Trace eyeblink conditioning is impaired in α7 but not in β2 nicotinic acetylcholine receptor knockout mice
- 1 Neuroscience Program and Department of Psychology, Temple University, Philadelphia, PA, USA
- 2 Department of Neuroscience, Baylor College of Medicine, Houston, TX, USA
Nicotinic acetylcholine receptors (nAChRs) are essentially involved in learning and memory. A neurobiologically and behaviorally well-characterized measure of learning and memory, eyeblink classical conditioning, is sensitive to disruptions in acetylcholine neurotransmission. The two most common forms of eyeblink classical conditioning – the delay and trace paradigms – differentially engage forebrain areas densely-populated with nAChRs. The present study used genetically modified mice to investigate the effects of selective nAChR subunit deletion on delay and trace eyeblink classical conditioning. α7 and β2 nAChR subunit knockout (KO) mice and their wild-type littermates were trained for 10 daily sessions in a 500-ms delay or 500-ms trace eyeblink conditioning task, matched for the interstimulus interval between conditioned stimulus and unconditioned stimulus onset. Impairments in conditioned responding were found in α7 KO mice trained in trace – but not delay – eyeblink conditioning. Relative to littermate controls, β2 KO mice were unimpaired in the trace task but displayed higher levels of conditioned responding in delay eyeblink conditioning. Elevated conditioned response levels in delay-conditioned β2 KOs corresponded to elevated levels of alpha responding in this group. These findings suggest that α7 nAChRs play a role in normal acquisition of 500 ms trace eyeblink classical conditioning in mice. The prominent distribution of α7 nAChRs in the hippocampus and other forebrain regions may account for these genotype-specific acquisition effects in this hippocampus-dependent trace paradigm.
Introduction
Nicotinic acetylcholine receptors (nAChRs) play an important role in learning and memory. However, the mechanisms by which nAChRs exert their actions require further elaboration. Neuronal nAChRs are pentameric, ligand-gated excitatory ion channels composed of α (α2 to α10) and β (β2 to β4) subunits (Lindstrom et al., 1991; Cordero-Erausquin et al., 2000; Hogg et al., 2003; De Biasi and Salas, 2008; Albuquerque et al., 2009). The α7 and α4β2 nAChRs are the most widespread in the brain (Zoli et al., 1998; Sihver et al., 2000; Gotti et al., 2006). However, α7 and α4β2 nAChRs possess distinct physiological properties and are distributed differentially in some brain regions (Cordero-Erausquin et al., 2000; Gotti et al., 2006; De Biasi and Salas, 2008; Albuquerque et al., 2009). Understanding of the functional significance of nAChRs has advanced through the use of α7 and β2 nAChR knockout (KO) mice in various behavioral paradigms (Franceschini et al., 2000; Walters et al., 2006; Cincotta et al., 2008; Maubourguet et al., 2008; Portugal et al., 2008), though performance on many tasks is largely unimpaired in young adult α7 and β2 nAChR KO mice (Paylor et al., 1998; Champtiaux and Changeux, 2004). Examination of these mutant mice in tasks such as eyeblink classical conditioning in which the relationship between brain function and behavior is well defined can advance our understanding of the functional significance of these nAChRs.
The neural substrates of eyeblink conditioning are conserved across species (Chen et al., 1999; Woodruff-Pak and Steinmetz, 2000a,b), and investigations of eyeblink conditioning in genetically modified mice have advanced our understanding of the cellular mechanisms underlying associative learning and memory (Chen et al., 1996; Woodruff-Pak et al., 2006; Kakegawa et al., 2008). Eyeblink classical conditioning requires the functional integrity of a well-defined cerebellum-brainstem circuit (Thompson, 1986; Christian and Thompson, 2003) and differentially engages various forebrain mechanisms as a function of conditioning parameters. In delay eyeblink conditioning in which the conditioned stimulus (CS) overlaps and coterminates with the unconditioned stimulus (US), the ipsilateral cerebellum is essential and the hippocampus plays a modulatory role (Solomon et al., 1983; Woodruff-Pak et al., 1997). In trace eyeblink conditioning, a stimulus-free (trace) interval is imposed between CS offset and US onset, and with sufficiently long trace intervals the hippocampus becomes essential for normal acquisition (Moyer et al., 1990; Tseng et al., 2004). The function of nAChRs is important in the acquisition of eyeblink conditioning, as nAChR antagonists retard conditioned response (CR) acquisition (Woodruff-Pak, 2003) and nAChR agonists enhance conditioning (Woodruff-Pak et al., 1994, 2007; Li et al., 2008). Furthermore, rabbits that acquire conditioned eyeblink responses rapidly have higher numbers of nAChRs than do rabbits that condition poorly (Woodruff-Pak et al., 2010).
To explore further the role of nAChR subunit function in learning and memory, we assessed the performance of α7 and β2 nAChR KO mice in delay and trace eyeblink conditioning tasks matched for the 500 ms interstimulus interval (ISI) between CS and US onset (see Figure 1 for an illustration of our delay and trace tasks). Previous work in our laboratory with the 750 ms ISI in New Zealand white rabbits (Woodruff-Pak et al., 2007) and the 500 ms ISI in C57BL/6 mice (Spath and Woodruff-Pak, 2004) indicated that when ISIs are matched in the trace and delay paradigms, acquisition in trace is slower and reaches a lower asymptote. ISI-matched delay and trace eyeblink conditioning tasks differentially engage forebrain circuitry associated with higher-order learning and memory paradigms (Moyer et al., 1990; Ivkovich and Stanton, 2001; Green and Arenos, 2007). The 500-ms trace eyeblink conditioning paradigm with a 250-ms trace interval is hippocampus-dependent in mice (Tseng et al., 2004). Because of the prominent distribution and known functional properties of α7 nAChRs in the hippocampus (Séguéla et al., 1993; Cincotta et al., 2008; De Biasi and Salas, 2008), we predicted greater impairments in the hippocampus-sensitive trace task in α7 nAChR KO mice. Additionally, due to the greater importance of hippocampal integrity for acquisition of trace relative to delay eyeblink conditioning, conditioning impairments in α7 nAChR KO mice were expected to be greater in trace than those in delay-conditioned α7 or β2 nAChR KO mice.
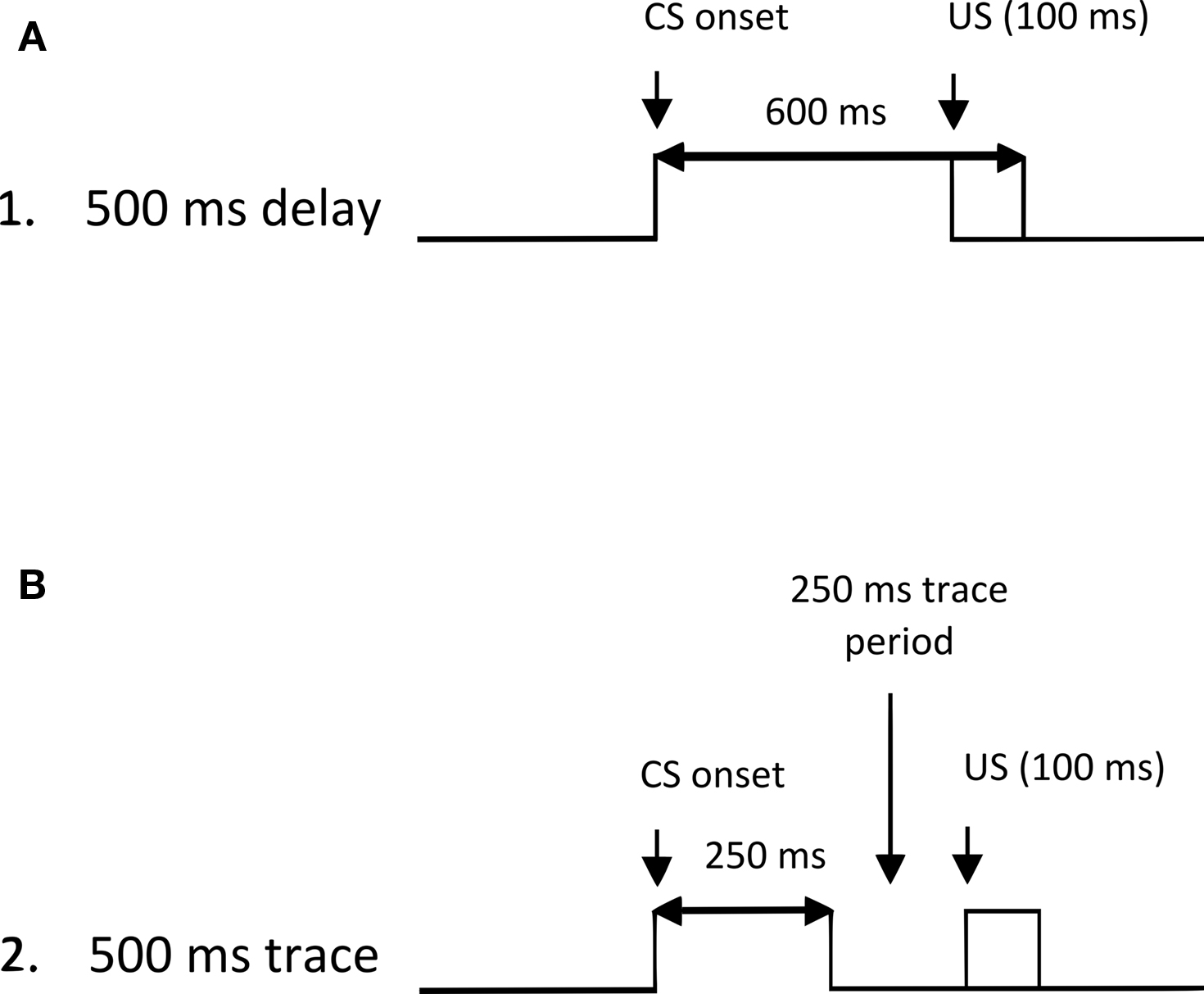
Figure 1. Illustration of the delay (A) and trace (B) eyeblink classical conditioning tasks used in the present study. The tasks were matched for the 500 ms interval between conditioned stimulus (CS) onset and unconditioned stimulus (US) onset. In delay conditioning, the CS precedes, overlaps, and coterminates with the 100 ms US. In trace conditioning, the CS precedes the US, but a 250-ms stimulus-free (trace) interval is imposed between the offset of the CS and the onset of the US.
Materials and Methods
Subjects
Eighty-seven mice (32 female; 55 male) were initially included in this study. Seventeen mice were excluded from analyses primarily due to excessive pre-CS activity (or “noise”, see Eyeblink Conditioning Analysis), leaving 70 mice (23 female; 47 male) used for subsequent data reporting. Twenty mice (5 female; 15 male) were α7 KOs, 24 mice (7 female; 17 male) were β2 KOs, and 26 mice (11 female; 15 male) were wild-type littermate controls. Both mutant strains were bred into a C57BL/6J strain for 10 generations. Of the mice studied, 37 were trained in the delay eyeblink conditioning paradigm (10 α7 KOs; 12 β2 KOs; 15 wild-type) while the remaining 33 were trained in the trace eyeblink conditioning paradigm (10 α7 KOs; 12 β2 KOs; 11 wild-type). α7 and β2 KO mice were genotyped after weaning as previously described (Orr-Urtreger et al., 1997). The genotype was reconfirmed at the end of the experiment. Both strains have normal life spans and do not display overt morphological or behavioral phenotypes. All mice weighed between 18 and 46 grams at the time of surgery, with wild-type controls (mean weight = 26.2 g; SD = 4.83) weighing significantly less than both the α7 (mean weight = 31.25 g; SD = 4.31; p < 0.05) and β2 KO mice (mean weight = 32.74 g; SD = 8.58; p < 0.05). At 4–9 months of age mice began eyeblink classical conditioning training. Mice were group-housed in standard polycarbonate cages and had ad libitum access to sterile food and water. Room lighting was timed for a 12:12-h light–dark cycle. All research methods were approved by the Institutional Animal Care and Use Committee of Albert Einstein Healthcare Network where they were bred and tested.
Surgery
All mice received surgery to implant recording and stimulating electrodes for eyeblink classical conditioning. For anesthesia, a “non-rebreathing” isoflurane administration system was used. Anesthesia was induced in a chamber with O2 + 3% isoflurane at a flow rate of 1 L/min. The isoflurane was then reduced to 2.5% as the mouse was placed on a surgical platform and fitted with a nose cone for anesthesia maintenance throughout the procedure. Opthalmic ointment was applied to each eye to prevent drying, and mice were covered with gauze strips to maintain normal thermoregulation. After a small surgical incision was made to expose the top of the skull and two screws were inserted into the skull, four Teflon-coated stainless steel wires (0.003-in bare, 0.0045-in coated; A-M Systems, Everett, WA, USA) soldered to a four-pin male header (Jameco Electronics, Belmont, CA, USA) were implanted intramuscularly in the orbicularis oculi of the left upper eyelid. Wires were stripped of Teflon and carefully placed such that only the muscle-embedded wire was bare. To ensure that the wires would not move or recede back into the periorbital cavity, wires were glued to the skull. The two wires most rostral were used to record differential electromyography (EMG) activity, and the two most caudal were used to deliver the eyeblink-eliciting stimulus. When all wires were placed, the four-pin headstage was cemented to the skull (with the glue adhering to the skull screws) and the incision was closed. Following surgery, mice were given Baytril antibiotic (85 mg/kg sc) to prevent infection and Buprenex (0.075 mg/kg sc) for analgesia. Mice were allowed a minimum of 5 days to recover from surgery.
Eyeblink Conditioning Apparatus and Procedure
The conditioned eyeblink training apparatus consisted of four sound- and light-attenuating chambers (Med Associates, St. Albans, VT, USA). Each chamber contained a beaker in which the mouse was placed, a copper Faraday cage covering the beaker, a ventilation fan, and a wall-mounted speaker. A shielded four-conductor wire was attached to the mouse’s headstage and was used to deliver a blink-eliciting stimulus to the orbicularis oculi and to record EMG activity. EMG activity was passed through a 300–5,000 Hz filter and amplified by 10 K. The signal was then integrated and digitized before being read into a system compatible with IBM (White Plains, NY, USA; described by Chen and Steinmetz, 1998) for processing. Data were collected in RAM and saved for offline analyses.
Mice were run for 10 days in 500 ms delay or 500 ms trace eyeblink classical conditioning (500 ms ISI between CS and US onset for both tasks) with each day consisting of one session of 90 paired CS–US and 10 CS alone trials. Each training session was controlled by a program written in C++ language (Chen and Steinmetz, 1998). The intertrial interval was random, ranging from 15 to 30-s at 1-s intervals. Mice were trained in groups of four. Each session lasted approximately 1 h and mice were allowed to move about freely within the beaker during testing. The ventilation fan generated a 70-dB background noise. Each daily session (10 sessions of acquisition total) consisted of 100 trials (presented in blocks of 10). Each 10-trial block consisted of nine paired trials and one CS-alone test trial. Delay eyeblink conditioning trials included a 600-ms 85-db white noise CS. Five hundred milliseconds after CS onset, a 100-ms 0.5 mA stimulation US was delivered, and this coterminated with the CS. Trace eyeblink conditioning paired trials included a 250-ms white noise CS also followed 500 ms after its onset (and 250 ms after its offset) by a 100-ms 0.5 mA stimulation US. The 250 ms period between CS offset and US onset represented a stimulus-free “trace” interval [see Figure 2 for sample EMG recordings from a wild-type control (Figure 2A) and an α7 KO (Figure 2B) trained in trace conditioning].
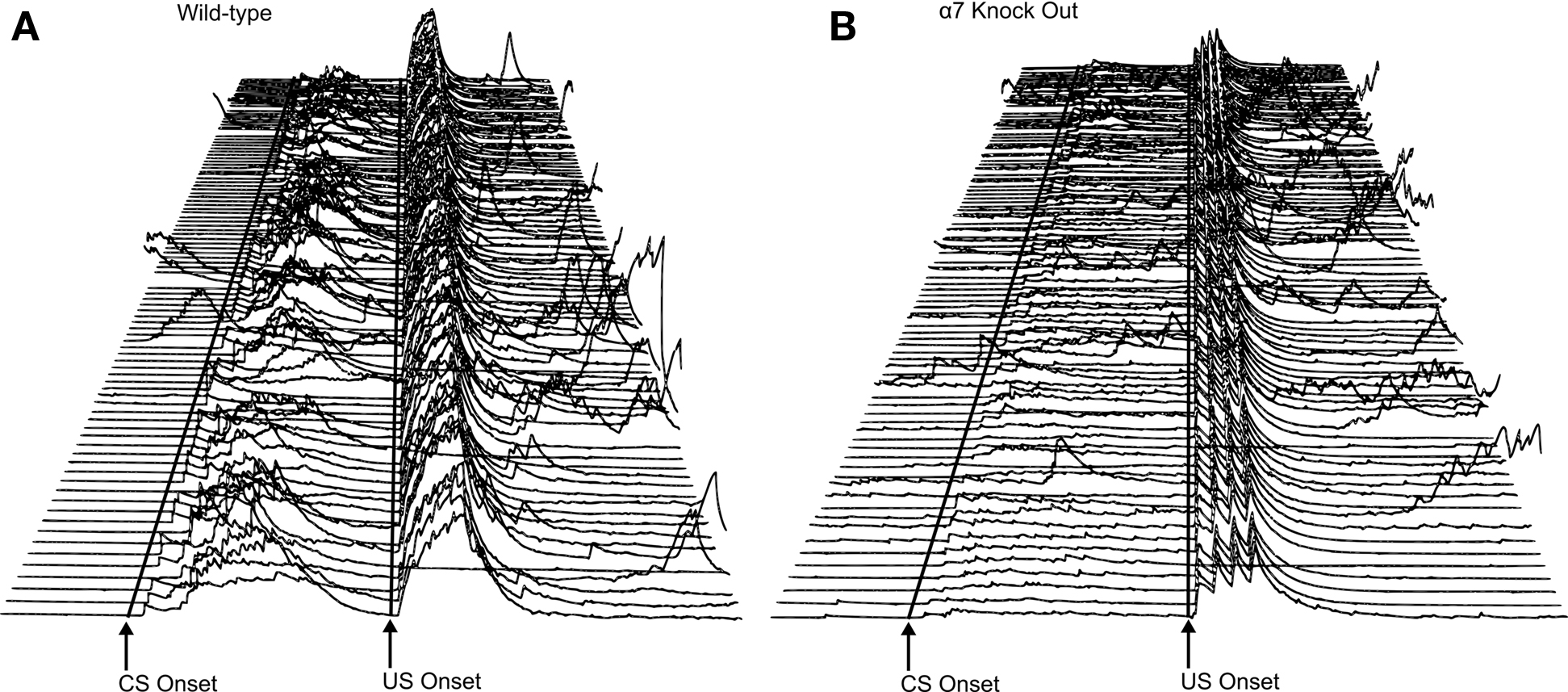
Figure 2. (A,B) Electromyography (EMG) recorded from eye muscles (orbicularis oculi) of the left upper eyelid during trace eyeblink conditioning. Each line represents EMG activity from an individual trial (1–100, with Trial 1 represented at the bottom of each figure and Trial 100 represented at the top of each figure) from Session 7 of 500-ms trace eyeblink classical conditioning. Total trial length was 1,350 ms. Lines are drawn to approximate the onset of the conditioned stimulus (CS) and unconditioned stimulus (US). There were 249 ms in the pre-CS period before CS onset. CS onset is marked, and then there were 500 ms between CS onset and US onset (marked). There were 601 ms in the post-US period. A response was scored if it exceeded the peak of pre-CS activity by 1.5 units. Performance is shown for a wild-type control mouse (A) and an α7 knockout (KO) mouse (B) that were representative of their respective groups in terms of CR production levels. For the wild-type control mouse shown here (A), 95 of the 100 trials were usable for analyses; the remaining 5 trials were excluded due to excessive pre CS EMG activity. During paired CS–US trials this subject (A) displayed conditioned responses (CRs) on 88% of the trials. Short latency alpha (or “startle”) responses (0–60 ms after CS onset) occurred in 58% of the paired CS–US trials. For the α7 KO subject (B), 93 of the 100 trials were usable for analysis. During paired CS–US trials there were 63% CRs and 24% alpha responses.
Eyeblink Conditioning Analysis
Each session was computer-scored with a macro written in Visual Basic, which analyzed each trial individually for responses. Response threshold was set to 1.5 units above the highest point of pre-CS activity. A startle (or “alpha”) response was scored if the response occurred in the first 60 ms after CS onset. A CR was scored if a response occurred after the 60 ms startle period and before the US onset (500 ms after CS onset) for paired CS–US trials. All data are reported from paired CS–US trials. Trials were excluded from analysis in cases where (a) excessive pre-CS activity (also termed “noise”) was present, or (b) activity that originated in the pre-CS period exceeded the response threshold after CS onset. Subjects were excluded from analyses if 30% or more of their trials (averaged across the 10 training sessions) were determined to be unusable.
Data were analyzed via analysis of variance (ANOVA) using SPSS statistical package with significance levels set at p < 0.05. The primary dependent measures of interest were (1) percentage of CRs and (2) peak amplitude of CRs. Additional measures of interest included “performance” measures assessing basic sensory and motor functions including startle response percentage and unconditioned response amplitude. Although we analyzed data for sex difference and found that none were significant, the analyses had low power due to unequal as well as small sample sizes. Previous studies with these KO mice have not found significant sex differences (De Biasi and Salas, 2008). Subsequent analyses combined male and female mice. The focus of subsequent analyses was on comparisons of each KO group (α7 or β2) with wild-type controls.
Results
The major aim of this study was to compare eyeblink classical conditioning in genetically engineered mice with selective deletions of nAChR subunits (α7 or β2 KOs) versus wild-type controls. Subjects were trained in either a delay or trace eyeblink conditioning paradigm, with the ISI between CS and US onset matched (500 ms) across tasks. For each task, a separate 2 (genotype; α7 KO or β2 KO versus wild-type) × 10 (sessions) ANOVA was performed on the dependent measures of interest.
CR Percentage and CR Peak Amplitude – Delay Conditioning
Significant differences as a function of genotype in CR generation measures were evident between both α7 KOs and wild-type controls and between β2 KOs and wild-type controls trained in the delay conditioning paradigm. However, the patterns of differences between KO and wild-type subjects differed across these genotype comparisons (see Figures 3 and 4 depicting CR percentage and CR peak amplitude across training, respectively). For the comparison between α7 KOs and wild-type controls, a 2 (genotype) × 10 (sessions) ANOVA revealed the expected significant main effect of sessions for both the CR percentage, F(9, 207) = 6.012, p < 0.001, and CR peak amplitude measures, F(9, 207) = 3.612, p < 0.001, suggesting that both α7 KOs and wild-types trained in delay conditioning showed improvements in learning across the 10-day acquisition period. For the CR percentage measure, both the main effect of genotype and the interaction of Genotype × Sessions failed to reach statistical significance (p values >0.1). The main effect of group was not significant for the CR peak amplitude measure (p > 0.1), but the interaction of Genotype × Sessions was significant, F(9, 207) = 2.375, p < 0.015. This interaction was driven by superior performance of α7 KOs relative to wild-types early in training, followed by a reversal in which wild-types outperformed α7 KOs later in training.
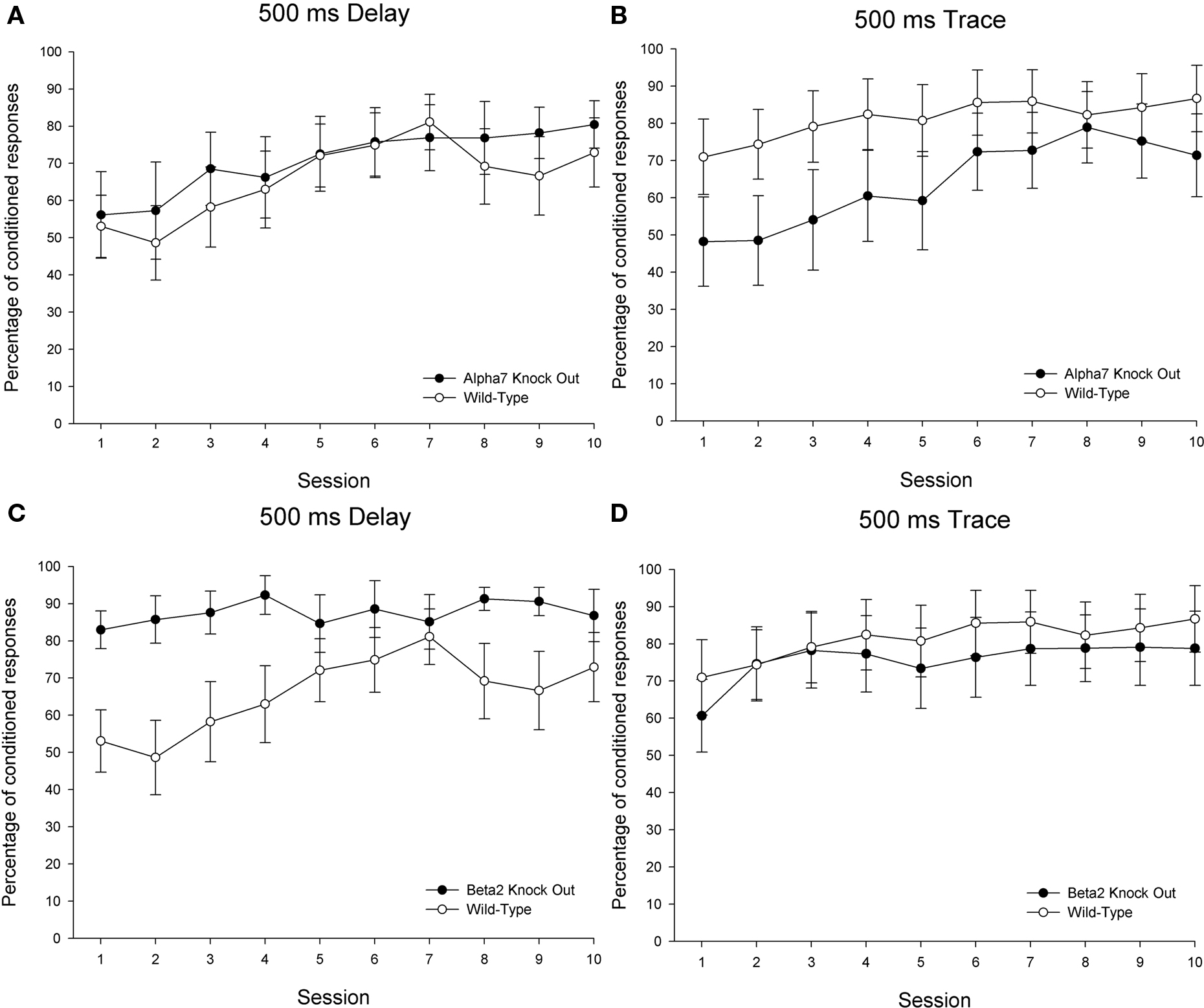
Figure 3. Delay and trace eyeblink classical conditioning over 10 sessions in 26 wild-type control mice (15 in delay and 11 trained in trace), 20 α7 KO mice (10 in delay and 10 trained in trace), and 24 β2 KO mice (12 in delay and 12 trained in trace). The dependent measure is percentage of conditioned responses. Each session included 90 paired and 10 conditioned stimulus (CS)-alone trials. Performance in the 90 paired trials/session is shown here. Delay eyeblink conditioning is depicted on the left side and trace eyeblink conditioning is shown on the right. Comparisons between wild-type controls and α7 KOs are shown on the top (A,B) panels, while comparisons between wild-type controls and β2 KOs are shown across the bottom (C,D) panels. The error bars indicate standard error of the mean.
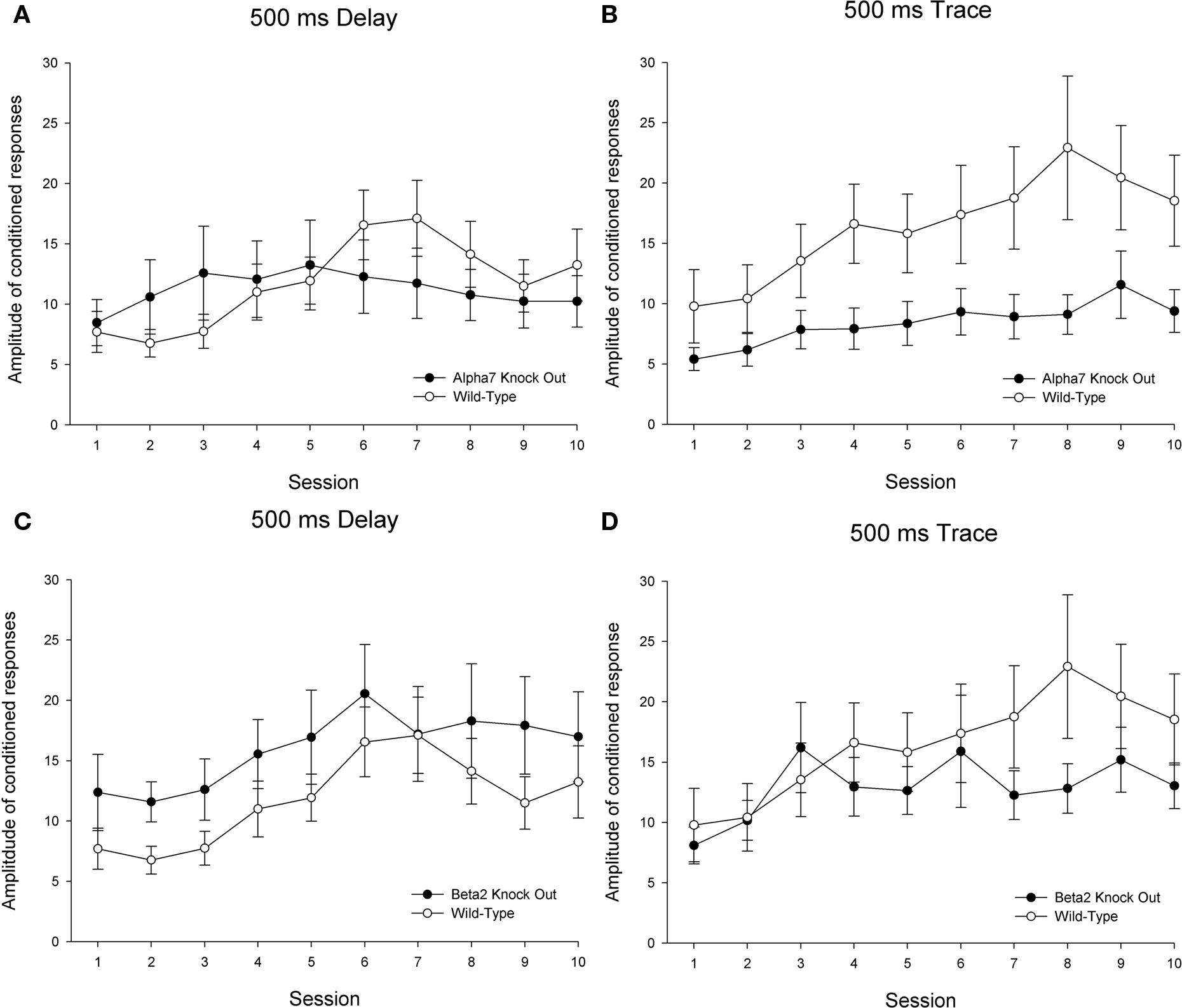
Figure 4. Delay and trace eyeblink classical conditioning over 10 sessions in 26 wild-type control mice (15 in delay and 11 trained in trace), 20 α7 KO mice (10 in delay and 10 trained in trace), and 24 β2 KO mice (12 in delay and 12 trained in trace). The dependent measure is peak amplitude of conditioned responses. Each session included 90 paired and 10 conditioned stimulus (CS)-alone trials. Performance in the 90 paired trials/session is shown here. Delay eyeblink conditioning is depicted on the left side and trace eyeblink conditioning is shown on the right. Comparisons between wild-type controls and α7 KOs are shown on the top (A,B) panels, while comparisons between wild-type controls and β2 KOs are shown across the bottom (C,D) panels. The error bars indicate standard error of the mean.
A 2 (genotype) × 10 (sessions) ANOVA comparing β2 KOs and wild-types trained in delay conditioning also revealed significant main effects of session for both the CR percentage, F(9, 225) = 2.577, p < 0.009, and CR peak amplitude measures, F(9, 225) = 8.337, p < 0.001. For the CR percentage measure, a significant main effect of genotype, F(1, 25) = 4.674, p < 0.041, as well as a significant interaction of Genotype × Sessions, F(9, 225) = 2.209, p < 0.023, indicated that β2 KOs outperformed their wild-type counterparts, particularly during the early stages of training. No significant effects involving the factor of genotype were found for the CR peak amplitude measure (p values >0.1).
CR Percentage and CR Peak Amplitude – Trace Conditioning
Significant differences as a function of genotype in CR generation measures were evident between α7 KOs and wild-types but not between β2 KOs and wild-types trained in the trace conditioning paradigm (see Figures 3 and 4). For the comparison between α7 KOs and wild-type controls, a 2 (genotype) × 10 (sessions) ANOVA revealed the expected significant main effect of sessions for both the CR percentage, F(9, 171) = 9.068, p < 0.001, and CR peak amplitude measures, F(9, 171) = 6.188, p < 0.001. A significant interaction of Genotype × Sessions was observed for the CR percentage measure, F(9, 171) = 1.964, p < 0.047, though the main effect of genotype did not achieve statistical significance (p > 0.1). The Genotype × Sessions interaction was driven by superior performance of wild-types relative to α7 KOs early, but not late in training. For the CR peak amplitude measure, the main effect of genotype achieved statistical significance – F(1, 19) = 4.371, p < 0.050. Consistent with findings in the CR percentage measure, this suggests that α7 KOs were impaired in conditioning relative to wild-type controls. The Genotype × Sessions interaction failed to reach significance in the CR peak amplitude measure (p > 0.1).
A 2 (genotype) × 10 (sessions) ANOVA comparing β2 KOs and wild-types trained in trace conditioning also revealed significant main effects of session for both the CR percentage, F(9, 189) = 3.616, p < 0.001, and CR peak amplitude measures, F(9, 189) = 4.066, p < 0.001, though no significant main or interactive effects were found involving the factor of genotype (p values >0.1).
Performance Measures
Levels of alpha responding (responses that exceeded threshold within the first 60 ms of CS onset) differed across some groups. Separate 2 (genotype) × 10 (sessions) ANOVAs revealed significant effects of genotype [F(1, 25) = 5.861, p < 0.024] and Genotype × Sessions [F(9. 225) = 2.488, p < 0.011] for the comparison between β2 KOs and wild-type controls trained in the delay eyeblink classical conditioning paradigm (the main effect of session failed to reach significance in this comparison – p > 0.1). These significant effects involving the factor of genotype were driven by significantly elevated levels of alpha (or “startle”) responding – particularly early in training – in β2 KOs relative to wild-types trained in delay conditioning. For the three remaining comparisons across genotype – α7 KOs versus wild-types in delay and in trace conditioning; β2 KOs versus wild-types in trace conditioning – no significant effects involving the factor of genotype were observed for the startle percentage measure (all p values >0.1). A significant main effect of sessions was observed across each of these three comparisons (all p values <0.007) (see Figure 5).
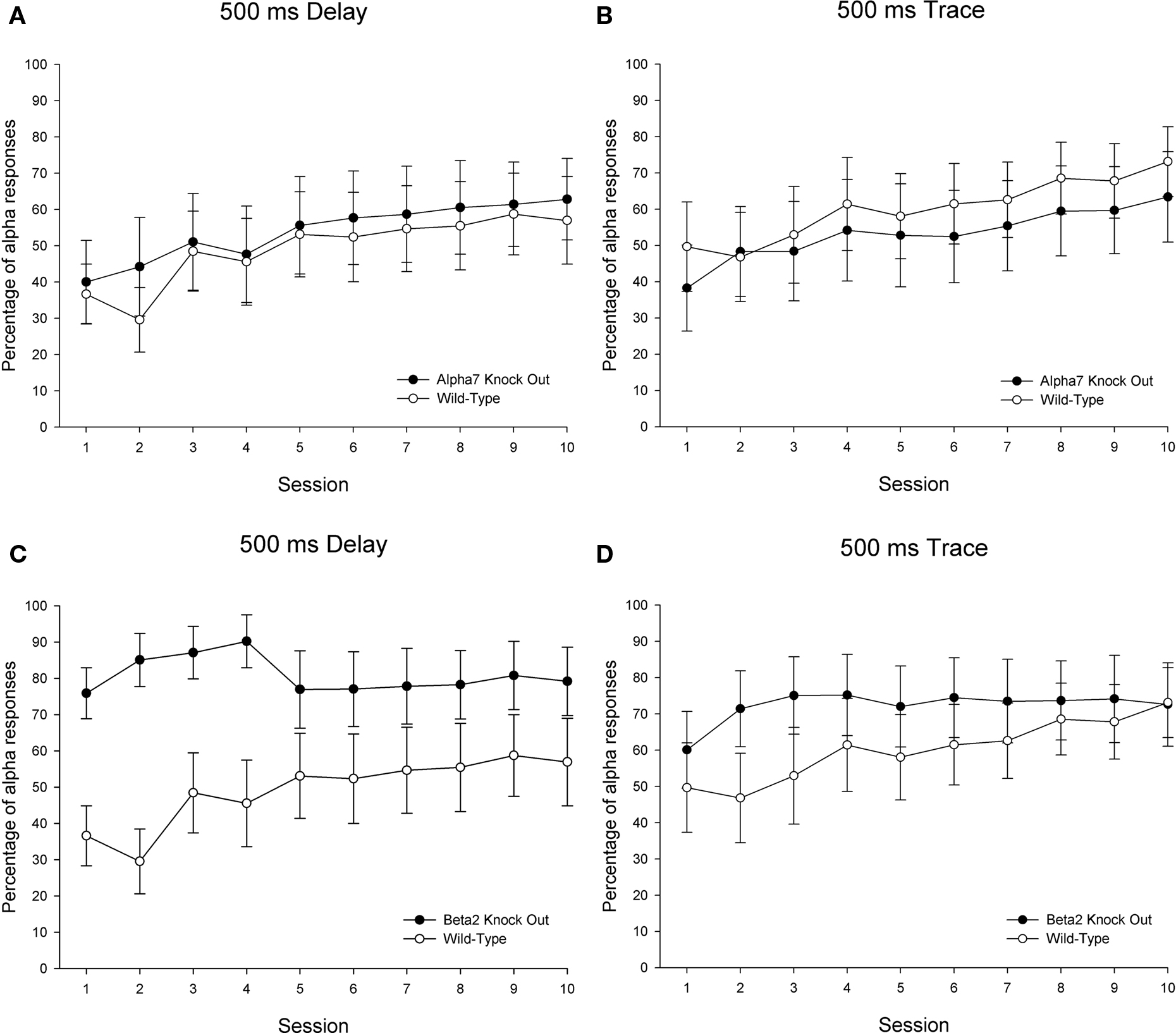
Figure 5. Percentage of alpha responding [responses occurring within the first 60 ms of conditioned stimulus – CS – onset] over 10 sessions in the same subjects and same sessions depicted in Figures 3 and 4: Twenty-six wild-type control mice (15 in delay and 11 trained in trace), 20 α7 KO mice (10 in delay and 10 trained in trace), and 24 β2 KO mice (12 in delay and 12 trained in trace). Delay eyeblink conditioning is depicted on the left side and trace eyeblink conditioning is shown on the right. Comparisons between wild-type controls and α7 KOs are shown on the top (A,B) panels, while comparisons between wild-type controls and β2 KOs are shown across the bottom (C,D) panels. The error bars indicate standard error of the mean.
Summary of Findings
As expected, differences in conditioning were found in a genotype- and task-specific fashion. Of primary interest were the impairments in CR generation observed in α7 KOs relative to wild-type controls trained in the trace conditioning task. Conversely, β2 KOs were unimpaired in trace eyeblink conditioning. These findings suggest that α7 – but not β2 – nAChRs play a role in the acquisition of trace eyeblink conditioning. Similar impairments were not observed between α7 KOs and wild-type controls trained in delay eyeblink conditioning. Significant differences were observed between β2 KOs and wild-types trained in delay conditioning. Specifically, β2 KOs showed enhanced CR levels (as measured by the CR percentage measure) relative to wild-type controls, though these differences coincided with enhanced levels of startle responding in β2 KOs relative to wild-type controls. In contrast to the aforementioned differences between α7 KOs and wild-type controls in trace eyeblink conditioning, these differences in CR levels in delay eyeblink conditioning may therefore be driven by non-associative effects.
Discussion
The present study is the first to report eyeblink classical conditioning performance in nAChR KO mice. Of primary significance are findings indicating that α7 nAChRs make a significant contribution to normal acquisition in hippocampus-dependent 500 ms trace eyeblink classical conditioning. Mice lacking α7 nAChRs were not impaired in delay eyeblink classical conditioning with an identical 500 ms ISI between CS and US onset, suggesting that the acquisition deficits in α7 KO mice do not reflect global associative learning impairments. Furthermore, β2 KO mice were unimpaired in both the delay and trace eyeblink conditioning tasks. Taken together, these findings suggest that the α7 and β2 nAChR subunits are differentially engaged in the 500 ms trace eyeblink conditioning paradigm.
Differential Roles of nAChR Subtypes in Eyeblink Classical Conditioning
The genotype- and task-specific effects observed in the present study suggest that the functional impairments were mediated primarily via the absence of functional α7 nAChRs in brain regions critically involved in trace eyeblink classical conditioning. Though the cerebellum is essential for all variants of eyeblink classical conditioning (Christian and Thompson, 2003), trace conditioning impairments in α7 KOs do not appear to reflect dysfunction arising from a lack of cerebellar nAChRs for at least two reasons. First, α7 nAChR distribution is sparse in the cerebellum relative to many other neural regions (Séguéla et al., 1993; Li et al., 2008). Second, cerebellum-dependent delay eyeblink conditioning was unimpaired in α7 nAChR KOs. In contrast, α7 nAChRs are abundant in the hippocampus (Séguéla et al., 1993; De Biasi and Salas, 2008), a region with strong cholinergic innervation that is essential for acquisition in trace eyeblink classical conditioning (Solomon et al., 1986; Moyer et al., 1990; Tseng et al., 2004). nAChR dysfunction in the hippocampus may therefore be primarily responsible for the task-specific impairments observed in the present study.
The hippocampus expresses many nAChRs, of which the α7 and β2 subtypes are among the most prominent (Zoli et al., 1998; Alkondon and Albuquerque, 2004; Yakel and Shao, 2004). Differences in the contributions of these nAChR subtypes to hippocampal functioning necessary for acquisition of trace eyeblink classical conditioning may arise from differences in physiological properties and/or differing levels of expression within particular areas of the hippocampal formation. High concentrations of functional α7 nAChRs are found on GABAergic hippocampal interneurons, levels that typically exceed those of non-α7 nAChRs (Frazier et al., 1998a,b; McQuiston and Madison, 1999; Buhler and Dunwiddie, 2001; Fabian-Fine et al., 2001). Through extensive signaling with hippocampal principal neurons (e.g., CA1 pyramidal cells), GABAergic interneuron activity controls inhibition and disinhibition of these excitatory output cells, thus coordinating hippocampal network dynamics critical for learning-related plasticity (Paulsen and Moser, 1998; Jones et al., 1999; Buzsáki, 2002). Fast-acting α7 nAChR-mediated neurotransmission with hippocampal interneurons – which in turn coordinates principal cell firing – may therefore modulate hippocampal activity essential for acquisition of trace eyeblink classical conditioning. Indeed, in vitro preparations using selective nAChR agonists and antagonists have consistently demonstrated robust activation and antagonism of hippocampal interneurons regulated primarily through α7 nAChRs (Alkondon et al., 1998; Frazier et al., 1998a,b; Dani and Bertrand, 2007; Wanaverbecq et al., 2007). The regulation of patterns of excitatory and inhibitory hippocampal activity is critical for many forms of learning and memory (Paulsen and Moser, 1998; Hasselmo et al., 2002), and the α7 nAChR may play a more substantial role than non-α7 nAChRs in this regard.
In line with this role in the regulation of patterns of excitatory and inhibitory hippocampal activity, α7 nAChRs may be preferentially involved in mediation of hippocampal theta (Siok et al., 2006; Lu and Henderson, 2010). The distinct rhythmic oscillatory pattern of neuronal activity called the “theta rhythm” is associated with rapid acquisition of eyeblink classical conditioning (Berry and Thompson, 1978; Berry and Seager, 2001; Seager et al., 2002; Griffin et al., 2004; Hoffmann and Berry, 2009). Whereas differences in nAChR subtype-mediated hippocampal oscillatory patterns may contribute to the present behavioral findings, we cannot discount the possibility that other features of hippocampal function may contribute as well. Furthermore, we cannot rule out the possibility of significant nAChR contributions from extra-hippocampal regions. One such candidate is the medial prefrontal cortex, a region critical for the acquisition of trace – but not delay – eyeblink conditioning in rabbits (Weible et al., 2000; Kalmbach et al., 2009). Like the hippocampus, nAChRs are abundant in the neocortex (Séguéla et al., 1993; De Biasi and Salas, 2008), and excitatory synaptic transmission is differentially modulated via α7 relative to β2 nAChRs in the rodent frontal cortex (Rousseau et al., 2005; Dickinson et al., 2008). The trace conditioning-specific acquisition impairments in α7 nAChR KOs may reflect impaired α7 nAChR function in the neocortex, in addition to aforementioned hippocampal contributions.
Alpha Responding
Eyeblink responses occurring within the first 60 ms of CS onset are identified as alpha responses, characterized as non-associative “startle” activity to the CS. The present findings demonstrate that β2 KOs exhibit aberrant, heightened reactivity to the auditory CS. Behavioral studies using selective β2 pharmacological agents and β2 KO mice have yielded inconsistent findings regarding β2 nAChR-mediated processing of sensory information (see Schreiber et al., 2002; Champtiaux and Changeux, 2004), though enhanced startle responding in our eyeblink tasks may be associated with previous findings of hyperactivity in β2 nAChR KO mice (cf., Maubourguet et al., 2008). Of the potential neural substrates underlying altered sensory processing in β2 KO mice, thalamocortical circuitry is a likely candidate due to its critical role in auditory processing (see Kawai et al., 2007). Since normal nicotinic functioning in the thalamus is critically dependent on the β2 subunit (Picciotto et al., 1995), augmented startle responding to the auditory CS in β2 KOs may primarily reflect dysregulation of thalamocortical circuitry associated with the lack of functional β2 nAChRs. Thus, enhanced CR levels in delay-conditioned β2 nAChR KOs (see Figure 3) may be driven largely by altered sensory processing of the CS rather than reflecting enhancements in associative learning of the CS–US relationship. Future studies exploring CS alone and/or unpaired CS and US training in these mice may be useful in identifying the nature of this aberrant responding. Furthermore, alpha responding increased across training for most groups in the present study, including controls. Similar findings were observed in control and APP mice in a previous report from our laboratory (Ewers et al., 2006), suggesting some influence of the paired CS–US relationship on this measure.
Conclusions
The present findings highlight the importance of task selection in identifying functional impairments mediated via nAChR dysfunction. α7 nAChR KO mice were impaired in a hippocampus and prefrontal cortex-dependent trace eyeblink classical conditioning task but not in a delay conditioning task. In delay eyeblink conditioning, the hippocampus plays a modulatory role and can facilitate or impair conditioning, but it is not essential. The failure to find impairments in β2 KOs in these tasks suggests that the distribution of α7 nAChRs, as well as some of their unique functional properties (e.g., enhanced calcium permeability), plays a critical role relative to other nAChRs in certain learning and memory processes. Given the relative lack of behavioral impairments previously observed in α7 nAChR KOs (e.g., Paylor et al., 1998; but see Franceschini et al., 2000), the present findings indicate that α7 nAChR-mediated functions in this forebrain-dependent trace task are engaged to a greater degree than in many of the other measures of learning and memory previously assessed in nAChR KOs, thus making eyeblink conditioning a useful paradigm for future studies investigating functional deficits resulting from nAChR loss and dysfunction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Max Chae and Sung Kim for technical assistance with surgeries to implant electrodes and Christy Spath for behavioral training. The authors also thank Andrey Mavrichev for designing the software used for visualization of EMG activity from individual sessions. This research was supported by grants from the National Institute on Aging, 1 R01 AG021925 and 1 R01 AG023742 to Diana S. Woodruff-Pak.
References
Albuquerque, E. X., Pereira, E. F., Alkondon, M., and Rogers, S. W. (2009). Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120.
Alkondon, M., and Albuquerque, E. X. (2004). The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog. Brain Res. 145, 109–120.
Alkondon, M., Pereira, E. F. R., and Albuquerque, E. X. (1998). α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 810, 257–263.
Berry, S. D., and Seager, M. A. (2001). Hippocampal theta oscillations and classical conditioning. Neurobiol. Learn. Mem. 76, 298–313.
Berry, S. D., and Thompson, R. F. (1978). Prediction of learning rate from the hippocampal electroencephalogram. Science 200, 1298–1300.
Buhler, A. V., and Dunwiddie, T. V. (2001). Regulation of the activity of hippocampal stratum oriens interneurons by α7 nicotinic acetylcholine receptors. Neuroscience 106, 55–67.
Champtiaux, N., and Changeux, J-P. (2004). Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Prog. Brain Res. 145, 235–251.
Chen, G., and Steinmetz, J. E. (1998). A general-purpose computer system for behavioral conditioning and neural recording experiments. Behav. Res. Methods Instrum. Comput. 30, 384–391.
Chen, L., Bao, S., Lockard J. M., Kim, J. J., and Thompson, R. F. (1996). Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J. Neurosci. 16, 2829–2838.
Chen, L., Bao, S., and Thompson, R. F. (1999). Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degeneration mutant mice. Behav. Neurosci. 113, 204–210.
Christian, K. M., and Thompson, R. F. (2003). Neural substrates of eyeblink conditioning: acquisition and retention. Learn. Mem. 11, 427–455.
Cincotta, S. L., Yorek, M. S., Moschak, T. M., Lewis, S. R., and Rodefer, J. S. (2008). Selective nicotinic acetylcholine receptor agonists: potential therapies for neuropsychiatric disorders with cognitive dysfunction. Curr. Opin. Invest. Drugs 9, 47–56.
Cordero-Erausquin, M., Marubio, L., Klink, R., and Changeux, J-P. (2000). Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 21, 211–217.
Dani, J. A., and Bertrand, D. (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47, 699–729.
De Biasi, M., and Salas, R. (2008). Influence of neuronal nicotinic receptors on nicotine addiction and withdrawal. Exp. Biol. Med. 233, 917–929.
Dickinson, J. A., Kew, J. N. C., and Wonnacott, S. (2008). Presynaptic α7- and β2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 74, 348–359.
Ewers, M., Morgan, D. G., Gordon, M. N., and Woodruff-Pak, D. S. (2006). Associative and motorlearning in 12-month-old transgenic APP + PS1 mice. Neurobiol. Aging 27, 1118–1128.
Fabian-Fine, R., Skehel, P., Errington, M. L., Davies, H. A., Sher, E., Stewart, M. G., and Fine, A. (2001). Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 21, 7993–8003.
Franceschini, D., Orr-Urtreger, A., Yu, W., Macket, L. Y., Armstrong, D., Patrick, J. W., Beaudet, A. L., and De Biasis, M. (2000). Altered baroreflex responses in alpha7 deficient mice. Behav. Brain Res. 113, 3–10.
Frazier, C. J., Buhler, A. V., Weiner, J. L., and Dunwiddie, T. V. (1998a). Synaptic potentials mediated via α-Bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal neurons. J. Neurosci. 18, 8228–8235.
Frazier, C. J., Rollins, Y. D., Breese, C. R., Leonard, S., Freedman, R., and Dunwiddie, T. V. (1998b). Acetylcholine activates an α-Bungarotoxin-sensitive nicotinic current in rat hippocampal neurons, but not pyramidal cells. J. Neurosci. 18, 1187–1195.
Gotti, C., Zoli, M., and Clementi, F. (2006). Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 27, 482–491.
Green, J. T., and Arenos, J. D. (2007). Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol. Learn. Mem. 87, 269–284.
Griffin, A. L., Asaka, Y., Darling, R. D., and Berry, S. D. (2004). Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav. Neurosci. 118, 403–411.
Hasselmo, M. E., Bodelón, C., and Wyble, B. P. (2002). A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817.
Hoffmann, L. C., and Berry, S. D. (2009). Cerebellar theta oscillations are synchronized during hippocampal-contingent trace conditioning. Proc. Natl. Acad. Sci. U.S.A 106, 21371–21376.
Hogg, R. C., Raggenbass, M., and Bertrand, D. (2003). Nicotinic acetylcholine receptors: from structure to function. Rev. Physiol. Biochem. Pharmacol. 147, 1–46.
Ivkovich, D., and Stanton, M. E. (2001). Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiol. Learn. Mem. 76, 426–446.
Jones, S., Sudweeks, S., and Yakel, J. L. (1999). Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 22, 555–561.
Kakegawa, W., Miyazaki, T., Emi, K., Matsuda, K., Kohda, K., Motohashi, J., Mishina, M., Kawahara, S., Watanabe, M., and Yuzaki, M. (2008). Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRδ2. J. Neurosci. 28, 1460–1468.
Kalmbach, B. E., Ohyama, T., Kreider, J. C., Riusech, F., and Mauk, M. D. (2009). Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn. Mem. 16, 86–95.
Kawai, H., Lazar, R., and Metherate, R. (2007). Nicotinic control of axon excitability regulates thalamocortical transmission. Nat. Neurosci. 10, 1168–1175.
Li, J-G., Lehr, M., Liu-Chen, L-Y., and Woodruff-Pak, D. S. (2008). Nicotinic acetylcholine receptors and modulation of learning in 4- and 27-month-old rabbits. Neuropsychopharmacology 33, 2820–2830.
Lindstrom, J., Schoepfer, R., Conroy, W., Whiting, P., Das, M., Saedi, M., and Anand, R. (1991). The nicotinic acetylcholine receptor gene family: structure of nicotinic receptors from muscle and neurons and neuronal α-bungarotoxin-binding proteins. Adv. Exp. Med. Biol. 287, 255–278.
Lu, C. B., and Henderson, Z. (2010). Nicotine inductions of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience 166, 84–93.
Maubourguet, N., Lesne, A., Changeux, J. P., Maskos, U., and Faure, P. (2008). Behavioral sequence analysis reveals a novel role for beta2* nicotinic receptors in exploration. PLoS Comput. Biol. 4, 1–12. doi: 10.1371/journal.pcbi.1000229.
McQuiston, A. R., and Madison, D. V. (1999). Nicotinic receptor activation excites distinct subtypes of interneuron in the rat hippocampus. J. Neurosci. 19, 2887–2896.
Moyer, J. R., Deyo, R. A., and Disterhoft, J. F. (1990). Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104, 243–252.
Orr-Urtreger, A., Göldner, F. M., Saeki, M., Lorenzo, I., Goldberg, L., De Biasi, M., Dani, J. A., Patrick, J. W., and Beaudet, A. L. (1997). Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic current. J. Neurosci. 17, 9165–9171.
Paulsen, O., and Moser, E. I. (1998). A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 21, 273–278.
Paylor, R., Nguyen, M., Crawley, J. N., Patrick, J., Beaudet, A., and Orr-Urtreger, A. (1998). α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn. Mem. 5, 302–316.
Picciotto, M. R., Zoli, M., Léna, C., Bessis, A., Lallemand, Y., Le Novère, N., Vincent, P., Pich, E. M., Brûlet, P., and Changeux, J. P. (1995). Abnormal avoidance learning in mice lacking high-affinity nicotine receptor in the brain. Nature 374, 65–67.
Portugal, G. S., Kenney, J. W., and Gould, T. J. (2008). β2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol. Learn. Mem. 89, 106–113.
Rousseau, S. J., Jones, I. W., Pullar, I. A., and Wonnacott, S. (2005). Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]D-aspartate release form rat frontal cortex in vitro. Neuropharmacology 49, 59–72.
Schreiber, R., Dalmus, M., and De Vry, J. (2002). Effects of α4/β2- and α7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology 159, 248–257.
Seager, M. A., Johnson, L. D., Chabot, E. S., Asaka, Y., and Berry, S. D. (2002). Oscillatory brain states and learning: impact of hippocampal theta-contingent training. Proc. Natl. Acad. Sci. U.S.A. 99, 1616–1620.
Séguéla, P., Wadiche, J., Dineley-Miller, K., Dani, J., and Patrick, J. W. (1993). Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 13, 596–604.
Sihver, W., Nordberg, A., Långström, B., Mukhin, A. G., Koren, A. O., Kimes, A. S., and London, E. D. (2000). Development of ligands for in vivo imaging of cerebral nicotinic receptors. Behav. Brain Res. 113, 143–157.
Siok, C. J., Rogers, J. A., Kocsis, B., and Hajós, M. (2006). Activation of α7 acetylcholine receptors augments stimulation-induced hippocampal theta oscillation. Eur. J. Neurosci. 23, 570–574.
Solomon, P. R., Solomon, S. D., Vander Schaff, E., and Perry, H. E. (1983). Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science 220, 329–331.
Solomon, P. R., Vander Schaaf, E. R., Thompson, R. F., and Weisz, D. J. (1986). Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 100, 729–744.
Spath, C. A., and Woodruff-Pak, D. S. (2004). Neuron loss and age-related learning deficits are more apparent in cerebellum and a cerebellum-dependent task than in hippocampus. Soc. Neurosci. Abstr. 30, 325.18.
Tseng, W., Guan, R., Disterhoft, J. F., and Weiss, C. (2004). Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus 14, 58–65.
Walters, C. L., Brown, S., Changeux, J-P., Martin, B., and Damaj, M. I. (2006). The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology 184, 339–344.
Wanaverbecq, N., Semyanov, A., Pavlov, I., Walker, M. C., and Kullmann, D. M. (2007). Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic α7 nicotinic receptors. J. Neurosci. 27, 5683–5693.
Weible, A. P., McEchron, M. D., and Disterhoft, J. F. (2000). Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav. Neurosci. 114, 1058–1067.
Woodruff-Pak, D. S. (2003). Mecamylamine reversal by nicotine and by a partial α7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits tested with delay eyeblink classical conditioning. Behav. Brain Res. 143, 159–167.
Woodruff-Pak, D. S., Green, J. T., Levin, S. I., and Meisler, M. H. (2006). Inactivation of sodium channel Scn8A (Nav1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav. Neurosci. 120, 229–240.
Woodruff-Pak, D. S., Lehr, M. A., Li, J.-G., and Liu-Chen, L.-Y. (2010). Good learners have higher levels of brain nicotinic receptor binding than poor learners. Neurobiol. Aging 31, 1032–1043.
Woodruff-Pak, D. S., Li, Y.-T., Hinchliffe, R. M., and Port, R. L. (1997). Hippocampus in delay eyeblink classical conditioning: Essential for nefiracetam amelioration of leaning in older rabbits. Brain Res. 747, 207–218.
Woodruff-Pak, D. S., Li, Y-T., Kazmi, A., and Kem, W. (1994). Nicotinic cholinergic system involvement in eyeblink classical conditioning in rabbits. Behav. Neurosci. 108, 486–493.
Woodruff-Pak, D. S., Seta, S., Roker, L. A., and Lehr, M. A. (2007). Effects of age and inter-stimulus interval in delay and trace eyeblink classical conditioning in rabbits. Learn. Mem. 14, 287–294.
Woodruff-Pak D. S., and Steinmetz J. E. (2000a). Eyeblink Classical Conditioning, Vol. 1, Applications in Humans. Boston: Kluwer Academic Publishers.
Woodruff-Pak, D. S., and Steinmetz, J. E. (2000b). Eyeblink Classical Conditioning, Vol. 2, Animal Models. Boston: Kluwer Academic Publishers.
Woodruff-Pak, D. S., Tobia, M. J., Jiao, X., Beck, K. D., and Servatius, R. (2007). Preclinical investigation of the functional effects of memantine, and memantine combined with galantamine or donepezil. Neuropsychopharmacology 32, 1284–1294.
Yakel, J. L., and Shao, Z. (2004). Functional and molecular characterization of neuronal nicotinic ACh receptors in rat hippocampal neurons. Prog. Brain Res. 145, 95–107.
Keywords: knockout mice, cerebellum, hippocampus, learning, memory
Citation: Brown KL, Comalli DM, De Biasi M and Woodruff-Pak DS (2010) Trace eyeblink conditioning is impaired in α7 but not in β2 nicotinic acetylcholine receptor knockout mice. Front. Behav. Neurosci. 4:166. doi: 10.3389/fnbeh.2010.00166
Received: 07 June 2010;
Accepted: 16 September 2010;
Published online: 08 October 2010
Edited by:
Jeansok J. Kim, University of Washington, USAReviewed by:
Jeansok J. Kim, University of Washington, USAJune-Seek Choi, Korea University, South Korea
Copyright: © 2010 Brown, Comalli, De Biasi and Woodruff-Pak. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Diana S. Woodruff-Pak, Temple University, 1701 North 13th Street/Weiss Hall, Philadelphia, PA 19122, USA. e-mail: pak@temple.edu