Deletion of P2X2 and P2X3 receptor subunits does not alter motility of the mouse colon
- 1 Department of Pharmacology and Toxicology, Michigan State University, East Lansing, MI, USA
- 2 The Neuroscience Program, Michigan State University, East Lansing, MI, USA
Purinergic P2X receptors contribute to neurotransmission in the gut. P2X receptors are ligand-gated cation channels that mediate synaptic excitation in subsets of enteric neurons. The present study evaluated colonic motility in vitro and in vivo in wild type (WT) and P2X2 and P2X3 subunit knockout (KO) mice. The muscarinic receptor agonist, bethanechol (0.3–3 μM), caused similar contractions of the longitudinal muscle in colon segments from WT, P2X2 and P2X3 subunit KO mice. Nicotine (1–300 μM), acting at neuronal nicotinic receptors, caused similar longitudinal muscle relaxations in colonic segments from WT and P2X2 and P2X3 subunit KO mice. Nicotine-induced relaxations were inhibited by nitro-L-arginine (NLA, 100 μM) and apamin (0.1 μM) which block inhibitory neuromuscular transmission. ATP (1–1000 μM) caused contractions only in the presence of NLA and apamin. ATP-induced contractions were similar in colon segments from WT, P2X2 and P2X3 KO mice. The mouse colon generates spontaneous migrating motor complexes (MMCs) in vitro. The MMC frequency was higher in P2X2 KO compared to WT tissues; other parameters of the MMC were similar in colon segments from WT, P2X2 and P2X3 KO mice. 5-Hydroxytryptophan-induced fecal output was similar in WT, P2X2 and P2X3 KO mice. These data indicate that nicotinic receptors are located predominately on inhibitory motor neurons supplying the longitudinal muscle in the mouse colon. P2X2 or P2X3 subunit containing receptors are not localized to motor neurons supplying the longitudinal muscle. Synaptic transmission mediated by P2X2 or P2X3 subunit containing receptors is not required for propulsive motility in the mouse colon.
Introduction
ATP is an excitatory and inhibitory neurotransmitter in the gastrointestinal tract (LePard et al., 1997; LePard and Galligan, 1999; Galligan et al., 2000; Nurgali et al., 2003; Serio et al., 2003). ATP relaxes gut smooth muscle via activation of metabotropic P2Y receptors coupled to small conductance Ca2+-gated K+ channels (Koh et al., 1997; Giaroni et al., 2002). P2X ionotropic receptors contribute to fast synaptic excitation in the small intestine and colon (Galligan and Bertrand, 1994; LePard et al., 1997; LePard and Galligan, 1999; Giaroni et al., 2002; Bian et al., 2003; Nurgali et al., 2003).
P2X receptors are multimeric proteins composed of three subunits that form a ligand-gated cation channel. There are seven P2X receptor subunits (P2X1–P2X7). Functional P2X receptors are monomeric or heteromeric trimers and the functional and pharmacological properties of the receptors are determined by the specific subunit composition (Surprenant and North, 2009). Receptors containing P2X2 and P2X3 subunits have been localized to enteric neurons in the small intestine and colon where they contribute to fast synaptic excitation (Castelucci et al., 2002; Poole et al., 2002; Van Nassauw et al., 2002; Nurgali et al., 2003; Monro et al., 2004). P2X subunits have also been localized to colonic circular and longitudinal smooth muscle cells from mouse (Giaroni et al., 2002) and dog (Lee et al., 2005). Studies of P2X receptors in the mouse colon would be important because the mouse colon generates spontaneous migrating contractions, the migrating motor complex (MMC), in vitro and this facilitates pharmacological studies of the neural circuitry controlling this complex motor pattern. The colonic MMC is a cyclic motor pattern that propagates the length of a colonic segment every 1–3 min in vitro. The propagating contractions are associated with phasic depolarizations of circular smooth muscle cells. The depolarizations and contractions are caused by acetylcholine acting at muscarinic receptors (Spencer et al., 2005). The MMC is inhibited by nicotinic receptor and 5-HT3 receptor antagonists indicating that cholinergic and serotonergic synaptic transmission in myenteric ganglia contribute to initiation and propagation of the colonic MMC (Fida et al., 1997; Bush et al., 2001). The contribution of P2X receptor-mediated synaptic transmission to the colonic MMC has not been investigated.
While some P2X receptor subtypes can be discriminated using somewhat selective agonists and antagonists, most drugs have affinity for more than one P2X receptor subtype (Jarvis and Khakh, 2009; Surprenant and North, 2009). The unavailability of drugs that are highly selective for P2X receptor subtypes complicates interpretation of pharmacological studies designed to determine the P2X receptor subtype mediating a particular response. In the present study, we used P2X2 and P2X3 gene knockout (KO) mice (Cockayne et al., 2000; Bian et al., 2003) and wild type (WT) control mice to investigate the contributions of receptors containing these subunits to neurotransmission controlling of colonic motility in vitro and in vivo.
Materials and Methods
Animals
All protocols for the use of animals were approved by the Institutional Animal Use and Care Committee on Animal Use at Michigan State University. P2X2 and P2X3 subunit KO mice were derived as described previously (Cockayne et al., 2000). The mice used in this study were on a C57BL/6 background and therefore WT C57BL/6 mice (Harlan Laboratories1) were used as controls.
Tissues used for in vitro isometric longitudinal muscle contraction and colonic MMC experiments were harvested from animals that were first anesthetized with halothane then killed by cervical dislocation. The distal colon was removed and placed in Krebs’ solution composed of (in mM): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, and glucose 11. The tissue was kept oxygenated (95% O2, 5% CO2) at room temperature.
Isometric Contractions of Longitudinal Muscle
Segments (2 cm) of distal colon were suspended between stationary hooks and isometric force transducers (FT03C; Grass Instruments, Quincy, MA, USA) in 20 ml jacketed baths containing oxygenated, 37°C Krebs’ solution. Tissues were placed under 10 mN initial tension and allowed 30 min equilibration time before experiments. Mechanical activity of the colonic segments was recorded with a chart recorder (7DAE; Grass Instruments). Drugs were added to the baths in volumes of 2–20 μl. Agonist concentration-response curves were constructed in a non-cumulative manner with 15-min intervals between agonist doses. Two preparations were obtained from each animal. One served as a control for agonist concentration response curves while the other was used to test antagonists versus the agonist concentration response curve.
Preliminary studies of circular muscle strips from the mouse colon yielded inconsistent results and many preparations did not respond to drugs. Therefore, we used whole colonic segments in which circular muscle contractions were recorded during propagated MMCs (see below).
Colonic Migrating Motor Complexes
Methods for assay of colonic MMCs were adapted from previously published work (Fida et al., 1997; Bush et al., 2000, 2001; Brierley et al., 2001). The colon was removed from the cecum to the rectum, fecal contents were gently flushed with Krebs’ solution and the mesentery dissected away. An 18 gauge stainless steel rod was inserted into the lumen and then secured at the bottom of a heated (37°C) chamber (35 ml volume) containing oxygenated Krebs’ solution. A microserfine clip (Fine Science Tools2) clip was placed at the end of the proximal colon, 5 mm aboral to the end of the haustra. A second clip was placed 2 cm distal to the first. The clips were connected to isometric force transducers (FT03C; Grass Instruments) with surgical silk. The tissue was allowed 60 min to equilibrate. The MMC frequency, oral amplitude, anal amplitude and velocity of transmission from oral to anal clip were measured (Bush et al., 2001).
Fecal Output
WT, P2X2 and P2X3 KO mice were injected with 5-hydroxytryptophan (100 mg/kg, i.p.) to induce defecation (Bourin et al., 1996). Fecal output was assayed by counting the number of fecal pellets produced in the 60 min post injection. Fecal pellet wet and dry weight over the same time was also measured. Dry weight was determined after the pellets had been dried for 1 h in a laboratory oven at 80°C.
Chemicals
All drugs were obtained from Sigma Chemical Company, St. Louis, MO, USA.
Statistical Analysis
Data are expressed as mean ± SEM; “n” refers to number of animals from which the data were obtained. Statistical analysis was preformed using Student’s t-test for unpaired data when comparing two groups, a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test or the Kruskal–Wallis and Dunn’s non-parametric tests for multiple group comparisons when standard deviations were not uniform across groups (InStat and Prism 4.0, Graphpad Software, San Diego, CA, USA). Potential differences in agonist concentration response curves were analyzed using a two-way ANOVA (Prism 4.0, Graphpad Software, San Diego, CA, USA).
Results
Drug-Induced Motor Responses of Colon Segments in vitro
In the first set of experiments, we tested the effects of deletion of the P2X2 or P2X3 subunits on longitudinal muscle contractility. We used the muscarinic receptor agonist, bethanechol, to cause longitudinal muscle contractions. Bethanechol caused concentration-dependent contractions in tissues from WT, P2X2 and P2X3 KO mice but there were no differences between the concentration response curves across groups (Figure 1A). The muscarinic antagonist scopolamine (1 μM) blocked bethanechol-induced contractions in all groups and bethanechol-induced contractions were unaffected by the sodium channel antagonist, tetrodotoxin (TTX, 0.3 μM) in all groups (not shown).
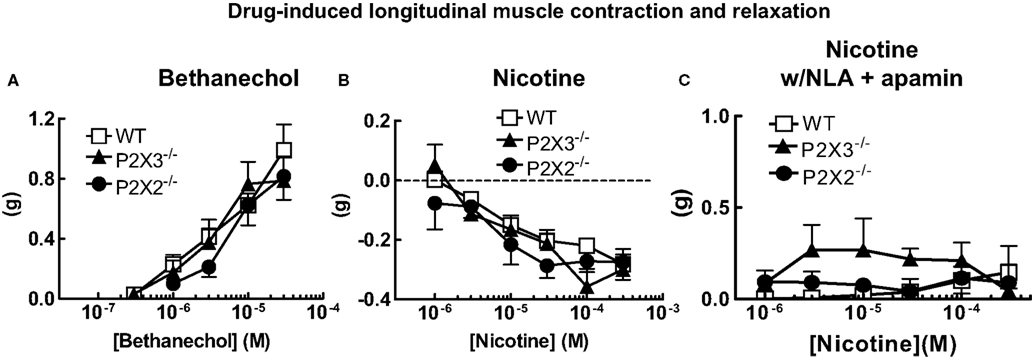
Figure 1. Cholinergic agonist-induced longitudinal muscle motor responses in the isolated colon of mice. (A) Concentration response curves for bethanechol-induced longitudinal muscle contractions were similar in colonic segments from WT (n = 21) and P2X2 (n = 10) and P2X3 (n = 17) subunit KO mice. (B) Nicotine-induced relaxations of the longitudinal muscle were similar colonic segments from WT (n = 23) and P2X2 (n = 14) and P2X3 (n = 18) subunit KO mice. (C) Relaxations of the longitudinal muscle caused by nicotine were blocked by NLA (100 μM) and apamin (0.1 μM) in tissues from WT (n = 14), P2X2 (n = 9) and P2X3 (n = 7) subunit KO mice. Data are mean ± SEM. There were no differences between responses obtained in tissues from WT, P2X2 and P2X3 subunit KO mice (two-way ANOVA, P > 0.05).
Motor neurons controlling contraction/relaxation of longitudinal smooth muscle are activated by acetylcholine acting at nicotinic receptors. To investigate the effect of P2X receptor subunit gene deletion on neurogenic responses, nicotine was used to active myenteric neurons. Nicotine caused similar concentration-dependent relaxations in colonic segments from WT, P2X2 and P2X3 KO mice (Figure 1B). When tissues were incubated with nitro-L-arginine (NLA, 100 μM) and apamin (0.1 μM) to block inhibitory neuromuscular transmission, the nicotine-induced relaxations were blocked revealing small amplitude contractions (Figure 1C). There were no differences in contraction amplitude in tissues from WT, P2X2 and P2X3 KO mice.
ATP is the endogenous ligand for P2X receptors. Therefore, we tested the effects of ATP on longitudinal muscle contractions. ATP did not affect resting tone of the colon preparations in normal Krebs’ solution (Figure 2A). However, ATP did cause concentration-dependent contractions of the longitudinal muscle when NLA (100 μM) and apamin (0.1 μM) were added to the buffer (Figure 2B). There was no difference in contraction amplitude in tissues from WT, P2X2 or P2X3 subunit KO mice (Figure 2B). The ATP-induced contractions were not blocked by TTX (0.3 μM, Figure 2C).
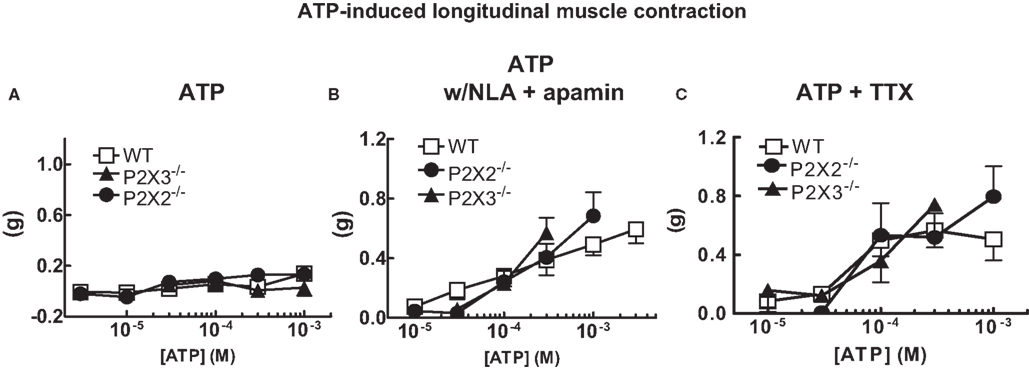
Figure 2. ATP induced longitudinal muscle contractions in the mouse colon. (A) ATP does not change the resting tone of longitudinal muscle in colonic segments from WT (n = 14) and P2X2 (n = 7) and P2X3 (n = 12) subunit KO mice. (B). ATP caused longitudinal muscle contractions in colonic segments of WT (n = 8) and P2X2 (n = 7) and P2X3 (n = 4) subunit KO mice when NLA (100 μM) and apamin (0.1 μM) were added to the Krebs’ solution. (C) ATP-induced contractions (NLA and apamin present) were not blocked by TTX (0.3 μM) in colonic segments of WT (n = 9) and P2X2 (n = 6) and P2X3 (n = 3) subunit KO mice. Data are mean ± SEM. In all of these studies there were no differences between responses obtained in tissues from WT, P2X2 and P2X3 subunit KO mice (two-way ANOVA, P > 0.05).
Colonic MMCs
The isolated colon of the mouse generates spontaneous migrating contractions that originate in the proximal colon and migrate to the distal colon (Figure 3). Time control studies revealed that the pattern was stable during a 90-min recording period. We measured MMC frequency, the speed of propagation and the amplitude of contractions at oral and anal recording sites. There was a small but statistically significant increase in MMC frequency in colon segments from P2X2 KO mice (Figure 4A, P < 0.05) but there were no differences in other MMC parameters measured in colon segments from WT, P2X2 and P2X3 subunit KO mice (Figures 4B–D).
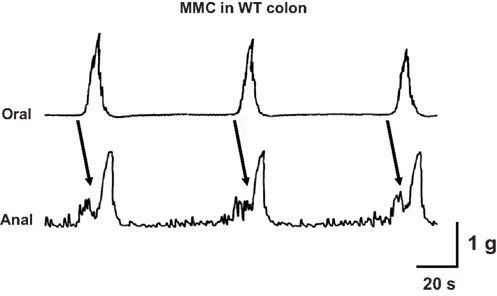
Figure 3. Representative recording of three cycles of the colonic MMC in vitro. Contractions originated at the oral end of a colonic segment and propagated to the anal recording site. Arrows indicate propagated contractions. Tissue obtained from a WT mouse.
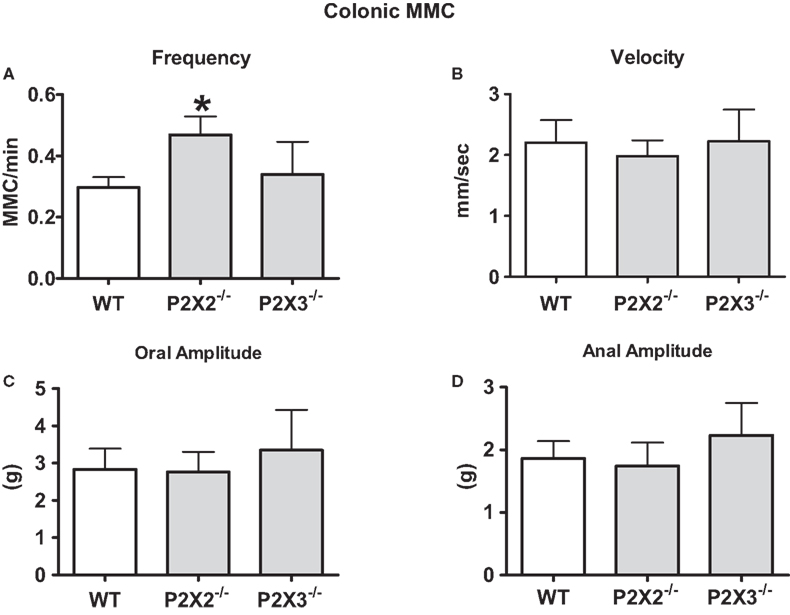
Figure 4. Properties of MMCs were similar in colon segments from WT and P2X2 and P2X3 KO mice. (A) The frequency of colonic MMC was increased in colonic segments from P2X2 but not P2X3 subunit KO mice when compared to WT colonic segments (P < 0.05). (B) The velocity of colonic MMC propagation was not altered by P2X2 or P2X3 subunit KO mice. The amplitude of contractions at the oral end (C) or anal end (D) of colonic segments was not altered by P2X2 or P2X3 subunit KO (P > 0.05). Data are mean ± SEM. Data analyzed by the Kruskal–Wallis and Dunn non-parametric multiple comparisons tests.
P2X3 receptors are expressed by colonic myenteric neurons (Poole et al., 2002; Xiang and Burnstock, 2004) and P2X3 receptors may contribute to fast synaptic excitation in the enteric nervous system (Ren and Galligan, 2007). Therefore, we anticipated that colonic MMCs might be more sensitive to inhibition by the nicotinic acetylcholine receptor antagonist, mecamylamine, in the colon of P2X3 subunit KO mice compared to WT mice. However, it was found that the number of MMCs that propagated fully along the length of the colon, their frequency and speed of propagation were equally inhibited by mecamylamine in colonic segments from WT and P2X3 subunit KO mice (Figure 5).
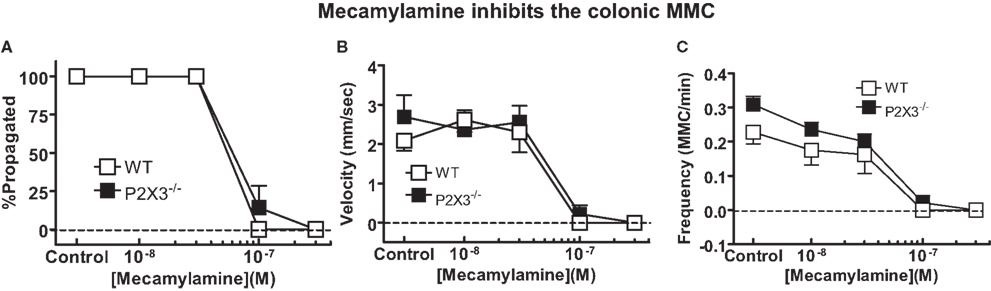
Figure 5. Mecamylamine caused equivalent inhibition of MMC efficiency of propagation (A), speed of propagation (B) and MMC frequency (C) in isolated colonic segments from WT (n = 4), and P2X3 (n = 7) subunit KO mice. Data are mean ± SEM. There were no differences among groups (P > 0.05) in any of these measures (two-way ANOVA).
Fecal Output in vivo
To assess the contribution of P2X2 and P2X3 receptor subunits to colonic motility in vivo, 5-HTP was used to induce fecal output. 5-HTP induces an increase in fecal pellet output within 60 min (Bourin et al., 1996) allowing more accurate assessment of drug- or other treatment-induced changes in colonic motility. A saline-treated group was not studied in this protocol as we were only interested in differences between WT and P2X subunit KO mice. However, others have shown that in control mice treated with saline fecal output ranges between one and three pellets per hour (Wang et al., 2007, 2009). 5-HTP treatment stimulated output of soft but formed fecal pellets. 5-HTP induced fecal output during the 1-h period post-injection was similar in WT, P2X2 and P2X3 KO mice (Figure 6A). There were no differences in the dry and wet weight of fecal pellets produced by WT, P2X2 and P2X3 subunit KO mice (Figures 6B,C).
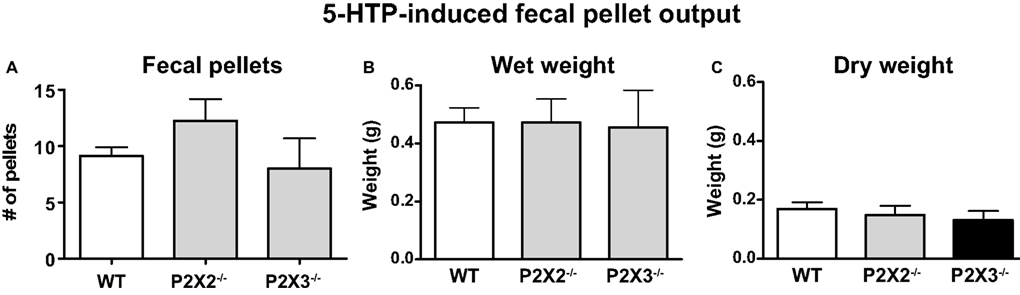
Figure 6. 5-HTP induced fecal pellet output is similar in WT (n = 8) and P2X2 (n = 4) and P2X3 (n = 4) subunit KO mice. (A) Number of fecal pellets expelled. (B) Total wet weight of fecal pellets expelled by each group. (C) Dry weight of fecal pellets expelled by each group. Data are mean ± SEM of the number of fecal pellets produced in the 60-min period after 5-HTP injection (100 mg/kg, i.p.). There were no differences among groups (P > 0.05, one-way ANOVA).
Discussion
P2X receptors contribute to fast synaptic excitation in the enteric nervous system (Galligan and Bertrand, 1994; LePard et al., 1997; Johnson et al., 1999). Immunohistochemical studies show that P2X2 immunoreactive neurons are found in guinea pig and mouse colon (Castelucci et al., 2002; Giaroni et al., 2002). P2X3 immunoreactivity is also localized to myenteric neurons in the small and intestine and colon in the guinea pig (Poole et al., 2002; Van Nassauw et al., 2002). Few electrophysiological studies of synaptic transmission have been done in mouse colon, with early experiments failing to observe a non-cholinergic component (Furukawa et al., 1986). However, Nurgali et al. (2003) identified purinergic synaptic potentials in the guinea pig colon that were mediated by P2X2 and other P2X subunit containing receptors (Nurgali et al., 2003).
Functional studies of the relative contributions of P2X receptors of different subunit composition are hampered by the shortage P2X subunit selective drugs. P2X subunit KO mice provide an alternative approach to studies of the integrative function of P2X subunit containing receptors in the gut. Previous studies have shown that small intestinal peristalsis studied in vitro is impaired tissues from P2X2 and P2X3 KO mice (Bian et al., 2003; Ren et al., 2003). The present study was designed to assess the functional role of P2X2 and P2X3 receptor subunits on colonic motility in vitro and in vivo.
Drug-Induced Contractions and Relaxations of Colonic Longitudinal Muscle
In the first set of experiments, we determined if P2X2 or P2X3 gene KO produced a general impairment of drug-induced contractions of the colon in vitro. Bethanechol activates muscarinic receptors on smooth muscle to stimulate contraction. Bethanechol-induced longitudinal muscle contractions were similar in colonic segments from WT, P2X2 or P2X3 subunit KO mice. This result indicates that P2X2 and P2X3 gene deletion does not compromise overall longitudinal muscle function.
Ganglionic transmission may also be affected by P2X receptor gene deletion. We used nicotine to activate nicotinic acetylcholine receptors on myenteric neurons to assess the function of motor neurons supplying the longitudinal muscle. In normal Krebs’ solution nicotine caused a concentration-dependent relaxation of the longitudinal muscle that was similar in WT, P2X2 and P2X3 subunit KO mice. Nicotine-induced relaxations were blocked when the nitric oxide synthase inhibitor, NLA, and the SK channel blocker, apamin, were added to the Krebs’ solution. However, even when inhibitory neuromuscular transmission was blocked, nicotine failed to cause significant contractions of the longitudinal muscle. These data indicate that although excitatory muscarinic cholinergic receptors are localized to the longitudinal muscle in the mouse colon (see above); there is relatively sparse functional innervation of these receptors.
ATP is an endogenous agonist for P2X receptors. ATP did not change the resting tone of colonic longitudinal muscle of WT, P2X2 or P2X3 subunit KO mice in normal Krebs’ solution. However, in the presence of NLA and apamin, ATP caused concentration-dependent contractions of the muscle. These data are consistent with ATP activated inhibitory neurotransmission to the longitudinal muscle in the mouse colon (Koh et al., 1997; Giaroni et al., 2002). ATP induced contractions were similar in tissues from WT, P2X2 or P2X3 subunit KO mice and these contractions were resistant to TTX. This result suggests that ATP-induced contraction were caused either by activation of enteric nerve endings (which would be resistant to TTX) or that the receptors for ATP were localized to longitudinal smooth muscle cells. This latter suggestion is consistent with data showing localization of P2X3, P2X5 and P2X7 receptors to the longitudinal muscle of the canine colon (Lee et al., 2005). P2X2 receptors were also localized to longitudinal muscle of the mouse colon (Giaroni et al., 2002). These investigators concluded that P2X2 receptors mediate ATP-induced contractions of the longitudinal muscle, a result that is inconsistent with our findings. It is possible that the drugs used in the previous study did not have sufficient selectivity to identify a P2X2-mediated response. Alternatively, P2X2 receptors may normally mediate ATP-induced contractions of the longitudinal muscle but that there are compensatory changes in purinergic receptor expression in P2X2 KO mice. However, others have concluded that P2Y receptors mediate ATP induced contractions of the longitudinal muscle in the mouse colon (Serio et al., 2003; Zizzo et al., 2007). Taken together these data indicate that ATP activates both excitatory and inhibitory mechanisms controlling longitudinal muscle tone in the mouse colon. It is unlikely that these responses are mediated by receptors composed of P2X2 or P2X3 subunits.
P2X2 but Not P2X3 Subunit KO Increases Colonic MMC Frequency
The colonic MMC is a cyclic motor pattern consisting of regularly occurring propagating contractions in the mouse colon maintained in vitro (Fida et al., 1997; Bush et al., 2000). This pattern may provide the contractions required to mix and knead the solid and semi-solid contents of the colon (Fida et al., 1997). Colonic MMCs require the interaction of myenteric nerve circuits and multiple neurotransmitters (Fida et al., 1997; Bush et al., 2000, 2001; Brierley et al., 2001). Our data obtained in the colon from P2X2 KO mice revealed that this subunit contributes to neurotransmission that regulates the MMC frequency. P2X2 subunit KO results in an increase in MMC frequency suggesting that these subunits are localized to inhibitory mechanisms that suppress the circuitry responsible for MMC initiation. Neurotransmission at receptors containing these subunits is not essential for MMC propagation. As discussed above, it is possible that P2X receptors normally contribute to MMC propagation but in P2X subunit KO mice there are compensatory changes in receptor expression that maintain normal MMC propagation.
Nicotinic acetylcholine receptor function is required for most motor patterns in the gut. We tested the possibility that nicotinic receptor function was sufficient to maintain the MMC in P2X3 subunit KO mice but the safety factor would be reduced. This would be revealed by an increase in the sensitivity of the MMC to inhibition by mecamylamine in P2X3 KO mice because it has been shown that P2X3 subunits are expressed by murine colonic myenteric neurons (Ruan and Burnstock, 2005). In addition, MMC propagation does not require P2X2 subunits (see above). However, MMC properties were similar in colonic segments from WT and P2X3 KO mice in the absence and presence of mecamylamine. These data suggest that receptors composed of P2X3 subunits are not required for normal MMC function.
P2X2 and P2X3 Subunit KO Does Not Alter Fecal Pellet Propulsion in vivo
We used 5-HTP treatment to enhance fecal output in WT and P2X2 and P2X3 subunit KO mice. P2X2 or P2X3 KO did not alter fecal pellet output or water content of fecal pellets compared to those produced by WT mice. These data indicate that KO of P2X2 or P2X3 subunits does not impair propulsive motility of the mouse colon in vivo.
Summary and Conclusions
Previous studies have shown that KO of P2X2 and P2X3 subunits causes impaired peristalsis in the mouse ileum maintained in vitro. The same KO’s do not alter colonic motility patterns in vitro or colonic propulsion in vivo. These data suggest that purinergic neurotransmission at P2X2 or P2X3 subunit containing receptors does not contribute to propulsive motility patterns in vitro or in vivo. This does not rule out potential contributions of other P2X subunits. However, only P2X2, P2X3 and P2X5 subunits have been localized to enteric neurons in the mouse gut (Ruan and Burnstock, 2005). P2X5 subunits can form functional homomeric receptors (North, 2002) so it is possible that these subunits can sustain purinergic neurotransmission in P2X2 or P2X3 subunit KO mice. It is also possible that the contributions of P2X2 and P2X3 subunits to neurotransmission in the colon might only be revealed under pathophysiological conditions. For example, colonic inflammation can alter colonic motility and a role for P2X2 or P2X3 subunits might become apparent under these conditions (Leng et al., 2008).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Debra Cockayne at Roche Bioscience (Palo Alto, CA, USA) for providing us with the P2X2 and P2X3 KO mice. Supported by DK057039.
Footnotes
References
Bian, X., Ren, J., DeVries, M., Schnegelsberg, B., Cockayne, D. A., Ford, A. P., and Galligan, J. J. (2003). Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J. Physiol. 551, 309–322.
Bourin, M., Hascoet, M., and Deguiral, P. (1996). 5-HTP-induced diarrhea as a carcinoid syndrome model in mice? Fundam. Clin. Pharmacol. 10, 450–457.
Brierley, S. M., Nichols, K., Grasby, D. J., and Waterman, S. A. (2001). Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br. J. Pharmacol. 132, 507–517.
Bush, T. G., Spencer, N. J., Watters, N., Sanders, K. M., and Smith, T. K. (2000). Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton. Neurosci. 84, 162–168.
Bush, T. G., Spencer, N. J., Watters, N., Sanders, K. M., and Smith, T. K. (2001). Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am. J. Physiol. 281, G974–G983.
Castelucci, P., Robbins, H. L., Poole, D. P., and Furness, J. B. (2002). The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem. Cell Biol. 117, 415–422.
Cockayne, D. A., Hamilton, S. G., Zhu, Q. M., Dunn, P. M., Zhong, Y., Novakovic, S., Malmberg, A. B., Cain, G., Berson, A., Kassotakis, L., Hedley, L., Lachnit, W. G., Burnstock, G., McMahon, S. B., and Ford, A. P. (2000). Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015.
Fida, R., Lyster, D. J., Bywater, R. A., and Taylor, G. S. (1997). Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol. Motil. 9, 99–107.
Furukawa, K., Taylor, G. S., and Bywater, R. A. (1986). An intracellular study of myenteric neurons in the mouse colon. J. Neurophysiol. 55, 1395–1406.
Galligan, J. J., and Bertrand, P. P. (1994). ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 14, 7563–7571.
Galligan, J. J., LePard, K. J., Schneider, D. A., and Zhou, X. (2000). Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 81, 97–103.
Giaroni, C., Knight, G. E., Ruan, H. Z., Glass, R., Bardini, M., Lecchini, S., Frigo, G., and Burnstock, G. (2002). P2 receptors in the murine gastrointestinal tract. Neuropharmacology 43, 1313–1323.
Jarvis, M. F., and Khakh, B. S. (2009). ATP-gated P2X cation-channels. Neuropharmacology 56, 208–215.
Johnson, P. J., Shum, O. R., Thornton, P. D., and Bornstein, J. C. (1999). Evidence that inhibitory motor neurons of the guinea-pig small intestine exhibit fast excitatory synaptic potentials mediated via P2X receptors. Neurosci. Lett. 266, 169–172.
Koh, S. D., Dick, G. M., and Sanders, K. M. (1997). Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. 273, C2010–C2021.
Lee, H. K., Ro, S., Keef, K. D., Kim, Y. H., Kim, H. W., Horowitz, B., and Sanders, K. M. (2005). Differential expression of P2X-purinoceptor subtypes in circular and longitudinal muscle of canine colon. Neurogastroenterol. Motil. 17, 575–584.
Leng, Y., Yamamoto, T., and Kadowaki, M. (2008). Alteration of cholinergic, purinergic and sensory neurotransmission in the mouse colon of food allergy model. Neurosci. Lett. 445, 195–198.
LePard, K. J., and Galligan, J. J. (1999). Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am. J. Physiol. 276, G529–G538.
LePard, K. J., Messori, E., and Galligan, J. J. (1997). Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology 113, 1522–1534.
Monro, R. L., Bertrand, P. P., and Bornstein, J. C. (2004). ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J. Physiol. 556, 571–584.
Nurgali, K., Furness, J. B., and Stebbing, M. J. (2003). Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience 116, 335–347.
Poole, D. P., Castelucci, P., Robbins, H. L., Chiocchetti, R., and Furness, J. B. (2002). The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton. Neurosci. 101, 39–47.
Ren, J., and Galligan, J. J. (2007). A novel calcium-sensitive potassium conductance is coupled to P2X3 subunit containing receptors in myenteric neurons of guinea pig ileum. Neurogastroenterol. Motil. 19, 912–922.
Ren, J., Bian, X., DeVries, M., Schnegelsberg, B., Cockayne, D. A., Ford, A. P., and Galligan, J. J. (2003). P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J. Physiol. 552, 809–821.
Ruan, H. Z., and Burnstock, G. (2005). The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res. 319, 191–200.
Serio, R., Alessandro, M., Zizzo, M. G., Tamburello, M. P., and Mule, F. (2003). Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur. J. Pharmacol. 460, 183–190.
Spencer, N. J., Hennig, G. W., Dickson, E., and Smith, T. K. (2005). Synchronization of enteric neuronal firing during the murine colonic MMC. J. Physiol. 564, 829–847.
Surprenant, A., and North, R. A. (2009). Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359.
Van Nassauw, L., Brouns, I., Adriaensen, D., Burnstock, G., and Timmermans, J. P. (2002). Neurochemical identification of enteric neurons expressing P2X3 receptors in the guinea-pig ileum. Histochem. Cell Biol. 118, 193–203.
Wang, L., Gourcerol, G., Yuan, P. Q., Wu, S. V., Million, M., Larauche, M., and Taché, Y. (2009). Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am. J. Physiol. PMC2806102 [Epub ahead of print].
Wang, L., Martínez, V., Kimura, H., and Taché, Y. (2007). 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am. J. Physiol. 292, G419–G428.
Xiang, Z., and Burnstock, G. (2004). P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem. Cell Biol. 121, 169–179.
Keywords: enteric nervous system, purinergic receptors, knockout mice
Citation: DeVries MP, Vessalo M and Galligan JJ (2010) Deletion of P2X2 and P2X3 receptor subunits does not alter motility of the mouse colon. Front. Neurosci. 4:22. doi: 10.3389/fnent.2010.00001
Received: 16 December 2009;
Paper pending published: 24 January 2010;
Accepted: 04 March 2010;
Published online: 19 March 2010.
Edited by:
Paul P. Bertrand, University of New South Wales, AustraliaReviewed by:
Eamonn Dickson, University of Nevada School of Medicine, USAPaul P. Bertrand, University of New South Wales, Australia
Copyright: © 2010 DeVries, Vessalo and Galligan. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: James J. Galligan, Department of Pharmacology and Toxicology, Michigan State University, East Lansing, MI 48824, USA. e-mail: galliga1@msu.edu