Specific cognitive functions and depressive symptoms as predictors of activities of daily living in older adults with heterogeneous cognitive backgrounds
- 1Faculdade de Medicina, Instituto Nacional de Ciências e Tecnologia e em Medicina Molecular, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
- 2Department of Psychology, Faculdade de Ciências Médicas de Minas Gerais, Belo Horizonte, Brazil
- 3Department of Mental Health, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
- 4Department of Internal Medicine, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
- 5Department of Physical Education, Universidade Federal de Viçosa, Viçosa, Brazil
Cognitive functioning influences activities of daily living (ADL). However, studies reporting the association between ADL and neuropsychological performance show inconsistent results regarding what specific cognitive domains are related to each specific functional domains. Additionally, whether depressive symptoms are associated with a worse functional performance in older adults is still under explored. We investigated if specific cognitive domains and depressive symptoms would affect different aspects of ADL. Participants were 274 older adults (96 normal aging participants, 85 patients with mild cognitive impairment, and 93 patients probable with mild Alzheimer’s disease dementia) with low formal education (∼4 years). Measures of ADL included three complexity levels: Self-care, Instrumental-Domestic, and Instrumental-Complex. The specific cognitive functions were evaluated through a factorial strategy resulting in four cognitive domains: Executive Functions, Language/Semantic Memory, Episodic Memory, and Visuospatial Abilities. The Geriatric Depression Scale measured depressive symptoms. Multiple linear regression analysis showed executive functions and episodic memory as significant predictors of Instrumental-Domestic ADL, and executive functions, episodic memory and language/semantic memory as predictors of Instrumental-Complex ADL (22 and 28% of explained variance, respectively). Ordinal regression analysis showed the influence of specific cognitive functions and depressive symptoms on each one of the instrumental ADL. We observed a heterogeneous pattern of association with explained variance ranging from 22 to 38%. Different instrumental ADL had specific cognitive predictors and depressive symptoms were predictive of ADL involving social contact. Our results suggest a specific pattern of influence depending on the specific instrumental daily living activity.
Introduction
Cognitive and functional impairments are hallmarks of cognitive disorders and defining features of mild cognitive impairment (MCI) and dementia. In MCI, the cognitive deficits do not impair the capacity to live independently, in contrast to individuals with dementia that present pronounced functional deficits, such as the ones observed in Alzheimer’s disease (AD; (Pereira et al., 2010; Brown et al., 2011; Seelye et al., 2013). The most usual form to assess functional performance in older adults is the investigation of activities of daily living (ADL), common activities performed by the majority of older adults in a specific cultural setting (Lawton and Brody, 1969).
Prior studies have investigated the relationship between cognitive and functional performance in older adults with MCI or AD. Longitudinal changes in cognition are related to longitudinal changes in ADL (Farias et al., 2009). In a comprehensive review, Royall et al. (2007) showed a weak to moderate association between global cognitive measures and functional impairment. However, their results are heterogeneous with cognitive features responding for 0 to 80% of the variance in functional performance (mean of 21% with a SD of 20%; Gold, 2012). Methodological differences and sample characteristics might explain part of this excessive variability.
Gold (2012) discuss some of the methodological issues. The definition and the type of ADL investigated varies between studies. Some studies focuses on a unitary construct of ADL, in contrast with several evidences from the literature of a multidimensional construct involving activities of different levels of complexity (Thomas et al., 1998; Niti et al., 2007; Gold, 2012; de Paula et al., 2014). Beyond the usual distinction between Basic (BADL) and Instrumental (IADL) activities, some studies found different latent structures in ADL questionnaires and scales. Thomas et al. (1998) reported a multidimensional structure for BADL and IADL combined, proposing its interpretation based on levels of complexity (basic, intermediate, and complex). Niti et al. (2007) found a bifactorial structure for IADL (“physical” and “cognitive”) in a sample of Asian older adults with a significant influence of specific cultural aspects on participants’ responses. In a sample of low educated older adults from Brazil, de Paula et al. (2014) observed a different factorial solution with a “Domestic” IADL component and a “Complex” IADL component, in addition to a third component classified as BADL. Therefore, ADL may be a multidimensional construct with different components varying according to sample characteristics (Bootsma-van der Wiel et al., 2001; Cabrero-García and López-Pina, 2008; Fieo et al., 2011). Such differences might account for some of the high heterogeneity between studies reported in Royall et al. (2007). This emphasizes the importance of investigating specificities in ADL structure (Gold, 2012).
Methodological difficulties include instrument bias. Sikkes et al. (2009) reviewed the literature concerning the different measures of IADL focusing on its psychometric properties. Their results suggest that most of the scales adopted in clinical and research settings still lack of psychometric studies, reducing its validity and reliability for the functional assessment, although great effort is being dispend on this matter (Fieo et al., 2011). The source of information (e.g., patient vs. caregiver) is also not consensual in studies including cases of dementia, which is of extreme importance to the use and interpretation of scales measurement (Farias et al., 2005). Bootsma-van der Wiel et al. (2001) also highlight the conceptual difference between a “can do” or an “actually do” score in specific activities. Additionally, there is no consensus in the literature indicating scales and questionnaires as adequate methods to functional level measurement. There are more ecological measures of ADL, which involve the observation of the patient’s behavior on real life or simulated settings (a more precise estimation of functional performance; Chaytor and Schmitter-Edgecombe, 2003). However, these procedures also have limitations such as the higher costs of execution and the inherent complexity of the assessment, and might not be well suited for most of the clinical and research settings. The suitability of these ecological measures for studies involving cognitive performance is controversial. There are studies showing a stronger association of cognitive measures with ecological measures of ADL (Burton et al., 2006; Tam et al., 2008). However, the opposite pattern was also demonstrated with the relation between cognitive performance with ADL being stronger for ADL measured by scales/questionnaires (Cahn-Weiner et al., 2002; Mariani et al., 2008; de Paula et al., 2014). The cognitive processes assessed by the neuropsychological tests used in these studies might be associated with ADL in different and more specific ways (Gold, 2012).
Taking specific cognitive domains as predictors of functional performance most of the studies report associations with executive functions (Royall et al., 2007; Pereira et al., 2008; de Paula and Malloy-Diniz, 2013). This complex cognitive construct usually shows the strongest correlations with functional performance. Executive functions involve planning, initiation, monitoring, inhibition, and flexibility of goal-oriented behavior (Diamond, 2013). These specific aspects of executive functions may contribute differently to the ADL (Jefferson et al., 2006). However, other cognitive functions may contribute to specific aspects of ADL. Cognitive measures of spatial processing predicted participants’ performance in an ecological measure of visuospatial abilities in which the subject had to estimate distances, positions, and directions in a “real-life” setting developed by Farley et al. (2011). Activities demanding communicative skills were related to semantic process and language (Razani et al., 2011). Schmitter-Edgecombe et al. (2009) investigated different aspects of episodic memory and its association with ADL reporting significant associations of specific memory components with specific functional components. In this sense, although most of the studies have focused on executive functions or global cognitive measures, different kinds of ADL may depend on different cognitive abilities.
Depressive symptoms can also impair functional performance in older adults, usually in more complex activities (Bombin et al., 2012; Tomita and Burns, 2013; Zahodne et al., 2013; Park et al., 2014). Our group reported a weak association between depressive symptoms and functional performance in older adults with low formal education diagnosed with MCI or AD (de Paula and Malloy-Diniz, 2013). A stronger association was recently reported in a similar sample (Assis et al., 2014). Cahn-Weiner et al. (2002) found an association of depressive symptoms with ADL independent from cognitive functioning. Remission of depressive symptoms is associated with improvement of ADL (Nyunt et al., 2012). Nonetheless, there is evidence for the contrary, suggesting that depressive symptoms are not associated with IADL after controlling for cognitive symptoms (Reppermund et al., 2011; Wadsworth et al., 2012). Then, whether depressive symptoms affect functional performance by behavioral symptoms (depressed mood, lack of pleasure, apathy, and vegetative symptoms) or through cognitive impairment associated with depression remains unclear.
Functional deficit is one of the hallmarks of MCI and AD in older adults. Improving cognitive functions and mood symptoms may result in gains in functional performance. Therefore, a better understanding of how specific cognitive abilities and depressive symptoms contribute to the performance of specific ADL could be important to the development of tailored rehabilitation programs to improve daily functioning in individuals with cognitive disorders in a personalized way. The objective of the present study is to assess how specific cognitive abilities and symptoms of depression are associated to different aspects of ADL.
Materials and Methods
Participants
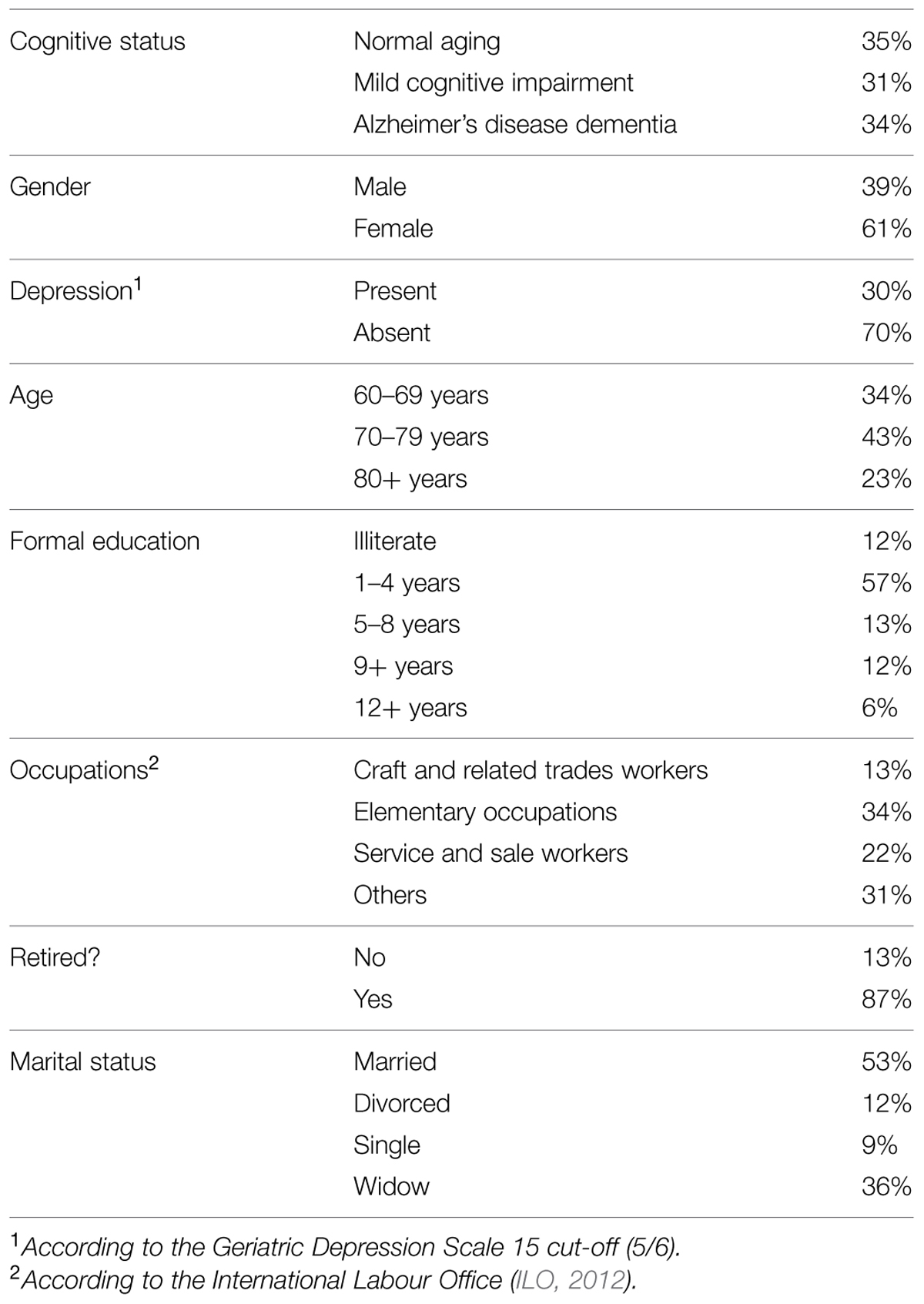
We evaluated 274 older adults from a public outpatient clinic specialized in cognitive disorders and frailty. The center usually receives elderly patients referred from primary-care physicians when they suspect of cognitive impairment, mental disorders, or multiple chronic diseases. Patients usually have a very low socioeconomic status and less than 4 years of formal education. Participants’ sociodemographic characteristics are shown in Table 1. A more detailed description of the typical profile of patients assessed in this center was published elsewhere (Bicalho et al., 2013; de Paula et al., 2013a).
The participants underwent a detailed clinical, cognitive, and behavioral assessment for diagnostic purposes as described below. During the geriatrician examination and the clinical neuropsychological assessment, the patients underwent cognitive, functional and behavioral assessment to determine their cognitive status. Ninety-three participants were diagnosed with mild Alzheimer’s disease dementia (AD), 85 patients were diagnosed with amnestic MCI (MCI) and 96 older adults were normal aging participants without clinical history, cognitive, or functional status suggestive of dementia or AD. AD was diagnosed by the NINCDS-ADRDA criteria for probable dementia (McKhann et al., 1984). Only patients with mild dementia, according to Clinical Dementia Rating (CDR; Morris, 1993), were invited for participation in this study. MCI diagnosis was based on a modified version of the Mayo Clinic diagnostic criteria (Petersen et al., 2001). Criteria for MCI was as follow:
(1) Subjective cognitive complaint, preferably corroborated by an informant/caregiver.
(2) Objective impairment on specific cognitive measures of the assessment battery for diagnosis according to Brazilian norms and cut-off scores (Porto et al., 2003; Nitrini et al., 2004). The cognitive battery includes the Verbal Learning Test of the CERAD Neuropsychological Battery (Morris et al., 1989), the memory test from the Brief Cognitive Battery (Nitrini et al., 2007), and subscales of the Mattis Dementia Rating Scale (Mattis, 1988).
(3) Normal global cognitive functioning (MMSE above the cut-off for dementia and CDR < 1).
(4) Preserved or minimal impairments in ADL assessed by a clinical interview and the CDR.
(5) Not demented based on the DSM-IV-TR criteria (American Psychiatric Association [APA], 2000).
All groups (i.e., AD, MCI, and control) were combined in a unique heterogeneous sample. This strategy was adopted to increase statistical power and to avoid detection loss of cognitive processes that are likely to underlie functional performance (Farias et al., 2009; Gold, 2012).
Cognitive, Functional, and Mood Assessment
We adopted an unstructured protocol of neuropsychological tests designed for the assessment of older adults with low formal education. The tests were not included in patients’ diagnosis. Cognitive composite factors were obtained through factor analysis from our sample (statistical procedures detailed below). This approach allows the assessment of different aspects of cognitive functioning (here, the core domains recommended for the MCI and AD diagnosis) with greater specificity. The protocol comprised tests of executive functions (Frontal Assessment Battery, Verbal Fluency tests, and Digit Span); language and semantic memory (Laboratory of Neuropsychological Investigations Naming Test – Nouns, Verbs and Professions, Token Test verbal comprehension component); episodic memory (components of learning, recognition, immediate, and delayed recall of the Rey Auditory-Verbal Learning Test); and visuospatial abilities (Clock Drawing Test, Stick Design Test, and Token Test visual attention components). These tests are valid and reliable for the assessment of older adults with a low educational background (de Paula et al., 2013a). The test measures are shown in Table 2.
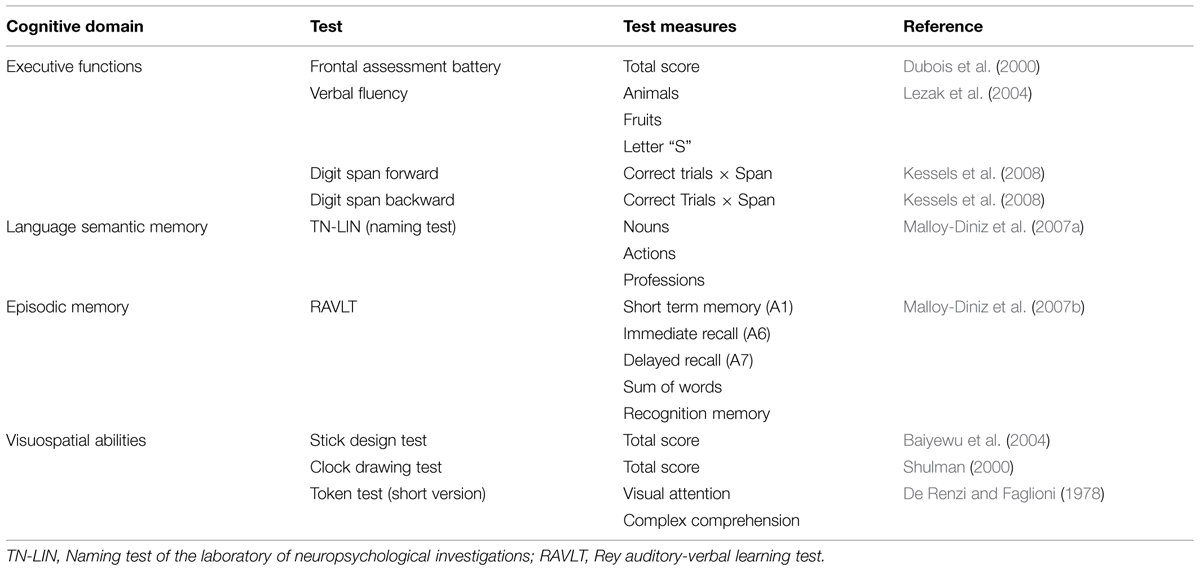
TABLE 2. Neuropsychological measures used to extract the four cognitive factors according to de Paula et al. (2013a).
The assessment of ADL occurred during the clinical and neuropsychological assessment by an interview with participants’ caregivers. For this study, we used the ‘General Activities of Daily Living Scale’ (GADL) (de Paula et al., 2014), a multidimensional functional measure of BADL/IADL based on the Lawton and Brody (1969) and Katz et al. (1970) indexes of ADL. The GADL shows a hierarchical structure with a general score and three components of more specific activities: a measure of BADL (Self-care: ability to change clothes, use the toilet, use the shower, transference from bed or chair, and feed itself) and two components of IADL. Instrumental Domestic ADL include ability to perform domestic chores, use the telephone, prepare meals, and do the laundry. Instrumental Complex ADL include ability to manage financial matters, shopping, adequate use of medication, and go out alone using transportation. This structure was determined by factor analysis on the same sample of the present study and showed evidence of reliability and validity (de Paula et al., 2014). We scored each activity using a 3-point Lickert scale (dependent, partially dependent, or independent of assistance to perform the activity). The GADL subscores of Self-care (0–10), Instrumental-Domestic (0–8), Instrumental-Complex (0–8), and the Global score (0–26) represented the general ADL measures in our study. To investigate the association of different cognitive functions with specific measures of functional performance, we also used each item of the scale (13 different ADL) independently.
We assessed the depressive symptoms with the Brazilian version of the Geriatric Depression Scale-15 (GDS-15; Sheikh and Yeasavage, 1986). A validation study conducted in Brazil attested its sensitivity and specificity for the detection of depression (Almeida and Almeida, 1999). However, since our focus was not to identify patients with major depressive disorder, but to use a dimensional measure of its symptoms, we used the GDS-15 total score in this research. The GDS use for depression diagnosis in dementia is controversial. To reduce biases we selected only patients with mild dementia for the AD group (CDR ≤ 1). Due to participants’ low formal education, the examiner read the GDS questions aloud to ensure the comprehension and validity of patients’ report.
Statistical Procedures
Our four cognitive domains were extracted by factor analysis (principal axis factoring with an oblique rotation of the neuropsychological tests described in Cognitive Assessment) from our sample. The procedures were described in detail elsewhere (de Paula et al., 2013a). Briefly, the cognitive factors were saved by a regression method and standardized (Z-Score) based on the performance of our cognitively normal non-depressed participants. The four factors were executive functions, episodic memory, language/semantic memory, and visuospatial Abilities. These factors showed high internal consistency and reliability (Cronbach’s alpha > 0.800 for all factors).
We carried out univariate analysis of variance (continuous variables) or chi-square tests (categorical variables) to evaluate baseline differences in sociodemographic, clinical, cognitive, and ADL measures between the AD, MCI, and control groups. Part of this data was previously published (de Paula et al., 2013a). For a preliminary assessment of the relationship between cognitive functioning, depressive level and ADL, we correlated each measure with the GADL global score. The influence of age, education, and gender in ADL was investigated by linear regression models (forced entry) containing each ADL measure as dependent variables. We also explored the pattern of association between cognitive performance and depressive symptoms through Pearson correlations to evaluate if the contribution of cognitive abilities and symptoms of depression to ADL performance is independent.
We used multiple linear regressions with a forced entry model to test whether the performance on specific cognitive domains and the intensity of depressive symptoms could predict the scores of the GADL components. Z-scores of executive functions, episodic memory, language/semantic memory, and visuospatial abilities, along with depressive symptoms (GDS-15 total score), were entered as independent variables in the models. In addition, we carried out ordinal regression analysis to assess whether the cognitive factors and depressive level predict the performance on each specific item of the GADL. Effect sizes were estimated by the adjusted R2 (linear regression) or Nagelkerke Pseudo-R2 (ordinal regression).
Results
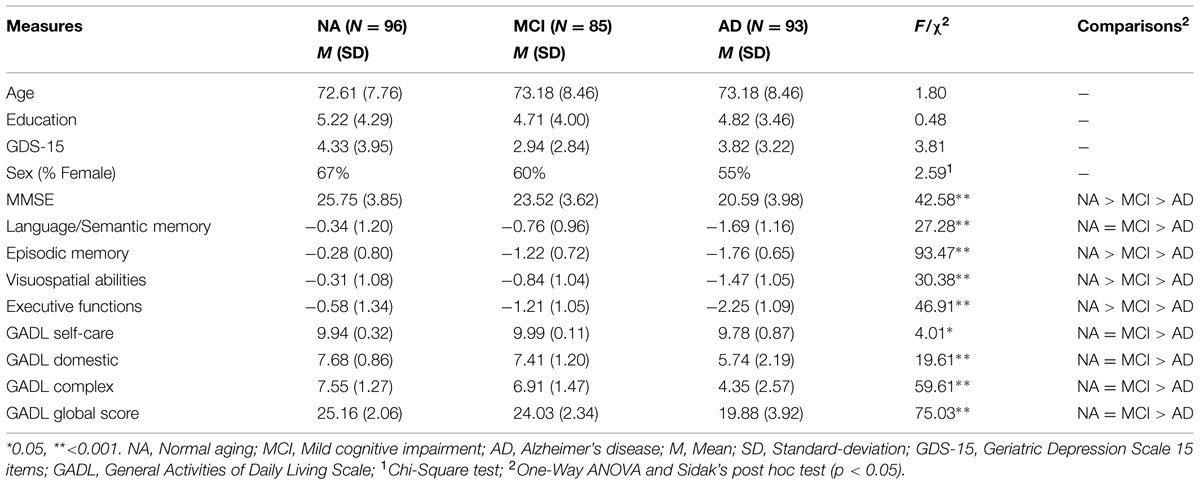
Table 3 shows the sociodemographic, clinical data, ADL, and Z-scores for individual cognitive domains, according to diagnosis. Group comparisons indicate no differences in sociodemographic measures (p > 0.05), but significant differences in cognitive (p < 0.01), and ADL (p < 0.05) measures. The normal aging group outperformed both MCI and AD groups in cognitive measures, except for language/semantic memory compared with MCI patients. MCI patients showed higher scores than AD patients in all cognitive measures. Differences in ADL occurred only between AD and the other groups.
Correlations between each cognitive factor, depressive symptoms and the global score of the GADL are show in Figures 1 and 2. All correlations between cognitive measures and ADL were significant (p < 0.001) and moderate, but we found only a weak correlation between depressive symptoms and the functional measure (r = -0.151, p = 0.013). The correlations between depressive symptoms and cognitive performance were significant for executive functions (r = -0.192, p = 0.001), but we found no association with episodic memory (r = -0.019, p = 0.753), language/semantic memory (r = -0.085, p = 0.162) and visuospatial abilities (r = -0.039, p = 0.525). The influence of sociodemographic factors (age, education and gender) on ADL performance was not significant: GADL Complex (F = 1.31, p = 0.272), GADL Domestic (F = 1.13, p = 0.336), GADL Self-care (F = 1.56, p = 0.200), and the global score (F = 2.40, p = 0.068).
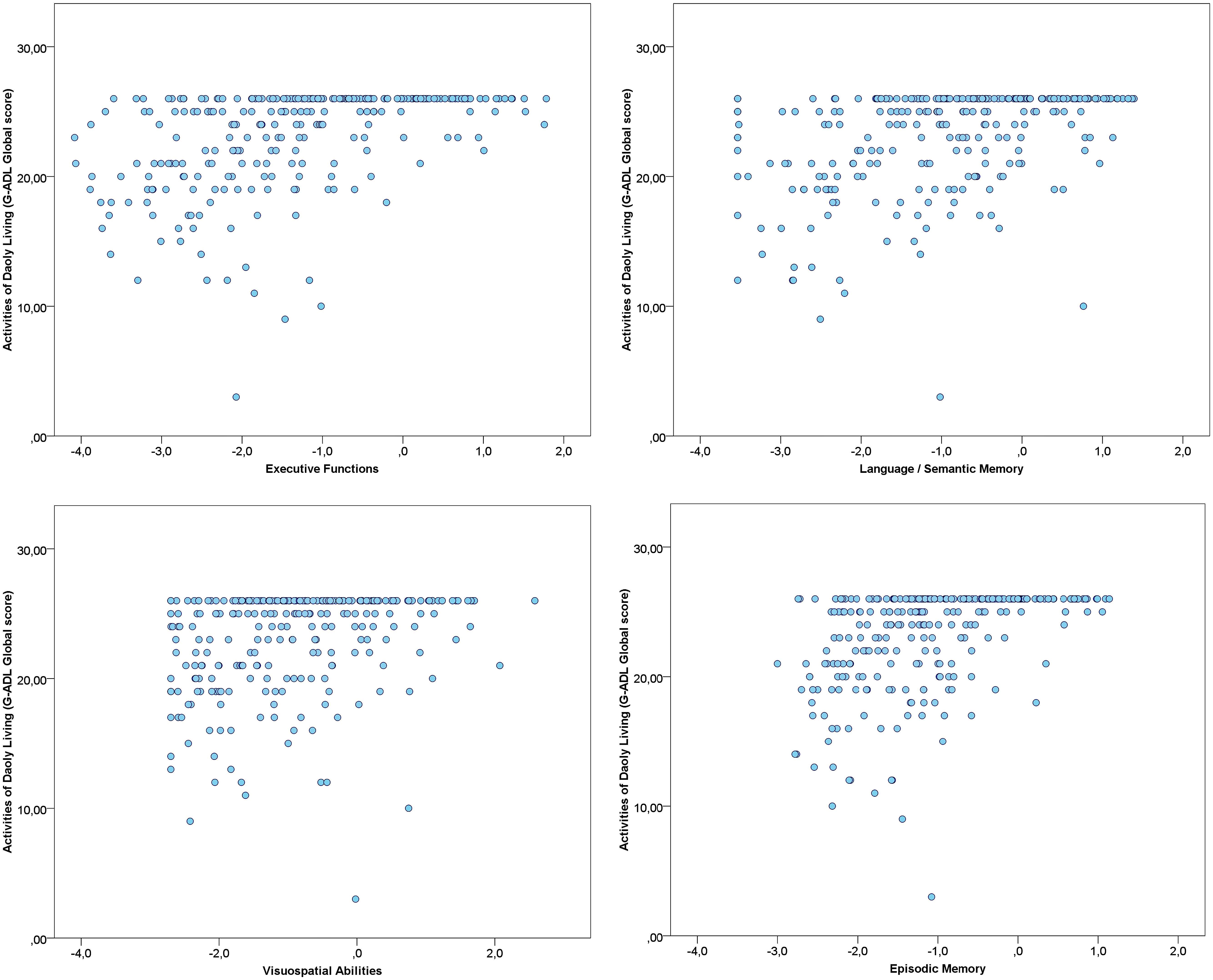
FIGURE 1. Association between Cognitive Factors and General Activities of Daily Living Scale (GADL). The correlations of cognitive factors with functional performance were all significant (p < 0.001). Executive functions showed the strongest correlation with GADL total score (r = 0.478), followed by episodic memory (r = 0.449), language/semantic memory (r = 0.410), and visuospatial abilities (r = 0.302). The number of dots in the scatterplot differs from the sample size due to superposed values.
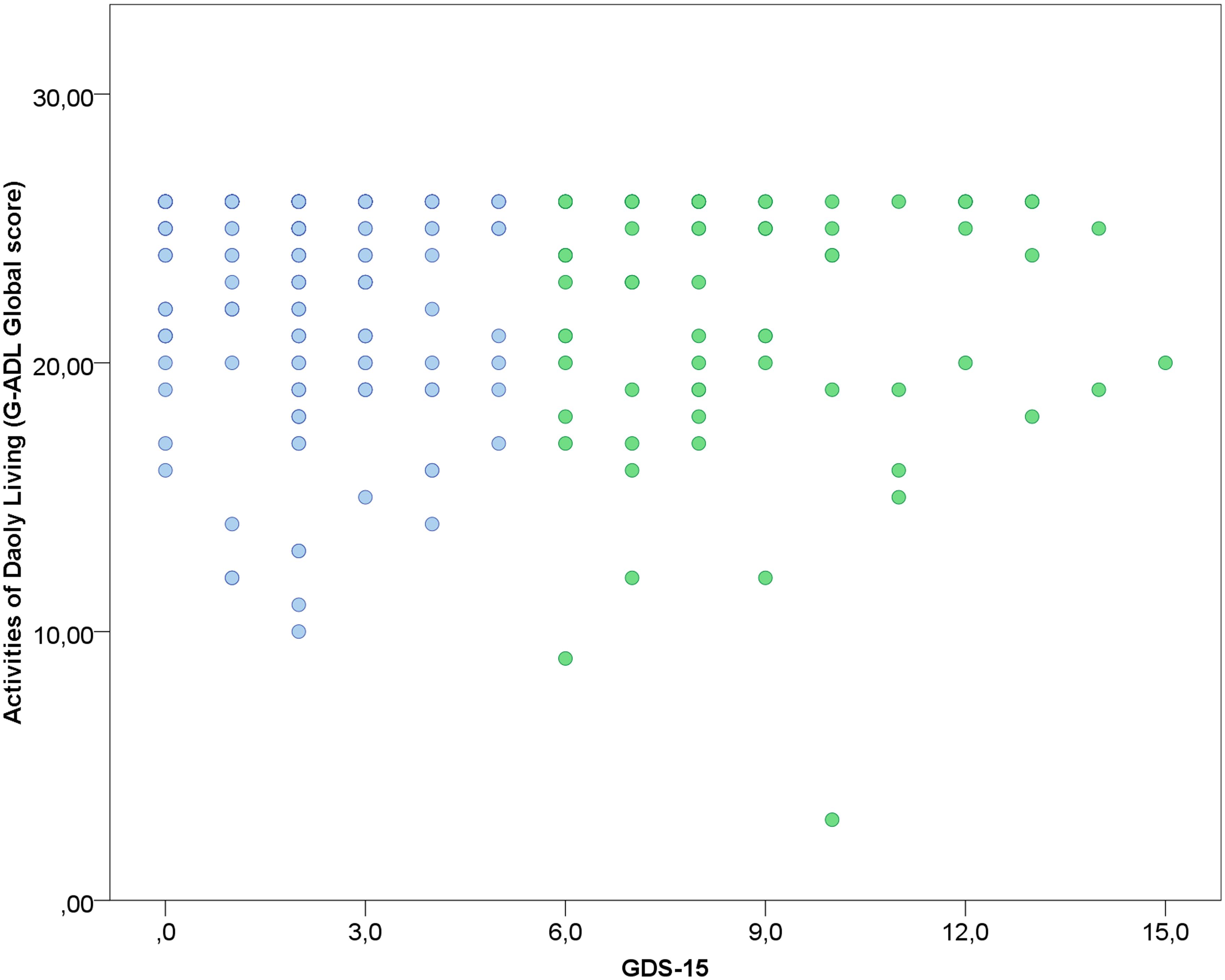
FIGURE 2. Association between depression and GADL scores. A weak correlation was observed between depressive status and GADL total score (r = -0.151, p = 0.013). In the scatterplot, blue dots represent the participants under the cut-off for depression according to the Geriatric Depression Scale and green dots the participants above the cut-off for depression. The number of dots in the scatterplot differs from the sample size due to superposed values.
Table 4 shows the predictors of GADL subscales scores. For the global score of the GADL the model was significant (F = 22.90, p < 0.001, R2 = 0.29) and contained as predictors executive functions (p < 0.001), episodic memory (p < 0.001), and language/semantic memory (p = 0.011). The Self-Care model was not significant (F = 1.37, p = 0.234, R2 < 0.01). The model for Instrumental-Complex ADL was significant (F = 24.66, p < 0.001, R2 = 0.30) and contained as predictors executive functions (p = 0.007), episodic memory (p < 0.001), and language/semantic memory (p = 0.001). The model for Instrumental-Domestic was also significant (F = 14.39, p < 0.001, R2 = 0.19) and involved executive functions (p < 0.001) and episodic memory (p-0.009) as significant predictors.
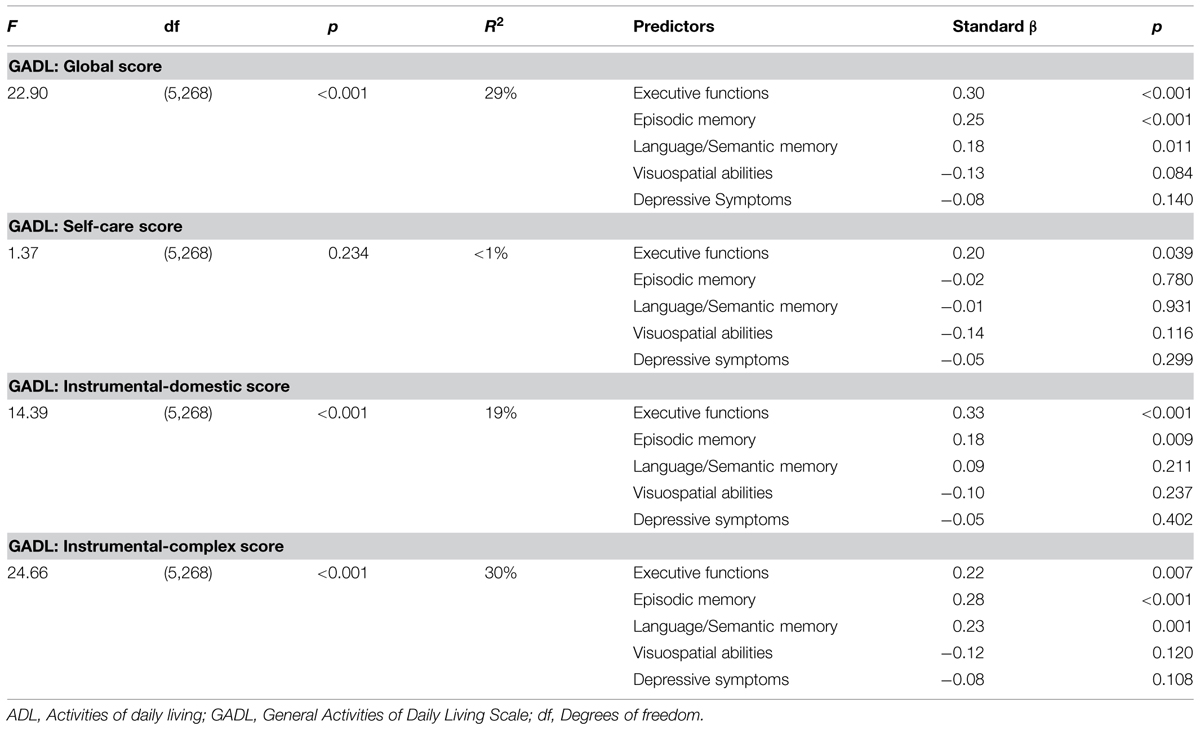
TABLE 4. Linear regression models of cognitive function and depressive symptoms as predictors of different ADL.
Tables 5 and 6 show the role of specific cognitive factors and depressive symptoms as predictors of specific IADL. Since the cognitive factors and depressive symptoms were unrelated to GADL Self-Care scores, the analyses were carried out for Instrumental-Domestic and Instrumental-Complex activities only.
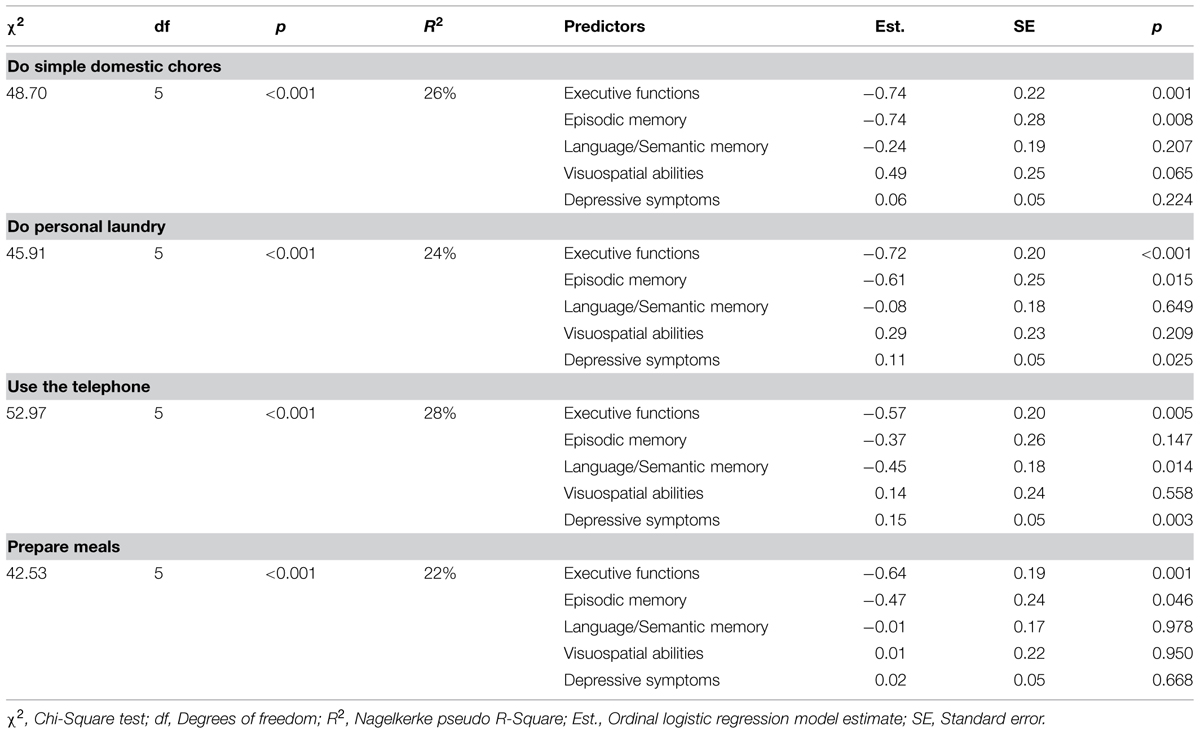
TABLE 5. Ordinal regression analysis of cognitive functions and depressive symptoms as predictors of Instrumental-Domestic activities of daily living.
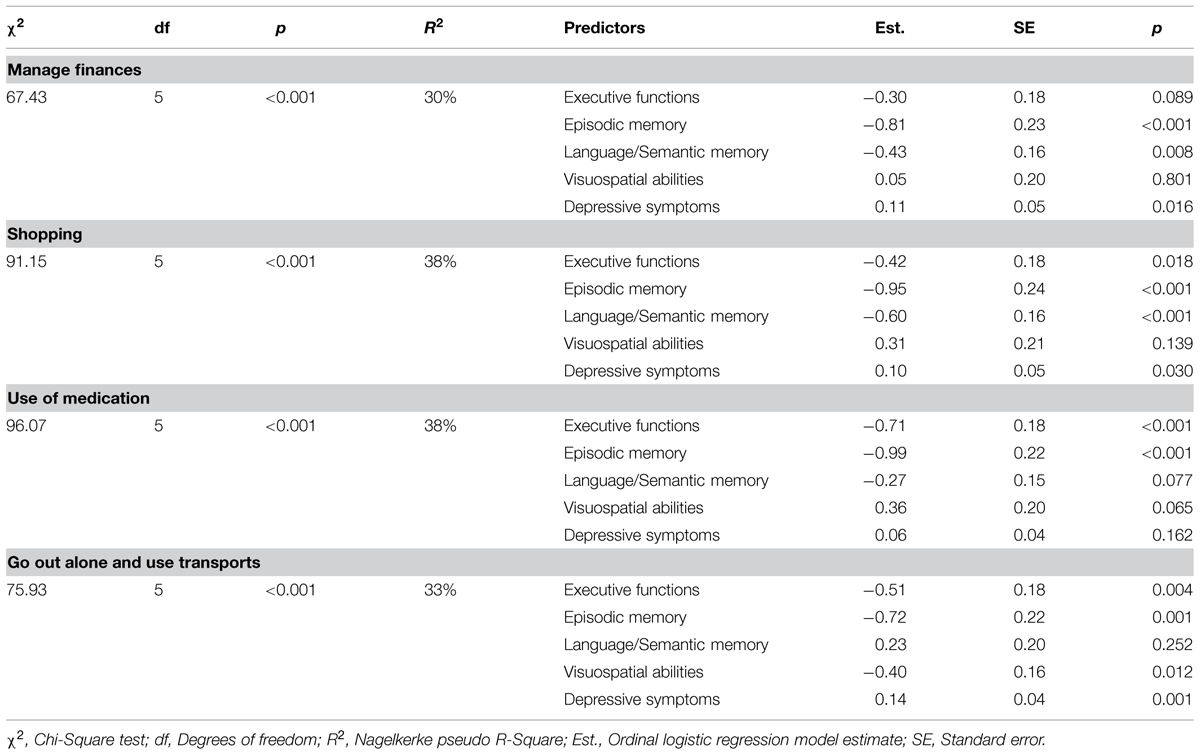
TABLE 6. Ordinal regression analysis of cognitive functions and depressive symptoms as predictors of Instrumental-Complex activities of daily living.
All Instrumental-Domestic activities were associated with executive functions (p < 0.05) and, except for the independence in doing personal laundry, with episodic memory (p < 0.05). Language/semantic memory was related only to the correct use of the telephone (p = 0.014). Visuospatial abilities were not significantly related to any Domestic ADL (all p > 0.05). Depressive symptoms were predictors of doing personal laundry (p = 0.025) and difficulties using the telephone (p = 0.003). The effect sizes for the comparisons were moderate-large, ranging from 22 to 28% of explained variance.
Episodic memory was a significant predictor of all Instrumental-Complex ADL (p < 0.05). Executive Functions followed a similar pattern (p < 0.05), but was not predictive of the individual’s ability to manage finances (p = 0.089). Language/Semantic Memory was a significant predictor of independence in performing simple shopping (p < 0.001) and managing finances (p = 0.008). The Visuospatial abilities were a significant predictor of the ability to go out alone and use transportation (p = 0.012). Depressive symptoms predicted the ability to shop (p = 0.030), handle financial matters (p = 0.016), and go out alone using transportation (p < 0.001), but not the medication management (p = 0.162). The effect sizes of these models were large, ranging from 30 to 38% of explained variance.
Discussion
In the present study, we showed distinct cognitive domains having a significant impact on ADL in older adults with a wide range of cognitive deficits. Executive functioning and Episodic Memory showed the strongest significant association with functional performance. Language/Semantic Memory contributed to complex aspects of ADL and visuospatial abilities contributed only to a specific instrumental activity. Depressive symptoms had a significant influence on more complex ADL such as handling finances. Self-care ADL were not related to cognitive performance. Executive functions showed only a weak correlation with depressive symptoms. The results are in agreement with previous studies and highlight the close relationship between deficits in specific cognitive domains and functional loss.
Episodic memory and executive functions were the most important predictors of domestic ADL performance. The execution of these activities requires skills related to the identification and ordering of different steps necessary to achieve the final goal (e.g., different steps to prepare a meal) or recalling information after a period of time or in face of distractors (e.g., remembering what of house cleaning was already done and what was not). These behaviors are intrinsically related to different aspects of executive functioning and episodic memory.
Similar to our findings, previous studies showed executive functions and episodic memory tests as significant predictors of the ability to cook and to do household chores (Farias et al., 2003; Matsuda and Saito, 2005; Mariani et al., 2008). Jefferson et al. (2006) found association between verbal fluency and food preparing, cognitive flexibility, and selective attention with doing laundry, but no correlations between executive functions and house cleaning. An interesting research using extrapyramidal signs and structural brain imaging found that, controlling these previous factors and sociodemographic aspects, the performance in tests of memory and executive functions was still associated with cooking, and specific measures of executive functions with the ability to perform simple domestic chores (Bennett et al., 2006). We found an association between depressive symptoms with doing laundry. We hypothesize that this might be a sample bias. Most of the older adults assessed in our study had a very low socioeconomic level and usually do not have laundry machines. Since they do the laundry manually, the association with depressive symptoms may be due to lack of energy or apathy since this activity is very physically demanding.
We found significant associations between executive functions, language/semantic memory, depressive symptoms, and telephone use. The engagement of these specific cognitive functions may reflect the necessity of communication to perform this activity. The neuropsychological battery used in this study included instruments related to expressive language, comprehension, and access to semantic and phonological lexicons. Therefore, we expected that ADL related to communicative skills would be influenced by the performance on these cognitive domains (Taler and Phillips, 2008; Razani et al., 2011). Farias et al. (2003), however, found motor praxis as the only predictor of telephone use. Depressive symptoms also influenced telephone use in our study. Social isolation, a common characteristic of elderly persons with depression (Corcoran et al., 2013), may reduce the individual willingness to actively pursue contact with other people, leading to impairments in this specific activity.
Two of the Complex-ADL activities involve management of finances. Episodic memory and language/semantic memory predicted financial management, while executive functions, episodic memory, and language/semantic memory predicted shopping ability. These are complex activities and involve several cognitive processes (Marson et al., 2009). Sherod et al. (2009) decomposed financial managing it in basic financial skills, financial conceptual knowledge, financial transactions, checkbook control, banking control, and financial judgment. The authors identified the cognitive predictors of financial capacity in the spectrum of normal aging, MCI, and AD using a specific questionnaire for financial management and a comprehensive battery of neuropsychological tests. Their findings suggest that arithmetic skills (which relies on working memory) are the main predictor of financial capacity. Jefferson et al. (2006) found an association between selective attention and the ability to shop and to control finances. Tasks related to episodic memory, basic math skills, and a test related to language/semantic memory predicted financial control in Matsuda and Saito (2005) study. Razani et al. (2011) evaluated skills related to the management of money and found similar predictors to the present study: how to write out checks was associated with language, control the checkbook was related to executive functions, and shopping ability was associated with memory and executive functions. Motor praxis also might be related to financial management (Farias et al., 2003). Additionally, we observed depressive symptoms predicting worse performance in the management of finances. This finding is in contrast to Farias et al. (2003) who found no significant association between depressive symptoms and management of finances.
The correct use of medications was associated with executive functions and episodic memory in the present research in accordance with previous studies (Maddigan et al., 2003; Sino et al., 2014). A very common complaint by patients with memory impairment is to forget when to take medications or difficult to remember if he/she has already take it or not. This emphasizes the importance of different aspects of the episodic memory for the correct maintenance of medical care routine (Matsuda and Saito, 2005). Complex medication routines may demand more executive control (Maddigan et al., 2003). A study reported that performance in executive functions tests (including working memory) was a significant predictor of medication use in older adults (Insel et al., 2006). However, Jefferson et al. (2006) did not find any significant associations in this direction. Compensatory strategies might explain these discrepancies. Carlson et al. (2005) tested two objective measures of medication use capacity (schedule and pillbox) and found a significant association between memory performance and the schedule strategy and between executive functions and the pillbox.
We found executive functions, episodic memory, visuospatial skills, and depressive symptoms as predictors of ADL related to going out of home alone to distant locations using transportation. Different aspects of executive functions such as planning, cognitive flexibility, and selective attention were associated with transportation in the study of Jeferson et al. (2006). Deficits in these functions were associated with impairment in “visually” dependent activities such as driving, orientation, and transport use (Silva et al., 2009; Farley et al., 2011). However, despite the expected relationship between visuospatial abilities and the ability to travel long distances, few studies have found a significant direct association between the neuropsychological performance and its functional counterpart. Our findings provide a model in which visuospatial and navigation abilities depend on executive functions, episodic memory, and visuospatial skills. Matsuda and Saito (2005) found similar results in the Japanese population. Depressive symptoms also predicted performance in the ability to travel. As in telephone use, social isolation might mediate the association between depressive symptoms and functional performance on this task.
In our view, the strengths of the current study are the relatively large sample size, the heterogeneity of the participants and the use of fine-grained cognitive and functional measures. The use of cognitive factors validated for this population instead of tests’ raw scores allow the construction of more precise conceptual models and is easier to be generalized for other settings, since the data is analyzed in the cognitive construct level and can be represented by different cognitive tests. However, the present results should be viewed in light of its limitations. Although the neuropsychological measures used comprise four specific cognitive domains, the protocol had no specific measure of processing speed, a cognitive domain related to functional performance and a potential mediator of depression influence on daily functioning (Brown et al., 2013). The executive functions factor adopted in this study may be related to processing speed in our population since processing speed and executive functions influence verbal fluency in an independent way in older adults with low formal education (de Paula et al., 2013b). Therefore, the relationship between the executive function domain and functional performance could be secondary to its processing speed component. Additional studies including specific measures of processing speed should evaluate its impact on ADL. Additionally, the present study has a cross-sectional design, which limits the interpretation of our findings. Future studies with a longitudinal design are necessary to assess the impact of changes in specific cognitive functions on the performance of specific ADL. Another aspect is the lack of an ecological functional measure since our work relies on scales of caregiver report, which may result in different results.
Conclusion
We found executive function and episodic memory as the cognitive domains most frequently related to impairment in general constructs of ADL. Nonetheless, language, semantic memory, and visuospatial abilities may influence specific functional aspects of ADL. The development of better predictive models can aid to the development of tailored and personalized rehabilitation programs to improve functional performance of subjects with neurocognitive disorders.
Funding
This work was supported by the following grants: APQ-01972/12-10, APQ-02755-10, APQ-04706-10, CBB-APQ-00075-09 from FAPEMIG, and 573646/2008-2 from CNPq. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Almeida, O. P., and Almeida, S. A. (1999). Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int. J. Geriatr. Psychiatry 14, 858–865. doi: 10.1002/(SICI)1099-1166(199910)14:10<858::AID-GPS35>3.0.CO;2-8
American Psychiatric Association [APA]. (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: Author.
Assis, L. O., de Paula, J. J., Assis, M. G., Moraes, E. N., and Malloy-Diniz, L. F. (2014). Psychometric properties of the Brazilian version of Pfeffer’s Functional Activities Questionnaire. Front. Aging Neurosci. 6:255. doi: 10.3389/fnagi.2014.00255
Baiyewu, O., Unverzagt, F. W., Lane, K. A., Gureje, O., Ogunniyi, A., Musick, B., et al. (2004). The stick design test: a new measure of visuoconstructional ability. J. Int. Neuropsychol. Soc. 11, 598–605.
Bennett, H. P., Piguet, O., Grayson, D. A., Creasey, H., Waite, L. M., Lye, T., et al. (2006). Cognitive, extrapyramidal, and magnetic resonance imaging predictors of functional impairment in nondemented older community dwellers: the sydney older person study. J. Am. Geriatrc. Soc. 54, 3–10. doi: 10.1111/j.1532-5415.2005.00532.x
Bicalho, M. A. C., Pimenta, F. A., Bastos-Rodrigues, L., Hansen, E. O., Neves, S. N., Melo, M., et al. (2013). Sociodemographic characteristics, clinical factors, and genetic polymorphisms associated with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 28, 640–646. doi: 10.1002/gps.3875
Bombin, I., Santiago-Ramajo, S., Garolera, M., Vega-González, E. M., Cerulla, N., Caracuel, A., et al. (2012). Functional impairment as a defining feature of: amnestic MCI cognitive, emotional, and demographic correlates. Int. Psychogeriatr. 24, 1494–1504. doi: 10.1017/S1041610212000622
Bootsma-van der Wiel, A., Gussekloo, J., de Craen, A. J., van Exel, E., Knook, D. L., Lagaay, A. M., et al. (2001). Disability in the oldest old: “can do” or “do do”? Am. Geriatr. Soc. 49, 909–914. doi: 10.1046/j.1532-5415.2001.49181.x
Brown, P. J., Devanand, D. P., Liu, X., Caccappolo, E., and Alzheimer’s disease Neuroimaging Initiative. (2011). Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch. Gen. Psychiatry 68, 617–626. doi: 10.1001/archgenpsychiatry.2011.57
Brown, P. J., Liu, X., Sneed, J. R., Pimontel, M. A., Devanand, D. P., and Roose, S. P. (2013). Speed of processing and depression affect function in older adults with mild cognitive impairment. Am. J. Geriatr. Psychiatry 21, 675–684. doi: 10.1016/j.jagp.2013.01.005
Burton, C. L., Strauss, E., Hultsch, D. F., and Hunter, M. A. (2006). Cognitive functioning and everyday problem solving in older adults. Clin. Neuropsychol. 20, 432–452. doi: 10.1080/13854040590967063
Cabrero-García, J., and López-Pina, J. A. (2008). Aggregated measures of functional disability in a nationally representative sample of disabled people: analysis of dimensionality according to gender and severity of disability. Qual. Life Res. 17, 425–436. doi: 10.1007/s11136-008-9313-x
Cahn-Weiner, D. A., Boyle, P. A., and Malloy, P. F. (2002). Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl. Neuropsychol. 9, 187–191. doi: 10.1207/S15324826AN0903_8
Carlson, M. C., Fried, L. P., Xue, Q. L., Tekwe, C., and Brandt, J. (2005). Validation of the Hopkins Medication Schedule to identify difficulties in taking medications. J. Gerontol. A Biol. Sci. Med. Sci. 60, 201–223. doi: 10.1093/gerona/60.2.217
Chaytor, N., and Schmitter-Edgecombe, M. (2003). The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychol. Rev. 13, 181–197. doi: 10.1023/B:NERV.0000009483.91468.fb
Corcoran, J., Brown, E., Davis, M., Pineda, M., Kadolph, J., and Bell, H. (2013). Depression in older adults: a Meta-Synthesis. J. Gerontol. Soc. Work 56, 509–534. doi: 10.1080/01634372.2013.811144
de Paula, J. J., Bertola, L., Ávila, R. T., Moreira, L., Coutinho, G., de Moraes, E. N., et al. (2013a). Clinical applicability and cutoff values for an unstructured neuropsychological assessment protocol for older adults with low formal education. PLoS ONE 8:e73167. doi: 10.1371/journal.pone.0073167
de Paula, J. J., Costa, D. S., Bertola, L., Moraes, E. N., and Malloy-Diniz, L. F. (2013b). Verbal fluency in older adults with low educational level: what is the role of executive functions and processing speed? Rev. Bras. Psiquiatr. 35, 440–442. doi: 10.1590/1516-4446-2013-1118
de Paula, J. J., Bertola, L., Ávila, R. T., Assis Lde, O., Albuquerque, M., and Bicalho, M. A. et al. (2014). Development validity and reliability of the General Activities of Daily Living Scale: a multidimensional measure of activities of daily living for older people. Rev. Bras. Psiquiatr. 36, 143–152. doi: 10.1590/1516-4446-2012-1003
de Paula, J. J., and Malloy-Diniz, L. F. (2013). Executive functions as predictors of functional performance in mild Alzheimer’s dementia and mild cognitive impairment elderly. Estud. Psicol. (Natal) 18, 117–124. doi: 10.1590/S1413-294X2013000100019
De Renzi, E., and Faglioni, P. (1978). Normative Data and screening power of a shortened version of the token test. Cortex 14, 41–49. doi: 10.1016/S0010-9452(78)80006-9
Diamond, A. (2013). Executive Functions. Annu. Rev. Psychol. 64, 35–68. doi: 10.1146/annurev-psych-113011-143750
Dubois, B., Slachevsky, A., Litvan, I., and Pilon, B. (2000). The FAB: a frontal assessment battery at bedside. Neurology 55, 1621–1626. doi: 10.1212/WNL.55.11.1621
Farias, S., Cahn-Weiner, D. A., Harvey, D. J., Reed, B. R., Mungas, D., Kramer, J. H., et al. (2009). Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. Clin. Neuropsychol. 23, 446–461. doi: 10.1080/13854040802360558
Farias, S. T., Harrell, E., Neumann, C., and Houtz, A. (2003). The relationship between neuropsychological performance and daily functioning in individuals with Alzheimer’s disease: ecological validity of neuropsychological tests. Arch. Clin. Neuropsychol. 18, 655–672. doi: 10.1016/S0887-6177(02)00159-2
Farias, S., Mungas, D., and Jagust, W. (2005). Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int. J. Geriatr. Psychiatry 20, 827–834. doi: 10.1002/gps.1367
Farley, K. L., Higginson, C. I., Sherman, M. F., and MacDougall, E. (2011). The ecological validity of clinical tests of visuospatial function in community-dwelling older adults. Arch. Clin. Neuropsychol. 26, 728–738. doi: 10.1093/arclin/acr069
Fieo, R. A., Austin, E. J., Starr, J. M., and Deary, I. J. (2011). Calibrating ADL-IADL scales to improve measurement accuracy and to extend the disability construct into the preclinical range: a systematic review. BMC Geriatr. 11:42. doi: 10.1186/1471-2318-11-42
Gold, D. A. (2012). An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J. Clin. Exp. Neuropsychol. 34, 11–34. doi: 10.1080/13803395.2011.614598
Insel, K., Morrow, D., Brewer, B., and Figueredo, A. (2006). Executive function, working memory, and medication adherence among older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 61, 102–107. doi: 10.1093/geronb/61.2.P102
International Labour Office (ILO). (2012). International Standard Classification of Occupations. Geneva: ILO Publications.
Jefferson, A. L., Paul, R. H., Ozonoff, A., and Cohen, R. A. (2006). Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs). Arch. Clin. Neuropsychol. 21, 311–320. doi: 10.1016/j.acn.2006.03.007
Katz, S., Downs, T. D., Cash, H. R., and Grotz, R. C. (1970). Progress in the development of the index of ADL. Gerontologist 10, 20–30. doi: 10.1093/geront/10.1_Part_1.20
Kessels, R. P., van den Berg, E., Ruis, C., and Brands, A. (2008). The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment 15, 426–434. doi: 10.1177/1073191108315611
Lawton, M. P., and Brody, E. M. (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. doi: 10.1093/geront/9.3_Part_1.179
Lezak, M. D., Howieson, D. B., and Loring, D. W. (2004). Neuropsychological Assessment, 3rd Edn. New York, NY: Oxford University Press.
Maddigan, S. L., Farris, K. B., Keating, N., Wiens, C. A., and Johnson, J. A. (2003). Predictors of older adults’ capacity for medication management in a self-medication program: a retrospective chart review. J. Aging Health 15, 332–352. doi: 10.1177/0898264303251893
Malloy-Diniz, L. F., Bentes, R. C., Figueiredo, P. M., Brandão-Bretas, D., Costa-Abrantes, S., Parizzi, A. M., et al. (2007a). Normalización de una batería de tests para evaluar las habilidades de comprensión del lenguaje, fluidez verbal y denominación en niños brasileños de 7 a 10 años: resultados preliminares. Rev. Neurol. 44, 275–280.
Malloy-Diniz, L. F., Lasmar, V. A. P., Gazinelli, L. S. R., Fuentes, D., and Salgado, J. V. (2007b). The Rey Auditory-Verbal Learning Test: applicability for the Brazilian elderly population. Rev. Bras. Psiquiatr. 29, 324–329. doi: 10.1590/S1516-44462006005000053
Mariani, E., Monastero, R., Ercolani, S., Rinaldi, P., Mangialasche, F., Costanzi, E., et al. (2008). Influence of comorbidity and cognitive status on instrumental activities of daily living in amnestic mild cognitive impairment: results from the ReGA1 project. Int. J. Geriatr. Psychiatry 23, 523–530. doi: 10.1002/gps.1932
Marson, D. C., Martin, R. C., Wadley, V., Griffith, H. R., Snyder, S., Goode, P. S., et al. (2009). Clinical Interview assessment of financial capacity in older adults with mild cognitive impairment and Alzheimer’s disease. Am. Geriatr. Soc. 57, 806–814. doi: 10.1111/j.1532-5415.2009.02202.x
Matsuda, O., and Saito, M. (2005). Functional competence and Cognitive ability in mild Alzheimer’s disease: relationship between ADL assessed by a relative/carer-rated scale and neuropsychological performance. Int. Psychogeriatr. 17, 275–288. doi: 10.1017/S1041610205001304
Mattis, S. (1988). Dementia Rating Scale. Professional Manual. Florida: Psychological Assessment Resources.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/WNL.34.7.939
Morris, J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/WNL.43.11.2412-a
Morris, J. C., Heyman, A., Mohs, R. C., Hughes, J. P., van Belle, G., Fillenbaum, G., et al. (1989). The Consortium to Establish a Registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. doi: 10.1212/WNL.39.9.1159
Niti, M., Ng, T. P., Chiam, P. C., and Kua, E. H. (2007). Item bias was present in instrumental activities of daily living scale in Asian older adults. J. Clin. Epidemiol. 60, 366–374. doi: 10.1016/j.jclinepi.2006.07.012
Nitrini, R., Caramelli, P., Herrera, E. Jr., Porto, C. S., Charchat-Fichman, H., Carthery, M. T., et al. (2004). Performance of illiterate and literate nondemented elderly subjects in two tests of long-term memory. J. Int. Neuropsychol. Soc. 10, 634–638. doi: 10.1017/S1355617704104062
Nitrini, R., Caramelli, P., Porto, C. S., Charchat-Fichman, H., Formigoni, A. P., Carthery-Goulart, M. T., et al. (2007). Brief cognitive battery in the diagnosis of mild Alzheimer’s disease in subjects with medium and high levels of education. Dement. Neuropsychol. 1, 32–36.
Nyunt, M. S., Lim, M. L., Yap, K. B., and Ng, T. P. (2012). Changes in depressive symptoms and functional disability among community-dwelling depressive older adults. Int. Psychogeriatr. 24, 1633–1641. doi: 10.1017/S1041610212000890
Park, B., Jun, J. K., and Park, J. (2014). Cognitive impairment and depression in the early 60s: which is more problematic in terms of instrumental activities of daily living? Geriatr. Gerontol. Int. 14, 62–70. doi: 10.1111/ggi.12055
Pereira, F. S., Yassuda, M. S., Oliveira, A. M., Diniz, B. S., Radanovic, M., Talib, L. L., et al. (2010). Profiles of functional deficits in mild cognitive impairment and dementia: benefits from objective measurement. J. Int. Neuropsychol. Soc. 16, 297–305. doi: 10.1017/S1355617709991330
Pereira, F. S., Yassuda, M. S., Oliveira, A. M., and Forlenza, O. V. (2008). Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. Int. Psychogeriatr. 20, 1104–1115. doi: 10.1017/S1041610208007631
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Porto, C. S., Fichman, H. C., Caramelli, P., Bahia, V. S., and Nitrini, R. (2003). Brazilian version of the Mattis Dementia Rating Scale: Diagnosis of mild dementia in Alzheimer’s disease. Arq. Neuropsiquiatr. 61, 339–345. doi: 10.1590/S0004-282X2003000300004
Razani, J., Bayan, S., Funes, C., Mahmoud, N., Torrence, N., Wong, J., et al. (2011). Pattern of deficits in daily functioning and cognitive performance of patients with Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 24, 23–32. doi: 10.1177/0891988710390812
Reppermund, S., Brodaty, H., Crawford, J. D., Kochan, N. A., Slavin, M. J., Trollor, J. N., et al. (2011). The relationship of current depressive symptoms and past depression with cognitive impairment and instrumental activities of daily living in an elderly population: the Sydney Memory and Ageing Study. J. Psychiatr. Res. 45, 1600–1607. doi: 10.1016/j.jpsychires.2011.08.001
Royall, D. R., Lauterbach, E. C., Kaufer, D., Malloy, P., Coburn, K. L., Black, K. J., et al. (2007). The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J. Neuropsychiatry Clin. Neurosci. 19, 249–265. doi: 10.1176/appi.neuropsych.19.3.249
Schmitter-Edgecombe, M., Woo, E., and Greeley, D. R. (2009). Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology 23, 168–177. doi: 10.1037/a0014186
Seelye, A. M., Schmitter-Edgecombe, M., Cook, D. J., and Crandall, A. (2013). Naturalistic assessment of everyday activities and prompting technologies in mild cognitive impairment. J. Int. Neuropsychol. Soc. 19, 442–452. doi: 10.1017/S135561771200149X
Sheikh, J. I., and Yeasavage, J. A. (1986). Geriatric Depression Scale (GDS): Recent Evidence and Development of a shorter version. Clin. Gerontol. 5, 165–172. doi: 10.1300/J018v05n01_09
Sherod, M. G., Griffith, H. R., Copeland, J., Belue, K., Krzywanski, S., Zamrini, E. Y., et al. (2009). Neurocognitive predictors of financial capacity across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer’s disease. J. Int. Neuropsychol. Soc. 15, 528–267. doi: 10.1017/S1355617709090365
Shulman, K. (2000). Clock-drawing: is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 15, 548–561. doi: 10.1002/1099-1166(200006)15:6<548::AID-GPS242>3.0.CO;2-U
Sikkes, S. A., de Lange-de Klerk, E. S., Pijnenburg, Y. A., Scheltens, P., and Uitdehaag, B. M. (2009). A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement. J. Neurol. Neurosurg. Psychiatry 80, 7–12. doi: 10.1136/jnnp.2008.155838
Silva, M. T., Laks, J., and Engeldardt, E. (2009). Neuropsychological tests and driving in dementia: a review of the recent literature. Rev. Assoc. Med. Bras. 55, 484–488. doi: 10.1590/S0104-42302009000400027
Sino, C. G., Sietzema, M., Egberts, T. C., and Schuurmans, M. J. (2014). Medication management capacity in relation to cognition and self-management skills in older people on polypharmacy. J. Nutr. Health Aging 18, 44–49. doi: 10.1007/s12603-013-0359-2
Taler, V., and Phillips, N. A. (2008). Language performance in Alzheimer’s disease and mild cognitive impairment: a comparative review. J. Clin. Exp. Neuropsychol. 30, 501–556. doi: 10.1080/13803390701550128
Tam, C. W., Lam, L. C., Lui, V. W., Chan, W. C., Chan, S. S., Chiu, H. F., et al. (2008). Clinical correlates of functional performance in community-swelling Chinese older persons with mild cognitive impairment. Int. Psychogeriatr. 20, 1059–1070. doi: 10.1017/S1041610208007345
Thomas, V. S., Rockwood, K., and McDowell, I. (1998). Multidimensionality in instrumental and basic activities of daily living. J. Clin. Epidemiol. 51, 315–321. doi: 10.1016/S0895-4356(97)00292-8
Tomita, A., and Burns, J. K. (2013). Depression, disability and functional status among community-dwelling older adults in South Africa: evidence from the first South African National Income Dynamics Study. Int. J. Geriatr. Psychiatry 28, 1270–1279. doi: 10.1002/gps.3954
Wadsworth, L. P., Lorius, N., Donovan, N. J., Locascio, J. J., Rentz, D. M., Johnson, K. A., et al. (2012). Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement. Geriatr. Cogn. Disord. 34, 96–111. doi: 10.1159/000342119
Keywords: activities of daily living, functional performance, neuropsychological assessment, depression, dementia, mild cognitive impairment, executive functions
Citation: de Paula JJ, Diniz BS, Bicalho MA, Albuquerque MR, Nicolato R, de Moraes EN, Romano-Silva MA and Malloy-Diniz LF (2015) Specific cognitive functions and depressive symptoms as predictors of activities of daily living in older adults with heterogeneous cognitive backgrounds. Front. Aging Neurosci. 7:139. doi: 10.3389/fnagi.2015.00139
Received: 08 February 2015; Accepted: 06 July 2015;
Published: 20 July 2015.
Edited by:
Manuel Menéndez-González, Hospital Álvarez Buylla, SpainReviewed by:
Paul Gerson Unschuld, University of Zürich, SwitzerlandRena Li, Roskamp Institute, USA
Copyright © 2015 de Paula, Diniz, Bicalho, Albuquerque, Nicolato, de Moraes, Romano-Silva and Malloy-Diniz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonas J. de Paula, Faculdade de Medicina, Instituto Nacional de Ciências e Tecnologia e em Medicina Molecular, Universidade Federal de Minas Gerais, Avenue Alfredo Balena 190, Belo Horizonte, Minas Gerais 30130-100, Brazil, jonasjardim@gmail.com
 Jonas J. de Paula
Jonas J. de Paula Breno S. Diniz1,3
Breno S. Diniz1,3  Maria A. Bicalho
Maria A. Bicalho Maicon Rodrigues Albuquerque
Maicon Rodrigues Albuquerque Rodrigo Nicolato
Rodrigo Nicolato Marco A. Romano-Silva
Marco A. Romano-Silva Leandro F. Malloy-Diniz
Leandro F. Malloy-Diniz