5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders
- 1Department of Biomedical Sciences, University of Catania, Catania, Italy
- 2Institute of Neurological Sciences, the National Research Council of Italy (CNR), Catania, Italy
- 3Laboratory of Neurobiology, IRCCS Oasi Maria SS, Troina, Italy
Serotonin type 7 receptors (5-HT7) are expressed in several brain areas, regulate brain development, synaptic transmission and plasticity, and therefore are involved in various brain functions such as learning and memory. A number of studies suggest that 5-HT7 receptors could be potential pharmacotherapeutic target for cognitive disorders. Several abnormalities of serotonergic system have been described in patients with autism spectrum disorder (ASD), including abnormal activity of 5-HT transporter, altered blood and brain 5-HT levels, reduced 5-HT synthesis and altered expression of 5-HT receptors in the brain. A specific role for 5-HT7 receptors in ASD has not yet been demonstrated but some evidence implicates their possible involvement. We have recently shown that 5-HT7 receptor activation rescues hippocampal synaptic plasticity in a mouse model of Fragile X Syndrome, a monogenic cause of autism. Several other studies have shown that 5-HT7 receptors modulate behavioral flexibility, exploratory behavior, mood disorders and epilepsy, which include core and co-morbid symptoms of ASD. These findings further suggest an involvement of 5-HT7 receptors in ASD. Here, we review the physiological roles of 5-HT7 receptors and their implications in Fragile X Syndrome and other ASD.
Introduction
The monoamine serotonin (5-HT) is widely distributed in the central nervous system (CNS), where it functions as neurotransmitter and neuro-hormone to control mood, circadian rhythm, nociception, hormone secretion, feeding and sexual behavior (Hannon and Hoyer, 2008; Nichols and Nichols, 2008). 5-HT and its receptors are also present in peripheral tissues where they influence several functions including intestinal motility (Foxx-Orenstein et al., 1996), immune/inflammatory response (Ahern, 2011), modulation of nociception (Cervantes-Duran et al., 2013). Seven families of 5-HT receptors have been identified to date in mammals, 5-HT1 through 5-HT7, each including distinct receptor subtypes. 5-HT3 receptors are ligand-gated ion channels mediating fast depolarization (Sugita et al., 1992). All the other 5-HT receptors are G protein-coupled metabotropic receptors: 5-HT1 and 5-HT5 receptors inhibit adenylate cyclase, 5-HT4, 5-HT6 and 5-HT7 receptors instead stimulate adenylate cyclase, whereas the 5-HT2 receptor family is positively linked to phospholipase C (Hannon and Hoyer, 2008; Millan et al., 2008; Pytliak et al., 2011).
5-HT7 receptors were the last to be cloned in 1993 by different independent laboratories (Lovenberg et al., 1993; Monsma et al., 1993; Ruat et al., 1993) and their role was initially poorly understood. In the last decade, evidence has emerged that 5-HT7 receptors play an important role in the control of body temperature, sleep/wake cycle, nociception, learning and memory (Table 1). 5-HT7 receptors are also involved in mood disorders, epilepsy and pain and are starting to be considered as possible targets for these disorders.
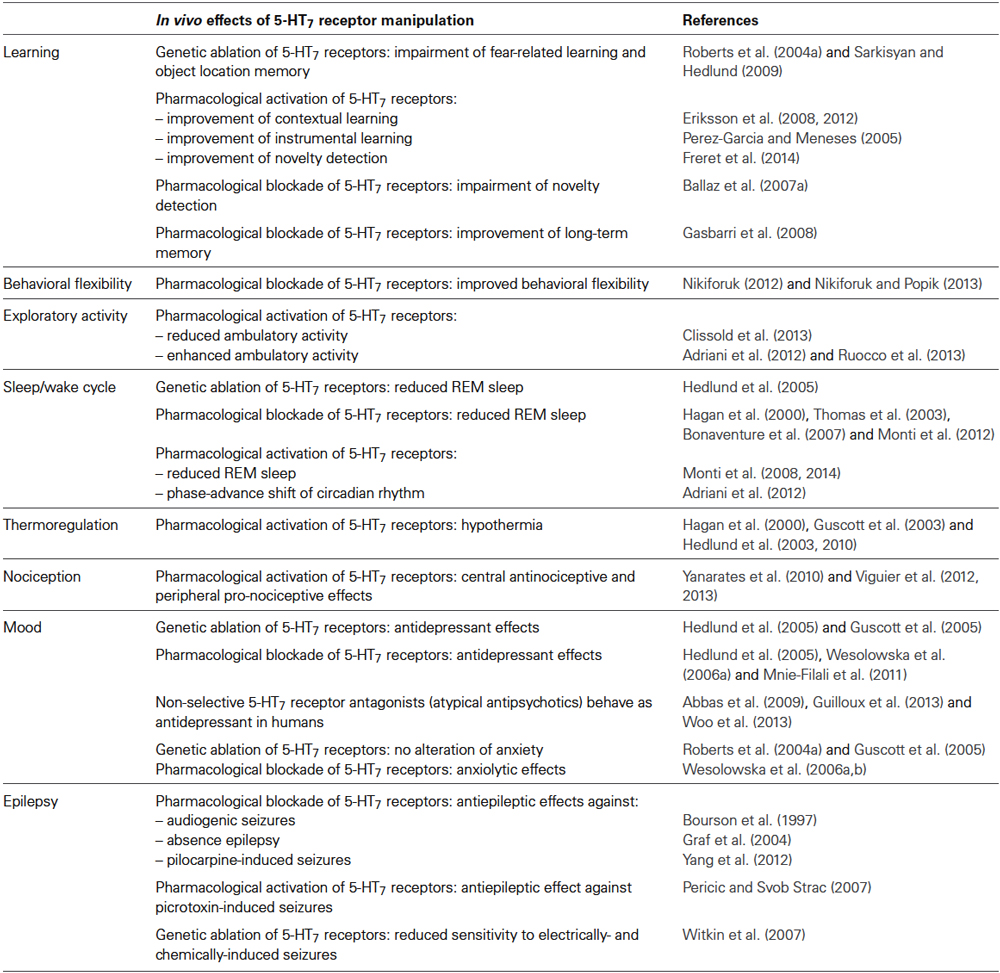
Table 1. In vivo effects of pharmacological activation, pharmacological blockade or genetic ablation of 5-HT7 receptors.
The structural, pharmacological and functional features of 5-HT7 receptors have been extensively illustrated in recent reviews (Matthys et al., 2011; Gellynck et al., 2013); thus, we will only briefly outline these receptor properties in the first part of the present work. A core part of our review is dedicated to the physiological functions regulated by 5-HT7 receptors and particularly to 5-HT7 receptor-mediated effects on cognition and mood regulation, two higher brain functions that are strictly related and mutually influence each other. Higher brain functions depend on the activity of brain neuronal networks, which are profoundly affected by changes in neuronal excitability and synaptic efficacy. For this reason, we will describe the effects of 5-HT7 receptor activation on intrinsic neuronal excitability, synaptic transmission and synaptic plasticity, and will discuss the possible functional consequences of these 5-HT7 receptor-mediated effects.
In the last part, we will review the current literature showing an impairment of the brain serotonin system in autism spectrum disorders (ASD) and its involvement in their pathophysiology. A possible malfunction of 5-HT7 receptors in ASD has not yet been investigated. In this respect, we will highlight a number of reports indicating that 5-HT7 receptors regulate many physiological functions that are altered in ASD. In addition, we will provide indication from different research groups and from our laboratories that pharmacological manipulation of 5-HT7 receptors might be considered as a therapeutic strategy in ASD.
Characterization of 5-HT7 Receptors
Localization, Structure and Pharmacological Profile
5-HT7 receptors are highly expressed in the thalamus, hypothalamus and hippocampus of mice and rats; significant amounts were detected in cerebral cortex, amygdala, striatum, cerebellum and spinal cord (Belenky and Pickard, 2001; Neumaier et al., 2001; Bickmeyer et al., 2002; Geurts et al., 2002; Muneoka and Takigawa, 2003; Doly et al., 2005; reviewed by Hedlund and Sutcliffe, 2004).
In the human brain, 5-HT7 receptor mRNA was detected in many CNS areas, with highest expression levels in thalamus, hypothalamus, amygdala and hippocampus (Hagan et al., 2000). Autoradiographic studies confirmed that 5-HT7 receptor levels in human brain are in good correlation with those found in rodents, with high density in thalamus, dorsal raphe, hippocampus (Martin-Cora and Pazos, 2004; Varnäs et al., 2004) and hypothalamus (Varnäs et al., 2004). Unlike in rodents, in the human brain 5-HT7 receptor binding sites were also found at high levels in caudate nucleus, putamen and substantia nigra (Martin-Cora and Pazos, 2004).
In the rodent brain, the immunoreactivity for 5-HT7 receptors was high in several regions at birth and then decreased progressively during postnatal development (Muneoka and Takigawa, 2003; García-Alcocer et al., 2006; Kobe et al., 2012). However, in spite of the reported age-related reduction in their brain expression levels, 5-HT7 receptors exert important functions also in the adult (see Section Physiological Functions Regulated by 5-HT7 Receptors). This conclusion is supported by several data: first of all, 5-HT7 receptors have been detected in the brain of adult animals (Neumaier et al., 2001; Geurts et al., 2002; Muneoka and Takigawa, 2003). In the human brain, the presence of 5-HT7 receptors was confirmed by post-mortem studies on adult subjects (age range 22–73 years; Martin-Cora and Pazos, 2004; Varnäs et al., 2004). Moreover, systemic administration of 5-HT7 receptor agonists affected body temperature (Naumenko et al., 2011) and circadian rhythms (Adriani et al., 2012; Monti et al., 2014; Romano et al., 2014) in adult animals and improved learning in young adults (Eriksson et al., 2008; Freret et al., 2014). All these data indicate that 5-HT7 receptor-mediated effects are still present at adult age and exert an important control on various physiological functions.
5-HT7 receptors display the typical structure of G protein-coupled receptors (GPCRs) with seven transmembrane domains and were initially characterized for their ability to stimulate adenylate cyclase (Bard et al., 1993; Lovenberg et al., 1993; Ruat et al., 1993; Shen et al., 1993).
Among all 5-HT receptor subtypes, 5-HT7 receptors display the highest affinity (in the low nanomolar range) for the natural agonist serotonin (Ruat et al., 1993). Other high affinity agonists for 5-HT7 receptors are 5-carboxamidotryptamine (5-CT) and 8-hydroxy-N,N-dipropyl-aminotetralin (8-OH-DPAT), both also showing high affinity for 5-HT1A receptors (Bard et al., 1993; Lovenberg et al., 1993; Ruat et al., 1993). Selective agonists for 5-HT7 receptors were lacking until recently (Di Pilato et al., 2014). The first compound described as a 5-HT7 receptor agonist was AS-19, a partial agonist with high affinity but moderate selectivity for 5-HT7 receptors (Brenchat et al., 2009). The most selective 5-HT7 receptor agonists described to date are the compounds E-55888 and E-57431 (not commercially available), produced by Esteve pharmaceutical company (Brenchat et al., 2009, 2012). Another high-affinity and selective 5-HT7 receptor agonist is LP-211 (indicated as compound 25 in the first publication Leopoldo et al., 2008), that shows excellent brain permeation properties (Hedlund et al., 2010). In vivo administration of LP-211 induced hypothermia in wild-type but not in 5-HT7 KO mice (Hedlund et al., 2010) and shifted the sleep-wake cycle (Adriani et al., 2012), two typical 5-HT7 receptor-mediated effects. Other analogs of LP-211 with improved selectivity and pharmacokinetic properties have been synthesized (Leopoldo, personal communication) and are currently under investigation in our laboratories as possible novel 5-HT7 receptor agonists.
Concerning antagonists, several antidepressant and antipsychotic drugs showed high affinity for 5-HT7 receptors and behaved as antagonists on a cyclic adenosine monophosphate (cAMP) formation assay (Shen et al., 1993; Roth et al., 1994; see Section Mood Disorders). Selective and high-affinity antagonists of 5-HT7 receptors are also available, among which the compound SB-269970 is to date considered the most reliable (Hagan et al., 2000; Guscott et al., 2003; Hedlund et al., 2003).
Coupling to Intracellular Transduction Mechanisms
5-HT7 receptors display interesting transduction properties, being able to couple to Gs and G12 GTP-binding proteins, which activate divergent signaling pathways (reviewed by Woehler and Ponimaskin, 2009; Matthys et al., 2011; Gellynck et al., 2013).
Since their discovery, 5-HT7 receptors were found to be coupled to Gs and induce adenylate cyclase activation, cAMP formation and activation of protein kinase A (PKA; Bard et al., 1993; Lovenberg et al., 1993; Ruat et al., 1993).
Downstream to cAMP formation, 5-HT7 receptors can activate the extracellular signal-regulated kinase (ERK), as shown in transfected cells expressing 5-HT7 receptors (Lin et al., 2003; Norum et al., 2003) and in native rat hippocampal neurons (Errico et al., 2001; Lin et al., 2003). 5-HT7 receptor-induced activation of ERK was mediated either by PKA (Norum et al., 2003) or by a cAMP-dependent, PKA-independent pathway involving exchange proteins directly activated by cAMP (Epacs) (Lin et al., 2003). In line with this result, cAMP-dependent but PKA-independent 5-HT7 receptor-mediated effects have been described (Chapin and Andrade, 2001; Bonsi et al., 2007).
5-HT7 receptors can activate additional intracellular biochemical cascades, among which the kinase Akt (also known as protein kinase B; Hoffman and Mitchell, 2011; Johnson-Farley et al., 2005).
As mentioned above, 5-HT7 receptors can also couple to G12 (Kvachnina et al., 2005), a heterotrimeric G protein that modulates the activity of “small” monomeric GTPases (Hall, 1998), such as members of the Rho family Rho, Rac and Cdc42. Through the G12-dependent activation of RhoA and Cdc42 5-HT7 receptor activation regulates gene transcription and neuronal morphology (Kvachnina et al., 2005).
The mechanisms regulating 5-HT7 receptor coupling to Gs or G12 are not clear. Recent evidence suggests that agonist-induced dynamic palmitoylation of 5-HT7 receptors affects its Gs-mediated constitutive activity with no effect on G12-mediated activity (Kvachnina et al., 2009; Gorinski and Ponimaskin, 2013). This result implies that pathways inducing palmitoylation of 5-HT7 receptors might modify their constitutive activity and switch their intracellular coupling, ultimately changing their final effect.
Another interesting finding is that the expression of Gs remains constant during development, whereas the expression level of G12 is higher at early post natal age and parallels the expression level of 5-HT7 receptors; consistently, 5-HT7 receptor-mediated effects on synapse formation and function were observed in the hippocampus of juvenile but not adult mice, indicating a crucial role of the 5-HT7/G12 pathway in the development of brain synaptic circuitry (Kobe et al., 2012).
A particular feature of 5-HT7 receptors, similar to other GPCRs, is the ability to form receptor complexes, either homo- or heterodimers, in which monomers reciprocally modulate receptor trafficking, ligand binding affinity and coupling to intracellular signaling cascades (Renner et al., 2012; Teitler and Klein, 2012). For example, it has been suggested that a 5-HT1A/5-HT7 heterodimer interaction plays a modulatory role in the control of body temperature (Matthys et al., 2011). This is a further element of complexity in 5-HT receptor-mediated signal transduction mechanisms.
5-HT7 Receptors Regulate Synapse Development
5-HT plays a crucial role in shaping brain structure and circuits during development through modulation of neural cell proliferation, migration and differentiation, as well as neurite outgrowth, axonal guidance and synaptogenesis (Gaspar et al., 2003). Accordingly, early changes in 5-HT brain levels during development affect the functional organization of brain networks and may underlie the pathogenesis of neurodevelopmental disorders including autism (reviewed by Lesch and Waider, 2012). While the role of some 5-HT receptors during brain development has been ascertained (Sodhi and Sanders-Bush, 2004), the involvement of 5-HT7 receptors has only recently emerged. As mentioned above, the group of Ponimaskin demonstrated that in mouse hippocampal neurons 5-HT7 receptor activation stimulated the small GTP-ases RhoA and Cdc42 and enhanced neurite elongation, dendritic spine density, the number of synaptic contacts and the amount of AMPA receptors expressed at synapses, leading to increased synaptic efficacy (Kvachnina et al., 2005; Kobe et al., 2012). In line with these results, it was recently shown that the 5-HT7 receptor agonists 8-OH-DPAT and LP-211 stimulated neurite outgrowth in primary cultures of mouse and rat striatal and cortical neurons by activation of the cyclin-dependent protein kinase 5 (Cdk5), a kinase playing an important role in microtubule assembly and cytoarchitecture rearrangements (Speranza et al., 2013).
Modulation of Neuronal Excitability by 5-HT7 Receptors
5-HT7 Receptor Activation Exerts Depolarizing Effects
Brain 5-HT7 receptors modulate neuronal networks by playing a crucial role in brain wiring during development. However, as mentioned before, 5-HT7 receptor-mediated effects are not restricted to a defined developmental window, as a large number of studies show that 5-HT7 receptors modulate neuronal excitability, synaptic transmission and synaptic plasticity both during development and adult life. 5-HT7 receptors control neuronal intrinsic excitability by modulating non-synaptic membrane ion currents which directly affect neuronal firing. 5-HT7 receptor agonists exerted slow depolarizing effects and increased neuronal firing in several brain areas (Figure 1), among which the anterodorsal nucleus of the rat thalamus (Chapin and Andrade, 2001), rat globus pallidus (Chen et al., 2008), mouse hippocampal CA1 region (Bickmeyer et al., 2002) and mouse trigeminal nucleus caudalis (Yang et al., 2014). Activation of 5-HT7 receptors enhanced the firing rate of rat CA1 pyramidal neurons (Tokarski et al., 2003), CA3 pyramidal neurons (Gill et al., 2002), striatal cholinergic interneurons (Bonsi et al., 2007), prefrontal cortex pyramidal neurons (Zhang, 2003; Béïque et al., 2004a) and nucleus accumbens (NAc) neurons (Ishihara et al., 2013). In a spinal cord slice preparation, 5-HT application induced a locomotor-like rhythmic firing activity of motoneurons in wild type but not in 5-HT7 KO mice, indicating that the excitability of spinal motoneurons was enhanced by 5-HT7 receptors (Liu et al., 2009).
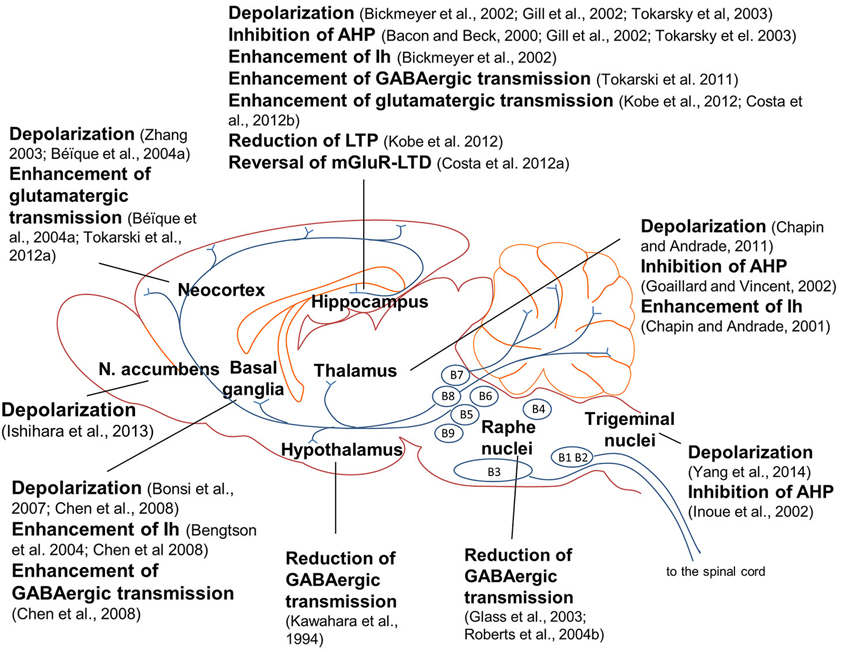
Figure 1. Effects of 5-HT7 receptor activation on neuronal excitability, synaptic transmission and synaptic plasticity in the rodent brain. Activation of 5-HT7 receptors induced depolarization in several brain regions; in many cases, depolarization was mediated by inhibition of a post-spike afterhyperpolarization (AHP) and/or enhancement of a hyperpolatization-activated cation current (Ih; see explanations in the text). Activation of 5-HT7 receptors modulated glutamate-mediated synaptic transmission and plasticity in frontal cortex and hippocampus. 5-HT7 receptors differently modulated GABAergic synaptic transmission in distinct brain areas; notably, activation of 5-HT7 receptors in raphe nuclei inhibited GABAergic interneurons, enhancing the activity of serotonergic raphe neurons and 5-HT release in target structures. Most of the results illustrated here were obtained in the rat, with the following exceptions: mouse (Bickmeyer et al., 2002; Costa et al., 2012a; Yang et al., 2014), hamster (Glass et al., 2003) and guinea pig (Roberts et al., 2004b).
In many of the studies above cited, 5-HT7 receptor-induced depolarization was mediated by inhibition of a post-spike AHP or by enhancement of a hyperpolarization-activated inward cation current (Ih).
5-HT7 Receptor Activation Inhibits Post-Spike Afterhyperpolarization (AHP)
Action potentials are followed by a negative shift in membrane potential named afterhyperpolarization (AHP), which is mediated by a Ca2+-dependent K+ current triggered by Ca2+ influx. The subsequent efflux of K+ ions hyperpolarizes the membrane during a few seconds reducing neuronal firing rate, a phenomenon called “spike frequency adaptation” (Sah, 1996). Activation of 5-HT7 receptors inhibited a slow AHP (sAHP) in thalamic neurons (Goaillard and Vincent, 2002), in CA3 pyramidal neurons (Bacon and Beck, 2000; Gill et al., 2002) and in CA1 pyramidal neurons (Tokarski et al., 2003; Figure 1). Likewise, in rat trigeminal motoneurones 5-HT7 receptor activation reduced a Ca2+-dependent K+ current responsible for a medium-duration afterhyperpolarization (mAHP), another type of AHP with a time-course faster than the sAHP and with similar inhibitory effects on neuronal excitability (Inoue et al., 2002). Modulation of the AHP by 5-HT7 receptors was mediated by activation of the cAMP/PKA pathway (Goaillard and Vincent, 2002; Inoue et al., 2002).
Modulation of the AHP has profound effects on neuronal firing. By inhibiting post-spike mAHP and sAHP, 5-HT7 receptor activation enhances neuronal firing rate and reduces spike frequency adaptation. In addition, 5-HT7 receptor-mediated modulation of sAHP is likely to involve broader functional consequences, as intrinsic excitability and synaptic inputs mutually influence each other. In fact, Ca2+ influx through N-methyl-D-aspartate (NMDA) channels can also activate the sAHP (Lancaster et al., 2001), which implies that synaptic activation also modulates the AHP. Changes in AHP amplitude have been observed following activation of NMDA and kainate receptors (Cherubini et al., 1990) and group I metabotropic glutamate receptors (mGluRs; Ireland and Abraham, 2002).
The AHP in turn reduces NMDA-mediated synaptic transmission: it has been proposed that activation of AHP, by hyperpolarizing the membrane, would favor Mg2+ blockade of NMDA channels and reduce NMDA-mediated transmission and plasticity (Wu et al., 2004; Fernández de Sevilla et al., 2007). Vice-versa, conditions reducing the AHP lead to depolarization and removal of Mg2+ blockade, thus are likely to enhance NMDA-mediated long term plasticity and learning. Consistent with this hypothesis, it was shown that the amplitude of AHP in hippocampal neurons was decreased after learning (Disterhoft et al., 1996; Moyer et al., 1996; Oh et al., 2009) and age-related learning deficits are accompanied by increased AHP (Disterhoft et al., 1996; Tombaugh et al., 2005).
Since 5-HT7 receptor activation is able to inhibit post-spike AHP in many brain regions, as described above, the subsequent depolarization might favor NMDA receptor-mediated synaptic plasticity and ultimately enhance learning. As a matter of fact, several studies indicate that 5-HT7 receptor activation exerts pro-cognitive effects (see Section Learning and Memory).
5-HT7 Receptor Activation Enhances a Hyperpolarization-Activated Cation Current (Ih)
Another non-synaptic ion current modulated by 5-HT7 receptors is a hyperpolarization-activated cation current (Ih). Ih is carried by Na+ and K+ ions and is activated by hyperpolarization beyond −60 mV, thus is already active at resting membrane potential. The voltage-dependence of Ih activation is modulated by cAMP, with increases in cAMP levels facilitating Ih activation (Chen et al., 2001). The physiological consequences of Ih activation are very complex, as it differently affects membrane excitability and synaptic responsiveness. Concerning membrane excitability, Ih shifts the value of resting membrane potential towards depolarization. With respect to synaptic responsiveness, Ih instead exerts a membrane-shunting effect that reduces the amplitude and duration of excitatory post-synaptic potentials. Thus, an enhancement of Ih, although producing a depolarizing shift in membrane potential, ultimately decreases synaptic responsiveness, as it reduces neuronal ability to elicit action potentials in response to synaptic inputs. Ih also reduces the temporal summation of synaptic signals. Notably, the channels responsible for Ih (hyperpolarization-activated cyclic nucleotide-gated non-specific cation channels, HCN) are maximally expressed in distal dendrites, where integration of synaptic signals mostly occurs (Magee, 1998, 1999); reviewed by Mozzachiodi and Byrne (2010).
It was shown that 5-HT7 receptor activation enhanced Ih in dorsal root ganglion neurons (Cardenas et al., 1999), in mouse hippocampus (Bickmeyer et al., 2002), in the anterodorsal thalamic nucleus (Chapin and Andrade, 2001) and in rat globus pallidus (Bengtson et al., 2004; Chen et al., 2008; Figure 1). In most cases, 5-HT7 receptor-mediated enhancement of Ih was mediated by an increase in cAMP levels (Cardenas et al., 1999; Chapin and Andrade, 2001; Bickmeyer et al., 2002; Bengtson et al., 2004).
Neurotransmitter-mediated modulation of Ih has important functional consequences on neuronal rhythmic firing (McCormick and Pape, 1990). Ih is also suggested to be involved in epilepsy, since either an increase (Surges et al., 2012) or a decrease (Jung et al., 2007, 2011) of Ih have been reported in different types of epilepsy.
Changes in Ih also occur during long-term synaptic plasticity (reviewed by Mozzachiodi and Byrne, 2010). Ih was increased during NMDA-mediated long term potentiation (LTP; Fan et al., 2005) and decreased after the induction of metabotropic glutamate receptor-mediated long term depression (mGluR-LTD; Brager and Johnston, 2007). Interestingly, it was recently shown that hippocampal Ih is altered in Fmr1KO mice, the mouse model of Fragile X syndrome, in which mGluR-mediated signaling is deregulated. In Fmr1 KO mice, Ih is abnormally enhanced in the apical dendrites but not in the soma of CA1 pyramidal neurons and NMDA-mediated modulation of Ih is disrupted (Brager et al., 2012; Brager and Johnston, 2014). The authors suggested that the subsequent reduction in dendritic synaptic integration together with enhanced intrinsic excitability may participate to epilepsy, a typical feature of Fmr1KO mice as well as of Fragile X patients.
In summary, modulation of Ih by 5-HT7 receptors is likely to have important consequences on neuronal firing and synaptic responsiveness and might account for the effects exerted by 5-HT7 receptor activation on synaptic transmission and on epilepsy (see below).
Modulatory Role of 5-HT7 Receptors on Synaptic Transmission and Plasticity
Effects of 5-HT7 Receptor Activation on GABAergic Synaptic Transmission
5-HT7 receptors differently modulate the activity of GABAergic inhibitory interneurons in distinct brain areas (Figure 1). An early study showed that 5-HT7 receptor activation reduced GABAA receptor-activated current in cultured rat SCN neurons acting through a post-synaptic cAMP-mediated mechanism (Kawahara et al., 1994). Also in raphe nuclei 5-HT7 receptor activation reduced GABAergic transmission. In raphe nuclei, GABAergic interneurons exert a negative control on the activity of serotonergic neurons, inhibiting 5-HT release from raphe efferent fibers. When a 5-HT7 receptor antagonist was applied in raphe nuclei, 5-HT efflux onto target structures was reduced (Glass et al., 2003; Roberts et al., 2004b). Thus, activation of 5-HT7 receptors in raphe nuclei reduces GABA-mediated inhibition of raphe serotonergic neurons and consequently enhances 5-HT release in target structures.
In the hippocampus, 5-HT7 receptor activation was instead shown to stimulate the activity of GABAergic interneurons. Application of a 5-HT7 receptor agonist enhanced the frequency of GABA-mediated spontaneous inhibitory post-synaptic currents (sIPSCs) recorded from rat CA1 pyramidal neurons, indicating an increased GABA release from interneurons (Tokarski et al., 2011). The authors suggest that 5-HT7 receptors exerted two effects, both at a pre-synaptic level: an enhancement of glutamate release from fibers targeting GABAergic interneurons and an increase of GABA release from interneuron terminals. In the CA1 region, GABAergic interneurons represent a system of feed-forward and feedback inhibition of pyramidal neurons, being activated by afferent fibers (among which the raphe-hippocampal serotonergic pathway), by Schaffer collaterals as well as by recurrent collaterals from pyramidal neurons (Freund and Buzsaki, 1996). Therefore, 5-HT7 receptors exert a very complex modulation of hippocampal circuits, as they directly depolarize pyramidal neurons (Tokarski et al., 2003) and simultaneously regulate their firing by enhancing GABAergic inhibitory control.
5-HT7 receptor activation enhanced GABAergic transmission also in rat globus pallidus (Chen et al., 2008). To summarize, in the hippocampus and globus pallidus 5-HT7 receptor activation stimulates GABA release from interneurons, differing from results observed in suprachiasmatic nucleus (SCN) and dorsal raphe, where 5-HT7 receptor activation instead reduces GABAergic transmission.
Effects of 5-HT7 Receptor Activation on Glutamate-Mediated Synaptic Transmission and Plasticity
Two different reports indicate that 5-HT7 receptor activation can stimulate glutamate release from glutamatergic terminals. In rat frontal cortex, glutamate-mediated synaptic transmission was enhanced by activation of 5-HT7 receptors (Béïque et al., 2004b) and decreased by the 5-HT7 receptor antagonist SB-269970 (Tokarski et al., 2012a; Figure 1). In both studies, 5-HT7 receptor-mediated effect was exerted at a pre-synaptic level on glutamatergic terminals.
In the hippocampus, 5-HT7 receptors modulate glutamate-mediated transmission acting at a post-synaptic level. Field recordings of excitatory post-synaptic potentials (EPSPs) from rat hippocampal slices have shown that serotonin inhibits the perforant path input to the CA1 region, an effect partially mediated by 5-HT7 receptors and exerted at a post-synaptic level (Otmakhova et al., 2005). The authors suggest that 5-HT7 receptor-mediated reduction of EPSP amplitude might be due to an enhancement of the hyperpolarization-activated current Ih, similar to what observed in several brain regions (see above).
The other main input to CA1 pyramidal neurons is represented by Schaffer collaterals from CA3 pyramidal neurons. We have studied the effects of serotonin on glutamatergic transmission in the CA3-CA1 synapse in mouse and rat hippocampal slices and showed that activation of post-synaptic 5-HT7 receptors enhances the amplitude of the AMPA receptor-mediated component of the excitatory post synaptic current (EPSCAMPA), which is responsible for basal glutamatergic transmission (Costa et al., 2012b). In the same study, we observed instead that 5-HT1A receptors inhibit AMPA-mediated CA3-CA1 synaptic transmission. These data provide a physiological substrate to the previous observation that 5-HT7 receptor activation exerts a pro-cognitive action and is able to counteract 5-HT1A-mediated impairment of learning (Eriksson et al., 2008).
In the last few years, evidence has emerged that 5-HT7 receptors can also modulate long-term synaptic plasticity (Figure 1). As already mentioned, in mice hippocampal neurons activation of 5-HT7 receptors enhanced basal synaptic transmission by increasing the number of AMPA receptors at synapses but also modulates glutamate-mediated long-term synaptic plasticity, reducing the amount of LTP in the CA3-CA1 synapse (Kobe et al., 2012). The authors suggested that 5-HT7 receptor-mediated enhancement of basal glutamatergic transmission might have prevented further potentiation, thus reducing LTP (Kobe et al., 2012). In contrast with this report, mice lacking 5-HT7 receptors (5-HT7 KO) display decreased LTP in the CA3-CA1 hippocampal synapse (Roberts et al., 2004a), suggesting that 5-HT7 receptors are necessary for LTP. LTP is induced by activation of NMDA receptors and 5-HT7 receptors were shown to exert different short- and long-term effects on NMDA-mediated currents in hippocampal neurons. In particular, acute activation of 5-HT7 receptors enhanced the amplitude of NMDA-mediated currents, whereas a long-term activation of 5-HT7 receptors reduced the cell membrane expression of NMDA receptors through an indirect mechanism involving platelet-derived growth factor (PDGF), an endogenous neurotrophin that protects against NMDA-induced excitotoxicity (Vasefi et al., 2013). This dual action of 5-HT7 receptors, namely a short-term enhancement and a long-term inhibition of NMDA receptors, might also explain different 5-HT7 receptor-mediated effects on LTP.
Another form of long-term depression, mGluR-LTD, is induced by activation of group I mGluRs and is mainly expressed through removal of AMPA receptors from synaptic membrane surface by endocytosis (Luscher and Huber, 2010). We have shown that 5-HT7 receptor activation prevented mGluR-induced endocytosis of AMPA receptors and reversed mGluR-LTD in the CA3-CA1 synapse in mouse hippocampal slices (Costa et al., 2012a). Interestingly, we found that 5-HT7 receptor activation reversed mGluR-mediated endocytosis of AMPA receptors and mGluR-LTD also in Fmr1 KO mice, a mouse model of Fragile X Syndrome, the most common form of inherited intellectual disability associated with epilepsy and autism. Our result that 5-HT7 receptor activation can correct abnormal mGluR-mediated synaptic plasticity in the mouse model of Fragile X Syndrome might suggest new strategies for the therapy of this disorder (see below).
Physiological Functions Regulated by 5-HT7 Receptors
5-HT7 receptors control several body functions including the sleep-wake cycle, body temperature and nociception, and strongly influence mood and learning, two higher brain functions that are strictly related (Table 1). Most of the functions regulated by 5-HT7 receptors have been studied using the 5-HT7 receptor knock-out (5-HT7 KO) mice that were generated and characterized by the research group of Dr. Hedlund (Roberts et al., 2004a; Sarkisyan and Hedlund, 2009) and later in other laboratories (Guscott et al., 2005; Witkin et al., 2007).
Sleep/Wake Cycle
Consistent with their abundant expression in the hypothalamus (see Section Localization, Structure and Pharmacological Profile), 5-HT7 receptors play an important role in the regulation of circadian rhythm and sleep (Monti and Jantos, 2014). In the SCN, which is the main regulator of circadian rhythm, 5-HT7 receptors are abundantly expressed (Belenky and Pickard, 2001) and regulate glutamatergic input from the retina (Smith et al., 2001) and serotonergic input from raphe nuclei (Glass et al., 2003).
The role of 5-HT7 receptors in sleep has emerged from studies using both pharmacological and genetic approaches. 5-HT7 KO mice displayed a reduced duration of REM sleep, whereas time spent during wake or non-REM sleep was not different from wild-type mice (Hedlund et al., 2005). Consistently, in vivo administration of a 5-HT7 receptor antagonist to wild-type animals reduced the duration of REM sleep (Hagan et al., 2000; Thomas et al., 2003; Bonaventure et al., 2007; Monti et al., 2012). However, in contradiction with these results, systemic administration of a 5-HT7 receptor agonist also enhanced the wake period and reduced the duration of REM sleep (Monti et al., 2008, 2014), together with inducing a phase-shift in the sleep-wake cycle (Adriani et al., 2012). As a possible explanation, it has been proposes that 5-HT7 receptors do not behave according to the classical model of two-state on-off (activation/blockade) ligand-receptor interaction (Monti and Jantos, 2014). In addition, 5-HT7 receptors differently modulate distinct brain areas (dorsal raphe, locus coeruleus, basal forebrain) involved in sleep control (Monti et al., 2012; Monti and Jantos, 2014) and their precise role in each area should be further investigated.
Body Temperature
5-HT7 receptors exert a well-established role in thermoregulation (for extensive review on this topic, see Hedlund and Sutcliffe, 2004; Matthys et al., 2011). Administration of 5-HT7 receptor agonists induced hypothermia in wild type guinea pigs and mice (Hagan et al., 2000; Guscott et al., 2003; Hedlund et al., 2003, 2010) but not in 5-HT7 KO mice (Guscott et al., 2003; Hedlund et al., 2003).
Two other 5-HT receptors are involved on thermoregulation, namely 5-HT1A and 5-HT3 receptors, both also inducing hypothermic effects (Hedlund et al., 2004; Naumenko et al., 2011). It has been proposed that 5-HT7 receptors, possessing the highest affinity for 5-HT, are activated by low doses of 5-HT and are responsible for the fine-tuning of body temperature. On the other hand, higher agonist concentrations activate 5-HT1A receptors, which might play a role as a defense against hyperthermia (Hedlund et al., 2004).
Nociception
Immunohistochemical studies provide evidence for localization of 5-HT7 receptors in the superficial laminae of the dorsal horn and in small and medium sized dorsal root ganglion cells, which is consistent with a role of 5-HT7 receptors in nociception (Doly et al., 2005).
The role of 5-HT7 receptors in the control of pain transmission is multifaceted and has been described in details in recent reviews (Matthys et al., 2011; Viguier et al., 2013). Overall, 5-HT7 receptor agonists induce pro-nociceptive effects on peripheral 5-HT7 receptors and antinociceptive effects on central 5-HT7 receptors (Yanarates et al., 2010; Viguier et al., 2012), with a different outcome depending on preexisting conditions (health vs. neuropathic pain; Viguier et al., 2013).
Learning and Memory
In the last decade, an important role of 5-HT7 receptors on learning and memory has emerged (Roberts and Hedlund, 2012; Meneses, 2013). As pointed out in the previous paragraphs, 5-HT7 receptors are expressed at high levels in the hippocampus, one of the brain regions most crucially involved in learning, and modulate hippocampal synaptic transmission and plasticity. Behavioral studies performed on mice lacking 5-HT7 receptors (Roberts et al., 2004a; Sarkisyan and Hedlund, 2009) and on wild-type animals (Manuel-Apolinar and Meneses, 2004; Perez-Garcia and Meneses, 2005; Eriksson et al., 2008, 2012) show that 5-HT7 receptor activation exerts a pro-cognitive action on different types of memory.
5-HT7 KO mice showed no memory impairment in operant food conditioning tests (involving a hippocampus-independent type of memory) and Barnes maze (involving hippocampus-dependent spatial learning) but displayed a memory deficit in the fear conditioning test, which involves hippocampus-dependent contextual learning with emotional aspects. These results indicate that the lack of 5-HT7 receptors did not affect hippocampus-independent memory and among different types of hippocampus-dependent learning, only one with a strong emotional component (fear conditioning) was selectively impaired in 5-HT7 KO mice. The specific contextual learning impairment of 5-HT7 KO mice was accompanied by a decrease of LTP in the CA1 region of the hippocampus (Roberts et al., 2004a).
Further studies from the same laboratory indicated that 5-HT7 KO mice displayed normal recognition of novel objects (Sarkisyan and Hedlund, 2009), a type of visual episodic memory that depends on brain cortex and is believed to correspond to human declarative episodic memory. 5-HT7 KO mice instead showed a selective impairment in the recognition of a novel location, particularly in allocentric spatial memory (a hippocampus-dependent type of memory concerning the location of objects independent from the observer), without any defect in egocentric memory (a striatum-dependent type of memory concerning the position of objects with respect to the observer). The same result was observed in wild-type mice following pharmacological blockade of 5-HT7 receptors (Sarkisyan and Hedlund, 2009).
Behavioral studies on wild-type animals confirmed that activation of 5-HT7 receptors exerts a pro-cognitive effect on hippocampus-dependent contextual learning and revealed that other types of memory are also modulated by 5-HT7 receptors. In mice submitted to the passive avoidance test (involving contextual learning), in vivo activation of 5-HT7 receptors was able to exert pro-cognitive effects and counteract a learning impairment induced by 5-HT1A receptor activation (Eriksson et al., 2008, 2012).
5-HT7 receptor stimulation enhanced memory formation in adult rats trained to a learning task involving both conditioning (Pavlonian) and instrumental learning modes: in this experimental protocol, a food reward was delivered with a short delay following a light signal (conditioned learning) but was delivered immediately if the rat pressed a lever (instrumental learning). When the 5-HT7 receptor agonist AS-19 was injected subcutaneously to animals immediately after training, the latency of responses was reduced, indicating enhanced memory performance. AS-19 administration was also able to reverse a memory impairment induced by scopolamine (an anticholinergic agent) or dizocilpine (an NMDA antagonist), suggesting that 5-HT7 receptor activation might exert a pro-cognitive effect by modulating cholinergic and glutamatergic transmission (Perez-Garcia and Meneses, 2005).
Concerning hippocampus-independent memory, different from results on 5-HT7 KO mice (Sarkisyan and Hedlund, 2009), in wild-type mice novel object recognition was enhanced by administration of the 5-HT7 receptor agonist 5-CT and impaired by administration of the 5-HT7 receptor antagonist SB-269970 (Freret et al., 2014), consistent with previous data showing that administration of SB-269970 impaired novel object recognition in wild-type rats (Ballaz et al., 2007a). These data show that 5-HT7 receptor activation exert a pro-cognitive effect also on cortex-dependent memory corresponding to human episodic memory.
To summarize, studies on wild-type animals using 5-HT7 receptor agonists and antagonists have shown that 5-HT7 receptor activation enhances many different types of memory, including some (operant conditioning, novel object recognition) that were not impaired in 5-HT7 KO mice.
5-HT7 receptor-mediated pro-cognitive effects were observed in behavioral tests performed on adult animals, reinforcing the conclusion that 5-HT7 receptors are functional in the adult hippocampus and exert life-long effects on learning and memory.
In one study, blockade rather than activation of 5-HT7 receptors exerted a pro-cognitive effect: in vivo administration of the 5-HT7 receptor antagonist SB-269970 to rats submitted to the radial arm maze enhanced long-term reference memory without affecting short-term working memory (Gasbarri et al., 2008).
Heterogeneous results are not surprising considering that different models have been used (either rats or mice; wild-type or 5-HT7 KO animals) and different types of memory were tested, involving different brain areas and distinct circuits. Furthermore, considering that learning and memory are strictly related to stress, 5-HT7 receptor-mediated effects on learning might also be influenced by 5-HT7 receptor-mediated effects on mood (see below).
Involvement of 5-HT7 Receptors in Pathology
Mood Disorders
Since the first pharmacological characterization of 5-HT7 receptors, it was evident that several antipsychotics and antidepressants bind these receptors with high affinity (Monsma et al., 1993; Roth et al., 1994). In line with these early results, several studies have later shown that 5-HT7 receptors play a role in mood regulation and thus are potential targets for the therapy of anxiety and depression (Hedlund, 2009; Sarkisyan et al., 2010).
Mice lacking 5-HT7 receptors displayed reduced levels of depression when submitted to forced swimming and tail suspension tests (Guscott et al., 2005; Hedlund et al., 2005). Consistently, pharmacological blockade of 5-HT7 receptors exerted antidepressant effects in wild-type mice and rats submitted to the same stress protocols (Hedlund et al., 2005; Wesolowska et al., 2006a; Mnie-Filali et al., 2011). In addition, 5-HT7 receptor blockade was found to potentiate the effect of other antidepressant drugs: synergistic antidepressant effects were observed when a low and ineffective dose of SB-269970 was administered in combination with a low and ineffective dose of citalopram, a selective serotonin reuptake inhibitor (SSRI; Sarkisyan et al., 2010). In the same study, a synergistic interaction, although to a lesser extent, was also observed between SB-269970 and reuptake inhibitors of norepinephrine (but not dopamine).
With respect to anxiety, 5-HT7 KO mice did not show any alteration in anxiety-related behavior, evaluated by light-dark transfer test (Roberts et al., 2004a) and elevated plus maze (Guscott et al., 2005). On the other side, in wild-type rodents tested in different anxiety protocols (Vogel conflict drinking test; elevated plus maze; four-plate test; open field), administration of the 5-HT7 receptor antagonist SB-269970 induced anxiolytic effects (Wesolowska et al., 2006a,b).
Interestingly, anxiolytic and antidepressant effects were observed also when SB-269970 was administered by local intra-hippocampal injection, indicating the involvement of 5-HT7 receptors located in the hippocampus (Wesolowska et al., 2006a).
It was recently found that second-generation antipsychotic drugs, named atypical antipsychotics, also behave as antagonists of 5-HT7 receptors. Among these substances, the D2/D3 receptor antagonist amisulpride is a high-affinity competitive antagonist of 5-HT7 receptors. Interestingly, the antidepressant effect of amisulpride was absent in 5-HT7 KO mice, indicating that it was mediated exclusively by inhibition of 5-HT7 receptors (Abbas et al., 2009). Lurasidone, another atypical antipsychotic behaving as an antagonist at dopamine D2, 5-HT2, and 5-HT7 receptors (Ishibashi et al., 2010), was shown to exert significant antidepressant effects in patients with bipolar 1 disorder (Woo et al., 2013). Another atypical antipsychotic is vortioxetine, also named Lu AA21004, a multimodal compound that behaves as an antagonist at 5-HT3 and 5-HT7 receptors, a partial agonist at 5-HT1B, a full agonist at 5-HT1A receptors and an inhibitor of the serotonin transporter (Guilloux et al., 2013). Vortioxetine proved effective as antidepressant in several clinical studies and was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of major depressive disorder (Citrome, 2014), confirming that an enhancement of serotonergic transmission associated with 5-HT7 receptor antagonism exerts synergistic antidepressant effects.
These data together indicate that 5-HT7 receptors regulate the balance between anxiety and depression. Mood regulation has crucial consequences on cognition, as stress can improve or impair learning depending on the duration and intensity of stressful conditions. Acute stress enhances learning, particularly when experienced in the same context of learning acquisition (Joëls et al., 2006; Bangasser and Shors, 2010). As underlined above, 5-HT7 receptor activation improves fear-related learning, in which an acute stressor is presented during the paradigms of fear conditioning and passive avoidance (Roberts et al., 2004a; Eriksson et al., 2008). Therefore, an enhancement of anxiety levels by 5-HT7 receptor activation during a stress condition might contribute to 5-HT7 receptor-mediated improvement of stress-related learning.
Epilepsy
In view of the depolarizing effects exerted by 5-HT7 receptors (see above), their activation is likely to enhance seizure sensitivity. In line with this, a pharmacological study using different mixed antagonists suggested that blockade of 5-HT7 receptors may protect against audiogenic seizures in DBA/2J mice (Bourson et al., 1997). Pharmacological blockade using selective 5-HT7 receptor antagonists decreased cortical epileptic activity in a rat genetic model of absence epilepsy (Graf et al., 2004) and in a pilocarpine-induced rat model of temporal lobe epilepsy (Yang et al., 2012).
In contrast, 5-HT7 receptor activation was instead suggested to exert anticonvulsant effects in other experimental models of epilepsy. Systemic administration of the 5-HT7 receptor agonist 5-CT reduced picrotoxin-induced seizures in mice, an effect abolished by a selective 5-HT7 receptor antagonist (Pericic and Svob Strac, 2007). Consistently, mice lacking 5-HT7 receptors display enhanced sensitivity to electrically and chemically-induced seizures (Witkin et al., 2007).
Thus, 5-HT7 receptor-mediated effects on epilepsy are heterogeneous and apparently controversial, probably due to differences in the models used. Systemic administration of 5-HT7 receptor ligands exerts a complex modulation of the brain serotonin system because, besides directly acting on 5-HT7 receptors on target cells, they also modulate the activity of GABAergic interneurons. As pointed out in the previous paragraphs, in raphe nuclei 5-HT7 receptor activation inhibits GABAergic interneurons targeting serotonergic neurons and subsequently enhance 5-HT release in other brain areas. In the hippocampus, GABAergic interneurons are instead stimulated by 5-HT7 receptor activation. Therefore, the understanding of systemic effects exerted by 5-HT7 receptor ligands is complicated by 5-HT7 receptor-mediated modulation of GABAergic interneurons in distinct brain areas.
Concerning systemic administration of 5-HT7 receptor agonists, it should also be considered that 5-HT7 receptors undergo desensitization following prolonged activation (Shimizu et al., 1998). Desensitization of 5-HT7 receptors was also observed after antagonist treatment (Tokarski et al., 2012b). Thus, systemic treatments of animals should be carefully designed to minimize 5-HT7 receptor desensitization; in this respect, a preferential use of agonists with a short half-life, such as LP-211 and related compounds (Hedlund et al., 2010) might be considered preferentially.
The Serotonin Hypothesis of Autism
A large body of evidence has led to the serotonin hypothesis of autism, which points out a deficiency in the brain serotonin system as a causal mechanism in ASD (Whitaker-Azmitia, 2005; Harrington et al., 2013). Early experimental data (Schain and Freedman, 1961), later confirmed by many research groups (Anderson et al., 1990; Piven et al., 1991; McBride et al., 1998; Mulder et al., 2004), have documented an increase of serotonin levels in blood platelets (hyperserotonemia) in one third of autistic patients. Conversely, a decreased uptake of tryptophan (the precursor of 5-HT) and a reduced 5-HT synthesis were detected in the brain of autistic children by positron emission tomography (PET) using the radioligand tracer alpha-methyl-tryptophan (Chugani et al., 1997, 1999; Chandana et al., 2005). These studies have evidenced that global brain 5-HT synthesis was reduced in autistic patients in an age-dependent manner, with local differences in cortical regions. The localization of 5-HT defect was related to the severity of language problems, as children with the most severe language delay displayed the lowest amount of tryptophan uptake in the left cortex. Overall, in very young autistic children (2–5 years old) the rate of 5-HT synthesis in frontal, temporal and parietal cortex was significantly lower with respect to healthy children of the same age and was gradually increased only later in development (5–14 years old). The authors suggested that a high brain serotonin synthesis normally occurring during early childhood is disrupted in autism (Chugani et al., 1999).
A lack of 5-HT during early stages of development is likely to disrupt the wiring architecture of the brain. Consistently, post mortem observation of brains from young autistic patients showed an abnormal morphology of serotonergic fibers directed to the amygdala and temporal cortex and an increased density of the serotonin transporter (named either 5-HTT or SERT) on these fibers (Azmitia et al., 2011).
The 5-HTT is responsible for 5-HT uptake in CNS serotonergic nerve terminals, reducing the amount of 5-HT in the synaptic cleft. The 5-HTT is also located on blood platelets and up-regulation of 5-HTT activity participates to their increased uptake of serotonin, leading to hyperserotonemia (Marazziti et al., 2000). The gene coding for the 5-HTT, named SLC6A4, exists in various alleles related to different degrees of 5-HTT expression and/or activity. Polymorphism of the SLC6A4 gene has been correlated with autism, although results from different groups are heterogeneous with respect to the polymorphic sites involved and the type of allele associated with autism (Devlin et al., 2005; Cho et al., 2007; Coutinho et al., 2007; Wassink et al., 2007; Cross et al., 2008); however, others did not find any significant association (Ramoz et al., 2006). A SLC6A4 variant coding for an overactive form of 5-HTT has been identified in families of autistic patients (Sutcliffe et al., 2005). Mutant mice expressing this high functioning 5-HTT variant show hyperserotonemia, hypersensitivity of 5-HT receptors (as a result of reduced serotonergic transmission) and autistic behavior (Veenstra-VanderWeele et al., 2012).
Alterations in brain 5-HT receptor density were found in autistic patients. A reduction of 5-HT2A binding sites in the cingulate, frontal, and temporal cortex was detected in adults with Asperger syndrome by single photon emission computed tomography (SPECT; Murphy et al., 2006), although a later PET study found contrasting results (Girgis et al., 2011). A reduced density of 5-HT1A and 5-HT2 receptors in posterior cingulate cortex and fusiform cortex, brain regions involved in social and emotional behaviors, was observed in post-mortem brain tissue from young adults diagnosed with autism other than Asperger syndrome (Oblak et al., 2013).
To summarize, a large number of clinical studies indicate abnormal synthesis and increased uptake of 5-HT, morphological alteration of serotonergic fibers and reduced expression of 5-HT receptors in the brain of ASD patients. Consistently, neonatal mice depleted of forebrain serotonin by neurotoxin injection at birth (Boylan et al., 2007) and mice genetically depleted of serotonin (Kane et al., 2012) show a delay in development and typical autistic features, and have been proposed as animal models of ASD. Other animal models of ASD were instead generated by manipulations inducing pre-natal hyperserotonemia and postnatal loss of brain 5-HT, such as fetal exposure to sodium valproate (Dufour-Rainfray et al., 2011) or to 5-methoxytryptamine (5-MT), a non-selective agonist of all metabotropic 5-HT receptor types (McNamara et al., 2008). According to a developmental theory of autism, prenatal hyperserotonemia causes increased 5-HT levels in the fetal brain, as the immature blood-brain barrier is permeable to 5-HT; this in turn would cause a loss of serotonergic fibers as a negative feedback mechanism (Whitaker-Azmitia, 2005; McNamara et al., 2008). Thus, prenatal hyperserotonemia would ultimately reduce the function of the brain serotonin system and lead to consequences similar to post-natal serotonin depletion.
Animal studies have confirmed the involvement of 5-HT in ASD and provided some indication about the 5-HT-dependent mechanisms disrupted in ASD. In 5-HTT knockout animals, the lack of 5-HTT during early development altered the connectivity between raphe nuclei and prefrontal cortex (Witteveen et al., 2013), as well as cortical cell density and layer thickness (Altamura et al., 2007; Witteveen et al., 2013). Also neonatal 5-HT depletion in mouse forebrain caused a disruption in neocortical architecture, namely an increase of cortical thickness (Boylan et al., 2007) resembling the increased cortical volume observed in autistic patients (Carper and Courchesne, 2005).
In a hyperserotonemia rat model of autism, a reduced number of oxytocin neurons was detected in the hypothalamic paraventricular nucleus (PVN; McNamara et al., 2008). The PVN projects oxytocin-containing fibers to the NAc, where pre-synaptic oxytocin receptors stimulate 5-HT release from serotonergic nerve terminals arising from dorsal raphe. Thus, a reduced activity of this pathway might have important implication in the pathophysiology of autistic behavior. As a matter of fact, a recent study shows that 5-HT and oxytocin cooperate in the NAc and their combined activity is crucially involved in social reward, a mechanism disrupted in autism (Dölen et al., 2013).
5-HT7 Receptors as a Possible Novel Therapeutic Target in Autism Spectrum Disorders
Little is known about a possible malfunction of 5-HT7 receptors in ASD. A single base polymorphism was detected in the gene coding for the 5-HT7 receptor, giving rise to two different alleles; however, analysis of transmission disequilibrium in autistic patients did not evidence any correlation of either allele to autism (Lassig et al., 1999). The expression level of 5-HT7 receptors in the brain of autistic patients has not been specifically investigated and no information is presently available about the functional properties of 5-HT7 receptors in animal models of autism.
Although a dysfunction of 5-HT7 receptors has not been causally linked to ASD, we propose that 5-HT7 receptor ligands might be considered as valuable pharmacological tools in ASD. This suggestion is mainly based on our findings but is also supported by other studies addressing the role of 5-HT7 receptors in behavioral flexibility and repetitive behavior, as discussed below. We have shown that 5-HT7 receptor activation reverses mGluR-LTD in Fmr1KO mice (Costa et al., 2012a), a model of Fragile X Syndrome also considered as an animal model of autism (Bernardet and Crusio, 2006; Pietropaolo et al., 2011). This result might open new perspectives for therapy if pharmacological activation of 5-HT7 receptors, besides correcting the most prominent synaptic defect in the mouse model of FXS, is also able to rescue the symptoms of FXS such as learning deficits, epilepsy and autistic behavior.
A typical feature of ASD is a reduced behavioral flexibility, i.e., a reduced ability to replace a previously acquired rule with a new one, in adaptation to a new environmental context. Behavioral flexibility depends on brain circuits involving pre-frontal cortex (Floresco et al., 2009; Wolfensteller and Ruge, 2012; Logue and Gould, 2014) and can be evaluated in animals as well as in humans using an attentional set-shifting task (Birrell and Brown, 2000; Colacicco et al., 2002). In this test, rodents are trained to discriminate between different stimuli (odor, surface texture, digging medium) as a cue to obtain a reward. After a training period, the rules are changed and animals must learn that a previously relevant cue has become irrelevant and vice-versa. Using attentional set-shifting protocols, recent studies have shown an involvement of 5-HT7 receptors in behavioral flexibility, especially in conditions of stress (Nikiforuk, 2012; Nikiforuk and Popik, 2013). Rats exposed to restraint stress showed reduced behavioral flexibility; administration of amisulpride, an atypical antipsychotic drug and a 5-HT7 receptor antagonist, reversed the restraint-induced cognitive inflexibility and also improved attention in unstressed animals. Administration of the 5-HT7 receptor agonist AS-19 abolished the pro-cognitive effect of amisulpride. The same authors have shown that behavioral inflexibility induced by another protocol (ketamine administration) was reduced by amisulpride and by the 5-HT7 receptor antagonist SB-269970 (Nikiforuk et al., 2013). These results suggest that a lack of behavioral flexibility is associated with increased activation of 5-HT7 receptors. In this respect, antagonists rather than agonist of 5-HT7 receptors might be beneficial to correct the reduced behavioral flexibility observed in ASD. However, further studies are necessary to test the effects of 5-HT7 receptor agonists and antagonist on cognitive flexibility in different pathological conditions. In particular, it would be useful to study the relation between altered synaptic plasticity and behavioral flexibility in different models of intellectual disability and autism. For example, the Fmr1 KO mouse model of Fragile X Syndrome exhibit an abnormally enhanced mGluR-LTD (Huber et al., 2002) and show autistic features and reduced behavioral flexibility (Casten et al., 2011). On the other side, mGluR-LTD is abnormally reduced in other mouse models of syndromic and non syndromic forms of autism, which also show reduced behavioral flexibility. We are currently studying if systemic administration of a 5-HT7 receptor agonist can rescue learning deficits and altered behavior in Fmr1 KO mice. Our hypothesis is that 5-HT7 receptor activation, although reducing behavioral flexibility in wild-type animals (Nikiforuk, 2012; Nikiforuk and Popik, 2013), might restore behavior and thus be beneficial in Fragile X syndrome, a condition in which mGluR-LTD is exaggerated.
Repetitive and stereotypic behavior is considered a core symptom of ASD. A behavioral study has shown that the stereotypical behavior of marble burying, an experimental protocol used to evaluate anxiety and obsessive-compulsive behavior, was reduced in 5-HT7 KO mice and in wild-type mice following pharmacological blockade of 5-HT7 receptors (Hedlund and Sutcliffe, 2007). The authors suggest that this result might also be due to the anxiolytic effect of 5-HT7 receptor blockade (Hedlund, 2009).
Co-morbid symptoms of ASD, observed only in a fraction of patients, include sleep disorders, anxiety, depression, epilepsy, attention deficit and hyperactivity disorder (Spooren et al., 2012). As pointed out in the previous paragraphs, 5-HT7 receptors are crucially involved in the regulation of the sleep/wake cycle, exert either pro- or anti-convulsant effects in different models of epilepsy and play an important role in mood control (reviewed by Bagdy et al., 2007; Matthys et al., 2011). Since 5-HT7 receptor inactivation or blockade exert anxiolytic effects (see above), 5-HT7 receptor activation is likely to enhance the level of anxiety, thus might be expected to reduce hyperactivity. In line with this hypothesis, in highly active rats showing explorative behavior and low levels of anxiety, the expression level of 5-HT7 receptors in the thalamus and in the hippocampus was significantly lower than in low-activity rats (Ballaz et al., 2007b). Accordingly, another study shows that microinjections of the 5-HT7 receptor agonist AS-19 into the NAc decreased ambulatory activity in the rat (Clissold et al., 2013).
Other publications instead show that systemic administration of the 5-HT7 receptor agonist LP-211 enhanced locomotion in wild-type mice (Adriani et al., 2012) and exerted anxiolytic effects, enhancing the exploratory attitude, in a rat model of hyperactivity and attention deficit (Ruocco et al., 2013). Therefore, it would be interesting to further investigate the role of 5-HT7 receptors on different types of anxiety-related behaviors.
Concluding Remarks
Central 5-HT7 receptors modulate neuronal excitability and synaptic function and are important physiological modulators of learning and mood. Based on these properties, 5-HT7 receptors have been proposed as a pharmacological target in cognitive impairment, depression and epilepsy.
Core and co-morbid symptoms of ASD involve a disruption of several functions, some of which are physiologically regulated by 5-HT7 receptors. In light of this, it would be interesting to investigate the role of 5-HT7 receptors on different features disrupted in ASD. Cognitive functions are likely to be enhanced by 5-HT7 receptor agonists; on the other side, behavioral flexibility, hyperactivity and epilepsy might benefit from blockade of 5-HT7 receptors. In many cases, predictions of a possible outcome by pharmacological manipulation of 5-HT7 receptors are complicated by heterogeneous data present in literature and a direct investigation would be necessary.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Dr. Derek Bowie (McGill University, Montreal, QC, Canada) for critical reading of the manuscript. The present work was financed by FRAXA Research Foundation (call 2013) and Telethon Foundation (grant GGP13145). Part of images from Motifolio drawing toolkit1 were utilized in the figure preparation.
Footnotes
References
Abbas, A. I., Hedlund, P. B., Huang, X. P., Tran, T. B., Meltzer, H. Y., and Roth, B. L. (2009). Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl) 205, 119–128. doi: 10.1007/s00213-009-1521-8
Adriani, W., Travaglini, D., Lacivita, E., Saso, L., Leopoldo, M., and Laviola, G. (2012). Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology 62, 833–842. doi: 10.1016/j.neuropharm.2011.09.012
Ahern, G. P. (2011). 5-HT and the immune system. Curr. Opin. Pharmacol. 11, 29–33. doi: 10.1016/j.coph.2011.02.004
Altamura, C., Dell’Acqua, M. L., Moessner, R., Murphy, D. L., Lesch, K. P., and Persico, A. M. (2007). Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb. Cortex 17, 1394–1401. doi: 10.1093/cercor/bhl051
Anderson, G. M., Horne, W. C., Chatterjee, D., and Cohen, D. J. (1990). The hyperserotonemia of autism. Ann. N Y Acad. Sci. 600, 331–340; discussion 341–332. doi: 10.1111/j.1749-6632.1990.tb16893.x
Azmitia, E. C., Singh, J. S., and Whitaker-Azmitia, P. M. (2011). Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology 60, 1347–1354. doi: 10.1016/j.neuropharm.2011.02.002
Bacon, W. L., and Beck, S. G. (2000). 5-Hydroxytryptamine7 receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 294, 672–679.
Bagdy, G., Kecskemeti, V., Riba, P., and Jakus, R. (2007). Serotonin and epilepsy. J. Neurochem. 100, 857–873. doi: 10.1111/j.1471-4159.2006.04277.x
Ballaz, S. J., Akil, H., and Watson, S. J. (2007a). The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience 149, 192–202. doi: 10.1016/j.neuroscience.2007.07.043
Ballaz, S. J., Akil, H., and Watson, S. J. (2007b). Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience 147, 428–438. doi: 10.1016/j.neuroscience.2007.04.024
Bangasser, D. A., and Shors, T. J. (2010). Critical brain circuits at the intersection between stress and learning. Neurosci. Biobehav. Rev. 34, 1223–1233. doi: 10.1016/j.neubiorev.2010.02.002
Bard, J. A., Zgombick, J., Adham, N., Vaysse, P., Branchek, T. A., and Weinshank, R. L. (1993). Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 268, 23422–23426.
Béïque, J. C., Campbell, B., Perring, P., Hamblin, M. W., Walker, P., Mladenovic, L., et al. (2004a). Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A and 5-HT7 receptors. J. Neurosci. 24, 4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004
Béïque, J. C., Chapin-Penick, E. M., Mladenovic, L., and Andrade, R. (2004b). Serotonergic facilitation of synaptic activity in the developing rat prefrontal cortex. J. Physiol. 556, 739–754. doi: 10.1113/jphysiol.2003.051284
Belenky, M. A., and Pickard, G. E. (2001). Subcellular distribution of 5-HT(1B) and 5-HT7 receptors in the mouse suprachiasmatic nucleus. J. Comp. Neurol. 432, 371–388. doi: 10.1002/cne.1109
Bengtson, C. P., Lee, D. J., and Osborne, P. B. (2004). Opposing electrophysiological actions of 5-HT on noncholinergic and cholinergic neurons in the rat ventral pallidum in vitro. J. Neurophysiol. 92, 433–443. doi: 10.1152/jn.00543.2003
Bernardet, M., and Crusio, W. E. (2006). Fmr1 KO mice as a possible model of autistic features. ScientificWorldJournal 6, 1164–1176. doi: 10.1100/tsw.2006.220
Bickmeyer, U., Heine, M., Manzke, T., and Richter, D. W. (2002). Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur. J. Neurosci. 16, 209–218. doi: 10.1046/j.1460-9568.2002.02072.x
Birrell, J. M., and Brown, V. J. (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 20, 4320–4324.
Bonaventure, P., Kelly, L., Aluisio, L., Shelton, J., Lord, B., Galici, R., et al. (2007). Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 321, 690–698. doi: 10.1124/jpet.107.119404
Bonsi, P., Cuomo, D., Ding, J., Sciamanna, G., Ulrich, S., Tscherter, A., et al. (2007). Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT2C, 5-HT6 and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32, 1840–1854. doi: 10.1038/sj.npp.1301294
Bourson, A., Kapps, V., Zwingelstein, C., Rudler, A., Boess, F. G., and Sleight, A. J. (1997). Correlation between 5-HT7 receptor affinity and protection against sound-induced seizures in DBA/2J mice. Naunyn Schmiedebergs Arch. Pharmacol. 356, 820–826. doi: 10.1007/PL00005123
Boylan, C. B., Blue, M. E., and Hohmann, C. F. (2007). Modeling early cortical serotonergic deficits in autism. Behav. Brain Res. 176, 94–108. doi: 10.1016/j.bbr.2006.08.026
Brager, D. H., Akhavan, A. R., and Johnston, D. (2012). Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell Rep. 1, 225–233. doi: 10.1016/j.celrep.2012.02.002
Brager, D. H., and Johnston, D. (2007). Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J. Neurosci. 27, 13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007
Brager, D. H., and Johnston, D. (2014). Channelopathies and dendritic dysfunction in fragile X syndrome. Brain Res. Bull. 103, 11–17. doi: 10.1016/j.brainresbull.2014.01.002
Brenchat, A., Rocasalbas, M., Zamanillo, D., Hamon, M., Vela, J. M., and Romero, L. (2012). Assessment of 5-HT7 receptor agonists selectivity using nociceptive and thermoregulation tests in knockout versus wild-type mice. Adv. Pharmacol. Sci. 2012:312041. doi: 10.1155/2012/312041
Brenchat, A., Romero, L., Garcia, M., Pujol, M., Burgueno, J., Torrens, A., et al. (2009). 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 141, 239–247. doi: 10.1016/j.pain.2008.11.009
Cardenas, C. G., Mar, L. P., Vysokanov, A. V., Arnold, P. B., Cardenas, L. M., Surmeier, D. J., et al. (1999). Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J. Physiol. 518(Pt. 2), 507–523. doi: 10.1111/j.1469-7793.1999.0507p.x
Carper, R. A., and Courchesne, E. (2005). Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry 57, 126–133. doi: 10.1016/j.biopsych.2004.11.005
Casten, K. S., Gray, A. C., and Burwell, R. D. (2011). Discrimination learning and attentional set formation in a mouse model of fragile X. Behav. Neurosci. 125, 473–479. doi: 10.1037/a0023561
Cervantes-Duran, C., Rocha-Gonzalez, H. I., and Granados-Soto, V. (2013). Peripheral and spinal 5-HT receptors participate in the pronociceptive and antinociceptive effects of fluoxetine in rats. Neuroscience 252, 396–409. doi: 10.1016/j.neuroscience.2013.08.022
Chandana, S. R., Behen, M. E., Juhasz, C., Muzik, O., Rothermel, R. D., Mangner, T. J., et al. (2005). Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int. J. Dev. Neurosci. 23, 171–182. doi: 10.1016/j.ijdevneu.2004.08.002
Chapin, E. M., and Andrade, R. (2001). A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h). J. Pharmacol. Exp. Ther. 297, 403–409.
Chen, S., Wang, J., and Siegelbaum, S. A. (2001). Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 117, 491–504. doi: 10.1085/jgp.117.5.491
Chen, L., Yung, K. K., Chan, Y. S., and Yung, W. H. (2008). 5-HT excites globus pallidus neurons by multiple receptor mechanisms. Neuroscience 151, 439–451. doi: 10.1016/j.neuroscience.2007.11.003
Cherubini, E., Rovira, C., Ben-Ari, Y., and Nistri, A. (1990). Effects of kainate on the excitability of rat hippocampal neurones. Epilepsy Res. 5, 18–27. doi: 10.1016/0920-1211(90)90062-Z
Cho, I. H., Yoo, H. J., Park, M., Lee, Y. S., and Kim, S. A. (2007). Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios. Brain Res. 1139, 34–41. doi: 10.1016/j.brainres.2007.01.002
Chugani, D. C., Muzik, O., Behen, M., Rothermel, R., Janisse, J. J., Lee, J., et al. (1999). Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 45, 287–295. doi: 10.1002/1531-8249(199903)45:3<287::AID-ANA3>3.0.CO;2-9
Chugani, D. C., Muzik, O., Rothermel, R., Behen, M., Chakraborty, P., Mangner, T., et al. (1997). Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann. Neurol. 42, 666–669. doi: 10.1002/ana.410420420
Citrome, L. (2014). Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 68, 60–82. doi: 10.1111/ijcp.12350
Clissold, K. A., Choi, E., and Pratt, W. E. (2013). Serotonin 1A, 1B, and 7 receptors of the rat medial nucleus accumbens differentially regulate feeding, water intake and locomotor activity. Pharmacol. Biochem. Behav. 112, 96–103. doi: 10.1016/j.pbb.2013.10.002
Colacicco, G., Welzl, H., Lipp, H. P., and Wurbel, H. (2002). Attentional set-shifting in mice: modification of a rat paradigm and evidence for strain-dependent variation. Behav. Brain Res. 132, 95–102. doi: 10.1016/S0166-4328(01)00391-6
Costa, L., Spatuzza, M., D’Antoni, S., Bonaccorso, C. M., Trovato, C., Musumeci, S. A., et al. (2012a). Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of fragile X syndrome. Biol. Psychiatry 72, 924–933. doi: 10.1016/j.biopsych.2012.06.008
Costa, L., Trovato, C., Musumeci, S. A., Catania, M. V., and Ciranna, L. (2012b). 5-HT1A and 5-HT7 receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus 22, 790–801. doi: 10.1002/hipo.20940
Coutinho, A. M., Sousa, I., Martins, M., Correia, C., Morgadinho, T., Bento, C., et al. (2007). Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Hum. Genet. 121, 243–256. doi: 10.1007/s00439-006-0301-3
Cross, S., Kim, S. J., Weiss, L. A., Delahanty, R. J., Sutcliffe, J. S., Leventhal, B. L., et al. (2008). Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology 33, 353–360. doi: 10.1038/sj.npp.1301406
Devlin, B., Cook, E. H. Jr., Coon, H., Dawson, G., Grigorenko, E. L., McMahon, W., et al. (2005). Autism and the serotonin transporter: the long and short of it. Mol. Psychiatry 10, 1110–1116. doi: 10.1038/sj.mp.4001724
Di Pilato, P., Niso, M., Adriani, W., Romano, E., Travaglini, D., Berardi, F., et al. (2014). Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: an overview. Rev. Neurosci. 25, 401–415. doi: 10.1515/revneuro-2014-0009
Disterhoft, J. F., Thompson, L. T., Moyer, J. R. Jr., and Mogul, D. J. (1996). Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 59, 413–420. doi: 10.1016/0024-3205(96)00320-7
Dölen, G., Darvishzadeh, A., Huang, K. W., and Malenka, R. C. (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. doi: 10.1038/nature12518
Doly, S., Fischer, J., Brisorgueil, M. J., Verge, D., and Conrath, M. (2005). Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J. Comp. Neurol. 490, 256–269. doi: 10.1002/cne.20667
Dufour-Rainfray, D., Vourc’h, P., Tourlet, S., Guilloteau, D., Chalon, S., and Andres, C. R. (2011). Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci. Biobehav. Rev. 35, 1254–1265. doi: 10.1016/j.neubiorev.2010.12.013
Eriksson, T. M., Golkar, A., Ekstrom, J. C., Svenningsson, P., and Ogren, S. O. (2008). 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT1A receptor stimulation on contextual learning in mice. Eur. J. Pharmacol. 596, 107–110. doi: 10.1016/j.ejphar.2008.08.026
Eriksson, T. M., Holst, S., Stan, T. L., Hager, T., Sjogren, B., Ogren, S. O., et al. (2012). 5-HT1A and 5-HT7 receptor crosstalk in the regulation of emotional memory: implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology 63, 1150–1160. doi: 10.1016/j.neuropharm.2012.06.061
Errico, M., Crozier, R. A., Plummer, M. R., and Cowen, D. S. (2001). 5-HT7 receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience 102, 361–367. doi: 10.1016/S0306-4522(00)00460-7
Fan, Y., Fricker, D., Brager, D. H., Chen, X., Lu, H. C., Chitwood, R. A., et al. (2005). Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat. Neurosci. 8, 1542–1551. doi: 10.1038/nn1568
Fernández de Sevilla, D., Fuenzalida, M., Porto Pazos, A. B., and Buño, W. (2007). Selective shunting of the NMDA EPSP component by the slow afterhyperpolarization in rat CA1 pyramidal neurons. J. Neurophysiol. 97, 3242–3255. doi: 10.1152/jn.00422.2006
Floresco, S. B., Zhang, Y., and Enomoto, T. (2009). Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav. Brain Res. 204, 396–409. doi: 10.1016/j.bbr.2008.12.001
Foxx-Orenstein, A. E., Kuemmerle, J. F., and Grider, J. R. (1996). Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology 111, 1281–1290. doi: 10.1053/gast.1996.v111.pm8898642
Freret, T., Paizanis, E., Beaudet, G., Gusmao-Montaigne, A., Nee, G., Dauphin, F., et al. (2014). Modulation of 5-HT7 receptor: effect on object recognition performances in mice. Psychopharmacology (Berl) 231, 393–400. doi: 10.1007/s00213-013-3247-x
Freund, T. F., and Buzsaki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I
García-Alcocer, G., Segura, L. C., Garcia Pena, M., Martinez-Torres, A., and Miledi, R. (2006). Ontogenetic distribution of 5-HT2C, 5-HT5A and 5-HT7 receptors in the rat hippocampus. Gene Expr. 13, 53–57. doi: 10.3727/000000006783991935
Gasbarri, A., Cifariello, A., Pompili, A., and Meneses, A. (2008). Effect of 5-HT7 antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav. Brain Res. 195, 164–170. doi: 10.1016/j.bbr.2007.12.020
Gaspar, P., Cases, O., and Maroteaux, L. (2003). The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012. doi: 10.1038/nrn1256
Gellynck, E., Heyninck, K., Andressen, K. W., Haegeman, G., Levy, F. O., Vanhoenacker, P., et al. (2013). The serotonin 5-HT7 receptors: two decades of research. Exp. Brain Res. 230, 555–568. doi: 10.1007/s00221-013-3694-y
Geurts, F. J., De Schutter, E., and Timmermans, J. P. (2002). Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum. J. Chem. Neuroanat. 24, 65–74. doi: 10.1016/S0891-0618(02)00020-0
Gill, C. H., Soffin, E. M., Hagan, J. J., and Davies, C. H. (2002). 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology 42, 82–92. doi: 10.1016/S0028-3908(01)00149-6
Girgis, R. R., Slifstein, M., Xu, X., Frankle, W. G., Anagnostou, E., Wasserman, S., et al. (2011). The 5-HT2 A receptor and serotonin transporter in Asperger’s disorder: a PET study with [11C]MDL 100907 and [11 C]DASB. Psychiatry Res. 194, 230–234. doi: 10.1016/j.pscychresns.2011.04.007
Glass, J. D., Grossman, G. H., Farnbauch, L., and DiNardo, L. (2003). Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J. Neurosci. 23, 7451–7460.
Goaillard, J. M., and Vincent, P. (2002). Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT7 receptors. J. Physiol. 541, 453–465. doi: 10.1113/jphysiol.2001.013896
Gorinski, N., and Ponimaskin, E. (2013). Palmitoylation of serotonin receptors. Biochem. Soc. Trans. 41, 89–94. doi: 10.1042/BST20120235
Graf, M., Jakus, R., Kantor, S., Levay, G., and Bagdy, G. (2004). Selective 5-HT1A and 5-HT7 antagonists decrease epileptic activity in the WAG/Rij rat model of absence epilepsy. Neurosci. Lett. 359, 45–48. doi: 10.1016/j.neulet.2004.01.072
Guilloux, J. P., Mendez-David, I., Pehrson, A., Guiard, B. P., Reperant, C., Orvoen, S., et al. (2013). Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 73, 147–159. doi: 10.1016/j.neuropharm.2013.05.014
Guscott, M., Bristow, L. J., Hadingham, K., Rosahl, T. W., Beer, M. S., Stanton, J. A., et al. (2005). Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 48, 492–502. doi: 10.1016/j.neuropharm.2004.11.015
Guscott, M. R., Egan, E., Cook, G. P., Stanton, J. A., Beer, M. S., Rosahl, T. W., et al. (2003). The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology 44, 1031–1037. doi: 10.1016/S0028-3908(03)00117-5
Hagan, J. J., Price, G. W., Jeffrey, P., Deeks, N. J., Stean, T., Piper, D., et al. (2000). Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 130, 539–548.
Hall, A. (1998). G proteins and small GTPases: distant relatives keep in touch. Science 280, 2074–2075. doi: 10.1126/science.280.5372.2074
Hannon, J., and Hoyer, D. (2008). Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213. doi: 10.1016/j.bbr.2008.03.020
Harrington, R. A., Lee, L. C., Crum, R. M., Zimmerman, A. W., and Hertz-Picciotto, I. (2013). Serotonin hypothesis of autism: implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Res. 6, 149–168. doi: 10.1002/aur.1288
Hedlund, P. B. (2009). The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 206, 345–354. doi: 10.1007/s00213-009-1626-0
Hedlund, P. B., Danielson, P. E., Thomas, E. A., Slanina, K., Carson, M. J., and Sutcliffe, J. G. (2003). No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. U S A 100, 1375–1380. doi: 10.1073/pnas.0337340100
Hedlund, P. B., Huitron-Resendiz, S., Henriksen, S. J., and Sutcliffe, J. G. (2005). 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 58, 831–837. doi: 10.1016/j.biopsych.2005.05.012
Hedlund, P. B., Kelly, L., Mazur, C., Lovenberg, T., Sutcliffe, J. G., and Bonaventure, P. (2004). 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 487, 125–132. doi: 10.1016/j.ejphar.2004.01.031
Hedlund, P. B., Leopoldo, M., Caccia, S., Sarkisyan, G., Fracasso, C., Martelli, G., et al. (2010). LP-211 is a brain penetrant selective agonist for the serotonin 5-HT7 receptor. Neurosci. Lett. 481, 12–16. doi: 10.1016/j.neulet.2010.06.036
Hedlund, P. B., and Sutcliffe, J. G. (2004). Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 25, 481–486. doi: 10.1016/j.tips.2004.07.002
Hedlund, P. B., and Sutcliffe, J. G. (2007). The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci. Lett. 414, 247–251. doi: 10.1016/j.neulet.2006.12.054
Hoffman, M. S., and Mitchell, G. S. (2011). Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J. Physiol. 589, 1397–1407. doi: 10.1113/jphysiol.2010.201657
Huber, K. M., Gallagher, S. M., Warren, S. T., and Bear, M. F. (2002). Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U S A 99, 7746–7750. doi: 10.1073/pnas.122205699
Inoue, T., Itoh, S., Wakisaka, S., Ogawa, S., Saito, M., and Morimoto, T. (2002). Involvement of 5-HT7 receptors in serotonergic effects on spike afterpotentials in presumed jaw-closing motoneurons of rats. Brain Res. 954, 202–211. doi: 10.1016/S0006-8993(02)03286-9
Ireland, D. R., and Abraham, W. C. (2002). Group I mGluRs increase excitability of hippocampal CA1 pyramidal neurons by a PLC-independent mechanism. J. Neurophysiol. 88, 107–116. doi: 10.1152/jn.00679.2001
Ishibashi, T., Horisawa, T., Tokuda, K., Ishiyama, T., Ogasa, M., Tagashira, R., et al. (2010). Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 334, 171–181. doi: 10.1124/jpet.110.167346
Ishihara, K., Takahashi, N., Komoto, N., Yoshikawa, C., Fukumoto, S., Ide, S., et al. (2013). Serotonergic modulation of neuronal activity in the nucleus accumbens following repeated methamphetamine administration in rats. J. Pharmacol. Sci. 123, 140–146. doi: 10.1254/jphs.13100FP
Joëls, M., Pu, Z., Wiegert, O., Oitzl, M. S., and Krugers, H. J. (2006). Learning under stress: how does it work? Trends Cogn. Sci. 10, 152–158. doi: 10.1016/j.tics.2006.02.002
Johnson-Farley, N. N., Kertesy, S. B., Dubyak, G. R., and Cowen, D. S. (2005). Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J. Neurochem. 92, 72–82. doi: 10.1111/j.1471-4159.2004.02832.x
Jung, S., Jones, T. D., Lugo, J. N. Jr., Sheerin, A. H., Miller, J. W., D’Ambrosio, R., et al. (2007). Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J. Neurosci. 27, 13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007
Jung, S., Warner, L. N., Pitsch, J., Becker, A. J., and Poolos, N. P. (2011). Rapid loss of dendritic HCN channel expression in hippocampal pyramidal neurons following status epilepticus. J. Neurosci. 31, 14291–14295. doi: 10.1523/JNEUROSCI.1148-11.2011
Kane, M. J., Angoa-Perez, M., Briggs, D. I., Sykes, C. E., Francescutti, D. M., Rosenberg, D. R., et al. (2012). Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One 7:e48975. doi: 10.1371/journal.pone.0048975
Kawahara, F., Saito, H., and Katsuki, H. (1994). Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J. Physiol. 478(Pt. 1), 67–73.
Kobe, F., Guseva, D., Jensen, T. P., Wirth, A., Renner, U., Hess, D., et al. (2012). 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J. Neurosci. 32, 2915–2930. doi: 10.1523/JNEUROSCI.2765-11.2012
Kvachnina, E., Dumuis, A., Wlodarczyk, J., Renner, U., Cochet, M., Richter, D. W., et al. (2009). Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7a receptor is modulated by the palmitoylation of its C-terminal domain. Biochim. Biophys. Acta 1793, 1646–1655. doi: 10.1016/j.bbamcr.2009.08.008
Kvachnina, E., Liu, G., Dityatev, A., Renner, U., Dumuis, A., Richter, D. W., et al. (2005). 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J. Neurosci. 25, 7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005
Lancaster, B., Hu, H., Ramakers, G. M., and Storm, J. F. (2001). Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J. Physiol. 536, 809–823. doi: 10.1111/j.1469-7793.2001.00809.x
Lassig, J. P., Vachirasomtoon, K., Hartzell, K., Leventhal, M., Courchesne, E., Courchesne, R., et al. (1999). Physical mapping of the serotonin 5-HT7 receptor gene (HTR7) to chromosome 10 and pseudogene (HTR7P) to chromosome 12 and testing of linkage disequilibrium between HTR7 and autistic disorder. Am. J. Med. Genet. 88, 472–475. doi: 10.1002/(SICI)1096-8628(19991015)88:5<472::AID-AJMG7>3.0.CO;2-G
Leopoldo, M., Lacivita, E., De Giorgio, P., Fracasso, C., Guzzetti, S., Caccia, S., et al. (2008). Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J. Med. Chem. 51, 5813–5822. doi: 10.1021/jm800615e
Lesch, K. P., and Waider, J. (2012). Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76, 175–191. doi: 10.1016/j.neuron.2012.09.013
Lin, S. L., Johnson-Farley, N. N., Lubinsky, D. R., and Cowen, D. S. (2003). Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J. Neurochem. 87, 1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x
Liu, J., Akay, T., Hedlund, P. B., Pearson, K. G., and Jordan, L. M. (2009). Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J. Neurophysiol. 102, 337–348. doi: 10.1152/jn.91239.2008
Logue, S. F., and Gould, T. J. (2014). The neural and genetic basis of executive function: attention, cognitive flexibility and response inhibition. Pharmacol. Biochem. Behav. 123C, 45–54. doi: 10.1016/j.pbb.2013.08.007
Lovenberg, T. W., Baron, B. M., de Lecea, L., Miller, J. D., Prosser, R. A., Rea, M. A., et al. (1993). A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11, 449–458. doi: 10.1016/0896-6273(93)90149-L
Luscher, C., and Huber, K. M. (2010). Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459. doi: 10.1016/j.neuron.2010.01.016
Magee, J. C. (1998). Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. 18, 7613–7624.
Magee, J. C. (1999). Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 848. doi: 10.1038/12229
Manuel-Apolinar, L., and Meneses, A. (2004). 8-OH-DPAT facilitated memory consolidation and increased hippocampal and cortical cAMP production. Behav. Brain Res. 148, 179–184. doi: 10.1016/S0166-4328(03)00186-4
Marazziti, D., Muratori, F., Cesari, A., Masala, I., Baroni, S., Giannaccini, G., et al. (2000). Increased density of the platelet serotonin transporter in autism. Pharmacopsychiatry 33, 165–168. doi: 10.1055/s-2000-7588
Martin-Cora, F. J., and Pazos, A. (2004). Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br. J. Pharmacol. 141, 92–104. doi: 10.1038/sj.bjp.0705576
Matthys, A., Haegeman, G., Van Craenenbroeck, K., and Vanhoenacker, P. (2011). Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol. Neurobiol. 43, 228–253. doi: 10.1007/s12035-011-8175-3
McBride, P. A., Anderson, G. M., Hertzig, M. E., Snow, M. E., Thompson, S. M., Khait, V. D., et al. (1998). Effects of diagnosis, race and puberty on platelet serotonin levels in autism and mental retardation. J. Am. Acad. Child Adolesc. Psychiatry 37, 767–776. doi: 10.1097/00004583-199807000-00017
McCormick, D. A., and Pape, H. C. (1990). Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 431, 291–318.
McNamara, I. M., Borella, A. W., Bialowas, L. A., and Whitaker-Azmitia, P. M. (2008). Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 1189, 203–214. doi: 10.1016/j.brainres.2007.10.063
Meneses, A. (2013). 5-HT systems: emergent targets for memory formation and memory alterations. Rev. Neurosci. 24, 629–664. doi: 10.1515/revneuro-2013-0026
Millan, M. J., Marin, P., Bockaert, J., and la Cour, C. M. (2008). Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 29, 454–464. doi: 10.1016/j.tips.2008.06.007
Mnie-Filali, O., Faure, C., Lambas-Senas, L., El Mansari, M., Belblidia, H., Gondard, E., et al. (2011). Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36, 1275–1288. doi: 10.1038/npp.2011.13
Monsma, F. J. Jr., Shen, Y., Ward, R. P., Hamblin, M. W., and Sibley, D. R. (1993). Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 43, 320–327.
Monti, J. M., and Jantos, H. (2014). The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev. Neurosci. 25, 429–437. doi: 10.1515/revneuro-2014-0016
Monti, J. M., Leopoldo, M., and Jantos, H. (2008). The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav. Brain Res. 191, 184–189. doi: 10.1016/j.bbr.2008.03.025
Monti, J. M., Leopoldo, M., and Jantos, H. (2014). Systemic administration and local microinjection into the central nervous system of the 5-HT7 receptor agonist LP-211 modify the sleep-wake cycle in the rat. Behav. Brain Res. 259, 321–329. doi: 10.1016/j.bbr.2013.11.030
Monti, J. M., Leopoldo, M., Jantos, H., and Lagos, P. (2012). Microinjection of the 5-HT7 receptor antagonist SB-269970 into the rat brainstem and basal forebrain: site-dependent effects on REM sleep. Pharmacol. Biochem. Behav. 102, 373–380. doi: 10.1016/j.pbb.2012.05.012
Moyer, J. R. Jr., Thompson, L. T., and Disterhoft, J. F. (1996). Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 16, 5536–5546.
Mozzachiodi, R., and Byrne, J. H. (2010). More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 33, 17–26. doi: 10.1016/j.tins.2009.10.001.
Mulder, E. J., Anderson, G. M., Kema, I. P., de Bildt, A., van Lang, N. D., den Boer, J. A., et al. (2004). Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 43, 491–499. doi: 10.1097/00004583-200404000-00016
Muneoka, K. T., and Takigawa, M. (2003). 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int. J. Dev. Neurosci. 21, 133–143. doi: 10.1016/S0736-5748(03)00029-7
Murphy, D. G., Daly, E., Schmitz, N., Toal, F., Murphy, K., Curran, S., et al. (2006). Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger’s syndrome: an in vivo SPECT study. Am. J. Psychiatry 163, 934–936. doi: 10.1176/appi.ajp.163.5.934
Naumenko, V. S., Kondaurova, E. M., and Popova, N. K. (2011). On the role of brain 5-HT7 receptor in the mechanism of hypothermia: comparison with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology 61, 1360–1365. doi: 10.1016/j.neuropharm.2011.08.022
Neumaier, J. F., Sexton, T. J., Yracheta, J., Diaz, A. M., and Brownfield, M. (2001). Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization and agonist stimulated cFos expression. J. Chem. Neuroanat. 21, 63–73. doi: 10.1016/S0891-0618(00)00092-2
Nichols, D. E., and Nichols, C. D. (2008). Serotonin receptors. Chem. Rev. 108, 1614–1641. doi: 10.1021/cr078224o
Nikiforuk, A. (2012). Selective blockade of 5-HT7 receptors facilitates attentional set-shifting in stressed and control rats. Behav. Brain Res. 226, 118–123. doi: 10.1016/j.bbr.2011.09.006
Nikiforuk, A., Kos, T., Fijal, K., Holuj, M., Rafa, D., and Popik, P. (2013). Effects of the selective 5-HT7 receptor antagonist SB-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS One 8:e66695. doi: 10.1371/journal.pone.0066695
Nikiforuk, A., and Popik, P. (2013). Amisulpride promotes cognitive flexibility in rats: the role of 5-HT7 receptors. Behav. Brain Res. 248, 136–140. doi: 10.1016/j.bbr.2013.04.008
Norum, J. H., Hart, K., and Levy, F. O. (2003). Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a). J. Biol. Chem. 278, 3098–3104. doi: 10.1074/jbc.M206237200
Oblak, A., Gibbs, T. T., and Blatt, G. J. (2013). Reduced serotonin receptor subtypes in a limbic and a neocortical region in autism. Autism Res. 6, 571–583. doi: 10.1002/aur.1317
Oh, M. M., McKay, B. M., Power, J. M., and Disterhoft, J. F. (2009). Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc. Natl. Acad. Sci. U S A 106, 1620–1625. doi: 10.1073/pnas.0807708106
Otmakhova, N. A., Lewey, J., Asrican, B., and Lisman, J. E. (2005). Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J. Neurophysiol. 94, 1413–1422. doi: 10.1152/jn.00217.2005
Perez-Garcia, G. S., and Meneses, A. (2005). Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav. Brain Res. 163, 136–140. doi: 10.1016/j.bbr.2005.04.014
Pericic, D., and Svob Strac, D. (2007). The role of 5-HT7 receptors in the control of seizures. Brain Res. 1141, 48–55. doi: 10.1016/j.brainres.2007.01.019
Pietropaolo, S., Guilleminot, A., Martin, B., D’Amato, F. R., and Crusio, W. E. (2011). Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One 6:e17073. doi: 10.1371/journal.pone.0017073
Piven, J., Tsai, G. C., Nehme, E., Coyle, J. T., Chase, G. A., and Folstein, S. E. (1991). Platelet serotonin, a possible marker for familial autism. J. Autism Dev. Disord. 21, 51–59. doi: 10.1007/BF02206997
Pytliak, M., Vargova, V., Mechirova, V., and Felsoci, M. (2011). Serotonin receptors—from molecular biology to clinical applications. Physiol. Res. 60, 15–25.
Ramoz, N., Reichert, J. G., Corwin, T. E., Smith, C. J., Silverman, J. M., Hollander, E., et al. (2006). Lack of evidence for association of the serotonin transporter gene SLC6A4 with autism. Biol. Psychiatry 60, 186–191. doi: 10.1016/j.biopsych.2006.01.009
Renner, U., Zeug, A., Woehler, A., Niebert, M., Dityatev, A., Dityateva, G., et al. (2012). Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 125, 2486–2499. doi: 10.1242/jcs.101337
Roberts, A. J., and Hedlund, P. B. (2012). The 5-HT7 receptor in learning and memory. Hippocampus 22, 762–771. doi: 10.1002/hipo.20938
Roberts, A. J., Krucker, T., Levy, C. L., Slanina, K. A., Sutcliffe, J. G., and Hedlund, P. B. (2004a). Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur. J. Neurosci. 19, 1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x
Roberts, C., Thomas, D. R., Bate, S. T., and Kew, J. N. (2004b). GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology 46, 935–941. doi: 10.1016/j.neuropharm.2004.01.010
Romano, E., Ruocco, L. A., Nativio, P., Lacivita, E., Ajmone-Cat, M. A., Boatto, G., et al. (2014). Modulatory effects following subchronic stimulation of brain 5-HT7-R system in mice and rats. Rev. Neurosci. 25, 383–400. doi: 10.1515/revneuro-2014-0007
Roth, B. L., Craigo, S. C., Choudhary, M. S., Uluer, A., and Monsma, F. J. Jr. (1994). Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 268, 1403–1410.
Ruat, M., Traiffort, E., Leurs, R., Tardivel-Lacombe, J., Diaz, J., Arrang, J. M., et al. (1993). Molecular cloning, characterization and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U S A 90, 8547–8551. doi: 10.1073/pnas.90.18.8547
Ruocco, L. A., Romano, E., Treno, C., Lacivita, E., Claudio, A., Gironi-Carnevale, U. A., et al. (2013). Emotional and risk seeking behavior after prepuberal subchronic or adult acute stimulation of 5-HT7-Rs in naples high excitability rats. Synapse 68, 159–167. doi: 10.1002/syn.21724
Sah, P. (1996). Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 19, 150–154. doi: 10.1016/S0166-2236(96)80026-9
Sarkisyan, G., and Hedlund, P. B. (2009). The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav. Brain Res. 202, 26–31. doi: 10.1016/j.bbr.2009.03.011
Sarkisyan, G., Roberts, A. J., and Hedlund, P. B. (2010). The 5-HT7 receptor as a mediator and modulator of antidepressant-like behavior. Behav. Brain Res. 209, 99–108. doi: 10.1016/j.bbr.2010.01.022
Schain, R. J., and Freedman, D. X. (1961). Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 58, 315–320.
Shen, Y., Monsma, F. J. Jr., Metcalf, M. A., Jose, P. A., Hamblin, M. W., and Sibley, D. R. (1993). Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 268, 18200–18204.
Shimizu, M., Nishida, A., Zensho, H., Miyata, M., and Yamawaki, S. (1998). Agonist-induced desensitization of adenylyl cyclase activity mediated by 5-hydroxytryptamine7 receptors in rat frontocortical astrocytes. Brain Res. 784, 57–62.
Smith, B. N., Sollars, P. J., Dudek, F. E., and Pickard, G. E. (2001). Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J. Biol. Rhythms 16, 25–38. doi: 10.1177/074873040101600104
Sodhi, M. S., and Sanders-Bush, E. (2004). Serotonin and brain development. Int. Rev. Neurobiol. 59, 111–174. doi: 10.1016/S0074-7742(04)59006-2
Speranza, L., Chambery, A., Di Domenico, M., Crispino, M., Severino, V., Volpicelli, F., et al. (2013). The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology 67, 155–167. doi: 10.1016/j.neuropharm.2012.10.026
Spooren, W., Lindemann, L., Ghosh, A., and Santarelli, L. (2012). Synapse dysfunction in autism: a molecular medicine approach to drug discovery in neurodevelopmental disorders. Trends Pharmacol. Sci. 33, 669–684. doi: 10.1016/j.tips.2012.09.004
Sugita, S., Shen, K. Z., and North, R. A. (1992). 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron 8, 199–203. doi: 10.1016/0896-6273(92)90121-S
Surges, R., Kukley, M., Brewster, A., Ruschenschmidt, C., Schramm, J., Baram, T. Z., et al. (2012). Hyperpolarization-activated cation current Ih of dentate gyrus granule cells is upregulated in human and rat temporal lobe epilepsy. Biochem. Biophys. Res. Commun. 420, 156–160. doi: 10.1016/j.bbrc.2012.02.133
Sutcliffe, J. S., Delahanty, R. J., Prasad, H. C., McCauley, J. L., Han, Q., Jiang, L., et al. (2005). Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 77, 265–279. doi: 10.1086/432648
Teitler, M., and Klein, M. T. (2012). A new approach for studying GPCR dimers: drug-induced inactivation and reactivation to reveal GPCR dimer function in vitro, in primary culture and in vivo. Pharmacol. Ther. 133, 205–217. doi: 10.1016/j.pharmthera.2011.10.007
Thomas, D. R., Melotto, S., Massagrande, M., Gribble, A. D., Jeffrey, P., Stevens, A. J., et al. (2003). SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 139, 705–714. doi: 10.1038/sj.bjp.0705290
Tokarski, K., Kusek, M., and Hess, G. (2011). 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J. Physiol. Pharmacol. 62, 535–540.
Tokarski, K., Kusek, M., and Hess, G. (2012a). Repeated blockade of 5-HT7 receptors depresses glutamatergic transmission in the rat frontal cortex. J. Physiol. Pharmacol. 63, 173–177.
Tokarski, K., Zahorodna, A., Bobula, B., and Hess, G. (2003). 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. 993, 230–234. doi: 10.1016/j.brainres.2003.09.015
Tokarski, K., Zelek-Molik, A., Duszynska, B., Satala, G., Bobula, B., Kusek, M., et al. (2012b). Acute and repeated treatment with the 5-HT7 receptor antagonist SB 269970 induces functional desensitization of 5-HT7 receptors in rat hippocampus. Pharmacol. Rep. 64, 256–265. doi: 10.1016/S1734-1140(12)70763-6
Tombaugh, G. C., Rowe, W. B., and Rose, G. M. (2005). The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J. Neurosci. 25, 2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005
Varnäs, K., Thomas, D. R., Tupala, E., Tiihonen, J., and Hall, H. (2004). Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 367, 313–316. doi: 10.1016/j.neulet.2004.06.025
Vasefi, M. S., Kruk, J. S., Heikkila, J. J., and Beazely, M. A. (2013). 5-Hydroxytryptamine type 7 receptor neuroprotection against NMDA-induced excitotoxicity is PDGFβ receptor dependent. J. Neurochem. 125, 26–36. doi: 10.1111/jnc.12157
Veenstra-VanderWeele, J., Muller, C. L., Iwamoto, H., Sauer, J. E., Owens, W. A., Shah, C. R., et al. (2012). Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. U S A 109, 5469–5474. doi: 10.1073/pnas.1112345109
Viguier, F., Michot, B., Hamon, M., and Bourgoin, S. (2013). Multiple roles of serotonin in pain control mechanisms–implications of 5-HT7 and other 5-HT receptor types. Eur. J. Pharmacol. 716, 8–16. doi: 10.1016/j.ejphar.2013.01.074
Viguier, F., Michot, B., Kayser, V., Bernard, J. F., Vela, J. M., Hamon, M., et al. (2012). GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology 63, 1093–1106. doi: 10.1016/j.neuropharm.2012.07.023
Wassink, T. H., Hazlett, H. C., Epping, E. A., Arndt, S., Dager, S. R., Schellenberg, G. D., et al. (2007). Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch. Gen. Psychiatry 64, 709–717. doi: 10.1001/archpsyc.64.6.709
Wesolowska, A., Nikiforuk, A., and Stachowicz, K. (2006a). Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur. J. Pharmacol. 553, 185–190. doi: 10.1016/j.ejphar.2006.09.064
Wesolowska, A., Nikiforuk, A., Stachowicz, K., and Tatarczynska, E. (2006b). Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 51, 578–586. doi: 10.1016/j.neuropharm.2006.04.017
Whitaker-Azmitia, P. M. (2005). Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int. J. Dev. Neurosci. 23, 75–83. doi: 10.1016/j.ijdevneu.2004.07.022
Witkin, J. M., Baez, M., Yu, J., Barton, M. E., and Shannon, H. E. (2007). Constitutive deletion of the serotonin-7 (5-HT7) receptor decreases electrical and chemical seizure thresholds. Epilepsy Res. 75, 39–45. doi: 10.1016/j.eplepsyres.2007.03.017
Witteveen, J. S., Middelman, A., van Hulten, J. A., Martens, G. J., Homberg, J. R., and Kolk, S. M. (2013). Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front. Cell. Neurosci. 7:143. doi: 10.3389/fncel.2013.00143
Woehler, A., and Ponimaskin, E. G. (2009). G protein–mediated signaling: same receptor, multiple effectors. Curr. Mol. Pharmacol. 2, 237–248. doi: 10.2174/1874467210902030237
Wolfensteller, U., and Ruge, H. (2012). Frontostriatal mechanisms in instruction-based learning as a hallmark of flexible goal-directed behavior. Front. Psychol. 3:192. doi: 10.3389/fpsyg.2012.00192
Woo, Y. S., Wang, H. R., and Bahk, W. M. (2013). Lurasidone as a potential therapy for bipolar disorder. Neuropsychiatr. Dis. Treat. 9, 1521–1529. doi: 10.2147/NDT.S51910
Wu, W. W., Chan, C. S., and Disterhoft, J. F. (2004). Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J. Neurophysiol. 92, 2346–2356. doi: 10.1152/jn.00977.2003
Yanarates, O., Dogrul, A., Yildirim, V., Sahin, A., Sizlan, A., Seyrek, M., et al. (2010). Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-Desmethyltramadol, via activation of descending serotonergic pathways. Anesthesiology 112, 696–710. doi: 10.1097/ALN.0b013e3181cd7920
Yang, E. J., Han, S. K., and Park, S. J. (2014). Functional expression of 5-HT7 receptor on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Brain Res. 1543, 73–82. doi: 10.1016/j.brainres.2013.10.041
Yang, Z., Liu, X., Yin, Y., Sun, S., and Deng, X. (2012). Involvement of 5-HT7 receptors in the pathogenesis of temporal lobe epilepsy. Eur. J. Pharmacol. 685, 52–58. doi: 10.1016/j.ejphar.2012.04.011
Keywords: serotonin, 5-HT7 receptor, synaptic function, autism spectrum disorders, Fragile X Syndrome
Citation: Ciranna L and Catania MV (2014) 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front. Cell. Neurosci. 8:250. doi: 10.3389/fncel.2014.00250
Received: 29 May 2014; Accepted: 06 August 2014;
Published online: 27 August 2014.
Edited by:
Hansen Wang, University of Toronto, CanadaReviewed by:
Hansen Wang, University of Toronto, CanadaMolly-Maureen Huntsman, University of Colorado Denver Anschutz Medical Campus, USA
Evgeni Ponimaskin, Hannover Medical School, Germany
Copyright © 2014 Ciranna and Catania. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Ciranna, Department of Biomedical Sciences, University of Catania, 6, Viale Andrea Doria, Catania 95125, Italy e-mail: ciranna@unict.it
 Lucia Ciranna
Lucia Ciranna Maria Vincenza Catania
Maria Vincenza Catania