Physiological consequences of doublet discharges on motoneuronal firing and motor unit force
- 1Department of Neurobiology, University School of Physical Education, Poznań, Poland
- 2Institute of Biophysics and Biomedical Engineering, Bulgarian Academy of Sciences, Sofia, Bulgaria
The double discharges are observed at the onset of contractions of mammalian motor units (MUs), especially during their recruitment to strong or fast movements. Doublets lead to MU force increase and improve ability of muscles to maintain high force during prolonged contractions. In this review we discuss an ability to produce doublets by fast and slow motoneurons (MNs), their influence on the course of action potential afterhyperpolarization (AHP) as well as its role in modulation of the initial stage of the firing pattern of MNs. In conclusion, a generation of doublets is an important strategy of motor control, responsible for fitting the motoneuronal firing rate to the optimal for MUs at the start of their contraction, necessary for increment of muscle force.
Introduction
A pair of action potentials at short interspike intervals (ISIs, below 10 ms) called “doublet” (Simpson, 1969) has been frequently observed at the beginning of discharge pattern of motoneurons (MNs). Such initial doublets in trains of action potentials of motor units (MUs) have been recorded from numerous human muscles during different types of voluntary activity (Person and Kudina, 1972; Kudina, 1974; Bawa and Calancie, 1983; Kudina and Alexeeva, 1992; Garland and Griffin, 1999) or from animal muscles during locomotion (Zajac and Young, 1980b; Hennig and Lømo, 1985; Hoffer et al., 1987; Gorassini et al., 2000). Existence of doublets has also been confirmed in electrophysiological studies performed on MNs innervating inspiratory (Kirkwood and Munson, 1996) and locomotor muscles (Spielmann et al., 1993) of cat or hind limb muscles of rat (Mrówczyński et al., 2010; Bączyk et al., 2013) during their activation with intracellular current injection.
Many experiments on human muscles (Bawa and Calancie, 1983; Kirkwood and Munson, 1996; Van Cutsem et al., 1998; Garland and Griffin, 1999; Christie and Kamen, 2006) and MUs of various animal species (Zajac and Young, 1980a; Hennig and Lømo, 1987; Sandercock and Heckman, 1997) have suggested that doublets are responsible for considerable enhancement of muscle output force. From this reason, doublets are considered as a special strategy of the central nervous system, which improves efficiency of a motor task requiring large force especially at early stage of muscle contraction (Garland and Griffin, 1999; Kudina and Andreeva, 2013).
The occurrence of doublets is also an important mechanism of adaptation to increasing level of muscle activity. Binder-Macleod and Barker (1991) have demonstrated that effects of doublet in force enhancement are greater in fatigued than in unfatigued muscles. Furthermore, a substantial increase of a number of doublets in muscles of trained athletes during dynamic voluntary contractions have suggested their contribution to an increase in the speed of contraction after the dynamic training (Griffin et al., 1998; Van Cutsem et al., 1998).
The paper aims to describe physiological consequences of the doublet occurrence for the afterhyperpolarization (AHP) parameters following the action potentials and consequently for initial ISIs in a pattern of motoneuronal discharges, which have not been described in previous reviews concerning the doublets (Garland and Griffin, 1999; Kudina and Andreeva, 2013). The consequences of the doublet discharges are discussed in relation to the MU force development.
The Incidence of Doublets in Motoneurones
Experimental data obtained in animal studies suggest that the ability to produce doublets is attributed rather to fast than to slow MNs (Gorassini et al., 2000). However, electrophysiological studies with the intracellular injection of depolarizing current into spinal MNs of cat (Spielmann et al., 1993) and rat (Mrówczyński et al., 2010; Bączyk et al., 2013) have shown that doublets were produced by both slow and fast MNs. Therefore, it is likely that specific organization of supraspinal pathways descending rather to fast than slow MNs is responsible for doublet discharges during strong contractions in natural conditions.
Some studies indicate that the occurrence of doublets in the pattern of motoneuronal discharges depends on motoneuronal excitability (Christie and Kamen, 2006) and on power of synaptic inputs to MNs (Gorassini et al., 2000). Experiments with intracellular injection of a depolarizing current to rat MNs have demonstrated that doublet discharges are generated at current intensity 2.1–2.4 and 2.1–3.24 times higher than the rheobase of fast and slow MNs, respectively (Mrówczyński et al., 2010; Bączyk et al., 2013). In electrophysiological experiments, the depolarizing current is considered as a physical equivalent of the total synaptic input (Baldissera et al., 1987; Binder and Powers, 1999). From this point of view, a rapid increase of postsynaptic activity evoked through descending drive in MNs seems to be a major factor enabling generation of doublets during strong movements.
Changes in the Firing Pattern and the After Hyperpolarization After the Doublet Discharge
The neuronal firing rate is regulated by several mechanisms. The spike frequency adaptation (SFA) is one of fundamental neuronal properties influencing their repetitive firing. It indicates a decrease in action potentials discharge rate over time (Miles et al., 2005). A period including the first few spikes of a motoneuronal firing has been determined as an “initial” phase of SFA and it is followed by an “early” (up to 250 ms) and “late” (from seconds to even minutes) adaptations (Granit et al., 1963; Kernell and Monster, 1982; Sawczuk et al., 1995; Powers et al., 1999). The initial high rate of motoneuronal firing is responsible for the increase of speed of force development at the onset of a MU contraction, and despite a decreased firing rate observed during the early and late adaptation phases MUs are still able maintain relatively steady level of force (Burke et al., 1976; Stein and Parmiggiani, 1979; Bigland-Ritchie et al., 1983).
The firing rate depends also on the excitation intensity. Studies with the intracellular injection of depolarization current into cat (Spielmann et al., 1993) or rat motoneurones (Mrówczyński et al., 2010) have demonstrated an increase of the overall firing frequency of a MN with increasing intensity of applied current (Figure 1A). However, after doublet, a prolongation of the following ISI is observed, causing a transient decrease of firing rate (compare a2 vs. a1 in Figure 1A). This reduction of the firing rate of MNs appears despite higher intensity of depolarization current applied. After all, direct comparison of discharge patterns without doublets (evoked at a lower intensity of intracellular depolarizing current or a weaker synaptic input to MNs) to those with doublets (evoked at a higher intensity of depolarizing current or a stronger synaptic input to MNs) seems insufficient to explain functional consequences of initial doublets.
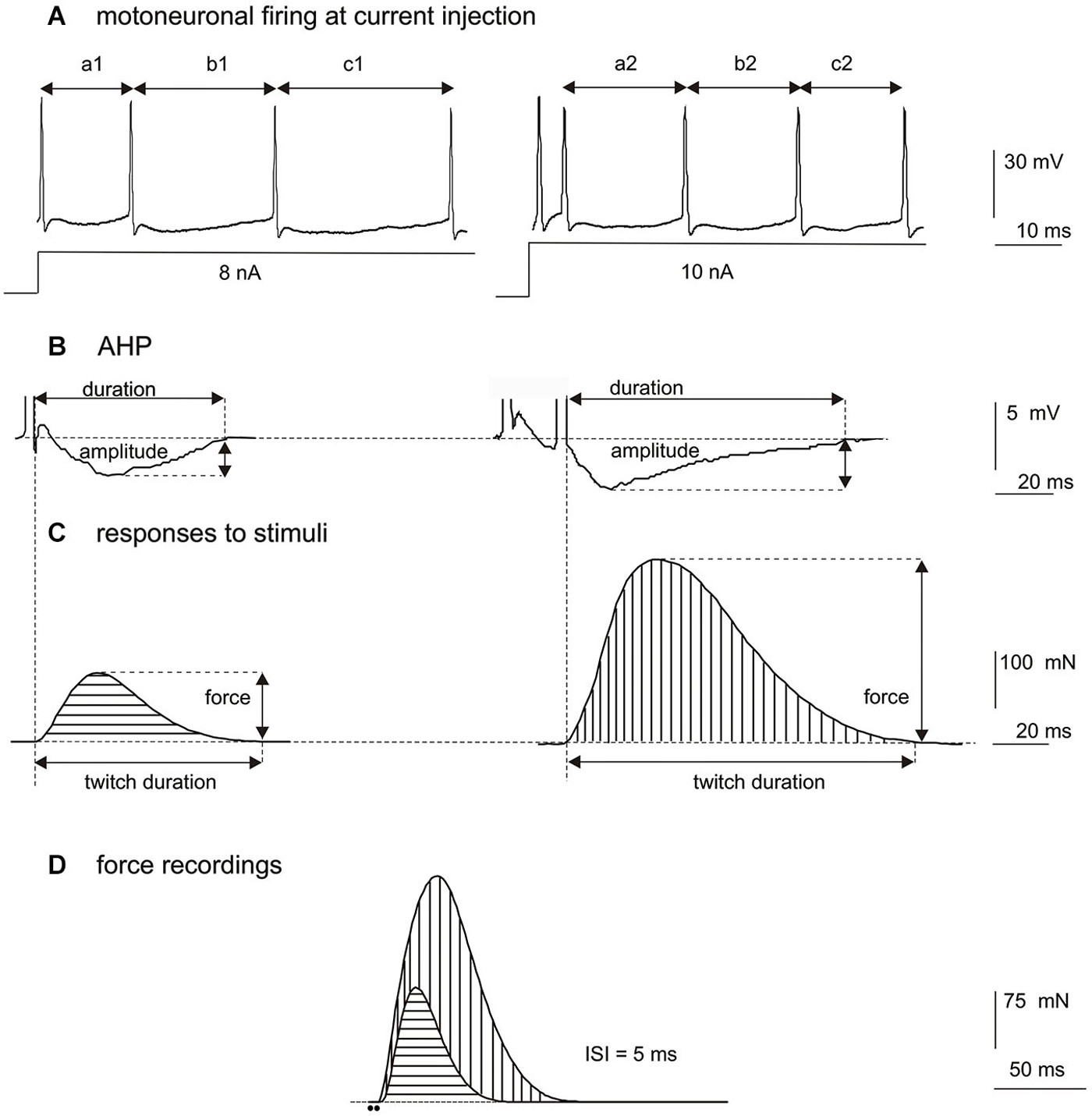
Figure 1. (A) Comparison of discharge rates of a single hind-limb MN of rat under pentobarbital anesthesia, after intracellular injection of depolarizing current of 8 nA (left record, without a doublet) and 10 nA (right record, with a doublet at about 5 ms ISI). Note the increased firing frequency of the MN with increased depolarizing current (the mean of a1 + b1 + c1 is longer than the mean of a2 + b2 + c2), however, the ISI immediately following the doublet (a2) is longer than the ISI after a single pulse below the doublet threshold (a1), and is longer than mean ISI calculated for later discharges (b2 + c2). (B) Comparison of the afterhyperpolarization (AHP) amplitude and duration after a single pulse (left record) or a doublet of action potentials (right record), showing a prolongation of the AHP and an increase of the AHP amplitude following a doublet at 5 ms ISI. (C) Models of twitch-shape contractions mathematically subtracted from the contraction obtained by two consecutive pulses at 5 ms ISI, for a fast-type MU. On the left, the twitch record in response to a single stimulus, on the right the response to the second stimulus calculated as a difference between the two superimposed recordings in (D). Note higher force and longer duration of twitch-shape response to the second stimulus. The beginning of each record corresponds to the appearance of a stimulus delivered to the axon. (D) Superimposed MU force records (the same MU as in C) obtained by application of one pulse (horizontal hatching) or by two consecutive pulses delivered at 5 ms ISI (vertical hatching). Note evidently increased MU force after the doublet. The time position of two stimuli at 5 ms ISI is indicated by dots below the record.
A considerable variability of ISIs in motoneuronal firing evoked by stretching of a muscle and activation of proprioceptors has been observed (Kostyukov et al., 2009, 2011). According to these reports, this is related to non-linear processes of summation of consecutive AHPs, described earlier by Baldissera and Gustafsson (1974).
Studies on rat MNs stimulated antidromically by one pulse and by doublet of pulses at intervals from 5 to 10 ms have shown that doublet modulates the AHP duration and amplitude in both types of MNs (Figure 1B; Mrówczyński et al., 2007). In MNs antidromically stimulated with trains of stimuli, from one to five, applied at 5 ms ISIs, an evident increase of the AHP amplitude and a significant prolongation of the AHP duration have been demonstrated after a doublet or sometimes also after a triplet of stimuli (Mrówczyński et al., 2011; Krutki et al., 2014). The AHP duration has not been considerably modified by the following (4th and 5th) pulses in the train. The results indicate that at high stimulation frequency the second activation has the strongest effect on the AHP course.
Duration of the AHP is an important factor influencing a rate of neuronal discharges (Eccles et al., 1958). A considerable decrease of motoneuronal excitability during the AHP reduces the probability of occurrence of subsequent action potential (Kernell, 1965). From this reason the AHP duration is extremely important property of MNs, which controls their firing rate (Piotrkiewicz et al., 2007) and in addition can be used to differentiate fast (with short AHP) and slow MNs (with long AHP) (Gardiner, 1993).
The AHP is an effect of increased potassium conductance in a neuron following the action potential (Barrett et al., 1980). Therefore, it is supposed that the post-doublet prolongation of the AHP duration is an effect of increase in the potassium conductance. However, many studies on spinal MNs and ascending neurons (Kuno et al., 1970; Baldissera and Gustafsson, 1974; Baldissera et al., 1978; Gustafsson, 1984; Mrówczyński et al., 2008) have demonstrated a non-linear summation of the AHPs after the doublet activation. These results have implied that additional ionic mechanisms are involved in prolongation of the AHP duration after the doublet than those responsible for the AHP following a single action potential. It is likely that activation of special types of potassium channels responding to increased intracellular concentration of sodium ions (KNa+ channels) may contribute to the increase of potassium conductance. Such channels have low sensitivity to normal cytoplasmatic concentration of sodium ions and are not involved in production of a single action potential. According to Safronov and Vogel (1996), a short train of stimuli delivered to the neuron can evoke an intracellular accumulation of sodium ions that is necessary to activate the KNa+ channels.
The increase of the AHP duration following the doublet seems to be responsible for a temporary reduction of motoneuronal firing rate, and therefore may be considered as important physiological mechanism fitting the motoneuronal firing rate to that optimal for MUs. Thus, this is an additional internal mechanism of motoneuronal firing rate reduction to previously described mechanisms related to the neuronal network activity, as reciprocal inhibition from the Renshaw cells, inhibition by Ib interneurons (from Golgi tendon organs) or inhibition from interneurons receiving information from descending pathways (Jankowska and Roberts, 1972; Hultborn et al., 1988; Jami, 1992).
The Influence of a Doublet on MU Force Development
The doublet of stimuli at the beginning of a train of pulses leads to an increase of the force output of contracting MU. Such effects have been frequently observed in experiments with doublets of pulses (in a range of 5–10 ms) on isolated fast or slow MUs of hind limb muscles of cat (Burke et al., 1976; Stein and Parmiggiani, 1979; Zajac and Young, 1980a) and rat (Hennig and Lømo, 1987; Celichowski and Grottel, 1998). A potentiation of MUs force in response to doublet has resulted from non-linear summation of twitch forces (Duchateau and Hainaut, 1986a) and could be twice to three times higher than the force of a twitch evoked by a single pulse (Parmiggiani and Stein, 1981; Kamavuako and Farina, 2010).
Simultaneous recording of the action potential from a single MN and the twitch of muscle fibers innervated by that neuron has revealed a positive correlation between the AHP duration and the twitch duration, and this correlation has been documented in several species, as cat (Zengel et al., 1985; Cope et al., 1986), rat (Gardiner and Kernell, 1990) or mouse (Meehan et al., 2010). All these studies have revealed that the activation with a single stimulus results in longer AHP and longer twitch of slow MUs in relation to fast ones. However, the amplitude and duration of both the AHP (Gustafsson, 1984; Mrówczyński et al., 2011) and twitch-shape responses (obtained by a mathematical decomposition of the recorded tetanus) to successive activations (Celichowski et al., 2008) are not constant. Recently, Krutki et al. (2014) have demonstrated in rat a parallelism in modification of the AHP as well as the contraction time and amplitude of the twitch-shape responses to individual stimuli. Parameters collected in one series of experiments with intracellular recordings of MNs (AHP amplitude and duration) (Figure 1B) have been compared to data from another series of experiments with the MU force recordings (Figure 1D) and to results of their mathematical decomposition (amplitude and duration of twitch-like responses to individual stimuli) (Figure 1C). In both series of experiments MNs as well as MUs were activated by trains of stimuli with the increasing numbers of pulses, from one to five, delivered at 5 ms ISIs. The most noticeable changes (the increase in the amplitude and the duration) have been observed in both the AHP and twitch-shape response parameters as an effect of activation with two stimuli (Figures 1B,C).
According to Krutki et al. (2014) an increase of twitch force in MUs after the doublet results rather from intracellular processes within muscle fibers than from electromechanical-coupling mechanisms. Duchateau and Hainaut (1986b) have suggested that an intensification of membrane processes in muscle fibers, leading to an increase of calcium concentration in the cell cytosol, is a cause of post-doublet twitch potentiation. Recently, Cheng et al. (2013) have pointed out that doublets evoked an increase of the Ca2+ release from sarcoplasmic reticulum, which is accompanied by greater force production in unfatigued muscle fibers of mouse. Thus, the increase of Ca2+ release enabling the phosphorylation of myosin light chain is responsible for facilitated formation of additional force-bearing cross bridges in the vicinity of already attached cross bridges leading to increase of MUs twitch force following the doublet (Sweeney et al., 1993; Abbate et al., 2002).
Functional Implications of Doublet
The initial doublet is a specific pattern of MN discharges described by Binder-Macleod (1995) as “high to low” strategy, with a transition from high to low discharge rate. Such patterns contain a strong initial dynamic component followed by the steady state activity. According to the hypothesis by Kostyukov and Korchak (1998), dynamic components in the efferent commands play a decisive role in coding the final position of limbs in real movements.
However, it should be stressed that during voluntary activity, a strong descending drive to MNs causes recruitment of many additional MUs (Aagaard, 2003). Some studies concerning human training have demonstrated that strong MUs may be included into the muscle contraction at an early stage of force development (Van Cutsem et al., 1998; Kamen and Knight, 2004; Vila-Chã et al., 2010). Therefore, although the recruitment is the main mechanism of force regulation, doublets add an extra force to the muscle contraction. However, Sandercock and Heckman (1997) have reported that muscle movement completely abolishes muscle potentiation evoked by doublet after about 0.4 s of eccentric or concentric contractions of cat soleus muscle. Such result suggests that the doublet can evoke an initial force increment, but this effect does not remain high throughout the movement (Garland and Griffin, 1999). Thus, the physiological meaning of doublets in the force increase could be less significant during voluntary activity than it appears from the traditional scheme based on comparison of linear summation of two isolated isometric twitches. Moreover, doublets are not unique components responsible for adding force at the beginning of MUs contraction. Gorassini et al. (2000) have noticed a variety of high-frequency firing patterns started with triplets in fast MUs during locomotion of rats. Thus, during natural activity different initial high-frequency trains of action potentials may lead to faster and stronger contractions.
In conclusion, the doublet at the beginning of motoneuronal activity can be observed in various mammals, and should be considered as a universal mechanism that enables rapid enhancement of force developed by muscles at the beginning of their activity, which lasts despite the passing after-doublet decrease of motoneuronal discharge frequency. Apart from this observation, the influence of doublets on further discharges in motoneuronal firing pattern, especially during voluntary movements, remains unclear.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aagaard, P. (2003). Training-induced changes in neural function. Exerc. Sport Sci. Rev. 31, 61–67. doi: 10.1097/00003677-200304000-00002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Abbate, F., Bruton, J. D., De Haan, A., and Westerblad, H. (2002). Prolonged force increase following a high-frequency burst is not due to a sustained elevation of [Ca2+]i. Am. J. Physiol. Cell Physiol. 283, C42–C47. doi: 10.1152/ajpcell.00416.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bączyk, M., Hałuszka, A., Mrówczyński, W., Celichowski, J., and Krutki, P. (2013). The influence of a 5-wk whole body vibration on electrophysiological properties of rat hindlimb spinal motoneurons. J. Neurophysiol. 109, 2705–2711. doi: 10.1152/jn.00108.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baldissera, F., Campadelli, P., and Piccinelli, L. (1987). The dynamic response of cat gastrocnemius motor units investigated by ramp-current injection into their motoneurones. J. Physiol. 387, 317–330. doi: 10.1113/jphysiol.1987.sp016575
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baldissera, F., and Gustafsson, B. (1974). Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol. Scand. 92, 27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baldissera, F., Gustafsson, B., and Parmiggiani, F. (1978). Saturating summation of the afterhyperpolarization conductance in spinal motoneurones: a mechanism for secondary range repetitive firing. Brain Res. 146, 69–82. doi: 10.1016/0006-8993(78)90218-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barrett, E. F., Barett, N. J., and Crill, W. E. (1980). Voltage-sensitive outward currents in cat motoneurones. J. Physiol. 304, 251–276. doi: 10.1113/jphysiol.1980.sp013323
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bawa, P., and Calancie, B. (1983). Repetitive doublets in human flexor carpi radialis muscle. J. Physiol. 339, 123–132. doi: 10.1113/jphysiol.1983.sp014707
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bigland-Ritchie, B., Johansson, R., Lippold, O. C., Smith, S., and Woods, J. J. (1983). Changes in motoneurone firing rates during sustained maximal voluntary contractions. J. Physiol. 340, 335–346. doi: 10.1113/jphysiol.1983.sp014765
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Binder, M. D., and Powers, R. K. (1999). Synaptic integration in spinal motoneurones. J. Physiol. Paris 93, 71–79. doi: 10.1016/s0928-4257(99)80137-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Binder-Macleod, S. A. (1995). Variable-frequency stimulation patterns for the optimization of force during muscle fatigue. Muscle wisdom and the catch-like property. Adv. Exp. Med. Biol. 384, 227–240. doi: 10.1007/978-1-4899-1016-5_18
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Binder-Macleod, S. A., and Barker, C. B. (1991). Use of a catchlike property of human skeletal muscle to reduce fatigue. Muscle Nerve 14, 850–857. doi: 10.1002/mus.880140909
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Burke, R. E., Rudomin, P., and Zajac, F. E. (1976). The effect of activation history on tension production by individual muscle units. Brain Res. 109, 515–529. doi: 10.1016/0006-8993(76)90031-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Celichowski, J., and Grottel, K. (1998). The influence of a doublet of stimuli at the beginning of the tetanus on its time course. Acta Neurobiol. Exp. (Wars) 58, 47–53.
Celichowski, J., Raikova, R., Drzymała-Celichowska, H., Ciechanowicz-Kowalczyk, I., Krutki, P., and Rusev, R. (2008). Model-generated decomposition of unfused tetani of motor units evoked by random stimulation. J. Biomech. 41, 3448–3454. doi: 10.1016/j.jbiomech.2008.09.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheng, A. J., Place, N., Bruton, J. D., Holmberg, H. C., and Westerblad, H. (2013). Doublet discharge stimulation increases sarcoplasmic reticulum Ca2+ release and improves performance during fatiguing contractions in mouse muscle fibres. J. Physiol. 591, 3739–3748. doi: 10.1113/jphysiol.2013.257188
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Christie, A., and Kamen, G. (2006). Doublet discharges in motoneurons of young and older adults. J. Neurophysiol. 95, 2787–2795. doi: 10.1152/jn.00685.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cope, T. C., Bodine, S. C., Fournier, M., and Edgerton, V. R. (1986). Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J. Neurophysiol. 55, 1202–1220.
Duchateau, J., and Hainaut, K. (1986a). Nonlinear summation of contractions in striated muscle. I. Twitch potentiation in human muscle. J. Muscle Res. Cell Motil. 7, 11–17. doi: 10.1007/bf01756197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Duchateau, J., and Hainaut, K. (1986b). Nonlinear summation of contractions in striated muscle. II. Potentiation of intracellular Ca2+ movements in single barnacle muscle fibres. J. Muscle Res. Cell Motil. 7, 18–24. doi: 10.1007/bf01756198
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eccles, J. C., Eccles, R. M., and Lundberg, A. (1958). The action potentials of the alpha motoneurones supplying fast and slow motor muscles. J. Physiol. 142, 275–291. doi: 10.1113/jphysiol.1958.sp006015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gardiner, P. F. (1993). Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J. Neurophysiol. 69, 1160–1170.
Gardiner, P. F., and Kernell, D. (1990). The “fastness” of rat motoneurones: time-course of afterhyperpolarization in relation to axonal conduction velocity and muscle unit contractile speed. Pflugers Arch. 415, 762–766. doi: 10.1007/bf02584018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garland, S. J., and Griffin, L. (1999). Motor unit double discharges: statistical anomaly or functional entity? Can. J. Appl. Physiol. 24, 113–130. doi: 10.1139/h99-010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gorassini, M., Eken, T., Bennett, D. J., Kiehn, O., and Hultborn, H. (2000). Activity of hindlimb motor units during locomotion in the conscious rat. J. Neurophysiol. 83, 2002–2011.
Granit, R., Kernell, D., and Shortess, G. K. (1963). Quantitative aspects of repetitive firing of mammalian motoneurones, caused by injected currents. J. Physiol. 168, 911–931. doi: 10.1113/jphysiol.1963.sp007230
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Griffin, L., Garland, S. J., and Ivanova, T. (1998). Discharge patterns in human motor units during fatiguing arm movements. J. Appl. Physiol. (1985) 85, 1684–1692.
Gustafsson, B. (1984). Afterpotentials and transduction properties in different types of central neurones. Arch. Ital. Biol. 122, 17–30.
Hennig, R., and Lømo, T. (1985). Firing patterns of motor units in normal rats. Nature 314, 164–166. doi: 10.1038/314164a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hennig, R., and Lømo, T. (1987). Gradation of force output in normal fast and slow muscles of the rat. Acta Physiol. Scand. 130, 133–142. doi: 10.1111/j.1748–1716.1987.tb08119.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hoffer, J. A., Sugano, N., Loeb, G. E., Marks, W. B., O’Donovan, M. J., and Pratt, C. A. (1987). Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J. Neurophysiol. 57, 530–553.
Hultborn, H., Katz, R., and Mackel, R. (1988). Distribution of recurrent inhibition within a motor nucleus. II. Amount of recurrent inhibition in motoneurones to fast and slow units. Acta Physiol. Scand. 134, 363–374. doi: 10.1111/j.1748–1716.1988.tb08503.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jami, L. (1992). Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol. Rev. 73, 623–666.
Jankowska, E., and Roberts, W. J. (1972). Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J. Physiol. 222, 623–642. doi: 10.1113/jphysiol.1972.sp009818
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kamavuako, E. N., and Farina, D. (2010). Time-dependent effects of pre-conditioning activation on muscle fiber conduction velocity and twitch torque. Muscle Nerve 42, 547–555. doi: 10.1002/mus.21726
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kamen, G., and Knight, C. A. (2004). Training-related adaptations in motor unit discharge rate in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 59, 1334–1338. doi: 10.1093/gerona/59.12.1334
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kernell, D. (1965). The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol. Scand. 65, 87–100. doi: 10.1111/j.1748-1716.1965.tb04252.x
Kernell, D., and Monster, A. W. (1982). Time course and properties of late adaptation in spinal motoneurones of the cat. Exp. Brain Res. 46, 191–196. doi: 10.1007/bf00237176
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kirkwood, P. A., and Munson, J. B. (1996). The incidence of initial doublets in the discharges of motoneurones of two different inspiratory muscles in the cat. J. Physiol. 493, 577–587. doi: 10.1113/jphysiol.1996.sp021405
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kostyukov, A. I., and Korchak, O. E. (1998). Length changes of the cat soleus muscle under frequency-modulated distributed stimulation of efferents in isotony. Neuroscience 82, 943–955. doi: 10.1016/s0306-4522(97)00105-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kostyukov, A. I., Lytvynenko, S. V., Bulgakova, N. V., and Gorkovenko, A. V. (2009). Subthreshold activation of spinal motoneurones in the stretch reflex: experimental data and modeling. Biol. Cybern. 100, 307–318. doi: 10.1007/s00422-009-0303-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kostyukov, A. I., Lytvynenko, S. V., Bulgakova, N. V., and Gorkovenko, A. V. (2011). Changes in the threshold of generation of action potentials by spinal motoneurons under conditions of their natural activation. Neurophysiology 43, 182–191. doi: 10.1007/s11062-011-9201-9
Krutki, P., Mrówczyński, W., Raikova, R., and Celichowski, J. (2014). Concomitant changes in afterhyperpolarization and twitch following repetitive stimulation of fast motoneurones and motor units. Exp. Brain Res. 232, 443–452. doi: 10.1007/s00221-013-3752-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kudina, L. P., and Alexeeva, N. L. (1992). Repetitive doublets of human motoneurones: analysis of interspike intervals and recruitment pattern. Electroencephalogr. Clin. Neurophysiol. 85, 243–247. doi: 10.1016/0168-5597(92)90112-o
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kudina, L. P., and Andreeva, R. E. (2013). Motoneuron double discharges: only one or two different entities? Front. Cell. Neurosci. 7:75. doi: 10.3389/fncel.2013.00075
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kuno, M., Miyahara, J. T., and Weakly, J. N. (1970). Post-tetanic hyperpolarization produced by an electrogenic pump in dorsal spinocerebellar tract neurones of the cat. J. Physiol. 210, 839–855. doi: 10.1113/jphysiol.1970.sp009245
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meehan, C. F., Sukiasyan, N., Zhang, M., Nielsen, J. B., and Hultborn, H. (2010). Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. J. Neurophysiol. 103, 2599–25610. doi: 10.1152/jn.00668.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miles, G. B., Dai, Y., and Brownstone, R. M. (2005). Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J. Physiol. 566, 519–532. doi: 10.1113/jphysiol.2005.086033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mrówczyński, W., Krutki, P., and Celichowski, J. (2007). Double stimulation modulates afterhyperpolarization phase following action potentials evoked in rat motoneurones. Acta Neurobiol. Exp. (Wars) 67, 439–446.
Mrówczyński, W., Krutki, P., Chakarov, V., and Celichowski, J. (2008). Summation of afterhyperpolarization after a doublet in fast motoneurones of the rat spinal cord. Arch. Ital. Biol. 146, 63–73.
Mrówczyński, W., Krutki, P., Chakarov, V., and Celichowski, J. (2010). Doublet of action potentials evoked by intracellular injection of rectangular depolarization current into rat motoneurones. Exp. Brain Res. 205, 95–102. doi: 10.1007/s00221-010-2339-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mrówczyński, W., Krutki, P., Chakarov, V., and Celichowski, J. (2011). Modulation of afterhyperpolarization by various stimulation patterns in rat motoneurones. J. Mot. Behav. 43, 63–71. doi: 10.1080/00222895.2010.542507
Parmiggiani, F., and Stein, R. B. (1981). Nonlinear summation of contractions in cat muscles. II. Later facilitation and stiffness changes. J. Gen. Physiol. 78, 295–311. doi: 10.1085/jgp.78.3.295
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Person, R. S., and Kudina, L. P. (1972). Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr. Clin. Neurophysiol. 32, 471–483. doi: 10.1016/0013-4694(72)90058-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piotrkiewicz, M., Kudina, L., Mierzejewska, J., Jakubiec, M., and Hausmanowa-Petrusewicz, I. (2007). Age-related change in duration of afterhyperpolarization of human motoneurones. J. Physiol. 585, 483–490. doi: 10.1113/jphysiol.2007.142356
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Powers, R. K., Sawczuk, A., Musick, J. R., and Binder, M. D. (1999). Multiple mechanisms of spike-frequency adaptation in motoneurones. J. Physiol. Paris 93, 101–114. doi: 10.1016/s0928-4257(99)80141-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Safronov, B. V., and Vogel, W. (1996). Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. J. Physiol. 497, 727–734. doi: 10.1113/jphysiol.1996.sp021803
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sandercock, T. G., and Heckman, C. J. (1997). Doublet potentiation during eccentric and concentric contractions of cat soleus muscle. J. Appl. Physiol. (1985) 82, 1219–1228.
Sawczuk, A., Powers, R. K., and Binder, M. D. (1995). Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J. Neurophysiol. 73, 1799–1810.
Simpson, J. A. (1969). Terminology of electromyogram. Electroencephalogr. Clin. Neurophysiol. 26, 224–226. doi: 10.1016/0013-4694(69)90217-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spielmann, J. M., Laouris, Y., Nordström, M. A., Robinson, G. A., Reinking, R. M., and Stuart, D. G. (1993). Adaptation of cat motoneurones to sustained and intermittent extracellular activation. J. Physiol. 464, 75–120. doi: 10.1113/jphysiol.1993.sp019625
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stein, R. B., and Parmiggiani, F. (1979). Optimal motor patterns for activating mammalian muscles. Brain Res. 175, 372–376. doi: 10.1016/0006-8993(79)91019-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sweeney, H. L., Bowman, B. F., and Stull, J. T. (1993). Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am. J. Physiol. 264, C1085–C1095.
Van Cutsem, M., Duchateau, J., and Hainaut, K. (1998). Changes in single motor unit behavior contribute to the increase in contraction speed after dynamic training in humans. J. Physiol. 513, 295–305. doi: 10.1111/j.1469-7793.1998.295by.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vila-Chã, C., Falla, D., and Farina, D. (2010). Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J. Appl. Physiol. (1985) 109, 1455–1466. doi: 10.1152/japplphysiol.01213.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zajac, E., and Young, J. L. (1980a). Properties of stimulus trains producing maximum tension-time area per pulse from single motor units in medial gastrocnemius muscle of the cat. J. Neurophysiol. 43, 1206–1220.
Zajac, F. E., and Young, J. L. (1980b). Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J. Neurophysiol. 43, 1221–1235.
Keywords: doublet, motoneuron, interspike interval, motor unit, force development
Citation: Mrówczynski W, Celichowski J, Raikova R and Krutki P (2015) Physiological consequences of doublet discharges on motoneuronal firing and motor unit force. Front. Cell. Neurosci. 9:81. doi: 10.3389/fncel.2015.00081
Received: 28 November 2014; Accepted: 23 February 2015;
Published online: 10 March 2015.
Edited by:
Sergey M. Korogod, International Center for Molecular Physiology, National Academy of Sciences of Ukraine, UkraineReviewed by:
Oliver Röhrle, Universität Stuttgart, GermanyAlexander Kostyukov, A. A. Bogomoletz Institute of Physiology, Ukraine
Copyright © 2015 Mrówczynski, Celichowski, Raikova and Krutki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Włodzimierz Mrówczyński, Department of Neurobiology, University School of Physical Education, 27/39 Królowej Jadwigi St., 61-871 Poznań, Poland mrowczynski@awf.poznan.pl
 Włodzimierz Mrówczyński
Włodzimierz Mrówczyński Jan Celichowski1
Jan Celichowski1  Piotr Krutki
Piotr Krutki