Hematopoietic cytokines as therapeutic players in early stages Parkinson’s disease
- Department of Neuroscience, Carleton University, Ottawa, ON, Canada
Parkinson’s disease (PD) is a devastating age related neurodegenerative disease that is believed to have a lengthy prodromal state. It is critical to find methods to harness compensatory recovery processes in order to slow or prevent the eventual progression of clinical symptoms. The current perspective paper argues that immune system signaling molecules represent such a promising therapeutic approach. Two cytokines of interest are granulocyte macrophage-colony stimulating factor (GM-CSF) and erythropoietin (EPO). These hematopoietic cytokines have been protective in models of stroke, neuronal injury, and more recently PD. It is our belief that these trophic cytokines can be used not only for cell protection but also regeneration. However, success is likely dependent on early intervention. This paper will outline our perspective on the development of novel trophic recovery treatments for PD. In particular, we present new data from our lab suggesting that EPO and GM-CSF can foster neural re-innervation in a “mild” or partial lesion PD model that could be envisioned as reflecting the early stages of the disease.
Parkinson’s Disease: Animal Model of Early Stages
Parkinson’s disease (PD) is characterized by a loss of dopamine (DA) neurons within the nigrostriatal pathway and the presence of Lewy body pathological protein aggregates (Farrer et al., 2001; Sherer et al., 2001). Clinically, PD is diagnosed based on tremors within distal limbs, muscle rigidity, and bradykinesia. By the time patients present with these motor symptoms, there has already been significant degeneration of DA neurons, with up to an 80% loss of striatal DA innervation (Bezard et al., 2001). There are also extensive non-motor symptoms, which present long before the cardinal motor symptoms (McDonald et al., 2003).
Current PD treatments only manage symptom severity and are not able to reverse or even appreciably slow the neurodegenerative processes. Thus, it is of interest to investigate potential treatments that could stabilize these surviving neurons and possibly induce some degree of neuronal recovery. It might be advantageous to target processes linked to the early or prodromal stages of PD, as neuronal plasticity would likely be more amenable to modulation at such times. However, models of early stage PD are less common and not as well understood as the late stage typically used. The neurotoxin, 6-hydroxydopamine (6-OHDA), is routinely used to induce PD-like pathology, inducing a loss of substantia nigra pars compacta (SNc) DA neurons and downstream striatal terminals (Alvarez-Fischer et al., 2008). 6-OHDA infused directly into the SNc rapidly produces a robust degeneration of SNc DA neurons, coupled with striatal DA depletion within 48–72 h (Blandini et al., 2008; Thiele et al., 2012). However, this method has the obvious caveat of not reflecting the chronic slow course of degeneration. A more progressive lesion has been observed with lower doses of 6-OHDA infused into the striatum rather than SNc. Indeed, intra-striatal 6-OHDA administration induced a lesion, which gradually increased in size over several weeks (Sauer and Oertel, 1994) and more closely mimicked the progression from early to later stages of PD.
Novel Treatment Strategies
One exciting new avenue for treating PD involves the use of trophic factors to stabilize neuronal viability and even promote some degree of recovery. In fact, recent studies have revealed a reduction of brain derived neurotrophic factor (BDNF) within the SNc of PD patients (Mogi et al., 1999; Salehi and Mashayekhi, 2009). Accordingly, BDNF can promote the survival and differentiation of mesencephalic DA neurons, as well as protect against the DA toxicants, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-OHDA (Murer et al., 2001). Likewise, glial derived neurotrophic factor (GDNF), has also emerged as a potential candidate for neuroprotection in PD patients, based on success in various animal models (Fox et al., 2001; Ai et al., 2003). However, the improvements observed in clinical trials were restricted to the immediate area surrounding the site of infusion (Gill et al., 2003) and a randomized placebo-controlled study was unsuccessful at replicating these beneficial effects (Lang et al., 2006). Moreover, BDNF and GDNF do not readily cross the blood brain barrier (BBB) and have numerous side effects (Pezet and McMahon, 2006; Pilakka-Kanthikeel et al., 2013).
Finding well-tolerated factors with trophic properties, which cross the BBB represents a considerable challenge. Two cytokines that may hold potential therapeutic significance are the hematopoietic cytokines, erythropoietin (EPO) and granulocyte macrophage-colony stimulating factor (GM-CSF). Indeed, GM-CSF had protective effects in models of Alzheimer’s disease (Boyd et al., 2010), and in MPTP and paraquat models of PD (Kim et al., 2009; Mangano et al., 2011). Moreover, GM-CSF administration induced spontaneous axonal regeneration and functional recovery from traumatic spinal cord injury (Ha et al., 2005; Bouhy et al., 2006) and reduced infarct volume following ischemia (Nakagawa et al., 2006; Schäbitz et al., 2008). Similarly, EPO has been investigated extensively for use in stroke, traumatic head injury and more recently, in toxin based animal models of PD (Sargin et al., 2010; Merelli et al., 2013; Bond and Rex, 2014). EPO was also shown to protect hippocampal neurons from stressor-induced apoptosis, and increased adult hippocampal neurogenesis (Merelli et al., 2015).
GM-CSF and EPO have well-documented trophic actions in the periphery and can infiltrate and accumulate within the brain (Enzler and Dranoff, 2003). Receptors for GM-CSF and EPO have been found on mature DA neurons and neural progenitor cells, suggesting that they might influence adult neuronal functioning, as well as stimulate maturation (Kim et al., 2004; Ha et al., 2005).
The peripheral function of GM-CSF is to promote the differentiation and maturation of innate immune cells, and it is routinely administered to cancer patients to modify neutrophil production (Dale et al., 1998). Similarly, EPO has potent mitogenic effects on immune cells, as well as red blood cells and is routinely prescribed for anemia and in the context of certain cancer treatments (Debeljak et al., 2014). Thus, both also have well established clinical track records.
GM-CSF and EPO Promote Striatal Re-Innervation
It is thought that a slow progressive “wave” of neurodegeneration occurs over many years before a critical threshold of neuronal loss is reached and clinical PD pathology is manifested. Accordingly, it is of interest to study the effects of potential treatments while the disease is still in an early or possibly even a prodromal stage. In the present paper, it was of interest to assess whether EPO or GM-CSF treatment could influence striatal innervation following the establishment of a partial or “mild” lesion that might be analogous to the early onset of the disease. To this end, male Sprague Dawley rats received a single intra-striatum (1.0 mm anterior, 3.0 mm lateral, 5.0 mm ventral relative to bregma) infusion of 6-OHDA (20 μg). Animals were then randomly divided into three groups (n = 7): Saline, EPO, and GM-CSF. On post-6-OHDA infusion Days 13 and 28, the animals received an intraperitoneal injection of either saline, recombinant human EPO solution (rhEPO; 50 μg/kg) or recombinant rat granulocyte-macrophage colony-stimulating factor solution (rat GM-CSF; 10 μg/kg). Animals were sacrificed on Day 30.
As shown in Figure 1, we did indeed find that a modest [~10% of striatal area, as determined using tyrosine hydroxylase (TH) staining] lesion was induced in rats following intra-striatal infusion of a single moderate dose of 6-OHDA. However, no significant neuronal loss was evident within the SNc using this relatively mild paradigm, although it is possible that some of these SNc neurons would eventually die if we explored longer time intervals. Indeed, many surviving neurons had irregular shaped soma (Figure 2).
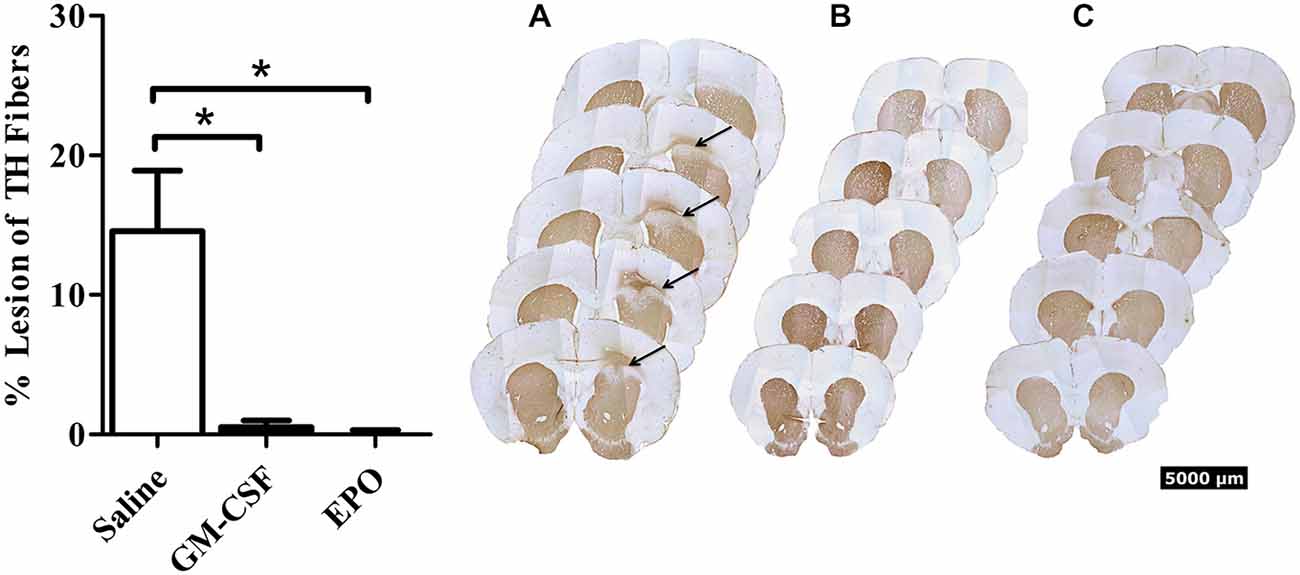
Figure 1. Thirty days after the intra-striatal infusion of 6-OHDA, saline treated animals (graph—white bars; A) had a modest but statistically significant loss of TH+ striatal fibers. The GM-CSF (graph—black bars; B) and EPO (graph—black bars; C) treated animals displayed no visible lesion at the 30-day sacrifice time. Data is expressed as mean ± 1 SEM, *p < 0.01.
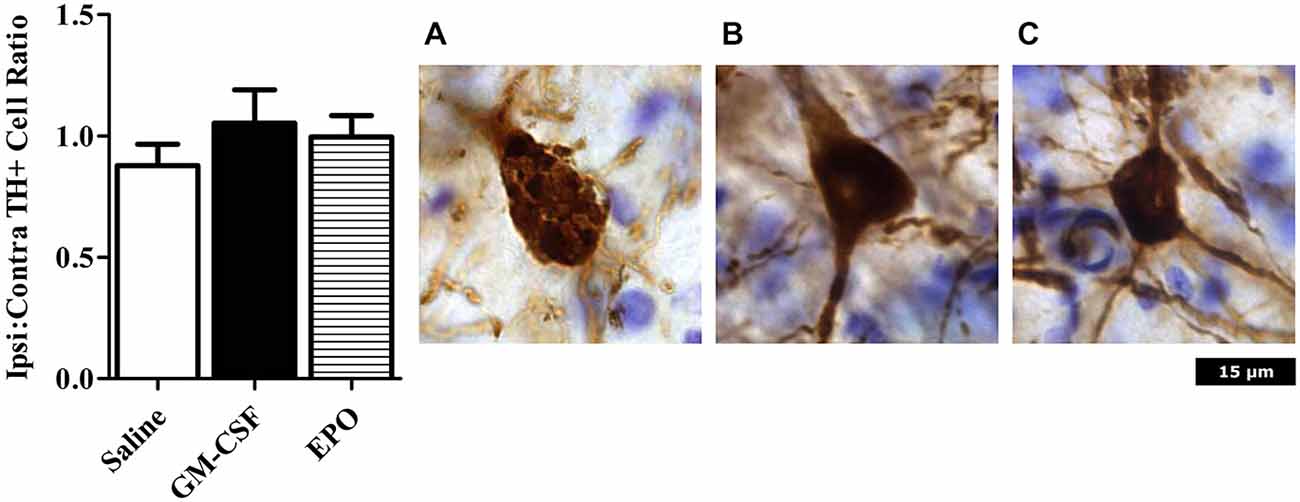
Figure 2. Neurons were counted in an unbiased manner using MBF Stereo investigator optical fractionator probe. There were no significant differences between treatment groups (left graph). Photomicrographs are representative of animals treated with (A) Saline; (B) GM-CSF; or (C) EPO in conjunction with 6-OHDA infusion. The saline (but not GM-CSF or EPO) treated rats that received 6-OHDA displayed TH+ neurons with an abnormal shaped nucleus, with a reduction of projections. Images were taken at 40 × magnification.
Importantly, the two EPO and GM-CSF injections that were given after the presumed establishment of the lesion (i.e., on Days 13 and 28 following 6-OHDA) provoked a significant recovery (presumed re-innervation) of striatal terminals. The statistical analysis revealed a significant treatment effect, F(2,15) = 10.7, P < 0.01, with regards to striatal lesion size (Figure 1), such that the cytokines prevented the striatal lesion by Day 30 following 6-OHDA.
The present findings are consistent with our own previous data and those of others showing beneficial effects of GM-CSF (Mangano et al., 2011; Kosloski et al., 2013) and EPO (Xue et al., 2007; Dhanushkodi et al., 2013; Qi et al., 2014) in toxicant animal models. However, since GM-CSF or EPO were administered after lesion establishment, the effects of the cytokine treatments in the current study would be expected to reflect some degree of recovery involving DA fiber re-growth rather than the prevention of fiber loss in the first place. This is a particularly novel finding given that the majority of studies typically focus on neuroprotective effects, rather than addressing the more clinically relevant issue of promoting recovery following some degree of neuronal damage.
Impact and Future Directions
It is important to underscore that the 6-OHDA paradigm presently used provoked a very modest loss of striatal terminals and future studies are required to ascertain whether GM-CSF and EPO might also have reparative properties in PD models with more significantly sized lesions. Nonetheless, GM-CSF and EPO may be ideal trophic treatment candidates based on their biological profiles, preclinical data and track record of clinical applicability. Mechanistically, these cytokines are potent inducers of BDNF and GDNF (Bouhy et al., 2006; Mengozzi et al., 2012), which we posit to be fundamental for their beneficial neural consequences. Targeting trophic processes to boost plasticity may be a critically important shift in treatment modalities away from failed attempts to translate neuroprotective approaches to the clinic; this strategy would also work well in tandem with recent efforts to identify biomarkers of disease state.
Author Contributions
CR and SH conceived the study. KF, CR, and SH designed the study. KF and CR performed the in vivo procedures. KF performed the immunohistochemical stains and data analysis. NP performed the stereological SNc counts. KF and SH prepared the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ai, Y., Markesbery, W., Zhang, Z., Grondin, R., Elseberry, D., Gerhardt, G. A., et al. (2003). Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J. Comp. Neurol. 461, 250–261. doi: 10.1002/cne.10689
Alvarez-Fischer, D., Henze, C., Strenzke, C., Westrich, J., Ferger, B., Höglinger, G. U., et al. (2008). Characterization of the striatal 6-OHDA model of Parkinson’s disease in wild type and alpha-synuclein-deleted mice. Exp. Neurol. 210, 182–193. doi: 10.1016/j.expneurol.2007.10.012
Bezard, E., Dovero, S., Prunier, C., Ravenscroft, P., Chalon, S., Guilloteau, D., et al. (2001). Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J. Neurosci. 21, 6853–6861.
Blandini, F., Armentero, M. T., and Martignoni, E. (2008). The 6-hydroxydopamine model: news from the past. Parkinsonism Relat. Disord. 14(Suppl. 2, S124–S129. doi: 10.1016/j.parkreldis.2008.04.015
Bond, W. S., and Rex, T. S. (2014). Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes and microglia. Front. Immunol. 5:523. doi: 10.3389/fimmu.2014.00523
Bouhy, D., Malgrange, B., Multon, S., Poirrier, A.-L., Scholtes, F., Schoenen, J., et al. (2006). Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 20, 1239–1241. doi: 10.1096/fj.05-4382fje
Boyd, T. D., Bennett, S. P., Mori, T., Governatori, N., Runfeldt, M., Norden, M., et al. (2010). GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers. Dis. 21, 507–518. doi: 10.3233/JAD-2010-091471
Dale, D. C., Liles, W. C., Llewellyn, C., and Price, T. H. (1998). Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) on neutrophil kinetics and function in normal human volunteers. Am. J. Hematol. 57, 7–15. doi: 10.1002/(sici)1096-8652(199801)57:1<7::aid-ajh2>3.0.co;2-0
Debeljak, N., Solár, P., and Sytkowski, A. J. (2014). Erythropoietin and cancer: the unintended consequences of anemia correction. Front. Immunol. 5:563. doi: 10.3389/fimmu.2014.00563
Dhanushkodi, A., Akano, E. O., Roguski, E. E., Xue, Y., Rao, S. K., Matta, S. G., et al. (2013). A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism. Genes Brain Behav. 12, 224–233. doi: 10.1111/gbb.12001
Farrer, M., Chan, P., Chen, R., Tan, L., Lincoln, S., Hernandez, D., et al. (2001). Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 50, 293–300. doi: 10.1002/ana.1132
Fox, C. M., Gash, D. M., Smoot, M. K., and Cass, W. A. (2001). Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 896, 56–63. doi: 10.1016/s0006-8993(00)03270-4
Gill, S. S., Patel, N. K., Hotton, G. R., O’Sullivan, K., McCarter, R., Bunnage, M., et al. (2003). Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595. doi: 10.1038/nm850
Ha, Y., Kim, Y. S., Cho, J. M., Yoon, S. H., Park, S. R., Yoon, D. H., et al. (2005). Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J. Neurosurg. Spine 2, 55–61. doi: 10.3171/spi.2005.2.1.0055
Kim, N. K., Choi, B. H., Huang, X., Snyder, B. J., Bukhari, S., Kong, T.-H., et al. (2009). Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson’s disease model. Eur. J. Neurosci. 29, 891–900. doi: 10.1111/j.1460-9568.2009.06653.x
Kim, J. K., Choi, B. H., Park, H. C., Park, S. R., Kim, S., Park, H. S., et al. (2004). Effects of GM-CSF on the neural progenitor cells. Neuroreport 15, 2161–2165. doi: 10.1097/00001756-200410050-00003
Kosloski, L. M., Kosmacek, E. A., Olson, K. E., Mosley, R. L., and Gendelman, H. E. (2013). GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J. Neuroimmunol. 265, 1–10. doi: 10.1016/j.jneuroim.2013.10.009
Lang, A. E., Gill, S., Patel, N. K., Lozano, A., Nutt, J. G., Penn, R., et al. (2006). Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466. doi: 10.1002/ana.20737
Mangano, E. N., Peters, S., Litteljohn, D., So, R., Bethune, C., Bobyn, J., et al. (2011). Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson’s disease. Neurobiol. Dis. 43, 99–112. doi: 10.1016/j.nbd.2011.02.011
McDonald, W. M., Richard, I. H., and DeLong, M. R. (2003). Prevalence, etiology and treatment of depression in Parkinson’s disease. Biol. Psychiatry 54, 363–375. doi: 10.1016/s0006-3223(03)00530-4
Mengozzi, M., Cervellini, I., Villa, P., Erbayraktar, Z., Gökmen, N., Yilmaz, O., et al. (2012). Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc. Natl. Acad. Sci. U S A 109, 9617–9622. doi: 10.1073/pnas.1200554109
Merelli, A., Czornyj, L., and Lazarowski, A. (2013). Erythropoietin: a neuroprotective agent in cerebral hypoxia, neurodegeneration and epilepsy. Curr. Pharm. Des. 19, 6791–6801. doi: 10.2174/1381612811319380011
Merelli, A., Czornyj, L., and Lazarowski, A. (2015). Erythropoietin as a new therapeutic opportunity in brain inflammation and neurodegenerative diseases. Int. J. Neurosci. doi: 10.3109/00207454.2014.989321 [Epub ahead of print].
Mogi, M., Togari, A., Kondo, T., Mizuno, Y., Komure, O., Kuno, S., et al. (1999). Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 270, 45–48. doi: 10.1016/s0304-3940(99)00463-2
Murer, M. G., Yan, Q., and Raisman-Vozari, R. (2001). Brain-derived neurotrophic factor in the control human brain and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 63, 71–124. doi: 10.1016/s0301-0082(00)00014-9
Nakagawa, T., Suga, S., Kawase, T., and Toda, M. (2006). Intracarotid injection of granulocyte-macrophage colony-stimulating factor induces neuroprotection in a rat transient middle cerebral artery occlusion model. Brain Res. 1089, 179–185. doi: 10.1016/j.brainres.2006.03.059
Pezet, S., and McMahon, S. B. (2006). Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538. doi: 10.1146/annurev.neuro.29.051605.112929
Pilakka-Kanthikeel, S., Atluri, V. S. R., Sagar, V., and Nair, M. (2013). Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in vitro study. PLoS One 8:e62241. doi: 10.1371/journal.pone.0062241
Qi, C., Xu, M., Gan, J., Yang, X., Wu, N., Song, L., et al. (2014). Erythropoietin improves neurobehavior by reducing dopaminergic neuron loss in a 6-hydroxydopamine-induced rat model. Int. J. Mol. Med. 34, 440–450. doi: 10.3892/ijmm.2014.1810
Salehi, Z., and Mashayekhi, F. (2009). Brain-derived neurotrophic factor concentrations in the cerebrospinal fluid of patients with Parkinson’s disease. J. Clin. Neurosci. 16, 90–93. doi: 10.1016/j.jocn.2008.03.010
Sargin, D., Friedrichs, H., El-Kordi, A., and Ehrenreich, H. (2010). Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract. Res. Clin. Anaesthesiol. 24, 573–594. doi: 10.1016/j.bpa.2010.10.005
Sauer, H., and Oertel, W. H. (1994). Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59, 401–415. doi: 10.1016/0306-4522(94)90605-x
Schäbitz, W.-R., Krüger, C., Pitzer, C., Weber, D., Laage, R., Gassler, N., et al. (2008). A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 28, 29–43. doi: 10.1038/sj.jcbfm.9600496
Sherer, T., Betarbet, R., and Greenamyre, J. (2001). Pathogenesis of Parkinson’s disease. Curr. Opin. Investig. drugs 2, 654–662.
Thiele, S. L., Warre, R., and Nash, J. E. (2012). Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J. Vis. Exp. 60:3234. doi: 10.3791/3234
Keywords: Parkinson’s disease, early stage, cytokines, granulocyte macrophage-colony stimulating factor, erythropoietin
Citation: Farmer K, Rudyk C, Prowse NA and Hayley S (2015) Hematopoietic cytokines as therapeutic players in early stages Parkinson’s disease. Front. Aging Neurosci. 7:126. doi: 10.3389/fnagi.2015.00126
Received: 01 May 2015; Accepted: 22 June 2015;
Published: 03 July 2015.
Edited by:
Fei Yin, University of Southern California, USAReviewed by:
Nicola B. Mercuri, University of Rome Tor Vergata - IRCCS Fondazione Santa Lucia, ItalyBjörn Spittau, Albert-Ludwigs-University Freiburg, Germany
Copyright © 2015 Farmer, Rudyk, Prowse and Hayley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shawn Hayley, Department of Neuroscience, Carleton University, 327 Life Sciences Research Building, 1123 Colonel By Drive, Ottawa, ON K1S5B6, Canada, shawn_hayley@carleton.ca
 Kyle Farmer
Kyle Farmer Christopher Rudyk
Christopher Rudyk Natalie A. Prowse
Natalie A. Prowse Shawn Hayley
Shawn Hayley