Neurobehavioral phenotyping of Gαq knockout mice reveals impairments in motor functions and spatial working memory without changes in anxiety or behavioral despair
- 1Neuroscience Graduate Program, Vanderbilt University School of Medicine, Nashville, TN, USA
- 2Vanderbilt Brain Institute, Vanderbilt University School of Medicine, Nashville, TN, USA
- 3Department of Pharmacology, Vanderbilt University School of Medicine, Nashville, TN, USA
- 4Vanderbilt Kennedy Center for Research on Human Development, Vanderbilt University School of Medicine, Nashville, TN, USA
Many neurotransmitters, hormones, and sensory stimuli elicit their cellular responses through the targeted activation of receptors coupled to the Gαq family of heterotrimeric G proteins. Nevertheless, we still understand little about the consequences of loss of this signaling activity on brain function. We therefore examined the effects of genetic inactivation of Gnaq, the gene that encode for Gαq, on responsiveness in a battery of behavioral tests in order to assess the contribution of Gαq signaling capacity in the brain circuits mediating expression of affective behaviors (anxiety and behavioral despair), spatial working memory, and locomotor output (coordination, strength, spontaneous activity, and drug-induced responses). First, we replicated and extended findings showing clear motor deficits in Gαq knockout mice as assessed on an accelerating rotarod and the inverted screen test. We then assessed the contribution of the basal ganglia motor loops to these impairments, using open field testing and analysis of drug-induced locomotor responses to the psychostimulant cocaine, the benzazepine D1 receptor agonists SKF83822 and SKF83959, and the NMDA receptor antagonist MK-801. We observed significant increases in drug-induced locomotor activity in Gαq knockout mice from the dopaminergic agonists but not MK-801, indicating that basal ganglia locomotor circuitry is largely intact in the absence of Gαq. Additionally, we observed normal phenotypes in both the elevated zero maze and the forced swim test indicating that anxiety and depression-related circuitry appears to be largely intact after loss of Gnaq expression. Lastly, use of the Y-maze revealed spatial memory deficits in Gαq knockout mice, indicating that receptors signaling through Gαq are necessary in these circuits for proficiency in this task.
Introduction
A large number of neurotransmitter receptors containing seven transmembrane domains, including the group I metabotropic glutamate receptors mGluR1 and mGluR5, α1 adrenergic receptors, and 5HT2 serotonergic receptors, mediate their physiological responses by activating heterotrimeric GTP-binding (G-) proteins with alpha subunits in the Gq family (Gαq) (Pin and Duvoisin, 1995; Millan et al., 2008; Cotecchia, 2010; Ribeiro et al., 2010). The Gαq family consists of four members: Gαq, Gα11, Gα14, and Gα15/16; of which Gαq and Gα11 represent the major isoforms in the adult brain (Strathmann and Simon, 1990; Wilkie et al., 1991). These proteins are co-expressed almost ubiquitously in the central nervous system, share 88% amino acid sequence homology and couple receptor stimulation to the activation of phospholipase C (PLC)-β isoforms, phosphatidylinositol (PI) hydrolysis, and downstream second messenger signaling systems (Strathmann and Simon, 1990; Smrcka et al., 1991; Taylor et al., 1991; Mailleux et al., 1992; Offermanns et al., 1994; Exton, 1996). Although Gαq and Gα11 are expressed together in almost every cell type, the relative levels of expression vary across brain regions with Gαq expression being 2–5 times higher than Gα11 in most areas (Milligan, 1993). Given that the functions of these two proteins are largely redundant, genetic inactivation of both Gnaq and Gna11, the genes that encode for Gαq and Gα11 respectively, results in embryonic lethality at embryonic day 10.5 (Offermanns et al., 1998). Genetic inactivation of either Gαq or Gα11 results in mice that are viable with more pronounced phenotypes observed in the Gαq knockouts, which harbor impairments in cerebellar maturation, motor coordination, and primary hemostasis (Offermanns et al., 1997a,b, 1998).
Gross anatomical deficits in the morphology and development of the nervous system have not been reported in Gαq knockout mice other than postnatal alterations in the innervation of the cerebellum (Offermanns et al., 1997b). As such, loss of Gαq-mediated synaptic pruning in the cerebellum has been suggested to underlie the altered behavioral output in motor function and ataxia observed in these animals. These findings raise the question of whether deficits in Gαq signaling in forebrain locomotor circuitry may also be involved. In these circuits, dopaminergic axons from the substantia nigra (SN) pars compacta innervate GABAergic medium spiny neurons (MSNs) in the dorsal striatum (caudate putamen). Also forming synaptic contacts on the dendritic arbors of the MSNs are descending corticostriatal glutamatergic inputs and those projections coming from the thalamus. MSNs in the dorsal striatum are largely segregated into two populations based on expression of dopamine receptors and axonal projections. MSNs expressing dopamine D1 receptors project directly to the SN pars reticulata while those expressing D2 receptors project to the SN pars reticulata via an indirect route involving intermediate synapses in the globus pallidus and the subthalamic nucleus. The SN pars reticulata then relays signals to the thalamus and motor cortex (stimulatory in the case of the direct pathway or inhibitory from the indirect) for the control of voluntary movement (Gerfen, 1992; Albin et al., 1995; Shuen et al., 2008). The thalamus and motor cortex are points of convergence between the cerebellar and basal ganglia circuits (Nakano, 2000), suggesting that deficits in either or both of these circuits could result in motor deficits from loss of Gαq signaling. In fact, Gαq is highly expressed in both the caudate putamen and the cerebellum (Mailleux et al., 1992).
Recent evidence also suggests that dopamine D1-like receptors, which are typically thought of as coupling with Gαs/olf, may also be capable of coupling to Gαq (Wang et al., 1995; Jin et al., 2001). Additionally, given the broad diversity in receptors that couple to Gαq and the range in biological processes in which these receptors are involved, there may be other circuits that are behaviorally relevant that might be impacted by constitutive loss of Gαq signaling. For example, the prefrontal cortex, a brain region that has been shown to directly regulate working memory and other cognitive functions (Goldman-Rakic, 1995; Chudasama, 2011; Kesner and Churchwell, 2011) as well as influencing a variety of other behaviors including mood regulation and emotional processing (Drevets et al., 2008; White et al., 2009; Etkin, 2010), also expresses high levels of Gαq protein (Milligan, 1993). The functions of the prefrontal cortex have been previously shown to be sensitive to local changes in neurochemical content and receptor signaling (Rinaldi et al., 2007; Vijayraghavan et al., 2007; Arnsten, 2011). Here, we address these questions and more precisely define the phenotype and drug responsivity of Gαq knockout mice using a systems-level approach.
Methods
Animals
The generation of Gαq knockout mice has previously been described (Offermanns et al., 1997b). For the present experiments, heterozygous Gαq males were mated to heterozygous females to generate litters containing wildtype, heterozygous, and knockout mice. The genotypes of all mice were determined by polymerase chain reaction (PCR) analysis of genomic DNA obtained from tail biopsies using methods previously described with minor adaptations (Offermanns et al., 1997b; Stanwood et al., 2005). For each experiment, an average of 6–24 wildtype, 6–20 heterozygous, and 6–15 knockout mice were analyzed with the exception of the forced swim test in which four knockout mice were analyzed. Tail biopsies were obtained at the time of weaning, postnatal day (P)21, for initial assignment of genotypes and once again at the time of sacrifice for confirmation.
Male mice were housed in cages of 2–5 with their littermates under standard housing conditions on a 12 h light/dark cycle (lights on 0600–1800 h) with ad libitum food and water. Their cages contained environmental enrichment huts (Otto Environmental, Milwaukee, WI) and their diet was high-energy irradiated LabDiet 5LJ5 (Tusculum, Nashville, TN). All behavioral testing was conducted during the light phase on mice that were at least (P)60 at the time of initial testing. Mice were extensively handled for at least 1 week prior to the beginning of testing and were habituated to the testing rooms for ~30 min prior to beginning of every experiment. Mice were also weighed prior to the beginning of each experiment and there were no significant changes in weight as a result of the behavioral testing. All procedures were approved by the Vanderbilt University Animal Care and Use Committee.
Drugs
The drugs used in this study were the dopamine D1-like receptor agonists SKF83959 (3-methyl-6-chloro-7,8-dihydroxy-1-[3-methylphenyl]-2,3,4,5-tetrahydro-1H-3-benzazepine; Tocris Biosciences, Minneapolis, MN) used at 1 mg/kg and SKF83822 ([R/S]-6-chloro-7, 8-dihydroxy-3-allyl-1-[3-methyl-phenyl]-2,3,4,5-tetrahydro-1H-3-benzazepine, NIMH Chemical Synthesis Program, Research Triangle Park, NC) used at 0.4 mg/kg. Additionally, the NMDA receptor antagonist MK-801 ([5R,10S]-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate, Sigma, St. Louis, MO) was used at 0.2 mg/kg and cocaine HCl (NIH/NIDA, Bethesda, MD) was used at 30 mg/kg. All drugs were dissolved in 0.9% saline and injected intraperiotoneally (i.p.).
Rotarod
Motor coordination and balance were measured using a commercially available accelerating rotarod apparatus (Ugo Basile model 7650, Collegeville, PA). Mice were placed on the rotating cylinder (3 cm in diameter) and confined to a section approximately 6.0 cm long by gray plastic dividers. The rotational speed of the cylinder was increased from 5 to 40 rpm over a 5 min period. The latency at which mice fell off the rotating cylinder was measured. Each mouse was tested on three independent trials per day (with a 15 min inter-trial period) over a 3 day testing period.
Inverted Screen
For the inverted screen test, 2–4 littermates were placed on a metal grid screen (10 × 14 cm) with separate compartments. After placement, the mice were allowed time to grip the grid before it was inverted 60 cm over a plastic cage containing fresh bedding. Latency to fall was recorded up to 60 s, at which point mice were removed from the apparatus and returned to the home cage. Three independent trials were conducted approximately 15 min apart on 1 day of testing, and data from all three trials were averaged together.
Elevated Zero Maze
The elevated zero maze is a modification of the elevated plus maze used for assessing anxiety-related behaviors. Use of the circular maze removes any ambiguity in data interpretation as there is no center zone (Lister, 1990; Shepherd et al., 1994; Rodgers et al., 1997). The elevated circular platform (40 cm off the ground, 50 cm in diameter) had two enclosed arenas opposite each other (5 cm wide with 15 cm high walls) and two open arenas (5 cm wide). At the start of the test, each mouse was lowered by its tail into the open arena of the maze and allowed to explore the maze for 300 s. Activity of the mouse was monitored via an overhead camera connected to a computer in a separate room using video acquisition and ANY-maze analysis software (Stoelting, Wood Dale, IL). Data analyzed included percentage of time spent in the open versus closed arenas and the total distance traveled in the maze.
Y-Maze
The Y-maze containing three clear arms (34.5 × 5.2 cm) joined in the center was placed on an opaque table about 91 cm above the ground in a room containing several large immovable objects to use as spatial cues. At the start of the test, each mouse was lowered by its tail into the center junction of the maze and allowed to explore the maze for 360 s. Activity of the mouse was monitored via an overhead camera connected to a computer in a separate room using video acquisition and ANY-maze analysis software (Stoelting, Wood Dale, IL). The sequence of individual arm entries was scored by the observer in real time and used to calculate the percentage of spontaneous alternations for each animal (consecutive entry into each of the three arms) as previously described (Thompson et al., 2005). The Y-maze assesses spatial working memory as animals tend to alternate between arms based on their memory of the previously visited arms. Chance performance is 22.2% in this paradigm.
Forced Swim Test
Behavioral despair was assessed in the forced swim test using plastic cylinders (50 cm in diameter, 21 cm in height) filled approximately three-fourth full with room temperature water. Mice were individually placed into the cylinder for a 6 min test and were recorded on video for the duration of the test. After testing, the mice were placed into a heating cage to dry before returning to the home cage. The water was changed between tests and the temperature of the water was recorded. Videos were later analyzed for time spent immobile for each mouse by an observer blinded to genotype.
Open Field
Locomotor activity in a novel open field and locomotor responses to SKF83959, SKF83822, cocaine, and MK-801 were measured using commercial open field activity chambers (Med Associates, 29 × 29 × 20.5 cm) that were contained within light- and air-controlled environmental chambers (Med Associates, St. Albans, VT; 64 × 45 × 42 cm) (Stanwood and Levitt, 2007). Location and movement were detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal direction, as well as 16 photocells elevated 4 cm to measure rearing) and were measured by the Med Associates Activity Monitoring program. On Days 1 and 2 of testing, mice were placed into activity chambers for 30 min for baseline measurements, removed from the chambers, injected with 0.9% saline, and returned to the chambers for 60 min. On Day 3 of testing, the mice were injected with SKF83959 (1 mg/kg) instead of 0.9% saline. This extended protocol was designed to extensively habituate the mice to the chambers before drug administration. For testing of additional compounds, a 2-day protocol was implemented where the mice received 0.9% saline on Day 1 and the test compound (0.4 mg/kg SKF83822, 0.2 mg/kg MK-801 or 30 mg/kg cocaine; i.p.) on Day 2. Experiments were conducted at least 1 week apart and animals were handled during the period of time during which they were not tested. For simplicity of analysis and display, the baseline and post-injection periods were averaged and are represented as bar graphs.
Data Analysis and Statistics
Except when otherwise noted, data were subjected to one- or-two way analysis of variance (ANOVA) using genotype as a between-group factor using GraphPad Prism. Post-hoc Tukey's Multiple Comparison Tests were used to compare groups to each other except for the rotarod analysis, where Bonferroni comparisons were employed. Normality was not observed within the inverted screen dataset, due to many null mice immediately falling from the screen. For these data, therefore, a nonparametric Kruskal-Wallace test and post-hoc Dunn's comparisons were employed. Graphs are marked with an asterisk (*) to denote statistical significance (p < 0.05). For data with p < 0.01 or p < 0.001, the graphs are marked with two (**) or three (***) asterisks, respectively. For data with a p > 0.05 but less than p = 0.20, the data was noted as exhibiting a trend. In the inverted screen test, genotype differences were assessed by unpaired Student's t-test with significance defined as two-tailed p < 0.05.
Results
Gαq Knockout Mice Exhibit Alterations in Body Weight
Visual inspection revealed that Gαq knockout mice are significantly smaller than their wildtype littermates (Figure 1A). Figure 1B shows the average weights of adult Gαq null, heterozygous, and wildtype mice at the start of behavioral testing. Consistent with their smaller sizes, Gαq knockout mice weigh almost half as much as wildtype mice [F(2, 28) = 12.33, p < 0.001] and this phenotype is maintained across their lifespan (data not shown).
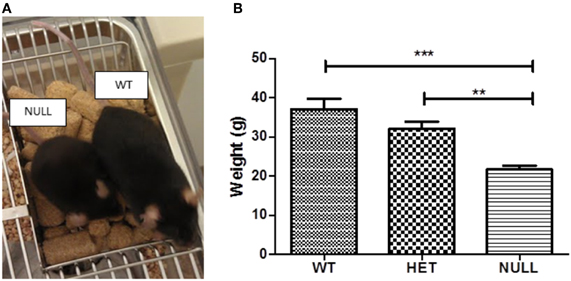
Figure 1. Weight analysis. Gαq knockout mice are smaller than their wildtype littermates as shown in the photomicrograph in (A). (B) shows the average weight of each genotype at the time of initial testing (~2–3 months of age). Gαq knockout mice weigh significantly less than wildtype [F(2, 28) = 12.33, p < 0.001] and heterozygous [F(2, 28) = 12.33, p < 0.01] mice and this phenotype is maintained throughout the life of the animals (data not shown). Each column represents the average of 8–12 animals.
Abnormal Motor Function in Gαq Knockout Mice
Mice homozygous for a deletion in Gαq have previously been described as exhibiting deficits in motor function including loss of balance during walking and rearing, spastic, and uncontrolled movements and ataxia upon visual inspection (Offermanns et al., 1997b and data not shown). Quantifiable deficits in motor function and coordination are revealed on an accelerating rotarod where Gαq knockout mice fell from the device in significantly less time than controls on each of three consecutive testing days (Figure 2A; factorial ANOVA, post-hoc Bonferroni comparisons p < 0.05 on Day 1, p < 0.01 on Days 2 and 3), confirming previous findings (Offermanns et al., 1997b). There were also significant differences in performance observed between the heterozygous and null mice on each day of testing (Figure 2A; p < 0.05 on Day 1, p < 0.001 on Days 2 and 3) with no significant differences between the heterozygous and wildtype mice. Similarly, Gαq knockout mice performed significantly worse than wildtype and heterozygous animals on an inverted screen test, confirming motor, and/or coordination impairments in the null animals (p < 0.05; Figure 2B).
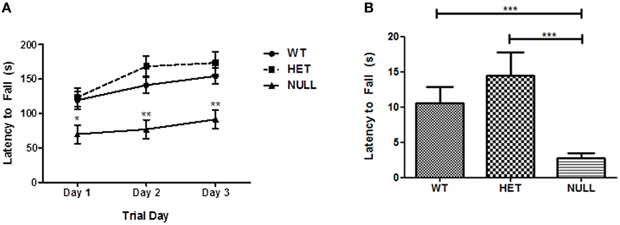
Figure 2. Rotarod and inverted screen tests. Gαq knockout mice spend significantly less time on an accelerating rotarod than their wildtype and heterozygous littermates (A; p < 0.05 between the knockouts and the other two genotypes on day 1 of testing; p < 0.01 on days 2 and 3 by Two-Way ANOVA with Bonferroni post-hoc comparison test). n = 6 for each genotype and each individual animal was tested in three trials on three consecutive days of testing. The data shown here represents the three trial averages across genotypes on each day of testing. (B) shows the latency of each genotype to fall from an inverted screen. Wildtype and heterozygous mice are able to grip the screen almost three times longer than Gαq null mice indicating reduced muscle and/or grip strength in the knockouts (overall p < 0.0001 by Kruskal–Wallis test; null mice are significantly different from both wildtype and heterozygote mice by Dunn's test, p < 0.05). Each column represents the average of 13–22 animals.
Gαq Knockout Mice Appear Normal in Tests of Anxiety and Behavioral Despair
Gαq knockout mice exhibited a significant hypolocomotive phenotype in the elevated zero maze as evidenced by the reduction in total ambulatory distance traveled in the maze compared to wildtype and heterozygote animals (Figure 3A). Both Gαq knockout and wildtype mice spent significantly more time in the closed arenas than the open arenas with no significant difference between the genotypes with respect to the percentage of time spent in the open (33.9 ± 3.8% for wildtype, 25.3 ± 6.1% for Gαq knockout) or closed arenas (66.1 ± 3.8% for wildtype, 74.7 ± 6.1% for Gαq knockout) of the maze (Figure 3B). This is consistent with previous reports suggesting that wildtype mice spend approximately 20–30% of their time in the open arenas of the zero maze (Shepherd et al., 1994). Although not statistically significant [F(2, 32) = 2.3, p = 0.11], heterozygous animals spent somewhat more time in the open arenas (42.1 ± 6.8%) than the other two genotypes. While in the open arenas, wildtype, and heterozygous animals traveled at significantly faster speeds than the knockout mice (Figure 3C; F(5, 30) = 8.5, p < 0.001) which moved slower in the maze overall (Figure 3D; F(2, 33) = 11.96, p < 0.001) compared to the other two genotypes.
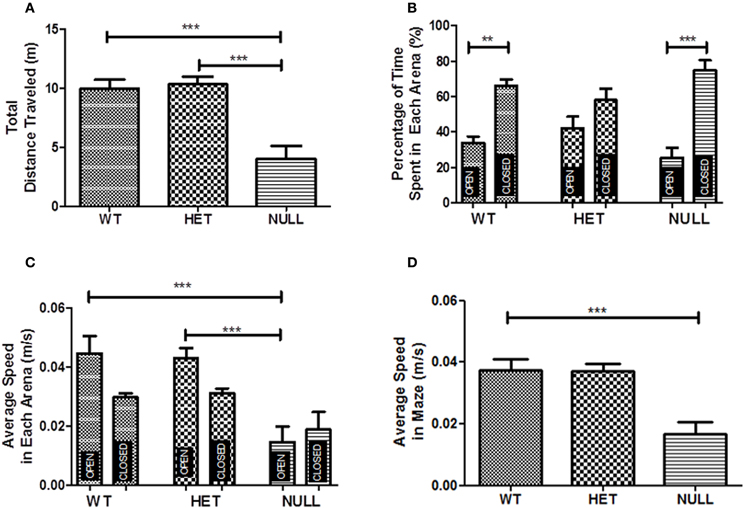
Figure 3. Elevated zero maze. Gαq knockout mice exhibit a hypoactive phenotype on the elevated zero maze and travel significantly less distance in the maze than their wildtype and heterozygous littermates (A; F(2, 15) = 16.55, p < 0.001). There is not a significant difference between the genotypes in the percentage of time spent in the open areas (B) indicating a normal anxiety phenotype in the null mice. n = 12 for wildtype and heterozygous mice, n = 11 for knockout mice in these experiments. While in the open arenas, wildtype, and heterozygous mice move at significantly faster speeds than knockout mice (C; F(5, 30) = 8.5, p < 0.001), which move slower in the maze overall (D; F(2, 33) = 11.96, p < 0.001).
Figure 4 displays the results of the forced swim test, a commonly used assay of behavioral despair used to predict the antidepressant potential of compounds or drug targets in animal models (Porsolt et al., 1977; Shepherd et al., 1994). The forced swim test revealed no significant differences between the genotypes, indicating that loss of Gαq protein expression does not confer antidepressant effects.
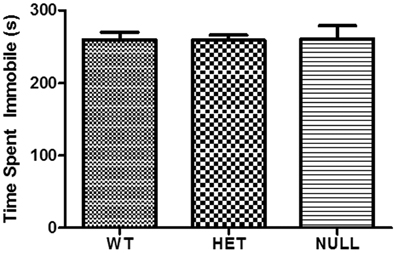
Figure 4. Forced swim test. Forced swim test analysis reveals no significant differences (p > 0.05) between the genotypes in this assay of behavioral despair. Each column represents the average of 4–6 animals.
Gαq Knockout Mice Exhibit Deficits in Spatial Working Memory
Gαq knockout mice again exhibited significant hypoactivity in the Y-maze as evidenced by the significant reduction in total arm entries committed by this genotype compared heterozygous and wildtype littermates (Figure 5A). In addition, acquisition of this spatial task was severely impaired in Gαq knockouts compared to wildtype [F(2, 4) = 13.3, p < 0.001]. Gαq knockout mice exhibited a significant reduction in the number of spontaneous alternations (entry of the maze's three arms in sequence), and the percentage of alternations, a measure which takes into account the hypoactive phenotype of the Gαq null mice in this task (Figures 5B,C). Wildtype animals spontaneously alternated at 62.5 ± 2.9% compared to 44.1 ± 7.5% observed in Gαq knockout mice.
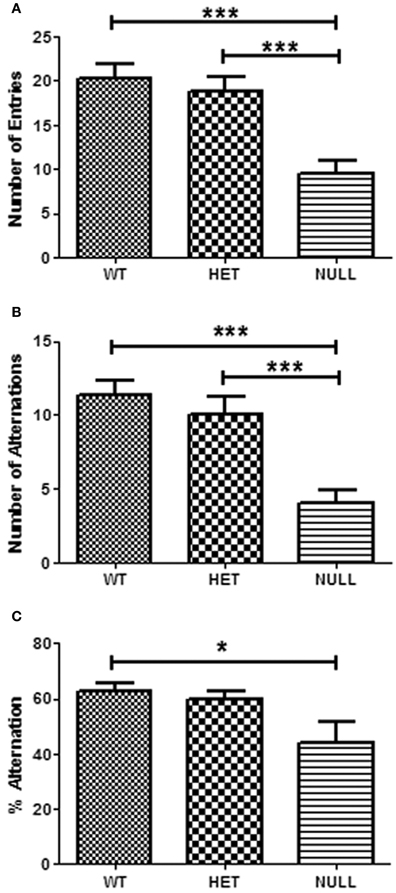
Figure 5. Y-maze test. In this test of spatial working memory, the reduced number of arms entered by Gαq knockout mice compared to wildtype and heterozygous littermates again reveals a hypoactive phenotype (A). In addition, acquisition of this spatial task was severely impaired in Gαq knockout mice which exhibited a significant reduction in the number of spontaneous alternations (B; F(2, 44) = 14.42, p < 0.001) and percentage of alternations (C; F(2, 44) = 4.44, p < 0.05 between wildtype and null) in this task. Percentage of alternations was determined by dividing the number of alternations by the number of possible alternations [(# total arm entries − 2) × 100], thereby taking into account the locomotor phenotype of Gαq knockout mice. Each column represents the average of 12–18 animals.
Drug-Induced Locomotor Responses Appear Intact in Gαq Knockout Mice
In order to gauge the integrity of basal ganglia locomotor circuitry, we investigated the spontaneous locomotor activity of Gαq knockout, heterozygous, and wildtype mice in open field chambers and their acute locomotor response to pharmacological compounds known to modulate locomotor output (primarily by modulating dopaminergic signaling in basal ganglia circuits). Our data show that in a novel open field, Gαq knockout mice are initially hypoactive compared to their wildtype and heterozygous littermates (as assessed by distance traveled) and travel significantly less distance than wildtype animals during the 90 min session (Figure 6A; F(2, 30) = 4.2, p < 0.05). These data further support our earlier observations regarding the activity level of the null animals in both the elevated zero and Y-maze tasks. On the second day of habituation (Figure 6B), as the wildtype and heterozygous animals acclimate further to the chambers and reduce their level of activity, there is no longer a significant difference in the total distance traveled by each genotype, although there is still a trend [F(2, 31) = 4.2, p = 0.16] toward hypoactivity in the null mice.
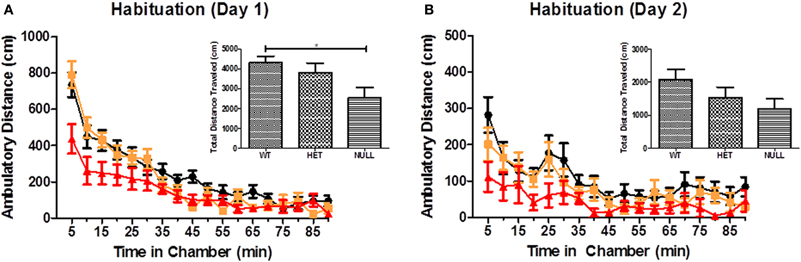
Figure 6. Open field test. Locomotor activity in a novel open field was assessed. (A,B) show the ambulatory distance traveled as a function of time (5 min blocks over a 90 min testing period) by each genotype on 2 days of habituation. Mice were removed from the chamber after 30 min and administered 0.9% saline (i.p.) before returning to the chamber for the last 60 min of testing. The inset in each panel shows the total distance traveled by each genotype, which is significantly reduced for Gαq knockout mice on day 1 (A; F(2, 30) = 4.2, p < 0.05). n = 12–18 for each genotype.
Drug-induced locomotor responses were first assessed using cocaine, a prototypical psychomotor stimulant that increases locomotor activity by blocking high affinity monoamine transporters. Figure 7A shows the ambulatory distance traveled by each genotype as a function of time in the open field chamber. The first 30 min represent the baseline period during which the animals were allowed time to habituate to the chamber and the last 60 min (minutes 35–90 on the graph) represent the post-injection period. The data for the baseline and post-injection periods are then averaged and displayed as Figure 7B. These data illustrate that injection of 30 mg/kg cocaine (i.p.) elicited a significant locomotor response in all three genotypes relative to the pre-injection baseline period [F(5, 21) = 186.0, p < 0.001]. The raw distance traveled for the knockout mice in the post-injection period, however, was significantly reduced compared to responses observed in both the wildtype and heterozygous animals [F(5, 21) = 186.0, p < 0.001]. There was also a small but significant blunting of the post-injection response of the heterozygous animals [F(5, 21) = 186.0, p < 0.001]. However, when normalized for percentage change from baseline, there were no significant differences between the genotypes. If anything, there was a trend [F(2, 25) = 2.23, p = 0.13] toward a greater percentage change from baseline in the null animals because their baseline activity was very low (Figure 7C). Taken together, these results indicate that the cocaine-induced locomotor response is largely intact in Gαq knockout mice, despite their profound basal hypoactivity.
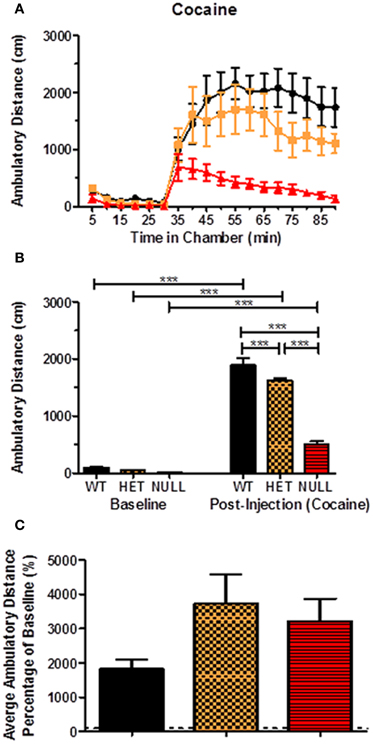
Figure 7. Locomotor response to acute cocaine. We investigated the integrity of locomotor responses to variety of pharmacological compounds with the ability to modulate dopaminergic signaling (directly or indirectly) and motor output. (A) shows the ambulatory distance traveled as a function of time (5 min blocks over a 90 min testing period) by each genotype during a 30 min baseline period and 60 min after the animals were injected with cocaine (30 mg/kg; i.p.). The data is represented as bar graphs in (B) with the data from min 10–30 collapsed for each genotype as the baseline measurement and min 40–60 representing the post-injection period. There is a significant increase in locomotor activity in each genotype following exposure to cocaine, however there is a significant difference between the post-injection locomotor response of the knockout mice compared to the other two genotypes [F(5, 21) = 186.0, p < 0.001]. The percentage change from baseline for each genotype is displayed in (C) with no significant changes. n = 6–12 for each genotype in these experiments.
Next, we assessed the locomotor responses to direct stimulation of dopamine D1 receptors by the high affinity benzazepine-derived agonist SKF83822. SKF83822 has been reported to activate dopamine D1 receptors coupled to Gαs/olf and downstream cyclase activity (Undie et al., 1994; Rashid et al., 2007). Behaviorally, SKF83822 has been shown to produce a locomotor response in both rodent and non-human primate models without affecting stereotypy, intense grooming, or dyskinesia (Peacock and Gerlach, 2001; O'Sullivan et al., 2004). In our analyses, an acute injection of SKF83822 (0.4 mg/kg; i.p.) induced a greater than threefold increase in locomotor activity relative to the baseline period for each genotype (Figures 8A,B; F(5, 18) = 139.2, p < 0.001). Again, as observed with acute cocaine, there were significant differences between the post-injection response of the knockout animals compared to their wildtype and heterozygous littermates (Figure 8B; F(5, 18) = 139.2, p < 0.001) without significant changes in the percentage change from baseline between the genotypes (Figure 8C).
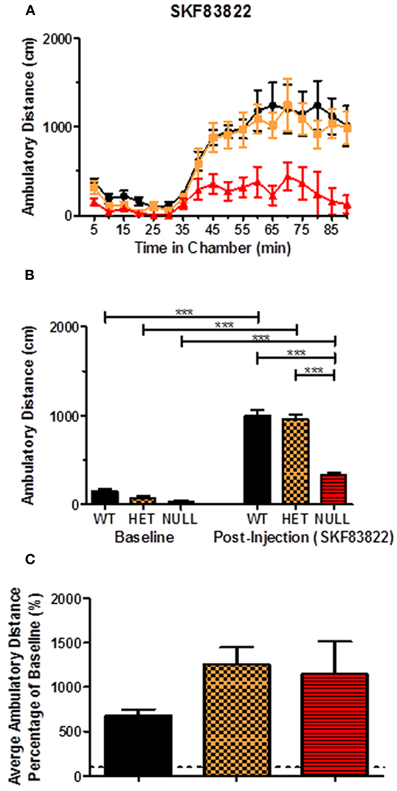
Figure 8. Locomotor response to acute SKF83822. (A,B) represent the ambulatory distance traveled by each genotype in response to an acute injection of the dopamine D1 receptor agonist SKF83822 (0.4 mg/kg; i.p.). There is a significant increase in locomotor activity in each genotype following exposure to SKF83822 [F(5, 18) = 139.2, p < 0.001]. There is, however, a significant difference between the locomotor response of the knockouts compared to the other two genotypes [F(5, 18) = 139.2, p < 0.001] without significant changes in the percentage change from baseline between the genotypes (C). n = 6–12 for each genotype in these experiments.
We then assessed a second benzazepine-derived D1 receptor agonist, SKF83959, for activity in the open field. Unlike SKF83822, SKF83959 has been reported to antagonize dopamine-mediated stimulation of adenylyl cyclase (Arnt et al., 1992; Andringa et al., 1999; Cools et al., 2002; Jin et al., 2003) and may preferentially activate D1 receptors linked to stimulation of PI hydrolysis (Arnt et al., 1992; Panchalingam and Undie, 2001; Jin et al., 2003). Initial studies assessing the locomotor response to varying doses of SKF83959 suggested that 1 mg/kg (i.p.) elicited a maximal response in wildtype mice (data not shown). This response was still fairly modest, however, and increased locomotor activity approximately twofold over the baseline level (data not shown). In response to an acute injection of SKF83959 (1 mg/kg; i.p.), we again observed significant increases in post-injection locomotor responses in wildtype, heterozygous, and knockout mice (Figures 9A,B; F(5, 18) = 123.9, p < 0.001). We did observe significant differences in the post-injection response of the knockout animals compared to the wildtype and heterozygous animals [F(5, 18) = 123.9, p < 0.001], however the magnitude of the locomotor response was more robust in the knockout [F(2, 33) = 7.0, p < 0.05] and heterozygous [F(2, 33) = 7.0, p < 0.001] animals compared to wildtype when considering the percentage change from baseline (Figure 9C).
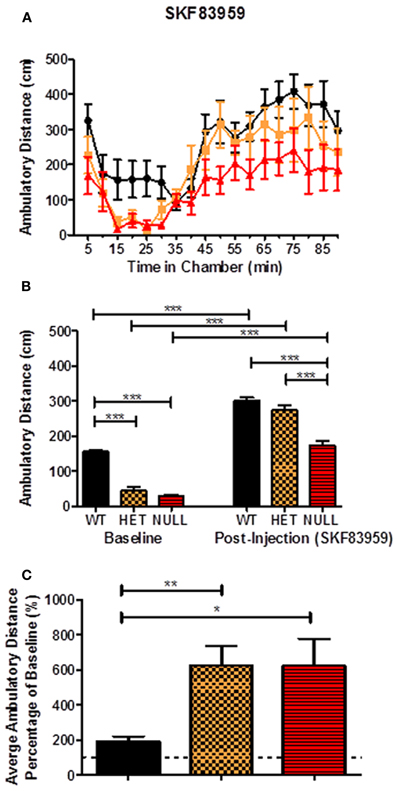
Figure 9. Locomotor response to acute SKF83959. SKF83959 (1 mg/kg; i.p.), a dopamine D1 receptor agonist that has been reported to activate D1 receptors coupled to Gαq, significantly increases locomotor activity in Gαq knockout, heterozygous, and wildtype mice (A,B; F(5, 18) = 123.9, p < 0.001). There is also a significant difference in the post-injection locomotor response of the null mice compared to their wildtype and heterozygous littermates (B; F(5, 18) = 123.9, p < 0.001) and a significant difference in the percentage change from baseline of the knockouts [C; F(2, 33) = 7.0, p < 0.05] and heterozygotes [F(2, 33) = 7.0, p < 0.001] compared to wildtype. n = 12–18 for each genotype.
Lastly, we set out to evaluate locomotor activity by modulating glutamatergic input with the non-competitive N-Methyl-D-Aspartate (NMDA) receptor antagonist MK-801. In response to acute MK-801 (0.2 mg/kg; i.p.), we observed significant increases in post-injection locomotor responses in wildtype and Gαq heterozygotes [F(5, 36) = 9.8, p < 0.05] but not in Gαq knockout mice (Figures 10A,B). There were however, as observed previously with all compounds tested, significant differences between the post-injection response of the knockout animals compared to their wildtype [F(5, 36) = 9.8, p < 0.001] and heterozygous [F(5, 36) = 9.8, p < 0.01] littermates (Figure 10B) following acute MK-801. Similarly, as observed with all compounds tested except SKF83959, there were no significant changes in the percentage change from baseline between the genotypes (Figure 10C).
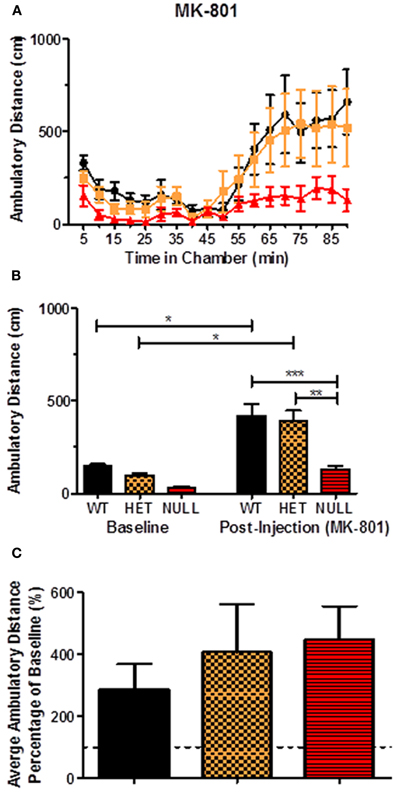
Figure 10. Locomotor response to acute MK-801. The NMDA receptor antagonist MK-801 (0.2 mg/kg; i.p.) induced a significant locomotor response in wildtype and heterozygous mice [F(5, 36) = 9.8, p < 0.05] but not in Gαq knockouts (A,B). Additionally, there were no significant changes observed in the percentage change from baseline between the genotypes (C). n = 6–12 for each genotype in these experiments.
Discussion
As predicted, Gαq knockout mice performed significantly worse than wildtype mice on tests of motor coordination and strength revealing a phenotypic motor dysfunction in these animals. These results confirm the visual phenotype of the null mice which hints at ataxia, inability to coordinate movements, and uncontrolled locomotion. Gαq knockout mice are also much smaller and weigh less than heterozygous or wildtype mice.
Motor impairments in Gαq knockout mice could result from deficits in motor circuits controlled by cerebellar output as previously hypothesized (Offermanns et al., 1997b) and/or the involvement of other motor pathways including those involving the basal ganglia locomotor circuitry, which we assessed indirectly using locomotor stimulant drugs. In attempting to holistically evaluate Gαq knockout mice for circuit-level deficits in brain function, we used simple, well-defined behavioral paradigms to probe the contribution of Gαq signaling capacity in complex behaviors that may be relevant to mental health disorders.
Anxiety and depression, for example, are two common emotional disorders accounting for a substantial proportion of the burden of mental health disorders in the United States (Weissman et al., 1996; Kessler et al., 2005). Although the neural circuits underlying these disorders are not completely understood, dysfunctions in the amygdala, hippocampus, basal ganglia, and prefrontal cortex are commonly implicated (Clark et al., 2009; Aupperle and Paulus, 2010; Clark and Beck, 2010; Harro et al., 2011; McEwen et al., 2012). In the elevated zero maze, a useful task for assessing anxiety-related behavior in rodent models, we saw no significant differences between the genotypes with respect to the percentage of time spent in the open arenas. These results indicate a normal anxiety-like phenotype in the knockout mice although we do not know if there are compensations within the circuit from global loss of Gαq that result in lack of an observed phenotype. Additionally, we observed no significant differences in the forced swim test assessing depressive-like phenotypes. These results were unexpected as there is evidence in the literature suggesting that inhibiting the PLC—protein kinase C signaling transduction pathway or intracellular calcium release (which can be activated by Gαq-coupled receptors) produces antidepressant effects in the forced swim test (Galeotti et al., 2006; Galeotti and Ghelardini, 2011). There is also evidence suggesting that chronic stress in rodent models alters transcript levels of Gnaq (Alfonso et al., 2006); another implication of alterations in Gαq signaling in rodent models of depression. It is possible, however, to activate these signaling pathways via other mechanisms including signaling initiated by Gα11 coupling which remains intact in the Gαq knockout animals.
The Y-maze task is often employed as a test of spatial working memory whereby mice will alternate between the three arms of the maze based on their interest in exploring the novel environment and their memory of the last arm entered. The influence of the prefrontal cortex in spontaneous alternation behaviors has previously been demonstrated in rodent models (Kolb, 1984) in addition to the roles of other brain regions including the hippocampus, basal forebrain, dorsal striatum, and cerebellum in mediating this behavior (Lalonde, 2002). We observed significant differences in the ability of the Gαq knockout mice to perform this task indicating that functional Gαq signaling in the prefrontal cortex may be necessary for acquisition of this task. These results are interesting with respect to the extensive literature detailing the importance of catecholamine signaling, in particular the role of dopamine, in mediating prefrontal cortex function, and working memory (Vijayraghavan et al., 2007; Arnsten, 2011). Additionally, there is evidence in the literature suggesting that dopamine D1 receptors in the prefrontal cortex are able to couple to Gαq (Jin et al., 2001) and thus the performance of the Gαq null mice could be explained by lack of dopamine signaling in this pathway. Alternatively, lack of signaling through other Gαq-coupled receptors in the cortex could be contributing to the observed phenotype.
In evaluating the intactness of the basal ganglia locomotor circuitry, we assessed the drug-induced locomotor responses of wildtype, heterozygous, and Gαq null animals to a variety of pharmacologic compounds. The psychostimulant cocaine acts indirectly to increase dopaminergic signaling by blocking the dopamine transporter, thus inhibiting dopamine re-uptake into pre-synaptic nerve terminals. As a result, dopamine accumulates at MSN synapses in the dorsal striatum, thus increasing and prolonging receptor activation primarily through D1 receptors signaling in the direct motor pathway to increase locomotor output (Kolb, 1984; Karasinska et al., 2005; Bateup et al., 2010). We were successful in generating locomotor responses in all three genotypes in response to acute cocaine suggesting that the functioning of the basal ganglia motor pathways remains largely intact in the absence of Gαq. Although Gαq protein expression does not appear to be necessary for the acute locomotor response to cocaine, it does appear to be involved in the expression of cocaine withdrawal in rodent models. It has previously been shown that rats undergoing withdrawal for 2 days after receiving twice-daily cocaine injections (15 mg/kg; i.p.) exhibited increased levels of membrane-associated Gα11 and Gαq proteins in the amygdala after 1 or 3 days (for peak expression, respectively) of cocaine exposure (Carrasco et al., 2004). Additionally, following 5 days of cocaine treatment, membrane-bound Gα11 and Gαq were also increased in the paraventricular nucleus of the hypothalamus (Carrasco et al., 2004), but no changes were observed in brain regions such as the frontal cortex even after 14 days of cocaine exposure (Carrasco et al., 2003, 2004). These changes in Gα11 and Gαq protein expression are transient, however, and are reversed back to baseline levels when assessed after 7 days of withdrawal (Carrasco et al., 2003).
We were also successful in generating a locomotor response in Gαq knockout mice by directly stimulating dopamine D1 receptors in the direct motor output pathway with the D1 receptor partial agonists SKF83822 and SKF83959. These results were expected with SKF83822 as this compound has been previously shown to produce a locomotor response typical of classical dopamine agonists stimulating adenylyl cyclase activity (Peacock and Gerlach, 2001; O'Sullivan et al., 2004). Interestingly, however, we also observed a locomotor response in Gαq knockout mice following administration of SKF83959, a dopamine D1 receptor agonist that has been reported to activate D1 receptors coupled to PI hydrolysis, and intracellular calcium mobilization. Previous reports assessing the behavioral effects of SKF83959 have shown the drug to elicit intense grooming behavior and orofacial movements in rodent models (Downes and Waddington, 1993; Fujita et al., 2010) and prove effective in reversing parkinsonian symptoms in rodent (unilateral 6-OHDA lesioned) and non-human primate (MPTP treated) models of Parkinson's disease (Arnt et al., 1992; Gnanalingham et al., 1995a,b,c; Zhang et al., 2007).
One recent hypothesis in the literature detailing a mechanism by which SKF83959 stimulates PI activity involves activating a D1/D2 receptor dimer complex coupled to Gαq protein (Rashid et al., 2007) and subsequent signaling systems. We therefore expected to observe minimal SKF83959-induced locomotor responses in Gαq knockout mice, if in fact, SKF83959 does activate D1 receptors signaling through Gαq. Contrary to our hypotheses, however, SKF83959-induced locomotor responses were intact in Gαq null animals. In fact, when their different baselines are taken into account, Gαq heterozygous and knockout mice may actually be more sensitive to the effects of the SKF83959 in that both genotypes exhibited a significantly greater percentage change from baseline in their post-injection locomotor response compared to wildtype animals. Taken together, these results suggest that SKF83959 may not be exclusively activating receptors coupled to the Gαq signaling pathway. The most likely explanation is that SKF83959 is not a selective as thought, and may activate D1 receptors coupled to Gαs/olf signaling pathways. It is conceivable that even if SKF83959 does activate D1-Gαs/olf coupled receptors, there could be alternative cyclase-independent pathways feeding into IP3 dependent-calcium mobilization that would support the initial biochemical characterization of this drug (Arnt et al., 1992; Andringa et al., 1999; Jin et al., 2003).
Our attempts to induce a significant locomotor response in Gαq knockout mice by modulating glutamatergic tone with the non-competitive NMDA receptor antagonist MK-801 proved unsuccessful. Experiments using this MK-801 were designed to indirectly modulate locomotor output as descending glutamatergic inputs from the cortex project to the striatum where they synapse on the dendritic spines of MSNs in close proximity to the synaptic contacts of the ascending midbrain dopamine neurons from the SN pars compacta. The glutamatergic and dopaminergic nerve terminals form synaptic triads where they contact MSNs: points of contact whereby the two signaling systems likely converge to modulate MSN output (Schmidt and Kretschmer, 1997; Jason et al., 2011). Additionally, there is evidence suggesting that MK-801 produces indirect activation of dopaminergic neurons to dose-dependently induce locomotor activity up to 0.5 mg/kg with a peak response around 0.2 mg/kg. At higher doses of MK-801 (>0.5 mg/kg) a characteristic motor syndrome is produced involving ataxia and stereotypic behaviors including head weaving and body rolling (Liljequist et al., 1991). Although the locomotor response induced by MK-801 was not statistically significant in the null animals in our assessments, there was a trend toward increased activity in these mice. In addition, the response observed in the wildtype animals was fairly modest after a 0.2 mg/kg exposure and it may be necessary to move to slightly higher doses for a more robust response. At higher doses, however, other behaviors are also elicited which could confound the clarity of the locomotor response. Furthermore, there is some evidence suggesting that proper postnatal signaling of the Gαq-coupled mGluR1 receptor is required for proper maturation of glutamatergic synapses in the ventral tegmental area, a midbrain nucleus containing dopaminergic cell bodies (Bellone et al., 2011). A mechanism such as this could potentially be necessary in other brain regions and may partially explain the results observed in the Gαq knockout mice.
Conclusions
We have replicated and extended findings showing clear motor deficits in Gαq knockout mice as assessed on an accelerating rotarod and the inverted screen test. Also, we have shown that Gαq knockout mice exhibit a significant hypoactive phenotype in the Y-maze, elevated zero maze and the open field, further supporting deficits in motor output. Drug-induced locomotor activity in Gαq knockout mice, however, remains intact with stimulation by dopaminergic agonists but not with the glutamatergic antagonist, MK-801. These findings indicate that basal ganglia locomotor circuitry is largely functional in the absence of Gαq signaling capacity. Motor impairments in these animals, therefore, likely originate in the cerebellum or other brain regions important in initiating motor output or discrete regions in areas such as the thalamus that are involved in signal integration and relay of motor signals to the cortex.
Additionally, we observed normal phenotypes in both the elevated zero maze and the forced swim test indicating that anxiety and depression-related circuitry appears intact after loss of Gαq expression. Lastly, use of the Y-maze revealed spatial memory deficits in Gαq knockout mice, indicating that functional Gαq-coupled receptor signaling is necessary for proficiency in this task, most likely in the prefrontal cortex. However, our use a global mutant line and systemic injections clearly requires a very cautious interpretation, particularly with regard to specific brain regions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by RO1MH086629 (Gregg D. Stanwood), F31DA029499 (Aliya L. Frederick), the Vanderbilt Kennedy Center and the Vanderbilt Brain Institute. Behavioral work was performed at the Vanderbilt Mouse Neurobehavioral Core which is supported in part by P30HD15052. We thank Dr. Stefan Offermanns (University of Heidelberg, Germany) for supplying the Gαq mutant line. We also thank Matt Buendia, Heather Durai and Dr. John Allison for excellent technical assistance and Dr. Devon Graham for reading the manuscript.
References
Albin, R. L., Young, A. B., and Penney, J. B. (1995). The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 18, 63–64.
Alfonso, J., Frick, L. R., Silberman, D. M., Palumbo, M. L., Genaro, A. M., and Frasch, A. C. (2006). Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biol. Psychiatry 59, 244–251.
Andringa, G., Drukarch, B., Leysen, J. E., Cools, A. R., and Stoof, J. C. (1999). The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur. J. Pharmacol. 364, 33–41.
Arnsten, A. F. (2011). Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 69, e89–e99.
Arnt, J., Hyttel, J., and Sanchez, C. (1992). Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur. J. Pharmacol. 213, 259–267.
Aupperle, R. L., and Paulus, M. P. (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin. Neurosci. 12, 517–531.
Bateup, H. S., Santini, E., Shen, W., Birnbaum, S., Valjent, E., Surmeier, D. J., Fisone, G., Nestler, E. J., and Greengard, P. (2010). Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl. Acad. Sci. U.S.A. 107, 14845–14850.
Bellone, C., Mameli, M., and Luscher, C. (2011). In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat. Neurosci. 14, 1439–1446.
Carrasco, G. A., Damjanoska, K. J., D'Souza, D. N., Zhang, Y., Garcia, F., Battaglia, G., Muma, N. A., and van de Kar, L. D. (2004). Short-term cocaine treatment causes neuroadaptive changes in Galphaq and Galpha11 proteins in rats undergoing withdrawal. J. Pharmacol. Exp. Ther. 311, 349–355.
Carrasco, G. A., Zhang, Y., Damjanoska, K. J., D'Souza, D. N., Garcia, F., Battaglia, G., Muma, N. A., and van de Kar, L. D. (2003). A region-specific increase in Galphaq and Galpha11 proteins in brains of rats during cocaine withdrawal. J. Pharmacol. Exp. Ther. 307, 1012–1019.
Chudasama, Y. (2011). Animal models of prefrontal-executive function. Behav. Neurosci. 125, 327–343.
Clark, D. A., and Beck, A. T. (2010). Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends Cogn. Sci. 14, 418–424.
Clark, L., Chamberlain, S. R., and Sahakian, B. J. (2009). Neurocognitive mechanisms in depression: implications for treatment. Annu. Rev. Neurosci. 32, 57–74.
Cools, A. R., Lubbers, L., van Oosten, R. V., and Andringa, G. (2002). SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: a key to its antiparkinsonian effect in animals? Neuropharmacology 42, 237–245.
Cotecchia, S. (2010). The alpha1-adrenergic receptors: diversity of signaling networks and regulation. J. Recept. Signal Transduct. Res. 30, 410–419.
Downes, R. P., and Waddington, J. L. (1993). Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine ‘D1-like’ receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur. J. Pharmacol. 234, 135–136.
Drevets, W. C., Price, J. L., and Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118.
Etkin, A. (2010). Functional neuroanatomy of anxiety: a neural circuit perspective. Curr. Top. Behav. Neurosci. 2, 251–277.
Exton, J. H. (1996). Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 36, 481–509.
Fujita, S., Kiguchi, M., Kobayashi, M., Kinsella, A., Koshikawa, N., and Waddington, J. L. (2010). Assessment of jaw movements by magnetic sensor in relation to topographies of orofacial behaviour in freely moving rats: studies with the dopamine D(1)-like receptor agonists SKF 83822 vs SKF 83959. Eur. J. Pharmacol. 632, 39–44.
Galeotti, N., Bartolini, A., and Ghelardini, C. (2006). Blockade of intracellular calcium release induces an antidepressant-like effect in the mouse forced swimming test. Neuropharmacology 50, 309–316.
Galeotti, N., and Ghelardini, C. (2011). Antidepressant phenotype by inhibiting the phospholipase Cbeta(1)–protein kinase Cgamma pathway in the forced swim test. Neuropharmacology 60, 937–943.
Gerfen, C. R. (1992). The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 15, 133–139.
Gnanalingham, K. K., Erol, D. D., Hunter, A. J., Smith, L. A., Jenner, P., and Marsden, C. D. (1995c). Differential anti-parkinsonian effects of benzazepine D1 dopamine agonists with varying efficacies in the MPTP-treated common marmoset. Psychopharmacology (Berl.) 117, 275–286.
Gnanalingham, K. K., Hunter, A. J., Jenner, P., and Marsden, C. D. (1995a). Stimulation of adenylate cyclase activity by benzazepine D-1 dopamine agonists with varying efficacies in the 6-hydroxydopamine lesioned rat–relationship to circling behaviour. Biochem. Pharmacol. 49, 1185–1193.
Gnanalingham, K. K., Hunter, A. J., Jenner, P., and Marsden, C. D. (1995b). The differential behavioural effects of benzazepine D1 dopamine agonists with varying efficacies, co-administered with quinpirole in primate and rodent models of Parkinson's disease. Psychopharmacology (Berl.) 117, 287–297.
Harro, J., Kanarik, M., Matrov, D., and Panksepp, J. (2011). Mapping patterns of depression-related brain regions with cytochrome oxidase histochemistry: relevance of animal affective systems to human disorders, with a focus on resilience to adverse events. Neurosci. Biobehav. Rev. 35, 1876–1889.
Jason, K., Ariel, D., Roger, C., and Danny, W. (2011). “Synaptic triad in the neostriatum,” in Dopamine – Glutamate Interactions in the Basal Ganglia, ed S. Jones (New York, NY: CRC Press), 71–104.
Jin, L. Q., Goswami, S., Cai, G., Zhen, X., and Friedman, E. (2003). SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J. Neurochem. 85, 378–386.
Jin, L. Q., Wang, H. Y., and Friedman, E. (2001). Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J. Neurochem. 78, 981–990.
Karasinska, J. M., George, S. R., Cheng, R., and O'Dowd, B. F. (2005). Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur. J. Neurosci. 22, 1741–1750.
Kesner, R. P., and Churchwell, J. C. (2011). An analysis of rat prefrontal cortex in mediating executive function. Neurobiol. Learn. Mem. 96, 417–431.
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602.
Kolb, B. (1984). Functions of the frontal cortex of the rat: a comparative review. Brain Res. 320, 65–98.
Lalonde, R. (2002). The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 26, 91–104.
Liljequist, S., Ossowska, K., Grabowska-Anden, M., and Anden, N. E. (1991). Effect of the NMDA receptor antagonist, MK-801, on locomotor activity and on the metabolism of dopamine in various brain areas of mice. Eur. J. Pharmacol. 195, 55–61.
Lister, R. G. (1990). Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 46, 321–340.
Mailleux, P., Mitchell, F., Vanderhaeghen, J. J., Milligan, G., and Erneux, C. (1992). Immunohistochemical distribution of neurons containing the G-proteins Gq alpha/G11 alpha in the adult rat brain. Neuroscience 51, 311–316.
McEwen, B. S., Eiland, L., Hunter, R. G., and Miller, M. M. (2012). Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62, 3–12.
Millan, M. J., Marin, P., Bockaert, J., and Mannoury la Cour, C. (2008). Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 29, 454–464.
Milligan, G. (1993). Regional distribution and quantitative measurement of the phosphoinositidase C-linked guanine nucleotide binding proteins G11 alpha and Gq alpha in rat brain. J. Neurochem. 61, 845–851.
Nakano, K. (2000). Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 1(Suppl. 22), S5–S16.
O'Sullivan, G. J., Roth, B. L., Kinsella, A., and Waddington, J. L. (2004). SK&F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioural topography. Eur. J. Pharmacol. 486, 273–280.
Offermanns, S., Heiler, E., Spicher, K., and Schultz, G. (1994). Gq and G11 are concurrently activated by bombesin and vasopressin in Swiss 3T3 cells. FEBS Lett. 349, 201–204.
Offermanns, S., Toombs, C. F., Hu, Y. H., and Simon, M. I. (1997a). Defective platelet activation in G alpha(q)-deficient mice. Nature 389, 183–186.
Offermanns, S., Hashimoto, K., Watanabe, M., Sun, W., Kurihara, H., Thompson, R. F., Inoue, Y., Kano, M., and Simon, M. I. (1997b). Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc. Natl. Acad. Sci. U.S.A. 94, 14089–14094.
Offermanns, S., Zhao, L. P., Gohla, A., Sarosi, I., Simon, M. I., and Wilkie, T. M. (1998). Embryonic cardiomyocyte hypoplasia and craniofacial defects in G alpha q/G alpha 11-mutant mice. EMBO J. 17, 4304–4312.
Panchalingam, S., and Undie, A. S. (2001). SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology 40, 826–837.
Peacock, L., and Gerlach, J. (2001). Aberrant behavioral effects of a dopamine D1 receptor antagonist and agonist in monkeys: evidence of uncharted dopamine D1 receptor actions. Biol. Psychiatry 50, 501–509.
Pin, J. P., and Duvoisin, R. (1995). The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26.
Porsolt, R. D., Bertin, A., and Jalfre, M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336.
Rashid, A. J., So, C. H., Kong, M. M., Furtak, T., El-Ghundi, M., Cheng, R., O'Dowd, B. F., and George, S. R. (2007). D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A. 104, 654–659.
Ribeiro, F. M., Paquet, M., Cregan, S. P., and Ferguson, S. S. (2010). Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol. Disord. Drug Targets 9, 574–595.
Rinaldi, A., Mandillo, S., Oliverio, A., and Mele, A. (2007). D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology 32, 309–319.
Rodgers, R. J., Cao, B. J., Dalvi, A., and Holmes, A. (1997). Animal models of anxiety: an ethological perspective. Braz. J. Med. Biol. Res. 30, 289–304.
Schmidt, W. J., and Kretschmer, B. D. (1997). Behavioural pharmacology of glutamate receptors in the basal ganglia. Neurosci. Biobehav. Rev. 21, 381–392.
Shepherd, J. K., Grewal, S. S., Fletcher, A., Bill, D. J., and Dourish, C. T. (1994). Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl.) 116, 56–64.
Shuen, J. A., Chen, M., Gloss, B., and Calakos, N. (2008). Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J. Neurosci. 28, 2681–2685.
Smrcka, A. V., Hepler, J. R., Brown, K. O., and Sternweis, P. C. (1991). Regulation of polyphos-phoinositide-specific phospholipase C activity by purified Gq. Science 251, 804–807.
Stanwood, G. D., and Levitt, P. (2007). Waved-1 mutant mice are hypersensitive to the locomotor actions of cocaine. Synapse 61, 259–262.
Stanwood, G. D., Parlaman, J. P., and Levitt, P. (2005). Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J. Comp. Neurol. 487, 270–282.
Strathmann, M., and Simon, M. I. (1990). G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc. Natl. Acad. Sci. U.S.A. 87, 9113–9117.
Taylor, S. J., Chae, H. Z., Rhee, S. G., and Exton, J. H. (1991). Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature 350, 516–518.
Thompson, B. L., Levitt, P., and Stanwood, G. D. (2005). Prenatal cocaine exposure specifically alters spontaneous alternation behavior. Behav. Brain Res. 164, 107–116.
Undie, A. S., Weinstock, J., Sarau, H. M., and Friedman, E. (1994). Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J. Neurochem. 62, 2045–2048.
Vijayraghavan, S., Wang, M., Birnbaum, S. G., Williams, G. V., and Arnsten, A. F. (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 10, 376–384.
Wang, H. Y., Undie, A. S., and Friedman, E. (1995). Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol. Pharmacol. 48, 988–994.
Weissman, M. M., Bland, R. C., Canino, G. J., Faravelli, C., Greenwald, S., Hwu, H. G., Joyce, P. R., Karam, E. G., Lee, C. K., Lellouch, J., Lepine, J. P., Newman, S. C., Rubio-Stipec, M., Wells, J. E., Wickramaratne, P. J., Wittchen, H., and Yeh, E. K. (1996). Cross-national epidemiology of major depression and bipolar disorder. JAMA 276, 293–299.
White, L. K., Helfinstein, S. M., Reeb-Sutherland, B. C., Degnan, K. A., and Fox, N. A. (2009). Role of attention in the regulation of fear and anxiety. Dev. Neurosci. 31, 309–317.
Wilkie, T. M., Scherle, P. A., Strathmann, M. P., Slepak, V. Z., and Simon, M. I. (1991). Characterization of G-protein alpha subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc. Natl. Acad. Sci. U.S.A. 88, 10049–10053.
Keywords: activity, coordination, G protein, Gq, inverted screen, learning, mood, rotarod
Citation: Frederick AL, Saborido TP and Stanwood GD (2012) Neurobehavioral phenotyping of Gαq knockout mice reveals impairments in motor functions and spatial working memory without changes in anxiety or behavioral despair. Front. Behav. Neurosci. 6:29. doi: 10.3389/fnbeh.2012.00029
Received: 09 March 2012; Accepted: 29 May 2012;
Published online: 19 June 2012.
Edited by:
Alcino J. Silva, University of California, Los Angeles, USAReviewed by:
Marie H. Monfils, University of Texas at Austin, USAAlicia Izquierdo, California State University, Los Angeles, USA
Martijn Schonewille, Erasmus MC, Netherlands
Copyright: © 2012 Frederick, Saborido and Stanwood. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Gregg D. Stanwood, Department of Pharmacology, Vanderbilt University School of Medicine, 8405 MRBIV, 2213 Garland Ave, Nashville, TN 37232-6600, USA. e-mail: gregg.stanwood@vanderbilt.edu