Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus
- 1Doctorado en Ciencias en Biología Molecular en Medicina (DCBMM), CUCS, Universidad de Guadalajara, Guadalajara, Jalisco, México
- 2Instituto de Investigación en Ciencias Biomédicas (IICB), CUCS, Universidad de Guadalajara, Guadalajara, Jalisco, México
- 3Tecnológico de Monterrey, División de Biotecnología y Salud, Escuela de Medicina, Campus Guadalajara, Guadalajara, Jalisco, México
- 4Department of Pharmacology and Medical Chemistry, Faculty of Pharmacy School of Pharmacy, Institute of Biomedicine (IBUB), Centros de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), University of Barcelona, Barcelona, Spain
- 5Departamento de Ciencias Ambientales, Instituto de Neurociencias, CUCBA, Universidad de Guadalajara, Guadalajara, Jalisco, México
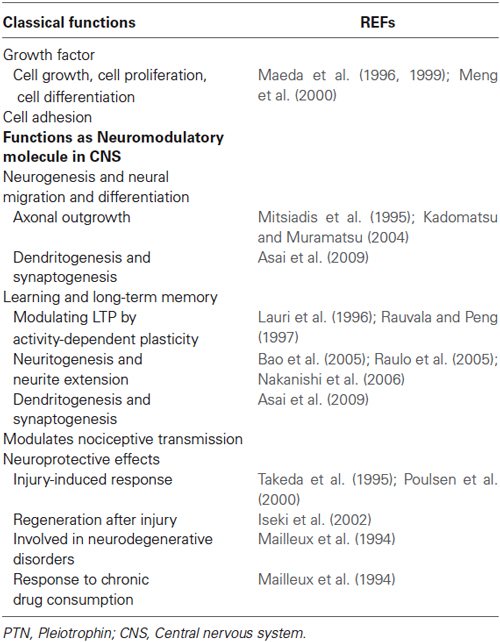
Pleiotrophin (PTN) is a secreted growth factor, and also a cytokine, associated with the extracellular matrix, which has recently starting to attract attention as a significant neuromodulator with multiple neuronal functions during development. PTN is expressed in several tissues, where its signals are generally related with cell proliferation, growth, and differentiation by acting through different receptors. In Central Nervous System (CNS), PTN exerts post-developmental neurotrophic and -protective effects, and additionally has been involved in neurodegenerative diseases and neural disorders. Studies in Drosophila shed light on some aspects of the different levels of regulatory control of PTN invertebrate homologs. Specifically in hippocampus, recent evidence from PTN Knock-out (KO) mice involves PTN functioning in learning and memory. In this paper, we summarize, discuss, and contrast the most recent advances and results that lead to proposing a PTN as a neuromodulatory molecule in the CNS, particularly in hippocampus.
Introduction
Pleiotrophin (PTN) is a secreted cell signaling cytokine that acts as growth factor associated with the extracellular matrix, which has recently started to come to the fore as a significant neuromodulator with multiple neuronal functions. PTN is an 18-KDa protein and has 168 amino acids. It was discovered practically simultaneously by several laboratories nearly 25 years ago; thus, it initially received several names as follows: HBGF-8 (Heparin-binding growth factor; Milner et al., 1989); HB-GAM (Heparin-binding growth-associated molecule; Rauvala, 1989; Merenmies and Rauvala, 1990); HBNF (Heparin-binding neutrophil factor; Kovesdi et al., 1990); OSF-1 (Osteoblast-specific factor 1; Tezuka et al., 1990), and HARP (Heparin affinity regulatory peptide; Courty et al., 1991).
PTN shares high homology (>50%) with another peptide, denominated Midkine (MK); both are highly conserved throughout evolution and are found in species ranging from Drosophila to humans (Kadomatsu and Muramatsu, 2004). This means that although both have many functions in common and participate in similar functions, they also possess more particular, specific, and non-redundant functions. It is evident when both are simultaneously knocked out in mice, they display severe abnormality phenotypes. However, when independently knocked out, PTN−/− and MDK−/− mice are far from being completely normal and exhibit moderate but different abnormalities (Muramatsu et al., 2006; Zou et al., 2006; Gramage and Herradón, 2010; Himburg et al., 2012; Vicente-Rodríguez et al., 2013), which denotes that although both peptides could present overlapping or similar functions, they are also clearly involved in different roles.
PTN could Signal Through a Multi-Receptor Complex
PTN signals are generally related with cell proliferation, growth and differentiation, but PTN has also has been involved in other functions by acting through different receptors (Figure 1). Mainly, PTN can bind and signal via Receptor protein tyrosine phosphatase ζ (RPTPζ), EC = 3.1.3.48 (Maeda et al., 1996, 1999; Meng et al., 2000), which is a transmembrane chondroitin sulfate proteoglycan present in two isoforms (shorter and full-length), which in turn also binds with various cell adhesion molecules (NrCAM, L1/Ng-CAM, contactin, N-CAM, and TAG1), growth factors (PTN, MK, and fibroblast growth factor (FGF-2), and extracellular matrix molecules (amphoterin, tenascin-C, and tenascin-R) (reviewed in Maeda et al., 2010). Under certain circumstances, PTN can act via Anaplastic Lymphoma Kinase (ALK) receptor (Stoica et al., 2001, 2002; Powers et al., 2002), although some evidences suggest that the action of PTN on ALK could occur through its previous interaction with RPTPζ (Perez-Pinera et al., 2007). Additionally, PTN; (1) promote neurite outgrowth via N-syndecan receptor (Raulo et al., 1994) or via Neuroglycan-C (NGC; Nakanishi et al., 2010), (2) interact with integrin ανβ3 (alpha nu beta 3) receptor, which is a mechano-sensitive cell membrane receptor, for cell adhesion (Mikelis et al., 2009), and (3) interact with Low-density lipoprotein (LDL) Receptor-related protein (LRP; Kadomatsu and Muramatsu, 2004). Additionally, two different species of PTN, PTN15 and PTN18, have been described (Lu et al., 2005), but their differential interaction or their affinities to different receptors has not yet been established, which adds another level of complexity to their physiological functioning.
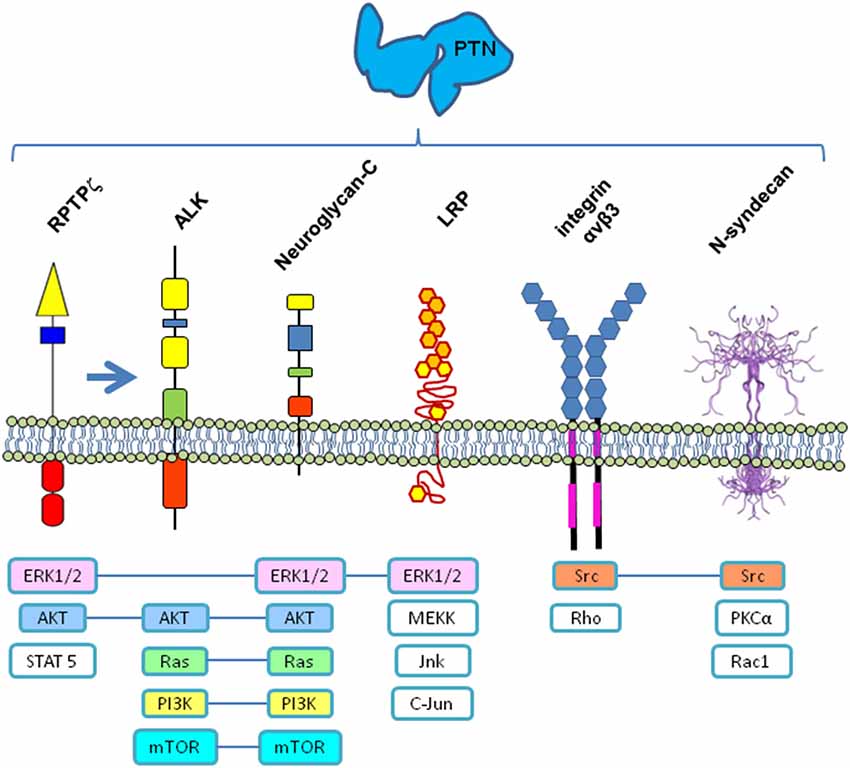
Figure 1. Receptors and signaling pathways possibly involved in PTN signaling. All or some of these membrane receptors could function as a multi-molecular complex coordinated to transduce the PTN signal into the cell by different signaling pathways. RPTPζ—Receptor protein tyrosine phosphatase ζ, EC = 3.1.3.48); ALK—Anaplastic Lymphoma Kinase; LRP—Low-density lipoprotein receptor-related protein; ERK1/2—Extracellular-Signal-Regulated Kinase; AKT—Serine/Threonine-specific protein kinase; STAT5—Signal transducer and activator of transcription 5; Ras—Rat sarcoma small GTP-ase; PI3K—Phosphatidylinositol-4,5-bisphosphate 3-kinase; mTOR—Mechanistic target of Rapamycin (serine/threonine kinase); MEKK—mitogen-activated protein Kinase/ERK kinase kinase 3; Jnk—c-Jun N-terminal kinase; Src—Sarcoma tyrosin kinase; Rho—Ras homology small GTPase; PKCα—Protein kinase C alpha; Rac1—Ras related small GTPase. N-syndecan structure from www.ebi.ac.uk
It has been recently proposed that PTN signaling may function through a multi-receptor complex (Xu et al., 2014), combining the previously mentioned receptors, and most probably other adaptor proteins, which interact under certain circumstances inside particular cell membrane microdomains, probably also associated with lipids in raft configuration, which could explain the variety of functions in different tissues, in terms of the combinatorial analysis of the elements present at each time and place. Then, PTN action over previously mentioned receptors could in turn signal through different signal pathways (Figure 1). Increasing our knowledge of the intricate molecular mechanisms involved would clarify the receptor complexes and signaling pathways implicated, as well as advance the discovery of other molecules involved, which in turn will lead us to fully explain its variety of functions.
Differential Expression of PTN Receptors During Development and in Adult could Indicate its Dissimilar Participation in Different Functions
Although during early development PTN expression is widely distributed in Central Nervous System (CNS; Li et al., 1990), expression of PTN in adult brain appears to be constitutive and apparently limited to only a few cell types in brain cortex, hippocampus, cerebellum and olfactory bulb (Wanaka et al., 1993; Lauri et al., 1996; Basille-Dugay et al., 2013), as well as in some striatal interneurons (Taravini et al., 2005). At these locations, the differential expression of its receptors could exist, which might partially explain its diverse actions. RPTPζ is expressed in glial cells as well as neurons. In hippocampal cells, it is located at the postsynaptic membrane of pyramidal neurons in adult (Hayashi et al., 2005), and its expression is modulated by spatial learning (Robles et al., 2003); therefore, it is involved in learning and long-term memory. Additionally, it is highly expressed following injury in areas of axonal sprouting and glial scarring (Snyder et al., 1996), and its expression is induced in inner molecular-layer astrocytes of the dentate gyrus of the sclerotic hippocampus in patients with epilepsy (Perosa et al., 2002). Also, it is involved in regulating dendritogenesis and synaptogenesis of hippocampal neurons in vitro (Asai et al., 2009). Therefore, PTN signaling through RPTPζ could be involved in modulation of hippocampal plasticity during learning and also during recovery after a lesion or in neuropathological situations, by modulating dendritogenesis and synaptogenesis. RPTP-ζ/β might be implicated in plastic rearrangements of nigrostriatal connections, such as sprouting of dopaminergic terminals or postsynaptic changes triggered by L-DOPA treatment in a model of Parkinson disease (Ferrario et al., 2008).
Likewise, ALK receptor is expressed in adult mammalian hippocampus and has also been implicated in neurogenesis, memory, and learning (Weiss et al., 2012). In addition, it has been involved in basal hippocampal progenitor proliferation and its deficiency induces alterations in behavioral tests (Bilsland et al., 2008). Although MK has been postulated to be the ligand for ALK receptor, at least in controlling sympathetic neurogenesis (Reiff et al., 2011), PTN also appears to be able to interact with this receptor (Stoica et al., 2001), although this remains controversial (Mathivet et al., 2007), and it appears that PTN performs its action on ALK thought its previous interaction with RPTPζ (Perez-Pinera et al., 2007).
It is clear that overlapping of PTN and MK activities can occur in some cases, but certainly not under all circumstances, as mistakenly suggested by Xu et al. (see Figure 3 in Xu et al., 2014). Although both peptides exhibit similar actions under certain physiological conditions, at least in the CNS, each also exerts diverse effects and performs different actions, depending on the cerebral region, as mentioned later.
Studies in Drosophila Enlighten some Aspects of the Different Levels of Regulatory Control of PTN Expression and Function in their Invertebrate Homologs
Drosophila homologs to MK and PTN are Miple1 and Miple2, with 20 and 24% identical to human MK and human PTN, respectively (Englund et al., 2006). However, they cannot be assigned as respective homologs, but only as members of the same family. Respective genes are arranged in tandem, suggesting that they have arisen as a result of a gene duplication event at some point of evolution. However, these secreted proteins are expressed in restricted, non-overlapping patterns, with Miple1 mainly expressed in the developing embryonic nervous system, while miple2 is strongly expressed in the developing gut endoderm (Englund et al., 2006). Therefore, had they been generated by gene duplication, they were clearly submitted to different selective pressure expression regulation, and consequently diverge in their expression pattern, and most probably in functioning. Thus, it will be relevant to elucidate, in useful model such as Drosophila, the molecular interactions of these peptides during complex developmental processes.
The messenger RNA (mRNA) 3′-Untranslated region (UTR) binding protein HOW (Held out wing) is able to post-translationally repress miple, downregulating its mRNA levels in mesoderm in order to enable proper mesoderm spreading during early embryogenesis in Drosophila (Toledano-Katchalski et al., 2007). This suggests that a similar mechanism could drive some regulatory action over PTN and MK expression in vertebrates.
Another point of regulation corresponds to the interaction of miple, as a signaling peptide, with other proteins. For example, by affecting the affinity of HTL ligands to the HTL receptor (Heartless, a Drosophila FGF receptor), thereby modulating the strength of HTL-dependent signaling (Toledano-Katchalski et al., 2007). Thus, it is feasible that PTN could interact with other peptides being a key modulator in the binding process to different complexes of receptors.
Interestingly, the combined expression pattern of Miple1 and Miple2 complements the expression pattern of the Drosophila ALK homolog (DAlk; Lorén et al., 2001, 2003). However, its ligand has been identified as a different peptide, namely Jelly belly (Jeb), which play roles in neuromuscular junction growth and function, early mesoderm development, and also in axon targeting of photoreceptors (Weiss et al., 2001; Englund et al., 2003; Lee et al., 2003; Bazigou et al., 2007; Rohrbough and Broadie, 2010). It is relevant to mention that Drosophila Jeb is not able to activate mouse ALK (Yang et al., 2007), and Jeb homologs in vertebrates have not yet been described. However, it is noteworthy that secreted Jeb contains a LDL receptor class A domain that contains 6 disulphide-bound cysteines (Bieri et al., 1995), and could constitute a binding site for LDL and calcium (Yamamoto et al., 1984). Given that LRP is a LDL receptor-related protein involved in PTN action in vertebrates (Kadomatsu and Muramatsu, 2004), it would be possible that Jeb signaling could be related with miple signaling and their vertebrate counterpart is unveiled to date.
Based on all previous cited evidences, and given the complexity of the molecular interactions in which PTN is clearly involved, it will be necessary to widely divulge approaches for disclosing its functioning. In this respect, one of the most useful approaches could be analysis by microarrays of the gene profile expression in PTN-defective Knock-out (KO) mice. Recently, in our laboratory, we performed these experiments and established the differential gene expression in the hippocampus of PTN KO mice (In preparation).
Differential Effects of PTN vs. MDK Indicate it as a Neuromodulatory Peptide in CNS, Particularly in Hippocampus
PTN and MK have been shown to induce and stimulate neuronal differentiation (Jung et al., 2004; Ishikawa et al., 2009; Luo et al., 2012). More specifically, PTN has been involved in lineage-specific differentiation of glial progenitor cells, axonal outgrowth, synaptic plasticity, and angiogenesis (Mitsiadis et al., 1995; Kadomatsu and Muramatsu, 2004). PTN participates in axon regeneration after injury, being highly expressed by reactive astrocytes (Iseki et al., 2002) as a source of trophic support for neurons in brain (Dugas et al., 2008) and rescuing nigral dopaminergic neurons from degeneration (Hida et al., 2007; Moses et al., 2008). However, its precise molecular mechanisms remain unknown.
In addition to these widely recognized roles of PTN, functionally, PTN−/− mice exhibited a delayed response to nociceptive stimulus in the tail-flick test (Gramage and Herradón, 2010), and clonidine-induced analgesia was significantly enhanced (Vicente-Rodríguez et al., 2013) when compared with MK−/− and Wild-type (WT+/+) mice. These evidences strongly suggest that endogenous PTN modulates nociceptive transmission at the spinal level.
In addition, PTN has been involved in neurodegenerative disorders and in response to chronic drug consumption. PTN is upregulated in cortex and caudate-putamen after injection of a cannabinol (Mailleux et al., 1994), and in nucleus accumbens after acute administration of amphetamine (Le Grevès, 2005); in addition, it is also highly upregulated in substantia nigra of patients with Parkinson disease (Marchionini et al., 2007) and treatment with L-Dopa increases PTN levels in striatum (Ferrario et al., 2004). Thus, it has been involved, as is MK (Prediger et al., 2011), in regulation of the survival and function of dopaminergic neurons (Jung et al., 2004). Taken together, this evidence supports the hypothesis that PTN is upregulated in neurodegenerative and addictive disorders in order to induce trophic or neuroprotective effects on dopaminergic neurons (Herradón and Pérez-García, 2014).
After PTN expression was described in hippocampus (Bloch et al., 1992; Vanderwinden et al., 1992; Wanaka et al., 1993), it was suggested that it plays a role in injury-induced response (Takeda et al., 1995; Poulsen et al., 2000) and activity-dependent plasticity (Lauri et al., 1996; Rauvala and Peng, 1997) in rat hippocampus, by affecting early, synapse-specific stages of LTP production (Lauri et al., 1998). Later, it was demonstrated, in PTN-deficient mice, that hippocampal slices exhibit a lowered threshold for induction of LTP (Amet et al., 2001) and that LTP was attenuated in mice overexpressing PTN (Pavlov et al., 2002), possibly by enhancing GABAergic inhibition in CA1 (Pavlov et al., 2006) and affecting recognition memory (del Olmo et al., 2009). Together, these evidences indicate that PTN could act as inducible signal to inhibit LTP in the hippocampus. Therefore, taken collectively, these evidences add a new role to the previous functions referred for PTN, thus functioning as a neuromodulatory factor in the hippocampus (Table 1). However, molecular evidence continues to be incomplete regarding the complex signaling system involved in PTN modulation.
To complete a whole view and to fully understand the modulatory role of PTN in CNS, and particularly in hippocampus, it is necessary first to establish which elements of the molecular machinery are present, and second, which are the ways in which they interact with each other. In this respect, immunohistochemical analyses reveal that RPTPζ and its substrate, GIT1/Cat-1, are co-localized in the processes of pyramidal cells in hippocampus and neocortex in rat brain, and PTN increases tyrosine phosphorylation of GIT1/Cat-1 in neuroblastoma B103 cells (Kawachi et al., 2001). Also, PSD-95/SAP90 family proteins, along with RPTPζ, are distributed in the dendrites of pyramidal neurons of hippocampus and neocortex (Kawachi et al., 1999). Additionally, it has been demonstrated that P190 RhoGAP activity, regulated by PTN/RPTPζ pathway, is involved in hippocampus-dependent memory formation through the downstream Rho/Rock pathway, which plays an important role in cell migration, axonal growth, and synaptic plasticity (Tamura et al., 2006). Another receptor involved in PTN signaling is N-syndecan receptor (Raulo et al., 1994), which due to deficiency in hippocampus exhibits enhanced LTP and altered hippocampus-dependent memory (Kaksonen et al., 2002). Moreover, this KO mouse is not responsive to PTN.
On the other hand, PTN regulates neurite extension and plasticity in pig hippocampal neurons in vitro, signaling through chondroitin sulfate/dermatan sulfate hybrid chains (Bao et al., 2005; Raulo et al., 2005); this action could involve chondroitin sulfate E as a binding partner, co-receptor, or genuine receptor for PTN (Deepa et al., 2002), but it is also reasonable to speculate that this could involve NGC, a brain-specific chondroitin sulfate proteoglycan involved in neuritogenesis (Nakanishi et al., 2006) and which interacts with PTN (Nakanishi et al., 2010).
Concluding Remarks
Therefore, the different actions of PTN as a neuromodulatory peptide (Table 1) could vary during development depending on the signaling pathways that it mainly activates. During early brain development, PTN implication in regulating neurogenesis and neural migration and differentiation, regulating axonal outgrowth, dendritogenesis, and synaptogenesis, could principally involve signaling through PTN/RPTPζ, and also through integrin αν β3, possibly acting coordinately. Later, in adult, the participation of PTN in learning and long-term memory, by modulating LTP by activity-dependent plasticity memory process in hippocampus, can again be principally mediated by its signaling through PTN/RPTPζ, possibly in combination with its signaling through N-syndecan pathway. Finally, its neuroprotective effects constitute a relevant role, suggesting that PTN signaling pathways are involved in neurodegenerative disorders, as well as in response to injuries and chronic drug consumption. Those signaling pathways may be functioning through a multi-molecular complex of receptors, combining previously mentioned receptors and other adaptor proteins, which interact inside membrane microdomains in raft configuration, which could explain each of these functions.
We are sure that a lot of molecules involved in PTN signaling pathways remain unknown to date. It is necessary to perform more integral studies, such as the use of proteomics and genomics approaches, as well as studies in vivo (employing PTN-KO) and in vitro (by mean of experiments with small interfering RNA [siRNA]), which will undoubtedly elucidate the complete molecular mechanisms involved.
Author Statement
Daniel Ortuño-Sahagún, Argelia E. Rojas-Mayorquín, Mercè Pallàs Conceived the work, drafted and revised critically.
Celia González-Castillo, Carolina Guzmán-Brambila, Acquire and compilates information.
All authors, Write and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by Universidad de Guadalajara (UdeG) grant 222769 PRO-SNI 2014 to Daniel Ortuño-Sahagún, and CONACyT-México grants 2012-180268 and PROMEP/103.5/12/8143 to Argelia E. Rojas-Mayorquín. Authors wish to thank Dra. Consuelo Morgado and to Frontiers reviewers for their critical review of the manuscript and constructive comments. Our apologies to authors whose works have not been reviewed and to those whose papers have not received the emphasis that they merit. We also apologize to authors whose work has not been appropriately cited due to limitations of space and/or to limitations of our knowledge.
References
Amet, L. E., Lauri, S. E., Hienola, A., Croll, S. D., Lu, Y., Levorse, J. M., et al. (2001). Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol. Cell. Neurosci. 17, 1014–1024. doi: 10.1006/mcne.2001.0998
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Asai, H., Yokoyama, S., Morita, S., Maeda, N., and Miyata, S. (2009). Functional difference of receptor-type protein tyrosine phosphatase zeta/beta isoforms in neurogenesis of hippocampal neurons. Neuroscience 164, 1020–1030. doi: 10.1016/j.neuroscience.2009.09.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bao, X., Mikami, T., Yamada, S., Faissner, A., Muramatsu, T., and Sugahara, K. (2005). Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J. Biol. Chem. 280, 9180–9191. doi: 10.1074/jbc.m413423200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Basille-Dugay, M., Hamza, M. M., Tassery, C., Parent, B., Raoult, E., Bénard, M., et al. (2013). Spatio-temporal characterization of the pleiotrophinergic system in mouse cerebellum: evidence for its key role during ontogenesis. Exp. Neurol. 247, 537–551. doi: 10.1016/j.expneurol.2013.02.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bazigou, E., Apitz, H., Johansson, J., Lorén, C. E., Hirst, E. M., Chen, P. L., et al. (2007). Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell 128, 961–975. doi: 10.1016/j.cell.2007.02.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bieri, S., Djordjevic, J. T., Daly, N. L., Smith, R., and Kroon, P. A. (1995). Disulfide bridges of a cysteine-rich repeat of the LDL receptor ligand-binding domain. Biochemistry 34, 13059–13065. doi: 10.1021/bi00040a017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bilsland, J. G., Wheeldon, A., Mead, A., Znamenskiy, P., Almond, S., Waters, K. A., et al. (2008). Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology 33, 685–700. doi: 10.1038/sj.npp.1301446
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloch, B., Normand, E., Kovesdi, I., and Bohlen, P. (1992). Expression of the HBNF (heparin-binding neurite-promoting factor) gene in the brain of fetal, neonatal and adult rat: an in situ hybridization study. Brain Res. Dev. Brain Res. 70, 267–278. doi: 10.1016/0165-3806(92)90206-c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Courty, J., Dauchel, M. C., Caruelle, D., Perderiset, M., and Barritault, D. (1991). Mitogenic properties of a new endothelial cell growth factor related to pleiotrophin. Biochem. Biophys. Res. Commun. 180, 145–151. doi: 10.1016/s0006-291x(05)81267-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Deepa, S. S., Umehara, Y., Higashiyama, S., Itoh, N., and Sugahara, K. (2002). Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 277, 43707–43716. doi: 10.1074/jbc.m207105200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
del Olmo, N., Gramage, E., Alguacil, L. F., Pérez-Pinera, P., Deuel, T. F., and Herradón, G. (2009). Pleiotrophin inhibits hippocampal long-term potentiation: a role of pleiotrophin in learning and memory. Growth Factors 27, 189–194. doi: 10.1080/08977190902906859
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dugas, J. C., Mandemakers, W., Rogers, M., Ibrahim, A., Daneman, R., and Barres, B. A. (2008). A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J. Neurosci. 28, 8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Englund, C., Birve, A., Falileeva, L., Grabbe, C., and Palmer, R. H. (2006). Miple1 and miple2 encode a family of MK/PTN homologues in Drosophila melanogaster. Dev. Genes Evol. 216, 10–18. doi: 10.1007/s00427-005-0025-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Englund, C., Lorén, C. E., Grabbe, C., Varshney, G. K., Deleuil, F., Hallberg, B., et al. (2003). Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature 425, 512–516. doi: 10.1038/nature01950
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ferrario, J. E., Rojas-Mayorquín, A. E., Saldaña-Ortega, M., Salum, C., Gomes, M. Z., Hunot, S., et al. (2008). Pleiotrophin receptor RPTP-zeta/beta expression is up-regulated by L-DOPA in striatal medium spiny neurons of parkinsonian rats. J. Neurochem. 107, 443–452. doi: 10.1111/j.1471-4159.2008.05640.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ferrario, J. E., Taravini, I. R., Mourlevat, S., Stefano, A., Delfino, M. A., Raisman-Vozari, R., et al. (2004). Differential gene expression induced by chronic levodopa treatment in the striatum of rats with lesions of the nigrostriatal system. J. Neurochem. 90, 1348–1358. doi: 10.1111/j.1471-4159.2004.02595.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gramage, E., and Herradón, G. (2010). Genetic deletion of pleiotrophin leads to disruption of spinal nociceptive transmission: evidence for pleiotrophin modulation of morphine-induced analgesia. Eur. J. Pharmacol. 647, 97–102. doi: 10.1016/j.ejphar.2010.08.029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hayashi, N., Oohira, A., and Miyata, S. (2005). Synaptic localization of receptor-type protein tyrosine phosphatase zeta/beta in the cerebral and hippocampal neurons of adult rats. Brain Res. 1050, 163–169. doi: 10.1016/j.brainres.2005.05.047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herradón, G., and Pérez-García, C. (2014). Targeting midkine and pleiotrophin signalling pathways in addiction and neurodegenerative disorders: recent progress and perspectives. Br. J. Pharmacol. 171, 837–848. doi: 10.1111/bph.12312
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hida, H., Masuda, T., Sato, T., Kim, T. S., Misumi, S., and Nishino, H. (2007). Pleiotrophin promotes functional recovery after neural transplantation in rats. Neuroreport 18, 179–183. doi: 10.1097/wnr.0b013e328011398e
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Himburg, H. A., Harris, J. R., Ito, T., Daher, P., Russell, J. L., Quarmyne, M., et al. (2012). Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2, 964–975. doi: 10.1016/j.celrep.2012.09.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Iseki, K., Hagino, S., Mori, T., Zhang, Y., Yokoya, S., Takaki, H., et al. (2002). Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue. Glia 39, 1–9. doi: 10.1002/glia.10078
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ishikawa, E., Ooboshi, H., Kumai, Y., Takada, J., Nakamura, K., Ago, T., et al. (2009). Midkine gene transfer protects against focal brain ischemia and augments neurogenesis. J. Neurol. Sci. 285, 78–84. doi: 10.1016/j.jns.2009.05.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jung, C. G., Hida, H., Nakahira, K., Ikenaka, K., Kim, H. J., and Nishino, H. (2004). Pleiotrophin mRNA is highly expressed in neural stem (progenitor) cells of mouse ventral mesencephalon and the product promotes production of dopaminergic neurons from embryonic stem cell-derived nestin-positive cells. FASEB J. 18, 1237–1239. doi: 10.1096/fj.03-0927fje
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kadomatsu, K., and Muramatsu, T. (2004). Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 204, 127–143. doi: 10.1016/s0304-3835(03)00450-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kaksonen, M., Pavlov, I., Võikar, V., Lauri, S. E., Hienola, A., Riekki, R., et al. (2002). Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 21, 158–172. doi: 10.1006/mcne.2002.1167
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kawachi, H., Fujikawa, A., Maeda, N., and Noda, M. (2001). Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase zeta/beta by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. U S A 98, 6593–6598. doi: 10.1073/pnas.041608698
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kawachi, H., Tamura, H., Watakabe, I., Shintani, T., Maeda, N., and Noda, M. (1999). Protein tyrosine phosphatase ζ/RPTPα interacts with PSD-95/SAP90 family. Brain Res. Mol. Brain Res. 72, 47–54. doi: 10.1016/s0169-328x(99)00204-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kovesdi, I., Fairhurst, J. L., Kretschmer, P. J., and Böhlen, P. (1990). Heparin-binding neurotrophic factor (HBNF) and MK, members of a new family of homologous, developmentally regulated proteins. Biochem. Biophys. Res. Commun. 172, 850–854. doi: 10.1016/0006-291x(90)90753-a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lauri, S. E., Rauvala, H., Kaila, K., and Taira, T. (1998). Effect of heparin-binding growth-associated molecule (HB-GAM) on synaptic transmission and early LTP in rat hippocampal slices. Eur. J. Neurosci. 10, 188–194. doi: 10.1046/j.1460-9568.1998.00039.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lauri, S. E., Taira, T., Kaila, K., and Rauvala, H. (1996). Activity-induced enhancement of HB-GAM expression in rat hippocampal slices. Neuroreport 7, 1670–1674. doi: 10.1097/00001756-199607080-00029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, H. H., Norris, A., Weiss, J. B., and Frasch, M. (2003). Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature 425, 507–512. doi: 10.1038/nature01916
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Le Grevès, P. (2005). Pleiotrophin gene transcription in the rat nucleus accumbens is stimulated by an acute dose of amphetamine. Brain Res. Bull. 65, 529–532. doi: 10.1016/j.brainresbull.2005.03.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, Y. S., Milner, P. G., Chauhan, A. K., Watson, M. A., Hoffman, R. M., Kodner, C. M., et al. (1990). Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science 250, 1690–1694. doi: 10.1126/science.2270483
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lorén, C. E., Englund, C., Grabbe, C., Hallberg, B., Hunter, T., and Palmer, R. H. (2003). A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 4, 781–786. doi: 10.1038/sj.embor.embor897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lorén, C. E., Scully, A., Grabbe, C., Edeen, P. T., Thomas, J., McKeown, M., et al. (2001). Identification and characterization of DAlk: a novel Drosophila melanogaster RTK which drives ERK activation in vivo. Genes Cells 6, 531–544. doi: 10.1046/j.1365-2443.2001.00440.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lu, K. V., Jong, K. A., Kim, G. Y., Singh, J., Dia, E. Q., Yoshimoto, K., et al. (2005). Differential induction of glioblastoma migration and growth by two forms of pleiotrophin. J. Biol. Chem. 280, 26953–26964. doi: 10.1074/jbc.m502614200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luo, J., Uribe, R. A., Hayton, S., Calinescu, A. A., Gross, J. M., and Hitchcock, P. F. (2012). Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev. 7:33. doi: 10.1186/1749-8104-7-33
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maeda, N., Fukazawa, N., and Ishii, M. (2010). Chondroitin sulfate proteoglycans in neural development and plasticity. Front. Biosci. (Landmark Ed.) 15, 626–644. doi: 10.2741/3637
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maeda, N., Ichihara-Tanaka, K., Kimura, T., Kadomatsu, K., Muramatsu, T., and Noda, M. (1999). A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J. Biol. Chem. 274, 12474–12479. doi: 10.1074/jbc.274.18.12474
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maeda, N., Nishiwaki, T., Shintani, T., Hamanaka, H., and Noda, M. (1996). 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM). J. Biol. Chem. 271, 21446–21452. doi: 10.1074/jbc.271.35.21446
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mailleux, P., Preud’homme, X., Albala, N., Vanderwinden, J. M., and Vanderhaeghen, J. J. (1994). Δ-9-Tetrahydrocannabinol regulates gene expression of the growth factor pleiotrophin in the forebrain. Neurosci. Lett. 175, 25–27. doi: 10.1016/0304-3940(94)91069-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marchionini, D. M., Lehrmann, E., Chu, Y., He, B., Sortwell, C. E., Becker, K. G., et al. (2007). Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson’s disease. Brain Res. 1147, 77–88. doi: 10.1016/j.brainres.2007.02.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mathivet, T., Mazot, P., and Vigny, M. (2007). In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell. Signal. 19, 2434–2443. doi: 10.1016/j.cellsig.2007.07.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Meng, K., Rodriguez-Peña, A., Dimitrov, T., Chen, W., Yamin, M., Noda, M., et al. (2000). Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc. Natl. Acad. Sci. U S A 97, 2603–2608. doi: 10.1073/pnas.020487997
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Merenmies, J., and Rauvala, H. (1990). Molecular cloning of the 18-kDa growth-associated protein of developing brain. J. Biol. Chem. 265, 16721–16724.
Mikelis, C., Sfaelou, E., Koutsioumpa, M., Kieffer, N., and Papadimitriou, E. (2009). Integrin alpha(v)beta(3) is a pleiotrophin receptor required for pleiotrophin-induced endothelial cell migration through receptor protein tyrosine phosphatase beta/zeta. FASEB J. 23, 1459–1469. doi: 10.1096/fj.08-117564
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Milner, P. G., Li, Y. S., Hoffman, R. M., Kodner, C. M., Siegel, N. R., and Deuel, T. F. (1989). A novel 17 kD heparin-binding growth factor (HBGF-8) in bovine uterus: purification and N-terminal amino acid sequence. Biochem. Biophys. Res. Commun. 165, 1096–1103. doi: 10.1016/0006-291x(89)92715-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mitsiadis, T. A., Salmivirta, M., Muramatsu, T., Muramatsu, H., Rauvala, H., Lehtonen, E., et al. (1995). Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121, 37–51.
Moses, D., Drago, J., Teper, Y., Gantois, I., Finkelstein, D. I., and Horne, M. K. (2008). Fetal striatum- and ventral mesencephalon-derived expanded neurospheres rescue dopaminergic neurons in vitro and the nigro-striatal system in vivo. Neuroscience 154, 606–620. doi: 10.1016/j.neuroscience.2008.03.058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Muramatsu, H., Zou, P., Kurosawa, N., Ichihara-Tanaka, K., Maruyama, K., Inoh, K., et al. (2006). Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells 11, 1405–1417. doi: 10.1111/j.1365-2443.2006.01028.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nakanishi, K., Aono, S., Hirano, K., Kuroda, Y., Ida, M., Tokita, Y., et al. (2006). Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J. Biol. Chem. 281, 24970–24978. doi: 10.1074/jbc.m601498200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nakanishi, K., Tokita, Y., Aono, S., Ida, M., Matsui, F., Higashi, Y., et al. (2010). Neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, interacts with pleiotrophin, a heparin-binding growth factor. Neurochem. Res. 35, 1131–1137. doi: 10.1007/s11064-010-0164-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pavlov, I., Rauvala, H., and Taira, T. (2006). Enhanced hippocampal GABAergic inhibition in mice overexpressing heparin-binding growth-associated molecule. Neuroscience 139, 505–511. doi: 10.1016/j.neuroscience.2005.11.070
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pavlov, I., Võikar, V., Kaksonen, M., Lauri, S. E., Hienola, A., Taira, T., et al. (2002). Role of heparin-binding growth-associated molecule (HB-GAM) in hippocampal LTP and spatial learning revealed by studies on overexpressing and knockout mice. Mol. Cell. Neurosci. 20, 330–342. doi: 10.1006/mcne.2002.1104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Perez-Pinera, P., Zhang, W., Chang, Y., Vega, J. A., and Deuel, T. F. (2007). Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 282, 28683–28690. doi: 10.1074/jbc.m704505200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Perosa, S. R., Porcionatto, M. A., Cukiert, A., Martins, J. R., Passeroti, C. C., Amado, D., et al. (2002). Glycosaminoglycan levels and proteoglycan expression are altered in the hippocampus of patients with mesial temporal lobe epilepsy. Brain Res. Bull. 58, 509–516. doi: 10.1016/s0361-9230(02)00822-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poulsen, F. R., Lagord, C., Courty, J., Pedersen, E. B., Barritault, D., and Finsen, B. (2000). Increased synthesis of heparin affin regulatory peptide in the perforant path lesioned mouse hippocampal formation. Exp. Brain Res. 135, 319–330. doi: 10.1007/s002210000536
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Powers, C., Aigner, A., Stoica, G. E., McDonnell, K., and Wellstein, A. (2002). Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J. Biol. Chem. 277, 14153–14158. doi: 10.1074/jbc.m112354200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Prediger, R. D., Rojas-Mayorquin, A. E., Aguiar, A. S. Jr., Chevarin, C., Mongeau, R., Hamon, M., et al. (2011). Mice with genetic deletion of the heparin-binding growth factor midkine exhibit early preclinical features of Parkinson’s disease. J. Neural Transm. 118, 1215–1225. doi: 10.1007/s00702-010-0568-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raulo, E., Chernousov, M. A., Carey, D. J., Nolo, R., and Rauvala, H. (1994). Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J. Biol. Chem. 269, 12999–13004.
Raulo, E., Tumova, S., Pavlov, I., Pekkanen, M., Hienola, A., Klankki, E., et al. (2005). The two thrombospondin type I repeat domains of the heparin-binding growth-associated molecule bind to heparin/heparan sulfate and regulate neurite extension and plasticity in hippocampal neurons. J. Biol. Chem. 280, 41576–41583. doi: 10.1074/jbc.m506457200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rauvala, H. (1989). An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 8, 2933–2941.
Rauvala, H., and Peng, H. B. (1997). HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog. Neurobiol. 52, 127–144. doi: 10.1016/s0301-0082(97)00007-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Reiff, T., Huber, L., Kramer, M., Delattre, O., Janoueix-Lerosey, I., and Rohrer, H. (2011). Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development 138, 4699–4708. doi: 10.1242/dev.072157
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robles, Y., Vivas-Mejía, P. E., Ortiz-Zuazaga, H. G., Félix, J., Ramos, X., and Peña de Ortiz, S. (2003). Hippocampal gene expression profiling in spatial discrimination learning. Neurobiol. Learn. Mem. 80, 80–95. doi: 10.1016/s1074-7427(03)00025-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rohrbough, J., and Broadie, K. (2010). Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses is regulated by mind the gap. Development 137, 3523–3533. doi: 10.1242/dev.047878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Snyder, S. E., Li, J., Schauwecker, P. E., McNeill, T. H., and Salton, S. R. (1996). Comparison of RPTP ζ/beta, phosphacan and trkB mRNA expression in the developing and adult rat nervous system and induction of RPTP ζ/beta and phosphacan mRNA following brain injury. Brain Res. Mol. Brain Res. 40, 79–96. doi: 10.1016/0169-328x(96)00039-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stoica, G. E., Kuo, A., Aigner, A., Sunitha, I., Souttou, B., Malerczyk, C., et al. (2001). Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 276, 16772–16779. doi: 10.1074/jbc.m010660200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stoica, G. E., Kuo, A., Powers, C., Bowden, E. T., Sale, E. B., Riegel, A. T., et al. (2002). Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998. doi: 10.1074/jbc.m205749200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takeda, A., Onodera, H., Sugimoto, A., Itoyama, Y., Kogure, K., Rauvala, H., et al. (1995). Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience 68, 57–64. doi: 10.1016/0306-4522(95)00110-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tamura, H., Fukada, M., Fujikawa, A., and Noda, M. (2006). Protein tyrosine phosphatase receptor type Z is involved in hippocampus-dependent memory formation through dephosphorylation at Y1105 on p190 RhoGAP. Neurosci. Lett. 399, 33–38. doi: 10.1016/j.neulet.2006.01.045
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Taravini, I. R., Ferrario, J. E., Delbe, J., Ginestet, L., Debeir, T., Courty, J., et al. (2005). Immunodetection of heparin-binding growth associated molecule (pleiotrophin) in striatal interneurons. Brain Res. 1066, 196–200. doi: 10.1016/j.brainres.2005.10.055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tezuka, K., Takeshita, S., Hakeda, Y., Kumegawa, M., Kikuno, R., and Hashimoto-Gotoh, T. (1990). Isolation of mouse and human cDNA clones encoding a protein expressed specifically in osteoblasts and brain tissues. Biochem. Biophys. Res. Commun. 173, 246–251. doi: 10.1016/s0006-291x(05)81048-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Toledano-Katchalski, H., Nir, R., Volohonsky, G., and Volk, T. (2007). Post-transcriptional repression of the Drosophila midkine and pleiotrophin homolog miple by HOW is essential for correct mesoderm spreading. Development 134, 3473–3481. doi: 10.1242/dev.006080
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vanderwinden, J. M., Mailleux, P., Schiffmann, S. N., and Vanderhaeghen, J. J. (1992). Cellular distribution of the new growth factor pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat. Embryol. (Berl) 186, 387–406. doi: 10.1007/bf00185989
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vicente-Rodríguez, M., Gramage, E., Herradón, G., and Pérez-García, C. (2013). Phosphoproteomic analysis of the striatum from pleiotrophin knockout and midkine knockout mice treated with cocaine reveals regulation of oxidative stress-related proteins potentially underlying cocaine-induced neurotoxicity and neurodegeneration. Toxicology 314, 166–173. doi: 10.1016/j.tox.2013.09.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wanaka, A., Carroll, S. L., and Milbrandt, J. (1993). Developmentally regulated expression of pleiotrophin, a novel heparin binding growth factor, in the nervous system of the rat. Brain Res. Dev. Brain Res. 72, 133–144. doi: 10.1016/0165-3806(93)90166-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weiss, J. B., Suyama, K. L., Lee, H. H., and Scott, M. P. (2001). Jelly belly: a Drosophila LDL receptor repeat-containing signal required for mesoderm migration and differentiation. Cell 107, 387–398. doi: 10.1016/S0092-8674(01)00540-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weiss, J. B., Xue, C., Benice, T., Xue, L., Morris, S. W., and Raber, J. (2012). Anaplastic lymphoma kinase and leukocyte tyrosine kinase: functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol. Biochem. Behav. 100, 566–574. doi: 10.1016/j.pbb.2011.10.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xu, C., Zhu, S., Wu, M., Han, W., and Yu, Y. (2014). Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN). Biol. Pharm. Bull. 37, 511–520. doi: 10.1248/bpb.b13-00845
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yamamoto, T., Davis, C. G., Brown, M. S., Schneider, W. J., Casey, M. L., Goldstein, J. L., et al. (1984). The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell 39, 27–38. doi: 10.1016/0092-8674(84)90188-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yang, H. L., Eriksson, T., Vernersson, E., Vigny, M., Hallberg, B., and Palmer, R. H. (2007). The ligand Jelly Belly (Jeb) activates the Drosophila Alk RTK to drive PC12 cell differentiation, but is unable to activate the mouse ALK RTK. J. Exp. Zool. B Mol. Dev. Evol. 308, 269–282. doi: 10.1002/jez.b.21146
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zou, P., Muramatsu, H., Sone, M., Hayashi, H., Nakashima, T., and Muramatsu, T. (2006). Mice doubly deficient in the midkine and pleiotrophin genes exhibit deficits in the expression of beta-tectorin gene and in auditory response. Lab. Invest. 86, 645–653. doi: 10.1038/labinvest.3700428
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: pleiotrophin, neuromodulation, hippocampus, neuropeptide, miple
Citation: González-Castillo C, Ortuño-Sahagún D, Guzmán-Brambila C, Pallàs M and Rojas-Mayorquín AE (2015) Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus. Front. Cell. Neurosci. 8:443. doi: 10.3389/fncel.2014.00443
Received: 13 October 2014; Accepted: 10 December 2014;
Published online: 08 January 2015.
Edited by:
Victoria Campos, Instituto Nacional De Neurologia Y Neurocirugia, MéxicoReviewed by:
Corette J. Wierenga, Utrecht University, NetherlandsTakumi Takizawa, Gunma University, Japan
Copyright © 2015 González-Castillo, Ortuño-Sahagún, Guzmán-Brambila, Pallàs and Rojas-Mayorquín. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Argelia Esperanza Rojas-Mayorquín, Departamento de Ciencias Ambientales, Instituto de Neurociencias, CUCBA, Universidad de Guadalajara, Francisco de Quevedo 180, Col. Arcos Vallarta, Guadalajara, 44130 Jalisco, México e-mail: argelia.rojas@cucba.udg.mx
†These authors have contributed equally to this work.
 Celia González-Castillo
Celia González-Castillo Daniel Ortuño-Sahagún
Daniel Ortuño-Sahagún Carolina Guzmán-Brambila3
Carolina Guzmán-Brambila3  Mercè Pallàs
Mercè Pallàs Argelia Esperanza Rojas-Mayorquín
Argelia Esperanza Rojas-Mayorquín