
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 15 January 2015
Sec. Individual and Social Behaviors
Volume 8 - 2014 | https://doi.org/10.3389/fnbeh.2014.00453
This article is part of the Research Topic Further Understanding of Serotonin 7 Receptors’ Neuro-Psycho-Pharmacology View all 13 articles
 Jonathan Shelton1*
Jonathan Shelton1* Sujin Yun1
Sujin Yun1 Susan Losee Olson2
Susan Losee Olson2 Fred Turek2
Fred Turek2 Pascal Bonaventure1
Pascal Bonaventure1 Curt Dvorak1
Curt Dvorak1 Timothy Lovenberg1
Timothy Lovenberg1 Christine Dugovic1
Christine Dugovic1Recent reports have illustrated a reciprocal relationship between circadian rhythm disruption and mood disorders. The 5-HT7 receptor may provide a crucial link between the two sides of this equation since the receptor plays a critical role in sleep, depression, and circadian rhythm regulation. To further define the role of the 5-HT7 receptor as a potential pharmacotherapy to correct circadian rhythm disruptions, the current study utilized the selective 5-HT7 antagonist JNJ-18038683 (10 mg/kg) in three different circadian paradigms. While JNJ-18038683 was ineffective at phase shifting the onset of wheel running activity in mice when administered at different circadian time (CT) points across the circadian cycle, pretreatment with JNJ-18038683 blocked non-photic phase advance (CT6) induced by the 5-HT1A/7 receptor agonist 8-OH-DPAT (3 mg/kg). Since light induced phase shifts in mammals are partially mediated via the modulation of the serotonergic system, we determined if JNJ-18038683 altered phase shifts induced by a light pulse at times known to phase delay (CT15) or advance (CT22) wheel running activity in free running mice. Light exposure resulted in a robust shift in the onset of activity in vehicle treated animals at both times tested. Administration of JNJ-18038683 significantly attenuated the light induced phase delay and completely blocked the phase advance. The current study demonstrates that pharmacological blockade of the 5-HT7 receptor by JNJ-18038683 blunts both non-photic and photic phase shifts of circadian wheel running activity in mice. These findings highlight the importance of the 5-HT7 receptor in modulating circadian rhythms. Due to the opposite modulating effects of light resetting between diurnal and nocturnal species, pharmacotherapy targeting the 5-HT7 receptor in conjunction with bright light therapy may prove therapeutically beneficial by correcting the desynchronization of internal rhythms observed in depressed individuals.
Circadian rhythms are governed by a variety of environmental inputs including light, food, social interaction, and pharmacological agents (Lall et al., 2012; Bloch et al., 2013; Patton and Mistlberger, 2013; Pendergast and Yamazaki, 2014). Of these, light is the most powerful of the synchronizing agents (Klein et al., 1991) and exerts its influence on circadian rhythms by first exciting a subset of retinal ganglion cells that subsequently activate neurons within the master circadian clock located in the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (RHT; Moore and Lenn, 1972; Gooley et al., 2001; Hattar et al., 2002; Panda et al., 2002; Ruby et al., 2002). In addition to photic input, serotoninergic pathways can also exert non-photic influence over the synchronization of circadian rhythms either by direct projections from raphe nucleus onto the SCN or indirect via the intergeniculate leaflet onto the SCN (Moga and Moore, 1997; Pickard and Rea, 1997; Ciarleglio et al., 2011). Working in accordance with each other, the photic and non-photic pathways help ensure proper synchronization of circadian rhythms by responding to a variety of changes in the environment. Recent studies have demonstrated that the misalignment or the inability to adjust to such derivations in the environment give rise to mood disorders in humans (Sprouse, 2004; Grandin et al., 2006; Murray and Harvey, 2010; Salvadore et al., 2010).
Utilizing a “clock in a dish” model, a role for the 5-HT7 receptor in the modulation of the non-photic regulation of circadian rhythms was noted for the first time when the receptor was initially cloned (Lovenberg et al., 1993). This method demonstrated that the phase advance in neuronal activity of the SCN following administration of the 5-HT1A/7 receptor agonist 8-OH-DPAT was attenuated by the 5-HT7/2 receptor antagonist ritanserin, but not by pindolol, a 5-HT1A/1B receptor antagonist (Lovenberg et al., 1993). This finding was later confirmed with the more selective 5-HT7 receptor antagonist SB-269970 (Sprouse et al., 2004). In vivo studies later reported the translational aspects of this cell model by demonstrating that phase advances in wheel running activity induced by 8-OH-DPAT were blocked following the administration of the 5-HT7 receptor antagonist DR-4004 in hamsters and absent in the 5-HT7 receptor knockout (KO) mouse (Ying and Rusak, 1997; Ehlen et al., 2001; Horikawa and Shibata, 2004; Gardani and Biello, 2008; Horikawa et al., 2013). In addition to its effects on the non-photic regulation of circadian rhythms, there is evidence that the 5-HT7 receptor may also influence photic regulation of circadian rhythms either by altering the sensitivity to light or modulating the release of serotonin (Ying and Rusak, 1997; Smith et al., 2001).
In addition to regulating circadian rhythms, the 5-HT7 receptor has been studied extensively for its role in depression. Initial investigation noted that 5-HT7 receptor KO mice exhibited an antidepressant-like phenotype in models of depression such as the tail suspension and forced swim tests (Guscott et al., 2005; Hedlund et al., 2005). Comparable antidepressant-like effects were found in these behavioral tests with the selective 5-HT7 receptor antagonist SB-269970 (Wesołowska et al., 2006; Sarkisyan et al., 2010). While many studies have utilized SB-269970 as a tool compound to investigate a role for the 5-HT7 receptor in various physiologic systems and pathological states, its utility is hampered due to a short half-life and poor drug-like properties (Hagan et al., 2000). Our in-house efforts yielded JNJ-18038683, a selective 5-HT7 receptor antagonist that exhibits better pharmacokinetic properties than SB-269970. Pre-clinical and clinical evaluation of the compound demonstrated that JNJ-18038683 was efficacious in the mouse tail suspension test and also enhanced serotonin transmission, antidepressant-like properties, and REM sleep suppression induced by the selective serotonin reuptake inhibitor (SSRI) citalopram in rats (Bonaventure et al., 2012). The effects of JNJ-18038683 on REM sleep translated from rodents to humans whereas the antidepressant efficacy needed to be further assessed (Bonaventure et al., 2012). Interestingly, systemic administration of the selective 5-HT7 receptor agonist LP-211 significantly increased the time spent awake while the direct infusion of this compound into dorsal raphe nucleus, locus coeruleus, basal forebrain, or laterodorsal tegmental nucleus resulted in decreased duration of REM sleep (Monti et al., 2008, 2014; Monti and Jantos, 2014). Similar REM sleep suppressive effects were observed when another selective 5-HT7 receptor agonist LP-44 was injected directly into the dorsal raphe nucleus (Monti et al., 2008).
Given the association of the 5-HT7 receptor with mood and circadian rhythms, pharmacological manipulation of this receptor may provide a critical insight into the therapeutic link between depression and circadian disruption. Therefore, the current study was designed to examine a role for the 5-HT7 receptor in circadian rhythm regulation by administering the selective 5-HT7 receptor antagonist JNJ-18038683 in both photic and non-photic circadian paradigms. To determine if JNJ-18038683 exerts direct phase resetting properties, a phase response curve was generated by administering the compound to mice at select times throughout the circadian cycle. Second, mice were administered JNJ-18038683 to determine if the compound alters the non-photic phase shift of wheel running activity induced by 8-OH-DPAT. Finally, JNJ-18038683 was administered prior to a light pulse that occurred at times known to delay (circadian time (CT) 15) or advance (CT 22) the onset of wheel running activity (Daan and Pittendrigh, 1976; Takahashi et al., 1984) in mice to determine the effects of acute pharmacological blockade of the 5-HT7 receptor on photic induced phase shifts. Results of these studies demonstrate that the 5-HT7 receptor influences both photic and non-photic aspects of circadian regulation and therefore may provide a therapeutic avenue to alleviate circadian disruptions associated with depression.
Studies conducted for the current investigation were performed in accordance with the policies and regulations of the respective IACUC committees at Northwestern University and Janssen Research and Development, L.L.C. For the experiments outlined for the following studies, male C57Bl/6 J mice (average weight ~30 grams) were purchased from Jackson Labs (Sacramento, CA) and allowed to acclimate for at least 2 weeks before being moved to environmental chambers that allowed for the ability to control lighting schedules and modified cages that contained a running wheel. Mice were allowed access to food and water ad libitum and maintained under a 12 h light/12 h dark schedule.
JNJ-18038683 (1-Benzyl-3-(4-chlorophenyl)-1,4,5,6,7,8-hexahydropyrazolo[3,4-d]azepine) was synthesized by medicinal chemistry group at Janssen Research and Development, L.L.C. as the citrate salt form. For formulations, 20% (w/v) hydroxypropyl-β-cyclodextrin was used to solubilize the compound and a correction factor of 1.5690 was applied to compensate for the salt form of JNJ-18038683. 8-OH-DPAT ((±)-8-Hydroxy-2-dipropylaminotetralin hydrobromide) (Tocris Biosciences) was formulated in saline. A correction factor of 1.32 was applied to compensate for the hydrobromide salt form of 8-OH-DPAT. Both compounds were injected in a volume of 10 mL/kg body mass. With each compound, the pH of the solution was adjusted to neutral before injection.
A phase response curve for JNJ-18038683 (10 mg/kg, i.p.) was generated by administering the compound at various times across the circadian cycle (CT2, 6, 10, 14, 18, or 22) to separate groups of mice for each time point. For these studies, a single animal was used in more than one injection condition and at least 2 weeks were given between conditions to prevent carry-over, or interference effects from the previous trial.
To determine if JNJ-18038683 alters the phase advance of wheel running activity induced by the 5-HT1A/7 receptor agonist, 8-OH-DPAT, mice were first randomized into four separate groups. Animals then received an injection of JNJ-18038683 (3 mg/kg, i.p.) or vehicle. Thirty minutes later, mice were further designated to receive 8-OH-DPAT (3 mg/kg, i.p.,) or corresponding vehicle at CT6 which had been previously reported to result in a phase advance of the onset of locomotor rhythm by 8-OH-DPAT (Horikawa and Shibata, 2004).
To investigate the role of the 5-HT7 receptor on photic induced phase shifts, JNJ-18036863 (10 mg/kg, s.c.) or vehicle was administered 30 min prior to an acute light pulse (200 lux, 30 min) that occurred at times that are known to produce a photic phase delay (CT 15) or advance (CT 22) of the onset of wheel running activity. As a control for the light pulse, mice were placed in the chamber but not exposed to light. For each treatment, separate groups of mice were used.
Wheel running activity was recorded by a magnetic switch located on the wheel that transferred each revolution as an event to an IBM compatible computer in 5 min bins using ClockLab software. Following at least a one-week acclimation period to adjust to the running wheels and novel cage, mice were released into constant darkness to assess free-running conditions and the onset of activity was monitored. On designated experimental days, the onset of activity (designated by convention as CT 12) was calculated based on the free running phase advance of activity of the previous 7 days. All timing for injections and light exposure was predicated by the calculation of CT 12 for each animal. Wheel running activity was monitored for at least 1 week following treatment or corresponding control conditions for changes to the onset of activity. A phase shift was calculated using ClockLab software and defined as the difference in the onset of activity after treatment vs. onset of activity prior to treatment. Data are expressed as mean ± S.E.M. To determine if JNJ-18038683 resulted in a significant phase shift at various time points along the phase response curve, an unpaired t-test (Vehicle vs. JNJ-18038683 treated animals) was executed. A one factor, four-level ANOVA followed by post hoc analysis was performed for each of the remaining two studies to determine if the phase shift was significant with the four treatment groups (p < 0.05). For visualization, a phase advance is denoted as a positive value on the bar graph while a phase delay is graphed as a negative value.
To determine the effects of the pharmacological blockade of the 5-HT7 receptor by JNJ-18038683 (10 mg/kg, i.p.), the compound was administered at various circadian times throughout the subjective circadian cycle (CT 2, 6, 10, 14, 18, 22) and the resulting phase shift was calculated post administration and compared to vehicle. At all times tested, JNJ-18038683 failed to elicit a phase shift as determined by unpaired t-test (Table 1). In a separate study, negative results were also obtained following the administration of a higher dose of JNJ-18038683 (20 mg/kg, i.p.) (data not shown).
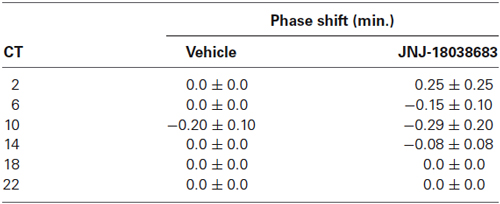
Table 1. Phase response curve following the administration of the 5-HT7 receptor antagonist JNJ-18038683 (10 mg/kg, i.p.) at various circadian times.
We then tested whether the 5-HT7 receptor antagonist JNJ-18038683 would attenuate the phase advance of wheel running locomotor activity elicited by the 5-HT1A/7 receptor agonist 8-OH-DPAT. Administration of the vehicle for JNJ-18038683 followed by 8-OH-DPAT resulted in a robust phase advance of the onset of locomotor activity during constant dark conditions (37.0 ± 6.3 min, F(3,58) = 18.49 p < 0.0001, one-way ANOVA, Tukey’s post hoc analysis) when compared to the other three treatment groups (Vehicle + Vehicle: −5.4 ± 4.1 min, JNJ-18038683 + Vehicle −0.9 ± 3.6 min, JNJ-18038683 + 8-OH-DPAT: −2.7 ± 3.0 min) (Figure 1). Comparable to what had been measured during the generation of the phase response curve, the administration of JNJ-18038683 in conjunction with the vehicle for 8-OH-DPAT did not produce any phase shift at CT 6. The administration of the 5-HT7 receptor antagonist JNJ-18038683 completely blocked the phase advance produced by 8-OH-DPAT (Figure 1).
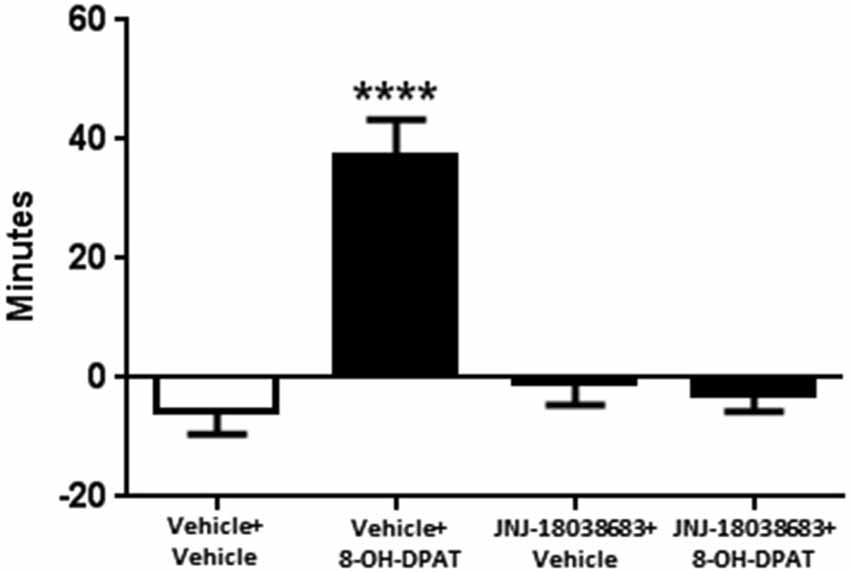
Figure 1. Phase shifting effects of JNJ-18038683 (3 mg/kg. i.p.) and 8-OH-DPAT (3 mg/kg, i.p.) on the circadian rhythms of wheel running activity in mice. Mice were randomly divided to receive one of four treatments: Vehicle + Vehicle (n = 14), Vehicle + 8-OH-DPAT (n = 21), JNJ-18038683 + Vehicle (n = 11), and JNJ-18038683 + 8-OH-DPAT (n = 16). After treatment, phase shifts of locomotor activity were calculated based upon the onset of activity following treatment compared to prior treatment. Results are expressed as mean ± S.E.M. and a one-way ANOVA followed by a Tukey’s post hoc test was performed to determine if a result was significant (****p < 0.0001 vs. Vehicle + Vehicle, JNJ-18038683 + Vehicle, and JNJ-18038683 + 8-OH-DPAT).
To determine the effects of pharmacological blockade of the 5-HT7 receptor on photic control of circadian rhythms, JNJ-18038683 was administered prior to a light pulse and the potential alterations in the onset of mouse wheel running activity was analyzed. In vehicle treated animals, light exposure resulted in a significant phase shift in the onset of activity at both times tested (CT 15: −2.65 ± 0.12 h: F(3,18) = 42.37; CT22: 0.76 ± 0.19 h: F(3,19) = 6.757 one way ANOVA, Tukey’s post hoc analysis) (Figures 2A–D). Administration of JNJ-18038683 attenuated the light-induced phase delay (−1.64 ± 0.29 h) (Figures 2C,D) while the resulting phase advance following light exposure at CT 22 was completely blocked by the compound (Figures 2A,B).
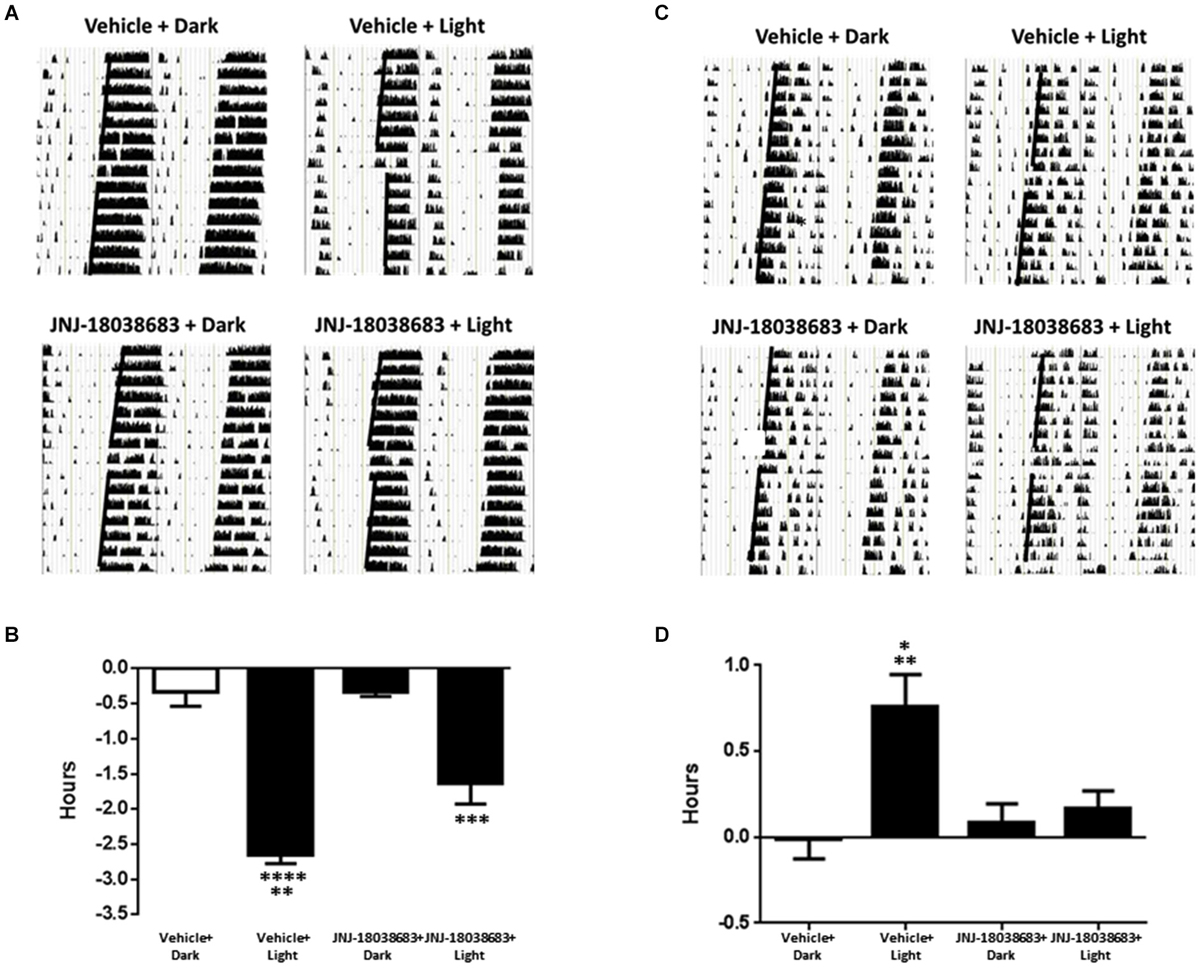
Figure 2. The impact of the 5-HT7 receptor antagonist JNJ-18038683 (10 mg/kg, s.c.) on light induced phase shifts. Representative actigrams and resulting phase shifts following administration of Vehicle/JNJ-18038683 or dark/light pulse on the circadian rhythms of wheel running activity in mice. Mice were randomly divided to receive one of four treatments: Vehicle + Dark (n = 5), Vehicle + Light Pulse (n = 6), JNJ-18038683 + Dark (n = 6), and JNJ-18038683 + Light Pulse (n = 5, CT 15; n = 6, CT 22). The light pulse was administered at times known to phase delay (CT15) (A,B) or advance (CT22) (C,D) the onset of wheel running activity in mice. After treatment, phase shifts of locomotor activity (onsets of activity indicated by bold black line) were calculated based upon the onset of activity following treatment compared to prior treatment. Results are expressed as mean ± S.E.M. and a one-way ANOVA followed by a Tukey’s post hoc test was performed to determine if a result was significant (Phase Delay—**p < 0.01 vs. JNJ-18038683 + Light, ***p < 0.001 vs. JNJ-18038683 + Dark, ****p < 0.0001 vs. Vehicle + Dark and JNJ-18038683 + Dark; Phase Advance—*p < 0.05 vs. JNJ-18038683 + Light, **p < 0.01 vs. Vehicle + Dark and JNJ-18038683 + Dark).
The present investigation examined the impact of the 5-HT7 receptor antagonist JNJ-18038683 on non-photic and photic regulation of the circadian rhythm of wheel running activity in mice. Results reported from these studies demonstrate that the compound did not exert direct non-photic or photic phase resetting properties by itself. However, JNJ-18038683 was able to block the non-photic phase advance of wheel running activity induced by the 5-HT1A/7 receptor agonist 8-OH-DPAT. In addition, JNJ-18038683 completely blocked the phase advance and significantly attenuated the phase delay of wheel running activity induced by a light pulse.
To determine if JNJ-18038683 exerts direct non-photic or photic phase resetting effects in free running mice, the compound was administered at specified times across the circadian cycle. At all times tested, JNJ-18038683 failed to elicit a phase shift when compared to vehicle treated mice. The lack of effect with JNJ-18038683 on phase shifts in mice was similar to what has been reported for the 5-HT2/7 receptor antagonist, ritanserin and a more selective 5-HT7 receptor antagonist SB-269970 in hamsters and rats (Antle et al., 1998; Duncan et al., 2004; Westrich et al., 2013). While antagonists for the 5-HT7 receptor do not modulate non-photic regulation of circadian rhythms, agonists for this receptor (8-OH-DPAT and LP-211) are capable of shifting the onset of wheel running activity in constant conditions (Horikawa and Shibata, 2004; Adriani et al., 2012). In addition, fluoxetine that inhibits the reuptake of serotonin and therefore, universally activates the serotonergic system has been shown to induce non-photic phase shifts (Cuesta et al., 2008, 2009). Therefore, global activation of the serotonergic system or direct pharmacological activation of the 5-HT7 receptor may be needed to induce photic or non-photic phase shifts.
The second study was designed to determine if the acute pharmacological blockade of the 5-HT7 receptor with the selective antagonist JNJ-18038683 impacted the phase advance of wheel running activity induced by 8-OH-DPAT in mice. The timing of 8-OH-DPAT administration was based on previous studies that demonstrated a robust phase advance when the compound was injected at CT 6 (Horikawa and Shibata, 2004). When JNJ-18038683 was administered before 8-OH-DPAT in the current study, the phase advance induced by the agonist was completely blocked demonstrating the impact of the 5-HT7 receptor on non-photic phase resetting of circadian rhythms. Since 8-OH-DPAT is an agonist for both the 5-HT1 and 5-HT7 receptors, future studies will utilize selective agonists for the 5-HT7 receptor (i.e., LP-44 or LP-211) to further elucidate the role of the 5-HT7 receptor on non-photic regulation of circadian rhythms.
Earlier in vivo and in vitro studies from other labs have provided some insight into a mechanism by which the 5-HT7 receptor regulates non-photic control of circadian rhythms. During mid-subjective day, micro-injection of 8-OH-DPAT or 5-Carboxamidotryptamine (5-CT) into the dorsal raphe nucleus activates 5-HT7 receptors and subsequently phase advances the circadian rhythms of wheel running activity in hamsters (Mintz et al., 1997; Duncan et al., 2004; Duncan and Davis, 2005). These phase shifts were blocked by the administration of the selective 5-HT7 receptor antagonist SB-269970 (Duncan et al., 2004). Further investigation delineated this model to show that the activation of the 5-HT7 receptor inhibits glutamate release within the dorsal raphe nucleus resulting in the inhibition of the release of GABA from interneurons which subsequently relieves the inhibition on serotonergic neurons located within the dorsal or medial raphe nucleus (Glass et al., 2003; Harsing et al., 2004; Duncan and Congleton, 2010). The subsequent release of serotonin onto the SCN results in non-photic phase modulation (Duncan and Congleton, 2010). Therefore, administration of JNJ-18038683 may be acting on 5-HT7 receptors within the dorsal raphe nucleus to inhibit glutamate release and thus blocking the non-photic phase shifts induced by 8-OH-DPAT. However, further studies are needed to define specific pathways in which the 5-HT7 receptor modulates non-photic circadian re-setting since the receptor is localized not only within the dorsal/median raphe nucleus to modulate GABA release, but also within the primary circadian oscillator of the SCN and other brain regions known to influence circadian rhythms such as the thalamus that could potentially impact NPY release which has been shown to alter non-photic phase shifts (Mrosovsky, 1996; Belenky and Pickard, 2001; Neumaier et al., 2001; Bonaventure et al., 2002; Gamble et al., 2006; Matthys et al., 2011; Hughes and Piggins, 2012).
In addition to the non-photic influence of circadian rhythms, serotonin can also alter photic responsiveness of the SCN possibly by acting via the 5-HT7 receptor. Early studies associated the 5-HT7 receptor with changes in the sensitivity of light in SCN neurons. Administration of serotonin, 5-CT, or 8-OH-DPAT reduced the firing of SCN neurons located in the hamster following a light pulse (Ying and Rusak, 1997). This decrease in neuronal firing was subsequently reversed by applying ritanserin or clozapine which are known to antagonize the 5-HT7 receptor but not the 5-HT1A/B/D receptor antagonist, cyanopindolol or the 5-HT1A receptor antagonist WAY-100635 (Ying and Rusak, 1997). In subsequent studies, hypothalamic excitatory post synaptic currents evoked by optic nerve stimulation which mimics a light pulse were reduced when the 5-HT1A/B agonist TFMPP was added to the bath (Smith et al., 2001). The change in these glutamate dependent currents within the hypothalamus were significantly attenuated with ritanserin, thus further implicating a role for the 5-HT7 receptor in photic control of circadian rhythms by modulating glutamate transmission (Smith et al., 2001). The additional findings that TFMPP had little effect in this model in 5-HT1B receptor KO mice and was minimally attenuated with the 5-HT1A receptor antagonist WAY-100635, provide some indirect evidence that the role of the 5-HT7 receptor in modulating photic control of circadian rhythms may be in coordination with other serotonergic receptors and glutamatergic neurotransmission.
Additional studies have investigated a role for the 5-HT7 receptor in the photic control of circadian rhythms by generating a phase response curve of light in 5-HT7 receptor KO and corresponding WT mice (Gardani and Biello, 2008). Overall, the phase shifts induced by the light pulse were comparable in magnitude and direction between WT and 5-HT7 receptor KO mice except at CT 22 (Gardani and Biello, 2008). At this time point, the light pulse resulted in the requisite phase advance in WT mice but a phase delay in the 5-HT7 receptor KO mice (Gardani and Biello, 2008). Interestingly, while the current study measured a significant attenuation of the light induced phase delay at CT 15 with the administration of JNJ-18038683, there was no difference in WT or 5-HT7 receptor KO mouse when the light pulse was administered at CT 14 or 16 which replicated an earlier report (Sprouse et al., 2003). The difference in regards to the light induced phase shift at these earlier time points between the pharmacological and the genetic deletion of the receptor may be the result of compensatory mechanisms involved in the phase resetting after a light pulse at these earlier time points of the subjective dark phase in the 5-HT7 receptor KO mouse.
Since this is the first report of attenuation of phase shifts following light pulses following the acute pharmacological blockade of the 5-HT7 receptor, additional studies are needed to further investigate the mechanism by which the 5-HT7 receptor is modulating the photic control of circadian rhythms. Results from these studies will provide an explanation as to why pharmacological blockade of the 5-HT7 receptor resulted in diminished photic induced phase shifts as opposed to an enhancement given the finding that activation of the 5-HT7 receptor inhibits the RHT input to the SCN by decreasing glutamate (Ying and Rusak, 1997; Smith et al., 2001). In addition, since antagonists selective for the 5-HT7 receptor lack direct photic-like phase shifting effects by themselves, the actions of these 5-HT7 receptor antagonists are probably due to the coordination with other serotonin receptors including the 5-HT1A and 5-HT1B receptors (Ying and Rusak, 1997; Rea, 1998; Smith et al., 2001).
Similar to our findings that JNJ-18038683 diminished photic induced phase resetting, pharmacological agents that are known to activate the serotonin system such as SSRIs (fluoxetine, citalopram, fluvoxamine, and paroxetine) also reduce the phase shift induced by a light pulse in the hamster (Gannon and Millan, 2007). Interestingly, fluoxetine potentiates the light induced phase shift in the diurnal species Arvicanthis ansorgei (Cuesta et al., 2008). These contrasting effects between nocturnal and diurnal species may be explained by the differential regulation of brain concentrations of serotonin corresponding to arousal state and also may be due to the changes in expression of circadian controlled genes following a light pulse in nocturnal and diurnal animals (Faradji et al., 1983; Poncet et al., 1993; Rea et al., 1994; Weber et al., 1998; Cuesta et al., 2008). While the current study did not measure the changes in the expression of such genes in response to pharmacological manipulation of the 5-HT7 receptor, Westrich and colleagues reported that the in vitro period as measured by a luciferase reporter linked to the PER2 gene, can be shortened by the 5-HT7 receptor agonist AS-19 and subsequently blocked by the 5-HT7 receptor antagonist SB-269970 (Westrich et al., 2013).
Due to the possible potentiation of the effects of light in diurnal species such as humans, 5-HT7 receptor antagonists may provide an interesting adjunctive therapy for the antidepressant effects associated with bright light therapy. The idea of adjunctive therapy with a 5-HT7 receptor antagonist arose from earlier studies in which JNJ-18038683 or SB-269970 enhanced the antidepressant-like effects of an SSRI in pre-clinical studies (Bonaventure et al., 2007, 2012; Wesołowska et al., 2007). When JNJ-18038683 was administered in combination with citalopram, there was an enhancement of serotonin transmission, antidepressant-like behavior, and REM sleep suppression induced by the SSRI in rodents (Bonaventure et al., 2012). In another study, Westrich et al. demonstrated that the administration of escitalopram or the 5-HT7 receptor antagonist SB-269970 was ineffective at phase shifting wheel running activity in rats, however, the co-administration of escitalopram with SB-269970 resulted in phase delays in rodent wheel running (Westrich et al., 2013). In addition to adjunctive therapy with an SSRI, a 5-HT7 receptor antagonist may also provide useful adjunctive therapy to bright light exposure in humans. While bright light therapy has been used for several decades as a stand-alone antidepressant therapy for seasonal affective disorder (Pail et al., 2011), it has also been used in conjunction with SSRI for major depressive disorder to bolster and hasten the antidepressant properties of SSRIs since the onset of mood improvement may take several weeks and also to increase the responsiveness in those individuals who are resistant to treatment with SSRIs by themselves (Benedetti et al., 2003; Blier, 2003; Martiny, 2004; Martiny et al., 2004; Wirz-Justice et al., 2005). There is clinical evidence that bright light therapy with sertraline can be therapeutically beneficial as an adjunct therapy for depression resulting in significant reductions in the HAM-D scale of depression (Martiny et al., 2005a,b). In addition, combining bright light therapy with citalopram has been shown to accelerate the onset of the anti-depressant properties of the SSRI (Benedetti et al., 2003). Therefore, combining bright light therapy with JNJ-18038683 may enhance the antidepressant effects of the light exposure in humans. Findings from these studies help provide insight into the translational therapeutic benefits of combining a 5-HT7 receptor antagonist with additional antidepressant modalities including bright light therapy that would correct the various circadian disruptions that are commonly associated with depression.
Jonathan Shelton, Sujin Yun, Curt Dvorak, Pascal Bonaventure, Timothy Lovenberg, and Christine Dugovic are full-time employees of Janssen Research and Development, L.L.C. Work performed by Dr. Fred Turek and Susan Losee Olson was part of a contract service in which they were compensated.
We gratefully acknowledge the contribution of Dr. Kevin Sharp and the vivarium staff.
Adriani, W., Travaglini, D., Lacivita, E., Saso, L., Leopoldo, M., and Laviola, G. (2012). Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology 62, 833–842. doi: 10.1016/j.neuropharm.2011.09.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Antle, M. C., Marchant, E. G., Niel, L., and Mistlberger, R. E. (1998). Serotonin antagonists do not attenuate activity-induced phase shifts of circadian rhythms in the Syrian hamster. Brain Res. 813, 139–149. doi: 10.1016/s0006-8993(98)01048-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Belenky, M. A., and Pickard, G. E. (2001). Subcellular distribution of 5-HT(1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J. Comp. Neurol. 432, 371–388. doi: 10.1002/cne.1109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Benedetti, F., Colombo, C., Pontiggia, A., Bernasconi, A., Florita, M., and Smeraldi, E. (2003). Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J. Clin. Psychiatry 64, 648–653. doi: 10.4088/jcp.v64n0605
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blier, P. (2003). The pharmacology of putative early-onset antidepressant strategies. Eur. Neuropsychopharmacol. 13, 57–66. doi: 10.1016/s0924-977x(02)00173-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloch, G., Herzog, E. D., Levine, J. D., and Schwartz, W. J. (2013). Socially synchronized circadian oscillators. Proc. Biol. Sci. 280:20130035. doi: 10.1098/rspb.2013.0035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bonaventure, P., Dugovic, C., Kramer, M., De Boer, P., Singh, J., Wilson, S., et al. (2012). Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J. Pharmacol. Exp. Ther. 342, 429–440. doi: 10.1124/jpet.112.193995
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bonaventure, P., Kelly, L., Aluisio, L., Shelton, J., Lord, B., Galici, R., et al. (2007). Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 321, 690–698. doi: 10.1124/jpet.107.119404
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bonaventure, P., Nepomuceno, D., Kwok, A., Chai, W., Langlois, X., Hen, R., et al. (2002). Reconsideration of 5-hydroxytryptamine (5-HT)(7) receptor distribution using [(3)H]5-carboxamidotryptamine and [(3)H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT(1A) knockout and 5-HT(1A/1B) double-knockout mice. J. Pharmacol. Exp. Ther. 302, 240–248. doi: 10.1124/jpet.302.1.240
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ciarleglio, C. M., Resuehr, H. E., and Mcmahon, D. G. (2011). Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 197, 8–16. doi: 10.1016/j.neuroscience.2011.09.036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cuesta, M., Clesse, D., Pévet, P., and Challet, E. (2009). New light on the serotonergic paradox in the rat circadian system. J. Neurochem. 110, 231–243. doi: 10.1111/j.1471-4159.2009.06128.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cuesta, M., Mendoza, J., Clesse, D., Pevet, P., and Challet, E. (2008). Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Exp. Neurol. 210, 501–513. doi: 10.1016/j.expneurol.2007.11.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Daan, S., and Pittendrigh, C. S. (1976). A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 106, 253–266. doi: 10.1007/bf01417857
Duncan, M. J., and Congleton, M. R. (2010). Neural mechanisms mediating circadian phase resetting by activation of 5-HT(7) receptors in the dorsal raphe: roles of GABAergic and glutamatergic neurotransmission. Brain Res. 1366, 110–119. doi: 10.1016/j.brainres.2010.09.103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Duncan, M. J., and Davis, V. A. (2005). Cyclic AMP mediates circadian phase shifts induced by microinjection of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 1058, 10–16. doi: 10.1016/j.brainres.2005.07.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Duncan, M. J., Grear, K. E., and Hoskins, M. A. (2004). Aging and SB-269970-A, a selective 5-HT7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 1008, 40–48. doi: 10.1016/j.brainres.2004.02.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ehlen, J. C., Grossman, G. H., and Glass, J. D. (2001). In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J. Neurosci. 21, 5351–5357.
Faradji, H., Cespuglio, R., and Jouvet, M. (1983). Voltammetric measurements of 5-hydroxyindole compounds in the suprachiasmatic nuclei: circadian fluctuations. Brain Res. 279, 111–119. doi: 10.1016/0006-8993(83)90168-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gamble, K. L., Paul, K. N., Karom, M. C., Tosini, G., and Albers, H. E. (2006). Paradoxical effects of NPY in the suprachiasmatic nucleus. Eur. J. Neurosci. 23, 2488–2494. doi: 10.1111/j.1460-9568.2006.04784.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gannon, R. L., and Millan, M. J. (2007). Evaluation of serotonin, noradrenaline and dopamine reuptake inhibitors on light-induced phase advances in hamster circadian activity rhythms. Psychopharmacology (Berl) 195, 325–332. doi: 10.1007/s00213-007-0903-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gardani, M., and Biello, S. M. (2008). The effects of photic and nonphotic stimuli in the 5-HT7 receptor knockout mouse. Neuroscience 152, 245–253. doi: 10.1016/j.neuroscience.2007.10.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glass, J. D., Grossman, G. H., Farnbauch, L., and Dinardo, L. (2003). Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J. Neurosci. 23, 7451–7460.
Gooley, J. J., Lu, J., Chou, T. C., Scammell, T. E., and Saper, C. B. (2001). Melanopsin in cells of origin of the retinohypothalamic tract. Nat. Neurosci. 4:1165. doi: 10.1038/nn768
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grandin, L. D., Alloy, L. B., and Abramson, L. Y. (2006). The social zeitgeber theory, circadian rhythms and mood disorders: review and evaluation. Clin. Psychol. Rev. 26, 679–694. doi: 10.1016/j.cpr.2006.07.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Guscott, M., Bristow, L. J., Hadingham, K., Rosahl, T. W., Beer, M. S., Stanton, J. A., et al. (2005). Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 48, 492–502. doi: 10.1016/j.neuropharm.2004.11.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hagan, J. J., Price, G. W., Jeffrey, P., Deeks, N. J., Stean, T., Piper, D., et al. (2000). Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br. J. Pharmacol. 130, 539–548. doi: 10.1038/sj.bjp.0703357
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harsing, L. G. Jr., Prauda, I., Barkoczy, J., Matyus, P., and Juranyi, Z. (2004). A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: evidence for a serotonergic-glutamatergic interaction. Neurochem. Res. 29, 1487–1497. doi: 10.1023/b:nere.0000029560.14262.39
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hattar, S., Liao, H. W., Takao, M., Berson, D. M., and Yau, K. W. (2002). Melanopsin-containing retinal ganglion cells: architecture, projections and intrinsic photosensitivity. Science 295, 1065–1070. doi: 10.1126/science.1069609
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hedlund, P. B., Huitron-Resendiz, S., Henriksen, S. J., and Sutcliffe, J. G. (2005). 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 58, 831–837. doi: 10.1016/j.biopsych.2005.05.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Horikawa, K., Fuji, K., Fukazawa, Y., and Shibata, S. (2013). Two distinct serotonin receptors co-mediate non-photic signals to the circadian clock. J. Pharmacol. Sci. 123, 402–406. doi: 10.1254/jphs.13170sc
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Horikawa, K., and Shibata, S. (2004). Phase-resetting response to (+)8-OH-DPAT, a serotonin 1A/7 receptor agonist, in the mouse in vivo. Neurosci. Lett. 368, 130–134. doi: 10.1016/j.neulet.2004.06.072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hughes, A. T., and Piggins, H. D. (2012). Feedback actions of locomotor activity to the circadian clock. Prog. Brain Res. 199, 305–336. doi: 10.1016/b978-0-444-59427-3.00018-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Klein, D. C., Moore, R. Y., and Reppert, S. M. (1991). Suprachiasmatic Nucleus. The Mind’s Clock. New York: Oxford University Press.
Lall, G. S., Atkinson, L. A., Corlett, S. A., Broadbridge, P. J., and Bonsall, D. R. (2012). Circadian entrainment and its role in depression: a mechanistic review. J. Neural Transm. 119, 1085–1096. doi: 10.1007/s00702-012-0858-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lovenberg, T. W., Baron, B. M., De Lecea, L., Miller, J. D., Prosser, R. A., Rea, M. A., et al. (1993). A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11, 449–458. doi: 10.1016/0896-6273(93)90149-l
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martiny, K. (2004). Adjunctive bright light in non-seasonal major depression. Acta Psychiatr. Scand. Suppl. 110, 7–28. doi: 10.1111/j.1600-0447.2004.00460_2.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martiny, K., Lunde, M., Simonsen, C., Clemmensen, L., Poulsen, D. L., Solstad, K., et al. (2004). Relapse prevention by citalopram in SAD patients responding to 1 week of light therapy. A placebo-controlled study. Acta Psychiatr. Scand. 109, 230–234. doi: 10.1046/j.1600-0447.2003.00256.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martiny, K., Lunde, M., Undén, M., Dam, H., and Bech, P. (2005a). Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr. Scand. 112, 117–125. doi: 10.1111/j.1600-0447.2005.00574.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martiny, K., Lunde, M., Undén, M., Dam, H., and Bech, P. (2005b). Adjunctive bright light in non-seasonal major depression: results from patient-reported symptom and well-being scales. Acta Psychiatr. Scand. 111, 453–459. doi: 10.1111/j.1600-0447.2005.00532.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Matthys, A., Haegeman, G., Van Craenenbroeck, K., and Vanhoenacker, P. (2011). Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol. Neurobiol. 43, 228–253. doi: 10.1007/s12035-011-8175-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mintz, E. M., Gillespie, C. F., Marvel, C. L., Huhman, K. L., and Albers, H. E. (1997). Serotonergic regulation of circadian rhythms in Syrian hamsters. Neuroscience 79, 563–569. doi: 10.1016/s0306-4522(96)00696-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moga, M. M., and Moore, R. Y. (1997). Organization of neural inputs to the suprachiasmatic nucleus in the rat. J. Comp. Neurol. 389, 508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Monti, J. M., and Jantos, H. (2014). The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev. Neurosci. 25, 429–437. doi: 10.1515/revneuro-2014-0016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Monti, J. M., Leopoldo, M., and Jantos, H. (2008). The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav. Brain Res. 191, 184–189. doi: 10.1016/j.bbr.2008.03.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Monti, J. M., Leopoldo, M., and Jantos, H. (2014). Systemic administration and local microinjection into the central nervous system of the 5-HT(7) receptor agonist LP-211 modify the sleep-wake cycle in the rat. Behav. Brain Res. 259, 321–329. doi: 10.1016/j.bbr.2013.11.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moore, R. Y., and Lenn, N. J. (1972). A retinohypothalamic projection in the rat. J. Comp. Neurol. 146, 1–14. doi: 10.1002/cne.901460102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mrosovsky, N. (1996). Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 71, 343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murray, G., and Harvey, A. (2010). Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 12, 459–472. doi: 10.1111/j.1399-5618.2010.00843.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Neumaier, J. F., Sexton, T. J., Yracheta, J., Diaz, A. M., and Brownfield, M. (2001). Localization of 5-HT(7) receptors in rat brain by immunocytochemistry, in situ hybridization and agonist stimulated cFos expression. J. Chem. Neuroanat. 21, 63–73. doi: 10.1016/s0891-0618(00)00092-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pail, G., Huf, W., Pjrek, E., Winkler, D., Willeit, M., Praschak-Rieder, N., et al. (2011). Bright-light therapy in the treatment of mood disorders. Neuropsychobiology 64, 152–162. doi: 10.1159/000328950
Panda, S., Sato, T. K., Castrucci, A. M., Rollag, M. D., Degrip, W. J., Hogenesch, J. B., et al. (2002). Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216. doi: 10.1126/science.1076848
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patton, D. F., and Mistlberger, R. E. (2013). Circadian adaptations to meal timing: neuroendocrine mechanisms. Front. Neurosci. 7:185. doi: 10.3389/fnins.2013.00185
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pendergast, J. S., and Yamazaki, S. (2014). Effects of light, food and methamphetamine on the circadian activity rhythm in mice. Physiol. Behav. 128, 92–98. doi: 10.1016/j.physbeh.2014.01.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pickard, G. E., and Rea, M. A. (1997). Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms. Biol. Cell 89, 513–523. doi: 10.1016/s0248-4900(98)80007-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poncet, L., Denoroy, L., and Jouvet, M. (1993). Daily variations in in vivo tryptophan hydroxylation and in the contents of serotonin and 5-hydroxyindoleacetic acid in discrete brain areas of the rat. J. Neural Transm. Gen. Sect. 92, 137–150. doi: 10.1007/bf01244873
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rea, M. A. (1998). Photic entrainment of circadian rhythms in rodents. Chronobiol. Int. 15, 395–423. doi: 10.3109/07420529808998699
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rea, M. A., Glass, J. D., and Colwell, C. S. (1994). Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J. Neurosci. 14, 3635–3642.
Ruby, N. F., Brennan, T. J., Xie, X., Cao, V., Franken, P., Heller, H. C., et al. (2002). Role of melanopsin in circadian responses to light. Science 298, 2211–2213. doi: 10.1126/science.1076701
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Salvadore, G., Quiroz, J. A., Machado-Vieira, R., Henter, I. D., Manji, H. K., and Zarate, C. A. Jr. (2010). The neurobiology of the switch process in bipolar disorder: a review. J. Clin. Psychiatry 71, 1488–1501. doi: 10.4088/JCP.09r05259gre
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sarkisyan, G., Roberts, A. J., and Hedlund, P. B. (2010). The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav. Brain Res. 209, 99–108. doi: 10.1016/j.bbr.2010.01.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, B. N., Sollars, P. J., Dudek, F. E., and Pickard, G. E. (2001). Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J. Biol. Rhythms 16, 25–38. doi: 10.1177/074873040101600104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sprouse, J. (2004). Pharmacological modulation of circadian rhythms: a new drug target in psychotherapeutics. Expert Opin. Ther. Targets 8, 25–38. doi: 10.1517/eott.8.1.25.26408
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sprouse, J., Reynolds, L., Fujiwara, R., and Siuciak, J. (2003). Circadian Rhythm Phenotype of 5-HT7 Receptor Knockout Mice: Greater Role for 5-HT1A in Mediating Drug-Induced Phase Shifts. Program No. 512.8. Washington, DC: Society for Neuroscience.
Sprouse, J., Reynolds, L., Li, X., Braselton, J., and Schmidt, A. (2004). 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 46, 52–62. doi: 10.1016/j.neuropharm.2003.08.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takahashi, J. S., DeCoursey, P. J., Bauman, L., and Menaker, M. (1984). Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308, 186–188. doi: 10.1038/308186a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weber, E. T., Gannon, R. L., and Rea, M. A. (1998). Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J. Biol. Rhythms 13, 209–218. doi: 10.1177/074873098129000057
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wesołowska, A., Nikiforuk, A., Stachowicz, K., and Tatarczyńska, E. (2006). Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 51, 578–586. doi: 10.1016/j.neuropharm.2006.04.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wesołowska, A., Tatarczyńska, E., Nikiforuk, A., and Chojnacka-Wójcik, E. (2007). Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur. J. Pharmacol. 555, 43–47. doi: 10.1016/j.ejphar.2006.10.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westrich, L., Sprouse, J., and Sánchez, C. (2013). The effects of combining serotonin reuptake inhibition and 5-HT7 receptor blockade on circadian rhythm regulation in rodents. Physiol. Behav. 110–111, 42–50. doi: 10.1016/j.physbeh.2012.12.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wirz-Justice, A., Benedetti, F., Berger, M., Lam, R. W., Martiny, K., Terman, M., et al. (2005). Chronotherapeutics (light and wake therapy) in affective disorders. Psychol. Med. 35, 939–944. doi: 10.1017/s003329170500437x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ying, S. W., and Rusak, B. (1997). 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 755, 246–254. doi: 10.1016/s0006-8993(97)00102-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: circadian rhythms, 5-HT7 receptor, phase shift, photic, non-photic, depression, mood, serotonin
Citation: Shelton J, Yun S, Losee Olson S, Turek F, Bonaventure P, Dvorak C, Lovenberg T and Dugovic C (2015) Selective pharmacological blockade of the 5-HT7 receptor attenuates light and 8-OH-DPAT induced phase shifts of mouse circadian wheel running activity. Front. Behav. Neurosci. 8:453. doi: 10.3389/fnbeh.2014.00453
Received: 22 September 2014; Accepted: 18 December 2014;
Published online: 15 January 2015.
Edited by:
Walter Adriani, Istituto Superiore di Sanita’, ItalyReviewed by:
J. M. Monti, Clinics Hospital, UruguayCopyright © 2015 Shelton, Yun, Losee Olson, Turek, Bonaventure, Dvorak, Lovenberg and Dugovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Shelton, Neuroscience, Janssen Research and Development, LLC, 3210 Merryfield Row, San Diego, CA 92121, USA e-mail:Jshelto5@its.jnj.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.