Differential modulation of repetitive firing and synchronous network activity in neocortical interneurons by inhibition of A-type K+ channels and Ih
- Department of Neurobiology, Civitan International Research Center and Evelyn F. McKnight Brain Institute, University of Alabama at Birmingham, Birmingham, AL, USA
GABAergic interneurons provide the main source of inhibition in the neocortex and are important in regulating neocortical network activity. In the presence 4-aminopyridine (4-AP), CNQX, and D-APV, large amplitude GABAA-receptor mediated depolarizing responses were observed in the neocortex. GABAergic networks are comprised of several types of interneurons, each with its own protein expression pattern, firing properties, and inhibitory role in network activity. Voltage-gated ion channels, especially A-type K+ channels, differentially regulate passive membrane properties, action potential (AP) waveform, and repetitive firing properties in interneurons depending on their composition and localization. HCN channels are known modulators of pyramidal cell intrinsic excitability and excitatory network activity. Little information is available regarding how HCN channels functionally modulate excitability of individual interneurons and inhibitory networks. In this study, we examined the effect of 4-AP on intrinsic excitability of fast-spiking basket cells (FS-BCs) and Martinotti cells (MCs). 4-AP increased the duration of APs in both FS-BCs and MCs. The repetitive firing properties of MCs were differentially affected compared to FS-BCs. We also examined the effect of Ih inhibition on synchronous GABAergic depolarizations and synaptic integration of depolarizing IPSPs. ZD 7288 enhanced the amplitude and area of evoked GABAergic responses in both cell types. Similarly, the frequency and area of spontaneous GABAergic depolarizations in both FS-BCs and MCs were increased in presence of ZD 7288. Synaptic integration of IPSPs in MCs was significantly enhanced, but remained unaltered in FS-BCs. These results indicate that 4-AP differentially alters the firing properties of interneurons, suggesting MCs and FS-BCs may have unique roles in GABAergic network synchronization. Enhancement of GABAergic network synchronization by ZD 7288 suggests that HCN channels attenuate inhibitory network activity.
Introduction
GABAergic interneurons are the main source of inhibition in the neocortex and regulate the output of neocortical networks. GABA released from interneurons acts on a variety of receptors located both pre- and post-synaptically (Martin and Olsen, 2000; Farrant and Kaila, 2007). Activation of GABAA receptors produces gating of Cl- permeable channels resulting in phasic inhibition via membrane hyperpolarization (Avoli and de Curtis, 2011). During the early postnatal period, GABAA receptor mediated responses can be depolarizing due to a lack of KCC2, a K+-Cl- co-transporter that extrudes Cl- (Ben-Ari et al., 1989; Rivera et al., 1999). These depolarizing responses are involved in network synchronization during development (Khazipov et al., 1997; Allene et al., 2008). In response to iontophoretic application of GABA, depolarizing GABAA-mediated responses can also be seen in mature animals (Andersen et al., 1980; Alger and Nicoll, 1982; Weiss and Hablitz, 1984). Application of the A-type K+ channel blocker 4-aminopyridine (4-AP) results in generation of a GABAergic, long-lasting depolarization (termed giant depolarizing potentials; Avoli and Perreault, 1987; Avoli et al., 1988). These responses persist when excitatory glutamatergic transmission is blocked with CNQX and D-APV (Aram et al., 1991; Michelson and Wong, 1991; Avoli et al., 1994; Benardo, 1997). These depolarizing GABA responses, which propagate across the neocortex (DeFazio and Hablitz, 2005), are assumed to result from synchronous firing of inhibitory interneurons. The present study examines activity in specific classes of neocortical interneurons during such depolarizing events.
GABAergic interneurons are involved in processes such as modulation of synaptic integration (Pouille and Scanziani, 2001; Klausberger and Somogyi, 2008), control of spike timing (Wehr and Zador, 2003), and synchronization of network activity (McBain and Fisahn, 2001). Subpopulations of GABAergic interneurons can be identified based on cell morphology, intrinsic excitability, and inherent protein expression patterns. Each GABAergic interneuron subtype has a unique synaptic target. The largest class of GABAergic interneurons in the neocortex are the parvalbumin (PV)-expressing cells, which constitute roughly 40% of the interneurons in the neocortex (Rudy et al., 2011). A subclass of PV-expressing cells, the fast-spiking basket cells (FS-BCs), have a multipolar morphology, vary in size, and target the proximal dendrite and soma of pyramidal cells (Kawaguchi and Kubota, 1997). These cells exhibit low input resistance, high frequency repetitive firing and no accommodation (Kawaguchi and Kubota, 1997). They have been shown to exhibit powerful feed-forward inhibition (Pouille and Scanziani, 2001) and initiate oscillatory activity (Traub et al., 2005). Importantly, FS-BCs also contribute to the control of the excitation/inhibition balance necessary to maintain the functional integrity of the cortical network (Haider and McCormick, 2009), induce gamma rhythm activity (Cardin et al., 2009), and entrain excitatory neurons (Fries et al., 2001; Kawaguchi, 2001; Hasenstaub et al., 2005). In contrast to FS-BCs, Martinotti cells (MCs) express the peptide somatostatin (SOM) and synaptically target the apical and basal dendrites of pyramidal neurons. MCs display vast arborized axons that densely innervate layer I across multiple columns. These cells demonstrate burst firing in response to depolarizing steps and show accommodation (Kawaguchi and Kubota, 1997). Excitatory inputs from pyramidal cells onto MCs are strongly facilitating and drive feedback inhibition (Silberberg and Markram, 2007).
4-aminopyridine sensitive, A-type K+ channels encompass a subset of voltage-gated K+ channels comprised of the Kv1, Kv3, and Kv4 subunit subfamilies (Jerng et al., 2004). Differential expression of these channels gives rise to the different characteristic repetitive firing patterns among interneurons (Martina et al., 1998; Serodio and Rudy, 1998; Wang et al., 1998; Chow et al., 1999; Erisir et al., 1999; Lau et al., 2000; Lien et al., 2002). In situ hybridization and immunofluorescent labeling demonstrate Kv3.1 and Kv3.2 transcripts and proteins co-localize with PV-positive interneurons (Weiser et al., 1994; Sekirnjak et al., 1997; Chow et al., 1999). Furthermore, pharmacological inhibition and genetic disruption of presynaptic Kv1 and somatodendritic Kv3 channels impairs fast-spiking firing patterns in interneurons (Martina et al., 1998; Erisir et al., 1999; Lau et al., 2000; Goldberg et al., 2008). Alternatively, SOM positive interneurons have been shown to contain a significant higher density of somatodendritic Kv4 channels and the associated K+ current, contributing to their characteristic firing pattern (Serodio and Rudy, 1998; Lien et al., 2002; Lai and Jan, 2006; Bourdeau et al., 2007). Kv3.2 channels are also highly expressed in non-fast-spiking SOM positive interneurons in the neocortex, where they may play a different role in repetitive firing (Weiser et al., 1994; Chow et al., 1999). Consistent with their role in regulating intrinsic excitability, the genetic loss or pharmacological blockade of A-type K+ channels is epileptogenic (Smart et al., 1998; Avoli et al., 2001; Bagetta et al., 2004; Monaghan et al., 2008). It remains unclear how the inhibition of A-type K+ channels induces interneuron synchronization.
Cortical network excitability can be modulated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, and their associated Ih current. In excitatory pyramidal cells, the Ih current contributes to the cell’s intrinsic excitability by depolarizing the membrane, increasing the membrane conductance, and decreasing dendritic excitability (Magee, 1998; Williams and Stuart, 2000; Berger et al., 2001; Robinson and Siegelbaum, 2003). During synaptic activation, Ih normalizes the decay time of distal excitatory postsynaptic potentials (EPSPs; Williams and Stuart, 2000) and decreases temporal summation (Berger et al., 2001). It also functions to constrain excitatory network activity (Albertson et al., 2013). Furthermore, loss of HCN channels has been reported in experimental epilepsy models (Jung et al., 2007; Powell et al., 2008; Shin et al., 2008; Albertson et al., 2011). Neocortical GABAergic interneurons do not typically stain with HCN channel antibodies (Lorincz et al., 2002), but do display varying amounts of Ih. FS-BCs demonstrate small or absent “sag” responses upon hyperpolarization (Okaty et al., 2009; Albertson et al., 2013). In contrast, MCs display a prominent “sag” response to hyperpolarizing current pulses and a “rebound” response to repolarization, characteristic of Ih (Lupica et al., 2001; Wang et al., 2004; Ma et al., 2006). The role of HCN channels in modulating GABAergic interneuron excitability and inhibitory network activity is not well established.
In the present study, we examined the influence of A-type K+ channels on AP and repetitive firing properties of L2/3 FS-BCs and MCs in the 4-AP model of interneuron network synchronization. We further investigated the role of HCN channel inhibition in modulating 4-AP induced GABAergic network synchronization. We found that 4-AP differentially alters the repetitive firing properties of FS-BCs and MCs. We also found that Ih inhibition enhances the magnitude of evoked and spontaneous depolarizing GABAergic potentials as well as the frequency of spontaneous depolarizing GABAergic potentials in L2/3 neocortical interneurons. These results indicate that interneuron excitability is both up- and down-regulated by voltage-gated ion channels.
Materials and Methods
Ethics Statement
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Slice Preparation
To prepare acute neocortical slices, vesicular GABA transporter (VGAT)-Venus-expressing Wistar rats (Uematsu et al., 2008) from PND 20 to 36 were anesthetized with isoflurane and rapidly decapitated. Brains were removed and immediately placed in ice-cold oxygenated (95% O2/5% CO2, pH 7.4) cutting solution consisting of (in mM): 135 N-Methyl-D-glucamine, 1.5 KCl, 23 NaHCO3, 0.4 ascorbic acid, 25 D-glucose, 1.5 KH2PO4, 1.25 CaCl2, and 8.75 MgCl2 (Tanaka et al., 2008). Using a Microm HM 650 vibratome (Microm, Walldorf, Germany), coronal brain slices (300 μm thick) of somatosensory cortex were made. After slicing was completed, neocortical slices were placed in a holding chamber in a water bath at 37°C for 45–60 min in saline consisting of (in mM) 125 NaCl, 3.5 KCl, 10 D-glucose, 26 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2. Slices were subsequently kept at room temperature until recording.
Whole Cell Recording
Slices were visualized using a Zeiss AxioExaminer D1 (Carl Zeiss Inc., Thornwood, NY, USA) microscope, equipped with Dodt contrast optics, a 40X-water immersion lens and infrared illumination. Individual slices were held in a submerged recording chamber continuously perfused with oxygenated saline (3 ml/min at 32°C). Whole-cell access was obtained using glass patch electrodes with an open tip resistance of 3–5 MΩ. Pipettes were filled with an intracellular solution consisting of (in mM): 125 K-gluconate, 10 KCl, 10 HEPES, 10 creatine phosphate, 2 Mg-ATP, 0.2 Na-GTP, 0.5 EGTA, with an adjusted pH of 7.3 and osmolarity of 290 mOsm. In most experiments, biocytin (0.5%; Sigma, St. Louis, MO, USA) was added to the intracellular solution for post hoc morphological analysis.
Data Acquisition and Analysis
Whole-cell recordings were obtained using an ELC-03XS npi bridge balance amplifier (npi Electronic GmbH, Tamm, Germany) with Clampex 8.2 software via a Digidata 1322A interface (Molecular Devices, Union City, CA, USA), filtered at 2 kHz and digitized at 10 kHz. Analysis of all recordings was performed using Clampfit 9.0 software (Molecular Devices). Interneurons were physiologically identified by the response to a series of 800 ms hyperpolarizing and depolarizing current pulses ranging from -200 – 350 pA. Resting membrane potential (RMP) was monitored and maintained throughout the recording process. Input resistance was measured in current clamp with 800 ms, 25 pA hyperpolarizing current steps. Initial and final firing frequencies were calculated from the time interval between the first and last two APs, respectively, in an 800 ms depolarizing current pulse. Accommodation ratio was defined as the initial firing frequency/final firing frequency in response to a depolarizing current pulse. The amplitude of the after-hyperpolariztion (AHP) was measured form the AP threshold the the peak of the AHP. The slow after-depolarization (sADP) following an 800 ms current pulse was measured from the RMP before the current pulse to the peak of the sADP. Synaptic responses were evoked using a bipolar nichrome electrode positioned 100–200 μm adjacent to the recording electrode. Evoked depolarizing GABAergic potentials were triggered using a single 50–320 μA current pulse of 100 μs duration. A train of 5 stimuli at 25 Hz was used to study synaptic integration. Amplitude of evoked depolarizing GABAergic potentials was measured from RMP at the time of stimulation to the peak of depolarization. Evoked and spontaneous responses area was measured from the time of stimulation to the point at which the membrane potential returned to baseline.
Drugs and Drug Application
Drugs were obtained from the following sources: 4-AP, Sigma, St. Louis, MO, USA; 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), Abcam, Cambridge, MA, USA; D-(-)-2-Amino-5-phosphonopentanoic acid (D-APV) and 4-Ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD 7288; Tocris, Ellisville, MO, USA).
To induce synchronous GABA-mediated events, slices were incubated in 100 μM 4-AP for at least 1 h prior to recording. After control recordings were obtained in the presence of 10 μM CNQX and 20 μM D-APV, 20 μM ZD 7288 was applied to inhibit HCN channels. All drugs were bath applied and each neuron served as its own control.
Statistics
All statistical analysis was performed using GraphPad Prism 4 (LaJolla, CA, USA). Data are expressed as mean ± SEM. Sample size (n) is the number of cells used for each experiment, with a maximum of three cells per animal. Statistical comparisons of responses before and during drug application was performed using a one- or two-tailed Student’s t-test, for which p < 0.05 was considered significant. For analysis of IPSP summation before and after Ih inhibition, a two-way ANOVA was used.
Results
Identification of FS-BC and MCs in Rat Neocortex
Neocortical GABAergic interneurons represent approximately 25% or less of the total cortical neuron population (Fairen et al., 1984; Peters and Jones, 1984) and are highly heterogeneous (Ascoli et al., 2008). FS-BCs and MCs are two major classes of inhibitory GABAergic interneurons in rat neocortex (Rudy et al., 2011). FS-BCs innervate somatic and perisomatic regions of pyramidal cells, forming dense and unspecific connections (Packer and Yuste, 2011). In contrast, MCs target apical, basal, and distal tuft dendrites of pyramidal cells and contact somas of L1 neurons (Wang et al., 2004; McGarry et al., 2010). In order to facilitate visual identification of specific cell types in L2/3, recordings were obtained in transgenic rats co-expressing the yellow fluorescent protein, Venus, with the vesicular GABA transporter (Uematsu et al., 2008).
The laminar distribution of Venus positive cells is shown in Figure 1A. Recordings were obtained from fluorescent cells in L2/3. It was possible to morphologically discriminate FS-BCs and MCs prior to recording. FS-BSs were identified on basis of depth below the pial surface and presence of a round soma. Neurons with oval shaped somas and bi-tufted appearance were classified as MCs. Biocytin was included in the patch electrode to allow for post hoc confirmation of cell identification. Examples of a Venus-positive GABAergic FS-BC and a MC labeled with biocytin are shown in Figure 1B. Cells were further identified by their firing properties and response to strong hyperpolarizing current pulses. Typical MC responses are shown in Figure 1C. In the present series, most MCs showed initial burst responses followed by accommodation. A marked “sag” upon hyperpolarization, indicative of Ih activation, was seen upon membrane hyperpolarization. In contrast, FS-BCs (Figure 1D) displayed high frequency, non-accommodating firing and lacked significant sag responses. The combination of anatomical and electrophysiological characteristics allowed for clear identification of these two classes of cells and allowed us to address the question of differential modulation by 4-AP and inhibitors of Ih channels.
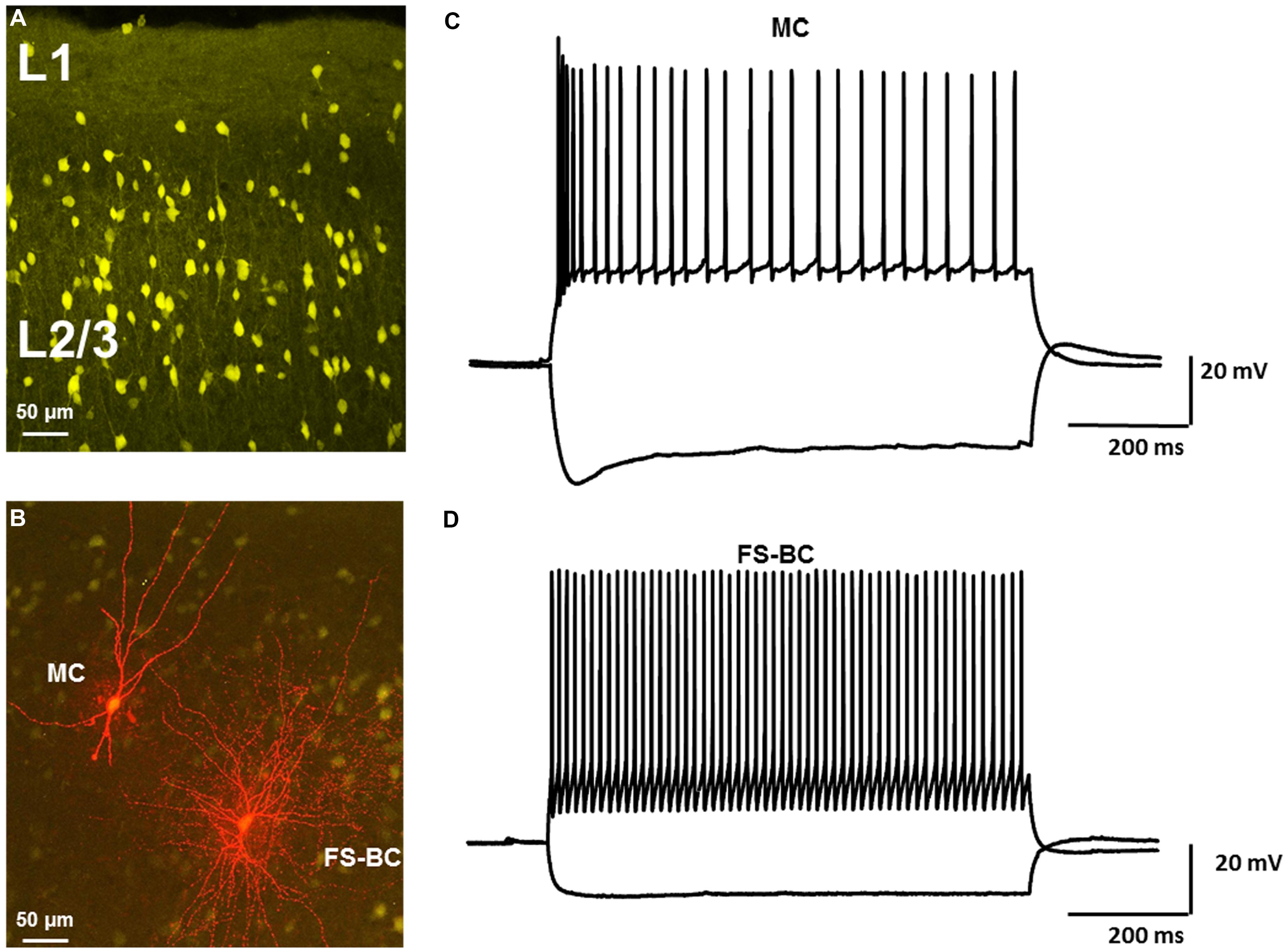
FIGURE 1. Identification of neocortical GABAergic interneurons in VGAT-Venus transgenic rats. (A) Confocal image of Venus-expressing interneurons in upper layers of rat neocortex. Morphologically distinct subtypes of interneurons can be identified. (B) Confocal image of Venus-expressing interneurons (yellow) and biocytin labeled interneurons labeled (red). Neurons were labeled with biocytin during whole cell recording. Distinct morphological differences were observed. Leftmost neuron had characteristics of a Martinotti cell (MC) whereas rightmost neuron was classified as a fast spiking basket cell (FS-BC). (C) Response of MC to depolarizing and hyperpolarizing current pulses demonstrate typical repetitive firing properties and prominent sag responses. (D) Representative response of FS-BC to depolarizing and hyperpolarizing current pulses. High frequency repetitive firing, prominent after hyperpolarizations and lack of a pronounced sag response were typical of fast spiking basket cells.
Alterations in the Intrinsic Excitability of GABAergic Interneurons Induced by 4-AP
Fast-Spiking Basket Cells
Previous studies of L1 fast-spiking interneurons have shown that low concentrations (50 μM) of 4-AP broadened APs and induced burst firing (Zhou and Hablitz, 1996). In the present study, under control conditions, L2/3 FS-BCs had an average RMP of -68.7 ± 0.7 mV and input resistance of 97.3 ± 18.1 MΩ (n = 10). APs were evoked by somatic injections of depolarizing current pulses before and after bath application of 100 μM 4-AP, 10 μM CNQX, and 20 μM D-APV. Under control conditions, suprathreshold current pulses elicited repetitive firing in L2/3 FS-BCs (Figure 2A, black). APs under control and 4-AP conditions are shown superimposed in Figure 2B. APs had a half-width of 0.42 ± 0.03 ms (n = 10) and were followed by a characteristic fast afterhyperpolarization (fAHP) with an amplitude of 14.8 ± 1.4 mV (n = 10; Figures 2A,D). In the presence of 4-AP, the shape of the AP was distinctly different with a significant increase in the AP half-width (0.9 ± 0.04 ms; t-test p < 0.05, n = 10). The fAHP was blocked and a slower AHP with a significantly lower amplitude was observed (5.5 ± 0.1 mV, t-test p < 0.05, n = 10). The effects of 4-AP on AP duration and AHP amplitude are summarized in Figures 2C,D, respectively.
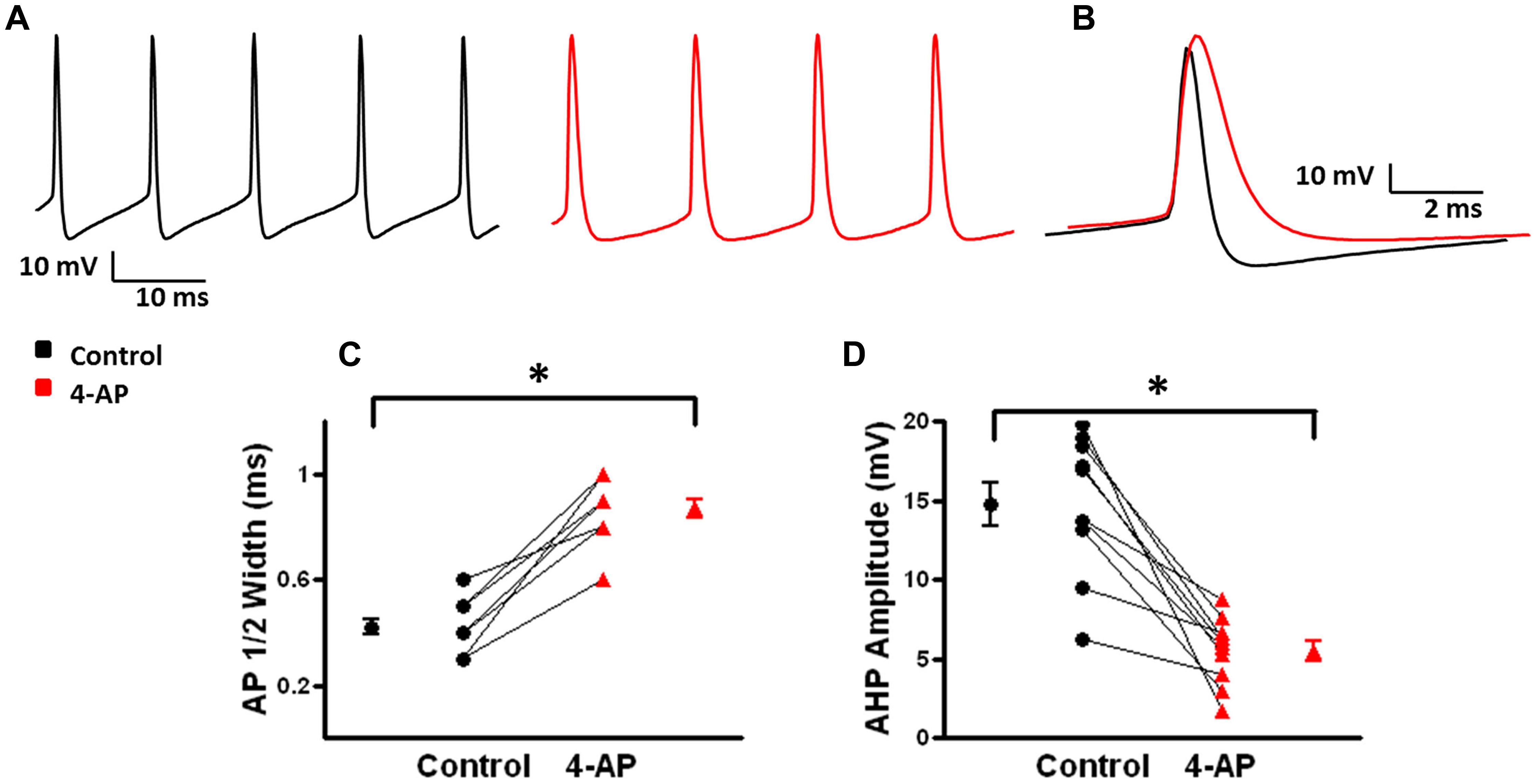
FIGURE 2. Effects of 4-AP on action potential (AP) properties of FS-BCs. (A) Representative firing from a L2/3 FS-BC during a 400 pA depolarizing current pulse before (black) and after (red) bath application of 100 uM 4-AP. 4-AP induced spike widening and decreases in fAHP amplitude. (B) Superimposition of APs from A before and after 4-AP demonstrates significantly increased AP duration and decreased AHP amplitude. (C) Summary plot showing that bath application of 4-AP significantly increases AP half width of FS-BCs (n = 10). (D) Summary plot illustrating that 4-AP significantly decreases AHP amplitudes in FS-BCs (n = 10). ∗p < 0.05.
A-type K+ channels have been implicated in the repolarization of APs and, therefore, repetitive firing properties of certain subclasses of interneurons (Massengill et al., 1997; Erisir et al., 1999; Lau et al., 2000; Rudy and McBain, 2001). These alterations in repetitive firing properties can lead to changes in network activity (Lau et al., 2000; Harvey et al., 2012). To determine if 4-AP induced similar alterations in repetitive firing properties in neocortical FS-BCs and possibly inhibitory network activity, suprathreshold depolarizing current pulses were applied for 800 ms to measure repetitive firing properties. L2/3 FS-BCs demonstrated sustained firing throughout the current pulse with no significant accommodation (Figures 3A,B). Upon application of 4-AP, burst firing was induced at the onset of the depolarizing current pulse (Figures 3A,B). As shown in Figure 3C, the initial firing frequency, calculated from the first interspike interval, significantly increased in the presence of 4-AP (control: 81.7 ± 12.2 Hz, 4-AP: 172 ± 16.4; t-test p < 0.05, n = 10). A ratio of the first and last interspike intervals was determined before and after wash-in of 4-AP. As shown in Figure 3D, this ratio was near 1 under control conditions indicating little or no accommodation. The ratio was significantly decreased showing firing frequency was significantly decreased in the presence of 4-AP, indicating increased accommodation. These results suggest that inhibition of A-type K+ channels with 4-AP significantly alters the AP and repetitive firing properties of neocortical L2/3 FS-BCs.
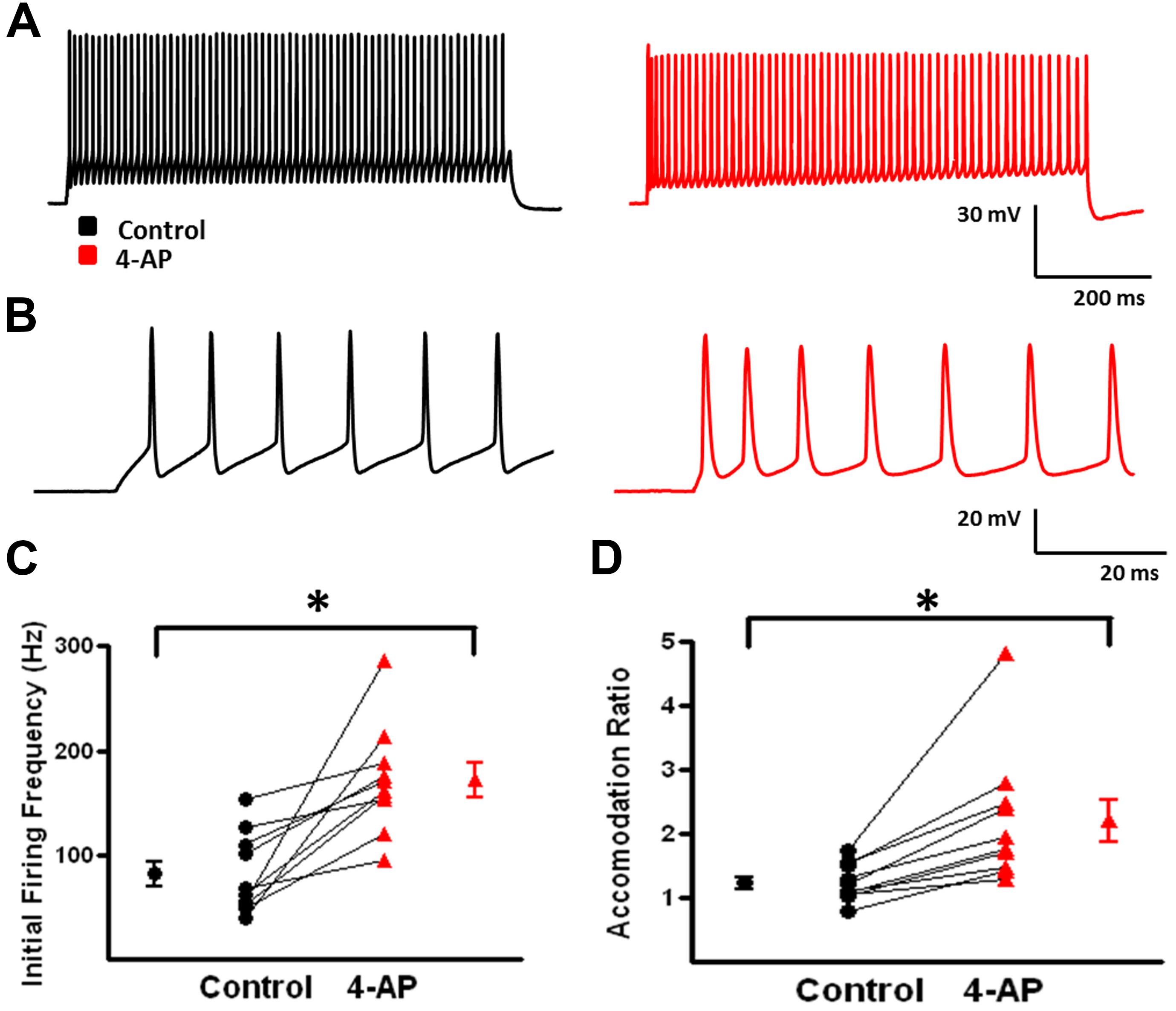
FIGURE 3. 4-aminopyridine induced changes in the repetitive firing properties of FS-BCs. (A) Representative response of a FS-BC to a 400 pA depolarizing current pulses before (black) and after (red) bath application of 4-AP. (B) Expanded view of the initial firing at the onset of responses shown in A. 4-AP induced an increase in the initial firing frequency. (C) Summary plot illustrating that bath application of 4-AP significantly increases the initial firing frequency at the onset of a depolarizing current (n = 10). (D) Summary plot showing that the accommodation ratio, calculated by dividing the first interspike interval by the last interspike interval, was significantly increased after bath application of 4-AP, indicating that accommodation was increased (n = 10). ∗p < 0.05.
Martinotti Cells
Martinotti cells express mRNA for all three major subclasses of A-type K+ channels (Wang et al., 2004), but it is unclear how these channels contribute to the intrinsic and repetitive firing properties of these critical feed-back inhibitory cells. To address this, recordings were made from L2/3 Venus-positive MCs. It is well established that MCs are a heterogeneous subclass of interneurons and can be further classified based on their repetitive firing properties (Wang et al., 2004; Xu et al., 2006; McGarry et al., 2010). In this series of experiments, two electrophysiologically distinct classes of MCs were encountered: classical accommodating (c-AC; 3/13) and burst accommodating (b-AC) MCs (10/13 cells; Wang et al., 2004). Only b-AC MCs were examined and analyzed for the following set of experiments.
Under control conditions, b-AC MCs fired a burst of APs at onset of a depolarizing current pulse followed by a slowly accommodating train of single APs (Figure 5A, black). Following application of 4-AP, the same current pulse elicited repetitive burst firing (Figure 5A, red). Initial burst responses are shown at higher time resolution in Figure 5B. In the presence of 4-AP, single APs were transformed into bursts in a subset of cells; small, prolonged APs were observed following the initial AP in these burst (Figures 4A,B). AP half width was significantly increased by 4-AP (control: 0.8 ± 0.1 ms, 4-AP: 1.3 ± 0.2; t-test p < 0.05, n = 10; Figure 4C). The amplitude of the AHP following individual APs was also significantly decreased in the presence of 4-AP (control: 8.1 ± 0.7 mV, 4-AP: 6.2 ± 0.8 mV; t-test p > 0.05, n = 10; Figure 4D). In contrast to FS-BCs, the initial firing frequency at the onset of the depolarizing current injection did not change following 4-AP application (control: 130.4 ± 14.1 Hz, 4-AP: 112.3 ± 18.7 Hz; t-test p > 0.05, n = 10; Figure 5C). However, firing following the initial burst displayed increased accommodation with cells firing at a lower frequency (control: 27.7 ± 1.7, 4-AP: 18. 8 ± 1.6 Hz; t-test p < 0.05, n = 10; Figure 5D). In addition to the above described changes, a slow afterdepolarization (sADP) was observed in presence of 4-AP (control: 1.2 ± 0.2 mV, 4-AP: 7.6 ± 1.9 mV; t-test p < 0.05, n = 9; Figure 6). These results suggest that alterations in the firing patterns of b-AC MCs could alter network excitability and increase synchronization.
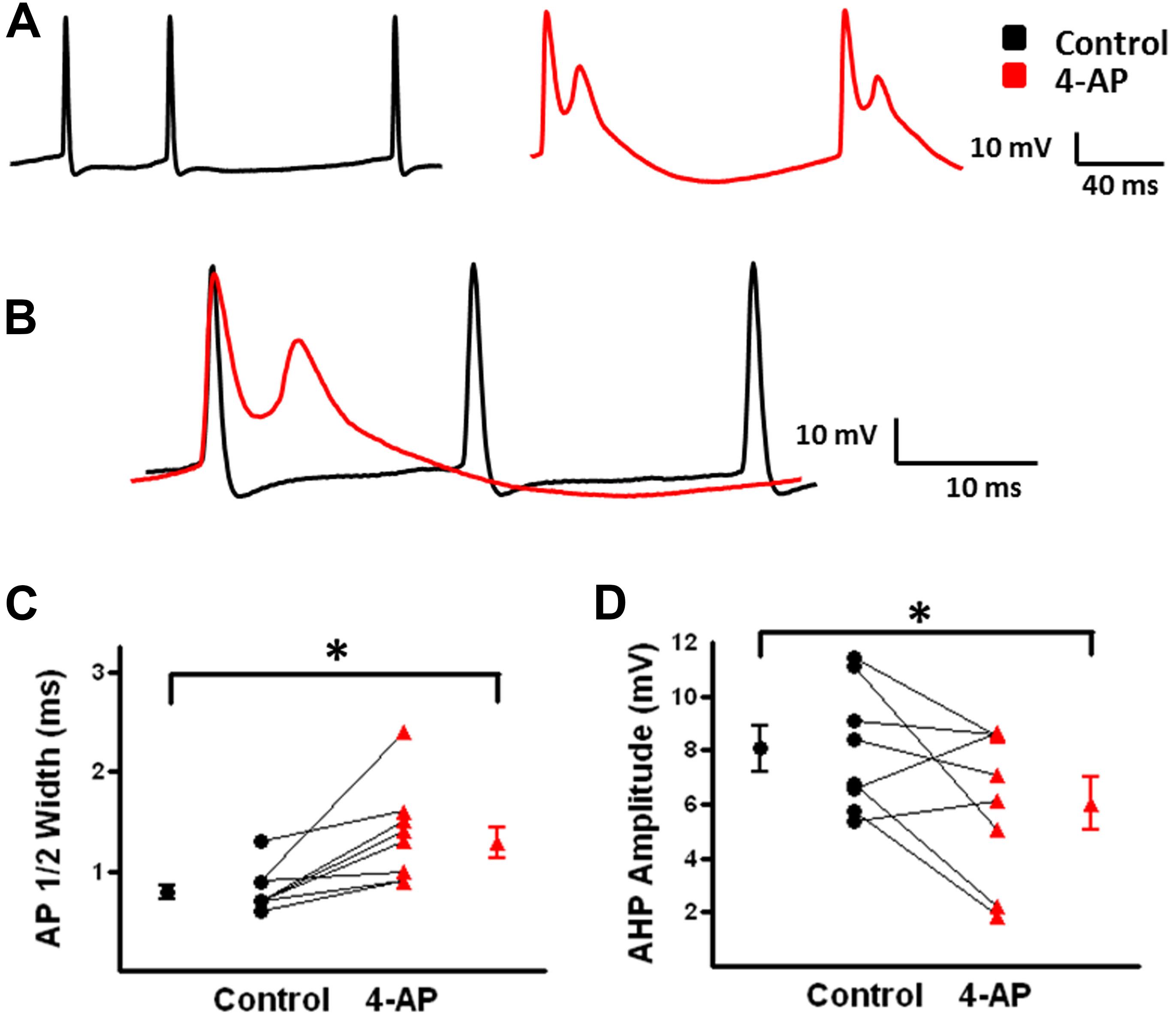
FIGURE 4. Action potential (AP) properties of b-AC Martinotti cells (b-AC MCs) are modified in the presence of 4-AP. (A) Representative AP firing of a L2/3 b-MC during a 475 pA depolarizing current pulse before (black) and after (red) bath application of 4-AP. (B) Superimposition of APs from A before and after bath application of 4-AP showing spike widening and burst firing in presence of 4-AP. (C) Summary plot demonstrates that bath application of 4-AP significantly increases AP duration in MCs (n = 10). (D) Summary graph illustrating that the AHP after each AP in b-AC MCs was significantly reduced by bath application of 4-AP (n = 10). ∗p < 0.05.
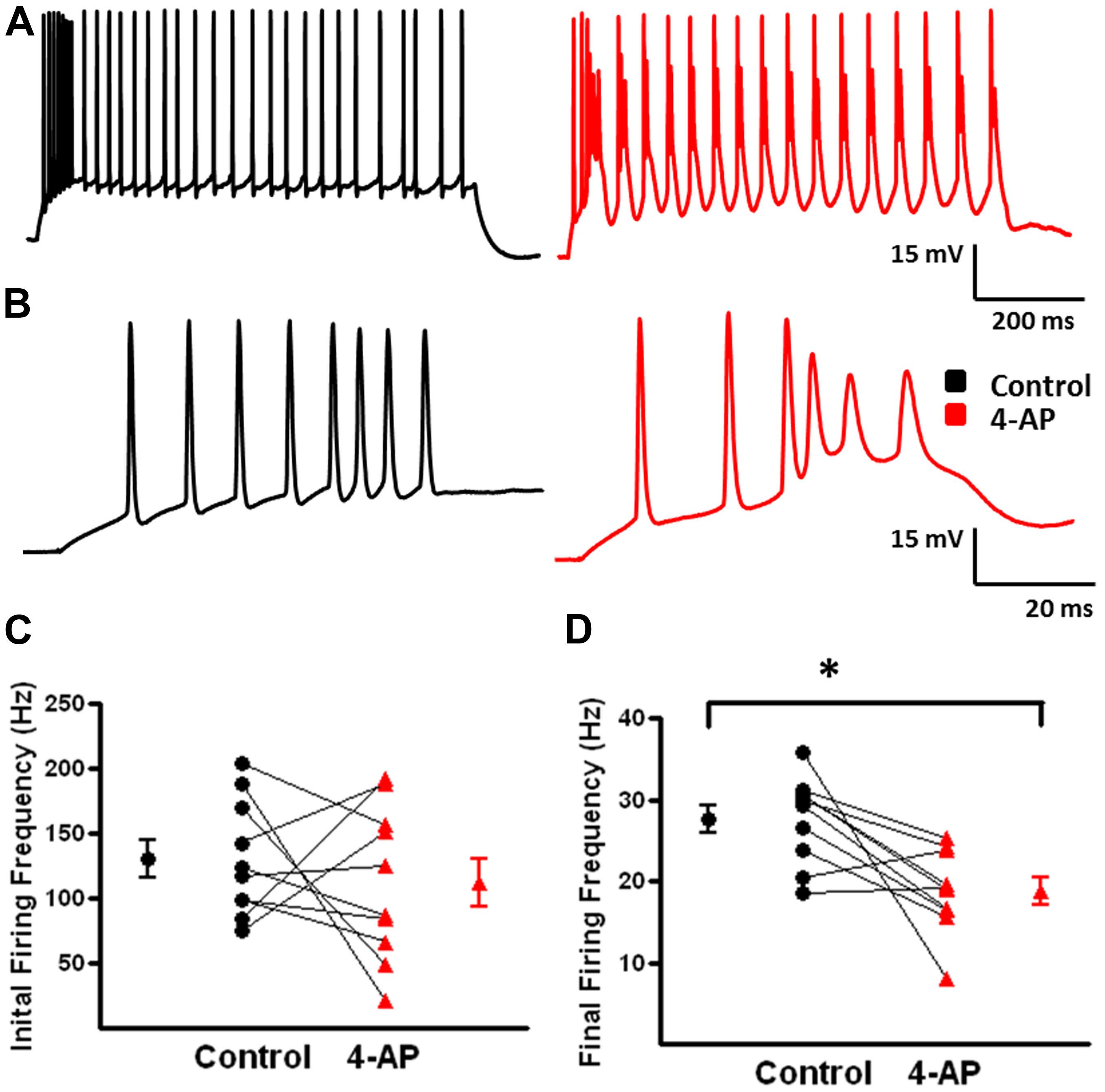
FIGURE 5. Alterations in repetitive firing of b-AC MCs following bath application of 4-AP. (A) Representative response of a b-AC MC to a 475 pA depolarizing current pulse before (black) and after (red) bath application of 4-AP. (B) An expanded view of the initial firing pattern at the onset of a 475 pA depolarizing current pulse before and after bath application of 4-AP. Enhanced, continuous, burst firing was seen in presence of 4-AP. (C) Summary plot illustrating that the initial firing frequency at the onset of a depolarizing current pulse is not altered by 4-AP (n = 10). (D) Summary plot showing that the final firing frequency of b-AC MCs is significantly decreased after bath application of 4-AP (n = 10). ∗p < 0.05.
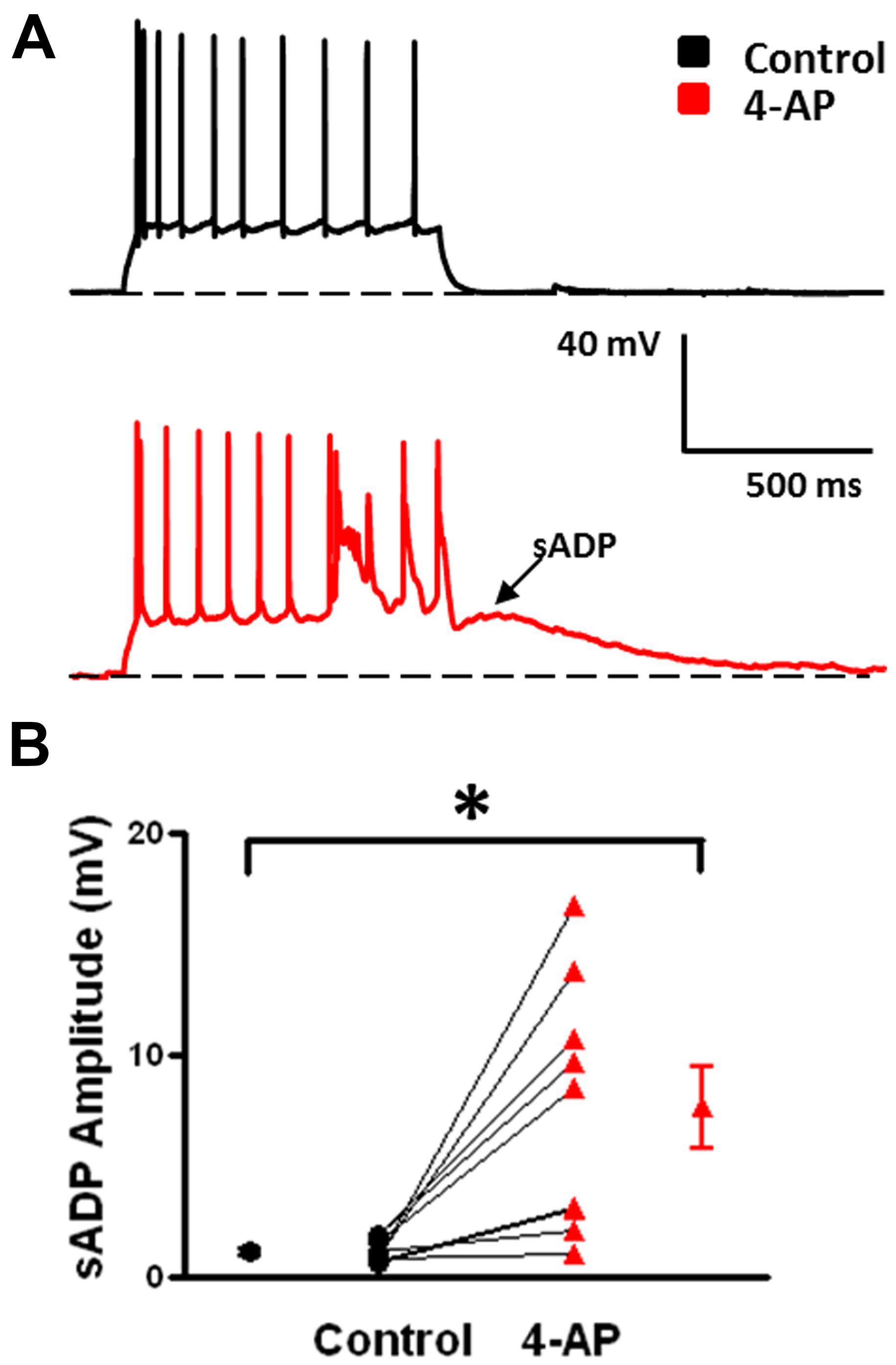
FIGURE 6. Emergence of slow afterdepolarizations (sADPs) in b-AC MCs in presence of 4-AP. (A) Representative response of a L2/3 b-AC MC to a 475 pA depolarizing current pulse before (black) and after (red) bath application of 4-AP. Following application of 4-AP, a sADP was observed following repetitive firing. (B) Summary plot showing that a significant s-ADP was observed in all b-AC MCs in presence of 4-AP (n = 10). *p < 0.05.
Inhibition of Ih Increases Synchronous GABAergic Network Activity
GABAergic interneurons in rat neocortex do not typically stain with HCN channel antibodies (Lorincz et al., 2002; Notomi and Shigemoto, 2004). However, there is electrophysiological evidence for the presence of functional HCN channels in specific subclasses of neocortical interneurons (Lupica et al., 2001; Aponte et al., 2006; Ma et al., 2006). The role of HCN channels in excitability of GABAergic interneurons and synchronous GABAergic network activity is poorly understood. In neocortex and hippocampus, application of 4-AP, in the presence of CNQX and D-APV to block excitatory glutamatergic transmission, induces synchronized bursting of inhibitory interneurons (Avoli, 1990; Aram et al., 1991). We first examined the effects of Ih inhibition on inhibitory network activity in MCs, which express the largest Ih current among interneurons (Ma et al., 2006).
Martinotti Cells
Ih activation is associated with a “sag” in the peak voltage response evoked by large hyperpolarizing current pulses coupled with a rebound depolarization on pulse offset (Lupica et al., 2001; Ma et al., 2006). Figure 7A shows a sag (arrow) and rebound (arrow head) response in an identified MC. ZD 7288, a specific HCN channel antagonist, reduced both sag and rebound responses when bath applied at 20 μM (Figure 7A). In the presence of 4-AP, CNQX, and D-APV, electrical stimulation in L2/3 reliably produced a depolarizing GABAergic response in the recorded MCs. With the given intracellular Cl- concentration of the pipette solution and the extracellular Cl- concentration of the bath solution, the calculated reversal potential for evoked GABAergic responses is -66 mV. These responses are inhibited by bicuculline (20 uM), indicating they are GABAergic in nature (data not shown; see also Aram et al., 1991). Inhibition of HCN channels with ZD 7288 enhanced evoked depolarizing GABAergic responses (Figure 7B). Summary graphs in Figures 7C,D show that ZD 7288 significantly increased evoked depolarizing GABAergic response amplitude and area, respectively. These results suggest that HCN channels, presumably via Ih, normally restrict network activity in this model of GABAergic synchronization.
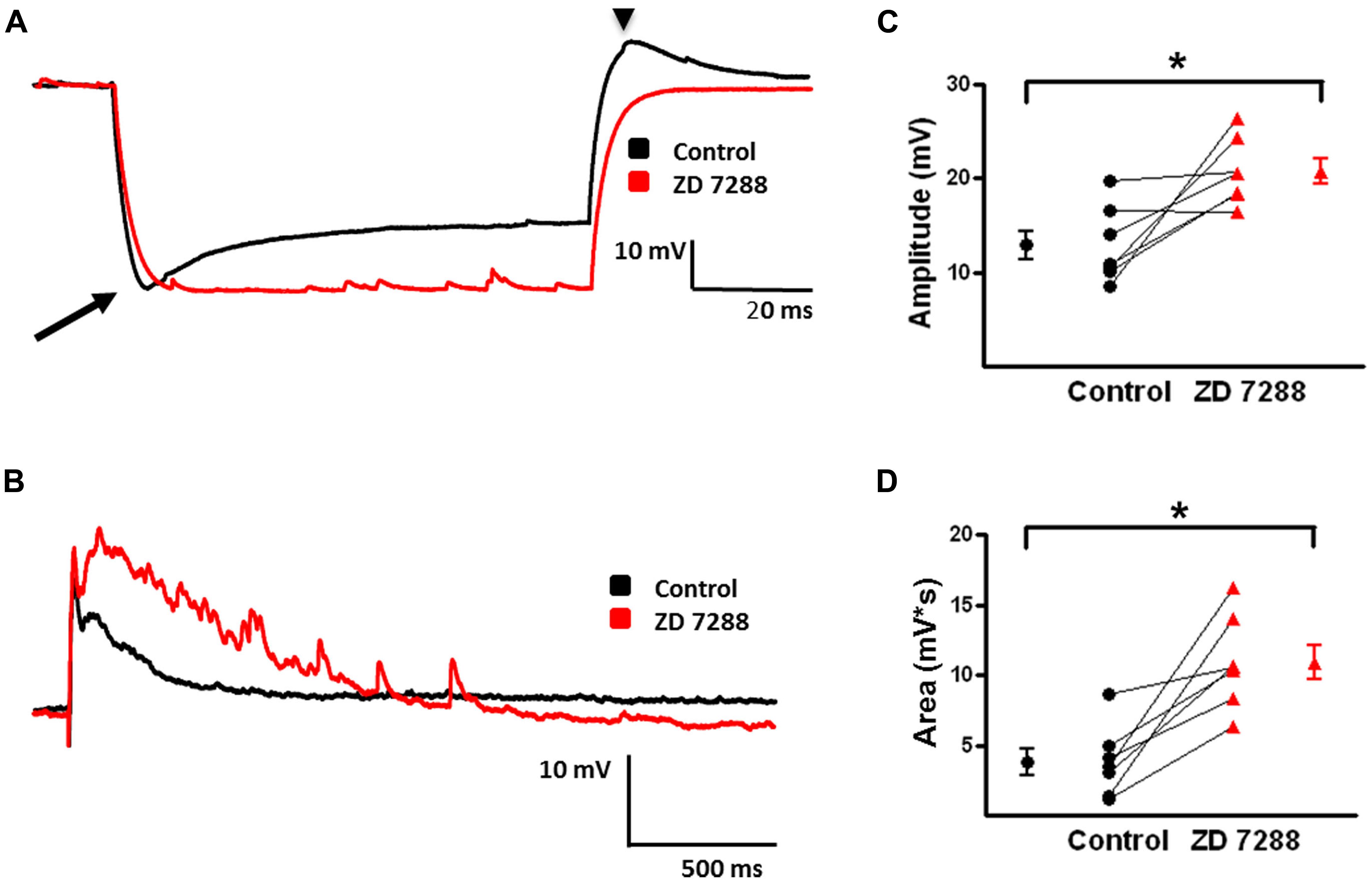
FIGURE 7. Effect of Ih inhibition with ZD 7288 on magnitude of evoked depolarizing GABAergic potentials in MCs. (A) Superimposition of representative responses of a L2/3 MC to a 250 pA hyperpolarizing current pulse. Characteristic sag (arrow) and rebound responses (arrow head) observed under control conditions (black) were inhibited following application of ZD 7288 (red). (B) Representative responses of evoked depolarizing GABAergic potentials in a L2/3 MC before (black) and after (red) bath application of ZD 7288. (C) Summary graph showing a significant increase in the amplitude of evoked depolarizing GABAergic potentials in MCs upon inhibition of Ih. (D) Summary plot indicating that ZD 7288 significantly increases the area of evoked depolarizing GABAergic potential responses in MCs (n = 7). ∗p < 0.05.
We further investigated the effect of Ih inhibition on network activity by measuring spontaneously occurring depolarizing GABAergic responses in L2/3 MCs. Examples of spontaneous depolarizing GABAergic responses are shown in Figures 8A,B. In the presence of 4-AP, CNQX, and D-APV, spontaneous events occurred at a low rate. When examined at higher time resolution, spontaneous events in MCs were seen to consist of a membrane depolarization with few or no superimposed APs (Figure 8B). ZD 7288 significantly increased the frequency of spontaneous depolarizing GABAergic responses (control: 0.009 ± 0.002 Hz, ZD: 0.018 ± 0.003 Hz; t-test p < 0.05, n = 9; Figure 8C). Similar to the effects on evoked depolarizing GABAergic responses, bath application of ZD 7288 significantly increased the area of spontaneous depolarizing GABAergic responses (control: 2585 ± 556 mv∗ms, ZD: 9079 ± 3126 mV∗ms; t-test p < 0.05, n = 5; Figure 8D). These results show that inhibition of HCN channels leads to an increase in the amplitude and area of evoked and spontaneously occurring depolarizing GABAergic responses, as well as an increase the frequency of spontaneous depolarizing GABAergic responses in L2/3 MCs.
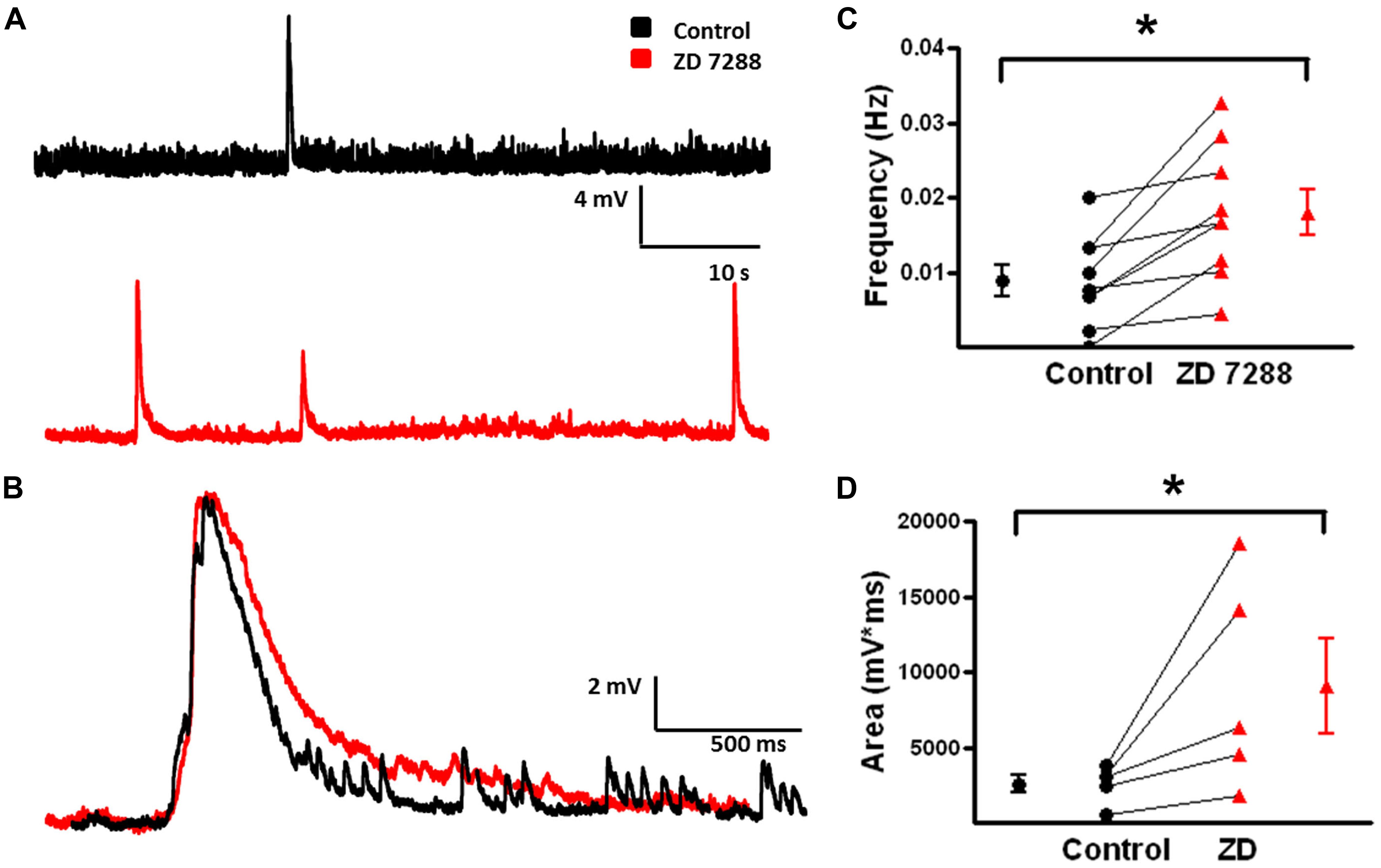
FIGURE 8. Frequency and area of spontaneous depolarizing GABAergic potentials in L2/3 MCs increase with inhibition of Ih. (A) Specimen records showing spontaneous depolarizing GABAergic potentials before (black) and after (red) bath application of ZD 7288. A significant increase in the frequency of depolarizing GABAergic potentials was observed following inhibition of Ih. (B) An expanded view of a single spontaneous depolarizing GABAergic potential before (black) and after (red) bath application of ZD 7288. (C) Summary plot demonstrating a significant increase in the frequency of spontaneous GABAergic events following application of ZD 7288 (n = 9). (D) Summary graph demonstrating that inhibition of Ih significantly increases the area of spontaneous depolarizing GABAergic potentials. (n = 5). ∗p < 0.05.
Fast-Spiking Basket Cells
These cells display small Ih current upon hyperpolarization (Aponte et al., 2006). Nonetheless, it was hypothesized that HCN channel inhibition would also enhance activity in FS-BCs due to enhanced network activity. Recordings were obtained from YFP-positive cells that displayed FS-BC intrinsic and repetitive firing properties, as described above. Figure 9A shows that hyperpolarizing current pulses were associated with small rapid sag responses (arrow) and rebound response (arrow head). ZD 7288 (20 μM) application abolished both sag and rebound responses (Figure 9A).
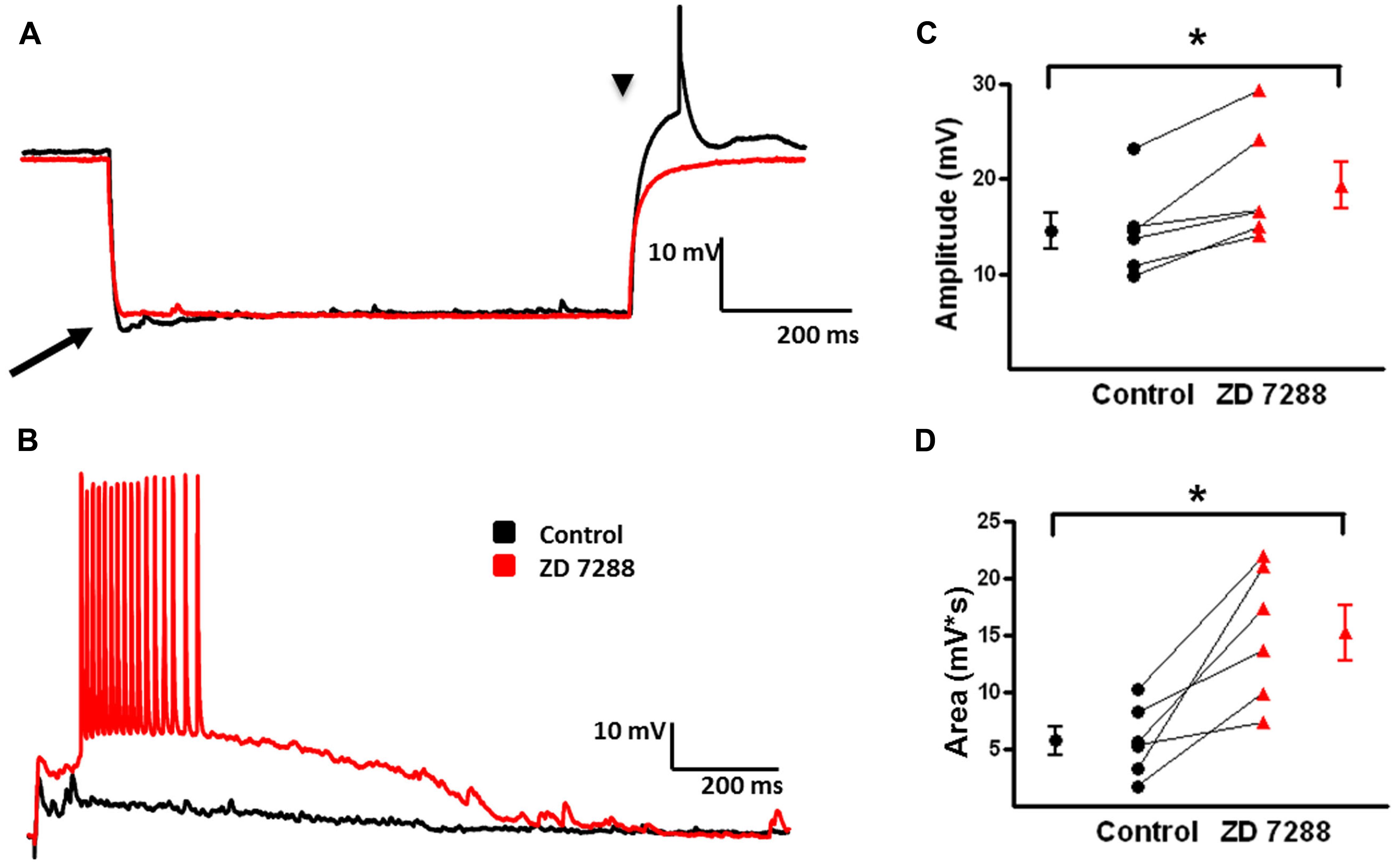
FIGURE 9. Evoked depolarizing GABAergic potentials are enhanced in FS-BCs following Ih inhibition. (A) Superimposition of representative responses of a L2/3 FS-BC to a 250 pA hyperpolarizing current pulse. Characteristic small amplitude sag (arrow) responses and rebound depolarizations (arrow head) were observed in FS-BCs under control conditions (black). Sag and rebound responses were blocked in presence of ZD 7288 (red). (B) Superimposed specimen records of evoked depolarizing GABAergic potentials in a L2/3 FS-BC before (black) and after (red) bath application of ZD 7288. (C) Summary plot demonstrating a significant increase in the amplitude of evoked depolarizing GABAergic potentials in FS-BCs upon Ih inhibition (n = 6). (D) Summary plot demonstrating ZD 7288 significantly increases the area of evoked depolarizing GABAergic potential responses in FS-BCs (n = 6). ∗p < 0.05.
Slices were incubated in 4-AP for >1 h to develop robust synchronous network activity. In the presence of 4-AP, CNQX, and D-APV, electrical stimulation evoked depolarizing GABA-mediated responses when cells were held at -80 mV in current clamp (Figure 9, black). Depolarizing GABAergic responses were reliably evoked in FS-BCs (Figure 9B) with an average amplitude of 14.5 ± 1.9 mV and area under the curve of 5783 ± 1270 mV∗ms (Figures 9C,D). Bath application of ZD 7288 significantly increased the amplitude (ZD: 19.3 ± 2.5 mV; t-test p < 0.05, n = 6; Figure 9C) and area (ZD: 15269. ± 2428 mV*ms; t-test, p < 0.05, n = 7; Figure 9D) of evoked depolarizing GABAergic responses. In 6/7 cells, the previously subthreshold stimulation initiated large amplitude events with superimposed APs.
Spontaneous depolarizing GABAergic responses with superimposed APs were observed in FS-BCs (Figure 10A, arrow). In addition, FS-BCs demonstrated a persistent membrane potential oscillation often accompanied by APs on the depolarizing phase (Figure 10A). These baseline oscillations and bursts of APs were not accompanied by a significant membrane depolarization and were not included in the analysis of depolarizing GABAergic responses. This activity was not observed following application of ZD 7288 whereas spontaneous depolarizing GABA responses were significantly enhanced (Figure 10B). ZD 7288 significantly increased the frequency of spontaneous depolarizing GABAergic responses in FS-BCs (control: 0.007 ± 0.002 Hz, ZD: 0.014 ± 0.003 Hz; t-test, p < 0.05, n = 7; Figure 10C). HCN channel inhibition also significantly increased the area of spontaneous depolarizing GABAergic responses (control: 14709 ± 2257 mV∗ms, ZD: 23951 ± 3306 mV∗ms; t-test p < 0.05, n = 7; Figure 10D). This increase in depolarizing GABAergic response frequency and area was accompanied by a decrease or loss of baseline oscillations observed under control conditions.
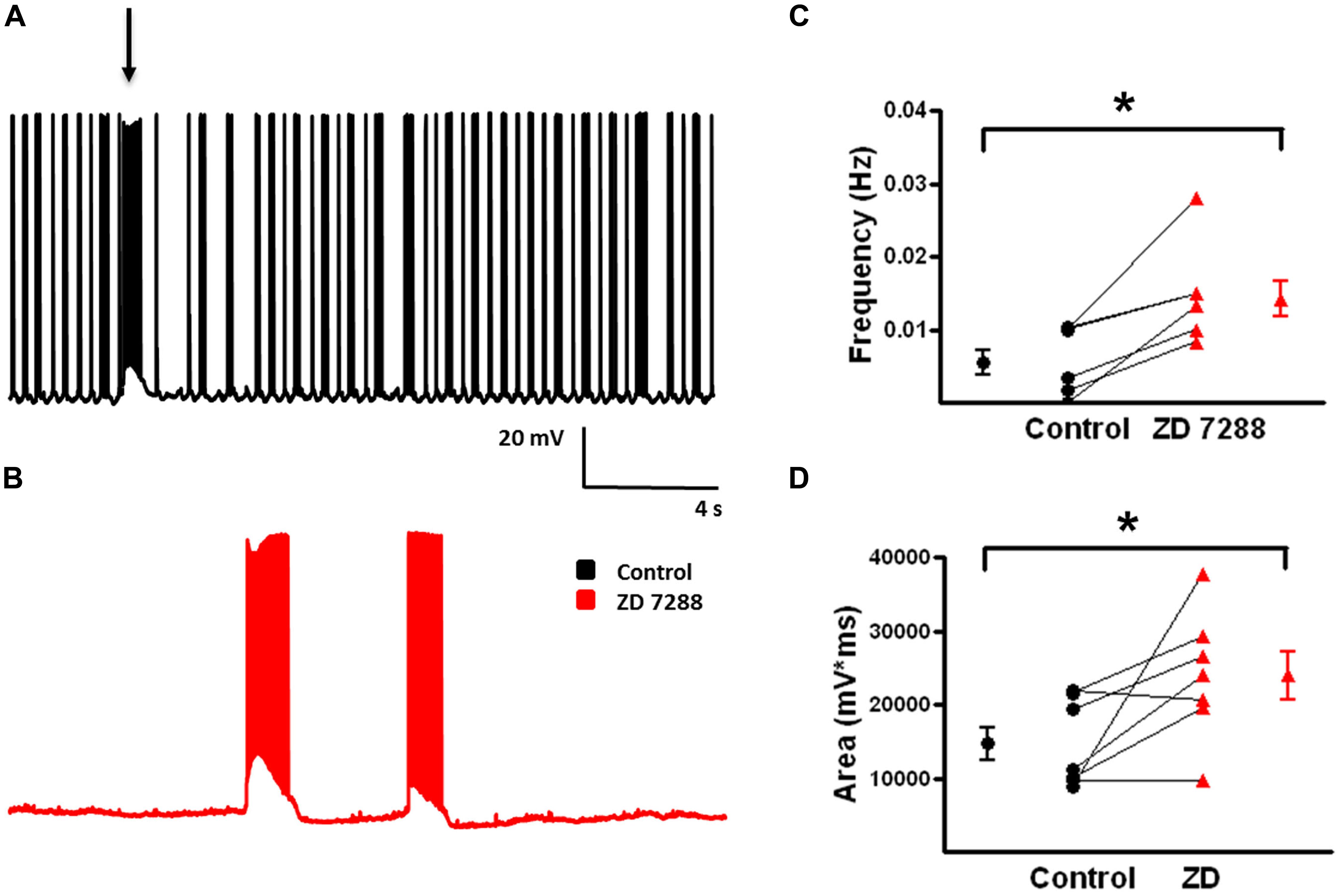
FIGURE 10. Frequency and area of spontaneous depolarizing GABAergic potentials in L2/3 FS-BCs increase with Ih inhibition. (A) Representative trace of a spontaneous depolarizing GABAergic potential (arrow) in a L2/3 FS-BC. Specimen record also shows characteristic 4-AP induced baseline burst firing in FS-BC, which precede and follow spontaneous depolarizing GABAergic potentials. (B) Representative trace of spontaneous events following bath application of ZD 7288. (C) Summary plot demonstrates that inhibition of Ih with ZD 7288 significantly increases the frequency of spontaneous depolarizing GABAergic potentials in FS-BCs (n = 7). (D) Summary plot demonstrates that the area of spontaneous depolarizing GABAergic potentials significantly increases with inhibition of Ih (n = 7). ∗p < 0.05.
Temporal IPSP Summation in GABAergic Interneurons
In neocortical pyramidal cells, temporal integration of EPSPs is reduced or prevented by Ih and Ih inhibition results in enhanced synaptic integration (Berger et al., 2001). Modulation of synaptic integration in interneurons is relatively unexplored. However, it has been observed that facilitating EPSPs from pyramidal cells onto MCs were only slightly changed in the presence of ZD 7288 to block Ih (Berger et al., 2010). In the present study, IPSPs are depolarizing due to alterations in the Cl- equilibrium potential (Aram et al., 1991). It was hypothesized that the increased network excitability observed in the presence of the HCN channel inhibitor ZD 7288 was due to changes in temporal integration. To test this, five IPSPs were evoked at 25 Hz in both MCs and FS-BCs. EPSPs in MCs typically show temporal integration (Berger et al., 2010). In MCs, when depolarizing IPSPs were evoked at 25 Hz, modest temporal integration was observed (Figure 11A, control). These IPSPs were associated with a pronounced underlying depolarization. Inhibition of Ih significantly increased the amplitude of IPSPs 1-5 (Two-way ANOVA, p < 0.05; Figure 11B). Paired pulse ratios (PPRs) for IPSPs1 and 5 in MCs were significantly increased in the presence of ZD 7288 (1.67 ± 0.3 in Control and 2.4 ± 0.3, t-test, p < 0.05, n = 6). The area of the underlying depolarization was significantly enhanced (Figure 11C) and was occasionally associated with the onset of a depolarizing GABAergic response (Control: 1566 ± 189 mV∗s, ZD: 4806 ± 803 mV∗ s; t-test, p = 0.05).
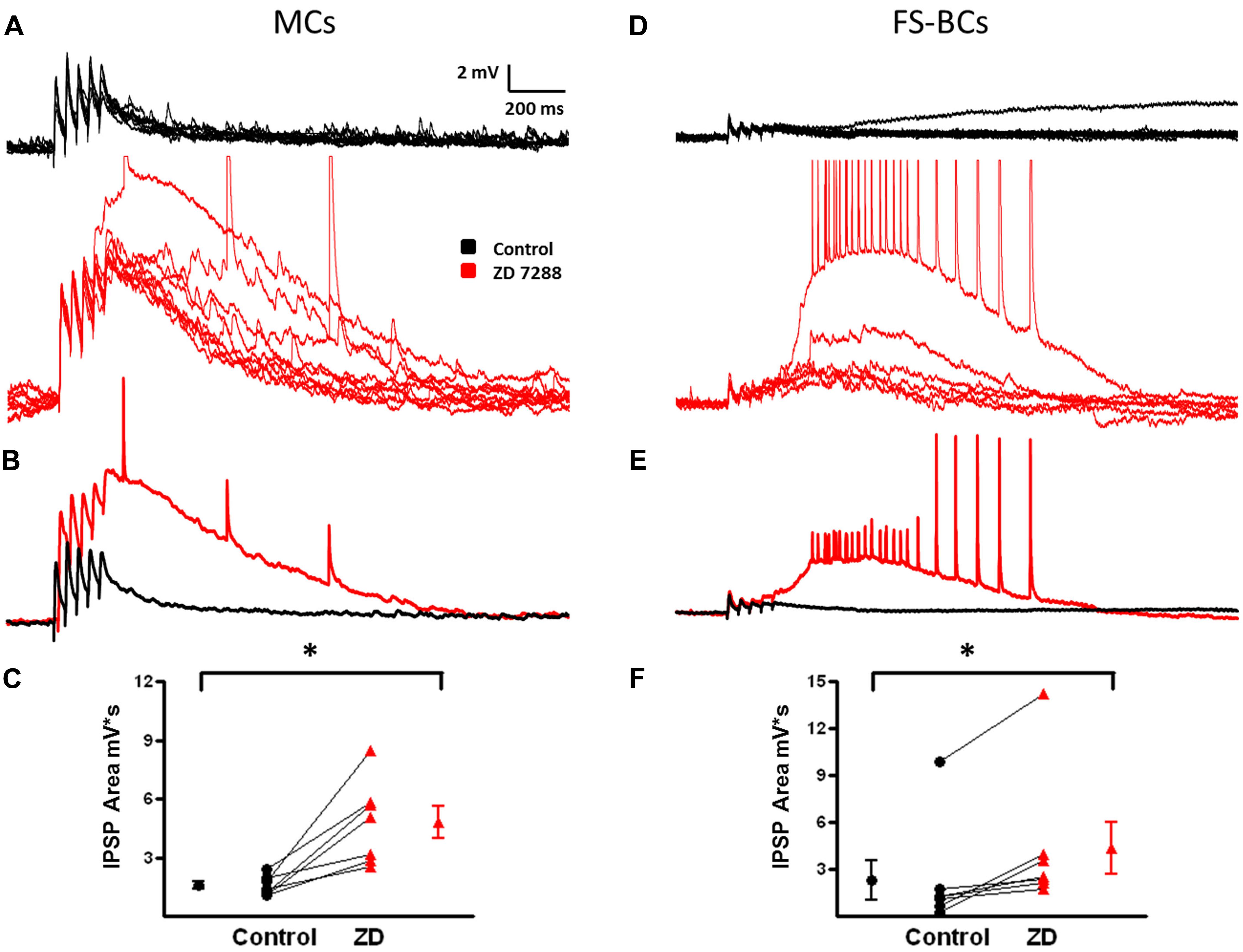
FIGURE 11. Changes in temporal integration of depolarizing IPSP in MCs and FS-BCs after Ih inhibition. (A) Ten superimposed responses of a L2/3 MC to a train of five stimuli at 25 Hz (20–50 μA) before (top, black) and after (bottom, red) bath application of Z7288. Temporal integration of IPSPs was observed under control conditions. This was significantly enhanced upon inhibition of Ih. (B) Superimposed averaged responses of the individual responses shown in (A). (C) Summary plot demonstrating ZD 7288 significantly increases in IPSP area in L2/3 MCs (n = 7). (D) Superimposed individual responses of a L2/3 FS-BC to a similar 25 Hz train before (top, black) and after (bottom, red) bath application of ZD7288. Characteristic IPSP depression was observed in the control condition. (E) Averaged responses of the individual traces shown in (D). Alterations in IPSP depression was not observed in FS-BCs following inhibition of Ih. However, ZD 7288 did cause the appearance of a late depolarization that could initiate depolarizing GABAergic potentials. (F) Summary plot illustrating that bath application of ZD 7288 significantly increases the area of depolarization after 25 Hz stimulation (n = 7). ∗p < 0.05.
Ih currents and the effect of Ih on synaptic integration have not been extensively examined in FS-BCs. The small sag responses observed here (Figure 9A) led us to hypothesis that synaptic integration would not be affected in FS-BCs, but an enhanced underlying depolarization would be seen following Ih inhibition. Superimposed records in Figure 11D show that IPSPs displayed depression during the train of 25 Hz stimulation. Averages of these responses are shown in Figure 11E. Inhibition of Ih had no significant effect on the amplitude of first IPSP (Control: 3.3 ± 0.4 mV, ZD: 3.2 ± 0.4 mV; t-test, p = 0.25) or IPSPs 1–5 (two-way ANOVA, p = 0.08). PPRs were not significantly changed in presence of ZD 7288 (0.575 ± 0.1 Control and 0.86 ± 0.1 in presence of ZD 7288; t-test, p < 0.05, n = 4). Ih inhibition was, however, accompanied by a increased late depolarization and associated with evoked depolarizing GABAergic responses (Control: 2314 ± 1276 mV∗ s, ZD: 4354 ± 1671 mV∗ s; t-test, p = 0.05; Figure 11F). These results suggest that HCN channels differentially modulate synaptic integration of depolarizing IPSPs in MCs compared to FS-BCs.
Discussion
This study investigated the effect of 4-AP on the firing properties of L2/3 FS-BCs and MCs. In the presence of 4-AP, AP durations increased and AHP amplitudes decreased in both L2/3 FS-BCs and MCs. FS-BCs demonstrated an increase in initial firing frequency upon suprathreshold depolarization and an increase in accommodation in the presence of 4-AP. Initial burst firing was maintained in b-AC MCs and persistent repetitive burst firing was also observed. Additionally, we investigated the effect of HCN channel inhibition on GABAergic network synchronization. In the presence of ZD 7288 to inhibit Ih, the area of evoked GABAergic depolarizations was increased in both FS-BCs and b-AC MCs. Similarly, inhibition of Ih increased the area and frequency of spontaneous depolarizing GABAergic events in these cells. These results indicate that 4-AP differentially alters the intrinsic firing patterns of interneurons. Acute inhibition of HCN channels enhances GABAergic interneuron synchronization indicating that Ih modulates inhibitory network excitability.
4-AP Alters the Intrinsic Firing Properties of FS-BCs and b-MCs
The mechanism by which application of 4-AP in the presence of CNQX and D-APV induces GABAA receptor-mediated synchronization of neocortical GABAergic interneurons remains unclear. Early studies with 4-AP examined role of potassium channels in synaptic transmission at the squid giant synapse (Llinas et al., 1976). It was later shown to have convulsant effects in vitro and in vivo (Spyker et al., 1980; Thesleff, 1980; Jack et al., 1981; Voskuyl and Albus, 1985; Rutecki et al., 1987; Szente and Baranyi, 1987; Mihaly et al., 1990; Perreault and Avoli, 1991). Subsequent investigation into the mechanism of action concluded that 4-AP blocks transient outward K+ currents through A-type K+ channels in a variety of systems (Hermann and Gorman, 1981; Choquet and Korn, 1992; Mei et al., 1995). Additionally, 4-AP blocks the delayed K+ current ID (Storm, 1988; Wu and Barish, 1992; Barish et al., 1996; Coetzee et al., 1999).
Application of 4-AP enhances release of both excitatory and inhibitory neurotransmitters (Rutecki et al., 1987; Tibbs et al., 1989; Perreault and Avoli, 1991; Otis and Mody, 1992). A-type K+ channels are predominantly expressed in the soma and dendrites of pyramidal neurons, increasing in density distal to the soma, with sparse expression in axons and terminals (Hoffman et al., 1997; Bekkers, 2000; Korngreen and Sakmann, 2000; Johnston et al., 2003; Dodson and Forsythe, 2004; Foust et al., 2011). These channels, mainly consisting of Kv4 subunits, in addition to shaping the AP waveform, regulate back propagation of APs into distal dendrites, and control excitability and output of pyramidal neurons (Stuart et al., 1997; Bekkers, 2000; Kang et al., 2000; Yuan et al., 2005). The isoform composition and cellular localization of A-type K+ channels is more variable in interneurons compared to pyramidal cells (Weiser et al., 1995; Du et al., 1996; Martina et al., 1998; Chow et al., 1999; Coetzee et al., 1999).
The dissimilar effects of 4-AP on AP and repetitive firing properties in FS-BCs and MCs observed here may be attributable to differential expression and localization of A-type K+ channels in various classes of interneurons (Chow et al., 1999; Erisir et al., 1999; Jerng et al., 2004; Goldberg et al., 2008; Rudy et al., 2011). In the presence of 4-AP, we observed that AP duration was similarly increased in both FS-BCs and MCs, consistent with a known global enhancement of synaptic transmission (Jankowska et al., 1977; Buckle and Haas, 1982; Otis and Mody, 1992). The characteristic fAHP of FS-BCs was markedly reduced and accommodation was increased. Axons and nerve terminals of FS-BCs express Kv3 and Kv1 which may directly affect the AP repolarization in these structures (Massengill et al., 1997; Erisir et al., 1999; Lau et al., 2000; Goldberg et al., 2008). The blockade of the Kv1 current is most likely responsible for the switch from a single AP to a burst of APs at the onset of a depolarizing current pulse (Wu and Barish, 1992; Goldberg et al., 2008). Furthermore, Kv3 channels are necessary for the FS phenotype of FS-BCs (Rudy and McBain, 2001; Lien and Jonas, 2003). The inhibition of Kv3 channels likely resulted in the observed increase in AP accommodation in the present study.
In contrast to FS-BCs, MCs express the Kv4 family of channels at a higher density in the somatodendritic region. It remains unknown if these channels are expressed in a similar gradient pattern along the dendrites in interneurons as seen in pyramidal neurons. However, they function to tightly regulate dendritic Ca2+ spikes and back-propagating APs in other cells (Serodio and Rudy, 1998; Lien et al., 2002; Chen and Johnston, 2004; Lai and Jan, 2006; Bourdeau et al., 2007; Sun et al., 2011). Our observed effects of 4-AP on repetitive firing properties in neocortical MCs can be attributed to blockade of Kv4 channels. By blocking Kv4 channels, back-propagating APs and Ca2+ spikes would be able to antidromically invade the dendrites causing a prolonged depolarization and possibly initiating further AP firing, directly contributing the transformation of single APs to repetitive burst responses observed in MCs. Additionally, the dendritic hyperexcitability resulting from inhibition of somatodendritic Kv4 channels could underlie the unmasking of a sADP following repetitive firing in the presence of 4-AP.
4-AP Induced GABAergic Network Synchronization
GABAergic synapse formation often precedes that of excitatory glutamatergic synapses (Ben-Ari et al., 1989). Since immature neurons have higher intracellular chloride concentrations due to early lack of KCC2 chloride transporters, GABA depolarizes and excites immature neurons (Ben-Ari et al., 2007). Early in development, excitatory GABAergic transmission is associated with spontaneous giant depolarizing potentials (Garaschuk et al., 1998; Bonifazi et al., 2009). Similar appearing responses are seen in more mature animals under appropriate pharmacological conditions. In the presence of glutamatergic antagonists, bath application of 4-AP induces a GABAA-receptor mediated network synchronization of interneurons (Aram et al., 1991; Avoli et al., 1998). This hyperexcitability in inhibitory networks produces large amplitude depolarizing potentials that propagate through the cortex (DeFazio and Hablitz, 2005). The enhancement in inhibitory synaptic transmission associated with broadened APs could produce an increase in the intracellular Cl- concentration through the activation of GABA-gated chloride channels producing an activity-dependent shift in the concentration gradient for Cl- resulting in the observed membrane depolarization (Ling and Benardo, 1995; Staley et al., 1995). The synchronous firing of neurons associated with depolarizing GABA responses produces an elevation in the extracellular K+ concentration contributing to the persistence of interneuron synchronization (Gilbert et al., 1984; MacVicar et al., 1989; Avoli et al., 2002). Synchronous GABAergic discharges have been predominantly investigated in brain slice preparations. However, co-perfusion of 4AP and glutamate receptor blockers in an in vitro isolated guinea pig brain preparation produced spontaneous synchronized propagating events that exhibited sensitivity to the GABAA receptor antagonist bicuculline (Uva et al., 2009). It is likely that synchronization of GABAergic networks also occurs in vivo. The intrinsic changes that occur in interneurons to initiate this form of synchronous activity are not well understood. The subclasses of interneurons that initiate and participate in network synchronization have not been clearly established.
We observed robust spontaneous and evoked GABAergic depolarizations in FS-BCs and MCs in the presence of 4-AP and glutamatergic antagonists. The GABAergic depolarizations observed in FS-BCs were noticeably different from those seen in MCs. Large GABAergic depolarizations in FS-BCs were accompanied by high frequency firing. Similar firing has been seen in other fast spiking interneurons (Benardo, 1997; Keros and Hablitz, 2005). GABAergic depolarizations rarely initiated AP firing in MCs. Differential expression and localization of 4-AP sensitive channels in FS-BCs versus MCs may contribute to this variability. The inhibition of Kv1 and Kv3 currents in the axons of FS-BCs can lower threshold and increase the onset of AP firing (Rudy and McBain, 2001; Goldberg et al., 2008). The repetitive firing of FS-BCs in association with GABAergic depolarizations in the presence of 4-AP may contribute to network synchronization and support propagation of synchronous activity.
In addition to firing APs in coordination with GABAergic depolarizations, FS-BCs repeatedly fired single APs or bursts of APs from baseline. These ectopic APs (EAPs) are generated distally in either the axons or dendrites and propagate into the soma (Gutnick and Prince, 1974; Stasheff et al., 1993). EAPs are known to be associated with 4-AP induced spontaneous GABAergic depolarizations (Perreault and Avoli, 1989; Avoli et al., 1998) and have been observed previously in GABAergic interneurons (Benardo, 1997; Keros and Hablitz, 2005). FS-BC are known to rapidly synchronize due to the high incidence of reciprocal connections and gap junctions, allowing them to initiate cortical gamma oscillations and control theta oscillations in the hippocampus (Pouille and Scanziani, 2001; Connors and Long, 2004; Cardin et al., 2009; Pouille et al., 2009). The enhanced output of FS-BCs associated with GABAergic depolarizations in combination with their electrical coupling through gap junctions suggests a role in the initiation and propagation of the depolarizing GABA responses observed here.
Fast-spiking basket cells and MCs are only two classes of interneurons out of many in the neocortex. A third interneuron subclass of great interest in the regulation of network synchronization is the neurogliaform (NGF) cell. Unlike many other interneuron subclasses, NGFs form electrical synapses with homologous and heterologous interneurons (Zsiros and Maccaferri, 2005), making them critical in the generation of synchronized neuronal network activity (Price et al., 2005). Also, this subclass of interneuron can uniquely modulate local network activity through volume transmission or non-synaptic transmission (Olah et al., 2009). Further investigation of the effects of 4-AP on the firing properties of NGFs and of 4-AP induced synchronous events in these cells would provide insight into their role in modulating GABAergic network synchronization.
Ih Modulation of Network GABAergic Synchronization
The inhibitory role of Ih in regulating the excitability of pyramidal neurons is well established (Magee, 1999; Williams and Stuart, 2000; Berger et al., 2001). Ih also acts to constrain epileptiform activity in the L5 pyramidal cells (Albertson et al., 2013). Genetic deletion of HCN1 channels in the forebrain enhances theta oscillations in the hippocampus (Nolan et al., 2004). Additionally, loss of HCN channels enhances oscillatory activity related to epileptic activity in the neocortex (Strauss et al., 2004; Kole et al., 2007). How HCN channels modulate synaptic integration in GABAergic interneurons and synchronous neocortical inhibitory network activity remains unclear.
Hyperpolarization-activated cyclic nucleotide-gated channels and Ih have been shown to be present in both FS-BCs and MCs at varying densities (Kilb and Luhmann, 2000; Santoro et al., 2000; Wang et al., 2004; Luján et al., 2005; Wu and Hablitz, 2005; Aponte et al., 2006; Albertson et al., 2013). In the present study, MCs displayed robust Ih activation upon hyperpolarization whereas minimal Ih responses were observed in FS-BCs. Despite the difference in Ih expression between FS-BCs and MCs, a significant enhancement of the amplitude and area of evoked and spontaneous synchronous GABAergic events was observed in both cell types after inhibition of HCN channels. Furthermore, the frequency of spontaneous events was also enhanced in both types of interneurons. The increase in amplitude and area suggests an effect on the presynaptic release of GABA possibly caused by a depolarization of the terminals after Ih inhibition, consistent with previous findings (Southan et al., 2000; Aponte et al., 2006; Boyes et al., 2007). The increase in frequency may be attributable to enhanced intrinsic excitability, perhaps resulting in recruitment of small synchronized groups of interneurons to a point where a propagating network event occurs. Ih inhibition was associated with a loss or decrease in spontaneous EAP firing between synchronous depolarizing GABAergic events. In rodent pain models, HCN channel inhibition has been shown to decrease spontaneous APs and burst firing in dorsal root ganglion cells (Chaplan et al., 2003; Lee et al., 2005; Sun et al., 2005).
We further investigated the mechanism by which Ih inhibition modulated network activity by examining changes in synaptic integration. Excitatory inputs in MCs show facilitation whereas FS-BCs show depression (Buhl et al., 1997; Galarreta and Hestrin, 1998; Reyes et al., 1998; Gil et al., 1999; Wang et al., 2004; Silberberg and Markram, 2007; Thomson and Lamy, 2007). Similar findings were observed here with depolarizing IPSPs. Depolarizing IPSPs evoked at 25 Hz in MCs were facilitating. Facilitation and the underlying depolarization were enhanced by inhibition of Ih with ZD 7288. In FS-BCs, synaptic responses showed depression. Following inhibition of Ih, no significant effect on synaptic depression was observed in FS-BCs. However, a delayed synchronous GABAergic network event was reliably evoked. This suggests that inhibition of Ih was associated with significant excitability changes in other neurons which resulted in a propagating response that was seen in FS-BCs.
The subcellular distribution of HCN channels in cortical MCs and FS-BCs may be the cause for the differences we observed in IPSP summation in these two cell types. Given that MCs exhibit large Ih currents upon hyperpolarization, it is assumed that there is a corresponding high density of HCN channels. Since ZD 7288 caused an increase in summation of depolarizing IPSPs, it is possible that HCN channels are located in the somatodendritic region of MCs where they would be able to exert control over synaptic regulation. We also observed an increase in IPSP area with summation in MCs, consistent with somatodendritic localization of HCN channels. Conversely, presynaptic localization of HCN channels in cortical FS-BC may account for the small density of Ih recorded in the soma and lack of an effect on somatodendritic summation of synaptic inputs. Functional HCN channels have been localized in the axon and presynaptic terminal of interneurons in the hippocampus, cerebellum, and basal ganglia and have been shown to regulate GABAergic synaptic transmission (Southan et al., 2000; Aponte et al., 2006; Boyes et al., 2007). Inhibition of Ih with ZD 7288 results in an increase in miniature inhibitory postsynaptic currents (mIPSCs), suggesting a presynaptic mechanism of action (Southan et al., 2000; Boyes et al., 2007). It is possible that the inhibition of HCN channels in the synaptic terminal causes hyperexcitability of the terminal resulting in an increase of GABA release, thus leading to a further enhancement of GABAergic network synchronization. This is consistent with our results showing that the robust firing associated with depolarizing GABAergic events in FS-BCs is significantly enhanced following the inhibition of Ih. Further studies are needed to directly determine if release probability is regulated by HCN channels.
In summary, the data presented here show that 4-AP differentially alters the AP and repetitive firing properties of FS-BC and MCs in the neocortex. MCs and FS-BCs display different patterns of activity during depolarizing GABA responses suggesting different classes of interneurons subserve diverse roles in generation and propagation of these responses. Although the role of HCN channels in regulating normal GABAergic synaptic transmission needs to be established, our results suggest that HCN channels restrict inhibitory synaptic transmission as well as network activity in the presence of 4-AP, CNQX, and D-APV.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH grants NS090041 and NS0474666. VGAT-Venus transgenic rats were generated by Drs. Y. Yanagawa, M. Hirabayashi, and Y. Kawaguchi in the Institute for Physiological Sciences, Okazaki, Japan, using pCS2-Venus provided by Dr. A. Miyawaki. We thank Alison Margolies for excellent technical assistance.
References
Albertson, A. J., Williams, S. B., and Hablitz, J. J. (2013). Regulation of epileptiform discharges in rat neocortex by HCN channels. J. Neurophysiol. 110, 1733–1743. doi: 10.1152/jn.00955.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Albertson, A. J., Yang, J., and Hablitz, J. J. (2011). Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia. J. Neurophysiol. 106, 2189–2200. doi: 10.1152/jn.00164.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alger, B. E., and Nicoll, R. A. (1982). Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J. Physiol. (Lond.) 328, 125–141. doi: 10.1113/jphysiol.1982.sp014256
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Allene, C., Cattani, A., Ackman, J. B., Bonifazi, P., Aniksztejn, L., Ben-Ari, Y.,et al. (2008). Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J. Neurosci. 28, 12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Andersen, P., Dingledine, R., Gjerstad, L., Langmoen, I. A., and Laursen, A. M. (1980). Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J. Physiol. 305, 279–296. doi: 10.1113/jphysiol.1980.sp013363
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Aponte, Y., Lien, C. C., Reisinger, E., and Jonas, P. (2006). Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J. Physiol. 574, 229–243. doi: 10.1113/jphysiol.2005.104042
Aram, J. A., Michelson, H. B., and Wong, R. K. S. (1991). Synchronized GABAergic IPSCs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J. Neurophysiol. 65, 1034–1041.
Ascoli, G. A., Alonso-Nanclares, L., Anderson, S. A., Barrionuevo, G., Benavides-Piccione, R., Burkhalter, A.,et al. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568. doi: 10.1038/nrn2402
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M. (1990). Epileptiform discharges and a synchronous GABAergic potential induced by 4-aminopyridine in the rat immature hippocampus. Neurosci. Lett. 117, 93–98. doi: 10.1016/0304-3940(90)90125-S
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., D’Antuono, M., Louvel, J., Kohling, R., Biagini, G., Pumain, R.,et al. (2002). Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog. Neurobiol. 68, 167–207. doi: 10.1016/S0301-0082(02)00077-1
Avoli, M., and de Curtis, M. (2011). GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog. Neurobiol. 95, 104–132. doi: 10.1016/j.pneurobio.2011.07.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., Mattia, D., Siniscalchi, P., Perreault, P., and Tomaiuolo, F. (1994). Pharmacology and electrophysiology of a synchronous GABA-mediated potential in the human neocortex. Neuroscience 62, 655–666. doi: 10.1016/0306-4522(94)90467-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., Methot, M., and Kawasaki, H. (1998). GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur. J. Neurosci. 10, 2714–2722. doi: 10.1046/j.1460-9568.1998.00275.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., and Perreault, P. (1987). A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 400, 191–195. doi: 10.1016/0006-8993(87)90671-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., Perreault, P., Olivier, A., and Villemure, J.-G. (1988). 4-Aminopyridine induces a long-lasting depolarizing GABA-ergic potential in human neocortical and hippocampal neurons maintained in vitro. Neurosci. Lett. 94, 327–332. doi: 10.1016/0304-3940(88)90039-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avoli, M., Rogawski, M. A., and Avanzini, G. (2001). Generalized epileptic disorders: an update. Epilepsia 42, 445–457. doi: 10.1046/j.1528-1157.2001.39800.x
Bagetta, G., Palma, E., Piccirilli, S., Del Duca, C., Morrone, A. L., Nappi, G.,et al. (2004). Involvement of a glutamatergic mechanism in δ-dendrotoxin-induced hippocampal neuronal cell loss in the rat. Basic Clin. Pharmacol. Toxicol. 94, 132–138. doi: 10.1111/j.1742-7843.2004.pto940306.x
Barish, M. E., Ichikawa, M., Tominaga, T., Matsumoto, G., and Iijima, T. (1996). Enhanced fast synaptic transmission and a delayed depolarization induced by transient potassium current blockade in rat hippocampal slice as studied by optical recording. J. Neurosci. 16, 5672–5687.
Bekkers, J. M. (2000). Properties of voltage-gated potassium currents in nucleated patches from large layer 5 cortical pyramidal neurons of the rat. J. Physiol. 525, 593–609. doi: 10.1111/j.1469-7793.2000.t01-1-00593.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ben-Ari, Y., Cherubini, E., Corradetti, R., and Gaiarsa, J. L. (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. (Lond.) 416, 303–325. doi: 10.1113/jphysiol.1989.sp017762
Ben-Ari, Y., Gaiarsa, J. L., Tyzio, R., and Khazipov, R. (2007). GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87,1215–1284. doi: 10.1152/physrev.00017.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Benardo, L. S. (1997). Recruitment of GABAergic inhibition and synchronization of inhibitory interneurons in rat neocortex. J. Neurophysiol. 77, 3134–3144.
Berger, T., Larkum, M. E., and Lüscher, H.-R. (2001). High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J. Neurophysiol. 85, 855–868.
Berger, T. K., Silberberg, G., Perin, R., and Markram, H. (2010). Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol. 8:e1000473. doi: 10.1371/journal.pbio.1000473
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bonifazi, P., Goldin, M., Picardo, M. A., Jorquera, I., Cattani, A., Bianconi, G.,et al. (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326,1419–1424. doi: 10.1126/science.1175509
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bourdeau, M. L., Morin, F., Laurent, C. E., Azzi, M., and Lacaille, J. C. (2007). Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J. Neurosci. 27, 1942–1953. doi: 10.1523/JNEUROSCI.3208-06.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Boyes, J., Bolam, J. P., Shigemoto, R., and Stanford, I. M. (2007). Functional presynaptic HCN channels in the rat globus pallidus. Eur. J. Neurosci. 25, 2081–2092. doi: 10.1111/j.1460-9568.2007.05463.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Buckle, P. J., and Haas, H. L. (1982). Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J. Physiol. (Lond.) 326, 109–122. doi: 10.1113/jphysiol.1982.sp014180
Buhl, E. H., Tamís, G., Szilígyi, T., Stricker, C., Paulsen, O., and Somogyi, P. (1997). Effect, number and location of synapses made by single pyramidal cells onto aspiny interneurones of cat visual cortex. J. Physiol. (Lond.) 500, 689–713. doi: 10.1113/jphysiol.1997.sp022053
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cardin, J. A., Carlen, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K.,et al. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. doi: 10.1038/nature08002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chaplan, S. R., Guo, H. Q., Lee, D. H., Luo, L., Liu, C., Kuei, C.,et al. (2003). Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 23, 1169–1178.
Chen, X., and Johnston, D. (2004). Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J. Physiol. (Lond.) 559, 187–203. doi: 10.1113/jphysiol.2004.068114
Choquet, D., and Korn, H. (1992). Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J. Gen. Physiol. 99, 217–240. doi: 10.1085/jgp.99.2.217
Chow, A., Erisir, A., Farb, C., Nadal, M. S., Ozaita, A., Lau, D.,et al. (1999). K+ channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J. Neurosci. 19, 9332–9345.
Coetzee, W. A., Amarillo, Y., Chiu, J., Chow, A., Lau, D., McCormack T.,et al. (1999). Molecular Diversity of K+ Channels. Ann. N. Y. Acad. Sci. 868, 233–255. doi: 10.1111/j.1749-6632.1999.tb11293.x
Connors, B. W., and Long, M. A. (2004). Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27, 393–418. doi: 10.1146/annurev.neuro.26.041002.131128
DeFazio, R. A., and Hablitz, J. J. (2005). Horizontal spread of activity in neocortical inhibitory networks. Dev. Brain Res. 157, 83–92. doi: 10.1016/j.devbrainres.2005.03.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dodson, P. D., and Forsythe, I. D. (2004). Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 27, 210–217. doi: 10.1016/j.tins.2004.02.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Du, J., Zhang, L., Weiser, M., Rudy, B., and McBain, C. J. (1996). Develpomental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J. Neurosci. 16, 506–518.
Erisir, A., Lau, D., Rudy, B., and Leonard, C. S. (1999). Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J. Neurophysiol. 82, 2476–2489.
Fairen, A., DeFelipe, J., and Regidor, J. (1984). “Nonpyramidal neurons General account,” in Cerebral Cortex: Cellular Components of the Cerebral Cortex, Vol. 1, eds A. Peters and E. G. Jones (New York: Plenum Press), 201–245.
Farrant, M., and Kaila, K. (2007). “Progress in Brain Research“ in Gaba and the Basal Ganglia From Molecules to Systems, Vol. 160, ed. M. T. James (Amsterdam: Elsevier), 59–87. doi: 10.1016/S0079-6123(06)60005-8
Foust, A. J., Yu, Y., Popovic, M., Zecevic, D., and McCormick, D. A. (2011). Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons. J. Neurosci. 31, 15490–15498. doi: 10.1523/JNEUROSCI.2752-11.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fries, P., Reynolds, J. H., Rorie, A. E., and Desimone, R. (2001). Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563. doi: 10.1126/science.1055465
Galarreta, M., and Hestrin, S. (1998). Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat. Neurosci. 1, 587–594. doi: 10.1038/2882
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garaschuk, O., Hanse, E., and Konnerth, A. (1998). Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. (Lond.) 507, 219–236. doi: 10.1111/j.1469-7793.1998.219bu.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gil, Z., Connors, B. W., and Amitai, Y. (1999). Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23, 385–397. doi: 10.1016/S0896-6273(00)80788-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gilbert, P., Kettenmann, H., and Schachner, M. (1984). gamma-Aminobutyric acid directly depolarizes cultured oligodendrocytes. J. Neurosci. 4, 561–569.
Goldberg, E. M., Clark, B. D., Zagha, E., Nahmani, M., Erisir, A., and Rudy, B. (2008). K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58, 387–400. doi: 10.1016/j.neuron.2008.03.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gutnick, M. J., and Prince, D. A. (1974). Effects of projected cortical epileptiform discharges on neuronal activities in cat VPL. I. Interictal discharge. J. Neurophysiol. 37, 1310–1327.
Haider, B., and McCormick, D. A. (2009). Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189. doi: 10.1016/j.neuron.2009.04.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harvey, M., Lau, D., Civillico, E., Rudy, B., and Contreras, D. (2012). Impaired long-range synchronization of gamma oscillations in the neocortex of a mouse lacking Kv3.2 potassium channels. J. Neurophysiol. 108, 827–833. doi: 10.1152/jn.00102.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hasenstaub, A., Shu, Y., Haider, B., Kraushaar, U., Duque, A., and McCormick, D. A. (2005). Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47, 423–435. doi: 10.1016/j.neuron.2005.06.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hermann, A., and Gorman, A. L. (1981). Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J. Gen. Physiol. 78, 63–86. doi: 10.1085/jgp.78.1.63
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hoffman, D. A., Magee, J. C., Colbert, C. M., and Johnston, D. (1997). K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875. doi: 10.1038/42571
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jack, J. J., Redman, S. J., and Wong, K. (1981). Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J. Physiol. (Lond.) 321, 111–126. doi: 10.1113/jphysiol.1981.sp013974
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jankowska, E., Lundberg, A., Rudomin, P., and Sykova, E. (1977). Effects of 4-aminopyridine on transmission in excitatory and inhibitory synapses in the spinal cord. Brain Res. 136, 387–392. doi: 10.1016/0006-8993(77)90816-2
Jerng, H. H., Pfaffinger, P. J., and Covarrubias, M. (2004). Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 27, 343–369. doi: 10.1016/j.mcn.2004.06.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johnston, D., Christie, B. R., Frick, A., Gray, R., Hoffman, D. A., Schexnayder, L. K.,et al. (2003). Active dendrites, potassium channels and synaptic plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 667–674. doi: 10.1098/rstb.2002.1248
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jung, S., Jones, T. D., Lugo, J. N. Jr., Sheerin, A. H., Miller, J. W., D’Ambrosio, R.,et al. (2007). Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J. Neurosci. 27, 13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kang, J., Huguenard, J. R., and Prince, D. A. (2000). Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J. Neurophysiol. 83, 70–80.
Kawaguchi, Y. (2001). Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J. Neurosci. 21, 7261–7272.
Kawaguchi, Y., and Kubota, Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486. doi: 10.1093/cercor/7.6.476
Keros, S., and Hablitz, J. J. (2005). Ectopic action potential generation in cortical interneurons during synchronized GABA responses. Neuroscience 131, 833–842. doi: 10.1016/j.neuroscience.2004.12.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Khazipov, R., Leinekugel, X., Khalilov, I., Gaiarsa, J.-L., and Ben-Ari, Y. (1997). Synchronization of GABAergic interneuronal network in CA3 subfield of neonatal rat hippocampal slices. J. Physiol. (Lond.) 498, 763–772. doi: 10.1113/jphysiol.1997.sp021900
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kilb, W., and Luhmann, H. J. (2000). Characterization of a hyperpolarization-activated inward current in Cajal-Retzius cells in rat neonatal neocortex. J. Neurophysiol. 84, 1681–1691.
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kole, M. H. P., Brauer, A. U., and Stuart, G. J. (2007). Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J. Physiol. (Lond.) 578, 507–525. doi: 10.1113/jphysiol.2006.122028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Korngreen, A., and Sakmann, B. (2000). Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: Subtypes and gradients. J. Physiol. (Lond.) 525, 621–639. doi: 10.1111/j.1469-7793.2000.00621.x
Lai, H. C., and Jan, L. Y. (2006). The distribution and targeting of neuronal voltage-gated ion channels. Nat. Rev. Neurosci. 7, 548–562. doi: 10.1038/nrn1938
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lau, D., de Miera, E. V.-S., Contreras, D., Ozaita, A., Harvey, M., Chow, A.,et al. (2000). Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J. Neurosci. 20, 9071–9085.
Lee, D. H., Chang, L., Sorkin, L. S., and Chaplan, S. R. (2005). Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. J. Pain 6, 417–424. doi: 10.1016/j.jpain.2005.02.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lien, C. C., and Jonas, P. (2003). Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J. Neurosci. 23, 2058–2068.
Lien, C. C., Martina, M., Schultz, J. H., Ehmke, H., and Jonas, P. (2002). Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J. Physiol. (Lond.) 538, 405–419. doi: 10.1113/jphysiol.2001.013066
Ling, D. S. F., and Benardo, L. S. (1995). Activity-dependent depression of monosynaptic fast IPSCs in hippocampus: contributions from reductions in chloride driving force and conductance. Brain Res. 670, 142–146. doi: 10.1016/0006-8993(94)01298-V
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Llinas, R., Walton, K., and Bohr, V. (1976). Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys. J. 16, 83–86. doi: 10.1016/S0006-3495(76)85664-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lorincz, A., Notomi, T., Tamas, G., Shigemoto, R., and Nusser, Z. (2002). Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat. Neurosci. 5, 1185–1193. doi: 10.1038/nn962
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Luján, R., Albasanz, J. L., Shigemoto, R., and Juiz, J. M. (2005). Preferential localization of the hyperpolarization-activated cyclic nucleotide-gated cation channel subunit HCN1 in basket cell terminals of the rat cerebellum. Eur. J. Neurosci. 21, 2073–2082. doi: 10.1111/j.1460-9568.2005.04043.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lupica, C. R., Bell, J. A., Hoffman, A. F., and Watson, P. L. (2001). Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J. Neurophysiol. 86, 261–268.
Ma, Y., Hu, H., Berrebi, A. S., Mathers, P. H., and Agmon, A. (2006). Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 26, 5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
MacVicar, B. A., Tse, F. W., Crichton, S. A., and Kettenmann, H. (1989). GABA-activated Cl- channels in astrocytes of hippocampal slices. J. Neurosci. 9, 3577–3583.
Magee, J. C. (1998). Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. 18, 7613–7624.
Magee, J. C. (1999). Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 508–514. doi: 10.1038/9158
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin, D. L., and Olsen, R. W. (2000). GABA in the Nervous System: The View at Fifty Years Philadelphia: Lippincott Williams and Wilkins.
Martina, M., Schultz, J. H., Ehmke, H., Monyer, H., and Jonas, P. (1998). Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J. Neurosci. 18, 8111–8125.
Massengill, J. L., Smith, M. A., Son, D. I., and O’Dowd, D. K. (1997). Differential expression of K4-AP currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. J. Neurosci. 17, 3136–3147.
McBain, C. J., and Fisahn, A. (2001). Interneurons unbound. Nat. Neurosci. 2, 11–23. doi: 10.1038/35049047
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McGarry, L. M., Packer, A. M., Fino, E., Nikolenko, V., Sippy, T., and Yuste, R. (2010). Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front. Neural Circuits 4:12. doi: 10.3389/fncir.2010.00012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mei, Y. A., Louiset, E., Vaudry, H., and Cazin, L. (1995). A-type potassium current modulated by A1 adenosine receptor in frog melanotrophs. J. Physiol. (Lond.) 489, 431–442. doi: 10.1113/jphysiol.1995.sp021063
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Michelson, H. B., and Wong, R. K. S. (1991). Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science 253, 1420–1423. doi: 10.1126/science.1654594
Mihaly, A., Bencsik, K., and Solymosi, T. (1990). Naltrexone potentiates 4-aminopyridine seizures in the rat. J. Neural Transm. Gen. Sect. 79, 59–67. doi: 10.1007/BF01251001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Monaghan, M. M., Menegola, M., Vacher, H., Rhodes, K. J., and Trimmer, J. S. (2008). Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience 156, 550–562. doi: 10.1016/j.neuroscience.2008.07.057
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nolan, M. F., Malleret, G., Dudman, J. T., Buhl, D. L., Santoro, B., Gibbs, E.,et al. (2004). A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119, 719–732.
Notomi, T., and Shigemoto, R. (2004). Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 471, 241–276. doi: 10.1002/cne.11039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Okaty, B. W., Miller, M. N., Sugino, K., Hempel, C. M., and Nelson, S. B. (2009). Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci. 29, 7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Olah, S., Fule, M., Komlosi, G., Varga, C., Baldi, R., Barzo, P.,et al. (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. doi: 10.1038/nature08503
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Otis, T. S., and Mody, I. (1992). Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J. Neurophysiol. 67, 227–235.
Packer, A. M., and Yuste, R. (2011). Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J. Neurosci. 31, 13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Perreault, P., and Avoli, M. (1989). Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J. Neurophysiol. 61, 953–970.
Perreault, P., and Avoli, M. (1991). Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J. Neurophysiol. 65, 771–785.
Peters, A., and Jones, E. G. (1984). Cellular Components of the Cerebral Cortex. New York, NY: Plenum Press.
Pouille, F., Marin-Burgin, A., Adesnik, H., Atallah, B. V., and Scanziani, M. (2009). Input normalization by global feedforward inhibition expands cortical dynamic range. Nat. Neurosci. 12, 1577–1587. doi: 10.1038/nn.2441
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pouille, F., and Scanziani, M. (2001). Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163. doi: 10.1126/science.1060342
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Powell, K. L., Ng, C., O’Brien, T. J., Xu, S. H., Williams, D. A., Foote, S. J.,et al. (2008). Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia 49, 1686–1695. doi: 10.1111/j.1528-1167.2008.01593.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Price, C. J., Cauli, B., Kovacs, E. R., Kulik, A., Lambolez, B., Shigemoto, R.,et al. (2005). Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J. Neurosci. 25, 6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reyes, A., Lujan, R., Rozov, A., Burnashev, N., Somogyi, P., and Sakmann, B. (1998). Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1, 279–285. doi: 10.1038/1092
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rivera, C., Voipo, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K.,et al. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. doi: 10.1038/16697
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Robinson, R. B., and Siegelbaum, S. A. (2003). Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65, 453–480. doi: 10.1146/annurev.physiol.65.092101.142734
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rudy, B., Fishell, G., Lee, S., and Hjerling-Leffler, J. (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. doi: 10.1002/dneu.20853
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rudy, B., and McBain, C. J. (2001). Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526. doi: 10.1016/S0166-2236(00)01892-0
Rutecki, P. A., Lebeda, F. J., and Johnston, D. (1987). 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J. Neurophysiol. 57, 1911–1924.
Santoro, B., Chen, S., Luthi, A., Pavlidis, P., Shumyatsky, G. P., Tibbs, G. R.,et al. (2000). Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 20, 5264–5275.
Sekirnjak, C., Martone, M. E., Weiser, M., Deerinck, T., Bueno, E., Rudy, B.,et al. (1997). Subcellular localization of the K+ channel subunit Kv3.1b in selected rat CNS neurons. Brain Res. 766, 173–187. doi: 10.1016/S0006-8993(97)00527-1
Serodio, P., and Rudy, B. (1998). Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J. Neurophysiol. 79, 1081–1091.
Shin, M., Brager, D., Jaramillo, T. C., Johnston, D., and Chetkovich, D. M. (2008). Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol. Dis. 32, 26–36. doi: 10.1016/j.nbd.2008.06.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silberberg, G., and Markram, H. (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti Cells. Neuron 53, 735–746. doi: 10.1016/j.neuron.2007.02.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smart, S. L., Lopantsev, V., Zhang, C. L., Robbins, C. A., Wang, H., Chiu, S. Y.,et al. (1998). Deletion of the Kv 1.1 potassium channel causes epilepsy in mice. Neuron 20, 809–819. doi: 10.1016/S0896-6273(00)81018-1
Southan, A. P., Morris, N. P., Stephens, G. J., and Robertson, B. (2000). Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J. Physiol. (Lond.) 526, 91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x
Spyker, D. A., Lynch, C., Shabanowitz, J., and Sinn, J. A. (1980). Poisoning with 4-Aminopyridine: report of three cases. Clin. Toxicol. 16, 487–497. doi: 10.3109/15563658008989978
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Staley, K. J., Soldo, B. L., and Proctor, W. R. (1995). Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269, 977–981. doi: 10.1126/science.7638623
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stasheff, S. F., Hines, M., and Wilson, W. A. (1993). Axon terminal hyperexcitability associated with epileptogenesis in vitro. I. Origin of ectopic spikes. J. Neurophysiol. 70, 961–975.
Storm, J. F. (1988). Temporal integration by a slowly inactivating K+ current in rat hippocampal neurons. Nature 336, 379–381. doi: 10.1038/336379a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strauss, U., Kole, M. H. P., Brauer, A. U., Pahnke, J., Bajorat, R., Rolfs, A.,et al. (2004). An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur. J. Neurosci. 19, 3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stuart, G., Spruston, N., Sakmann, B., and Häusser, M. (1997). Action potential initiation and back propagation in neurons of the mammalian CNS. Trends Neurosci. 20, 125–131. doi: 10.1016/S0166-2236(96)10075-8
Sun, Q., Xing, G. G., Tu, H. Y., Han, J. S., and Wan, Y. (2005). Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. Brain Res. 1032, 63–69. doi: 10.1016/j.brainres.2004.10.033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, W., Maffie, J. K., Lin, L., Petralia, R. S., Rudy, B., and Hoffman, D. A. (2011). DPP6 establishes the A-type K+ current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 71, 1102–1115. doi: 10.1016/j.neuron.2011.08.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Szente, M., and Baranyi, A. (1987). Mechanism of aminopyridine-induced ictal seizure activity in the cat neocortex. Brain Res. 413, 368–373. doi: 10.1016/0006-8993(87)91031-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanaka, Y., Tanaka, Y., Furuta, T., Yanagawa, Y., and Kaneko, T. (2008). The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices of adult mice. J. Neurosci. Meth. 171, 118–125. doi: 10.1016/j.jneumeth.2008.02.021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thesleff, S. (1980). Aminopyridines and synaptic transmission. Neuroscience 5, 1413–1419. doi: 10.1016/0306-4522(80)90002-0
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1:19–42. doi: 10.3389/neuro.01.1.1.002.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tibbs, G. R., Barrie, A. P., Van Miegham, F. J. E., McMahon, H. T., and Nicholls, D. G. (1989). Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: effects on cytosolic free Ca2+ and glutamate release. J. Neurochem. 53, 1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Traub, R. D., Pais, I., Bibbig, A., LeBeau, F. E. N., Buhl, E. H., Garner, H.,et al. (2005). Transient depression of excitatory synapses on interneurons contributes to epileptiform bursts during gamma oscillations in the mouse hippocampal slice. J. Neurophysiol. 94, 1225–1235. doi: 10.1152/jn.00069.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Uematsu, M., Hirai, Y., Karube, F., Ebihara, S., Kato, M., Abe, K.,et al. (2008). Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb. Cortex 18, 315–330. doi: 10.1093/cercor/bhm056
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Uva, L., Avoli, M., and de Curtis, M. (2009). Synchronous GABAA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur. J. Neurosci. 29, 911–920. doi: 10.1111/j.1460-9568.2009.06672.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Voskuyl, R. A., and Albus, H. (1985). Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 342, 54–66. doi: 10.1016/0006-8993(85)91352-6
Wang, L.-Y., Gan, L., Forsythe, I. D., and Kaczmarek, L. K. (1998). Contribution of the Kv3.1 potassium channel to high-frequency firing in the mouse auditory neurones. J. Physiol. (Lond.) 509, 183–194. doi: 10.1111/j.1469-7793.1998.183bo.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Y., Toledo-Rodriguez, M., Gupta, A., Wu, C., Silberberg, G., Luo, J.,et al. (2004). Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. (Lond.) 561, 65–90. doi: 10.1113/jphysiol.2004.073353
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wehr, M., and Zador, A. M. (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446. doi: 10.1038/nature02116
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Weiser, M., Bueno, E., Sekirnjak, C., Martone, M. E., Baker, H., Hillman, D.,et al. (1995). The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J. Neurosci. 15, 4298–4314.
Weiser, M., Vega-Saenz de Miera, E., Kentros, C., Moreno, H., Franzen, L., Hillman, D.,et al. (1994). Differential expression of Shaw-related K+ channels in the rat central nervous system. J. Neurosci. 14, 949–972.
Weiss, D. S., and Hablitz, J. J. (1984). Interaction of penicillin and pentobarbital with inhibitory synaptic mechanisms in neocortex. Cell Mol. Neurobiol. 4, 301–317. doi: 10.1007/BF00733594
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Williams, S. R., and Stuart, G. J. (2000). Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J. Neurophysiol. 83, 3177–3182.
Wu, J., and Hablitz, J. J. (2005). Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J. Neurosci. 25, 6322–6328. doi: 10.1523/JNEUROSCI.1405-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wu, R. L., and Barish, M. E. (1992). Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J. Neurosci. 12, 2235–2246.
Xu, X., Roby, K. D., and Callaway, E. M. (2006). Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Comp. Neurol. 499, 144–160. doi: 10.1002/cne.21101
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yuan, W., Burkhalter, A., and Nerbonne, J. M. (2005). Functional role of the fast transient outward K+ current IA in pyramidal neurons in (Rat) primary visual cortex. J. Neurosci. 25, 9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, F.-M., and Hablitz, J. J. (1996). Layer I neurons of the rat neocortex. II. Voltage-dependent outward currents. J. Neurophysiol. 76, 668–682.
Zsiros, V., and Maccaferri, G. (2005). Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J. Neurosci. 25, 8686–8695. doi: 10.1523/JNEUROSCI.2810-05.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: HCN channels, A-type K+ channels, 4-AP, synchronization, Ih, neocortex, GABAergic interneurons, Martinotti cells, basket cells
Citation: Williams SB and Hablitz JJ (2015) Differential modulation of repetitive firing and synchronous network activity in neocortical interneurons by inhibition of A-type K+ channels and Ih. Front. Cell. Neurosci. 9:89. doi: 10.3389/fncel.2015.00089
Received: 22 October 2014; Accepted: 26 February 2015;
Published online: 18 March 2015
Edited by:
Andreas Frick, Institut National de la Santé et de la Recherche Médicale, FranceReviewed by:
Dirk Feldmeyer, Rheinisch-Westfälische Technische Hochschule Aachen University, GermanyCorette J. Wierenga, Utrecht University, Netherlands
Copyright © 2015 Williams and Hablitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John J. Hablitz, Department of Neurobiology, Civitan International Research Center and Evelyn F. McKnight Brain Institute, University of Alabama at Birmingham, Birmingham, AL 35294, USA jhablitz@uab.edu
 Sidney B. Williams
Sidney B. Williams John J. Hablitz
John J. Hablitz