Association of pancreatic polypeptide with mild cognitive impairment varies by APOE ε4 allele
- 1Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
- 2Department of Neurology, Mayo Clinic, Rochester, MN, USA
- 3Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
- 4Center for Clinical and Translational Science, Mayo Clinic, Rochester, MN, USA
- 5Department of Psychiatry and Psychology, Mayo Clinic, Scottsdale, AZ, USA
- 6Department of Neurology, Mayo Clinic, Scottsdale, AZ, USA
We conducted a preliminary case–control investigation of the association of pancreatic polypeptide (PP) with mild cognitive impairment (MCI) in 202 MCI cases (mean age, 81.6 years) and 202 age- and sex-matched cognitively normal controls in the Mayo Clinic Study of Aging. Plasma PP was measured and examined as the natural logarithm (continuous) and dichotomized at the median. The OR (95% CI) of MCI increased with increasing PP [1.46 (1.04–2.05)]. There was a negative interaction of PP with apolipoprotein E (APOE) ε4 allele; compared to the reference group (no APOE ε4 allele and low PP), the OR (95% CI) for combinations of ε4 and PP were: 2.64 (1.39–5.04) for APOE ε4 plus low PP; 2.09 (1.27–3.45) for no APOE ε4 plus high PP; and 1.91 (1.04–3.53) for no APOE ε4 plus high PP (P for interaction = 0.017). There was also a trend toward a negative interaction with type 2 diabetes (P for interaction = 0.058). Compared to no diabetes and low PP, the OR (95% CI) was 3.02 (1.22–7.46) for low PP plus diabetes but 1.80 (1.01–3.22) for high PP plus diabetes. Participants with high PP had a greater mean (SD) weight loss (kilograms per decade) than persons with low PP [−2.27 (4.07) vs. −1.61 (5.24); P = 0.016]. MCI cases had a non-significantly greater weight loss per decade compared to controls. These findings suggest that high PP alone or jointly with APOE ε4 allele or type 2 diabetes is associated with MCI, and that high PP may mitigate some effects of APOE ε4 allele and type 2 diabetes on cognition. Potential mechanisms may involve PP-related weight loss and centrally mediated effects of PP on cognition. These findings remain to be validated in other studies.
Introduction
The need for diagnostic and prognostic biomarkers remains essential for early detection and prevention of Alzheimer’s disease (AD). Blood-based (non-genetic) biomarkers are important because they are easily acquired, relatively inexpensive compared to brain imaging biomarkers, less invasive than cerebrospinal fluid (CSF) acquisition, and more amenable to large-scale screening. Blood-based biomarkers may have utility for early detection or enhance recruitment and monitoring in clinical trials (Doecke et al., 2012).
Pancreatic polypeptide (PP) has been associated with mild cognitive impairment (MCI) and AD dementia in blood-based biomarker panels for these conditions (Craig-Schapiro et al., 2011; O’Bryant et al., 2011; Doecke et al., 2012). PP is produced by F cells of the pancreatic islets of Langerhans and released into the circulation after food ingestion. It is one of several neuropeptides with activity both in the gut and in the brain regions affected by AD dementia, such as the hippocampus, and hypothalamus (Asakawa et al., 2003). The effects of secreted PP are mediated by neuropeptide receptors (Y4 and Y5) in the brain and in the gut through vagal signaling. In response to PP, there is vagal signaling to neuroendocrine regions of the brain, such as the hypothalamus, locus ceruleus, area postrema, dorsal vagal complex, and brain stem regions that control gastrointestinal functions and regulate food intake (Holzer et al., 2012; Asakawa et al., 2003; McTigue et al., 1993). Physiologic effects of PP in response to vagal signaling from the brain include decreased gastric emptying, appetite suppression, decreased food intake, and a negative energy balance (i.e., energy expenditure exceeds energy intake) (Asakawa et al., 2003; Holzer et al., 2012). Low physiologic levels of PP and low food-induced PP are associated with hyperphagia and morbid obesity (Lassmann et al., 1980; Marco et al., 1980); in contrast, administration of PP stimulates weight loss (Berntson et al., 1993). Because of the association of high levels of PP with MCI and AD dementia in biomarker panels using multiplex platforms, the association of high levels of PP with reduced food intake and weight loss, and the importance of diet and caloric intake in risk of MCI and AD, we hypothesized that abnormally increased PP levels may be involved in the pathogenesis of MCI. The objective of this study was to conduct a preliminary cross-sectional investigation of plasma PP with MCI among participants in the Mayo Clinic study of aging (MCSA).
Materials and Methods
Study Design and Participants
The details of the study design and methodology for participant recruitment have been published (Roberts et al., 2008). Briefly, the MCSA is a population-based study established in Olmsted County, MN, USA, in 2004. Participants aged 70–89 years were randomly selected from an enumeration of the county population through the Rochester Epidemiology Project (Rocca et al., 2012), a medical records linkage system. We excluded subjects who were terminally ill or in hospice, or had previously diagnosed dementia. Participants were clinically evaluated to assess cognitive status (normal cognition, MCI, or dementia). Ongoing recruitment was established in 2008 to maintain the sample size, and follow-up is performed every 15 months. All study protocols were approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. All participants provided written informed consent prior to participation.
The clinical evaluation included an interview of the participant (using questions about memory) and an informant (using the Functional Activities Questionnaire and Clinical Dementia Rating Scale) (Pfeffer et al., 1982; Morris, 1993) by a study coordinator to evaluate memory and functioning; a complete neurologic evaluation by a physician, which included the Short Test of Mental Status – a global test of memory (Kokmen et al., 1987) and a full neurological evaluation; and a neuropsychological testing battery consisting of nine tests to assess performance in four cognitive domains: memory (three tests), executive function (two tests), visuospatial skills (two tests), and language (two tests) (Roberts et al., 2008; Petersen et al., 2010). Data from the evaluation were reviewed for a diagnosis of MCI defined as: (i) cognitive concern; (ii) impairment in one or more cognitive domains; (iii) essentially normal functional activities; and (iv) absence of dementia (Petersen, 2004; Roberts et al., 2008; Petersen et al., 2009, 2010), taking into account level of education and longest held occupation. A diagnosis of dementia was based on DSM IV criteria (American Psychiatric Association, 2000; Petersen, 2004; Roberts et al., 2008; Petersen et al., 2009, 2010); diagnosis of normal cognition was made in persons who performed in the normal cognitive range and did not meet criteria for MCI or dementia (Roberts et al., 2008; Petersen et al., 2010).
MCI Cases and Controls
Cases and controls for the present study were selected from among participants who were evaluated prior to December 31, 2011, and had stored blood samples available for measurement of PP. Among 1,323 eligible participants, 202 had MCI. Each case was matched to a cognitively normal control by sex, age (±2 years), number of clinical visits in the MCSA (±1), and duration of follow-up (±1 year; 1:1 matching).
Measurement of PP
Pancreatic polypeptide was measured using a radioimmunoassay technique developed in the Mayo Clinic Endocrine Laboratory (Schwartz, 1983; Koch et al., 1985) The assay included a commercial antibody from Peninsula Laboratories International, Inc., radioactive reagents from PerkinElmer Inc., and calibration material from Sigma-Aldrich. The assay system utilized rabbit antihuman PP antiserum, a standard or patient plasma specimen, and radiolabeled human PP, which has been iodinated by a modified Hunter–Greenwood technique. The sample and antibody (both primary and secondary) and the radioactive label were diluted in the same buffer. The buffer consisted of 0.02 M sodium 5,5-diethylbarbiturate (Sigma B-050), 4.12 g, 0.006 M barbituric acid (Fisher CAS#67527), 0.02% bovine serum albumin and some preservatives dissolved in water, and the pH was adjusted to 8.2. The buffer and precipitating antibody and validation were performed in the laboratory. The standard (patient) HPP competes with the radioactive HPP for binding sites on the primary antibody, which is rabbit anti-human pancreatic polypeptide. This antibody complex was precipitated with Goat Anti Rabbit serum along with normal rabbit serum and polyethylene glycol. The mixture was centrifuged and the supernate discarded. The radioactive counts in the pellet are inversely proportional to the amount of HPP present in the tube. The 2.5 SD detection limit based on 20 determinations from one assay was 28.5 pg/mL. The coefficients of variation were 3.65% for intra-assay precision and 8.05% for interassay precision.
Other Covariates
Demographic variables were assessed at baseline by interview. Medical comorbidities (type 2 diabetes mellitus, hypertension, stroke, and coronary artery disease) and maximum adult weight (age 40–65 years) were ascertained from medical records of each participant using the Rochester Epidemiology Project. Apolipoprotein E (APOE) genotyping was performed from a blood draw using standard methods (Hixson and Vernier, 1990), and depressive symptoms were ascertained from the Neuropsychiatric Inventory Questionnaire (Kaufer et al., 2000).
Statistical Analyses
The distribution of PP in cases and controls was skewed, so we characterized PP using the natural logarithm. We also examined PP as a dichotomous variable, with a high PP (considered abnormal) arbitrarily defined using a median split and the 90th percentile based on the distribution of PP levels for cognitively normal controls. We examined the associations of PP with MCI using conditional logistic regression models matched on sex and age (basic model) and also adjusted for age at sample draw to account for any residual confounding by age. In separate models, we investigated potential confounding of the association of PP with MCI by covariates that were significantly associated with MCI [education, depression, hypertension, coronary artery disease, type 2 diabetes, and APOE ε4 genotype (any ε4 vs. no ε4 allele)]; there was no association with body mass index (BMI). We examined effect modification by including interaction terms of each covariate with PP (dichotomized as ≤median vs. >median) in models with the main effects. To determine whether PP was associated with weight loss prior to the date of blood draw, we computed the average weight loss (in kilograms) per decade from the maximum weight in midlife to date of blood draw for PP assessment in MCI cases vs. controls and for participants with high vs. low PP levels. Comparisons across groups were made using Wilcoxon rank sum tests. All P values were considered significant at alpha of 0.05. Analyses were performed by SAS version 9.3 (SAS Institute).
Results
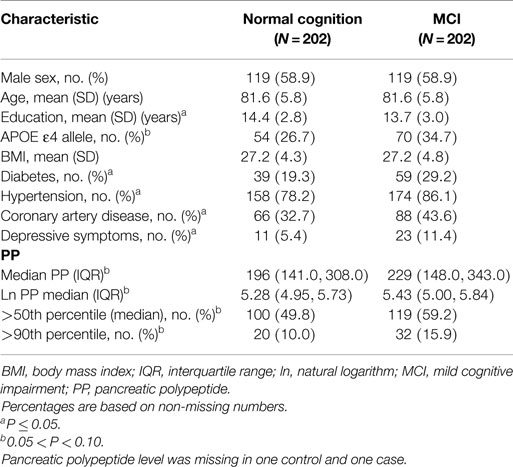
The characteristics of MCI cases and controls are described in Table 1. The mean age was 81.6 years and 58.9% were men. Cases had significantly fewer years of education; higher frequency of type 2 diabetes, hypertension, coronary artery disease, depressive symptoms, and APOE ε4 allele status; and marginally higher PP levels compared to controls (P = 0.073).
Cross-Sectional Association of PP with MCI
In age- and sex-adjusted models, the OR of MCI increased with increasing PP level [β (SE), 0.379 (0.173); P = 0.029] (Table 2). When participants were characterized by dichotomous cut points, the associations of high PP with MCI persisted but were marginally significant: OR, 1.47 (P = 0.069) for PP greater than median and OR, 1.89 (P = 0.051) for PP >90th percentile (Table 2). There was no confounding by coronary artery disease, type 2 diabetes, hypertension, depressive symptoms, or APOE ε4 allele when each variable was separately included in a model with age and sex (data not shown). When all the potential confounders were included in the model, the OR for MCI remained elevated but decreased to 1.33 (P = 0.116).
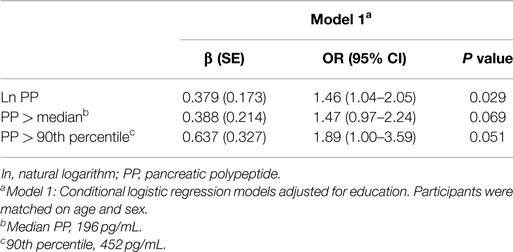
Table 2. Conditional logistic regression models for association of pancreatic polypeptide with mild cognitive impairment.
We observed a significant interaction of PP with APOE ε4 allele (P for interaction = 0.017). The joint effects of high PP and APOE ε4 allele were indicative of a negative (antagonistic) interaction. Compared to the reference group (no APOE ε4 allele and low PP), the ORs (95% CIs) for combinations of APOE and PP were as follows: 2.64 (1.39–5.04), P = 0.003 for APOE ε4 plus low PP; 2.09 (1.27–3.45), P = 0.004 for no APOE ε4 plus high PP; and 1.91 (1.04–3.53), P = 0.038 for APOE ε4 plus high PP (Figure 1). There was also a marginal negative interaction with type 2 diabetes (P for interaction = 0.058). Compared to the reference group (no type 2 diabetes and low PP), the ORs (95% CIs) of MCI were: 3.02 (1.22–7.46), P = 0.017 for diabetes plus low PP; 1.69 (1.07–2.68), P = 0.026 for no diabetes plus high PP; and 1.80 (1.01–3.22), P = 0.046 for diabetes plus high PP (Figure 2).
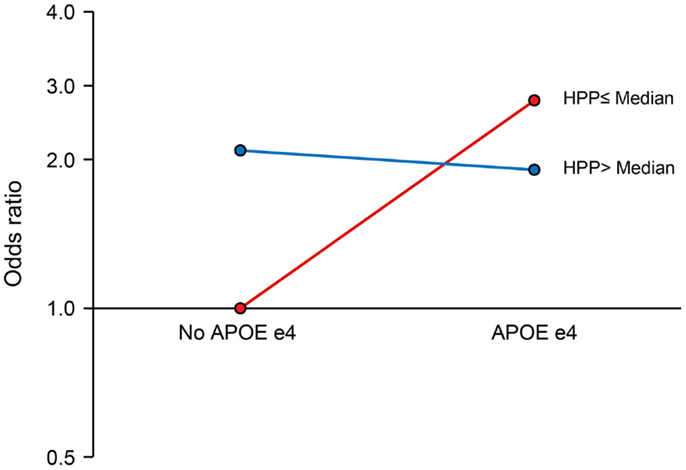
Figure 1. Interaction of pancreatic polypeptide (PP) with APOE ε4 Allele. Compared to persons without an APOE ε4 allele and low PP level (reference), the ORs (95% CIs) were as follows: 2.64 (1.39–5.04), P = 0.003 for low PP plus APOE ε4 allele; 2.09 (1.27–3.45), P = 0.004 for high PP without APOE ε4 allele; and 1.91 (1.04–3.53), P = 0.038 for high PP plus APOE ε4 allele. PP was dichotomized at the median as low PP (≤196 pg/mL) and high PP (>196 pg/mL).
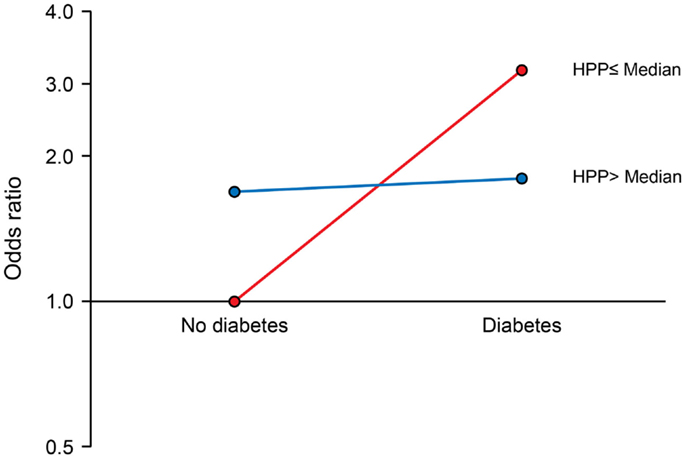
Figure 2. Interaction of pancreatic polypeptide (PP) with type 2 diabetes. Compared to persons without type 2 diabetes and low PP level (reference), the ORs (95% CIs) were as follows: 3.02 (1.22–7.46), P = 0.017 for low PP plus diabetes; 1.69 (1.07–2.68), P = 0.026 for high PP without diabetes; and 1.80 (1.01–3.22), P = 0.046 for high PP plus diabetes. PP was dichotomized at the median as low PP (≤196 pg/mL) and high PP (>196 pg/mL).
Weight Loss
Mild cognitive impairment cases had a non-significantly greater mean (SD) weight loss (kilograms per decade) assessed from midlife to date of blood draw for measurement of PP compared to controls [−2.40 (5.14) vs. −1.53 (4.03); P = 0.124]. However, participants with high PP had a greater mean weight loss per decade than persons with low PP [−2.27 (4.07) vs. −1.61 (5.24); P = 0.016] and a non-significantly lower BMI [26.906 (4.73) vs. 27.534 (4.37); P = 0.212].
Discussion
In this preliminary case–control study of elderly persons, elevated PP levels were associated with an increased OR of MCI. However, the association of PP with MCI varied significantly by APOE ε4 allele, and non-significantly, by diabetes status. High PP was also associated with greater weight loss per decade, and MCI cases had a non-significantly greater weight loss per decade compared to controls. The findings suggest that PP may be involved in the pathogenesis of MCI and should be examined in a definitive prospective study.
The negative interaction of PP with APOE ε4 allele and marginally significant interaction with diabetes are interesting, but the implications are unclear. The interactions showed that the OR for the joint effects of an APOE ε4 allele or diabetes with high PP was lower than that for APOE ε4 or diabetes with low PP (i.e., APOE ε4 or diabetes alone), but comparable to the elevated estimates of OR in APOE ε4 allele non-carriers and in non-diabetics.
Our findings are in keeping with findings from other investigators (Doecke et al., 2012; Hu et al., 2012; Soares et al., 2012; O’Bryant et al., 2013; Burnham et al., 2014). In the Australian Imaging Biomarker and Lifestyle study, PP levels were elevated 1.54-fold in AD dementia cases compared to cognitively normal controls (Doecke et al., 2012). In AD Neuroimaging Initiative samples/cohorts plasma, PP levels were higher in AD dementia or MCI cases compared to controls (Kiddle et al., 2012; Soares et al., 2012). In the Texas Alzheimer’s Research and Care Consortium, PP was overexpressed in a biomarker panel and improved the diagnostic accuracy for AD in whites (O’Bryant et al., 2011) and in Mexican Americans (O’Bryant et al., 2013). In two independent samples from the University of Pennsylvania and Washington University, elevated PP levels were associated with impaired cognition (MCI, AD dementia, or Clinical Dementia Rating of 0.5 or 1) (Hu et al., 2012).
Mechanisms that mitigate effects of elevated PP levels on MCI risk in APOE ε4 carriers may involve the need to maintain a balance in inhibitory and excitatory input in the hippocampus for memory encoding and spatial recognition (Andrews-Zwilling et al., 2010). This balance is maintained by somatostatin-expressing cells, which colocate with γ-aminobutyric acid-ergic (GABAergic) expression to provide inhibitory input on pyramidal cell activity in the hippocampus (Freund and Buzsaki, 1996). With aging, there is a decrease in somatostatin and GABAergic interneuron expression in the hippocampus; this results in increased excitatory activity at rest that is hypothesized to contribute to memory impairment in amnestic MCI (Yassa et al., 2010, 2011). This decreased somatostatin expression is also observed in AD brains and in APOE ε4 allele carriers (Kumar, 2005). Functional imaging studies have reported greater default brain network activation at rest and during memory tasks and greater hippocampal activation in persons at increased risk of genetic or familial AD, APOE ε4 allele carriers (Bookheimer et al., 2000; Bassett et al., 2006; Filippini et al., 2009), and in individuals with MCI (Dickerson et al., 2004, 2005; Celone et al., 2006; Hamalainen et al., 2007). These findings suggest that there is increased imbalance in inhibitory–excitatory signals and impaired inhibition of GABAergic interneurons in persons at risk for AD, such as persons with MCI and ε4 carriers. This APOE ε4 effect may be exacerbated in elderly ε4 carriers who also have age-related decrease in somatostatin, resulting in greater excitatory activity in granule and pyramidal cells in the hippocampal formation and impaired memory encoding compared to non-carriers (Andrews-Zwilling et al., 2010). In aMCI patients, administration of antiepileptic drugs reduced CA3/dentate overactivity and improved memory performance (Bakker et al., 2012).
Similarly, animal studies have demonstrated decreased somatostatin in mouse models of age-related neurodegeneration (Andrews-Zwilling et al., 2010) and in the CA3/dentate gyrus of aged rats with age-related loss of spatial memory involving the hippocampus (Spiegel et al., 2013). A recent study in mice demonstrated that PP activates somatostatin-expressing cells in the hippocampus (Kim et al., 2014). Other animal studies have also reported beneficial effects of antiepileptic drugs and inhibitory neuropeptides that target loss of GABAergic activity on memory performance in memory-impaired rats compared to controls (Koh et al., 2010, 2013). APOE ε4 impairs GABAergic interneurons, increases excitatory activity in the hippocampus, and impairs memory encoding (Andrews-Zwilling et al., 2010). Pentobarbital was observed to improve performance in APOE ε4 knock-in mice with deficits in hippocampal inhibitory activity (Andrews-Zwilling et al., 2010). Together, these human and animals studies are highly relevant to our findings and are in keeping with the interaction of PP with APOE ε4 allele observed in our study. If indeed PP (a neuropeptide) activates somatostatin-containing cells in persons at risk for AD (e.g., APOE ε4 carriers), elevated levels of PP in APOE ε4 carriers may reduce excitatory signals, reduce the inhibitory–excitatory imbalance to GABAergic neurons, and promote memory encoding.
In persons with diabetes, elevated PP levels may reduce the likelihood of MCI by effects on weight reduction, negative energy balance, and improved glycemic control. Effects of PP are mediated by binding to neuropeptide Y (NPY) receptors in the gastrointestinal tract, pancreas, liver, and brain (hippocampus and hypothalamus). High PP levels may increase insulin secretion from the pancreatic islets of Langerhans cells through inhibition of somatostatin release (Mandarino et al., 1981; D’Alessio et al., 1989; Kim et al., 2014). Elevated levels of PP secretion observed in diabetics (Floyd et al., 1976) is hypothesized to be a compensatory mechanism to increase insulin release and improve glycemic control (Floyd et al., 1976; Kim et al., 2014); this may also enhance brain insulin signaling and glucose metabolism, and thereby reduce MCI risk (Roberts et al., 2013). Central effects of PP involve the hypothalamus, dorsal vagal complex (McTigue et al., 1993). High circulating PP may enhance the brain control of gastrointestinal functions (McTigue et al., 1993; Asakawa et al., 2003). PP inhibits hypothalamic release of neuropeptides that stimulate eating, enhances release of anorexigenic hypothalamic peptides, and suppresses gastric release of ghrelin (an appetite stimulant) (Asakawa et al., 2003), with potentially beneficial effects in diabetics.
In APOE ε4 non-carriers and non-diabetics, the potential mechanisms for the association of high PP levels with MCI are unclear, but may relate to dietary effects of PP. Overexpression of PP may lead to decreased food intake, excessive weight loss, anemia, and deficiencies in micronutrients required for neuronal function. Consistent with this, we observed a non-significantly greater weight loss per decade in MCI cases compared to controls, in keeping with reported declines in weight prior to dementia (Nourhashemi et al., 2003; Knopman et al., 2007; Besser et al., 2014; Sobow et al., 2014). Persons with high PP levels in the study also had a greater average weight loss and lower BMI than persons with lower PP levels. Nutritional deficiencies have adverse implications for cognition. Several dietary nutrients and antioxidants including vitamins (e.g., A, C, D, E, folate, B12), monounsaturated and polyunsaturated fatty acids, dietary antioxidants, and phospholipids (Blok et al., 1996; Engelhart et al., 2002; Chrysohoou et al., 2004; Feart et al., 2009; Roberts et al., 2010a,b) are beneficial for cognitive function. Deficiencies in micronutrients reportedly occur prior to the protein-calorie malnutrition observed in patients with AD dementia (Lopes da Silva et al., 2014). Dietary nutrients are the basis for ongoing AD dementia prevention and treatment trials (Scheltens et al., 2010; Hartmann et al., 2014; Swaminathan and Jicha, 2014). Elevated PP levels have been observed in persons with both AD and non-AD dementia (Hu et al., 2012). The association with non-AD dementia, which may have a vascular etiology, suggests that vascular mechanisms, possibly involving diet-related metabolic or signaling abnormalities, may be involved in the association of PP with cognitive impairment. There are also suggestions that elevated PP levels may be part of an immune signature that may adversely affect MCI risk (Hallgren and Lundqvist, 1980; Ray et al., 2007; Burnham et al., 2014).
The cause of the abnormally elevated levels of PP is uncertain. There are suggestions that this could result from a dysfunction in cholinergic tone that precedes and is present in clinical AD dementia (Mufson et al., 2008; Doecke et al., 2012; Hu et al., 2012; Soares et al., 2012; O’Bryant et al., 2013; Burnham et al., 2014). Elevated levels of PP observed in CSF of patients with AD suggest that impaired transport across the blood–brain and CSF barriers may occur (Hu et al., 2010, 2012). A disruption in the blood–brain barrier in the aging hippocampus could also lead to elevated brain PP levels (Hu et al., 2012; Montagne et al., 2015).
A limitation of our study is that the case–control design precludes our ability to assess causality. However, these preliminary findings have generated hypotheses for further investigations. Another limitation is that several gut hormones that influence energy balance may interact with PP; however, as an initial step, we specifically examined only PP. Another limitation is that due to the imbalance in MCI risk factors between cases and controls, the ORs were attenuated in the multivariable models and the associations were no longer significant. Finally, several complex metabolic and neuronal pathways and mechanisms are involved in the association of PP with cognition and we are limited in our ability to fully investigate these associations using a case–control design.
Our study has several strengths. Participants were well characterized for cognitive outcomes. We adjusted for the effects of vascular risk factors using information from the medical record. In contrast to several studies, we measured PP levels from overnight fasting blood samples to eliminate potential confounding by food intake. Given the prospective design of the MCSA, we are able to conduct a definitive study to test the hypotheses generated by this preliminary investigation.
Author Contributions
RR designed the study; RR, MM, DK, RP, WK, JA, RC acquired, analyzed, and interpreted the data; RR drafted the manuscript; RR, MM, DK, RP, WK, JA, YG, SV critically reviewed the manuscript for intellectual content; WK, RC, JA performed the statistical analyses; RP, RR, MM, DK obtained the funding; RP and RR provided administrative, technical, or material support; RR and WK provided study supervision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The study was supported by the National Institute on Aging (U01 AG006786, P50 AG016574), the Mayo Foundation for Medical Education and Research, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Acknowledgments
The authors thank Mrs. Sondra Buehler for her editorial support, Ms. Connie Fortner, RN and Ms. Mary Dugdale, RN for abstraction of data from the medical record, Ms. Mary Eastvold for performing pancreatic polypeptide assays, and the Mayo Clinic Study of Aging participants and staff.
Abbreviations
AD, Alzheimer’s disease; APOE, apolipoprotein E; BMI, body mass index; CF, cerebrospinal fluid; MCI, mild cognitive impairment; MCSA, Mayo Clinic Study of Aging; PP, pancreatic polypeptide.
References
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association.
Andrews-Zwilling, Y., Bien-Ly, N., Xu, Q., Li, G., Bernardo, A., Yoon, S. Y., et al. (2010). Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 30, 13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010
Asakawa, A., Inui, A., Yuzuriha, H., Ueno, N., Katsuura, G., Fujimiya, M., et al. (2003). Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 124, 1325–1336. doi:10.1016/S0016-5085(03)00216-6
Bakker, A., Krauss, G. L., Albert, M. S., Speck, C. L., Jones, L. R., Stark, C. E., et al. (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. doi:10.1016/j.neuron.2012.03.023
Bassett, S. S., Yousem, D. M., Cristinzio, C., Kusevic, I., Yassa, M. A., Caffo, B. S., et al. (2006). Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain 129, 1229–1239. doi:10.1093/brain/awl089
Berntson, G. G., Zipf, W. B., O’Dorisio, T. M., Hoffman, J. A., and Chance, R. E. (1993). Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides 14, 497–503. doi:10.1016/0196-9781(93)90138-7
Besser, L. M., Gill, D. P., Monsell, S. E., Brenowitz, W., Meranus, D. H., Kukull, W., et al. (2014). Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 28, 36–43. doi:10.1097/WAD.0000000000000005
Blok, W. L., Katan, M. B., and van der Meer, J. W. (1996). Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J. Nutr. 126, 1515–1533.
Bookheimer, S. Y., Strojwas, M. H., Cohen, M. S., Saunders, A. M., Pericak-Vance, M. A., Mazziotta, J. C., et al. (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 343, 450–456. doi:10.1056/NEJM200008173430701
Burnham, S. C., Faux, N. G., Wilson, W., Laws, S. M., Ames, D., Bedo, J., et al. (2014). A blood-based predictor for neocortical Abeta burden in Alzheimer’s disease: results from the AIBL study. Mol. Psychiatry 19, 519–526. doi:10.1038/mp.2013.40
Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L., et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J. Neurosci. 26, 10222–10231. doi:10.1523/JNEUROSCI.2250-06.2006
Chrysohoou, C., Panagiotakos, D. B., Pitsavos, C., Das, U. N., and Stefanadis, C. (2004). Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J. Am. Coll. Cardiol. 44, 152–158. doi:10.1016/j.jacc.2004.03.039
Craig-Schapiro, R., Kuhn, M., Xiong, C., Pickering, E. H., Liu, J., Misko, T. P., et al. (2011). Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PLoS ONE 6:e18850. doi:10.1371/journal.pone.0018850
D’Alessio, D. A., Sieber, C., Beglinger, C., and Ensinck, J. W. (1989). A physiologic role for somatostatin 28 as a regulator of insulin secretion. J. Clin. Invest. 84, 857–862. doi:10.1172/JCI114246
Dickerson, B. C., Salat, D. H., Bates, J. F., Atiya, M., Killiany, R. J., Greve, D. N., et al. (2004). Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 56, 27–35. doi:10.1002/ana.20163
Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M., et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi:10.1212/01.wnl.0000171450.97464.49
Doecke, J. D., Laws, S. M., Faux, N. G., Wilson, W., Burnham, S. C., Lam, C. P., et al. (2012). Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch. Neurol. 69, 1318–1325. doi:10.1001/archneurol.2012.1282
Engelhart, M. J., Geerlings, M. I., Ruitenberg, A., van Swieten, J. C., Hofman, A., Witteman, J. C., et al. (2002). Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 287, 3223–3229. doi:10.1001/jama.287.24.3223
Feart, C., Samieri, C., Rondeau, V., Amieva, H., Portet, F., Dartigues, J. F., et al. (2009). Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 302, 638–648. doi:10.1001/jama.2009.1146
Filippini, N., MacIntosh, B. J., Hough, M. G., Goodwin, G. M., Frisoni, G. B., Smith, S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U.S.A. 106, 7209–7214. doi:10.1073/pnas.0811879106
Floyd, J. C. Jr., Fajans, S. S., Pek, S., and Chance, R. E. (1976). A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog. Horm. Res. 33, 519–570.
Freund, T. F., and Buzsaki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi:10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I
Hallgren, R., and Lundqvist, G. (1980). Elevated levels of circulating pancreatic polypeptide in inflammatory and infectious disorders. Regul. Pept. 1, 159–167. doi:10.1016/0167-0115(80)90269-4
Hamalainen, A., Pihlajamaki, M., Tanila, H., Hanninen, T., Niskanen, E., Tervo, S., et al. (2007). Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging 28, 1889–1903. doi:10.1016/j.neurobiolaging.2006.08.008
Hartmann, T., van Wijk, N., Wurtman, R. J., Olde Rikkert, M. G., Sijben, J. W., Soininen, H., et al. (2014). A nutritional approach to ameliorate altered phospholipid metabolism in Alzheimer’s disease. J. Alzheimers Dis. 41, 715–717. doi:10.3233/JAD-141137
Hixson, J. E., and Vernier, D. T. (1990). Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 31, 545–548.
Holzer, P., Reichmann, F., and Farzi, A. (2012). Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46, 261–274. doi:10.1016/j.npep.2012.08.005
Hu, W. T., Chen-Plotkin, A., Arnold, S. E., Grossman, M., Clark, C. M., Shaw, L. M., et al. (2010). Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 119, 669–678. doi:10.1007/s00401-010-0667-0
Hu, W. T., Holtzman, D. M., Fagan, A. M., Shaw, L. M., Perrin, R., Arnold, S. E., et al. (2012). Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 79, 897–905. doi:10.1212/WNL.0b013e318266fa70
Kaufer, D. I., Cummings, J. L., Ketchel, P., Smith, V., MacMillan, A., Shelley, T., et al. (2000). Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J. Neuropsychiatry Clin. Neurosci. 12, 233–239. doi:10.1176/jnp.12.2.233
Kiddle, S. J., Thambisetty, M., Simmons, A., Riddoch-Contreras, J., Hye, A., Westman, E., et al. (2012). Plasma based markers of [11C] PiB-PET brain amyloid burden. PLoS ONE 7:e44260. doi:10.1371/journal.pone.0044260
Kim, W., Fiori, J. L., Shin, Y. K., Okun, E., Kim, J. S., Rapp, P. R., et al. (2014). Pancreatic polypeptide inhibits somatostatin secretion. FEBS Lett. 588, 3233–3239. doi:10.1016/j.febslet.2014.07.005
Knopman, D. S., Edland, S. D., Cha, R. H., Petersen, R. C., and Rocca, W. A. (2007). Incident dementia in women is preceded by weight loss by at least a decade. Neurology 69, 739–746. doi:10.1212/01.wnl.0000267661.65586.33
Koch, M. B., Go, V. L., and DiMagno, E. P. (1985). Can plasma human pancreatic polypeptide be used to detect diseases of the exocrine pancreas? Mayo Clin. Proc. 60, 259–265. doi:10.1016/S0025-6196(12)60319-X
Koh, M. T., Haberman, R. P., Foti, S., McCown, T. J., and Gallagher, M. (2010). Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology 35, 1016–1025. doi:10.1038/npp.2009.207
Koh, M. T., Rosenzweig-Lipson, S., and Gallagher, M. (2013). Selective GABA(A) alpha5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology 64, 145–152. doi:10.1016/j.neuropharm.2012.06.023
Kokmen, E., Naessens, J. M., and Offord, K. P. (1987). A short test of mental status: description and preliminary results. Mayo Clin. Proc. 62, 281–288. doi:10.1016/S0025-6196(12)61905-3
Kumar, U. (2005). Expression of somatostatin receptor subtypes (SSTR1-5) in Alzheimer’s disease brain: an immunohistochemical analysis. Neuroscience 134, 525–538. doi:10.1016/j.neuroscience.2005.04.001
Lassmann, V., Vague, P., Vialettes, B., and Simon, M. C. (1980). Low plasma levels of pancreatic polypeptide in obesity. Diabetes 29, 428–430. doi:10.2337/diabetes.29.6.428
Lopes da Silva, S., Vellas, B., Elemans, S., Luchsinger, J., Kamphuis, P., Yaffe, K., et al. (2014). Plasma nutrient status of patients with Alzheimer’s disease: systematic review and meta-analysis. Alzheimers Dement. 10, 485–502. doi:10.1016/j.jalz.2013.05.1771
Mandarino, L., Stenner, D., Blanchard, W., Nissen, S., Gerich, J., Ling, N., et al. (1981). Selective effects of somatostatin-14, -25 and -28 on in vitro insulin and glucagon secretion. Nature 291, 76–77. doi:10.1038/291076a0
Marco, J., Zulueta, M. A., Correas, I., and Villanueva, M. L. (1980). Reduced pancreatic polypeptide secretion in obese subjects. J. Clin. Endocrinol. Metab. 50, 744–747. doi:10.1210/jcem-50-4-744
McTigue, D. M., Edwards, N. K., and Rogers, R. C. (1993). Pancreatic polypeptide in dorsal vagal complex stimulates gastric acid secretion and motility in rats. Am. J. Physiol. 265, G1169–G1176.
Montagne, A., Barnes, S. R., Sweeney, M. D., Halliday, M. R., Sagare, A. P., Zhao, Z., et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. doi:10.1016/j.neuron.2014.12.032
Morris, J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi:10.1212/WNL.43.11.2412-a
Mufson, E. J., Counts, S. E., Perez, S. E., and Ginsberg, S. D. (2008). Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev. Neurother. 8, 1703–1718. doi:10.1586/14737175.8.11.1703
Nourhashemi, F., Deschamps, V., Larrieu, S., Letenneur, L., Dartigues, J. F., and Barberger-Gateau, P. (2003). Body mass index and incidence of dementia: the PAQUID study. Neurology 60, 117–119. doi:10.1212/01.WNL.0000038910.46217.AA
O’Bryant, S. E., Xiao, G., Barber, R., Huebinger, R., Wilhelmsen, K., Edwards, M., et al. (2011). A blood-based screening tool for Alzheimer’s disease that spans serum and plasma: findings from TARC and ADNI. PLoS ONE 6:e28092. doi:10.1371/journal.pone.0028092
O’Bryant, S. E., Xiao, G., Edwards, M., Devous, M., Gupta, V. B., Martins, R., et al. (2013). Biomarkers of Alzheimer’s disease among Mexican Americans. J. Alzheimers Dis. 34, 841–849. doi:10.3233/JAD-122074
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. doi:10.1111/j.1365-2796.2004.01388.x
Petersen, R. C., Roberts, R. O., Knopman, D. S., Boeve, B. F., Geda, Y. E., Ivnik, R. J., et al. (2009). Mild cognitive impairment: ten years later. Arch. Neurol. 66, 1447–1455. doi:10.1001/archneurol.2009.266
Petersen, R. C., Roberts, R. O., Knopman, D. S., Geda, Y. E., Cha, R. H., Pankratz, V. S., et al. (2010). Prevalence of mild cognitive impairment is higher in men: the Mayo clinic study of aging. Neurology 75, 889–897. doi:10.1212/WNL.0b013e3181f11d85
Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H. Jr., Chance, J. M., and Filos, S. (1982). Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329. doi:10.1093/geronj/37.3.323
Ray, S., Britschgi, M., Herbert, C., Takeda-Uchimura, Y., Boxer, A., Blennow, K., et al. (2007). Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat. Med. 13, 1359–1362. doi:10.1038/nm1653
Roberts, R. O., Cerhan, J. R., Geda, Y. E., Knopman, D. S., Cha, R. H., Christianson, T. J., et al. (2010a). Polyunsaturated fatty acids and reduced odds of MCI: the Mayo clinic study of aging. J. Alzheimers Dis. 21, 853–865. doi:10.3233/JAD-2010-091597
Roberts, R. O., Geda, Y. E., Cerhan, J. R., Knopman, D. S., Cha, R. H., Christianson, T. J., et al. (2010b). Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 29, 413–423. doi:10.1159/000305099
Roberts, R. O., Geda, Y. E., Knopman, D. S., Cha, R. H., Pankratz, V. S., Boeve, B. F., et al. (2008). The Mayo clinic study of aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69. doi:10.1159/000115751
Roberts, R. O., Geda, Y. E., Knopman, D. S., Cha, R. H., Pankratz, V. S., Boeve, B. F., et al. (2013). Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA Neurol. 70, 374–382. doi:10.1001/jamaneurol.2013.607
Rocca, W. A., Yawn, B. P., St Sauver, J. L., Grossardt, B. R., and Melton, L. J. III (2012). History of the Rochester epidemiology project: half a century of medical records linkage in a United States population. Mayo Clin. Proc. 87, 1202–1213. doi:10.1016/j.mayocp.2012.08.012
Scheltens, P., Kamphuis, P. J., Verhey, F. R., Olde Rikkert, M. G., Wurtman, R. J., Wilkinson, D., et al. (2010). Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimers Dement. 6, e11. doi:10.1016/j.jalz.2009.10.003
Schwartz, T. W. (1983). Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 85, 1411–1425.
Soares, H. D., Potter, W. Z., Pickering, E., Kuhn, M., Immermann, F. W., Shera, D. M., et al. (2012). Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch. Neurol. 69, 1310–1317. doi:10.1001/archneurol.2012.1070
Sobow, T., Fendler, W., and Magierski, R. (2014). Body mass index and mild cognitive impairment-to-dementia progression in 24 months: a prospective study. Eur. J. Clin. Nutr. 68, 1216–1219. doi:10.1038/ejcn.2014.167
Spiegel, A. M., Koh, M. T., Vogt, N. M., Rapp, P. R., and Gallagher, M. (2013). Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol. 521, 3508–3523. doi:10.1002/cne.23367
Swaminathan, A., and Jicha, G. A. (2014). Nutrition and prevention of Alzheimer’s dementia. Front. Aging Neurosci. 6:282. doi:10.3389/fnagi.2014.00282
Yassa, M. A., Lacy, J. W., Stark, S. M., Albert, M. S., Gallagher, M., and Stark, C. E. (2011). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, 968–979. doi:10.1002/hipo.20808
Keywords: cognition, mild cognitive impairment, case–control study, pancreatic polypeptide, neuropeptide, type 2 diabetes, apolipoprotein E
Citation: Roberts RO, Aakre JA, Cha RH, Kremers WK, Mielke MM, Velgos SN, Geda YE, Knopman DS and Petersen RC (2015) Association of pancreatic polypeptide with mild cognitive impairment varies by APOE ε4 allele. Front. Aging Neurosci. 7:172. doi: 10.3389/fnagi.2015.00172
Received: 24 June 2015; Accepted: 19 August 2015;
Published: 08 September 2015
Edited by:
P. Hemachandra Reddy, Texas Tech University, USAReviewed by:
Ramesh Kandimalla, Texas Tech University, USARenu Bajaj, Thomas Jefferson University Hospital, USA
Copyright: © 2015 Roberts, Aakre, Cha, Kremers, Mielke, Velgos, Geda, Knopman and Petersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosebud O. Roberts, Department of Health Sciences Research, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, USA, roberts.rosebud@mayo.edu
 Rosebud O. Roberts
Rosebud O. Roberts Jeremiah A. Aakre
Jeremiah A. Aakre Ruth H. Cha
Ruth H. Cha Walter K. Kremers
Walter K. Kremers Michelle M. Mielke
Michelle M. Mielke Stefanie N. Velgos
Stefanie N. Velgos Yonas E. Geda
Yonas E. Geda David S. Knopman
David S. Knopman Ronald C. Petersen1,2
Ronald C. Petersen1,2