Aluminum and its potential contribution to Alzheimer's disease (AD)
- 1LSU Neuroscience Center, Louisiana State University Health Sciences Center, Louisiana State University, New Orleans, LA, USA
- 2Department of Ophthalmology, Louisiana State University Health Sciences Center, Louisiana State University, New Orleans, LA, USA
- 3Department of Microbiology, Louisiana State University Health Sciences Center, Louisiana State University, New Orleans, LA, USA
- 4Departments of Physiology and Obstetrics and Gynaecology, University of Toronto, Toronto, ON, Canada
- 5Neurogenetics Laboratory, Surrey Place Centre, Toronto, ON, Canada
- 6Department of Neurology, Louisiana State University Health Sciences Center, New Orleans, LA, USA
Alzheimer's disease (AD) is perhaps the principal example of cognitive failure in humans, and currently over 5.5 million Americans suffer from this incapacitating and progressive disorder of thought, reasoning and memory. Our laboratory has been evaluating the potential contribution of environmentally bioavailable neurotoxic metals to the onset, development and progression of AD for about 30 years (Lukiw et al., 1987). Largely because of its known multiple and potent neurotoxic effects, much of our research has focused on the potential contribution of aluminum to the AD process: (i) because of aluminum's remarkable abundance and bioavailability in the biosphere—in fact it is the most abundant naturally occurring neurotoxic element to which we are exposed; (ii) because of aluminum's remarkable cellular toxicity and genotoxicity at low nanomolar concentrations toward brain genetic processes, and (iii) because of aluminum's highly structured, specific and unique interactions with the phosphate-rich nucleic acids associated with the expression of genetic information in the human brain (Lukiw et al., 1989; Bryant et al., 2004; Alexandrov et al., 2005, 2013; Lukiw and Pogue, 2007; Pogue et al., 2009, 2012; Lukiw, 2010; Percy et al., 2011; Bhattacharjee et al., 2013; De Sole et al., 2013).
Aluminum's contribution to AD is based upon at least seven independently derived observations: (i) that at physiologically realistic concentrations, aluminum strongly promotes amyloid aggregation and accumulation, a key feature of AD neuropathology (Exley, 2005; Rodella et al., 2008; Walton and Wang, 2009; Yumoto et al., 2009); (ii) that both in vitro and in vivo aluminum promotes inflammatory signaling via the pro-inflammatory transcription factor NF-kB, another prominent feature characteristic of AD brain (Bondy, 2013; Walton, 2013); (iii) that out of the many thousands of brain gene messenger RNA (mRNAs) and micro RNAs (miRNAs), the family of mRNAs and miRNAs induced by aluminum are also strikingly similar to those found to be increased in AD; (iv) that in transgenic animal models of AD dietary aluminum enhances the development of pathological markers such as lipid peroxidation, oxidative stress, apoptosis, and gene expression deficits (Praticò et al., 2002; Bharathi et al., 2008; Zhang et al., 2012); (v) that many of the observed deficits in AD such as chromatin compaction, impaired energy utilization, impaired signaling involving chemical messengers such as adenine triphosphate (ATP) are recapitulated in aluminum-treated cellular or animal models of AD (Alexandrov et al., 2005; Lukiw and Pogue, 2007; Pogue et al., 2012; Bhattacharjee et al., 2013); (vi) that a very significant number of studies link the amount of aluminum in drinking water to the incidence of AD [worldwide, aluminum is added to drinking water as hydrated aluminum potassium sulfate KAl(SO4)2·12H2O, or alum, as a clarification and “finishing” agent (Flaten, 2001; Frisardi et al., 2010; Walton, 2013)], and (vii) perhaps most importantly, that of all pharmaceutical treatment approaches directed against AD to date, chelation using the anti-oxidant and trivalent iron/aluminum chelator desferrioxamine has been shown to be one of the most effective therapeutic strategies yet devised (Crapper McLachlan et al., 1991; Percy et al., 2011).
Abundant research indicates that aluminum is a particularly reactive metal toward multiple aspects of human neurobiology and the altered genetics that are associated with the development and propagation of sporadic AD (Lukiw et al., 1989; Lukiw, 2010; Bhattacharjee et al., 2013; Bondy, 2013; Shaw and Tomljenovic, 2013; Walton, 2013). Thirty years of research since the potent effects of aluminum on the genetic apparatus in AD were first described, the most recent evidence suggests a strong linkage between aluminum sulfates and induction of NF-kB-sensitive pro-inflammatory miRNAs (Lukiw et al., 1987; Alexandrov et al., 2013; Zhao et al., 2013). Aluminum has been previously shown to significantly induce the transcription factor NF-kB (Pogue et al., 2009; Bondy, 2013), and up-regulation of NF-kB drives synthesis of NF-kB-sensitive miRNAs which in turn down regulate the expression of many AD-relevant genes, including complement factor H (CFH) and neurotropic signaling in human brain cells (Pogue et al., 2009; Zhao et al., 2013).
We would like here to briefly include some recent genetic data on aluminum and its effects on miRNA abundance in a highly relevant transgenic animal model for AD that shows strong parallels to miRNA profiles which are found in AD brain (Figure 1). There are currently over 90 transgenic mouse models of AD (http://www.alzforum.org/research-models/). A commonly used Tg2576 mouse model overexpresses a mutant form of beta amyloid precursor protein (β APP), APPK670/671L, linked to early-onset familial AD, and develops amyloid plaques and progressive cognitive deficits as the mice age. Tg2576 mice exposed to dietary aluminum have been shown to develop oxidative stress and robust amyloidogenesis, key features of AD neuropathology (Praticò et al., 2002). Animals provided a 2 mg/kg aluminum-supplemented diet were analyzed for miRNA speciation and complexity in their brains using GeneChip and miRNA array technologies; intriguingly, the same quintet of up-regulated pro-inflammatory miRNAs (miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155) as found in (i) AD and (ii) in aluminum-treated human brain cells in primary culture were also found to be amongst the most up-regulated in these aluminum-supplemented Tg2576 mice (Alexandrov et al., 2005; Lukiw and Pogue, 2007; Pogue et al., 2012; Bhattacharjee et al., 2013; Hill et al., 2014; Figure 1; unpublished observations). Up-regulated miRNAs are known to target susceptible mRNAs and down-regulate the expression of many AD-relevant brain genes as is widely observed in AD brain tissues (Colangelo et al., 2002; Guo et al., 2010; Ginsberg et al., 2012). Interestingly, these findings suggest some common miRNA-induced mechanism between two important in vitro and in vivo models for AD with AD itself. Indeed, the abundance of specific miRNAs are highly selective, and potential indicators and predictors of human health and disease, including progressive neurological disorders such as AD (Alexandrov et al., 2005, 2013; Lukiw and Pogue, 2007; Pogue et al., 2009; Maciotta et al., 2013; Zhao et al., 2013).
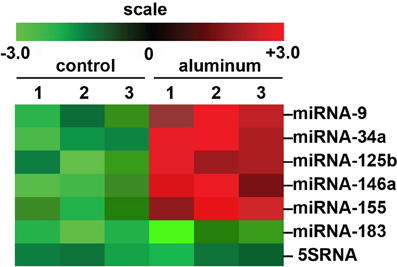
Figure 1. Array-based cluster analysis of miRNA abundance in aluminum-fed Tg2576 mice vs. controls. In these experiments the brain (cortex) of 3 month-old Tg2576 mice fed aluminum-enriched diets were analyzed for miRNA speciation compared to age-matched controls (receiving standard diets); methodologies for aluminum-treatment of transgenic animals have been previously described in detail (Praticò et al., 2002; Zhang et al., 2012). Aluminum treatment of other transgenic murine AD models such as the amyloid over-expressing APP/PS1 show similar intensification of AD-type changes (Zhang et al., 2012). The up-regulation of the pro-inflammatory quintet of miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155, depicted here, were amongst the most significantly increased miRNAs found to be 2- to 5-fold above normal diet, age-matched controls (compared to an unchanging internal control miRNA-183 and 5SRNA in the same samples). The results strongly suggest a potential contribution of aluminum to the AD processes associated with miRNA-mediated down-regulation of gene expression in the sporadic AD brain as is widely observed (Colangelo et al., 2002; Lukiw and Pogue, 2007; Ginsberg et al., 2012; Pogue et al., 2012; Alexandrov et al., 2013). Importantly, other common environmental neurotoxic divalent metals such as calcium, cadmium, copper, iron (2+), mercury, nickel, and lead, and neurotoxic trivalent metals such as boron, chromium, gadolinium, indium, iron (3+), and yttrium do not exhibit this potentially pathogenic effect (Walker et al., 1989; Alexandrov et al., 2005; Lukiw, 2010; unpublished observations); N = 3 control and N = 3 aluminum-treated mice; methodologies and data analysis have been extensively described elsewhere (Lukiw and Pogue, 2007; Alexandrov et al., 2013).
Lastly, more research into the potential contribution of aluminum to the AD process is clearly warranted. There are currently no treatments for AD that effectively prevent or cure AD's insidious onset or propagation. We think it important to emphasize that the most effective clinical treatment yet devised for moderate- to late-stage AD patients was the implementation of the first generation anti-oxidant and trivalent iron/aluminum chelator desferrioxamine to attempt to remove aluminum from the brains of AD patients (Crapper McLachlan et al., 1991; Percy et al., 2011). Second generation aluminum chelators such as Feralex-G, either alone or in combination with other chelators, has shown higher specificity, increased selectivity and higher efficacy in aluminum sequestration and chelation in preliminary in vitro studies (Kruck et al., 2004, 2008; Percy et al., 2011; unpublished observations). Certainly, laboratory experimentation in cultured primary human brain cells, in transgenic AD animal models, and clinical studies employing next-generation aluminum chelators, perhaps in combination with other drug strategies, are one of the research areas needing more focused attention—to more effectively address the exact role and mechanism of aluminum neurotoxicity in this rapidly expanding healthcare concern.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
These studies were presented in part at the Tenth Keele Meeting on Aluminum, Winchester England, 23–27 February 2013. Research on human brain and retinal RNA in the Lukiw laboratory involving non-coding RNA, miRNA, the innate-immune response in AD, metal-induced neurotoxicity, amyloidogenesis and neuro-inflammation was supported through a COBRE III Pilot Project, an unrestricted Departmental grant from Research to Prevent Blindness (RPB) and NIH grants NEI EY006311 and NIA AG038834.
References
Alexandrov, P. N., Zhao, Y., Jones, B. M., Bhattacharjee, S., and Lukiw, W. J. (2013). Expression of the phagocytosis-essential protein TREM2 is down-regulated by an aluminum-induced miRNA-34a in a microglial cell line. J. Inorg. Biochem. 128, 267–269. doi: 10.1016/j.jinorgbio.2013.05.010
Alexandrov, P. N., Zhao, Y., Pogue, A. I., Tarr, M. A., Kruck, T. P., Percy, M. E., et al. (2005). Synergistic effects of iron and aluminum on stress-related gene expression in primary human neural cells. J. Alzheimers Dis. 8, 117–127; discussion 209–215.
Bharathi, V., Vasudevaraju, P., Govindaraju, M., Palanisamy, A. P., Sambamurti, K., and Rao, K. S. (2008). Molecular toxicity of aluminium in relation to neurodegeneration. Indian J. Med. Res. 128, 545–556.
Bhattacharjee, S., Zhao, Y., Hill, J. M., Culicchia, F., Kruck, T. P., Percy, M. E., et al. (2013). Selective accumulation of aluminum in cerebral arteries in Alzheimer's disease. J. Inorg. Biochem. 126, 35–37. doi: 10.1016/j.jinorgbio.2013.05.007
Bondy, S. C. (2013). Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology 315, 1–7. doi: 10.1016/j.tox.2013.10.008
Bryant, P. L., Lukiw, W. J., Gan, Z., Hall, R. W., and Butler, L. G. (2004). High-field 19.6T 27Al solid-state MAS NMR of in vitro aluminated brain tissue. J. Magn. Reson. 170, 257–262. doi: 10.1016/j.jmr.2003.12.013
Colangelo, V., Schurr, J., Ball, M. J., Pelaez, R. P., Bazan, N. G., and Lukiw, W. J. (2002). Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 70, 462–473. doi: 10.1002/jnr.10351
Crapper McLachlan, D. R., Dalton, A. J., Kruck, T. P., Bell, M. Y., Smith, W. L., Kalow, W., et al. (1991). Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337, 1304–1308. doi: 10.1016/0140-6736(91)92978-B
De Sole, P., Rossi, C., Chiarpotto, M., Ciasca, G., Bocca, B., Alimonti, A., et al. (2013). Possible relationship between Al/ferritin complex and Alzheimer's disease. Clin. Biochem. 46, 89–93. doi: 10.1016/j.clinbiochem.2012.10.023
Exley, C. (2005). The aluminium-amyloid cascade hypothesis and Alzheimer's disease. Subcell. Biochem. 38, 225–234. doi: 10.1007/0-387-23226-5_11
Flaten, T. P. (2001). Aluminium as a risk factor in Alzheimer's disease. Brain Res. Bull. 55, 187–196. doi: 10.1016/S0361-9230(01)00459-2
Frisardi, V., Solfrizzi, V., Capurso, C., Kehoe, P. G., Imbimbo, B. P., Santamato, A., et al. (2010). Aluminum in the diet and Alzheimer's disease: from current epidemiology to possible disease-modifying treatment. J. Alzheimers Dis. 20, 17–30. doi: 10.3233/JAD-2009-1340
Ginsberg, S. D., Alldred, M. J., and Che, S. (2012). Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer's disease. Neurobiol. Dis. 45, 99–107. doi: 10.1016/j.nbd.2011.07.013
Guo, H., Ingolia, N. T., Weissman, J. S., and Bartel, D. P. (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840. doi: 10.1038/nature09267
Hill, J. M., Zhao, Y., Bhattacharjee, S., and Lukiw, W. J. (2014). miRNAs and viroids utilize common strategies in genetic signal transfer. Front. Mol. Neurosci. 7:10. doi: 10.3389/fnmol.2014.00010
Kruck, T. P., Cui, J. G., Percy, M. E., and Lukiw, W. J. (2004). Molecular shuttle chelation: the use of ascorbate, desferrioxamine and Feralex-G in combination to remove nuclear bound aluminum. Cell. Mol. Neurobiol. 24, 443–459. doi: 10.1023/B:CEMN.0000022773.70722.b2
Kruck, T. P., Percy, M. E., and Lukiw, W. J. (2008). Metal sulfate-mediated induction of pathogenic genes and repression by phenyl butyl nitrone and Feralex-G. Neuroreport 19, 245–249. doi: 10.1097/WNR.0b013e3282f4cb7e
Lukiw, W. J. (2010). Evidence supporting a biological role for aluminum in brain chromatin compaction and epigenetics. J. Inorg. Biochem. 104, 1010–1012. doi: 10.1016/j.jinorgbio.2010.05.007
Lukiw, W. J., Kruck, T. P., and McLachlan, D. R. (1987). Alterations in human linker histone-DNA binding in the presence of aluminum salts in vitro and in Alzheimer's disease. Neurotoxicology 8, 291–301.
Lukiw, W. J., Kruck, T. P., and McLachlan, D. R. C. (1989). Aluminium and the nucleus of nerve cells. Lancet 1, 781. doi: 10.1016/S0140-6736(89)92596-8
Lukiw, W. J., and Pogue, A. I. (2007). Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J. Inorg. Biochem. 101, 1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004
Maciotta, S., Meregalli, M., and Torrente, Y. (2013). The involvement of microRNAs in neurodegenerative diseases. Front. Cell Neurosci. 7:265. doi: 10.3389/fncel.2013.00265
Percy, M. E., Kruck, T. P., Pogue, A. I., and Lukiw, W. J. (2011). Towards the prevention of potential aluminum toxic effects and an effective treatment for Alzheimer's disease. J. Inorg. Biochem. 105, 1505–1512. doi: 10.1016/j.jinorgbio.2011.08.001
Pogue, A. I., Jones, B. M., Bhattacharjee, S., Percy, M. E., Zhao, Y., and Lukiw, W. J. (2012). Metal-sulfate induced generation of ROS in human brain cells: detection using an isomeric mixture of 5- and 6-carboxy-2',7'-dichlorofluorescein diacetate (carboxy-DCFDA) as a cell permeant tracer. Int. J. Mol. Sci. 13, 9615–9626. doi: 10.3390/ijms13089615
Pogue, A. I., Li, Y. Y., Cui, J. G., Zhao, Y., Kruck, T. P., Percy, M. E., et al. (2009). Characterization of an NF-kB-regulated, miRNA-146a-mediated down-regulation of CFH in metal- stressed brain cells. J. Inorg. Biochem. 103, 1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012
Praticò, D., Uryu, K., Sung, S., Tang, S., Trojanowski, J. Q., and Lee, V. M. (2002). Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 16, 1138–1140. doi: 10.1096/fj.02-0012fje
Rodella, L. F., Ricci, F., Borsani, E., Stacchiotti, A., Foglio, E., Favero, G., et al. (2008). Aluminium exposure induces Alzheimer's disease-like histopathological alterations in mouse brain. Histol. Histopathol. 23, 433–439.
Shaw, C. A., and Tomljenovic, L. (2013). Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 56, 304–316. doi: 10.1007/s12026-013-8403-1
Walker, P. R., LeBlanc, J., and Sikorska, M. (1989). Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 28, 3911–3915. doi: 10.1021/bi00435a043
Walton, J. R. (2013). Aluminum involvement in the progression of Alzheimer's disease. J. Alzheimers Dis. 35, 7–43. doi: 10.3233/JAD-121909. Review Erratum in: J. Alzheimers Dis. (2013), 35:875.
Walton, J. R., and Wang, M. X. (2009). APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer's disease. J. Inorg. Biochem. 103, 1548–1554. doi: 10.1016/j.jinorgbio.2009.07.027
Yumoto, S., Kakimi, S., Ohsaki, A., and Ishikawa, A. (2009). Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer's disease. J. Inorg. Biochem. 103, 1579–1584. doi: 10.1016/j.jinorgbio.2009.07.023
Zhang, Q. L., Jia, L., Jiao, X., Guo, W. L., Ji, J. W., Yang, H. L., et al. (2012). APP/PS1 transgenic mice treated with aluminum: an update of Alzheimer's disease model. Int. J. Immunopathol. Pharmacol. 25, 49–58.
Zhao, Y., Bhattacharjee, S., Jones, B. M., Hill, J., Dua, P., and Lukiw, W. J. (2013). Regulation of neurotropic signaling by the inducible, NF-kB-sensitive miRNA-125b in Alzheimer's disease (AD) and in primary human neuronal-glial (HNG) cells. Mol Neurobiol. doi: 10.1007/s12035-013-8595-3. [Epub ahead of print].
Keywords: aluminum, Alzheimer's disease, micro RNA (miRNA), Tg2576, genetic variability, Feralex-G, desferrioxamine
Citation: Bhattacharjee S, Zhao Y, Hill JM, Percy ME and Lukiw WJ (2014) Aluminum and its potential contribution to Alzheimer's disease (AD). Front. Aging Neurosci. 6:62. doi: 10.3389/fnagi.2014.00062
Received: 06 February 2014; Accepted: 21 March 2014;
Published online: 08 April 2014.
Edited by:
Paul Adlard, The Mental Health Research Institute, AustraliaReviewed by:
Kurt A. Jellinger, Institute of Clinical Neurobiology, AustriaJ. R. Walton, University of New South Wales Faculty of Medicine, Australia
Copyright © 2014 Bhattacharjee, Zhao, Hill, Percy and Lukiw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: wlukiw@lsuhsc.edu
 Surjyadipta Bhattacharjee
Surjyadipta Bhattacharjee Yuhai Zhao
Yuhai Zhao James M. Hill1,2,3
James M. Hill1,2,3  Walter J. Lukiw
Walter J. Lukiw