Wiring of divergent networks in the central auditory system
- 1 Department of Comparative Biomedical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
- 2 Harvard Medical School, Boston, MA, USA
- 3 Division of Neurobiology, Department of Molecular and Cell Biology, University of California, Berkeley, CA, USA
Divergent axonal projections are found throughout the central auditory system. Here, we evaluate these branched projections in terms of their types, distribution, and putative physiological roles. In general, three patterns of axon collateralization are found: intricate local branching, long-distance collaterals, and branched axons (BAs) involved in feedback-control loops. Local collaterals in the auditory cortex may be involved in local processing and modulation of neuronal firing, while long-range collaterals are optimized for wide-dissemination of information. Rarely do axons branch to both ascending and descending targets. Branched projections to two or more widely separated nuclei or areas are numerically sparse but widespread. Finally, branching to contralateral targets is evident at multiple levels of the auditory pathway and may enhance binaural computations for sound localization. These patterns of axonal branching are comparable to those observed in other modalities. We conclude that the operations served by BAs are area- and nucleus-specific and may complement the divergent unbranched projections of local neuronal populations.
Introduction
A cardinal feature of axons is their divergent projections, which range from sparse branching in the thalamic input to different auditory cortex (AC) areas (Morel and Imig, 1987; Lee et al., 2004a; Kishan et al., 2008) to the many collaterals and thousand of boutons of single spiral Ia cochlear ganglion axons (Brown, 1981). Branched axons (BAs) are present throughout the auditory system (Fekete et al., 1984; Willard and Martin, 1984; Ojima, 1994; Hazama et al. 2004; Coomes et al., 2005; Kimura et al., 2005; Lee and Winer, 2008a,b,c) and can take many forms, from local (Brown et al., 1988a,b) to very distant (Hashikawa et al., 1995; Cetas et al., 1999; Huang and Winer, 2000), presumably allowing neurons to synchronize remote events or form multiple feature-specific representations.
Different patterns of axonal branching prevail at different levels of the auditory system (Figures 1–3). For instance, branching between different nuclei is common in the pathways to and from the medial nucleus of the trapezoid body (MTB; Morest, 1968; Spirou et al., 1990; Kuwabara and Zook, 1991, 1992; Kuwabara et al., 1991; Smith et al., 1991), while thalamocortical axons rarely project to different cortical fields, such as the primary auditory cortex (AI) and the anterior auditory field (AAF; Morel and Imig, 1987; Lee et al., 2004a,b). Other axons have both descending and ascending projections, e.g., from MTB cell axons projecting to the cochlear nucleus (CoN) and the inferior colliculus (IC), <1% project to both (Schofield, 1994).
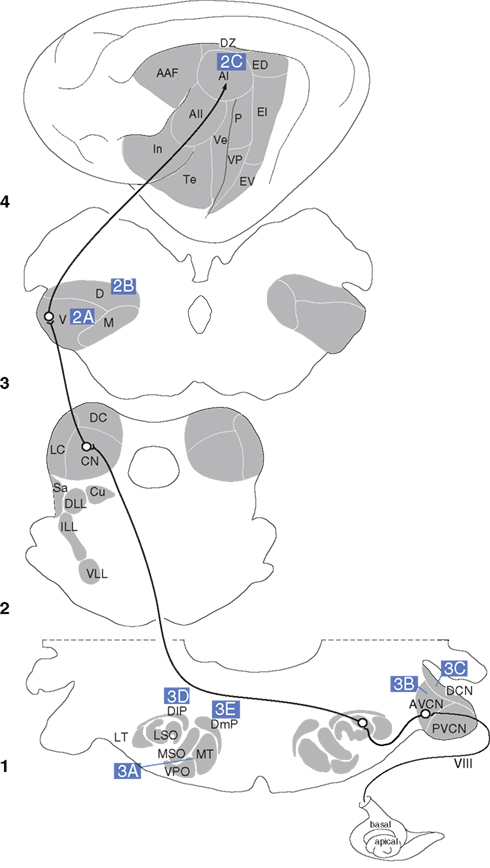
Figure 1. The central auditory pathway. Key nuclei in the feline auditory system and elements of the lemniscal pathway from the medulla (1), midbrain (2), thalamus (3), and auditory cortex (4). Letters in blue boxes indicate the pathways depicted in Figures 2 and 3.
In discussing the wide variety of branching patterns present in the auditory system, it is imperative to acknowledge that various methods allow the detection of different patterns of axonal branching, and that these different methods have inherent limitations in terms of the conclusions that can be drawn from their use. Thus, we review the technical considerations inherent in assessing axonal branching. An especially important caveat to establish at the outset, however, is that dual retrograde injections can only ascertain axonal branching to the specific regions within the nuclei injected; conclusions cannot be drawn about other forms of axonal branching from these studies. Nonetheless, the use of dual retrograde tracing has been useful in formulating hypotheses about neural function.
Although the functional implications of BAs are numerous (Morest, 1968; Kuwabara et al., 1991; Ojima et al., 1991, 1992; Li and Mizuno, 1997a,b; Kuwabara and Zook, 1999; Ye et al., 2000; Mulders and Robertson, 2002, 2003; Mulders et al., 2007), we are treating the function of BAs from the perspective of general organizational principles.
In the first two sections (see Branched Axons in the Auditory Cortical System, Branched Axons in the Auditory Brainstem and Midbrain), we review the existence, magnitude, and possible functions of BAs in the auditory cortex and thalamus as compared with those at earlier levels of the auditory system. These initial sections review the specifics of axonal branching in the auditory system, which the general reader may wish to skim in favor of the final sections (see Technical Considerations, Thematic Perspective, Alternatives to Collateralization in the Auditory Cortex, Collaterals in Other Modalities, and Summary), where we examine principles of axonal branching and evaluate the technical difficulties inherent in detecting BAs.
Branched Axons in the Auditory Cortical System
Thalamocortical System
All regions of the auditory cortex (AC) receive an input from the thalamus (Lee and Winer, 2008a). The principal source of auditory thalamocortical (TC) input, the medial geniculate body (MG), has tonotopic ventral (MGv) and rostral pole (RP) divisions, and non-tonotopic dorsal (MGd) and medial (MGm) divisions, which project in varying degrees to each of the 13 auditory cortical (AC) areas in the cat (Huang and Winer, 2000). Although focal regions within a thalamic nucleus can project broadly to multiple cortical areas based on anterograde tracing studies (Huang and Winer, 2000), axonal divergence of single neurons beyond a few millimeters is quite rare based on retrograde double labeling studies (Kishan et al., 2008). Thus, axonal branching in the auditory thalamocortical system is highly local, but with unique topographical features.
One of these features is the patchy distribution of TC BAs, which extend over 300–500 μm in layers IIIb and IV of the primate AC core (Hashikawa et al., 1995). In the lateral and posteromedial auditory cortical areas, larger (1000–1500 μm) patches arise from the MG anterodorsal and/or posterodorsal nuclei. In the rabbit, TC BAs form patches 1–2 mm apart in AI layers III and IV, with tangential layer I BAs up to 7 mm long (Cetas et al., 1999; Figure 2A). In the cat, similar patches are seen in AI, AAF, ventral, and the posterior AC (P) following injections of anterograde tracers into the MGv (Huang and Winer, 2000). More divergence occurs after similar MGd and MGm deposits, though not explicitly from BAs. Thick MGm axons in AC layer Ia project laterally across wide expanses, and vertical branches in layers II, IVb, and Va have fewer lateral BAs (Huang and Winer, 2000). Axons in layer IIIb also have many local BAs shorter than those in layers Ia and VIb.
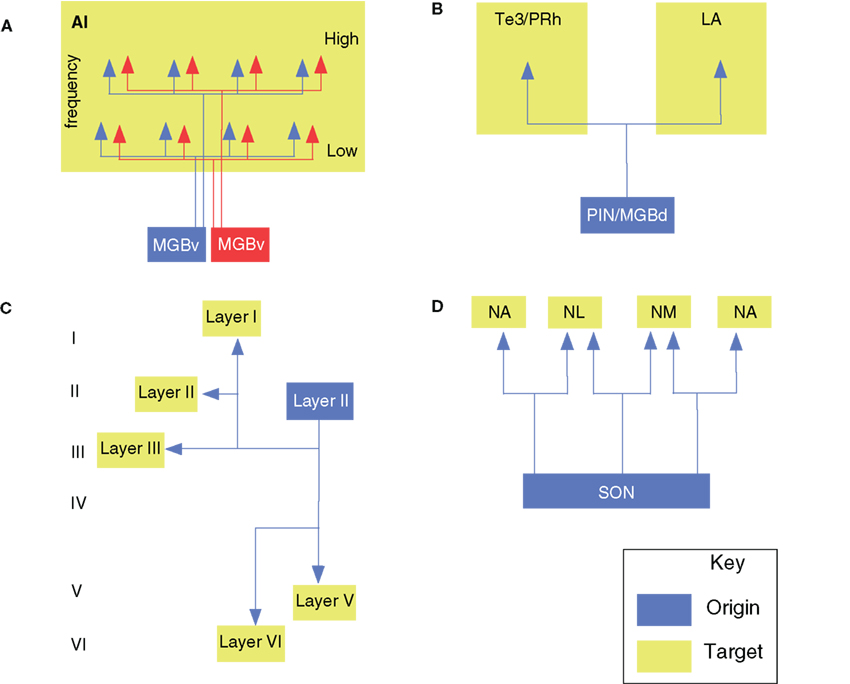
Figure 2. Branched axonal projections in the auditory forebrain. (A) Clustered and periodic thalamocortical projections from medial geniculate body subdivisions to area AI (Velenovsky et al., 2003). (B) Posterior intralaminar (PIN) and dorsal division of the medial geniculate body branched projections to the non-primary auditory cortex (Te3/PRh) and lateral amygdala (LA; Doron and LeDoux, 2000). (C) Local, interlaminar, and collateral projections of an intracellularly labeled layer II pyramidal cell in AI (Ojima et al., 1991). (D) Avian olivary (SON) branched input to the laminaris (NL) and angularis (NA) nuclei (Burger et al., 2005).
The patchy distribution of MG afferents in AC may correlate with parvalbumin immunoreactivity and perhaps with modules of broadly and narrowly tuned neurons (Read et al., 2008) or binaural excitatory–excitatory/inhibitory (EE, EI) modules, though physiological–anatomical studies suggest that EE and EI columns are not linked by BAs (Middlebrooks and Zook, 1983). Similar patchy distributions in AC areas ostensibly lacking a binaural columnar arrangement imply that BAs are unrelated to binaurality. Intraareal BAs linking EE or EI columns are also sparse (Middlebrooks and Zook, 1983, but see Brandner and Redies, 1990).
Another canonical feature of the primary auditory cortical areas is the orderly spatial arrangement of neurons according to characteristic frequency (CF), i.e., tonotopy. A question that naturally arises is whether TC BAs contribute to the creation of the multiple AC CF maps (Morel and Imig, 1987) from the two representations in the MG (Imig and Morel, 1985a,b, 1985a,b)? Based on retrograde studies where different tracers are placed into matched isofrequency loci in different primary cortical areas, few double-labeled thalamic neurons are found (Morel and Imig, 1987), with some differences among the MGv and RP (Lee et al., 2004a; Kishan et al., 2008). Due to the paucity of double labeling in such studies, it appears that TC BAs do not create multiple CF maps in these areas.
Finally, the MG and intralaminar nuclei also project widely to non-auditory cortex. Thalamic BAs targeting both the lateral amygdaloid nucleus and the perirhinal or primary AC could influence autonomic and affective responses to auditory and multisensory stimuli (Namura et al., 1997). BAs may link some intralaminar nuclei with the dorsal (and, less so) ventral perirhinal cortex, and rarely arise from MGd/m neurons, though up to 17% of MGm cells project to perirhinal cortex and to the lateral amygdaloid nucleus (Figure 2B; Table 1). Although MGd cells project to both the frontal cortex and primary/non-primary AC, these originate from unbranched sources (Kurokawa and Saito, 1995). Thus, these TC parts of the auditory and motor pathways are segregated, despite extensive interdigitation of the projection cells. Overall, the few studies and diversity of relevant pathways make it difficult to specify the role of BAs in TC projections to non-auditory cortex.
Corticocortical System
Every area of the auditory cortex receives extensive input from local intrinsic cortical connections and extrinsic connections from other cortical areas in both hemispheres (Winer and Lee, 2007; Lee and Winer, 2008b,c), which provide ∼95% of the total input to an area (Lee et al., 2004a; Lee and Winer, 2011). As with the thalamocortical system, anterograde, axon-filling, and retrograde studies each provide complementary evidence about BAs in the corticocortical system.
On a local level, neurons in the auditory cortex branch within an area to create extensive divergent laminar circuits. In particular, layer II and III pyramidal cell axons branch proximally and distally to the cell body (Ojima et al., 1991; Figure 2C), forming an axonal network that extends across layers I–V, with two-to-five thick collaterals in layer III or V in addition to the main axon descending to the white matter for other cortical targets (Ojima et al., 1992). The horizontal branches in layer III or V run parallel to the pia for 500–2500 μm and emit, at a few distant points, local plexuses of secondary branches extending to upper and lower layers. This collateralization as a whole forms a columnar terminal field in layers I through V with a branch-sparse gap in layer IV (Figure 2C). Each neuron has a number of vertical branches distributing around its cell body, forming a columnar terminal field, which is similar to that formed at distant points. Some non-projecting pyramidal neurons have thick, bifurcated axons with recurrent oblique or horizontal BAs; the latter extend 1–2 mm in layer V, and oblique branches project heavily in layers II–IV, with weaker input to layers I or II. Such cells may interact with those producing the synchronized oscillations arising in layer V (Silva et al., 1991). Several long-range dorsoventrally oriented BAs may link or segregate AI isofrequency loci in the cat (Read et al., 2001). Alternatively, they may synchronize cells with similar CF response properties, analogously to pyramidal neurons in visual cortex (Gray and Singer, 1989; Gray et al., 1989). Perhaps TC BAs complement these rich, local periodic projections.
As with the thalamocortical system, branched corticocortical projections that link similar CF regions are sparse, comprising <1% of AI and AAF cells projecting to matched CF regions (Lee et al., 2004a), although earlier studies using anterograde methods found extensive interconnections among matched CF regions (Imig and Reale, 1980), perhaps accounted for by neuronal populations that project in an unbranched manner to matched CF regions in different areas. Thus, long-range cortical BAs may be more rare than axon filling studies suggest. This implies that cortical BAs do not contribute significantly to spectral maps in different AC areas and illustrates a fundamental difference between the auditory forebrain and the brainstem, where axons subdivide profusely to innervate many different targets (Irvine, 1986). Intrinsic intraareal BAs across frequency laminae are also rare (Kishan et al., 2008), but may be more prevalent along an isofrequency contour.
Commissural AI axons may also target disparate areas, with homo- and hetero-topic terminal sites; a dual retrograde study found that some rat BAs target both sites (Rüttgers et al., 1990). However, <1% of AI or AAF neurons project commissurally to frequency-matched loci in both fields, and <4% of non-primary (AII, Te, and In) neurons project to two loci in their contralateral counterparts (Kishan et al., 2008).
Corticofugal Projections
The auditory corticofugal system targets many thalamic, midbrain, and brainstem nuclei (Winer, 2006). Of these, the corticothalamic (CT) system is massive, with each major MG division receiving input from four or more AC areas (Winer et al., 2001). Two types of terminals arise from AI: small endings from thin axons of layer VI pyramidal neurons and large boutons from thick axons of layer V pyramidal neurons (Ojima, 1994; Winer et al., 1999; Llano and Sherman, 2008). Layer VI CT neurons typically project in a feedback manner to the thalamic nucleus from which they receives their major TC input, while layer V CT neurons project in a feedforward manner to a higher order thalamic nucleus (Winer et al., 2001; Sherman and Guillery, 2006).
Layer V CT pyramidal cell targets include MGm, MGd, and ventrolateral MGv, with thick horizontal BAs occurring in cortical layers V and VI forming heterogeneous en passant and spine-like boutons, and thin vertical axons ending above layer IV (Ojima et al., 1992), and with no BAs to the contralateral AI (Wong and Kelly, 1981), reserving collateralization to the ipsilateral AC. BAs crossing the cortical CF axis may enhance inhibition at other CFs, while those parallel to the isofrequency contours could have local roles (Ojima et al., 1991; Song et al., 2006).
Layer VI CT neurons branch extensively in both thalamus and cortex. Some layer VI CT cells have recurrent branches in cortical layer VI, then ascend to layers III and IV, where their processes form a dense plexus. In the thalamus, thin fiber BAs form dorsoventrally elongated bands parallel to MGv CF laminae (Rouiller and de Ribaupierre, 1990). Layer VI CT cells may activate local columnar neurons, while layer V CT neurons target more remote columns at the same or different CF. In addition, anterograde tracer deposits at separate frequency loci in the cat label terminals segregated in the MG, suggesting that microtopography complements BAs (Takayanagi and Ojima, 2006).
Corticothalamic projections include BAs to the thalamic reticular nucleus (TRN; Lam and Sherman, 2010). Layer V or VI axons traverse the TRN (Hazama et al., 2004); forming elongated slabs; these may be BAs of cells targeting the MGv. High- and low-CF loci in rat primary and non-primary AC areas converge in the MGv and target different TRN regions (Kimura et al., 2005). The TRN has inhibitory input to much of the MG, and some TRN neurons project to both the ventrolateral MGv and MGd, or to both the MGv pars ovoidea and MGm (Crabtree, 1998). This branching pattern might enable two AC tonotopic areas to convergently excite one MG region via direct CT projections, while divergently inhibiting separate MG regions via indirect reticulothalamic projections (Kimura et al., 2005). The AC also targets the midbrain, medulla, and striatum (Winer, 2006), and these corticofugal cells may also have intracortical BAs. Layer V corticostriatal neurons have vertical and short-range horizontal BAs. The vertical BAs form a dense network of terminal arbors in layers III and IV, perhaps reinforcing supragranular, reciprocal connections between AC CF loci projecting to similar striatal targets.
The corticocollicular system is also a rich substrate for axonal branching (Winer et al., 1998; Winer, 2006). Rat corticocollicular cells project to the caudal striatum (Moriizumi and Hattori, 1991b), and some corticofugal cells target the superior olivary complex (SOC) and IC, or the IC and the CoN, via BAs (Doucet et al., 2002, 2003). Some corticocollicular cells send BAs to the nucleus of the brachium of the IC (Saldaña et al., 1996). Retrograde experiments indicate that ∼5% of layer V neurons project to both IC (Willard and Martin, 1984; Coomes et al., 2005). Almost half of contralaterally projecting corticocollicular cells project bilaterally. Given the conservative estimates provided by retrograde tracers, all contralaterally projecting cells may target both ICs (Coomes et al., 2005), though no neurons appear to have BAs targeting both the IC and MG (Wong and Kelly, 1981).
Branched Axons in the Auditory Brainstem and Midbrain
Brainstem Projections
Now, we consider the axonal branching patterns observed in the auditory brainstem and midbrain, in comparison with those of the auditory cortical systems described previously. Do similar branching patterns and principles apply across multiple stages of the auditory pathway? The numerous connections among brainstem and midbrain nuclei might suggest different patterns of axonal branching exist at these stages. As noted in morphological studies, auditory BAs begin in the periphery (Lorente de Nó, 1981). At the earliest levels, type I auditory nerve fibers branch extensively in the CoN (Fekete et al., 1984). One main branch targets the ventral cochlear nucleus (VCoN) and the other ends in the dorsal cochlear nucleus (DCoN). Near this bifurcation, the parent trunk has few collaterals at low CFs, while axons at higher CFs have more numerous and complex axonal branches. Descending axons have 14–30 collaterals and, in the DCoN, the main trunk often makes parallel branches ending within 100 μm. Many BAs end in simple, en passant swellings, and others terminate diffusely in the neuropil. BAs have regional morphologic variations, e.g., in the posteroventral cochlear nucleus, some have en passant swellings, while in the central part of the nucleus, fibers with a CF >4 kHz have many BAs that extend for hundreds of microns. These are parallel to octopus cell primary dendrites and could enhance the sharpness of tuning near the intensity threshold and broaden tuning at higher intensities. The ascending branch has 4–16 collaterals and ends in calyces of Held. These collaterals form complex, endbulb-like endings or en passant swellings and often remain within 100 μm of the parent branch, though one-third end in the anteroventral CoN. Small branches from high- and low-CF fiber may create heterotopic high frequency response zones in the VCoN (Fekete et al., 1984).
Cochlear nucleus afferents also branch. VCoN neurons send branches to matching frequency loci in the cat IC and contralateral DCoN (Adams, 1983a). Planar and radiate multipolar cells (T- and D-stellate cells, respectively) in the anterior VCoN branch to the DCoN and posterior VCoN, mainly to the multipolar cell area (Oertel et al., 1990). Radiate multipolar cells project to both the ipsilateral DCoN and the contralateral CoN (Doucet and Ryugo, 2006). Up to half the cells projecting to CoN also target the thalamic ventrobasal complex and may provide information about head and body position useful in sound localization or for somatic sensory–auditory interactions (Li and Mizuno, 1997a).
A prominent CoN target is the contralateral MTB (Morest, 1968), whose principal cells provide glycinergic input to the ipsilateral lateral superior olive (LSO) for interaural intensity difference computations (Smith et al., 1998). CoN projections form calyces of Held endings on MTB principal cells (Smith et al., 1991) and collateralize ipsilateral to the CoN of origin, targeting the lateral nucleus of the trapezoid body (LTB), posterior periolivary nucleus, or ventrolateral periolivary nucleus and end in large terminal swellings of variable shapes (Figure 3B; Spirou et al., 1990). En passant swellings are rare.
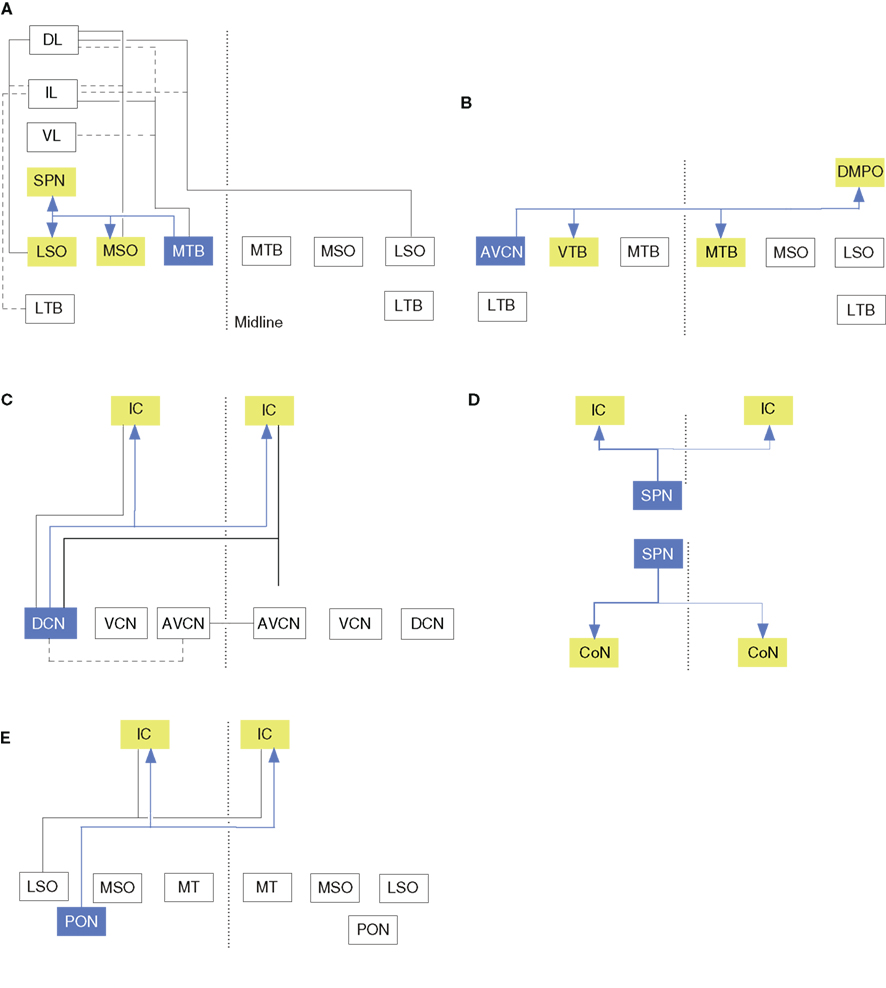
Figure 3. Branched axonal projections in the auditory brainstem and midbrain. (A) Collateral projections from the medial nucleus of the trapezoid body to olivary and lateral lemniscal targets (Kuwabara and Zook, 1992). (B) Anteroventral cochlear nucleus collateral input to the ventral nucleus of the trapezoid body (VTB) and dorsomedial periolivary nucleus (Smith et al., 1991). (C) Cochlear nucleus branched projections to the inferior colliculus (IC; Schofield and Cant, 1996a; Schofield, 2002). (D) Branched ascending and descending projections from the superior paraolivary nucleus to the cochlear nucleus (CoN) and IC (Schofield, 1995). (E) Periolivary (PON) projections to the inferior colliculus (IC) and CoN. Dashed line in all panels represents the midline.
Most CoN BAs are precalycine. These traverse the MTB and ventral nucleus of the trapezoid body (VTB) toward the lateral lemniscus, forming branches in the anterolateral periolivary nucleus, the rostral LTB, and the VTB. Some fibers form collaterals at their branch point near the abducens nerve root, and branch sparsely before ending in the nucleus paragigantocellularis lateralis. Other precalycine collaterals target the dorsomedial and ventral periolivary nuclei and branch repeatedly within it (Kuwabara et al., 1991). About 40% of ipsilateral calyciferous branches end axosomatically in the ventral periolivary nucleus (VPO), 20% in the LTB and LSO, and 7% near the MTB in an area associated with the medial olivocochlear system (MOC). All axons have extensive BAs within the MTB, perhaps contributing to lateral inhibition. Other BAs end diffusely in the adjacent periolivary nuclei, the LTB, and the LSO, and 25% reach the lateral lemniscus (Figure 3A). Of the calycine collaterals, all terminate 20–80 μm from their origin in varicosities. Thus, ascending input to the MTB reaches parts of the ipsilateral lateral and medial olivocochlear system and diverse contralateral brain stem nuclei. MOC BAs to the CoN often converge with type II auditory nerve fiber endings (Benson and Brown, 2004), and areas targeted by such axons also project to the MOC, forming another prospective feedback-gain loop (Ye et al., 2000).
Perhaps unsurprisingly for brainstem projections, MTB axons are also collateralized (Figure 3A; Morest, 1968; Kuwabara et al., 1991). Principal cell axons send 2–6 BAs to the periolivary nuclei, superior paraolivary nucleus (SPN; the rodent homolog of the cat dorsomedial periolivary nucleus), and the VTB. Half of these axons also branch to the medial superior olive (MSO), and 25% branch to the lateral lemniscus. Recurrent MTB collaterals are also seen. The main axon often ends in a cascade of terminal BAs in the LSO; sometimes forming 1–2 thick perpendicular branches and then arborizing in the neuropil. MTB branches to the MSO are tonotopically organized (Smith et al., 1998).
Many brain stem neurons sample both the outputs of the MTB as well as collaterals bifurcating from input to the MTB, perhaps for monitoring or instructing gain control (Morest, 1968; Kuwabara et al., 1991). LSO-projecting neurons from the LTB also have collaterals to MSO (except in big brown bats), which, like MTB BAs, have axosomatic input on bipolar cells (Kuwabara and Zook, 1992). These inhibitory inputs may complement excitatory CoN afferents, perhaps preceding excitatory inputs because the contralateral calyciferous axons are much thicker than the CoN axons directly projecting to the contralateral MSO. Cell filling experiments in gerbil brain stem slices demonstrate that the MSO input to the SPN is highly branched, with >40% of thick, ascending MSO axons having one or more short BAs from their main trunk that ramify sparsely in the SPN (Kuwabara and Zook, 1999).
Not all brain stem projections have BAs. While some CoN efferent axons in the guinea pig target both CoN-projecting and IC-projecting cells in the SPN, their BAs may not be extensive (Schofield, 1995). Further, <1% of MTB neurons project to both the IC and CoN ipsilaterally, contralaterally, or have one ipsilateral and one contralateral target (Schofield, 1994).
Projections of the Inferior Colliculus
The IC is the midbrain target for auditory input arising from earlier brainstem sources, e.g., the CoN, SOC, lateral lemniscal nuclei, AC, and many other non-auditory structures. The tonotopic central nucleus of the IC (CN) contains narrowly tuned neurons, while the cells in the dorsal cortex and lateral cortex (La) have broader frequency-tuning and multisensory properties. The IC projects to the MG, CoN, SOC, dorsal column nuclei, superior colliculi (SC), and other nuclei (for review see Winer and Schreiner, 2005).
The projection from the ventral nucleus of the lateral lemniscus to the CN has few BAs to different high- and low-frequency regions in the rat CN (Merchán and Berbel, 1996).
Such tonotopic precision is implicit in the narrow frequency tuning of anteroventral CoN cells (Bourk et al., 1981). In addition, in the rat lateral lemniscal nuclei, no neurons project to both the IC and the SC, or to both SCs, though cells in the dorsal nucleus of the lateral lemniscus may project to the SC deep layers for acoustic motor reflexes and head orientation (Tanaka et al., 1985).
The proportion of brainstem afferents that target both ICs via BAs may be species specific. In the cat IC, only 2% of LSO olivocollicular neurons project to both IC, while surprisingly, in the opossum, 20–25% of LSO olivocollicular neurons and almost all MSO olivocollicular cells project to both (Willard and Martin, 1984). Similar work in the guinea pig finds no branched projections in the LSO, MSO, or VCoN, but in the DCoN, 68% of ipsilateral IC-projecting cells have BAs to the contralateral IC (Figure 3C; Schofield and Cant, 1996b). Compared with the corticofugal system (see above), in both the guinea pig and the opossum, ∼6% of AC neurons project bilaterally to the IC.
Branched brainstem projections to the IC and other targets are also rare. In the SPN, ∼2% of IC-projecting cells branch to the CoN (Figure 3D; Schofield, 1991). Similarly, in the guinea pig SOC, only 1% of IC-projecting neurons send axons to the CoN (Figure 3E; Schofield, 2002). These few branched projections originate in the ventral periolivary region, including the anteroventral periolivary nucleus and the VTB, but no cells project to both targets contralaterally, or to one ipsilaterally – and the other contralaterally. In addition, some non-auditory afferents also have BAs (Moriizumi and Hattori, 1991a,b; Li and Mizuno, 1997a,b).
Within the IC itself, local connections are highly collateralized as revealed by intracellular filling studies in the cat (Oliver et al., 1991). These intrinsic BAs sometimes parallel the dendrites, extending for hundreds of microns (as in the CoN), while other IC neurons have non-oriented CN BAs. This diversity suggests extensive IC computational roles for local BAs and interneurons (Oliver et al., 1991). Axons of these cells extend toward the brachium of the IC, and many likely project to the MG (Winer et al., 1996). Some of these tectothalamic neurons are inhibitory (Winer et al., 1996; Peruzzi et al., 1997; Bartlett and Smith, 2002; Lee and Sherman, 2010), providing a source of feedforward inhibition that is unique to the auditory system.
However, most long-range IC projections have few BAs. Few colliculobulbar neurons target both CoNs in the guinea pig (Schofield, 2001) and rat (Okoyama et al., 2006). Instead, the IC may exert descending divergent influence disynaptically through contact with cells that projecting bilaterally to the CoN, particularly in the VTB and anteroventral periolivary nucleus (Schofield and Cant, 1999). As in the brain stem, IC neurons with ascending and descending projections are rare, with reports suggesting that no or few cells project to both the CoN and the MG in the cat (Hashikawa, 1983), rat (Okoyama et al., 2006), and guinea pig (Coomes and Schofield, 2004), and <1% project to both the SC and the pontine nuclei (Hashikawa, 1983). IC neurons branching to the MG and the contralateral IC also target the contralateral CoN, and comprise 1–10% of all tectothalamic cells (González-Hernández et al., 1991; Okoyama et al., 2006). Similarly, few axons target both the contralateral IC and the SOC or CoN.
Technical Considerations
Many approaches have been used to characterize BAs. Dual retrograde tract tracing (Hayes and Rustioni, 1979; Kuypers et al., 1980; Jones, 1983) can provide a profile of BA projections, as the many labeled cells permit quantitative analyses (Table 1). However, these studies presume equivalent uptake affinity, injection site size and efficacy, visualization methods, the interaction of damage with uptake, and transport rate (Schofield et al., 2007). To label significant numbers of cells, sufficiently large deposits can complicate the collection of quantitative data. Thus, for example, injections restricted to a single binaural response bands may be too small to label sufficient cells to provide reliable statistically appropriate estimates of double-labeled cells (DLs; Kishan et al., 2008).
Negative results are also problematic. Few DLs suggest that the injected regions do not receive BAs, though other areas might, or that the tracers were neither equivalent spatially nor equally likely to be transported. If BAs are oriented selectively, and the injections are not aligned appropriately, DL estimates would be spurious. Finally, dual retrograde tracing methods are limited since BAs to only a few sites can be detected, even if multiple targets are present. Thus, dual retrograde tracing likely underestimates the divergence of axonal projections.
In comparison, focal anterograde injections may overestimate the degree of single axon divergence by labeling fibers-of-passage or filling closely apposed neurons that project to separate loci. However, both anterograde and axon filling studies can demonstrate recurrent, local, and distant BAs. Some BAs are too near their source to be detected reliably by retrograde means (Winer, 1986), and anterograde or filling approaches do not require a precise or systematic injection orientation to reveal them. Anterograde studies may not reveal the full range of targets since incomplete filling of fine or long processes or insufficient transport time may confound estimates.
Intracellular filling studies are highly constrained by sample size (Fekete et al., 1984; Ojima et al., 1991). While a few axons may have collaterals (or, alternatively, lack branches), it is uncertain whether these are representative. As in anterograde studies, incomplete staining or insufficient transport time can constrain firm conclusions or population values except when many axons can be filled and their targets visualized (Brown, 1981; Humphrey et al., 1985).
Thematic Perspective
Branched axons are common in the auditory cortical system, as well as in the auditory midbrain and brainstem. However, several general principles are evident from a comparison across processing levels. First, most axons branch according to one of three patterns: intricate local BAs, long-distance collaterals, and BAs involved in feedback-control loops. Second, cells rarely project to both ascending and descending targets, suggesting that these streams are well segregated and that descending projections play specific roles rather than merely feedback or modulatory ones (Guinan, 2006; Winer, 2006). Third, some neurons have both ascending and contralateral targets, e.g., CoN neurons projecting to the IC and the contralateral CoN (Adams, 1983b), and IC neurons targeting the MG and the contralateral IC (González-Hernández et al., 1991). Most projections are contra- or ipsi-lateral because of the acoustic chiasm (Glendenning and Masterton, 1983); thus, these BAs may enhance binaural computations for sound localization or otherwise modulate ascending input from an ear with contralateral influence. This may not pertain to descending projections since corticothalamic neurons are not commissural (Wong and Kelly, 1981). Fourth, bilateral projection neurons are part of at least the corticofugal and olivocochlear streams, with ∼5% of corticocollicular neurons projecting to both IC (Willard and Martin, 1984; Coomes and Schofield, 2004), and a similar proportion of MOC cells targeting both cochleae (Thompson and Thompson, 1986; Robertson et al., 1987a,b; Aschoff and Ostwald, 1988). Such bilaterally projecting neurons in ascending pathways are differentially distributed in various nuclei.
Alternatives to Collateralization in the Auditory Cortex
In the auditory cortex, one might predict that BAs would be an ideal way to create multiple independent representations of frequency, aurality, amplitopy, or other dimensions required for computation (Ehret, 1997). It is somewhat unexpected that BAs to matched frequency regions are comparatively rare, especially in the forebrain (Lee et al., 2004a), where the emergence of multiple CF maps (Reale and Imig, 1980) suggest that they might be more common.
A robust alternative mechanism is provided by heterotopic projections that arise from interleaved thalamic and cortical neurons situated in close proximity and serving presumably similar physiologic roles but whose targets are separated widely (Lee et al., 2004b; Lee and Winer, 2005). Three obvious advantages accrue to this arrangement. First, precise branching to multiple targets is unnecessary, and neurons that target multiple cortical areas can migrate as a group and assemble their connectivity with comparative ease relative to the precision required by multiple branches that must terminate in exact register in different targets. Second, and perhaps most critically, heterotopic arrangements enable easy coordination of activity across large spatial territories, a prospectively problematic issue when coordinating diverse spatiotemporal patterns across vast expanses of brain (Lee et al., 2004b; Winer et al., 2004). Third, they provide a simple mechanism enabling the precise coordination of discharge patterns among resident thalamic or cortical neurons, either via local circuit neurons or, in their absence (Winer and Larue, 1996), via the BAs of excitatory neurons.
A second alternative is that the terminal plexus of many axons is highly divergent, and can span wide arrays, as in the TC axons in visual (Ferster and LeVay, 1978), somatic sensory (Landry and Deschênes, 1981), and auditory (Velenovsky et al., 2003) cortex. Such axons engage large areas and could readily initiate or sustain parallel intracortical and corticocortical modularity (DeFelipe et al., 1986) in networks larger than the comparatively finer scale of interneuronal projections (Kisvárday et al., 1994). The complexity of these axons belies point-to-point models of connectivity (Brandner and Redies, 1990).
Collaterals in Other Modalities
Comparable, and perhaps even more extensive, collaterals systems exist in other modalities. The complexity of the subcortical auditory pathway frustrates direct comparisons with the visual, somatic sensory, or autonomic systems. Nonetheless, some comparisons can be drawn. For example, primary phrenic afferents send BAs to different spinal cord laminae (Goshgarian and Roubal, 1986), as do Ia muscle spindle (Brown and Fyffe, 1978), and Ib Golgi tendon organ (Brown and Fyffe, 1978) afferents. Many cuneate nucleus inputs are collateralized (Weinberg et al., 1990), resembling type I ganglion cell axons near the CoN. Retinofugal fibers to the lateral geniculate nucleus (LGN) ramify within the LGN (Conley and Fitzpatrick, 1989), resembling type I ganglion axons within the CoN.
Forebrain connections are compared more readily. The visual TC system may have more interareal BAs and intraareal BAs to matched functional domains than the somatic sensory or auditory systems. Retinotopically matched deposits in areas 17 and 18 double label 3–16% of neurons in the LGN A lamina (Bullier, 1984; Birnbacher and Albus, 1987; Salin et al., 1989), while matched somatotopic injections (SI) in the primary and secondary somatosensory areas only double label 2.3% of cells (Fisher et al., 1983).
Horizontal BAs are also present in all modalities. In the visual system, extensive lateral collaterals, similar to those seen in AI link loci with similar functional properties (Gilbert and Wiesel, 1979; Michalski et al., 1983; LeVay, 1988). There are also horizontal connections in higher-level areas such as the macaque inferior temporal cortex (Tanigawa et al., 2005), and long-range horizontal collaterals from SI pyramidal cells may target neurons in other fields (DeFelipe et al., 1986).
As in AI, some rat SI CT cells have local collaterals to neurons in the same column, while others project remotely (Zhang and Deschênes, 1997). Mirroring the absence of AI corticofugal BAs to diverse targets, <2% of SI cells have BAs to the corticostriatal, corticorubral, corticopontine, and corticospinal pathways (Akintunde and Buxton, 1992). In the somatic sensory (Bourassa et al., 1995) and visual (Bourassa and Deschênes, 1995) systems, the CT fibers arising from axons of layer V neurons in V1 or SI were collaterals of corticotectal or corticopontine axons, unlike the auditory CT system. This suggests modality specific rules for BAs, whose ontogeny and functional specificity remain for further investigation.
Summary
The floridness of axonal branching throughout the central auditory system, and other modalities, is indicative of the functional importance of divergent processing in sensory systems. Such branching ranges across scales, from intrinsic branches that modulate firing in local circuits, to long-range collaterals that widely disseminate information. Yet, it remains an open question whether BAs as a wiring principle is more efficient from an ontological and developmental standpoint, compared with the targeting of separate loci by unbranched neuronal ensembles. In addition, the degree to which separate branches have similar synaptic properties and efficacy in terms of transmitting auditory information remains to be investigated. Indeed, widely varying synaptic properties at separate axonal branches would have profound effects on the divergent dissemination of auditory information. Thus, defining the functional role of axonal divergence will require a convergence of future theory and experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is dedicated to the late Jeffery A. Winer, who served as a mentor and inspirational figure for innumerable students, colleagues, and friends, and without whom this work would not have reached fruition. The void left by his absence testifies to the breadth and depth of his scholarship, friendship, and humanity. Jeff, we miss you greatly. We also thank David T. Larue for helpful discussions and assistance with the figures. This work was supported by NIH Grants R03 DC 11361 (Charles C. Lee) and R01 DC 2319 (Jeffery A. Winer).
References
Adams, J. C. (1983a). Cytology of periolivary cells and the organization of their projections in the cat. J. Comp. Neurol. 215, 275–289.
Adams, J. C. (1983b). Multipolar cells in the ventral cochlear nucleus project to the dorsal cochlear nucleus and the inferior colliculus. Neurosci. Lett. 37, 205–208.
Akintunde, A., and Buxton, D. F. (1992). Origins and collateralization of corticospinal, corticopontine, corticorubral and corticostriatal tracts: a multiple retrograde fluorescent tracing study. Brain Res. 586, 208–218.
Aschoff, A., and Ostwald, J. (1988). Different origins of cochlear efferents in some bat species, rats, and guinea pigs. J. Comp. Neurol. 264, 56–72.
Bartlett, E. L., and Smith, P. H. (2002). Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience 113, 957–974.
Benson, T. E., and Brown, M. C. (2004). Postsynaptic targets of type II auditory nerve fibers in the cochlear nucleus. J. Assoc. Res. Otolaryngol. 5, 111–125.
Birnbacher, D., and Albus, K. (1987). Divergence of single axons in afferent projections to the cat’s visual cortical areas 17, 18, and 19: a parametric study. J. Comp. Neurol. 261, 543–561.
Bourassa, J., and Deschênes, M. (1995). Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66, 253–263.
Bourassa, J., Pinault, D., and Deschênes, M. (1995). Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur. J. Neurosci. 7, 19–30.
Bourk, T. R., Mielcarz, J. P., and Norris, B. E. (1981). Tonotopic organization of the anteroventral cochlear nucleus of the cat. Hear. Res. 4, 215–241.
Brandner, S., and Redies, H. (1990). The projection of the medial geniculate body to field AI: organization in the isofrequency dimension. J. Neurosci. 10, 50–61.
Brown, A. G., and Fyffe, R. W. (1978). The morphology of group Ia muscle afferent fibre collaterals. J. Physiol. (Lond.) 278, 111–127.
Brown, M. C., Berglund, A. M., Kiang, N. Y. S., and Ryugo, D. K. (1988a). Central trajectories of type II spiral ganglion cells. J. Comp. Neurol. 278, 581–590.
Brown, M. C., Liberman, M. C., Benson, T. E., and Ryugo, D. K. (1988b). Brainstem branches from olivocochlear axons in cats and rodents. J. Comp. Neurol. 278, 591–603.
Bullier, J. (1984). “Axonal bifurcation in the afferents to cortical areas of the visual system,” in Visual Neuroscience, eds J. D. Pettigrew, K. J. Sanderson and W. R. Levick (London: Cambridge University Press), 239–259.
Burger, R. M., Cramer, K. S., Pfeiffer, J. D., and Rubel, E. W. (2005). Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J. Comp. Neurol. 481, 6–18.
Cetas, J. S., de Venecia, R. K., and McMullen, N. T. (1999). Thalamocortical afferents of Lorente de Nó: medial geniculate axons that project to primary auditory cortex have collateral branches to layer I. Brain Res. 830, 203–208.
Conley, M., and Fitzpatrick, D. (1989). Morphology of retinogeniculate axons in the macaque. Vis. Neurosci. 2, 287–296.
Coomes, D. L., and Schofield, B. R. (2004). Separate projections from the inferior colliculus to the cochlear nucleus and thalamus in guinea pigs. Hear. Res. 191, 67–78.
Coomes, D. L., Schofield, R. M., and Schofield, B. R. (2005). Unilateral and bilateral projections from cortical cells to the inferior colliculus in guinea pigs. Brain Res. 1042, 62–72.
Crabtree, J. W. (1998). Organization in the auditory sector of the cat’s thalamic reticular nucleus. J. Comp. Neurol. 390, 167–182.
DeFelipe, J., Conley, M., and Jones, E. G. (1986). Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J. Neurosci. 6, 3749–3766.
Doron, N. N., and LeDoux, J. E. (2000). Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J. Comp. Neurol. 425, 257–274.
Doucet, J. R., Molavi, D. L., and Ryugo, D. K. (2003). The source of corticocollicular and corticobulbar projections in area Te1 of the rat. Exp. Brain Res. 153, 477–485.
Doucet, J. R., Rose, L., and Ryugo, D. K. (2002). The cellular origin of corticofugal projections to the superior olivary complex in the rat. Brain Res. 925, 28–41.
Doucet, J. R., and Ryugo, D. K. (2006). Structural and functional classes of multipolar cells in the ventral cochlear nucleus. Anat. Rec. 288, 331–344.
Fekete, D. M., Rouiller, E. M., Liberman, M. C., and Ryugo, D. K. (1984). The central projections of intracellularly labeled auditory nerve fibers in cats. J. Comp. Neurol. 229, 432–450.
Ferster, D., and LeVay, S. (1978). The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J. Comp. Neurol. 182, 923–944.
Fisher, G. R., Freeman, B., and Rowe, M. J. (1983). Organization of parallel projections from Pacinian afferent fibers to somatosensory cortical areas I and II in the cat. J. Neurophysiol. 49, 75–97.
Gilbert, C. D., and Wiesel, T. N. (1979). Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280, 120–125.
Glendenning, K. K., and Masterton, R. B. (1983). Acoustic chiasm: efferent projections of the lateral superior olive. J. Neurosci. 3, 1521–1537.
González-Hernández, T. H., Galindo-Mireles, D., Castañeyra-Perdomo, A., and Ferres-Torres, R. (1991). Divergent projections of projecting neurons of the inferior colliculus to the medial geniculate body and the contralateral inferior colliculus in the rat. Hear. Res. 52, 17–22.
Goshgarian, H. G., and Roubal, P. J. (1986). Origin and distribution of phrenic primary afferent nerve fibers in the spinal cord of the adult rat. Exp. Neurol. 92, 624–638.
Gray, C. M., Konig, P., Engel, A. K., and Singer, W. (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–338.
Gray, C. M., and Singer, W. (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 86, 1698–1702.
Guinan, J. J. (2006). Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects. Ear Hear. 27, 589–607.
Hashikawa, T. (1983). The inferior colliculopontine neurons of the cat in relation to other collicular descending neurons. J. Comp. Neurol. 219, 241–249.
Hashikawa, T., Molinari, M., Rausell, E., and Jones, E. G. (1995). Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. J. Comp. Neurol. 362, 195–208.
Hayes, N. L., and Rustioni, A. (1979). Dual projections of single neurons are visualized simultaneously: use of enzymatically inactive [3]HRP. Brain Res. 165, 321–326.
Hazama, M., Kimura, A., Donishi, T., Sakoda, T., and Tamai, Y. (2004). Topography of corticothalamic projections from the auditory cortex of the rat. Neuroscience 124, 655–667.
Huang, C. L., and Winer, J. A. (2000). Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J. Comp. Neurol. 427, 302–331.
Humphrey, A. L., Sur, M., Uhlrich, D. J., and Sherman, S. M. (1985). Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J. Comp. Neurol. 233, 159–189.
Imig, T. J., and Morel, A. (1985a). Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J. Neurophysiol. 53, 309–340.
Imig, T. J., and Morel, A. (1985b). Tonotopic organization in lateral part of posterior group of thalamic nuclei in the cat. J. Neurophysiol. 53, 836–851.
Imig, T. J., and Reale, R. A. (1980). Patterns of cortico-cortical connections related to tonotopic maps in cat auditory cortex. J. Comp. Neurol. 192, 293–332.
Irvine, D. R. F. (1986). “The auditory brainstem. A review of the structure and function of auditory brainstem processing mechanisms,” in Progress in Sensory Physiology, eds H. Autrum, D. Ottoson, E. R. Perl, R. F. Schmidt, H. Shimazu and W. D. Willis (Berlin: Springer-Verlag), 1–279.
Jones, E. G. (1983). Lack of collateral thalamocortical projections to fields of the first somatic sensory cortex in monkeys. Exp. Brain Res. 52, 375–384.
Kimura, A., Donishi, T., Okamoto, K., and Tamai, Y. (2005). Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience 135, 1325–1342.
Kishan, A. U., Lee, C. C., and Winer, J. A. (2008). Branched projections in the auditory thalamocortical and corticocortical systems. Neuroscience 154, 283–293.
Kisvárday, Z. F., Kim, D.-S., Eysel, U. T., and Bonhoeffer, T. (1994). Relationship between lateral inhibitory connections and the topography of the orientation map in cat visual cortex. Eur. J. Neurosci. 6, 1619–1632.
Kurokawa, T., and Saito, H. (1995). Retrograde axonal transport of different fluorescent tracers from the neocortex to the suprageniculate nucleus in the rat. Hear. Res. 85, 103–108.
Kuwabara, N., DiCaprio, R. A., and Zook, J. M. (1991). Afferents to the medial nucleus of the trapezoid body and their collateral projections. J. Comp. Neurol. 314, 684–706.
Kuwabara, N., and Zook, J. M. (1991). Classification of the principal cells of the medial nucleus of the trapezoid body. J. Comp. Neurol. 314, 707–720.
Kuwabara, N., and Zook, J. M. (1992). Projections to the medial superior olive from the medial and lateral nuclei of the trapezoid body in rodents and bats. J. Comp. Neurol. 324, 522–538.
Kuwabara, N., and Zook, J. M. (1999). Local collateral projections from the medial superior olive to the superior paraolivary nucleus in the gerbil. Brain Res. 846, 59–71.
Kuypers, H. G. J. M., Bentivoglio, M., Catsman-Berrevoets, C. E., and Bharos, A. T. (1980). Double retrograde labeling through divergent axons collaterals, using two fluorescent tracers with the same excitation wavelength which label different features of the cell. Exp. Brain Res. 40, 383–392.
Lam, Y. W., and Sherman, S. M. (2010). Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb. Cortex 20, 13–24.
Landry, P., and Deschênes, M. (1981). Intracortical arborizations and receptive fields of identified ventrobasal thalamocortical afferents to the primary somatic sensory cortex in the cat. J. Comp. Neurol. 199, 345–372.
Lee, C. C., Imaizumi, K., Schreiner, C. E., and Winer, J. A. (2004a). Concurrent tonotopic processing streams in auditory cortex. Cereb. Cortex 14, 441–451.
Lee, C. C., Schreiner, C. E., Imaizumi, K., and Winer, J. A. (2004b). Tonotopic and heterotopic projection systems in physiologically defined auditory cortex. Neuroscience 128, 871–887.
Lee, C. C., and Sherman, S. M. (2010). Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 372–377.
Lee, C. C., and Winer, J. A. (2005). Principles governing auditory forebrain connections. Cereb. Cortex 15, 1804–1814.
Lee, C. C., and Winer, J. A. (2008a). Connections of cat auditory cortex: I. Thalamocortical system. J. Comp. Neurol. 507, 1879–1900.
Lee, C. C., and Winer, J. A. (2008b). Connections of cat auditory cortex: II. Commissural system. J. Comp. Neurol. 507, 1901–1919.
Lee, C. C., and Winer, J. A. (2008c). Connections of cat auditory cortex: III. Corticocortical system. J. Comp. Neurol. 507, 1920–1943.
Lee, C. C., and Winer, J. A. (2011). Convergence of thalamic and cortical pathways in cat auditory cortex. Hear. Res. 274, 85–94.
Li, H., and Mizuno, N. (1997a). Collateral projections from single neurons in the dorsal column nuclei to the inferior colliculus and the ventrobasal thalamus: a retrograde double-labeling study in the rat. Neurosci. Lett. 225, 21–24.
Li, H., and Mizuno, N. (1997b). Direct projections from nucleus X to the external cortex of the inferior colliculus in the rat. Brain Res. 774, 200–206.
Llano, D. A., and Sherman, S. M. (2008). Evidence for non-reciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. J. Comp. Neurol. 507, 1209–1227.
Merchán, M. A., and Berbel, P. (1996). Anatomy of the ventral nucleus of the lateral lemniscus in rats: a nucleus with a concentric laminar organization. J. Comp. Neurol. 372, 245–263.
Michalski, A., Gerstein, G. L., Czarkowska, J., and Tarnecki, R. (1983). Interactions between cat striate cortex neurons. Exp. Brain Res. 51, 97–107.
Middlebrooks, J. C., and Zook, J. M. (1983). Intrinsic organization of the cat’s medial geniculate body identified by projections to binaural response-specific bands in the primary auditory cortex. J. Neurosci. 3, 203–225.
Morel, A., and Imig, T. J. (1987). Thalamic projections to fields A, AI, P, and VP in the cat auditory cortex. J. Comp. Neurol. 265, 119–144.
Morest, D. K. (1968). The collateral system of the medial nucleus of the trapezoid body of the cat, its neuronal architecture and relation to the olivo-cochlear bundle. Brain Res. 9, 288–311.
Moriizumi, T., and Hattori, T. (1991a). Non-dopaminergic projection from the subparafascicular area to the temporal cortex in the rat. Neurosci. Lett. 129, 127–130.
Moriizumi, T., and Hattori, T. (1991b). Pyramidal cells in rat temporoauditory cortex project to both striatum and inferior colliculus. Brain Res. Bull. 27, 141–144.
Mulders, W. H. A. M., Harvey, A. R., and Robertson, D. (2007). Electrically evoked responses in onset chopper neurons in guinea pig cochlear nucleus. J. Neurophysiol. 97, 3288–3297.
Mulders, W. H. A. M., and Robertson, D. (2002). Inputs from the cochlea and the inferior colliculus converge on olivocochlear neurones. Hear. Res. 167, 206–213.
Mulders, W. H. A. M., and Robertson, D. (2003). Olivocochlear collaterals evoke excitatory effects in onset neurones of the rat cochlear nucleus. Hear. Res. 176, 113–121.
Namura, S., Takada, M., Kikuchi, H., and Mizuno, N. (1997). Collateral projections of single neurons in the posterior thalamic region to both the temporal cortex and the amygdala: a fluorescent retrograde double-labeling study in the rat. J. Comp. Neurol. 384, 59–70.
Oertel, D., Wu, S. H., Garb, M. W., and Dizack, C. (1990). Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J. Comp. Neurol. 295, 136–154.
Ojima, H. (1994). Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb. Cortex 6, 646–663.
Ojima, H., Honda, C. N., and Jones, E. G. (1991). Patterns of axon collateralization of identified supragranular pyramidal neurons in the cat auditory cortex. Cereb. Cortex 1, 80–94.
Ojima, H., Honda, C. N., and Jones, E. G. (1992). Characteristics of intracellularly injected infragranular pyramidal neurons in cat primary auditory cortex. Cereb. Cortex 2, 197–216.
Okoyama, S., Ohbayashi, M., Ito, M., and Harada, S. (2006). Neuronal organization of the rat inferior colliculus participating in four major auditory pathways. Hear. Res. 218, 72–80.
Oliver, D. L., Kuwada, S., Yin, T. C. T., Haberly, L. B., and Henkel, C. K. (1991). Dendritic and axonal morphology of HRP-injected neurons in the inferior colliculus of the cat. J. Comp. Neurol. 303, 75–100.
Peruzzi, D., Bartlett, E., Smith, P. H., and Oliver, D. L. (1997). A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J. Neurosci. 17, 3766–3777.
Read, H. L., Miller, L. M., Schreiner, C. E., and Winer, J. A. (2008). Two thalamic pathways to primary auditory cortex. Neuroscience 152, 151–159.
Read, H. L., Winer, J. A., and Schreiner, C. E. (2001). Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 98, 8042–8047.
Reale, R. A., and Imig, T. J. (1980). Tonotopic organization in auditory cortex of the cat. J. Comp. Neurol. 192, 265–291.
Robertson, D., Anderson, C. J., and Cole, K. S. (1987a). Segregation of efferent projections to different turns of the guinea pig cochlea. Hear. Res. 25, 69–76.
Robertson, D., Cole, K. S., and Corbett, K. (1987b). Quantitative estimates of bilaterally projecting medial olivocochlear neurones in the guinea pig brainstem. Hear. Res. 27, 177–181.
Rouiller, E. M., and de Ribaupierre, F. (1990). Arborization of corticothalamic axons in the auditory thalamus of the cat: a PHA-L tracing study. Neurosci. Lett. 108, 29–35.
Rüttgers, K., Aschoff, A., and Friauf, E. (1990). Commissural connections between the auditory cortices of the rat. Brain Res. 509, 71–79.
Saldaña, E., Feliciano, M., and Mugnaini, E. (1996). Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J. Comp. Neurol. 371, 15–40.
Salin, P. A., Bullier, J., and Kennedy, H. (1989). Convergence and divergence in the afferent projections to cat area 17. J. Comp. Neurol. 283, 486–512.
Schofield, B. R. (1991). Superior paraolivary nucleus in the pigmented guinea pig: separate classes of neurons project to the inferior colliculus and the cochlear nucleus. J. Comp. Neurol. 312, 68–76.
Schofield, B. R. (1994). Projections to the cochlear nuclei from principal cells in the medial nucleus of the trapezoid body in guinea pigs. J. Comp. Neurol. 344, 83–100.
Schofield, B. R. (1995). Projections from the cochlear nucleus to the superior paraolivary nucleus in guinea pigs. J. Comp. Neurol. 360, 135–149.
Schofield, B. R. (2001). Origins of projections from the inferior colliculus to the cochlear nucleus in guinea pigs. J. Comp. Neurol. 429, 206–220.
Schofield, B. R. (2002). Ascending and descending projections from the superior olivary complex in guinea pigs: different cells project to the cochlear nucleus and the inferior colliculus. J. Comp. Neurol. 453, 217–225.
Schofield, B. R., and Cant, N. B. (1992). Organization of the superior olivary complex in the guinea pig: II. Patterns of projection from the periolivary nuclei to the inferior colliculus. J. Comp. Neurol. 317, 438–455.
Schofield, B. R., and Cant, N. B. (1996a). Projections from the ventral cochlear nucleus to the inferior colliculus and the contralateral cochlear nucleus in guinea pigs. Hear. Res. 102, 1–14.
Schofield, B. R., and Cant, N. B. (1996b). Origins and targets of commissural connections between cochlear nuclei in guinea pigs. J. Comp. Neurol. 375, 128–146.
Schofield, B. R., and Cant, N. B. (1999). Descending auditory pathways: projections from the inferior colliculus contact superior olivary cells that project bilaterally to the cochlear nuclei. J. Comp. Neurol. 409, 210–223.
Schofield, B. R., Schofield, R. M., Sorensen, K. A., and Motts, S. D. (2007). On the use of retrograde tracers for identification of axon collaterals with multiple fluorescent retrograde tracers. Neuroscience 146, 773–783.
Sherman, S. M., and Guillery, R. W. (2006). Exploring the Thalamus and Its Role in Cortical Function. MIT Press, Cambridge.
Silva, L. R., Amitai, Y., and Connors, B. W. (1991). Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251, 432–435.
Smith, P. H., Joris, P. X., Carney, L. H., and Yin, T. C. T. (1991). Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J. Comp. Neurol. 304, 387–407.
Smith, P. H., Joris, P. X., and Yin, T. C. T. (1998). Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J. Neurophysiol. 79, 3127–3142.
Song, W.-J., Kawaguchi, H., Totoki, S., Inoue, Y., Katura, T., Maeda, S., Inagaki, S., Shirasawa, H., and Nishimura, M. (2006). Cortical intrinsic circuits can support activity propagation through an isofrequency strip of the guinea pig primary auditory cortex. Cereb. Cortex 16, 718–729.
Spirou, G. A., Brownell, W. E., and Zidanic, M. (1990). Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J. Neurophysiol. 63, 1169–1190.
Takayanagi, M., and Ojima, H. (2006). Microtopography of the dual corticothalamic projections originating from domains along the frequency axis of the cat primary auditory cortex. Neuroscience 142, 769–780.
Tanaka, K., Katsumi, O., Tokunaga, A., and Sugita, S. (1985). The organization of neurons in the nucleus of the lateral lemniscus projecting to the superior and inferior colliculi in the rat. Brain Res. 341, 252–260.
Tanigawa, H., Wang, Q., and Fujita, I. (2005). Organization of horizontal axons in the inferior temporal cortex and primary visual cortex of the macaque monkey. Cereb. Cortex 15, 1887–1899.
Thompson, G. C., and Thompson, A. M. (1986). Olivocochlear neurons in the squirrel monkey brainstem. J. Comp. Neurol. 254, 246–258.
Velenovsky, D. S., Cetas, J. S., Price, R. O., Sinex, D. G., and McMullen, N. T. (2003). Functional subregions in primary auditory cortex defined by thalamocortical terminal arbors: an electrophysiological and anterograde labeling study. J. Neurosci. 23, 308–316.
Weinberg, R., Pierce, J., and Rustioni, A. (1990). Single fiber studies of ascending input to the cuneate nucleus of cats: I. Morphometry of primary afferent fibers. J. Comp. Neurol. 300, 113–133.
Willard, F. H., and Martin, G. F. (1984). Collateral innervation of the inferior colliculus in the North American opossum: a study using fluorescent markers in the double-labeling paradigm. Brain Res. 303, 171–182.
Winer, J. A. (1986). Neurons accumulating [3H]gamma-aminobutyric acid (GABA) in supragranular layers of cat primary auditory cortex (AI). Neuroscience 19, 771–793.
Winer, J. A., Diehl, J. J., and Larue, D. T. (2001). Projections of auditory cortex to the medial geniculate body of the cat. J. Comp. Neurol. 430, 27–55.
Winer, J. A., and Larue, D. T. (1996). Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc. Natl. Acad. Sci. U.S.A. 93, 3083–3087.
Winer, J. A., Larue, D. T., Diehl, J. J., and Hefti, B. J. (1998). Auditory cortical projections to the cat inferior colliculus. J. Comp. Neurol. 400, 147–174.
Winer, J. A., Larue, D. T., and Huang, C. L. (1999). Two systems of giant axon terminals in the cat medial geniculate body: convergence of cortical and GABAergic inputs. J. Comp. Neurol. 413, 181–197.
Winer, J. A., Lee, C. C., Imaizumi, K., and Schreiner, C. E. (2004). “Challenges to a theory of neuroanatomical theory of forebrain auditory plasticity,” in Plasticity and Signal Representation in the Auditory System, eds J. Syka and M. M. Merzenich (New York: Kluwer/Academic Plenum Publishers), 99–107.
Winer, J. A., Saint Marie, R. L., Larue, D. T., and Oliver, D. L. (1996). GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc. Natl. Acad. Sci. U.S.A. 93, 8005–8010.
Winer, J. A., and Schreiner, C. E. (2005). “The central auditory system: a functional analysis,” in The Inferior Colliculus, eds J. A. Winer and C. E. Schreiner (New York: Springer-Verlag), 1–68.
Wong, D., and Kelly, J. P. (1981). Differentially projecting cells in individual layers of the auditory cortex: a double-labeling study. Brain Res. 230, 362–366.
Ye, Y., Machado, D. G., and Kim, D. O. (2000). Projection of the marginal shell of the anteroventral cochlear nucleus to olivocochlear neurons in the cat. J. Comp. Neurol. 420, 127–138.
Keywords: branched axon, auditory system, collaterals, cortical, thalamocortical, brainstem
Citation: Lee CC, Kishan AU, and Winer JA (2011) Wiring of divergent networks in the central auditory system. Front. Neuroanat. 5:46. doi: 10.3389/fnana.2011.00046
Received: 12 May 2011; Accepted: 09 July 2011;
Published online: 28 July 2011.
Edited by:
Julian Budd, University of Sussex, UKReviewed by:
Sarah L. Pallas, Georgia State University, USAHisayuki Ojima, Tokyo Medical and Dental University, Japan
Copyright: © 2011 Lee, Kishan and Winer. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Charles C. Lee, Department of Comparative Biomedical Sciences, School of Veterinary Medicine, Louisiana State University, Skip Bertman Drive, Baton Rouge, LA 70803, USA. e-mail: cclee@lsu.edu