines the outcome. To rule out toxicity due to higher concentration of these proteins we performed MTT assay in RAW264.7 cells and found that these proteins are not toxic at 3 μg/ml concentration. IFN-γ is a well-known Th1 cytokine and its production is an important factor involved in protection against TB (Smeltz et al., 2002). This finding also suggests that PE32 and PPE65 suppresses T-cell activity and likely favors Th2 pathway. Th1 response being not the only signature of protection, the importance of Th17 to protection is also emerging. Effect of these PE/PPE proteins on Th1/Th17 immunomodulation will be an interesting aspect of work that remains to be explored.
We also tried to assess for the change in cellular components of splenocytes, as it is known that CD4+ T cells are central to the defense and CD8+ cells have important role in clearance of pathogens in several diseases including M. tuberculosis (van Meijgaarden et al., 2015). We investigated their effect on multifunctional CD4+ and CD8+ cells expressing IFN-γ and IL-2. There was significant decrease in both antigen specific CD4+ and CD8+ cells double positive for IFN-γ and IL-2. These poly functional cells are considered as important contributors to protective immunity as these can lyse infected host cells, or can directly kill M. tuberculosis by secreting perforin, granzymes, and granulysin (Canaday et al., 2001; Pitabut et al., 2013). The decrease in the number of these poly functional cells was consistent with the earlier findings and ratifies a diminishing Th1 response that in turn escalates pathogen friendly Th2 response. Thus, these PE/PPE proteins seem to be directly involved in dampening of the host immune response in order to help M. tuberculosis for its survival.
Our observations suggest that this PE/PPE operon plays distinct and complementary roles in infection process, acting in concert to facilitate adaptation to the hostile host environment. These proteins along with many others could prove to be critical mediators of host responses and determine the outcome of infection.
Author Contributions
NE and SH conceptualized and designed the research; MK, JS, BS, and SP performed experiments; JS, SP, and MB carried out data analysis; NK contributed in reagents/analytical tools; MK, JS, SH, and NE wrote the manuscript.
Funding
This work was supported by a Centre of Excellence Grant to SH and NE (Grant No. BT/PR12817/COE/34/23/2015) from the Department of Biotechnology, Ministry of Science and Technology, GoI.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MK, SP, and BS received JRF/SRF from CSIR, New Delhi, India. JS is a recipient of Research Associateship from DBT, India. MB is a recipient of a Research Associateship of the ICMR, Ministry of Health, Government of India (GoI).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00719
References
Ahmed, A., Das, A., and Mukhopadhyay, S. (2015). Immunoregulatory functions and expression patterns of PE/PPE family members: roles in pathogenicity and impact on anti tuberculosis vaccine and drug design. IUBMB Life 67, 414–427. doi: 10.1002/iub.1387
Ahmed, N., Dobrindt, U., Hacker, J., and Hasnain, S. E. (2008). Genomic fluidity and pathogenic bacteria: applications in diagnostics, epidemiology and intervention. Nat. Rev. Microbiol. 6, 387–394. doi: 10.1038/nrmicro1889
Akhter, Y., Ehebauer, M. T., Mukhopadhyay, S., and Hasnain, S. E. (2012). The PE/PPE multigene family codes for virulence factors and is a possible source of mycobacterial antigenic variation: perhaps more? Biochimie 94, 110–116. doi: 10.1016/j.biochi.2011.09.026
Banerjee, S., Nandyala, A., Podili, R., Katoch, V. M., Murthy, K. J. R., and Hasnain, S. E. (2004). Mycobacterium tuberculosis (Mtb) isocitrate dehydrogenases show strong B cell response and distinguish vaccinated controls from TB patients. Proc. Natl. Acad. Sci. U.S.A. 101, 12652–12657. doi: 10.1073/pnas.0404347101
Banu, S., Honore, N., Saint-Joanis, B., Philpott, D., Prevost, M. C., and Cole, S. T. (2002). Are the PE-PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol. Microbiol. 44, 9–19. doi: 10.1046/j.1365-2958.2002.02813.x
Canaday, D. H., Wilkinson, R. J., Li, Q., Harding, C. V., Silver, R. F., and Boom, W. H. (2001). CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J. Immunol. 167, 2734–2742. doi: 10.4049/jimmunol.167.5.2734
Chakhaiyar, P., Nagalakshmi, Y., Aruna, B., Murthy, K. J. R., Katoch, V. M., and Hasnain, S. E. (2004). Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open-reading frame, Rv2608, show a differential humoral response and a low T cell response in various categories of patients with tuberculosis. J. Infect. Dis. 190, 1237–1244.
Choudhary, R., Mukhopadhyay, S., Chakhaiyar, P., Sharma, N., Murthy, K. J. R., Katoch, V. M., et al. (2003). PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect. Immun. 71, 6338–6343. doi: 10.1128/IAI.71.11.6338-6343.2003
Clay, H., Davis, J. M., Beery, D., Huttenlocher, A., Lyons, S. E., and Ramakrishnan, L. (2007). Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2, 29–39. doi: 10.1016/j.chom.2007.06.004
Cole, S. T. (1998). Comparative mycobacterial genomics. Curr. Opin. Microbiol. 1, 567–571. doi: 10.1016/S1369-5274(98)80090-8
Copin, R., Coscollá, M., and Seiffert, S. N. (2014). Sequence diversity in the PE-PGRS genes of M. tuberculosis is independent of human T-cell recognition. MBio 5:e00960-13. doi: 10.1128/mBio.00960-13
Delogu, G., Pusceddu, C., Bua, A., Fadda, G., Brennan, M. J., and Zanetti, S. (2004). Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol. Microbiol. 52, 725–733. doi: 10.1111/j.1365-2958.2004.04007.x
Delogu, G., Sanguinetti, M., Pusceddu, C., Bua, A., Brennan, M. J., Zanetti, S., et al. (2006). PE_PGRS proteins are differentially expressed by Mycobacterium tuberculosis in host tissues. Microbes Infect. 8, 2061–2067. doi: 10.1016/j.micinf.2006.03.015
Deng, W., Li, W., Zeng, J., Zhao, Q., Li, C., Zhao, Y., et al. (2014). Mycobacterium tuberculosis PPE family protein Rv1808 manipulates cytokines profile via co-activation of MAPK and NF-kappaB signaling pathways. Cell Physiol. Biochem. 33, 273–288. doi: 10.1159/000356668
Finkelman, F. D., Svetic, A., Gresser, I., Snapper, C., Holmes, J., Trotta, P. P., et al. (1991). Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J. Exp. Med. 174, 1179–1188. doi: 10.1084/jem.174.5.1179
Fishbein, S., van Wyk, N., Warren, R. M., and Sampson, S. L. (2015). Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol. Microbiol. 96, 901–916. doi: 10.1111/mmi.12981
Gey van Pittius, N. C., Sampson, S. L., Lee, H., Kim, Y., van Helden, P. D., and Warren, R. M. (2006). Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6:95. doi: 10.1186/1471-2148-6-95
Goldstone, R. M., Goonesekera, S. D., Bloom, B. R., and Sampson, S. L. (2009). The transcriptional regulator Rv0485 modulates the expression of a pe and ppe gene pair and is required for Mycobacterium tuberculosis virulence. Infect. Immun. 77, 4654–4667. doi: 10.1128/IAI.01495-08
Houben, E. N. G., Korotkov, K. V., and Bitter, W. (2014). Take five - Type VII secretion systems of Mycobacteria. Biochim. Biophys. Acta 1843, 1707–1716. doi: 10.1016/j.bbamcr.2013.11.003
Jackson-Sillah, D., Cliff, J. M., Mensah, G. I., Dickson, E., Sowah, S., Tetteh, J. K. A., et al. (2013). Recombinant ESAT-6-CFP10 fusion protein induction of Th1/Th2 cytokines and FoxP3 expressing treg cells in pulmonary TB. PLoS ONE 8:e68121. doi: 10.1371/journal.pone.0068121
Jha, S. S., Danelishvili, L., Wagner, D., Maser, J., Li, Y., Moric, I., et al. (2010). Virulence-related Mycobacterium avium subsp hominissuis MAV_2928 gene is associated with vacuole remodeling in macrophages. BMC Microbiol. 10:100. doi: 10.1186/1471-2180-10-100
Kasturi, S. P., Skountzou, I., Albrecht, R. A., Koutsonanos, D., Hua, T., Nakaya, H. I., et al. (2011). Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547. doi: 10.1038/nature09737
Kohli, S., Singh, Y., Sharma, K., Mittal, A., Ehtesham, N. Z., and Hasnain, S. E. (2012). Comparative genomic and proteomic analyses of PE/PPE multigene family of Mycobacterium tuberculosis H37Rv and H37Ra reveal novel and interesting differences with implications in virulence. Nucleic Acids Res. 40, 7113–7122. doi: 10.1093/nar/gks465
Lewis, K. N., Liao, R., Guinn, K. M., Hickey, M. J., Smith, S., Behr, M. A., et al. (2003). Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J. Infect. Dis. 187, 117–123. doi: 10.1086/345862
Mangtani, P., Abubakar, I., Ariti, C., Beynon, R., Pimpin, L., Fine, P. E. M., et al. (2014). Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 58, 470–480. doi: 10.1093/cid/cit790
Mohareer, K., Tundup, S., and Hasnain, S. E. (2011). Transcriptional regulation of Mycobacterium tuberculosis PE/PPE genes: a molecular switch to virulence? J. Mol. Microbiol. Biotechnol. 21, 97–109. doi: 10.1159/000329489
Nair, S., Ramaswamy, P. A., Ghosh, S., Joshi, D. C., Pathak, N., Siddiqui, I., et al. (2009). The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J. Immunol. 183, 6269–6281. doi: 10.4049/jimmunol.0901367
Parra, M., Cadieux, N., Pickett, T., Dheenadhayalan, V., and Brennan, M. J. (2006). A PE protein expressed by Mycobacterium avium is an effective T cell immunogen. Infect. Immun. 74, 786–789. doi: 10.1128/IAI.74.1.786-789.2006
Pitabut, N., Sakurada, S., Tanaka, T., Ridruechai, C., Tanuma, J., Aoki, T., et al. (2013). Potential function of granulysin, other related effector molecules and lymphocyte subsets in patients with TB and HIV/TB coinfection. Int. J. Med. Sci. 10, 1003–1014. doi: 10.7150/ijms.6437
Rahman, S. A., Singh, Y., Kohli, S., Ahmad, J., Ehtesham, N. Z., Tyagi, A. K., et al. (2014). Comparative analyses of nonpathogenic, opportunistic, and totally pathogenic mycobacteria reveal genomic and biochemical variabilities and highlight the survival attributes of Mycobacterium tuberculosis. MBio 5:e02020-14. doi: 10.1128/mBio.02020-14
Rao, K. R., Kauser, F., Srinivas, S., Zanetti, S., Sechi, L. A., Ahmed, N., et al. (2005). Analysis of genomic downsizing on the basis of region-of-difference polymorphism profiling of Mycobacterium tuberculosis patient isolates reveals geographic partitioning. J. Clin. Microbiol. 43, 5978–5982. doi: 10.1128/JCM.43.12.5978-5982.2005
Ravindran, R., Khan, N., Nakaya, H. I., Li, S., Loebbermann, J., Weiss, D. S., et al. (2014). Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343, 313–317. doi: 10.1126/science.1246829
Saini, V., Raghuvanshi, S., Khurana, J. P., Ahmed, N., Hasnain, S. E., and Tyagi, A. K. (2012). Massive gene acquisitions in Mycobacterium indicus pranii provide a perspective on mycobacterial evolution. Nucleic Acids Res. 40, 10832–10850. doi: 10.1093/nar/gks793
Saini, V., Raghuvanshi, S., Talwar, G. P., Ahmed, N., Khurana, J. P., Hasnain, S. E., et al. (2009). Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS ONE 4:e6263. doi: 10.1371/journal.pone.0006263
Sampson, S. L. (2011). Mycobacterial PE/PPE proteins at the host-pathogen interface. Clin. Dev. Immunol. 2011:497203. doi: 10.1155/2011/497203
Singh, Y., Kohli, S., Sowpati, D. T., Rahman, S. A., Tyagi, A. K., and Hasnain, S. E. (2014). Gene cooption in mycobacteria and search for virulence attributes: comparative proteomic analyses of Mycobacterium tuberculosis, Mycobacterium indicus pranii and other mycobacteria. Int. J. Med. Microbiol. 304, 742–748. doi: 10.1016/j.ijmm.2014.05.006
Smeltz, R. B., Chen, J., Ehrhardt, R., and Shevach, E. M. (2002). Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J. Immunol. 168, 6165–6172. doi: 10.4049/jimmunol.168.12.6165
Strong, M., Sawaya, M. R., Wang, S., Phillips, M., Cascio, D., and Eisenberg, D. (2006). Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 103, 8060–8065. doi: 10.1073/pnas.0602606103
Tiwari, B. M., Kannan, N., Vemu, L., and Raghunand, T. R. (2012). The Mycobacterium tuberculosis PE proteins Rv0285 and Rv1386 modulate innate immunity and mediate bacillary survival in macrophages. PLoS ONE 7:e51686. doi: 10.1371/journal.pone.0051686
Tundup, S., Akhter, Y., Thiagarajan, D., and Hasnain, S. E. (2006). Clusters of PE and PPE genes of Mycobacterium tuberculosis are organized in operons: evidence that PE Rv2431c is co-transcribed with PPE Rv2430c and their gene products interact with each other. FEBS Lett. 580, 1285–1293. doi: 10.1016/j.febslet.2006.01.042
Tundup, S., Mohareer, K., and Hasnain, S. E. (2014). Mycobacterium tuberculosis PE25/PPE41 protein complex induces necrosis in macrophages: role in virulence and disease reactivation? FEBS Open Bio. 4, 822–828. doi: 10.1016/j.fob.2014.09.001
Tundup, S., Pathak, N., Ramanadham, M., Mukhopadhyay, S., Murthy, K. J. R., Ehtesham, N. Z., et al. (2008). The co-operonic PE25/PPE41 protein complex of Mycobacterium tuberculosis elicits increased humoral and cell mediated immune response. PLoS ONE 3:e3586. doi: 10.1371/journal.pone.0003586
van Meijgaarden, K. E., Haks, M. C., Caccamo, N., Dieli, F., Ottenhoff, T. H. M., and Joosten, S. A. (2015). Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS Pathog. 11:e1004671. doi: 10.1371/journal.ppat.1004671
Keywords: M. tuberculosis, PE32/PPE65, CD4+ T cells, CD8+ T cells, IgG subtyping
Citation: Khubaib M, Sheikh JA, Pandey S, Srikanth B, Bhuwan M, Khan N, Hasnain SE and Ehtesham NZ (2016) Mycobacterium tuberculosis Co-operonic PE32/PPE65 Proteins Alter Host Immune Responses by Hampering Th1 Response. Front. Microbiol. 7:719. doi: 10.3389/fmicb.2016.00719
Received: 08 April 2016; Accepted: 29 April 2016;
Published: 17 May 2016.
Edited by:
Miguel Cacho Teixeira, University of Lisbon, PortugalReviewed by:
Noman Ahmad Siddiqi, Harvard T.H. Chan School of Public Health, USAAstrid Lewin, Robert Koch Institute, Germany
Copyright © 2016 Khubaib, Sheikh, Pandey, Srikanth, Bhuwan, Khan, Hasnain and Ehtesham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed E. Hasnain, seyedhasnain@gmail.com; Nasreen Z. Ehtesham, nzehtesham@gmail.com
†These authors have contributed equally to this work.
 Mohd Khubaib
Mohd Khubaib Javaid A. Sheikh
Javaid A. Sheikh Saurabh Pandey
Saurabh Pandey Battu Srikanth
Battu Srikanth Manish Bhuwan
Manish Bhuwan Nooruddin Khan
Nooruddin Khan Seyed E. Hasnain2,4*
Seyed E. Hasnain2,4* Nasreen Z. Ehtesham
Nasreen Z. Ehtesham