Mice lacking Ras-GRF1 show contextual fear conditioning but not spatial memory impairments: convergent evidence from two independently generated mouse mutant lines
- 1 Division of Neuroscience, Institute of Experimental Neurology, San Raffaele Scientific Institute and University, Milano, Italy
- 2 Institute of Membrane and Systems Biology, University of Leeds, Leeds, UK
- 3 Institute of Anatomy and Zurich Center for Integrative Human Physiology, Institute of Human Movement Sciences, University of Zurich, Zurich, Switzerland
- 4 Institute of Psychiatry, King’s College London, London, UK
- 5 School of Biosciences, Cardiff University, Cardiff, UK
Ras-GRF1 is a neuronal specific guanine exchange factor that, once activated by both ionotropic and metabotropic neurotransmitter receptors, can stimulate Ras proteins, leading to long-term phosphorylation of downstream signaling. The two available reports on the behavior of two independently generated Ras-GRF1 deficient mouse lines provide contrasting evidence on the role of Ras-GRF1 in spatial memory and contextual fear conditioning. These discrepancies may be due to the distinct alterations introduced in the mouse genome by gene targeting in the two lines that could differentially affect expression of nearby genes located in the imprinted region containing the Ras-grf1 locus. In order to determine the real contribution of Ras-GRF1 to spatial memory we compared in Morris Water Maze learning Brambilla’s mice with a third mouse line (GENA53) in which a non-sense mutation was introduced in the Ras-GRF1 coding region without additional changes in the genome and we found that memory in this task is normal. Also, we measured both contextual and cued fear conditioning, which were previously reported to be affected in Brambilla’s mice, and we confirmed that contextual learning but not cued conditioning is impaired in both mouse lines. In addition, we also tested both lines for the first time in conditioned place aversion in the Intellicage, an ecological and remotely controlled behavioral test, and we observed normal learning. Finally, based on previous reports of other mutant lines suggesting that Ras-GRF1 may control body weight, we also measured this non-cognitive phenotype and we confirmed that both Ras-GRF1 deficient mutants are smaller than their control littermates. In conclusion, we demonstrate that Ras-GRF1 has no unique role in spatial memory while its function in contextual fear conditioning is likely to be due not only to its involvement in amygdala functions but possibly to some distinct hippocampal connections specific to contextual learning.
Memory is a high level brain function that allows organisms to modify their behavior in a way that is adaptive to the environment, increasing hence their probability to survive. It can be categorized in many subtypes and in different processes, but its main subdivision is the one in short-term memory (STM) and long-term memory (LTM), based on the duration of memory retention (Squire et al., 1993; Milner et al., 1998; Squire, 2004; D’isa et al., 2011). The first one, STM, which has a duration ranging from minutes to hours, does not require protein synthesis and is mainly based on the regulation of already existing proteins. However, in order to form a LTM, which can last days, months, years, or even decades, gene expression and de novo protein synthesis are required (Davis and Squire, 1984; McGaugh, 2000).
One of the major molecular cascades implicated in LTM formation is the Ras-controlled mitogen-activated protein kinase (MAPK)–extracellular regulated kinase (ERK) pathway (Blum and Dash, 2009). The Ras-ERK pathway was originally described as regulating cell proliferation and differentiation (Boulton et al., 1991; Marshall, 1995; Malumbres and Barbacid, 2003; Fernandez-Medarde and Santos, 2011a), but in the last 15 years its role in synaptic plasticity and memory consolidation has become evident. The canonical cascade is either brought about by neurotransmitters and their ionotropic and metabotropic receptors or by growth factors that act on receptor tyrosine kinases. This leads to the sequential activation of the Ras subfamily of small GTPases, Raf (the MAP kinase kinase kinase, MAPKKK), MEK (MAPKK) and finally ERK1 and ERK2, the two main MAPKs. ERK1/2 then translocate into the nucleus where they cause either directly or indirectly the activation of transcription factors like Elk-1, c-Myc, and CREB.
ERK1/2 activation is an essential requirement for synaptic plasticity (Orban et al., 1999; Mazzucchelli and Brambilla, 2000; Adams and Sweatt, 2002; Thomas and Huganir, 2004; Davis and Laroche, 2006; Peng et al., 2010; Fasano and Brambilla, 2011). The first evidence that this MAPK signaling is necessary for long-term potentiation (LTP) came in late 1990s from the work of English and Sweatt on hippocampal area CA1 (English and Sweatt, 1996, 1997), but since then similar findings have been made in many brain areas, including dentate gyrus (Coogan et al., 1999), amygdala (Huang et al., 2000), and visual cortex (Di Cristo et al., 2001).
Likewise, MAPK–ERK signaling is a fundamental requisite for LTM formation in the hippocampus. Pharmacological and genetic manipulations have demonstrated its role in several different types of memory. Administration of MEK inhibitor PD98059, for example, in entorhinal cortex impaired inhibitory avoidance (Walz et al., 1999) and spatial memory in Morris water maze (MWM; Hebert and Dash, 2002), while injected intracerebroventricularly it caused a deficit in conditioned taste aversion (Swank, 2000) and object recognition memory (Kelly et al., 2003). Systemic administration of SL327 (a MEK inhibitor which is able to cross the blood–brain barrier) led to impairments in MWM spatial memory and fear memory (contextual fear conditioning was completely blocked, while cued fear conditioning was attenuated; Atkins et al., 1998; Selcher et al., 1999). Some genetic manipulations confirm these results, such as the MEK1 KO mice which have reduced fear memory (Shalin et al., 2004) or mice with a homozygous null mutation for RIN1, a negative regulator of Ras, that have an enhancement in fear conditioning (Dhaka et al., 2003). In other cases, genetic ablation of components of the Ras-ERK pathway have led to more complex scenarios, see for instance the ERK1 KO mice or the NF1 mutants (Silva et al., 1997; Costa et al., 2002; Mazzucchelli et al., 2002; Cui et al., 2008; Fasano and Brambilla, 2011).
In the present work, we used mutant mice lacking the Ras guanine nucleotide-releasing factor 1 (Ras-GRF1; Fernandez-Medarde and Santos, 2011b). Ras is a small G-protein that is inactive until it is bound to GDP (Ras-GDP) and becomes active when it binds to GTP (Ras-GTP), acting as a molecular switch for the MAPK–ERK pathway (Boguski and McCormick, 1993). The shift from the inactive to the active state is mediated by guanine nucleotide exchange factors (GEFs), while the reverse change is promoted by GTPase-activating proteins (GAPs). Ras-GRF1 is a neuronal specific Ras-GEF, exclusively expressed in post-natal, fully differentiated neurons. The behavioral phenotype of Ras-GRF1 deficient mice (MGI nomenclature: Ras-grf1tm1Kln) was first described in a paper by Brambilla et al. (1997). Mutant mice showed a clear dissociation between amygdala-dependent memory (fear conditioning and inhibitory avoidance), which was impaired, and hippocampus-dependent spatial memory in MWM, which resulted normal. Electrophysiology confirmed that LTP was compromised in basolateral amygdala (BLA) but not in CA1 region of hippocampus. Few years later, a study by Giese et al. (2001) found different results testing another knock-out mouse line for Ras-GRF1 (MGI nomenclature: Ras-grf1tm1Sva). Amygdala-dependent memory (inhibitory avoidance and contextual fear conditioning) was normal, while a memory deficit was present in MWM. These mutants were also impaired in hippocampus-dependent contextual discrimination and the social transmission of food preference tasks.
To clarify this controversy, we used a third mutant line, GENA53 (MGI nomenclature: Ras-grf1enu1H), which are ENU mutated mice with a non-sense point mutation that makes the Ras-GRF1 gene inactive with the minimum disturbance to the genome (Clapcott et al., 2003). The aim of this study is to conclusively define the role of Ras-GRF1 in learning and memory by simultaneously testing the two independent mutant lines Ras-GRF1 KO and GENA53 for spatial memory in MWM, contextual and cued fear conditioning, and conditioned corner avoidance in the recently developed system Intellicage.
Materials and Methods
Animal Care
Male and female virgin mice were housed in separate sex and mixed genotype groups of 2–5 each in standard transparent polypropylene cages for mice (L × W × H: 33 cm × 15 cm × 13 cm) endowed with a sawdust bedding. Commercial pellet mice food and bottled tap water were available ad libitum. Animals were kept in a fixed light/dark cycle of 12 h:12 h, with lights on at 9:00 AM. Environment was temperature-controlled (21.5 ± 1°C) and humidity-controlled (40 ± 10%). Cages were inspected twice a week and changed weekly, but only before a day without behavioral testing. Adult (3–5 months) mice were used for the experiments. For the MWM both males and females, in the 50% proportion for the four genotypes, were used. Subsequent statistical analysis confirmed that no sex differences existed. In Intellicage, only females were used because less aggressive. Finally, in fear conditioning only males were used. All the behavioral tests were performed in a quiet and dimly lit room, different from the housing room. Animal care and experiments were conducted in accordance to the ethical guidelines expressed in the European Union directives, approved by the local Institutional Animal Care and Use Committee (IACUC) of the Istituto Scientifico San Raffaele and communicated to the national Ministry of Health as required by the Italian law and by the relevant European regulations.
Morris Water Maze
The MWM test was performed in a circular pool of 1.5 m in diameter, which was filled with water at the temperature of 26 ± 2°C. The water was made opaque by the addition of 2 L of full-cream long-life (UHT) milk, in order to render the submerged platform invisible to the mice. Extra-maze spatial cues were fixed on the walls surrounding the pool. During the training phase a transparent Plexiglas square platform (12 cm × 12 cm) was hidden in the target quadrant (TQ) 1 cm below the level of the water (the choice of the TQ was randomized across mice). In each trial animals were placed in the pool at specific release points (which were randomized across trials and mice) and were let to swim until they found the hidden platform or they reached 120 s of time. Escape latencies and swim tracks were recorded with the videotracking software SMART version 2.0 (Panlab, Barcelona, Spain)1. The spatial training lasted 24 trials (4 days with 6 trials per day). On the fifth day the test phase for memory consolidation was carried out. The hidden platform was removed and mice were tested in a single probe trial, which lasted 60 s and during which the swim tracks and the times spent in the TQ, zone, and platform were recorded with SMART. On the sixth day memories were reactivated in a 60-s session, during which the animals could freely swim in the pool without platform. Finally, 24 h later, on the seventh day, in order to evaluate memory reconsolidation mice were re-tested in a new probe trial identical to the one performed on day 5.
Intellicage
Apparatus and software
The Intellicage apparatus (NewBehavior AG, Zurich, Switzerland)2 is placed in a polycarbonate type III cage (20.5 cm high, 58 cm × 40 cm top, 55 cm × 37.5 cm bottom, Tecniplast, 2000P, Buguggiate, Italy) and accommodated up to 16 mice. It is covered by an aluminum top with a food rack. Its floor is covered with bedding and four central triangular red shelters (Tecniplast, Buguggiate, Italy) are provided. Four triangular conditioning chambers (15 cm × 15 cm × 21 cm) are fitted in the four cage corners and can be accessed by one mouse at a time. Each chamber contains two drinking bottles, accessible via two round openings (13 mm diameter) with motorized doors. Three multi-color LEDs are mounted above each door and the ceiling of each chamber contains a motorized valve that can deliver air puffs. Mice that access a chamber are identified by a circular RIFD antenna at its entrance (30 mm inner diameter) and the duration of their visit is monitored by a temperature sensor. During a visit, number and duration of individual nosepokes at each door are recorded using IR-beam sensors. Licking episodes at each bottle are monitored using lickometers (duration of the episode, number of licks, total contact time). Intellicages have an individual controller and are connected to a central PC running the software that permits to design and run experiments, as well as to analyze the recorded data (Designer, Controller, and Analyzer version 2.2.2, NewBehavior AG).
General procedures
RFID transponders (Datamars SA, Bedano, Switzerland) were injected subcutaneously in the dorso-cervical region under Isoflurane inhalation anesthesia. This was followed by 1 week recovery in groups of 10–12 mice in standard Type III cages (Tecniplast, Buguggiate, Italy) with food ad libitum. During the first week in Intellicage all doors were open providing free access to all eight drinking bottles (free adaptation). During the second week, all doors were closed but could be opened with a nosepoke for 5 s once per visit (nosepoke adaptation). This was followed by the corner avoidance task. During all phases of the experiment, mice were fed ad libitum with standard mouse food (Kliba Nafag 3430; Provimi Kliba AG, Kaiseraugst, Switzerland) and kept on aspen bedding (5 mm × 5 mm × 1 mm, Tapvei OY, Kortteinen, Finland) under controlled environmental conditions (temperature 21 ± 1°C, humidity 50 ± 5%, ambient lights off between 8:00 and 20:00).
Corner avoidance task
This task consisted of a training trial followed by two probe trials (test and re-test). All trials lasted 24 h and were separated from the previous trial by a 24-h retention interval outside Intellicage in a regular type III cage. During the last 18 h of the retention interval water was removed. During the training trial, each mouse was assigned a target corner (avoiding the most and least visited corner during pre-training baseline) in which nosepokes triggered a 1-s air puff instead of opening the door. During probe trials, no air puffs were given and doors opened in response to nosepokes in all corners, as during nosepoke adaptation. After the second probe trial, the mice were left in the Intellicage without air puffs for further 3 days. To assess extinction of the learned response, avoidance of the target corner was again evaluated during the last 24 h of this period. Avoidance of the target corner was quantified as 100% × nosepokes to target corner/total nosepokes. The distribution of poke-less visits was also evaluated but was not affected by training. Within a visit to a corner only the first nosepoke was counted since multiple nosepokes during the same visit are not related to memory.
Fear Conditioning
Apparatus and procedures
Four mice were tested in parallel in an Actimetrics FreezeFrame video-based Conditioned Fear System3. The conditioning chambers (175 mm deep × 180 mm wide × 280 mm high) are enclosed in ventilated and sound-attenuated cabinets and have a floor consisting of stainless steel rods permitting the application of current. The training session consisted of a 60-s pre-exposure to the training context immediately followed by three 60-s training trials consisting of a 2500-Hz 85 dB tone conditioned stimulus (CS) lasting 30 s, co-terminating with a 2-s 0.25 mA foot shock (US), and followed by a 300-s interval. To assess memory consolidation 24 h after training, the mice were re-exposed to the training chamber for 120 s without activation of the CS or US (context test). Thereafter the floor of the conditioning chamber was covered with plastic and some bedding material and a pebble were added. The mice were pre-exposed to this modified context for 60 s which was immediately followed by a 60-s CS presentation (tone test). To assess memory reconsolidation, both test sessions were repeated 6 days later (context and tone re-test).
Data analysis
Freezing (absence of movement aside from respiration) was quantified automatically by the FreezeFrame software subtracting subsequent images recorded by the ceiling-mounted IR video cameras in the conditioning chambers. Bouts of 1.0 s were used to define percentage freezing and movement thresholds were set at 20 (training and context test) or 8 units (tone test). The training effect was evaluated by comparison of percentage freezing during the last training cycle with freezing during the first CS presentation plus the last 30 s of pre-exposure. The US-context association was assessed by comparing percentage freezing during pre-exposure with percentage freezing during the context test. The US–CS association (consolidation and reconsolidation) was evaluated by comparing percentage freezing during the pre-CS and CS phases of the tone test. Additional measures were: percentage freezing during context pre-exposure (unconditioned freezing to the training context), percentage freezing during the first tone presentation (unconditioned freezing to the tone), percentage freezing during the pre-CS phase of the tone test (generalized freezing to the new context).
Results
Morris Water Maze Learning is Normal in both Ras-GRF1 Deficient Mouse Lines
In order to assess spatial memory mice were tested in the (MWM; Morris et al., 1982), in which animals have to swim in a circular pool with opaque water and learn to find a submerged platform that is hidden under the water surface of one of the four quadrants of the pool, called the “TQ.” Mice were trained for 24 trials (4 days with six trials per day). Figure 1 illustrates the learning curves during the 4 days of training (data are shown as couples of trials). One-way ANOVA for repeated measures performed on data from each of the four genotype groups revealed that all the four groups learned, since there is a significant decrease in the escape latencies [Ras-GRF1 KO: F(11,121) = 9.323, p < 0.0001; Ras-GRF1 WT: F(11,121) = 19.268, p < 0.0001; GENA53: F(11,121) = 17.799, p < 0.0001; GENA53 WT: F(11,121) = 9.030, p < 0.0001]. Comparing the learning curves by means of a two-way ANOVA for repeated measures, Ras-GRF1 KO and GENA53 mutant mice resulted both undistinguishable from their respective WT littermates (Bonferroni’s post hoc, Ras-GRF1 KO vs. Ras-GRF1 WT: p = 1; GENA53 vs. GENA53 WT: p = 1), demonstrating their learning was of the same entity and velocity. Moreover they were comparable between themselves (Ras-GRF1 KO vs. GENA53: p = 0.621), as were the wild-type controls (Ras-GRF1 WT vs. GENA53 WT: p = 0.264). There was instead a significant main effect of the training [Trial: F(11,484) = 47.100, p < 0.0001].
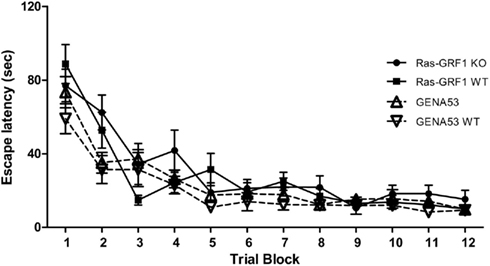
Figure 1. Spatial learning in the Morris water maze. Escape latencies (mean ± SEM) during the 4 days of training are illustrated (results are shown by couples of trials). Ras-GRF1 WT (n = 12); Ras-GRF1 KO (n = 12); GENA53 WT (n = 12); GENA53 (n = 12).
After the 4 days of training, on the fifth day the hidden platform was removed and the animals were challenged with the memory test, which consisted in a single probe trial. Figure 2A shows the percentages of time spent swimming in the TQ. Learning is considered significant if the percentage of time for the TQ is significantly superior than the chance level (which is 25%, since the pool is divided into four equal quadrants) and if this percentage is significantly higher than the ones for the remaining three control quadrants (opposite quadrant, OQ; right quadrant, RQ; left quadrant, LQ). All four genotypes showed a significant preference for the TQ, demonstrating to have learned the task. For each genotype the percentage of time spent in the TQ was significantly higher than chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 5.652, p < 0.0001; Ras-GRF1 KO: t(11) = 5.838, p < 0.0001; GENA53 WT: t(11) = 6.555, p < 0.0001; GENA53: t(11) = 6.507, p < 0.0001] and than the percentages for the other quadrants (One-way ANOVA for repeated measures, Bonferroni’s post hoc, TQ vs. OQ: all p < 0.0001; TQ vs. LQ: all p < 0.009; TQ vs. RQ: all p < 0.013). Moreover one-way ANOVA revealed that the percentages of time spent in the TQ did not differ between genotypes, indicating that the levels of learning of the mutants were analogous to the ones of the wild-type littermates [F(3,44) = 0.487, p = 0.693].
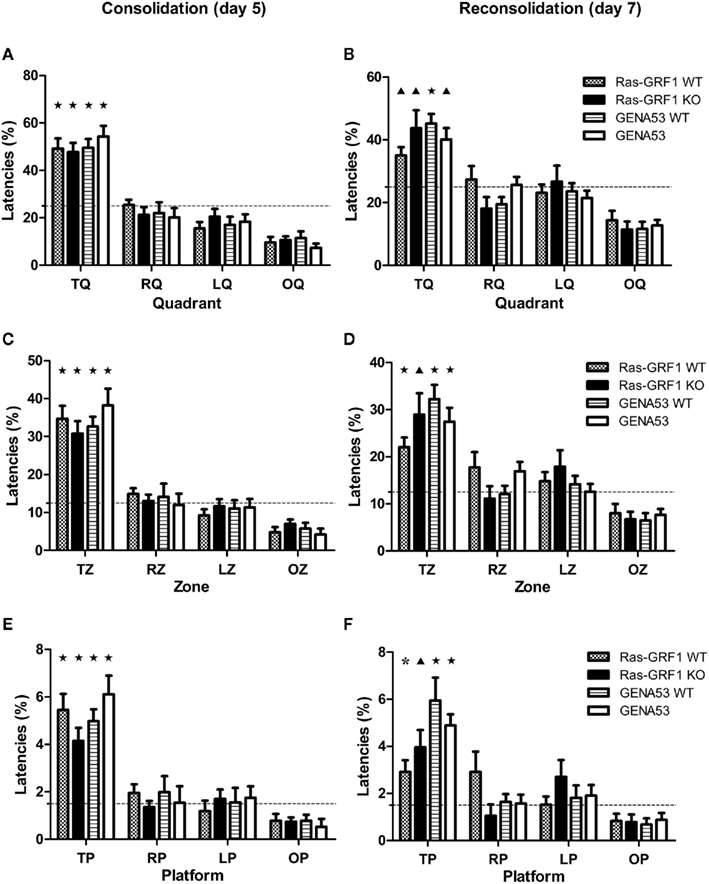
Figure 2. Spatial memory in the Morris water maze. Percentages (mean ± SEM) of time spent in target quadrant (first row: A,B), target zone (second row: C,D), and target platform (third row: E,F). Spatial memory consolidation (day 5 probe trial) is shown in the left column (A,C,E), while spatial memory reconsolidation (day 7 probe trial) is shown in the right column (B,D,F). Ras-GRF1 WT (n = 12); Ras-GRF1 KO (n = 12); GENA53 WT (n = 12); GENA53 (n = 12). Percentages in target area are compared against chance level. Dashed lines represent chance level for each area (quadrant: 25%; zone: 12.5%; platform: 1.5%). T, target; R, right; L, left; O, opposite. P-value symbols: black star = p < 0.001; black triangle = p < 0.01; asterisk = p < 0.05.
These data prove that Ras-GRF1 KO and GENA53 have no impairment in spatial memory consolidation. As consolidation and reconsolidation often dissociate in their molecular bases (Alberini, 2005), we also wanted to test these mutant mice for spatial memory reconsolidation. On the sixth day, animals underwent memory reactivation and 24 h later, on the seventh day, they were exposed to a new probe trial. All the mice still maintained memory of the location of the hidden platform (Figure 2B), as times spent in TQ were again above chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 3.828, p = 0.003; Ras-GRF1 KO: t(11) = 3.269, p = 0.007; GENA53 WT: t(11) = 6.500, p < 0.0001; GENA53: t(11) = 4.122, p = 0.002] and superior than the times spent in the opposite quadrant (One-way ANOVA for repeated measures, Bonferroni’s post hoc, Ras-GRF1 WT: p = 0.005; Ras-GRF1 KO: p = 0.011; GENA53 WT: p < 0.0001; GENA53: p = 0.002). One-way ANOVA confirmed that Ras-GRF1 KO and GENA53 did not have their memory disrupted after reactivation and had intact reconsolidation processes, since the times spent in the TQ by the four genotypes resulted equivalent [F(3,44) = 1.294, p = 0.288].
As Ras-GRF1 KO and GENA53 mutants, although lacking Ras-GRF1, resulted normal in memory consolidation and reconsolidation considering the TQ, we decided to analyze the performance of these animals with a more demanding requirement, learning within the target zone (TZ), which represents only 12.5% of the total area of the pool (half a quadrant), in order to detect even slight memory impairments. On the fifth day, in the probe trial for consolidation, mice of all four genotypes revealed a significant preference for the TZ (Figure 2C). Percentages of time spent in it were significantly superior than chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 6.526, p < 0.0001; Ras-GRF1 KO: t(11) = 5.491, p < 0.0001; GENA53 WT: t(11) = 7.818, p < 0.0001; GENA53: t(11) = 5.805, p < 0.0001] and than the percentages of time spent in the other zones (One-way ANOVA for repeated measures, Bonferroni’s post hoc, TZ vs. OZ: all p < 0.001; TZ vs. LZ: all p < 0.014; TZ vs. RZ: all p < 0.018). In addition the comparison of the animals’ performances by means of one-way ANOVA indicated that the levels of learning of Ras-GRF1 KO and GENA53 were equal to the ones of their wild-type littermates [F(3,44) = 0.833, p = 0.483].
On the seventh day, in the probe trial for reconsolidation, none of the four genotypes showed to have its memory disrupted by the reactivation of the sixth day (Figure 2D). Times spent in TZ were still higher than chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 4.674, p = 0.001; Ras-GRF KO: t(11) = 3.607, p = 0.004; GENA53 WT: t(11) = 6.410, p < 0.0001; GENA53: t(11) = 4.975, p < 0.0001] and than the times spent in the opposite zone (One-way ANOVA for repeated measures, Bonferroni’s post hoc, Ras-GRF1 WT: p = 0.006; Ras-GRF1 KO: p = 0.018; GENA53 WT: p < 0.0001; GENA53: p = 0.002). Levels of memory were equivalent across genotypes [One-way ANOVA, F(3,44) = 1.654, p = 0.191].
Finally, since also in the TZ test GENA53 and Ras-GRF1 KO mice demonstrated to be able to learn, we decided to challenge the animals with a most stringent analysis, the target platform test, in which a significantly smaller surface is considered, merely the area that would have been occupied by the platform that has been removed for the probe trial, which represents only 1.5% of the pool, hence a threshold that is over 16 times more stringent than the TQ and over eight times more than the TZ. We found that the mutant mice passed also this test (Figure 2E). All genotypes spent in the target platform a percentage of time that was higher than chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 5.779, p < 0.0001; Ras-GRF1 KO: t(11) = 4.777, p = 0.001; GENA53 WT: t(11) = 7.099, p < 0.0001; GENA53: t(11) = 5.867, p < 0.0001] and than the one spent in the opposite platform (One-way ANOVA for repeated measures, Bonferroni’s post hoc, Ras-GRF1 WT: p = 0.002; Ras-GRF1 KO: p = 0.001; GENA53 WT: p < 0.0001; GENA53: p < 0.0001). Performances of Ras-GRF1 KO and GENA53 mice did not differ from the ones of their wild-type littermates even in this stringent test [One-way ANOVA, F(3,44) = 1.669, p = 0.187].
In the probe trial for reconsolidation the results were the same as for consolidation (Figure 2F). All the animals passed the test, given that the time spent in the target platform was superior than chance level [One-sample t-test, Ras-GRF1 WT: t(11) = 2.847, p = 0.016; Ras-GRF1 KO: t(11) = 3.390, p = 0.006; GENA53 WT: t(11) = 4.582, p = 0.001; GENA53: t(11) = 7.241, p < 0.0001] and than the one spent in the opposite platform (One-way ANOVA for repeated measures, Bonferroni’s post hoc, Ras-GRF1 WT: p = 0.034; Ras-GRF1 KO: p = 0.030; GENA53 WT: p = 0.003; GENA53: p < 0.0001). Again, performances of the mutants were comparable to the ones of the wild-types and between them (One-way ANOVA, Bonferroni’s post hoc, Ras-GRF1 KO vs. Ras-GRF1 WT: p = 1; GENA53 vs. GENA53 WT: p = 1; Ras-GRF1 KO vs. GENA53: p = 1).
Normal Conditioned Place Avoidance Learning in the Intellicage of the Two Ras-GRF1 Mutant Lines
Mice were tested in Intellicage for conditioned place avoidance. Intellicage is a recently developed system (Galsworthy et al., 2005; Lipp, 2005; see text footnote 2) that allows automated measurement of mice’s spontaneous behavior and cognitive abilities (Knapska et al., 2006; Kiryk et al., 2008; Jaholkowski et al., 2009; Viosca et al., 2009; Barlind et al., 2010; Voikar et al., 2010). With this system animals are tested while living in their cage, without being stressed by the manipulation of human experimenters and without altering their normal social life. Each Intellicage contains fours identical corners, which can be accessed by only one mouse at a time. The identity of mice entering a corner is revealed by passive transponders implanted subcutaneously.
In the conditioned place avoidance each corner contains two water bottles located behind a door that can be opened by a nosepoke. After an adaptation phase in which the animals learn that the doors can be opened by nosepokes (nosepoke adaptation), for each mouse a corner is selected randomly for avoidance conditioning. In this corner the nosepoke, instead of opening the door to allow drinking, triggers an air puff, a non-painful punishment. After 24 h of conditioning mice are removed from Intellicages. The day after the animals, water-deprived for 18 h, undergo the test session for their 24 h-memory, which lasts other 24 h. In this session in all corners the doors can be opened by a nosepoke and there are no punishments. Mice that have learned the task avoid the corner associated with the air puffs and drink from the other three. Percentages of visits with nosepokes (or first nosepokes; see Materials and Methods) to the trained corner are recorded as an index of learning (visits without nosepokes are not influenced by training).
Learning did not appear to be affected by the mutations, as all genotypes developed a strong avoidance for the trained corner (Figure 3). Percentage of nosepokes in the conditioned corner during the test phase was significantly lower than the one during the nosepoke adaptation phase [T-test for paired-samples, Ras-GRF1 WT: t(11) = 5.271, p = 0.0003; Ras-GRF1 KO: t(11) = 7.710, p < 0.0001; GENA53 WT: t(11) = 4.809, p = 0.0006; GENA53: t(10) = 6.186, p = 0.0001], as it was already the one of the training phase [T-test for paired-samples, Ras-GRF1 WT: t(11) = 6.090, p < 0.0001; Ras-GRF1 KO: t(11) = 9.333, p < 0.0001; GENA53 WT: t(11) = 5.056, p = 0.0004; GENA53: t(10) = 8.464, p < 0.0001]. Furthermore, the percentages of nosepokes were comparable between the mutant animals and their wild-type control littermates in the training phase (One-way ANOVA, Bonferroni’s post hoc, Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.184; GENA53 WT vs. GENA53: p = 1) and in the test phase (One-way ANOVA, Bonferroni’s post hoc, Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.596; GENA53 WT vs. GENA53: p = 1).
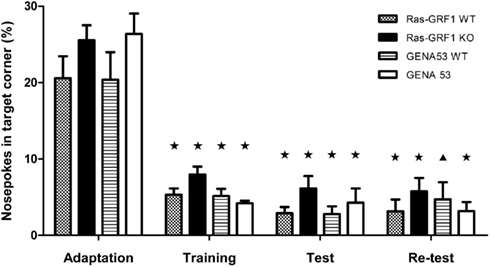
Figure 3. Conditioned corner avoidance in Intellicage. Percentages (mean ± SEM) of first nosepokes (i.e., visits with a nosepoke) in the trained corner during the adaptation, training, test, and re-test phases. Ras-GRF1 WT (n = 12); Ras-GRF1 KO (n = 12); GENA53 WT (n = 12); GENA53 (n = 11). Percentages of first nosepokes are compared with the basal percentages observed during the adaptation phase. P-value symbols: black star = p < 0.001; black triangle = p < 0.01; asterisk = p < 0.05.
After the memory consolidation test the mice were removed again from Intellicages, placed in standard cages for 24 h (water-deprived in the last 18 h) and then returned to Intellicages for a new 24-h probe trial, this time to test memory reconsolidation. Subsequently, animals were kept in Intellicages for other 3 days to assess extinction.
In the second probe trial the mutated mice were again comparable to their wild-type littermates, revealing that Ras-GRF1 KO and GENA53 mutants do not have impairments in reconsolidation (One-way ANOVA, Bonferroni’s post hoc, Ras-GRF1 WT vs. Ras-GRF1 KO: p = 1; GENA53 WT vs. GENA53: p = 1). For all four genotypes learning was still significant, compared with the baseline level of nosepokes recorded during the adaptation phase [T-test for paired-samples, Ras-GRF1 WT: t(11) = 10.632, p < 0.0001; Ras-GRF1 KO: t(11) = 8.263, p < 0.0001; GENA53 WT: t(11) = 3.969, p = 0.002; GENA53: t(10) = 8.148, p < 0.0001]. Finally, after the 3-day extinction protocol, in which the air puff punishment had been removed, we found the mutants’ percentages of nosepokes in the trained corner did not differ from the ones of their littermates, demonstrating that Ras-GRF1 KO and GENA53 mice are not impaired in extinction learning (One-way ANOVA, Bonferroni’s post hoc, Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.740; GENA53 WT vs. GENA53: p = 1).
Impaired Learning of Fear Conditioning in the Two Mutant Lines
Fear conditioning is a procedure based on Pavlovian associative learning, widely used to study emotional memory (Fendt and Fanselow, 1999; LeDoux, 2000; Maren, 2001; Fanselow and Poulos, 2005). Mice are exposed to an electric shock and learn to associate it with a neutral stimulus like the chamber in which they received the shock (contextual fear conditioning) or an auditory tone (cued fear conditioning). Both contextual and cued fear conditioning require the BLA whereas only contextual fear conditioning is hippocampus-dependent (Phillips and LeDoux, 1992). After having trained the animals we tested them for LTM and memory reconsolidation in both contextual and cued fear conditioning.
In the training phase both Ras-GRF1 KO and GENA53 mutants showed a deficit in learning (Figure 4A). Comparing the conditioned fear responses (measured as percentage of freezing) during the first presentation of the tone used as CS and the fear responses during the last CS, two-way ANOVA for repeated measures revealed a significant effect of training [F(1,40) = 38.979, p < 0.0001], a significant interaction training × genotype [F(3,40) = 2.997, p = 0.042], and a significant effect of genotype [F(3,40) = 3.388, p = 0.027]. LSD post hoc made clear that Ras-GRF1 KO mutants have a memory deficit (Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.028), as GENA53 mutants have (GENA53 WT vs. GENA53: p = 0.034). Moreover, one-way ANOVA performed on the rates of learning (freezing to last CS – first CS) indicated a significant genotype effect [F(3,40) = 3.201, p = 0.033] and LSD post hoc analysis showed that both mutants have a memory impairment (Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.035; GENA53 WT vs. GENA53: p = 0.048).
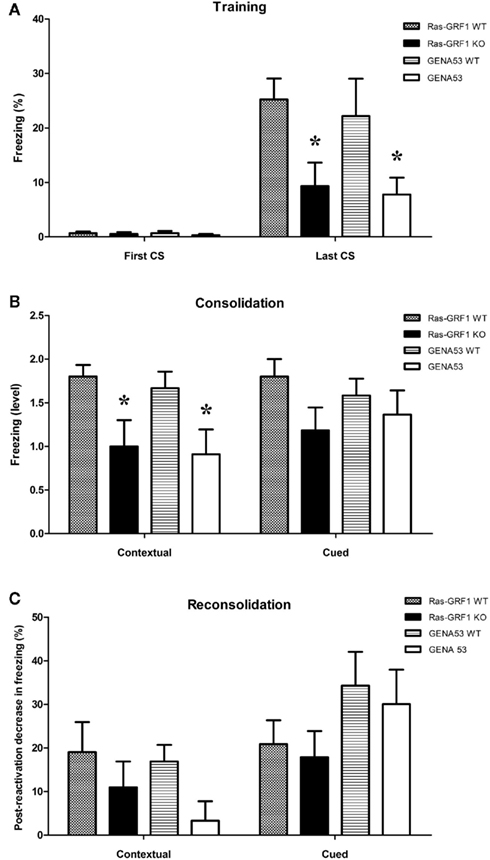
Figure 4. Contextual and cued fear conditioning. Fear memory (means ± SEM) in the training (A), test (B), and re-test (C) phases. (A) Percentage of freezing at the beginning (first CS) and at the end (last CS) of the training. (B) Freezing level in the memory consolidation test (categorized by performance: 0 = non-learner; 1 = bad learner; 2 = good learner). (C) Decrease of freezing percentage in the memory reconsolidation test (percentage of freezing before reactivation minus percentage of freezing post-reactivation). Ras-GRF1 WT (n = 10); Ras-GRF1 KO (n = 11); GENA53 WT (n = 12); GENA53 (n = 11). Each mutant is compared with its respective wild-type by means of a post hoc comparison. P-value symbols: black star = p < 0.001; black triangle = p < 0.01; asterisk = p < 0.05.
Twenty-four hours after the training mice were tested for contextual and cued fear memory consolidation (Figure 4B). In contextual fear conditioning we found a significant effect of context [Two-way ANOVA for repeated measures: F(1,40) = 81.181, p < 0.001], a significant context × genotype interaction [F(3,40) = 3.112, p = 0.037], and a significant genotype effect [F(3,40) = 3.039, p = 0.040]. For both mutants consistently, LSD post hoc displayed only a trend toward significance when they were compared with their respective wild-types (Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.075; GENA53 WT vs. GENA53: p = 0.068). Nevertheless, when freezing responses of the mice were categorized on the basis of the performance (<5%: non-learners; 5–19,99%: bad learners; >20%: good learners), we discovered that the Ras-GRF1 KO and GENA53 mutants had both a strikingly greater proportion of non-learners (Ras-GRF1 KO: 45.5%; Ras-GRF1 WT: 0%; GENA53: 45.5%; GENA53 WT: 8.3%) and a much lower proportion of good learners (Ras-GRF1 KO: 45.5%; Ras-GRF1 WT: 80%; GENA53: 36.4%; GENA53 WT: 75%). Furthermore, comparison of the mean ratings of the performance (non-learners: 0; bad learners: 1; good learners: 2) by means of one-way ANOVA revealed a significant effect of genotype [F(3,40) = 3.554, p = 0.023]. LSD post hoc clarified that Ras-GRF1 KO and GENA53 mutants had both a significantly lower learning than their wild-type littermates (Ras-GRF1 WT vs. Ras-GRF1 KO: p = 0.026; GENA53 WT vs. GENA53: p = 0.028).
In cued fear conditioning instead Ras-GRF1 KO and GENA53 mice did not show any memory consolidation impairment. Effect of tone presentation was significant, but the effect of genotype was not [Two-way ANOVA for repeated measures, Tone effect: F(1,40) = 95.354, p < 0.0001; Genotype effect: F(3,40) = 0.810, p = 0.496]. Mean ratings of performance were also comparable across genotypes [One-way ANOVA: F(3,40) = 0.803, p = 0.499].
Six days after the memory consolidation tests mice were re-tested to evaluate also memory reconsolidation (which was assessed by comparing the memory levels shown in the test after 24 h with the ones of the re-test). For what concerns contextual fear memory reconsolidation, two-way ANOVA for repeated measures proved a significant effect of time [F(1,40) = 22.706, p < 0.001], but the effect of genotype was no more significant [F(3,40) = 2.846, p = 0.050] and neither was the time × genotype interaction [F(3,40) = 1.764, p = 0.170].
Similar results were obtained in the test for tone fear memory reconsolidation [Two-way ANOVA for repeated measures, Time: F(1,40) = 54.198, p < 0.001; Genotype: F(3,40) = 1.515, p = 0.225; Time × Genotype interaction: F(3,40) = 1.238, p = 0.309; Figure 4C].
Ras-GRF1 Controls Body Weight Development
It has previously been reported that the lack of Ras-GRF1 can lead to reduced body growth and development (Itier et al., 1998; Clapcott et al., 2003). Thus, we decided to test our mutant mice also from this point of view and compare the effect of the two mutations on a non-cognitive phenotype like body weight. However, in order to understand the involvement of our mutations in ponderal alterations, we measured the adult body weight of mutant mice from three genetic lines: the already mentioned Ras-GRF1 and GENA53 lines and a third additional line in which the mutants over-express Ras-GRF1, that is the Ras-GRF1, OE line (MGI nomenclature: Ras-grf1tm2Pds; Yoon et al., 2005; Fasano et al., 2009; Figure 5).
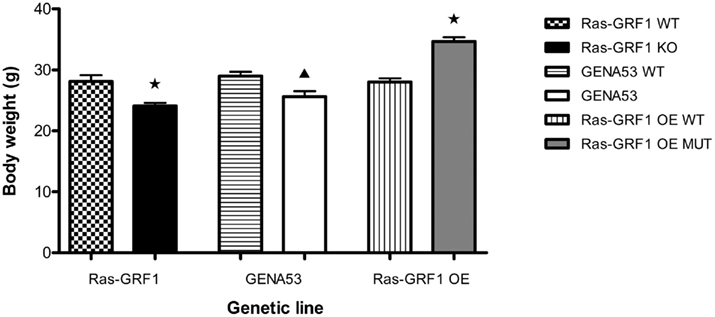
Figure 5. Body weight. Total body weight (mean ± SEM) of adult mice from the three genetic lines Ras-GRF1, GENA53, and Ras-GRF1 OE. Ras-GRF1 WT (n = 24); Ras-GRF1 KO (n = 24); GENA53 WT (n = 24); GENA53 (n = 24); Ras-GRF1 OE WT (n = 24); Ras-GRF1 OE MUT (n = 24). Each mutant is compared with its respective wild-type by means of an independent-samples t-test. P-value symbols: black star = p < 0.001; black triangle = p < 0.01; asterisk = p < 0.05.
For what concerns the Ras-GRF1 line, wild-type mice (4-month-old males only) showed a mean weight of 28.1 ± 1.0 g, ranging from a minimum of 19.1 g to a maximum of 40.0 g. Ras-GRF1 KO mice instead had a mean weight of 24.1 ± 0.5 g (range: 18.7 – 29.9 g), revealing a reduction of approximately 14% [Independent-samples t-test, t(46) = -3.508, p = 0.001]. Similarly, in the GENA53 line the wild-type mice exhibited a mean weight of 29.0 ± 0.7 g (range: 21.7 – 37.4 g), while the littermate GENA53 mutants showed a mean weight of 25.6 ± 0.9 g (range: 21.0 – 37.2 g), demonstrating a reduction of roughly 12% [Independent-samples t-test, t(46) = -3.031, p = 0.004]. Finally, in the Ras-GRF1 OE line the wild-types had a mean weight of 28.0 ± 0.6 g (range: 22.8 – 33.6 g), whereas the Ras-GRF1 OE mutants developed a mean weight of 34.7 ± 0.7 g (range: 29.8 – 41.4 g). In this case, in the mutants we observed an opposite effect on body weight: the range was shifted (both minimum and maximum were higher) and the mean weight was increased by approximately 24% [Independent-samples t-test, t(46) = 7.181, p < 0.0001].
Interestingly, the mean weight reductions observed in the two loss of function mutants, Ras-GRF1 KO and GENA53, were comparable among them [Independent-samples t-test, t(46) = 0.422, p = 0.675]. As a control, we also checked that the weights of the wild-type mice from the three different lines were all equivalent between them [One-way ANOVA: F(2,71) = 0.466, p = 0.630].
Discussion
The present manuscript addresses a 10-year-old controversy originated by the contrasting results reported by Brambilla et al. (1997) and Giese et al. (2001). Brambilla et al. (1997) showed that spatial memory was intact in a mouse line deficient for Ras-GRF1 while both contextual and cued fear conditioning as well as fear-related forms of instrumental learning were found severely impaired. Consistently, Brambilla et al. (1997) found that two forms of hippocampal LTP were intact in these mouse mutants while LTP in the BLA was lost. On the contrary, Giese et al. (2001) reported that MWM learning was impaired in an independently generated deficient line for Ras-GRF1. In addition, Giese et al. (2001) also showed impaired learning in two additional hippocampal dependent tests, social transmission of food preference and contextual discrimination. These later findings suggested significant differences among the two mouse lines which were not strictly dependent on the loss of p140 Ras-GRF1 but rather ascribable to genomic alterations, including the different position of the mutation caused within the Ras-grf1 locus and/or the insertion of the neomycin resistance cassette. Indeed, the Ras-grf1 gene is located within a genomic region which is maternally repressed through an imprinting mechanism and we and others demonstrated that the Ras-grf1 gene is silenced in maternally derived alleles (Plass et al., 1996; Brambilla et al., 1997; Yoon et al., 2002). In order to conclusively define the role of Ras-GRF1 in various forms of LTM, we took advantage of a third mouse line, GENA53, in which Ras-GRF1 expression was abolished by chemical mutagenesis that inserted in the locus a single non-sense mutation, without introducing the neomycin resistance cassette which may potentially interfere with the expression of nearby genes (Clapcott et al., 2003). This line was preliminarily tested for MWM performance in 2009 and did not show deficits but it did manifest a loss of memory in a form of instrumental learning (passive avoidance), as for Brambilla’s mice (Fasano et al., 2009). Therefore, in the present work we compared side by side MWM learning of both Brambilla’s mice and the GENA53 line. We did not find any sign of impairment, neither in the acquisition, nor in the consolidation/reconsolidation processes, even considering very stringent learning criteria such as time swimming in the target zone area or in the target platform area. These data conclusively indicate that Ras-GRF1 dependent signaling is not per se essential for the formation of the memory trace in spatial learning and that the phenotype originally detected in the Giese’s mice is likely due to the peculiar genomic location of the targeted mutation. However, since we did not tested Brambilla’s and GENA53 mice in social transmission of food preference and in contextual discrimination, we cannot formally exclude that Ras-GRF1 may be implicated in these two tests which may depend on other components of the hippocampal circuitry, as we recently showed for novel object recognition and the perirhinal cortex (Berardi et al., 2011). Interestingly, at the cellular level, a more recent report on the Giese’s mice indicated that hippocampal LTP was normal, while long-term depression (LTD) was found reduced (Li et al., 2006). These data were interpreted as a compensatory effect of Ras-GRF2, a close homolog, on hippocampal LTP, and our new data tend to confirm this interpretation also at the behavioral level. The role of the LTD effect found in the Giese’s mice in behavior remains to be elucidated but clearly does not seem to be crucial for spatial learning.
The major emphasis of the original paper on Brambilla’s mice was on the function of Ras-GRF1 in amygdala since both LTP in this structure and fear conditioning were found altered. A close comparison of Brambilla’s and the GENA53 mice in the present manuscript essentially confirmed the phenotype associated with contextual fear but not with cued conditioning, suggesting interesting differences. Traditionally, both forms of fear conditioning have been linked to amygdala functions but the contextual version is also dependent on the hippocampus (Kim and Fanselow, 1992; Maren and Fanselow, 1996). Based on this interpretation we should suggest that hippocampal but not amygdalar functions are altered in both Brambilla’s and the GENA53 mice. However, a possible alternative interpretation is that the protocol for cued conditioning used here is not sensitive enough to detect subtle differences. Indeed, here we used an intermediate protocol, with a three-tone/shock pairing, between the original Brambilla et al. (1997) report (five tone/shock pairings) and the Giese et al. (2001) one (one tone/shock pairing). Possibly, with a stronger protocol we would have been able to detect a phenotype in the cued version of the task as well. That would be more in line with the idea that MWM performance and hippocampal LTP are normal in most Ras-GRF1 mutant lines (Brambilla’s and GENA53 are normal in MWM, Brambilla’s and Giese’s have normal LTP) and that MWM is also normal in a Ras-GRF1 over-expressing line, whose behavioral characterization has already been reported (Fasano et al., 2009). However, it is also important to consider the fact that the hippocampal circuitry and/or the hippocampal signaling mechanisms involved in contextual fear conditioning may be distinct from those used in spatial learning, leaving the possibility that other components of the hippocampal formation may be specifically impaired in fear learning in the Ras-GRF1 deficient mice, as previously suggested for other mutant lines (Mizuno and Giese, 2005) or based on lesion studies (Phillips and LeDoux, 1992, 1994, 1995).
Concerning this point, the fact that, at least in the Giese mice, LTD was found affected, already provides an interesting cellular phenotype which may underlie the contextual fear conditioning impairment.
The strongest behavioral phenotype detected in Brambilla’s mice which was further confirmed in the GENA53 mice is the impairment of LTM in fear-related instrumental learning tasks such as passive and active avoidance (Brambilla et al., 1997; Fasano et al., 2009). Interestingly, an opposite phenotype (i.e., a memory gain) was found in the above mentioned Ras-GRF1 over-expressing mice, further supporting the notion that this signaling modulator of the Ras-ERK pathway is crucial for these types of learning. At present, although we cannot exclude a contribution of amygdala on the effect seen in instrumental learning, the available data suggest that the behavioral alterations of all Ras-GRF1 mutations are likely to be linked to an altered synaptic plasticity and abnormal cell signaling in the striatum, as we recently reported (Fasano et al., 2009). Thus, our observation that learning in the Intellicage is normal in both Brambilla’s and the GENA53 mice is not entirely surprising. Certainly, conditioned place avoidance as assessed in this fully automated system is rather different than active and passive avoidance learning and indeed it may rely on different neural circuitry which may also include the hippocampus but not the dorsal striatum (Voikar et al., 2010).
One final comment is about the growth reduction originally observed in yet another Ras-GRF1 KO line (MGI nomenclature: Ras-grf1tm1Toc) which was also confirmed in the original GENA53 report (Itier et al., 1998; Clapcott et al., 2003). Here, we show that also Brambilla’s mice have a reduced body weight in the adult stage, as previously suggested but not formally reported (Orban et al., 1999). In addition, we have also included for the first time the body weight data of the Ras-GRF1 over-expressing mice (Fasano et al., 2009). The fact that the over-expressing mice weight more than controls is an independent confirmation that Ras-GRF1 is important for post-natal development of the body and that this may also be linked to its role in aging, as recently suggested (de Magalhaes, 2010; Borras et al., 2011).
Altogether, our data indicate that Ras-GRF1 is important for contextual fear-related memories but its involvement in spatial memory is likely to be masked by the presence of Ras-GRF2 in the hippocampus. Indeed, our previously published expression data of Ras-GRF1/2 (Fasano et al., 2009) and in situ hybridization data by the Allen Institute for Brain Science4 indicate that Ras-GRF2 is abundantly expressed in the hippocampus and thus may vicariate for the absence of Ras-GRF1 in this brain structure.
In the near future it will be important to gain access to the behavioral data of both Ras-GRF2 KO and double Ras-GRF1 and 2 deficient mice, as well as from transgenic mice in which both Ras-GRF1 and 2 may be knocked down (via RNA interference) without altering the genome, in order to better understand the role of Ras-GRF1/2 dependent signaling in memory processing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors would like to thank Prof. Chris Graham (University of Oxford) for encouragement and guidance. This work was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s UK as well as by the Italian Ministry of Health, the Fondazione CARIPLO, and the Compagnia di San Paolo (to Riccardo Brambilla), by a Royal Society postdoctoral fellowship award (to Steven J. Clapcote), by the Medical Research Council of the UK (to Karl Peter Giese), and by Swiss NF and NCCR Neural Plasticity and Repair (to David P. Wolfer).
Footnotes
References
Adams, J. P., and Sweatt, J. D. (2002). Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu. Rev. Pharmacol. Toxicol. 42, 135–163.
Alberini, C. M. (2005). Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56.
Atkins, C. M., Selcher, J. C., Petraitis, J. J., Trzaskos, J. M., and Sweatt, J. D. (1998). The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609.
Barlind, A., Karlsson, N., Bjork-Eriksson, T., Isgaard, J., and Blomgren, K. (2010). Decreased cytogenesis in the granule cell layer of the hippocampus and impaired place learning after irradiation of the young mouse brain evaluated using the IntelliCage platform. Exp. Brain Res. 201, 781–787.
Berardi, N., Silingardi, D., Angelucci, A., De Pasquale, R., Borsotti, M., Squitieri, G., Putignano, E., Brambilla, R., and Pizzorusso, T. (2011). ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex. Front. Behav. Neurosci. (in press).
Blum, S., and Dash, P. K. (2009). “MAP kinase signaling in learning and memory,” in Encyclopedia of Neuroscience, Vol. 5, ed. L. R. Squire (San Diego: Elsevier), 657–662.
Boguski, M. S., and McCormick, F. (1993). Proteins regulating Ras and its relatives. Nature 366, 643–654.
Borras, C., Monleon, D., Lopez-Grueso, R., Gambini, J., Orlando, L., Pallardo, F. V., Santos, E., Vina, J., and Font de Mora, J. (2011). RasGrf1 deficiency delays aging in mice. Aging (Albany N. Y.) 3, 262–276.
Boulton, T. G., Nye, S. H., Robbins, D. J., Ip, N. Y., Radziejewska, E., Morgenbesser, S. D., De, P. R., Panayotatos, N., Cobb, M. H., and Yancopoulos, G. D. (1991). ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65, 663–675.
Brambilla, R., Gnesutta, N., Minichiello, L., White, G., Roylance, A. J., Herron, C. E., Ramsey, M., Wolfer, D. P., Cestari, V., Rossi-Arnaud, C., Grant, S. G., Chapman, P. F., Lipp, H. P., Sturani, E., and Klein, R. (1997). A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature 390, 281–286.
Clapcott, S. J., Peters, J., Orban, P. C., Brambilla, R., and Graham, C. F. (2003). Two ENU-induced mutations in Rasgrf1 and early mouse growth retardation. Mamm. Genome 14, 495–505.
Coogan, A. N., O’Leary, D. M., and O’Connor, J. J. (1999). P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro. J. Neurophysiol. 81, 103–110.
Costa, R. M., Federov, N. B., Kogan, J. H., Murphy, G. G., Stern, J., Ohno, M., Kucherlapati, R., Jacks, T., and Silva, A. J. (2002). Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530.
Cui, Y., Costa, R. M., Murphy, G. G., Elgersma, Y., Zhu, Y., Gutmann, D. H., Parada, L. F., Mody, I., and Silva, A. J. (2008). Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560.
Davis, H. P., and Squire, L. R. (1984). Protein synthesis and memory: a review. Psychol. Bull. 96, 518–559.
Davis, S., and Laroche, S. (2006). Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav. 5(Suppl. 2), 61–72.
de Magalhaes, J. P. (2010). Paternal genome effects on aging: evidence for a role of Rasgrf1 in longevity determination? Mech. Ageing Dev. 132, 72–73.
Dhaka, A., Costa, R. M., Hu, H., Irvin, D. K., Patel, A., Kornblum, H. I., Silva, A. J., O’Dell, T. J., and Colicelli, J. (2003). The RAS effector RIN1 modulates the formation of aversive memories. J. Neurosci. 23, 748–757.
Di Cristo, G., Berardi, N., Cancedda, L., Pizzorusso, T., Putignano, E., Ratto, G. M., and Maffei, L. (2001). Requirement of ERK activation for visual cortical plasticity. Science 292, 2337–2340.
D’isa, R., Solari, N., and Brambilla, R. (2011). “Biological memory in animals and in man,” in Memory Mass Storage, eds G. Campardo, F. Tiziani, and M. Iaculo (Berlin-Heidelberg: Springer), 417–441.
English, J. D., and Sweatt, J. D. (1996). Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J. Biol. Chem. 271, 24329–24332.
English, J. D., and Sweatt, J. D. (1997). A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J. Biol. Chem. 272, 19103–19106.
Fanselow, M. S., and Poulos, A. M. (2005). The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 56, 207–234.
Fasano, S., and Brambilla, R. (2011). Ras-ERK signaling in behavior: old questions and new perspectives. Front. Behav. Neurosci. 5:79. doi: 10.3389/fnbeh.2011.00079
Fasano, S., D’Antoni, A., Orban, P. C., Valjent, E., Putignano, E., Vara, H., Pizzorusso, T., Giustetto, M., Yoon, B., Soloway, P., Maldonado, R., Caboche, J., and Brambilla, R. (2009). Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol. Psychiat. 66, 758–768.
Fendt, M., and Fanselow, M. S. (1999). The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 23, 743–760.
Fernandez-Medarde, A., and Santos, E. (2011a). Ras in cancer and developmental diseases. Genes Cancer 2, 344–358.
Fernandez-Medarde, A., and Santos, E. (2011b). The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim. Biophys. Acta 1815, 170–188.
Galsworthy, M. J., Amrein, I., Kuptsov, P. A., Poletaeva, II Zinn, P., Rau, A., Vyssotski, A., and Lipp, H. P. (2005). A comparison of wild-caught wood mice and bank voles in the Intellicage: assessing exploration, daily activity patterns and place learning paradigms. Behav. Brain Res. 157, 211–217.
Giese, K. P., Friedman, E., Telliez, J. B., Fedorov, N. B., Wines, M., Feig, L. A., and Silva, A. J. (2001). Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1). Neuropharmacology 41, 791–800.
Hebert, A. E., and Dash, P. K. (2002). Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn. Mem. 9, 156–166.
Huang, Y. Y., Martin, K. C., and Kandel, E. R. (2000). Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J. Neurosci. 20, 6317–6325.
Itier, J. M., Tremp, G. L., Leonard, J. F., Multon, M. C., Ret, G., Schweighoffer, F., Tocque, B., Bluet-Pajot, M. T., Cormier, V., and Dautry, F. (1998). Imprinted gene in postnatal growth role. Nature 393, 125–126.
Jaholkowski, P., Kiryk, A., Jedynak, P., Ben Abdallah, N. M., Knapska, E., Kowalczyk, A., Piechal, A., Blecharz-Klin, K., Figiel, I., Lioudyno, V., Widy-Tyszkiewicz, E., Wilczynski, G. M., Lipp, H. P., Kaczmarek, L., and Filipkowski, R. K. (2009). New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn. Mem. 16, 439–451.
Kelly, A., Laroche, S., and Davis, S. (2003). Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23, 5354–5360.
Kim, J. J., and Fanselow, M. S. (1992). Modality-specific retrograde amnesia of fear. Science 256, 675–677.
Kiryk, A., Aida, T., Tanaka, K., Banerjee, P., Wilczynski, G. M., Meyza, K., Knapska, E., Filipkowski, R. K., Kaczmarek, L., and Danysz, W. (2008). Behavioral characterization of GLT1(+/−) mice as a model of mild glutamatergic hyperfunction. Neurotox. Res. 13, 19–30.
Knapska, E., Walasek, G., Nikolaev, E., Neuhausser-Wespy, F., Lipp, H. P., Kaczmarek, L., and Werka, T. (2006). Differential involvement of the central amygdala in appetitive versus aversive learning. Learn. Mem. 13, 192–200.
Li, S., Tian, X., Hartley, D. M., and Feig, L. A. (2006). Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J. Neurosci. 26, 1721–1729.
Lipp, H. P. (2005). High-throughput and automated behavioural screening of normal and genetically modified mice. Bus. Brief. Future Drug Discov. 1–5.
Malumbres, M., and Barbacid, M. (2003). RAS oncogenes: the first 30 years. Nat. Rev. Cancer 3, 459–465.
Maren, S., and Fanselow, M. S. (1996). The amygdala and fear conditioning: has the nut been cracked? Neuron 16, 237–240.
Marshall, C. (1995). “The Ras/Raf/ERK pathway,” in Guidebook to the Small GTPases, eds M. Zerial, and L. A. Hubery (Oxford: Oxford University Press), 65–73.
Mazzucchelli, C., and Brambilla, R. (2000). Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell. Mol. Life Sci. 57, 604–611.
Mazzucchelli, C., Vantaggiato, C., Ciamei, A., Fasano, S., Porrazzo, A., Orban, P. C., Pakhotin, P., Krezel, W., Wezl, H., Wolfer, D. P., Pagès, G., Valverde, O., Marowsky, A., Porrazzo, A., Orban, P. C., Maldonado, R., Ehrengruber, M. U., Cestari, V., Lipp, H. P., Chapman, P. F., Pouysségur, J., and Brambilla, (2002). Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34, 807–820.
Milner, B., Squire, L. R., and Kandel, E. R. (1998). Cognitive neuroscience and the study of memory. Neuron 20, 445–468.
Mizuno, K., and Giese, K. P. (2005). Hippocampus-dependent memory formation: do memory type-specific mechanisms exist? J. Pharmacol. Sci. 98, 191–197.
Morris, R. G., Garrud, P., Rawlins, J. N., and O’Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683.
Orban, P. C., Chapman, P. F., and Brambilla, R. (1999). Is the Ras-MAPK signalling pathway necessary for long-term memory formation? Trends Neurosci. 22, 38–44.
Peng, S., Zhang, Y., Zhang, J., Wang, H., and Ren, B. (2010). ERK in learning and memory: a review of recent research. Int. J. Mol. Sci. 11, 222–232.
Phillips, R. G., and LeDoux, J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285.
Phillips, R. G., and LeDoux, J. E. (1994). Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1, 34–44.
Phillips, R. G., and LeDoux, J. E. (1995). Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contexual fear conditioning. J. Neurosci. 15, 5308–5315.
Plass, C., Shibata, H., Kalcheva, I., Mullins, L., Kotelevtseva, N., Mullins, J., Kato, R., Sasaki, H., Hirotsune, S., Okazaki, Y., Held, W. A., Hayashizaki, Y., and Chapman, V. M. (1996). Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS-M. Nat. Genet. 14, 106–109.
Selcher, J. C., Atkins, C. M., Trzaskos, J. M., Paylor, R., and Sweatt, J. D. (1999). A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 6, 478–490.
Shalin, S. C., Zirrgiebel, U., Honsa, K. J., Julien, J. P., Miller, F. D., Kaplan, D. R., and Sweatt, J. D. (2004). Neuronal MEK is important for normal fear conditioning in mice. J. Neurosci. Res. 75, 760–770.
Silva, A. J., Frankland, P. W., Marowitz, Z., Friedman, E., Lazlo, G., Cioffi, D., Jacks, T., and Bourtchuladze, R. (1997). A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat. Genet. 15, 281–284.
Squire, L. R. (2004). Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177.
Squire, L. R., Knowlton, B., and Musen, G. (1993). The structure and organization of memory. Annu. Rev. Psychol. 44, 453–495.
Swank, M. W. (2000). Conditioned c-Fos in mouse NTS during expression of a learned taste aversion depends on contextual cues. Brain Res. 862, 138–144.
Thomas, G. M., and Huganir, R. L. (2004). MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183.
Viosca, J., Schuhmacher, A. J., Guerra, C., and Barco, A. (2009). Germline expression of H-Ras(G12V) causes neurological deficits associated to Costello syndrome. Genes Brain Behav. 8, 60–71.
Voikar, V., Colacicco, G., Gruber, O., Vannoni, E., Lipp, H. P., and Wolfer, D. P. (2010). Conditioned response suppression in the IntelliCage: assessment of mouse strain differences and effects of hippocampal and striatal lesions on acquisition and retention of memory. Behav. Brain Res. 213, 304–312.
Walz, R., Roesler, R., Quevedo, J., Rockenbach, I. C., Amaral, O. B., Vianna, M. R., Lenz, G., Medina, J. H., and Izquierdo, I. (1999). Dose-dependent impairment of inhibitory avoidance retention in rats by immediate post-training infusion of a mitogen-activated protein kinase kinase inhibitor into cortical structures. Behav. Brain Res. 105, 219–223.
Yoon, B., Herman, H., Hu, B., Park, Y. J., Lindroth, A., Bell, A., West, A. G., Chang, Y., Stablewski, A., Piel, J. C., Loukinov, D. I., Lobanenkov, V. V., and Soloway, P. D. (2005). Rasgrf1 imprinting is regulated by a CTCF-dependent methylation-sensitive enhancer blocker. Mol. Cell. Biol. 25, 11184–11190.
Keywords: Ras-GRF1, Ras-ERK, spatial memory, fear conditioning, intellicage, body weight reduction
Citation: d’Isa R, Clapcote SJ, Voikar V, Wolfer DP, Giese KP, Brambilla R and Fasano S (2011) Mice lacking Ras-GRF1 show contextual fear conditioning but not spatial memory impairments: convergent evidence from two independently generated mouse mutant lines. Front. Behav. Neurosci. 5:78. doi: 10.3389/fnbeh.2011.00078
Received: 24 August 2011; Accepted: 31 October 2011;
Published online: 06 December 2011.
Edited by:
Gilberto Fisone, Karolinska Institutet, SwedenReviewed by:
Irini Skaliora, Biomedical Research Foundation of the Academy of Athens, GreecePeter Vanhoutte, Centre National de la Recherche Scientifique, France
Copyright: © 2011 d’Isa, Clapcote, Voikar, Wolfer, Giese, Brambilla and Fasano. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Stefania Fasano, Division of Neuroscience, Institute of Experimental Neurology, San Raffaele Scientific Institute and University, Via Olgettina 58, 20132 Milano, Italy. e-mail: fasano.stefania@hsr.it
†Present address: Vootele Voikar, Neuroscience Center, University of Helsinki, P.O. Box 56 (Viikinkaari 4), Helsinki FI-00014, Finland.
‡Raffaele d’Isa and Steven J. Clapcote have contributed equally to this work.