New tools for investigating astrocyte-to-neuron communication
- 1Biophysics of Gliotransmitter Release Team, Laboratory of Neurophysiology and New Microscopies, INSERM U603, CNRS UMR 8154, University Paris Descartes, Paris, France
- 2Glia-Glia and Glia-Neuron Interactions in Neurophysiopathology Team, Laboratory of Neurophysiology and New Microscopies, INSERM U603, CNRS UMR 8154, University Paris Descartes, Paris, France
Gray matter protoplasmic astrocytes extend very thin processes and establish close contacts with synapses. It has been suggested that the release of neuroactive gliotransmitters at the tripartite synapse contributes to information processing. However, the concept of calcium (Ca2+)-dependent gliotransmitter release from astrocytes, and the release mechanisms are being debated. Studying astrocytes in their natural environment is challenging because: (i) astrocytes are electrically silent; (ii) astrocytes and neurons express an overlapping repertoire of transmembrane receptors; (iii) the size of astrocyte processes in contact with synapses are below the resolution of confocal and two-photon microscopes (iv) bulk-loading techniques using fluorescent Ca2+ indicators lack cellular specificity. In this review, we will discuss some limitations of conventional methodologies and highlight the interest of novel tools and approaches for studying gliotransmission. Genetically encoded Ca2+ indicators (GECIs), light-gated channels, and exogenous receptors are being developed to selectively read out and stimulate astrocyte activity. Our review discusses emerging perspectives on: (i) the complexity of astrocyte Ca2+ signaling revealed by GECIs; (ii) new pharmacogenetic and optogenetic approaches to activate specific Ca2+ signaling pathways in astrocytes; (iii) classical and new techniques to monitor vesicle fusion in cultured astrocytes; (iv) possible strategies to express specifically reporter genes in astrocytes.
Introduction
The concept of gliotransmission at the tripartite synapse developed more than 10 years ago (Araque et al., 1999; Perea and Araque, 2010) is very attractive: it suggests that cerebral gray matter protoplasmic astrocytes are not only supportive cells with homeostatic functions, but that they also play a role in information processing by responding to neuronal synaptic activity with Ca2+ elevations that induce the subsequent release of gliotransmitters and modulate neuronal excitability and synaptic plasticity [reviewed in (Angulo et al., 2008; Bergersen and Gundersen, 2009; Cali et al., 2009; Perea et al., 2009; Santello and Volterra, 2009; Halassa and Haydon, 2010; Perea and Araque, 2010; Parpura et al., 2011; Gucek et al., 2012; Zorec et al., 2012)]. However, the concept of gliotransmission is debated. First, the ability of astrocytes to release neuroactive compounds in a Ca2+-dependent manner has been questioned (Agulhon et al., 2008; Fiacco et al., 2009). Second, there is no consensus concerning the mechanisms of gliotransmitter release. In fact, studying astrocytes in situ is very challenging and an agreement is emerging that new methods are needed to selectively activate (Fiacco et al., 2009; Hamilton and Attwell, 2010) and read out Ca2+ signals in astrocytes in situ (Shigetomi et al., 2013b).
By gliotransmission, we mean Ca2+-dependant release of fast-acting neuroactive compounds, the gliotransmitters. Astrocytes can also release other molecules acting not only on neighboring neurons but also on nearby glial cells such as microglia, NG2 cells, and on cellular constituents of the blood brain barrier. Our review will focus on the fast acting gliotransmitter candidates, glutamate mostly, as well as D-serine, ATP, and GABA. Several pathways have been suggested for glutamate release: exocytosis, hemichannels, sodium-dependent transporters, volume-regulated anion channels, purine P2X7 receptor channel [reviewed in (Hamilton and Attwell, 2010)], and more recently the bestrophin 1 (Best1) chloride channel and the two-pore domain potassium TREK1 channel (Woo et al., 2012). The release of glutamate by Ca2+-regulated vesicular fusion is considered as an important pathway for gliotransmitter release because, by analogy with neuronal exocytosis, it appears to be the most suitable pathway for rapid information processing by astrocytes. Experimental evidence in favor of glutamate exocytosis has been provided using (i) dihydroxyphenylglycine (DHPG), mechanical stimulation, inositol triphosphate (IP3) and Ca2+ uncaging to activate astrocytes; (ii) Ca2+ buffering with BAPTA, VAMP2/3 cleaving with tetanus toxin (TeNT) or botulinum toxin (BoNT), and generating a dominant negative SNARE (dnSNARE)-expressing mouse line to inactivate vesicular release in astrocytes; (iii) fluoroacetate to inactivate astrocyte metabolism [reviewed in Bergersen and Gundersen, 2009; Cali et al., 2009; Gucek et al., 2012; Zorec et al., 2012], but contrasts with the relative absence on electron micrographs of small vesicles in astrocyte processes, when compared to the neuronal presynaptic terminal.
In this review, we discuss several new genetically encoded tools to read out astrocytic Ca2+ activity, to activate Ca2+ signals in astrocytes, and to monitor gliotransmitter release from astrocytes. Optical methods for astrocyte photoactivation and imaging, and strategies to selectively target the genes in astrocytes in their native environment are also reviewed.
Imaging Astrocyte Activity
Organic vs. Genetically Encoded Ca2+ Indicators
Protoplasmic astrocytes are electrically silent. However, they may be considered as excitable cells in the sense that they show Ca2+ signals, both spontaneously and in response to neuronal activity. In spite of the evidence suggesting that Ca2+ signals are necessary and sufficient to induce gliotransmitter release, many questions remain, concerning both the role and sources of Ca2+ signals in astrocytes (Agulhon et al., 2008; Fiacco et al., 2009; Parpura et al., 2011). One limiting factor to study Ca2+ signaling has been methodological. So far, most studies in acute brain slices and in vivo have been based on bulk-loaded membrane-permeable chemical Ca2+ indicators. There exist many organic Ca2+ indicators with different spectral properties and affinity for Ca2+ which can monitor either Ca2+-related fluorescence changes or, for the ratiometric probes, can be calibrated to provide absolute Ca2+ concentration [reviewed in (Paredes et al., 2008)]. The membrane-permeable acetoxymethyl (AM) esters, Fluo4-AM and Oregon Green BAPTA1-AM (OGB1-AM), the most popular dyes used to image Ca2+ activity in populations of astrocytes, allow imaging the somatic region and the larger proximal processes but leave the very thin distant processes that participate to the tripartite synapse unsampled (Reeves et al., 2011). At the laser powers, dye concentrations and integration times typically used and with the spatial resolution available, fluorescence changes in fine processes are generally not resolved (see below for a detailed discussion).
Another limiting factor has been the lack of specificity of these membrane-permeable Ca2+ indicators, which label both neurons and astrocytes (Garaschuk et al., 2006) with cell-type preferences, depending on the indicator, the protocol of application, and the age of the animal. Therefore, sulforhodamine 101 (SR101) that is specifically taken up by astrocytes has been generally used as a secondary fluorescent marker for astrocyte identification. The deep-red emission of SR101 can be detected with negligible spectral overlap with GFP or green fluorescein-based membrane-permeable Ca2+ indicators (Nimmerjahn et al., 2004). However, SR101 uptake is age-dependent (Kafitz et al., 2008) and it does not work in all brain regions (Schnell et al., 2012). Also, at concentrations needed for astrocyte labeling, SR101 leads to increases of neuronal excitability and long-term potentiation (Kang et al., 2010; Garaschuk, 2013), and thus might affect functional studies. As an alternative, transgenic (Tg) mouse lines (Nolte et al., 2001; Vives et al., 2003; Heintz, 2004; Zuo et al., 2004; Regan et al., 2007) expressing a green/yellow fluorescent protein (GFP/YFP) or Discosoma red protein (DsRed) under astrocyte-specific promoters (GFAP, S100β, GLT-1, ALDH1L1) can be used to identify astrocytes.
In this context, the recent genetically encoded Ca2+ indicators (GECI) provide a new alternative for non-invasive imaging of Ca2+ activity in vivo and in brain slices [reviewed in (Knopfel, 2012; Looger and Griesbeck, 2012)]. Their level of expression can be stable for months in the absence of apparent adverse effect (Zariwala et al., 2012). GECIs can be targeted to the plasma membrane of astrocytes (Shigetomi et al., 2010) and their specific expression by astrocytes in vivo is being developed using viral constructs and Tg mouse lines (see below). Finally new GECI variants are being generated having greater signal to noise ratio, different Ca2+-binding affinities, and different spectral properties (Horikawa et al., 2010; Zhao et al., 2011; Ohkura et al., 2012; Akerboom et al., 2013; Chen et al., 2013b) which further enlarge their utility for studying the role of astrocytes on synaptic transmission (Tong et al., 2013).
Among the most recent GECIs, several variants of the original GFP-based Ca2+ sensor GCaMP1 (Nakai et al., 2001) have been tested in astrocytes: GCaMP2 (Hoogland et al., 2009), GCaMP3 (Shigetomi et al., 2010, 2013b; Tong et al., 2013), GCaMP5 (Akerboom et al., 2012), and red GECIs (Akerboom et al., 2013), as well as Case12 (Souslova et al., 2007; Gourine et al., 2010), and yellow Cameleon YC3.60 (Atkin et al., 2009). In neurons GCaMP5G and GCaMP6 variants have been shown to produce a higher signal-to-noise ratio than GCaMP3 and can detect Ca2+ changes evoked by single action potential (Akerboom et al., 2012). GCaMP3, GCaMP5G, and GCaMP6 are all compatible with two-photon excitation at 910-930 nm (Akerboom et al., 2012; Mutze et al., 2012). Recently, Khakh's group (Tian et al., 2009; Shigetomi et al., 2010, 2013b) compared Ca2+ changes in astrocytes using a membrane-permeable Ca2+ indicator (Fluo4-AM) with those detected with two GECIs, the cytosolic GCaMP3 and a membrane-targeted Lck-GCaMP3 (Figure 1). Ca2+ signals were recorded with a confocal microscope at the surface of acute hippocampal slices from adult mice. Specific astrocytic targeting of GCaMP3 and Lck-GCaMP3 was obtained using a short version (gfaABC1D) of the human glial fibrillary acidic (hGFAP) promoter. Unlike Fluo4-AM which diffuses poorly to the thin astrocyte processes (Reeves et al., 2011), both GCaMP3 and Lck-GCaMP3 reported a wealth of Ca2+ signals in distant thin astrocytic processes with relatively less activity in the soma and proximal processes. Interestingly, (i) spontaneous Ca2+ rises recorded with the GCaMP3 are highly localized and desynchronized, as suggested previously from whole-cell dye loading single astrocytes with higher dye concentrations through the patch pipette (Nett et al., 2002; Di Castro et al., 2011; Panatier et al., 2011); (ii) spontaneous somatic activity does not appear to integrate the signals generated locally in the thin processes; (iii) using Lck-GCaMP3, a new Ca2+ signaling pathway has been suggested in astrocytes involving the A1 transient receptor potential (TRPA1) channel (Shigetomi et al., 2011) that has been proposed to contribute to D-serine release (Shigetomi et al., 2013a). In summary, earlier studies using membrane-permeable Ca2+ indicators may have underestimated the variety of Ca2+ signaling mechanisms, and missed local interactions between astrocytes and neurones.
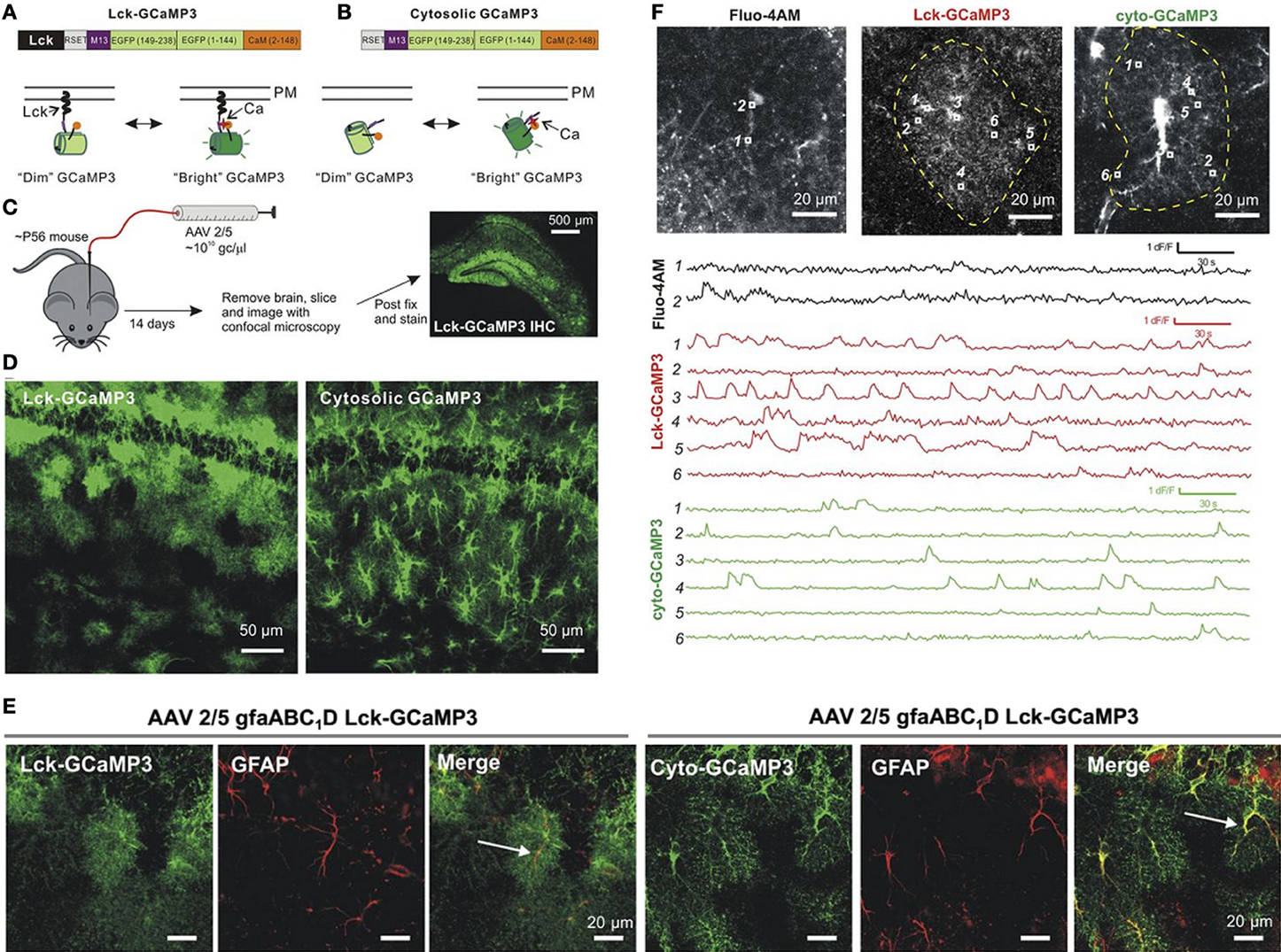
Figure 1. Expression of GCAMP3 in astrocytes and comparison of Ca2+ signals detected with Fluo-4, cyto-GCAMP3, and Lck-GCAMP3. (A,B) Illustration of the membrane-targeted Lck-GCAMP3 and cytosolic non-targeted Cyto-GCAMP3. (C) Protocol of AAV2/5 injections into a mouse hippocampus. (D) Confocal images in CA1 stratum radiatum for Lck-GCaMP3 and cyto-GCaMP3. (E) Colocalization between Lck-GCaMP3 and cyto-GCaMP3 with the astrocytic marker GFAP. (F) Ca2+ signals imaged with Fluo-4 (black traces), cyto-GCaMP3 (green traces), and Lck-GCaMP3 (red traces). Top, representative images of astrocytes loaded with Fluo-4AM, Lck-GCaMP3 or cyto-GCaMP3. ROIs are shown in each image, and their time-lapse intensities are shown below. Adapted from (Shigetomi et al., 2013b).
GECIs have been introduced only recently compared to chemical Ca2+ indicators that have been used since the 80s. Therefore, their photophysical properties and impact on intracellular Ca2+ homeostasis have not yet been characterized to the same extent as their chemical counterparts (Perez Koldenkova and Nagai, 2013). For example, the Ca2+affinity of many GECIs has not been yet determined in the complex intracellular milieu; their binding kinetics (on- and off-rates for Ca2+ binding), as well as their aggregation and bleaching rates are not well established. Open questions concern their precise mobility, local concentration, Ca2+ buffer capacity and subcellular localization, which can be engineered by adding genetically encoded targeting sequences, as done with Lck-GCaMP3 (Shigetomi et al., 2010). However, the capacity of GECIs to specifically detect local Ca2+ signals in a population of astrocytes together with their low photobleaching and high signal-to-noise ratio seem to outweigh these limitations. Indeed experiments become feasible that were simply not possible with earlier small-molecule chemical indicators. The new Ca2+ data with the GECIs provide intriguing clues to explore astrocyte functions but also present new challenges. New tools will be needed to reliably detect and quantify the wealth of rapid asynchronous and local fluorescence changes. The mechanisms and functional significance of these Ca2+ signals are far from being understood. Given the striking difference between fluorimetric Ca2+ signals detected with chemical Ca2+ indicators and GECIs, the relation between neuronal activity and astrocytic Ca2+ signals needs to be re-investigated under physiological and pathological conditions using acute brain slices, and anesthetized or non-anesthetized mouse preparations.
Optical Methods for Imaging Astrocyte Activity
Imaging GECIs with two-photon microscopy holds important potential for monitoring astrocytic Ca2+ signals in situ. However, major challenges remain when imaging astrocyte signals in the neuropil. The limited spatial resolution is an issue when it comes to tell apart morphological changes and Ca2+ signals. An astrocyte process occupies only a small fraction of the two-photon excitation volume and, this fraction will become smaller with increasing imaging depth, for which two-photon resolution degrades due to scattering and wave front aberrations (Chaigneau et al., 2011). Thus, subtle morphological changes expected to modulate synapse coverage and modulate astrocyte-neuron interactions are unlikely to be resolved in two-photon microscopy and will be confounded with Ca2+ changes.
Two-photon microscopy combined with two-photon stimulated emission depletion (STED) (Ding et al., 2009; Li et al., 2009b; Moneron and Hell, 2009; Nägerl and Bonhoeffer, 2010) increases lateral resolution and super-resolution imaging of dendritic spines in live mouse brain in vivo has been recently reported (Berning et al., 2012). Two-photon-STED is attractive because it brings two-photon resolution closer to typical dimensions of astrocyte processes but it aggravates the temporal resolution problem inherent to scanning microscopies where temporal resolution depends on the number of pixels (i.e., the sampling rate imposed by the spatial resolution) and the dwell time per pixel (i.e., the number of photons available). As spatial resolution is privileged in 2PE-STED, the number of image pixels increases as the resolution gain squared, and the temporal resolution drops accordingly. Simultaneous multi-spot detection (Cheng et al., 2011; Grosberg et al., 2012; Ducros et al., 2013) speeds up image acquisition by an order of magnitude compared to conventional raster scanning, but does not fundamentally address the temporal undersampling problem. Finally, STED resolution scales with the square-root of power of the depletion beam so that STED increases the already high light burden of two-photon imaging (Koester et al., 1999). Faster imaging, i.e., shorter pixel dwell times will thus require better fluorophores with higher fluorescence quantum yield, greater depletion efficiency, and higher photostability, all at the same time. Also, while these arguments already hold for imaging a single focal plane, their weight increases when it comes to imaging Ca2+ changes in three dimensions along a branch or an entire astrocyte. In summary, major technological advances are still needed to image small signals in fine astrocyte processes and track their subtle morphological changes.
Activation of Astrocytes
Following neuronal activity, the activation of astrocytes is mediated by neurotransmitter released from synaptic terminals (Porter and McCarthy, 1996; Wang et al., 2006). The subsequent release of gliotransmitters from mature protoplasmic astrocytes has been reported to depend upon Gq GPCR activation leading to astrocytic type-2 IP3 receptor (IP3R2) activation and Ca2+ release from the endoplasmic reticulum [reviewed in (Halassa et al., 2007)]. While this pathway has been implicated in gliotransmitter release, the mechanisms and the concept of gliotransmission remains debated (Agulhon et al., 2008; Fiacco et al., 2009; Hamilton and Attwell, 2010) in part because of our inability to selectively activate Ca2+ signals in astrocytes. The exogenous generation of Ca2+ signals that mimic those evoked by neuronal stimuli should clarify the interactions between neurons and astrocytes.
Pharmacogenetics
Since most cell types in the brain express an overlapping array of GPCRs, conventional pharmacological approaches consisting of bath application or local pipette perfusion of Gq GPCR agonists to evoke Ca2+ elevations in astrocytes in situ lack cellular selectivity, For instance, one of the agonists most frequently used to stimulate astrocytes, dihydroxyphenylglycine (DHPG), a group I metabotropic receptor (mGluR) agonist, has direct effects on neurons, and elicits neuronal Ca2+ elevations, long-term depolarization (Mannaioni et al., 2001; Rae and Irving, 2004), and potentiation of N-methyl-D-aspartate (NMDA) receptor-mediated currents (Benquet et al., 2002). Therefore, the use of Gq GPCR agonists, not only DHPG but also many other agonists, will lead to direct activation of neuronal receptors.
To overcome these limitations, a novel Tg mouse model was created (Fiacco et al., 2007) using the Gq GPCR MrgA1 receptor normally expressed by nociceptive sensory neurons (Dong et al., 2001). In the MrgA1 mouse model: (i) the GFP-tagged MrgA1 receptor is expressed selectively by astrocytes using an inducible tet-off system transcribed from a tet (tetO) minimal promoter; (ii) it is not activated by endogenous ligands found in brain; (iii) its ligand, the FMRF peptide, does not activate any endogenous brain Gq GPCRs. These mice were crossed with mice in which the tetracycline transactivator (tTA) is targeted to astrocytes using the hGFAP promoter. In the absence of doxycycline, tTA binds to tetO and drives expression of the MrgA1-GFP construct selectively in astrocytes and the MrgA1 receptor is functional in most astrocytes (Fiacco et al., 2007).
MrgA1-receptor-mediated Ca2+ release from astrocytic internal Ca2+ stores did not affect synaptic transmission and plasticity (Fiacco et al., 2007; Agulhon et al., 2010), raising questions about the ability of astrocytes to undergo Ca2+-dependent gliotransmitter release. Using the same MrgA1 mouse model, it was later shown that instead, astrocytic MrgA1R-mediated Ca2+ elevations potentiate glutamate and K+ uptake (Wang et al., 2012; Devaraju et al., 2013). The MrgA1 Tg mouse model demonstrates the interest of a pharmacogenetic approach to investigate the role of astrocytic Ca2+ in acute slices and in vivo. However, astrocytes are heterogeneous (Zhang and Barres, 2010) and they are likely to exhibit different functions depending on the brain area. Consequently, continued improvements are needed to activate discrete populations of astrocytes in specific brain areas. The combination of pharmacogenetic with the use of adeno-associated viral technology to deliver the expression of genetically engineered new Gq GPCRs (Wess et al., 2013) is a promising approach (see below). Additionally, two-photon uncaging of caged FMRF at the vicinity of thin astrocyte processes should better mimic synaptically-induced Gq GPCR activation and therefore help addressing further the role of this signaling pathway in astrocyte physiology.
Optogenetics
Over the last decade, the development of new photoswitchable genetically encoded channels and receptors to activate and inactivate specific neuronal subtypes had a significant impact on Neuroscience. The simultaneous methodological advances in several fields: (i) optics for photoactivation and imaging in situ, (ii) molecular engineering for developing new photoswitchable proteins, (iii) molecular biology for specific targeting of the light sensitive proteins, have been instrumental for the success of optogenetics in elucidating the function of neuronal circuits (Szobota and Isacoff, 2010; Fenno et al., 2011; Miesenbock, 2011). The most popular photoswitchable channel to activate neurons is the H314R channelrhodopsin 2 [ChR2(H314R)], a variant of the wild type ChR2 with reduced desensitization (Nagel et al., 2005). ChR2 is a cationic channel highly permeable to proton (P+H/P+Na ~ 106) but weakly permeable to Ca2+ (P2+Ca/P+Na ~ 0.117) (Nagel et al., 2003; Lin et al., 2009). In neurons, its photoactivation triggers Ca2+ elevations which depend mainly on the secondary activation of voltage-gated Ca2+ channels (VGCC) (Nagel et al., 2003; Zhang and Oertner, 2007; Li et al., 2012).
Attempts have been made to photoactivate protoplasmic astrocytes. In situ experiments suggest that the photoactivation of ChR2-expressing astrocytes can trigger gliotransmitter release (Gradinaru et al., 2009; Gourine et al., 2010; Sasaki et al., 2012; Chen et al., 2013a). In the rat brain stem retrotapezoid nucleus, ChR2-expressing astrocytes responded to long lasting (20–60 s) illumination by slow Ca2+ rises that lasted for minutes (Gourine et al., 2010). In the hippocampal CA1 region, blue light pulses induce rapid time-locked Ca2+ signals in astrocytes (Chen et al., 2013a). However, our own experiments using mouse cortical astrocytes in culture, show that ChR2 activation induces variable and weak Ca2+ elevations (Li et al., 2012). Instead we found that the activation of the Ca2+-permeable light-gated glutamate receptor (LiGluR) [reviewed in (Szobota and Isacoff, 2010)], and the Ca2+-translocating ChR2 (CatCh) (Kleinlogel et al., 2011) evokes reliable and robust Ca2+ signals in astrocytes (Figure 2). We attributed the low efficacy of ChR2 in astrocytes to its relatively weak Ca2+ permeability (Nagel et al., 2003; Lin et al., 2009), and to the absence of VGCC in protoplasmic astrocytes (Carmignoto et al., 1998; Parpura and Verkhratsky, 2012). Interestingly, LiGluR can be rapidly switched ON and OFF to mimic endogenous Ca2+ signals recorded with the GCaMP3 (Shigetomi et al., 2013b). Finally, LiGluR activation induces a large Ca2+ influx that is further shaped by internal stores, while CatCh activation generates a Ca2+ influx insensitive to internal Ca2+ store depletion, indicating that LiGluR and CatCh are interesting tools to activate differentially selective Ca2+ signaling pathways and to study their downstream effects.
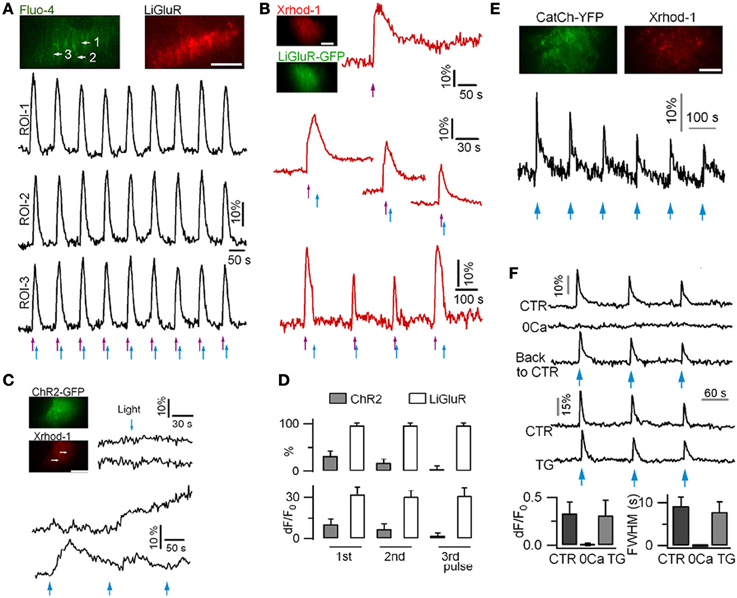
Figure 2. Ca2+ signals recorded in mouse cortical astrocytes in culture using LiGluR, ChR2(H134R) and CatCh photoactivation. (A) Light-gated Ca2+ rises in an astrocyte expressing LiGluR-mRFP. Ca2+ rises were imaged with dual-color TIRFM and repetitively switched on and off by 385-nm (violet arrows, 0.3 mW/mm2, 50 ms) and 488-nm (blue arrows, 39.1 mW/mm2, 200 ms) light pulses, respectively. (B) LiGluR(GFP)-gated astrocytic Ca2+ elevations monitored with the red-fluorescent Ca2+ dye Xrhod-1. (C) In astrocytes expressing ChR2(H134R), short photoactivation (458-nm, 27.3 mW/mm2, 500 ms) of ChR2 failed to evoke near-membrane Ca2+ elevation (top). Longer light pulses (458-nm, 1 s) evoked variable Ca2+ signals (bottom). (D) Comparison of the percentage of astrocytes showing light-gated Ca2+ rises, and of the amplitude of Ca2+ responses in LiGluR- and ChR2-expressing astrocytes. LiGluR evokes more reliable and reproducible Ca2+ rises in astrocytes. (E) CatCh-evoked astrocyte Ca2+ elevations following blue light photoactivation (1 s, 458-nm). (F) CatCh-induced Ca2+ signaling was abolished in the absence of extracellular Ca2+, but unaffected when ER Ca2+ store is perturbed by thapsigargin (TG). Bars, 10 μm. Adapted from (Li et al., 2012).
Astrocytes express a rich repertoire of metabotropic Gq, Gi/o, and Gs GPCRs (Porter and McCarthy, 1997). New light-gated proteins that mimic these GPCR-mediated pathways have been developed (Schroder-Lang et al., 2007; Airan et al., 2009; Ryu et al., 2010; Gutierrez et al., 2011; Stierl et al., 2011; Levitz et al., 2013), but they have not yet been tested on astrocytes. Since astrocytes express store-operated Ca2+ channels Orai1 (Akita and Okada, 2011; Linde et al., 2011; Moreno et al., 2012), it should also be of interest to activate them with the new photosensitive synthetic protein LOVS1K that reversibly translocates to Orai1 channels and generates either local Ca2+ signals at the plasma membrane or global Ca2+ signals upon repeated photoactivation (Pham et al., 2011).
Optical Methods to Photoactivate Astrocytes
While imaging morphological dynamics of astrocytic fine processes may not yet be possible, stimulating astrocytes locally with light is more promising because the astrocyte-specific expression of light-sensitive Ca2+-permeable ion channels circumvents the optical resolution problem, and therefore even one-photon whole-field illumination is sufficient to stimulate specifically the astrocytes. A more specific photoactivation of a subset of cells, and the local subcellular stimulation of a single astrocyte can be achieved using spatial light modulators [reviewed in (Maurer et al., 2011)] to shape the light (Shoham, 2010; Vaziri and Emiliani, 2012; Papagiakoumou, 2013). One-photon digital holography allows photoactivation within precisely shaped regions of interest at or near the tissue surface.
Combining digital holography and two-photon excitation with temporal focusing to modulate the temporal width of the pulsed laser, several groups reported shaped two-photon excitation deep inside scattering tissue (Andrasfalvy et al., 2010; Papagiakoumou et al., 2010). The spatial patterns thus generated are robust against scattering and remained confined at depths of 100 μm (Papagiakoumou, 2013). Combining optogenetics, shaped photoactivation and two-photon imaging for the optical readout of astrocytes (combined with electrophysiology for recording neuronal signals) holds important promises for interrogating interactions between neurons and astrocytes in intact brain tissue. To probe specific signaling pathways, wave-front light shaping can be combined with uncaging of classical IP3 and Ca2+ cages (Ellis-Davies, 2011), and new endothelin cage (Bourgault et al., 2007).
Monitoring Gliotransmitter Release
Among the mechanisms of gliotransmitter release, Ca2+-regulated exocytosis of synaptic-like small vesicles has been proposed as a major pathway (Cali et al., 2009). Total internal reflection fluorescence microscopy (TIRFM) is a powerful technique to monitor single-vesicle behavior and to study the mechanisms of vesicular docking and fusion in cultured cells (Holz and Axelrod, 2008). Since cultured astrocytes may differ from their in situ counterparts, the physiological relevance of the findings made in culture with TIRFM need to be validated in situ using other approaches.
TIRFM has been used to visualize near membrane single vesicles and monitor single vesicle fusion in cultured astrocytes (Bezzi et al., 2004; Zhang et al., 2004; Bowser and Khakh, 2007; Li et al., 2008; Malarkey and Parpura, 2011; Potokar et al., 2013). In early experiments (Bezzi et al., 2004), the fluorescent weak base acridine orange (AO) was used to report exocytosis, and the vesicular glutamate transporter (VGLUT) tagged with the enhanced GFP (EGFP) was overexpressed to identify the AO-positive vesicles. Following DHPG application, rapid millisecond Ca2+-dependent flashes of AO-labeled vesicles were detected and interpreted as the exocytosis of glutamatergic vesicles (Bezzi et al., 2004; Domercq et al., 2006). This interpretation was soon complicated by studies showing that AO metachromasy results in its simultaneous emission of green and red fluorescence, which invalidates the identification of the AO-positive vesicles with EGFP labeling (Nadrigny et al., 2006, 2007). It has also been shown that the flash events of AO-loaded astrocyte vesicles are not solely due to exocytosis but also reflect intracellular vesicle photolysis (Jaiswal et al., 2007; Li et al., 2008), due to the action of AO as a photosensitizer. Styryl pyridinium FM dyes, established markers of vesicular release in neurons (Rizzoli and Betz, 2005), were also used to label the astrocytic vesicular compartments and report exocytosis. However, FM dyes are handled differently by neurons and astrocytes (Li et al., 2009a) and they label mainly lysosomes (Zhang et al., 2007; Li et al., 2008; Liu et al., 2011).
Later, the genetically encoded exocytotic reporter, pHluorin, has emerged as a valuable tool to monitor astrocyte vesicle exocytosis. As a pH-sensitive GFP mutant, pHluorin fluorescence is quenched in the acidic vesicle lumen and becomes bright upon vesicle fusion when the fluorescent protein is exposed to external neutral pH (Miesenbock et al., 1998). Since there was evidence that astrocytes release glutamate via Ca2+-regulated exocytosis (Cali et al., 2009), pHluorin was targeted to the lumen of putative glutamatergic vesicles in astrocytes by using the fusion protein VGLUT1-pHluorin (Marchaland et al., 2008). TIRFM imaging of single vesicles in cultured astrocytes labeled with VGLUT1-pHluorin revealed fusion events occurring within hundreds of milliseconds after Ca2+ rise evoked by either mGluR (Marchaland et al., 2008) or purinergic P2Y1 receptor activation (Santello et al., 2011). These results were consistent with those obtained by the same lab using AO-labeled (Bezzi et al., 2004; Domercq et al., 2006), and FM-labelled astrocytes (Cali et al., 2008).
Different kinetics have been reported for the exocytosis of the putative glutamatergic vesicles in astrocytes when using another pHluorin-based exocytotic reporter synaptopHluorin (spH), a chimeric construct tagging the luminal side of synaptobrevin 2 (Burrone et al., 2006). As synaptobrevin 2 appears to colocalize with VGLUT1 on the same vesicles in astrocytes (Montana et al., 2004; Zhang et al., 2004; Liu et al., 2011), expressing spH in astrocytes leads to the labeling of VGLUT1-positive vesicles (Bowser and Khakh, 2007; Liu et al., 2011). However, unlike VGLUT1-phluorin that reports fast millisecond kinetics of exocytosis (Cali et al., 2008; Santello et al., 2011), spH-labeled vesicles undergo slow exocytosis that is loosely coupled to stimulation, with most events occurring ~2 min after P2 receptor-mediated Ca2+ rise (Malarkey and Parpura, 2011), and within hundreds of milliseconds following Ca2+ increase evoked by mechanical stimulation (Liu et al., 2011; Malarkey and Parpura, 2011).
Genetically encoded reporters of exocytosis set the stage for investigating the mechanisms of astrocyte exocytosis and for addressing several remaining questions. First, the reasons for the variable fusion kinetics of putative VGLUT-positive vesicles remain to be elucidated. Second, several studies failed to detect the presence of VGLUT expression in astrocytes (Cahoy et al., 2008; Juge et al., 2010; Li et al., 2013), therefore, new experiments are needed to clarify the molecular identity of the VGLUT-positive vesicles. Recently, a new genetically encoded red pH-sensitive probe, pHTomato, has been introduced to image single vesicle exocytosis (Li and Tsien, 2012). It should allow monitoring exocytosis, and, simultaneously, activating Ca2+ signal with optogenetic tools that typically require blue light illumination (Li et al., 2012). Finally, the new genetically encoded glutamate sensor, iGluSnFR, shows fast kinetics (Marvin et al., 2013) and is potentially suitable for fast real-time recording of glutamate release from astrocytes. Combining it with TIRFM detection of single vesicle exocytosis would help to clarify the relative contribution of vesicular vs. non-vesicular release pathways to glutamate release (Kimelberg et al., 2006; Li et al., 2012; Woo et al., 2012). Fourth, a combination of fast two-photon imaging, local photoactivation and genetically targeted expression of pHluorins in slice and in vivo must validate earlier findings from cell-culture studies.
Targeting Genetically Encoded Proteins to Astrocytes
Most optical techniques available today lack the spatial resolution for imaging thin astrocyte processes. Bulk-loaded Ca2+ indicators that label both astrocytes and neurons, report mixed signals. However, using GECIs targeted selectively to astrocytes, it becomes possible to record astrocyte-specific Ca2+ signals with standard imaging techniques, like confocal, spinning-disk confocal, and two-photon microscopies. Similarly by targeting the light-gated proteins, selective photoactivation of astrocytes can be achieved (see below). Therefore, targeting the GECIs and the light-gated channels/receptors to astrocytes in situ is a critical step, and several strategies have been used. First, plasmids can be electroporated in utero (Yoshida et al., 2010) but the yield of electroporation is variable, therefore, protocols using intracerebral and intravenous injections of viral constructs and Tg mouse lines are being favored.
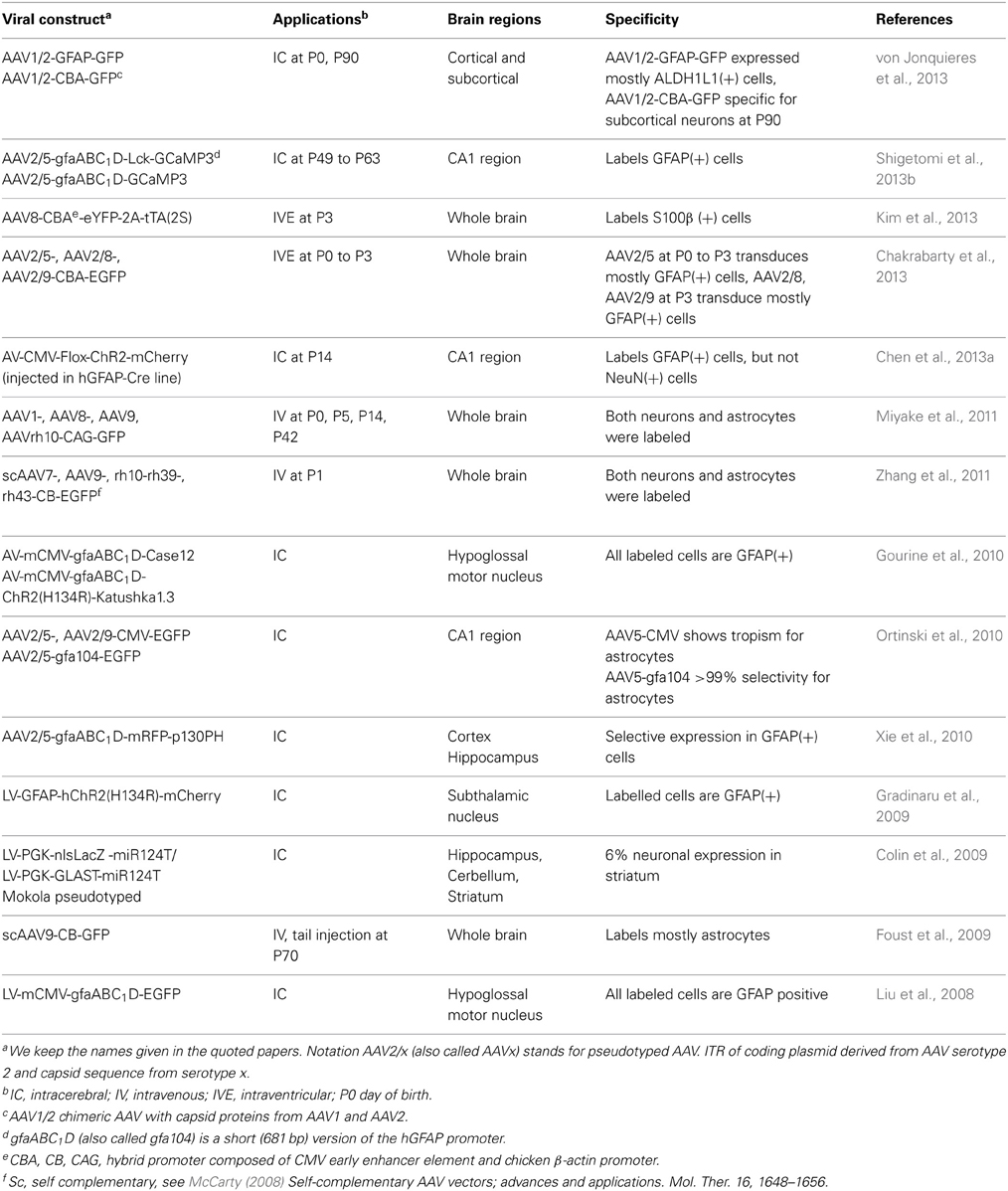
Viral constructs are relatively easy to generate and adeno-associated virus (AAV) are the most widely used for astrocyte infection (Table 1). Intracerebral injections of lentivirus (LV) and adenovirus (AV) (Liu et al., 2008; Colin et al., 2009; Gourine et al., 2010) have been limited due to their possible toxic effects. Among the AAV variants, the serotype 5 with a high tropism for astrocytes (Ortinski et al., 2010), is commonly used for packaging the DNA constructs. Following intracerebral viral injections, the area of infection extends well beyond the injection site and the expression of the reporter genes is stable for months. In adult mice, intravenous injections of AAV serotype 9 labels mostly astrocytes rather than neurons (Foust et al., 2009). Intraventricular injection of AAV serotype 8 at postnatal day 3 (P3) labels preferentially astrocytes (Kim et al., 2013). New serotypes have been developed using a directed evolution approach with higher transduction level for astrocytes leading to 94% specific expression in retinal Müller glial cells after intravitreal injection (Klimczak et al., 2009). This seems to be promising to reduce the virus titer needed for expression. More specific targeting can be achieved by inserting an astrocyte-specific promoter. The 2.2 kb hGFAP promoter targets selectively ChR2 to astrocytes using LV constructs (Gradinaru et al., 2009). However, since AAVs cannot carry constructs larger than 4.7 kb, a shorter 681 bp hGFAP promoter (gfaABC1D also called gfa104) (Lee et al., 2008) has been used to target selectively EGFP, ChR2, GECIs (case12, GCaMP3, Lck-GCaMP3), and a pleckstrin homology (PH) domain of phospholipase C-like protein p130 (p130PH) to astrocytes (Ortinski et al., 2010; Xie et al., 2010; Shigetomi et al., 2013b).
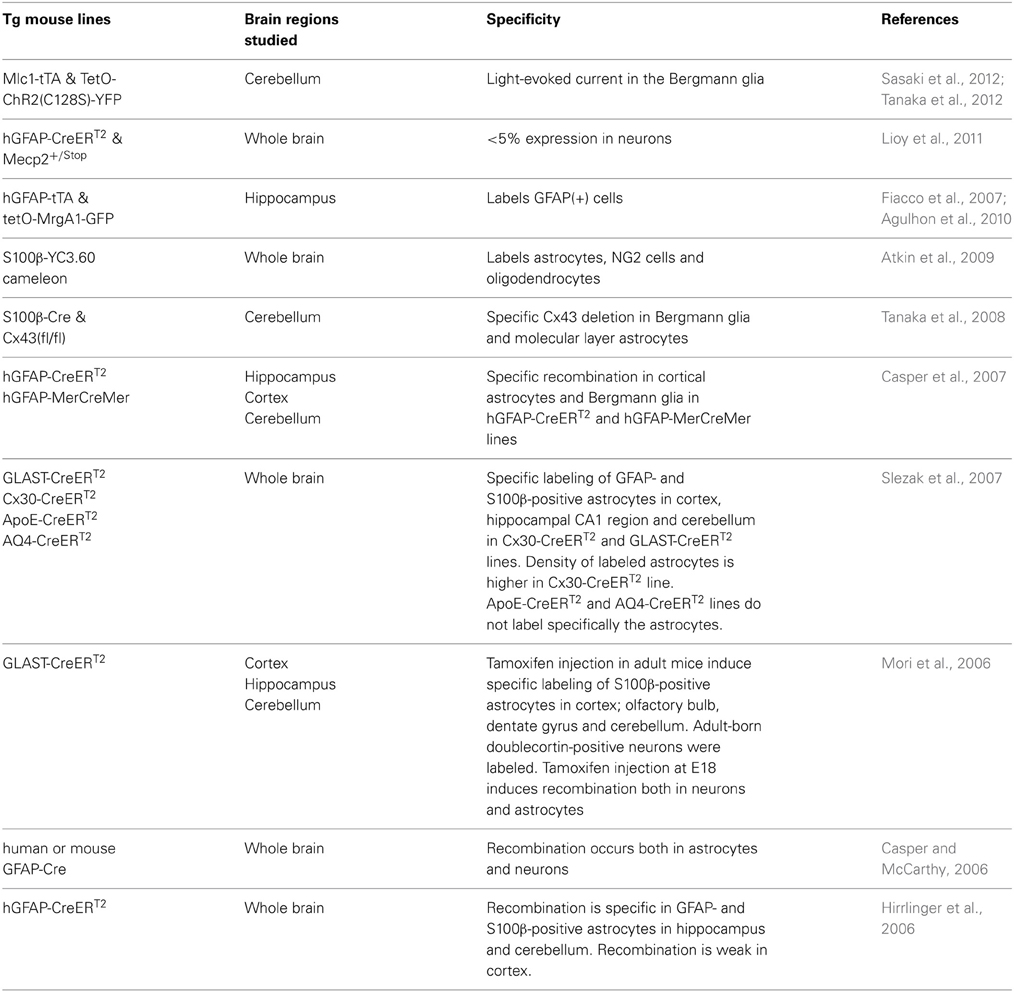
Gene targeting of viral constructs can also be achieved by the Cre-Lox or tetO-tTA strategies injecting floxed (or flexed) viral constructs in Cre mouse lines, or tetO virus in the tTA mouse lines (Pfrieger and Slezak, 2012). Several astrocyte-specific Cre- and tTA-expressing mouse lines have been generated (Table 2). When a Cre-dependent ChR2-expressing virus was injected in hippocampus (Chen et al., 2013a) of a P14 hGFAP mouse line (Casper and McCarthy, 2006), selective expression of ChR2 was obtained in the astrocytes.
Yet an important limitation of the viral delivery strategies needs to be taken into account. Following intracerebral injection of AAV2/5-gfa104-EGFP construct, a significant dose-dependent reactive gliosis has been observed (Reimsnider et al., 2007; Klein et al., 2008; Ortinski et al., 2010). Since gliosis is associated with changes of several signaling pathways in astrocytes (Hamby et al., 2012), it will be important to develop alternative approaches to study the role of astrocytes in physiological conditions. Introducing a sequence encoding the VIVIT peptide that interferes with the calcineurin/nuclear factor of activated T-cells signaling pathway and down regulates GFAP overexpression (Furman et al., 2012), may reduce AAV-induced gliosis. Replacing viral constructs by Tg floxed/tetO mouse lines is a very promising approach to control gliosis. Several floxed (Slezak et al., 2012; Zariwala et al., 2012) and tetO (Fiacco et al., 2007; Agulhon et al., 2010) mouse lines of interest have been generated. With a hGFAP-CreERT2 mouse line in which the recombination can be induced in juvenile or adult mice by tamoxifen injections, astrocyte-specific targeting has been obtained in cortex, hippocampal CA1 region, cerebellum, diencephalon and brain stem, with weaker levels of recombination in cortex (Hirrlinger et al., 2006; Lioy et al., 2011). In glutamate-aspartate transporter (GLAST)- and connexin 30 (Cx30)-CreERT2 mouse lines, astrocyte-specific recombination occurs in cortex, hippocampal CA1 region, and cerebellum. But in GLAST-CreERT2 and hGFAP-CreERT2 mouse lines, the recombination is not astrocyte-specific in brain regions including the olfactory bulb and hippocampal dentate gyrus, where neurons are also labeled (Mori et al., 2006; Slezak et al., 2007). The floxed/tetO strategy is advantageous since it does not require surgery for viral injections. A Ai38 floxed GCaMP3 reporter mouse line was generated by a knockin strategy to insert GCaMP3 into the ROSA26 locus (Zariwala et al., 2012). When the Ai38 mouse was crossed with an inducible Wfs1-Tg2-CreERT2 mouse line, a uniform expression of the reporter genes was obtained in cortical excitatory neurons without over expression of GCaMP3 in the nucleus as observed after cortical injection of an AAV-syn-GCaMP3 construct (Figure 3). Importantly, it appears that various Cre- and tTA-dependent mouse lines differ in their ability to induce recombination, and also the specificity of recombination can vary with the brain region, and with the age. Therefore, in order to ascertain specificity of astrocytic signal measurement and photoactivation, it will be critical to carefully validate astrocyte-specific expression, for example, by using cell type-specific antibodies and confocal microscopy.
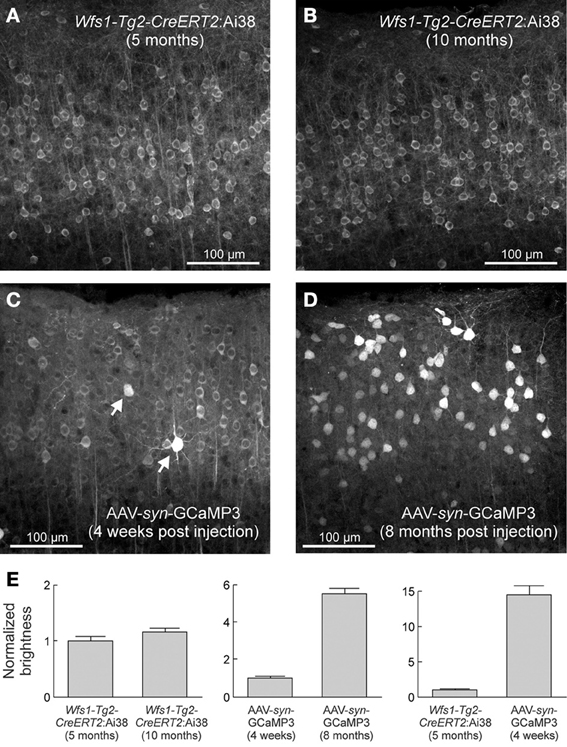
Figure 3. Stable expression levels in the Ai38 ROSA26-GCaMP3 mouse line over months. Native GCaMP3 fluorescence in layer2/3 excitatory neurons of visual cortex from Wfs1-Tg2-CreERT2:Ai38 mice (A,B) and adult wild-type mice injected with AAV-syn-GCaMP3 (C,D). (E) Quantification of neuronal brightness. Error bars correspond to SEM. From (Zariwala et al., 2012), with permission.
Perspectives
In conclusion, new genetically targeted optical and pharmacological tools allow the selective measurement and activation of astrocytic Ca2+ signals. These tools should be of value for studying the mechanisms of gliotransmitter release, the role of astrocytes, and more specifically the bidirectional communication between astrocytes and neurons.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Karine Hérault for her permanent support, Jonathan Bradley and Deniz Dalkara for their advice on viral vectors, Ehud Isacoff for his expertise on light-gated channels, Christian Giaume and Etienne Audinat for discussions, Florent Jean and Patrice Jegouzo for technical assistance. Our work is supported by grants from the Agence Nationale de la Recherche (ANR) to Nicole Ropert; France-BioImaging (FBI) to Martin Oheim; a Ph.D. fellowship from the Fondation pour la Recherche Médicale (FRM) to Elke Schmidt; a Chair of Excellence from the Paris School of Neuroscience (Ecole des Neurosciences de Paris, ENP) and a Marie Curie Career Integration grant to Cendra Agulhon.
Abbreviations
AAV, adeno-associated virus; ACR, astrocyte Cre reporter; ALDH1L1, aldehyde dehydrogenase 1 L1; AM, acetoxymethyl; AV, adenovirus; CatCh calcium-translocating channelrhodopsin 2; ChR, channelrhodopsin; CMV, cytomegalovirus; Cx30, connexin 30; DHPG, dihydroxyphenylglycine; DsRed, discosoma red protein; EGFP, enhanced green fluorescent protein; GCaMP, green fluorescent protein-based Ca2+ sensor; GECI, genetically encoded calcium indicator; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; GLAST, glutamate-aspartate transporter; GLT-1, glutamate transporter 1; hGFAP, human glial fibrillary acidic protein; IP3, inositol triphosphate; LiGluR, light-gated glutamate receptor; LimGluR, light-gated metabotropic glutamate receptor; LV, lentivirus; mGluR, metabotropic glutamate receptor; Mlc1, megalencephalic leukoencephalopathy with subcortical cysts 1; MrgA1, Mas-related gene A1; OGB1, oregon green BAPTA1; STED, stimulated emission depletion; TIRF(M), total internal reflection fluorescence (microscopy); TA, tetracycline transactivator; TetO, tetracycline operator; Tg, transgenic; YFP, yellow fluorescent protein
References
Agulhon, C., Fiacco, T. A., and McCarthy, K. D. (2010). Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. doi: 10.1126/science.1184821
Agulhon, C., Petravicz, J., McMullen, A. B., Sweger, E. J., Minton, S. K., Taves, S. R., et al. (2008). What is the role of astrocyte calcium in neurophysiology. Neuron 59, 932–946. doi: 10.1016/j.neuron.2008.09.004
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H., and Deisseroth, K. (2009). Temporally precise in vivo control of intracellular signaling. Nature 458, 1025–1029. doi: 10.1038/nature07926
Akerboom, J., Chen, T. W., Wardill, T. J., Tian, L., Marvin, J. S., Mutlu, S., et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012
Akerboom, J., Carreras Calderón, N., Tian, L., Wabnig, S., Prigge, M., Tolö, J., et al. (2013). Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6:2. doi: 10.3389/fnmol.2013.00002
Akita, T., and Okada, Y. (2011). Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J. Physiol. 589, 3909–3927. doi: 10.1113/jphysiol.2011.208173
Andrasfalvy, B. K., Zemelman, B. V., Tang, J., and Vaziri, A. (2010). Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. 107, 11981–11986. doi: 10.1073/pnas.1006620107
Angulo, M. C., Le Meur, K., Kozlov, A. S., Charpak, S., and Audinat, E. (2008). GABA, a forgotten gliotransmitter. Prog. Neurobiol. 86, 297–303. doi: 10.1016/j.pneurobio.2008.08.002
Araque, A., Parpura, V., Sanzgiri, R. P., and Haydon, P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. doi: 10.1016/S0166-2236(98)01349-6
Atkin, S. D., Patel, S., Kocharyan, A., Holtzclaw, L. A., Weerth, S. H., Schram, V., et al. (2009). Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo. J. Neurosci. Methods 181, 212–226. doi: 10.1016/j.jneumeth.2009.05.006
Benquet, P., Gee, C. E., and Gerber, U. (2002). Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci. 22, 9679–9686.
Bergersen, L. H., and Gundersen, V. (2009). Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience 158, 260–265. doi: 10.1016/j.neuroscience.2008.03.074
Berning, S., Willig, K. I., Steffens, H., Dibaj, P., and Hell, S. W. (2012). Nanoscopy in a living mouse brain. Science 335, 551. doi: 10.1126/science.1215369
Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhauser, C., Pilati, E., et al. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. doi: 10.1038/nn1246
Bourgault, S., Letourneau, M., and Fournier, A. (2007). Development of photolabile caged analogs of endothelin-1. Peptides 28, 1074–1082. doi: 10.1016/j.peptides.2007.02.013
Bowser, D. N., and Khakh, B. S. (2007). Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc. Natl. Acad. Sci. U.S.A. 104, 4212–4217. doi: 10.1073/pnas.0607625104
Burrone, J., Li, Z., and Murthy, V. N. (2006). Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat Protoc 1, 2970–2978. doi: 10.1038/nprot.2006.449
Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Zamanian, J. L., Christopherson, K. S., et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278. doi: 10.1523/JNEUROSCI.4178-07.2008
Cali, C., Marchaland, J., Regazzi, R., and Bezzi, P. (2008). SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy. J. Neuroimmunol. 198, 82–91. doi: 10.1016/j.jneuroim.2008.04.015
Cali, C., Marchaland, J., Spagnuolo, P., Gremion, J., and Bezzi, P. (2009). Regulated exocytosis from astrocytes physiological and pathological related aspects. Int. Rev. Neurobiol. 85, 261–293. doi: 10.1016/S0074-7742(09)85020-4
Carmignoto, G., Pasti, L., and Pozzan, T. (1998). On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J. Neurosci. 18, 4637–4645.
Casper, K. B., Jones, K., and McCarthy, K. D. (2007). Characterization of astrocyte-specific conditional knockouts. Genesis 45, 292–299. doi: 10.1002/dvg.20287
Casper, K. B., and McCarthy, K. D. (2006). GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol. Cell. Neurosci. 31, 676–684. doi: 10.1016/j.mcn.2005.12.006
Chaigneau, E., Wright, A. J., Poland, S. P., Girkin, J. M., and Silver, R. A. (2011). Impact of wavefront distortion and scattering on 2-photon microscopy in mammalian brain tissue. Opt. Express 19, 22755–22774. doi: 10.1364/OE.19.022755
Chakrabarty, P., Rosario, A., Cruz, P., Siemienski, Z., Ceballos-Diaz, C., Crosby, K., et al. (2013). Capsid serotype and timing of injection determines AAV transduction in the neonatal mice brain. PLoS ONE 8:e67680. doi: 10.1371/journal.pone.0067680
Chen, J., Tan, Z., Zeng, L., Zhang, X., He, Y., Gao, W., et al. (2013a). Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia 61, 178–191. doi: 10.1002/glia.22425
Chen, T. W., Wardill, T. J., Sun, Y., Pulver, S. R., Renninger, S. L., Baohan, A., et al. (2013b). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. doi: 10.1038/nature12354
Cheng, A., Gonçalves, J. T., Golshani, P., Arisaka, K., and Portera-Cailliau, C. (2011). Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nat. Methods 8, 139–142. doi: 10.1038/nmeth.1552
Colin, A., Faideau, M., Dufour, N., Auregan, G., Hassig, R., Andrieu, T., et al. (2009). Engineered lentiviral vector targeting astrocytes in vivo. Glia 57, 667–679. doi: 10.1002/glia.20795
Devaraju, P., Sun, M. Y., Myers, T. L., Lauderdale, K., and Fiacco, T. A. (2013). Astrocytic group I mGluR dependent potentiation of astrocytic glutamate and potassium uptake. J. Neurophysiol. 109, 2404–2414. doi: 10.1152/jn.00517.2012
Di Castro, M. A., Chuquet, J., Liaudet, N., Bhaukaurally, K., Santello, M., Bouvier, D., et al. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284. doi: 10.1038/nn.2929
Ding, J., Takasaki, K. T., and Sabatini, B. L. (2009). Supraresolution imaging in brain slices using stimulated-emission depletion 2-photon laser scanning microscopy. Neuron 63, 429. doi: 10.1016/j.neuron.2009.07.011
Domercq, M., Brambilla, L., Pilati, E., Marchaland, J., Volterra, A., and Bezzi, P. (2006). P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 281, 30684–30696. doi: 10.1074/jbc.M606429200
Dong, X., Han, S., Zylka, M. J., Simon, M. I., and Anderson, D. J. (2001). A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106, 619–632. doi: 10.1016/S0092-8674(01)00483-4
Ducros, M., Goulam Hossen, Y., Bradley, J., de Sars, V., and Charpak, S. (2013). Encoded multi-site two-photon microscopy. Proc. Natl. Acad. Sci. U.S.A. 110, 13138–13143. doi: 10.1073/pnas.1307818110
Ellis-Davies, G. C. (2011). “Are caged compounds still useful,” in Photosensitive Molecules for Controlling Biological Function, eds J. J. Chambers and R. H. Kramer (New York, NY: Springer Humana Press), 39–56. doi: 10.1007/978-1-61779-031-7_3
Fenno, L., Yizhar, O., and Deisseroth, K. (2011). The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412. doi: 10.1146/annurev-neuro-061010-113817
Fiacco, T. A., Agulhon, C., and McCarthy, K. D. (2009). Sorting out astrocyte physiology from pharmacology. Annu. Rev. Pharmacol. Toxicol. 49, 151–174. doi: 10.1146/annurev.pharmtox.011008.145602
Fiacco, T. A., Agulhon, C., Taves, S. R., Petravicz, J., Casper, K. B., Dong, X., et al. (2007). Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626. doi: 10.1016/j.neuron.2007.04.032
Foust, K. D., Nurre, E., Montgomery, C. L., Hernandez, A., Chan, C. M., and Kaspar, B. K. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65. doi: 10.1038/nbt.1515
Furman, J. L., Sama, D. M., Gant, J. C., Beckett, T. L., Murphy, M. P., Bachstetter, A. D., et al. (2012). Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J. Neurosci. 32, 16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012
Garaschuk, O. (2013). Imaging microcircuit function in healthy and diseased brain. Exp. Neurol. 242, 41–49. doi: 10.1016/j.expneurol.2012.02.009
Garaschuk, O., Milos, R. I., and Konnerth, A. (2006). Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nat. Protoc. 1, 380–386. doi: 10.1038/nprot.2006.58
Gourine, A. V., Kasymov, V., Marina, N., Tang, F., Figueiredo, M. F., Lane, S., et al. (2010). Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575. doi: 10.1126/science.1190721
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., and Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359. doi: 10.1126/science.1167093
Grosberg, L. E., Chen, B. R., and Hillman, E. (2012). Simultaneous multiplane in vivo nonlinear microscopy using spectral encoding. Opt. Lett. 37, 2967–2969. doi: 10.1364/OL.37.002967
Gucek, A., Vardjan, N., and Zorec, R. (2012). Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem. Res. 37, 2351–2363. doi: 10.1007/s11064-012-0773-6
Gutierrez, D. V., Mark, M. D., Masseck, O., Maejima, T., Kuckelsberg, D., Hyde, R. A., et al. (2011). Optogenetic control of motor coordination by Gi/o protein-coupled vertebrate rhodopsin in cerebellar Purkinje cells. J. Biol. Chem. 286, 25848–25858. doi: 10.1074/jbc.M111.253674
Halassa, M. M., Fellin, T., and Haydon, P. G. (2007). The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13, 54–63. doi: 10.1016/j.molmed.2006.12.005
Halassa, M. M., and Haydon, P. G. (2010). Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355. doi: 10.1146/annurev-physiol-021909-135843
Hamby, M. E., Coppola, G., Ao, Y., Geschwind, D. H., Khakh, B. S., and Sofroniew, M. V. (2012). Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J. Neurosci. 32, 14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012
Hamilton, N. B., and Attwell, D. (2010). Do astrocytes really exocytose neurotransmitters. Nat. Rev. Neurosci. 11, 227–238. doi: 10.1038/nrn2803
Heintz, N. (2004). Gene expression nervous system atlas (GENSAT). Nat. Neurosci. 7, 483. doi: 10.1038/nn0504-483
Hirrlinger, P. G., Scheller, A., Braun, C., Hirrlinger, J., and Kirchhoff, F. (2006). Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia 54, 11–20. doi: 10.1002/glia.20342
Holz, R. W., and Axelrod, D. (2008). Secretory granule behavior adjacent to the plasma membrane before and during exocytosis: total internal reflection fluorescence microscopy studies. Acta Physiol. (Oxf.) 192, 303–307. doi: 10.1111/j.1748-1716.2007.01818.x
Hoogland, T. M., Kuhn, B., Gobel, W., Huang, W., Nakai, J., Helmchen, F., et al. (2009). Radially expanding transglial calcium waves in the intact cerebellum. Proc. Natl. Acad. Sci. U.S.A. 106, 3496–3501. doi: 10.1073/pnas.0809269106
Horikawa, K., Yamada, Y., Matsuda, T., Kobayashi, K., Hashimoto, M., Matsu-ura, T, et al. (2010). Spontaneous network activity visualized by ultrasensitive Ca(2+) indicators, yellow Cameleon-Nano. Nat. Methods 7, 729–732. doi: 10.1038/nmeth.1488
Jaiswal, J. K., Fix, M., Takano, T., Nedergaard, M., and Simon, S. M. (2007). Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 104, 14151–14156. doi: 10.1073/pnas.0704935104
Juge, N., Gray, J. A., Omote, H., Miyaji, T., Inoue, T., Hara, C., et al. (2010). Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112. doi: 10.1016/j.neuron.2010.09.002
Kafitz, K. W., Meier, S. D., Stephan, J., and Rose, C. R. (2008). Developmental profile and properties of sulforhodamine 101–labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 169, 84–92. doi: 10.1016/j.jneumeth.2007.11.022
Kang, J., Kang, N., Yu, Y., Zhang, J., Petersen, N., Tian, G. F., et al. (2010). Sulforhodamine 101 induces long-term potentiation of intrinsic excitability and synaptic efficacy in hippocampal CA1 pyramidal neurons. Neuroscience 169, 1601–1609. doi: 10.1016/j.neuroscience.2010.06.020
Kim, J. Y., Ash, R. T., Ceballos-Diaz, C., Levites, Y., Golde, T. E., Smirnakis, S. M., et al. (2013). Viral transduction of the neonatal brain delivers controllable genetic mosaicism for visualising and manipulating neuronal circuits in vivo. Eur. J. Neurosci. 37, 1203–1220. doi: 10.1111/ejn.12126
Kimelberg, H. K., Macvicar, B. A., and Sontheimer, H. (2006). Anion channels in astrocytes: biophysics, pharmacology, and function. Glia 54, 747–757. doi: 10.1002/glia.20423
Klein, R. L., Dayton, R. D., Tatom, J. B., Henderson, K. M., and Henning, P. P. (2008). AAV8 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol. Ther. 16, 89–96. doi: 10.1038/sj.mt.6300331
Kleinlogel, S., Feldbauer, K., Dempski, R. E., Fotis, H., Wood, P. G., Bamann, C., et al. (2011). Ultra light-sensitive and fast neuronal activation with the Ca(2)+-permeable channelrhodopsin CatCh. Nat. Neurosci. 14, 513–518. doi: 10.1038/nn.2776
Klimczak, R. R., Koerber, J. T., Dalkara, D., Flannery, J. G., and Schaffer, D. V. (2009). A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS ONE 4:e7467. doi: 10.1371/journal.pone.0007467
Knopfel, T. (2012). Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700. doi: 10.1038/nrn3293
Koester, H. J., Baur, D., Uhl, R., and Hell, S. W. (1999). Ca2+ fluorescence imaging with pico-and femtosecond two-photon excitation: signal and photodamage. Biophys. J. 77, 2226. doi: 10.1016/S0006-3495(99)77063-3
Lee, Y., Messing, A., Su, M., and Brenner, M. (2008). GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493. doi: 10.1002/glia.20622
Levitz, J., Pantoja, C., Gaub, B., Janovjak, H., Reiner, A., Hoagland, A., et al. (2013). Optical control of metabotropic glutamate receptors. Nat. Neurosci. 16, 507–516. doi: 10.1038/nn.3346
Li, D., Herault, K., Isacoff, E. Y., Oheim, M., and Ropert, N. (2012). Optogenetic activation of LiGluR-expressing astrocytes evokes anion channel-mediated glutamate release. J. Physiol. 590, 855–873. doi: 10.1113/jphysiol.2011.219345
Li, D., Herault, K., Oheim, M., and Ropert, N. (2009a). FM dyes enter via a store-operated calcium channel and modify calcium signaling of cultured astrocytes. Proc. Natl. Acad. Sci. U.S.A. 106, 21960–21965. doi: 10.1073/pnas.0909109106
Li, Q., Wu, S. S. H., and Chou, K. C. (2009b). Subdiffraction-limit two-photon fluorescence microscopy for GFP-tagged cell imaging. Biophys. J. 97, 3224. doi: 10.1016/j.bpj.2009.09.038
Li, D., Herault, K., Silm, K., Evrard, A., Wojcik, S., Oheim, M., et al. (2013). Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J. Neurosci. 33, 4434–4455. doi: 10.1523/JNEUROSCI.3667-12.2013
Li, D., Ropert, N., Koulakoff, A., Giaume, C., and Oheim, M. (2008). Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J. Neurosci. 28, 7648–7658. doi: 10.1523/JNEUROSCI.0744-08.2008
Li, Y., and Tsien, R. W. (2012). pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat. Neurosci. 15, 1047–1053. doi: 10.1038/nn.3126
Lin, J. Y., Lin, M. Z., Steinbach, P., and Tsien, R. Y. (2009). Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814. doi: 10.1016/j.bpj.2008.11.034
Linde, C. I., Baryshnikov, S. G., Mazzocco-Spezzia, A., and Golovina, V. A. (2011). Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein. Am. J. Physiol. Cell Physiol. 300, C1502–C1512. doi: 10.1152/ajpcell.00379.2010
Lioy, D. T., Garg, S. K., Monaghan, C. E., Raber, J., Foust, K. D., Kaspar, B. K., et al. (2011). A role for glia in the progression of Rett's syndrome. Nature 475, 497–500. doi: 10.1038/nature10214
Liu, B., Paton, J. F., and Kasparov, S. (2008). Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol. 8:49. doi: 10.1186/1472-6750-8-49
Liu, T., Sun, L., Xiong, Y., Shang, S., Guo, N., Teng, S., et al. (2011). Calcium triggers exocytosis from two types of organelles in a single astrocyte. J. Neurosci. 31, 10593–10601. doi: 10.1523/JNEUROSCI.6401-10.2011
Looger, L. L., and Griesbeck, O. (2012). Genetically encoded neural activity indicators. Curr. Opin. Neurobiol. 22, 18–23. doi: 10.1016/j.conb.2011.10.024
Malarkey, E. B., and Parpura, V. (2011). Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J. Physiol. 589, 4271–4300. doi: 10.1113/jphysiol.2011.210435
Mannaioni, G., Marino, M. J., Valenti, O., Traynelis, S. F., and Conn, P. J. (2001). Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 21, 5925–5934.
Marchaland, J., Cali, C., Voglmaier, S. M., Li, H., Regazzi, R., Edwards, R. H., et al. (2008). Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J. Neurosci. 28, 9122–9132. doi: 10.1523/JNEUROSCI.0040-08.2008
Marvin, J. S., Borghuis, B. G., Tian, L., Cichon, J., Harnett, M. T., Akerboom, J., et al. (2013). An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170. doi: 10.1038/nmeth.2333
Maurer, C., Jesacher, A., Bernet, S., and Ritsch−Marte, M. (2011). What spatial light modulators can do for optical microscopy. Laser Photon. Rev. 5, 81–101. doi: 10.1002/lpor.200900047
McCarty, D. M. (2008). Self-complementary AAV vectors; advances and applications. Mol. Ther. 16, 1648–1656. doi: 10.1038/mt.2008.171
Miesenbock, G. (2011). Optogenetic control of cells and circuits. Annu. Rev. Cell Dev. Biol. 27, 731–758. doi: 10.1146/annurev-cellbio-100109-104051
Miesenbock, G., De Angelis, D. A., and Rothman, J. E. (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. doi: 10.1038/28190
Miyake, N., Miyake, K., Yamamoto, M., Hirai, Y., and Shimada, T. (2011). Global gene transfer into the CNS across the BBB after neonatal systemic delivery of single-stranded AAV vectors. Brain Res. 1389, 19–26. doi: 10.1016/j.brainres.2011.03.014
Moneron, G., and Hell, S. W. (2009). Two-photon excitation STED microscopy. Opt. Express 17, 14567–14573. doi: 10.1364/OE.17.014567
Montana, V., Ni, Y., Sunjara, V., Hua, X., and Parpura, V. (2004). Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24, 2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004
Moreno, C., Sampieri, A., Vivas, O., Pena-Segura, C., and Vaca, L. (2012). STIM1 and Orai1 mediate thrombin-induced Ca(2+) influx in rat cortical astrocytes. Cell Calcium 52, 457–467. doi: 10.1016/j.ceca.2012.08.004
Mori, T., Tanaka, K., Buffo, A., Wurst, W., Kuhn, R., and Gotz, M. (2006). Inducible gene deletion in astroglia and radial glia–a valuable tool for functional and lineage analysis. Glia 54, 21–34. doi: 10.1002/glia.20350
Mutze, J., Iyer, V., Macklin, J. J., Colonell, J., Karsh, B., Petrasek, Z., et al. (2012). Excitation spectra and brightness optimization of two-photon excited probes. Biophys. J. 102, 934–944. doi: 10.1016/j.bpj.2011.12.056
Nadrigny, F., Li, D., Kemnitz, K., Ropert, N., Koulakoff, A., Rudolph, S., et al. (2007). Systematic colocalization errors between acridine orange and EGFP in astrocyte vesicular organelles. Biophys. J. 93, 969–980. doi: 10.1529/biophysj.106.102673
Nadrigny, F., Rivals, I., Hirrlinger, P. G., Koulakoff, A., Personnaz, L., Vernet, M., et al. (2006). Detecting fluorescent protein expression and co-localisation on single secretory vesicles with linear spectral unmixing. Eur. Biophys. J. 35, 533–547. doi: 10.1007/s00249-005-0040-8
Nagel, G., Brauner, M., Liewald, J. F., Adeishvili, N., Bamberg, E., and Gottschalk, A. (2005). Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284. doi: 10.1016/j.cub.2005.11.032
Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 100, 13940–13945. doi: 10.1073/pnas.1936192100
Nägerl, U. V., and Bonhoeffer, T. (2010). Imaging living synapses at the nanoscale by STED microscopy. J. Neurosci. 30, 9341–9346. doi: 10.1523/JNEUROSCI.0990-10.2010
Nakai, J., Ohkura, M., and Imoto, K. (2001). A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141. doi: 10.1038/84397
Nett, W. J., Oloff, S. H., and McCarthy, K. D. (2002). Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 87, 528–537. doi: 10.1152/jn.00268.2001
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N., and Helmchen, F. (2004). Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37. doi: 10.1038/nmeth706
Nolte, C., Matyash, M., Pivneva, T., Schipke, C. G., Ohlemeyer, C., Hanisch, U. K., et al. (2001). GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86. doi: 10.1002/1098-1136(20010101)33:1<72::AID-GLIA1007>3.3.CO;2-1
Ohkura, M., Sasaki, T., Sadakari, J., Gengyo-Ando, K., Kagawa-Nagamura, Y., Kobayashi, C., et al. (2012). Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE 7:e51286. doi: 10.1371/journal.pone.0051286
Ortinski, P. I., Dong, J., Mungenast, A., Yue, C., Takano, H., Watson, D. J., et al. (2010). Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat. Neurosci. 13, 584–591. doi: 10.1038/nn.2535
Panatier, A., Vallee, J., Haber, M., Murai, K. K., Lacaille, J. C., and Robitaille, R. (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. doi: 10.1016/j.cell.2011.07.022
Papagiakoumou, E. (2013). Optical developments for optogenetics. Biol. Cell. 105, 443–464. doi: 10.1111/boc.201200087
Papagiakoumou, E., Anselmi, F., Bègue, A., de Sars, V., Glückstad, J., Isacoff, E. Y., et al. (2010). Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854. doi: 10.1038/nmeth.1505
Paredes, R. M., Etzler, J. C., Watts, L. T., Zheng, W., and Lechleiter, J. D. (2008). Chemical calcium indicators. Methods 46, 143–151. doi: 10.1016/j.ymeth.2008.09.025
Parpura, V., Grubisic, V., and Verkhratsky, A. (2011). Ca(2+) sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta 1813, 984–991. doi: 10.1016/j.bbamcr.2010.11.006
Parpura, V., and Verkhratsky, A. (2012). Homeostatic function of astrocytes: Ca(2+) and Na(+) signaling. Transl Neurosci 3, 334–344. doi: 10.2478/s13380-012-0040-y
Perea, G., and Araque, A. (2010). GLIA modulates synaptic transmission. Brain Res. Rev. 63, 93–102. doi: 10.1016/j.brainresrev.2009.10.005
Perea, G., Navarrete, M., and Araque, A. (2009). Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431. doi: 10.1016/j.tins.2009.05.001
Perez Koldenkova, V., and Nagai, T. (2013). Genetically encoded Ca(2+) indicators: properties and evaluation. Biochim. Biophys. Acta 1833, 1787–1797. doi: 10.1016/j.bbamcr.2013.01.011
Pfrieger, F. W., and Slezak, M. (2012). Genetic approaches to study glial cells in the rodent brain. Glia 60, 681–701. doi: 10.1002/glia.22283
Pham, E., Mills, E., and Truong, K. (2011). A synthetic photoactivated protein to generate local or global Ca(2+) signals. Chem. Biol. 18, 880–890. doi: 10.1016/j.chembiol.2011.04.014
Porter, J. T., and McCarthy, K. D. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 16, 5073–5081.
Porter, J. T., and McCarthy, K. D. (1997). Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 51, 439–455. doi: 10.1016/S0301-0082(96)00068-8
Potokar, M., Vardjan, N., Stenovec, M., Gabrijel, M., Trkov, S., Jorgacevski, J., et al. (2013). Astrocytic vesicle mobility in health and disease. Int. J. Mol. Sci. 14, 11238–11258. doi: 10.3390/ijms140611238
Rae, M. G., and Irving, A. J. (2004). Both mGluR1 and mGluR5 mediate Ca2+ release and inward currents in hippocampal CA1 pyramidal neurons. Neuropharmacology 46, 1057–1069. doi: 10.1016/j.neuropharm.2004.02.002
Reeves, A. M., Shigetomi, E., and Khakh, B. S. (2011). Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J. Neurosci. 31, 9353–9358. doi: 10.1523/JNEUROSCI.0127-11.2011
Regan, M. R., Huang, Y. H., Kim, Y. S., Dykes-Hoberg, M. I., Jin, L., Watkins, A. M., et al. (2007). Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J. Neurosci. 27, 6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007
Reimsnider, S., Manfredsson, F. P., Muzyczka, N., and Mandel, R. J. (2007). Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol. Ther. 15, 1504–1511. doi: 10.1038/sj.mt.6300227
Rizzoli, S. O., and Betz, W. J. (2005). Synaptic vesicle pools. Nat. Rev. Neurosci. 6, 57–69. doi: 10.1038/nrn1583
Ryu, M. H., Moskvin, O. V., Siltberg-Liberles, J., and Gomelsky, M. (2010). Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J. Biol. Chem. 285, 41501–41508. doi: 10.1074/jbc.M110.177600
Santello, M., Bezzi, P., and Volterra, A. (2011). TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. doi: 10.1016/j.neuron.2011.02.003
Santello, M., and Volterra, A. (2009). Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158, 253–259. doi: 10.1016/j.neuroscience.2008.03.039
Sasaki, T., Beppu, K., Tanaka, K. F., Fukazawa, Y., Shigemoto, R., and Matsui, K. (2012). Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. U.S.A. 109, 20720–20725. doi: 10.1073/pnas.1213458109
Schnell, C., Hagos, Y., and Hulsmann, S. (2012). Active sulforhodamine 101 uptake into hippocampal astrocytes. PLoS ONE 7:e49398. doi: 10.1371/journal.pone.0049398
Schroder-Lang, S., Schwarzel, M., Seifert, R., Strunker, T., Kateriya, S., Looser, J., et al. (2007). Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42. doi: 10.1038/nmeth975
Shigetomi, E., Jackson-Weaver, O., Huckstepp, R. T., O'Dell, T. J., and Khakh, B. S. (2013a). TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153.
Shigetomi, E., Bushong, E. A., Haustein, M. D., Tong, X., Jackson-Weaver, O., Kracun, S., et al. (2013b). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 141, 633–647. doi: 10.1085/jgp.201210949
Shigetomi, E., Kracun, S., Sofroniew, M. V., and Khakh, B. S. (2010). A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 13, 759–766. doi: 10.1038/nn.2557
Shigetomi, E., Tong, X., Kwan, K. Y., Corey, D. P., and Khakh, B. S. (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80. doi: 10.1038/nn.3000
Shoham, S. (2010). Optogenetics meets optical wavefront shaping. Nat. Methods 7, 798–799. doi: 10.1038/nmeth1010-798
Slezak, M., Grosche, A., Niemiec, A., Tanimoto, N., Pannicke, T., Münch, T. A., et al. (2012). Relevance of exocytotic glutamate release from retinal glia. Neuron 74, 504–516. doi: 10.1016/j.neuron.2012.03.027
Slezak, M., Goritz, C., Niemiec, A., Frisen, J., Chambon, P., Metzger, D., et al. (2007). Transgenic mice for conditional gene manipulation in astroglial cells. Glia 55, 1565–1576. doi: 10.1002/glia.20570
Souslova, E. A., Belousov, V. V., Lock, J. G., Stromblad, S., Kasparov, S., Bolshakov, A. P., et al. (2007). Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol. 7:37. doi: 10.1186/1472-6750-7-37
Stierl, M., Stumpf, P., Udwari, D., Gueta, R., Hagedorn, R., Losi, A., et al. (2011). Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 286, 1181–1188. doi: 10.1074/jbc.M110.185496
Szobota, S., and Isacoff, E. Y. (2010). Optical control of neuronal activity. Annu. Rev. Biophys. 39, 329–348. doi: 10.1146/annurev.biophys.093008.131400
Tanaka, K. F., Matsui, K., Sasaki, T., Sano, H., Sugio, S., Fan, K., et al. (2012). Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell Rep. 2, 397–406. doi: 10.1016/j.celrep.2012.06.011
Tanaka, M., Yamaguchi, K., Tatsukawa, T., Theis, M., Willecke, K., and Itohara, S. (2008). Connexin43 and bergmann glial gap junctions in cerebellar function. Front. Neurosci. 2, 225–233. doi: 10.3389/neuro.01.038.2008
Tian, L., Hires, S. A., Mao, T., Huber, D., Chiappe, M. E., Chalasani, S. H., et al. (2009). Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881. doi: 10.1038/nmeth.1398
Tong, X., Shigetomi, E., Looger, L. L., and Khakh, B. S. (2013). Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19, 274–291. doi: 10.1177/1073858412468794
Vaziri, A., and Emiliani, V. (2012). Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137. doi: 10.1016/j.conb.2011.11.011
Vives, V., Alonso, G., Solal, A. C., Joubert, D., and Legraverend, C. (2003). Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J. Comp. Neurol. 457, 404–419. doi: 10.1002/cne.10552
von Jonquieres, G., Mersmann, N., Klugmann, C. B., Harasta, A. E., Lutz, B., Teahan, O., et al. (2013). Glial promoter selectivity following AAV-delivery to the immature brain. PLoS ONE 8:e65646. doi: 10.1371/journal.pone.0065646
Wang, F., Smith, N. A., Xu, Q., Fujita, T., Baba, A., Matsuda, T., et al. (2012). Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26. doi: 10.1126/scisignal.2002334
Wang, X., Lou, N., Xu, Q., Tian, G. F., Peng, W. G., Han, X., et al. (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9, 816–823. doi: 10.1038/nn1703
Wess, J., Nakajima, K., and Jain, S. (2013). Novel designer receptors to probe GPCR signaling and physiology. Trends Pharmacol. Sci. 34, 385–392. doi: 10.1016/j.tips.2013.04.006
Woo, D. H., Han, K. S., Shim, J. W., Yoon, B. E., Kim, E., Bae, J. Y., et al. (2012). TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40. doi: 10.1016/j.cell.2012.09.005
Xie, Y., Wang, T., Sun, G. Y., and Ding, S. (2010). Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170, 992–1003. doi: 10.1016/j.neuroscience.2010.08.034
Yoshida, A., Yamaguchi, Y., Nonomura, K., Kawakami, K., Takahashi, Y., and Miura, M. (2010). Simultaneous expression of different transgenes in neurons and glia by combining in utero electroporation with the Tol2 transposon-mediated gene transfer system. Genes Cells 15, 501–512. doi: 10.1111/j.1365-2443.2010.01397.x
Zariwala, H. A., Borghuis, B. G., Hoogland, T. M., Madisen, L., Tian, L., De Zeeuw, C. I., et al. (2012). A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012
Zhang, H., Yang, B., Mu, X., Ahmed, S. S., Su, Q., He, R., et al. (2011). Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 19, 1440–1448. doi: 10.1038/mt.2011.98
Zhang, Q., Pangrsic, T., Kreft, M., Krzan, M., Li, N., Sul, J. Y., et al. (2004). Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 279, 12724–12733. doi: 10.1074/jbc.M312845200
Zhang, Y. P., and Oertner, T. G. (2007). Optical induction of synaptic plasticity using a light-sensitive channel. Nat. Methods 4, 139–141. doi: 10.1038/nmeth988
Zhang, Y., and Barres, B. A. (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594. doi: 10.1016/j.conb.2010.06.005
Zhang, Z., Chen, G., Zhou, W., Song, A., Xu, T., Luo, Q., et al. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953. doi: 10.1038/ncb1620
Zhao, Y., Araki, S., Wu, J., Teramoto, T., Chang, Y. F., Nakano, M., et al. (2011). An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333, 1888–1891. doi: 10.1126/science.1208592
Zorec, R., Araque, A., Carmignoto, G., Haydon, P. G., Verkhratsky, A., and Parpura, V. (2012). Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signaling route. ASN Neuro 4, 103–119. doi: 10.1042/AN20110061
Keywords: photoactivation, pharmacogenetics, optogenetics, gliotransmission, GCaMP, LiGluR, CatCh, ChR2
Citation: Li D, Agulhon C, Schmidt E, Oheim M and Ropert N (2013) New tools for investigating astrocyte-to-neuron communication. Front. Cell. Neurosci. 7:193. doi: 10.3389/fncel.2013.00193
Received: 03 July 2013; Accepted: 07 October 2013;
Published online: 29 October 2013.
Edited by:
Carole Escartin, MIRCen, FranceReviewed by:
Baljit S. Khakh, Brain Research Institute, USARichard Robitaille, Université de Montréal, Canada
Frank Kirchhoff, University of Saarland, Germany
Copyright © 2013 Li, Agulhon, Schmidt, Oheim and Ropert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole Ropert, Laboratory of Neurophysiology and New Microscopies, Université Paris Descartes, INSERM U603, CNRS UMR8154, 45 rue des Saints Pères, 75006, Paris, France e-mail: nicole.ropert@parisdescartes.fr
 Dongdong Li
Dongdong Li Cendra Agulhon
Cendra Agulhon Elke Schmidt
Elke Schmidt Martin Oheim
Martin Oheim Nicole Ropert
Nicole Ropert