Adenosine A2A Receptor Blockade Prevents Rotenone-Induced Motor Impairment in a Rat Model of Parkinsonism
- 1Faculty of Medicine, Department of Pharmacology, Suez Canal University, Ismailia, Egypt
- 2Center for Aging and Associated Diseases, Zewail City of Science and Technology, Giza, Egypt
- 3Department of Veterans Affairs, New Jersey Health Care System, East Orange, NJ, USA
- 4School of Social Sciences and Psychology and Marcs Institute for Brain and Behaviour, Western Sydney University, Sydney, NSW, Australia
Pharmacological studies implicate the blockade of adenosine receptorsas an effective strategy for reducing Parkinson’s disease (PD) symptoms. The objective of this study is to elucidate the possible protective effects of ZM241385 and 8-cyclopentyl-1, 3-dipropylxanthine, two selective A2A and A1 receptor antagonists, on a rotenone rat model of PD. Rats were split into four groups: vehicle control (1 ml/kg/48 h), rotenone (1.5 mg/kg/48 h, s.c.), ZM241385 (3.3 mg/kg/day, i.p) and 8-cyclopentyl-1, 3-dipropylxanthine (5 mg/kg/day, i.p). After that, animals were subjected to behavioral (stride length and grid walking) and biochemical (measuring concentration of dopamine levels using high performance liquid chromatography, HPLC). In the rotenone group, rats displayed a reduced motor activity and disturbed movement coordination in the behavioral tests and a decreased dopamine concentration as foundby HPLC. The effect of rotenone was partially prevented in the ZM241385 group, but not with 8-cyclopentyl-1,3-dipropylxanthine administration. The administration of ZM241385 improved motor function and movement coordination (partial increase of stride length and partial decrease in the number of foot slips) and an increase in dopamine concentration in the rotenone-injected rats. However, the 8-cyclopentyl-1,3-dipropylxanthine and rotenone groups were not significantly different. These results indicate that selective A2A receptor blockade by ZM241385, but not A1 receptor blockadeby 8-cyclopentyl-1,3-dipropylxanthine, may treat PD motor symptoms. This reinforces the potential use of A2A receptor antagonists as a treatment strategy for PD patients.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by motor dysfunction (Kramberger et al., 2010). The loss of dopaminergic (DA) neurons is responsible for the development of PD motor symptoms (Liu, 2006). DA therapies, such as L-DOPA and dopamine agonists, either have a short half-life or may induce psychiatric side effects (Olanow et al., 2004; Moustafa et al., 2014). These issues raise the urgent need for an alternative form of therapeutic intervention.
Rotenone is a neurotoxin that replicates most of PD motor symptoms and leads toa loss of nigrostriatal DA neurons (Thiffault et al., 2000; von Wrangel et al., 2015). In this study, we used a rotenone model, as done in prior studies (Zaitone et al., 2012; Samim et al., 2014).
Adenosine is a neuromodulator in the striatum (Schiffmann et al., 2007), acting through four subtypes of G-protein coupled receptors, A1, A2A, A2B and A3 receptors (Fredholm, 2010). A2A receptors are co-localized with dopamine D2 receptors inthe indirect pathway of the basal ganglia (Morelli et al., 2007). The blocking of A2A receptors causes locomotor activation by lowering the inhibitory function of the indirect pathway of the basal ganglia, which is similar to the effects of blocking dopamine D2 receptors activation (Jenner, 2014; Pinna et al., 2014). Thus, adenosine A2A receptor antagonists are considered a promising strategy to treat PD (Schwarzschild et al., 2006; Pinna et al., 2014).
The adenosine A1 receptors are localized in the striatum presynaptically of dopamine axon terminals where they inhibit dopamine release (Borycz et al., 2007). ZM241385(4-(2-[7-amino-2-(2-furyl)[1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-yl amino]ethyl) phenol) is an antagonist with high affinity at the adenosine A2A receptor subtype in the brain (Cunha et al., 1997).
In this study, we test the protective effects of 8-cyclopentyl-1,3-dipropylxanthine as a selective A1 receptor antagonist and ZM241385 as a selective A2A receptor antagonist in a rat model of PD induced by rotenone.
Materials and Methods
Animals
Thirty two adult male albino rats weighing 200 ± 20 g were used for the current study. Animals were purchased from the National Research Center for Experimental Animals, Cairo, Egypt. Animals were housed under standardized conditions away from any stressful stimuli with normal day/night cycle, 25 ± 2°C temperature, in plastic polyethelyne cages with free access to food and water and were permitted for acclimatization for 1 week before starting the study. The behavioral tests were conducted after rotenone injections at 4 p.m. to minimize circadian influence on behavior. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Suez Canal University. All efforts were exerted to reduce animal suffering and to minimize the number of animals used.
Drugs
ZM241385 and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in saline solution (Chen et al., 2001). ZM241385 or DPCPX were administered intraperitoneally (IP) at a dose of 3.3 or 5 mg/kg/day, respectively, for 12 consecutive days in a volume of 1 ml/kg (Chen et al., 2001).
Rotenone was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 1:1 (v/v) dimethylsulfoxide (DMSO) and polyethyleneglycol (PEG-300; Thiffault et al., 2000). Rats received six subcutaneous injections of rotenone (1.5 mg/kg/48 h, s.c.) in a volume of 1 ml/kg. The rotenone-treated animals showed signs of akinesia and rigidity starting from the third injection (Thiffault et al., 2000).
ZM241385 and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in a saline solution (Chen et al., 2001). ZM241385 or DPCPX were administered IP at a dose of 3.3 or 5 mg/kg/day, respectively, for 12 consecutive days in a volume of 1 ml/kg (Chen et al., 2001).
Study Design
Rats were randomly divided into four groups, each has eight animals: (a) (vehicle-control group): rats received six intraperitoneal injections of the vehicle in a volume of 1 ml/kg; (b) (rotenone group): rats received subcutaneous rotenone (1.5 mg/kg/48 h) and received normal saline in a volume of 1 ml/kg daily for 12 days; (c) (ZM241385-treated group): 10 min before rotenone injection, rats received daily doses of intraperitoneal ZM241385 at a dose of 3.3 mg/kg daily for 12 days; and (d) (8-cyclopentyl-1,3-dipropylxanthine-treated group): 10 min before rotenone injection, rats received daily doses of intraperitoneal 8-cyclopentyl-1,3-dipropylxanthine at a dose of 5 mg/kg daily for 12 days.
Tasks and Functional Assessment
Rats were screened for motor impairment using the stride length and grid walking tests.
Stride Length Quantitative Gait Analysis Test (Fernagut et al., 2002)
Rats were habituated to the apparatus for 3 days before the beginning of the experiment. The apparatus was composed of an open field (60 × 60 × 40 cm) illuminated by a light, in which a runway (4.5 cm wide, 42 cm long, borders 12 cm height) was prepared to lead out into a dark wooden box (20 × 17 × 10 cm). Stride length was measured by wetting animal fore- and then hind-paws with black ink; animals were then allowed to trot on a paper strip (4.5 cm wide, 40 cm long) down the brightly lit runway towards the dark goal box. First, the length of the forelimb stride was measured in all animals, followed by the hind-limbs on a new strip of paper, directly after drying of the forelimb inked paws. The manual measurement of stride length was performed as the distance between two paw-prints. The mean of the longest three of the measured stride length (corresponding to maximal velocity) were measured in each run. We excluded paw-prints made at the beginning (7 cm) and the end (7 cm) of the run due to velocity changes. Any runs in which the rats stopped or made an obvious decelerations observed by the experimenter were excluded from analysis.
Grid Walking Test (Menet et al., 2003)
This test assesses the ability of accurate placing the forepaws during spontaneous exploration of an elevated grid by calculating the frequency of failure to accurately hold the rungs. Here, rats were placed on a wire grid (330 mm in diameter with 15 × 15 mm grid squares) and allowed to freely move for 3 min. The rats were videotaped and subsequently an experimenter blinded to the treatment group scored the number of foot slips in the first 50 steps, with the left and right fore- and hind-paws. A foot slip was recorded either when the paw completely fails to hold a rung, thus the limb dropped in between the rungs, or when the paw was accurately placed on the rung but fell during weight bearing. No pre-training of animals was required but they were put on the grid twice prior to injection for habituation and to obtain baseline scores.
Brain Tissue Preparation for Measuring Dopamine Levels in the Midbrain
At 4 p.m. of the following day (24 h after the last assessment of motor performance), rats were anesthetized by injection of thiopental sodium (30 mg/kg, intraperitoneal; Flecknell, 1993) and sacrificed by decapitation. Their brains were quickly dissected, the midbrain was surgically dissected and washed with ice-cold saline, and then weighed and rapidly frozen (−80°C of liquid nitrogen) until used for determination of dopamine by high performance liquid chromatography (HPLC) according to the method of Hussein et al. (2012). Frozen tissues were cut into small pieces and homogenized in phosphate buffer (pH 7.4), then centrifuged at 4000 rpm for 15 min at 4°C to spin down tissue fragments, nuclei and mitochondria. The supernatant was removed and filtered through a 0.2 micrometer teflon syringe filter for HPLC analysis. The measurement of dopamine levels was carried out by using a HPLC system, Agilent technologies 1100 series, equipped with an aquaternary pump (Quat pump, G131A model). The separation of dopamine was carried out by means of ODS-reverse-phase column (c18, 25 × 0.46 cm i.d. 5 μm). The mobile phase consists of 50 mM potassium phosphate buffer/methanol 97/3 (v/v), pH 3.5 and was delivered at flow rate of 1.5 ml/min. The substrates were detected by UV at 270 nm. The injection volume was 20 microliter. Serial dilutions of dopamine HPLC standard were injected, followed by a determination of their peak areas. A linear standard curve was drawn by plotting peak areas vs. the corresponding concentrations. The concentration of samples was obtained from Hussein et al. (2012).
Statistical Analysis
Data were expressed as mean ± SEM and analyzed using the statistical package of social sciences (SPSS program, version 17, SPSS Inc., Chicago, IL, USA). The assessment of difference of mean values among groups was conducted using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons test. p < 0.05 was considered significant.
Results
Behavioral Results
Stride Length Quantitative Gait Analysis Test
Systemic administration of rotenone (1.5 mg/kg, s.c. every other day, 6 doses) produced a significant difference between the stride length of forelimbs and hindlimbs starting from the third injection (Figure 1, p < 0.05). After the last injection, the mean of longest three of the measured stride was (6.47 ± 0.213 cm) in the in the rotenone group and (8.97 ± 0.60 cm) in the vehicle-control group. Compared to rotenone, ZM241385 significantly increased the stride length of forelimbs and hindlimbs of rats (8.6 ± 0.41 cm; Figure 1, p < 0.05), whereas 8-cyclopentyl-1,3-dipropylxanthine was devoid of effects (6.6 ± 0.17 cm; Figure 1, p > 0.05).
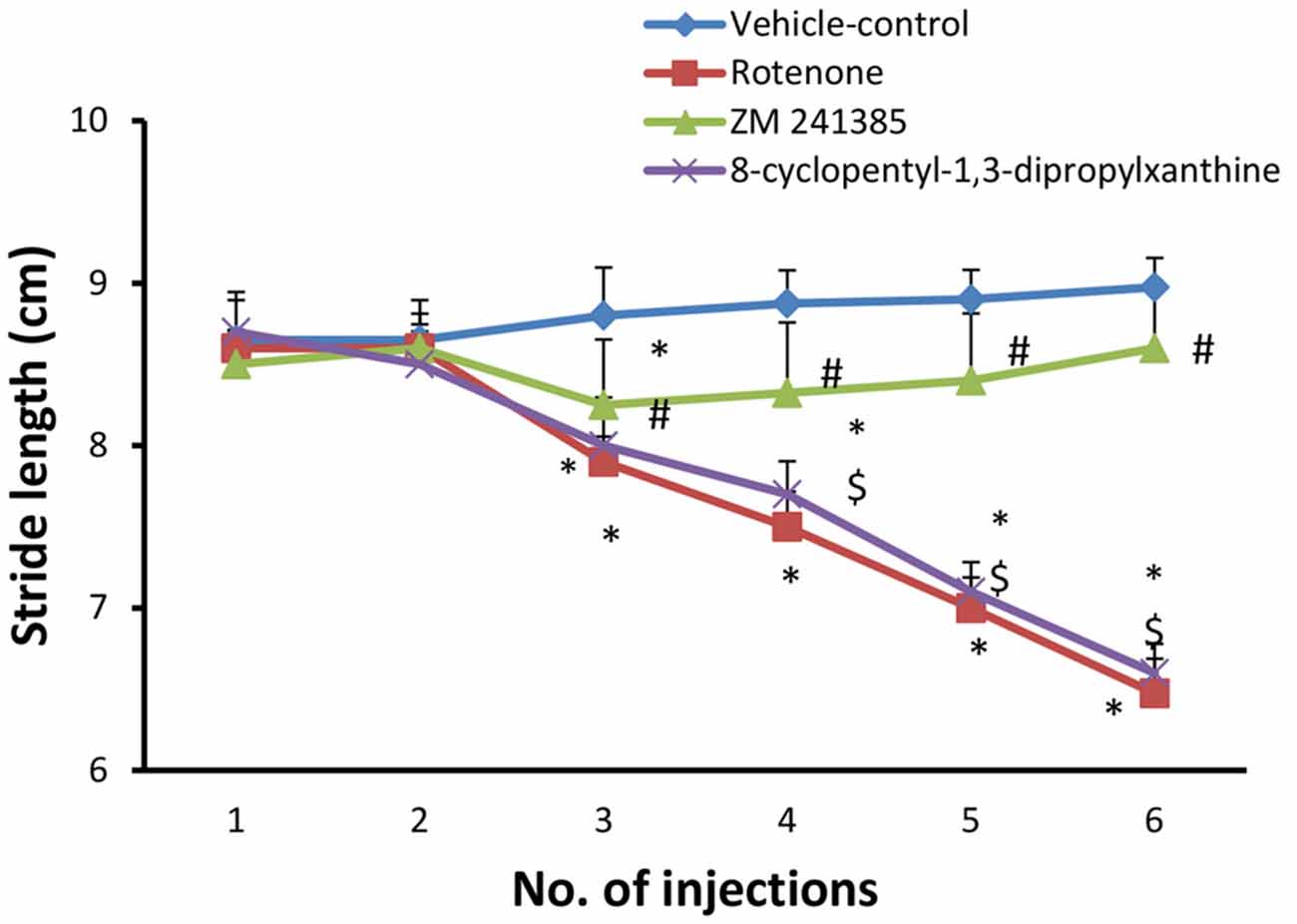
Figure 1. Stride length test in the experimental groups. The figure shows forelimbs and hindlimbs stride length (cm) in the experimental groups. Rotenone induced a significant difference in the mean stride length between the forelimbs and hindlimbs, while ZM241385 improved it significantly and 8-cyclopentyl-1,3-dipropylxanthine did not improve it compared to the rotenone group. Data were expressed as mean ± SE, analyzed using one way analysis of variance (ANOVA) followed by Bonferroni post hoc test, n = 8. *p < 0.05 compared to the vehicle-control group, #p < 0.05 compared to rotenone group, $p< 0.05 compared to the ZM241385-treated group.
Grid Walking Test
The number of foot slips in the first 50 steps was measured. In the present study, grid walking test was performed after each injection with either vehicle (group 1) or rotenone (groups 2, 3 and 4). when compared to the vehicle-control group (4 ± 0.001), rotenone significantly (p < 0.05) increased the number of foot slips beginning from the third injection (13.5 ± 0.95) throughout the study. Compared to the rotenone group, ZM241385 decreased in a sustained and significant manner the number of foot slips (4 ± 0.5 at third injection), whereas DPCPX was devoid of effects (13 ± 0.57 at third injection) compared to rotenone group (Figure 2, p > 0.05).
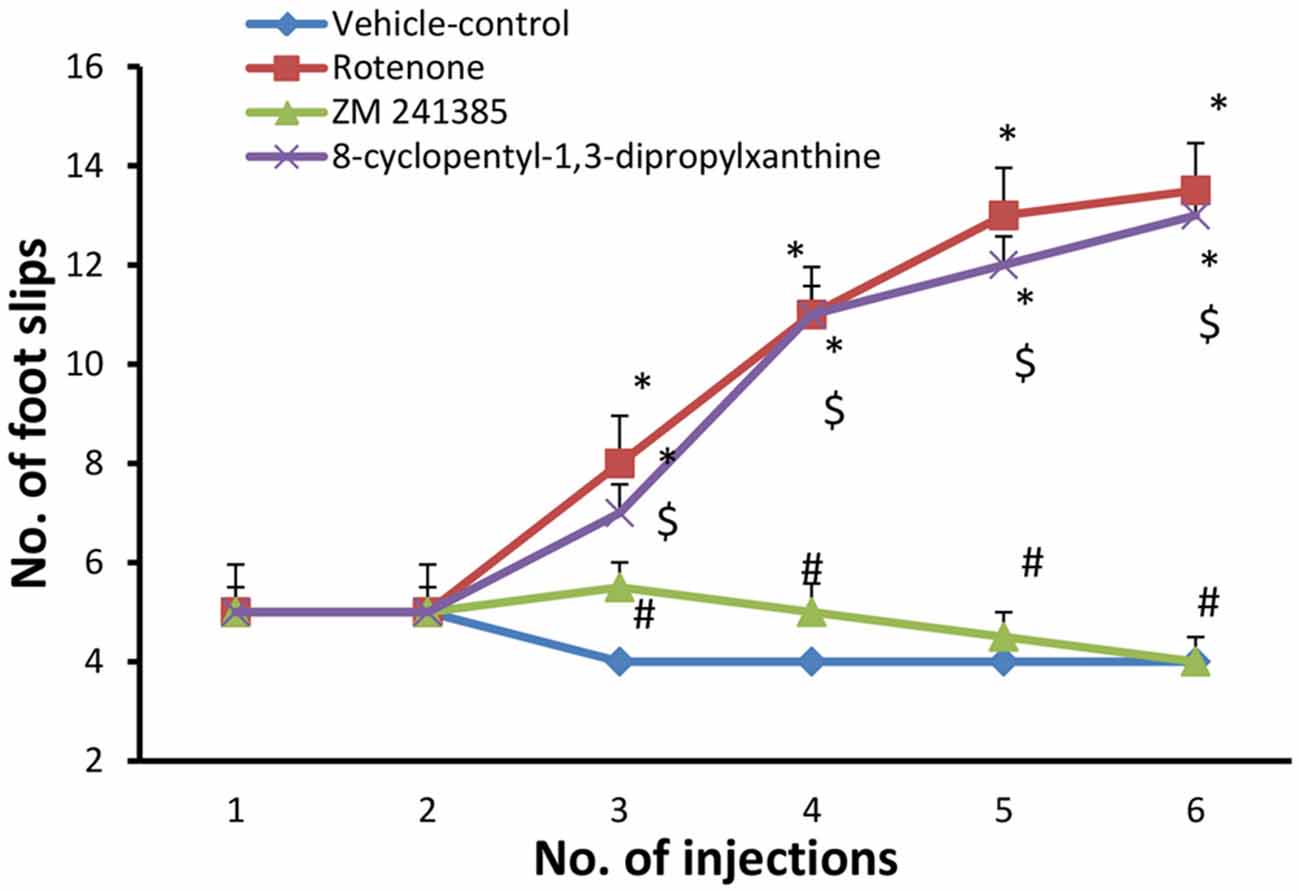
Figure 2. Grid walking test in the experimental groups. Here, we show the number of foot slips in the experimental groups in the grid walking test. Rotenone induced higher foot slip errors compared to vehicle-control group starting from the third injection. The treatment with ZM241385 decreased the number of foot slip errors compared to the rotenone group. Data were expressed as mean ± SE, analyzed using one way ANOVA followed by Bonferroni post hoc test. n = 8. *p < 0.05 compared to vehicle-control group, #p < 0.05 compared to rotenone group, $p < 0.05, compared to the ZM241385-treated group.
Dopamine Level Analysis Results
Dopamine levelsin the midbrain in the vehicle-control group were 3.15 ± 0.02 μg/g wet tissue and were significantly (p < 0.05) reduced to 2.16 ± 0.01 μg/g wet tissue by rotenone. ZM241385 significantly (p < 0.05) attenuated the impact of rotenone on dopamine levels in the midbrain (2.87 ± 0.02 μg/g wet tissue), whereas DPCPX was devoid of effects (2.2 ± 0.081 μg/g wet tissue; Figure 3, p > 0.05).
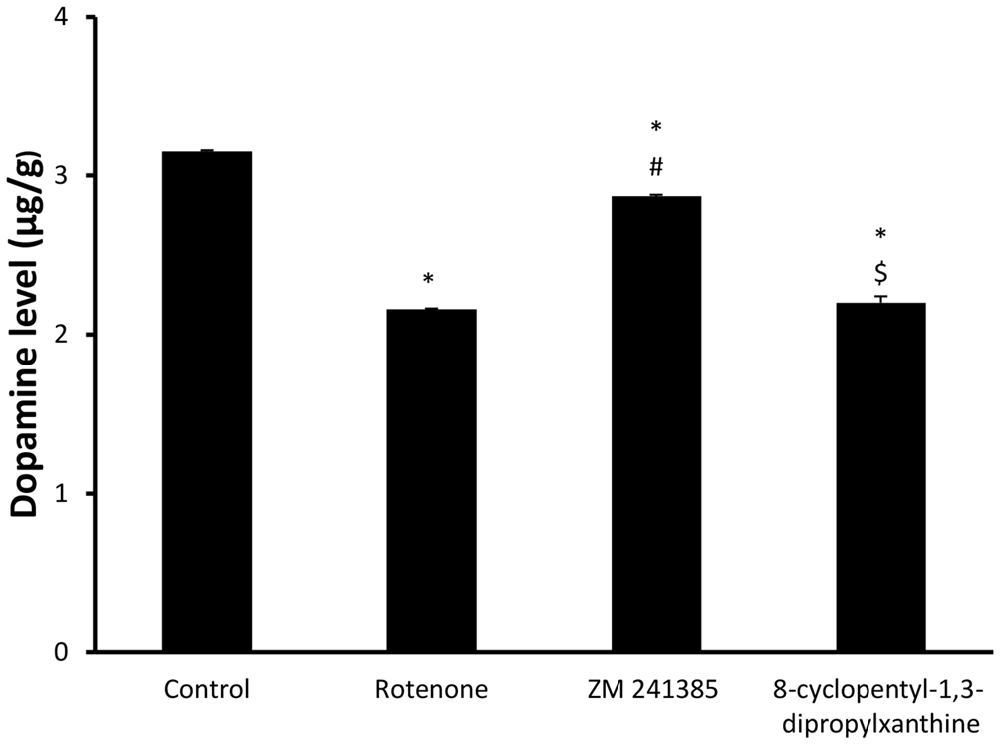
Figure 3. Dopamine levels in all four groups. Here, we show DA concentration in the different experimental groups. The administration of rotenone resulted in a significant decrease in DA levels, while ZM241385 increased DA levels, in comparison to the rotenone group. Data were expressed as mean ± SE, analyzed using one way ANOVA followed by Bonferroni post hoc test, n = 8. *p < 0.05 compared to the vehicle-treated group, #p < 0.05 compared to the rotenone group, $p < 0.05 compared to the ZM241385-treated group.
Discussion
Our results demonstrate that rotenone-treated rats exhibited motor deficits in the stride length and grid walking tests, as described by others (Hisahara and Shimohama, 2010; Li et al., 2012; von Wrangel et al., 2015), as well as lower dopamine levels in the midbrain (Höglinger et al., 2003; Sharma and Nehru, 2013), supporting its validity as a PD model. Notably, the A2AR antagonist ZM241385 attenuated all these alterations induced by rotenone, whereas the A1 receptor antagonist, DPCPX was devoid of effects. These findings, using a different animal model of PD and different behavioral tests of motor function, re-enforce the benefits afforded by A2A receptor blockade in different tests and animal models of PD (reviewed in Schwarzschild et al., 2006; Pinna et al., 2014), which are not mimicked by A1 receptor antagonists (Chen et al., 2001). This efficiency of A2A receptors to control motor dysfunction in PD, probably result from the ability of A2AR to control a series of concurrent processes, such as the release of glutamate from corticostriatal terminals that engage striatal circuits (Quiroz et al., 2009), the processing of information by medium spiny striatal neurons (Higley and Sabatini, 2010; Shen et al., 2013), the control microglia reactivity and neuroinflammation (Gyoneva et al., 2014) and the astrocytic support of neuronal function (Matos et al., 2015), the control the trophic support of DA terminal in the striatum (Gomes et al., 2009), the loss of nerve terminals and apoptosis of neurons (Silva et al., 2007), as well as the aggregation of α-synuclein (Ferreira et al., 2015).
Our study is not without limitations. First, future histopathological studies should investigate the effects of A2A receptors blockade on the levels of dopamine metabolites to confirm or disconfirm our findings. Second, additional experimental studies are needed to explore the possible preventive and curative molecular mechanisms of adenosine A2A receptors antagonists. Also, additional long-term studies with a large sample size should be carried out for further assessment of the effects of long-term duration of adenosine A2A receptor antagonists on different PD models. Finally, as this is a pharmacological study, it is assumed that our results are related to adenosine antagonism, based on prior findings (Cunha et al., 1997).
Author Contributions
Study was conducted by AMF and AMS. Writing was done by all co-authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Borycz, J., Pereira, M. F., Melani, A., Rodrigues, R. J., Köfalvi, A., Panlilio, L., et al. (2007). Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J. Neurochem. 101, 355–363. doi: 10.1111/j.1471-4159.2006.04386.x
Chen, J. F., Xu, K., Petzer, J. P., Staal, R., Xu, Y. H., Beilstein, M., et al. (2001). Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 21:RC143.
Cunha, R. A., Constantino, M. D., and Ribeiro, J. A. (1997). ZM241385 is an antagonist of the facilitatory responses produced by the A2A adenosine receptor agonists CGS21680 and HENECA in the rat hippocampus. Br. J. Pharmacol. 122, 1279–1284. doi: 10.1038/sj.bjp.0701507
Fernagut, O., Elsa, D., Bertrand, L., and Franc, T. (2002). A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J. Neurosci. Methods 113, 123–130. doi: 10.1016/s0165-0270(01)00485-x
Ferreira, D. G., Batalha, V. L., Vicente Miranda, H., Coelho, J. E., Gomes, R., Gonçalves, F. Q., et al. (2015). Adenosine A2A receptors modulate α-synuclein aggregation and toxicity. Cereb. Cortex bhv268. doi: 10.1093/cercor/bhv268 [Epub ahead of print].
Flecknell, P. A. (1993). Anesthesia of animals for biomedical research. Br. J. Anaesth. 71, 885–894. doi: 10.1093/bja/71.6.885
Fredholm, B. (2010). Adenosine receptors as drug targets. Exp. Cell Res. 316, 1284–1288. doi: 10.1016/j.yexcr.2010.02.004
Gomes, C. A., Simões, P. F., Canas, P. M., Quiroz, C., Sebastião, A. M., Ferré, S., et al. (2009). GDNF control of the glutamatergic cortico-striatal pathway requires tonic activation of adenosine A2A receptors. J. Neurochem. 108, 1208–1219. doi: 10.1111/j.1471-4159.2009.05876.x
Gyoneva, S., Shapiro, L., Lazo, C., Garnier-Amblard, E., Smith, Y., Miller, G. W., et al. (2014). Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson’s disease. Neurobiol. Dis. 67, 191–202. doi: 10.1016/j.nbd.2014.03.004
Higley, M. J., and Sabatini, B. L. (2010). Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci. 13, 958–966. doi: 10.1038/nn.2592
Hisahara, S., and Shimohama, S. (2010). Toxin-induced and genetic animal models of Parkinson’s disease. Parkinsons Dis. 2011:951709. doi: 10.4061/2011/951709
Höglinger, G. U., Féger, J., Prigent, A., Michel, P. P., Parain, K., Champy, P., et al. (2003). Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 84, 491–502. doi: 10.1046/j.1471-4159.2003.01533.x
Hussein, J., Abo El-Matty, D., El-Khayat, Z., and Abdel-Lativ, Y. (2012). Brain neurotransmitters in diabetic rats treated with coenzyme Q10. Int. J. Pharm. Pharm. Sci. 4, 554–556.
Jenner, P. (2014). An overview of adenosine A2A receptor antagonists in Parkinson’s disease. Int. Rev. Neurobiol. 119, 71–86. doi: 10.1016/b978-0-12-801022-8.00003-9
Kramberger, M., Stukovnik, V., Cus, A., Repovs, G., Tomse, P., Meglic, N., et al. (2010). Parkinson’s disease dementia: clinical correlates of brain spect perfusion and treatment. Psychiatr. Danub. 22, 446–449.
Li, C., Chen, X., Zhang, N., Song, Y., and Mu, Y. (2012). Gastrodin inhibits neuroinflammation in rotenone-induced Parkinson’s disease model rats. Neural Regen. Res. 7, 325–331. doi: 10.3969/j.issn.1673-5374.2012.05.001
Liu, B. (2006). Modulation of microglia pro-inflammatory and neurotoxic activity for the treatment of Parkinson’s disease. AAPS J. 8, E606–E621. doi: 10.1208/aapsj080369
Matos, M., Shen, H. Y., Augusto, E., Wang, Y., Wei, C. J., Wang, Y. T., et al. (2015). Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol. Psychiatry 78, 763–774. doi: 10.1016/j.biopsych.2015.02.026
Menet, V., Prieto, M., Privat, A., and Giménez y Ribotta, M. (2003). Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl. Acad. Sci. U S A 100, 8999–9004. doi: 10.1073/pnas.1533187100
Morelli, M., Di Paolo, T., Wardas, J., Calon, F., Xiao, D., and Schwarzschild, M. A. (2007). Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog. Neurobiol. 83, 293–309. doi: 10.1016/j.pneurobio.2007.07.001
Moustafa, A. A., Krishna, R., Frank, M. J., Eissa, A. M., and Hewedi, D. H. (2014). Cognitive correlates of psychosis in patients with Parkinson’s disease. Cogn. Neuropsychiatry 19, 381–398. doi: 10.1080/13546805.2013.877385
Olanow, C. W., Agid, Y., Mizuno, Y., Albanese, A., Bonuccelli, U., and Damier, P. (2004). Levodopa in the treatment of Parkinson’s disease: current controversies. Mov. Disord. 19, 997–1005. doi: 10.1002/mds.20243
Pinna, A., Bonaventura, J., Farré, D., Sánchez, M., Simola, N., Mallol, J., et al. (2014). L-DOPA disrupts adenosine A2A-cannabinoid CB1 dopamine D2 receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: biochemical and behavioral studies. Exp. Neurol. 253, 180–191. doi: 10.1016/j.expneurol.2013.12.021
Quiroz, C., Luján, R., Uchigashima, M., Simoes, A. P., Lerner, T. N., Borycz, J., et al. (2009). Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal 9, 1321–1344. doi: 10.1100/tsw.2009.143
Samim, M., Yajamanam, S. H., Bano, N., Veeresh, B., and Reddy, M. B. (2014). Neuroprotective effect of ocimum sanctum linn on rotenone induced Parkinsonism in rats. IJPRS 3, 772–784.
Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A., and Ferré, S. (2007). Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 83, 277–292. doi: 10.1016/j.pneurobio.2007.05.001
Schwarzschild, M. A., Agnati, L., Fuxe, K., Chen, J. F., and Morelli, M. (2006). Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 29, 647–654. doi: 10.1016/j.tins.2006.09.004
Sharma, N., and Nehru, B. (2013). Beneficial effect of vitamin E in rotenone induced model of PD: behavioral, neurochemical and biochemical study. Exp. Neurobiol. 22, 214–223. doi: 10.5607/en.2013.22.3.214
Shen, H. Y., Canas, P. M., Garcia-Sanz, P., Lan, J. Q., Boison, D., Moratalla, R., et al. (2013). Adenosine A2A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One 8:e80902. doi: 10.1371/journal.pone.0080902
Silva, C. G., Porciúncula, L. O., Canas, P. M., Oliveira, C. R., and Cunha, R. A. (2007). Blockade of adenosine A2A receptors prevents staurosporine-induced apoptosis of rat hippocampal neurons. Neurobiol. Dis. 27, 182–189. doi: 10.1016/j.nbd.2007.04.018
Thiffault, C., Langston, J., and Di Monte, D. (2000). Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res. 885, 283–288. doi: 10.1016/s0006-8993(00)02960-7
von Wrangel, C., Schwabe, K., John, N., Krauss, J. K., and Alam, M. (2015). The rotenone-induced rat model of Parkinson’s disease: behavioral and electrophysiological findings. Behav. Brain Res. 279, 52–61. doi: 10.1016/j.bbr.2014.11.002
Keywords: Parkinson’s disease, dopamine, grid walk, stride length, rotenone, adenosine receptors
Citation: Fathalla AM, Soliman AM, Ali MH and Moustafa AA (2016) Adenosine A2A Receptor Blockade Prevents Rotenone-Induced Motor Impairment in a Rat Model of Parkinsonism. Front. Behav. Neurosci. 10:35. doi: 10.3389/fnbeh.2016.00035
Received: 05 November 2015; Accepted: 15 February 2016;
Published: 29 February 2016.
Edited by:
Nuno Sousa, University of Minho, PortugalReviewed by:
Gregg Stanwood, Florida State University, USARodrigo A. Cunha, University of Coimbra, Portugal
Copyright © 2016 Fathalla, Soliman, Ali and Moustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. Moustafa, a.moustafa@westernsydney.edu.au
 Ahmed M. Fathalla1
Ahmed M. Fathalla1  Mohamed H. Ali
Mohamed H. Ali Ahmed A. Moustafa
Ahmed A. Moustafa