- Department of Biotechnology and Microbiology, Kannur University, Kannur, India
Fungal laccases are involved in a variety of physiological functions such as delignification, morphogenesis, and parasitism. In addition to these functions, we suggest that fungal laccases are involved in defense mechanisms. When the laccase secreting Trichoderma viride was grown in the presence of a range of microorganisms including bacteria and fungi, laccase secretion was enhanced in response to antagonistic organisms alone. In addition, growth of antagonistic microbes was restricted by the secreting fungi. Besides, our study for the first time shows the inability of the secreting fungi (T. viride) to compete with antagonistic organism when laccase activity is inhibited, further emphasizing its involvement in rendering a survival advantage to the secreting organism. When laccase inhibitor was added to the media, the zone of inhibition exerted by the antagonist organism was more pronounced and consequently growth of T. viride was significantly restricted. Based on these observations we accentuate that, laccase plays an important role in defense mechanism and provides endurance to the organism when encountered with an antagonistic organism in its surrounding.
Introduction
Laccases (E.C.1.10.3.2) are oxidoreductases that contain copper ions at the catalytic center (Kiiskinen et al., 2002) and are one of the few microbial enzymes employed in number of industrial applications (Abadulla et al., 2000; Cuoto and Herrera, 2006; Kidwai et al., 2012; Sole et al., 2012; Divya et al., 2013). Fungal laccases are unique in that they exhibit low substrate specificity and strong oxidative abilities and are involved in a variety of physiological functions such as delignification, morphogenesis, and parasitism (Worrall et al., 1986; Williamson, 1997; Missall et al., 2005; Camarero et al., 2007). In addition to these functions, our study suggests that fungal laccases are involved in conferring the secreting organism a resistance to antagonistic microorganisms.
Besides directly oxidizing a variety of phenolic compounds, laccases catalyze the indirect oxidation of chemicals that are not phenols or amines in the presence of a redox mediator or Laccase-mediator system (LMS), which can be of natural or synthetic origin (Eggert et al., 1998). The combination of the laccase with low molecular weight mediators not only lead to higher rates and yields in the transformation of laccase substrates but also add new oxidative reactions to the laccase repertory toward substrates in which the enzyme alone had no or only marginal activity. Thus, LMS enlarges substrate range being able to oxidize compounds with redox potential (E°) higher than that of laccase.
Most fungi will come across competitive or antagonistic organisms in their natural population and communities. Though these interactions may not produce noticeable morphological response between the intermingling fungi, they can form mutual inhibition zones (Rayner and Boddy, 1988; Cooke and Whipps, 1993). These fungal interactions can be seen both in culture as well as in their natural environment (Crowe and Olsson, 2001; Wei et al., 2010). Studies shows that fungi involved in such competition often produce secondary metabolites, extracellular phenol-oxidizing enzymes like laccase, and differentiated structures in the zone of interaction (Iakovlev and Stenlid, 2000; Crowe and Olsson, 2001; Zhang et al., 2006; Wei et al., 2010). The present study was undertaken to analyze the probable role of laccase in fungal defense mechanisms.
Laccase Secretion Was Enhanced When Trichoderma viride NFCCI-2745 Was Grown in the Presence of Antagonistic Fungi and Bacteria
The laccase producing Trichoderma viride Pers NFCCI-2745, which was isolated from a highly saline and phenolic rich environment (Divya et al., 2014) was used for studying the role of laccase in antagonistic microbial interaction. Identification of the strain was done based on Inter Transcribed Spacer (ITS) sequencing of rDNA of fungal genome from the National Fungal Culture Collection of India (NFCCI), Pune, India and the sequence deposited in GenBank under the GenBank accession ID: KF638399.
Top soil along with decomposing leaves and twigs near the canteen premises of the Kannur University Campus, Kerala, India, were sampled for laccase inducing microorganisms. Serially diluted samples were spread plated onto agar plates containing 1 mM guaiacol. Plates were incubated at room temperature for 6 h and then one colony plug of 2 mm diameter were cut out from actively growing edge of T. viride NFCCI-2745 and then transferred onto the same screening plate at the center. Only two strains of microbes were found to induce laccase secretion and interestingly both exhibited antifungal properties (Figure 1). These two strains were identified as Bacillus sp. (Figure 1A) and Aspergillus ochraceus (Figure 1B) based on biochemical and morphological characteristics, respectively. Bacillus sp. and A. ochraceus were unable to oxidize guaiacol directly but induced laccase secretion of T. viride (Figures 1A,B). An enhanced laccase secretion was observed around the clear inhibitory zone of Bacillus sp. and at the contact junction of A. ochraceus. The pattern of induction suggested that the T. viride mycelium was reacting to agents diffusing from the antagonistic microbes within the agar medium. The similar form of laccase induction during inter-specific microbial interactions was reported with many fungi belonging to Basidiomycete family as well (Iakovlev and Stenlid, 2000; Crowe and Olsson, 2001; Baldrian, 2004; Qian and Chen, 2012).
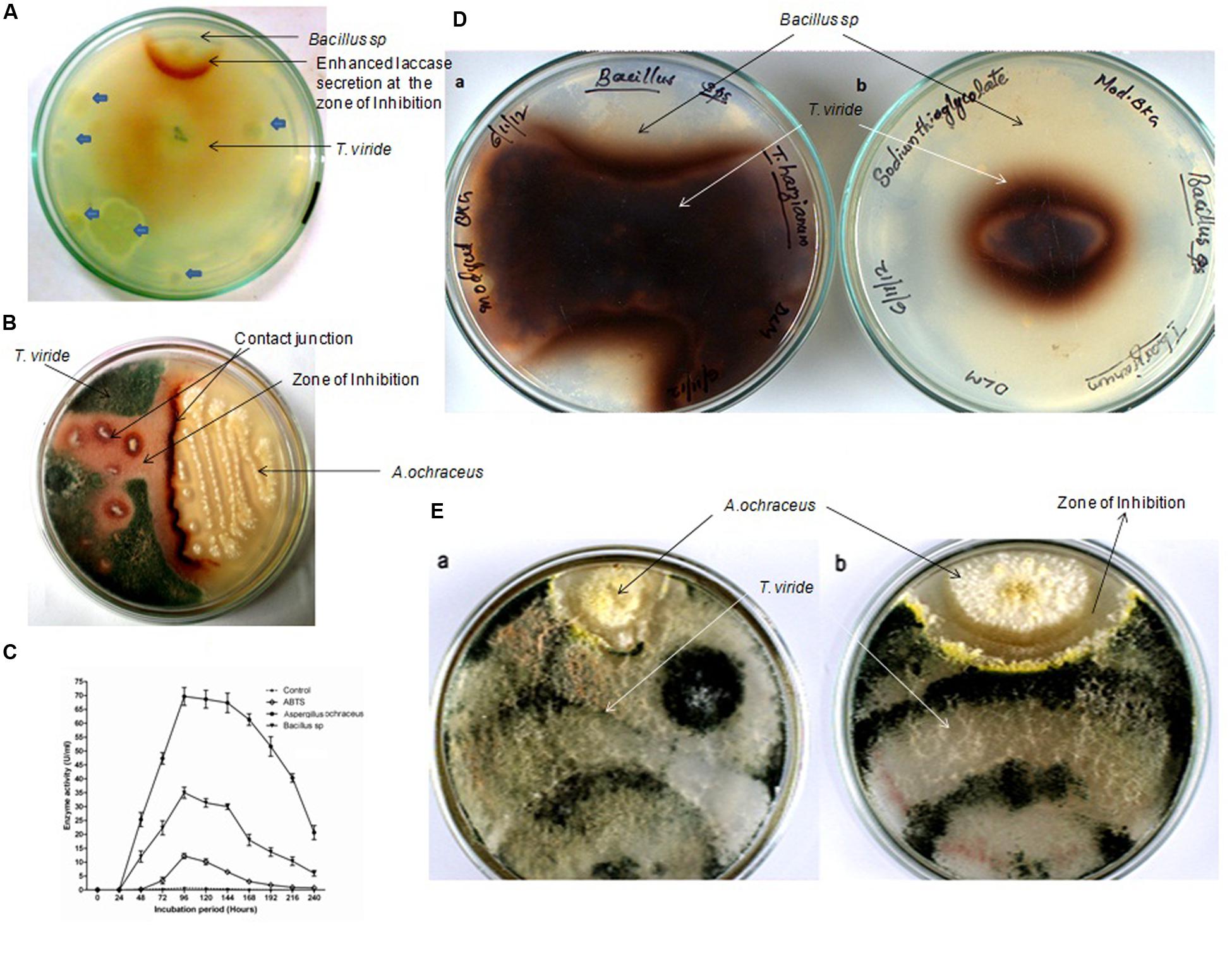
FIGURE 1. Representative pictures of the pairing experiments of Trichoderma viride with Bacillus sp. and Aspergillus ochraceus. (A) Backside of the co-cultured screening plate (1 mM Guaiacol) showing enhanced laccase secretion by T. viride in response to the zone of inhibition produced by Bacillus sp. Other microorganisms failed to induce any sort of laccase induction (is shown with blue block arrows). (B) Plate showing enhanced laccase secretion by T. viride response to the antagonistic effects exerted by the A. ochraceus. (C) Quantitative assay of total laccase activity in the presence of antifungal metabolites. (D) Rear view of the Co-Culture of T. viride –Bacillus in 3 mM Guaiacol media (a) Control without Laccase inhibitor (b) with Laccase inhibitor. In the control where laccase inhibitor was not added, T. viride exhibited normal growth pattern and restrict the growth of Bacillus sp., whereas when laccase is inhibited, the growth of T. viride was significantly inhibited. Also the pattern of growth exhibited by T. viride showed marked difference in the presence and absence of laccase. (E) Front view of the Co-Culture of T. viride –A.ochraceus; (a) Control without Laccase inhibitor (b) with Laccase inhibitor. In the control where laccase inhibitor was not added, T. viride could restrict the growth of A. ochraceus. Also A. ochraceus failed to exert a prominent inhibition zone. Whereas when laccase was inhibited, the zone of inhibition produced by A. ochraceus was remarkably prominent when compared to control.
In the interaction combinations, laccase activity was high in the contact zone but low or not detectable in other parts of mycelia from 2nd to 4th day in guaiacol (1 mM) supplemented agar medium. After 4 days, laccase activities were distributed more homogeneously over the entire mycelium and became higher in the contact zone compared to initial days of interactions. After 4 days of incubation, T. viride started growing above the zone of inhibition formed by the antagonistic organism, restricting the growth of antagonistic microorganism to a great extent, with both forming mutual inhibitory zones. The qualitative assays were confirmed by quantitative assay of total laccase activity (Figure 1C). It was found that enzyme activity was induced to almost 50 times in the case of Bacillus sp. and almost 100 times with A. ochraceus. In the control the laccase activity reached its maximum within 96 h of incubation and slowly declined thereafter. But with the addition of cell free supernatant of the antagonistic culture, the laccase activity reached its maximum within 96 h and remained the same for almost 144 h and then declined slowly. The results of both quantitative and qualitative assays confirmed enhanced laccase secretion as a result of inter-specific fungal interactions.
In contrast to previous studies where Trichoderma sp. are reported as inducers, enhancing the laccase production of Basidiomycetes such as Pleurotus ostreatus, Agaricus bisporus, Lentinula edodes, Serpula lacrymans and Trametes versicolor (Score et al., 1997; Savoie et al., 1998; Savoie and Mata, 1999; Hatvani et al., 2002; Baldrian, 2004, 2006; Velázquez-Cedeño et al., 2004; Flores et al., 2009, 2010; Bertrand et al., 2013; Sjaarda et al., 2015), our study for the first time reports enhanced laccase activity in Trichoderma sp. in response to other co-cultures. Though many early studies on inter-specific and intra-specific microbial interactions have observed similar induction in laccase secretion in presence of competing microbes (Savoie et al., 1998; Iakovlev and Stenlid, 2000; Crowe and Olsson, 2001; Baldrian, 2004; Velázquez-Cedeño et al., 2004; Snajdr et al., 2011; Qian and Chen, 2012; Sjaarda et al., 2015), its role in conferring a survival advantage to the secreting organism was not probed.
Is Laccase Secretion in Trichoderma viride NFCCI-2745 Necessary for Combating Antagonistic Microbes?
In order to check whether enhanced production of laccase by T. viride in the co-culture is necessary for its survival, we decided to conduct a study with laccase inhibitors. Interestingly in the pairing experiments, when laccase inhibitor, sodium thioglycolate (10 mM) was added to the media, we observed a marked difference in the growth pattern of T. viride (Figures 1D,E). When T. viride was paired with Bacillus sp. we observed that in the control where laccase inhibitor was not added, T. viride exhibited normal growth pattern and restricted the growth of Bacillus sp., whereas when laccase is inhibited, the growth of T. viride was significantly reduced (Figure 1D). The diameter of T. viride colony in its pairing with Bacillus sp. is shown in Table 1. In the pairing experiment with A. ochraceus, in the control where laccase inhibitor was not added, T. viride could restrict the growth of A. ochraceus. Also A. ochraceus failed to exert a prominent inhibition zone. Whereas when laccase was inhibited, the zone of inhibition produced by A. ochraceus was remarkably prominent (Figure 1E). The measurement of the zone of inhibition exerted during this pairing experiment is shown in Table 1. These results suggest that the formation of this inhibition zone is influenced by laccase. This result was not influenced by the presence of guaiacol (laccase indicator) in the media. Both the cultures with and without guaiacol exhibited almost the same pattern of interaction. The above results suggest that laccase secretion may be a requisite for T. viride to compete with other antagonistic microbes. The results point toward the possibility of the role laccase in conferring defense against antifungal secreting microorganisms. This result supported the previous interpretations, where the changes in laccases production due to interaction of the laccase secreting fungi (Basidiomycetes) and the antagonists (Trichoderma sp.) was linked to defense reaction of the secreting fungi which limits the progression of antagonists (Savoie et al., 2001; Velázquez-Cedeño et al., 2007; Sjaarda et al., 2015). In addition, our study for the first time shows the inability of the secreting fungi (T. viride) to compete with antagonistic organism when laccase activity is inhibited, further emphasizing its involvement in rendering a survival advantage to the secreting organism.
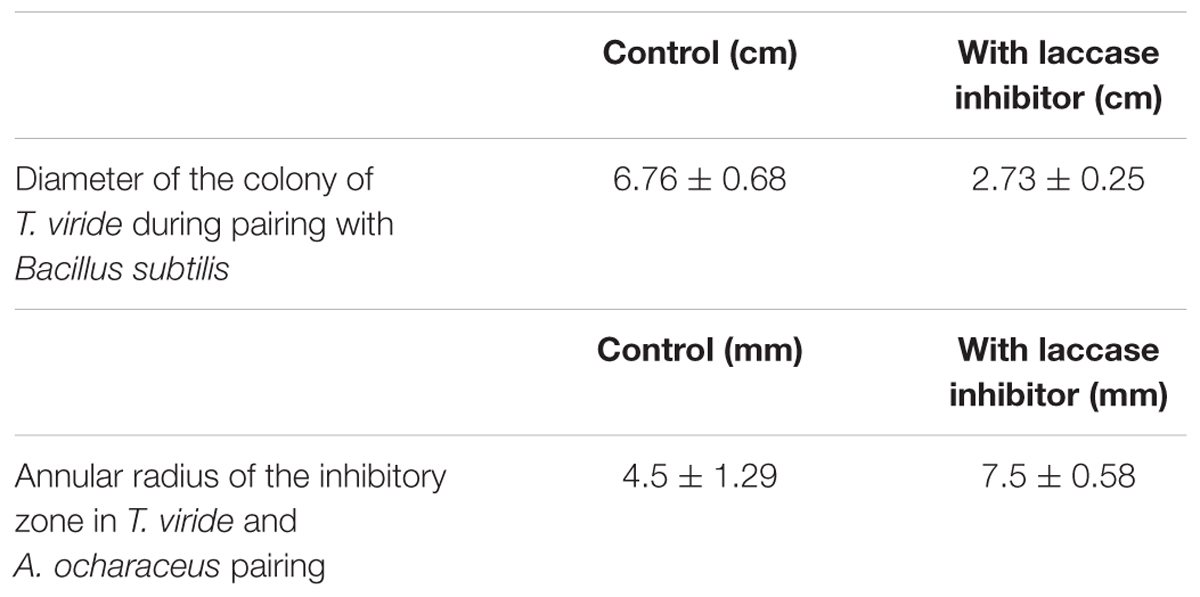
TABLE 1. Measured parameters of the pairing experiments of Trichoderma viride with Bacillus sp. and Aspergillus ochraceus.
We also observed that only lyophilized crude cell free supernatant enhanced laccase secretion whereas synthetic antagonistic compounds [Ketoconazole and clotrimazole (50 μl of 150 mg/ml)] as well partially purified antagonistic fraction [chloroform: methanol (7:3)] fraction from culture supernatant of Bacillus sp. failed to induce laccase secretion. A previous study by Crowe and Olsson (2001) supports our observations which states that purified antagonistic compound couldn’t induce laccase secretion while the organism (from which the substance was purified) enhanced laccase secretion (Crowe and Olsson, 2001). The authors concluded that it is the calcium or heat shock signaling in response to the effects of bacterial metabolites which induces laccase secretion (Crowe and Olsson, 2001). Though we couldn’t explain the nature of induction of laccase, we believed that laccase may be neutralizing the effects of these toxins, with help of mediators. Some of the earlier studies reports that laccase mediators synthetic as well as natural aids in oxidizing non phenolic substrates by laccase (Thurston, 1994; Eggert et al., 1998). Based on the previous evidences from literatures, we speculate the possibility of certain metabolites secreted by the antagonistic organism in acting as a mediator for laccase, in oxidizing the anti- fungal compound and thus eliminating its antagonistic effect. This may perhaps explain the inability of the purified antagonistic compound to induce laccase secretion. Thus in conclusion laccase may be exerting a significant role in defense mechanism and might be indispensable for the survival of the secreting organism when encountered with an antagonistic organism in its surrounding.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Department of Biotechnology and Microbiology, Kannur University, India is gratefully acknowledged for the facilities provided to conduct this study.
References
Abadulla, E., Tzanov, T., Costa, S., Robra, K. H., Cavaco, P. A., and Guebitz, G. M. (2000). Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 66, 3357–3362. doi: 10.1128/AEM.66.8.3357-3362.2000
Baldrian, P. (2004). Increase of laccase activity during inter-specific interactions of white-rot fungi. FEMS Microbiol. Ecol. 50, 245–255. doi: 10.1016/j.femsec.2004.07.005
Baldrian, P. (2006). Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 30, 215–242. doi: 10.1111/j.1574-4976.2005.00010.x
Bertrand, B., Martínez-Morales, F., and Trejo-Hernandez, M. R. (2013). Fungal laccases: induction and production. Rev. Mexicana Ingeniería Química 12, 473–488.
Camarero, S., Ibarsa, D., Martinez, A., Romero, J., Gutierrez, A., and del Rio, C. J. (2007). Paper pulp delignification using laccase and natural mediators. Enzyme Microbial. Technol. 40, 1264–1267. doi: 10.1016/j.enzmictec.2006.09.016
Crowe, J. D., and Olsson, S. (2001). Induction of laccase activity in rhizoctonia solani by antagonistic Pseudomonas fluorescens strains and a range of chemical treatments. Appl. Environ. Microbiol. 67, 2088–2094. doi: 10.1128/AEM.67.5.2088-2094.2001
Cuoto, S., and Herrera, J. L. (2006). Fungal laccases: biotechnology application. Biotechnol. Adv. 2, 500–513.
Divya, L. M., Prasanth, G. K., and Sadasivan, C. (2013). Elimination of estrogenic activity of thermal paper using laccase from Trichoderma sp NFCCI-2745. Appl. Biochem. Biotechnol. 169, 1126–1133. doi: 10.1007/s12010-012-0016-y
Divya, L. M., Prasanth, G. K., and Sadasivan, C. (2014). Potential of salt tolerant laccase producing strain of Trichoderma viride Pers NFCCI-2745 from estuary in bioremediation of phenol polluted environments. J. Basic Microbiol. 54, 542–547. doi: 10.1002/jobm.201200394
Eggert, C., LaFayette, P. R., Temp, U., Eriksson, K. L., and Dean, J. F. D. (1998). Molecular analysis of a laccase gene from the white rot fungus Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 64, 1766–1772.
Flores, C., Casasanero, R., Trejo-Hernández, M. R., Galindo, E., and Serrano-Carreón, L. (2010). Production of laccases by Pleurotus ostreatus in submerged fermentation in co-culture with Trichoderma viride. J. Appl. Microbiol. 108, 810–817. doi: 10.1111/j.1365-2672.2009.04493.x
Flores, C., Vidal, C., Trejo-Hernández, M. R., Galindo, E., and Serrano-Carreón, L. (2009). Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J. Appl. Microbiol. 106, 249–257. doi: 10.1111/j.1365-2672.2008.03998.x
Hatvani, N., Kredics, L., Antal, Z., and Mecs, I. (2002). Changes in activity of extracellular enzymes in dual cultures of Lentinula edodes and mycoparasitic Trichoderma strains. J. Appl. Microbiol. 92, 415–423. doi: 10.1046/j.1365-2672.2002.01542.x
Iakovlev, A., and Stenlid, J. (2000). Spatiotemporal patterns of laccase activity in interacting mycelia of wood-decaying basidiomycete fungi. Microb. Ecol. 39, 236–245.
Kidwai, M., Jain, A., Sharma, A., and Kuhad, R. C. (2012). Ecofriendly approach for detection of phenols in water using laccase from different fungi. Water Sci. Technol. 66, 385–393. doi: 10.2166/wst.2012.198
Kiiskinen, L., Viikari, L., and Kruus, K. (2002). Purification and characterization of a novel laccase from the ascomycete Melanocarpus albomyces. Appl. Microbiol. Biotechnol. 59, 198–204. doi: 10.1007/s00253-002-1012-x
Missall, T. A., Moran, J. M., Corbett, J. A., and Lodge, J. K. (2005). Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot. Cell 4, 202–208.
Qian, L., and Chen, B. (2012). Enhanced oxidation of benzo[a]pyrene by crude enzyme extracts produced during interspecific fungal interaction of Trametes versicolor and Phanerochaete chrysosporium. J. Environ. Sci. (China), 24, 1639–1646. doi: 10.1016/S1001-0742(11)61056-5
Rayner, A. D. M., and Boddy, L. (1988). Fungal communities in the decay of wood. Adv. Microb. Ecol. 10, 115–166. doi: 10.1007/978-1-4684-5409-3_4
Savoie, J. M., and Mata, G. (1999). The antagonistic action of Trichoderma sp. hyphae to Lentinula edodes hyphae changes lignocellulolytic activities during cultivation in wheat straw. World J. Microbiol. Biotechnol. 15, 369–373. doi: 10.1023/A:1008979701853
Savoie, J. M., Mata, G., and Billette, C. (1998). Extracellular laccase production during hyphal interactions between Trichoderma sp. and shiitake. Lentinula edodes. Appl. Microbiol. Biotechnol. 49, 589–593. doi: 10.1007/s002530051218
Savoie, J.-M., Mata, G., and Mamoun, M. (2001). Variability in brown line formation and extracellular laccase production during interaction between white-rot basidiomycetes and Trichoderma harzianum biotype Th2. Mycologia 93, 243–248. doi: 10.2307/3761644
Score, A. J., Palfreyman, J. W., and White, N. A. (1997). Extracellular phenoloxidase and peroxidase enzyme production during interspecific fungal interactions. Int. Biodeterior Biodegradat. 39, 225–233. doi: 10.1016/S0964-8305(97)00012-7
Sjaarda, C. P., Abubaker, K. S., and Castle, A. J. (2015). Induction of lcc2 expression and activity by Agaricus bisporus provides defence against Trichoderma aggressivum toxic extracts. Microb Biotechnol. 8, 918–929. doi: 10.1111/1751-7915.12277
Snajdr, J., Dobiasova, P., Vetrovsky, T., Valaskova, V., Alawi, A., Boddy, L., et al. (2011). Saprotrophic basidiomycete mycelia and their inter-specific interactions affect the spatial distribution of extracellular enzymes in soil. FEMS Microbiol. Ecol. 78, 80–90. doi: 10.1111/j.1574-6941.2011.01123.x
Sole, M., Muller, I., Pecyna, M. J., Fetzer, I., Harms, H., and Schlosser, D. (2012). Differential regulation by organic compounds and heavy metals of multiple laccase genes in the aquatic hyphomycete Clavariopsis aquatica. Appl. Environ. Microbiol. 78, 4732–4739. doi: 10.1128/AEM.00635-12
Thurston, C. F. (1994). The structure and function of fungal laccases. Microbiology 140, 19–26. doi: 10.1099/13500872-140-1-19
Velázquez-Cedeño, M., Farnet, A. M., Billette, C., Mata, G., and Savoie, J.-M. (2007). Interspecific interactions with Trichoderma longibrachiatum induce Pleurotus ostreatus defence reactions based on the production of laccase isozymes. Biotechnol. Lett. 29, 1583–1590. doi: 10.1007/s10529-007-9445-z
Velázquez-Cedeño, M., Farnet, A. M., Ferré, E., and Savoie, J.-M. (2004). Variation of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia 96, 712–719. doi: 10.2307/3762105
Wei, F., Hong, Y., Liu, J., Yuan, J., Fang, W., Peng, H., et al. (2010). Gongronella sp induces overproduction of laccase in Pannus rudis. J. Basic Microbiol. 50, 98–103. doi: 10.1002/jobm.200900155
Williamson, P. R. (1997). Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front. Biosci. 2:e99.
Worrall, J. J., Chet, I., and Huttermann, A. (1986). Association of rhizomorph formation with laccase activity in Armillaria sp. J. Gen. Microbiol. 132, 2527–2533.
Keywords: antifungal, defense mechanism, inter-specific interaction, laccase, Trichoderma sp.
Citation: Lakshmanan D and Sadasivan C (2016) Trichoderma viride Laccase Plays a Crucial Role in Defense Mechanism against Antagonistic Organisms. Front. Microbiol. 7:741. doi: 10.3389/fmicb.2016.00741
Received: 07 January 2016; Accepted: 03 May 2016;
Published: 17 May 2016.
Edited by:
Caroline Westwater, Medical University of South Carolina, USAReviewed by:
Alan Castle, Brock University, CanadaJulia Ines Fariña, Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina
Copyright © 2016 Lakshmanan and Sadasivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Sadasivan, csadasivan@gmail.com; Divya Lakshmanan, divyalmangalath@gmail.com
†Present address: Divya Lakshmanan, Yenepoya Research Centre, Yenepoya University, Mangalore, Karnataka 575018, India
 Divya Lakshmanan
Divya Lakshmanan C. Sadasivan*
C. Sadasivan*