- 1Division of Plant Pathology and Plant Protection, Department of Crop Sciences, Georg-August-Universität Göttingen, Göttingen, Germany
- 2Department of Biotechnology, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
- 3Institute of Organic and Biomolecular Chemistry, Georg-August-Universität Göttingen, Göttingen, Germany
The release of zoospores from sporangia and motility of the released zoospores are critical in the disease cycle of the Peronosporomycetes that cause devastating diseases in plants, fishes, animals and humans. Disruption of any of these asexual life stages eliminates the possibility of pathogenesis. In the course of screening novel bioactive secondary metabolites, we found that extracts of some strains of marine Streptomyces spp. rapidly impaired motility and caused subsequent lysis of zoospores of the grapevine downy mildew pathogen Plasmopara viticola at 10 μg/ml. We tested a number of secondary metabolites previously isolated from these strains and found that macrotetrolide antibiotics such as nonactin, monactin, dinactin and trinactin, and nactic acids such as (+)-nonactic acid, (+)-homonactic acid, nonactic acid methyl ester, homonactic acid methyl ester, bonactin and feigrisolide C impaired motility and caused subsequent lysis of P. viticola zoospores in a dose- and time-dependent manners with dinactin being the most active compound (MIC 0.3 μg/ml). A cation channel-forming compound, gramicidin, and a carrier of monovalent cations, nigericin also showed similar biological activities. Among all 12 compounds tested, gramicidin most potently arrested the motility of zoospores at concentrations starting from 0.1 μg/ml. All macrotetrolide antibiotics also displayed similar motility impairing activities against P. viticola, Phytophthora capsici, and Aphanomyces cochlioides zoospores indicating non-specific biological effects of these compounds toward peronosporomyctes. Furthermore, macrotetrolide antibiotics and gramicidin also markedly suppressed the release of zoospores from sporangia of P. viticola in a dose-dependent manner. As macrotetrolide antibiotics and gramicidin are known as enhancers of mitochondrial ATPase activity, inhibition of zoosporogenesis and motility of zoospores by these compounds are likely linked with hydrolysis of ATP through enhanced ATPase activity in mitochondria. This is the first report on motility inhibitory and lytic activities of macrotetrolide antibiotics and nactic acids against the zoospores of peronosporomycete phytopathogens.
Introduction
The Peronosporomycete genera such as Plasmopara, Phytophthora, Pythium, and Aphanomyces are notorious pathogens of plants, fishes and vertebrates (Agrios, 1997). Although, morphologically and physiologically they have similarities to fungi, phylogenetically they are relatives of brown algae and diatoms and thus belong to the kingdom of Straminipila (Dick, 2001). One of the unique features of the peronosporomycete pathogens is that in favorable environment they asexually produce motile zoospores from sporangia that develop at the tip of branched sporangiophores (Judelson and Blanco, 2005). Sporangia are globose to lemon-shaped containers capable of converting their cytoplasm into multiple wall-less zoospores. The release of zoospores from sporangia (zoosporogenesis) involves cleavage of the sporangial cytoplasm by nucleus-enveloping membrane networks and an assembly of two flagellae per zoospore (Hardham and Hyde, 1997). The produced zoospores are expelled from the sporangium through sporangial papillae by turgor pressure, resulting in part from high concentration of proline that accumulates in the cytoplasm of cleaving sporangia (Ambikapathy et al., 2002). The zoospores swim after their release, using an anterior tinsel-type flagellum ornamented with tripartite tubular hairs to pull the cell and a posterior whiplash-type flagellum for steering that accomplish “thrust reversal” (Islam et al., 2002b; Judelson and Blanco, 2005). The primary goal of swimming zoospores is to find potential infection sites of the host guided by host-mediated signaling cues (Islam and Tahara, 2001), followed by morphological change into round cystospores by cellular encystment and shedding of flagellae (Islam et al., 2002b, 2003). The cystospores rapidly germinate to form hyphal germ tubes, which penetrate host tissues for infection. Although, little is known about the underlying molecular mechanisms of zoosporogenesis and motility pathways, it has been found that substantial energy from reserve β-1,3-glucan (mycolaminarin) is used during zoosporogenesis and for motility of zoospores (Bimpong, 1975; Judelson and Blanco, 2005). Zoospores are highly energy demanding life stages as ATPase activity in zoospores is similar to that in contracting skeletal muscles (Holker et al., 1993; Stienen et al., 1996). Inhibition of enzymes that maintain ATP concentration, or shuttling of ATP from mitochondria to sites of high utilization such as flagellar kinetosomes of zoospores or depletion of ATP by enhancement of ATPase activity would result in impairment of motility of zoospores and suppression of zoosporogenesis (Judelson and Blanco, 2005).
Plasmopara viticola is a devastating downy mildew pathogen of grapevine, which causes severe economic losses worldwide (Agrios, 1997). This pathogen spreads by an extremely efficient cycle of asexual propagation (Kiefer et al., 2002; Riemann et al., 2002). The success of this obligate biotrophic pathogen can be attributed in part to the speed of asexual differentiation to generate biflagellate motile zoospores from airborne sporangia (zoosporogenesis). It has been observed that zoospores locate the stomata being guided by biochemical host cues followed by encystment and germination to form germ tubes to initiate infection through stomata (Kiefer et al., 2002). Disruption of zoospore release from sporangia (zoosporogenesis) and/or motility of zoospores by any kind of inhibitor eliminates the potential for pathogenesis (Judelson and Blanco, 2005; Islam et al., 2011). Control of downy mildew in practice is difficult as host plant resistance is generally low and the polycyclic life style of the pathogen requires frequent treatments with fungicides. Novel biorational approaches may help to improve control of this notorious phytopathogen. Discovery of inhibitory chemical substances that can affect the pathways of zoosporogenesis and/or motility of zoospores might be useful for development of innovative and effective strategies for controlling the disease (Judelson and Blanco, 2005; Islam et al., 2011).
Due to the biotrophic nature, P. viticola is recalcitrant to cultivation on culture media and thus it is difficult to test the inhibitory potential of novel chemical compounds on zoospore release from sporangia or the motility of zoospores. We developed in vitro methods to produce high quantities of sporangia on excised grapevine leaves and get copious amounts of biflagellate motile zoospores in a host-free system (Islam and von Tiedemann, 2008). The released zoospores remain motile in sterilized water for 12–16 h. These in vitro methods allow screening secondary metabolites from antagonistic environmental microorganisms using convenient bioassay protocols (Islam et al., 2011). In this way, we recently isolated several new secondary metabolites from plants and microorganisms that suppress zoosporogenesis, inhibit motility and/or cause lysis of P. viticola zoospores (Abdalla et al., 2011; Islam et al., 2011; Zinad et al., 2011; Talontsi et al., 2012a,b; Dame et al., 2016). Furthermore, using a natural product, staurosporine and some further kinase inhibitors, we recently demonstrated that protein kinase C is involved in both flagellar motility and zoosporogenesis of the Peronosporomyces (Islam et al., 2011).
Secondary metabolites from marine microorganisms especially Streptomyces spp. are known to possess diverse biological activities through inhibiting specific enzyme or proteins in the signaling pathways (Islam et al., 2011). In the course of screening for novel secondary metabolites from marine Streptomyces spp., we found that extracts of some marine Streptomyces spp. (such as strains Act 8970, ACT 7619) rapidly impaired motility and caused subsequent lysis of zoospores at 10 μg/ml. We then tested all previously isolated compounds from these Streptomyces strains and found that macrotetrolide antibiotics such as dinactin and nactic acids (Al-Refai, 2008; Mahmoud, 2008), displayed motility inhibitory and lytic activities against zoospores which were identical to the effects in crude extracts. Dinactin is a member of the macrotetrolide complex produced by a range of Streptomyces species, which also includes nonactin, monactin, trinactin and tetranactin (Beck et al., 1962; Al-Refai, 2008; Mahmoud, 2008). These nactins are known to enhance ATPase activity in the mitochondria and cause rapid hydrolysis of ATP. They were also shown to act as monovalent cation ionophores with high selectivity for ammonium and potassium (Graven et al., 1966, 1967). The novel biological activities of macrotetrolides found in this study prompted us to further test structurally related compounds of dinactin and some known ionophores to understand the structure-activity relationships as well as to get information on the mode of action of these natural products. Therefore, the objectives of our study were (i) to screen extracts of marine Streptomyces spp. on motility and viability of zoospores of P. viticola; (ii) to test compounds previously isolated from extracts of Streptomyces spp. that exhibited motility inhibitory and lytic activities against zoospores; (iii) to test compounds structurally related to (+)-nonactic acid and dinactin on motility and lysis of zoospores of P. viticola, Phytophthora capsici and Aphanomyces cochlioides; (iv) to evaluate the effect of further known compounds such as nigericin and gramicidin having similar mode of action on zoospores; and (v) to evaluate the effects of zoospore motility inhibitors on zoosporogenesis of P. viticola.
Materials and Methods
Materials and Experimental Procedure
Macrotetrolide antibiotics such as monactin and trinactin and further ionophoric compounds, nigericin and gramicidin were purchased from Tebu-bio and Sigma-Aldrich, respectively. Dinactin, nonactin, bonactin, feigrisolide, (+)-nonactic acid, (+)-homonactic acid, nonactic acid methyl ester, and homonactic acid methyl ester available in the laboratory were either previously isolated from marine Streptomyces spp. (Act 8970 and ACT 7619) or synthesized. All other chemicals were of at least reagent grade. Stock solutions of test compounds were prepared in small amounts of dimethyl sulfoxide (DMSO) and then diluted with water. The concentration of DMSO in the incubation medium never exceeded 1%, a condition that does not affect motility and viability of peronosporomycete zoospore (Islam et al., 2011).
Cultivation of Marine Streptomyces spp. and Extraction
The marine Streptomyces spp. strains (such as Act8970) used in this research were obtained from the collection of the Institute of Organic and Biomolecular Chemistry, University of Göttingen, Germany. These strains were pre-cultivated on medium (+ 50% sea water) agar plates at 28°C for 3 days. To upscale these strains, pieces of well colonized agar were added to 1 l shaker cultures. Each strain was propagated in 5 1l-Erlenmyer flasks each containing 200 ml of M2+ (50% sea water) for 11 days at 28°C on a linear shaker with 110 rpm. After extraction of water phase and cell mass with ethyl acetate, the obtained extract was subjected to the bioassay.
Peronosporomycete Strains, Production of Zoospores and Bioassay
Sporangia of P. viticola were isolated from infected leaves of grapevine (Vitis vinifera cv. Müller-Thurgau) (Islam et al., 2011). This strain was originally gained from infected leaf materials of the grapevine cv. Riesling in 1996 and since then maintained and propagated on fresh leaves of cv. Müller-Thurgau kept on Petri dishes containing 1.5% agar at 25°C and 95% relative humidity (Islam and von Tiedemann, 2008, 2011). At day 6 of cultivation, the sporangiophores bearing lemon-shaped sporangia were harvested into an Eppendorf vial by a micro-vacuum cleaner. The freshly harvested sporangia were separated from sporangiophores by filtration through a nylon sieve (50 μm mesh), washed twice with distilled water and then incubated in sterilized tap water (3 × 104 sporangia/mL) in the dark for 6 h at room temperature (23°C) to release zoospores. These zoospores remained motile for 10–12 h in sterilized water and were used for the bioassay (Islam et al., 2011). The bioassay for testing the effects of pure compounds on release of zoospores from sporangia was carried out as described earlier (Islam et al., 2011).
The bioassay on motility and lysis of zoospores in presence of varying doses of pure compounds was carried out as described earlier (Islam et al., 2002a, 2011). Briefly, 40 μL of sample solution was directly added to 360 μL of zoospore suspension (ca. 105/mL) taken in a dish of a plant tissue culture multi-well plate to make a final volume of 400 μL and then quickly mixed with a glass rod; 1% aqueous DMSO was used as a control. The motility of zoospores was observed under a light microscope at 100-fold magnification. Quantification of time-course changes of motility and lysis of zoospores were carried out as described earlier (Islam et al., 2004). Each treatment was replicated five times. The mean value (%) ± SE (standard error) of the affected spores in each treatment was calculated.
Cultivation of Aphanomyces cochlioides and Phytophthora capsici, Production of Zoospores and Bioassay
The damping-off pathogen of sugar beet and spinach, Aphanomyces cochlioides, was obtained from the Sugar Beet Research Institute (IFZ) in Goettingen, Germany. The culture of this strain and protocol for production of zoospores are described elsewhere (Islam and von Tiedemann, 2011; Zohara et al., 2016). P. capsici was provided by Prof. W. Yuancaho of Nanjing Agricultural University, China, which was isolated from soil of Nanjing, China. This organism was cultured on V8 juice agar. Production of zoospores and bioassays were carried out following protocols reported earlier (Tareq et al., 2014; Zohara et al., 2016). Each treatment was replicated five times. The mean value (%) ± SE (standard error) of the affected spores in each treatment was calculated.
Statistical Analysis, Experimental Design/Replications
Experiments for evaluating biological activities of the pure compounds were carried out using a complete randomized design (CRD). Data were analyzed by one way analysis of variance (ANOVA) and the mean values were separated by Tukey's HSD (honest significant difference) posthoc statistic. All the analyses were performed using SPSS (IBM SPSS statistics 21, Georgia, USA). Mean value ± standard error of 5 replications were used in Tables and Figures.
Results
Motility Inhibitory and Lytic Activities of Extracts of Marine Streptomyces spp. Strains
To see whether marine Streptomyces spp. produce inhibitory substances against notorious phytopathogenic peronosporomycetes, we tested crude extracts of a large number of previously studied strains on motility behavior of P. viticola zoospores that produce diverse bioactive secondary metabolites. Out of 89 crude extracts from different strains of marine Streptomyces spp. tested, strains Act 8970, B6167, B7857, ACT7619, and Gt-2005/009 displayed significantly higher (p ≤ 0.001) motility inhibitory and subsequent lytic activities against P. viticola zoospores at 10 μg/ml or lower concentrations (Table 1). The antibiotic activity of the crude extracts of strains Act 8970, B6167, B7857, Act7619, and Gt2005/2009 was due to the presence of macrotetrolide antibiotics as dinactin and nactic acids displayed identical motility inhibitory and lytic activities against the zoospores in a dose- and time-dependant manners. These compounds were isolated from all these strains in our laboratory (Al-Refai, 2008; Mahmoud, 2008; Rahman, 2008). Therefore, homologs of dinactin and nactic acids were used in further detailed inhibition bioassays toward zoosporogenesis and motility of zoospores of P. viticola. To see whether the inhibitory activities of macrotetrolides and nactic acids are specific to P. viticola or general to other economically important phytopathogenic peronosporomycetes, we included a damping-off pathogen of sugar beet and spinach, Aphanomyces cochlioides and a late blight pathogen of chili and several vegetables, Phytophthora capsici.
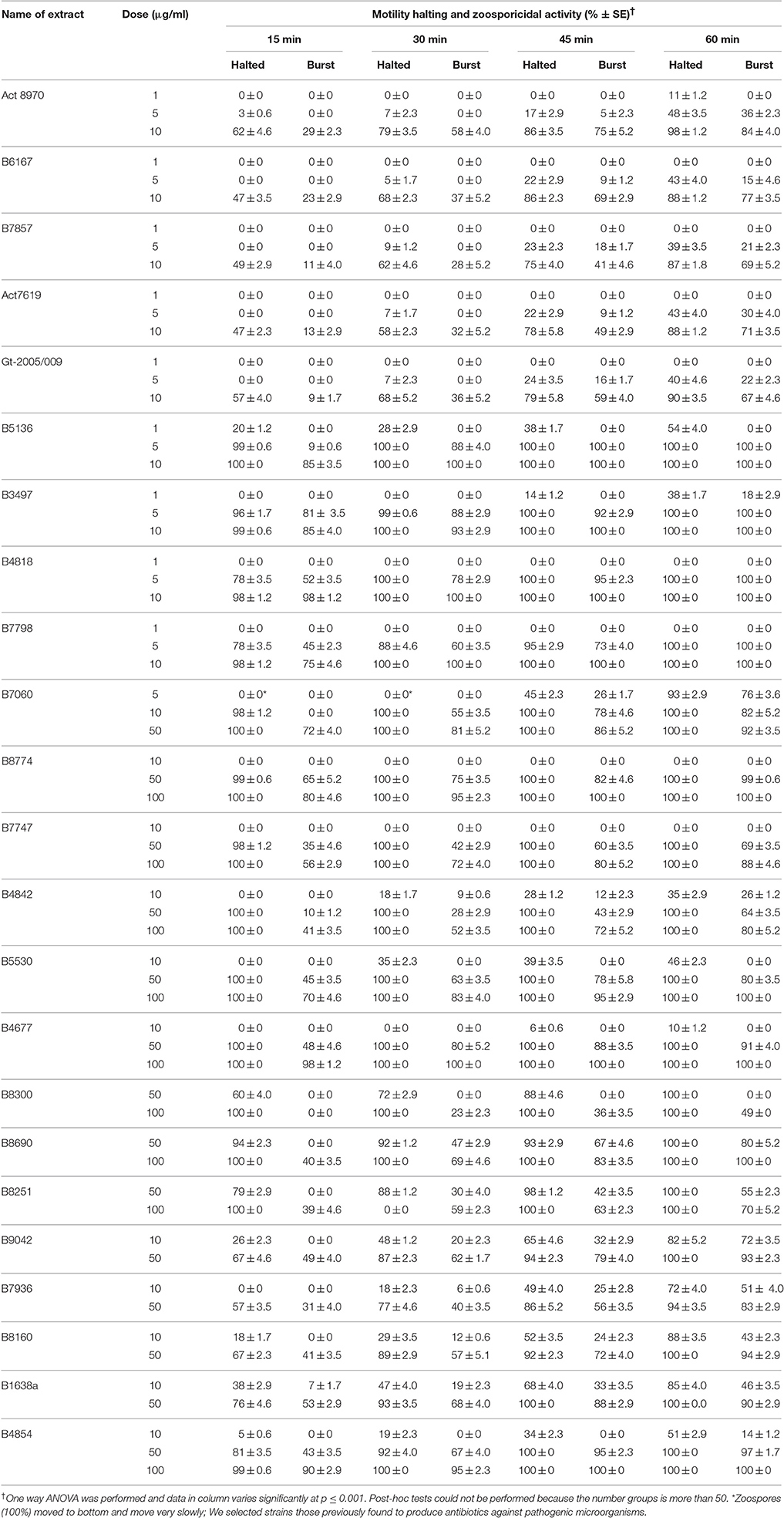
Table 1. Motility halting and zoosporicidal activity of marine Streptomyces spp. extracts against the downy mildew pathogen Plasmopara viticola.
Motility Inhibitory and Lytic Activities of Macrotetrolide Antibiotics and Nactic Acids against P. viticola Zoospores
Compounds previously isolated from strains of Act 8970 viz. dinactin, (+)-nonactic acid, (+)-homononactic acid, homononactic acid methyl ester, and nonactic acid methyl ester, were tested on motility of P. viticola zoospores (Table 2 and Figure 1). Among them, the macrotetrolide antibiotic dinactin displayed the highest potency in arresting motility and caused subsequent lysis of P. viticola zoospores starting from 0.3 μg/ml (Table 2). The activities of the linear nactic acids and their methyl esters were 5–50-fold lower in inhibition of zoospore motility than of dinactin. Dinactin inhibited the motility completely and caused lysis of all stopped zoospores (100%) within 15 min exposure to the compound at 1 μg/ml. One way ANOVA revealed that zoospore motility inhibitory activities of the varying concentrations of the tested compounds varied significantly at p ≤ 0.05. Microscopic observation revealed that in presence of dinactin, swimming of zoospores was rapidly impaired or slowed down and/or zoospores spun in tight circles for a short time (Figure 2). Finally, all affected zoospores stopped moving and most of them subsequently lysed within several minutes of treatment, depending on the concentration of the compound. Before lysis, the cellular materials in halted zoospores rapidly became granulated and then gradually fragmented and dispersed into the surrounding water upon burst of their cell membranes (Figure 2f). In contrast, P. viticola zoospores in untreated control dishes exhibited the characteristic helical swimming almost following a straight line for several hours. Other nactic acids and their methyl esters also impaired motility of zoospores and caused subsequent lysis in an identical manner but at varying concentrations (Table 2). Among them, (+)-homonactic acid exhibited the strongest activity in both inhibition of motility and lysis of zoospores followed by nonactic acid methyl ester, homononactic methyl ester and (+)-nonactic acid in decreasing order. (+)-Homononactic acid inhibited motility of 100% zoospores at 5 μg/ml by 60 min of treatment, which was 10-fold stronger activity compared to the activity of (+)-nonactic acid (Table 2). The zoospore lytic activities of the tested compounds also varied significantly at p ≤ 0.05.
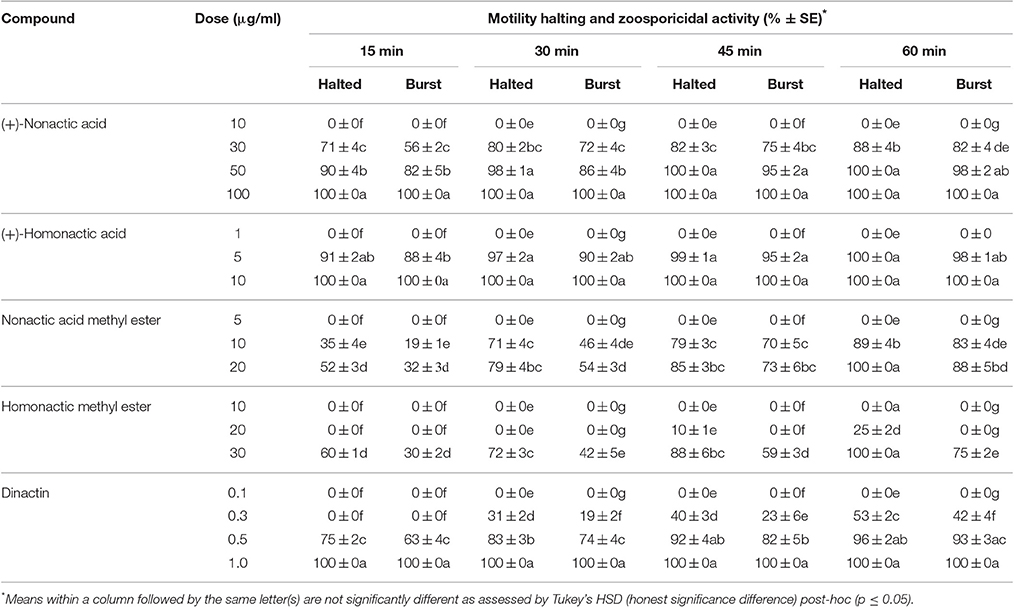
Table 2. Motility halting and zoosporicidal activity of nactic acids and their esters and dinactin isolated from marine Streptomyces sp. Act 8970 against the grapevine downy mildew pathogen Plasmopara viticola.
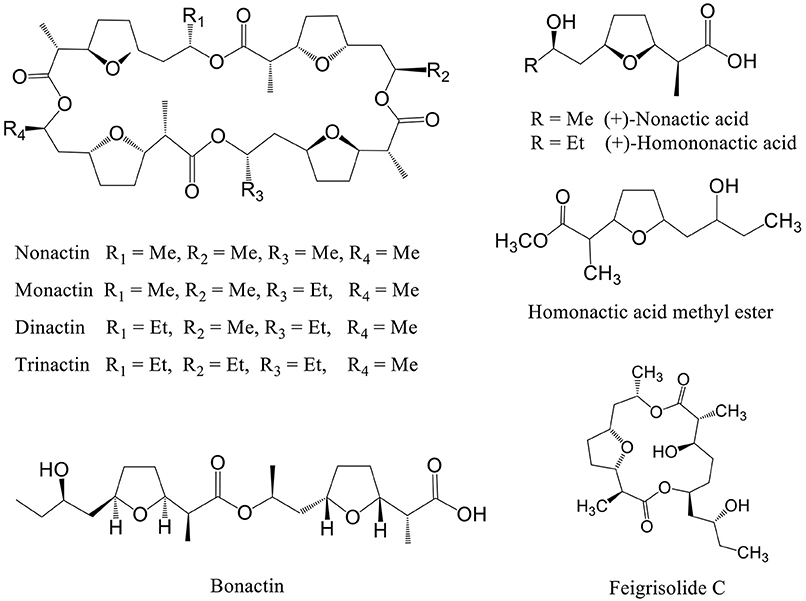
Figure 1. Structure of secondary metabolites isolated from marine Streptomyces spp. having motility inhibitory and lytic activities against peronosporomycete zoospores.
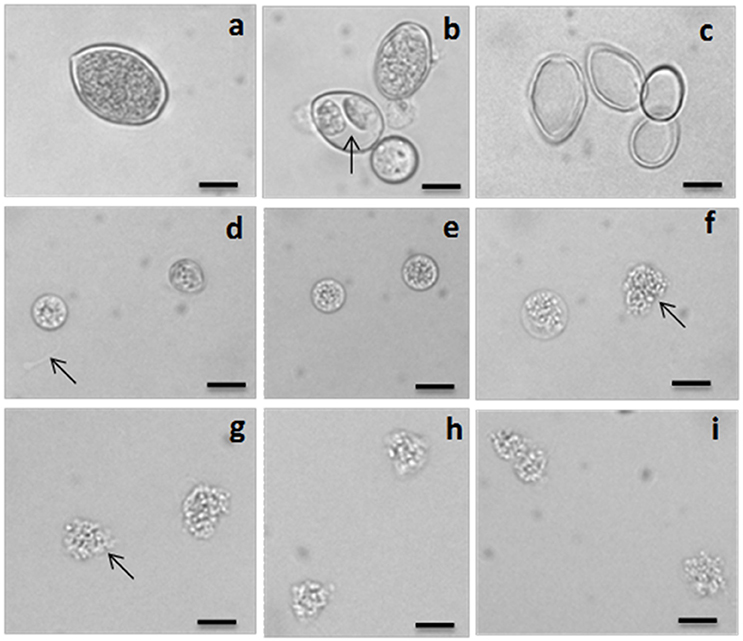
Figure 2. Light micrographs showing sporangium, differentiating sporangium, empty sporangium (ghost) and inhibitory effects of dinactin, trinactin and gramicidin on zoospores of the grapevine downy mildew pathogen Plasmopara viticola. (a) A mature freshly harvested sporangium, (b) differentiated sporangia with developed zoospores (arrow) inside, (c) empty sporangia (ghosts) after release of zoospores, (d) two halted zoospores at the bottom of the dish just after addition of dinactin (1 μg/ml). Arrow indicates the trace of a halted zoospore, (e) both halted zoospores become round by 5 min after treatment with dinactin (1 μg/ml), (f) disruption of membrane and granulation of cell organelle and lysis (arrow) of zoospores by dinactin 10 min after treatment (1 μg/ml), (g) lysis of zoospores by dinactin 15 min after treatment (1 μg/ml), (h) lysis of zoospores by trinactin 15 min after treatment (1 μg/ml), (i) lysis of zoospores by gramicidin 15 min after treatment (1 μg/ml). Bar represents 10 μm.
Biological Activity of Compounds Structurally Related to Dinactin and Dinactic Acids
Dinactin is a member of the macrotetrolide complex produced by a range of Streptomyces species which includes several homologs such as nonactin, monactin, and trinactin (Beck et al., 1962). Early literature reported that these compounds almost equally enhance mitochondrial ATPase activity and cause rapid hydrolysis of ATP (Graven et al., 1966). They are also known to act as monovalent cation ionophore with high selectivity for ammonium and potassium (Graven et al., 1967) and have diverse biological activities (Zizka, 1998). To get insight into the structure-activity relationships, we tested some homologs of dinactin such as nonactin, monactin and trinactin together with two linear compounds such as bonactin and feigrisolide C previously isolated from marine Streptomyces species (Figure 1).
All three homologs (nonactin, monactin, and trinactin) of dinactin displayed motility impairing and lytic activities against P. viticola zoospores in an identical fashion and dose- and time-dependent manner (Table 3). One way ANOVA revealed that zoospore motility inhibitory activities of the tested compounds and their different concentrations varied significantly at p ≤ 0.05. The strengths of activity of all these homologs were similar. Bonactin and feigrisolide C also exhibited motility inhibitory and lytic activities in a similar manner but required almost 10-fold higher doses compared to dinactin. Time-course investigation revealed that dinactin and trinactin caused 100% inhibition of zoospore motility within 15 min exposure to the tested compounds at 1 μg/ml (Figure 3A). At the same concentration, nonactin and monactin also inhibited motility by 100% but required longer time, i.e., 30 and 60 min, respectively. All compounds showed almost similar phenomena in causing lysis of halted zoospores (Figure 3B). Initially, zoospores became paralyzed or moved very slowly in tight circles, stopped and then rapidly immobilized (Figure 2). The zoospore lytic activities of the varying concentration of the tested compounds also varied significantly at p ≤ 0.05.
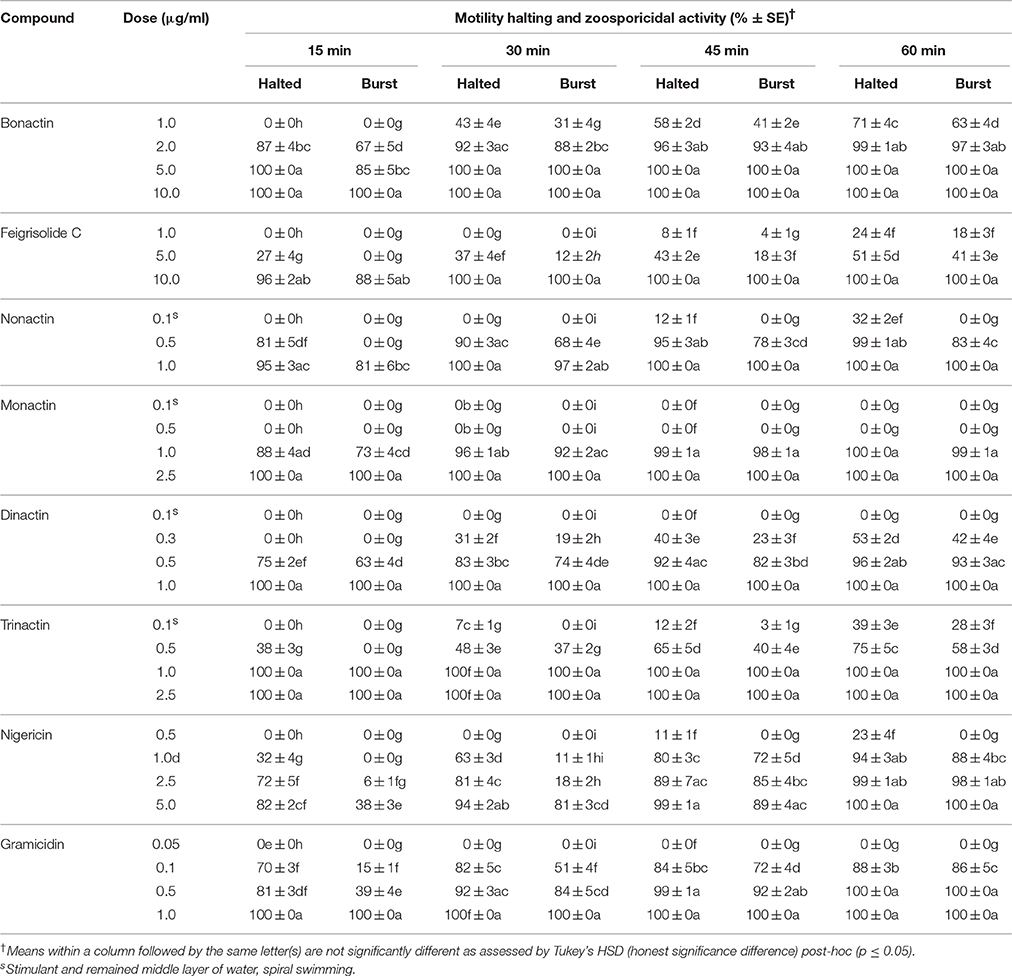
Table 3. Motility inhibitory and zoosporicidal activity of bonactin, feigrisolide C, macrotetrolide antibiotics (nonactin, monactin, dinactin and trinactin), nigericin and gramicidin against the grapevine downy mildew pathogen Plasmopara viticola.
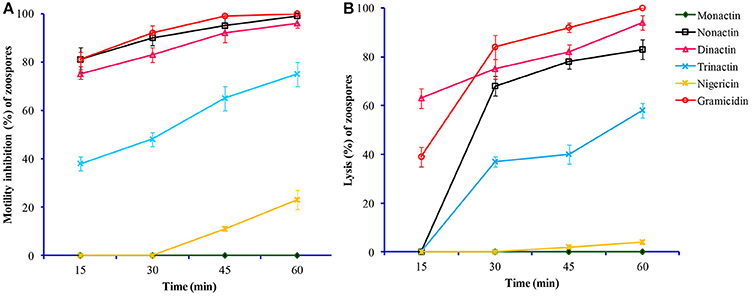
Figure 3. Time-course comparative motility inhibitory (A) and lytic activities (B) of macrotetrolide antibiotics against zoospores of the grapevine downy mildew pathogen Plasmopara viticola at 0.5 μg/ml (p ≤ 0.05).
Motility of Zoospores in Presence of Gramicidin and Nigericin
To better assess whether induction of ATPase activity and hydrolysis of ATP in mitochondria or any other mechanism is associated with motility inhibitory and lytic activities of zoospores by macrotetrolide antibiotics, a channel-forming ionophore, gramicidin and a mobile carrier of cations through plasma membranes, nigericin were tested (Graven et al., 1966, 1967). Gramicidin showed the highest activity (p ≤ 0.05) among the tested compounds, which was 2-fold stronger than those of macrotetrolides for inhibition of zoospore motility by 100% (Table 3). On the other hand, nigericin also displayed zoospore motility arresting activity but had 10-fold weaker efficacy compared with gramicidin. In both cases, halted zoospores were lysed in a similar manner as shown by the macrotetrolide antibiotics. On the other hand, nigericin displayed almost equal strength to bonactin in arresting motility of 100% zoospores at 5 μg/ml, while the dose required for equivalent efficacy by feigrisolide was 10 μg/ml (Table 3). Motility inhibitory and lytic activities against zoospores by gramicidin and nigericin were statistically significant (p ≤ 0.05).
Effects of Macrotetrolide Antibiotics on Motility of Aphanomyces cochlioides Zoospores
To evaluate whether motility inhibitory and lytic activities of marotetrolides are common phenomena in Peronosporomycete zoospores, we tested all homologs of dinactin and other bioactive compounds evaluated on P. viticola against a sugar beet damping-off pathogen Aphanomyces cochlioides (Peronosporomycete). An almost identical phenomenon was observed when motile A. cochlioides zoospores were exposed to macrotetrolide antibiotics or other inhibitors, but surprisingly none of the compounds caused any lysis of the halted zoospores until 60 min (Table 4). One way ANOVA revealed that zoospore motility inhibitory and lytic activities of the tested compounds and their varying concentrations varied significantly at p ≤ 0.001. Irrespective of the test compounds, all motility-impaired zoospores rapidly became round cystospores instead of lysis, however, none of them germinated until 60 min after the treatment (data not shown).
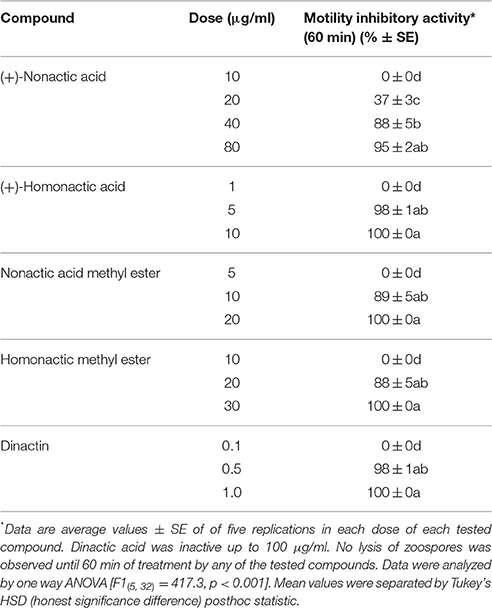
Table 4. Motility inhibitory activity of nactic acids and their esters, and dinactin isolated from marine Streptomyces sp. Act 8970 against the sugar beet damping-off pathogen Aphanomyces cochlioides AC-1.
Motility Inhibitory and Lytic Activities of Dinactin and Trinactin against Phytophthora capsici Zoospores
Two homologs of macroterolide antibiotics, dinactin and trinactin were also tested against the zoospores of another notorious peronosporomycete phytopathogen, Phytophthora capsici. As expected, both compounds displayed inhibitory activity against P. capsici zoospores in a dose- and time-dependant manner. Trinactin showed significantly (p ≤ 0.001) stronger inhibitory activity compared with dinactin (Figure 4). The zoospores of P. capsici seemed less sensitive to the macrotetrolides compared to P. viticola zoospores. The IC50 values for motility inhibition of zoospores by dinactin and trinactin were ca. 1.0 and 0.5 μg/ml, respectively. Unlike P. viticola zoospores, only a small fraction of motility impaired zoospores became lysed by the treatment of dinactin and trinactin. Approximately, 50% of the halted zoospores became round cystospores and failed to germinate until 60 min after the treatments.
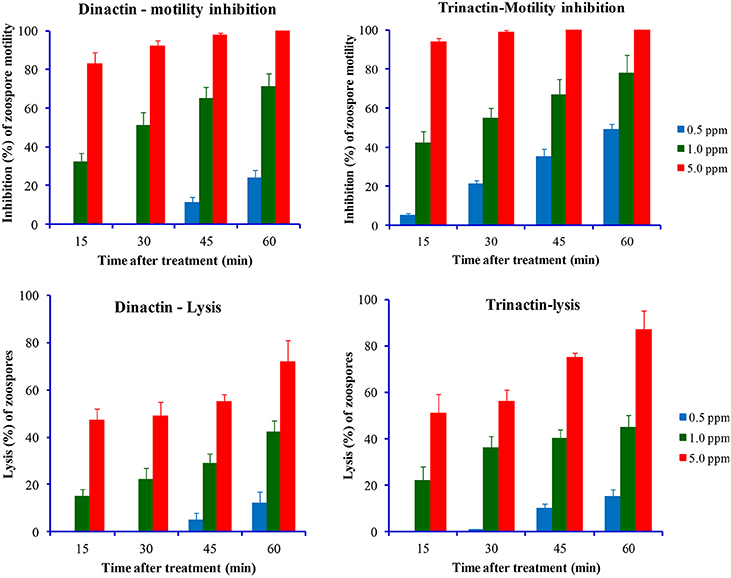
Figure 4. Time-course comparative motility inhibitory (upper panel) and lytic activities (lower panel) of dinactin and trinactin against zoospores of the late blight pathogen of chili and cucumber, Phytophthora capsici at varying doses of tested compounds.
Inhibition of Zoosporogenesis by Nonactic Acid and Macroterolides
Freshly harvested and washed P. viticola sporangia (3 × 105/ml) typically release zoospores up to 1 × 106/ml in sterilized water within 5–6 h. We tested whether macrotetrolide antibiotics (nonactin, monactin, dinactin, and trinactin), bonactin, feigrisolide, nigericin and gramicidin, have an effect on the process of zoospore release (i.e., zoosporogenesis). The bioassay revealed that all compounds significantly (p ≤ 0.001) inhibited zoosporogenesis in a dose-dependent manner but in varying concentrations (Table 5 and Figure 5). Zoosporogenesis was completely blocked by monactin, dinactin, trinactin and gramicidin at 5 μg/ml. At lower doses of these compounds, the release of zoospores still occurred but most zoospores became immobilized soon after release. Nonactin also displayed a similar inhibitory effect but required a 2-fold higher concentration for equivalent activity to other macrotetrolides. The bonactin and feigrisolide also suppressed the release of zoospores but required several fold higher concentrations compared with dinactin. Nigericin had weak activity (IC50 10 μg/ml) in suppressing zoosporogenesis of P. viticola.
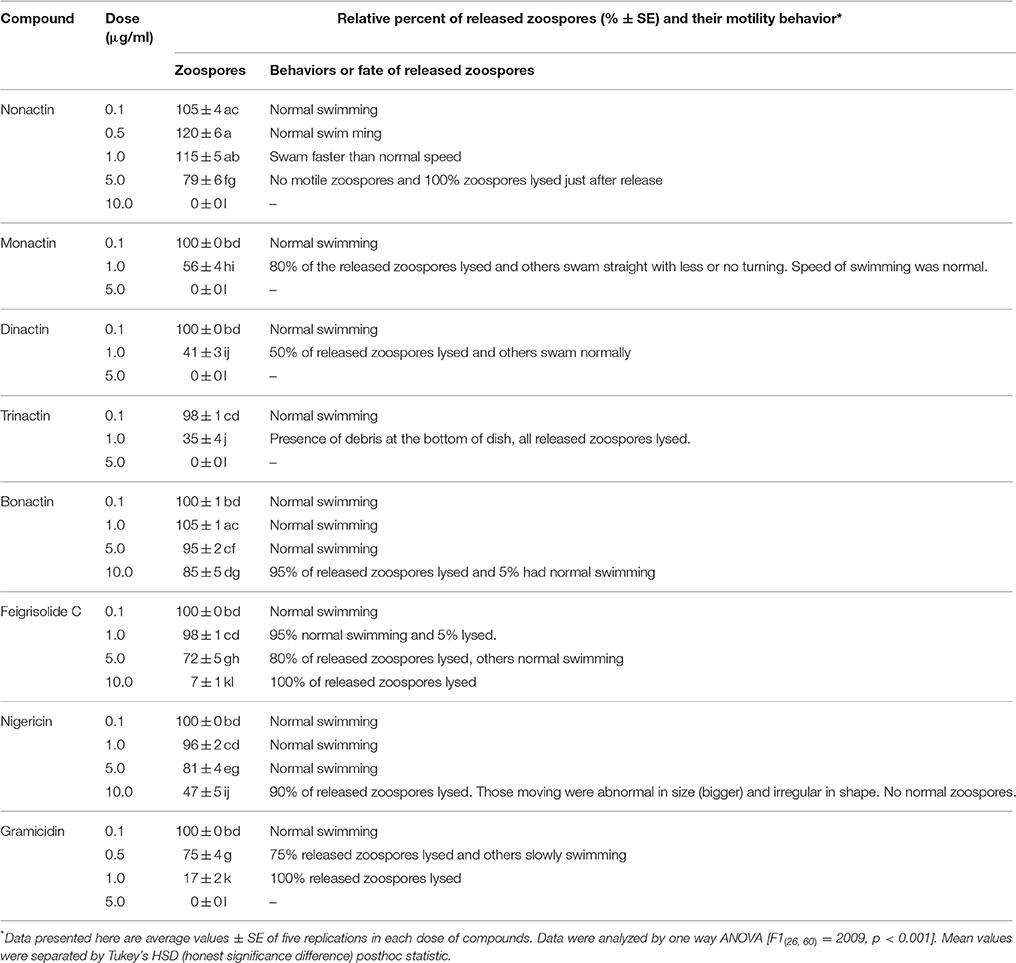
Table 5. Effects of macrotetrolide antibiotics, and bonactin, feigrisolide C, nigericin and gramicidin on the release of zoospores from sporangia (zoosporogenesis) of the grapevine downy mildew pathogen Plasmopara viticola.
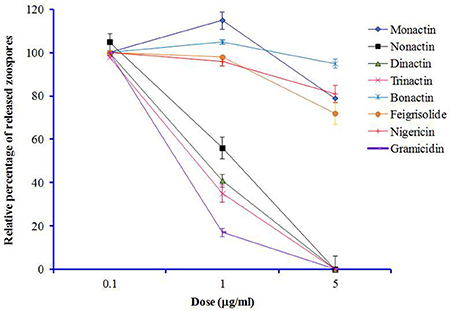
Figure 5. Effects of macrotetrolide antibiotics, and bonactin, feigrisolide C, nigericin and gramicidin on relative percentage (%) of release of zoospores from sporangia (zoosporogenesis) of grapevine downy mildew pathogen Plasmopara viticola over untreated control (p ≤ 0.001).
Discussion
In this study, we demonstrated that macrotetrolide antibiotics such as dinactin and nactic acids isolated from marine Streptomyces spp. impaired motility and caused lysis of P. viticola zoospores that are key stages of this devastating pathogen of grapevine (Tables 2, 3). These bioactive compounds also inhibited motility of P. capsici and A. cochlioides zoospores in a similar way (Table 4) and suppressed the release of zoospores from P. viticola sporangia in a dose-dependent manner (Table 5). In addition, the homologs of dinactin (e.g., nonactin, monactin, trinactin), which are known as enhancers of mitochondrial ATPase activity similar to nigericin and gramicidin also suppressed zoosporogenesis, impaired motility and caused lysis of P. viticola zoospores (Table 5, Figures 3, 4). Taken together, our results show for the first time that macroterolide antibiotics and nactic acids from marine Streptomyces spp. and other bacteria suppress zoosporogenesis and impair motility of peronosporomycete zoospores. Furthermore, preliminary bioassay revealed that one of the macrotetrolide, dinactin suppressed the sporulation of P. viticola in artificially inoculated grapevine leaf disks (data not shown) indicating its potential as a natural peronosporomicide. A further in vivo study is warranted to test this hypothesis. As zoosporogenesis and motility of zoospores are high energy demanding processes, the mode of action of these inhibitory activities by macroterolides is likely linked with the hydrolysis of mitochondrial ATP through enhanced ATPase activity. Motility inhibition and subsequent lysis of Peronosporomycete zoospores by various kinds of natural products such as indolocarbazole alkaloid, staurosporine (Islam et al., 2011), khatmiamycin from Streptomyces sp. ANK313 (Abdalla et al., 2011), isocoumarins from Streptomyces sp. ANK302 (Zinad et al., 2011), macrocyclic lactam antibiotics from Lysobacter sp. SB-K88 (Islam et al., 2005), polyflavonoid tannins from the bark of Lannea coromandelica (Islam et al., 2002a), anacardic acids from Ginkgo biloba (Begum et al., 2002), and polyketides and depsidones from the fungal endophyte Cryptosporiopsis sp. CAFT122-2 and Phomopsis sp. CAFT69, respectively (Talontsi et al., 2012a,b) have been reported previously. Impairment of motility and lysis of peronosporomycete zoospores by oligomycins and pamamycin homologs from marine Streptomyces have also been reported (Dame et al., 2016)
A hallmark of our finding is that all four macrotetrolide antibiotics (nonactin, monactin, dinactin, and trinactin) produced by marine Streptomyces spp. displayed qualitatively and quantitatively similar motility-impairing activities against the phytopathogenic peronosporomycete zoospores (Table 3 and Figures 3, 4). Furthermore, an ion-forming peptide gramicidin also displayed strong motility-impairing effects against the zoospores. As zoospores are unable to take up nutrients from their environment for maintenance of motility, they require steady supply of energy (ATP) from the internal cellular energy reserves (β-1,3-glucan or mycolaminarins) (Bimpong, 1975). The ATPase activity per volume of zoospores is similar to that of contracting skeletal muscles (Holker et al., 1993; Stienen et al., 1996). Therefore, disruption of energy supply from mitochondria causes impairment of swimming behavior of the zoospores. Both macrotetrolide antibiotics and gramicidin have been found to enhance ATPase activity and cause rapid hydrolysis of ATP in the mitochondria (Graven et al., 1966). Therefore, the motility inhibitory effect of same macrotetrolide antibiotics and gramicidin shown in this study is likely to be linked to the depletion of ATP by enhanced ATPase activity in the mitochondria of treated zoospores. The underlying molecular mechanism of maintenance of zoospore motility is still poorly understood. As antibiotics have been used as effective tools for many metabolic studies, a further quantitative study on ATPase activity in the mitochondria of zoospores treated with varying doses of macrotetrolide antibiotics should shed light on motility pathway of zoospores and may be used in the search for new targets for controlling this notorious class of phytopathogens. Inhibition of zoospore motility through disruption of cytoskeletal filamentous actin by microbial metabolites such as latrunculin B, 2,4-diacetylphloroglucinol and macrolyclic lactam antibiotic, xanthobaccin A has been reported (Islam, 2008; Islam and von Tiedemann, 2011).
Another novel finding of this study was suppression of zoospore release from sporangia of P. viticola by macrotetrolide antibiotics (Table 5 and Figure 5). The cation channel-former and inducer of mitochondrial ATPase activity, gramicidin also suppressed zoosporogenesis with very high efficacy. Although, the underlying molecular mechanisms of zoosporogenesis are still poorly understood (Judelson and Blanco, 2005), cleavage of nuclei and differentiation of sporangia during zoosporogenesis require supply of energy from mitochondria. Moreover, intracellular Ca2+ ions play important roles in zoosporogenesis (Islam and Tahara, 2001). Therefore, suppression of zoosporogenesis by known inducers (macrotetrolides and gramicidin) of mitochondrial ATPase activity and ionophores suggests that depletion of ATP in mitochondria by hydrolysis of ATP in concert with translocation (efflux/influx) of cations from the cells might be involved in this process. Suppression of zoosporogenesis in P. viticola by staurosporine from a marine Streptomyces sp. B5136 (Islam et al., 2011) and 2,4-diacetylphloroglucinol from the soil bacterium Pseudomonas fluorescens have previously been reported (Islam and von Tiedemann, 2011). Moreover, a selective inhibitor of protein kinase C (PKC), chelerythrine also suppressed zoosporogenesis which indicated the involvement of PKC in the process of zoospore release from sporangia (Islam et al., 2011).
The experimental results reported in the present study do not clarify the precise mechanism involved but they point out that induction of ATPase activity in mitochondria and/or translocation/imbalance of cations in the cells might suppress zoosporogenesis and impair motility of the zoospores. Therefore, elucidation of the role of ATPase in the swimming pattern and motility of zoospores will obviously help to advance our understanding of the biology and pathogenicity of the peronosporomycete phytopathogens. In this study, some linear tetrahydrofurans such as (+)-homonactic acid and bonactin also impaired motility of zoospores qualitatively and quantitatively similar to the macrotetrolide antibiotics. Therefore, naturally occurring low molecular weight inducers of ATPase might have high potential as lead compounds for designing novel effective agrochemicals against the peronosporomycete phytopathogens.
One of the important findings of our study is that the zoospores of a biotrophic phytopathogen, P. viticola halted by macrotetrolide antibiotics or gramicidin or nigericin were rapidly immobilized (Figures 2, 3B). A similar phenomenon was observed when zoospores of P. capsici were treated with higher concentration of dinactin and trinactin. In an earlier study, Graven et al. (1966) reported that nonactin, monactin, dinactin, trinactin and gramicidin induced swelling of mitochondria through induction of alterations in the ion translocation system. Nigericin has also been found to inhibit mitochondrial respiration by blocking the uptake of both K+ and inorganic phosphorus (Pi) ions (Graven et al., 1967). Therefore, rapid lysis of halted P. viticola zoospores by the macrotetrolides, gramicidin, or nigericin might be associated with alteration in the ion translocation system in the mitochondria of zoospores and/or imbalance of osmotic balance in the zoospores. It appears from this study that inhibition of mitochondrial respiration by any chemical inhibitor might impair motility of zoospores. Experiments using gramicidin and nigericin ionophores, Appiah et al. (2005) demonstrated that altering potassium homeostasis during zoospore swimming significantly influenced speed, swimming pattern, and encystment of zoospores of Phytophthora and Pythium species (Appiah et al., 2005). Although, almost all P. viticola zoospores stopped by nactic acids or macrotetrolides were lysed, the stopped A. cochlioides zoospores by the same compounds became, however, round cystospores instead of lysing. A. cochlioides is a soilborne phytopathogen and hence sensitivity of zoospores to surrounding complex heterogenous signals in soils might be different compared to a biotrophic leaf pathogen such as P. viticola. In soilborne pathogens, those zoospores failing to find their host during their motile stage or exposed to toxic substances, rapidly become cystospores by developing a cell wall (Islam, 2011). These cystospores can regenerate secondary zoospores under favorable conditions to search the host plant. This adaptive strategy may be absent or not essential in the leaf pathogen P. viticola and zoospores released on a leaf can easily find stomata for infection, and hence zoospores halted by nactic acids and macrotetrolides are immediately lysed. Motility inhibition of A. cochlioides zoospores without lysis by a nonhost metabolite, nicotinamide has been reported (Islam et al., 2004).
Nonactin, dinactin, trinactin and teranactin isolated from a variety of Streptomyces species are cyclotetralactones derived from nonactic acid and homononactic acid as building units of ionophoric character. Nonactin and its homologs such as monactin, dinactin, trinactin, and tetranactin were isolated as bioactive compounds from Streptomyces spp. by many researchers (Beck et al., 1962; Dominguez et al., 1962; Meyers et al., 1965; Haneda et al., 1974; Callewaert et al., 1988; Sobolevskaya et al., 2004; Hashida et al., 2012). The precursor of nonactin and other marcotetrolides, nonactic acid is biosynthesized in Streptomyces spp. by a type II polyketide synthase (PKS) system (Walczak et al., 2000). In our study, both cyclotetralactones and their precursor, nonactic acid and homononactic acids displayed inhibitory effects against zoosporogenesis and motility of zoospores but in varying concentrations. Among the nactic acids, the homonactic acid showed a higher activity than nonactin. A further study is needed to establish precise structure-activity relationships of these bioactive compounds which might lead to the development of an effective biopesticide against the peronosporomycete phytopathogens.
Zoosporogenesis and motility of zoospores are two life stages critically important for disease cycles and also virulence of the peronosporomycete phytopathogens (Latijnhouwers et al., 2004; Judelson and Blanco, 2005; Islam et al., 2011). A motility inhibitory compound, staurosporine has recently been found to successfully suppress development of downy mildew on treated grapevine leaves (Islam et al., 2011). Macrotetrolide antibiotics exhibit a very wide range of effects, ranging from antimicrobial to insecticidal, acaricidal, anticancer, antiprotozoan (coccidiostatic), immunosuppressive and antiparasitic (antihelminthic) (Meyers et al., 1965; Borrel et al., 1994; Zizka, 1998; Kusche et al., 2009). Plant growth promotion and exhibition of specific insecticidal effects of precursors of macrotetrolide antibiotics, nonactic and homononactic acids have been reported (Jizba et al., 1992). The zoospore motility inhibitory substances found in this study such as dinactin, and nactic acids produced by several Streptomyces spp. including a marine strain Act 8970 might help us to design strategies for biorational management of the notorious Peronosporomycete phytopathogens. This study reveals that macrotetrolide antibiotics are not only potential candidates for development of antiperonosporomycete agrochemicals but could also be used as tools for dissecting underlying molecular mechanisms governing zoosporogenesis and motility functions of zoospores of the fungus-like peronosporomycetes.
Author Contributions
MTI: Involved in conceived idea, designed and executed experiments, analyzed data and writing manuscript; AvT and HL: Involved in conceived idea, designed experiments, and critically edited manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Alexander von Humboldt Foundation for the Georg Forster Fellowship to MTI and partial financial support of the research; B. Berkelmann Geisenheim Research Center, Germany, for kindly donating the Plasmopara viticola strain. The financial support from the World Bank through CP # 2071 under HEQEP is highly acknowledged. Our sincere thanks are due to Prof. Ruhul Amin of Bangabandhu Sheikh Mujibur Rahman Agricultural University for generously helping us for statistical analysis of the data.
References
Abdalla, M. A., Win, H. Y., Islam, M. T., von Tiedemann, A., Schüffler, A., and Laatsch, H. (2011). Khatmiamycin, a motility inhibitor and zoosporicide against the grapevine downy mildew pathogen Plasmopara viticola from Streptomyces sp. ANK313. J. Antibiot. 64, 655–659. doi: 10.1038/ja.2011.68
Al-Refai, M. H. I. (2008). New and Bioactive Secondary Metabolites from Marine and Terrestrial Bacteria: Ramthacin A, B, C, and Polyene Macrolides from Genetically Modified Bacteria. Ph D. dissertation, Georg-August-Universitaet Goettingen.
Ambikapathy, J., Marshall, J. S., Hocart, C. H., and Hardham, A. R. (2002). The role of proline in osmoregulation in Phytophthora nicotianae. Fungal Genet. Biol. 35, 287–299. doi: 10.1006/fgbi.2001.1327
Appiah, A. A., van West, P., Osborne, M. C., and Gow, A. N. R. (2005). Potassium homeostasis influences the locomotion and encystment of zoospores of plant pathogenic oomycetes. Fungal Genet. Biol. 42, 213–223. doi: 10.1016/j.fgb.2004.11.003
Beck, J., Gerlach, H., Prelog, V., and Voser, W. (1962). Metabolic products of actinomycetes. XXXV. Structure of monactin, dinactin and trinactin. Helv. Chim. Acta 45, 620–630.
Begum, P., Hashidoko, Y., Islam, M. T., and Tahara, S. (2002). Zoosporicidal activity of anacardic acids against Aphanomyces cochlioides. Z. Naturforsch. 57, 874–882. doi: 10.1515/znc-2002-9-1020
Bimpong, C. E. (1975). Changes in metabolic reserves activities during zoospore motility and cyst germination in Phytophthora palmivora. Can. J. Bot. 53, 1411–1416. doi: 10.1139/b75-170
Borrel, M. N., Pereira, E., Fiallo, M., and Garnier-Suillerot, A. (1994). Mobile ionophores are novel class of P-glycoprotein inhibitors. J. Biochem. 223, 125–133.
Callewaert, D. M., Radcliff, G., Tanouchi, Y., and Shichi, H. (1988). Tetranactin, a macrotetrolide antibiotic, suppresses in vitro proliferation of human lymphocytes and generation of cytotoxicity. Immunopharmacology 16, 25–32. doi: 10.1016/0162-3109(88)90047-1
Dame, Z. T., Islam, M. T., Helmke, E., von Tiedemann, A., and Laatsch, H. (2016). Oligomycins and pamamycin homologs impair motility and induce lysis of zoospores of the grapevine downy mildew pathogen, Plasmopara viticola. FEMS Microbiol. Lett. 363:fnw167. doi: 10.1093/femsle/fnw167
Dick, M. W. (2001). “The Peronosporomycetes,” in The Mycota VII, Part A, Systematics and Evolution, eds D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke (Berlin: Springer-Verlag), 39–72.
Dominguez, J., Dunitz, D., Gerlach, H., and Preleog, V. (1962). Stoffwechselprodukte von Actinomyceten: über die Konstitution von Nonactin. Helv. Chim. Acta 45, 129–138.
Graven, S. N., Lardy, H. A., and Estrada, O. S. (1967). Antibiotics as tools for metabolic studies. VIII. Effect of nonactin homologs on alakali metal cations transport and rate of respiration in mitochondria. Biochemistry 6, 365–371. doi: 10.1021/bi00854a001
Graven, S. N., Lardy, H. A., Johnson, D., and Rutter, A. (1966). Antibiotics as tools for metabolic studies. V. Effect of nonactin, monactin, dinactin, and trinactin on oxidative phosphorylation and adenosine triphosphatase induction. Biochemistry 5, 1729–1735. doi: 10.1021/bi00869a040
Haneda, M., Nawata, Y., Hayashi, T., and Ando, K. (1974). Tetranactin, a new miticidal antibiotic. VI Determination of dinactin, trinactin, and tetranactin in their mixtures by NMR spectroscopy. J. Antibiot. 27, 555–557. doi: 10.7164/antibiotics.27.555
Hardham, A. R., and Hyde, G. J. (1997). Asexual sporulation in the oomycetes. Adv. Bot. Res. 24, 353–398. doi: 10.1016/S0065-2296(08)60079-8
Hashida, J., Niitsuma, M., Iwatsuki, M., Mori, M., Ishiyama, A., Namatame, M., et al. (2012). Panowamycin A and B, new antitrypanosomal isochromans produced by Streptomyces sp. K07-0010. J. Antibiot. (Tokyo). 65, 197–202. doi: 10.1038/ja.2011.139
Holker, U., Ersek, T., and Hofer, M. (1993). Changes in ion fluxes and the energy demand during spore development in Phytophthora infestans. Folio Microbiol. 38, 193–200. doi: 10.1007/BF02814376
Islam, M. T. (2008). Disruption of ultrastructure and cytoskeletal network is involved with biocontrol of damping-off pathogen Aphanomyces cochlioides by Lysobacter sp. strain SB-K88. Biol. Control 46, 312–321. doi: 10.1016/j.biocontrol.2008.02.006
Islam, M. T. (2011). “Potentials for biological control of plant diseases by Lysobacter spp., with special reference to strain SB-K88,” in Bacteria in Agrobiology: Plant Growth Responses, ed D. K. Maheshwari (Berlin; Heidelberg: Springer), 335–365.
Islam, M. T., Hashidoko, Y., Deora, A., Ito, T., and Tahara, S. (2005). Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes. Appl. Environ. Microb. 71, 3786–3796. doi: 10.1128/AEM.71.7.3786-3796.2005
Islam, M. T., Hashidoko, Y., Ito, T., and Tahara, S. (2004). Interruption of the homing sequence of phytopathogenic Aphanomyces cochlioides zoospores by secondary metabolites from nonhost Amaranthus gangeticus. J. Pestic. Sci. 29, 6–14. doi: 10.1584/jpestics.29.6
Islam, M. T., Ito, T., Sakasal, M., and Tahara, S. (2002a). Zoosporicidal activity of polyflavonoid tannin identified in Lannea coromandelica stem bark against phytopathogenic oomycete Aphanomyces cochlioides. J. Agric. Food Chem. 50, 6697–6703. doi: 10.1021/jf020554g
Islam, M. T., Ito, T., and Tahara, S. (2002b). Microscopic studies on attachment and differentiation of zoospores of the phytopathogenic fungus Aphanomyces cochlioides. J. Gen. Plant Pathol. 68, 111–117. doi: 10.1007/PL00013063
Islam, M. T., Ito, T., and Tahara, S. (2003). Host-specific plant signal and G-protein activator, mastoparan, triggers differentiation of zoospores of the phytopathogenic oomycete Aphanomyces cochlioides. Plant Soil 255, 131–142. doi: 10.1023/A:1026114731718
Islam, M. T., and Tahara, S. (2001). Chemotaxis of fungal zoospores, with special reference to Aphanomyces cochlioides. Biosci. Biotechnol. Biochem. 65, 1933–1948. doi: 10.1271/bbb.65.1933
Islam, M. T., and von Tiedemann, A. (2008). Zoosporogenesis and differentiation of grapevine downy mildew pathogen Plasmopara viticola in host-free system. Phytopathology 98, S72.
Islam, M. T., and von Tiedemann, A. (2011). 2,4-Diacetylphloroglucinol suppresses zoosporogenesis and impairs motility of the Peronospotomycete zoospores. World J. Microbiol. Biotechnol. 27, 2071–2079. doi: 10.1007/s11274-011-0669-7
Islam, M. T., von Tiedemann, A., and Laatsch, H. (2011). Protein kinase C is likely to be involved in zoosporogenesis and maintenance of flagellar motility in the peronosporomycete zoospores. Mol. Plant-Microbe Interact. 8, 938–947. doi: 10.1094/MPMI-12-10-0280
Jizba, J., Přikrylová, V., Ujhelyiová, L., and Varkonda, Š. (1992) Insecticidal properties of nonactic acid homononactic acid, the precursors of macrotetrolide antibiotics. Folia Microbiol. 37, 299–303. doi: 10.1007/BF02814568
Judelson, H. S., and Blanco, F. A. (2005). The spores of Phytophthora: weapons of plant destroyer. Nat. Rev. Microbiol. 3, 47–58. doi: 10.1038/nrmicro1064
Kiefer, B., Riemann, M., Büche C, Kassemeyer, H. H., and Nick, P. (2002). The host guides morphogenesis and stomatal targeting in the grapevine pathogen Plasmopara viticola. Planta 215, 387–393. doi: 10.1007/s00425-002-0760-2
Kusche, B. R., Smith, A. E., McGuirl, M. A., and Priestley, N. D. (2009). Alternating pattern of stereochemistry in the nonactin macrocycle is required for antibacterial activity and efficient ion binding. J. Am. Chem. Soc. 131, 17155–17165. doi: 10.1021/ja9050235
Latijnhouwers, M., Ligterink, W., Vleeshouwers, V. G. A. A., van West, P., and Govers, F. A. (2004). G-alpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans. Mol. Microbiol. 51, 925–936. doi: 10.1046/j.1365-2958.2003.03893.x
Mahmoud, K. A. S. (2008). Nafisamycin, Cyclisation Product of a New Enediyne Precursor, Highly Cytotoxic Mansuramycins, Karamycins, Possessing a Novel Heterocyclic Skeleton and Further Unusual Secondary Metabolites from Terrestrial and Marine Bacteria. Ph D. dissertation, Georg-August-Universitaet Goettingen.
Meyers, E., Pansy, F. E., Perlman, D., Smith, D. A., and Weisenborn, F. L. (1965). The in vitro activity of nonactin and its homologs: monactin, dinactin, and trinactin. J. Antibiot. 18, 128–129.
Rahman, M. H. (2008). Unusual Sesquiterpenes: Gorgonenes and Further Bioactive Secondary Metabolites Derived from Marine and Terrestrial Bacteria. Ph D. dissetation, Georg-August-Universitaet Goettingen.
Riemann, M., Büche, C., Kassemmeyer, H.-H., and Nick, P. (2002). Cytoskeletal responses during early development of the downy mildew of grapevine (Plasmopara viticola). Protoplasma 219, 13–22. doi: 10.1007/s007090200001
Sobolevskaya, M. P., Fotso, S., Havash, U., Denisenko, V. A., Helmke, E., Prokofeva, N. G., et al. (2004). Metabolites of the sea isolate of bacteria Streptomyces sp. 6167. Chem. Nat. Comp. 40, 282–285. doi: 10.1023/B:CONC.0000039143.59854.6e
Stienen, G. J., Kiers, J. L., Bottinelli, R., and Reggiani, C. (1996). Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J. Physiol. 493, 503–519. doi: 10.1113/jphysiol.1996.sp021384
Talontsi, F. M., Facey, P., Tatong, M. D., Islam, M. T., Frauendorf, H., Draeger, S., et al. (2012a). Zoosporicidal metabolites from an endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii. Phytochemistry 83, 87–94. doi: 10.1016/j.phytochem.2012.06.006
Talontsi, F. M., Islam, M. T., Facey, P., Douanla-Meli, C., von Tiedemann, A., and Laatsch, H. (2012b). Depsidones and other constituents from Phomopsis sp. CAFT69 and its host plant Endodesmia calophylloides with potent inhibitory effect on motility of zoospores of grapevine pathogen Plasmopara viticola. Phytochem. Lett. 5, 657–664. doi: 10.1016/j.phytol.2012.06.017
Tareq, F. S., Hasan, C. M., Lee, M. A., Islam, M. T., Lee, H. S., Lee, Y. J., et al. (2014). Gageotetrins A-C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 16, 928–931. doi: 10.1021/ol403657r
Walczak, R. J., Woo, A. J., Strohl, W. R., and Priestley, N. D. (2000). Nonactin biosynthesis: the potential nonactin biosynthesis gene cluster contains type II polyketide synthase-like genes. FEMS Microbiol. Lett. 183, 171–175. doi: 10.1111/j.1574-6968.2000.tb08953.x
Zinad, D. S., Shaaban, K. A., Abdalla, M. A., Islam, M. T., Schüffler, A., and Laatsch, H. (2011). Bioactive isocoumarins from terrestrial Streptomyces sp. ANK302. Nat. Prod. Commun. 6, 45–48.
Zizka, Z. (1998). Biological effects of macrotetrolide antibiotics and nonactic acids. Folia Microbiol. 43, 7–14. doi: 10.1007/BF02815533
Keywords: ionophore, dinactin, zoosporocides, mitochondrial ATPase activity, biological control
Citation: Islam MT, Laatsch H and von Tiedemann A (2016) Inhibitory Effects of Macrotetrolides from Streptomyces spp. On Zoosporogenesis and Motility of Peronosporomycete Zoospores Are Likely Linked with Enhanced ATPase Activity in Mitochondria. Front. Microbiol. 7:1824. doi: 10.3389/fmicb.2016.01824
Received: 27 June 2016; Accepted: 31 October 2016;
Published: 18 November 2016.
Edited by:
Learn-Han Lee, Monash University Malaysia Campus, MalaysiaReviewed by:
Esteban Marcellin, University of Queensland, AustraliaMika Tapio Tarkka, Helmholtz Centre for Environmental Research (HZ), Germany
Janice Lorraine Strap, University of Ontario Institute of Technology, Canada
Copyright © 2016 Islam, Laatsch and von Tiedemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Tofazzal Islam, tofazzalislam@yahoo.com
 Md. Tofazzal Islam
Md. Tofazzal Islam Hartmut Laatsch3
Hartmut Laatsch3 Andreas von Tiedemann
Andreas von Tiedemann