Control of striatal signaling by G protein regulators
- The Scripps Research Institute, Jupiter, FL, USA
Signaling via heterotrimeric G proteins plays a crucial role in modulating the responses of striatal neurons that ultimately shape core behaviors mediated by the basal ganglia circuitry, such as reward valuation, habit formation, and movement coordination. Activation of G protein-coupled receptors (GPCRs) by extracellular signals activates heterotrimeric G proteins by promoting the binding of GTP to their α subunits. G proteins exert their effects by influencing the activity of key effector proteins in this region, including ion channels, second messenger enzymes, and protein kinases. Striatal neurons express a staggering number of GPCRs whose activation results in the engagement of downstream signaling pathways and cellular responses with unique profiles but common molecular mechanisms. Studies over the last decade have revealed that the extent and duration of GPCR signaling are controlled by a conserved protein family named regulator of G protein signaling (RGS). RGS proteins accelerate GTP hydrolysis by the α subunits of G proteins, thus promoting deactivation of GPCR signaling. In this review, we discuss the progress made in understanding the roles of RGS proteins in controlling striatal G protein signaling and providing integration and selectivity of signal transmission. We review evidence on the formation of a macromolecular complex between RGS proteins and other components of striatal signaling pathways, their molecular regulatory mechanisms and impacts on GPCR signaling in the striatum obtained from biochemical studies and experiments involving genetic mouse models. Special emphasis is placed on RGS9-2, a member of the RGS family that is highly enriched in the striatum and plays critical roles in drug addiction and motor control.
Striatal G Protein Signaling: An Overview
Striatum is a large part of the subcortical basal ganglia system consisting of caudate and putamen nuclei in the dorsal side that receives dopaminergic input from substantia nigra and nucleus accumbens on the ventral side that receives dopaminergic input from the ventral tegmental area. In addition, both ventral and dorsal striatum receive major glutamatergic input from cortical areas as well as inputs from other nuclei of basal ganglia such as subthalamic nucleus and the external globus pallidus (Graybiel, 2000).
Striatal neurons play critical roles in initiating and maintaining movement, mood control, reward behavior, and drug addiction (Graybiel, 2000; Kreitzer and Malenka, 2008). Dysfunctions in the neurocircuitry of the striatum have been implicated in the development of a wide range of disorders, including Parkinson’s disease (Picconi et al., 2005), Huntington’s disease (Cepeda et al., 2007), depression (Nestler and Carlezon, 2006), and drug addiction (Everitt and Robbins, 2005). The striatum contains a relatively heterogeneous neuronal population. Medium spiny neurons (MSNs) are the most abundant type, accounting for 90–95% of neurons in this region (Graveland and DiFiglia, 1985). Despite their morphological similarities, MSNs are categorized into two different subtypes based on differences in their gene expression and axonal projections (Gerfen et al., 1990; Surmeier et al., 2007; Heiman et al., 2008). The first group, striatonigral MSNs, selectively expresses dynorphin and dopamine D1 receptors and sends projections directly to basal ganglia output nuclei. The second group, striatopallidal MSNs, selectively expresses enkephalin and dopamine D2 receptors and sends axons to the external globus pallidus. In addition to MSNs, the striatum contains small populations of giant cholinergic interneurons, easily distinguished by their large cell bodies and tonic activities (Zhou et al., 2002), as well as parvalbumin and calretinin interneurons (Cicchetti et al., 2000).
One of the striking features of the striatum is the wide variety of neurotransmitter inputs that converge onto striatal neurons. The first major excitatory input is provided by glutamatergic afferents, most notably from the cerebral and prefrontal cortexes but also from several other brain regions, such as the thalamus, amygdala, and hippocampus (McGeorge and Faull, 1989; Bolam et al., 2000). The second major input to the striatum is provided by neurons from the substantia nigra and ventral tegmental area that release dopamine (Smith and Kieval, 2000). The glutamate and dopamine systems intimately overlap, and their interaction is thought to be essential for striatal signaling (for reviews see Graybiel, 1990; Chase and Oh, 2000; David et al., 2005). In addition, the striatum receives several other neurotransmitter inputs that play important modulatory roles. Despite their scarcity, striatal cholinergic interneurons densely innervate both the ventral and dorsal striatum; they form an extensive local axon collateral system, making contacts with MSNs and other interneurons (Koos and Tepper, 2002; Zhou et al., 2002). Changes in the amount of acetylcholine (ACh) released by these neurons are crucial for determining the activity of the striatal output neurons (Calabresi et al., 2000). Striatal neurons also receive substantial projections from noradrenergic (Nestler et al., 1999; Sara, 2009) and serotonergic neurons (Azmitia and Segal, 1978; Moukhles et al., 1997) located in the locus coeruleus and the dorsal raphe nuclei, respectively. Several locally synthesized and released neuromodulators also play important roles in striatal signaling. These neuromodulators include endogenous opioids, such as endorphins, enkephalins, dynorphins, and orphanin FG, which are found in abundance in the nucleus accumbens and the surrounding ventral striatum and are known to be involved in motivational aspects of behavior (Van Ree et al., 2000; Kelley et al., 2002), as well as endocannabinoids, which act in a retrograde manner to inhibit neurotransmitter release (Bisogno et al., 2005; Basavarajappa, 2007). Adenosine, a ubiquitous homeostatic substance released from most cells, including neurons and glia (Cunha, 2001; Dunwiddie and Masino, 2001), has important neuromodulatory effects on dopamine and glutamate transmission in the striatum. Presynaptically, adenosine inhibits or facilitates transmitter release while causing hyperpolarization or depolarization in postsynaptic neurons (Dunwiddie and Masino, 2001; Ribeiro et al., 2002). Finally, MSNs signal by releasing the inhibitory neurotransmitter GABA (Bolam et al., 2000; Graybiel, 2000). While their projections target a range of effector nuclei in the mesolimbic system, they also form extensive local contacts with neighboring neurons, making GABA important for regulating the spiking timing of spiny outputs (Tepper et al., 2004).
Thus, multiple neurotransmitter systems act simultaneously in the striatum both pre and postsynaptically to shape neuronal activity. This creates an environment where individual neurons integrate multiple inputs that often act cooperatively to affect excitability and the long-term adaptive effects that underlie their physiological function. This organization places a significant emphasis on the principles and mechanisms of intracellular signaling pathways that interpret, coordinate, and integrate signals carried by multiple neurotransmitters.
One of the central signaling systems that mediate the effects of neurotransmitters is the seven-transmembrane G protein-coupled receptors (GPCRs). In the canonical pathway, upon binding to neurotransmitter molecules, GPCRs undergo conformational changes that activate their ability to catalyze the exchange of GDP with GTP (Gilman, 1987; Pierce et al., 2002). From this perspective, GPCRs act as GDP/GTP guanine nucleotide exchange factors (GEFs) for G proteins. G proteins comprise three subunits: α, β and γ. GTP binding to the α subunit causes it to dissociate from the βγ complex. Both the active (GTP-bound) form of Gα and free Gβγ interact with a range of downstream signaling molecules (“effectors”) and modulate their activity to generate cellular responses. Recent studies have also revealed an additional G protein-independent signaling mechanism of GPCRs: neurotransmitter binding induces phosphorylation of GPCRs, followed by the binding of an adaptor protein, β-arrestin, that recruits a host of signaling factors to transmit the signal (Lefkowitz and Shenoy, 2005; Lefkowitz et al., 2006; Premont and Gainetdinov, 2007). While the importance of this non-canonical GPCR signaling pathway for striatal signaling is emerging, the scope of our review is limited to the traditional G protein-based signaling mechanisms.
Mammalian genomes contain more than 800 genes that encode GPCRs (Lander et al., 2001; Venter et al., 2001), and many different types of GPCRs are expressed in striatal neurons (see Versatility of the GPCR Repertoire in the Striatum). Despite such diversity, all GPCRs transduce signals via a limited set of G proteins. There are four subfamilies of Gα subunits (Gαs, Gαi/o, Gαq, and Gα12) that have selective preferences for the activation of different GPCR classes (Oldham and Hamm, 2008). However, many GPCRs can couple to more than one Gα protein. Gα subunits also show selectivity in their regulation of downstream effectors (Gilman, 1987). Canonically, Gαs and Gαi proteins regulate adenylyl cyclase, Gαq family proteins activate phospholipase Cβ (PLCβ), and Gα12 protein control members of the RhoGEF superfamily (Pierce et al., 2002; Oldham and Hamm, 2008). Four signal transducing beta subunits and 13 gamma subunits form constitutive complexes with poorly understood selectivity and, when released from the heterotrimer, can also regulate a range of effectors, most notably phospholipases (PLCβ2), and ion channels (Dupre et al., 2009). Signaling is terminated when the neurotransmitter dissociates from the receptor; the receptor’s GEF activity is quenched, and the GTP on the Gα subunit is hydrolyzed to allow reformation of the inactive Gαβγ heterotrimer and dissociates from the effectors.
G protein-coupled receptor signaling pathways are organized in a way that allows tremendous integration of signaling events at the G protein level. The balance between reactions that lead to activation or deactivation plays a central role in dictating the extent of the effectors’ activity regulation and is ultimately responsible for shaping neuronal responses. Intracellular factors that regulate G protein activity status are, thus, expected to play essential roles in controlling the responses of striatal neurons to neurotransmitters.
Versatility of the GPCR Repertoire in the Striatum
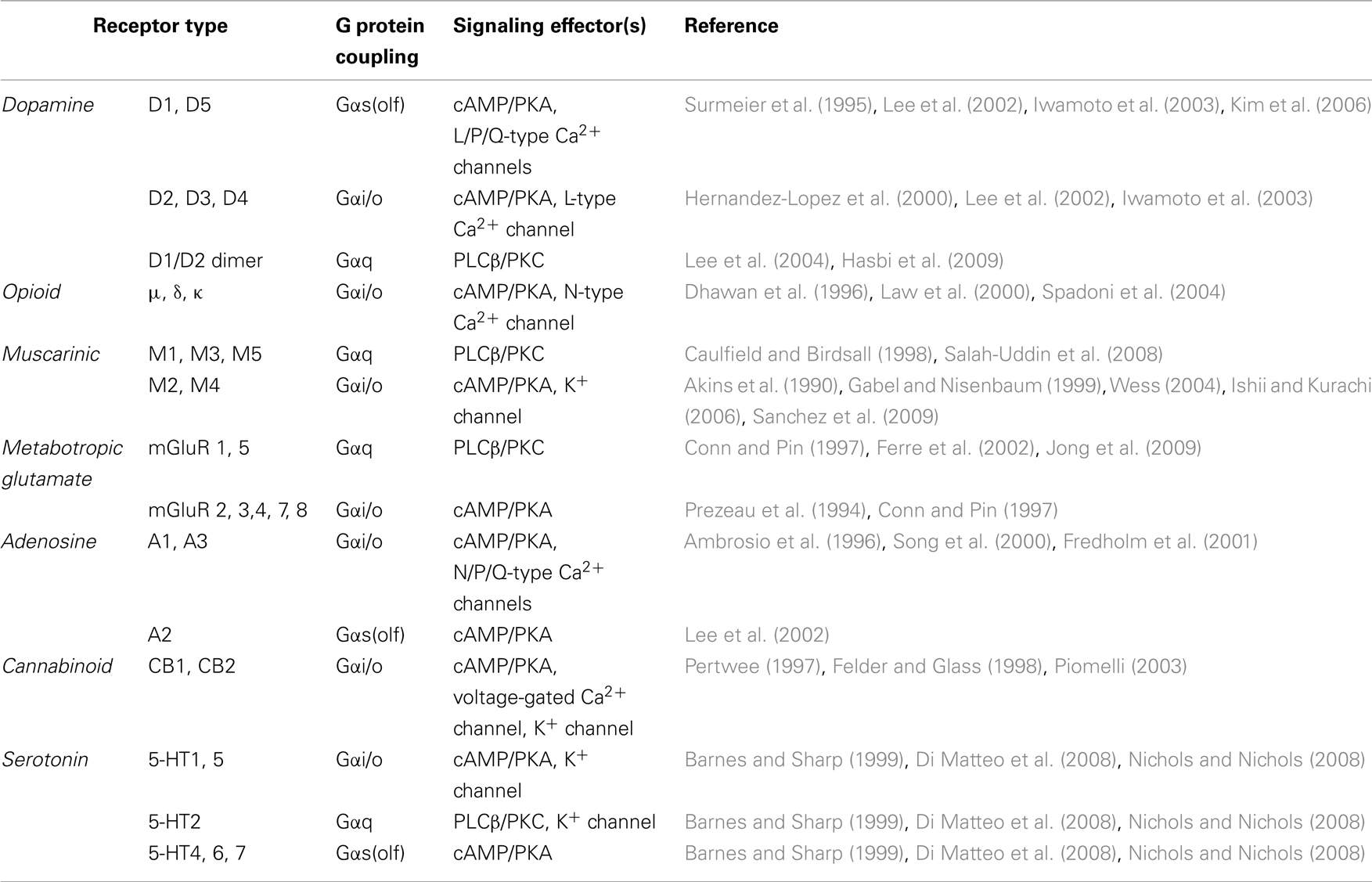
Consistent with multiple neurotransmitter inputs, striatal neurons utilize many GPCRs, which are abundantly expressed and play essential roles in striatal physiology. It is extremely difficult to provide a comprehensive overview about all of the GPCR systems involved in the physiology of the striatal neurons. Several orphan GPCRs for as yet unknown ligands are also present in the region (Mizushima et al., 2000; Marazziti et al., 2007; Logue et al., 2009). Our account below provides an overview of the GPCR systems that significantly contribute to G protein activation and have firmly established physiological roles (Table 1).
Dopamine receptors are perhaps the most studied GPCRs in the striatum and have firmly established physiological roles (Jaber et al., 1996; Missale et al., 1998; Glickstein and Schmauss, 2001; El-Ghundi et al., 2007). Although all five dopamine receptors are present in the striatum, the roles of D1R and D2R receptors have received the greatest attention. Both D1R and D2R are abundantly expressed in striatal neurons; however, they show remarkable segregation among cell types. D1R is expressed in the striatonigral MSNs, constituting the direct pathway, whereas D2R is expressed in the striatopallidal or indirect pathway (Gerfen et al., 1990; Graybiel, 2000; Shuen et al., 2008; Matamales et al., 2009). D2Rs are also located presynaptically on dopaminergic terminals and participate in the autoregulation of dopamine release (Jaber et al., 1996). A small population of MSNs (∼5%) co-expresses both D1R and D2R (Falk et al., 2006; Shuen et al., 2008), which have been shown to form heterodimers (Lee et al., 2004). Remarkably, D1R–D2R dimers can activate Gαq, creating an additional signaling modality (Lee et al., 2004; Hasbi et al., 2009).
Our knowledge regarding the involvement of D3R, D4R, and D5R is much more limited, in part due to their relatively low abundance. D5R is highly expressed in cholinergic neurons in the striatum and is involved in the induction of long-term potentiation (LTP; Suzuki et al., 2001). Although present at low levels in the striatum, the D3R receptor has approximately 200-fold higher affinity for dopamine than does D2R and is thought to be primarily involved in regulating dopamine release at lower dopamine concentrations (Joseph et al., 2002) by acting as an autoreceptor. While little information about D4R is available, it is known to play an important role in the regulation of striatal function because genetic ablation impairs locomotor sensitization to cocaine and amphetamine (Rubinstein et al., 1997; Kruzich et al., 2004; Thanos et al., 2010).
Opioid receptors account for the actions of both endogenous opioid peptides and exogenous opiates and are thought to be one of the central molecular substrates that modulate reward signaling in the striatum. Opioid receptors are involved in the modulation of dopaminergic transmission in the striatum. Blockage of opioid receptors, especially μ and δ, attenuates psychostimulant-induced behavior sensitization (Heidbreder et al., 1993; Schad et al., 1996; Balcells-Olivero and Vezina, 1997; Diaz-Otanez et al., 1997). μopioid receptors are specifically enriched in striosomes and have been shown to inhibit corticostriatal EPSCs (Jiang and North, 1992), and IPSCs (Miura et al., 2007), indicating that they play a critical role in modulation of corticostriatal excitatory and inhibitory synaptic transmission. μopioid receptors have also been recently found to be expressed in a subset of cholinergic neurons in the dorsal striatum, and activation of μopioid receptors inhibits ACh release (Jabourian et al., 2005; Perez et al., 2007). κ and δ opioid receptors in striatum were also shown to modulate dopamine (Spanagel et al., 1992) and glutamate (Rawls and McGinty, 2000) release and subsequently regulate stimulant-induced behavior (Gray et al., 1999; Gonzalez-Nicolini et al., 2003).
Muscarinic receptors (mAChR) are expressed in the striatum in a complex, overlapping manner where they mediate the slow-acting response to Ach (Weiner et al., 1990; Levey et al., 1991; Bernard et al., 1992; Hersch et al., 1994). The M1, M2, and M4 receptors are the predominant muscarinic receptors in the striatum (Levey et al., 1991; Abrams et al., 2006). When activated, muscarinic receptors modulate the excitability of striatal MSNs via the enhancement of NMDA receptor-mediated currents (Calabresi et al., 1998a) or the inhibition of voltage-activated N-, P-, and L-type Ca2+ currents (Howe and Surmeier, 1995). Muscarinic receptors also modulate the functions of striatal cholinergic interneurons; activation of M2 and M4 receptors has been shown to inhibit both N- and P-type Ca2+ currents (Yan and Surmeier, 1996). The inhibition of Ca2+ channels located at presynaptic sites ultimately leads to the inhibition of ACh or glutamate release, which plays a crucial role in coordinated changes in striatal neuronal activity (Calabresi et al., 1998b; Rawls and McGinty, 1998). In addition, most mAChRs are expressed in the terminals of dopaminergic neurons, where they bidirectionally regulate dopamine release (Xu et al., 1989; De Klippel et al., 1993; Smolders et al., 1997; Zhang et al., 2002).
Metabotropic glutamate receptors (mGluRs) modulate relatively slow glutamate transmission through second messenger systems (Nakanishi et al., 1998). mGluR1/5 are abundantly expressed postsynaptically in the medium spiny projection neurons of the striatum (Testa et al., 1995; Paquet and Smith, 2003). The activation of group I mGluRs in MSNs induces synaptic plasticity by modulating NMDA receptor activity (Pisani et al., 1997, 2001; Gubellini et al., 2001, 2003; Swanson et al., 2001). Group II and group III mGluRs are mostly located at presynaptic glutamatergic terminals (Bradley et al., 1999; Kosinski et al., 1999; Tamaru et al., 2001; Corti et al., 2002) and serve as autoreceptor decreasing glutamate release (Cartmell and Schoepp, 2000; Schoepp, 2001).
In addition to these major GPCR players, many other receptors expressed in this region are recognized to impact striatal signaling. Two cannabinoid receptors (CB1 and CB2) have been characterized to date. In the striatum, presynaptically expressed CB1 receptors control both glutamate and GABA release in a retrograde manner (Huang et al., 2001; Fernandez-Ruiz et al., 2002; Basavarajappa, 2007). Several G protein coupled serotonin (5-HT) receptors are also expressed in the striatum and mediate diverse physiological functions including modulation of stress response, depression, and anxiety (Di Matteo et al., 2008; Nichols and Nichols, 2008). Adenosine A2a receptors are highly enriched in the striatal neurons, especially in the GABAergic striatopallidal neurons, where adenosine A2a and dopamine D2 are colocalized (Fink et al., 1992; Augood and Emson, 1994). The physiological function of the adenosine A2a receptor is characterized by its antagonistic effect on the dopamine D2 receptor, indicating a critical fine-tuning effect on indirect signaling pathway. There are several excellent reviews of the basal ganglia functions of the adenosine A2a receptor (Fuxe et al., 2007; Schiffmann et al., 2007).
In summary, the presence of multiple neurotransmitter receptors in striatal neurons creates an environment where many GPCRs signal to several effector molecules that uniquely control various aspects of striatal function. At the same time, a tremendous integration of the signaling occurs downstream from the GPCRs as many receptors share limited number of the effector molecules ultimately responsible for generating responses in the striatum.
Regulator of G Protein Signaling Proteins: Key Focal Points of the Regulatory Influence
The convergent organization of the G protein pathways suggests that the factors that exert regulatory influence downstream from the GPCR and upstream of the effector molecules provide universal control over the extent and duration of signaling and are key in shaping cellular responses. Recent research has uncovered a number of proteins capable of altering the kinetics and dynamics of G protein signal transduction (Ross and Wilkie, 2000; Siderovski and Willard, 2005). Among these molecules, there has been substantial interest in proteins that facilitate termination of signaling, providing a major opposing force to GPCR-driven activation.
Once activated, G proteins transmit the signal until GTP hydrolysis occurs. G protein α subunits are inefficient GTPases, and their intrinsic inactivation takes up to several minutes. Nevertheless, signaling termination in many well-studied G protein pathways have been known to occur at substantially more rapid timescales (Zerangue and Jan, 1998). While some effectors can stimulate GTPase activity (Biddlecome et al., 1996; Paulssen et al., 1996; Hart et al., 1998; Kozasa et al., 1998) and serve as GTPase activating proteins (GAPs), these examples cannot account for the entire range of signaling situations. Over the last decade, it has become clear that the GAP function of heterotrimeric G proteins is driven by a conserved family of proteins called regulators of G protein signaling (RGS). RGS proteins associate with the GTP-bound form of the Gα subunits and stabilize the transition state, dramatically accelerating GTP hydrolysis (Ross and Wilkie, 2000). There are more than 30 members in the mammalian RGS family. They all share a conserved “catalytic” RGS domain (∼130 amino acid residues) and are categorized into six subfamilies (R4, RZ, R7, R12, RA, and RL) based on RGS domain homology (Ross and Wilkie, 2000; Hollinger and Hepler, 2002). It should be noted that not all RGS domains are capable of accelerating GTP hydrolysis on G proteins. Moreover, for many RGS proteins, the relative efficiency of their GAP function varies, depending on the identity of the Gα subunit substrate.
In addition to the RGS domain, many RGS proteins possess other, “non-catalytic” domains. Several functions of these non-catalytic domains have been uncovered. First, they serve as regulatory elements for other signaling pathways (e.g., small GTPases), endowing RGS proteins with multifunctionality in the regulation of cellular signaling (Hart et al., 1998; De Vries and Gist Farquhar, 1999; Siderovski et al., 1999; Schiff et al., 2000; Willard et al., 2007; Shu et al., 2010). Non-catalytic domains can also modify the functions of the constituent catalytic domains of RGS proteins by recruiting additional cellular factors and regulating their GAP activity (Chen and Lin, 1998; Martemyanov et al., 2003a), G protein selectivity (He et al., 2000; Martemyanov and Arshavsky, 2002), and localization (Srinivasa et al., 1998; De Vries et al., 2000b; Martemyanov et al., 2003b).
During the last decade, the physiological functions of RGS proteins in controlling a multitude of cellular reactions have been extensively studied in a number of animal models by eliminating RGS protein function (Chen et al., 2000; Rahman et al., 2003; Martin-McCaffrey et al., 2004; Sun et al., 2005; Cho et al., 2008; Cifelli et al., 2008; Xie et al., 2008, 2010; Posokhova et al., 2010). Phenotypic analysis varies, depending on the expression profile of the targeted RGS protein, and allows elucidation of the contributions of RGS proteins to the regulation of cellular processes and specific G protein pathways. One of the earliest examples is RGS9-1, a short splice isoform of the RGS9 gene exclusively expressed in photoreceptors of the retina (Martemyanov and Arshavsky, 2009). In the absence of RGS9-1, inactivation of the G protein transducin was shown to be severely delayed, causing dramatic delays in the deactivation of cellular responses to light (Chen et al., 2000). Another example is RGS2, a small, relatively ubiquitous RGS protein that shows high selectivity for Gαq subunits (Heximer, 2004). Given the importance of Gαq-coupled GPCRs in the regulation of smooth muscle tone, an RGS2 knockout model has been extensively evaluated in vascular systems and linked to the development of hypertension (Heximer et al., 2003).
An alternative strategy to investigate the physiological functions of endogenous RGS proteins is to create strains of mice carrying RGS-insensitive (RGSi) G proteins (Lan et al., 1998; Jeong and Ikeda, 2000; Huang et al., 2006; Talbot et al., 2010). This approach is based on the introduction of a point mutation in the Gα region that mediates RGS binding, rendering them insensitive to the GAP actions of RGS proteins without affecting their interactions with GPCRs and effectors (DiBello et al., 1998; Lan et al., 1998). Genomic knock-in of RGSi G184S Gαi2 resulted in severe dysfunctions in multiple organ systems, including the heart and myeloid and central nervous systems (Huang et al., 2006; Talbot et al., 2010).
RGS9-2, a Key Striatal RGS Protein with Effects on Dopamine and Opioid Signaling
Perhaps one of the most studied RGS proteins from the perspective of striatal function is the long splice isoform of RGS9 (RGS9-2). It first attracted attention due to its selective enrichment in the striatum (Gold et al., 1997; Thomas et al., 1998; Zhang et al., 1999). This was followed by extensive behavioral studies in mice lacking RGS9 that firmly established RGS9-2 as the key player in many critical aspects of striatal function. Specifically, RGS9 knockout mice display increased sensitivity to the reward and motor stimulatory properties of addictive drugs, including cocaine and amphetamine (Rahman et al., 2003; Zachariou et al., 2003). Elimination of RGS9 exaggerates physical dependence on morphine; mice lacking RGS9 showed heightened antagonist-precipitated withdrawal following chronic morphine administration (Zachariou et al., 2003). Furthermore, knockout of RGS9 delayed the development of analgesic tolerance to morphine (Zachariou et al., 2003), suggesting that RGS9-2 also controls long-term adaptation responses that adjust GPCR responsiveness following persistent stimulation and may contribute to the molecular changes underlying addiction. The changes in adaptive responses to drug administration in this model do not seem to be limited to morphine. RGS9 knockouts also display enhanced sensitization to repeated cocaine injections (Rahman et al., 2003). In contrast, overexpression of RGS9 in the striatum has the opposite effect and blunts responsiveness to cocaine (Rahman et al., 2003).
Consistent with the importance of the striatum in motor control, the role of RGS9 in this process was also examined. RGS9-2 elimination was found to be detrimental to the coordination of gross movements and led to delayed acquisition of motor learning in the rotarod test (Blundell et al., 2008; Anderson et al., 2010). RGS9-2 also appears to play a critical role in controlling the development of dyskinesia, a disorder in which patients involuntarily perform complex movements and which is often triggered by chronic treatment with drugs targeting dopamine signaling pathways (Rascol and Fabre, 2001; Jenner, 2008). RGS9 knockouts develop abnormal involuntary movements in response to chronic treatment with either antipsychotics (Kovoor et al., 2005) or L-DOPA in 6-OHDA model of Parkinson’s disease (Gold et al., 2007) much more rapidly than wild type animals. In contrast, virus-mediated overexpression of RGS9-2 in the striatum of 6-OHDA-treated rats and monkeys reduced the symptoms of L-DOPA-induced dyskinesia (Gold et al., 2007). Because drug-induced dyskinesia is a common debilitating side effect associated with many current anti-Parkinson’s and antipsychotic therapies, RGS9-2 is an attractive candidate for the development of pharmaceuticals aimed at ameliorating this condition.
Observations from mouse models are consistent with the involvement of RGS9-2 in regulating motor and reward behaviors. These processes are controlled by dopamine and opioid neurotransmitter systems (Hyman et al., 2006; Arbuthnott and Wickens, 2007; Koob and Le Moal, 2008). Several groups have investigated the role of RGS9-2 in regulating responses mediated by dopamine and opioid receptors at the molecular and cellular levels. Knockdown of RGS9-2 in vivo enhances the potency and duration of antinociception mediated by μ-opioid, but not the other opioid receptors (Sanchez-Blazquez et al., 2003). Similarly, RGS9 knockout mice develop prominent involuntary movements in response to D2R- but not D1R-selective agonists (Kovoor et al., 2005). Further supporting the involvement of RGS9-2 in D2R and μ-opioid actions, agonist-induced internalization of these receptors is strongly inhibited by co-expression with RGS9-2 in transfected cells (Psifogeorgou et al., 2007; Celver et al., 2010). This regulation appears to be specific to RGS9-2 as RGS4 does not have any appreciable effects on the internalization of D2R receptors (Celver et al., 2010). This selectivity could be explained by the formation of a macromolecular complex between RGS9-2 and the receptors it regulates. Indeed, RGS9-2 coprecipitates with μ-opioid from striatal lysates (Garzon et al., 2005a; Charlton et al., 2008) and is recruited to the plasma membrane by co-expression with D2R in transfected cells (Kovoor et al., 2005).
These findings imply that RGS9-2 is a negative regulator that acts downstream of the D2R and μ-opioid in striatal neurons. Both of these GPCRs are coupled to the inhibitory Gαi/o G proteins. Consistent with this notion, biochemical studies confirm that RGS9-2 is a potent and selective GAP, acting primarily on Gαo and, to a lesser extent, Gαi (Hooks et al., 2003; Martemyanov et al., 2003a). In this context, it is worth noting that several other Gαi/o-coupled GPCRs are expressed in striatal neurons and play critical roles in striatal functions. It is possible that RGS9-2 is also involved in regulating the responses of other Gαi/o GPCRs in this region, acting as a general G protein signaling inhibitor. However, the specificity of RGS9-2 action in the striatum is virtually unexplored and will need to be addressed in future studies.
The effectors regulated by RGS9-2 downstream from striatal GPCRs have not been fully identified. Existing evidence points to the involvement of RGS9-2 in the regulation of at least two ion channels in the striatum: Cav2.2 and NMDAR. The introduction of RGS9-2 into striatal cholinergic neurons reduced D2R receptor-mediated modulation of Cav2.2 channels (Cabrera-Vera et al., 2004). In striatal MSNs, knockout of RGS9 resulted in augmentation of D2R-mediated suppression of NMDA currents (Kovoor et al., 2005). Interestingly, RGS9-2 can physically bind to NMDAR by association with the adaptor protein α-actin-2 to regulate Ca2+-dependent NMDAR inactivation (Bouhamdan et al., 2006). Because both μ-opioid and D2R are known to control a variety of effector systems, it is possible that the role of RGS9-2 is not limited to the regulation of Cav2.2 and NMDAR channels. Indeed, RGS9-2 expression in transfected cells inhibits ERK phosphorylation in response to μ-opioid activation (Psifogeorgou et al., 2007). However, the full range of effector systems regulated by RGS9-2 in striatal neurons remains to be established.
The regulatory influence exerted by RGS9-2 in striatal neurons adjusts in response to changes in striatal signaling. Intriguingly, the expression of RGS9-2 changes in response to activation of signaling pathways that are normally regulated by RGS9-2. As discussed above, RGS9-2 regulates the extent of the effects produced by cocaine and morphine. On the other hand, administration of both cocaine and morphine leads to changes in RGS9-2 levels in the striatum. Acute morphine treatment increases, whereas chronic exposure decreases, the RGS9-2 protein content in the mouse ventral striatum (Zachariou et al., 2003; Psifogeorgou et al., 2007). Chronic cocaine exposure increases RGS9-2 levels in the same region (Rahman et al., 2003). RGS9-2 levels are also altered in response to several other stimuli and some neuropathological conditions. For example, repeated estradiol administration reduces both RGS9-2 mRNA and protein levels in the shell of the nucleus accumbens (Silverman and Koenig, 2007). RGS9-2 mRNA levels were significantly decreased in the striatum of a rat schizophrenia model and in the brains of schizophrenia patients (Seeman et al., 2007). RGS9-2 protein levels were elevated in the striata of Parkinson’s disease patients (Tekumalla et al., 2001). Finally, ischemia and neuronal depolarization modeled in acutely cultured slices also result in rapid downregulation of RGS9-2 (Anderson et al., 2009a). Changes in RGS9-2 levels in response to RGS9-2-regulated pathways may provide a mechanism for adaptive physiological feedback, where the extent of regulation is adjusted based on the volume of signaling.
At the molecular level, the regulation of RGS9 function is achieved by controlling its association with auxiliary proteins. RGS9-2 contains multiple domains (Anderson et al., 2009b; Martemyanov and Arshavsky, 2009): the N-terminal DEP (Disheveled, Egl-10, Pleckstrin) and DHEX (DEP helical extension) domains are followed by the GGL (G protein γ-like) and C-terminal RGS catalytic domains. The GGL domain binds to the type 5 Gβ subunit (Gβ5). The DEP/DHEX module mediates the interaction of R7 RGS with the anchor protein R7BP (R7 family binding protein). Both Gβ5 and R7BP simultaneously interact with RGS9-2 and are considered bona fide subunits within the complex. The knockout of Gβ5 in mice results in severe downregulation of RGS9-2 in the striatum (Chen et al., 2003). Similarly, levels of striatal RGS9-2 are dramatically reduced upon R7BP elimination (Anderson et al., 2007b). Conversely, R7BP overexpression in the striatum leads to the upregulation of RGS9-2 (Anderson et al., 2007a). In addition to ensuring proteolytic stability of the RGS9-2 complex, R7BP also controls the localization of RGS9-2 in the plasma membrane and targets the postsynaptic dendrites of striatal neurons (Anderson et al., 2007a).
Consistent with the roles of RGS9-2 in striatal functions and R7BP in maintaining a high expression level of RGS9-2, elimination of R7BP in mice results in a marked elevation in the sensitivity to the motor-stimulation actions of opioids and deficits in motor coordination and learning that are similar to those reported for RGS9-2 knockouts (Anderson et al., 2010). Interestingly, the sensitivity of dopamine receptors to stimulation remained unchanged in R7BP knockouts. These mice showed normal behavioral responses to cocaine, suggesting the existence of selective compensatory mechanisms (Anderson et al., 2010) that may involve other RGS proteins binding to R7BP. The behavioral consequences and effects on striatal signaling due to the loss of Gβ5 are largely unknown. Gβ5 knockout in mice alters the timing of GABA(B)-mediated synaptic transmission in CA1 hippocampal synapses (Xie et al., 2010), and similar deficits may occur in striatal neurons.
Other RGS Proteins in the Striatum
While severely affecting the levels of RGS9-2, the elimination of R7BP does not reproduce the behavior of RGS9-2 knockouts, raising the possibility that other RGS proteins in the striatum can bind to R7BP and be involved in controlling striatal signaling in parallel with RGS9-2. Indeed, both R7BP and Gβ5 have been shown to bind to RGS7. RGS7 belongs to the same R7 RGS family as RGS9-2 and shares the same domain organization (Anderson et al., 2009a, 2010). Although critical for RGS9-2 stability, R7BP is not necessary for RGS7 expression, as RGS7 protein levels are unchanged in R7BP knockout mice (Anderson et al., 2007a, 2009a). Moreover, RGS9-2 couples to R7BP with an affinity that is an order of magnitude greater than that of RGS7, resulting in a higher prevalence of RGS9-2/R7BP complexes when compared to RGS7/R7BP, despite equal concentrations of both RGS9-2 and RGS7 in striatal neurons (Anderson et al., 2009a). Elimination of RGS9-2 changes this balance and results in marked upregulation of R7BP/RGS7 complexes and their recruitment to postsynaptic densities (Anderson et al., 2009a). Therefore, the higher sensitivity to cocaine observed in RGS9 knockout mice might involve the alteration of RGS7 function, concurrent with the alteration of signaling directly controlled by RGS9-2. Indeed, viral knockdown of RGS7 in the striatum enhances behavioral sensitivity to cocaine (Anderson et al., 2010).
Several other RGS family members, including RGS2, RGS4, RGS10, and RGS17, are expressed in the striatum and play roles in signal processing in this region. The interest in these members is primarily driven by the observation that their expression levels change in response to different stimuli, most prominently psychostimulants, suggesting that they are involved in neuronal plasticity in this region. Specifically, RGS2 is rapidly induced in the striatum in response to maximal electroconvulsive seizure stimulation that evokes neuronal plasticity (Ingi et al., 1998). RGS2 is also upregulated upon administration of amphetamine (Burchett et al., 1998, 1999; Ingi et al., 1998; Robinet et al., 2001). Amphetamine-induced upregulation of RGS2 can be mimicked by D1 agonist administration and blocked by the D1 receptor antagonist, SCH 23390 (Taymans et al., 2003). In contrast, a D2 antagonist, haloperidol, upregulates RGS2 levels, whereas a D2 agonist, quinpirole, inhibits its expression (Ingi et al., 1998; Burchett et al., 1999; Taymans et al., 2003). Thus, D1 and D2 dopamine receptors play an antagonistic role in modulating the abundance of RGS2. RGS4 is another protein that belongs to the same R4 subfamily and shows prominent signal-dependent regulation of expression in the striatum. Its levels are generally downregulated by the excess dopamine produced by amphetamine (Gonzalez-Nicolini and McGinty, 2002; Schwendt et al., 2006) or cocaine (Zhang et al., 2005a; Schwendt et al., 2007) and upregulated when dopamine is depleted (e.g., in a Parkinson’s model; Geurts et al., 2002, 2003; Taymans et al., 2003; Zhang et al., 2005b; Ding et al., 2006). Interestingly, modulation of RGS4 expression levels has been linked to behavioral sensitization in response to repeated psychostimulant administration (Schwendt and McGinty, 2007) and upregulation of acetylcholine release with Parkinson’s due to decreased autoinhibition of the striatal cholinergic interneurons (Ding et al., 2006). The molecular details of the RGS2 and RGS4 actions are beginning to emerge. Both RGS proteins are potent negative modulators of Gαq signaling and affect signaling downstream from mGluR5 receptors, influencing synaptic plasticity (Schwendt and McGinty, 2007). In addition, RGS4 and, to a lesser extent, RGS2 are capable of controlling G protein signaling downstream from Gαi/o-coupled receptors. By this action, RGS4 serves as an inhibitor of M4 autoreceptors in striatal cholinergic neurons and μ-opioid receptors in the nucleus accumbens (Ding et al., 2006; Han et al., 2010). RGS2 was also shown to modulate synaptic vesicle release by controlling Gαi/o-mediated presynaptic Ca2+ channel inhibition (Han et al., 2006). RGS2 has additional GAP-independent effects that directly inhibit AC activity (Sinnarajah et al., 2001). However, the role of this striatal signaling mechanism remains unexplored.
Among other striatal RGS proteins, RGS17 (Stanwood et al., 2006) is upregulated in the nucleus accumbens by ontogenetic treatment with the D2R agonist, quinpirole (Maple et al., 2007), and has been shown to regulate three different neurotransmitter systems, including muscarinic M2, μ-opioid, and dopamine D2 receptors (Mao et al., 2004; Garzon et al., 2005b). Finally, RGS10 is enriched in microglia where it regulates the expression of inflammation-related genes (Lee et al., 2008). By regulating the survival of dopaminergic neurons in the substantia nigra, RGS10 plays a critical role in setting the dopaminergic tone in the striatum, the central target nucleus innervated by dopaminergic neurons (Lee et al., 2008).
Novel Proteins Influencing G Protein Cycle and Their Emerging Roles in the Striatum
Regulator of G protein signaling proteins could be viewed as general inhibitors of G protein signaling because they negatively regulate both Gα and Gβγ subunit signaling. In recent years, there has been an explosion of research on protein factors that affect G protein signaling at the same universal focal points as RGS proteins but exert differential effects on the activation of G protein subunits. Overall, these proteins can be separated into two groups. The first group is represented by molecules with guanine dissociation inhibitor (GDI) activity that stabilize Gα in the inactive GDP-bound form, preventing spontaneous and GPCR-dependent activation (Ma et al., 2003; Willard et al., 2004). At the same time, binding of GDI proteins to Gα–GDP inhibits reassociation with βγ, promoting βγ-selective signaling. All known GDI proteins contain a G protein regulatory (GPR, also known as GoLoco) domain that specifically binds and stabilizes Gα–GDP (Kimple et al., 2002; Natochin et al., 2002). Among several GDI proteins, AGS3 is known to have effects on striatal signaling by regulating the activation and deactivation of the Gαi protein and associated βγ subunits (Takesono et al., 1999; De Vries et al., 2000a). AGS3 levels are upregulated during withdrawal from chronic cocaine (Bowers et al., 2003, 2004) and morphine (Fan et al., 2009) administration. In accordance with these observations, knockdown of AGS3 prevents cAMP superactivation during morphine withdrawal in the rat nucleus accumbens neurons (Peterson et al., 2000; Fan et al., 2009). Finally, introduction of AGS3 GPR motifs in vivo prevent Gαi signaling and promote drug seeking and locomotor sensitization (Bowers et al., 2004).
The second group of G protein regulators with emerging striatal functions is represented by proteins that catalyze GPCR-independent activation of G protein α subunits. These proteins act as a GEF for the Gα subunit by interacting with GDP-bound α subunits in the absence of βγ. They were initially discovered in mutant screens and suppression assays in C. elegans and termed RIC (resistant to inhibitors of cholinesterase; Miller et al., 1996; Hajdu-Cronin et al., 1999). Mammalian homologs of Ric-8, termed Ric-8A and Ric-8B, were found through a yeast two-hybrid screen with Gαo as bait (Tall et al., 2003) and shown to be a powerful GEF for the Gα subunits. Although the role of Ric-8A in the striatum has not been addressed specifically, initial evidence indicates that this protein serves as a modulator of both AGS3 (Thomas et al., 2008) and AC5 (Wang et al., 2007), suggesting its involvement in key steps of striatal signaling.
In conclusion, emerging picture indicates that numerous G protein pathways in the striatum are controlled by the host of the regulatory proteins, that affect the dynamics of signaling in the region and thus play critical roles in shaping behavioral responses mediated by the striatal neurons.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrams, P., Andersson, K. E., Buccafusco, J. J., Chapple, C., de Groat, W. C., Fryer, A. D., Kay, G., Laties, A., Nathanson, N. M., Pasricha, P. J., and Wein, A. J. (2006). Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 148, 565–578.
Akins, P. T., Surmeier, D. J., and Kitai, S. T. (1990). Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature 344, 240–242.
Ambrosio, A. F., Malva, J. O., Carvalho, A. P., and Carvalho, C. M. (1996). Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci. Lett. 220, 163–166.
Anderson, G. R., Cao, Y., Davidson, S., Truong, H. V., Pravetoni, M., Thomas, M. J., Wickman, K., Giesler, G. J. Jr., and Martemyanov, K. A. (2010). R7BP complexes with RGS9-2 and RGS7 in the striatum differentially control motor learning and locomotor responses to cocaine. Neuropsychopharmacology 35, 1040–1050.
Anderson, G. R., Lujan, R., and Martemyanov, K. A. (2009a). Changes in striatal signaling induce remodeling of RGS complexes containing Gbeta5 and R7BP subunits. Mol. Cell. Biol. 29, 3033–3044.
Anderson, G. R., Posokhova, E., and Martemyanov, K. A. (2009b). The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem. Biophys. 54, 33–46.
Anderson, G. R., Lujan, R., Semenov, A., Pravetoni, M., Posokhova, E. N., Song, J. H., Uversky, V., Chen, C. K., Wickman, K., and Martemyanov, K. A. (2007a). Expression and localization of RGS9-2/G 5/R7BP complex in vivo is set by dynamic control of its constitutive degradation by cellular cysteine proteases. J. Neurosci. 27, 14117–14127.
Anderson, G. R., Semenov, A., Song, J. H., and Martemyanov, K. A. (2007b). The membrane anchor R7BP controls the proteolytic stability of the striatal specific RGS protein, RGS9-2. J. Biol. Chem. 282, 4772–4781.
Augood, S. J., and Emson, P. C. (1994). Adenosine A2a receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Brain Res. Mol. Brain Res. 22, 204–210.
Azmitia, E. C., and Segal, M. (1978). An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641–667.
Balcells-Olivero, M., and Vezina, P. (1997). Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology (Berl.) 131, 230–238.
Barnes, N. M., and Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152.
Basavarajappa, B. S. (2007). Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr. Neuropharmacol. 5, 81–97.
Bernard, V., Normand, E., and Bloch, B. (1992). Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J. Neurosci. 12, 3591–3600.
Biddlecome, G. H., Berstein, G., and Ross, E. M. (1996). Regulation of phospholipase C-beta1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J. Biol. Chem. 271, 7999–8007.
Bisogno, T., Ligresti, A., and Di Marzo, V. (2005). The endocannabinoid signalling system: biochemical aspects. Pharmacol. Biochem. Behav. 81, 224–238.
Blundell, J., Hoang, C. V., Potts, B., Gold, S. J., and Powell, C. M. (2008). Motor coordination deficits in mice lacking RGS9. Brain Res. 1190, 78–85.
Bolam, J. P., Hanley, J. J., Booth, P. A., and Bevan, M. D. (2000). Synaptic organisation of the basal ganglia. J. Anat. 196(Pt 4), 527–542.
Bouhamdan, M., Yan, H. D., Yan, X. H., Bannon, M. J., and Andrade, R. (2006). Brain-specific regulator of G-protein signaling 9-2 selectively interacts with alpha-actinin-2 to regulate calcium-dependent inactivation of NMDA receptors. J. Neurosci. 26, 2522–2530.
Bowers, M. S., Lake, R. W., McFarland, K., Peterson, Y. K., Lanier, S. M., Lapish, C. C., and Kalivas, P. W. (2003). AGS3: a G-Protein regulator of addiction-associated behaviors. Ann. N. Y. Acad. Sci. 1003, 356–357.
Bowers, M. S., McFarland, K., Lake, R. W., Peterson, Y. K., Lapish, C. C., Gregory, M. L., Lanier, S. M., and Kalivas, P. W. (2004). Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42, 269–281.
Bradley, S. R., Standaert, D. G., Rhodes, K. J., Rees, H. D., Testa, C. M., Levey, A. I., and Conn, P. J. (1999). Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J. Comp. Neurol. 407, 33–46.
Burchett, S. A., Bannon, M. J., and Granneman, J. G. (1999). RGS mRNA expression in rat striatum: modulation by dopamine receptors and effects of repeated amphetamine administration. J. Neurochem. 72, 1529–1533.
Burchett, S. A., Volk, M. L., Bannon, M. J., and Granneman, J. G. (1998). Regulators of G protein signaling: rapid changes in mRNA abundance in response to amphetamine. J. Neurochem. 70, 2216–2219.
Cabrera-Vera, T. M., Hernandez, S., Earls, L. R., Medkova, M., Sundgren-Andersson, A. K., Surmeier, D. J., and Hamm, H. E. (2004). RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc. Natl. Acad. Sci. U.S.A. 101, 16339–16344.
Calabresi, P., Centonze, D., Gubellini, P., Pisani, A., and Bernardi, G. (1998a). Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur. J. Neurosci. 10, 2887–2895.
Calabresi, P., Centonze, D., Pisani, A., Sancesario, G., North, R. A., and Bernardi, G. (1998b). Muscarinic IPSPs in rat striatal cholinergic interneurones. J. Physiol. (Lond.) 510(Pt 2), 421–427.
Calabresi, P., Centonze, D., Gubellini, P., Pisani, A., and Bernardi, G. (2000). Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 23, 120–126.
Cartmell, J., and Schoepp, D. D. (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 75, 889–907.
Caulfield, M. P., and Birdsall, N. J. (1998). International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 50, 279–290.
Celver, J., Sharma, M., and Kovoor, A. (2010). RGS9-2 mediates specific inhibition of agonist-induced internalization of D2-dopamine receptors. J. Neurochem. 114, 739–749.
Cepeda, C., Wu, N., Andre, V. M., Cummings, D. M., and Levine, M. S. (2007). The corticostriatal pathway in Huntington’s disease. Prog. Neurobiol. 81, 253–271.
Charlton, J. J., Allen, P. B., Psifogeorgou, K., Chakravarty, S., Gomes, I., Neve, R. L., Devi, L. A., Greengard, P., Nestler, E. J., and Zachariou, V. (2008). Multiple actions of spinophilin regulate mu opioid receptor function. Neuron 58, 238–247.
Chase, T. N., and Oh, J. D. (2000). Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 23, S86–S91.
Chen, C., and Lin, S. C. (1998). The core domain of RGS16 retains G-protein binding and GAP activity in vitro, but is not functional in vivo. FEBS Lett. 422, 359–362.
Chen, C. K., Burns, M. E., He, W., Wensel, T. G., Baylor, D. A., and Simon, M. I. (2000). Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature 403, 557–560.
Chen, C. K., Eversole-Cire, P., Zhang, H. K., Mancino, V., Chen, Y. J., He, W., Wensel, T. G., and Simon, M. I. (2003). Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc. Natl. Acad. Sci. U.S.A. 100, 6604–6609.
Cho, H., Park, C., Hwang, I. Y., Han, S. B., Schimel, D., Despres, D., and Kehrl, J. H. (2008). Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol. Cell. Biol. 28, 2590–2597.
Cicchetti, F., Prensa, L., Wu, Y., and Parent, A. (2000). Chemical anatomy of striatal interneurons in normal individuals and in patients with Huntington’s disease. Brain Res. Brain Res. Rev. 34, 80–101.
Cifelli, C., Rose, R. A., Zhang, H., Voigtlaender-Bolz, J., Bolz, S. S., Backx, P. H., and Heximer, S. P. (2008). RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ. Res. 103, 527–535.
Conn, P. J., and Pin, J. P. (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237.
Corti, C., Aldegheri, L., Somogyi, P., and Ferraguti, F. (2002). Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110, 403–420.
Cunha, R. A. (2001). Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 38, 107–125.
David, H. N., Ansseau, M., and Abraini, J. H. (2005). Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res. Brain Res. Rev. 50, 336–360.
De Klippel, N., Sarre, S., Ebinger, G., and Michotte, Y. (1993). Effect of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism: an in vivo microdialysis study comparing normal and 6-hydroxydopamine-lesioned rats. Brain Res. 630, 57–64.
De Vries, L., Fischer, T., Tronchere, H., Brothers, G. M., Strockbine, B., Siderovski, D. P., and Farquhar, M. G. (2000a). Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc. Natl. Acad. Sci. U.S.A. 97, 14364–14369.
De Vries, L., Zheng, B., Fischer, T., Elenko, E., and Farquhar, M. G. (2000b). The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40, 235–271.
De Vries, L., and Gist Farquhar, M. (1999). RGS proteins: more than just GAPs for heterotrimeric G proteins. Trends Cell Biol. 9, 138–144.
Dhawan, B. N., Cesselin, F., Raghubir, R., Reisine, T., Bradley, P. B., Portoghese, P. S., and Hamon, M. (1996). International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol. Rev. 48, 567–592.
Di Matteo, V., Pierucci, M., Esposito, E., Crescimanno, G., Benigno, A., and Di Giovanni, G. (2008). Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson’s disease and other motor disorders. Prog. Brain Res. 172, 423–463.
Diaz-Otanez, C. S., Capriles, N. R., and Cancela, L. M. (1997). D1 and D2 dopamine and opiate receptors are involved in the restraint stress-induced sensitization to the psychostimulant effects of amphetamine. Pharmacol. Biochem. Behav. 58, 9–14.
DiBello, P. R., Garrison, T. R., Apanovitch, D. M., Hoffman, G., Shuey, D. J., Mason, K., Cockett, M. I., and Dohlman, H. G. (1998). Selective uncoupling of RGS action by a single point mutation in the G protein alpha-subunit. J. Biol. Chem. 273, 5780–5784.
Ding, J., Guzman, J. N., Tkatch, T., Chen, S., Goldberg, J. A., Ebert, P. J., Levitt, P., Wilson, C. J., Hamm, H. E., and Surmeier, D. J. (2006). RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 9, 832–842.
Dunwiddie, T. V., and Masino, S. A. (2001). The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 24, 31–55.
Dupre, D. J., Robitaille, M., Rebois, R. V., and Hebert, T. E. (2009). The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 49, 31–56.
El-Ghundi, M., O’Dowd, B. F., and George, S. R. (2007). Insights into the role of dopamine receptor systems in learning and memory. Rev. Neurosci. 18, 37–66.
Everitt, B. J., and Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489.
Falk, T., Zhang, S., Erbe, E. L., and Sherman, S. J. (2006). Neurochemical and electrophysiological characteristics of rat striatal neurons in primary culture. J. Comp. Neurol. 494, 275–289.
Fan, P., Jiang, Z., Diamond, I., and Yao, L. (2009). Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol. Pharmacol. 76, 526–533.
Felder, C. C., and Glass, M. (1998). Cannabinoid receptors and their endogenous agonists. Annu. Rev. Pharmacol. Toxicol. 38, 179–200.
Fernandez-Ruiz, J., Lastres-Becker, I., Cabranes, A., Gonzalez, S., and Ramos, J. A. (2002). Endocannabinoids and basal ganglia functionality. Prostaglandins Leukot. Essent. Fatty Acids 66, 257–267.
Ferre, S., Karcz-Kubicha, M., Hope, B. T., Popoli, P., Burgueno, J., Gutierrez, M. A., Casado, V., Fuxe, K., Goldberg, S. R., Lluis, C., Franco, R., and Ciruela, F. (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc. Natl. Acad. Sci. U.S.A. 99, 11940–11945.
Fink, J. S., Weaver, D. R., Rivkees, S. A., Peterfreund, R. A., Pollack, A. E., Adler, E. M., and Reppert, S. M. (1992). Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 14, 186–195.
Fredholm, B. B., AP, I. J., Jacobson, K. A., Klotz, K. N., and Linden, J. (2001). International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552.
Fuxe, K., Marcellino, D., Genedani, S., and Agnati, L. (2007). Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov. Disord. 22, 1990–2017.
Gabel, L. A., and Nisenbaum, E. S. (1999). Muscarinic receptors differentially modulate the persistent potassium current in striatal spiny projection neurons. J. Neurophysiol. 81, 1418–1423.
Garzon, J., Rodriguez-Munoz, M., Lopez-Fando, A., and Sanchez-Blazquez, P. (2005a). Activation of mu-opioid receptors transfers control of Galpha subunits to the regulator of G-protein signaling RGS9-2: role in receptor desensitization. J. Biol. Chem. 280, 8951–8960.
Garzon, J., Rodriguez-Munoz, M., Lopez-Fando, A., and Sanchez-Blazquez, P. (2005b). The RGSZ2 protein exists in a complex with mu-opioid receptors and regulates the desensitizing capacity of Gz proteins. Neuropsychopharmacology 30, 1632–1648.
Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., Monsma, F. J. Jr., and Sibley, D. R. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432.
Geurts, M., Hermans, E., and Maloteaux, J. M. (2002). Opposite modulation of regulators of G protein signalling-2 RGS2 and RGS4 expression by dopamine receptors in the rat striatum. Neurosci. Lett. 333, 146–150.
Geurts, M., Maloteaux, J. M., and Hermans, E. (2003). Altered expression of regulators of G-protein signaling (RGS) mRNAs in the striatum of rats undergoing dopamine depletion. Biochem. Pharmacol. 66, 1163–1170.
Gilman, A. G. (1987). G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649.
Glickstein, S. B., and Schmauss, C. (2001). Dopamine receptor functions: lessons from knockout mice [corrected]. Pharmacol. Ther. 91, 63–83.
Gold, S. J., Hoang, C. V., Potts, B. W., Porras, G., Pioli, E., Kim, K. W., Nadjar, A., Qin, C., LaHoste, G. J., Li, Q., Bioulac, B. H., Waugh, J. L., Gurevich, E., Neve, R. L., and Bezard, E. (2007). RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J. Neurosci. 27, 14338–14348.
Gold, S. J., Ni, Y. G., Dohlman, H. G., and Nestler, E. J. (1997). Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J. Neurosci. 17, 8024–8037.
Gonzalez-Nicolini, M. V., Berglind, W., Cole, K. S., Keogh, C. L., and McGinty, J. F. (2003). Local mu and delta opioid receptors regulate amphetamine-induced behavior and neuropeptide mRNA in the striatum. Neuroscience 121, 387–398.
Gonzalez-Nicolini, V., and McGinty, J. F. (2002). Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Res. Gene Expr. Patterns 1, 193–198.
Graveland, G. A., and DiFiglia, M. (1985). The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 327, 307–311.
Gray, A. M., Rawls, S. M., Shippenberg, T. S., and McGinty, J. F. (1999). The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J. Neurochem. 73, 1066–1074.
Graybiel, A. M. (1990). Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 13, 244–254.
Gubellini, P., Saulle, E., Centonze, D., Bonsi, P., Pisani, A., Bernardi, G., Conquet, F., and Calabresi, P. (2001). Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology 40, 839–846.
Gubellini, P., Saulle, E., Centonze, D., Costa, C., Tropepi, D., Bernardi, G., Conquet, F., and Calabresi, P. (2003). Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology 44, 8–16.
Hajdu-Cronin, Y. M., Chen, W. J., Patikoglou, G., Koelle, M. R., and Sternberg, P. W. (1999). Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev. 13, 1780–1793.
Han, J., Mark, M. D., Li, X., Xie, M., Waka, S., Rettig, J., and Herlitze, S. (2006). RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. Neuron 51, 575–586.
Han, M. H., Renthal, W., Ring, R. H., Rahman, Z., Psifogeorgou, K., Howland, D., Birnbaum, S., Young, K., Neve, R., Nestler, E. J., and Zachariou, V. (2010). Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol. Psychiatry 67, 761–769.
Hart, M. J., Jiang, X., Kozasa, T., Roscoe, W., Singer, W. D., Gilman, A. G., Sternweis, P. C., and Bollag, G. (1998). Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280, 2112–2114.
Hasbi, A., Fan, T., Alijaniaram, M., Nguyen, T., Perreault, M. L., O’Dowd, B. F., and George, S. R. (2009). Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U.S.A. 106, 21377–21382.
He, W., Lu, L., Zhang, X., El-Hodiri, H. M., Chen, C. K., Slep, K. C., Simon, M. I., Jamrich, M., and Wensel, T. G. (2000). Modules in the photoreceptor RGS9-1.GBETA 5L GTPase-accelerating protein complex control effector coupling, GTPase acceleration, protein folding, and stability. J. Biol. Chem. 275, 37093–37100.
Heidbreder, C., Goldberg, S. R., and Shippenberg, T. S. (1993). Inhibition of cocaine-induced sensitization by the delta-opioid receptor antagonist naltrindole. Eur. J. Pharmacol. 243, 123–127.
Heiman, M., Schaefer, A., Gong, S., Peterson, J. D., Day, M., Ramsey, K. E., Suarez-Farinas, M., Schwarz, C., Stephan, D. A., Surmeier, D. J., Greengard, P., and Heintz, N. (2008). A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748.
Hernandez-Lopez, S., Tkatch, T., Perez-Garci, E., Galarraga, E., Bargas, J., Hamm, H., and Surmeier, D. J. (2000). D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J. Neurosci. 20, 8987–8995.
Hersch, S. M., Gutekunst, C. A., Rees, H. D., Heilman, C. J., and Levey, A. I. (1994). Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 14, 3351–3363.
Heximer, S. P., Knutsen, R. H., Sun, X., Kaltenbronn, K. M., Rhee, M. H., Peng, N., Oliveira-dos-Santos, A., Penninger, J. M., Muslin, A. J., Steinberg, T. H., Wyss, J. M., Mecham, R. P., and Blumer, K. J. (2003). Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Invest. 111, 445–452.
Hollinger, S., and Hepler, J. R. (2002). Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 54, 527–559.
Hooks, S. B., Waldo, G. L., Corbitt, J., Bodor, E. T., Krumins, A. M., and Harden, T. K. (2003). RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J. Biol. Chem. 278, 10087–10093.
Howe, A. R., and Surmeier, D. J. (1995). Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J. Neurosci. 15, 458–469.
Huang, C. C., Lo, S. W., and Hsu, K. S. (2001). Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J. Physiol. (Lond.) 532, 731–748.
Huang, X., Fu, Y., Charbeneau, R. A., Saunders, T. L., Taylor, D. K., Hankenson, K. D., Russell, M. W., D’Alecy, L. G., and Neubig, R. R. (2006). Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol. Cell. Biol. 26, 6870–6879.
Hyman, S. E., Malenka, R. C., and Nestler, E. J. (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598.
Ingi, T., Krumins, A. M., Chidiac, P., Brothers, G. M., Chung, S., Snow, B. E., Barnes, C. A., Lanahan, A. A., Siderovski, D. P., Ross, E. M., Gilman, A. G., and Worley, P. F. (1998). Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 18, 7178–7188.
Ishii, M., and Kurachi, Y. (2006). Muscarinic acetylcholine receptors. Curr. Pharm. Des. 12, 3573–3581.
Iwamoto, T., Okumura, S., Iwatsubo, K., Kawabe, J., Ohtsu, K., Sakai, I., Hashimoto, Y., Izumitani, A., Sango, K., Ajiki, K., Toya, Y., Umemura, S., Goshima, Y., Arai, N., Vatner, S. F., and Ishikawa, Y. (2003). Motor dysfunction in type 5 adenylyl cyclase-null mice. J. Biol. Chem. 278, 16936–16940.
Jaber, M., Robinson, S. W., Missale, C., and Caron, M. G. (1996). Dopamine receptors and brain function. Neuropharmacology 35, 1503–1519.
Jabourian, M., Venance, L., Bourgoin, S., Ozon, S., Perez, S., Godeheu, G., Glowinski, J., and Kemel, M. L. (2005). Functional mu opioid receptors are expressed in cholinergic interneurons of the rat dorsal striatum: territorial specificity and diurnal variation. Eur. J. Neurosci. 21, 3301–3309.
Jenner, P. (2008). Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 9, 665–677.
Jeong, S. W., and Ikeda, S. R. (2000). Endogenous regulator of G-protein signaling proteins modify N-type calcium channel modulation in rat sympathetic neurons. J. Neurosci. 20, 4489–4496.
Jiang, Z. G., and North, R. A. (1992). Pre- and postsynaptic inhibition by opioids in rat striatum. J. Neurosci. 12, 356–361.
Jong, Y. J., Kumar, V., and O’Malley, K. L. (2009). Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J. Biol. Chem. 284, 35827–35838.
Joseph, J. D., Wang, Y. M., Miles, P. R., Budygin, E. A., Picetti, R., Gainetdinov, R. R., Caron, M. G., and Wightman, R. M. (2002). Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience 112, 39–49.
Kelley, A. E., Bakshi, V. P., Haber, S. N., Steininger, T. L., Will, M. J., and Zhang, M. (2002). Opioid modulation of taste hedonics within the ventral striatum. Physiol. Behav. 76, 365–377.
Kim, K. S., Lee, K. W., Im, J. Y., Yoo, J. Y., Kim, S. W., Lee, J. K., Nestler, E. J., and Han, P. L. (2006). Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc. Natl. Acad. Sci. U.S.A. 103, 3908–3913.
Kimple, R. J., Kimple, M. E., Betts, L., Sondek, J., and Siderovski, D. P. (2002). Structural determinants for GoLoco-induced inhibition of nucleotide release by Galpha subunits. Nature 416, 878–881.
Koob, G. F., and Le Moal, M. (2008). Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53.
Koos, T., and Tepper, J. M. (2002). Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci. 22, 529–535.
Kosinski, C. M., Risso Bradley, S., Conn, P. J., Levey, A. I., Landwehrmeyer, G. B., Penney, J. B. Jr., Young, A. B., and Standaert, D. G. (1999). Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J. Comp. Neurol. 415, 266–284.
Kovoor, A., Seyffarth, P., Ebert, J., Barghshoon, S., Chen, C. K., Schwarz, S., Axelrod, J. D., Cheyette, B. N., Simon, M. I., Lester, H. A., and Schwarz, J. (2005). D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J. Neurosci. 25, 2157–2165.
Kozasa, T., Jiang, X., Hart, M. J., Sternweis, P. M., Singer, W. D., Gilman, A. G., Bollag, G., and Sternweis, P. C. (1998). p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280, 2109–2111.
Kreitzer, A. C., and Malenka, R. C. (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554.
Kruzich, P. J., Suchland, K. L., and Grandy, D. K. (2004). Dopamine D4 receptor-deficient mice, congenic on the C57BL/6J background, are hypersensitive to amphetamine. Synapse 53, 131–139.
Lan, K. L., Sarvazyan, N. A., Taussig, R., Mackenzie, R. G., DiBello, P. R., Dohlman, H. G., and Neubig, R. R. (1998). A point mutation in Galphao and Galphai1 blocks interaction with regulator of G protein signaling proteins. J. Biol. Chem. 273, 12794–12797.
Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin, J., Devon, K., Dewar, K., Doyle, M., FitzHugh, W., Funke, R., Gage, D., Harris, K., Heaford, A., Howland, J., Kann, L., Lehoczky, J., LeVine, R., McEwan, P., McKernan, K., Meldrim, J., Mesirov, J. P., Miranda, C., Morris, W., Naylor, J., Raymond, C., Rosetti, M., Santos, R., Sheridan, A., Sougnez, C., Stange-Thomann, N., Stojanovic, N., Subramanian, A., Wyman, D., Rogers, J., Sulston, J., Ainscough, R., Beck, S., Bentley, D., Burton, J., Clee, C., Carter, N., Coulson, A., Deadman, R., Deloukas, P., Dunham, A., Dunham, I., Durbin, R., French, L., Grafham, D., Gregory, S., Hubbard, T., Humphray, S., Hunt, A., Jones, M., Lloyd, C., McMurray, A., Matthews, L., Mercer, S., Milne, S., Mullikin, J. C., Mungall, A., Plumb, R., Ross, M., Shownkeen, R., Sims, S., Waterston, R. H., Wilson, R. K., Hillier, L. W., McPherson, J. D., Marra, M. A., Mardis, E. R., Fulton, L. A., Chinwalla, A. T., Pepin, K. H., Gish, W. R., Chissoe, S. L., Wendl, M. C., Delehaunty, K. D., Miner, T. L., Delehaunty, A., Kramer, J. B., Cook, L. L., Fulton, R. S., Johnson, D. L., Minx, P. J., Clifton, S. W., Hawkins, T., Branscomb, E., Predki, P., Richardson, P., Wenning, S., Slezak, T., Doggett, N., Cheng, J. F., Olsen, A., Lucas, S., Elkin, C., Uberbacher, E., Frazier, M., Gibbs, R. A., Muzny, D. M., Scherer, S. E., Bouck, J. B., Sodergren, E. J., Worley, K. C., Rives, C. M., Gorrell, J. H., Metzker, M. L., Naylor, S. L., Kucherlapati, R. S., Nelson, D. L., Weinstock, G. M., Sakaki, Y., Fujiyama, A., Hattori, M., Yada, T., Toyoda, A., Itoh, T., Kawagoe, C., Watanabe, H., Totoki, Y., Taylor, T., Weissenbach, J., Heilig, R., Saurin, W., Artiguenave, F., Brottier, P., Bruls, T., Pelletier, E., Robert, C., Wincker, P., Smith, D. R., Doucette-Stamm, L., Rubenfield, M., Weinstock, K., Lee, H. M., Dubois, J., Rosenthal, A., Platzer, M., Nyakatura, G., Taudien, S., Rump, A., Yang, H., Yu, J., Wang, J., Huang, G., Gu, J., Hood, L., Rowen, L., Madan, A., Qin, S., Davis, R. W., Federspiel, N. A., Abola, A. P., Proctor, M. J., Myers, R. M., Schmutz, J., Dickson, M., Grimwood, J., Cox, D. R., Olson, M. V., Kaul, R., Raymond, C., Shimizu, N., Kawasaki, K., Minoshima, S., Evans, G. A., Athanasiou, M., Schultz, R., Roe, B. A., Chen, F., Pan, H., Ramser, J., Lehrach, H., Reinhardt, R., McCombie, W. R., de la Bastide, M., Dedhia, N., Blöcker, H., Hornischer, K., Nordsiek, G., Agarwala, R., Aravind, L., Bailey, J. A., Bateman, A., Batzoglou, S., Birney, E., Bork, P., Brown, D. G., Burge, C. B., Cerutti, L., Chen, H. C., Church, D., Clamp, M., Copley, R. R., Doerks, T., Eddy, S. R., Eichler, E. E., Furey, T. S., Galagan, J., Gilbert, J. G., Harmon, C., Hayashizaki, Y., Haussler, D., Hermjakob, H., Hokamp, K., Jang, W., Johnson, L. S., Jones, T. A., Kasif, S., Kaspryzk, A., Kennedy, S., Kent, W. J., Kitts, P., Koonin, E. V., Korf, I., Kulp, D., Lancet, D., Lowe, T. M., McLysaght, A., Mikkelsen, T., Moran, J. V., Mulder, N., Pollara, V. J., Ponting, C. P., Schuler, G., Schultz, J., Slater, G., Smit, A. F., Stupka, E., Szustakowski, J., Thierry-Mieg, D., Thierry-Mieg, J., Wagner, L., Wallis, J., Wheeler, R., Williams, A., Wolf, Y. I., Wolfe, K. H., Yang, S. P., Yeh, R. F., Collins, F., Guyer, M. S., Peterson, J., Felsenfeld, A., Wetterstrand, K. A., Patrinos, A., Morgan, M. J., de Jong, P., Catanese, J. J., Osoegawa, K., Shizuya, H., Choi, S., Chen, Y. J., and International Human Genome Sequencing Consortium. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921.
Law, P. Y., Wong, Y. H., and Loh, H. H. (2000). Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 40, 389–430.
Lee, J. K., McCoy, M. K., Harms, A. S., Ruhn, K. A., Gold, S. J., and Tansey, M. G. (2008). Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J. Neurosci. 28, 8517–8528.
Lee, K. W., Hong, J. H., Choi, I. Y., Che, Y., Lee, J. K., Yang, S. D., Song, C. W., Kang, H. S., Lee, J. H., Noh, J. S., Shin, H. S., and Han, P. L. (2002). Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J. Neurosci. 22, 7931–7940.
Lee, S. P., So, C. H., Rashid, A. J., Varghese, G., Cheng, R., Lanca, A. J., O’Dowd, B. F., and George, S. R. (2004). Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678.
Lefkowitz, R. J., Rajagopal, K., and Whalen, E. J. (2006). New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell 24, 643–652.
Lefkowitz, R. J., and Shenoy, S. K. (2005). Transduction of receptor signals by beta-arrestins. Science 308, 512–517.
Levey, A. I., Kitt, C. A., Simonds, W. F., Price, D. L., and Brann, M. R. (1991). Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 11, 3218–3226.
Logue, S. F., Grauer, S. M., Paulsen, J., Graf, R., Taylor, N., Sung, M. A., Zhang, L., Hughes, Z., Pulito, V. L., Liu, F., Rosenzweig-Lipson, S., Brandon, N. J., Marquis, K. L., Bates, B., and Pausch, M. (2009). The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol. Cell. Neurosci. 42, 438–447.
Ma, H., Peterson, Y. K., Bernard, M. L., Lanier, S. M., and Graber, S. G. (2003). Influence of cytosolic AGS3 on receptor – G protein coupling. Biochemistry 42, 8085–8093.
Mao, H., Zhao, Q., Daigle, M., Ghahremani, M. H., Chidiac, P., and Albert, P. R. (2004). RGS17/RGSZ2, a novel regulator of Gi/o, Gz, and Gq signaling. J. Biol. Chem. 279, 26314–26322.
Maple, A. M., Perna, M. K., Parlaman, J. P., Stanwood, G. D., and Brown, R. W. (2007). Ontogenetic quinpirole treatment produces long-lasting decreases in the expression of Rgs9, but increases Rgs17 in the striatum, nucleus accumbens and frontal cortex. Eur. J. Neurosci. 26, 2532–2538.
Marazziti, D., Mandillo, S., Di Pietro, C., Golini, E., Matteoni, R., and Tocchini-Valentini, G. P. (2007). GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc. Natl. Acad. Sci. U.S.A. 104, 9846–9851.
Martemyanov, K. A., and Arshavsky, V. Y. (2002). Noncatalytic domains of RGS9-1.GBETA 5L play a decisive role in establishing its substrate specificity. J. Biol. Chem. 277, 32843–32848.
Martemyanov, K. A., and Arshavsky, V. Y. (2009). Chapter 7 biology and functions of the RGS9 isoforms. Prog. Mol. Biol. Transl. Sci. 86, 205–227.
Martemyanov, K. A., Hopp, J. A., and Arshavsky, V. Y. (2003a). Specificity of G protein-RGS protein recognition is regulated by affinity adapters. Neuron 38, 857–862.
Martemyanov, K. A., Lishko, P. V., Calero, N., Keresztes, G., Sokolov, M., Strissel, K. J., Leskov, I. B., Hopp, J. A., Kolesnikov, A. V., Chen, C. K., Lem, J., Heller, S., Burns, M. E., and Arshavsky, V. Y. (2003b). The DEP domain determines subcellular targeting of the GTPase activating protein RGS9 in vivo. J. Neurosci. 23, 10175–10181.
Martin-McCaffrey, L., Willard, F. S., Oliveira-dos-Santos, A. J., Natale, D. R., Snow, B. E., Kimple, R. J., Pajak, A., Watson, A. J., Dagnino, L., Penninger, J. M., Siderovski, D. P., and D’Souza, S. J. (2004). RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote. Dev. Cell 7, 763–769.
Matamales, M., Bertran-Gonzalez, J., Salomon, L., Degos, B., Deniau, J. M., Valjent, E., Herve, D., and Girault, J. A. (2009). Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS ONE 4, e4770. doi: 10.1371/journal.pone.0004770
McGeorge, A. J., and Faull, R. L. (1989). The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29, 503–537.
Miller, K. G., Alfonso, A., Nguyen, M., Crowell, J. A., Johnson, C. D., and Rand, J. B. (1996). A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. U.S.A. 93, 12593–12598.
Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998). Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225.
Miura, M., Saino-Saito, S., Masuda, M., Kobayashi, K., and Aosaki, T. (2007). Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J. Neurosci. 27, 9721–9728.
Mizushima, K., Miyamoto, Y., Tsukahara, F., Hirai, M., Sakaki, Y., and Ito, T. (2000). A novel G-protein-coupled receptor gene expressed in striatum. Genomics 69, 314–321.
Moukhles, H., Bosler, O., Bolam, J. P., Vallee, A., Umbriaco, D., Geffard, M., and Doucet, G. (1997). Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 76, 1159–1171.
Nakanishi, S., Nakajima, Y., Masu, M., Ueda, Y., Nakahara, K., Watanabe, D., Yamaguchi, S., Kawabata, S., and Okada, M. (1998). Glutamate receptors: brain function and signal transduction. Brain Res. Brain Res. Rev. 26, 230–235.
Natochin, M., Gasimov, K. G., and Artemyev, N. O. (2002). A GPR-protein interaction surface of Gi(alpha): implications for the mechanism of GDP-release inhibition. Biochemistry 41, 258–265.
Nestler, E. J., Alreja, M., and Aghajanian, G. K. (1999). Molecular control of locus coeruleus neurotransmission. Biol. Psychiatry 46, 1131–1139.
Nestler, E. J., and Carlezon, W. A. Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159.
Oldham, W. M., and Hamm, H. E. (2008). Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71.
Paquet, M., and Smith, Y. (2003). Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J. Neurosci. 23, 7659–7669.
Paulssen, R. H., Woodson, J., Liu, Z., and Ross, E. M. (1996). Carboxyl-terminal fragments of phospholipase C-beta1 with intrinsic Gq GTPase-activating protein (GAP) activity. J. Biol. Chem. 271, 26622–26629.
Perez, S., Tierney, A., Deniau, J. M., and Kemel, M. L. (2007). Tachykinin regulation of cholinergic transmission in the limbic/prefrontal territory of the rat dorsal striatum: implication of new neurokinine 1-sensitive receptor binding site and interaction with enkephalin/mu opioid receptor transmission. J. Neurochem. 103, 2153–2163.
Pertwee, R. G. (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 74, 129–180.
Peterson, Y. K., Bernard, M. L., Ma, H., Hazard, S. III, Graber, S. G., and Lanier, S. M. (2000). Stabilization of the GDP-bound conformation of Gialpha by a peptide derived from the G-protein regulatory motif of AGS3. J. Biol. Chem. 275, 33193–33196.
Picconi, B., Pisani, A., Barone, I., Bonsi, P., Centonze, D., Bernardi, G., and Calabresi, P. (2005). Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology 26, 779–783.
Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650.
Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884.
Pisani, A., Calabresi, P., Centonze, D., and Bernardi, G. (1997). Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br. J. Pharmacol. 120, 1007–1014.
Pisani, A., Gubellini, P., Bonsi, P., Conquet, F., Picconi, B., Centonze, D., Bernardi, G., and Calabresi, P. (2001). Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106, 579–587.
Posokhova, E., Wydeven, N., Allen, K. L., Wickman, K., and Martemyanov, K. A. (2010). RGS6/Gss5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ. Res. 107, 1350–1354.
Premont, R. T., and Gainetdinov, R. R. (2007). Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 69, 511–534.
Prezeau, L., Carrette, J., Helpap, B., Curry, K., Pin, J. P., and Bockaert, J. (1994). Pharmacological characterization of metabotropic glutamate receptors in several types of brain cells in primary cultures. Mol. Pharmacol. 45, 570–577.
Psifogeorgou, K., Papakosta, P., Russo, S. J., Neve, R. L., Kardassis, D., Gold, S. J., and Zachariou, V. (2007). RGS9-2 is a negative modulator of mu-opioid receptor function. J. Neurochem. 103, 617–625.
Rahman, Z., Schwarz, J., Gold, S. J., Zachariou, V., Wein, M. N., Choi, K. H., Kovoor, A., Chen, C. K., DiLeone, R. J., Schwarz, S. C., Selley, D. E., Sim-Selley, L. J., Barrot, M., Luedtke, R. R., Self, D., Neve, R. L., Lester, H. A., Simon, M. I., and Nestler, E. J. (2003). RGS9 modulates dopamine signaling in the basal ganglia. Neuron 38, 941–952.
Rascol, O., and Fabre, N. (2001). Dyskinesia: L-dopa-induced and tardive dyskinesia. Clin. Neuropharmacol. 24, 313–323.
Rawls, S. M., and McGinty, J. F. (1998). Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: an in vivo microdialysis study. J. Pharmacol. Exp. Ther. 286, 91–98.
Rawls, S. M., and McGinty, J. F. (2000). Delta opioid receptors regulate calcium-dependent, amphetamine-evoked glutamate levels in the rat striatum: an in vivo microdialysis study. Brain Res. 861, 296–304.
Ribeiro, J. A., Sebastiao, A. M., and de Mendonca, A. (2002). Adenosine receptors in the nervous system: pathophysiological implications. Prog. Neurobiol. 68, 377–392.
Robinet, E. A., Geurts, M., Maloteaux, J. M., and Pauwels, P. J. (2001). Chronic treatment with certain antipsychotic drugs preserves upregulation of regulator of G-protein signalling 2 mRNA in rat striatum as opposed to c-fos mRNA. Neurosci. Lett. 307, 45–48.
Ross, E. M., and Wilkie, T. M. (2000). GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827.
Rubinstein, M., Phillips, T. J., Bunzow, J. R., Falzone, T. L., Dziewczapolski, G., Zhang, G., Fang, Y., Larson, J. L., McDougall, J. A., Chester, J. A., Saez, C., Pugsley, T. A., Gershanik, O., Low, M. J., and Grandy, D. K. (1997). Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90, 991–1001.
Salah-Uddin, H., Thomas, D. R., Davies, C. H., Hagan, J. J., Wood, M. D., Watson, J. M., and Challiss, R. A. (2008). Pharmacological assessment of m1 muscarinic acetylcholine receptor-gq/11 protein coupling in membranes prepared from postmortem human brain tissue. J. Pharmacol. Exp. Ther. 325, 869–874.
Sanchez, G., Colettis, N., Vazquez, P., Cervenansky, C., Aguirre, A., Quillfeldt, J. A., Jerusalinsky, D., and Kornisiuk, E. (2009). Muscarinic inhibition of hippocampal and striatal adenylyl cyclase is mainly due to the M(4) receptor. Neurochem. Res. 34, 1363–1371.
Sanchez-Blazquez, P., Rodriguez-Diaz, M., Lopez-Fando, A., Rodriguez-Munoz, M., and Garzon, J. (2003). The GBeta5 subunit that associates with the R7 subfamily of RGS proteins regulates mu-opioid effects. Neuropharmacology 45, 82–95.
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223.
Schad, C. A., Justice, J. B. Jr., and Holtzman, S. G. (1996). Differential effects of delta- and mu-opioid receptor antagonists on the amphetamine-induced increase in extracellular dopamine in striatum and nucleus accumbens. J. Neurochem. 67, 2292–2299.
Schiff, M. L., Siderovski, D. P., Jordan, J. D., Brothers, G., Snow, B., De Vries, L., Ortiz, D. F., and Diverse-Pierluissi, M. (2000). Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature 408, 723–727.
Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A., and Ferre, S. (2007). Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 83, 277–292.
Schoepp, D. D. (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 299, 12–20.
Schwendt, M., Gold, S. J., and McGinty, J. F. (2006). Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J. Neurochem. 96, 1606–1615.
Schwendt, M., Hearing, M. C., See, R. E., and McGinty, J. F. (2007). Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport 18, 1261–1265.
Schwendt, M., and McGinty, J. F. (2007). Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor subtype 5 in rat striatum: relevance to amphetamine behavioral sensitization. J. Pharmacol. Exp. Ther. 323, 650–657.
Seeman, P., Ko, F., Jack, E., Greenstein, R., and Dean, B. (2007). Consistent with dopamine supersensitivity, RGS9 expression is diminished in the amphetamine-treated animal model of schizophrenia and in postmortem schizophrenia brain. Synapse 61, 303–309.
Shu, F. J., Ramineni, S., and Hepler, J. R. (2010). RGS14 is a multifunctional scaffold that integrates G protein and Ras/Raf MAPkinase signalling pathways. Cell. Signal. 22, 366–376.
Shuen, J. A., Chen, M., Gloss, B., and Calakos, N. (2008). Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J. Neurosci. 28, 2681–2685.
Siderovski, D. P., Strockbine, B., and Behe, C. I. (1999). Whither goest the RGS proteins? Crit. Rev. Biochem. Mol. Biol. 34, 215–251.
Siderovski, D. P., and Willard, F. S. (2005). The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 1, 51–66.
Silverman, J. L., and Koenig, J. I. (2007). Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm. Behav. 52, 146–155.
Sinnarajah, S., Dessauer, C. W., Srikumar, D., Chen, J., Yuen, J., Yilma, S., Dennis, J. C., Morrison, E. E., Vodyanoy, V., and Kehrl, J. H. (2001). RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature 409, 1051–1055.
Smith, Y., and Kieval, J. Z. (2000). Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 23, S28–S33.
Smolders, I., Bogaert, L., Ebinger, G., and Michotte, Y. (1997). Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J. Neurochem. 68, 1942–1948.
Song, W. J., Tkatch, T., and Surmeier, D. J. (2000). Adenosine receptor expression and modulation of Ca(2+) channels in rat striatal cholinergic interneurons. J. Neurophysiol. 83, 322–332.
Spadoni, F., Martella, G., Martorana, A., Lavaroni, F., D’Angelo, V., Bernardi, G., and Stefani, A. (2004). Opioid-mediated modulation of calcium currents in striatal and pallidal neurons following reserpine treatment: focus on kappa response. Synapse 51, 194–205.
Spanagel, R., Herz, A., and Shippenberg, T. S. (1992). Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U.S.A. 89, 2046–2050.
Srinivasa, S. P., Bernstein, L. S., Blumer, K. J., and Linder, M. E. (1998). Plasma membrane localization is required for RGS4 function in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 95, 5584–5589.
Stanwood, G. D., Parlaman, J. P., and Levitt, P. (2006). Genetic or pharmacological inactivation of the dopamine D1 receptor differentially alters the expression of regulator of G-protein signalling (Rgs) transcripts. Eur. J. Neurosci. 24, 806–818.
Sun, X., Kaltenbronn, K. M., Steinberg, T. H., and Blumer, K. J. (2005). RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol. Pharmacol. 67, 631–639.
Surmeier, D. J., Bargas, J., Hemmings, H. C. Jr., Nairn, A. C., and Greengard, P. (1995). Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron 14, 385–397.
Surmeier, D. J., Ding, J., Day, M., Wang, Z., and Shen, W. (2007). D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235.
Suzuki, T., Miura, M., Nishimura, K., and Aosaki, T. (2001). Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J. Neurosci. 21, 6492–6501.
Swanson, C. J., Baker, D. A., Carson, D., Worley, P. F., and Kalivas, P. W. (2001). Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J. Neurosci. 21, 9043–9052.
Takesono, A., Cismowski, M. J., Ribas, C., Bernard, M., Chung, P., Hazard, S. III, Duzic, E., and Lanier, S. M. (1999). Receptor-independent activators of heterotrimeric G-protein signaling pathways. J. Biol. Chem. 274, 33202–33205.
Talbot, J. N., Jutkiewicz, E. M., Graves, S. M., Clemans, C. F., Nicol, M. R., Mortensen, R. M., Huang, X., Neubig, R. R., and Traynor, J. R. (2010). RGS inhibition at G(alpha)i2 selectively potentiates 5-HT1A-mediated antidepressant effects. Proc. Natl. Acad. Sci. U.S.A. 107, 11086–11091.
Tall, G. G., Krumins, A. M., and Gilman, A. G. (2003). Mammalian Ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J. Biol. Chem. 278, 8356–8362.
Tamaru, Y., Nomura, S., Mizuno, N., and Shigemoto, R. (2001). Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106, 481–503.
Taymans, J. M., Leysen, J. E., and Langlois, X. (2003). Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: clues for RGS2 and RGS4 functions. J. Neurochem. 84, 1118–1127.
Tekumalla, P. K., Calon, F., Rahman, Z., Birdi, S., Rajput, A. H., Hornykiewicz, O., Di Paolo, T., Bedard, P. J., and Nestler, E. J. (2001). Elevated levels of DeltaFosB and RGS9 in striatum in Parkinson’s disease. Biol. Psychiatry 50, 813–816.
Tepper, J. M., Koos, T., and Wilson, C. J. (2004). GABAergic microcircuits in the neostriatum. Trends Neurosci. 27, 662–669.
Testa, C. M., Standaert, D. G., Landwehrmeyer, G. B., Penney, J. B. Jr., and Young, A. B. (1995). Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J. Comp. Neurol. 354, 241–252.
Thanos, P. K., Bermeo, C., Rubinstein, M., Suchland, K. L., Wang, G. J., Grandy, D. K., and Volkow, N. D. (2010). Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J. Psychopharmacol. (Oxford) 24, 897–904.
Thomas, C. J., Tall, G. G., Adhikari, A., and Sprang, S. R. (2008). Ric-8A catalyzes guanine nucleotide exchange on G alphai1 bound to the GPR/GoLoco exchange inhibitor AGS3. J. Biol. Chem. 283, 23150–23160.
Thomas, E. A., Danielson, P. E., and Sutcliffe, J. G. (1998). RGS9: a regulator of G-protein signalling with specific expression in rat and mouse striatum. J. Neurosci. Res. 52, 118–124.
Van Ree, J. M., Niesink, R. J., Van Wolfswinkel, L., Ramsey, N. F., Kornet, M. M., Van Furth, W. R., Vanderschuren, L. J., Gerrits, M. A., and Van den Berg, C. L. (2000). Endogenous opioids and reward. Eur. J. Pharmacol. 405, 89–101.
Venter, J. C., Adams, M. D., Myers, E. W., Li, P. W., Mural, R. J., Sutton, G. G., Smith, H. O., Yandell, M., Evans, C. A., Holt, R. A., Gocayne, J. D., Amanatides, P., Ballew, R. M., Huson, D. H., Wortman, J. R., Zhang, Q., Kodira, C. D., Zheng, X. H., Chen, L., Skupski, M., Subramanian, G., Thomas, P. D., Zhang, J., Gabor Miklos, G. L., Nelson, C., Broder, S., Clark, A. G., Nadeau, J., McKusick, V. A., Zinder, N., Levine, A. J., Roberts, R. J., Simon, M., Slayman, C., Hunkapiller, M., Bolanos, R., Delcher, A., Dew, I., Fasulo, D., Flanigan, M., Florea, L., Halpern, A., Hannenhalli, S., Kravitz, S., Levy, S., Mobarry, C., Reinert, K., Remington, K., Abu-Threideh, J., Beasley, E., Biddick, K., Bonazzi, V., Brandon, R., Cargill, M., Chandramouliswaran, I., Charlab, R., Chaturvedi, K., Deng, Z., Di Francesco, V., Dunn, P., Eilbeck, K., Evangelista, C., Gabrielian, A. E., Gan, W., Ge, W., Gong, F., Gu, Z., Guan, P., Heiman, T. J., Higgins, M. E., Ji, R. R., Ke, Z., Ketchum, K. A., Lai, Z., Lei, Y., Li, Z., Li, J., Liang, Y., Lin, X., Lu, F., Merkulov, G. V., Milshina, N., Moore, H. M., Naik, A. K., Narayan, V. A., Neelam, B., Nusskern, D., Rusch, D. B., Salzberg, S., Shao, W., Shue, B., Sun, J., Wang, Z., Wang, A., Wang, X., Wang, J., Wei, M., Wides, R., Xiao, C., Yan, C., Yao, A., Ye, J., Zhan, M., Zhang, W., Zhang, H., Zhao, Q., Zheng, L., Zhong, F., Zhong, W., Zhu, S., Zhao, S., Gilbert, D., Baumhueter, S., Spier, G., Carter, C., Cravchik, A., Woodage, T., Ali, F., An, H., Awe, A., Baldwin, D., Baden, H., Barnstead, M., Barrow, I., Beeson, K., Busam, D., Carver, A., Center, A., Cheng, M. L., Curry, L., Danaher, S., Davenport, L., Desilets, R., Dietz, S., Dodson, K., Doup, L., Ferriera, S., Garg, N., Gluecksmann, A., Hart, B., Haynes, J., Haynes, C., Heiner, C., Hladun, S., Hostin, D., Houck, J., Howland, T., Ibegwam, C., Johnson, J., Kalush, F., Kline, L., Koduru, S., Love, A., Mann, F., May, D., McCawley, S., McIntosh, T., McMullen, I., Moy, M., Moy, L., Murphy, B., Nelson, K., Pfannkoch, C., Pratts, E., Puri, V., Qureshi, H., Reardon, M., Rodriguez, R., Rogers, Y. H., Romblad, D., Ruhfel, B., Scott, R., Sitter, C., Smallwood, M., Stewart, E., Strong, R., Suh, E., Thomas, R., Tint, N. N., Tse, S., Vech, C., Wang, G., Wetter, J., Williams, S., Williams, M., Windsor, S., Winn-Deen, E., Wolfe, K., Zaveri, J., Zaveri, K., Abril, J. F., Guigó, R., Campbell, M. J., Sjolander, K. V., Karlak, B., Kejariwal, A., Mi, H., Lazareva, B., Hatton, T., Narechania, A., Diemer, K., Muruganujan, A., Guo, N., Sato, S., Bafna, V., Istrail, S., Lippert, R., Schwartz, R., Walenz, B., Yooseph, S., Allen, D., Basu, A., Baxendale, J., Blick, L., Caminha, M., Carnes-Stine, J., Caulk, P., Chiang, Y. H., Coyne, M., Dahlke, C., Mays, A., Dombroski, M., Donnelly, M., Ely, D., Esparham, S., Fosler, C., Gire, H., Glanowski, S., Glasser, K., Glodek, A., Gorokhov, M., Graham, K., Gropman, B., Harris, M., Heil, J., Henderson, S., Hoover, J., Jennings, D., Jordan, C., Jordan, J., Kasha, J., Kagan, L., Kraft, C., Levitsky, A., Lewis, M., Liu, X., Lopez, J., Ma, D., Majoros, W., McDaniel, J., Murphy, S., Newman, M., Nguyen, T., Nguyen, N., Nodell, M., Pan, S., Peck, J., Peterson, M., Rowe, W., Sanders, R., Scott, J., Simpson, M., Smith, T., Sprague, A., Stockwell, T., Turner, R., Venter, E., Wang, M., Wen, M., Wu, D., Wu, M., Xia, A., Zandieh, A., and Zhu, X. (2001). The sequence of the human genome. Science 291, 1304–1351.
Wang, S. C., Lai, H. L., Chiu, Y. T., Ou, R., Huang, C. L., and Chern, Y. (2007). Regulation of type V adenylate cyclase by Ric8a, a guanine nucleotide exchange factor. Biochem. J. 406, 383–388.
Weiner, D. M., Levey, A. I., and Brann, M. R. (1990). Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. U.S.A. 87, 7050–7054.
Wess, J. (2004). Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 44, 423–450.
Willard, F. S., Kimple, R. J., and Siderovski, D. P. (2004). Return of the GDI: the GoLoco motif in cell division. Annu. Rev. Biochem. 73, 925–951.
Willard, M. D., Willard, F. S., Li, X., Cappell, S. D., Snider, W. D., and Siderovski, D. P. (2007). Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J. 26, 2029–2040.
Xie, K., Allen, K. L., Kourrich, S., Colon-Saez, J., Thomas, M. J., Wickman, K., and Martemyanov, K. A. (2010). Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat. Neurosci. 13, 661–663.
Xie, Z., Geiger, T. R., Johnson, E. N., Nyborg, J. K., and Druey, K. M. (2008). RGS13 acts as a nuclear repressor of CREB. Mol. Cell 31, 660–670.
Xu, M., Mizobe, F., Yamamoto, T., and Kato, T. (1989). Differential effects of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism in freely moving rats. Brain Res. 495, 232–242.
Yan, Z., and Surmeier, D. J. (1996). Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 16, 2592–2604.
Zachariou, V., Georgescu, D., Sanchez, N., Rahman, Z., DiLeone, R., Berton, O., Neve, R. L., Sim-Selley, L. J., Selley, D. E., Gold, S. J., and Nestler, E. J. (2003). Essential role for RGS9 in opiate action. Proc. Natl. Acad. Sci. U.S.A. 100, 13656–13661.
Zerangue, N., and Jan, L. Y. (1998). G-protein signaling: fine-tuning signaling kinetics. Curr. Biol. 8, R313–R316.
Zhang, D., Zhang, L., Tang, Y., Zhang, Q., Lou, D., Sharp, F. R., Zhang, J., and Xu, M. (2005a). Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology 30, 1443–1454.
Zhang, Y., James, M., Middleton, F. A., and Davis, R. L. (2005b). Transcriptional analysis of multiple brain regions in Parkinson’s disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. Am. J. Med. Genet. B Neuropsychiatr. Genet. 137B, 5–16.
Zhang, K., Howes, K. A., He, W., Bronson, J. D., Pettenati, M. J., Chen, C., Palczewski, K., Wensel, T. G., and Baehr, W. (1999). Structure, alternative splicing, and expression of the human RGS9 gene. Gene 240, 23–34.
Zhang, W., Yamada, M., Gomeza, J., Basile, A. S., and Wess, J. (2002). Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J. Neurosci. 22, 6347–6352.
Keywords: G proteins, GPCR, RGS proteins, striatum
Citation: Xie K and Martemyanov KA (2011) Control of striatal signaling by G protein regulators. Front. Neuroanat. 5:49. doi: 10.3389/fnana.2011.00049
Received: 14 May 2011;
Accepted: 23 July 2011;
Published online: 08 August 2011.
Edited by:
Emmanuel Valjent, Université Montpellier 1 & 2, FranceReviewed by:
Jacqueline McGinty, University of South Carolina, USAVenetia Zachariou, University of Crete, Greece
Copyright: © 2011 Xie and Martemyanov. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Kirill A. Martemyanov, Department of Neuroscience, The Scripps Research Institute, 130 Scripps Way, #3C2, Jupiter, FL 33458, USA. e-mail: kirill@scripps.edu