- 1 Institute of Membrane and Systems Biology, University of Leeds, Leeds, UK
- 2 Division of Cardiovascular and Neuronal Remodelling, University of Leeds, Leeds, UK
Modulatory influences on sympathetic nervous system activity are diverse and far reaching, acting at select points in the complex pathways controlling sympathetic outflow to enable subtle changes or more global effects. Changes in the degree of sympathetic neuromodulation can have serious consequences on homeostatic variables such as heart rate, blood pressure and gut motility. At the level of the spinal cord, the sympathetic preganglionic neurons (SPNs) can be modulated by activation of presynaptic GABAB heteroreceptors on glutamatergic terminals and by postsynaptic GABAB receptors. Here we show that a low concentration of the GABAB agonist baclofen (1 μM) attenuated GABAergic inhibitory postsynaptic potentials in SPNs elicited from stimulation of either the central autonomic area or descending fibers in the lateral funiculus. This low baclofen concentration also elicited three categories of postsynaptic response: a large hyperpolarization with a decrease in input resistance, a moderate hyperpolarization with no change in input resistance and no response. Using cesium-loaded, tetraethylammonium chloride containing electrodes (to block potassium conductance), baclofen elicited moderate hyperpolarizations with no change in input resistance in 50% of SPNs; the remainder were unaffected. These modest hyperpolarizations were reduced in Ca2+ free solution or cadmium. Hyperpolarizing responses were also observed in interneurons in the vicinity of SPNs. These studies provide the first evidence for GABAB autoreceptors involved in inhibitory GABAergic transmission onto SPNs and for postsynaptic GABAB receptors on interneurons. The data also indicate that there is heterogeneity in the postsynaptic responses of SPNs.
Introduction
Sympathetic outflow to the many organs controlled by the autonomic nervous system is regulated at a number of levels within the central nervous system. The final point at which sympathetic activity can be influenced by the CNS is at the spinal cord level where the sympathetic preganglionic neurons (SPNs) reside. Continuing research into the spinal cord circuitry that regulates sympathetic output has revealed a high degree of complexity of control of these SPNs which enables fine tuning of the sympathetic activity influencing end organs. For example, studies from our laboratory have revealed that at least two GABAergic inhibitory influences on SPNs exist; one a descending monosynaptic inhibitory influence originating in the brainstem (Deuchars et al., 1997; Deuchars et al., 2001a) and another more local input from GABAergic interneurons located in the central autonomic area of the spinal cord (Deuchars et al., 2005). Thus one avenue for selective regulation of SPN activity would be at a presynaptic level since receptors located on specific presynaptic terminals would influence synaptic transmission exclusively from that input. Such input-specific modulation is observed in SPNs where A1 adenosine receptors are located on excitatory terminals and their activation decreases excitatory transmission (Deuchars et al., 2001a) whilst A2A adenosine receptors are confined solely to inhibitory GABAergic terminals and increase GABAergic transmission (Brooke et al., 2004).
GABAB receptors, like adenosine receptors, are also important modulators of neuronal excitability (Kerr and Ong, 1995). Their widespread actions and involvement in mechanisms underlying pathologies such as epilepsy and chronic pain (Bowery, 2006) have led to an increased interest in regulating these receptors as potential therapeutic targets. GABAB receptors are present postsynaptically on SPNs and high concentrations of the GABAB agonist, baclofen (10–100 μM) elicited hyperpolarizing responses associated with a decrease in input resistance that was attributed to opening of potassium channels (Whyment et al., 2004). However, a proportion of these responses may be due to activation of other second messenger mediated channels since in splanchnic SPNs, 5 μM baclofen also increased potassium channel opening but with an accompanying decrease in voltage gated calcium conductance, that together caused inconsistent effects on input resistance (Cheng et al., 2005). These discrepancies in findings regarding the nature of postsynaptic GABAB mediated responses may be due to differences in concentration of baclofen used.
Presynaptic GABAB heteroreceptors have been reported on excitatory terminals onto SPNs by Cheng’s group and others (Cheng et al., 2005; Wu and Dun, 1992). Here applications of baclofen (1–30 μM) decreased excitatory amino acid mediated transmission from lateral funiculus (Lf) stimulation (which comprises descending inputs to SPNs) although Wu and Dun (1992) reported that baclofen was without effect on the postsynaptic membrane. However, to date no evidence exists of GABAB autoreceptors on descending or local inhibitory inputs onto SPNs. Such autoreceptors are common in other regions of the CNS and may display different pharmacological profiles to heteroreceptors (Bonanno and Raiteri, 1992). In fact, differences in the sensitivity of pre versus postsynaptic receptors to baclofen are also common findings – in neurons of the nucleus tractus solitarius, among others, the sensitivity of presynaptic receptors was at least 20-fold higher than that of the postsynaptic receptors (Brooks and Glaum, 1995).
The lack of data regarding a role for GABAB autoreceptors in spinal cord circuitry underlying sympathetic control led us to examine how GABAB receptors may affect synaptic inhibitory transmission from both descending and local inputs onto SPNs. Furthermore the intriguing possibility of GABAB receptors with specific sensitivities to baclofen prompted us to determine how very low concentrations of baclofen affects both pre- and postsynaptic GABAB receptors on SPNs in the thoracic spinal cord slice preparation.
Materials and Methods
Patch Clamp Recordings
All experiments were performed under UK Home Office License and in accordance with the regulations of the UK Animals (Scientific Procedures) Act 1986.
Wistar rats of either sex aged 10–15 days were anesthetized with urethane (2 g/kg, i.p.) and transcardially perfused with ice-cold sucrose aCSF, composition (in mM); sucrose (217), NaHCO3 (26), KCl (3), MgSO4 (2), NaH2PO4 (2.5), CaCl2 (1), and glucose (10). After decapitation, the upper and middle thoracic spinal cord was isolated and the dura and pia mater were carefully removed. Coronal slices of 300 μm were cut using a Vibroslice (Campden Instruments) and kept in a holding chamber containing aCSF, composition (in mM): NaCl (124), NaHCO3 (26), KCl (3), MgSO4 (2), NaH2PO4 (2.5), CaCl2 (2), and glucose (10), bubbled in 95% O2/5%CO2.
Slices were transferred to a submerged recording chamber and continually perfused with aCSF also containing the non-selective glutamate receptor antagonist kynurenic acid (2 mM) at a rate of 2–5 ml/min at room temperature. For experiments carried out in calcium-free aCSF, CaCl2 was replaced with 2 mM MgCl2. Whole-cell patch clamp recordings were made in current-clamp mode using an Axopatch 1D (Axon Instruments, Foster City, CA, USA). Patch electrodes (tip diameter, 3 μm; resistance 3–6 MΩ) were filled with either K-Gluconate based intracellular solution, composition (in mM): K-Gluconate (110), EGTA (11), MgCl2 (2), CaCl2 (0.1), HEPES (10), Na2ATP (2), and NaGTP (0.3); or Cs-based intracellular solution, composition (in mM): Cs2SO4 (110), CaCl2 (0.5), MgCl2 (2), EGTA (5), HEPES (5), ATP-Na2 (5), TEA (5). Liquid junction potentials (14.8 mV for K-Gluconate intracellular; 10.9 mV for Cs2SO4 intracellular) were not corrected for in these experiments.
Neurons (SPNs and interneurons) within the IML were identified both electrophysiologically and anatomically as described previously (Deuchars et al., 2001b). When using Cs-based intracellular solution, the electrophysiological properties of the two populations were less prominent but their distinctive morphology still enabled identification post hoc. Depolarizing current pulses of +10 to +200 pA were applied to examine firing properties of the neurons.
Responses to bath applied drugs were determined. Neurons were held at −60 mV (not corrected for liquid junction potentials) to test the effects of the GABAB agonist baclofen and hyperpolarizing current pulses of −10 to −50 pA (1 s duration) injected to monitor changes in input resistance, represented by a change in the size of the voltage response to the injected current. Baclofen was repeatedly applied and during a response, the membrane potential was returned to −60 mV to check that the change in input resistance was due to an effect of the drug and not the voltage induced changes. To confirm that changes in membrane potential and input resistance were due to a specific action of the agonist at the GABAB receptor, the selective antagonist CGP55845 was applied for ≥5 to 10 min prior to application of the agonist.
The central autonomic area and the Lf were stimulated using bipolar stimulating electrodes and isolated stimulators (DS2A; Digitimer, Hertfordshire, UK). IPSPs were elicited at more depolarized potentials (−10 to +10 mV) to increase the chloride driving force. Paired pulse stimuli were used to investigate presynaptic actions of pharmacological tools.
Drugs, a GABAB agonist baclofen and an antagonist CGP55845 (both from Tocris Cookson, Bristol, UK), were applied in the superfusing solution and the concentration given is the final concentration in the bath. Both drugs were first dissolved in DMSO and the final concentration of DMSO in the bath was less than 1:1000. Bicuculline hydrochloride (Tocris Cookson) and cadmium chloride (Sigma, UK) were dissolved in water.
Data Analysis
Data capture was carried out using either Signal (version 1) and Spike 2 (version 2; Cambridge Electronic Design) or pClamp (version 9, Axon Instruments). The input resistance was calculated for each neuron by measurement of the voltage responses to injected current pulses (average of 3 sweeps). IPSP amplitudes were calculated by measuring their peak amplitude from the holding potential and were averaged for ≥10 consecutive sweeps and are given as the mean ± SEM.
Changes in IPSP amplitude as a consequence of drug application were expressed as a mean percentage of the control. Assessment of changes in the paired pulse ratio (PPR) was also carried out. PPR was derived from the ratio of the amplitude of the second IPSP to the first IPSP. The PPR during drug application was then compared with the control to note any significant change. The effects of the drugs were tested statistically using the paired Student’s t-test, and differences were considered significant when p < 0.05.
RNA Extraction and PCR
Total RNA was isolated from micropunches of IML and spinal cord slices of rats (10–15 days), anesthetized and prepared as described above. The RNA was reverse transcribed as previously described (Spary et al., 2008). PCR was performed with 0.4 μM primers (GABAB1a forward 5′-TAGCGATCGAGAAGGGGAGA-3′; GABAB1a reverse 5′-CTTCACCTGGTCGCGAGTCA-3′; GABAB1b forward 5′-GCGAACGTCTCCTGGCCCTA-3′; GABAB1b reverse 5′-GCGGGAGATGAGGGGAGTGA-3′; GABAB2 forward 5′-AGCAGATCCGCAACGAGTCA-3′; GABAB2 reverse 5′-GTGCTTCAGGAGCTTCAGGA-3′) (Spary et al., 2008).
Negative controls included dH2O and RNA, which had not undergone reverse transcription. The veracity of the PCR products was confirmed by DNA sequencing on an ABI 3100 genetic analyzer using BigDye terminator cycle sequencing version 3.1.
Results
Postsynaptic Effects of Low Concentrations of Baclofen Recorded in K-Gluconate Based Intracellular Solution
Firstly we determined how a low concentration of the GABAB receptor agonist baclofen affected postsynaptic responses of SPNs, using a concentration (1 μM) that has previously been shown in the nucleus of the tractus solitarius to selectively target pre- rather than postsynaptic receptors (Brooks et al., 1992). Using K-Gluconate based intracellular solution, this low concentration of baclofen (1 μM) elicited three types of responses in SPNs tested (n = 32), characterized by the degree of change in membrane potential and input resistance. Thus the SPNs were grouped according to their responses into (i) a large-response group (37.5%) where both parameters were significantly altered by drug application (Figure 1A); (ii) a modest response group (34.4%) with small hyperpolarizations observed but no significant alteration in input resistance (Figure 1B); and (iii) a non-responding group (28.1%) where neither membrane potential nor input resistance of neurons were changed after bath application of baclofen (Figure 1C).
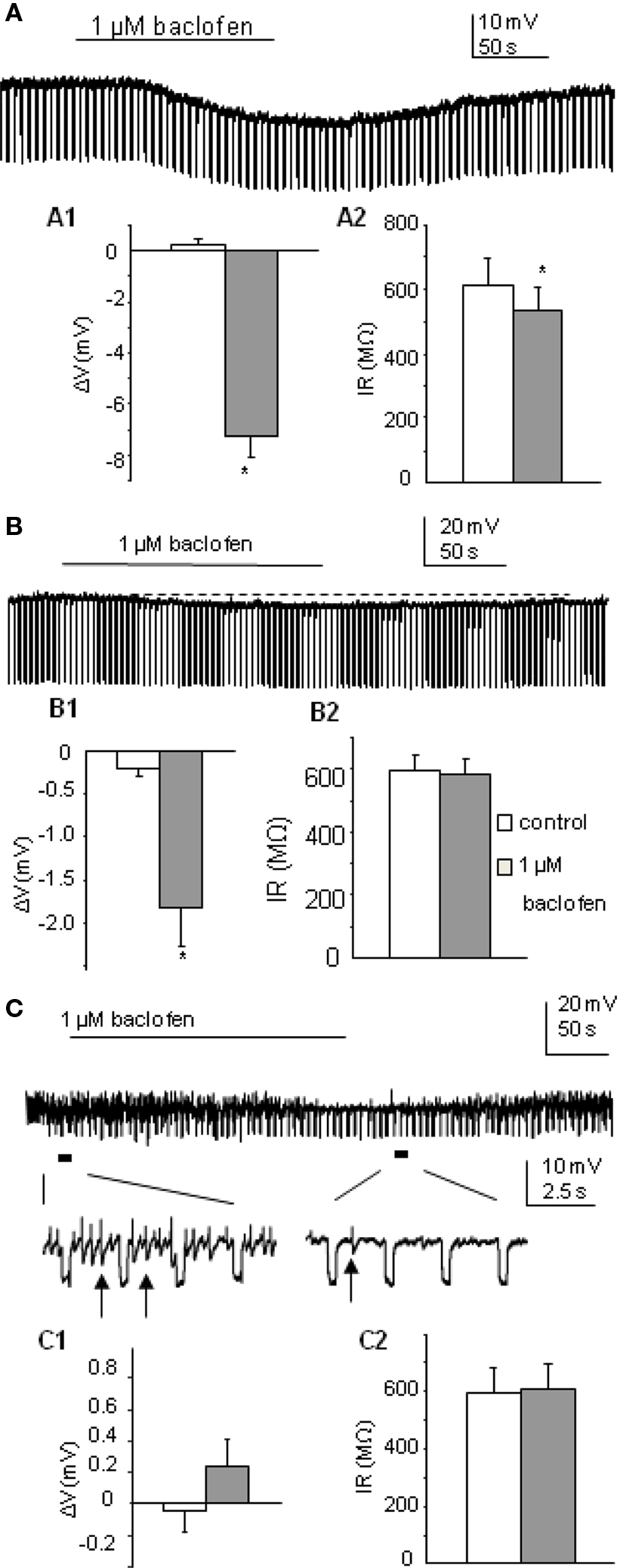
Figure 1. Postsynaptic responses to 1 μM baclofen differ in SPNs recorded in K-Gluconate based intracellular solution. (A) Bath application of baclofen induced hyperpolarization in this SPN, associated with a decrease in cell input resistance. (A1,A2) Pooled data (n = 12) showing membrane potential and input resistance changes before and during drug application. *p < 0.05, Student’s t-test. (B) Bath application of baclofen hyperpolarized this SPN slightly without changing cell input resistance. (B1,B2) Pooled data (n = 11) showing membrane potential and input resistance changes before and during drug application. (C) Bath application of baclofen did not induce any membrane potential or input resistance changes in this SPN. Expanded traces showing coupled activities (arrows) were reduced but not abolished during drug application. (C1,C2) Pooled data (n = 9) showing membrane potential and input resistance changes before and during drug application.
In the large-response group, the average change in cell membrane potential was −7.24 ± 0.9 mV associated with a decrease in input resistance to 87.6 ± 3% of control (612.7 ± 83 to 535.2 ± 72 MΩ, p < 0.005, n = 12). Responses were also maintained in the presence of 1 μM external TTX (n = 3) and antagonized by the GABAB receptor antagonist CGP55845 (mean hyperpolarization in baclofen alone was −8.9 ± 1.5 mV, mean hyperpolarization in baclofen + CGP55845 was −0.2 ± 0.2 mV; p < 0.005, n = 5; Figure 3A and see also Wang et al., 2008). An early study in SPNs also reported large hyperpolarizations associated with decreases in input resistance (Whyment et al., 2004) when using much higher concentrations of baclofen (10–100 μM) and suggested that this hyperpolarization was due to activation of GABAB receptor coupled K conductances. The moderate response group only showed hyperpolarizations of −1.80 ± 2.4 mV (n = 11; Figure 1B) which were significantly smaller than the large response group (p < 0.001) and the input resistances of these cells were not significantly altered (597.6 ± 50 to 586.4 ± 49 MΩ). Non-responding cells were not affected by low concentrations of baclofen (average change in membrane potential was 0.24 ± 0.2 mV, n = 9; Figure 1C). A lower concentration (0.5 μM) also showed a similar grouping of responses (data not shown) to these observed with 1 μM baclofen but with smaller sample sizes.
Electrically coupled activities (observed as spikelets described by Logan et al., 1996; Nolan et al., 1999) were recorded from 10 of the 32 SPNs, where a strong degree of coupled activity was noted. These strongly coupled cells were equally distributed between the three subgroups (4, 4, 2, in three groups respectively). Coupled events in all four cells in the large-response group were completely abolished during drug application. In the moderate response group, baclofen completely blocked spikelets in 2/4 cells and left the other two partially reduced in frequency. In the non-responding group, without altering the membrane properties of the recorded neurons, baclofen blocked the spikelets of one strongly coupled cell and reduced the frequency of the other (Figure 1C).
Postsynaptic Effects of Low Concentrations of Baclofen Recorded in CS-Based Intracellular Solution
Cs loaded TEA containing intracellular solution was used to internally block K+ conductance. Therefore, any membrane potential changes due to opening of K+ channels associated with activation of GABAB receptors should no longer be seen. TTX was also applied. Of the 18 neurons recorded, 50% were only slightly hyperpolarized to a mean value of −3.02 ± 0.3 mV (this is a significant change from control, p < 0.005; n = 9; Figure 2A) with no significant input resistance changes (346.0 ± 46 to 348.3 ± 46 MΩ), similar to that observed in the moderate response group with K-Gluconate based intracellular solution. Indeed there was no significant difference between these values and those moderate responses recorded in K-Gluconate, even though there were slight differences in liquid junction potentials between the two solutions. CGP55845 also antagonized the effects of baclofen with Cs-based intracellular solutions, similar to that observed with K-Gluconate containing electrodes (mean hyperpolarization to baclofen was −3.14 ± 0.5 mV, whilst in CGP + baclofen, the mean hyperpolarization was −0.02 ± 0.2 mV, n = 3; Figure 3B). The other SPNs did not respond to 1 μM baclofen application (Figure 2B; n = 9).
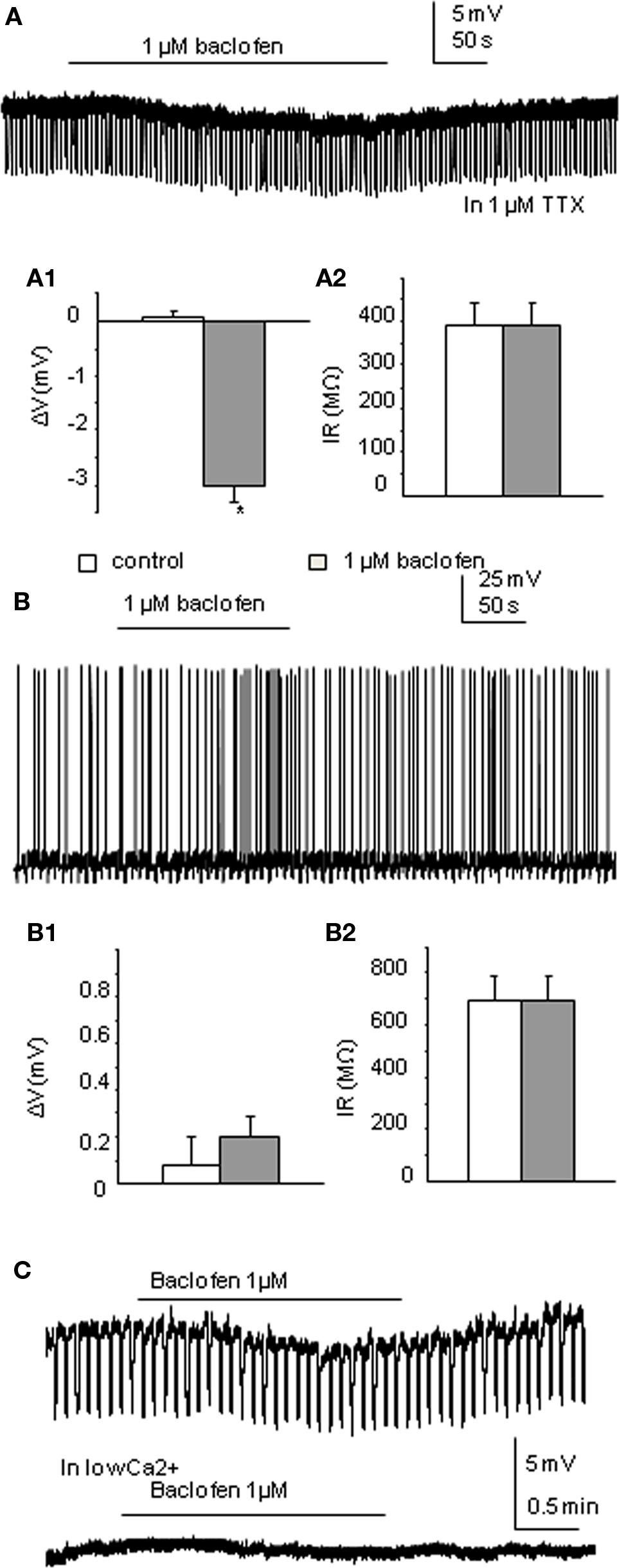
Figure 2. Low concentrations of baclofen elicit differential responses in neurons recorded in Cs-based intracellular solution. (A) Bath application of 1 μM baclofen hyperpolarized this neuron without changing cell input resistance in the presence of 1 μM TTX. (A1,A2) Pooled data (n = 9) showing membrane potential and input resistance changes before and during drug application. *p < 0.05, Student’s t-test. (B) Bath application of baclofen did not induce any membrane potential or cell input resistance changes in this neuron. (B1,B2) Pooled data (n = 9) showing membrane potential and input resistance changes before and during drug application. (C). Moderate responses to baclofen recorded in Cs-based intracellular solution could be further reduced by bathing in Ca2+-free solution. Upper trace shows the response to baclofen in normal aCSF, lower trace (without negative current pulses eliciting hyperpolarizing responses) in the same neuron shows a lack of response in Ca2+-free solution.
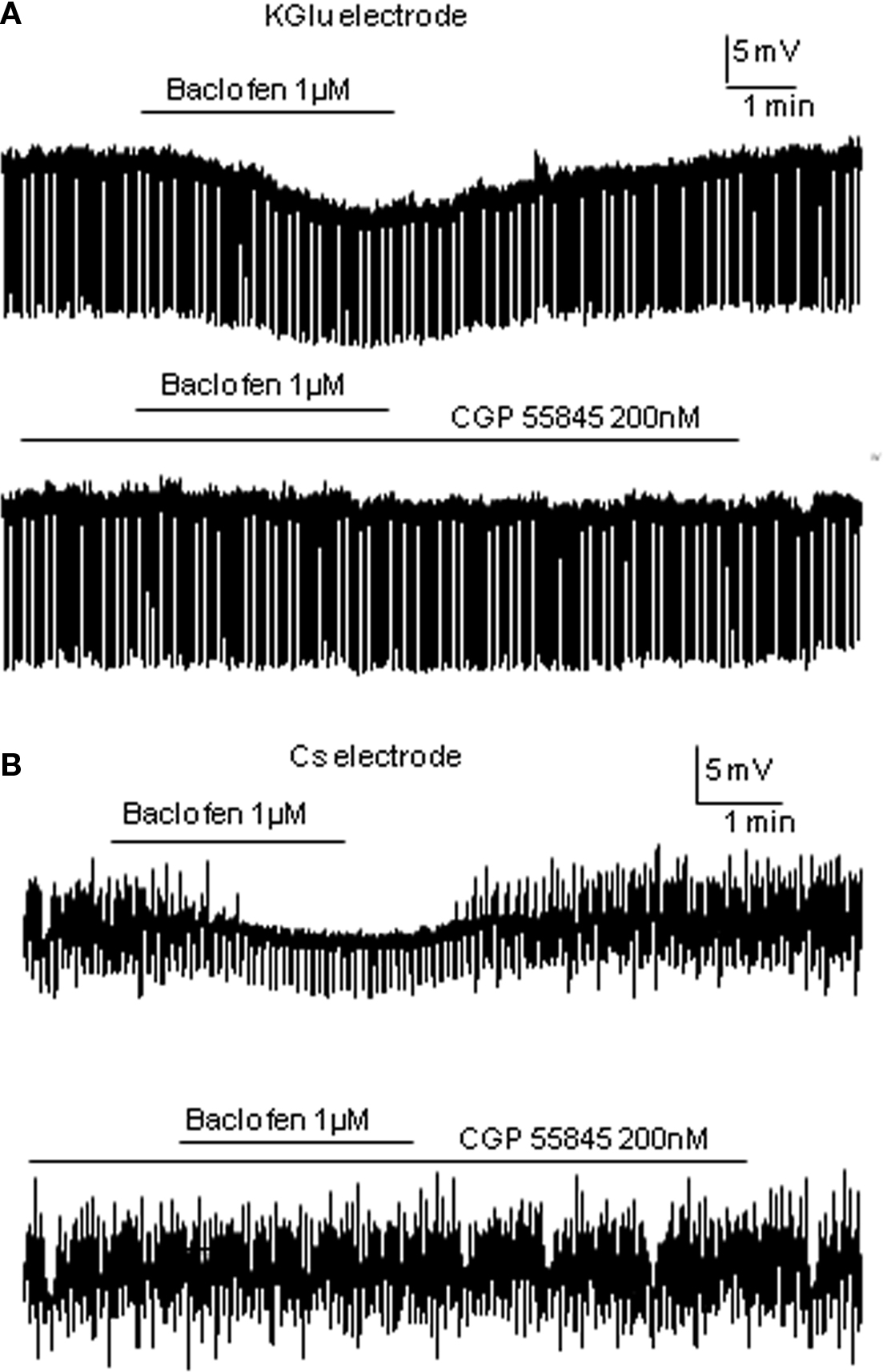
Figure 3. Baclofen responses are antagonized by CGP55845. (A) Using K-Gluconate electrodes, baclofen elicited a hyperpolarization that was antagonized completely by the preincubation of CGP55845. (B) A similar antagonism of baclofen induced hyperpolarizations was observed using TEA/Cs sulfate electrodes.
Of the 18 cells recorded, 13 were strongly coupled with their neighboring cells. In the non-responding group, bath application of baclofen completely blocked all the spikelets in two cells and reduced the frequency of spikelets in one cell. Coupled activities from three other cells stayed unchanged. The remaining two cells of the group only displayed occasional spikelets therefore were not compared. All coupled activities from the seven coupled cells of the small response group were abolished by baclofen application.
The effects of Ca2+-free solution or cadmium chloride (100 μM) was tested on baclofen responses using Cs-based intracellular solution. Both Ca2+-free solutions and CdCl2 significantly reduced the small hyperpolarizing effects of baclofen recorded in Cs intracellular solution from −2.3 ± 0.2 to −0.3 ± 0.1 mV (p < 0.005, n = 8; Figure 2C) with the extent of the blockade in the two situations not significantly different.
Postsynaptic Effects of Low Concentrations of Baclofen on Interneurons
In all five interneurons recorded using K-Gluconate based intracellular solution, bath application of 1 μM baclofen elicited an average membrane potential change to −7.2 ± 0.9 mV (p < 0.005), associated with a decrease in input resistance from 596 ± 102 to 463 ± 102 MΩ (p < 0.05; Figure 4). This indicates an effect of baclofen on GABAB receptors in these cells. By comparing the input resistance changes of the two cell populations (large-response group of SPNs and interneurons), the decrease in input resistance induced by low concentration of baclofen in interneurons is significantly higher than that of SPNs in the IML (p < 0.05).
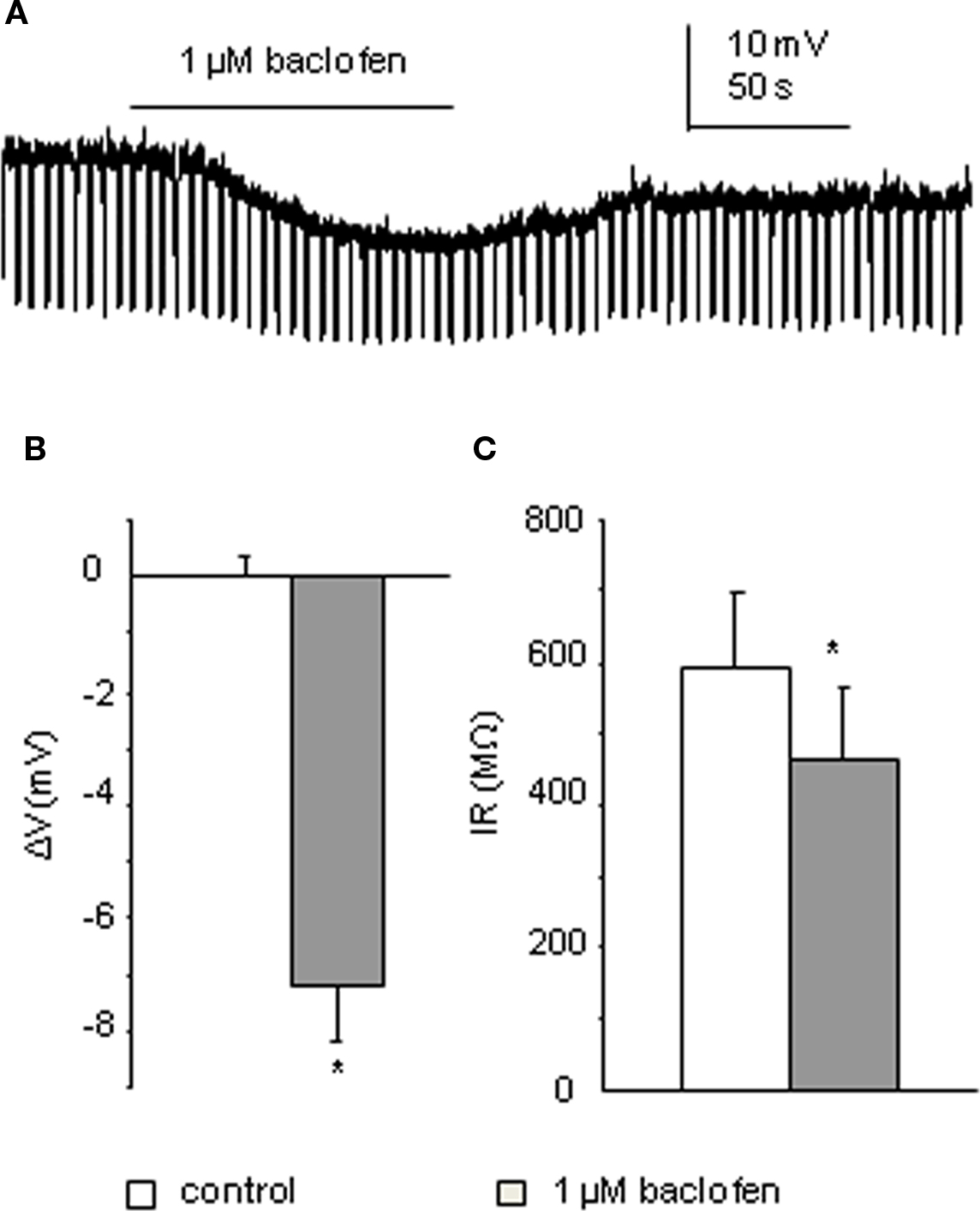
Figure 4. Interneurons also respond to 1 μM baclofen. (A) Bath application of baclofen hyperpolarized this interneuron associated with a decrease in cell input resistance (recorded with K-Gluconate based intracellular solution). (B,C) Pooled data (n = 4) showing membrane potential and input resistance changes before and during drug application. *p < 0.005, Student’s t-test.
GABAB Subunits are Expressed in the IML of Spinal Cord
To further verify the presence in the IML of the different subunits that may contribute to these effects, PCR was performed on punches of IML from spinal cord tissue of the same age, and prepared in the same way, as that used in the electrophysiological experiments.
PCR analysis was performed on cDNA samples taken from the spinal cord and IML. Expression of all GABAB subunits was observed in both the spinal cord and IML samples (n = 4; Figure 5), denoted by the presence of a single band corresponding to the predicted size of each subunit [B1a (319 bp), B1b (290 bp), B2 (319 bp)].
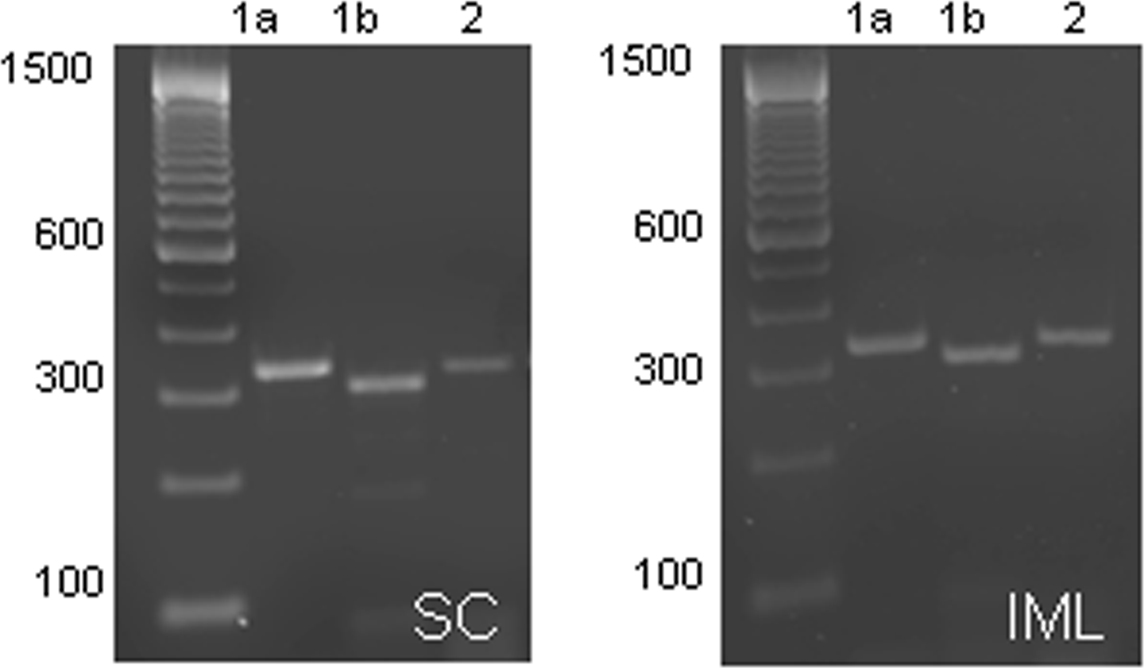
Figure 5. Expression of GABAB subunits in the spinal cord and IML. GABAB receptor subunit mRNA expression in the spinal cord (left panel) and IML (right panel) is denoted by the presence of a single band corresponding to the predicted size of the subunits [B1a (319 bp), B1b (290 bp), B2 (319 bp)]. It is clear that at this age, all three subunits are expressed in the IML.
Presynaptic Effects of Low Concentrations of Baclofen on Gabaergic Synaptic Transmission
In addition to activating at postsynaptic sites, GABAB receptors can also be present on presynaptic terminals as auto- or heteroreceptors. As presented above, blockade of potassium conductance with cesium eliminated the postsynaptic receptor mediated large response by 1 μM baclofen. Under these conditions, regardless of the membrane potential responses, which comprise either no effect or small hyperpolarizations, the whole-cell input resistance remains unchanged. Therefore with Cs intracellular solution, any alterations observed in evoked GABAergic IPSPs would likely be due to a presynaptic modulatory effect. A paired pulse stimulation protocol was used in both Lf and CAA monosynaptic GABAergic pathways in order to monitor the changes in PPR.
Bath application of 1 μM baclofen significantly reduced the peak amplitude of both CAA and Lf-IPSPs. The mean amplitudes of first IPSPs evoked via Lf stimulation were decreased to 75.4 ± 3.9% and the second IPSPs to 83.8 ± 3.9% of control values (p < 0.05, n = 11; Figure 6A). This decrease in amplitude was accompanied by a significant increase in PPR from 1.02 ± 0.0 to 1.16 ± 0.0 (p < 0.05). In four of the cells in which baclofen effects on Lf-IPSPs were observed and fully recovered upon washout, the GABAB receptor antagonist CGP55845 (200 nM) was then applied. Preincubation of CGP55845 alone had no effect on PPR (p > 0.1) or IPSP amplitude (p > 0.1). No significant changes were observed after further addition of baclofen (Figure 6B). These results together indicate a presynaptic depression on Lf-IPSPs due to activation of GABAB autoreceptors. In the CAA pathway, baclofen decreased the peak amplitudes of the first and second CAA-IPSPs to 70.3 ± 5.2% and 82.9 ± 9.4% (Figure 7A; p < 0.05) of control values, respectively with an increase in PPR (from 1.05 ± 0.1 to 1.28 ± 0.1, p < 0.05). The antagonist CGP55845 (200 nM) was used for incubation in four cells after recovery from the first application of baclofen. Bath application of CGP55845 alone had no significant effect on the amplitude or PPR of CAA-IPSPs. This preincubation antagonized the baclofen induced reduction in amplitudes and PPR facilitation (n = 4, p > 0.05; Figure 7B).
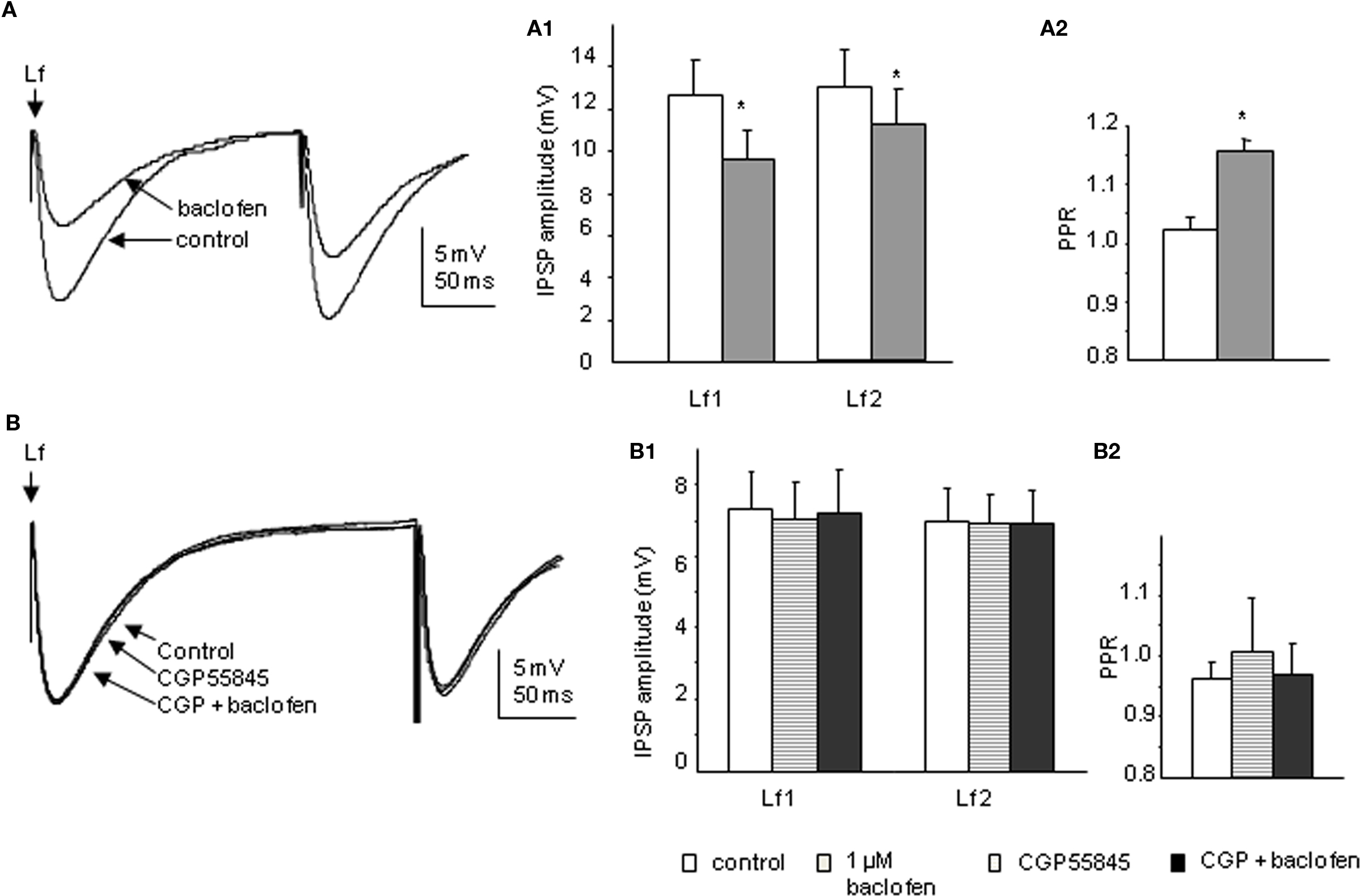
Figure 6. Baclofen decreases the amplitude of Lf-IPSPs elicited in SPNs via a presynaptic site of action. (A) Trace from a neuron with Lf-IPSPs elicited by paired pulse stimulation. Bath application of 1 μM baclofen reversibly decreased the amplitude of both Lf-IPSPs (average of 10 consecutive sweeps). Pooled data (n = 11) showing changes in the amplitude of Lf-IPSPs (A1) and paired pulse ratio (A2) before and during baclofen application. (B) Trace from a neuron with Lf-IPSPs elicited by paired pulse stimulation. Bath application of 200 nM CGP55845 alone did not significantly change the amplitude of Lf-IPSPs (average of 10 consecutive sweeps). Pooled data (n = 4) showing changes in the amplitudes of Lf-IPSPs (B1) and paired pulse ratios (B2). Under control conditions, incubation of CGP55845 and further addition of baclofen. *p < 0.05, Student’s t-test.
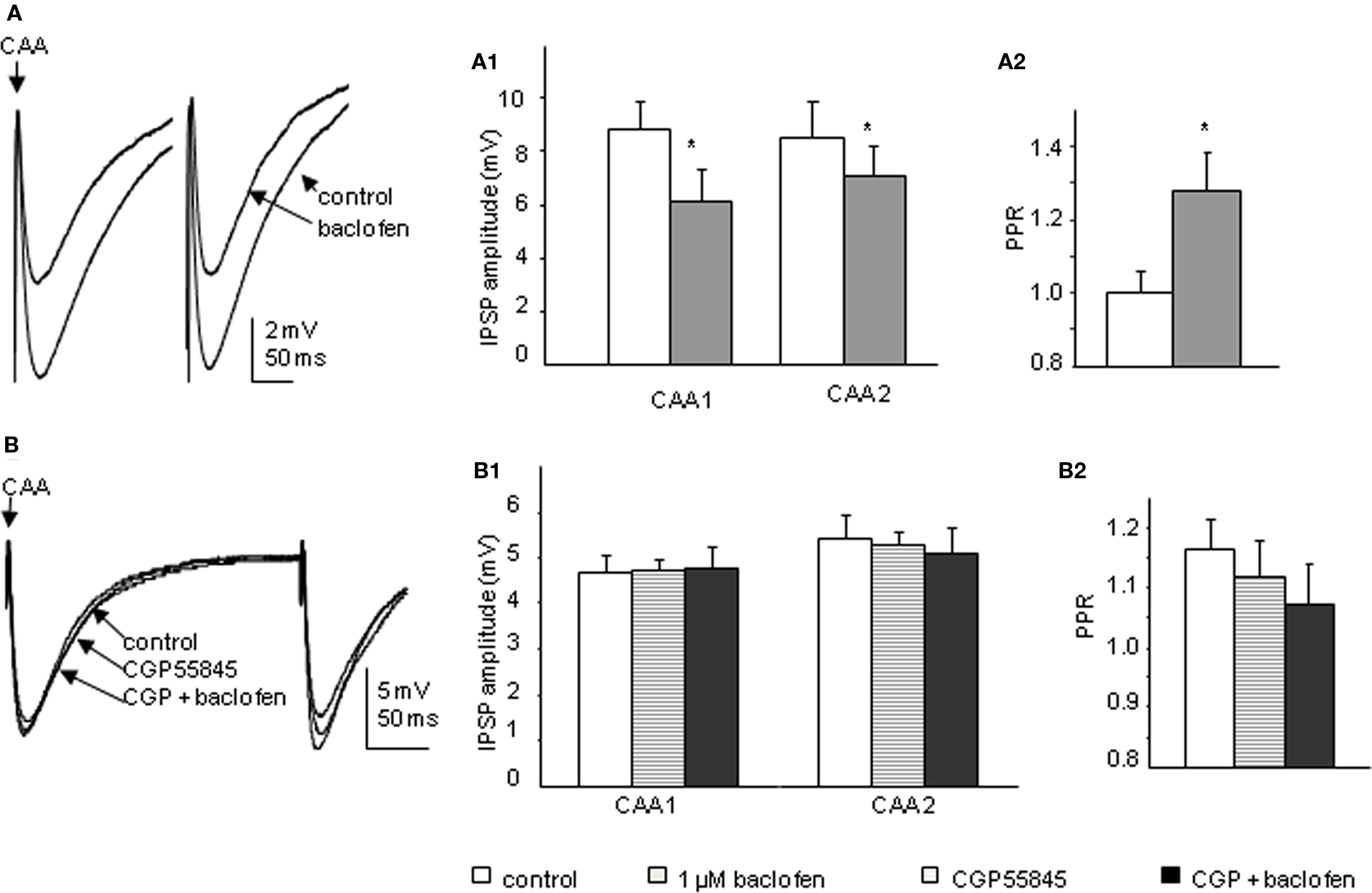
Figure 7. Baclofen decreases the amplitude of CAA-IPSPs elicited in SPNs via a presynaptic site of action. (A) Trace from a neuron with CAA-IPSPs elicited by paired pulse stimulation. Bath application of 1 μM baclofen reversibly decreased the amplitude of CAA-IPSPs (average of 10 consecutive sweeps). Pooled data (n = 13) showing changes in amplitude of CAA-IPSPs (A1) and paired pulse ratio (A2) before and during baclofen application. (B) Trace from a neuron with CAA-IPSPs elicited by paired pulse stimulation. Bath application of 200 nM CGP55845 alone did not significantly change the amplitude of CAA-IPSPs (average of 10 consecutive sweeps). (B1) Pooled data (n = 4) showing changes in the amplitudes of CAA-IPSPs (B1) and paired pulse ratio (B2). Under control conditions, incubation of CGP55845 and further addition of baclofen. *p < 0.05, Student’s t-test.
The sensitivity of evoked IPSPs to GABAA receptor antagonists was examined after blocking polysynaptic transmission and ionotropic glutamate receptors by kynurenic acid. Consistent with previous studies (Deuchars et al., 1997, 2005; Brooke et al., 2004), both Lf- and CAA-IPSPs were reduced in amplitude after bath application of 10 μM bicuculline (data not shown). The sensitivity to a GABAA receptor antagonist suggests the nature of these IPSPs to be GABAergic.
Spontaneous IPSPs were observed in two SPNs recorded. Bath application of 1 μM baclofen dramatically reduced the frequency of sIPSPs in both cells, leaving only a few detectable events (Figure 8). Further detailed analysis was not performed since the significant drug effect did not leave enough events to be compared. However, the spectacular decrease in the number of IPSPs also signifies a presynaptic site of action.
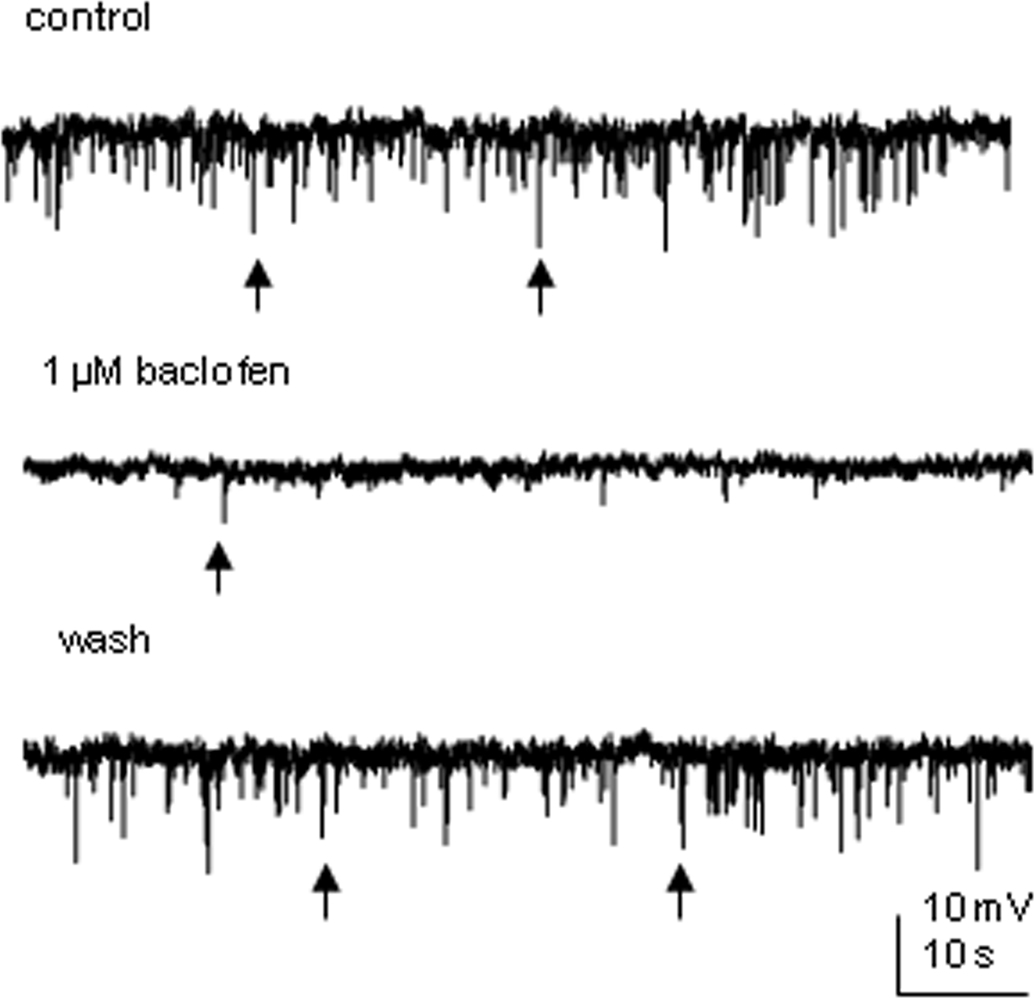
Figure 8. Baclofen decreases the frequency of spontaneous IPSPs in SPNs. An example from an SPN where bath application of 1 μM baclofen reversibly decreased the frequency of spontaneous IPSPs. Arrows indicate samples of synaptic events.
Discussion
There are two main observations from this study; firstly that activation of presynaptic GABAB autoreceptors caused attenuation of inhibitory postsynaptic potentials elicited by both Lf and CAA pathways. This suggests that there are GABAB autoreceptors located on terminals of both descending and local neurons influencing SPN activity. Secondly, very low concentrations of baclofen induced a number of responses in SPNs and also hyperpolarized interneurons – differences in responses may be due to the second messenger systems mediating these responses.
Presynaptic Modulation by GABAB Autoreceptors
Bath application of the GABAB receptor agonist baclofen decreased the amplitudes of evoked IPSPs in SPNs elicited from both GABAergic pathways. The reduction was accompanied by an increased PPR which indicates a presynaptic site of action, mediated through an inhibition of GABA release. Preincubation of the antagonist CGP55845 attenuated the agonist induced effects. The likelihood that this was a presynaptic action was strengthened by the observation that the frequency of spontaneous IPSPs, where observed, was also reduced by baclofen.
The concept of GABAB receptors serving as autoreceptors located at the GABAergic terminals in the CNS is well known. When activated, GABAB receptors cause inhibition of high voltage sensitive calcium channels (Mintz and Bean, 1993; Poncer et al., 1997) or can have a direct effect on synaptic vesicle priming (Sakaba and Neher, 2003) thus reducing neurotransmitter release. In our study, IPSPs elicited from Lf and CAA were affected to a similar degree by both the agonist baclofen (at low concentrations) and a broad spectrum antagonist CGP55845, indicating that the regulation of the two pathways is mediated through GABAB receptors with comparable pharmacological properties. GABAB receptors are composed of two subunits (GABAB1 and GABAB2) that form functional heterodimers (Marshall et al., 1999) and GABAB1 subunits can be further divided into GABAB1a and GABAB1b (Kaupmann et al., 1997; Kaupmann et al., 1998). In fact evidence suggests that presynaptic GABAB receptors are mainly comprised of GABAB1a/GABAB2 subunits (Billinton et al., 1999; Towers et al., 2000), so pharmacological differences would be unexpected. Hippocampal studies have reported that presynaptic GABAB receptors with similar properties are located on both stratum radiatum and stratum oriens interneurons, whilst mGluR receptors are limited to synaptic inputs from stratum oriens interneurons (Poncer et al., 2000). This further supports the idea that presynaptic GABAB receptors are quite homogenous. Stimulating within the CAA activates GABAergic interneurons located within this region (Deuchars et al., 2005) and this area receives dense descending inputs from the medial prefrontal cortex. This region of the cortex is a defined vasomotor center, contributing a hypotensive influence (Bacon and Smith, 1993) and this effect may be manifested through direct activation of these local inhibitory GABAergic interneurons in the spinal cord that consequently inhibit SPNs. LF stimulation activates direct descending inhibitory pathways from regions such as the rostral ventrolateral medulla (Miura et al., 1994; Deuchars et al., 1997). Activation of presynaptic GABAB receptors will reduce both the descending and local inhibitory influences on sympathetic outflow, regardless of their source or their target SPN. It seems that the heterogeneity is observed with the postsynaptic, rather than the presynaptic receptors onto SPN.
Presynaptic GABAB Receptors are Not Tonically Activated
The possibility that autoreceptors can be tonically activated at GABAergic terminals onto SPNs was examined in this study. Preincubation of GABAB receptor antagonist CGP55845 had no significant effect on evoked IPSPs from both pathways. Thus, the autoreceptors located at the GABAergic terminals innervating SPNs are unlikely to be stimulated by ambient GABA, even after synaptic release. Physiological stimulation of GABAB receptors seems to require strong synaptic stimulation which suggests that high GABA releases are required to reach and thus stimulate the receptors (Isaacson et al., 1993; Scanziani, 2000). This is likely due to the efficient uptake systems in place to remove GABA since uptake inhibitors increase the likelihood of activation of the GABAB receptors (Isaacson et al., 1993; Scanziani, 2000). The absence of tonic GABAB activation on SPNs has also been reported using a splanchnic nerve–spinal cord preparation (Cheng et al., 2005).
Distinct Postsynaptic Responses to Low Concentrations of Baclofen in SPNs
Postsynaptic GABAB receptors are predominantly coupled to G protein coupled inward rectifying potassium channels (GIRKs) and therefore cause an inhibitory effect on the cell by increasing K+ conductance. However, not all SPNs exhibited a typical large response after challenge with low concentrations of baclofen. Indeed, many exhibited much smaller responses that were not accompanied by changes in input resistance. One possible explanation for the more moderate responses observed in some SPNs is that the SPN itself is not affected directly by GABAB receptors located on its postsynaptic membrane but that the hyperpolarization is mediated by an effect on neighboring SPNS that are coupled by gap junctions to the recorded neuron and this hyperpolarization is relayed through the gap junctions. However, in non-responding neurons, spikelets were also observed that were reduced or abolished by application of baclofen with no accompanying change in membrane potential. This therefore makes the theory of the moderate response being due to conduction of hyperpolarizing responses from neighboring neurons less likely, although it cannot be totally discounted since some spikelets do remain.
Our data demonstrate that these moderate hyperpolarizations to low concentrations of baclofen were observed in some SPNs whether recordings were made either in K+ based or Cs-based intracellular solution. The moderate response is likely to be inhibition of voltage gated calcium channels since nominally Ca2+ free solution or cadmium further reduced this effect. This is similar to responses observed by (Cheng et al., 2005), who used higher concentrations of baclofen. We cannot rule out the possibility that the Ca2+ activated K+ channels contribute to the moderate response observed since these may not be blocked completely with the potassium blockers used, especially in more distal regions of the neuron where complete dialysis of the neuronal content is unlikely to be achieved. However in support of a role of calcium channels, recent studies (Vigot et al., 2006; Perez-Garci et al., 2006) using knockout mice (either 1b−/− or 1a−/−) concluded that activation of GABAB1(b) containing postsynaptic receptors directly blocks dendritic Ca2+ channels of layer 5 of cortical pyramidal neurons.
The distinct responses may reflect activation of different postsynaptic receptor subtypes or association with distinct second messenger systems. Such a suggestion is supported by data from substantia nigral cells that showed GABAB receptors linked to different GIRK channels (Koyrakh et al., 2005). Multiple subtypes of GABAB receptors have been suggested after observing the effects of different selective antagonists (Solis and Nicoll, 1992; Cunningham and Enna, 1996). Moreover, two types of pharmacologically distinct receptors were used to explain the residual responses of thalamocortical neurons mediated by baclofen (Guyon and Leresche, 1995). In fact evidence suggests that it is the GABAB1a/2 complex that exhibits the greater sensitivity to low concentrations of baclofen in the hippocampus that may be due mainly to differences in the density of those receptors over GABAB1b/2 complexes, rather than differences in their affinity (Guetg et al., 2009). Thus hyperpolarizations observed during application of 1 μM baclofen in some cells could be mediated through preferential activation of receptors present in a higher density. There are developmental changes in GABAB receptor subunits since GABAB1(a) is predominant during embryonic period and at birth whilst GABAB1(b) expression increases during the first postnatal month in the brain (Fritschy et al., 1994). Our PCR results show that all three subunits are in fact present at the age that we carried out the experiments, so absence of a subunit may not be a contributing factor to our findings. A detailed study of receptor subunit composition and subcellular localization would provide first hand information to understand whether they are related to the pharmacological distinctions observed. For example, GABAB1(b) subunit containing receptors have been reported to be present extrasynaptically in cerebellar Purkinje cells (Fritschy et al., 1999), dorsal cochlear nucleus (Lujan et al., 2004) and hippocampal CA1 pyramidal neurons (Kulik et al., 2006).
Functional Significance and Conclusion
The results of activation of GABAB receptors in sympathetic regulation are complicated and sometimes contradictory. Due to the multiple functions of GABAB receptors, it is not surprising that different experimental protocols lead to completely different conclusions. For example, intrathecal injection of baclofen in the thoracic spinal cord decreases cardiac sympathetic activity (Kim et al., 2000) whilst intracerebroventricular or intraperitoneal administration of baclofen leads to an increase in blood pressure and heart rate (Persson and Henning, 1980). In a spinal cord–splanchnic nerve preparation, bath application of baclofen suppresses splanchnic nerve activities possibly via two mechanisms: direct inhibition of membrane excitability by affecting K+ or Ca2+ currents and/or attenuation of excitatory synaptic events to SPNs (Cheng et al., 2005).
In this study, the effects of stimulation of postsynaptic receptors and presynaptic autoreceptors in SPNs were studied. Although pharmacologically distinct subtypes of postsynaptic GABAB receptors have not been proven, the fact that hyperpolarization was only observed in some SPNs (and possibly all interneurons) of the IML when using low concentrations of agonist might suggest a physiological significance underlying these differences in sensitivity. Indeed many previous studies examining characteristics of SPNs have reported differences in spontaneous activity (Spanswick and Logan, 1990), electrical coupling (Logan et al., 1996), other intrinsic membrane properties (Zimmerman and Hochman, 2010) and the synaptic inputs that they receive (Deuchars et al., 1997; Deuchars et al., 1995), that may reflect functional subgrouping of SPNs. Our study further supports this idea with distinct groups of SPN perhaps possessing specific GABAB receptors and suggests that such a difference may be a useful pharmacological target in the future. Presynaptic GABAB receptors regulating the sympathetic outflow at the spinal levels have been previously reported but mainly focused on its action at the excitatory nerve terminals (McKenna and Schramm, 1984; Wu and Dun, 1992). This study provides evidence that activation of GABAB autoreceptors suppresses GABAergic inhibitory transmission. The regulation of two distinct GABAergic pathways shows similar characteristics. These results somewhat improve the current knowledge on the function of GABAB receptors in sympathetic control and lend strength to the idea that these autoreceptors at least would not present a suitable target for differential control of the two pathways.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the British Heart Foundation (Grant No. PG/2001119 and PG/08/120/26338, Susan A. Deuchars and Jim Deuchars) and RCUK for their generous support. We also thank Brenda Frater for her skilled technical contribution.
References
Bacon, S., J., and Smith, A. D. (1993). A monosynaptic pathway from an identified vasomotor centre in the medial prefrontal cortex to an autonomic area in the thoracic spinal cord. Neuroscience 54, 719–728.
Billinton, A., Upton, N., and Bowery, N. G. (1999). GABA(B) receptor isoforms GBR1a and GBR1b, appear to be associated with pre- and post-synaptic elements respectively in rat and human cerebellum. Br. J. Pharmacol. 126, 1387–1392.
Bonanno, G., and Raiteri, M. (1992). Functional evidence for multiple gamma-aminobutyric acidB receptor subtypes in the rat cerebral cortex. J. Pharmacol. Exp. Ther. 262, 114–118.
Bowery, N. G. (2006). GABAB receptor: a site of therapeutic benefit. Curr. Opin. Pharmacol. 6, 37–43.
Brooke, R. E., Deuchars, J., and Deuchars, S. A. (2004). Input-specific modulation of neurotransmitter release in the lateral horn of the spinal cord via adenosine receptors. J. Neurosci. 24, 127–137.
Brooks, P. A., and Glaum, S. R. (1995). GABAB receptors modulate a tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat nucleus tractus solitarii in vitro. J. Auton. Nerv. Syst. 54, 16–26.
Brooks, P. A., Glaum, S. R., Miller, R. J., and Spyer, K. M. (1992). The actions of baclofen on neurons and synaptic transmission in the nucleus tractus solitarii of the rat in vitro. J. Physiol. (Lond.) 457, 115–129.
Cheng, Y. W., Ku, M. C., Ho, C. M., Chai, C. Y., and Su, C. K. (2005). GABAB-receptor-mediated suppression of sympathetic outflow from the spinal cord of neonatal rats. J. Appl. Physiol. 99, 1658–1667.
Cunningham, M. D., and Enna, S. J. (1996). Evidence for pharmacologically distinct GABAB receptors associated with cAMP production in rat brain. Brain Res. 720, 220–224.
Deuchars, S. A., Brooke, R. E., and Deuchars, J. (2001a). Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J. Neurosci. 21, 6308–6320.
Deuchars, S. A., Brooke, R. E., Frater, B., and Deuchars, J. (2001b). Properties of interneurons in the intermediolateral cell column of the rat spinal cord: role of the potassium channel subunit Kv3.1. Neuroscience 106, 433–446.
Deuchars, S. A., Milligan, C. J., Stornetta, R. L., and Deuchars, J. (2005). GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J. Neurosci. 25, 1063–1070.
Deuchars, S. A., Morrison, S. F., and Gilbey, M. P. (1995). Medullary-evoked EPSPs in neonatal rat sympathetic preganglionic neurons in vitro. J. Physiol. (Lond.) 487, 453–463.
Deuchars, S. A., Spyer, K. M., and Gilbey, M. P. (1997). Stimulation within the rostral ventrolateral medulla can evoke monosynaptic GABAergic IPSPs in sympathetic preganglionic neurons in vitro. J. Neurophysiol. 77, 229–235.
Fritschy, J. M., Meskenaite, V., Weinmann, O., Honer, M., Benke, D., and Mohler, H. (1999). GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci. 11, 761–768.
Fritschy, J. M., Paysan, J., Enna, A., and Mohler, H. (1994). Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J. Neurosci. 14, 5302–5324.
Guetg, N., Seddik, R., Vigot, R., Turecek, R., Gassmann, M., Vogt, K. E., Bräuner-Osborne, H., Shigemoto, R., Kretz, O., Frotscher, M., Kulik, A., and Bettler, B. (2009). The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J. Neurosci. 29, 1414–1423.
Guyon, A., and Leresche, N. (1995). Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurons. J. Physiol. 485, 29–42.
Isaacson, J. S., Solis, J. M., and Nicoll, R. A. (1993). Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10, 165–175.
Kaupmann, K., Huggel, K., Heid, J., Flor, P. J., Bischoff, S., Mickel, S. J., McMaster, G., Angst, C., Bittiger, H., Froestl, W., and Bettler, B. (1997). Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246.
Kaupmann, K., Malitschek, B., Schuler, V., Heid, J., Froestl, W., Beck, P., Mosbacher, J., Bischoff, S., Kulik, A., Shigemoto, R., Karschin, A., and Bettler, B. (1998). GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687.
Kim, B. S., Koh, H. C., Kang, J. S., Lee, H., Shin, I. C., Om, S. A., and Kang, J. H. (2000). Mediation of the cardiovascular response to spinal gamma-aminobutyric acid(B) receptor stimulation by adenosine A(1) receptors in anesthetized rats. Neurosci. Lett. 296, 153–157.
Koyrakh, L., Lujan, R., Colon, J., Karschin, C., Kurachi, Y., Karschin, A., and Wickman, K. (2005). Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J. Neurosci. 25, 11468–11478.
Kulik, A., Vida, I., Fukazawa, Y., Guetg, N., Kasugai, Y., Marker, C. L., Rigato, F., Bettler, B., Wickman, K., Frotscher, M., and Shigemoto, R. (2006). Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J. Neurosci. 26, 4289–4297.
Logan, S. D., Pickering, A. E., Gibson, I. C., Nolan, M. F., and Spanswick, D. (1996). Electrotonic coupling between rat sympathetic preganglionic neurons in vitro. J. Physiol. (Lond.) 495, 491–502.
Lujan, R., Shigemoto, R., Kulik, A., and Juiz, J. M. (2004). Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the rat. J. Comp. Neurol. 475, 36–46.
Marshall, F. H., White, J., Main, M., Green, A., and Wise, A. (1999). GABA(B) receptors function as heterodimers. Biochem. Soc. Trans. 27, 530–535.
McKenna, K. E., and Schramm, L. P. (1984). Baclofen inhibits sympathetic preganglionic neurons in an isolated spinal cord preparation. Neurosci. Lett. 47, 85–88.
Mintz, I. M., and Bean, B. P. (1993). GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron 10, 889–898.
Miura, M., Takayama, K., and Okada, J. (1994). Distribution of glutamate- and GABA-immunoreactive neurons projecting to the cardioacceleratory center of the intermediolateral nucleus of the thoracic cord of SHR and WKY rats: a double-labeling study. Brain Res. 638, 139–150.
Nolan, M. F., Logan, S. D., and Spanswick, D. (1999). Electrophysiological properties of electrical synapses between rat sympathetic preganglionic neurons in vitro. J. Physiol. (Lond.) 519 Pt 3, 753–764.
Perez-Garci, E., Gassmann, M., Bettler, B., and Larkum, M. E. (2006). The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron 50, 603–616.
Persson, B., and Henning, M. (1980). Central cardiovascular and biochemical effects of baclofen in the conscious rat. J. Pharm. Pharmacol. 32, 417–422.
Poncer, J. C., McKinney, R. A., Gahwiler, B. H., and Thompson, S. M. (1997). Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron 18, 463–472.
Poncer, J. C., McKinney, R. A., Gahwiler, B. H., and Thompson, S. M. (2000). Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J. Physiol. (Lond.) 528 Pt 1, 123–130.
Sakaba, T., and Neher, E. (2003). Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature 424, 775–778.
Scanziani, M. (2000). GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25, 673–681.
Solis, J. M., and Nicoll, R. A. (1992). Pharmacological characterization of GABAB-mediated responses in the CA1 region of the rat hippocampal slice. J. Neurosci. 12, 3466–3472.
Spanswick, D., and Logan, S. D. (1990). Spontaneous rhythmic activity in the intermediolateral cell nucleus of the neonate rat thoracolumbar spinal cord in vitro. Neuroscience 39, 395–403.
Spary, E. J., Maqbool, A., Saha, S., and Batten, T. F. (2008). Increased GABA(B) receptor subtype expression in the nucleus of the solitary tract of the spontaneously hypertensive rat. J. Mol. Neurosci. 35, 211–224.
Towers, S., Princivalle, A., Billinton, A., Edmunds, M., Bettler, B., Urban, L., Castro-Lopes, J., and Bowery, N. G. (2000). GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur. J. Neurosci. 12, 3201–3210.
Vigot, R., Barbieri, S., Brauner-Osborne, H., Turecek, R., Shigemoto, R., Zhang, Y. P., Lujan, R., Jacobson, L. H., Biermann, B., Fritschy, J. M., Vacher, C. M., Muller, M., Sansig, G., Guetg, N., Cryan, J. F., Kaupmann, K., Gassmann, M., Oertner, T. G., and Bettler, B. (2006). Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50, 589–601.
Wang, L., Spary, E., Deuchars, J., and Deuchars, S. A. (2008). Tonic GABAergic inhibition of sympathetic preganglionic neurons: a novel substrate for sympathetic control. J. Neurosci. 28, 12445–12452.
Whyment, A. D., Wilson, J. M., Renaud, L. P., and Spanswick, D. (2004). Activation and integration of bilateral GABA-mediated synaptic inputs in neonatal rat sympathetic preganglionic neurons in vitro. J. Physiol. (Lond.) 555, 189–203.
Wu, S. Y., and Dun, N. J. (1992). Presynaptic GABAB receptor activation attenuates synaptic transmission to rat sympathetic preganglionic neurons in vitro. Brain Res. 572, 94–102.
Keywords: sympathetic, spinal cord, interneuron, GABAB, baclofen, electrophysiology
Citation: Wang L, Bruce G, Spary E, Deuchars J and Deuchars SA (2010) GABAB mediated regulation of sympathetic preganglionic neurons: pre- and postsynaptic sites of action. Front. Neur. 1:142. doi: 10.3389/fneur.2010.00142
Received: 22 July 2010;
Accepted: 17 October 2010;
Published online: 11 November 2010.
Edited by:
James Brock, University of Melbourne, AustraliaCopyright: © 2010 Wang, Bruce, Spary, Deuchars and Deuchars. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Susan A. Deuchars, Institute of Membrane and Systems Biology, University of Leeds, Leeds LS2 9JT, UK. e-mail: s.a.deuchars@leeds.ac.uk
