Does cell lineage in the developing cerebral cortex contribute to its columnar organization?
- 1 Edmond and Lily Safra International Institute of Neuroscience of Natal, Natal, Rio Grande do Norte, Brazil
- 2 Universidade Federal do Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil
- 3 Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- 4 Laboratório de Neuroanatomia Celular, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
Since the pioneer work of Lorente de Nó, Ramón y Cajal, Brodmann, Mountcastle, Hubel and Wiesel and others, the cerebral cortex has been seen as a jigsaw of anatomic and functional modules involved in the processing of different sets of information. In fact, a columnar distribution of neurons displaying similar functional properties throughout the cerebral cortex has been observed by many researchers. Although it has been suggested that much of the anatomical substrate for such organization would be already specified at early developmental stages, before activity-dependent mechanisms could take place, it is still unclear whether gene expression in the ventricular zone (VZ) could play a role in the development of discrete functional units, such as minicolumns or columns. Cell lineage experiments using replication-incompetent retroviral vectors have shown that the progeny of a single neuroepithelial/radial glial cell in the dorsal telencephalon is organized into discrete radial clusters of sibling excitatory neurons, which have a higher propensity for developing chemical synapses with each other rather than with neighboring non-siblings. Here, we will discuss the possibility that the cell lineage of single neuroepithelial/radial glia cells could contribute for the columnar organization of the neocortex by generating radial columns of sibling, interconnected neurons. Borrowing some concepts from the studies on cell–cell recognition and transcription factor networks, we will also touch upon the potential molecular mechanisms involved in the establishment of sibling-neuron circuits.
Anatomic and Functional Organization of the Mammalian Cerebral Cortex
The cerebral cortex consists of distinct cytoarchitectonic areas, each serving a function ranging from sensory perception and motor control to symbolic thinking and language in humans. In fact, the anatomical observation of discontinuous architectural features of cerebral cortex uncovered very early by Ramón y Cajal and Lorente de Nó was followed by the realization that a functional architecture was also present probably as an emerging property of the underlying anatomical architecture. A striking feature of the neocortex, first unraveled in the work by Mountcastle (1957) describing the cortical representation of somatosensory perception, is its organization into functional columns.
The columnar organization of the cerebral cortex is a broadly documented principle of design preserved throughout mammalian evolution (Mountcastle, 1997 ), which has been proposed to be important to allow a large number of neurons to be connected without a significant increase in cortical volume. Indeed, it has been estimated that fusing 100 cortical columns would lead to a 10-fold increase in cortical volume (Mitchison, 1992 ). The explanation for such increase comes from the fact that neurons are locally connected within cortical columns and only restricted subsets of neurons are involved in long distance connections. Consequently, the length of axons that interconnect neurons is shortened, reducing also the cortical volume.
Within a given cortical column, discrete clusters of neurons project to a limited number of sites and tend to link columns of common functional properties (Mountcastle, 1997 ). The radial dispersion of these clusters is about 400 μm, similar to the spread of the dendritic arbor. Therefore, the cerebral cortex can be seen as a jigsaw of local neuronal microcircuits, which are interconnected by small subsets of neurons. Approaching the developmental building blocks of these microcircuits is an important step towards understanding the emergence of functional properties of cortical columns.
Is the Development of Cortical Columns Influenced by Molecular Cues Intrinsic to the Developing Cerebral Cortex?
It has long been though that both development and plasticity of cortical columns rely exclusively on activity-dependent mechanisms. Indeed, the development of ocular dominance columns is highly dependent on visual experience, as clearly evidenced by the physiological and anatomical shifts caused by monocular eye closure during the critical period (Wiesel and Hubel, 1963 , 1965 ; LeVay et al., 1978 , 1980 ). However, there has also been accumulating evidence indicating that the initial establishment of cortical columns may take place before the critical period. For instance, it has been shown that the basic structure of segregated lateral geniculate nucleus (LGN) afferents in the primary visual cortex (V1) of macaque monkeys is formed before birth (Rakic, 1976 ). Similarly, injection of anterograde tracers in the LGN of ferrets 2–3 weeks prior to the onset of the critical period reveals a clear ocular dominance segregation of the afferents (Crowley and Katz, 2000 ), indicating that molecular cues, intrinsic to the developing thalamocortical system, may be involved in the establishment of columns. Likewise, other systems which form discontinuous projections such as the interhemispheric cortico-cortical connections have also shown topographical precision in their innervation from the out start (Aggoun-Aouaoui et al., 1996 ; Hedin-Pereira et al., 1999 ). Moreover, laser-scanning photostimulation in brain slices combined with morphological analysis of axonal arbors has revealed that connections between layer 4 and layers 2/3 neurons develop with great specificity and without detectable pruning at the level of the cortical columns (Bureau et al., 2004 ).
These data prompt the question of which are the mechanisms governing this early columnar organization of neurons. As briefly noted above, cortical columns have been characterized by the existence of neurons sharing similar electrophysiological properties, involved in the processing of particular stimuli and distributed in discrete horizontal clusters along the cerebral cortex. Thus, the very first pre-requisite to link a given factor to the columnar organization of the cerebral cortex would be the capacity of this factor to organize neurons in discrete radial columns of neurons and to favor their interconnectivity.
Gap junctions have been recurrently implicated as players in the establishment of functional units (Yuste et al., 1992 , 1995 ; Kandler and Katz, 1995 ). The coordinated calcium fluctuation patterns underlying gap junctional mediated communication were suggested to form the basis of functional cell assemblies in postnatal cerebral cortex. Blocking activity did not eliminate calcium functional domains suggesting that gap junctions may promote metabolic rather than activity related assemblies (Kandler and Katz, 1998 ). Recently, it has been shown that glial cells in layer IV of the somatosensory cortex form gap-junction coupled ensembles correlated to barrels (anatomical structures that display eletrophysiological responses to individual whiskers) showing that non neuronal cells may also be important players in the formation of cortical units (Houades et al., 2008 ).
It has been shown previously that since very early, during embryonic neurogenesis, cells in different stages of the cell cycle (mainly S and G2 but also G1) are gap junctionally coupled with radial glia forming columnar functional units with 15 to 20 cells (Bittman et al., 1997 ). It is possible that these cells belong to the same clone and their functional and metabolic coupling at this stage could be important for the later establishment of connections among themselves (see discussion about neuronal clones in the following section). It is also known that gap junctions regulate neuronal migration (Elias et al., 2007 ; Marins et al., 2009 ) and adhesive connections formed by gap junctions between migrating neurons and radial glia were shown to be important for gliophilic migration. Thus, gap junctions and other molecules that regulate migration may be important at later stages helping connect columnar neuronal networks whose identity may have been primed early within the VZ.
An important aspect of the formation of columnar structures is the radial axon-dendrite polarity established from the very beginning by neurons migrating attached to radial glia with their leading and trailing processes. During migration neurons derived from the cortical VZ grow an axon directed towards the white matter and later an apical dendrite that ramifies in layer I. The sterotypical polarity of pyramidal neurons, an important feature of columnar organization appears to partially derive from the phosphorylation of neurogenin 2 (NGN2), a proneural gene, which has a double function – as an important pyramidal neuron phenotype determinant and as a cytoskeleton organizer for the emergence of dendrites in the apical portion of these cells (Schuurmans et al., 2004 ; Hand et al., 2005 ). These functions appear to be independent. Molecules that guide migration and axon growth such as semaphorins may also be important to orient axon-dendrite polarization (Polleux et al., 2000 ) influencing each of these components in a differential manner (repulsing axons and attracting dendrites).
Recently, it has also been shown that the radial distribution of clonally related neurons in the developing cerebral cortex depends on the expression levels of Ephrin A receptors (EphA) and ephrin-As (Torii et al., 2009 ). By using loss- and gain-of-function strategies to manipulate ephrin-As and EphA7 expression, Torii et al. (2009) have provided compelling evidence indicating that EphAs and ephrin-As signaling controls lateral dispersion of cortical excitatory neurons, likely contributing to the generation of cortical columns. Interestingly, ephrins and Eph receptors have also been involved in the establishment of other maps in the cerebral cortex, such as retinotopy (Flanagan and Vanderhaeghen, 1998 ).
Clones of Excitatory Neurons Disperse into Individual Cortical Columns
The lateral dispersion of clonally related neurons could also contribute to the early specification of cortical columns by generating spatially restricted radial clusters of neurons, which would later receive afferent connections and become involved in specific tasks. According to this scenario, we could expect that neurons generated from the same progenitor would disperse into unique functional cortical columns. To the best of our knowledge, this possibility has not been directly tested. However, there is accumulating evidence indicating that sibling neurons keep spatial relationships and display connection preferences at least compatible with their tentative role in the organization of cortical columns.
In order to investigate the degree of neuronal dispersion during development, a technique has been used which allows the infection of few progenitor cells at early developmental stages using a replication incompetent retroviral vector carrying a reporter gene (Luskin et al., 1988 , 1993 ; Price and Thurlow, 1988 ; Walsh and Cepko, 1988 , 1992 , 1993 ; Parnavelas et al., 1991 ; Luskin and McDermott, 1994 ; Mione et al., 1994 , 1997 ; Kornack and Rakic, 1995 ; Reid et al., 1995 ; Gaiano et al., 1999 ; McCarthy et al., 2001 ; Reid and Walsh, 2002 ; Costa et al., 2009 ). Later, the progeny of those few infected progenitors can be identified by the expression of the reporter gene, allowing measuring the dispersion of neuronal siblings. These studies have been termed “cell lineage” or “clonal analysis” studies and we may use both terms interchangeably.
The first cell lineage studies have found controversial results regarding the radial distribution of sibling neurons (Luskin et al., 1988 ; Price and Thurlow, 1988 ; Walsh and Cepko, 1988 ). While Luskin and co-workers suggested that clonally related neurons occur in columns, the works by Price and Walsh suggested that clones of neurons do not form radial columns, but rather disperse several hundreds of micrometers of cortex in the horizontal dimension. Several factors may have contributed for these divergent interpretations, such as the definition of clones based solely on the anatomical dispersion of cells (Luskin) or the expression of individual genetic tags by polymerase chain reaction (Walsh). For the reader interested in how these two variables may affect the conclusions of the cell lineage studies, we refer to a recent paper by Costa et al. (2009) where these issues were addressed.
Importantly, primary cell lineage studies were performed before the discovery of the massive tangential migration of GABAergic neurons in the developing brain (Marin and Rubenstein, 2001 ), what per se may have lead to misinterpretations about clonal relationship between cells. In fact, while the original population studies using tritiated thymidine to label post-mitotic cells have suggested that radial cell dispersion would be the primary mechanisms by which neurons could arrive to their final destination in the cerebral cortex (Angevine and Sidman, 1961 ; Rakic, 1974 ), it was only in the late 90s that tangential neuronal migration has been recognized as an important mechanism for settling GABAergic neurons in the cerebral cortex (Anderson et al., 1997 ). Nowadays, it is broadly accepted that, in the developing cerebral cortex of rodents, glutamatergic neurons migrate radially towards their final position in the cerebral cortex, whereas GABAergic neurons migrate tangentially (Marin and Rubenstein, 2003 ). Consequently, whilst radially migrating neurons could be distributed in arrays perpendicular to the pial surface, tangentially migrating ones disperse across different areas and no topographical relation has been detected between the ganglionic eminence VZ and the cortical destination of these cells. Rather the GE ventricular surface has been correlated to different types of interneurons (Fogarty et al., 2007 ; Xu et al., 2008 ). Therefore, it is possible that much of the tangential dispersion observed in early cell lineage studies was a consequence of tangential dispersion of GABAergic neurons (Walsh and Cepko, 1988 , 1992 , 1993 ; Tan et al., 1995 ).
At this point, it is important to cite that fate-mapping studies in the rodent cerebral cortex have provided compelling evidence indicating that glutamatergic and GABAergic neurons are indeed derived from separate pools of progenitors in the dorsal and ventral telencephalon, respectively. Using the Cre-lox system (Orban et al., 1992 ; Sauer, 1998 ), it has been shown that progenitors located in the dorsal telencephalon express the TF Emx1 and generate exclusively cortical excitatory neurons (Gorski et al., 2002 ), whereas those located in the ventral telencephalon express Nkx2.1, Lhx6, Gsh2 or Nkx6.2 and generate different subtypes of cortical interneurons (Fogarty et al., 2007 ; Xu et al., 2008 ). Thus, genetic fate-mapping studies indicate that the progeny of a single progenitor will comprise either glutamatergic or GABAergic neurons. Thus, these two types of neurons are very unlikely to be clonally related, at least in the rodent cerebral cortex.
Having that in mind, we have recently readdressed the issue of cell lineage in the developing cerebral cortex by using a combination of two silencing-resistant vectors carrying different reporter genes (Costa et al., 2009 ). We analyzed exclusively clones comprising spiny pyramidal neurons, so that the radial dispersion within clones of glutamatergic neurons, i.e. derived from dorsal telencephalic progenitors, could be accurately measured. Our results indicate that clonally related glutamatergic neurons generated from E13 progenitors do not disperse further than 280 μm in the adult cerebral cortex (Figure 1 ), what could very well be explained by the horizontal growth of the brain between the time of injection and analysis (Costa et al., 2009 ). In fact, we found that most neuronal clones derived from E13 progenitors span 150–250 μm in the horizontal axis and contribute to all cortical layers generated after that embryonic stage, namely layers V, IV, and II/III. Mathematical extrapolations for injections performed at the onset of neurogenesis in the cerebral cortex (E10-11) suggest that neuronal siblings would not disperse more than 400–500 μm. Thus, both the radial and horizontal dispersion of excitatory neuronal clones fits well with the possibility that they could help to create a structural basis for the future specification of columns.
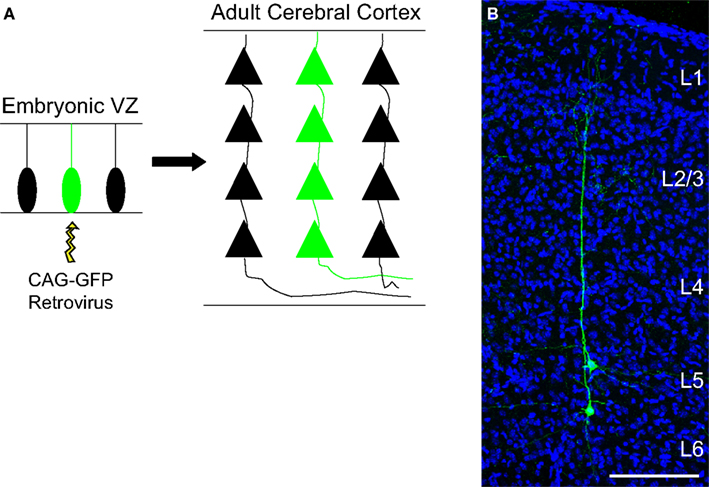
Figure 1. Columnar distribution of sister neurons in the cerebral cortex. (A) Schematic representation of a single progenitor cell transfected by a retroviral vector and its subsequent progeny (green). (B) Coronal section of the adult mouse cerebral cortex labeled for GFP (green) and DAPI (blue) where two neuronal siblings can be observed. For this experiment, the retrovirus carrying the gene for the protein GFP was injected into the lateral ventricle of an E13 animal. Abbreviations: VZ, ventricular zone; CAG-GFP, green fluorescent protein encoding plasmid; L, layer. Calibration bar: 100 μm.
Concurrently, it has also been suggested that excitatory neurons generated from the same progenitor are more likely to establish synaptic connections than non-sibling neurons (Yu et al., 2009 ). By injecting EGFP-expressing retroviruses through the uterus into the lateral ventricle of mouse embryos at early neurogenesis, the authors were able to identify individual clones of pyramidal neurons, similar to the cells shown in Figure 1 . Next, they performed simultaneous whole-cell recordings on two EGFP-expressing sister neurons and observed that these cells displayed unidirectional synaptic connections in 35% of pairs. In contrast, less than 7% of radially situated non-sister excitatory neurons were connected (Yu et al., 2009 ).
Taken together, these new lineage studies indicate that clonally related excitatory neurons not only keep a tight spatial relationship but are also capable of recognizing their siblings, either chemically or electrically, and establish functional synaptic connections.
The Link Between Progenitors and Post-Mitotic Neurons as the Cellular Basis for the Generation of Functional Circuits
Based on these findings, we would like to put forward a more tempting hypothesis, namely that transcriptional networks in cortical progenitors may help to establish functional units throughout the cerebral cortex by enabling these progenitors to generate neurons with similar electrochemical properties and high connectivity. According to this hypothesis, different levels and combinations of TFs expressed by discrete pools of progenitors would be responsible for the generation of individual microcircuits of sibling neurons, which would be able to recognize each other and establish synaptic connections. In other words, gene expression in individual cortical progenitors could influence the development of functional units throughout the cerebral cortex by generating small radial clusters of interconnected neurons (Figure 2 ), which in turn could be assembled together to generate functional minicolumns and columns.
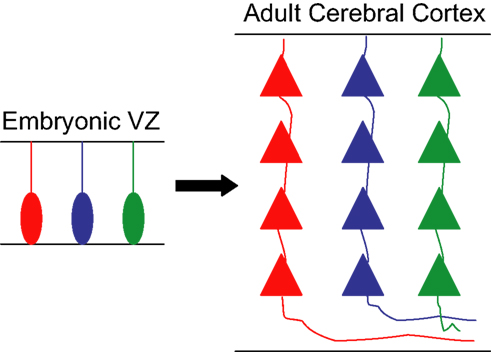
Figure 2. Hypothetic model for the generation of functional units from individual progenitors. Schematic drawing showing three progenitor cells in the embryonic ventricular zone (VZ) expressing different sets/levels of transcription factors, labeled in red, blue, and green. Each of these cells generates a clone of pyramidal neurons that inherit analogous genetic information from the founder progenitor and are organized in discrete radial arrays in the adult cerebral cortex. The similar genetic pedigree of sibling neurons allows their recognition and establishment of synaptic connections, creating a microcircuit of clonally related glutamatergic neurons.
The rationale behind our hypothesis is that neurons derived from the same progenitor are more likely to display similar chemical and physical properties, due to their genetic inheritance. Thus, sibling neurons would be more likely to recognize and respond stereotypically to the same molecular cues that could influence the early arrangement, metabolic coupling, and interconnectivity of those neurons within a single column. This ability is likely to rely on the expression of a similar set of surface molecules in sister neurons, which in turn could be controlled by TF networks operating in cortical progenitor cells.
But how molecules could contribute to specify the connection between sibling neurons? Sperry’s theory (1963) , known as chemoaffinity, proposed that molecules would be responsible for the wiring of neurons in the central nervous system. In fact, several groups have identified cell surface molecules involved in the patterning of neural circuits (Song and Poo, 2001 ). Yet, one important criticism to Sperry’s theory has been the fact that the number of neurons and connections in the brain (about 1012 and 1015, respectively, in humans) is far higher than the number of genes encompassed in the whole genome and, therefore, the number of different molecules would not suffice to specify all the neuronal connections. One possible solution envisioned by Sperry (1963) was the graded expression of cell surface molecules and their receptors, which has also been validated by recent findings (O’Leary et al., 1994 ). Such graded expression would lead to much higher combinatorial possibilities of cell responses than could be predicted by the number of signaling molecules and receptors. Indeed, it has been shown that a given ligand can elicit different responses in growing axons depending on the receptor complexes expressed in the target cell (Hong et al., 1999 ). Additional complexity is also added to the system when we consider the metabolic state of the axon. For example, the intracellular concentration of calcium can determine whether some axons are repelled or attracted by a given molecule (Hong et al., 2000 ). Thus, the number of genes in the cell genome clearly underestimates the repertoire of molecular combinations capable of dictating distinct cellular behaviors.
Furthermore, a large number of cell surface molecules can be generated from a limited number of genes in the nervous system through genetic rearrangements, such as the alternative splicing observed in the Drosophila gene Dscam1 (Down syndrome cell adhesion molecule) (Schmucker et al., 2000 ; Wojtowicz et al., 2004 ) and the mammal protocadherin family (Kohmura et al., 1998 ; Wu and Maniatis, 1999 ). Indeed, it has been proposed that Dscam1 gene gives rise to 18,048 proteins that could control self-avoidance between neurites through isoform-specific homophilic binding (Wojtowicz et al., 2007 ), what clearly indicates that a limited number of genes may generate a far broader variety of molecular tags responsible for cell-to-cell specific recognition in the nervous system. Therefore, it is not entirely absurd to suggest that the connectivity preference between sister neurons may be mediated by the expression of a given set of proteins involved in cell–cell recognition.
But now, how could gene expression in progenitor cells influence that? One possibility is that different expression levels of TFs could control the expression of distinct sets of surface molecules allowing the recognition of sibling neurons. Although we are just beginning to understand this phenomenon, increasing evidence support the notion that (i) distinct expression levels of a given TF; (ii) combination of TFs; or (iii) interactions between TF and its cofactors in the same cell type can in fact lead to completely different biological outcomes, likely reflecting differential gene expression induced by the TF.
For instance, it has been shown that the expression level Pax6 in the developing cerebral cortex is essential for controlling the balance between proliferation and differentiation (Sansom et al., 2009 ). By using Pax6 gain- and loss-of-function strategies, Sansom and colleagues have shown that the Pax6-regulated networks operating in cortical progenitors are highly dosage sensitive, so that relative levels of Pax6 are key determinants for controlling whether VZ progenitors will self-renew, generate neurons or basal progenitors. Therefore, it is not entirely absurd to suggest that different levels of Pax6 (or any other TF) within cortical progenitors might also kick off individual genetic programs by their neuronal lineage, leading to the expression of molecules responsible for the recognition and connectivity of these neurons.
Another example of such diversity in cell-response to a single TF is the activation of specific target genes by REST (repressor element-1 silencing transcription factor) during neuronal subtype specification (Abrajano et al., 2009 ). In this study, the authors have shown by chromatin immunoprecipitation on chip (Chip-chip) that REST and its cofactor CoREST (corepressor for element-1 silencing transcription factor) modulate the expression of largely distinct gene profiles responsible for inducing and maintaining different neuronal subtype identities, such as cholinergic, GABAergic, glutamatergic, and medium spiny neurons. These data clearly indicate that the balance between a single TF and its corepressor can regulate complex and distinct gene networks underlying important cell behaviors, such as neuronal subtype specification.
Collectively, these data support the idea that TF networks could modulate the expression of genes encoding proteins involved in cell–cell recognition and, consequently, contribute for the capacity of sibling neurons to recognize each other and establish synaptic connections. In the future, it will be interesting to investigate which genes and molecules subsidize the high probability of connection between sister neurons. Notably, we have observed a similar phenomenon in vitro, further suggesting that the capacity of clonally related neurons to recognize each other is a cell-intrinsic property.
Concluding Remarks
It has been suggested that one important phenomenon for the increased cerebral complexity during evolution may be the multiplication of neuronal columns throughout the cerebral cortex (Rakic and Caviness, 1995 ). Here, we further refine this conjecture by suggesting that discrete alterations in the gene expression pattern during development may support this phenomenon by allowing a larger number of individual progenitor cells to generate individual and highly interconnected neuronal clones. In other terms, neuronal clones could be seen as fundamental blocks in the construction of brain circuits, upon which later influences brought by axonal growth, synaptogenesis and activity will act to establish the functional anatomy of the cerebral cortex (Sur and Rubenstein, 2005 ). These fundamental blocks could also be influenced by earlier factors, such as incoming afferent systems to cerebral cortex that have been shown to regulate the cell cycle length in the germinal zone and contribute to generate areal differences in the germinal zones (Dehay and Kennedy, 2007 ). Also in that direction, recent work has shown that horizontal interconnectivity between columns is important to stabilize columnar size (Kaschube et al., 2009 ). Therefore, although lineage relationship could be at the base of the columnar organization of the cortex, several environmental factors are able to regulate column size and determine the properties that will be processed by the functional unit.
As can be deduced from our previous discussion about the origin of glutamatergic and GABAergic neurons, our hypothesis apply exclusively to the generation of glutamatergic neuronal clones. In fact, there is no evidence supporting the notion that tangentially migrating GABAergic neurons would settle in the cerebral cortex in an orderly manner, reflecting their original position in the VZ of the ventral telencephalon. Further support to this notion comes also from recent transplantation experiments indicating that GABAergic neurons are rather plastic and may develop functional inhibitory circuits in the visual primary cortex despite their site of origin (Southwell et al., 2010 ).
Concluding, we suggest here that the development of individual clones of glutamatergic neurons is a fundamental step for the parcellation of the cerebral cortex. These individual clones could be seen as singular functional units, which will be assembled into more complex parcels, such as minicolumns or columns, under the influence of intrinsic and extrinsic signals. According to this view, the number of independent functional units throughout the cerebral cortex would be increased not only by the enlargement of progenitor pools (Caviness et al., 1995 ), but also by discrete changes in the combinatory levels of TFs expressed in the progenitor cells. Consequently, this transcription network would represent an important target in brain evolution.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Work by Marcos R. Costa and Cecilia Hedin-Pereira is supported by CNPq, FAPERN and FAPERJ.
References
Abrajano, J. J., Qureshi, I. A., Gokhan, S., Zheng, D., Bergman, A., and Mehler, M. F. (2009). REST and CoREST modulate neuronal subtype specification, maturation and maintenance. PLoS ONE 4, e7936. doi: 10.1371/journal.pone.0007936.
Aggoun-Aouaoui, D., Kiper, D. C., and Innocenti, G. M. (1996). Growth of callosal terminal arbors in primary visual areas of the cat. Eur. J. Neurosci. 8, 1132–1148.
Anderson, S. A., Eisenstat, D. D., Shi, L., and Rubenstein, J. L. (1997). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476.
Angevine, J. B., Jr., and Sidman, R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768.
Bittman, K., Owens, D. F., Kriegstein, A. R., and LoTurco, J. J. (1997). Cell coupling and uncoupling in the ventricular zone of developing neocortex. J. Neurosci. 17, 7037–7044.
Bureau, I., Shepherd, G. M., and Svoboda, K. (2004). Precise development of functional and anatomical columns in the neocortex. Neuron 42, 789–801.
Caviness, V. S., Jr., Takahashi, T., and Nowakowski, R. S. (1995). Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18, 379–383.
Costa, M. R., Bucholz, O., Schroeder, T., and Gotz, M. (2009). Late origin of glia-restricted progenitors in the developing mouse cerebral cortex. Cereb. Cortex 19(Suppl. 1), i135–i143.
Crowley, J. C., and Katz, L. C. (2000). Early development of ocular dominance columns. Science 290, 1321–1324.
Dehay, C., and Kennedy, H. (2007). Cell-cycle control and cortical development. Nat. Rev. Neurosci. 8, 438–450.
Elias, L. A., Wang, D. D., and Kriegstein, A. R. (2007). Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448, 901–907.
Flanagan, J. G., and Vanderhaeghen, P. (1998). The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345.
Fogarty, M., Grist, M., Gelman, D., Marin, O., Pachnis, V., and Kessaris, N. (2007). Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946.
Gaiano, N., Kohtz, J. D., Turnbull, D. H., and Fishell, G. (1999). A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat. Neurosci. 2, 812–819.
Gorski, J. A., Talley, T., Qiu, M., Puelles, L., Rubenstein, J. L., and Jones, K. R. (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314.
Hand, R., Bortone, D., Mattar, P., Nguyen, L., Heng, J. I., Guerrier, S., Boutt, E., Peters, E., Barnes, A. P., Parras, C., Schuurmans, C., Guillemot, F., and Polleux, F. (2005). Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron 48, 45–62.
Hedin-Pereira, C., Lent, R., and Jhaveri, S. (1999). Morphogenesis of callosal arbors in the parietal cortex of hamsters. Cereb. Cortex 9, 50–64.
Hong, K., Hinck, L., Nishiyama, M., Poo, M. M., Tessier-Lavigne, M., and Stein, E. (1999). A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941.
Hong, K., Nishiyama, M., Henley, J., Tessier-Lavigne, M., and Poo, M. (2000). Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403, 93–98.
Houades, V., Koulakoff, A., Ezan, P., Seif, I., and Giaume, C. (2008). Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 28, 5207–5217.
Kandler, K., and Katz, L. C. (1995). Neuronal coupling and uncoupling in the developing nervous system. Curr. Opin. Neurobiol. 5, 98–105.
Kandler, K., and Katz, L. C. (1998). Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 18, 1419–1427.
Kaschube, M., Schnabel, M., Wolf, F., and Lowel, S. (2009). Interareal coordination of columnar architectures during visual cortical development. Proc. Natl. Acad. Sci. U.S.A. 106, 17205–17210.
Kohmura, N., Senzaki, K., Hamada, S., Kai, N., Yasuda, R., Watanabe, M., Ishii, H., Yasuda, M., Mishina, M., and Yagi, T. (1998). Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20, 1137–1151.
Kornack, D. R., and Rakic, P. (1995). Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron 15, 311–321.
LeVay, S., Stryker, M. P., and Shatz, C. J. (1978). Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. J. Comp. Neurol. 179, 223–244.
LeVay, S., Wiesel, T. N., and Hubel, D. H. (1980). The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol. 191, 1–51.
Luskin, M. B., and McDermott, K. (1994). Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia 11, 211–226.
Luskin, M. B., Parnavelas, J. G., and Barfield, J. A. (1993). Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J. Neurosci. 13, 1730–1750.
Luskin, M. B., Pearlman, A. L., and Sanes, J. R. (1988). Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron 1, 635–647.
Marin, O., and Rubenstein, J. L. (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790.
Marin, O., and Rubenstein, J. L. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441–483.
Marins, M., Xavier, A. L., Viana, N. B., Fortes, F. S., Froes, M. M., and Menezes, J. R. (2009). Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev. Neurobiol. 69, 715–730.
McCarthy, M., Turnbull, D. H., Walsh, C. A., and Fishell, G. (2001). Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J. Neurosci. 21, 6772–6781.
Mione, M. C., Cavanagh, J. F., Harris, B., and Parnavelas, J. G. (1997). Cell fate specification and symmetrical/asymmetrical divisions in the developing cerebral cortex. J. Neurosci. 17, 2018–2029.
Mione, M. C., Danevic, C., Boardman, P., Harris, B., and Parnavelas, J. G. (1994). Lineage analysis reveals neurotransmitter (GABA or glutamate) but not calcium-binding protein homogeneity in clonally related cortical neurons. J. Neurosci. 14, 107–123.
Mountcastle, V. B. (1957). Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J. Neurophysiol. 20, 408–434.
O’Leary, D. D., Ruff, N. L., and Dyck, R. H. (1994). Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr. Opin. Neurobiol. 4, 535–544.
Orban, P. C., Chui, D., and Marth, J. D. (1992). Tissue- and site-specific DNA recombination in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 89, 6861–6865.
Parnavelas, J. G., Barfield, J. A., Franke, E., and Luskin, M. B. (1991). Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb. Cortex 1, 463–468.
Polleux, F., Morrow, T., and Ghosh, A. (2000). Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404, 567–573.
Price, J., and Thurlow, L. (1988). Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development 104, 473–482.
Rakic, P. (1974). Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183, 425–427.
Rakic, P. (1976). Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261, 467–471.
Rakic, P., and Caviness, V. S., Jr. (1995). Cortical development: view from neurological mutants two decades later. Neuron 14, 1101–1104.
Reid, C. B., Liang, I., and Walsh, C. (1995). Systematic widespread clonal organization in cerebral cortex. Neuron 15, 299–310.
Reid, C. B., and Walsh, C. A. (2002). Evidence of common progenitors and patterns of dispersion in rat striatum and cerebral cortex. J. Neurosci. 22, 4002–4014.
Sansom, S. N., Griffiths, D. S., Faedo, A., Kleinjan, D. J., Ruan, Y., Smith, J., van Heyningen, V., Rubenstein, J. L., and Livesey, F. J. (2009). The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 5, e1000511. doi: 10.1371/journal.pgen.1000511.
Schmucker, D., Clemens, J. C., Shu, H., Worby, C. A., Xiao, J., Muda, M., Dixon, J. E., and Zipursky, S. L. (2000). Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 101, 671–684.
Schuurmans, C., Armant, O., Nieto, M., Stenman, J. M., Britz, O., Klenin, N., Brown, C., Langevin, L. M., Seibt, J., Tang, H., Cunningham, J. M., Dyck, R., Walsh, C., Campbell, K., Polleux, F., and Guillemot, F. (2004). Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 23, 2892–2902.
Southwell, D. G., Froemke, R. C., Alvarez-Buylla, A., Stryker, M. P., and Gandhi, S. P. (2010). Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148.
Sperry, R. W. (1963). Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. U.S.A. 50, 703–710.
Sur, M., and Rubenstein, J. L. (2005). Patterning and plasticity of the cerebral cortex. Science 310, 805–810.
Tan, S. S., Faulkner-Jones, B., Breen, S. J., Walsh, M., Bertram, J. F., and Reese, B. E. (1995). Cell dispersion patterns in different cortical regions studied with an X-inactivated transgenic marker. Development 121, 1029–1039.
Torii, M., Hashimoto-Torii, K., Levitt, P., and Rakic, P. (2009). Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature 461, 524–528.
Walsh, C., and Cepko, C. L. (1988). Clonally related cortical cells show several migration patterns. Science 241, 1342–1345.
Walsh, C., and Cepko, C. L. (1992). Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255, 434–440.
Walsh, C., and Cepko, C. L. (1993). Clonal dispersion in proliferative layers of developing cerebral cortex. Nature 362, 632–635.
Wiesel, T. N., and Hubel, D. H. (1963). Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017.
Wiesel, T. N., and Hubel, D. H. (1965). Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072.
Wojtowicz, W. M., Flanagan, J. J., Millard, S. S., Zipursky, S. L., and Clemens, J. C. (2004). Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 118, 619–633.
Wojtowicz, W. M., Wu, W., Andre, I., Qian, B., Baker, D., and Zipursky, S. L. (2007). A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell 130, 1134–1145.
Wu, Q., and Maniatis, T. (1999). A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97, 779–790.
Xu, Q., Tam, M., and Anderson, S. A. (2008). Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 506, 16–29.
Yu, Y. C., Bultje, R. S., Wang, X., and Shi, S. H. (2009). Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504.
Yuste, R., Nelson, D. A., Rubin, W. W., and Katz, L. C. (1995). Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron 14, 7–17.
Keywords: cortical columns; sister neurons; cell lineage; transcription factors
Citation: Costa MR and Hedin-Pereira C (2010) Does cell lineage in the developing cerebral cortex contribute to its columnar organization? Front. Neuroanat. 4:26. doi: 10.3389/fnana.2010.00026
Received: 01 March 2010;
Paper pending published: 19 March 2010;
Accepted: 26 May 2010;
Published online: 28 June 2010
Edited by:
Javier DeFelipe, Cajal Institute, SpainReviewed by:
Monique Esclapez, Institut National de la Santé et de la Recherche Médicale, FranceGeorge W. Huntley, Mt Sinai School of Medicine, USA
Copyright: © 2010 Costa and Hedin-Pereira. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Marcos R. Costa, Edmond and Lily Safra International Institute of Neuroscience of Natal, Rua Prof. Francisco Luciano de Oliveira, 2460 Natal, Rio Grande do Norte 59066-060, Brazil. e-mail: mrcosta@natalneuro.org.br