Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults
- 1Laboratory for Complex Brain Networks, Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 2Neuroscience Program, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 3Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 4MD Program, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 5Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC, USA
- 6Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC, USA
Recent census data has found that roughly 40% of adults 65 years and older not only consume alcohol but also drink more of it than previous generations. Older drinkers are more vulnerable than younger counterparts to the psychoactive effects of alcohol due to natural biological changes that occur with aging. This study was specifically designed to measure the effect of long-term moderate alcohol consumption on cognitive health in older adult drinkers. An extensive battery of validated tests commonly used in aging and substance use literature was used to measure performance in specific cognitive domains, including working memory and attention. An age (young, old) * alcohol consumption (light, moderate) factorial study design was used to evaluate the main effects of age and alcohol consumption on cognitive performance. The focus of the study was then limited to light and moderate older drinkers, and whether or not long-term moderate alcohol consumption exacerbated age-related cognitive decline. No evidence was found to support the idea that long-term moderate alcohol consumption in older adults exacerbates age-related cognitive decline. Findings were specific to healthy community dwelling social drinkers in older age and they should not be generalized to individuals with other consumption patterns, like heavy drinkers, binge drinkers or ex-drinkers.
Introduction
Alcohol consumption among older adults is on the rise in the United States. To illustrate this point, the cumulative probability of exposure to alcohol before the age of 100 years old was confined to approximately 50% of the US population between the years of 1894 and 1937, and in 1974 it rose to 75% by the age of 30 years old (USDA and USDHHS, 2010; Wang and Andrade, 2013). The most recent National Survey on Drug Use and Health conducted in 2012 found that approximately 40% of the estimated 43 million older adults in the US consumed at least one alcoholic drink within the last month and that the majority of these individuals were neither heavy drinkers nor binge drinkers (SAMHSA, 2013). In fact, findings from the National Longitudinal Alcohol Epidemiologic Survey (NLAES) (NIAAA, 1998) and the National Epidemiologic Survey on Alcohol and Alcohol Related Conditions (NESARC) (Fryar et al., 2006; NIAAA, 2006) show that the majority of older American drinkers consume light (≤3 drinks/week) to moderate (7–14 drinks/week) amounts of alcohol. Potential reasons for an overall increase in moderate alcohol consumption may stem from the belief that exposure to moderate levels of alcohol is associated with psychological (Peele and Brodsky, 2000) and physiological (Marchi et al., 2014) benefits as well as a lower risk of developing dementia syndromes (Panza et al., 2009, 2012).
In today's world, older adults enjoy longer lives and rely on their cognitive abilities for many years prior to the debilitating losses in brain function associated with dementia (Plassman et al., 2007; WHO, 2012). Older adults, however, are still subject to significant biological changes that occur with normal aging and that make them particularly vulnerable to alcohol exposure. For example, increases in the proportion of fat to water and decreases in the rate of liver metabolism expose older adults to higher blood alcohol content (BAC) levels relative to young adults with similar overall body mass (Vestal et al., 1977). Ethanol, which acts directly on the central nervous system, has psychoactive properties that are known to significantly affect behaviors like attention, working and short-term memory (Guillot et al., 2010; Dry et al., 2012; Magrys and Olmstead, 2014). These neurologic effects can be transient and reversible in the case of acute alcohol exposure (Gilbertson et al., 2009; Sklar et al., 2012). On the other hand, problematic drinking habits like those practiced by older heavy drinkers and alcoholics have been firmly associated with severe cognitive deficits that can persist even after years of abstinence (Thomas and Rockwood, 2001; Peters et al., 2008).
It is not clear, however, whether or not long-term moderate (i.e., social) drinking affects age-related cognitive decline. Research addressing this topic is primarily epidemiologic in nature and dominated by analyses that use secondary outcome measures from large-scale clinical trials, like the Framingham Heart Study (Elias et al., 1999), the Seattle Longitudinal Study (Zanjani et al., 2013) and the Women's Health Initiative (Espeland et al., 2005, 2006). These and similar trials collected summary measures related to alcohol consumption and overall cognitive health but were not specifically designed to measure the effect of moderate alcohol consumption on age-related cognitive decline as the primary outcome. See reviews for more detail (Solfrizzi et al., 2007; Peters et al., 2008; Panza et al., 2009, 2012; Kim et al., 2012).
This study was specifically designed to determine the effect of long-term moderate alcohol consumption on age-related cognitive decline in healthy community dwelling adults. A collection of validated tests commonly used in aging as well as substance use literature was used to evaluate attention, working memory, short-term memory, processing speed, planning, rule learning, and impulse control. The first study hypothesis was that younger drinkers would outperform older drinkers in all the cognitive domains tested. Heavy alcohol use in older age has been linked to severe deficits in cognitive health (Moriyama et al., 2006; Green et al., 2010) and because of this it was thought that the effect of alcohol consumption level on cognition would follow a continuum. Therefore, the second study hypothesis was that light older drinkers would outperform moderate older drinkers on all the cognitive domains tested. At present, there is no curative treatment or therapeutic approach to reversing age-related declines in cognitive function. Understanding whether or not older adults are more vulnerable to such declines as a result of long-term moderate alcohol consumption will help a growing segment of the older American population make informed decisions about their cognitive health.
Methods
Our main study question focused on the effect of moderate alcohol consumption on cognitive function in older adults, and to best address this question we adopted an age (young, old) * alcohol consumption (light, moderate) factorial study design. All participants were residents of Winston-Salem, North Carolina, and were recruited via local advertisements (physical flyers and internet) and by word-of-mouth. The Institutional Review Board of Wake Forest School of Medicine approved the study (IRB # 19961) and all participants gave written informed consent.
Study Population
A total of 63 individuals were enrolled in the study, and of these individuals 22 were younger adults (24–35 years old) and 41 were older adults (65–80 years old). The final study groups were as follows: 11 light young, 11 moderate young, 20 light old, and 21 moderate old drinkers. Census findings from the NLAES and the NESARC were used to help define light and moderate alcohol consumption criteria. Criteria were also defined such that light and moderate alcohol intake did not overlap. Light alcohol consumption was defined to be 1–8 drinks per month and did not exceed two drinks per week. Moderate alcohol consumption was defined as 7–21 drinks per week and did not exceed three drinks per day. Enrollment in the study was dependent on the self-report number of drinks per week as measured by the Time Line Follow Back (TLFB) questionnaire (Sobell and Sobell, 1992). Daily alcohol intake over 3 months was measured and only adults who reported maintaining either a light or moderate alcohol consumption pattern for at least 3 years were included.
Exclusion Criteria
Alcohol and other substance use
Participants were excluded if they reported more than one binge in the last 3 months (≥5 drinks for men and ≥4 drinks for women in less than 2 h) (NIAAA, 2004). Disqualification from the study also included a >0.00 alcohol reading as measured by an Intoxilyzer S-D5 breath alcohol screen (www.alcoholtest.com), or a positive test for illicit substance use as measured by an Alere iCassette 6 – panel drug screen at the start of any study visit (cocaine, THC, opiates, amphetamines, methamphetamines or benzodiazepines, www.alere.com). Use of illicit substances within the last 3 months was also grounds for disqualification. Subjects with a history of alcohol or drug abuse/dependence and/or current (within the last 6 months) Axis I disorders as determined by the Structured Interview for DSM Disorders (SCID) were also excluded (Fist et al., 2002). Individuals who consumed greater than 500 mg of caffeine per day were not included in this study so as to avoid the effects of excessive caffeine exposure or caffeine withdrawal on cognitive performance. Also, individuals who smoked ≤30 cigarettes (i.e., individuals defined by the World Health Organization as non-smokers – heavy smokers) were allowed to participate in the study so that during recruitment any potential confounding difference in cigarette smoking between light and moderate drinkers could be avoided.
Cognitive
Participants at high risk for dementia as measured by the Modified Mini Mental State Exam (3MSE score ≤ 80) (Jones et al., 2002), those with active neurological dysfunction that may affect cognitive processing (i.e., schizophrenia, Alzheimer's disease, Parkinson's disease, prior history of stroke, epilepsy, mental retardation and attention deficit-hyperactivity disorder) as well as those using antipsychotic and/or antiepileptic medications with known neurological/cognitive side effects were excluded. Individuals with previous brain surgery, serious CNS trauma as defined by a history of acquired sub- or epidural hematomas, or loss of consciousness for greater than 5 minutes were not included. Participants taking antidepressants were enrolled if they had been on stable treatment for more than 2 months and were not depressed according to the Center for Epidemiological Studies Depression Scale (CES-D) (Haringsma et al., 2004) or Profile of Moods Survey (POMS) (Shacham, 1983).
Physical
Participants with a body mass index (BMI kg/m2) (Keys et al., 1972) of less than 18 (normal) or greater than 35 (moderately obese) were excluded from the study in order to help control for the interaction of weight and alcohol metabolism (Sayon-Orea et al., 2011). Adults with diabetes (insulin-dependent) were also excluded, and individuals with high blood pressure were only included if they had been on stable treatment for at least 1 year (Hillbom et al., 2011). Other physical exclusions included visual acuity less than 20/40 (corrected), hearing loss, left-handed individuals, and positive pregnancy test results.
Procedure
Potential participants completed a phone screen with questions about their alcohol, drug and medication use. A modified version of the SHORT AUDIT-C (Babor et al., 2001) was administered as part of the phone screen. This modified version included Questions #1 and #2 of the original version but excluded Question #3. This question was removed because it directly related to binge drinking, which was assessed elsewhere in the phone screen. The sum of Questions #1 and #2 were used as an initial measure of alcohol consumption. Participants with a score between 1 and 2 were conditionally recruited as light drinkers, and a score between 4 and 6 as moderate drinkers. See Supplemental Figure 1 for additional scoring information. Participants were also asked how long they had maintained their particular drinking pattern and how long they had been exposed to alcohol. All participants were asked whether or not they would agree to submit to an alcohol and drug screen at the beginning of each study visit.
Individuals that met phone screen criteria were asked to come to the laboratory for formal screening. Participants were given time to record 3 months of alcohol consumption using the TLFB. A 12 oz. (beer), 5 oz. (wine), and a 1.5 oz. (hard liquor) glass were placed on the participant's desk; they were told that the glasses represented standard drink sizes. Participants were asked to use these glasses as reference when filling out the TLFB. The following were also administered during the formal screening visit: mood (POMS) and depression questionnaires (CES-D), vision and hearing tests, medical history and Axis I disorder assessments (SCID), and a dementia screen (3MSE).
Testing visit one
Participants that passed formal screening criteria were officially enrolled into the study and asked to return on a separate day for 2.5 h of cognitive testing separated by two 15-minute breaks. The domains that were tested included attention, working memory, short-term memory, processing speed, planning, rule learning, and motor control. For a list of the tasks and behavioral measures used to assess performance see Table 1A.
Testing visit two
Measures of performance on cognitive tests are the primary outcomes of this study. However, approximately one year after beginning recruitment the study became affiliated with a larger NIAAA-funded project grant focused on interactions of stress and anxiety with alcohol consumption. It was at that time that measures of stress, anxiety, and impulsivity were added as secondary outcome measures and used to more thoroughly characterize older adult drinkers (Table 1B). Of the 13 light older drinkers that had already completed Testing Visit One, 10 returned to complete measures of stress, anxiety and impulsivity as part of Testing Visit Two. All 14 moderate older drinkers that already completed Testing Visit One were able to return and completed Testing Visit Two. The TLFB was re-administered upon their return to laboratory to ensure that light and moderate drinkers had not altered their drinking habits. No significant differences in the number of drinks per week were found in either light or moderate drinkers between Testing Visit One and Testing Visit Two [Light Old: t(9) = −1.33, p = 0.22; Moderate Old: t(13) = 1.45, p = 0.16]. Individuals recruited after the addition of Testing Visit Two (seven light and seven moderate older drinkers) completed the entire study within one month of enrollment.
Table 1B. Questionnaires and measures used to assess stress, anxiety and impulsivity during Testing Visit Two.
Statistical Analysis
Multivariate analyses of covariance (MANCOVA) were used to assess the relationships between age (young, old) and alcohol consumption (light, moderate) with results from each cognitive test after controlling for four confounding variables: presence or absence of high blood pressure, BMI, total number of years consuming alcohol and the total number of years the individual maintained either a light or moderate alcohol consumption pattern. Additionally, sex differences were assessed and the drinking and age groups were collapsed across sex if found to be non-significant. Tests of normality were performed for each set of dependent variables (given the covariates) and any non-normally distributed variables were transformed appropriately (log transformed in our cases). Co-linearity and outlier assessments were also performed to ensure model validity. Step-down tests of the least square means were performed employing Tukey's adjustment for multiple comparisons if a significant main effect of alcohol, age or their interaction was found. All statistical analyses were performed using SAS software version 9.3.
Results
Demographics
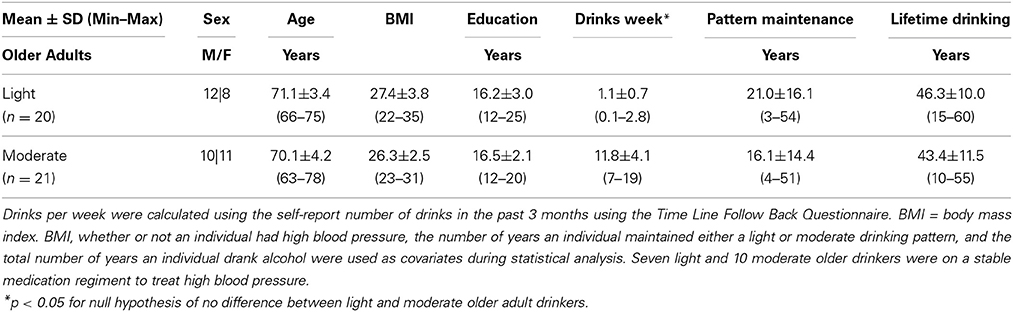
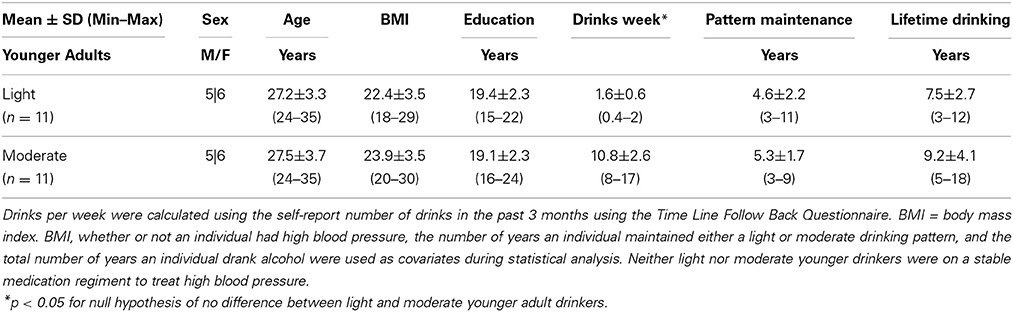
Sample characteristics for both light and moderate older drinkers can be found in Table 2. Light older drinkers consumed 1.1 ± 0.7 drinks per week and moderate older drinkers consumed 11.8 ± 4.1 drinks per week. An expected significant difference in the number of drinks per week consumed by light and moderate older drinkers was observed [t(39) = −11.6, p < 0.0001]. The two groups did not differ from each other in the total number of years they had maintained that consumption level [light: 21.0 ± 16.1 years, moderate: 16.1 ± 14.4 years; t(39) = 1.0, p = 0.31] or the total number of years they had been consuming alcohol [light: 46.3 ± 10.0 years, moderate: 43.4 ± 11.5 years; t(39) = 0.87, p = 0.39]. There were no statistical differences between light and moderate older drinkers in sex [light: 12 male, 8 female, moderate: 10 male, 11 female; χ2 = 0.63, p = 0.43], age [light: 71.1 ± 3.4 years old, moderate: 70.1 ± 4.2 years old; t(39) = 0.80, p = 0.43], BMI [light: 27.4 ± 3.8, moderate: 26.3 ± 2.5; t(39) = 1.12, p = 0.27], or years of education [light: 16.2 ± 3.0 years, moderate: 16.5 ± 2.1 years; t(39) = −0.40, p = 0.69]. Older drinkers scored similarly on depression [CES-D, light: 4.5 ± 3.6, moderate: 4.3 ± 4.9; t(39) = 0.12, p = 0.90] and mood screens [POMS, light: −3.4 ± 11.0, moderate: −1.6 ± 13.8; t(39) = −0.46, p = 0.65]. Older drinkers also performed similarly on the dementia screen [3MSE, light: 97.1 ± 3.4, moderate: 97.8 ± 3.0; t(39) = −0.71, p = 0.48]. No significant difference in the proportion of people on a stable regimen of prescription medication was found (light: 16/20, moderate: 17/21, χ2 = 0.01, p = 0.94). More specifically, no differences in the proportion of people on medication to treat high blood pressure (light: 7/20, moderate: 10/21, χ2 = 1.33, p = 0.25), high cholesterol (light: 4/20, moderate: 3/21, χ2 = 0.24, p = 0.67), Type II diabetes (light: 2/20, moderate: 1/21, χ2 = 0.42, p = 0.52) or depression were found (light:1/20, moderate: 5/21, χ2 = 2.9011, p = 0.09). Other commonly cited conditions that required prescription medication in both light and moderate older drinkers included hypothyroidism, arthritis, osteoporosis, and heartburn. In the light older drinker group, only one individual smoked (less than thirty cigarettes per day) and there were no ex-smokers. In the moderate older drinker group, no one actively smoked cigarettes; one individual was an ex-smoker.
For comparative purposes, sample characteristics for young adult drinkers can be found in Table 3. Light younger drinkers (27.2 ± 3.3 years old) consumed 1.6 ± 0.6 drinks per week and moderate younger drinkers (27.5 ± 3.7 years old) consumed 10.8 ± 2.6 drinks per week. These consumption patterns were statistically similar to the consumption patterns observed in light [t(29) = 1.89, p = 0.07] and moderate [t(30) = −0.70, p = 0.50] older adult drinker counterparts. The most notable medication taken by younger adult drinkers was birth control and none of the younger adult drinkers smoked cigarettes.
Cognitive Task Performance
Effects of age
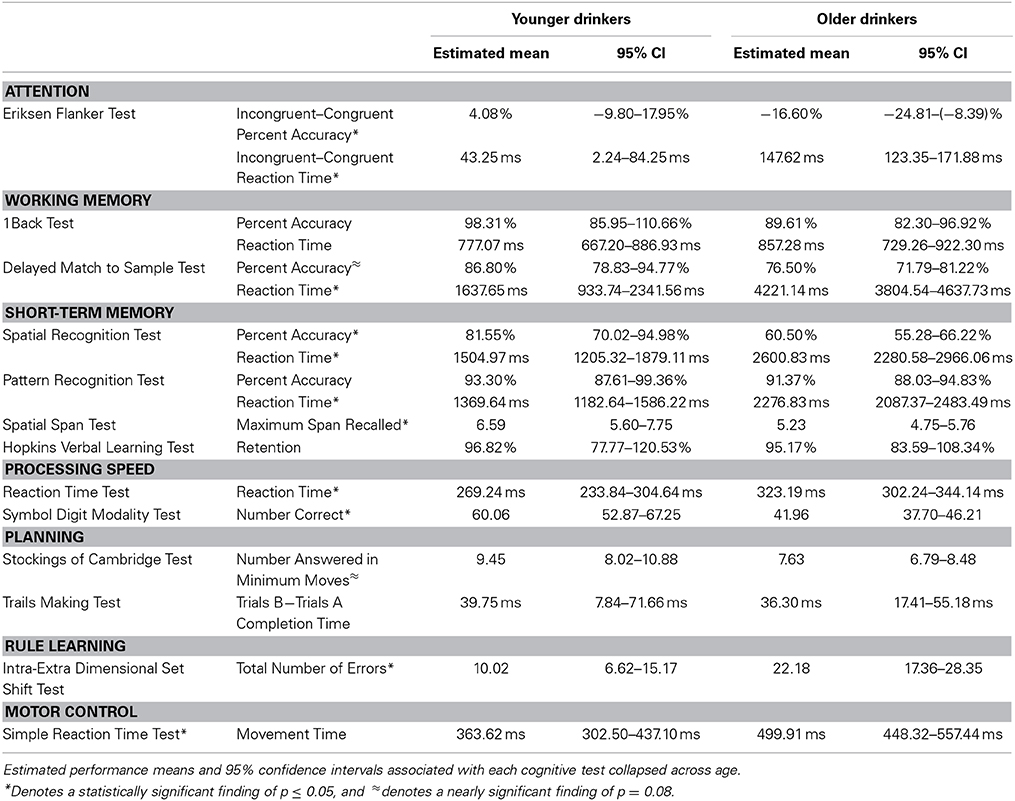
Age * alcohol consumption MANCOVA results are presented in Table 4. As expected, significant effects of age showed that younger adults outperformed older adults in several cognitive domains, including executive attention [F(54) = 7.25, p < 0.01], working-memory [F(52) = 7.29, p < 0.0001], short-term memory [F(50) = 4.58, p < 0.0001], processing speed [F(54) = 6.09, p = 0.01], and rule learning [F(52) = 9.73, p < 0. 01]. Younger adults trended toward faster movement times than older adults in the motor control domain [F(55) = 3.13, p = 0.08] but did similarly to older adults in the planning domain [F(54) = 1.31, p = 0.28]. See Table 5 for the estimated performance means and 95% confidence intervals for both younger and older drinkers.
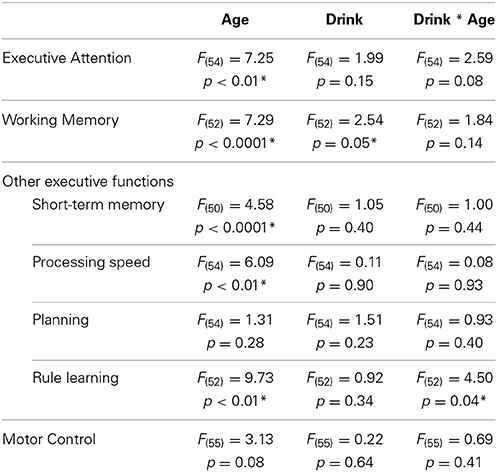
Table 4. Results of consumption level (low, moderate) * age group (young, old) MANCOVA analyses of cognitive performance.
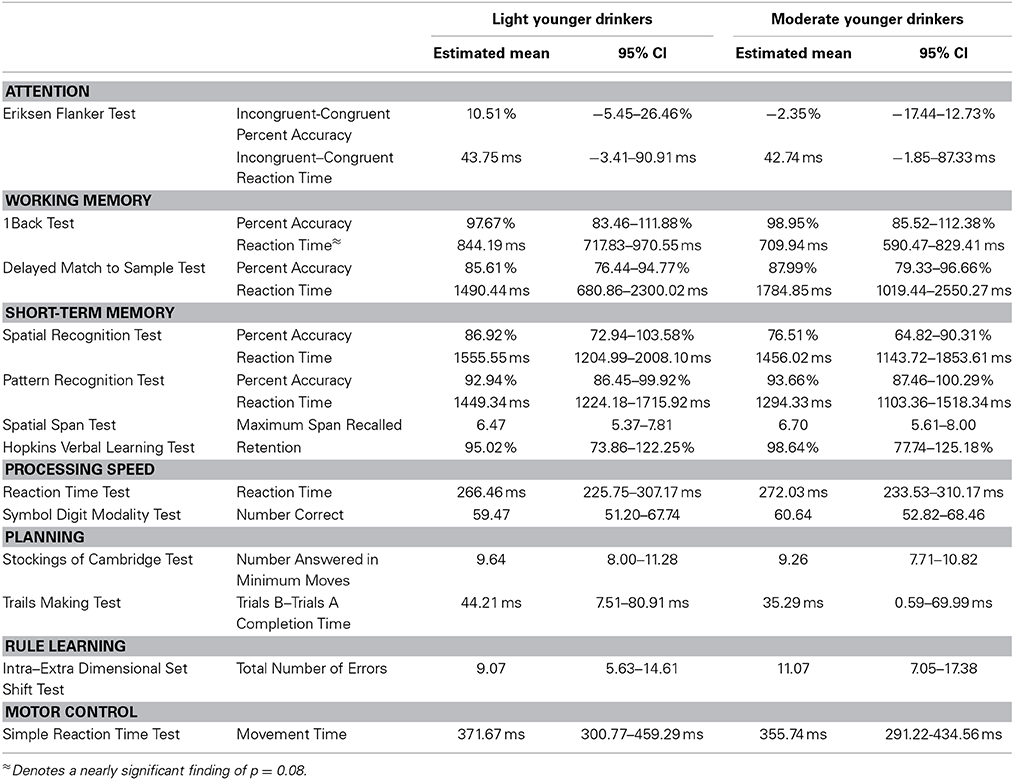
Effects of alcohol consumption
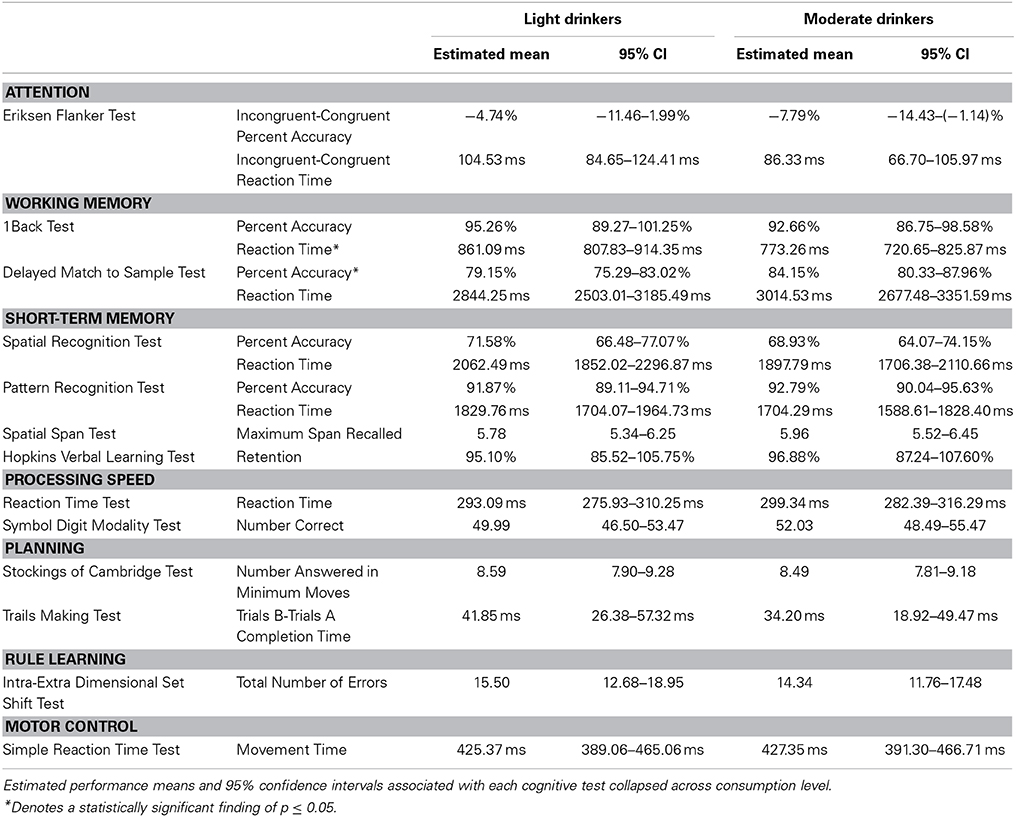
A modest effect of alcohol consumption was found in the working memory domain [F(52) = 2.54, p = 0.05]. No differences in 1Back Task percent accuracy were found [Light = 95.26%, 89.27–101.25%; Moderate = 92.66%, 86.75–98.58%; t(55) = 0.68, p = 0.50]. However, moderate drinkers responded faster during the 1Back Task [Light = 861.09 ms, 807.83–914.35 ms; Moderate = 773.26 ms, 720.65–825.87 ms; t(55) = 2.57, p = 0.01]. This finding was driven by a nearly significant difference between the reaction times of younger drinkers [Light Young = 844.19 ms, 717.83–970.55 ms; Moderate Young = 709.94 ms, 590.47–829.41 ms; t(55) = 2.44, p = 0.08] but not older drinkers [Light Old = 877.98 ms, 797.84–958.14 ms; Moderate Old = 836.58 ms, 762.57–910.58 ms; t(55) = 1.0, p = 0.75]. Moderate drinkers performed better on the Delayed Match to Sample Task [Light = 79.15%, 75.29–83.02%; Moderate = 84.15%, 80.33–87.96%; t(55) = −2.02, p = 0.05]. This finding was driven by a nearly significant difference between the percent accuracies of older adults [Light Old = 72.20%, 66.89–78.51%; Moderate Old = 80.31%, 74.94–85.67%; t(55) = −2.53, p = 0.07] but not younger adults [Light Young = 85.61%, 76.44–94.77%; Moderate Young = 87.99%, 79.33–96.66%; t(55) = −0.60, p = 0.93]. No difference between light and moderate drinker Delayed Match to Sample Task reaction times was found [Light = 2844.25 ms, 2503.01–3185.49 ms; Moderate = 3014.53 ms, 2677.48–3351.59; t(55) = −0.78, p = 0.44]. See Table 6 for the estimated performance means and 95% confidence intervals for both light and moderate drinkers.
Interactive effects of age and alcohol consumption
A modest age * alcohol consumption interaction effect was found in the rule learning domain [F(52) = 4.50, p = 0.04]. Further analysis showed that light old drinkers tended to make the most errors during the Intra-Extra Dimensional Set Shift Task. More specifically, light old drinkers (26.58 errors, 19.49–35.87 errors) were found to make significantly more errors than both light young [9.03 errors, 5.64–14.56 errors; t(55) = −3.12, p = 0.02] and moderate young drinkers [11.02 errors, 7.03–17.46 errors; t(55) = −2.65, p = 0.05]. Although the number of errors did not significantly differ between older drinkers [t(55) = 2.28, p = 0.11], moderate old drinkers [18.54 errors, 14.01–24.53 errors] performed just as well as light young [t(55) = −2.17, p = 0.15] and moderate young drinkers [t(55) = −1.63, p = 0.37].
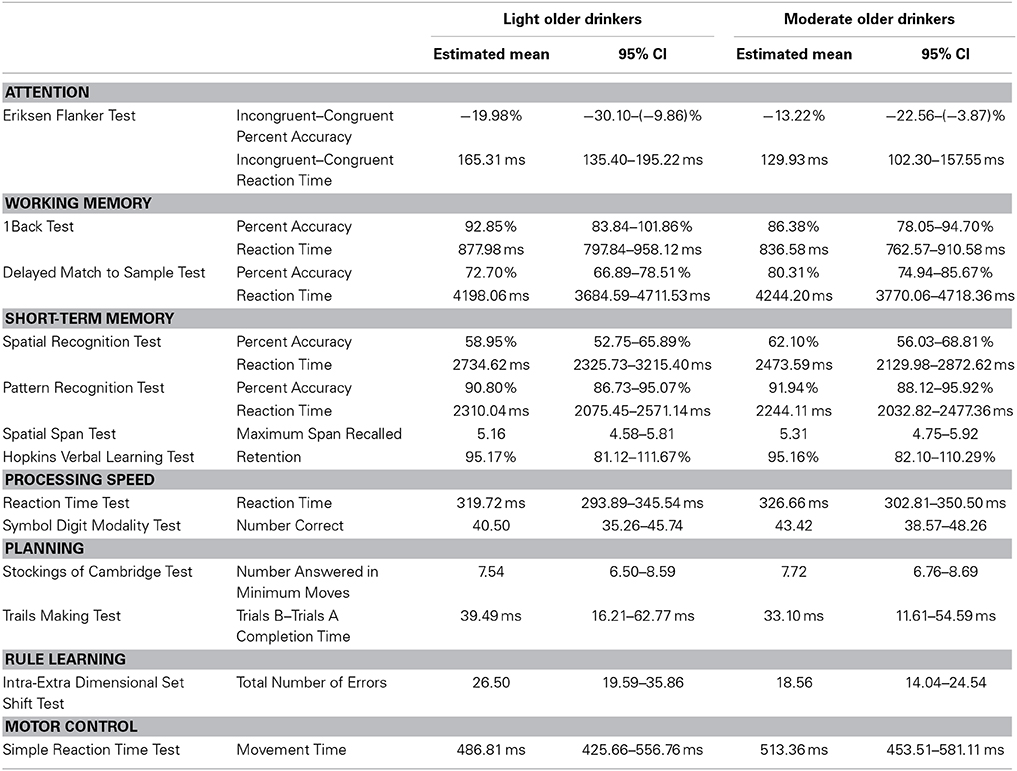
A nearly significant age * alcohol consumption interaction effect was found in the executive attention domain (F(54) = 2.59, p = 0.08). Light old drinkers tended to have the lowest percent accuracy on the Eriksen Flanker Task (Light Young = 10.51%, −5.45–26.46%; Moderate Young = −2.35%, −17.44–12.73%; Light Old = −19.98%, −30.10 to 9.86%; Moderate Old = −13.22%, −22.56 to −3.87%). In addition to this, light old drinkers tended to exhibit the slowest reaction times (Light Young = 43.75 ms, −3.41 to 90.91 ms; Moderate Young = 42.74 ms, −1.85–87.33 ms; Light Old = 165.31 ms, 135.40–195.22 ms; Moderate Old = 129.93 ms, 102.30–157.55 ms).
No significant age * alcohol consumption interaction effects were found in working memory [F(52) = 1.84, p = 0.14], short-term memory [F(50) = 1.00, p = 0.44], processing speed [F(54) = 0.08, p = 0.93], planning [F(54) = 0.93, p = 0.40] or motor control [F(55) = 0.69, p = 0.41] domains. See Table 7 for the estimated performance means and 95% confidence intervals for light and moderate older drinkers and Table 8 for light and moderate younger drinkers.
Stress, Anxiety, and Impulsivity
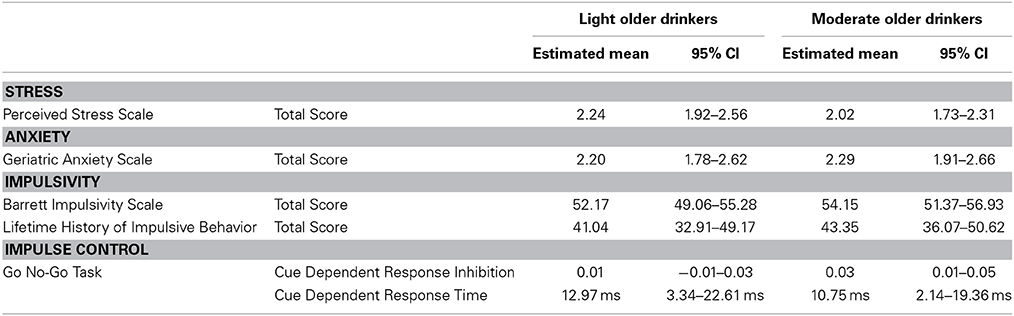
Light and moderate older drinkers did not differ from each other in measures known to influence cognition and overall alcohol consumption. Neither perceived stress nor anxiety showed a significant effect of alcohol consumption [Perceived Stress Score: F(32) = 2.02, p = 0.32 and Geriatric Anxiety Scale: F(31) = 0.02, 0.89]. No significant effects of alcohol consumption were found in either personality measures of impulsivity [Barrett Impulsivity Scale: F(30) = 2.18, p = 0.11; Lifetime History of Impulsive Behavior Inventory: F(31) = 0.07, p = 0.79] or the behavioral measure of impulse control [Go No-Go Task: F(31) = 0.53, p = 0.60]. See Table 9 for the estimated performance means and 95% confidence intervals for both light older and moderate older drinkers.
Discussion
This study was specifically designed to measure the effect of long-term moderate alcohol consumption on cognitive health in older adult drinkers. Expected age-related deficits in cognitive performance were observed but no evidence was found to suggest that long-term moderate alcohol consumption in older adults exacerbated age-related cognitive decline.
Interestingly, moderate older drinkers outperformed light older drinkers on the Delayed Match to Sample Task. This finding suggests that long-term moderate alcohol consumption in older age may be associated with select benefits to cognition, and in particular the short-term memory of learned relationships. However, when interpreting this finding it is important to make note of studies that show parameter-dependent performance in alcoholics on this task (Oscarberman and Bonner, 1985; Holden et al., 2012), and remember that potential benefits are likely to be highly specific and thus of have lower ecological relevance. This study also found that moderate younger drinkers responded to 1Back Task trials faster than light younger drinkers despite showing similar performance. This finding helps to support the idea that alcohol exposure can affect speed and accuracy on working memory tasks in different manners (Schweizer and Vogel-Sprott, 2008; Wilcox et al., 2014). It may also be indicative of a potential difference between younger drinkers in the interaction between impulsivity and different components of a working memory task. To help support this are previous alcohol research studies showing direct relationships between measures of impulsivity and working memory capacity (Gunn and Finn, 2013; Noel et al., 2013; Ellingson et al., 2014). Although studies were based on heavy alcohol drinkers, our findings may point to the sensitivity of these relationships to alcohol exposure at lower quantities in younger individuals.
Accounts detailing the effects of long-term moderate alcohol consumption on cognitive health were first published approximately 30 years ago (Goodwin et al., 1987; Herbert et al., 1993; Hendrie et al., 1996; Anstey et al., 2009). These and subsequent studies have been predominately epidemiologic in nature and dominated by analyses that use secondary outcome measures from large-scale clinical trials, like the Framingham Heart Study (Elias et al., 1999), the Seattle Longitudinal Study (Zanjani et al., 2013) and the Women's Health Initiative (Espeland et al., 2005, 2006). Although these analyses benefit from large sample sizes and longitudinal queries have been able to explore causal relationships between alcohol consumption and cognition (Edelstein et al., 1998; Virtaa et al., 2010), the parent clinical trials were not specifically designed to measure the effect of moderate alcohol consumption on age-related cognitive decline as the primary outcome. Consequently, there is little consensus in the literature, and the effect of moderate alcohol consumption on cognitive health in older age is unclear. To illustrate this point, studies have shown a negative effect (Dufouil et al., 2000; Zhou et al., 2003), a positive effect (Arntzen et al., 2010; Zanjani et al., 2013), as well as no consistent effect (Almeida et al., 2014; Sabia et al., 2014).
Strengths associated with this report include the definition of moderate alcohol consumption. In previous research, the definition of moderate has been broad and has ranged from 5 to 60 g of alcohol per day (approximately 1 to 6 drinks per day) depending on the tool used for assessment. In this study, the definition for moderate alcohol consumption was based on alcohol use trends observed in older American drinkers (NIAAA, 1998, 2006; Fryar et al., 2006) and helped make study criteria more generalizable. Another important addition to literature provided by this study was the attention paid to the quantity and frequency of alcohol consumption. Although the aggregate weekly intake volume for moderate consumption (approximately 91–273 g of alcohol) may seem lenient, the pattern with which it was consumed was considered in detail. For example, moderate consumption was not allowed to exceed 3 drinks (approximately 39 g of alcohol) per day and individuals that reported binge-drinking habits were not included in the study. Participants were also explicitly asked about their lifetime alcohol consumption habits, and were balanced in terms of the number of years they had maintained their consumption level as well as the total number of years they were exposed to alcohol. Individuals were thoroughly questioned about their drinking habits using a series of validated questionnaires to ensure those with present or past problematic drinking patterns were excluded. Furthermore, light drinkers were used as the control group. This bolstered ecological validity given recent census data and avoided any differences between non-drinkers and drinkers that are difficult to account for during analysis (e.g., religion or distaste for alcohol) (Green and Polen, 2001; Rehm et al., 2008). In addition, the study population was specifically restricted to healthy, active and community dwelling adults. Older adults who fulfilled these criteria but who were also on a stable medication regimen were also enrolled. This helped to make findings generalizable to a growing number of older American adults, who are living longer and healthier lives thanks to medications designed to stabilize conditions like high blood pressure, depression, and hypothyroidism. The extensive cognitive battery used in this study adds to current research that has predominately used global assessments of mild cognitive impairment and dementia (Dent et al., 1997; Dufouil et al., 2000; Bond et al., 2001, 2005; Chan et al., 2010). The assessments used in this study are validated measures of specific cognitive domains (i.e., short term memory vs. working memory) and have been widely used in aging and substance use literature. To our knowledge, this is the first study specifically designed to measure an extensive list of cognitive abilities in older social drinkers. Analyses included cognitive aspects but also explored stress, anxiety and impulsivity, which are directly relevant to the effects of alcohol on both physical and cognitive health.
Research limitations include the TLFB method used to quantify alcohol consumption in this study. Although underrepresentation is a common concern with self-report alcohol consumption questionnaires, it should be noted that this phenomenon is more common to populations that consume alcohol in larger quantities than those discussed in this study (Stockwell et al., 2004). Limitations to the study also include a relatively small sample size when compared to previous research based on large-scale clinical trials. Despite this, confidence in our findings was strengthened by the fact that the presented estimated performance means are similar to those previously reported in healthy older adults (Sahakian et al., 1988, 1990; Downes et al., 1989; Owen et al., 1990; Robbins et al., 1998; Shapiro et al., 1999; de Jager et al., 2002; Hogervorst et al., 2002; De Luca et al., 2003; Hoyer et al., 2004; Tombaugh, 2004; Van Gerven et al., 2008).
In summary, this study showed that long-term moderate alcohol consumption in older age is neither harmful nor beneficial to overall cognitive health. Given the fact that previous findings in this field have been inconsistent (i.e., both positive and negative) the finding of this tailored experiment likely represents the most probable effect of long-term light to moderate alcohol consumption on cognitive performance in older adults: little to none. It is important to note, of course, that this finding is specific to healthy, community-dwelling and social drinkers. Findings should not be generalized to individuals with other consumption patterns, like heavy drinkers, binge drinkers and ex-drinkers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA021639, AA007565, and AA021099), the National Institute on Drug Abuse (DA020074 and DA006634), the National Institute of Biomedical Imaging and Bioengineering (EB012236) and the Wake Forest University School of Medicine Venture Fund.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fnagi.2014.00341/abstract
References
Almeida, O. P., Hankey, G. J., Yeap, B. B., Golledge, J., and Flicker, L. (2014). Alcohol consumption and cognitive impairment in older men: a mendelian randomization study. Neurology 82, 1038–1044. doi: 10.1212/WNL.0000000000000255
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Anstey, K. J., Mack, H. A., and Cherbuin, N. (2009). Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry 17, 542–555. doi: 10.1097/JGP.0b013e3181a2fd07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arntzen, K. A., Schirmer, H., Wilsgaard, T., and Mathiesen, E. B. (2010). Moderate wine consumption is associated with better cognitive test results: a 7 year follow up of 5033 subjects in the Tromsø Study. Acta Neurol. Scand. Suppl. 122, 23–29. doi: 10.1111/j.1600-0404.2010.01371.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Babor, T. F., Higgins-Biddle, J. C., Saunders, J. B., and Monteiro, M. G. (2001). AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva: World Health Organization Department of Mental Health and Substance Dependance.
Benedict, R. H. B., Schretlen, D., Groninger, L., and Brandt, J. (1998). The Hopkins Verbal Learning Test–revised: normative data and analysis of interform and test-retest reliability. 12, 43–55.
Bond, G. E., Burr, R., McCurry, S. M., Graves, A. B., and Larson, E. B. (2001). Alcohol, aging, and cognitive performance in a cohort of Japanese Americans aged 65 and older: the Kame project. Int. Psychogeriatr. 13, 207–223. doi: 10.1017/S1041610201007591
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bond, G. E., Burr, R. L., McCurry, S. M., Rice, M. M., Borenstein, A. R., and Larson, E. B. (2005). Alcohol and cognitive performance: a longitudinal study of older Japanese Americans. The Kame Project. Int. Psychogeriatr. 17, 653–668. doi: 10.1017/S1041610205001651
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brandt, J. (1991). The hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 5, 125–142. doi: 10.1080/13854049108403297
Chan, K. K. K., Chiu, K. C., and Chu, L. W. (2010). Association between alcohol consumption and cognitive impairment in Southern Chinese older adults. Int. J. Geriatr. Psychiatry 25, 1272–1279. doi: 10.1002/gps.2470
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Coccaro, E. F., and Schmidt-Kaplan, C. A. (2012). Life history of impulsive behavior: development and validation of a new questionnaire. J. Psychiatr. Res. 46, 346–352. doi: 10.1016/j.jpsychires.2011.11.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. doi: 10.2307/2136404
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
de Jager, C. A., Milwain, E., and Budge, M. (2002). Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychol. Med. 32, 483–491. doi: 10.1017/S003329170200524X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Luca, C. R., Wood, S. J., Anderson, V., Buchanan, J.-A., Proffitt, T. M., Mahony, K., and Pantelis, C. (2003). Normative data from the CANTAB. I: development of executive function over the lifespan. J. Clin. Exp. Neuropsychol. 25, 242–254. doi: 10.1076/jcen.25.2.242.13639
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dent, O. F., Sulway, M. R., Broe, G. A., Creasey, H., Kos, S. C., Jorm, A. F., et al. (1997). Alcohol consumption and cognitive performance in a random sample of Australian soldiers who served in the Second World War. BMJ 314, 1655–1657. doi: 10.1136/bmj.314.7095.1655
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Derefinko, K. J., Adams, Z. W., Milich, R., Fillmore, M. T., Lorch, E. P., and Lynam, D. R. (2008). Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 36, 745–758. doi: 10.1007/s10802-007-9207-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Downes, J. J., Roberts, A. C., Sahakian, B. J., Evenden, J. L., Morris, R. G., and Robbins, T. W. (1989). Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction. Neuropsychologia 27, 1329–1343. doi: 10.1016/0028-3932(89)90128-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dry, M. J., Burns, N. R., Nettelbeck, T., Farquharson, A. L., and White, J. M. (2012). Dose-related effects of alcohol on cognitive functioning. PLoS ONE 7:e50977. doi: 10.1371/journal.pone.0050977
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dufouil, C., Tzourio, C., Brayne, C., Berr, C., Amouyel, P., and Alpérovitch, A. (2000). Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology 11, 280–284. doi: 10.1097/00001648-200005000-00009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Edelstein, S. L., Kritz-Silverstein, D., and Barrett-Connor, E. (1998). Prospective association of smoking and alcohol use with cognitive function in an elderly cohort. J. womens. Health 7, 1271–1281. doi: 10.1089/jwh.1998.7.1271
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Elias, P. K., Elias, M. F., D'Agostino, R. B., Silbershatz, H., and Wolf, P. A. (1999). Alcohol consumption and cognitive performance in the Framingham Heart Study. Am. J. Epidemiol. 150, 580–589. doi: 10.1093/oxfordjournals.aje.a010056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ellingson, J. M., Fleming, K. A., Verges, A., Bartholow, B. D., and Sher, K. J. (2014). Working memory as a moderator of impulsivity and alcohol involvement: testing the cognitive-motivational theory of alcohol use with prospective and working memory updating data. Addict. Behav. 39, 1622–1631. doi: 10.1016/j.addbeh.2014.01.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eriksen, B. A., and Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149. doi: 10.3758/BF03203267
Espeland, M. A., Gu, L., Masaki, K. H., Langer, R. D., Coker, L. H., Stefanick, M. L., et al. (2005). Association between reported alcohol intake and cognition: results from the Women's Health Initiative Memory Study. Am. J. Epidemiol. 161, 228–238. doi: 10.1093/aje/kwi043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Espeland, M. A., Coker, L. H., Wallace, R., Rapp, S. R., Resnick, S. M., Limacher, M., et al. (2006). Association between alcohol intake and domain-specific cognitive function in older women. Neuroepidemiology 27, 1–12. doi: 10.1159/000093532
Fillmore, M. T. (2003). Drug abuse as a problem of impaired control: current approaches and findings. Behav. Cogn. Neurosci. Rev. 2, 179–197. doi: 10.1177/1534582303257007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fist, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York: Biometrics Department, New York State Psychiatric Institute.
Fryar, C., Hirsch, R., Porter, K., Kottiri, B., Brody, D., and Louis, T. (2006). Smoking and Alcohol Behaviors Reported by Adults, United States, 1999-2002. Advance Data From Vital and Health Statistics, Vol. 378. Hyattsville, MD: National Center for Health Statistics.
Gilbertson, R., Ceballos, N. A., Prather, R., and Nixon, S. J. (2009). Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. J. Stud. Alcohol Drugs 70, 242–252.
Goodwin, J. S., Sanchez, C. J., Thomas, P., Hunt, C., Garry, P. J., and Goodwin, J. M. (1987). Alcohol intake in a healthy elderly population. Am. J. Public Health 77, 173–177. doi: 10.2105/AJPH.77.2.173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Green, A., Garrick, T., Sheedy, D., Blake, H., Shores, E. A., and Harper, C. (2010). The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol. Clin. Exp. Res. 34, 443–450. doi: 10.1111/j.1530-0277.2009.01108.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Green, C. A., and Polen, M. R. (2001). The health and health behaviors of people who do not drink alcohol. Am. J. Prev. Med. 21, 298–305. doi: 10.1016/S0749-3797(01)00365-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Guillot, C. R., Fanning, J. R., Bullock, J. S., McCloskey, M. S., and Berman, M. E. (2010). Effects of alcohol on tests of executive functioning in men and women: a dose response examination. Exp. Clin. Psychopharmacol. 18, 409–417. doi: 10.1037/a0021053
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gunn, R. L., and Finn, P. R. (2013). Impulsivity partially mediates the association between reduced working memory capacity and alcohol problems. Alcohol 47, 3–8. doi: 10.1016/j.alcohol.2012.10.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haringsma, R., Engels, G. I., Beekman, A. T. F., and Spinhoven, P. (2004). The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int. J. Geriatr. Psychiatry 19, 558–563. doi: 10.1002/gps.1130
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hendrie, H. C., Gao, S., Hall, K. S., Hui, S. L., and Unverzagt, F. W. (1996). The relationship between alcohol consumption, cognitive performance, and daily functioning in an urban sample of older black Americans. J. Am. Geriatr. Soc. 44, 1158–1165.
Herbert, L. E., Scherr, P. A., Beckett, L. A., Albert, M. S., Rosner, B., Taylor, J. O., et al. (1993). Relation of smoking and low-to-moderate alcohol consumption to change in cognitive function: a longitudinal study in a defined community of older persons. Am. J. Epidemiol. 137, 881–891.
Hillbom, M., Saloheimo, P., and Juvela, S. (2011). Alcohol consumption, blood pressure, and the risk of stroke. Curr. Hypertens. Rep. 13, 208–213. doi: 10.1007/s11906-011-0194-y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hogervorst, E., Combrinck, M., Lapuerta, P., Rue, J., Swales, K., and Budge, M. (2002). The Hopkins Verbal Learning Test and screening for dementia. Dement. Geriatr. Cogn. Disord. 13, 13–20. doi: 10.1159/000048628
Holden, H. M., Hoebel, C., Loftis, K., and Gilbert, P. E. (2012). Spatial pattern separation in cognitively normal young and older adults. Hippocampus 22, 1826–1832. doi: 10.1002/hipo.22017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hoyer, W. J., Stawski, R. S., Wasylyshyn, C., and Verhaeghen, P. (2004). Adult age and digit symbol substitution performance: a meta-analysis. Psychol. Aging 19, 211–214. doi: 10.1037/0882-7974.19.1.211
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jones, T. G., Schinka, J. A., Vanderploeg, R. D., Small, B. J., Graves, A. B., and Mortimer, J. A. (2002). 3MS normative data for the elderly. Arch. Clin. Neuropsychol. 17, 171–177. doi: 10.1093/arclin/17.2.171
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keys, A., Fidanza, F., Karvonen, M. J., Kimura, N., and Taylor, H. L. (1972). Indices of relative weight and obesity. J. Chronic Dis. 25, 329–343. doi: 10.1016/0021-9681(72)90027-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, J. W., Lee, D. Y., Lee, B. C., Jung, M. H., Kim, H., Choi, Y. S., et al. (2012). Alcohol and cognition in the elderly: a review. Psychiatry Investig. 9, 8–16. doi: 10.4306/pi.2012.9.1.8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 55, 352–358. doi: 10.1037/h0043688
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Magrys, S. A., and Olmstead, M. C. (2014). Alcohol intoxication alters cognitive skills mediated by frontal and temporal brain regions. Brain Cogn. 85, 271–276. doi: 10.1016/j.bandc.2013.12.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marchi, K. C., Muniz, J. J., and Tirapelli, C. R. (2014). Hypertension and chronic ethanol consumption: what do we know after a century of study? World J. Cardiol. 6, 283–294. doi: 10.4330/wjc.v6.i5.283
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Milner, B. (1971). Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 27, 272–277.
Moriyama, Y., Mimura, M., Kato, M., and Kashima, H. (2006). Primary alcoholic dementia and alcohol-related dementia. Psychogeriatrics 6, 114–118. doi: 10.1111/j.1479-8301.2006.00168.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
NIAAA. (1998). Drinking in the United States: Main Findings from the 1992 National Longitudinal Alcohol Epidemiologic Survey (NLAES). Bethesda, MD: U.S. Alcohol Epidemiologic Data Reference Manual Publication No. 99-3519.
NIAAA. (2004). National Institute on Alcohol Abuse and Alcoholism Council Approves Definition of Binge Drinking. Bethesda, MD: NIAAA Newsletter.
NIAAA. (2006). Alcohol Use and Alcohol Use Disorders in the United States: Main Findings from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Bethesda, MD: U.S. Alcohol Epidemiologic Data Reference Manual Publication No. 05-5737.
Noel, X., van der Linden, M., Brevers, D., Campanella, S., Verbanck, P., Hanak, C., et al. (2013). Separating intentional inhibition of prepotent responses and resistance to proactive interference in alcohol-dependent individuals. Drug Alcohol Depend. 128, 200–205. doi: 10.1016/j.drugalcdep.2012.08.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oscarberman, M., and Bonner, R. T. (1985). Matching-to-sample and delayed matching-to-sample performance as measures of visual processing, selective attention, and memory in aging and alcoholic individuals. Neuropsychologia 23, 639–651. doi: 10.1016/0028-3932(85)90065-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E., and Robbins, T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28, 1021–1034. doi: 10.1016/0028-3932(90)90137-D
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pachana, N. A., Byrne, G. J., Siddle, H., Koloski, N., Harley, E., and Arnold, E. (2007). Development and validation of the Geriatric Anxiety Inventory. Int. Psychogeriatr. 19, 103–114. doi: 10.1017/S1041610206003504
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panza, F., Capurso, C., D'Introno, A., Colacicco, A. M., Frisardi, V., Lorusso, M., et al. (2009). Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. J. Alzheimers Dis. 17, 7–31. doi: 10.3233/JAD-2009-1009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panza, F., Frisardi, V., Seripa, D., Logroscino, G., Santamato, A., Imbimbo, B. P., et al. (2012). Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int. J. Geriatr. Psychiatry 27, 1218–1238. doi: 10.1002/gps.3772
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774.
Peele, S., and Brodsky, A. (2000). Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug Alcohol Depend. 60, 221–247. doi: 10.1016/S0376-8716(00)00112-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Peters, R., Peters, J., Warner, J., Beckett, N., and Bulpitt, C. (2008). Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 37, 505–512. doi: 10.1093/ageing/afn095
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., et al. (2007). Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29, 125–132. doi: 10.1159/000109998
Rehm, J., Irving, H., Ye, Y., Kerr, W. C., Bond, J., and Greenfield, T. K. (2008). Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am. J. Epidemiol. 168, 866–871. doi: 10.1093/aje/kwn093
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Reitan, R. M., and Wolfson, D. (1985). The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Tucson, AZ: Neurophyschological Press.
Reitan, R. N. (1958). Validity of the Trail Making test as an indicator of organic brain damage. 8, 271–276.
Robbins, T. W., James, M., Owen, A. M., Sahakian, B. J., Lawrence, A. D., McInnes, L., et al. (1998). A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J. Int. Neuropsychol. Soc. 4, 474–490. doi: 10.1017/S1355617798455073
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sabia, S., Elbaz, A., Britton, A., Bell, S., Dugravot, A., Shipley, M., et al. (2014). Alcohol consumption and cognitive decline in early old age. Neurology 82, 332–339. doi: 10.1212/WNL.0000000000000063
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sahakian, B. J., Downes, J. J., Eagger, S., Evenden, J. L., Levy, R., Philpot, M. P., et al. (1990). Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia 28, 1197–1213. doi: 10.1016/0028-3932(90)90055-S
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sahakian, B. J., Morris, R. G., Evenden, J. L., Heald, A., Levy, R., Philpot, M., et al. (1988). A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain 111(Pt 3), 695–718.
SAMHSA. (2013). Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795, Rockville, MD: Substance Abuse and Mental Health Services Administration.
Sayon-Orea, C., Martinez-Gonzalez, M. A., and Bes-Rastrollo, M. (2011). Alcohol consumption and body weight: a systematic review. Nutr. Rev. 69, 419–431. doi: 10.1111/j.1753-4887.2011.00403.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schmidt, C. A., Fallon, A. E., and Coccaro, E. F. (2004). Assessment of behavioral and cognitive impulsivity: development and validation of the Lifetime History of Impulsive Behaviors Interview. Psychiatry Res. 126, 107–121. doi: 10.1016/j.psychres.2003.12.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schweizer, T. A., and Vogel-Sprott, M. (2008). Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp. Clin. Psychopharmacol. 16, 240–250. doi: 10.1037/1064-1297.16.3.240
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shacham, S. (1983). A shortened version of the Profile of Mood States. J. Pers. Assess. 47, 305–306. doi: 10.1207/s15327752jpa4703_14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shapiro, A. M., Benedict, R. H., Schretlen, D., and Brandt, J. (1999). Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin. Neuropsychol. 13, 348–358. doi: 10.1076/clin.13.3.348.1749
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sklar, A. L., Gilbertson, R., Boissoneault, J., Prather, R., and Nixon, S. J. (2012). Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcohol. Clin. Exp. Res. 36, 2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, A. (1968). “The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral disorders,” in Learning Disorders, ed J. Helmuth (Seattle: Special Child Publications), 83–91.
Sobell, L. C., and Sobell, M. B. (1992). “Timeline follow-back: a technique for assessing self-reported ethanol consumption,” in Measuring Alcohol Consumption: Psychosocial and Biological Methods, eds J. Allen and R. Z. Litten (Totowa, NJ: Humana Press), 41–72.
Solfrizzi, V., D'Introno, A., Colacicco, A. M., Capurso, C., Del Parigi, A., Baldassarre, G., et al. (2007). Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology 68, 1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stockwell, T., Donath, S., Cooper-Stanbury, M., Chikritzhs, T., Catalano, P., and Mateo, C. (2004). Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction 99, 1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thomas, V. S., and Rockwood, K. J. (2001). Alcohol abuse, cognitive impairment, and mortality among older people. J. Am. Geriatr. Soc. 49, 415–420. doi: 10.1046/j.1532-5415.2001.49085.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tombaugh, T. N. (2004). Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. doi: 10.1016/S0887-6177(03)00039-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
USDA and USDHHS. (2010). Dietary Guidelines for Americans, 7th Edn. Washington, DC: US Government Printing Ofice.
Van Gerven, P. W. M., Meijer, W. A., Prickaerts, J. H. M., and Van der Veen, F. M. (2008). Aging and focus switching in working memory: excluding the potential role of memory load. Exp. Aging Res. 34, 367–378. doi: 10.1080/03610730802274165
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vestal, R. E., McGuire, E. A., Tobin, J. D., Andres, R., Norris, A. H., and Mezey, E. (1977). Aging and ethanol metabolism. Clin. Pharmacol. Ther. 21, 343–354.
Virtaa, J. J., Järvenpää, T., Heikkilä, K., Perola, M., Koskenvuo, M., Räihä, I., et al. (2010). Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J. Alzheimers Dis. 22, 939–948. doi: 10.3233/JAD-2010-100870
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, Y.-P., and Andrade, L. H. (2013). Epidemiology of alcohol and drug use in the elderly. Curr. Opin. Psychiatry 26, 343–348. doi: 10.1097/YCO.0b013e328360eafd
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wilcox, C. E., Dekonenko, C. J., Mayer, A. R., Bogenschutz, M. P., and Turner, J. A. (2014). Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev. Neurosci. 25, 1–24. doi: 10.1515/revneuro-2013-0054
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zanjani, F., Downer, B. G., Kruger, T. M., Willis, S. L., and Schaie, K. W. (2013). Alcohol effects on cognitive change in middle-aged and older adults. Aging Ment. Health 17, 12–23. doi: 10.1080/13607863.2012.717254
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhou, H., Deng, J., Li, J., Wang, Y., Zhang, M., and He, H. (2003). Study of the relationship between cigarette smoking, alcohol drinking and cognitive impairment among elderly people in China. Age Ageing 32, 205–210. doi: 10.1093/ageing/32.2.205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: alcohol, moderate, chronic, aging, cognition
Citation: Moussa MN, Simpson SL, Mayhugh RE, Grata ME, Burdette JH, Porrino LJ and Laurienti PJ (2015) Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults. Front. Aging Neurosci. 6:341. doi: 10.3389/fnagi.2014.00341
Received: 16 October 2014; Accepted: 06 December 2014;
Published online: 05 January 2015.
Edited by:
P. Hemachandra Reddy, Texas Tech University, USAReviewed by:
Junming Wang, University of Mississippi Medical Center, USARamesh Kandimalla, Texas Tech University, USA
Copyright © 2015 Moussa, Simpson, Mayhugh, Grata, Burdette, Porrino, and Laurienti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malaak N. Moussa, Neuroscience Program, Laboratory for Complex Brain Networks, Medical Center Boulevard, Wake Forest University School of Medicine, 2000 West 1st Street, Suite #701, Winston-Salem, NC 27104, USA e-mail: mamoussa@wakehealth.edu
 Malaak N. Moussa
Malaak N. Moussa Sean L. Simpson
Sean L. Simpson Rhiannon E. Mayhugh
Rhiannon E. Mayhugh Michelle E. Grata
Michelle E. Grata Jonathan H. Burdette
Jonathan H. Burdette Linda J. Porrino6
Linda J. Porrino6  Paul J. Laurienti
Paul J. Laurienti