The endocannabinoid transport inhibitor AM404 differentially modulates recognition memory in rats depending on environmental aversiveness
- 1Department of Physiology and Pharmacology, Sapienza University of Rome, Rome, Italy
- 2Department of Biology, University of RomaTre, Rome, Italy
- 3Department of Cell Biology and Neuroscience, Istituto Superiore di Sanità, Rome, Italy
Cannabinoid compounds may influence both emotional and cognitive processes depending on the level of environmental aversiveness at the time of drug administration. However, the mechanisms responsible for these responses remain to be elucidated. The present experiments investigated the effects induced by the endocannabinoid transport inhibitor AM404 (0.5–5 mg/kg, i.p.) on both emotional and cognitive performances of rats tested in a Spatial Open Field task and subjected to different experimental settings, named High Arousal (HA) and Low Arousal (LA) conditions. The two different experimental conditions influenced emotional reactivity independently of drug administration. Indeed, vehicle-treated rats exposed to the LA condition spent more time in the center of the arena than vehicle-treated rats exposed to the HA context. Conversely, the different arousal conditions did not affect the cognitive performances of vehicle-treated animals such as the capability to discriminate a spatial displacement of the objects or an object substitution. AM404 administration did not alter locomotor activity or emotional behavior of animals exposed to both environmental conditions. Interestingly, AM404 administration influenced the cognitive parameters depending on the level of emotional arousal: it impaired the capability of rats exposed to the HA condition to recognize a novel object while it did not induce any impairing effect in rats exposed to the LA condition. These findings suggest that drugs enhancing endocannabinoid signaling induce different effects on recognition memory performance depending on the level of emotional arousal induced by the environmental conditions.
Introduction
The endocannabinoid system is a crucial regulator of central nervous system (CNS) function (Cravatt et al., 1996; Di Marzo and Matias, 2005; Pacher et al., 2006; Trezza et al., 2008b; Campolongo et al., 2009b,c, 2011; Bisogno and Di Marzo, 2010; Hill and McEwen, 2010). Endocannabinoids are released from post-synaptic neurons in an activity-dependent manner, travel retrogradely through the synaptic cleft and activate pre-synaptic cannabinoid type 1 receptors (CB1), thus suppressing neurotransmitter release from axon terminals (Wilson and Nicoll, 2002). Among the endogenous cannabimimetic signaling molecules, anandamide (N-arachidonoylethanolamine, AEA) and 2-arachidonoylglycerol (2-AG) stand out as the first identified and most intensively studied (Ueda et al., 1995, 2011; Di Marzo, 1998; Piomelli, 2003; Waku, 2006). Receptor activation by endocannabinoids ends by the removal from the synaptic cleft operated by a transport system present in neural and non-neural cells (Di Marzo et al., 1994; Beltramo et al., 1997; Hillard et al., 1997) followed by hydrolysis operated by fatty-acid amide hydrolase (FAAH, that hydrolyzes anandamide) or monoacylglycerol lipase (MAGL, that cleaves 2-AG) (Desarnaud et al., 1995; Hillard et al., 1995; Ueda et al., 1995; Cravatt et al., 1996). Interestingly, while the endocannabinoid hydrolyzing enzymes have been fully identified and cloned, the functional properties of the putative transporter have been only partially characterized (Hillard and Jarrahian, 2003; Yates and Barker, 2009; Fu et al., 2011) and its molecular identity remains still unknown.
CB1 receptor is crucially involved in neural plasticity mechanisms related to the processing, consolidation, and extinction of emotionally salient cognitive events (Marsicano et al., 2002; Laviolette and Grace, 2006a,b; Campolongo et al., 2009a,b; Mackowiak et al., 2009; Abush and Akirav, 2010; Akirav, 2011; Hauer et al., 2011). This fits well with the notion that CB1 receptors are highly expressed in brain structures including the basolateral amygdala (BLA), the medial prefrontal cortex (mPFC) and the hippocampus (Breivogel and Childers, 1998; Mackie, 2005; Katona, 2009), strictly associated with both cognitive and emotional processes (Laviolette and Grace, 2006a; Viveros et al., 2007; McLaughlin and Gobbi, 2011; Tan et al., 2011).
Animal studies have demonstrated that the endocannabinoid system modulates recognition memory by altering the mechanisms responsible for this process within the hippocampus and selectively affecting the encoding stage (Barna et al., 2007). Moreover, the important involvement of other structures, for instance the amygdala, in the modulation of memory consolidation and extinction for emotional events has been firmly established (McGaugh, 2000; Vianna et al., 2004; Clarke et al., 2008; de Oliveira Alvares et al., 2008, 2010; Campolongo et al., 2009b; Ganon-Elazar and Akirav, 2009; Manwell et al., 2009; Roozendaal and McGaugh, 2011). In line with the widespread distribution of CB1 receptors throughout the limbic system, it has been extensively demonstrated that cannabinoid compounds also induce diverse effects on anxiety- and fear-related behaviors (Trezza et al., 2008a, 2012; Micale et al., 2009; Moreira and Wotjak, 2010; Terzian et al., 2011). Interestingly, cannabinoid effects on emotionality are biphasic, as it is also reported by cannabis abusers (Fant et al., 1998; Hall and Solowij, 1998; Bolla et al., 2002; Curran et al., 2002). The classical explanation to this phenomenon is often provided by the use of different doses of cannabinoid drugs, with low doses generally inducing anxiolytic-like effects and high doses often causing the opposite. A new and appealing explanation to this phenomenon is now emerging, underlying that these opposite effects may also depend on previous experiences, the context of use and the level of emotional arousal at the time of drug administration/consumption (Akirav, 2011; Sciolino et al., 2011). Drugs that interfere with endocannabinoid degradation increase ongoing endocannabinoid signaling in a temporarily and spatially restricted manner (Janero et al., 2009). However, preclinical evidence has shown that indirect cannabinoid agonists can also induce biphasic effects on behavior, depending on the emotional state of the subject. For instance, it has been recently demonstrated that the FAAH inhibitor URB597 does not affect anxiety under mildly stressful circumstances but has robust anxiolytic-like effects in highly aversive testing conditions (Haller et al., 2009). This finding leaves open the possibility that inhibitors of endocannabinoid transport, which prolong endocannabinoid actions by preventing endocannabinoid access to intracellular hydrolyzing enzymes (Beltramo et al., 1997; Kathuria et al., 2003), may influence both emotional and cognitive processes depending on the level of environmental aversiveness at the time of drug administration.
To address this issue, in the present study we investigated the effect of the prototypical endocannabinoid transport inhibitor, AM404 in a non-aversive task, the Spatial Open Field test under two experimental conditions differing by the level of emotional arousal at the time of testing. The Spatial Open Field task has been extensively used (Poucet et al., 1986; Thinus-Blanc et al., 1987; Poucet, 1989, 1993; Ricceri et al., 1999, 2002; Scattoni et al., 2004; de Bartolo et al., 2010) and permits to assess both emotional and cognitive parameters, in terms of reactivity to a spatial or an object novelty, by exploiting the natural propensity of rodents to explore the environment. The High Arousal condition (HA) was obtained by testing rats in an empty arena under white light illumination without previous handling, while the Low Arousal condition (LA) was obtained by extensively handling the animals before testing in an arena with the ground loaded with familiar bedding, under a dim red lighted room.
By manipulating the experimental conditions and the tone of endogenous cannabinoids, this study may help to explain how the interaction between endocannabinoids and environment could influence recognition memory in rats.
Materials and Methods
Animals
Male adult Wistar rats (300 g at the time of testing, Charles River Laboratories, Italy) were housed in groups and maintained in a temperature-controlled environment (20 ± 1°C) under a 12 h light/12 h dark cycle (7:00 am to 7:00 pm lights on) with unlimited access to food and water. All procedures involving animal care or treatments were approved by the Italian Ministry of Health and performed in compliance with the guidelines of the US National Institutes of Health (NIH) and the Italian Ministry of Health (D.L. 116/92), the Declaration of Helsinki, the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2004) and the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Drug Treatments
N-(4-Hydroxyphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (AM404, 0.5–1–5 mg/kg), purchased from Tocris Bioscience (UK), was dissolved in a vehicle containing 10% polyethylene glycol, 10% Tween-80, and 80% saline. Drug solutions were freshly prepared before each experiment and administered by intraperitoneal injection in a volume of 1 ml/kg 15 min before the beginning of the task.
Spatial Open Field Procedures
The apparatus consisted in an open-field arena made of black Plexiglas (80 × 80 × 60 cm) surrounded with a visually uniform environment. A video camera above the field was connected to a video recorder. Experiments were performed between 10.00 am and 2.00 pm.
To assess the effect of the exogenous manipulation of the endocannabinoid tone on short-term memory performance, the test schedule consisted of a single task composed by six 5-min consecutive sessions taking place during the same day, separated by 3-min delays during which the subjects were returned to their home cage (Figure 1). During session 1, each rat was placed into the center of the empty arena to allow it to become familiar with the apparatus and to record baseline levels of locomotor and exploratory activity. Starting from session 2, three different objects were simultaneously present in the open field: Object A, a dark metal parallelepiped (4 cm high × 13 cm wide × 9 cm long); Object B, a transparent Plexiglas cube with holes regularly distributed on the sides (height = 10 cm); Object C, a gray plastic square (10 × 10 × 10 cm) with a central triangle forming a 90° angle. During sessions 2–3, the A, B, and C objects were placed in the arena. In session 4, the spatial test session, the configuration was changed by moving two objects: object B replaced object A which was itself displaced at the periphery of the apparatus. In session 5, the configuration of the objects was unchanged to let the rats habituate to the new arrangement of the objects. In the last session (session 6) one of the familiar, non-displaced objects (object C) was replaced by a new object (object D), which consisted of a black-and-white plastic cylinder, height = 13 cm; diameter = 6 cm (Figure 1).
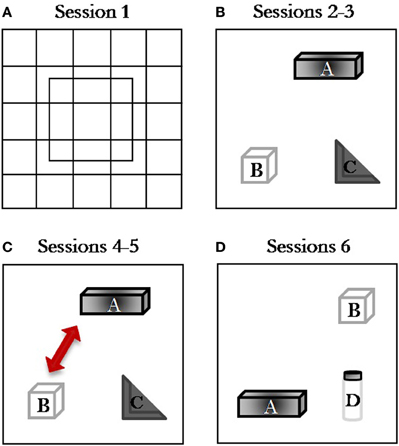
Figure 1. Spatial Open-Field procedure. Schematic diagram representing the object configuration in the Spatial Open-Field test: (A) session 1, open field without objects; (B) session 2, habituation session with three stable objects; (C) sessions 3–4, spatial change discrimination sessions where object B displaced object A (session 4); (D) session 5–6, object novelty sessions where object D replaced object C (session 6).
We exposed the rats to two experimental conditions, named HA and LA conditions. In the HA condition (Experiment 1), the test was performed under normal light (30–40 lux), rats were not handled and tested in an empty arena (no bedding). In the LA condition (Experiment 2), the test was performed under dim red light (2 lux) condition, rats were extensively habituated to the experimenter and to the injection procedure for one week before the experiment (every day, 1 min per each rat) and tested in an arena with the ground loaded with familiar bedding.
Statistical Analysis
Data collection was performed from the same observer who was unaware of animal treatment using the Observer XT software (Noldus, Netherland). During the first session, frequency and/or duration of the following responses were measured: crossings, rearings, and time spent in the center of the apparatus. From sessions 2–6, object exploration was measured as total time spent by the animal in contact with an object (1 s as minimal contact was considered) throughout all sessions 2–6.
The total time spent by rats investigating all objects throughout all sessions has been considered as an indicator of general investigative activity. A contact was defined as the subject's snout actually touching an object. In session 4, the spatial arrangement of the objects was modified and response to spatial change was assessed by comparing the mean time spent in contact with both Displaced (DO) and Non-Displaced (NDO) Objects in session 4 minus the mean time spent in contact with the same object in session 3. A discrimination index of the response to the spatial change was obtained by subtracting the NDO value to DO value. Finally, the response to the non-spatial novelty was assessed by comparing mean time in contact with the Substituted Object (SO, unfamiliar) and Non-Substituted Objects (NSO, familiar) in session 6 minus the mean time spent with objects located in the corresponding position in session 5. A discrimination index of the response to the non-spatial novelty was obtained by subtracting the NSO value to SO value. Unpaired t-test was used to compare the behavioral performance of vehicle groups. One-sample t-tests were used to determine whether the discrimination index was different from zero. Treatment (AM404) effects were analyzed by One-Way analysis of variance (ANOVA) or ANOVA for repeated measures (when appropriate), followed by Tukey's post-hoc comparison tests. A probability level of < 0.05 was accepted as statistically significant.
Results
Different Arousal Conditions Influenced Emotional Behavior and Object Exploration But did not Alter Cognitive Performances of Vehicle-Treated Animals
Unpaired t-test showed that the different arousal context did not affect locomotor activity of the vehicle groups. Both crossing (Figure 2A) and rearing (Figure 2B) frequencies did not statistically differ between the two groups (t = −0.66; p = 0.52; t = 1.09; p = 0.29, respectively). However, unpaired t-test showed that the different arousal conditions influenced the emotional behavior of vehicle-treated animals exposed to the different experimental contexts. Rats treated with vehicle and exposed to a High Arousal condition (HA group) spent less time in the center of the arena than vehicle-treated rats exposed to a Low Arousal context (LA group) (t = −4.11; p = 0.0005, Figure 2C).
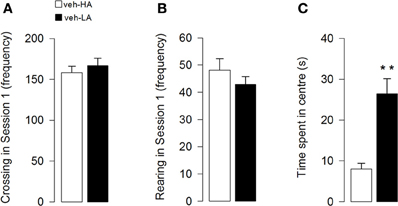
Figure 2. Effects induced by different arousal conditions on locomotor activity and emotional behavior of vehicle-treated rats. Locomotor activity: number of crossing (A) and rearing (B) in session 1. Emotional behavior: time spent in the center of the arena in session 1 (C). **P < 0.01. Data are expressed as mean ± SEM. (High Arousal: HA n = 10; Low Arousal: LA n = 15).
Unpaired t-test showed that rats treated with vehicle and exposed to a HA context spent less time investigating objects than vehicle-treated rats exposed to a LA context (t = −4.41; p < 0.0001, Figure 3A). Additionally, unpaired t-test showed that both vehicle groups did not differ in the discrimination index for a spatial object displacement in session 4 (t = 0.60; p = 0.55, Figure 3B) and for the substitution of the objects in session 6 (t = 0.47; p = 0.64, Figure 3C). However, One-sample t-tests revealed that while both vehicle groups were able to discriminate the object novelty (veh-HA, t9 = 4.49, P = 0.0015; veh-LA, t14 = 2.61, P = 0.02, Figure 3C) they did not respond to a spatial rearrangement (veh-HA, t9 = 1.10, P = 0.30; veh-LA, t14 = 0.16, P = 0.88, Figure 3B).
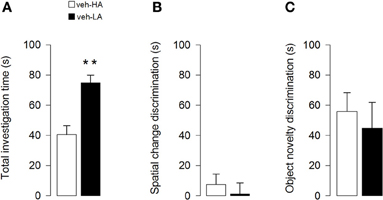
Figure 3. Effects induced by different arousal conditions on object investigation and cognitive performances of vehicle-treated rats. Total investigation time of all objects through sessions (A) spatial change discrimination (B) and object novelty discrimination (C). **P < 0.01. Data are expressed as mean ± SEM. (High Arousal: HA n = 10; Low Arousal: LA n = 15).
AM404 Administration did not Alter Locomotor Activity and Emotional Behavior in Rats Exposed to Different Arousal Conditions
AM404 administration did not alter locomotor activity of rats exposed to either a HA or LA condition. One–Way ANOVA for crossing (Figure 4A) and rearing (Figure 4B) frequencies in session 1 for AM404-treated rats exposed to a HA condition did not show a statistically significant difference (F3, 36 = 0.60; p = 0.62; F3, 36 = 1.44; p = 0.25, respectively). Moreover, One–Way ANOVA for the number of crossings (Figure 4C) or rearings (Figure 4D) in session 1 did not show a statistically significant difference between vehicle- and AM404-treated rats exposed to a LA condition (F3, 50 = 0.97; p = 0.42; F3, 50 = 2.21; p = 0.10, respectively).
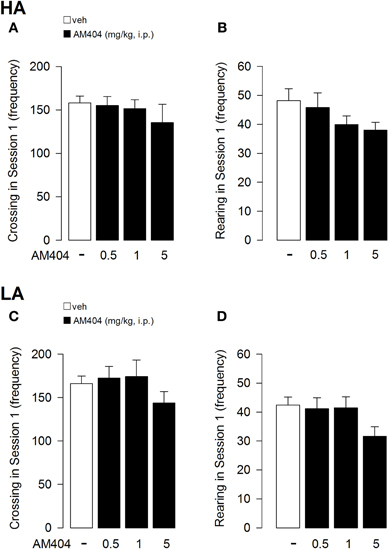
Figure 4. Effects of AM404 administration on locomotor activity in rats exposed to high arousal (HA) or low arousal (LA) conditions. Number of crossing (A) and rearing (B) of rats exposed to HA or LA conditions (C, D, respectively) in session 1. Data are expressed as mean ± SEM. (HA: veh n = 10, 0.5 mg/kg n = 11, 1 mg/kg n = 10, 5 mg/kg n = 9; LA: veh n = 15, 0.5 mg/kg n = 12, 1 mg/kg n = 14, 5 mg/kg n = 13).
AM404 administration did not affect emotional reactivity in rats exposed to either a HA or LA condition. Indeed, One-Way ANOVA showed that vehicle- and AM404-treated rats did not differ for the time spent in the center of the arena in session 1 (HA condition: F3, 36 = 1.25; p = 0.31; Figure 5A; LA condition: F3, 50 = 1.18; p = 0.33; Figure 5B).
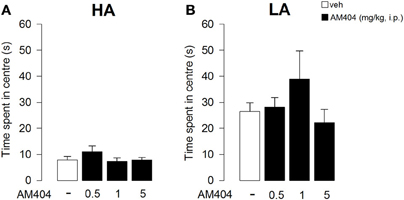
Figure 5. Effects of AM404 administration on emotional behavior in rats exposed to high arousal (HA) or low arousal (LA) conditions. Time spent in the center of the arena by rats exposed to HA (A) or LA conditions (B) in session 1. Data are expressed as mean ± SEM. (HA: veh n = 10, 0.5 mg/kg n = 11, 1 mg/kg n = 10, 5 mg/kg n = 9; LA: veh n = 15, 0.5 mg/kg n = 12, 1 mg/kg n = 14, 5 mg/kg n = 13).
AM404 Administration Influenced Object Exploration Depending on the Different Arousal Condition
The time spent in contact with objects throughout sessions 2–6 was analyzed with a mixed model-ANOVA taking treatment as one between-subject factor and sessions as one repeated measure factor. The mixed model-ANOVA for rats subjected to the HA condition gave the following differences: treatment F3, 33 = 2.72; p = 0.02; sessions F4, 132 = 39.78; p < 0.0001, interaction treatment × sessions F12, 132 = 1.50; p = 0.13. Since the interaction between treatment and sessions was not statistically significant, for a clearer representation of the results, data are represented in Figure 6 as the mean time spent in contact with the objects during all sessions. Individual comparisons (Tukey's test) performed on the main effect of treatment, revealed that AM404 (0.5 mg/kg) treated rats spent less time exploring the objects than control animals (p < 0.05, Figure 6A).
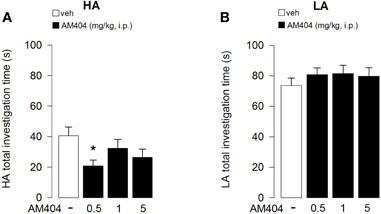
Figure 6. Effects of AM404 administration on object investigation in rats exposed to high arousal (HA) or low arousal (LA) conditions. Total investigation time of all objects by rats exposed to HA (A) or LA (B) conditions through sessions 2–6. *P < 0.05. Data are expressed as mean ± SEM. (HA: veh n = 10, 0.5 mg/kg n = 11, 1 mg/kg n = 10, 5 mg/kg n = 9; LA: veh n = 15, 0.5 mg/kg n = 12, 1 mg/kg n = 14, 5 mg/kg n = 13).
On the other hand, the mixed model-ANOVA for rats subjected to the LA condition revealed only a statistical significant effect for sessions without any statistical significance for either treatment or interaction between treatment and sessions: treatment F3, 46 = 0.12; p = 0.95; sessions F4, 184 = 20.82; p < 0.0001, interaction treatment × sessions F12, 132 = 1.20; p = 0.29 (Figure 6B), indicating that AM404 administration did not influence the total object investigation time in rats subjected to the LA arousal condition.
AM404 Administration did not Influence Spatial Change Discrimination while it Altered Object Novelty Recognition in Rats Exposed to Different Arousal Conditions
One–Way ANOVA showed that administration of AM404 did not influence the rat capability to discriminate the object displacement under both the HA (Figure 7A) or LA (Figure 7B) experimental conditions (F3, 36 = 1.176; p = 0.34; F3, 50 = 2.24; p = 0.095, respectively). However, One–Way ANOVA showed a statistical significant effect on the capability of the rats to discriminate a novel object under a HA condition (F3, 36 = 4.32; p = 0.01; Figure 8A). Post-hoc comparisons revealed that rats administered with AM404 0.5 and 1 mg/kg were not able to discriminate the new object as vehicle-treated rats did (p < 0.05). One–Way ANOVA revealed that AM404 administration to LA exposed rats did not influence the capability of the rats to discriminate the new object compared to the vehicle group (F3, 50 = 0.26; p = 0.85; Figure 8B).
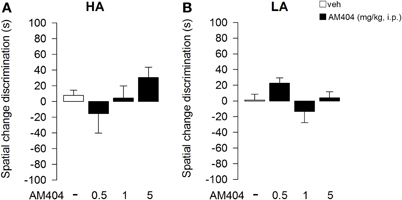
Figure 7. Effects of AM404 administration on spatial change discrimination in rats exposed to high arousal (HA) or low arousal (LA) conditions. Spatial change discrimination index of rats exposed to HA (A) or LA (B) conditions. Tim spent in contact with Displaced (DO) and Non-Displaced (NDO) Objects in session 4 minus the mean time spent in contact with the same object in session 3. A discrimination index was obtained by subtracting the NDO value to DO value. Data are expressed as mean ± SEM. (HA: veh n = 10, 0.5 mg/kg n = 11, 1 mg/kg n = 10, 5 mg/kg n = 9; LA: veh n = 15, 0.5 mg/kg n = 12, 1 mg/kg n = 14, 5 mg/kg n = 13).
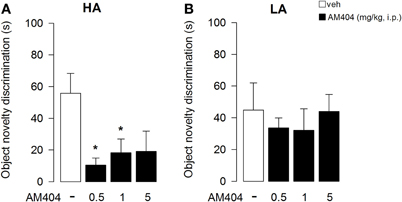
Figure 8. Effects of AM404 administration on object novelty discrimination in rats exposed to high arousal (HA) or low arousal (LA) conditions. Object novelty discrimination index of rats exposed to HA (A) or LA (B) conditions. Time spent in contact with Substituted Object (SO, unfamiliar) and Non-Substituted Objects (NSO, familiar) in session 6 minus the mean time spent with objects located in the corresponding position in session 5. A discrimination index was obtained by subtracting the NSO value to SO value. *P < 0.05. Data are expressed as mean ± SEM. (HA: veh n = 10, 0.5 mg/kg n = 11, 1 mg/kg n = 10, 5 mg/kg n = 9; LA: veh n = 15, 0.5 mg/kg n = 12, 1 mg/kg n = 14, 5 mg/kg n = 13).
Discussion
The present findings demonstrate that: (1) different levels of environmental aversiveness strongly influence the emotional reactivity of untreated rats without affecting the cognitive performance in the Spatial Open-Field test; (2) endocannabinoids affect recognition memory of rats in the Spatial Open Field test depending on the level of emotional arousal induced by the environmental conditions.
The Spatial Open-Field is a non-aversive test that permits to assess several behaviors which are indicative of the emotional state of the animal as well as the reactivity to both spatial rearrangement (spatial novelty) or the replacement of one familiar object with a new one (object novelty, as in the classical object recognition task) (Poucet et al., 1986; Thinus-Blanc et al., 1996). This test exploits the natural propensity of rodents to explore the environment without using rewards or punishments. Previous studies have shown that naive rodents respond to a new spatial displacement or substitution by renewed exploration of the entire environment and/or by selective reinvestigation of the displaced/substituted objects (Poucet et al., 1986; Thinus-Blanc et al., 1987; Poucet, 1989, 1993; Ricceri et al., 1999, 2000, 2002; Scattoni et al., 2004; de Bartolo et al., 2010). The one-day six-session assessment of the task used in our study permits to determine pharmacological effects on short-term memory as well as on emotional reactivity of the subject.
Activation of emotional responses, triggered by stressful stimuli, is crucial in the modulation of contextual learning and memory performances (McGaugh and Roozendaal, 2002; McGaugh, 2004; Morris, 2006; Campolongo et al., 2009b; Hill et al., 2010). There is evidence that behavioral responses to the environmental stimuli are strictly dependent on the emotional reactivity induced by the environment itself (Blanchard et al., 2001; Haller et al., 2009). The environmental–induced arousal is critically involved in assessing the novelty and salience of the external stimuli in terms of relevance for the adaptation and survival (Poucet, 1993; Biegler and Morris, 1996; Breivogel and Childers, 1998). Thus, when compared with a previous experience, a novel information recognized as highly relevant is committed to and stored by the memory (Lemaire et al., 1999). However, the mechanisms underlying the modulation of responsiveness to the environment and its evaluation in evolutionary terms both under LA or HA contexts remain to be elucidated.
Based on previous findings (Szeligo and Leblond, 1977; Sahakian et al., 1982; Morato and Castrechini, 1989; Griebel et al., 1993; Escorihuela et al., 1994; Hall et al., 1998; Varty et al., 2000; Haller et al., 2009), in order to characterize the behavioral responses to different environmental situations, we manipulated the experimental context to create two opposite arousal conditions by using two different protocols: (1) rats either extensively handled or not handled by the experimenter before testing, (2) isolated- or grouped-housed rats; (3) bright or dim red light conditions; (4) without or with familiar bedding during the testing phase for HA or LA conditions, respectively (for a comprehensive description see Materials and Methods). By using these different experimental conditions, we were able to induce a high or a low state in the animal, independently of any drug administration.
To first characterize the behavioral responses of rats to different environmental situations in the Spatial Open Field task, regardless of any drug administration, we analyzed the performance of vehicle-treated rats exposed to a HA or a LA context. The analysis of the first session of the Spatial Open Field task (when no objects were present) showed that locomotor activity was not influenced by the two different arousal conditions, while the different environmental situations influenced the level of emotional reactivity of the animals. Vehicle-treated rats, exposed to the LA context, spent indeed more time in the center of the open field than vehicle-treated rats exposed to the HA context. This result indicates that the LA environment may induce a lower level of emotional activation (Prut and Belzung, 2003).
The view that LA condition induces a lower level of emotional activation is also supported by behavioral analysis derived from sessions 2 to 6 of the task, in which the rats encountered different objects, also located in different positions in the open-field arena. Rats exposed to the LA context spent more time investigating the objects than rats exposed to the HA context, suggesting that a lower state of anxiety urges animals to better explore the objects (Crawley, 1985). Concerning the cognitive performance, the different level of emotional activation derived by exposure to the two environmental conditions did not influence the cognitive parameters measured in the task. Indeed, vehicle-treated rats exposed to either HA or LA conditions were equally able to recognize the object substitution but failed to respond to the object displacement. Interestingly, Ricceri and co-workers (Ricceri et al., 2000) showed that only 90-day-old mice were able to discriminate a spatial object rearrangement, while 46-day-old mice were not. In our study, we used young adult rats; this leaves open the possibility that the ability to discriminate a spatial change has to be still developed by rats at this age. Moreover, our findings are in accordance with the general assumption that the capability to recognize a new setting of the environment is important for the species survival, but the impact of the object novelty is more salient than a spatial rearrangement with the same objects (Mumby et al., 2002).
Extensive evidence demonstrates that the endocannabinoid system is a crucial regulator of emotionality and cognition (Marsicano et al., 2002; Laviolette and Grace, 2006a,b; Campolongo et al., 2009a,b; Mackowiak et al., 2009; Abush and Akirav, 2010; Akirav, 2011; Trezza et al., 2012). Although the neurobiological mechanisms underlying cannabinoid manipulation of emotional and cognitive functions have not yet been completely elucidated, previous evidence demonstrates that the anxiolytic effects induced by pharmacological enhancement of endocannabinoid tone strongly depend on the emotional state at the time of testing (Patel and Hillard, 2006) and that these effects are modulated by the level of emotional reactivity induced by high or low aversive experimental conditions (Haller et al., 2009).
To further shed light on the role of environmental aversiveness in cannabinoid modulation of emotionality and cognitive performance, we investigated whether exogenous manipulation of the endocannabinoid system influences rat behavior in the Spatial Open Field task in experimental conditions characterized by either a HA or LA state. Our findings clearly show that the effects of the endocannabinoid transport inhibitor AM404 on cognitive responses in the Spatial Open Field test strongly depend on the level of emotionality at the time of testing. Indeed, AM404 administration impaired the rat capability to discriminate between a familiar and a new object only in rats exposed to the HA condition.
Several studies have shown that CB1 receptor agonists produce anxiolytic- (Patel and Hillard, 2006; Scherma et al., 2008) or anxiogenic-like (Viveros et al., 2005; Patel and Hillard, 2006) effects, depending on the dose tested. Conversely, indirect cannabinoid agonists, that increase ongoing endocannabinoid signaling by interfering with their deactivation, induce anxiolytic-like effects without anxiogenic responses also when administered at high doses. For instance, the FAAH inhibitor URB597 produces anxiolytic-like effects in the elevated zero-maze and in the ultrasonic vocalization test in rats (Kathuria et al., 2003). In accordance with these findings, FAAH knockout mice exhibit an anxiolytic-like phenotype in the elevated plus-maze and in the light-dark box tests (Naidu et al., 2007; Moreira et al., 2008, 2009). Anxiolytic-like effects can also be induced by the inhibition of the endocannabinoid transport operated by endocannabinoid uptake inhibitors like AM404 (Beltramo et al., 1997; Beltramo and Piomelli, 2000). Thus, it has been demonstrated that the systemic administration of AM404 produces anxiolytic-like effects in three rat models of anxiety: elevated plus maze, defensive withdrawal, and separation-induced ultrasonic vocalization tests, and these effects are blocked by the administration of the CB antagonist rimonabant (Bortolato et al., 2006; Patel and Hillard, 2006). Nevertheless, it should be noted that in an another study Moreira and co-workers (Moreira et al., 2007) found that co-administration of anandamide and AM404 in the rat periaqueductal gray (a brain structure related to aversive response) elicited anxiolytic-like responses in the elevated plus maze test, whereas AM404 alone did not. In the present study, we found that administration of AM404 did not influence the emotional parameters taken onto consideration in the Spatial Open Field test, like the time spent in the central part of the arena during the first session of the task. However, it is important to note that, while AM404 administration did not influence the investigation of the objects through session 2–6 in a context characterized by a low-level of emotional activation, rats treated with the lower dose of AM404 and exposed to a stressful environment spent less time investigating objects, whereas the higher doses re-established the investigation activity at similar level of the vehicle-treated rats. The inhibition or the maintenance of the investigative behavior can be related to an anxiogenic or an anxiolytic phenotype, respectively (Crawley, 1985). It is possible to speculate that this biphasic effect may depend on a differential regulation activity on both GABAergic and gutamatergic neurons mediated by different doses of the endocannabinoid transport inhibitor (Foldy et al., 2007; Hashimotodani et al., 2007).
Regarding the cognitive performance, here we show for the first time that a pharmacologically-induced enhancement of endocannabinoid tone differentially modulates memory recognition in rats depending on different emotional states and different nature of the considered cognitive parameters (e.g., either spatial or novel object discrimination).
Concerning the object displacement, although the results did not reach any statistical significance it could be important to note that the treatment effect profile resemble a trend of a typical U-shaped dose response curve, in accordance with other results showing a similar dose-dependent biphasic response induced by cannabinoids, particularly by anandamide (Sulcova et al., 1998) and by the psychoactive constituent of Cannabis sativa preparation Δ9-tetrahydrocannabinol (Onaivi et al., 1990; Valjent et al., 2001). Concerning the object substitution, the lower doses of AM404 disrupted the ability to recognize a novel object in a stressful condition (HA) but not in a low arousal context (LA). It is well-established that the capability to recognize a new setting of the environment is important for species survival, but also that the impact of the object novelty is more salient than a spatial rearrangement with the same objects (Mumby et al., 2002). However, the capability to discriminate a novel object in the arena can be lost under particular circumstances such as in a more stressful context, after repeated exposure to an aversive environment and experimental manipulation of the endocannabinoid tone as in the present study (Save et al., 1992; Mumby et al., 2002; Hebda-Bauer et al., 2010).
These data confirm previous findings showing similar effects in humans and laboratory animals where acute or chronic exposure to the psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol, induces impairment in cognitive function (Egerton et al., 2006; Ranganathan and D'Souza, 2006; Solowij and Battisti, 2008; Campolongo et al., 2009c, 2011; D'Souza et al., 2009; Sofuoglu et al., 2010). In rodents, cannabinoid direct agonists induce impairment in several cognitive performances such as spatial learning, working memory, and attentional processes (Presburger and Robinson, 1999; Hampson and Deadwyler, 2000; Verrico et al., 2004; Robinson et al., 2007; Boucher et al., 2009, 2011). It is possible to speculate that these effects derive from cannabinoid-mediated disruption of cortical and hippocampal activity, crucially involved in encoding of the stimulus and making cognitive associations (Robbe et al., 2006; Deadwyler et al., 2007; Robbe and Buzsaki, 2009). The present results confirm the hypothesis that cannabinoid drugs, depending on the dose tested and the emotional state of the subject, could induce different effects on short-term memory parameters. The dissimilar effects induced by exposure to a different emotional state could depend on the activation of the hypothalamic-pituitary-adrenal (HPA) axis triggered by a HA context and to the subsequent release of stress hormones, such as glucocorticoids. It is well-known that this axis plays a crucial role in the stress response and that these hormones differentially modulate cognitive functions (Roozendaal and McGaugh, 1997; Mizoguchi et al., 2004; Atsak et al., 2011). In particular, de Quervain and co-workers (2009) reported that elevated glucocorticoid levels, elicited by aversive contexts, impair memory retrieval, and working memory. Moreover, further studies, conducted by our group, shed light on the crucial role of endocannabinoid signaling in the basolateral complex of the amygdala in modulating consolidation of aversive memory by an interaction with the glucocorticoid system (Campolongo et al., 2009a,b; Hill et al., 2010; Atsak et al., 2011).
Taken together, the present findings support the hypothesis of a fundamental role of the environment in influencing both the behavioral and cognitive outcomes in the Spatial Open Field task. Most importantly, it emerges that drugs that enhance endocannabinoid signaling by interfering with endocannabinoid deactivation induce different effects on short-term memory performance depending on the level of emotional arousal induced by different environmental settings.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by grants from Sapienza University “Progetti di Ricerca di Università” (to Patrizia Campolongo and Vincenzo Cuomo), from MIUR, FIRB Futuro in Ricerca to Patrizia Campolongo and Viviana Trezza and PRIN 2009 to Vincenzo Cuomo. The authors thank Daniela Valeri, Maria Chiara Bedetta, Chiara Pecci, and Veronica Carrara for technical help.
References
Abush, H., and Akirav, I. (2010). Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 20, 1126–1138.
Akirav, I. (2011). The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front. Behav. Neurosci. 5:34. doi: 10.3389/fnbeh.2011.00034
Atsak, P., Roozendaal, B., and Campolongo, P. (2011). Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience 204, 104–116.
Barna, I., Soproni, K., Arszovszki, A., Csabai, K., and Haller, J. (2007). WIN-55,212–2 chronically implanted into the CA3 region of the dorsal hippocampus impairs learning: a novel method for studying chronic, brain-area-specific effects of cannabinoids. Behav. Pharmacol. 18, 515–520.
Beltramo, M., and Piomelli, D. (2000). Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport 11, 1231–1235.
Beltramo, M., Stella, N., Calignano, A., Lin, S. Y., Makriyannis, A., and Piomelli, D. (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277, 1094–1097.
Biegler, R., and Morris, R. (1996). Landmark stability: studies exploring whether the perceived stability of the environment influences spatial representation. J. Exp. Biol. 199, 187–193.
Bisogno, T., and Di Marzo, V. (2010). Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 9, 564–573.
Blanchard, D. C., Griebel, G., and Blanchard, R. J. (2001). Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 25, 205–218.
Bolla, K. I., Brown, K., Eldreth, D., Tate, K., and Cadet, J. L. (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59, 1337–1343.
Bortolato, M., Campolongo, P., Mangieri, R. A., Scattoni, M. L., Frau, R., Trezza, V., La Rana, G., Russo, R., Calignano, A., Gessa, G. L., Cuomo, V., and Piomelli, D. (2006). Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 31, 2652–2659.
Boucher, A. A., Hunt, G. E., Micheau, J., Huang, X., McGregor, I. S., Karl, T., and Arnold, J. C. (2011). The schizophrenia susceptibility gene neuregulin 1 modulates tolerance to the effects of cannabinoids. Int. J. Neuropsychopharmacol. 14, 631–643.
Boucher, A. A., Vivier, L., Metna-Laurent, M., Brayda-Bruno, L., Mons, N., Arnold, J. C., and Micheau, J. (2009). Chronic treatment with Delta(9)-tetrahydrocannabinol impairs spatial memory and reduces zif268 expression in the mouse forebrain. Behav. Pharmacol. 20, 45–55.
Breivogel, C. S., and Childers, S. R. (1998). The functional neuroanatomy of brain cannabinoid receptors. Neurobiol. Dis. 5, 417–431.
Campolongo, P., Roozendaal, B., Trezza, V., Cuomo, V., Astarita, G., Fu, J., McGaugh, J. L., and Piomelli, D. (2009a). Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 106, 8027–8031.
Campolongo, P., Roozendaal, B., Trezza, V., Hauer, D., Schelling, G., McGaugh, J. L., and Cuomo, V. (2009b). Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl. Acad. Sci. U.S.A. 106, 4888–4893.
Campolongo, P., Trezza, V., Palmery, M., Trabace, L., and Cuomo, V. (2009c). Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterations. Int. Rev. Neurobiol. 85, 117–133.
Campolongo, P., Trezza, V., Ratano, P., Palmery, M., and Cuomo, V. (2011). Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology (Berl.) 214, 5–15.
Clarke, J. R., Rossato, J. I., Monteiro, S., Bevilaqua, L. R., Izquierdo, I., and Cammarota, M. (2008). Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol. Learn. Mem. 90, 374–381.
Cravatt, B. F., Giang, D. K., Mayfield, S. P., Boger, D. L., Lerner, R. A., and Gilula, N. B. (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87.
Crawley, J. N. (1985). Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 9, 37–44.
Curran, H. V., Brignell, C., Fletcher, S., Middleton, P., and Henry, J. (2002). Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl.) 164, 61–70.
D'Souza, D. C., Sewell, R. A., and Ranganathan, M. (2009). Cannabis and psychosis/schizophrenia: human studies. Eur. Arch. Psychiatry Clin. Neurosci. 259, 413–431.
de Bartolo, P., Cutuli, D., Ricceri, L., Gelfo, F., Foti, F., Laricchiuta, D., Scattoni, M. L., Calamandrei, G., and Petrosini, L. (2010). Does age matter? Behavioral and neuro-anatomical effects of neonatal and adult basal forebrain cholinergic lesions. J. Alzheimers Dis. 20, 207–227.
de Oliveira Alvares, L., Engelke, D. S., Diehl, F., Scheffer-Teixeira, R., Haubrich, J., de Freitas Cassini, L., Molina, V. A., and Quillfeldt, J. A. (2010). Stress response recruits the hippocampal endocannabinoid system for the modulation of fear memory. Learn. Mem. 17, 202–209.
de Oliveira Alvares, L., Genro, B. P., Diehl, F., and Quillfeldt, J. A. (2008). Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol. Learn. Mem. 90, 1–9.
de Quervain, D. J., Aerni, A., Schelling, G., and Roozendaal, B. (2009). Glucocorticoids and the regulation of memory in health and disease. Front. Neuroendocrinol. 30, 358–370.
Deadwyler, S. A., Goonawardena, A. V., and Hampson, R. E. (2007). Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav. Pharmacol. 18, 571–580.
Desarnaud, F., Cadas, H., and Piomelli, D. (1995). Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 270, 6030–6035.
Di Marzo, V. (1998). “Endocannabinoids” and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim. Biophys. Acta 1392, 153–175.
Di Marzo, V., Fontana, A., Cadas, H., Schinelli, S., Cimino, G., Schwartz, J. C., and Piomelli, D. (1994). Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691.
Di Marzo, V., and Matias, I. (2005). Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 8, 585–589.
Egerton, A., Allison, C., Brett, R. R., and Pratt, J. A. (2006). Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci. Biobehav. Rev. 30, 680–695.
Escorihuela, R. M., Tobena, A., and Fernandez-Teruel, A. (1994). Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behav. Brain Res. 61, 169–173.
Fant, R. V., Heishman, S. J., Bunker, E. B., and Pickworth, W. B. (1998). Acute and residual effects of marijuana in humans. Pharmacol. Biochem. Behav. 60, 777–784.
Foldy, C., Lee, S. Y., Szabadics, J., Neu, A., and Soltesz, I. (2007). Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat. Neurosci. 10, 1128–1130.
Fu, J., Bottegoni, G., Sasso, O., Bertorelli, R., Rocchia, W., Masetti, M., Guijarro, A., Lodola, A., Armirotti, A., Garau, G., Bandiera, T., Reggiani, A., Mor, M., Cavalli, A., and Piomelli, D. (2011). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci. 15, 64–69.
Ganon-Elazar, E., and Akirav, I. (2009). Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J. Neurosci. 29, 11078–11088.
Griebel, G., Moreau, J. L., Jenck, F., Martin, J. R., and Misslin, R. (1993). Some critical determinants of the behaviour of rats in the elevated plus-maze. Behav. Process. 29, 37–48.
Hall, F. S., Huang, S., Fong, G. W., Pert, A., and Linnoila, M. (1998). Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl.) 139, 203–209.
Haller, J., Barna, I., Barsvari, B., Gyimesi Pelczer, K., Yasar, S., Panlilio, L. V., and Goldberg, S. (2009). Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl.) 204, 607–616.
Hampson, R. E., and Deadwyler, S. A. (2000). Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J. Neurosci. 20, 8932–8942.
Hashimotodani, Y., Ohno-Shosaku, T., and Kano, M. (2007). Endocannabinoids and synaptic function in the CNS. Neuroscientist 13, 127–137.
Hauer, D., Ratano, P., Morena, M., Scaccianoce, S., Briegel, I., Palmery, M., Cuomo, V., Roozendaal, B., Schelling, G., and Campolongo, P. (2011). Propofol enhances memory formation via an interaction with the endocannabinoid system. Anesthesiology 114, 1380–1388.
Hebda-Bauer, E. K., Pletsch, A., Darwish, H., Fentress, H., Simmons, T. A., Wei, Q., Watson, S. J., and Akil, H. (2010). Forebrain glucocorticoid receptor overexpression increases environmental reactivity and produces a stress-induced spatial discrimination deficit. Neuroscience 169, 645–653.
Hill, M. N., and McEwen, B. S. (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 791–797.
Hill, M. N., Patel, S., Campolongo, P., Tasker, J. G., Wotjak, C. T., and Bains, J. S. (2010). Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 30, 14980–14986.
Hillard, C. J., Edgemond, W. S., Jarrahian, A., and Campbell, W. B. (1997). Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J. Neurochem. 69, 631–638.
Hillard, C. J., and Jarrahian, A. (2003). Cellular accumulation of anandamide: consensus and controversy. Br. J. Pharmacol. 140, 802–808.
Hillard, C. J., Wilkison, D. M., Edgemond, W. S., and Campbell, W. B. (1995). Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim. Biophys. Acta 1257, 249–256.
Janero, D. R., Vadivel, S. K., and Makriyannis, A. (2009). Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies. Int. Rev. Psychiatry 21, 122–133.
Kathuria, S., Gaetani, S., Fegley, D., Valino, F., Duranti, A., Tontini, A., Mor, M., Tarzia, G., La Rana, G., Calignano, A., Giustino, A., Tattoli, M., Palmery, M., Cuomo, V., and Piomelli, D. (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81.
Katona, I. (2009). Endocannabinoid receptors: CNS localization of the CB cannabinoid receptor. Curr. Top. Behav. Neurosci. 1, 65–86.
Laviolette, S. R., and Grace, A. A. (2006a). Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J. Neurosci. 26, 6458–6468.
Laviolette, S. R., and Grace, A. A. (2006b). The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell. Mol. Life Sci. 63, 1597–1613.
Lemaire, V., Aurousseau, C., Le Moal, M., and Abrous, D. N. (1999). Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur. J. Neurosci. 11, 4006–4014.
Mackie, K. (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 168, 299–325.
Mackowiak, M., Chocyk, A., Dudys, D., and Wedzony, K. (2009). Activation of CB1 cannabinoid receptors impairs memory consolidation and hippocampal polysialylated neural cell adhesion molecule expression in contextual fear conditioning. Neuroscience 158, 1708–1716.
Manwell, L. A., Satvat, E., Lang, S. T., Allen, C. P., Leri, F., and Parker, L. A. (2009). FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol. Biochem. Behav. 94, 154–162.
Marsicano, G., Wotjak, C. T., Azad, S. C., Bisogno, T., Rammes, G., Cascio, M. G., Hermann, H., Tang, J., Hofmann, C., Zieglgansberger, W., Di Marzo, V., and Lutz, B. (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534.
McGaugh, J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28.
McGaugh, J. L., and Roozendaal, B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 12, 205–210.
McLaughlin, R. J., and Gobbi, G. (2011). Cannabinoids and emotionality: a neuroanatomical perspective. Neuroscience 204, 134–144.
Micale, V., Cristino, L., Tamburella, A., Petrosino, S., Leggio, G. M., Drago, F., and Di Marzo, V. (2009). Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 34, 593–606.
Mizoguchi, K., Ishige, A., Takeda, S., Aburada, M., and Tabira, T. (2004). Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J. Neurosci. 24, 5492–5499.
Morato, S., and Castrechini, P. (1989). Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Br. J. Med. Biol. Res. 22, 707–710.
Moreira, F. A., Aguiar, D. C., Campos, A. C., Lisboa, S. F., Terzian, A. L., Resstel, L. B., and Guimaraes, F. S. (2009). Antiaversive effects of cannabinoids: is the periaqueductal gray involved? Neural Plast. 2009, 625469.
Moreira, F. A., Aguiar, D. C., and Guimaraes, F. S. (2007). Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology 52, 958–965.
Moreira, F. A., Kaiser, N., Monory, K., and Lutz, B. (2008). Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 54, 141–150.
Moreira, F. A., and Wotjak, C. T. (2010). Cannabinoids and anxiety. Curr. Top. Behav. Neurosci. 2, 429–450.
Morris, R. G. (2006). Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 23, 2829–2846.
Mumby, D. G., Gaskin, S., Glenn, M. J., Schramek, T. E., and Lehmann, H. (2002). Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 9, 49–57.
Naidu, P. S., Varvel, S. A., Ahn, K., Cravatt, B. F., Martin, B. R., and Lichtman, A. H. (2007). Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl.) 192, 61–70.
Onaivi, E. S., Green, M. R., and Martin, B. R. (1990). Pharmacological characterization of cannabinoids in the elevated plus maze. J. Pharmacol. Exp. Ther. 253, 1002–1009.
Pacher, P., Batkai, S., and Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462.
Patel, S., and Hillard, C. J. (2006). Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304–311.
Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884.
Poucet, B. (1989). Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behav. Neurosci. 103, 1009–1016.
Poucet, B. (1993). Spatial cognitive maps in animals: new hypotheses on their structure and neural mechanisms. Psychol. Rev. 100, 163–182.
Poucet, B., Chapuis, N., Durup, M., and Thinus-Blanc, C. (1986). A study of exploratory behavior as an index of spatial knowledge in hamsters. Learn. Behav. 14, 93–100.
Presburger, G., and Robinson, J. K. (1999). Spatial signal detection in rats is differentially disrupted by delta-9-tetrahydrocannabinol, scopolamine, and MK-801. Behav. Brain Res. 99, 27–34.
Prut, L., and Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33.
Ranganathan, M., and D'Souza, D. C. (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl.) 188, 425–444.
Ricceri, L., Colozza, C., and Calamandrei, G. (2000). Ontogeny of spatial discrimination in mice: a longitudinal analysis in the modified open-field with objects. Dev. Psychobiol. 37, 109–118.
Ricceri, L., Hohmann, C., and Berger-Sweeney, J. (2002). Early neonatal 192 IgG saporin induces learning impairments and disrupts cortical morphogenesis in rats. Brain Res. 954, 160–172.
Ricceri, L., Usiello, A., Valanzano, A., Calamandrei, G., Frick, K., and Berger-Sweeney, J. (1999). Neonatal 192 IgG-saporin lesions of basal forebrain cholinergic neurons selectively impair response to spatial novelty in adult rats. Behav. Neurosci. 113, 1204–1215.
Robbe, D., and Buzsaki, G. (2009). Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J. Neurosci. 29, 12597–12605.
Robbe, D., Montgomery, S. M., Thome, A., Rueda-Orozco, P. E., McNaughton, B. L., and Buzsaki, G. (2006). Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 9, 1526–1533.
Robinson, L., Goonawardena, A. V., Pertwee, R. G., Hampson, R. E., and Riedel, G. (2007). The synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br. J. Pharmacol. 151, 688–700.
Roozendaal, B., and McGaugh, J. L. (1997). Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol. Learn. Mem. 67, 176–179.
Sahakian, B. J., Burdess, C., Luckhurst, H., and Trayhurn, P. (1982). Hyperactivity and obesity: the interaction of social isolation and cafeteria feeding. Physiol. Behav. 28, 117–124.
Save, E., Poucet, B., Foreman, N., and Buhot, M. C. (1992). Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav. Neurosci. 106, 447–456.
Scattoni, M. L., Valanzano, A., Popoli, P., Pezzola, A., Reggio, R., and Calamandrei, G. (2004). Progressive behavioural changes in the spatial open-field in the quinolinic acid rat model of Huntington's disease. Behav. Brain Res. 152, 375–383.
Scherma, M., Medalie, J., Fratta, W., Vadivel, S. K., Makriyannis, A., Piomelli, D., Mikics, E., Haller, J., Yasar, S., Tanda, G., and Goldberg, S. R. (2008). The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54, 129–140.
Sciolino, N. R., Zhou, W., and Hohmann, A. G. (2011). Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 64, 226–234.
Sofuoglu, M., Sugarman, D. E., and Carroll, K. M. (2010). Cognitive function as an emerging treatment target for marijuana addiction. Exp. Clin. Psychopharmacol. 18, 109–119.
Solowij, N., and Battisti, R. (2008). The chronic effects of cannabis on memory in humans: a review. Curr. Drug Abuse Rev. 1, 81–98.
Sulcova, E., Mechoulam, R., and Fride, E. (1998). Biphasic effects of anandamide. Pharmacol. Biochem. Behav. 59, 347–352.
Szeligo, F., and Leblond, C. P. (1977). Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J. Comp. Neurol. 172, 247–263.
Tan, H., Lauzon, N. M., Bishop, S. F., Chi, N., Bechard, M., and Laviolette, S. R. (2011). Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J. Neurosci. 31, 5300–5312.
Terzian, A. L., Drago, F., Wotjak, C. T., and Micale, V. (2011). The dopamine and cannabinoid interaction in the modulation of emotions and cognition: assessing the role of cannabinoid CB1 receptor in neurons expressing dopamine D1 receptors. Front. Behav. Neurosci. 5:49. doi: 10.3389/fnbeh.2011.00049
Thinus-Blanc, C., Bouzouba, L., Chaix, K., Chapuis, N., Durup, M., and Poucet, B. (1987). A study of spatial parameters encoded during exploration in hamsters. J. Exp. Psychol. Anim. Behav. Process. 13, 418–427.
Thinus-Blanc, C., Save, E., Rossi-Arnaud, C., Tozzi, A., and Ammassari-Teule, M. (1996). The differences shown by C57BL/6 and DBA/2 inbred mice in detecting spatial novelty are subserved by a different hippocampal and parietal cortex interplay. Behav. Brain Res. 80, 33–40.
Trezza, V., Campolongo, P., Cassano, T., Macheda, T., Dipasquale, P., Carratu, M. R., Gaetani, S., and Cuomo, V. (2008a). Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl.) 198, 529–537.
Trezza, V., Campolongo, P., Manduca, A., Morena, M., Palmery, M., and Cuomo, V. (2012). Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front. Behav. Neurosci. 6:2. doi: 10.3389/fnbeh.2012.00002
Trezza, V., Cuomo, V., and Vanderschuren, L. J. (2008b). Cannabis and the developing brain: insights from behavior. Eur. J. Pharmacol. 585, 441–452.
Ueda, N., Kurahashi, Y., Yamamoto, S., and Tokunaga, T. (1995). Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J. Biol. Chem. 270, 23823–23827.
Ueda, N., Tsuboi, K., Uyama, T., and Ohnishi, T. (2011). Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors 37, 1–7.
Valjent, E., Pages, C., Rogard, M., Besson, M. J., Maldonado, R., and Caboche, J. (2001). Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 14, 342–352.
Varty, G. B., Paulus, M. P., Braff, D. L., and Geyer, M. A. (2000). Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol. Psychiatry 47, 864–873.
Verrico, C. D., Jentsch, J. D., Roth, R. H., and Taylor, J. R. (2004). Repeated, intermittent delta(9)-tetrahydrocannabinol administration to rats impairs acquisition and performance of a test of visuospatial divided attention. Neuropsychopharmacology 29, 522–529.
Vianna, M. R., Coitinho, A. S., and Izquierdo, I. (2004). Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr. Neurovasc. Res 1, 55–60.
Viveros, M. P., Marco, E. M., and File, S. E. (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 81, 331–342.
Viveros, M. P., Marco, E. M., Llorente, R., and Lamota, L. (2007). The role of the hippocampus in mediating emotional responses to nicotine and cannabinoids: a possible neural substrate for functional interactions. Behav. Pharmacol. 18, 375–389.
Waku, K. (2006). [Endogenous cannabinoid receptor ligands–anandamide and 2-arachidonoylglycerol]. Yakugaku Zasshi 126, 67–81.
Wilson, R. I., and Nicoll, R. A. (2002). Endocannabinoid signaling in the brain. Science 296, 678–682.
Keywords: cannabinoid system, endocannabinoids, emotionality, short-term memory, cognition
Citation: Campolongo P, Ratano P, Manduca A, Scattoni ML, Palmery M, Trezza V and Cuomo V (2012) The endocannabinoid transport inhibitor AM404 differentially modulates recognition memory in rats depending on environmental aversiveness. Front. Behav. Neurosci. 6:11. doi: 10.3389/fnbeh.2012.00011
Received: 13 February 2012; Paper pending published: 25 February 2012;
Accepted: 01 March 2012; Published online: 20 March 2012.
Edited by:
Antonella Gasbarri, University of L'Aquila, ItalyReviewed by:
Raquel V. Fornari, Universidade Federal do ABC, BrazilVincenzo Micale, Max-Planck Institute of Psychiatry, Germany
Copyright: © 2012 Campolongo, Ratano, Manduca, Scattoni, Palmery, Trezza and Cuomo. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Patrizia Campolongo, Department of Physiology and Pharmacology, Sapienza University of Rome, P.le A. Moro 5, 00185 Rome, Italy. e-mail: patrizia.campolongo@uniroma1.it
† These authors equally contributed to this work.