Impaired social behavior in 5-HT3A receptor knockout mice
- Center for Neuroscience, Swammerdam Institute for Life Sciences, University of Amsterdam, Amsterdam, Netherlands
The 5-HT3 receptor is a ligand-gated ion channel expressed on interneurons throughout the brain. So far, analysis of the 5-HT3A knockout mouse revealed changes in nociceptive processing and a reduction in anxiety related behavior. Recently, it was shown that the 5-HT3 receptor is also expressed on Cajal-Retzius cells which play a key role in cortical development and that knockout mice lacking this receptor showed aberrant growth of the dendritic tree of cortical layer II/III pyramidal neurons. Other mouse models in which serotonergic signaling was disrupted during development showed similar morphological changes in the cortex, and in addition, also deficits in social behavior. Here, we subjected male and female 5-HT3A knockout mice and their non-transgenic littermates to several tests of social behavior. We found that 5-HT3A knockout mice display impaired social communication in the social transmission of food preference task. Interestingly, we showed that in the social interaction test only female 5-HT3A knockout mice spent less time in reciprocal social interaction starting after 5 min of testing. Moreover, we observed differences in preference for social novelty for male and female 5-HT3A knockout mice during the social approach test. However, no changes in olfaction, exploratory activity and anxiety were detected. These results indicate that the 5-HT3A knockout mouse displays impaired social behavior with specific changes in males and females, reminiscent to other mouse models in which serotonergic signaling is disturbed in the developing brain.
Introduction
The serotonin 5-HT3 receptor is a ligand-gated ion channel comprising two subunits (5-HT3A and 5-HT3B) of which the 5-HT3A subunit is essential to form a functional receptor (Maricq et al., 1991; Davies et al., 1999). In the brain, the 5-HT3A receptor is mainly expressed on interneurons in limbic regions such as the cortex, amygdala and hippocampus (Morales and Bloom, 1997). Anxiolytic effects of 5-HT3 receptor antagonists suggest a role for the 5-HT3 receptor in anxiety regulation (Costall et al., 1989).
The generation of the 5-HT3A knockout mouse was initially described by Zeitz et al. (2002) who examined the role of the 5-HT3A subunit in nociceptive processing. Apart from a decreased sensitivity to formalin induced pain, 5-HT3A knockout mice did not exhibit abnormalities in body weight, food, or water intake, sexual behavior, or motor function during rotarod performance. Further behavioral tests designed to measure anxiety such as the elevated plus maze showed reduced anxiety in the 5-HT3A knockout mouse (Kelley et al., 2003; Bhatnagar et al., 2004). Recently, our group found that Cajal-Retzius cells, a population of cells which play an important role in cortical development by regulating dendritic maturation, also express 5-HT3 receptors (Chameau et al., 2009). In the same study, analysis of the 5-HT3A knockout mouse showed an increase in dendritic complexity of the apical dendrites of cortical layer II/III pyramidal neurons (Chameau et al., 2009). Another study investigating the effects of disrupted serotonergic innervation to these Cajal-Retzius cells, reported changes in cortical organization (Janusonis et al., 2004). One group that examined the effects of depletion of 5-HT in the developing brain, found in addition to changes in cortical width several deficits in social behavior in mice that were 5-HT depleted (Boylan et al., 2007; Hohmann et al., 2007). Together, these studies suggest a relation between changes in cortical morphology as a consequence of disturbed serotonin signaling during development and social behavior later in life.
In this study, we sought to determine whether the 5-HT3A receptor knockout mouse displays similar forms of impaired social behavior. Therefore, we subjected male and female 5-HT3A receptor knockout mice and their wildtype littermates to the social transmission of food preference task, social interaction test and social approach test. Whereas the social transmission of food preference test investigates social communication by analyzing the natural preference for food previously scented on an unfamiliar mouse during an interaction phase, the social interaction test primarily focuses on reciprocal social interactions (Kogan et al., 1997; Bolivar et al., 2007). The social approach test, in turn, measures the preference for social novelty in a three compartment cage during two distinct phases (Moy et al., 2004; Sankoorikal et al., 2006). To control for anxiety, which might be an important driving force for impaired social behavior, 5-HT3A knockout mice were in addition tested in the novelty suppressed feeding test.
Materials and Methods
Animals
The generation of the 5-HT3A knockout mouse was initially described by Zeitz et al. (2002). In this study, 5-HT3A knockout mice were maintained on the C57BL/6J background and back-crossed for at least 35 generations. Homozygotic 5-HT3A knockout mice were bred with C57BL/6J mice to obtain F1 heterozygous KO mice. Subsequently, male and female heterozygote breeding pairs were formed. Offspring of these breeders were earmarked, and a small piece of the tail was cut at 1 week of age. This tissue was used for genotyping. The following primers were used:
5-HT3KO-FOR = 5′-AGT CAG CCT CTT GCT GCC CAG TA-3′ (2051-2073)
5-HT3KO-REV = 5′-TCC ATC CTG AAC CCA GCT TCC A-3′ (2622-2603)
5-HT3KO-NEO = 5′-TCG ACG TTG TCA CTG AAG CGG-3′ (1126-1146)
Mice were weaned at day 28 and same sex littermates were housed in groups of 2–4 under a 12-h dark/light cycle with lights on at 08.00 h and with ad libitum access of food and water. Between 7 and 14 weeks of age, 12 male and 12 female mutants and 14 male and 12 female wildtype littermates originating from 15 litters were tested in a battery of behavioral tests in the following order: social transmission of food preference (7 weeks), social interaction (8 weeks), social approach (9 weeks), buried food finding (12 weeks), and novelty suppressed feeding test (14 weeks). When mice were excluded from one of the tests they were not further tested. Tests took place between 13:00 and 18:30 hours except for the social transmission of food preference and buried food finding which started at 09:00 hours. Experiments were performed according to the guidelines of the ethical committee of the University of Amsterdam and in accordance with the European Council Directive.
Behavioral Testing
Social transmission of food preference
The procedure was adapted from Kogan et al. (1997). Briefly, 5 days before the experiment animals were placed in novel home cages (32 cm × 16 cm × 14 cm) and transferred to the testroom. Three days before testing animals were given powdered food pellets from a plastic cup (8 cm diameter and 2 cm deep) placed in one corner of the cage. Testing of the mice comprised three phases. First, C57BL/6J demonstrator mice originating from a separate breeding that was not subject to any other test, but of the same sex and age as the test mice were removed from their cage and food restricted for 15 h. Second, test mice further indicated as observer mice were food restricted for 15 h. At the day of testing demonstrator mice were allowed to consume powdered food pellets scented with either 1% cinnamon or 2% cacao for 2 h which was randomly chosen and equally divided. Only mice eating more than 0.2 g were used as demonstrator mice. In the next phase, demonstrator mice were placed in the home cages of the observer mice were they could interact for 5 min. Immediately thereafter, observer mice were placed individually into novel cages (32 cm × 16 cm × 14 cm) for 30 min. These cages contained water and two plastic cups with pre weighed scented food on opposite sides of the cage. One of the cups contained food which was identical to the food the demonstrator mouse previously had eaten. At the end of the test total amount of food eaten was measured by weighing the amount of food left in both cups.
Social interaction
The procedure was adapted from Bolivar et al. (2007). Briefly, the chamber for testing was a clear Plexiglas box (36 cm × 20 cm × 19 cm) with fresh bedding on the bottom. After each test this box was cleaned with ethanol and fresh bedding was added. Pairs of unfamiliar mice of same sex, age, and genotype were formed. Prior to the test both mice were allowed to habituate to a similar box for 10 min. Immediately after this period, mice were placed at opposite ends of the test box and social interaction was recorded on videotape for 20 min. The following types of social behavior were analyzed off-line: sniffing (nose, body, or anogenital), mounting, following/chasing, wrestling/biting, and allogrooming. The amount of time during which pairs of mice were engaged in the above indicated social interactions was analyzed in blocks of 5 min. The number of times a mouse from a pair showed a specific type of behavioral response during the test was analyzed for each individual test mouse.
Social approach
The procedure was adapted from Moy et al. (2004) and Sankoorikal et al. (2006). The testing arena was a rectangular, three chambered Plexiglas box. Each chamber was 20 cm × 12 cm × 19 cm in size, separated from the other chambers by walls with rectangular openings (6 cm × 6 cm). In each end chamber, a transparent plastic cup (diameter 8 cm height 9 cm) with a total of 20 (12 mm) holes in it to allow nose contact was placed. The test consisted of three phases: (i) habituation, (ii) test phase one, and (iii) test phase two. During habituation a mouse was allowed to explore the center chamber for 5 min while the openings to the end chambers remained closed. For test phase one an unfamiliar C57BL/6J mouse originating from a separate breeding but of the same sex and age was placed in one of the plastic cups. This mouse had previously been habituated to the placement in the cup. To prevent climbing a glass bottle filled with water was placed on top of the cup. The cup in the other side of the chamber was left empty. Starting phase one of the test, both openings were unblocked and the test mouse was allowed to explore freely all chambers for 10 min. Behavior was recorded on videotape (Akai VHS and Sony camera) and the amount of time spent in each chamber and the number of transitions were analyzed off-line. At the end of phase one the animal was placed back in the center chamber while the other chambers were blocked again. For test phase two, another unfamiliar mouse was placed in the empty cup. In this phase, the mouse had a choice between the in the previous phase investigated mouse and a novel mouse. Again, after unblocking the openings the mouse was allowed to explore all chambers for 10 min. Similar to the previous phase the amount of time spent in each chamber and the number of transitions was recorded and analyzed afterward. After each trial the box was cleaned with ethanol and fresh bedding was added.
Buried food finding
The procedure was adapted from Jamain et al. (2008). Two days before testing, mice received 1.5 g of chocolate chip cookies and 1 g of powdered food pellets per mouse with water ad libitum each day. Fifteen hours before testing food was removed. At the day of testing mice were placed individually into a clear Plexiglas box (36 cm × 20 cm × 19 cm), in which a piece of chocolate chip cookie of 1.7 g was hidden under a 1.5-cm layer of standard bedding at the far end of the box. The mouse was introduced at the other end of the box and allowed to explore for 6 min. The latency retrieve the hidden cookie was recorded. All mice managed to find the hidden cookie within 6 min. After each experiment boxes were cleaned with ethanol and a new cookie and fresh bedding was added.
Novelty suppressed feeding
The procedure was adapted from Gross et al. (2002). Three days before testing mice were housed individually. Fifteen hours before the onset of the test mice were weighed and food was removed from the cage, while water remained available ad libitum. At the day of testing, mice were transferred to the testing room and at the onset of the test mice were placed in an open field (70 cm × 70 cm) with a pre weighed food pellet in the center. For this experiment latency to begin chewing food was measured. Immediately after beginning to eat or 5 min after being placed in the open field, mice returned to their homecage were they could continue to consume the pellet for 5 min. The amount of food consumed was measured by weighing the food pellet afterward. At the end of the test animals were weighed again.
Statistical Analysis
Statistical significance of all tests was determined as p < 0.05. In the social transmission of food preference task a two-way ANOVA with repeated measures for cue was used. After a main significant effect of genotype and/or cue, within-group post hoc Bonferroni comparisons were performed. For analysis of the social approach test we used a two-way ANOVA with repeated measures for chamber. After a main significant effect of genotype and/or chamber, within-group post hoc Bonferroni comparisons were performed. In the social interaction test, the buried food finding test and the novelty suppressed feeding test an unpaired two-tailed Student’s t-test or a Mann–Whitney test for non-parametric data was used. All data are expressed as mean ± S.E.M.
Results
Social Transmission of Food Preference and Buried Food Finding
After a short interaction period with a demonstrator mouse which had previously eaten “cued” scented food, male wildtype observer mice showed a preference for cued food (0.427 ± 0.048 g) over non-cued food (0.207 ± 0.036 g) in terms of the amount of both types of food eaten. In contrast, male 5-HT3A knockout observer mice did not show a preference for cued food (Figure 1A; post hoc Bonferroni comparisons following a RMANOVA significant main effect of cue F1,24 = 4.875, p = 0.0371 revealed for WT: p < 0.05, n = 14 and KO: p > 0.05, n = 12). Overall, the total amount of food eaten was comparable between genotypes (WT: 0.63 ± 0.084; KO: 0.59 ± 0.096).
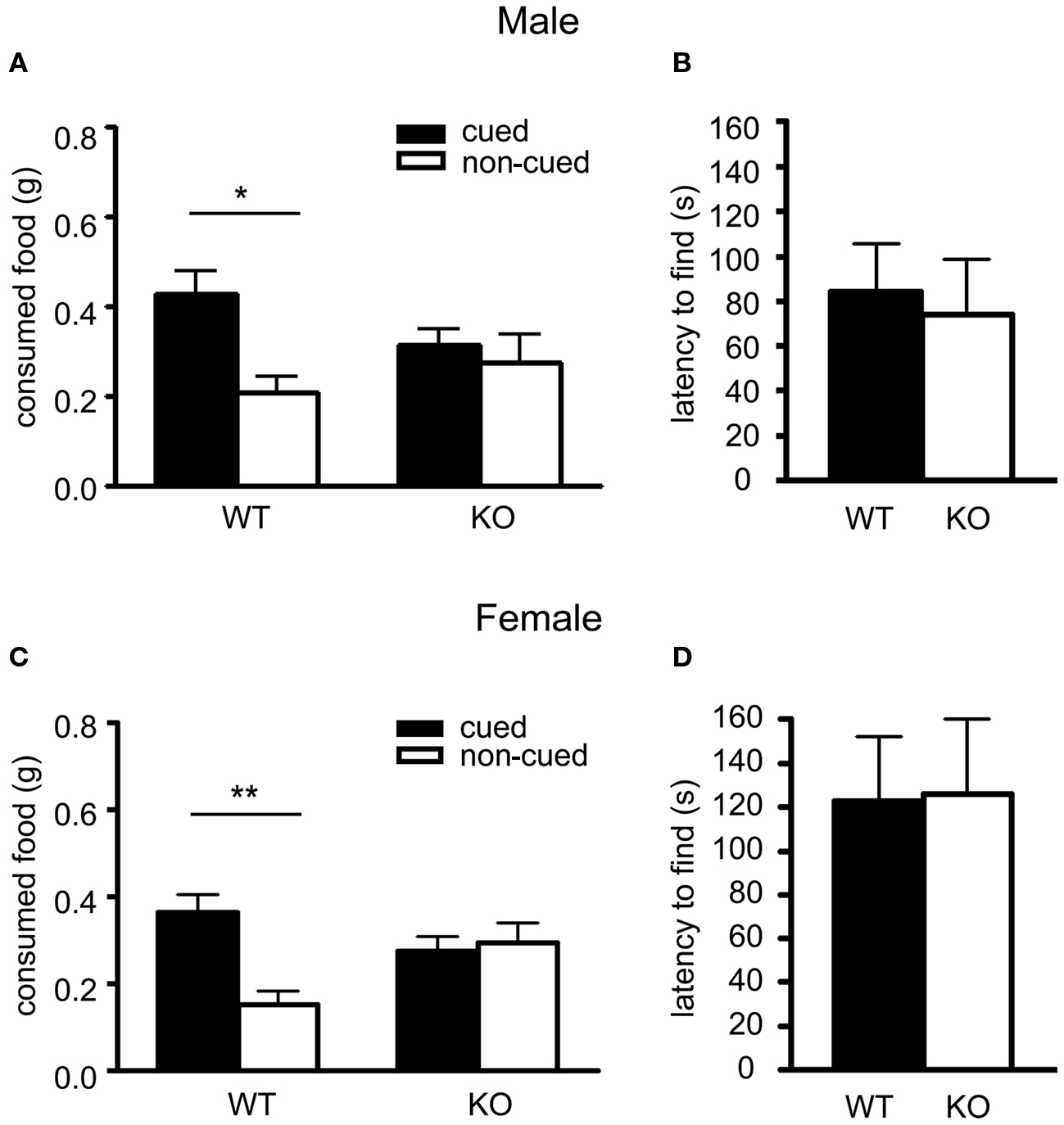
Figure 1. Social communication but not olfaction is impaired in the 5-HT3A knockout mouse. In the social transmission of food preference task both male (A) and female (C) 5-HT3A knockout mice ate equal amounts of both cued food previously smelled on a demonstrator mouse and non-cued food, whereas wildtype mice ate more cued food than non-cued food. In the buried food finding test both male (B) and female (D) 5-HT3A knockout mice showed a similar latency to find a hidden cookie as compared to wildtype mice. Data are expressed as the mean ± SEM (*p < 0.05, **p < 0.01).
Similar results were found for female wildtype and 5-HT3A knockout observer mice. Here, female wildtype mice ate (0.365 ± 0.042 g) of cued food over (0.151 ± 0.031 g) of non-cued food, while female 5-HT3A knockout mice ate equal amounts of both cued and non-cued food (Figure 1C; post hoc tests following a main effect of cue F1,22 = 4.921, p = 0.0372 and interaction cue × genotype F1,22 = 7.197, p = 0.0136 show for WT: p < 0.01, n = 12 and KO: p > 0.05, n = 12). Again, both wildtype and 5-HT3A knockout observer mice ate comparable amounts of food in total (WT: 0.52 ± 0.072; KO: 0.57 ± 0.08).
To ascertain that the lack of preference for cued food observed in the 5-HT3A knockout mouse was not due to impaired olfaction, mice were tested in the buried food finding test. In this test, both male and female 5-HT3A knockout mice showed no difference in latency to find a hidden cookie as compared to wildtype mice (Figure 1B; WT n = 13, KO n = 8) (Figure 1D; WT n = 12, KO n = 11).
Social Interaction
In this test, male 5-HT3A knockout mice spent equal amounts of time in reciprocal social interaction as compared to wildtype mice (Figure 2A; WT n = 7 pairs, KO n = 4 pairs). To investigate the frequency of reciprocal social interactions over time, interaction time was analyzed in blocks of 5 min. As shown in Figure 2B, both groups showed a similar reduction in time spent in social interaction as the test continued. Analysis of the number of times individual mice displayed a specific type of behavioral response during the test showed that male 5-HT3A knockout mice displayed less mounting (WT; 9 ± 1.5, KO; 4 ± 1; p = 0.03) behavior during the test (Figure 2C; WT n = 14, KO n = 8). None of the pairs showed allogrooming behavior. Although biting and wrestling behavior during test was observed for two wildtype and two knockout pairs, no differences between groups were detected.
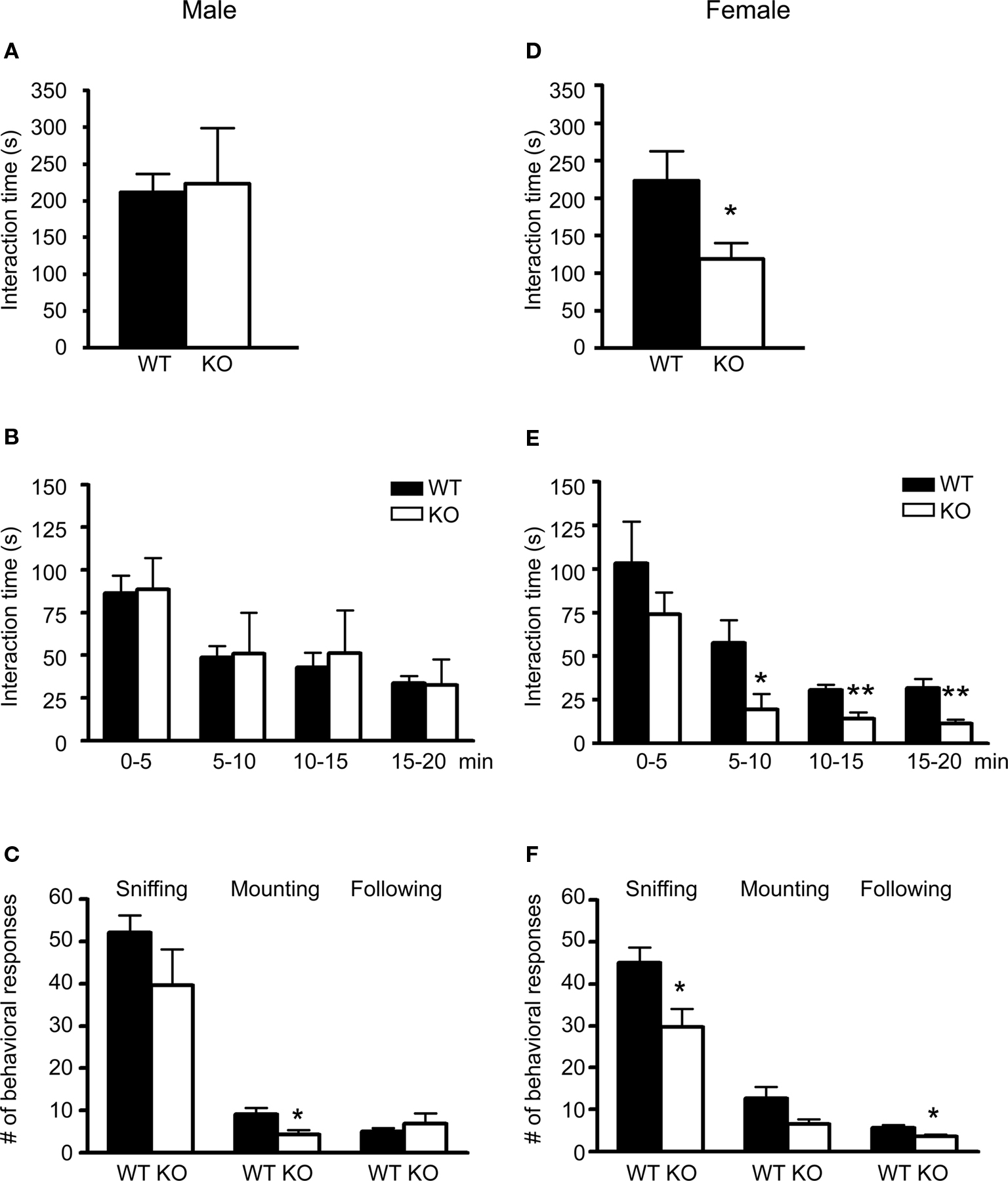
Figure 2. Only female 5-HT3A knockout mice show deficits in social interaction. (A) Pairs of male 5-HT3A knockout mice spent a similar amount of time in reciprocal social interaction as compared to pairs of wildtype mice. (B) When total interaction time was divided into 5 min blocks, both wildtype and 5-HT3A knockout mice showed a decrease in reciprocal social interactions over time. (C) Male 5-HT3A knockout mice only showed a lower number of mounting responses during the test as compared to wildtype mice. (D) Pairs of female 5-HT3A knockout mice spent less time in reciprocal social interaction than pairs of wildtype mice. (E) Although both groups showed a similar decrease in reciprocal social interactions over time, female 5-HT3A knockout mice spent less time in reciprocal social interaction between 5 and 20 min of testing than wildtype mice. (F) Female 5-HT3A knockout mice showed a lower number of sniffing and following responses during the test as compared to wildtype mice. Data are expressed as the mean ± SEM (*p < 0.05; **p < 0.01).
In contrast, female 5-HT3A knockout mice spent less time in reciprocal social interaction as compared to wildtype mice (WT; 223.8 ± 39.2 s, KO; 119.2 ± 20.9 s) (Figure 2D; WT n = 5 pairs, KO n = 5 pairs; p = 0.047). When time spent in social interaction was divided into 5 min blocks, it became clear that as the test continued time spent in social interaction reduced for both wildtype and 5-HT3A knockout mice. However, only during the first 5 min both wildtype and 5-HT3A knockout mice spent equal amounts of time in social interaction (Figure 2E; WT n = 5 pairs, KO n = 5 pairs; p = 0.043 for 5–10 min, p = 0.009 for 10–15 min, p = 0.007 for 15–20 min).
In addition, female 5-HT3A knockout mice displayed less sniffing (WT; 45 ± 4, KO; 30 ± 3; p = 0.015) and following (WT; 5.7 ± 0.7, KO; 3.6 ± 0.4; p = 0.018) responses during the test (Figure 2F). Again no allogrooming behavior was observed. Also, none of the pairs started to bite or wrestle during the test.
Social Approach
Analysis of the first phase of the experiment showed that, unlike wildtype mice, male 5-HT3A knockout mice did not show a significant preference for the chamber containing an unfamiliar mouse versus an empty cup (Figure 3A1; post hoc Bonferroni comparisons following a RMANOVA significant main effect of genotype F1,21 = 16.69, p = 0.0005 and chamber F1,21 = 11.11, p = 0.0032 revealed for WT: p < 0.01, n = 13 and KO: p > 0.05, n = 10). On the other hand, female 5-HT3A knockout mice did show a preference for the chamber containing an unfamiliar mouse over an empty cup (Figure 3C1; post hoc tests following a main effect of genotype F1,21 = 5.121, p = 0.0344 and chamber F1,21 = 28.8, p < 0.0001 show for WT: p < 0.05, n = 12 and KO: p < 0.001, n = 11).
Interestingly, in the second phase of the experiment male 5-HT3A knockout mice performed similar to wildtype mice by exhibiting a preference for the unfamiliar mouse over the familiar mouse (Figure 3A2; post hoc tests following a main effect of genotype F1,21 = 8.848, p = 0.0072 and chamber F1,21 = 18.65, p = 0.0003 show for WT: p < 0.05, n = 13 and KO: p < 0.01, n = 10). This time in female 5-HT3A knockout mice, the preference for the unfamiliar mouse over the familiar mouse did not reach significance (Figure 3C2; post hoc tests following a main effect of chamber F1,21 = 20.77, p = 0.0002 show for WT: p < 0.001, n = 12 and KO: p > 0.05, n = 11). However, the familiar-new effect for female KO mice in Figure 3C2 was significant when tested with a paired t-test (p = 0.01).
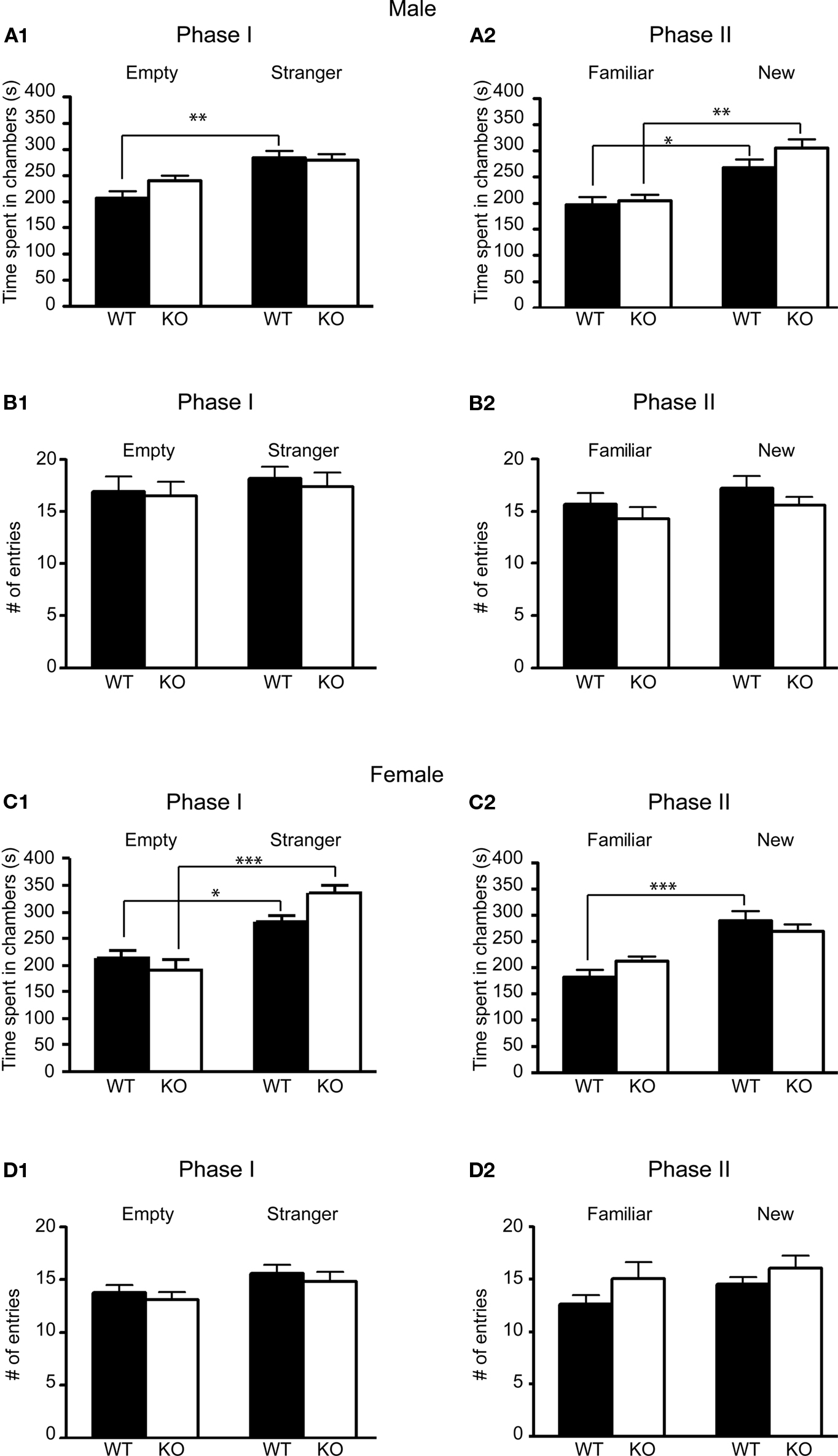
Figure 3. Male and Female 5-HT3A knockout mice show differences in preference for social novelty in the social approach test. (A1,C1) In the first phase (Phase I) of the social approach test only female and not male 5-HT3A knockout mice spent significantly more time in a chamber containing a wildtype stranger mouse than in a chamber containing an empty cup similar to wildtype mice. (A2,C2) In the second phase (Phase II) of the test only male and not female 5-HT3A knockout mice spent significantly more time in the chamber containing a novel stranger mouse than in the chamber containing the same mouse of the previous experiment similar to wildtype mice. (B,D) During both phases of the test both wildtype and 5-HT3A knockout mice of both sexes did not visit one of the end chambers more than the other. Data are expressed as the mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001).
Analysis of the number of visits to one of the end chambers showed that in both phases of the test none of the wildtype or 5-HT3A knockout mice visited one of the end chambers more than the other (Figures 3B,D). To compare exploratory activity of both groups, the total number of visits to both chambers was analyzed. However, analysis did not reveal a difference in total number of visits between wildtype and 5-HT3A knockout mice (Figures 3B,D).
Novelty Suppressed Feeding
Both male and female 5-HT3A knockout mice showed a similar latency to eat a food pellet placed in the center of an open field as compared to wildtype mice (Figure 4A; WT n = 13, KO n = 8) (Figure 4B; WT n = 12, KO n = 11). Also the time spent eating and the amount of food pellet eaten in the home cage after the experiment were not different. Furthermore, 5-HT3A knockout mice did not show more weight loss due to food deprivation as compared to wildtype mice.
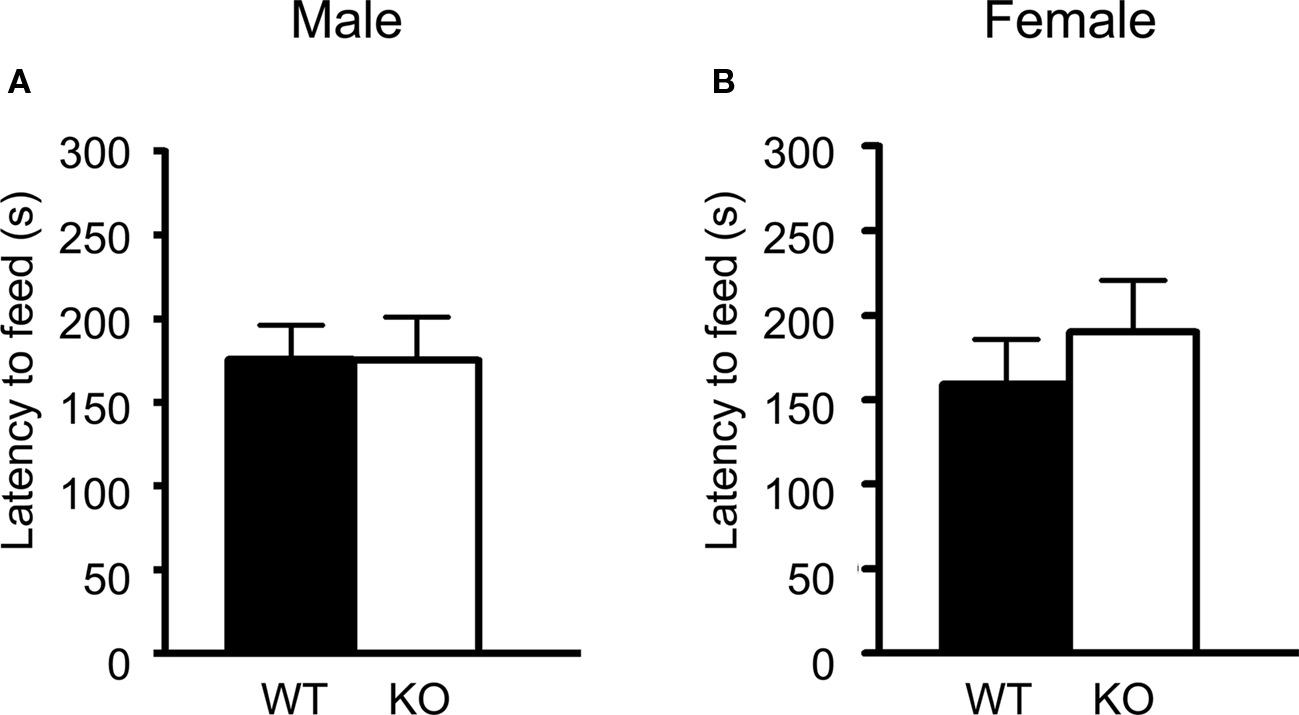
Figure 4. 5-HT3A knockout mice do not show changes in anxiety in the novelty suppressed feeding task. (A,B) Both male and female 5-HT3A knockout mice showed a similar latency to start eating a food pellet in the novelty suppressed feeding task as compared to wildtype mice. Data are expressed as the mean ± SEM.
Discussion
In this study, we found that 5-HT3A knockout mice displayed impaired social communication in the social transmission of food preference task, while olfaction was not affected. Moreover, we showed that female, but not male 5-HT3A knockout mice spent less time in reciprocal social interaction after 5 min of testing onwards. While male 5-HT3A knockout mice only showed a reduction in the number of mounting responses during this test, female 5-HT3A knockout mice showed a reduction in both sniffing and following responses. Interestingly, we found that in the social approach test male 5-HT3A did not show a significant preference for the cup in which a mouse was placed over an empty cup, whereas female 5-HT3A knockout mice did not show a significant preference for the chamber containing a novel mouse over the chamber containing a familiar mouse. Yet, no abnormalities in exploratory activity were observed for both groups in the social approach test. Also, no changes in anxiety in the novelty suppressed feeding task were detected. These results indicate that both male and female 5-HT3A knockout mice display specific forms of impaired social behavior, although some deficits could only be observed in male or female 5-HT3A knockout mice.
Social communication skills as investigated in the social transmission of food preference task could be measured based on the knowledge that communication in rodents primarily relies on olfactory cues and in general rodents are neophobic and avoid novel food (File, 2001; Wrenn et al., 2003; Ryan et al., 2008). We found that both male and female 5-HT3A knockout mice did not succeed in extracting the proper olfactory information from the demonstrator mouse during the 5-min interaction phase while not showing olfactory dysfunction based on the results of the buried food finding test. However, in the social interaction test both male and female 5-HT3A knockout mice did not spend less time in social interaction during the first 5 min. Together, these results support the interpretation that 5-HT3A knockout mice show impaired social behavior and failed to retrieve any olfactory information in the social transmission of food preference test because of communication deficits. Although olfactory dysfunction has been ruled out as an explanation for the observed effects, it cannot be ruled out that the observed impairments in social behavior are related to a global impairment in (sensory) information processing.
In the social interaction test, it became apparent that both male and female 5-HT3A knockout mice showed a reduction in time spent in reciprocal social interaction as the test continued, but that only female 5-HT3A knockout mice spent less time in reciprocal interaction than wildtype mice. Consistent with these results, only female 5-HT3A knockout mice showed less sniffing and following responses than wildtype mice. Male 5-HT3A knockout mice, on the other hand, only showed a reduction in mounting responses, which can be considered as negative social behavior (Bolivar et al., 2007). It is interesting to note that only male pairs showed biting and wrestling behavior during the test, although no differences between wildtype and 5-HT3A knockout mice could be observed. These results imply that deficits in reciprocal social interaction are specific for female 5-HT3A knockout mice, and that negative social behavior did not overrule the performance of the females.
Another frequently used test to investigate social behavior is the social approach test. This test focuses on the preference for social novelty while reciprocal social interactions are intentionally avoided by allowing the test mice to explore all chambers while target mice are caged within a plastic cup (Brodkin et al., 2004; Moy et al., 2004; Sankoorikal et al., 2006). In contrast to female mice, male 5-HT3A knockout mice did not show a significant preference for a mouse over a cup in the first phase. These results suggest male and female 5-HT3A knockout show different behaviors in terms of preference for social novelty. Another important observation in this study was that overall activity of the 5-HT3A knockout mouse was unaffected in terms of visits to each chamber in the social approach test. The fact both male and female 5-HT3A knockout mice did not show a reduction in overall activity, strengthens the interpretation that social behavior is not impaired as a result of changes in exploratory behavior.
However, also anxiety could be an important driving force for deficits in social behavior. Mice that are anxious will avoid novel situations and thus reciprocal social interactions (Crawley, 2007). On the other hand, mice that show reduced anxiety might initiate a novel social encounter more easily. Although some studies showed reduced anxiety in 5-HT3A knockout mice (Kelley et al., 2003; Bhatnagar et al., 2004), here we did not show differences in anxiety between wildtype and 5-HT3A knockout mice. Therefore, it is less likely that a change in anxiety influenced the performance of the 5-HT3A knockout mice involved in this study.
The deficits male and female 5-HT3A knockout mice displayed in the social transmission of food preference test and other social behavior tests are in concordance with other studies which investigated the effects of early developmental alterations in serotonergic signaling. One group that examined the effects of a depletion of 5-HT fibers in the neocortex of neonatal mice reported sex-dependent deficits in processing social, sensory, and environmental information apart from changes in the width of cortical layers. Strikingly, male but not female lesioned mice were more neophobic, while both sexes showed deficits in the social transmission of food preference task which is in line with our results (Boylan et al., 2007; Hohmann et al., 2007). Another group which reported overall changes in brain volume in a genetic mouse line that was haploinsufficient for PTEN and SLC6A4, two susceptibility genes for autism which have an indirect influence on serotonergic signaling, reported sex specific changes in the social approach test (Page et al., 2009). However, in contrast to the present study, they showed that females showed a lack of preference for the cup in which a mouse was placed over an empty cup. Together, these studies imply that disturbances in serotonergic signaling during early development lead to sex specific deficits in social behavior later in life. However, the underlying mechanism via which serotonin affects neurodevelopment in males and females specifically remains to be investigated.
Another important aspect these studies have in common is that the overall phenotype of these mice is reminiscent to autism, a severe neurodevelopmental disorder defined by deficits in social interaction, communication and repetitive behavior. Beside these behavioral symptoms, some consistent neuroanatomical changes in brain tissue of autistic individuals have been described such as a change in cortical minicolumn size, number and cellular distribution, and increased cortical volumes (Bailey et al., 1998; Casanova et al., 2002; Carper and Courchesne, 2005). Another interesting observation is that 30% of the autistic patients show an increase in blood levels of serotonin (Anderson et al., 1987; Chugani, 2002). According to the hyperserotonemia model an increase in blood serotonin in autistic patients could lead to a loss of serotonin terminals in the brain, thereby influencing ongoing developmental processes (Whitaker-Azmitia, 2005). Serotonin has an important regulatory function during neurodevelopment and changes in serotonergic signaling have been implicated to play a role in a number of neurodevelopmental disorders (Gaspar et al., 2003). Susceptibility genes for autism including PTEN and SLC6A4 have an indirect influence on serotonergic signaling and investigation of genetic mouse models interfering with the expression of these genes showed changes in brain volume, cortical thickness, and sex specific changes in social behavior reminiscent to the described changes in autism (Kwon et al., 2006; Page et al., 2009).
In the 5-HT3A knockout mouse an increase in dendritic complexity of the apical dendrites of cortical layer II/III pyramidal neurons together with a reduction in the glycoprotein reelin has been reported (Chameau et al., 2009). Similarly, in another study serotonergic innervation to Cajal-Retzius cells was disrupted resulting in aberrant cortical column formation and decreased reelin levels (Janusonis et al., 2004). Next to impaired serotonin signaling, also reduced reelin levels have been proposed to play an important role in several neurodevelopmental disorders such as autism (Fatemi, 2002). Likewise, a number of similarities between the morphological and behavioral phenotype of the 5-HT3A knockout mouse and other animal models investigating the effects of impaired serotonin signaling in relation to autism can be found. Nevertheless, it remains important to note that this does not imply that similar changes occur in autism. Taken together, by showing specific deficits in social behavior in both male and female 5-HT3A knockout mice we provide knowledge about the function of the 5-HT3 receptor and the behavioral consequences of deficits in serotonin receptor functioning during development.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Harm Krugers for help with the social transmission of food preference task, Martijn Rep for assistance with genotyping the mice, David Julius (University of San Francisco, San Fransisco, CA, USA) for providing the 5-HT3A knockout mice and Melly Oitzl for her comments on the manuscript.
References
Anderson, G. M., Freedman, D. X., Cohen, D. J., Volkmar, F. R., Hoder, E. L., McPhedran, P., Minderaa, R. B., Hansen, C. R., and Young, J. G. (1987). Whole blood serotonin in autistic and normal subjects. J. Child. Psychol. Psychiatry 28, 885–900.
Bailey, A., Luthert, P., Dean, A., Harding, B., Janota, I., Montgomery, M., Rutter, M., and Lantos, P. (1998). A clinicopathological study of autism. Brain 121, 889–905.
Bhatnagar, S., Nowak, N., Babich, L., and Bok, L. (2004). Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forces swim and defensive withdrawal test. Behav. Brain Res. 153, 527–535.
Bhatnagar, S., Sun, L. M., Raber, J., Maren, S., Julius, D., and Dallman, M. F. (2004). Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT3A receptor. Physiol. Behav. 81, 545–455.
Bolivar, V. J., Walters, S. R., and Phoenix, J. L. (2007). Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav. Brain Res. 176, 21–26.
Boylan, C. B., Blue, M. E., and Hohmann, C. F. (2007). Modeling early cortical serotonergic deficits in autism. Behav. Brain Res. 176, 94–108.
Brodkin, E. S., Hagemann, A., Nemetski, N. M, and Silver, L. M. (2004). Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 1002, 151–157.
Carper, R. A., and Courchesne. E. (2005). Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry 57, 126–133.
Casanova, M. F., Buxhoeveden, D. P., Switala, A. E., and Roy, E. (2002). Minicolumnar pathology in autism. Neurology 58, 428–432.
Chameau, P., Inta, D., Vitalis, T., Monyer, H., Wadman, W. J., and van Hooft, J. A. (2009). The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 106, 7227–7232.
Chugani, D. C. (2002). Role of altered brain serotonin mechanisms in autism. Mol. Pscychiatry. 7, S16–S17.
Costall, B., Jones, B. J., Kelley, M. E., Naylor, R. J., and Tomkins, D. M. (1989). Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol. Biochem. Behav. 32, 777–785.
Crawley, J. N. (2007). Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 17, 448–459.
Davies, P. A., Pistis, M., Hanna, M. C., Peters, J. A., Lambert, J. J., Hales, T. G., and Kirkness, E. F. (1999). The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397, 359–363.
File, S. E. (2001). Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 125, 151–157.
Gaspar, P., Cases, O., and Maroteaux, L. (2003). The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 12, 1002–1012.
Gross, C., Zhuang, X., Stark, K., Ramboz, S., Oosting, R., Kirby, L., Santarelli, L., Beck, S., and Hen, R. (2002). Serotonin1A receptor acts during development to establish normal anxiety-like behavior in the adult. Nature 416, 396–400.
Hohmann, C. F., Walker, E. M., Boylan, C. B., and Blue, M. E. (2007). Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Res. 1139, 163–177.
Jamain, S., Radyushkin, K., Hammerschmidt, K., Granon, S., Boretius, S., Varogueaux, F., Ramanantsoa, N., Gallego, J., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., Bourgeron, T., Ehrenreich, H., and Brose, N. (2008). Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. U.S.A. 105, 1710–1715.
Janusonis, S., Gluncic, V., and Rakic, P. (2004). Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J. Neurosci. 24, 1652–1659.
Kelley, S. P., Bratt, A. M., and Hodge, C. W. (2003). Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur. J. Pharmacol. 461, 19–25.
Kogan, J. H., Frankland, P. W., Blendy, J. A., Coblentz, J., Marowitz, Z., Schutz, G., and Silva, A. J. (1997). Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 7, 1–11.
Kwon, C. H., Luikart, B. W., Powell, C. M., Zhou, J., Matheny, S. A., Zhang, W., Li, Y., Baker, S. J., and Parada, L. F. (2006). Pten regulates neuronal arborization and social interaction in mice. Neuron 50, 377–388.
Maricq, A. V., Peterson, A. S., Brake, A. J., Myers, R. M., and Julius, D. (1991). Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254, 432–437.
Morales, M., and Bloom, F. E. (1997). The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J. Neurosci. 17, 3157–3167.
Moy, S. S., Nadler, J. J., Perez, A., Barbaro, R. P., Johns, J. M., Magnuson, T. R., Piven, J., and Crawley, J. N. (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 5, 287–302.
Page, D. T., Kuti, O. J., Prestia, C., and Sur, M. (2009). Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc. Natl. Acad. Sci. U.S.A. 106, 1989–1994.
Ryan, B. C., Young, N. B., Moy, S. S., and Crawley, J. N. (2008). Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav. Brain Res. 193, 235–242.
Sankoorikal, G. M., Kaercher, K. A., Boon, C. J., Lee, J. K., and Brodkin, E. S. (2006). A Mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry 59, 415–423.
Whitaker-Azmitia, P. M. (2005). Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int. J. Dev. Neurosci. 23, 75–83.
Wrenn, C. C., Harris, A. P., Saavedra, M. C., and Crawley J. N. (2003). Social transmission of food preference in mice: methodology and application to galanin- overexpressing transgenic mice. Behav Neurosci. 117, 21–31.
Zeitz, K. P., Guy, N., Malmberg, A. B., Dirajlal, S., Martin, W. J., Sun, L., Bonhaus, D. W., Stucky, C. L., Julius, D., and Basbaum, A. I. (2002). The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J. Neurosci. 22, 1010–1019.
Keywords: serotonin, knockout, cortex, development, autism, social interaction, anxiety
Citation: Smit-Rigter LA, Wadman WJ and van Hooft JA (2010) Impaired social behavior in 5-HT3A receptor knockout mice. Front. Behav. Neurosci. 4:169. doi: 10.3389/fnbeh.2010.00169
Received: 25 July 2010;
Accepted: 07 October 2010;
Published online: 01 November 2010.
Edited by:
John F. Cryan, University College Cork, IrelandReviewed by:
David Crews, University of Texas at Austin, USA;René Hurlemann, University of Bonn, Germany
Copyright: © 2010 Smit-Rigter, Wadman and van Hooft. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Johannes A. van Hooft, Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, P.O. Box 94232, 1090 GE Amsterdam, Netherlands. e-mail: j.a.vanhooft@uva.nl