Acutely increasing δGABAA receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus
- 1Institute of Medical Science, University of Toronto, Toronto, ON, Canada
- 2Department of Pharmacology, University of Toronto, Toronto, ON, Canada
- 3Department of Physiology, University of Toronto, Toronto, ON, Canada
- 4Department of Anesthesia, University of Toronto, Toronto, ON, Canada
- 5Department of Anesthesia, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Extrasynaptic γ-aminobutyric acid type A (GABAA) receptors that contain the δ subunit (δGABAA receptors) are expressed in several brain regions including the dentate gyrus (DG) and CA1 subfields of the hippocampus. Drugs that increase δGABAA receptor activity have been proposed as treatments for a variety of disorders including insomnia, epilepsy and chronic pain. Also, long-term pretreatment with the δGABAA receptor–preferring agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) enhances discrimination memory and increases neurogenesis in the DG. Despite the potential therapeutic benefits of such treatments, the effects of acutely increasing δGABAA receptor activity on memory behaviors remain unknown. Here, we studied the effects of THIP (4 mg/kg, i.p.) on memory performance in wild-type (WT) and δGABAA receptor null mutant (Gabrd−/−) mice. Additionally, the effects of THIP on long-term potentiation (LTP), a molecular correlate of memory, were studied within the DG and CA1 subfields of the hippocampus using electrophysiological recordings of field potentials in hippocampal slices. The results showed that THIP impaired performance in the Morris water maze, contextual fear conditioning and object recognition tasks in WT mice but not Gabrd−/− mice. Furthermore, THIP inhibited LTP in hippocampal slices from WT but not Gabrd−/− mice, an effect that was blocked by GABAA receptor antagonist bicuculline. Thus, acutely increasing δGABAA receptor activity impairs memory behaviors and inhibits synaptic plasticity. These results have important implications for the development of therapies aimed at increasing δGABAA receptor activity.
Introduction
γ-Aminobutyric acid type A (GABAA) receptors are the primary mediators of inhibitory neurotransmission in the mammalian central nervous system. These transmitter-gated ion channels are constituted from a wide array of subunits (α1–6, β1–3, γ1–3, δ, π, θ, ε) and mediate two distinct forms of inhibition: phasic and tonic (Farrant and Nusser, 2005). Phasic inhibition is generated by postsynaptic GABAA receptors, whereas tonic inhibition is mediated primarily by extrasynaptic GABAA receptors that contain either the δ subunit (δGABAA receptors) or α5 subunit (α5GABAA receptors) (Farrant and Nusser, 2005). Recently, δGABAA receptors have attracted considerable attention as therapeutic targets because these receptors significantly reduce neuronal excitability in vitro (Stell et al., 2003; Maguire et al., 2009) and also regulate neurogenesis (Whissell et al., 2013), memory (Wiltgen et al., 2005; Shen et al., 2010; Whissell et al., 2013), nociception (Bonin et al., 2011), maternal behaviors (Maguire and Mody, 2008) and responses to stress (Shen et al., 2007; Sarkar et al., 2011).
Drugs that directly activate δGABAA receptors, and those that act as positive allosteric modulators, are currently under investigation as potential treatments for a wide variety of disorders, including insomnia (Wafford and Ebert, 2006), pain (Bonin et al., 2011), cognitive dysfunction (Wang et al., 2007) and depression (Maguire and Mody, 2008; Christensen et al., 2012). The most widely studied of these compounds is 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), a δGABAA receptor–preferring agonist (Brown et al., 2002; Meera et al., 2011). THIP is considered a “super”-agonist of δGABAA receptors as the drug generates a greater peak response than GABA (Brown et al., 2002). The hypnotic properties of THIP were shown in studies of humans and laboratory animals (Faulhaber et al., 1997; Wafford and Ebert, 2006), and antinociceptive properties were observed in rodent models of acute and persistent pain (Bonin et al., 2011). Unlike other less selective positive modulators of GABAA receptors such as benzodiazepines and barbiturates, THIP may have a low risk of tolerance and addiction (Ebert et al., 2008; Tan et al., 2011) and thus is a promising candidate for long-term use.
THIP may also have memory-enhancing effects. We recently showed that pre-treatment with THIP for 7 days improved discrimination memory, when studied 14 days after drug treatment in a mouse model (Whissell et al., 2013). The memory-enhancing properties of THIP were associated with increased postnatal neurogenesis in the dentate gyrus (DG), a process whereby new cells are generated in the adult brain. Such adult-born neurons are thought to contribute to multiple forms of memory performance, including spatial memory, recognition memory and fear memory (Marin-Burgin and Schinder, 2012).
While long-term pre-treatment with THIP improves memory, several lines of evidence predict that an acute increase in δGABAA receptor activity will impair memory. First, enhanced δGABAA receptor activity constrains neuronal firing (Bonin et al., 2011), reduces network excitability (Maguire et al., 2009) and attenuates synaptic plasticity in the CA1 region of the hippocampus (Shen et al., 2010). Second, one of the primary molecular targets of THIP, the α4βδ GABAA receptor (Brown et al., 2002), constrains fear-associated memory (Wiltgen et al., 2005) as evidenced by studies of transgenic mice that lack either the δ subunit gene (Wiltgen et al., 2005) or the α4 subunit gene (Moore et al., 2010; Cushman et al., 2011). Interestingly, human studies have shown that THIP does not alter memory performance measured 12–24 h after drug treatment (Mathias et al., 2005; Boyle et al., 2009; Leufkens et al., 2009). However, these studies examined memory at a time point when THIP was likely to have been eliminated (Cremers and Ebert, 2007).
Here, we tested the hypothesis that acutely increasing δGABAA receptor activity impairs memory. Memory was studied in wild-type (WT) and δ subunit null mutant (Gabrd−/−) mice 30 min after treatment with THIP, a time point when THIP levels in the brain peak (Cremers and Ebert, 2007). Additionally, to identify the molecular basis of memory impairment, long-term potentiation (LTP), a putative molecular substrate of memory, was studied in the DG and CA1 subfields of the hippocampus. A decrease or increase in GABAA receptor activity enhances or depresses LTP, respectively (Wigstrom and Gustafsson, 1985; Snyder et al., 2001; Arima-Yoshida et al., 2011). Further, it has been demonstrated that selectively increasing tonic inhibition depresses LTP, even when synaptic inhibition remains unchanged (Arima-Yoshida et al., 2011). Given that δGABAA receptors are densely expressed in the DG, and also expressed in the CA1 subfield (Glykys et al., 2008), it was predicted that THIP would depress LTP. Consistent with our hypotheses, the results show that acutely increasing δGABAA receptor activity impairs memory, and inhibits LTP in hippocampal slices from WT but not Gabrd−/− mice.
Materials and Methods
Animals
All experiments were approved by the local Animal Care Committee. WT and Gabrd−/− mice were generously provided by Dr. Gregg Homanics (University of Pittsburgh) and were generated as previously described (Mihalek et al., 1999). These mice were bred in the animal facility at University of Toronto. Only male mice 3–6 months of age were used for behavioral experiments, as the estrous cycle influences δGABAA receptor expression and activity (Maguire et al., 2005). Researchers were blinded to the genotype and drug conditions.
Drugs
THIP was obtained from Tocris Bioscience (Bristol, UK). For behavioral experiments, THIP (4 mg/kg) was administered by intraperitoneal (i.p.) injection. This dose was selected because it has no sedative effects, although it may have a mild antinociceptive effect (Bonin et al., 2011). In electrophysiological experiments, hippocampal slices were treated with 1 μM THIP, as this concentration is expected to preferentially activate δGABAA receptors (Brown et al., 2002) and is within the range of the dose used to treat humans (Schultz et al., 1981; Madsen et al., 1983). The non-selective competitive GABAA receptor antagonists bicuculline methiodide (BIC) and SR-95531 were employed for some experiments (Bai et al., 2001; Nusser and Mody, 2002) as no selective δGABAA receptor antagonists are available. Both compounds were obtained from Tocris Bioscience (Bristol, UK). SR-95531 preferentially blocks synaptic rather than extrasynaptic GABAA receptors at low concentrations (Nusser and Mody, 2002).
Behavioral Assays
Morris water maze
This assay was used to assess hippocampus-dependent spatial memory. The water maze was a circular pool (ø = 1.2 m) that was surrounded by visual cues, filled with opaque white non-toxic paint and kept at 25 ± 2°C. The escape platform was a 10 × 10 cm square of Plexiglas that was positioned 0.5 cm below the pool surface so that it was not visible during the experiment. On the training day, the platform was placed in a random quadrant of the pool, and mice were given 4 trials to learn its location for memory acquisition. The total time (s) to locate and remain on the platform (escape latency) was recorded during each trial. If a mouse did not locate the platform within 60 s, it was gently guided to the platform, and the maximum value of 60 s was assigned. The next day, 24 h after acquisition, long-term recall of the platform location was tested using a 60-s probe trial. During this trial, the platform was removed and the percentage of time the mouse spent in the quadrant that formerly contained the platform (the “correct” quadrant) was calculated. Mouse position was recorded and analyzed using SMART video tracking software (San Diego Instruments, San Diego, CA, USA).
Fear conditioning
This assay was used to examine hippocampus-dependent contextual fear memory (Phillips and LeDoux, 1992) and amygdala-dependent auditory-cued fear memory that does not require the hippocampus (Phillips and LeDoux, 1992). An exposure chamber (20 × 20 × 30 cm) with a shock grid floor consisting of stainless steel bars (2 cm apart, ø = 2 mm) was used for this task (Med Associates Inc., St. Albans, VT, USA) (Wang et al., 2012). During acquisition, each mouse was allowed to explore the chamber for 180 s. A 4 kHz tone, created by a frequency generator, amplified to 100 dB and lasting 20 s, was then presented. The last 2 s of the auditory tone was paired with an electric footshock (0.7 mA). This tone–shock pairing was presented three times (designated S1, S2, S3), separated by 60-s intervals. The next day (i.e., day 2), 24 h after acquisition, contextual fear memory was assessed by returning the mouse to the context for 8 min and measuring the percentage of time that it spent freezing. On day 3, the conditioning chamber was modified to measure the freezing response to the auditory tone (auditory-cued fear memory). This modified context had a significantly different shape, scent and visual appearance than the original chamber. Mice were monitored for 180 s for freezing to the modified context, to rule out contextual influences. After this monitoring period, the auditory tone was presented for 5 min, and the percentage of time that each mouse spent freezing was determined using FreezeView software (Version 2.26, Actimetrics Inc., Wilmette, IL, USA).
Novel object recognition
This assay was used to study short-term working memory. Twenty-four hours before testing, each mouse was habituated for 15 min in a chamber (20 × 20 × 20 cm) marked with visual cues (Saab et al., 2009). During testing, the mouse was exposed to a set of three identical objects in the chamber for 2 min (Figure 3A). The mouse was then removed from the chamber for 2 min while the entire setup was cleaned with 70% ethanol and one of the objects was replaced with a novel object (NO). The mouse was then returned to the chamber and the interaction time with the two familiar objects (O1 + O2) and the NO was recorded. Total interaction time was the sum of these interaction times (O1 + O2 + NO). NO preference (%) was defined as NO/(O1 + O2 + NO) × 100. An interaction was defined as active investigation of the object while the mouse was within 1 cm of the object and oriented toward it. Mice with a total interaction time of less than 3 s were excluded from analysis.
Electrophysiology
Male mice were anesthetized deeply with isoflurane and then decapitated, and their brains were removed. Coronal hippocampal slices (350–400 μm thick) were cut with a vibratome (VT1000E; Leica, Deerfield, IL, USA), then immersed in ice-cold artificial cerebrospinal fluid (ACSF) that contained (in mM) 124 NaCl, 3 KCl, 1.3 MgCl2, 2.6 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 d-glucose. The ACSF was saturated with 95% O2 and 5% CO2, and osmolarity was adjusted to 300–310 mOsm. The slices were allowed to recover for at least 1 h at room temperature (23–25°C) before being transferred to the recording chamber, where they were perfused with ACSF at 3–4 ml/min. All recordings were performed at room temperature using a MultiClamp 700A amplifier (Molecular Devices, Sunnyvale, CA, USA) controlled with pClamp 9.0 software via a Digidata 1322A interface (Molecular Devices, Sunnyvale, CA, USA).
Extracellular field potential recordings
Hippocampal slices were obtained from 3- to 6-month-old mice. In experiments examining LTP in the DG, extracellular field postsynaptic potentials (fPSPs) were recorded from the stratum moleculare of the DG using an ACSF-filled borosilicate pipette (World Precision Instruments, Sarasota, FL, USA). The medial perforant pathway was stimulated with a bipolar tungsten electrode (Rhodes Medical Instruments, Summerland, CA, USA).
To study presynaptic plasticity and confirm correct placement of the stimulating electrode in the medial perforant pathway, a pair of stimuli was applied at various time intervals (50, 100, 150, 200, or 300 ms) to generate a pair of responses. The paired pulse ratio was defined as (the slope of response 2)/(the slope of response 1). The presence of paired pulse depression (ratio < 1), one of the criteria used to assess medial perforant pathway inputs (Christie and Abraham, 1994), was deemed to indicate successful stimulation of the medial perforant pathway. To record LTP, baseline fPSPs were measured for at least 10 min at 0.05 Hz using a stimulation intensity that produced a half-maximal response. LTP was induced with a stimulation protocol that consisted of 4 stimulus trains delivered every 20 s, with each train occurring at 100 Hz and lasting 500 ms. fPSPs were monitored for 60 min after the stimulation, and the average of the last 5 min of recording was compared with the average of the baseline fPSPs. All drugs were allowed to perfuse the slices for 15 min before recording.
In experiments examining LTP in CA1, the same procedure was followed except that the recording electrode was placed in the stratum radiatum of the CA1 subfield and the Schaffer collateral pathway was stimulated. The protocol for LTP induction consisted of 10 stimulus trains of 4 pulses at 100 Hz with an inter-train interval of 500 ms (Martin et al., 2009).
Whole-cell voltage-clamp recordings
Hippocampal slices were obtained from 14- to 21-day-old male mice. Mice of this age range were utilized as their brains exhibit are more resistant to the dissection process (Moyer and Brown, 1998) and offer a larger population of healthy cells for easier patching. These cells show significant δGABAA receptor expression and δGABAA receptor-mediated currents (Laurie et al., 1992; Shen et al., 2011). All recordings were obtained from cells located in the granule cell layer of the DG that were visually identified with a Olympus BX51WI microscope (Center Valley, PA, USA). Recording pipettes (3–5 MΩ) were filled with the intracellular solution containing (in mM) 140 CsCl, 11 ethylene glycol tetra-acetic acid, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 K2-ATP, 1 CaCl2, 2 MgCl2 and 2 tetraethylammonium with osmolarity adjusted to 290–295 mOsm and pH adjusted to 7.3. To block glutaminergic neurotransmission and voltage-dependent sodium channels, 6-Cyano-7-nitroquinoxaline-2,3-dione (10 μM), (2R)-amino-5-phosphonovaleric acid (40 μM), and tetrodotoxin (0.5 μM) were added to the ACSF. All recordings were performed at a holding potential of –70 mV, sampled at 10 kHz and filtered at 2 kHz by a low-pass Bessel filter. Cells were included in analysis only if they had an access resistance of ≤ 20 MΩ and this resistance did not vary by more than 20% during the recording period.
Statistical Analyses
Statistical analyses were conducted using Graphpad Prism 5.0 and SPSS17 for Windows. The acquisition data for the Morris water maze and fear conditioning assay were analyzed using repeated-measures analysis of variance (ANOVA). In other cases, either a Student's t-test or a standard One-Way or Two-Way ANOVA followed by Bonferroni post-hoc test was used. All values are expressed as mean ± SEM, and p < 0.05 was considered statistically significant. Performance scores more than 2 standard deviations from the mean were excluded from the analysis.
Results
THIP Impairs Spatial Memory in the Morris Water Maze
The effect of THIP on spatial memory was first examined in the Morris water maze, as performance of this task is hippocampus-dependent and is regulated by GABAA receptor activity (D'Hooge and De Deyn, 2001; Collinson et al., 2002; Myhrer, 2003; Cheng et al., 2006). WT and Gabrd−/− mice were treated with THIP (4 mg/kg, i.p.) or vehicle 30 min before being trained over 4 trials to locate a hidden platform for memory acquisition. All mice learned to locate the platform, as evidenced by reduced escape latencies over sequential trials, and there were no baseline differences in acquisition between vehicle-treated WT and Gabrd−/− mice (Figure 1A; genotype × trial, p > 0.2, n = 16–19). Notably, THIP-treated WT mice showed impaired acquisition relative to vehicle-treated WT mice, as evidenced by slower escape latencies on the third and fourth trials (Figure 1A, left; genotype × drug × trial, p < 0.05). In contrast, THIP had no effect in Gabrd−/− mice (Figure 1A, right).
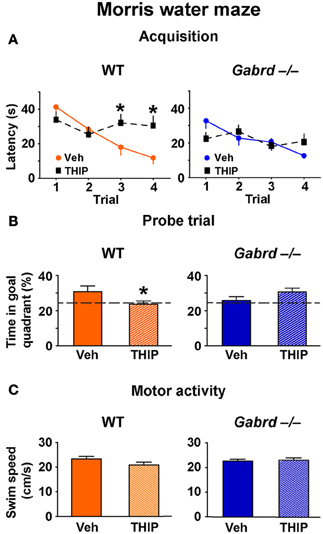
Figure 1. THIP impairs spatial memory in the Morris water maze. (A) THIP increased the escape latencies on the third and fourth trials in WT but not Gabrd−/− mice. (B) THIP decreased the preference for the goal quadrant formerly containing the escape platform in WT but not Gabrd−/− mice. (C) THIP did not affect swim speed in WT or Gabrd−/− mice. n = 16–19, *p < 0.05.
Next, to investigate whether THIP impairs long-term memory, recall of the platform location was tested in a probe trial that was performed 24 h after the acquisition trials. THIP-treated WT mice spent less time in the target quadrant that formerly contained the platform compared with vehicle-treated WT mice (Figure 1B, left; genotype × drug, p < 0.05, n = 14–17). In contrast, THIP- and vehicle-treated Gabrd−/− mice performed similarly (Figure 1B, right). THIP had no effect on motor activity in any of the groups, as swim speed was unchanged (Figure 1C; drug and drug × genotype, all p-values > 0.05). Collectively, these results indicate that THIP impaired spatial memory in WT but not Gabrd−/− mice.
THIP Impairs Contextual but not Auditory-Cued Fear Memory
To determine whether increased activity of δGABAA receptors regulates additional forms of hippocampus-dependent memory, the effect of THIP on aversive contextual fear conditioning (Phillips and LeDoux, 1992) was studied. Thirty minutes after injection, mice were trained to associate an electric footshock (an unconditioned stimulus) with a context and auditory cue (conditioned stimuli). A 1-day acquisition protocol that utilized three mild footshocks (designated S1, S2, and S3) was employed (Mihalek et al., 1999). During acquisition, all groups showed progressively increasing levels of freezing after each shock (Figure 2A), indicating they successfully acquired the task. There was no difference in acquisition between vehicle-treated WT and Gabrd−/− mice (genotype × shock, p > 0.5, n = 25–30), which is consistent with previous results obtained with this protocol (Mihalek et al., 1999). However, THIP-treated WT mice exhibited reduced freezing after the third shock compared with vehicle-treated controls (Figure 2A, left; genotype × drug × shock, p < 0.01, n = 25–30). In contrast, THIP had no effect on Gabrd−/− mice (Figure 2A, right). These results indicate that THIP impaired the acquisition of fear memory.
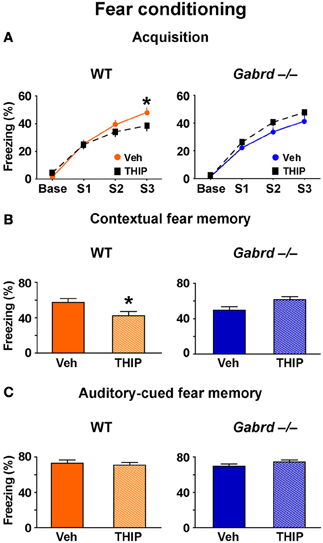
Figure 2. THIP impairs contextual but not auditory-cued fear memory. (A) THIP decreased the freezing score following the third tone-shock pairing in WT but not Gabrd−/− mice. (B) THIP reduced the freezing score for contextual fear memory in WT but not Gabrd−/− mice. (C) THIP did not affect the freezing score for auditory-cued fear memory in response to tone in both WT and Gabrd−/− mice. n = 25–30, *p < 0.05.
To measure contextual fear memory, mice were returned to the same training context 24 h after fear acquisition. THIP-treated WT mice showed reduced freezing scores relative to vehicle-treated controls, indicating reduced contextual fear memory (Figure 2A, left; genotype × drug, p < 0.01, n = 25–30). Gabrd−/− mice treated with THIP exhibited no memory deficits (Figure 2B, right). Next, the effects of THIP on auditory-cued fear memory, an amygdala-dependent task that does not normally require the hippocampus (Phillips and LeDoux, 1992), was examined. Interestingly, THIP did not impair auditory-cued fear memory in WT or Gabrd−/− mice (Figure 2C; p > 0.2 for main effects and interaction, n = 25–30). Collectively, these results show that THIP impairs contextual but not auditory-cued fear memory.
THIP Impairs Novel Object Recognition
To determine whether increased δGABAA receptor activity impairs short-term working memory, the NO recognition task was used. In this assay, mice must recognize a NO within a set of familiar objects to which they have been previously exposed (Figure 3A). Because animals are driven to investigate novelty, mice that recall the familiar objects will preferentially interact with the NO (Ennaceur and Meliani, 1992). NO recognition is a non-aversive task that depends primarily upon the perirhinal cortex (Winters et al., 2010) and is regulated by GABAA receptors (Zurek et al., 2012; Whissell et al., 2013).
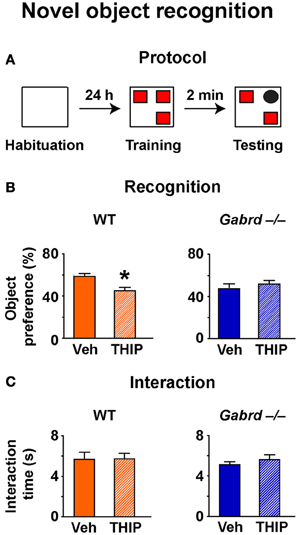
Figure 3. THIP impairs novel object recognition. (A) Schematic diagram showing the protocol. (B) THIP decreased the preference for the novel object in WT but not Gabrd−/− mice. (C) THIP had no effect on total interaction time in both WT and Gabrd−/− mice. n = 12–21, *p < 0.05.
NO preference was reduced in vehicle-treated Gabrd−/− mice relative to vehicle-treated WT mice (Figure 3B; WT + vehicle = 59.4 ± 2.2%, Gabrd−/− + vehicle = 47.6 + 3.2%; drug × genotype, p < 0.05, n = 12–21), a result that is consistent with our previous finding (Whissell et al., 2013). THIP-treated WT mice showed impaired NO recognition relative to vehicle-treated controls (Figure 3B, left; drug × genotype, p < 0.05, n = 12–21), whereas THIP had no effect in Gabrd−/− mice (Figure 3B, right). These results could not be attributed to an effect of either the genotype or THIP treatment on exploratory drive, as the total object interaction time was similar in all groups (Figure 3C).
THIP Depresses Long-Term Potentiation in the Dentate Gyrus
We next examined the effects of THIP on LTP in the DG of the hippocampus as δGABAA receptors are densely expressed in this region (Pirker et al., 2000). Before studying LTP, we confirmed that THIP (1 μM) increased a tonic δGABAA receptor–mediated conductance using whole-cell voltage-clamp recordings. Perfusion of THIP activated a significant inward current in granule cells from WT but not Gabrd−/− mice (Figure 4; p < 0.01, n = 6–7). The competitive GABAA receptor antagonist BIC (20 μM) completely blocked the effects of THIP. BIC also reduced the baseline holding current and revealed a tonic conductance generated by GABAA receptors. The tonic current was greater in WT neurons than Gabrd−/− neurons (Figure 4; p < 0.05, n = 6–7).
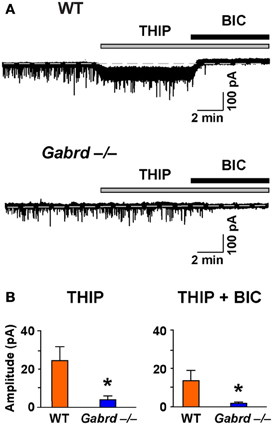
Figure 4. The effects of THIP, and the tonic inhibitory current revealed by BIC, in DG granule cells from WT and Gabrd−/− mice. (A) Representative recordings show the effects of THIP, and the tonic current revealed by BIC (20 μM). (B) Quantified data. n = 6–7, *p < 0.05.
The effect of THIP on LTP obtained in the stratum moleculare of the DG was examined in slices from WT and Gabrd−/− mice following tetanic stimulation of the medial perforant pathway. The slope of fPSPs after stimulation increased to 112.2 ± 6.7% (n = 10) and 110.0 ± 6.5% (n = 10) of baseline in WT and Gabrd−/− slices, respectively (Figure 5). There was no difference in the amplitude of LTP between genotypes (p > 0.4). Interestingly, THIP treatment completely blocked LTP in the DG in slices from WT mice (Figure 5A; WT + THIP = 91.5 ± 4.8%; genotype × drug, p < 0.05, n = 8–10) but not from Gabrd−/− mice (Figure 5B; Gabrd−/− + THIP = 109.2 ± 5.7%; genotype × drug, p > 0.05, n = 10–13). These results suggest that THIP depresses LTP in the DG by acting upon δGABAA receptors.
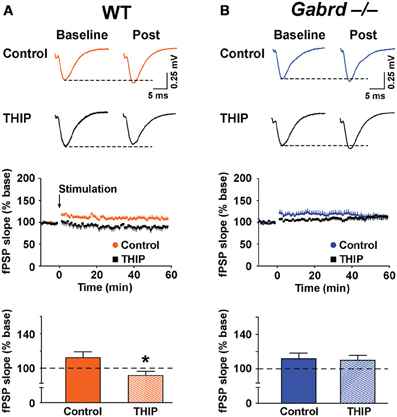
Figure 5. THIP inhibits long-term potentiation in the dentate gyrus. (A,B) THIP depressed LTP in the DG in slices from WT but not Gabrd−/− mice. Upper panels: Representative traces before and after tetanic stimulation. Middle panels: Normalized slope of fPSPs following tetanic stimulation. Bottom panels: Summarized data showing the last 5 min of recording. Note that THIP depressed LTP in DG only in WT mice. n = 8–13, *p < 0.05.
Extrasynaptic GABAA Receptors Mediate the Inhibitory Effect of THIP on LTP
To verify that GABAA receptors were involved in THIP effects on plasticity, LTP was studied in the presence of BIC (100 μM). Application of BIC alone increased LTP to 136.7 ± 8.4% and 142.3 ± 11.7% for WT and Gabrd−/− mice, respectively (Figure 6, n = 9–10). This marked increase in plasticity is consistent with results reported by others (Snyder et al., 2001). Co-application of THIP (1 μM) and BIC did not reduce LTP (Figure 6; WT + BIC + THIP = 135.9 ± 8.1%, Gabrd−/− + BIC + THIP = 144.6 ± 9.5%; drug and drug × genotype, p > 0.4, n = 9–10) suggesting THIP actions are mediated by GABAA receptors.
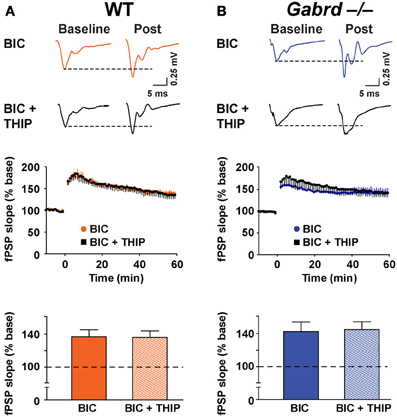
Figure 6. BIC occludes the inhibitory effects of THIP on long-term potentiation in the dentate gyrus. (A,B) THIP does not impair LTP in the DG in BIC-treated slices. BIC (100 μM) was perfused throughout the recordings. Upper panels: Representative traces before and after tetanic stimulation. Middle panels: Normalized slope of fPSPs following tetanic stimulation. Bottom panels: Summarized data showing the last 5 min of recording. Note that THIP did not depress LTP in the DG in both WT and Gabrd−/− mice. n = 9–10.
Next, LTP was studied in the presence of SR-95531 (1 μM), a compound that preferentially blocks synaptic GABAA receptors at low concentrations (Nusser and Mody, 2002). Application of SR-95531 alone did not significantly elevate LTP (WT = 112.2 ± 6.7% vs. WT + SR-95531 = 123.3 ± 5.2%, Gabrd−/− = 110.0 ± 6.5% vs. Gabrd−/− + SR-95531 = 125.7 ± 5.4%; p > 0.05, n = 10–12) (Figures 5, 7). THIP reduced LTP in SR-95531-treated slices from WT mice (Figure 7; WT + SR-95531 = 123.3 ± 5.2% and WT + SR-95531 + THIP = 107.8 ± 7.2%, drug effect, p < 0.05, n = 10–12), but not in slices from Gabrd−/− mice (Figure 7; Gabrd−/− + SR-95531 = 125.7 ± 5.4%, Gabrd−/− + SR-95531 + THIP = 124.4 ± 5.2%; drug effect, p > 0.05, n = 10–12). These results indicate that the inhibitory effects of THIP on LTP are mediated by extrasynaptic rather than synaptic GABAA receptors.
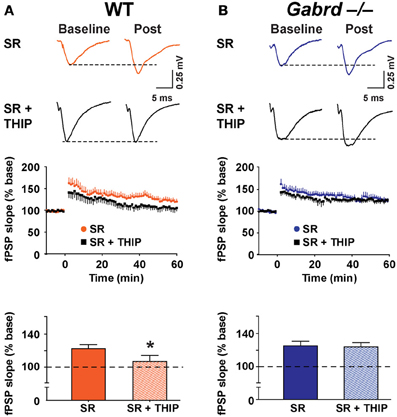
Figure 7. SR-95531 does not prevent THIP-mediated depression of long-term potentiation in the dentate gyrus. (A,B) THIP impairs LTP in the DG of SR-95531-treated slices from WT but not Gabrd−/− mice. SR-95531 (1 μM) was perfused throughout the recordings. Upper panels: Representative traces before and after tetanic stimulation. Middle panels: Normalized slope of fPSPs following tetanic stimulation. Bottom panels: Summarized data showing the last 5 min of recording. n = 10–12. *p < 0.05.
δGABAA Receptor Activity does not Alter Baseline Synaptic Transmission or Presynaptic Function in the Dentate Gyrus
We then examined whether δGABAA receptors modify baseline synaptic transmission in the DG by studying the input–output relationships for field potentials recorded in WT and Gabrd−/− slices. To generate an input–output plot, the stimulus intensity was increased incrementally to generate fPSPs of increasing strength. The amplitude of the presynaptic fiber volley vs. the slope of each fPSP was graphed as a scatter plot. The presynaptic fiber volley and the slope of each fPSP are indicative of presynaptic input (fiber activation) and postsynaptic output, respectively. A “best-fit line” representing the input–output relationship was then computed using linear regression (Figure 8A). There was no difference in the slope of the input–output relationship in relation to either genotype. Similarly, treating the slices with THIP did not alter the input-output relationship (all p-values > 0.05).
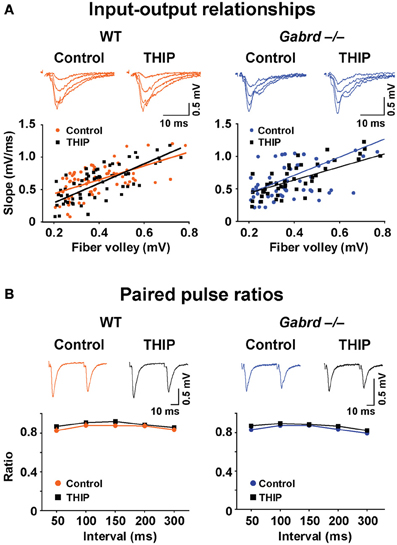
Figure 8. THIP has no effects on baseline synaptic transmission or presynaptic function in slices from both WT and Gabrd−/− mice. (A) Representative traces show fPSPs with increasing stimulus intensities. The input-output relationships beneath the traces, indicators of baseline synaptic transmission, were similar between genotypes, and between control and THIP treatment groups in either genotype. n = 9–10. (B) Sample traces show paired-pulse depression. Paired pulse ratios, indicators of presynaptic function, were also similar between genotypes, and between control and THIP treatment groups in either genotype. n = 9–10.
We next investigated the effects of THIP on the ratio of paired pulses in DG, which represents a presynaptic form of short-term plasticity. To generate paired pulses, two fPSPs were elicited by applying two stimuli to the medial perforant pathway at varying time intervals ranging from 50 to 300 ms. The ratio of the resulting responses was then computed (response 2/response 1). As reported previously (Christie and Abraham, 1994), paired pulse depression (ratio < 1) was observed in the DG with stimulation of the medial perforant pathway (Figure 8B). There was no difference in paired pulse ratios in relation to either genotype or THIP treatment (p > 0.05).
THIP Depresses Long-Term Potentiation in the CA1 Region
Finally, to determine if THIP depressed plasticity in other regions of the hippocampus, LTP was studied in the CA1 subfield. δGABAA receptors are expressed in this region (Pirker et al., 2000) but generate a lower magnitude current under baseline conditions when compared with the DG (Glykys et al., 2008). fPSPs were recorded in the stratum radiatum of the CA1 subfield before and after tetanic stimulation of the Schaffer collateral pathway. Stimulation increased the slope of fPSPs to 137.3 ± 10.3% (n = 8) and 135.4 ± 5.1% (n = 8) of baseline in WT and Gabrd−/− mice, respectively (Figure 9). There were no differences in LTP in CA1 between genotypes (p > 0.1). THIP treatment attenuated LTP in slices from WT mice (Figure 9A; WT + THIP = 109.6 ± 7.9%; genotype × drug, p < 0.05, n = 8–9) but not from Gabrd−/− mice (Figure 9B; Gabrd−/− + THIP = 138.7 ± 5.0%; genotype × drug, p > 0.05, n = 8–9).
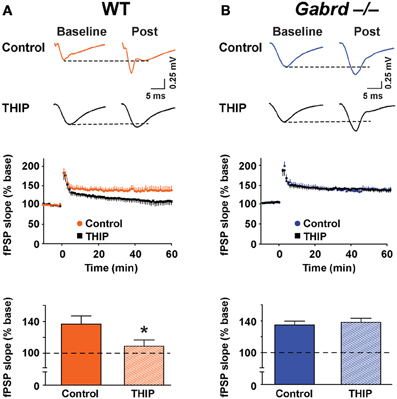
Figure 9. THIP inhibits long-term potentiation in the CA1 region. (A,B) THIP depressed LTP in CA1 in slices from WT but not Gabrd−/− mice. Upper panels: Representative traces before and after tetanic stimulation. Middle panels: Normalized slope of fPSPs following tetanic stimulation. Bottom panels: Summarized data showing the last 5 min of recording. Note that THIP depressed LTP in CA1 only in WT mice. n = 8–9, *p < 0.05.
Discussion
The above results show that THIP impaired multiple forms of memory in WT but not Gabrd−/− mice. THIP also depressed LTP, but only in slices from WT mice. Collectively, these results show that the neurodepressive effects of THIP were mediated by δGABAA receptors.
THIP Impairs Memory
THIP selectively impaired hippocampus-dependent memory, as evidenced by deficits in the Morris water maze and contextual fear conditioning tasks. In contrast, auditory-cued fear conditioning, a behavioral task which primarily depends upon the amygdala rather than the hippocampus (Phillips and LeDoux, 1992), was unaffected. The vulnerability of hippocampus-dependent memory to THIP may be attributed to high expression levels of δGABAA receptors in the hippocampus, relative to other brain regions involved in memory, such as the amygdala (Pirker et al., 2000).
THIP-mediated impairment of long-term memory was likely due to impaired memory acquisition. These effects of THIP are consistent with previous results as neuroactive steroids that act as positive allosteric modulators of the δGABAA receptor (Belelli and Lambert, 2005) reduce memory acquisition. Specifically, allopregnanolone impairs acquisition in the shock avoidance assay (Shen et al., 2010), while tetrahydroprogesterone impairs acquisition in the Y-maze recognition task (Mayo et al., 1993). Also, muscimol and other non-selective agonists of the GABAA receptor that increase δGABAA receptor activity impair acquisition in multiple memory tasks (Myhrer, 2003; Makkar et al., 2010). Whether THIP also impairs the consolidation or retrieval of memory is a topic for future study.
Interestingly, NO recognition was also impaired by THIP. This behavior is primarily regulated by the perirhinal cortex (Winters et al., 2010). δGABAA receptors expressed in this area (Pirker et al., 2000) and in other cortical regions (Drasbek and Jensen, 2006) may be involved in the effects of THIP. Alternatively, δGABAA receptors in the hippocampus may substantially contribute to the THIP effects. The hippocampus can modify recognition memory when a novel and/or complex testing environment is used (Oliveira et al., 2010; Sannino et al., 2012) or when the interval between the training and testing phases is short (Rose et al., 2012). The current experiments utilized a complex testing environment that included multiple visual cues and three objects. Further, there was a relatively short interval between the training and testing periods (2 min). Such testing conditions may facilitate the involvement of hippocampal δGABAA receptors in THIP impairment of NO recognition.
Baseline Memory is not Enhanced in GABRD−/− Mice
Gabrd−/− mice did not differ from WT mice in baseline contextual fear conditioning and Morris water maze performance. These data are consistent with previous results that showed no enhanced memory in male Gabrd−/− mice (Mihalek et al., 1999; Wiltgen et al., 2005). Interestingly, the unchanged memory performance of Gabrd−/− mice contrasts with the generally enhanced memory seen in other GABAA receptor subunit knockout mice. Notably, transgenic mice lacking the α4 subunit (Moore et al., 2010; Cushman et al., 2011) or α5 subunit (Collinson et al., 2002; Martin et al., 2010) exhibit enhanced memory performance, particularly in the contextual fear conditioning and Morris water maze tasks. While there was no evidence of enhanced memory in Gabrd−/− mice, the impairment in NO recognition is consistent with our previous report (Whissell et al., 2013).
Several potential explanations account for the lack of memory enhancement in Gabrd−/− mice. The deletion of the δGABAA receptor impedes neurogenesis in the DG (Whissell et al., 2013), a process that contributes to memory performance (Marin-Burgin and Schinder, 2012). Disruption of neurogenesis is associated with impaired memory performance in the Morris water maze, contextual fear conditioning and NO recognition tasks (Snyder et al., 2005; Saxe et al., 2006; Jessberger et al., 2009). Thus, reduced neurogenesis may counteract the potential enhancement of memory caused by reduction of tonic inhibition in Gabrd−/− mice. Alternatively, deletion of the δ subunit may induce a compensatory change in the expression or function of other ion channels which regulate memory, such as potassium channels (Brickley et al., 2001) or α5GABAA receptors (Glykys et al., 2008).
THIP Impairs Long-Term Potentiation in the Hippocampus
To identify the neurophysiological substrate of THIP-induced memory deficits, LTP was measured in the DG and CA1. LTP in the DG (~110% of baseline) was roughly one third the magnitude of LTP in the CA1 (~130% of baseline). The low LTP in the DG has been attributed to strong GABAA receptor-mediated inhibition (Wigstrom and Gustafsson, 1983; Snyder et al., 2001; Arima-Yoshida et al., 2011). Consistent with this postulate, BIC enhanced LTP in the DG nearly 4-fold (from ~110 to ~140% of baseline). In contrast, only subtle and variable effects of BIC on LTP were reported in the CA1 region; BIC either enhanced LTP 2-fold (Arima-Yoshida et al., 2011) or had no significant effect (Chen et al., 2011).
Interestingly, we observed no baseline differences in LTP in either the DG or CA1 between WT and Gabrd−/− slices. The lack of enhanced LTP in the DG of Gabrd−/− slices might be explained by the disruption of neurogenesis (Whissell et al., 2013). Neurogenesis facilitates baseline LTP in the DG (Snyder et al., 2001) likely because adult-born neurons show greater plasticity than older or developmentally-generated neurons (Ming and Song, 2011). We also observed no increase in LTP in the CA1 in Gabrd−/− mice, possibly due to the relatively low expression of δGABAA receptors in this region (Pirker et al., 2000). Additionally, as discussed above, compensatory changes in the expression of other receptors that constrain plasticity, such as α5GABAA receptors, might be contributing factors (Glykys et al., 2008; Martin et al., 2010).
THIP depressed LTP in the DG and CA1 in slices from WT mice but not Gabrd−/− mice, which is consistent with impaired memory in THIP-treated WT mice. Others showed that LTP in the DG is impaired by increasing tonic inhibition with low concentrations GABA (Arima-Yoshida et al., 2011). THIP also significantly attenuated LTP in the CA1 region, a result that was somewhat surprising given the relatively low expression and baseline activity of δGABAA receptors in this area (Pirker et al., 2000; Glykys et al., 2008). δGABAA receptors in the CA1 may play a more important role in memory processes than initially thought, particularly when these receptors are highly activated by drugs. Alternatively, impairment of LTP in the CA1 might be due to activation of other, non-δGABAA receptors. Low concentrations of THIP within the range employed in this study (~2 μM) also activate extrasynaptic α5GABAA receptors (Ebert et al., 1997; Lindquist et al., 2003), which are present in the CA1 subfield and constrain LTP (Martin et al., 2010).
Possible mechanisms for THIP-mediated depression of LTP include membrane hyperpolarization and shunting inhibition (Andersen et al., 1980; Staley and Mody, 1992). THIP-mediated membrane hyperpolarization would be expected to impair LTP via inhibition of channels critical for LTP, such as N-methyl-D-aspartate receptors (Morris et al., 1986). Alternatively, THIP may impair LTP via shunting inhibition. The opening of δGABAA receptor channels by THIP would decrease the neuronal input resistance and attenuate the membrane depolarization elicited by excitatory neurotransmitters, which would also impair LTP.
In the current study, THIP did not affect the input/output relationship, a correlate of neuronal excitability. This result contrasts with the finding that increases in δGABAA receptor activity with neurosteroids shift the input-output relationship to the right (Stell et al., 2003). Methodological factors may account for this discrepancy. In this study, stimulus intensity was incrementally increased to generate output fPSPs (Martin et al., 2010). In the previous report (Stell et al., 2003), stimulus intensity was kept constant but stimulus duration (i.e., the pulse width) was incrementally increased. These two inputs (stimulus intensity vs. pulse width) may not produce similar results. In addition, different compounds with distinct mechanisms of action were employed in the two studies. THIP is a “super”-agonist of the δGABAA receptor (Brown et al., 2002), whereas neurosteroids are positive allosteric modulators of the δGABAA receptor (Belelli and Lambert, 2005).
Potential Therapeutic Implications
Our current and previous findings (Whissell et al., 2013) show that THIP has two distinct effects on memory. A single acute treatment with THIP reduces memory performance, possibly due to increased tonic inhibitory conductance and reduced synaptic plasticity in the hippocampus. In contrast, long-term pre-treatment with THIP enhanced memory performance and neurogenesis weeks after THIP had been eliminated. THIP, administered as a single injection in the current study, was unlikely to influence neurogenesis, a process that occurs over a time period of many weeks (Zhao et al., 2008).
The acute memory-blocking properties of THIP may be desirable in several clinical contexts. For example, THIP could be used as an adjunct to facilitate the induction of general anesthesia or to prevent inadvertent recall of traumatic events during surgery (Mashour et al., 2011). Under other conditions, THIP-induced memory loss could be highly undesirable, such as during the performance of demanding memory tasks (e.g., studying) or during spatial navigation (e.g., driving) (Leufkens et al., 2009). Any long-term beneficial effects of THIP must be carefully weighed against acute effects that reduce memory performance. Future studies are required to determine an optimum dose and drug protocol that maximizes the therapeutic effects of THIP but minimizes undesired memory loss. It is also of interest to determine whether other off-target effects of THIP, such as ataxia (Bonin et al., 2011) or driving impairment (Leufkens et al., 2009), result from increased δGABAA receptor activity.
Finally, the present study demonstrates significant memory-blocking properties of THIP in healthy adult male WT mice. The sensitivity to THIP may vary with age, gender, physiologic state or other factors. Notably, δGABAA receptor expression is significantly increased during puberty (Shen et al., 2010), certain stages of the ovarian cycle (Maguire et al., 2005), stress (Sanna et al., 2011) and following traumatic brain injury (Kharlamov et al., 2011). Thus, the memory-blocking effects of THIP may be greatly enhanced in certain clinical populations, which is an additional consideration in the therapeutic use of this drug.
Author Contributions
Paul D. Whissell, Dave Eng, Loren J. Martin, and Beverley A. Orser designed the studies; Paul D. Whissell, Dave Eng, and Irene Lecker performed the experiments; Paul D. Whissell analyzed the data; Paul D. Whissell, Dian-Shi Wang, Irene Lecker, and Beverley A. Orser wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by grants from the Canadian Institutes of Health Research to Beverley A. Orser (MOP: 79428, 38028) and Loren J. Martin and grants from the Natural Sciences and Engineering Research Council of Canada to Paul D. Whissell and Dave Eng. Irene Lecker was supported by a Savoy Foundation Studentship. Beverley A. Orser holds a Canada Research Chair. The authors would like to thank Ella Czerwinska and Agnieszka A. Zurek for technical assistance.
References
Andersen, P., Dingledine, R., Gjerstad, L., Langmoen, I. A., and Laursen, A. M. (1980). Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J. Physiol. 305, 279–296.
Arima-Yoshida, F., Watabe, A. M., and Manabe, T. (2011). The mechanisms of the strong inhibitory modulation of long-term potentiation in the rat dentate gyrus. Eur. J. Neurosci. 33, 1637–1646. doi: 10.1111/j.1460-9568.2011.07657.x
Bai, D., Zhu, G., Pennefather, P., Jackson, M. F., MacDonald, J. F., and Orser, B. A. (2001). Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 59, 814–824.
Belelli, D., and Lambert, J. J. (2005). Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 6, 565–575. doi: 10.1038/nrn1703
Bonin, R. P., Labrakakis, C., Eng, D. G., Whissell, P. D., Koninck, Y. D., and Orser, B. A. (2011). Pharmacological enhancement of δ-subunit-containing GABAA receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain 152, 1317–1326. doi: 10.1016/j.pain.2011.02.011
Boyle, J., Wolford, D., Gargano, C., McCrea, J., Cummings, C., Cerchio, K., et al. (2009). Next-day residual effects of gaboxadol and flurazepam administered at bedtime: a randomized double-blind study in healthy elderly subjects. Hum. Psychopharmacol. 24, 61–71. doi: 10.1002/hup.986
Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W., and Farrant, M. (2001). Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92. doi: 10.1038/35051086
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J., and Wafford, K. A. (2002). Pharmacological characterization of a novel cell line expressing human α4β3δGABAA receptors. Br. J. Pharmacol. 136, 965–974. doi: 10.1038/sj.bjp.0704795
Chen, X., Whissell, P., Orser, B. A., and MacDonald, J. F. (2011). Functional modifications of acid-sensing ion channels by ligand-gated chloride channels. PLoS ONE 6: e21970. doi: 10.1371/journal.pone.0021970
Cheng, V. Y., Martin, L. J., Elliott, E. M., Kim, J. H., Mount, H. T., Taverna, F. A., et al. (2006). Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J. Neurosci. 26, 3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006
Christensen, T., Betry, C., Mnie-Filali, O., Etievant, A., Ebert, B., Haddjeri, N., et al. (2012). Synergistic antidepressant-like action of gaboxadol and escitalopram. Eur. Neuropsychopharmacol. 22, 751–760. doi: 10.1016/j.euroneuro.2012.02.001
Christie, B. R., and Abraham, W. C. (1994). Differential regulation of paired-pulse plasticity following LTP in the dentate gyrus. Neuroreport 5, 385–388. doi: 10.1097/00001756-199401120-00003
Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., et al. (2002). Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J. Neurosci. 22, 5572–5580.
Cremers, T., and Ebert, B. (2007). Plasma and CNS concentrations of Gaboxadol in rats following subcutaneous administration. Eur. J. Pharmacol. 562, 47–52. doi: 10.1016/j.ejphar.2007.01.017
Cushman, J. D., Moore, M. D., Jacobs, N. S., Olsen, R. W., and Fanselow, M. S. (2011). Behavioral pharmacogenetic analysis on the role of the alpha4 GABA(A) receptor subunit in the ethanol-mediated impairment of hippocampus-dependent contextual learning. Alcohol. Clin. Exp. Res. 35, 1948–1959. doi: 10.1111/j.1530-0277.2011.01546.x
D'Hooge, R., and De Deyn, P. P. (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 36, 60–90. doi: 10.1016/S0165-0173(01)00067-4
Drasbek, K. R., and Jensen, K. (2006). THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb. Cortex 16, 1134–1141. doi: 10.1093/cercor/bhj055
Ebert, B., Anderson, N. J., Cremers, T. I., Rasmussen, S., Vogel, V., Fahey, J. M., et al. (2008). Gaboxadol – a different hypnotic profile with no tolerance to sleep EEG and sedative effects after repeated daily dosing. Pharmacol. Biochem. Behav. 90, 113–122. doi: 10.1016/j.pbb.2008.01.021
Ebert, B., Thompson, S. A., Saounatsou, K., McKernan, R., Krogsgaard-Larsen, P., and Wafford, K. A. (1997). Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human gamma-aminobutyric acid type A receptors. Mol. Pharmacol. 52, 1150–1156.
Ennaceur, A., and Meliani, K. (1992). A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav. Brain Res. 51, 83–92. doi: 10.1016/S0166-4328(05)80315-8
Farrant, M., and Nusser, Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229. doi: 10.1038/nrn1625
Faulhaber, J., Steiger, A., and Lancel, M. (1997). The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl.). 130, 285–291. doi: 10.1007/s002130050241
Glykys, J., Mann, E. O., and Mody, I. (2008). Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 28, 1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008
Jessberger, S., Clark, R. E., Broadbent, N. J., Clemenson, G. D. Jr., Consiglio, A., Lie, D. C., et al. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154. doi: 10.1101/lm.1172609
Kharlamov, E. A., Lepsveridze, E., Meparishvili, M., Solomonia, R. O., Lu, B., Miller, E. R., et al. (2011). Alterations of GABAA and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 95, 20–34. doi: 10.1016/j.eplepsyres.2011.02.008
Laurie, D. J., Wisden, W., and Seeburg, P. H. (1992). The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 12, 4151–4172.
Leufkens, T. R., Lund, J. S., and Vermeeren, A. (2009). Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J. Sleep Res. 18, 387–396. doi: 10.1111/j.1365-2869.2009.00746.x
Lindquist, C. E., Ebert, B., and Birnir, B. (2003). Extrasynaptic GABA(A) channels activated by THIP are modulated by diazepam in CA1 pyramidal neurons in the rat brain hippocampal slice. Mol. Cell. Neurosci. 24, 250–257. doi: 10.1016/S1044-7431(03)00128-3
Madsen, S. M., Lindeburg, T., Folsgard, S., Jacobsen, E., and Sillesen, H. (1983). Pharmacokinetics of the gamma-aminobutyric acid agonist THIP (Gaboxadol) following intramuscular administration to man, with observations in dog. Acta Pharmacol. Toxicol. (Copenh). 53, 353–357. doi: 10.1111/j.1600-0773.1983.tb03434.x
Maguire, J., Ferando, I., Simonsen, C., and Mody, I. (2009). Excitability changes related to GABAA receptor plasticity during pregnancy. J. Neurosci. 29, 9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009
Maguire, J., and Mody, I. (2008). GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213. doi: 10.1016/j.neuron.2008.06.019
Maguire, J. L., Stell, B. M., Rafizadeh, M., and Mody, I. (2005). Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804. doi: 10.1038/nn1469
Makkar, S. R., Zhang, S. Q., and Cranney, J. (2010). Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35, 1625–1652. doi: 10.1038/npp.2010.53
Marin-Burgin, A., and Schinder, A. F. (2012). Requirement of adult-born neurons for hippocampus-dependent learning. Behav. Brain Res. 227, 391–399. doi: 10.1016/j.bbr.2011.07.001
Martin, L. J., Oh, G. H., and Orser, B. A. (2009). Etomidate targets alpha5 gamma-aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 111, 1025–1035. doi: 10.1097/ALN.0b013e3181bbc961
Martin, L. J., Zurek, A. A., MacDonald, J. F., Roder, J. C., Jackson, M. F., and Orser, B. A. (2010). α5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J. Neurosci. 30, 5269–5282. doi: 10.1523/JNEUROSCI.4209-09.2010
Mashour, G. A., Orser, B. A., and Avidan, M. S. (2011). Intraoperative awareness: from neurobiology to clinical practice. Anesthesiology 114, 1218–1233. doi: 10.1097/ALN.0b013e31820fc9b6
Mathias, S., Zihl, J., Steiger, A., and Lancel, M. (2005). Effect of repeated gaboxadol administration on night sleep and next-day performance in healthy elderly subjects. Neuropsychopharmacology 30, 833–841. doi: 10.1038/sj.npp.1300641
Mayo, W., Dellu, F., Robel, P., Cherkaoui, J., Le Moal, M., Baulieu, E. E., et al. (1993). Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Res. 607, 324–328. doi: 10.1016/0006-8993(93)91524-V
Meera, P., Wallner, M., and Otis, T. S. (2011). Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J. Neurophysiol. 106, 2057–2064. doi: 10.1152/jn.00450.2011
Mihalek, R. M., Banerjee, P. K., Korpi, E. R., Quinlan, J. J., Firestone, L. L., Mi, Z. P., et al. (1999). Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 12905–12910. doi: 10.1073/pnas.96.22.12905
Ming, G. L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. doi: 10.1016/j.neuron.2011.05.001
Moore, M. D., Cushman, J., Chandra, D., Homanics, G. E., Olsen, R. W., and Fanselow, M. S. (2010). Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA(A) receptor. Neurobiol. Learn. Mem. 93, 383–387. doi: 10.1016/j.nlm.2009.12.004
Morris, R. G., Anderson, E., Lynch, G. S., and Baudry, M. (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776. doi: 10.1038/319774a0
Moyer, J. R. Jr., and Brown, T. H. (1998). Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J. Neurosci. Methods 86, 35–54. doi: 10.1016/S0165-0270(98)00143-5
Myhrer, T. (2003). Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res. Brain Res. Rev. 41, 268–287. doi: 10.1016/S0165-0173(02)00268-0
Nusser, Z., and Mody, I. (2002). Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 87, 2624–2628.
Oliveira, A. M., Hawk, J. D., Abel, T., and Havekes, R. (2010). Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 17, 155–160. doi: 10.1101/lm.1625310
Phillips, R. G., and LeDoux, J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285. doi: 10.1037/0735-7044.106.2.274
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W., and Sperk, G. (2000). GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. doi: 10.1016/S0306-4522(00)00442-5
Rose, N. S., Olsen, R. K., Craik, F. I., and Rosenbaum, R. S. (2012). Working memory and amnesia: the role of stimulus novelty. Neuropsychologia 50, 11–18. doi: 10.1016/j.neuropsychologia.2011.10.016
Saab, B. J., Georgiou, J., Nath, A., Lee, F. J., Wang, M., Michalon, A., et al. (2009). NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron 63, 643–656. doi: 10.1016/j.neuron.2009.08.014
Sanna, E., Talani, G., Obili, N., Mascia, M. P., Mostallino, M. C., Secci, P. P., et al. (2011). Voluntary ethanol consumption induced by social isolation reverses the increase of alpha(4)/delta GABA(A) receptor gene expression and function in the hippocampus of C57BL/6J mice. Front Neurosci. 5:15. doi: 10.3389/fnins.2011.00015
Sannino, S., Russo, F., Torromino, G., Pendolino, V., Calabresi, P., and De Leonibus, E. (2012). Role of the dorsal hippocampus in object memory load. Learn. Mem. 19, 211–218. doi: 10.1101/lm.025213.111
Sarkar, J., Wakefield, S., MacKenzie, G., Moss, S. J., and Maguire, J. (2011). Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 31, 18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011
Saxe, M. D., Battaglia, F., Wang, J. W., Malleret, G., David, D. J., Monckton, J. E., et al. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 103, 17501–17506. doi: 10.1073/pnas.0607207103
Schultz, B., Aaes-Jorgensen, T., Bogeso, K. P., and Jorgensen, A. (1981). Preliminary studies on the absorption, distribution, metabolism, and excretion of THIP in animal and man using 14C-labelled compound. Acta Pharmacol. Toxicol. (Copenh). 49, 116–124. doi: 10.1111/j.1600-0773.1981.tb00879.x
Shen, H., Gong, Q. H., Aoki, C., Yuan, M., Ruderman, Y., Dattilo, M., et al. (2007). Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 10, 469–477. doi: 10.1038/nn1868
Shen, H., Sabaliauskas, N., Sherpa, A., Fenton, A. A., Stelzer, A., Aoki, C., et al. (2010). A critical role for α4βδGABAA receptors in shaping learning deficits at puberty in mice. Science 327, 1515–1518. doi: 10.1126/science.1184245
Shen, Y., Lindemeyer, A. K., Spigelman, I., Sieghart, W., Olsen, R. W., and Liang, J. (2011). Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol. Pharmacol. 79, 432–442. doi: 10.1124/mol.110.068650
Snyder, J. S., Hong, N. S., McDonald, R. J., and Wojtowicz, J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852. doi: 10.1016/j.neuroscience.2004.10.009
Snyder, J. S., Kee, N., and Wojtowicz, J. M. (2001). Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 85, 2423–2431.
Staley, K. J., and Mody, I. (1992). Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 68, 197–212.
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., and Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 100, 14439–14444. doi: 10.1073/pnas.2435457100
Tan, K. R., Rudolph, U., and Luscher, C. (2011). Hooked on benzodiazepines: GABA(A) receptor subtypes and addiction. Trends Neurosci. 34, 188–197. doi: 10.1016/j.tins.2011.01.004
Wafford, K. A., and Ebert, B. (2006). Gaboxadol–a new awakening in sleep. Curr. Opin. Pharmacol. 6, 30–36. doi: 10.1016/j.coph.2005.10.004
Wang, D. S., Zurek, A. A., Lecker, I., Yu, J., Abramian, A. M., Avramescu, S., et al. (2012). Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABA(A) receptors. Cell Rep. 2, 488–496. doi: 10.1016/j.celrep.2012.08.022
Wang, J. M., Irwin, R. W., Liu, L., Chen, S., and Brinton, R. D. (2007). Regeneration in a degenerating brain: potential of allopregnanolone as a neuroregenerative agent. Curr. Alzheimer Res. 4, 510–517. doi: 10.2174/156720507783018262
Whissell, P. D., Rosenzweig, S., Lecker, I., Wang, D. S., Wojtowicz, J. M., and Orser, B. A. (2013). δGABA receptors promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. doi: 10.1002/ana.23941. [Epub ahead of print].
Wigstrom, H., and Gustafsson, B. (1983). Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 275, 153–158. doi: 10.1016/0006-8993(83)90428-6
Wigstrom, H., and Gustafsson, B. (1985). Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol. Scand. 125, 159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x
Wiltgen, B. J., Sanders, M. J., Ferguson, C., Homanics, G. E., and Fanselow, M. S. (2005). Trace fear conditioning is enhanced in mice lacking the δ subunit of the GABAA receptor. Learn. Mem. 12, 327–333. doi: 10.1101/lm.89705
Winters, B. D., Saksida, L. M., and Bussey, T. J. (2010). Implications of animal object memory research for human amnesia. Neuropsychologia 48, 2251–2261. doi: 10.1016/j.neuropsychologia.2010.01.023
Zhao, C., Deng, W., and Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. doi: 10.1016/j.cell.2008.01.033
Keywords: extrasynaptic GABAA receptors, δ subunit, tonic inhibition, THIP, memory, long-term potentiation, dentate gyrus, CA1
Citation: Whissell PD, Eng D, Lecker I, Martin LJ, Wang D-S and Orser BA (2013) Acutely increasing δGABAA receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Front. Neural Circuits 7:146. doi: 10.3389/fncir.2013.00146
Received: 12 June 2013; Accepted: 29 August 2013;
Published online: 17 September 2013.
Edited by:
Matthew Walker, University College London, UKReviewed by:
Bryndis Birnir, Uppsala University, SwedenSheryl S. Smith, SUNY Downstate Medical Center, USA
Copyright © 2013 Whissell, Eng, Lecker, Martin, Wang and Orser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beverley A. Orser, Department of Physiology, University of Toronto, 1 King's College Circle, Medical Sciences Building,Room 3318, Toronto, ON M5S 1A8, Canada e-mail: beverley.orser@utoronto.ca