Identification of a specific assembly of the G protein Golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum
- 1 INSERM UMR-S839, Paris, France
- 2 Université Pierre et Marie Curie, Paris, France
- 3 Institut du Fer à Moulin, Paris, France
In the principal neurons of striatum (medium spiny neurons, MSNs), cAMP pathway is primarily activated through the stimulation of dopamine D1 and adenosine A2A receptors, these receptors being mainly expressed in striatonigral and striatopallidal MSNs, respectively. Since cAMP signaling pathway could be altered in various physiological and pathological circumstances, including drug addiction and Parkinson’s disease, it is of crucial importance to identify the molecular components involved in the activation of this pathway. In MSNs, cAMP pathway activation is not dependent on the classical Gs GTP-binding protein but requires a specific G protein subunit heterotrimer containing Gαolf/β2/γ7 in particular association with adenylyl cyclase type 5. This assembly forms an authentic functional signaling unit since loss of one of its members leads to defects of cAMP pathway activation in response to D1 or A2A receptor stimulation, inducing dramatic impairments of behavioral responses dependent on these receptors. Interestingly, D1 receptor (D1R)-dependent cAMP signaling is modulated by the neuronal levels of Gαolf, indicating that Gαolf represents the rate-limiting step in this signaling cascade and could constitute a critical element for regulation of D1R responses. In both Parkinsonian patients and several animal models of Parkinson’s disease, the lesion of dopamine neurons produces a prolonged elevation of Gαolf levels. This observation gives an explanation for the cAMP pathway hypersensitivity to D1R stimulation, occurring despite an unaltered D1R density. In conclusion, alterations in the highly specialized assembly of Gαolf/β2/γ7 subunits can happen in pathological conditions, such as Parkinson’s disease, and it could have important functional consequences in relation to changes in D1R signaling in the striatum.
Introduction
Dopamine, probably the best characterized neurotransmitter involved in slow synaptic neurotransmission, plays a prominent role in a variety of brain functions, including motor control, motivation, short-term memory, and reward (Schultz, 1998). Five genes encoding dopamine receptors have been cloned in mammals (see Sibley and Monsma, 1992 for review). All these receptors belong to the superfamily of G protein-coupled receptors with seven transmembrane domains and the comparison of their amino acid sequence, pharmacological profile, and biochemical properties has revealed two distinct categories, named respectively D1- and D2-type dopamine receptors. The D1-type receptors, comprising D1 and D5 subtypes, are positively coupled to cAMP production whereas the D2-type receptors, comprising D2, D3, and D4 subtypes, are able to inhibit cAMP production (see Missale et al., 1998 review). The D1 receptor (D1R) is the most abundantly expressed dopamine receptors and is present in virtually all the brain areas innervated by dopamine neurons (Boyson et al., 1986). Consistent with its dense dopamine innervation, the striatum contains the highest concentration of D1Rs in the brain. Different approaches using in situ hybridization, immunocytochemistry, and transgenic mice indicate that D1R in the striatum is highly expressed in a subpopulation of GABAergic medium spiny neurons (MSNs) projecting to the substantia nigra and entopeduncular nucleus (direct pathway of basal ganglia) and containing substance P and dynorphin as co-neurotransmitters (Gerfen et al., 1990; Le Moine and Bloch, 1995; Yung et al., 1995; Drago et al., 1998b; Gong et al., 2003; Lee et al., 2006; Bertran-Gonzalez et al., 2008). By contrast, D2 receptors are essentially present in the MSNs projecting to the globus pallidus and containing enkephalins (indirect pathway of basal ganglia). These neurons express abundantly the adenosine A2A receptors that are able to stimulate production of intracellular cAMP (Schiffmann et al., 2007). In a recent study using transgenic mouse lines, it was estimated that about 50% of GABA MSNs express exclusively D1Rs and 35–45% exclusively D2 receptors (Bertran-Gonzalez et al., 2008). The population of MSNS co-expressing both D1R and D2 receptor, is low in the dorsal striatum and core of nucleus accumbens (about 5%) but is slightly higher in the shell of nucleus accumbens (17%; Bertran-Gonzalez et al., 2008; Hasbi et al., 2009; Matamales et al., 2009).
Pharmacological studies and investigations on D1R-deleted mice have shown the importance of D1R in mediating the effects of DA neurotransmission (Drago et al., 1998a; El-Ghundi et al., 2007). The actions of D1R require the participation of heterotrimeric guanine nucleotide binding proteins (G proteins) whose roles in diverse signaling pathways may be determined by their specific αβγ subunit combinations. These heterotrimeric G proteins are molecular switches in which the agonist-activated receptors catalyze the exchange of GDP for GTP on the α-subunit of heterotrimeric G proteins (Gα), which in turn engages conformational and/or dissociational events between the Gα and dimeric Gβγ subunits (Bourne et al., 1991). In the case of D1R, it is well established that the GTP-bound Gα subunit initiates the activation of adenylyl cyclase (AC) leading to the intracellular production of cAMP and stimulation of cAMP-dependent protein kinase (PKA) and others cAMP-dependent proteins (Kebabian and Calne, 1979; Hervé and Girault, 2005). An extensive body of evidence indicates that D1-type receptors also couple via Gαq subunits to phospholipase C (Mahan et al., 1990; Arnt et al., 1992; Wang et al., 1995; Lezcano and Bergson, 2002; Mannoury La Cour et al., 2007) but a debate exists to know if the receptors involved are bona fide D1Rs (Mannoury La Cour et al., 2007), heteromers of D1R and D2 receptor (Hasbi et al., 2009), or different receptors with D1-type pharmacological properties (Friedman et al., 1997). Here, we will review the present knowledge about the nature of the G proteins able to couple D1R to AC in the striatum and about the regulatory processes at the level of these G proteins that control the D1R-mediated signaling and its functional consequences.
Gαolf Role in the Coupling of D1R and A2A Receptor to Adenylyl Cyclase in the Striatum
Since D1R is positively coupled to AC, a long-standing dogma stated that this action was mediated by the classical heterotrimeric stimulatory G protein containing the Gαs subunit. However, the Gαs expression is low in the striatum compared with that observed in many other brain areas (Figure 1A; Largent et al., 1988). In the striatum, Gαs is replaced by a high expression of Gαolf, an isoform of Gαs (Figure 1; Drinnan et al., 1991; Herve et al., 1993) which was first discovered in the olfactory epithelium and found crucial for olfaction by mediating the coupling of olfactory receptors to AC (Jones and Reed, 1989; Belluscio et al., 1998).
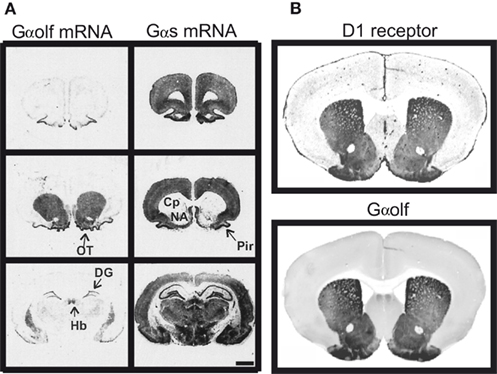
Figure 1. Similar distribution of D1 receptor and Gαolf protein in the striatum. (A) Distribution of Gαolf and Gαs mRNAs in rat forebrain. Gαolf mRNA (left) is highly expressed in striatal areas, including caudate putamen (Cp), nucleus accumbens (NA), and olfactory tubercle (OT). These areas show almost no expression of Gαs, except for some sparse neurons (right). On the contrary, Gαs mRNA was highly expressed in many brain areas including all cortical areas, septum, most of the hypothalamic and thalamic nuclei, hippocampus, and amygdala, in which Gαolf mRNA expression is very low. In few brain regions, substantial expression of both Gαolf and Gαs mRNAs is observed: piriform cortex (Pyr), medial habenula (Hb), and dentate gyrus (DG). Positive of X-ray films exposed to rat brain sections hybridized with 35S-labeled probes for Gαolf (left) and Gαs mRNAs (right). Scale bar, 5 mm. Adapted with permission from the work of Herve et al. (2001). (B) Mouse sections (30 μm-thick) have been incubated with mouse antibodies against D1R (generous gift of R. Luedtke) and rabbit antibodies against Gαolf. The primary antibodies were detected with IRDye700 conjugated anti-mouse IgG and IRDye800 conjugated anti-rabbit IgG. The sections were scanned using a LI-COR Odyssey infrared fluorescent detection system. The figure shows the scans of one representative section with anti-D1R (upper panel) and anti-Gαolf antibodies (lower panel). Adapted with permission from the work of E. Valjent.
In rodents Gαolf is highly expressed in all the MSNs in the striatum including those bearing the D1R (Kull et al., 2000; Herve et al., 2001). It is also expressed in cholinergic aspiny interneurons in the striatum. In human, high expression of Gαolf was also detected in the striatum and its decrease in patients with Huntington’s disease is a strong indication of its expression in the MSNs (Corvol et al., 2004). In the striatum of mice with a null targeted mutation of Gαolf-encoding gene, AC activation in response to D1R stimulation is absent, which demonstrates clearly that the D1R acts through the Gαolf protein to stimulate cAMP production (Zhuang et al., 2000; Corvol et al., 2001).
However, Gαolf and D1R are not systematically associated in the various neuronal types. D1R is present in neurons that do not express high level of Gαolf, such as the neurons of prefrontal cortex. In these neurons, AC activation to D1 agonist is mediated through Gαs as indicated by the lack of alteration of this response in Gαolf-deficient mutant mice (Corvol et al., 2001). In the other hand, Gαolf is present in high amount in the MSNs without D1R expression that project to the globus pallidus and contain D2 receptors. In these neurons, Gαolf is involved in the positive coupling of adenosine A2A receptors with AC since an A2A receptor agonist dose dependently activates Gαolf in striatal membranes (Kull et al., 2000) and the stimulatory effect of this agonist on AC activity is missing in the mutant mice deficient in Gαolf (Corvol et al., 2001).
Gαolf is Necessary for Dopamine Action in the Striatum
Mice homozygous for null mutation in Gαolf gene show a complete anosmia because of the crucial role played by Gαolf in the transduction of the olfactory receptor at the level of primary olfactory neurons (Belluscio et al., 1998). This profound anosmia produces an important postnatal lethality (more than 80% of the mutant mice do not feed properly and die within 3 days after birth). The rare surviving homozygous animals exhibit reduction in body weight and they display a marked hyperactive behavior, evoking a possible alteration of striatal functions (Belluscio et al., 1998). The psychostimulants, such as cocaine or D-amphetamine, produce in the striatum the activation of several D1R-dependent signaling events, including activation of PKA, extracellular signal-regulated kinase (ERK), or c-fos gene induction (Berretta et al., 1992; Valjent et al., 2000; Nairn et al., 2004). All these effects are absent when Gαolf gene is deleted (Zhuang et al., 2000; Corvol et al., 2007), showing the crucial role played by Gαolf in most of the known intracellular effects of D1R activation.
The D1 agonist-induced hyperlocomotor response is abolished in Gαolf knockout mice, indicating that Gαolf is necessary for behavior action of D1R stimulation (Zhuang et al., 2000). In addition, it is well established that the acute hyperlocomotion induced by cocaine is dependent on D1R stimulation (Drago et al., 1998a; Valjent et al., 2000). It is noteworthy that the acute locomotor response to cocaine is absent in Gαolf knockout mice (Zhuang et al., 2000). Altogether, these observations demonstrate that acute responses to cocaine and probably other psychostimulants are highly dependent on Gαolf-linked D1R signaling.
Comparison of Gαolf and Gαs
Gαolf shares 80% amino acid identity with Gαs and the exon/intron structures of genes encoding Gαolf and Gαs (Gnal and Gnas respectively in mouse) are very similar, the main difference being the absence in Gnal of the alternatively spliced exon 3 of Gnas (Jones and Reed, 1989; Wadhawan et al., 2008). Both genes are characterized by the use of alternate upstream promoters and first exons giving rise to “extra-large” variants of the proteins (XL–Gαolf and XL–Gαs) in addition to the classically described proteins (Corradi et al., 2005). XL–Gαolf, in which the N-terminal end of Gαolf is replaced by a longer polypeptide, is able to couple D1R to AC in transfected cells because it retains all the functional domains of Gαolf. In the human striatal areas, the expression of XL–Gαolf mRNA is low, about 10-fold less than that of Gαolf mRNA (Corradi et al., 2005). In striatal extracts of rodents, the protein is below the detection threshold in western blotting (personal observations). In the vicinity of the two alternatively used exons, both Gnal and Gnas locus contain CpG islands that could undergo differential methylation of DNA (Corradi et al., 2005; Wadhawan et al., 2008). DNA methylation most often results in repression of transcription and constitutes a hallmark of genomic imprinting. In the gene encoding Gαs, differential methylation of CpG islands was reported in the alleles of maternal or paternal origins, and distinct transcripts are either biallelically expressed, maternally imprinted, or paternally imprinted (Weinstein et al., 2001). For instance, the XL–Gαs mRNA is a transcript specific of the paternally derived allele (Plagge et al., 2008). It is possible that similar phenomenon exists for XL–Gαolf mRNA but the specific imprinting that affects the maternal and paternal alleles of Gαolf gene has not been precisely determined (Corradi et al., 2005).
The Gnal and Gnas genes are present in all the examined vertebrates, including mammals, amphibians, and fishes (Wadhawan et al., 2008). In contrast, studies in Drosophila indicate the existence only one Gnas ortholog (Wolfgang et al., 2001), suggesting that Gnas and Gnal result from a gene duplication after the divergence of vertebrates from invertebrates but before the divergence of tetrapods from fishes. The time point of this event is estimated at −570 millions of years (Wadhawan et al., 2008).
Gαolf displays some functional differences with Gαs. Particularly, its affinity for GDP is lower and its deactivation after GTP-binding is more rapid (Liu et al., 2001). Because of these properties, Gαolf has a higher constitutive activity than Gαs in vitro (Liu et al., 2001), which may explain the decrease of basal AC activity in the Gαolf knockout mice (Corvol et al., 2001). This relatively high constitutive activity of Gαolf could result in a tonic AC activity in vivo leading to a constant activation of cAMP pathway in both striatonigral and striatopallidal MSNs.
Some evidence shows also that the percentage of AC activation is higher when receptor is coupled to Gαolf than when it is coupled to Gαs (Liu et al., 2001). Basically the signal-to-noise ratio for Gαolf-coupled receptor appears considerably greater. In addition, because of its ability to deactivate more rapidly, Gαolf could give rise to more transient activation of AC than Gαs. It is conceivable that physiological functions of dopamine in the striatum require phasic AC activation and the fast deactivation of Gαolf could contribute to rapidly restore responsiveness of MSNs between two dopamine stimuli. Adenosine signaling is generally regarded as a slow modulator regulating A2A receptor-containing neurons in the striatum. However, evidence indicates that the formation of extracellular adenosine partly results from ATP released from nerve endings, which is dephosphorylated in adenosine by ecto-nucleotidases (Fredholm et al., 2005; Schiffmann et al., 2007). ATP is stored in synaptic vesicles together with most of neurotransmitters, including glutamate, and is co-released with the neurotransmitter upon nerve stimulation. Because Gαolf provides high signal-to-noise ratio and rapidly deactivates, the Gαolf-dependent signaling of A2A receptor could mediate more time-limited actions than it is generally believed and could quickly adapt MSN functions to transient variations in synaptic input.
Specific Assembly of Gαolf/β2/γ7 Mediates Coupling of D1R to Adenylyl Cyclase
The regional expression of the γ7 subunit of G protein (Gγ7) in the brain was found to mirror that of D1R and Gαolf, with a particularly high expression in MSNs (Watson et al., 1994), suggesting that Gγ7 subunit selectively associates with Gαolf to couple D1R to AC. In agreement with this hypothesis, the deletion of Gγ7 gene in mutant mice causes an important reduction in the levels of Gαolf and logically leads to drastic reduction of D1R or A2A responses on the cAMP production (Schwindinger et al., 2003, 2010). In the Gγ7 mutant mice, reduction of β2 subunit of G protein (Gβ2) was also observed and quantitative measurements have indicated that the decrease in Gγ7, Gαolf, and Gβ2 was very similar in term of molarity, strongly suggesting a specific assembly of Gαolf/β2/γ7 heterotrimer enabling D1R coupling to AC in the striatal MSNs (Figure 2; Schwindinger et al., 2010).
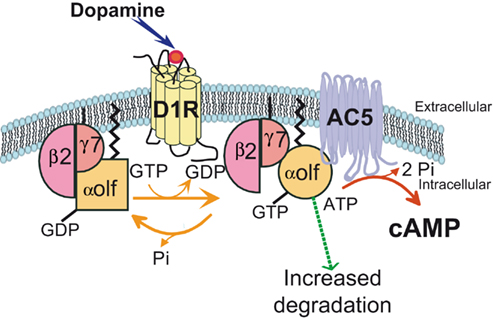
Figure 2. A specific assembly of Gαolf, Gβ2, and Gγ7 subunits of G protein mediates the coupling of D1 receptor to adenylyl cyclase 5. The expression of Gγ7 subunit in striatal neurons recruits and stabilizes Gαolf and Gβ2 subunits. They form a specific heterotrimeric protein that provides the signaling complex necessary for the coupling of D1R receptor to the adenylyl cyclase 5 (AC5), an isoform particularly enriched in the striatum. The D1R stimulation by dopamine activates the Gαolf/β2/γ7 heterotrimer by triggering substitution of GDP by GTP in the Gαolf subunit and changes in the subunit conformation. The current data indicate that the Gα activation does not necessary cause its dissociation from Gβγ complex as it was thought previously (Bunemann et al., 2003). It has been proposed a “clamshell” model according to which activated receptor provokes movements in Gαβγ complex that unmask previously buried interfaces and enable interaction of Gα and Gβγ with specific effectors (Robishaw and Berlot, 2004). In this model, the Gβγ subunits are not shared among several α subunits but can remain associated with a specific pool of α subunit (Robishaw and Berlot, 2004). Such stable association may contribute to the specificity of Gαolf interaction with Gβ2γ7 complex. In addition, the activation of the heterotrimeric complex could increase its vulnerability to degradation processes. This effect could explain why the receptor usage reduces the Gαolf levels in striatal neurons.
Interestingly, in the mice with targeted deletion of Gαolf gene, the levels of Gγ7 remain normal contrasting with the selective and coordinated reduction of Gαolf and Gβ2 observed in the mutant mice lacking Gγ7 (Schwindinger et al., 2010). Because the corresponding mRNAs are not altered, the simplest explanation is to postulate that Gγ7 is required at a post-transcriptional level for the stabilization and/or trafficking of the Gαolf and Gβ2 proteins to the plasma membrane. These results indicate that in the MSNs, the formation of the Gαolf/β2/γ7 heterotrimer is a hierarchical process that begins by the production of Gγ7 subunits and the later recruitment of Gαolf and Gβ2 subunits. These observations are surprising since MSNs express others types of γ subunits of G proteins, sometimes in higher abundance than Gγ7. The Gγ7 subunit appears to recruit selectively Gαolf and Gβ2 subunits to form a highly specialized heterotrimeric G protein in the MSNs, refuting the notion that G protein subunits are largely interchangeable (Schwindinger et al., 2010).
Medium spiny neurons are specially enriched in type 5 AC (AC5; Glatt and Snyder, 1993) that provides around 80% of basal AC activity in the striatum (Lee et al., 2002; Iwamoto et al., 2003). These observations suggest that this AC isoform is associated with the Gαolf/β2/γ7 heterotrimeric G protein in the MSNs. In agreement with this idea, the lack of AC5 in the striatum of mice homozygous for a null mutation of AC5 gene produces drastic decrease of Gαolf content in the striatum and AC activation in response to D1 agonist (Lee et al., 2002; Iwamoto et al., 2004). The mice deficient in AC5 display important deficit in appetitive pavlovian conditioning (Kheirbek et al., 2008) but surprisingly increased locomotor response to D1 agonist (Lee et al., 2002). This paradoxical response is related to D1R stimulation since it is blocked by D1R antagonist, but its understanding remains unclear (Lee et al., 2002). It was hypothesized that the D1R-dependent behavior seen in AC5 knockout mice is related to non-AC effectors but the identification of these D1R-activated signaling pathways remains to be determined. The ERK pathway appears to be excluded since the AC5 knockout mice show a profound decoupling of D1R from downstream activation of ERK, similar to that observed on cAMP pathway (Kheirbek et al., 2008). It remains the possible implication of D1R coupled to phospholipase C in the striatum (Wang et al., 1995), or D1Rs independent from AC5 expressed in extrastriatal motor regions in the brain. Alternatively, the absence of AC5 in the striatum could produce profound alterations of other signaling pathways leading to an exacerbation of D1R-related responses despite the low D1R-related responses on cAMP production. Particularly, the behavioral responses linked to D2 receptors are completely eliminated in the mutant mice (Lee et al., 2002), possibly leading to an enhancement of responses produced by D1 agonist.
In conclusion, the signaling machinery enabling D1R to activate cAMP pathway is made up of a highly specialized assembly of Gαolf/β2/γ7 heterotrimer and AC5. The consequences in term of functions, response dynamics, subcellular localization, or regulation remain largely unknown. Interestingly, the absence of Gγ7 or AC5 produces reduction of Gαolf levels in the striatum, most probably by shortening its half-life. In activated state (GTP liganded), Gαolf interacts with AC5 while in inactivated state, Gαolf is associated with Gβ2γ7 complex. Gαolf stability appears thus to depend on the cellular availability of its two main interacting molecules, suggesting the tight and coordinated regulation of Gαolf quantity in the neuron.
Gαolf Levels Control the Efficacy of the Adenylyl Cyclase Activation by D1R
The reductions of the levels of Gαolf or D1R have contrasting consequences on various D1R signaling responses in the mouse striatum. These diminutions of Gαolf or D1R can be obtained in mice heterozygous for targeted deletions of Gαolf or D1R genes (Drago et al., 1994; Corvol et al., 2001), in which the striatal contents of corresponding proteins are decreased by about 50%. The reduction in Gαolf levels induces a marked reduction of both basal and D1R-activated cAMP production in striatal membranes (Herve et al., 2001; Corvol et al., 2007). The AC activities in the presence of dopamine or in basal condition are reduced by approximately 50% and the D1R-related response (as estimated by the difference between the basal and stimulated activities) by about 35%. In contrast, the haplodeficiency in D1R leads to no significant change in D1 agonist response or in the basal and dopamine-stimulated activities (Corvol et al., 2007).
The levels of Gαolf are not only determinant for in vitro AC responses, but also for in vivo responses. The increased cAMP levels resulting from D1R stimulation activate PKA in striatal neurons, leading to the phosphorylation of numerous PKA substrates including the GluR1 subunit of AMPA glutamate receptors (Valjent et al., 2005). Acute injection of psychostimulants like cocaine or D-amphetamine activates this pathway by increasing extracellular levels of dopamine in the brain. This response is highly dependent on Gαolf in the striatum since it is strongly decreased when the Gαolf levels are reduced in the brain (mutant mice with heterozygous null mutation of Gαolf gene; Corvol et al., 2007). Reduction in D1R in mice heterozygous for null mutation of D1R gene did not alter significantly this response.
These results show that the levels of Gαolf protein, but not D1R, constitute a limiting factor determining the amplitude of cAMP pathway response upon D1R activation in the striatal neurons. This observation is consistent with the existence of “spare” D1Rs not coupled to AC in the striatum (Hess et al., 1987; Trovero et al., 1992). However, this is in apparent contradiction with the existence of a large excess of Gαolf/β2/γ7 heterotrimers in comparison with D1Rs in term of number of molecules present in striatal membranes. Thorough measurements using quantitative immunoblots indicate that the concentration of G protein in striatal membrane is around 70–80 pmol/mg of membrane protein (Schwindinger et al., 2010). In contrast, the content in D1R (and A2A receptors) would be almost two orders of magnitude lower (about 1 and 0.3 pmol/mg of membrane protein for D1R and A2A receptor, respectively; Hess et al., 1987; Schwindinger et al., 2010). The mechanisms of activation of G proteins by receptors are still a matter of debate. Depending on receptor/G protein systems, two opposing models have been proposed (Lohse et al., 2008): (1) in the “collision coupling” model, the receptor/G protein interactions occur as a result of free lateral diffusion within the plasma membrane, wherein G proteins only interact with activated receptors; (2) the alternative model suggests that G proteins can interact with receptors before agonist binding, in a “precoupling” state. The second model is attractive because it could explain the specificity of coupling of D1R with precise G proteins. However, in this model, decreasing levels of receptor and G protein should lead to similar reductions of cAMP production. “Collision coupling” model explains probably better the mechanisms occurring in the striatal membranes even though the kinetics data are not enough precise to really settle this issue. In this model, the high excess of G proteins in the MSNs in vivo can result in amplification of signal, activated receptors being able to switch on multiple G proteins. This notion has been well established in the retina for the rhodopsin–transducin system (similar to the receptor-G protein couple) in which studies have given rates of 1300 transducin molecules activated per rhodopsin molecule per second (Heck and Hofmann, 2001). Probably the amplification factor is lower in the striatal cells, but it is well conceivable that despite the high excess in Gαolf/β2/γ7 heterotrimers compared to D1R (or A2A receptor), partial activation of D1R can saturate the G proteins present in the plasma membrane and thus the levels of the G proteins can represent a limiting factor controlling the D1R coupling with AC.
Partial Reduction of Gαolf Levels Does Not Alter ERK Pathway
Surprisingly, the haplodeficiency of Gαolf gene does not affect D1R-dependent ERK pathway in the striatal neurons, contrary to what is observed for the cAMP pathway. Psychostimulants (cocaine or D-amphetamine) produce ERK activation specifically in D1R expressing striatal neurons (Valjent et al., 2000; Bertran-Gonzalez et al., 2008). This effect is dependent on D1R activation since it is prevented by pharmacological or genetic inaction of D1R (Valjent et al., 2000, 2005). Importantly this pathway appears critical for the long-lasting effects of cocaine or D-amphetamine, including conditioned place preference and locomotor sensitization (Valjent et al., 2000, 2006). In the heterozygous Gαolf mutant mice, psychostimulant-induced ERK activation is normal, similar to that observed in the wild type animals (Corvol et al., 2007).
Unexpectedly, this ERK response is impaired when the D1R levels are reduced by half in the mice heterozygous for null mutation of D1R gene (Pascoli et al., 2010). In the same mice cAMP/PKA response appears completely normal. In fact, the mechanisms of ERK activation following psychostimulants are complex since stimulation of D1R cannot activate ERK alone but potentiates the ERK activation initiated by calcium influx through glutamate-activated NMDA receptor (Pascoli et al., 2010). The mechanisms of this potentiation are probably multiple and combine PKA-dependent and independent processes. The cAMP/PKA-dependent potentiation of ERK pathway is mediated by the protein DARPP-32 via its ability to inhibit protein phosphatase 1 and striatal-enriched tyrosine phosphatase (STEP; Valjent et al., 2005). More recently, it has been uncovered an alternative pathway, by which D1R can stimulate glutamate-induced ERK activation by promoting D1R-dependent activation of Src family kinases and tyrosine phosphorylation of the NR2B subunit of NMDA receptor (Pascoli et al., 2010). This pathway is independent from the cAMP/PKA cascade and appears to be downregulated in mice heterozygous for null mutation of D1R gene (Pascoli et al., 2010). In these animals, the impairment of cocaine-induced ERK activation goes together with reduced activation of Src family kinase and phosphorylation of NR2B subunit.
In conclusion, D1R levels control the efficiency of the D1R-regulated ERK pathway whereas Gαolf levels controls that of the D1R-regulated cAMP/PKA pathway.
Reduction of Gαolf Levels has Contrasted Behavioral Consequences
As previously mentioned, the acute responses to cocaine and D-amphetamine are highly dependent on D1R-linked signaling. The Gαolf heterozygous mice display a clear reduction in acute locomotor response to cocaine or D-amphetamine, in agreement with the decreased cAMP signaling responses in vivo (Herve et al., 2001; Corvol et al., 2007). By contrast a partial decrease in D1R amounts did not significantly affect the acute locomotor response to cocaine or D-amphetamine in D1R heterozygous mice (Corvol et al., 2007). This is consistent with the unaltered biochemical responses of the PKA pathway in these mice.
In contrast, the partial deficiency of Gαolf does not prevent the development of locomotor sensitization to cocaine or D-amphetamine in Gαolf heterozygous mice (Corvol et al., 2007). Moreover, because the acute locomotor response is very low in these mice, the sensitized response appears proportionally higher than in wild type animals. Similarly, conditioned place preference to D-amphetamine is not altered in Gαolf heterozygous mice. The contrast between altered responses to acute administrations of psychostimulants and normal responses to repeated treatments in these mice suggests that different signaling pathways may be limiting for the two types of effects. Several factors could account for the quasi-normal sensitizing and conditioning properties of psychostimulants, including the possibility that these effects are partially independent from D1R activation (Salomon et al., 2006). One interesting possibility involves the ERK pathway which is normally activated by psychostimulants in Gαolf heterozygous mice. ERK appears essential for long-lasting effects of drugs since its pharmacological inhibition blocks both locomotor sensitization and conditioned place preference with only minor effects on acute responses (Valjent et al., 2000, 2006). The normal psychostimulant-induced ERK activation could enable these responses in the Gαolf heterozygous mutant mice. Some evidence argues in favor of this hypothesis. In particular, it has been found some alterations of the sensitization to cocaine in mice heterozygous for D1R gene, in which ERK activation, but not cAMP/PKA activation, is altered in response to cocaine (Valjent et al., 2010).
These studies indicate that variability in the levels of expression of specific genes involved in various aspects of D1R signaling can produce very different behavioral reactions in response to drugs. Depending on the element affected, genetic or environmental factors altering components of D1R signaling can have contrasted consequences leading to specific pathological or phenotypical traits.
Regulation of Gαolf Levels
Because Gαolf levels constitute an important parameter controlling D1R-dependent cAMP/PKA pathway, they could represent an ideal target for regulation in physiological and pathological conditions. Such regulations have been well demonstrated following degeneration of dopamine neurons in the experimental context or human pathology of Parkinson’s disease.
The dopamine denervation of the striatum produces an important hypersensitivity of the D1R signaling that could enable the therapeutic effects of L-DOPA in Parkinsonian patients but also promote averse secondary effects, essentially the abnormal involuntary movements or dyskinesia that develop after 5–10 years of L-DOPA treatments (Bezard et al., 2001). This hypersensitivity affects both the cAMP/PKA and ERK pathways, since both are highly activated by D1R agonists in the denervated striatum (Gerfen et al., 2002; Pavon et al., 2006; Santini et al., 2007; Westin et al., 2007). The denervation-induced hypersensitivity happens despite a lack of changes in the density of D1R in the striatum (Savasta et al., 1988; Herve et al., 1989; Missale et al., 1989) or minimal alterations in the intracellular distribution of D1R (Berthet et al., 2009). The most plausible mechanism is an increase in the coupling of D1R with G protein, which has been demonstrated in both rodents and non-human primates after dopamine denervation of the striatum (Cai et al., 2002; Aubert et al., 2005). This higher coupling is essentially linked to an increase of Gαolf levels in the denervated striatum. In the rat, 6-hydroxydopamine-induced lesions of dopamine neurons in adult or newborn animals lead to an increase by about 50% of Gαolf levels in the following weeks (Herve et al., 1993; Marcotte et al., 1994; Penit-Soria et al., 1997). Similar increase in Gαolf levels has been observed in 6-hydroxydopamine-lesioned mice (Alcacer et al., unpublished data). Upregulation of Gαolf was also observed in human putamen in Parkinsonian patients and, interestingly, this effect was associated with a parallel increase in Gγ7 levels (Corvol et al., 2004). In this study, there was a correlation between the increase in Gαolf levels and the duration of disease. In addition, the patients in whom the increase was the highest displayed intense L-DOPA-induced dyskinesia but there was no established causal relationship between the two effects.
In rat, the upregulation in the Gαolf protein levels is not linked to a parallel increase in Gαolf mRNA expression, showing that the regulation is post-transcriptional (Herve et al., 1993). An attractive possibility is that changes in Gαolf protein levels depend directly from its rate of activation. This hypothesis is supported by several studies on Gαs which is a protein very close to Gαolf. In cell culture, various long-lasting stimulations of Gαs by receptors, cholera toxin, or mutation induce a downregulation of Gαs at a post-translational level, which is independent of cAMP production and involves possibly an increased degradation rate of the protein (McKenzie and Milligan, 1990; Levis and Bourne, 1992; Milligan, 1993; Adie and Milligan, 1994). The chronic lack of D1R and Gαolf stimulation in dopamine-denervated striatum could lower the Gαolf degradation rate and lead to accumulation of the protein. In agreement with this hypothesis, total absence of D1R in mutant mice with targeted invalidation of D1R gene induces important increase of Gαolf protein levels without any modification of Gαolf mRNA expression (Herve et al., 2001). Conversely, reduced levels of Gαolf were observed in mutant mice devoid of dopamine transporter (Herve et al., 2001), in which extracellular concentration of dopamine is strongly increased, leading thus to a chronic stimulation of D1R (Giros et al., 1996). Interestingly, the lack of A2A receptors in mutant mice produces also an upregulation of Gαolf protein without any changes in the levels of Gαolf transcripts (Herve et al., 2001). Thus these results strongly suggest a homeostatic regulation of Gαolf in vivo, in which the intensity of Gαolf stimulation tends to reduce its levels. These variations are reminiscent, at the level of a G protein, of the classical “denervation hypersensitivity” and “agonist-induced desensitization,” well characterized at the level of receptors (Freedman and Lefkowitz, 1996; Bloch et al., 1999).
The mechanisms of elimination from membrane and degradation of Gαolf or Gαs are not known in detail. Upon stimulation of receptor, Gαs was shown to internalize in a vesicle pool, corresponding probably to recycling endosomes with minimal overlap with vesicles containing receptors (Hynes et al., 2004). Recently, Gαs was reported to be ubiquitinated and possibly degraded through proteasome (Nagai et al., 2010). Interestingly, Ric-8B, a protein highly expressed in the striatum (Von Dannecker et al., 2005), inhibits the Gαs ubiquitination, and increases the Gαs protein without affecting the Gαs mRNA level (Nagai et al., 2010). Ric-8B plays the same essential role on Gαolf and enhances the accumulation of Gαolf at the cytoplasmic membrane (Von Dannecker et al., 2006). However further studies are needed to determine the precise mechanisms important for upregulation of Gαolf following degeneration of dopamine neurons.
Conclusion
An assembly composed of Gαolf, Gβ2, and Gγ7 of G protein mediates the activation of AC5 by the D1R in the MSNs expressing this receptor while the same heterotrimer provides the coupling of adenosine A2A receptor to AC in the MSN population containing D2 receptors. The total absence of this assembly impairs all the biochemical and behavioral responses involving D1R. These studies provide the proof, probably unique in the literature, that the receptor recognizes a specific assembly of αβγ subunits of G protein in vivo. The Gαolf stability depends on the presence of the Gγ7 subunit and AC5 effector protein. The cellular concentration of Gαolf appears thus to be regulated by the availability of its interacting proteins. By contrast, D1R receptor exerts a negative regulation on Gαolf: more the receptor is stimulated more the cellular Gαolf levels decrease. As important consequences of this regulation, it was observed an increase of striatal levels of Gαolf following degeneration of dopamine neurons in both lesioned animals and Parkinsonian patients. Gαolf upregulation is certainly a major factor explaining the hypersensitivity of D1R-linked cAMP signaling detected after dopamine lesion since the Gαolf levels control in vivo the efficiency of D1Rs on AC activation. Alterations of levels of Gαolf or its interacting proteins because of genetic or pathological factors could play an important role in the physiology of Parkinson’s disease as well as in the individual responses to therapeutic drugs. In addition, because of the involvement of dopamine signaling in several mental diseases, such as schizophrenia or drug addiction, dysregulation of Gαolf could contribute to physiopathology of these diseases. The gene encoding Gαolf (GNAL) has been investigated as a candidate gene for several mental diseases. To date, no coding variants of Gαolf have been tested but a strong association with schizophrenia was reported for an intronic marker (Schwab et al., 1998). In contrast, other genetic studies on bipolar affective disorder and unipolar depression have yielded negative results (Tsiouris et al., 1996; Berrettini et al., 1998; Zill et al., 2002). More recently, two studies, one in human using intronic GNAL polymorphisms (Laurin et al., 2008) and the other using rat models (DasBanerjee et al., 2008), suggest a possible contribution of Gαolf in the susceptibility to attention deficit/hyperactivity disorder (ADHD) in children. These studies suggest that alterations or quantitative modifications of the components of specific signaling machinery associated with D1R in the striatum have the potential to affect the various behavioral responses linked to dopamine functions in human.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author wishes to thank Dr. Emmanuel Valjent and Dr. Catherine Le Moine for having provided immunofluorescence and in situ hybridization images as well as Jean-Antoine Girault and Lucile Marion-Poll for their support. The work was supported by INSERM and grants from the Fondation pour la Recherche Médical and the Agence Nationale de la Recherche (ANR09-MNPS-014).
References
Adie, E. J., and Milligan, G. (1994). Agonist regulation of cellular Gs alpha-subunit levels in neuroblastoma x glioma hybrid NG108-15 cells transfected to express different levels of the human beta 2 adrenoceptor. Biochem. J. 300(Pt 3), 709–715.
Arnt, J., Hyttel, J., and Sanchez, C. (1992). Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur. J. Pharmacol. 213, 259–267.
Aubert, I., Guigoni, C., Hakansson, K., Li, Q., Dovero, S., Barthe, N., Bioulac, B. H., Gross, C. E., Fisone, G., Bloch, B., and Bezard, E. (2005). Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 57, 17–26.
Belluscio, L., Gold, G. H., Nemes, A., and Axel, R. (1998). Mice deficient in G(olf) are anosmic. Neuron 20, 69–81.
Berretta, S., Robertson, H. A., and Graybiel, A. M. (1992). Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J. Neurophysiol. 68, 767–777.
Berrettini, W. H., Vuoristo, J., Ferraro, T. N., Buono, R. J., Wildenauer, D., and Ala-Kokko, L. (1998). Human G(olf) gene polymorphisms and vulnerability to bipolar disorder. Psychiatr. Genet. 8, 235–238.
Berthet, A., Porras, G., Doudnikoff, E., Stark, H., Cador, M., Bezard, E., and Bloch, B. (2009). Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J. Neurosci. 29, 4829–4835.
Bertran-Gonzalez, J., Bosch, C., Maroteaux, M., Matamales, M., Herve, D., Valjent, E., and Girault, J. A. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–5685.
Bezard, E., Brotchie, J. M., and Gross, C. E. (2001). Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat. Rev. Neurosci. 2, 577–588.
Bloch, B., Dumartin, B., and Bernard, V. (1999). In vivo regulation of intraneuronal trafficking of G protein-coupled receptors for neurotransmitters. Trends Pharmacol. Sci. 20, 315–319.
Bourne, H. R., Sanders, D. A., and Mccormick, F. (1991). The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127.
Boyson, S. J., Mcgonigle, P., and Molinoff, P. B. (1986). Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. 6, 3177–3188.
Bunemann, M., Frank, M., and Lohse, M. J. (2003). Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082.
Cai, G., Wang, H. Y., and Friedman, E. (2002). Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J. Pharmacol. Exp. Ther. 302, 1105–1112.
Corradi, J. P., Ravyn, V., Robbins, A. K., Hagan, K. W., Peters, M. F., Bostwick, R., Buono, R. J., Berrettini, W. H., and Furlong, S. T. (2005). Alternative transcripts and evidence of imprinting of GNAL on 18p11.2. Mol. Psychiatry 10, 1017–1025.
Corvol, J. C., Muriel, M. P., Valjent, E., Feger, J., Hanoun, N., Girault, J. A., Hirsch, E. C., and Herve, D. (2004). Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J. Neurosci. 24, 7007–7014.
Corvol, J. C., Studler, J. M., Schonn, J. S., Girault, J. A., and Herve, D. (2001). Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 76, 1585–1588.
Corvol, J. C., Valjent, E., Pascoli, V., Robin, A., Stipanovich, A., Luedtke, R. R., Belluscio, L., Girault, J. A., and Herve, D. (2007). Quantitative changes in Galphaolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology 32, 1109–1121.
DasBanerjee, T., Middleton, F. A., Berger, D. F., Lombardo, J. P., Sagvolden, T., and Faraone, S. V. (2008). A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 1554–1563.
Drago, J., Gerfen, C. R., Lachowicz, J. E., Steiner, H., Hollon, T. R., Love, P. E., Ooi, G. T., Grinberg, A., Lee, E. J., Huang, S. P., Bartlett, P. F., Jose, P. A., Sibley, D. R., and Westphal, H. (1994). Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl. Acad. Sci. U.S.A. 91, 12564–12568.
Drago, J., Padungchaichot, P., Accili, D., and Fuchs, S. (1998a). Dopamine receptors and dopamine transporter in brain function and addictive behaviors: insights from targeted mouse mutants. Dev. Neurosci. 20, 188–203.
Drago, J., Padungchaichot, P., Wong, J. Y., Lawrence, A. J., Mcmanus, J. F., Sumarsono, S. H., Natoli, A. L., Lakso, M., Wreford, N., Westphal, H., Kola, I., and Finkelstein, D. I. (1998b). Targeted expression of a toxin gene to D1 dopamine receptor neurons by cre-mediated site-specific recombination. J. Neurosci. 18, 9845–9857.
Drinnan, S. L., Hope, B. P., Snutch, T. P., and Vincent, S. R. (1991). Golf in the basal ganglia. Mol. Cell. Neurosci. 2, 66–70.
El-Ghundi, M., O’Dowd, B. F., and George, S. R. (2007). Insights into the role of dopamine receptor systems in learning and memory. Rev. Neurosci. 18, 37–66.
Fredholm, B. B., Chen, J. F., Masino, S. A., and Vaugeois, J. M. (2005). Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 45, 385–412.
Freedman, N. J., and Lefkowitz, R. J. (1996). Desensitization of G protein-coupled receptors. Recent Prog. Horm. Res. 51, 319–351; discussion 352–313.
Friedman, E., Jin, L. Q., Cai, G. P., Hollon, T. R., Drago, J., Sibley, D. R., and Wang, H. Y. (1997). D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol. Pharmacol. 51, 6–11.
Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., Monsma, F. J. Jr., and Sibley, D. R. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432.
Gerfen, C. R., Miyachi, S., Paletzki, R., and Brown, P. (2002). D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J. Neurosci. 22, 5042–5054.
Giros, B., Jaber, M., Jones, S. R., Wightman, R. M., and Caron, M. G. (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612.
Glatt, C. E., and Snyder, S. H. (1993). Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature 361, 536–538.
Gong, S., Zheng, C., Doughty, M. L., Losos, K., Didkovsky, N., Schambra, U. B., Nowak, N. J., Joyner, A., Leblanc, G., Hatten, M. E., and Heintz, N. (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925.
Hasbi, A., Fan, T., Alijaniaram, M., Nguyen, T., Perreault, M. L., O’Dowd, B. F., and George, S. R. (2009). Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U.S.A. 106, 21377–21382.
Heck, M., and Hofmann, K. P. (2001). Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J. Biol. Chem. 276, 10000–10009.
Hervé, D., and Girault, J. A. (2005). “Signal transduction of dopamine receptors,” in Handbook of Chemical Neuroanatomy, 21; Dopamine, eds S. B. Dunnett, M. Bentivoglio, A. Björklund, and T. Hökfelt (Amsterdam: Elsevier Science), 109–151.
Herve, D., Le Moine, C., Corvol, J. C., Belluscio, L., Ledent, C., Fienberg, A. A., Jaber, M., Studler, J. M., and Girault, J. A. (2001). Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 21, 4390–4399.
Herve, D., Levi-Strauss, M., Marey-Semper, I., Verney, C., Tassin, J. P., Glowinski, J., and Girault, J. A. (1993). G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J. Neurosci. 13, 2237–2248.
Herve, D., Trovero, F., Blanc, G., Thierry, A. M., Glowinski, J., and Tassin, J. P. (1989). Nondopaminergic prefrontocortical efferent fibers modulate D1 receptor denervation supersensitivity in specific regions of the rat striatum. J. Neurosci. 9, 3699–3708.
Hess, E. J., Battaglia, G., Norman, A. B., and Creese, I. (1987). Differential modification of striatal D1 dopamine receptors and effector moieties by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in vivo and in vitro. Mol. Pharmacol. 31, 50–57.
Hynes, T. R., Mervine, S. M., Yost, E. A., Sabo, J. L., and Berlot, C. H. (2004). Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J. Biol. Chem. 279, 44101–44112.
Iwamoto, T., Iwatsubo, K., Okumura, S., Hashimoto, Y., Tsunematsu, T., Toya, Y., Herve, D., Umemura, S., and Ishikawa, Y. (2004). Disruption of type 5 adenylyl cyclase negates the developmental increase in Galphaolf expression in the striatum. FEBS Lett. 564, 153–156.
Iwamoto, T., Okumura, S., Iwatsubo, K., Kawabe, J., Ohtsu, K., Sakai, I., Hashimoto, Y., Izumitani, A., Sango, K., Ajiki, K., Toya, Y., Umemura, S., Goshima, Y., Arai, N., Vatner, S. F., and Ishikawa, Y. (2003). Motor dysfunction in type 5 adenylyl cyclase-null mice. J. Biol. Chem. 278, 16936–16940.
Jones, D. T., and Reed, R. R. (1989). Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science 244, 790–795.
Kheirbek, M. A., Beeler, J. A., Ishikawa, Y., and Zhuang, X. (2008). A cAMP pathway underlying reward prediction in associative learning. J. Neurosci. 28, 11401–11408.
Kull, B., Svenningsson, P., and Fredholm, B. B. (2000). Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol. Pharmacol. 58, 771–777.
Largent, B. L., Jones, D. T., Reed, R. R., Pearson, R. C., and Snyder, S. H. (1988). G protein mRNA mapped in rat brain by in situ hybridization. Proc. Natl. Acad. Sci. U.S.A. 85, 2864–2868.
Laurin, N., Ickowicz, A., Pathare, T., Malone, M., Tannock, R., Schachar, R., Kennedy, J. L., and Barr, C. L. (2008). Investigation of the G protein subunit Galphaolf gene (GNAL) in attention deficit/hyperactivity disorder. J. Psychiatr. Res. 42, 117–124.
Le Moine, C., and Bloch, B. (1995). D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 355, 418–426.
Lee, K. W., Hong, J. H., Choi, I. Y., Che, Y., Lee, J. K., Yang, S. D., Song, C. W., Kang, H. S., Lee, J. H., Noh, J. S., Shin, H. S., and Han, P. L. (2002). Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J. Neurosci. 22, 7931–7940.
Lee, K. W., Kim, Y., Kim, A. M., Helmin, K., Nairn, A. C., and Greengard, P. (2006). Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 103, 3399–3404.
Levis, M. J., and Bourne, H. R. (1992). Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J. Cell Biol. 119, 1297–1307.
Lezcano, N., and Bergson, C. (2002). D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J. Neurophysiol. 87, 2167–2175.
Liu, H. Y., Wenzel-Seifert, K., and Seifert, R. (2001). The olfactory G protein G(alphaolf) possesses a lower GDP-affinity and deactivates more rapidly than G(salphashort): consequences for receptor-coupling and adenylyl cyclase activation. J. Neurochem. 78, 325–338.
Lohse, M. J., Nikolaev, V. O., Hein, P., Hoffmann, C., Vilardaga, J. P., and Bunemann, M. (2008). Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors. Trends Pharmacol. Sci. 29, 159–165.
Mahan, L. C., Burch, R. M., Monsma, F. J. Jr., and Sibley, D. R. (1990). Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 87, 2196–2200.
Mannoury La Cour, C., Vidal, S., Pasteau, V., Cussac, D., and Millan, M. J. (2007). Dopamine D1 receptor coupling to Gs/olf and Gq in rat striatum and cortex: a scintillation proximity assay (SPA)/antibody-capture characterization of benzazepine agonists. Neuropharmacology 52, 1003–1014.
Marcotte, E. R., Sullivan, R. M., and Mishra, R. K. (1994). Striatal G-proteins: effects of unilateral 6-hydroxydopamine lesions. Neurosci. Lett. 169, 195–198.
Matamales, M., Bertran-Gonzalez, J., Salomon, L., Degos, B., Deniau, J. M., Valjent, E., Herve, D., and Girault, J. A. (2009). Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS ONE 4, e4770. doi: 10.1371/journal.pone.0004770
McKenzie, F. R., and Milligan, G. (1990). Prostaglandin E1-mediated, cyclic AMP-independent, down-regulation of Gs alpha in neuroblastoma x glioma hybrid cells. J. Biol. Chem. 265, 17084–17093.
Milligan, G. (1993). Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol. Sci. 14, 413–418.
Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998). Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225.
Missale, C., Nisoli, E., Liberini, P., Rizzonelli, P., Memo, M., Buonamici, M., Rossi, A., and Spano, P. (1989). Repeated reserpine administration up-regulates the transduction mechanisms of D1 receptors without changing the density of [3H]SCH 23390 binding. Brain Res. 483, 117–122.
Nagai, Y., Nishimura, A., Tago, K., Mizuno, N., and Itoh, H. (2010). Ric-8B stabilizes the alpha subunit of stimulatory G protein by inhibiting its ubiquitination. J. Biol. Chem. 285, 11114–11120.
Nairn, A. C., Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., and Greengard, P. (2004). The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47(Suppl. 1), 14–23.
Pascoli, V., Besnard, A., Herve, D., Pages, C., Heck, N., Girault, J. A., Caboche, J., and Vanhoutte, P. (2010). Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol. Psychiatry 69, 218–227.
Pavon, N., Martin, A. B., Mendialdua, A., and Moratalla, R. (2006). ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol. Psychiatry 59, 64–74.
Penit-Soria, J., Durand, C., Besson, M. J., and Herve, D. (1997). Levels of stimulatory G protein are increased in the rat striatum after neonatal lesion of dopamine neurons. Neuroreport 8, 829–833.
Plagge, A., Kelsey, G., and Germain-Lee, E. L. (2008). Physiological functions of the imprinted Gnas locus and its protein variants Galpha(s) and XLalpha(s) in human and mouse. J. Endocrinol. 196, 193–214.
Robishaw, J. D., and Berlot, C. H. (2004). Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol. 16, 206–209.
Salomon, L., Lanteri, C., Glowinski, J., and Tassin, J. P. (2006). Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc. Natl. Acad. Sci. U.S.A. 103, 7476–7481.
Santini, E., Valjent, E., Usiello, A., Carta, M., Borgkvist, A., Girault, J. A., Herve, D., Greengard, P., and Fisone, G. (2007). Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 27, 6995–7005.
Savasta, M., Dubois, A., Benavides, J., and Scatton, B. (1988). Different plasticity changes in D1 and D2 receptors in rat striatal subregions following impairment of dopaminergic transmission. Neurosci. Lett. 85, 119–124.
Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A., and Ferre, S. (2007). Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 83, 277–292.
Schwab, S. G., Hallmayer, J., Lerer, B., Albus, M., Borrmann, M., Honig, S., Strauss, M., Segman, R., Lichtermann, D., Knapp, M., Trixler, M., Maier, W., and Wildenauer, D. B. (1998). Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis. Am. J. Hum. Genet. 63, 1139–1152.
Schwindinger, W. F., Betz, K. S., Giger, K. E., Sabol, A., Bronson, S. K., and Robishaw, J. D. (2003). Loss of G protein gamma 7 alters behavior and reduces striatal alpha(olf) level and cAMP production. J. Biol. Chem. 278, 6575–6579.
Schwindinger, W. F., Mihalcik, L. J., Giger, K. E., Betz, K. S., Stauffer, A. M., Linden, J., Herve, D., and Robishaw, J. D. (2010). Adenosine A2A receptor signaling and golf assembly show a specific requirement for the gamma7 subtype in the striatum. J. Biol. Chem. 285, 29787–29796.
Sibley, D. R., and Monsma, F. J. Jr. (1992). Molecular biology of dopamine receptors. Trends Pharmacol. Sci. 13, 61–69.
Trovero, F., Herve, D., Blanc, G., Glowinski, J., and Tassin, J. P. (1992). In vivo partial inactivation of dopamine D1 receptors induces hypersensitivity of cortical dopamine-sensitive adenylate cyclase: permissive role of alpha 1-adrenergic receptors. J. Neurochem. 59, 331–337.
Tsiouris, S. J., Breschel, T. S., Xu, J., Mcinnis, M. G., and Mcmahon, F. J. (1996). Linkage disequilibrium analysis of G-olf alpha (GNAL) in bipolar affective disorder. Am. J. Med. Genet. 67, 491–494.
Valjent, E., Bertran-Gonzalez, J., Aubier, B., Greengard, P., Herve, D., and Girault, J. A. (2010). Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35, 401–415.
Valjent, E., Corvol, J. C., Pages, C., Besson, M. J., Maldonado, R., and Caboche, J. (2000). Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 20, 8701–8709.
Valjent, E., Corvol, J. C., Trzaskos, J. M., Girault, J. A., and Herve, D. (2006). Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 7, 20. doi: 10.1186/1471-2202-7-20
Valjent, E., Pascoli, V., Svenningsson, P., Paul, S., Enslen, H., Corvol, J. C., Stipanovich, A., Caboche, J., Lombroso, P. J., Nairn, A. C., Greengard, P., Herve, D., and Girault, J. A. (2005). Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. U.S.A. 102, 491–496.
Von Dannecker, L. E., Mercadante, A. F., and Malnic, B. (2005). Ric-8B, an olfactory putative GTP exchange factor, amplifies signal transduction through the olfactory-specific G-protein Galphaolf. J. Neurosci. 25, 3793–3800.
Von Dannecker, L. E., Mercadante, A. F., and Malnic, B. (2006). Ric-8B promotes functional expression of odorant receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 9310–9314.
Wadhawan, S., Dickins, B., and Nekrutenko, A. (2008). Wheels within wheels: clues to the evolution of the Gnas and Gnal loci. Mol. Biol. Evol. 25, 2745–2757.
Wang, H. Y., Undie, A. S., and Friedman, E. (1995). Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol. Pharmacol. 48, 988–994.
Watson, J. B., Coulter, P. M. II, Margulies, J. E., De Lecea, L., Danielson, P. E., Erlander, M. G., and Sutcliffe, J. G. (1994). G-protein gamma 7 subunit is selectively expressed in medium-sized neurons and dendrites of the rat neostriatum. J. Neurosci. Res. 39, 108–116.
Weinstein, L. S., Yu, S., Warner, D. R., and Liu, J. (2001). Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr. Rev. 22, 675–705.
Westin, J. E., Vercammen, L., Strome, E. M., Konradi, C., and Cenci, M. A. (2007). Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol. Psychiatry 62, 800–810.
Wolfgang, W. J., Hoskote, A., Roberts, I. J., Jackson, S., and Forte, M. (2001). Genetic analysis of the Drosophila Gs(alpha) gene. Genetics 158, 1189–1201.
Yung, K. K., Bolam, J. P., Smith, A. D., Hersch, S. M., Ciliax, B. J., and Levey, A. I. (1995). Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65, 709–730.
Zhuang, X., Belluscio, L., and Hen, R. (2000). G(olf)alpha mediates dopamine D1 receptor signaling. J. Neurosci. 20, RC91.
Keywords: D1 receptor, A2A receptor, heterotrimeric G protein, cAMP pathway, extracellular signal-regulated kinase, Gnal gene, Parkinson’s disease, cocaine
Citation: Hervé D (2011) Identification of a specific assembly of the G protein Golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front. Neuroanat. 5:48. doi: 10.3389/fnana.2011.00048
Received: 14 June 2011;
Paper pending published: 27 June 2011;
Accepted: 20 July 2011;
Published online: 05 August 2011.
Edited by:
Emmanuel Valjent, Université Montpellier 1 & 2, FranceReviewed by:
Sergi Ferre, Karolinska Institute, SwedenSerge N. Schiffmann, Université Libre de Bruxelles, Belgium
Copyright: © 2011 Hervé. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Denis Hervé, INSERM UMR-S839, Institut du Fer à Moulin, 17, rue du Fer à Moulin, 75005 Paris, France. e-mail: denis.herve@inserm.fr