Nogo-A Neutralization Improves Graft Function in a Rat Model of Parkinson’s Disease
- 1Department of Neurosurgery, Neurocenter and Regenerative Neuroscience Cluster, University Hospital Bern, Switzerland
- 2Graduate School for Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland
Transplantation of fetal human ventral mesencephalic (VM) dopaminergic neurons into the striatum is a promising strategy to compensate for the characteristic dopamine deficit observed in Parkinson’s disease (PD). This therapeutic approach, however, is currently limited by the high number of fetuses needed for transplantation and the poor survival and functional integration of grafted dopaminergic neurons into the host brain. Accumulating evidence indicates that contrasting inhibitory signals endowed in the central nervous system (CNS) might support neuronal regeneration. Hence, in the present study we aimed at improving survival and integration of grafted cells in the host brain by neutralizing Nogo-A, one of the most potent neurite growth inhibitors in the CNS. For that purpose, VM tissue cultures were transplanted into rats with a partial 6-hydroxydopamine (6-OHDA) lesion causing a hemi-PD model and concomitantly treated for 2 weeks with intra-ventricular infusion of neutralizing anti-Nogo-A antibodies. Motor behavior using the cylinder test was assessed prior to and after transplantation as functional outcome. At the end of the experimental period the number of dopaminergic fibers growing into the host brain, the number of surviving dopaminergic neurons in the grafts as well as graft size was examined. We found that anti-Nogo-A antibody infusion significantly improved the asymmetrical forelimb use observed after lesions as compared to controls. Importantly, a significantly three-fold higher dopaminergic fiber outgrowth from the transplants was detected in the Nogo-A antibody treated group as compared to controls. Furthermore, Nogo-A neutralization showed a tendency for increased survival of dopaminergic neurons (by two-fold) in the grafts. No significant differences were observed for graft volume and the number of dopaminergic neurons co-expressing G-protein-coupled inward rectifier potassium channel subunit two between groups. In sum, our findings support the view that neutralization of Nogo-A in the host brain may offer a novel and therapeutically meaningful intervention for cell transplantation approaches in PD.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder mainly characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) with a subsequent loss of dopamine innervation in the striatum. To restore the imbalanced innervation in the striatum, cell replacement strategies have been evaluated as a long-term treatment for PD. Numerous clinical trials showed functional improvement after transplantation of fetal human ventral mesencephalic (VM) dopaminergic neurons into the striatum of PD patients (Lindvall et al., 1989, 1990; Kordower et al., 1995; Hauser et al., 1999) even without the support of additional pharmacological treatment (Kefalopoulou et al., 2014). It became clear, however, that before cell replacement strategies can be used in clinical practice, the criteria for patient selection (Freed et al., 2001; Barker et al., 2013) as well as the collection, the pre-treatment and the storage of donor tissue have to be optimized and standardized (Petit et al., 2014). Currently, a multicenter clinical trial called TRANSEURO is establishing a standardized protocol for transplantation approaches in PD (Moore et al., 2014). Two other major limitations associated with cell transplantation procedures in PD still include suboptimal survival and poor innervation of the host brain by grafted dopaminergic neurons. Several transplantation studies in animals revealed that most of the transplanted dopaminergic neurons die within the first week after transplantation and that many of the surviving dopaminergic neurons do not functionally integrate into the host brain (Emgard et al., 1999; Karlsson et al., 2000). In line with this notion, it has been shown that mainly the dopaminergic sub-population expressing the G-protein-coupled inward rectifier potassium channel subunit 2 (GIRK2) innervate the host brain after transplantation (Mendez et al., 2005; Thompson et al., 2005; Gaillard et al., 2009; Grealish et al., 2010).
Accumulating evidence points out that the tissue microenvironment plays a vital role in influencing the degree of engraftment of the transplant (Ourednik and Ourednik, 2005; Alsberg et al., 2006; Stefanova et al., 2009). Suppression of myelin associated proteins like Nogo-A, a major neurite growth inhibitor in the central nervous system (CNS) might therefore offer novel ways to improve functional recovery in various disease states. In line with this notion, several studies have demonstrated that neutralizing Nogo-A signaling with anti-Nogo-A antibodies promoted axonal sprouting as well as improved functional recovery in spinal cord injured rats and monkeys (Merkler et al., 2001; Freund et al., 2009). Moreover, Nogo-A antibodies increased neuronal remodeling and functional recovery after stroke in rodents (Cheatwood et al., 2008; Tsai et al., 2011). Hence, we hypothesized that this strategy may be considered a valuable approach in the context of cell transplantation in PD. Nogo-A, is widely distributed in the CNS and it is expressed in dopaminergic neurons of the rodent fetus as well as in dopaminergic neurons of the adult rodent SNc (Seiler et al., 2013; Kurowska et al., 2014; Schawkat et al., 2015). Notably, knock-out animals for leucine rich repeat neuronal protein 1 (LINGO-1), a co-receptor of the Nogo-receptor 1 (NgR1) complex, showed increased survival of dopaminergic neurons and functional improvements after induction of parkinsonian symptoms (Inoue et al., 2007). In line with this study, antagonizing the NgR1 significantly increased dopaminergic cell numbers and their morphological complexity in primary VM cultures (Seiler et al., 2013). Therefore, in the present work, we investigated the potential of Nogo-A neutralization on graft survival and function in a rat model with induced parkinsonian symptoms. We demonstrate here that neutralization of Nogo-A significantly promoted sprouting of grafted dopaminergic cells into the host brain and led to robust functional improvement of asymmetric forelimb use. Our results support the concept of Nogo-A inhibition as a relevant strategy for transplantation approaches in PD.
Materials and Methods
Animals
Female Wistar rats (Janvierlabs, France) were housed at 12 h light dark cycle with food and water ad libitum. For the preparation of the transplants, pregnant Wistar rats were purchased from Janvier Labs (France). All experiments were carried out in the light phase and in accordance with the guidelines of the Animal Research Ethics Committee of the Canton Berne, Switzerland, and the University of Bern Animal Care and Use Committee, Switzerland.
Experimental Design
To induce a unilateral reduction of dopaminergic neurons in the substantia nigra, animals received 6-hydroxydopamine (6-OHDA) injections into the right striatum. Six weeks after the lesion, each rat was grafted with half a fetal ventral mesencephalon transplant into the right striatum and received a mini-osmotic pump that injected Nogo-A neutralizing antibodies 11C7 or control antibodies (IgG; kind gifts from Dr. Anis Mir, Novartis, Switzerland) over a period of 2 weeks into the right lateral ventricle. Six weeks after the transplantation the rats were perfused and their brains used for histological analyses. Behavior (cylinder test) of the rats was assessed 1 week before (baseline) and 5 weeks after the lesion (lesioned) and 1, 3 and 5 weeks after the transplantation (Figure 1).
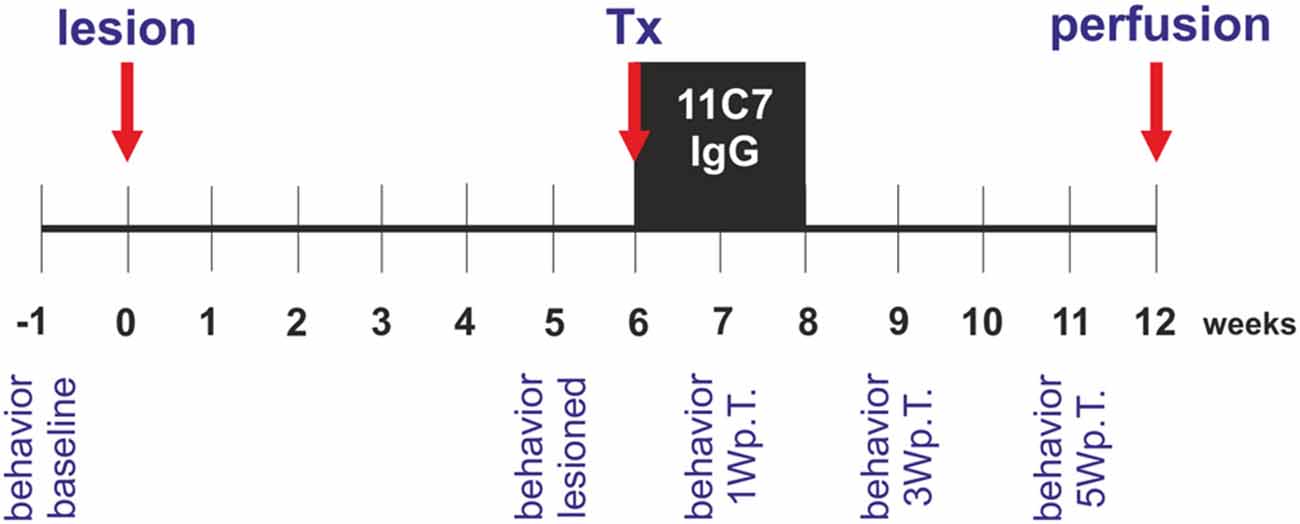
Figure 1. Experimental design of the study. Rats received a unilateral intra-striatal 6-hydroxydopamine (6-OHDA) injection (lesion) and 6 weeks later the animals were transplanted with ventral mesencephalic (VM) free-floating roller-tube cultures (Tx). Concomitantly, rats were implanted with mini-osmotic pumps releasing neutralizing Nogo-A antibodies (11C7) or control antibodies (IgG) for a period of 2 weeks (black box). Six weeks after the transplantation the rats were perfused (perfusion) and their brains removed for histological analyses. Behavior by means of the cylinder test was assessed 1 week before the lesion (behavior baseline), 5 weeks after the lesion (behavior lesioned) and 1, 3 and 5 weeks after the transplantation (behavior 1 Wp.T.; behavior 3 Wp.T.; behavior 5 Wp.T.).
Parkinson’s Disease Rat Model
Female Wistar rats (200–270 g; Janvierlabs, France) were anesthetized with Isoflurane (75% N2O, 20% O2, 4.5–5%) followed by an intraperitoneal (i.p.) injection of Narketan (75 mg/kg; Vétoquind AG, Ittigen, CH, Switzerland) and Xylaxine (5 mg/kg; Vétoquind AG, Ittigen, CH, Switzerland) and a subcutaneous (s.c.) injection of Buprenorphine (0.5 mg/kg; Reckitt Benckiser AG, Wallisellen, Switzerland) applied 30 min before surgical intervention. Thereafter, the rats were placed in a stereoscopic frame (Stoelting Co.) on a heating pad. Each rat received an injection of 4 μl of 6-OHDA (20 mM 6-OHDA; H116 Sigma-Aldrich Chemie GmbH) through a small burr hole created in the skull into the right striatum. The injection was performed over 4 min using a 10 μl Hamilton syringe. The following coordinates in relation to bregma were used (Paxinos Watson rat brain atlas): anterior 1.0 mm, lateral 3.0 mm and 5.0 mm ventral to the dura, the incisor bar was set at 0.0 mm. After the surgery rats were allowed to recover for 5 weeks.
Preparation of Transplants
The organotypic fetal rat VM free-floating roller-tube culture technique was used for the preparation of the grafts as previously described (Andereggen et al., 2009). In brief, pregnant Wistar rats were anesthetized with Isoflurane (75% N2O, 20% O2, 4.5–5%) followed by an i.p. injection of Narketan (120 mg/kg) and Xylaxine (20 mg/kg). After removal of the fetus by cesarean section, the ventral mesencephalon was dissected out of the fetal brain with the help of a stereoscopic microscope, cut into four equally sized pieces corresponding to two rostral and two caudal portions and individually placed into gas-permeable conical plastic tubes (Falcon), supplied with 1 ml of culture medium consisting of 55% DMEM, 32.5% Hank’s balanced salt solution (HBSS; Gibco), 0.3% glucose, 10% fetal calf serum (FCS; Gibco) and 1% 0.01 M HEPES (Merck) as well as antibiotics/antimycotics (No. 061-05240 D; Gibco). Thereafter, the tubes were placed into a roller drum and grown for 7 days in an incubator at 37°C in a 5% CO2 atmosphere as described in detail previously (Spenger et al., 1994). The medium was changed after 2 and 5 days in vitro.
Transplantation
Six weeks after the intrastriatal 6-OHDA lesions rats were anesthetized with Isoflurane (75% N2O, 20% O2, 4.5–5%) followed by an i.p. injection of Narketan (75 mg/kg) and Xylaxine (5 mg/kg). A s.c. injection of Buprenorphine (0.5 mg/kg) was given 30 min before the operations. Thereafter, the rats were mounted on a stereoscopic frame on a heating pad. Each rat received an intrastriatal graft consisting of one rostral and one caudal VM free-floating roller tube culture corresponding to half of a ventral mesencephalon from one embryo to assure roughly equal amounts of dopaminergic neurons in the grafts. The following coordinates in relation to bregma (Paxinos Watson rat brain atlas) were used: anterior 1.0 mm, lateral 2.7 mm and 4.5 mm ventral to the dura, the incisor bar was set at 0.0 mm. After the transplantation procedure, mini-osmotic pumps (2 ml2, Alzet osmotic pumps, DURECT Corporation ALZET Osmotic Pumps) were implanted under the skin and the cannulas subcutaneously connected to the skull into the right ventricle of each rat (Alzet brain infusion kit2), according to the following coordinates in relation to bregma (Paxinos Watson rat brain atlas): posterior 0.8 mm, lateral 1.6 mm and 3.5 mm ventral to the dura, the incisor bar was set at 0.0 mm. The mini-osmotic pumps were filled with either monoclonal mouse Nogo-A antibodies (11C7; 1 mg/ml) or control mouse IgG (1 mg/ml) and administered these substances continuously during the following 2 weeks (flow rate: 5 μg/h). The animals were randomly assigned to the two groups (n = 5 for each group) and let to recover for 1 week.
Cylinder Test
To analyze the asymmetry in forelimb use, as observed after unilateral lesions, the cylinder test is a reliable measure for analysis of 6-OHDA induced behavioral changes in animal models of PD (Brooks and Dunnett, 2009; Cordeiro et al., 2010; Schaar et al., 2010). Behavior was assessed 1 week before the lesion (baseline), 5 weeks after the lesion (lesioned) and 1, 3 and 5 weeks after the transplantation (1 Wp.T., 3 Wp.T. and 5 Wp.T., respectively). In brief, rats were placed in a transparent cylinder (diameter 30 cm and height 41 cm) and were video recorded for 10 min. Mirrors were placed behind the cylinder to allow a 360° view on the cylinder walls. The number of wall touches with the left, the right or both paws together was counted by a researcher blinded to the treatment groups. In order to discriminate between a meaningful physiological movement and an accidental touch, only contacts in which the rat supported its body weight on the forelimb with extended digits were counted. Furthermore, rats that touched the wall less than 20 times during the 10 min period were excluded from the analysis (Schallert et al., 2000; lesioned: one animal from the IgG group with 16 touches; 1 Wp.T.: one animal from the 11C7 group with 13 touches; 3 Wp.T.: one animal from the 11C7 group with 13 touches; 5 Wp.T.: one animal from the IgG group with 14 touches and one animal from the 11C7 group with 14 touches). The percentage of left wall touches are calculated according to the formula: [(left + of both paw touches)/(left + right + both paw touches)] * 100 as previously described (Boix et al., 2015).
Perfusions
Six weeks after the transplantation, the rats were anesthetized with Isoflurane (75% N2O, 20% O2, 4.5–5%) followed by an i.p. injection of Narketan (75 mg/kg) and Xylaxine (5 mg/kg). Just prior to opening the thorax the rats received an i.p. injection of Fentanyl (0.005mg/kg, Janssen-AG, Zug, CH, Switzerland). Thereafter, the rats were transcardinally perfused with 200 ml ice cold 0.1M phosphate buffer saline (PBS, pH 7.4) containing heparin (1000 I.E./100 ml, NOVO Nordisk) followed by 250 ml 4% paraformaldehyde in 0.1M PBS. The brains were removed from the skull and placed in 4% paraformaldehyde overnight and thereafter cryoprotected in 10% sucrose-PBS solution.
Immunohistochemistry
The brains were cut on a cryostat (Leica CM 1900) into 30 μm thick coronal slices and mounted on Superfrost slides (Thermo Scientific) so that on one slide 3 brain slices were mounted (one 180 μm apart from the next one). Brain sections were washed 4× in PBS and blocked with 10% horse serum in 0.1% Triton-PBS. Primary and secondary antibodies were incubated in a 0.1% Triton-PBS solution containing 2.5% horse serum. After overnight incubation with the mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (1:1000, Millipore) and/or the polyclonal rabbit anti-GIRK2 antibody (1:200, Alomone), the selected sections were washed 4× in PBS and incubated for 2 h with a secondary biotinylated anti-mouse IgG (1:200, Vector Laboratories) antibody. Subsequently, the slides for the TH staining only were washed in PBS, incubated in a solution of 10% methanol and 3% hydrogenperoxide in PBS to block the endogenous peroxidase and washed again in PBS. Following incubation with an avidin-biotin-complex (7 μl/ml; Vectastain ABC-Peroxidase KIT, Vector Labs) for 1 h, specifically bound antibodies were visualized with a metal-enhanced 3, 3′-diaminobenzidine substrate kit (Pierce, 34002, Life Technologies). Sections were dehydrated in alcohol, cleared in xylene and mounted in Eukitt (O. Kindler GmbH, Freiburg, Germany). The slides for the TH/GIRK2 co-localization analyses were washed 4× with PBS and incubated for 2 h with the Alexa-Fluor 594 nm donkey conjugated anti-mouse IgG (1:250, Molecular Probes) and the Alexa-Fluor 488 nm donkey conjugated anti-rabbit IgG (1:250, Molecular Probes) antibodies. Cell nuclei were counterstained with Hoechst 33342 (Invitrogen, Molecular Probes) at 1:10,000. Thereafter, the sections were washed in PBS 4 × 10 min and covered with 50% PBS-glycerol mounting media. Fluorescence pictures were taken using a Zeiss Laser scanning confocal microscope (LSM 710). Brightness, saturation, and sharpness of presented images were adjusted only as necessary to best replicate the immunostaining as viewed directly under the microscope (Gombash et al., 2012).
Histological Analyses
All analyses were done by a researcher blinded to the treatment groups using a calibrated neuron tracing software (Cellsense Dimension, Olympus) and a microscope (Olypmus DP72) equipped with a motorized stage that was connected to a digital camera (Olympus).
Estimation of TH Positive Cells in the SNc
The estimation of the extent of the lesion was done as described previously by Tronci et al. (2012). In brief, brain sections of each animal were selected corresponding to the following coordinates in relation to bregma (Paxinos Watson rat brain atlas): posterior 4.8 mm (three sections analyzed, range 4.7–4.9 mm), 5.3 mm (three sections analyzed, range 5.2–5.4 mm), and 5.8 mm (three sections analyzed, range 5.7–5.9 mm). TH positive cells were counted in the SNc of the lesioned as well as the unlesioned according to stereological methods (assisted by S.A. Tschanz, Microscopy Imaging Center, Institute of Anatomy, University of Bern, Switzerland) with the help of a microscope (Olypmus DP72) equipped with a motorized stage that was connected to a digital camera (Olympus). TH positive cells with a clearly stained cell body were counted at 20× magnification excluding the cells touching the right and lower boarder of the frames. Data are expressed as percentage of TH positive cells on the lesioned side as compared to the number of TH positive neurons on the unlesioned side.
Histological Analysis of the Graft
Every third section containing a graft was chosen by systematic randomized sampling to determine the size of the graft, the number of TH positive fibers growing 100 μm into the host brain and the number of surviving TH positive cells in the graft. The volume of the graft was calculated as previously described (Andereggen et al., 2009). In brief, graft boundaries were traced using a calibrated neuron tracing software (Cellsense Dimension, Olympus) and after automated computation of the areas integrated to yield the graft volume. Assessment of TH positive fibers growing into the host brain was done as previously described by Andereggen et al. (2009). In brief, TH positive fibers originating in the graft were followed up to a virtual line drawn 100 μm from the graft boundary with a length of 300 μm and counted if they crossed this line. The counts were done at four sites, i.e., medial, lateral, dorsal and ventral from the border of the graft using a 10× magnification. The number of TH positive cells throughout the grafts was counted according to stereological methods (assisted by S.A. Tschanz, Microscopy Imaging Center, Institute of Anatomy, University of Bern, Switzerland) with the help of a microscope (Olypmus DP72) equipped with a motorized stage that was connected to a digital camera (Olympus). The cells were counted at 20× magnification excluding the ones touching the right and lower boarder of the frames. Only cells with a distinct immunoreactivity and a clear neuronal shape were included for analysis. The co-localization rate of TH positive neurons with GIRK2 was analyzed as described previously (Andereggen et al., 2009). In brief, the most central slides containing three brain sections of each graft were selected and stained for TH and GIRK2. The analysis was done with the help of a microscope (Olypmus DP72) equipped with a motorized stage that was connected to a digital camera (Olympus) at 20× magnification excluding the cells touching the right and lower boarder of the frames and only positive cells with a clear immunoreactivity and a clear neuronal shape were chosen for co-localization analyses. Data are expressed as percentage of total TH positive cell numbers.
Estimation of TH Positive Fibers in the Striatum
The density of TH positive fibers in the striatum was assessed as described previously with slight modifications (Winkler et al., 1996; Hoglinger et al., 2001). In brief, pictures of the lesioned and unlesioned dorsal striatum, 540 μm rostral before the first graft was detected, were taken from all animals with the help of a microscope (Olypmus DP72) that was connected to a digital camera (Olympus). The pictures were converted to 8 bit black and white images and inverted using the Fiji software. The mean gray intensity of the dorsal striatum of the lesioned and unlesioned side was measured in a defined area (100,000 μm2). To account for non-specific background staining, the mean gray value in a defined area (30,000 μm2) in the corpus callosum was measured. First the mean gray intensity of the corpus callosum was substracted form the respective striatal mean gray intensity values. Thereafter the values of the lesioned striatum were expressed as percentage of the unlesioned side.
Statistical Analysis
For statistical analysis a commercially available software package was used (GraphPad Prism 6). To compare group means of several groups repeated two-way analysis of variance (ANOVA) was used, followed by Tukey’s or Bonferroni’s multiple comparison test where appropriate. Statistical significance of two groups only, was assessed by two-tailed unpaired t-test and the statistical significance levels are expressed as tα/υ, where α indicates the student’s t values and υ the relative degree of freedom. Statistical significance was set at p < 0.05. Data are presented as mean ± SEM.
Results
Estimated Extent of the Lesion
To determine the extent of the lesion, the surviving TH positive cells in the SNc of each rat were counted. As anticipated our lesions corresponded to an early stage of PD (Figure 2). Loss of TH positive neurons was 34.2 ± 3.0% on the lesioned side as compared to unlesioned control side with no differences observed for the three different levels analyzed in relation to bregma (34.2 ± 4.3%, 32.1 ± 4.6% and 32.9 ± 5.1%, for −4.8 mm, −5.3 mm and −5.8 mm, respectively).
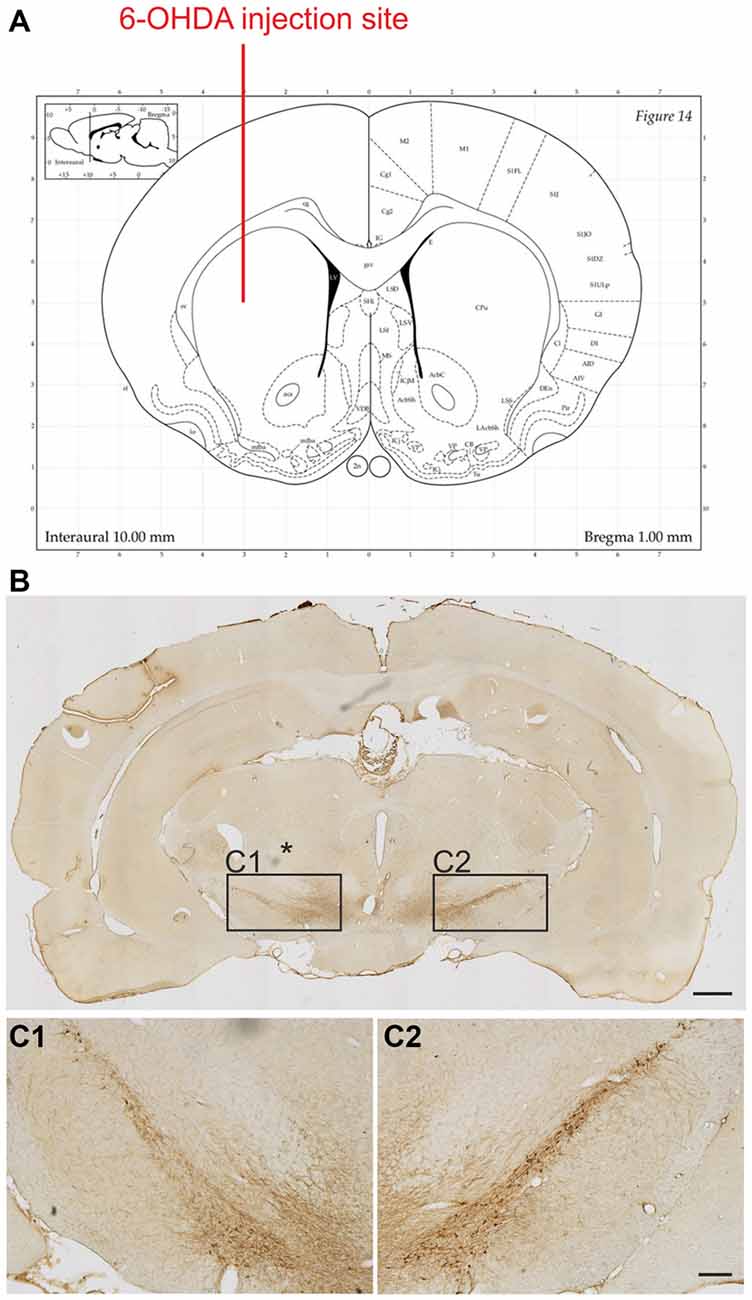
Figure 2. Extent of the 6-OHDA lesion on tyrosine hydroxylase (TH) positive cell numbers in the substantia nigra. 6-OHDA was injected into the right striatum at the following coordinates in relation to bregma (Paxinos Watson rat brain atlas): anterior 1.0 mm, lateral 3.0 mm and 5.0 mm ventral to the dura (A). Representative photomicrographs showing TH positive neurons on the control and lesion (*) sides of the substantia nigra (B). Boxed areas in (B) are shown at higher magnification (C1,C2). Scale bars: 1 mm (B) and 200 μm (C1,C2).
Behavioral Analysis
To determine the asymmetry in forelimb use, we analyzed the rat’s behavior in the cylinder test. As expected, after the lesion the rats used the left paw (contralateral to the lesion) to touch the wall significantly less often as compared to baseline (46.0 ± 3.2 vs. 19.2 ± 7.8, % of left paw use for baseline and lesioned in the IgG group, t3.2/6 ≤ 0.05 and 52.9 ± 2.3 vs. 30.1 ± 4.7, % of left paw use for baseline and lesioned in the 11C7 group, t4.4/8 ≤ 0.01; Figure 3A). No significant difference was found between the 11C7 and the IgG groups (data not shown). One week after the transplantation no significant improvement in using the left paw in either one of the treatment groups could be observed as compared to after the lesion (19.2 ± 7.9 vs. 25.9 ± 7.5 and 30.1 ± 4.7 vs. 31.7 ± 5.8, % of left paw use for lesioned, IgG and 11C7, respectively; Time point, F(2,14) = 25.48, p < 0.0001; post hoc, lesioned vs. IgG p = 0.47 and lesioned vs. 11C7 p = 0.94; Figure 3A). Similarly, 3 weeks after the transplantation no significant improvement in either one of the treatment groups could be detected as compared to after the lesion (19.2 ± 7.9 vs. 31.8 ± 7.0 and 27.2 ± 4.7 vs. 40.2 ± 7.9, % of left paw use for lesioned, IgG and 11C7, respectively; Time point, F(2,12) = 21.74, p = 0.0001; post hoc, lesioned vs. IgG p = 0.12 and lesioned vs. 11C7 p = 0.09; Figure 3B). Importantly, 5 weeks after the transplantation the asymmetrical forelimb use was significantly improved in the 11C7 treated rats compared to after the lesions (19.2 ± 7.8 vs. 24.2 ± 6.5 and 32.8 ± 4.9 vs. 44.8 ± 3.0, % of left paw use for lesioned, IgG and 11C7, respectively; Time point, F(2,12) = 29.04, p < 0.0001; post hoc, lesioned vs. IgG p = 0.53 and lesioned vs. 11C7 p = 0.05). Moreover, the 11C7 treated rats showed a trend towards more left paw uses compared to IgG treated rats (24.2 ± 6.5 vs. 44.8 ± 3.0, % of left paw use for IgG and 11C7, respectively; Treatment, F(1,6) = 5.534, p = 0.057; post hoc, IgG vs. 11C7 p < 0.05; Figure 3C). In contrast, the IgG treated rats never performed significantly better during the observed test period than after the lesion and showed no recovery as compared to baseline levels (Figures 3A–C).
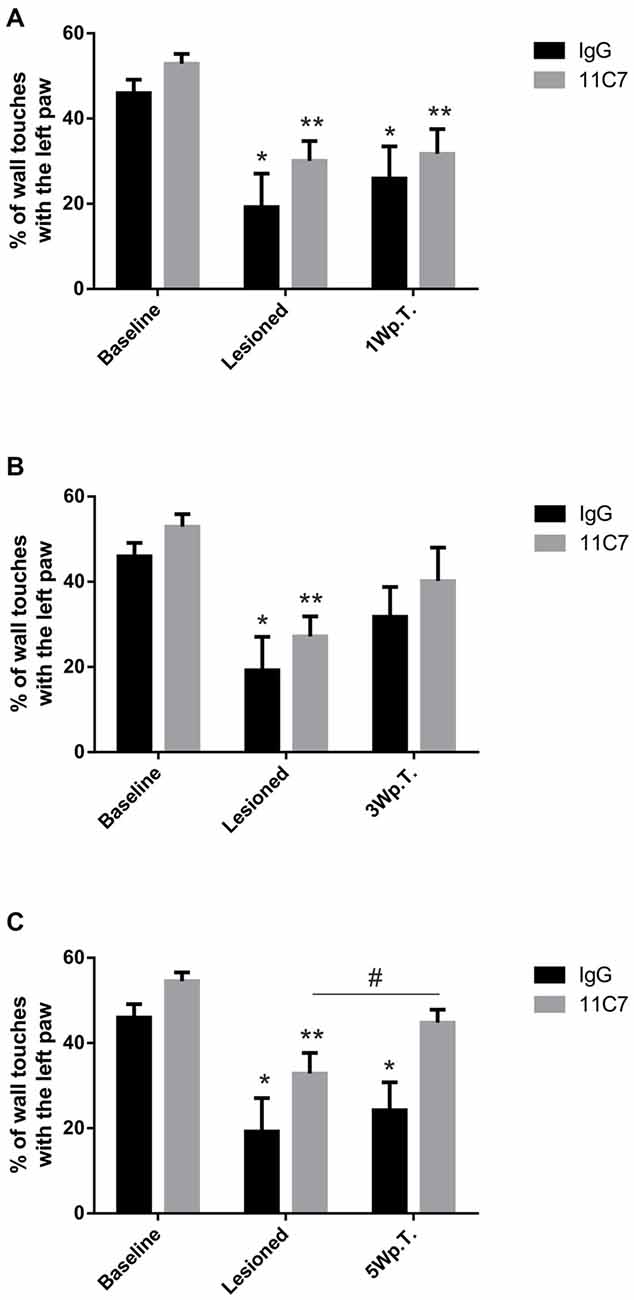
Figure 3. Behavioral assessment of IgG or 11C7 treated rats by means of the cylinder test. The outcome is shown before and after the 6-OHDA lesion as well as one (1 W.p.T.) (A), three (3 W.p.T.) (B) and 5 weeks after the transplantation (5 W.p.T.) (C). The 6-OHDA lesion resulted in significantly lower wall touches with the left paw (A–C). Notably, the 11C7 treatment improved asymmetrical forelimb use 5 Wp.T as compared to after the lesion. (C). In addition, 11C7 treated rats performed better than IgG treated rats 5 Wp.T. (C). In contrast, IgG treated rats did not improve their behavior (A–C). Data are given as mean ± SEM and expressed as percentage of corresponding baselines. *p < 0.05 vs. corresponding baselines IgG, **p < 0.05 vs. corresponding baselines 11C7, #p < 0.05 vs. corresponding lesion 11C7.
Histological Analysis of the Grafts
Both groups of treated rats showed surviving grafts that did not differ significantly in their volume (t0.8/8 = 0.448; Figure 4). Notably, 11C7 treatment significantly increased the number of TH positive fibers growing 100 μm into the host brain as compared to the IgG group (by 2.9 fold; t2.4/8 ≤ 0.05; Figure 5). Similarly, 11C7 treatment resulted in increased densities of TH positive cells in the grafts as compared to the IgG group (by 2.4 fold; t1.2/8 = 0.258) which, however, did not reach statistical significance (Figure 6). Moreover, to determine the sub-population of dopaminergic neurons that has been shown to integrate best into the host brain after transplantation, we investigated the percentage of grafted TH positive neurons co-localizing with GIRK2. The analysis revealed that about 75% of the grafted TH positive neurons co-expressed GIRK2, however, no significant differences were detected between groups (Figure 7).
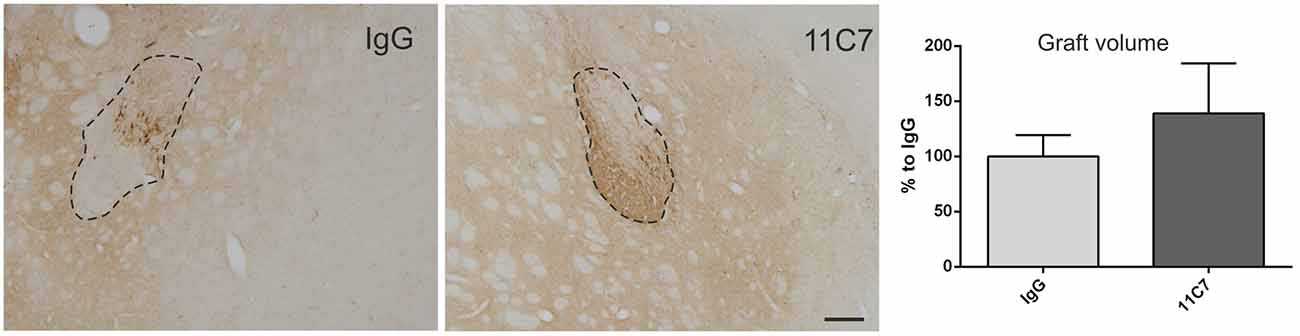
Figure 4. Effects of intra-ventricular infusion of IgG or 11C7 on graft volumes. Representative photomicrographs of grafts treated with IgG or 11C7 stained for TH. The graft boarder is highlighted with a dashed black line. Scale bar: 200 μm. No difference in graft volume was found between the two treatment groups, as demonstrated in the bar graph. Data are given as mean ± SEM and are presented as percentage of control (IgG).
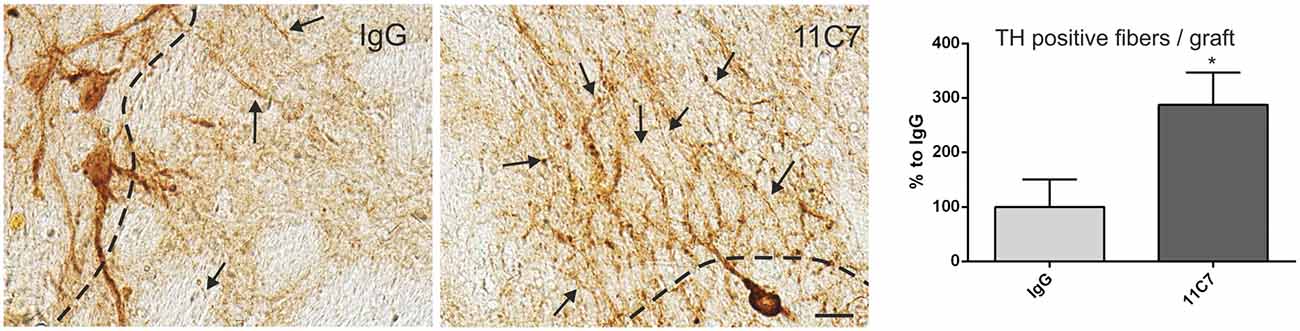
Figure 5. Effects of intra-ventricular infusion of IgG or 11C7 on TH positive fiber outgrowth. TH positive fibers from the grafts growing into the host brain (arrows) are shown on representative photomicrographs from IgG (left panel) and 11C7 (middle panel) treated animals. The black dashed line indicates the graft boarder. Scale bar: 20 μm. Note that 11C7 treatment resulted in a significantly increased number of TH positive fibers growing 100 μm into the host brain, as shown in the bar graph (right panel). Data are given as mean ± SEM and are presented as percentage of control (IgG). *p < 0.05.
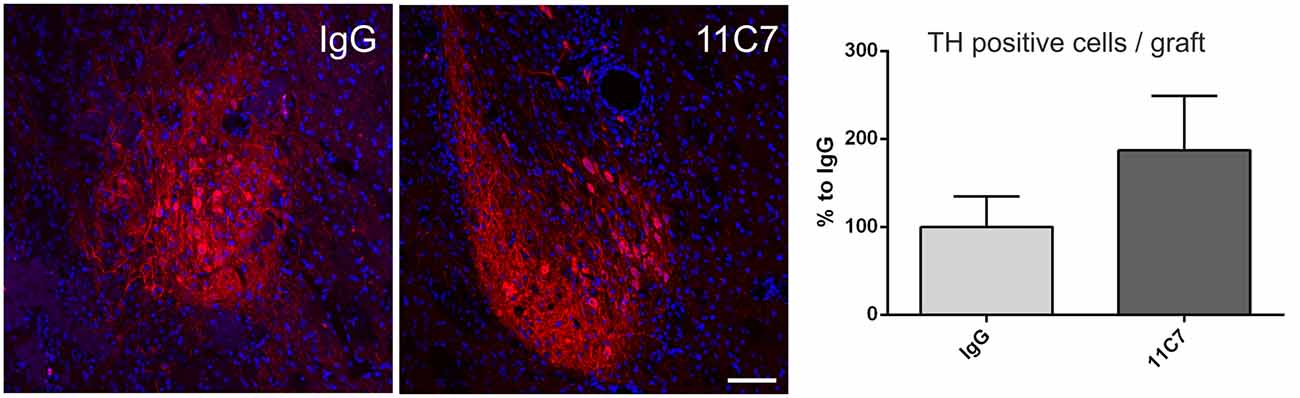
Figure 6. Effects of intra-ventricular infusion of IgG or 11C7 on TH positive cell numbers in the grafts. Representative photomicrographs showing TH positive cells (in red) in grafts from IgG and 11C7 treated animals. The sections were co-stained for the nuclear marker Hoechst (blue). Scale bar: 100 μm. Note that 11C7 treated grafts revealed a tendency towards higher numbers of TH positive cells in the graft, as shown in the bar graph. Data are given as mean ± SEM and are presented as percentage of control (IgG).
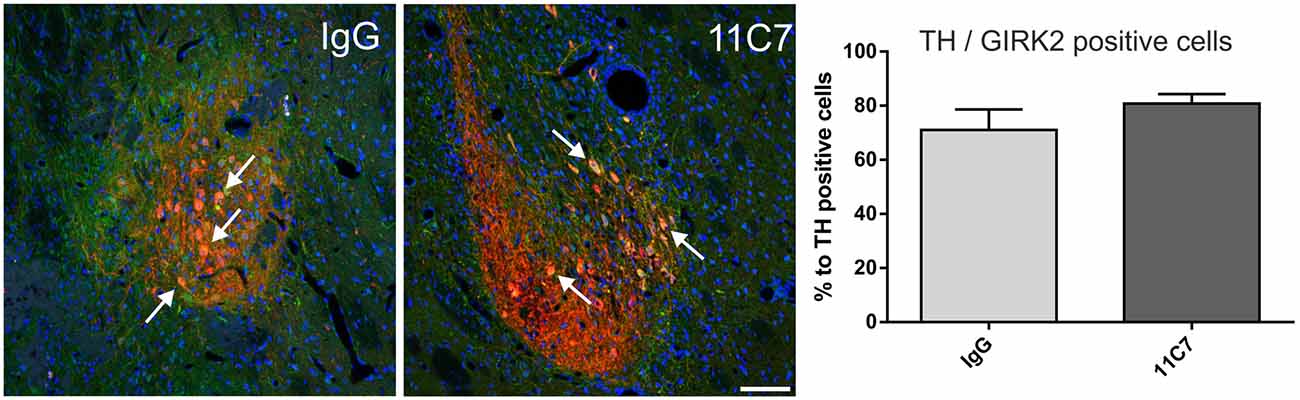
Figure 7. Effects of intra-ventricular infusion of IgG or 11C7 on TH positive cells co-localizing with G-protein-coupled inward rectifier potassium channel subunit 2 (GIRK2) in the grafts. Representative photomicrographs showing TH positive (red) and GIRK2 positive (green) cells in grafts from IgG and 11C7 treated animals. The sections were co-stained for the nuclear marker Hoechst (blue). Selected TH positive cells co-localizing with GIRK2 are highlighted with white arrows. Scale bar: 100 μm. No significant difference in number of TH positive cells co-expressing GIRK2 was observed between the two treatments, as demonstrated in the bar graph. Data are given as mean ± SEM and are presented as percentage of TH positive cells.
Estimation of TH Positive Fibers in the Striatum
To evaluate a potential effect of anti-Nogo-A antibody treatment on endogenous fiber growth of the surviving dopaminergic neurons, the TH positive fiber density in the dorsal striatum was analyzed. The densiometric analysis revealed, however, no difference of the mean gray intensity of TH positive fibers in the striatum of IgG and 11C7 treated rats (76.8 ± 18.9 vs. 62.7 ± 15.0, % of unlesioned side, IgG and 11C7, respectively; t0.6/7 = 0.573; Figure 8).
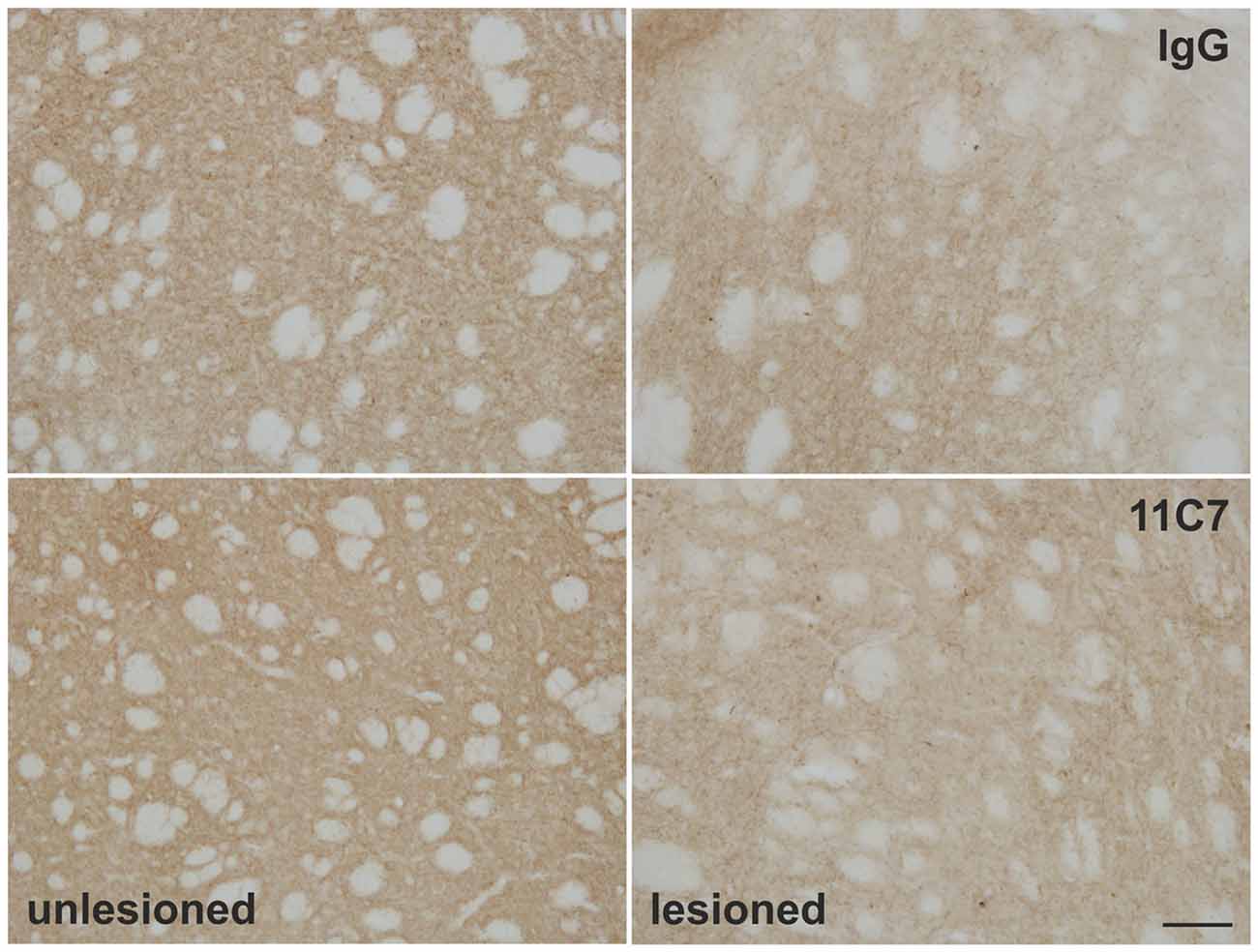
Figure 8. Effects of intra-ventricular infusion of IgG or 11C7 on TH positive fibers in the dorsolateral striatum. Representative photomicrographs showing TH positive fibers in the dorsolateral striatum on the control and lesion sides from IgG and 11C7 treated animals. Scale bar: 100 μm. No significant difference in the TH positive fiber density was observed between the two treatments.
Discussion
The present study shows for the first time, that transplantation of fetal VM neurons into the striatum of lesioned hemi-parkinsonian rats with concomitant neutralizing Nogo-A antibody treatment significantly increased graft derived dopaminergic fiber outgrowth and resulted in functional recovery.
In the present study, the rats were transplanted with only half a ventral mesencephalon, which corresponds to a sub-therapeutic treatment, since we have previously shown that transplantation of a whole ventral mesencephalon results in complete recovery (Meyer et al., 1998). We have chosen to transplant organotypic free-floating roller tube cultures as this method allows for effective storage of midbrain tissue, which is of importance in clinical settings where several fetuses have to be pooled to reach the amount of dopaminergic neurons needed for successful transplantations (Meyer et al., 1998; Studer, 2001). The observed lack of functional improvement after IgG treatment is thus not surprising and is consistent with our previously published data (Sautter et al., 1998; Matarredona et al., 2003). In contrast, neutralizing Nogo-A antibody treatment in a time dependent manner reversed the asymmetrical forelimb use induced by the 6-OHDA lesions over the observed period of 5 weeks. Even though we observed a roughly two fold higher survival of dopaminergic cell numbers in the graft of animals treated with neutralizing Nogo-A antibodies as compared to controls this difference did not reach statistical significance. We hence inferred that behavioral recovery was likely not due specifically to an increased survival of grafted DAergic neurons. This finding is somewhat surprising as Inoue et al. (2007) demonstrated that inhibition of LINGO-1 increases the survival of DAergic neurons in a mouse model of PD. Furthermore, blocking NgR1 or LINGO-1 was demonstrated to significantly increase the number of dopaminergic neurons in midbrain cultures (Inoue et al., 2007; Seiler et al., 2013). The difference in the outcome between our present study and the study by Inoue et al. (2007) may be due to the diverse signaling mechanisms of Nogo-A. While inhibition of LINGO-1 interferes directly with the NgR1, anti-Nogo-A antibodies target the B-20 domain of Nogo-A, thus a structure that signals independent of NgR1 (Kurowska et al., 2014). In both groups of animals, a substantial portion of surviving dopaminergic cells in the grafts co-expressed GIRK2, however, no difference was detected between groups hinting to the idea that Nogo-A neutralization did not favor a specific subpopulation of grafted neurons. Nevertheless, given the tendency for higher numbers of dopaminergic neurons in the Nogo-A group of animals one may assume that overall a larger portion of dopaminergic neurons of the GIRK2 phenotype are present in this grafts. Because graft size did not differ significantly between control and anti-Nogo-A treated rats, we suggest that the anti-Nogo-A promoted graft-derived dopaminergic fiber outgrowth contributed primarily to functional recovery. This hypothesis is supported by the observations from other studies demonstrating functional recovery in absence of higher survival of grafted dopaminergic neurons but in presence of increased fiber outgrowth and integration (Haque et al., 1996; Zhou et al., 1997; Yurek et al., 1998). Moreover, our results showing no difference of the fiber density in the striatum between both treatment groups supports the idea that mainly the fiber outgrowth of the transplanted dopaminergic neurons contributed to the improved behavior. Nevertheless, we cannot exclude that the treatment with anti-Nogo-A antibodies only accelerates the fiber outgrowth of the transplanted dopaminergic neurons. Whether at later time points the IgG treated rats would display increased densities of TH fibers sprouting from the graft remains to be investigated. Moreover, we cannot rule out the possibility that the graft itself influenced endogenous sprouting of remaining TH positive fibers, as several groups have reported particularly with adrenal gland tissue (Bankiewicz et al., 1990; Kordower et al., 1991) Promoting integration rather than the survival of grafted dopaminergic cells might be the relevant strategy to improve efficacy of transplants. Even though our study did not specifically address for adverse effects associated with the application of neutralizing Nogo-A antibodies we did not observe any obvious changes in the behavior of the animals. Given that it has been demonstrated that intra-ventricular administration of anti-Nogo-A antibodies leads to a widespread distribution of the antibodies in the brain (Wiessner et al., 2003) possible side effects of an intra-ventricular administration of anti-Nogo-A antibodies cannot completely be excluded. Nevertheless, a similar injection route, namely intrathecal administration of anti-Nogo-A antibodies in spinal cord injured patients, has been demonstrated to be feasible and safe (Zörner and Schwab, 2010).
Our findings showing augmented fiber outgrowth after Nogo-A neutralization, are in contrast to previous reports, showing unchanged neurite length and even decreased neurite numbers in dopaminergic neurons from Nogo-A knock-out animals (Kurowska et al., 2014). It is, however, important to note that the outcome of experiments employing Nogo-A knock-out animals differs from those using Nogo-A neutralization experiments. It has been reported that Nogo gene knockout results in inferior regeneration capacity as compared to treatment of animals with neutralizing agents (Teng and Tang, 2005). The authors proposed that these differences may be explained by the potential interactions of Nogo-A with other molecules, which do not take place in knock-out animals (Teng and Tang, 2005). In this respect, interactions between Nogo-A and neurotrophic factors (Raiker et al., 2010; Sepe et al., 2014) are of particular interest in view of cell transplantation approaches for PD, as glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor and nerve growth factor have been described to improve graft functions and behavior recovery in animal models of PD and in PD patients (Cunningham et al., 1994; Rosenblad et al., 1996; Yurek et al., 1996, 2009; Sautter et al., 1998; Torres et al., 2005; Emborg et al., 2008; Andereggen et al., 2009; Somoza et al., 2010; Deng et al., 2013). Hence, it is tempting to speculate that Nogo-A neutralization exerts its action not only through disinhibition of neurite outgrowth but also indirectly through elevation of neurotrophic factor signaling. Such assumptions, however, have yet to be proven in the context of cell transplantation approaches for PD and remain currently speculative.
Conclusion
Taken together, the present report demonstrates that the continuous delivery of Nogo-A neutralizing antibodies improves dopaminergic graft function likely by increasing sprouting of grafted dopaminergic neurons into the host brain. Our findings support the view that neutralization of Nogo-A in the host brain may offer a novel and therapeutically meaningful intervention for cell transplantation approaches in PD. Further studies, however, will be necessary to evaluate the clinical potential of this mode of intracerebral delivery of anti-Nogo-A antibodies to support graft survival and function.
Ethics Statement
The experimental animal procedure was approved by the Animal Research Ethics Committee of the Canton Bern, Switzerland.
Author Contributions
Author’s contribution to the study and manuscript preparation includes the following. Conception and design of the work was done by SS, SDS, HRW. Acquisition of data was performed by SS, SDS, HRW. Analysis and interpretation of data was carried out by SS, SDS, HRW. The draft of the article was performed by SS and critically revised by SDS, HRW. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The expert technical assistance from Susanne Wälchli is gratefully acknowledged. We like to thank Prof. Dr. Martin Schwab (University of Zurich, Switzerland) for critical reading of the manuscript and his interest in the study. Moreover, we would like to thank Dr. Stefan Tschanz (University of Bern, Switzerland) for his advice with the stereological analyses. The 11C7 antibody was kindly provided by Novartis Switzerland (Dr. Anis Mir). This research was supported by the Swiss Parkinson Foundation, the HANELA Foundation and the Swiss National Science Foundation (No. 31003A_135565).
Abbreviations
6-OHDA, 6-hydroxydopamine; CNS, central nervous system; GIRK2, G-protein-coupled inward rectifier potassium channel subunit 2; i.p., intraperitoneal; LINGO-1, leucine rich repeat neuronal protein 1; NgR1, Nogo-receptor 1; PBS, phosphate buffered saline; PD, Parkinson’s disease; s.c., subcutaneous; SNc, substantia nigra pars compacta; TH, tyrosine hydroxylase; VM, ventral mesencephalic.
References
Alsberg, E., von Recum, H. A., and Mahoney, M. J. (2006). Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin. Biol. Ther. 6, 847–866. doi: 10.1517/14712598.6.9.847
Andereggen, L., Meyer, M., Guzman, R., Ducray, A. D., and Widmer, H. R. (2009). Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson’s disease. Brain Res. 1276, 39–49. doi: 10.1016/j.brainres.2009.04.021
Bankiewicz, K. S., Plunkett, R. J., Jacobowitz, D. M., Porrino, L., di Porzio, U., London, W. T., et al. (1990). The effect of fetal mesencephalon implants on primate MPTP-induced parkinsonism. Histochemical and behavioral studies. J. Neurosurg. 72, 231–244. doi: 10.3171/jns.1990.72.2.0231
Barker, R. A., Barrett, J., Mason, S. L., and Bjorklund, A. (2013). Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 12, 84–91. doi: 10.1016/s1474-4422(12)70295-8
Boix, J., Padel, T., and Paul, G. (2015). A partial lesion model of Parkinson’s disease in mice–characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 284, 196–206. doi: 10.1016/j.bbr.2015.01.053
Brooks, S. P., and Dunnett, S. B. (2009). Tests to assess motor phenotype in mice: a user’s guide. Nat. Rev. Neurosci. 10, 519–529. doi: 10.1038/nrn2652
Cheatwood, J. L., Emerick, A. J., Schwab, M. E., and Kartje, G. L. (2008). Nogo-A expression after focal ischemic stroke in the adult rat. Stroke 39, 2091–2098. doi: 10.1161/STROKEAHA.107.507426
Cordeiro, K. K., Jiang, W., Papazoglou, A., Tenório, S. B., Döbrössy, M., and Nikkhah, G. (2010). Graft-mediated functional recovery on a skilled forelimb use paradigm in a rodent model of Parkinson’s disease is dependent on reward contingency. Behav. Brain Res. 212, 187–195. doi: 10.1016/j.bbr.2010.04.012
Cunningham, L. A., Short, M. P., Breakefield, X. O., and Bohn, M. C. (1994). Nerve growth factor released by transgenic astrocytes enhances the function of adrenal chromaffin cell grafts in a rat model of Parkinson’s disease. Brain Res. 658, 219–231. doi: 10.1016/s0006-8993(09)90029-4
Deng, X., Liang, Y., Lu, H., Yang, Z., Liu, R., Wang, J., et al. (2013). Co-transplantation of GDNF-overexpressing neural stem cells and fetal dopaminergic neurons mitigates motor symptoms in a rat model of Parkinson’s disease. PloS One 8:e80880. doi: 10.1371/journal.pone.0080880
Emborg, M. E., Ebert, A. D., Moirano, J., Peng, S., Suzuki, M., Capowski, E., et al. (2008). GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 17, 383–395. doi: 10.3727/096368908784423300
Emgard, M., Karlsson, J., Hansson, O., and Brundin, P. (1999). Patterns of cell death and dopaminergic neuron survival in intrastriatal nigral grafts. Exp. Neurol. 160, 279–288. doi: 10.1006/exnr.1999.7198
Freed, C. R., Greene, P. E., Breeze, R. E., Tsai, W. Y., DuMouchel, W., Kao, R., et al. (2001). Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344, 710–719. doi: 10.1056/NEJM200103083441002
Freund, P., Schmidlin, E., Wannier, T., Bloch, J., Mir, A., Schwab, M. E., et al. (2009). Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates–re-examination and extension of behavioral data. Eur. J. Neurosci. 29, 983–996. doi: 10.1111/j.1460-9568.2009.06642.x
Gaillard, A., Decressac, M., Frappé, I., Fernagut, P. O., Prestoz, L., Besnard, S., et al. (2009). Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol. Dis. 35, 477–488. doi: 10.1016/j.nbd.2009.07.003
Gombash, S. E., Lipton, J. W., Collier, T. J., Madhavan, L., Steece-Collier, K., Cole-Strauss, A., et al. (2012). Striatal pleiotrophin overexpression provides functional and morphological neuroprotection in the 6-hydroxydopamine model. Mol. Ther. 20, 544–554. doi: 10.1038/mt.2011.216
Grealish, S., Jonsson, M. E., Li, M., Kirik, D., Björklund, A., and Thompson, L. H. (2010). The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain 133, 482–495. doi: 10.1093/brain/awp328
Haque, N. S., Hlavin, M. L., Fawcett, J. W., and Dunnett, S. B. (1996). The neurotrophin NT4/5, but not NT3, enhances the efficacy of nigral grafts in a rat model of Parkinson’s disease. Brain Res. 712, 45–52. doi: 10.1016/0006-8993(95)01427-6
Hauser, R. A., Freeman, T. B., Snow, B. J., Nauert, M., Gauger, L., Kordower, J. H., et al. (1999). Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch. Neurol. 56, 179–187. doi: 10.1001/archneur.56.2.179
Hoglinger, G. U., Widmer, H. R., Spenger, C., Meyer, M., Seiler, R. W., Oertel, W. H., et al. (2001). Influence of time in culture and BDNF pretreatment on survival and function of grafted embryonic rat ventral mesencephalon in the 6-OHDA rat model of Parkinson’s disease. Exp. Neurol. 167, 148–157. doi: 10.1006/exnr.2000.7546
Inoue, H., Lin, L., Lee, X., Shao, Z., Mendes, S., Snodgrass-Belt, P., et al. (2007). Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure and function of dopaminergic neurons in Parkinson’s disease models. Proc. Natl. Acad. Sci. U S A 104, 14430–14435. doi: 10.1073/pnas.0700901104
Karlsson, J., Emgård, M., Gidö, G., Wieloch, T., and Brundin, P. (2000). Increased survival of embryonic nigral neurons when grafted to hypothermic rats. Neuroreport 11, 1665–1668. doi: 10.1097/00001756-200006050-00014
Kefalopoulou, Z., Politis, M., Piccini, P., Mencacci, N., Bhatia, K., Jahanshahi, M., et al. (2014). Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87. doi: 10.1001/jamaneurol.2013.4749
Kordower, J. H., Cochran, E., Penn, R. D., and Goetz, C. G. (1991). Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson’s disease. Ann. Neurol. 29, 405–412. doi: 10.1002/ana.410290411
Kordower, J. H., Freeman, T. B., Snow, B. J., Vingerhoets, F. J., Mufson, E. J., Sanberg, P. R., et al. (1995). Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 332, 1118–1124. doi: 10.1056/nejm199504273321702
Kurowska, Z., Brundin, P., Schwab, M. E., and Li, J. Y. (2014). Intracellular Nogo-A facilitates initiation of neurite formation in mouse midbrain neurons in vitro. Neuroscience 256, 456–466. doi: 10.1016/j.neuroscience.2013.10.029
Lindvall, O., Brundin, P., Widner, H., Rehncrona, S., Gustavii, B., Frackowiak, R., et al. (1990). Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 247, 574–577. doi: 10.1126/science.2105529
Lindvall, O., Rehncrona, S., Brundin, P., Gustavii, B., Astedt, B., Widner, H., et al. (1989). Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 46, 615–631. doi: 10.1001/archneur.1989.00520420033021
Matarredona, E. R., Meyer, M., Seiler, R. W., and Widmer, H. R. (2003). CGP 3466 increases survival of cultured fetal dopaminergic neurons. Restor. Neurol. Neurosci. 21, 29–37.
Mendez, I., Sanchez-Pernaute, R., Cooper, O., Viñuela, A., Ferrari, D., Björklund, L., et al. (2005). Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 128, 1498–1510. doi: 10.1093/brain/awh510
Merkler, D., Metz, G. A., Raineteau, O., Dietz, V., Schwab, M. E., and Fouad, K. (2001). Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 21, 3665–3673.
Meyer, M., Widmer, H. R., Wagner, B., Guzman, R., Evtouchenko, L., Seiler, R. W., et al. (1998). Comparison of mesencephalic free-floating tissue culture grafts and cell suspension grafts in the 6-hydroxydopamine-lesioned rat. Exp. Brain Res. 119, 345–355. doi: 10.1007/s002210050350
Moore, S. F., Guzman, N. V., Mason, S. L., Williams-Gray, C. H., and Barker, R. A. (2014). Which patients with Parkinson’s disease participate in clinical trials? One centre’s experiences with a new cell based therapy trial (TRANSEURO). J. Parkinsons. Dis. 4, 671–676. doi: 10.3233/JPD-140432
Ourednik, V., and Ourednik, J. (2005). Graft/host relationships in the developing and regenerating CNS of mammals. Ann. N Y Acad. Sci. 1049, 172–184. doi: 10.1196/annals.1334.016
Petit, G. H., Olsson, T. T., and Brundin, P. (2014). The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol. Appl. Neurobiol. 40, 60–70. doi: 10.1111/nan.12110
Raiker, S. J., Lee, H., Baldwin, K. T., Duan, Y., Shrager, P., and Giger, R. J. (2010). Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J. Neurosci. 30, 12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010
Rosenblad, C., Martinez-Serrano, A., and Björklund, A. (1996). Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience 75, 979–985.
Sautter, J., Tseng, J. L., Braguglia, D., Aebischer, P., Spenger, C., Seiler, R. W., et al. (1998). Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth and function of fetal dopaminergic grafts. Exp. Neurol. 149, 230–236. doi: 10.1006/exnr.1997.6718
Schaar, K. L., Brenneman, M. M., and Savitz, S. I. (2010). Functional assessments in the rodent stroke model. Exp. Transl. Stroke Med. 2:13. doi: 10.1186/2040-7378-2-13
Schallert, T., Fleming, S. M., Leasure, J. L., Tillerson, J. L., and Bland, S. T. (2000). CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777–787. doi: 10.1016/s0028-3908(00)00005-8
Schawkat, K., Di Santo, S., Seiler, S., Ducray, A. D., and Widmer, H. R. (2015). Loss of Nogo-A-expressing neurons in a rat model of Parkinson’s disease. Neuroscience 288, 59–72. doi: 10.1016/j.neuroscience.2014.12.035
Seiler, S., Pollini, D., Di Santo, S., and Widmer, H. R. (2013). Antagonizing Nogo-receptor 1 promotes the number of cultured dopaminergic neurons and elongates their neurites. Neuroreport 24, 1047–1052. doi: 10.1097/WNR.0000000000000063
Sepe, M., Lignitto, L., Porpora, M., Delle Donne, R., Rinaldi, L., Belgianni, G., et al. (2014). Proteolytic control of neurite outgrowth inhibitor NOGO-A by the cAMP/PKA pathway. Proc. Natl. Acad. Sci. U S A 111, 15729–15734. doi: 10.1073/pnas.1410274111
Somoza, R., Juri, C., Baes, M., Wyneken, U., and Rubio, F. J. (2010). Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinson’s disease. Biol. Blood Marrow Transplant. 16, 1530–1540. doi: 10.1016/j.bbmt.2010.06.006
Spenger, C., Studer, L., Evtouchenko, L., Egli, M., Burgunder, J. M., Markwalder, R., et al. (1994). Long-term survival of dopaminergic neurones in free-floating roller tube cultures of human fetal ventral mesencephalon. J. Neurosci. Methods 54, 63–73. doi: 10.1016/0165-0270(94)90160-0
Stefanova, N., Bücke, P., Duerr, S., and Wenning, G. K. (2009). Multiple system atrophy: an update. Lancet Neurol. 8, 1172–1178. doi: 10.1016/S1474-4422(09)70288-1
Studer, L. (2001). Culture of substantia nigra neurons. Curr. Protoc. Neurosci. Chapter 3:Unit 3.3. doi: 10.1002/0471142301.ns0303s00
Teng, F. Y., and Tang, B. L. (2005). Why do Nogo/Nogo-66 receptor gene knockouts result in inferior regeneration compared to treatment with neutralizing agents? J. Neurochem. 94, 865–874. doi: 10.1111/j.1471-4159.2005.03238.x
Thompson, L., Barraud, P., Andersson, E., Kirik, D., and Björklund, A. (2005). Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression and efferent projections. J. Neurosci. 25, 6467–6477. doi: 10.1523/jneurosci.1676-05.2005
Torres, E. M., Monville, C., Lowenstein, P. R., Castro, M. G., and Dunnett, S. B. (2005). Delivery of sonic hedgehog or glial derived neurotrophic factor to dopamine-rich grafts in a rat model of Parkinson’s disease using adenoviral vectors increased yield of dopamine cells is dependent on embryonic donor age. Brain Res. Bull. 68, 31–41. doi: 10.1016/j.brainresbull.2005.08.021
Tronci, E., Shin, E., Björklund, A., and Carta, M. (2012). Amphetamine-induced rotation and L-DOPA-induced dyskinesia in the rat 6-OHDA model: a correlation study. Neurosci. Res. 73, 168–172. doi: 10.1016/j.neures.2012.03.004
Tsai, S. Y., Papadopoulos, C. M., Schwab, M. E., and Kartje, G. L. (2011). Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke 42, 186–190. doi: 10.1161/STROKEAHA.110.590083
Wiessner, C., Bareyre, F. M., Allegrini, P. R., Mir, A. K., Frentzel, S., Zurini, M., et al. (2003). Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J. Cereb. Blood Flow Metab. 23, 154–165. doi: 10.1097/00004647-200302000-00003
Winkler, C., Sauer, H., Lee, C. S., and Bjorklund, A. (1996). Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 16, 7206–7215.
Yurek, D. M., Flectcher, A. M., Kowalczyk, T. H., Padegimas, L., and Cooper, M. J. (2009). Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 18, 1183–1196. doi: 10.3727/096368909x12483162196881
Yurek, D. M., Hipkens, S. B., Wiegand, S. J., and Altar, C. A. (1998). Optimal effectiveness of BDNF for fetal nigral transplants coincides with the ontogenic appearance of BDNF in the striatum. J. Neurosci. 18, 6040–6047.
Yurek, D. M., Lu, W., Hipkens, S., and Wiegand, S. J. (1996). BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp. Neurol. 137, 105–118. doi: 10.1006/exnr.1996.0011
Zhou, J., Bradford, H. F., and Stern, G. M. (1997). Influence of BDNF on the expression of the dopaminergic phenotype of tissue used for brain transplants. Brain Res. Dev. Brain Res. 100, 43–51. doi: 10.1016/s0165-3806(97)00019-9
Keywords: Parkinson’s disease, Nogo-A, cell transplantation, dopaminergic neurons, behavior, rat
Citation: Seiler S, Di Santo S and Widmer HR (2016) Nogo-A Neutralization Improves Graft Function in a Rat Model of Parkinson’s Disease. Front. Cell. Neurosci. 10:87. doi: 10.3389/fncel.2016.00087
Received: 30 December 2015; Accepted: 21 March 2016;
Published: 05 April 2016.
Edited by:
Alessandro Tozzi, University of Perugia, ItalyReviewed by:
Carmelo Sgobio, Deutsches Zentrum für Neurodegenerative Erkrankungen, GermanyDaniella Rylander Ottosson, Lund University, Sweden
Copyright © 2016 Seiler, Di Santo and Widmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans Rudolf Widmer, hanswi@insel.ch
 Stefanie Seiler
Stefanie Seiler Stefano Di Santo
Stefano Di Santo Hans Rudolf Widmer
Hans Rudolf Widmer